Abstract
Early life predictors of attention-deficit/hyperactivity disorder (ADHD) are critically needed; they could inform etiological theory and may help identify new prevention targets. The current study examined prospectively whether maternal cytokine levels during pregnancy predict offspring ADHD symptoms at age 4–6 years. Secondarily, we evaluated maternal cytokine levels as a possible common pathway through which prenatal risks exert influence on child ADHD. Data came from a sample of women recruited during the 2nd trimester of pregnancy (N=62) and followed postnatally until children were 4–6 years old. Maternal inflammation was assessed using 3rd trimester plasma concentrations of three indicators of nuclear factor kappa B signaling: interleukin-6, tumor necrosis factor-alpha, and monocyte chemoattractant protein-1 which were combined into a latent variable. Mothers and teachers reported on child ADHD symptoms, negative affect, and externalizing behaviors at 48–72 months of age. Maternal inflammation in the 3rd trimester predicted ADHD symptoms when children were 4–6 years old (β=0.53, 95% CI=0.154, 0.905, p=0.006). Further, maternal inflammation mediated the effect of prenatal distress on child ADHD (β=0.21, 95% CI=0.007, 0.419, p=0.04). The inflammation effect on ADHD was not explained by concurrent child negative affect, externalizing behavior, or familial ADHD status. This is the first human study to prospectively link maternal pregnancy cytokine levels and offspring ADHD symptoms, suggesting that cytokine levels are a possible marker of ADHD risk. Results also provide new evidence that maternal prenatal inflammation may be one common pathway by which prenatal risk factors influence offspring mental health outcomes.
Keywords: ADHD, Prenatal Distress, Inflammation, Cytokines
1. Introduction
Attention-deficit/hyperactivity disorder (ADHD) is hypothesized to have origins in early life, perhaps as early as prenatal development, influenced by both genetic and environmental factors (Nigg et al., 2010); emerging theories suggest prenatal origins and programming may be powerful factors in a range of psychopathologies (Monk et al., 2019). Candidate early risk markers for ADHD (and possibly other conditions) include maternal prenatal psychological distress (Grizenko et al., 2012; Ronald et al., 2011), maternal dietary intake, and maternal adiposity (Bolton and Bilbo, 2014; Rodriguez, 2010). Paternal pre-conception exposures, though not studied here to limit scope, may also be important (Chan et al., 2017; Yuan et al., 2016). Discovery of mechanisms associated with these early risks that might themselves predict offspring psychopathology, in this case ADHD, could open the door to earlier identification and prevention.
Inflammation, particularly involving cytokine function, is one critical yet understudied candidate mechanism (Bilbo and Schwarz, 2009; Dunn et al., 2019), though it operates in conjunction with associated systems, including glucocorticoid function (Osborne et al., 2018; Pariante, 2017). Whereas inflammation involves multiple molecule types, there is substantial theoretical and empirical justification for focusing on cytokines (Bilbo and Schwarz, 2009; Rees and Harding, 2004; Wadhwa, 2005). Maternal-fetal cytokine levels influence offspring brain development and behavior (Deverman and Patterson, 2009; Mehler and Kessler, 1998) and, suggestively, seem to be related in humans to infant negative affect (Gustafsson et al., 2018), toddler inhibition (Graham et al., 2018) and working memory (Rudolph et al., 2018). However, their association to child ADHD is yet untested.
A prospective association between maternal prenatal cytokine levels and child ADHD symptoms would suggest that maternal cytokines during pregnancy may be an etiological pathway as well as a potential marker of risk for future child ADHD. Because cytokine levels may be a “common pathway” for multiple risk factors, we modeled it with three common maternal risk factors—maternal distress, adiposity, and low circulating omega-3 levels. The current study examined maternal interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α) and monocyte chemoattractant protein-1 (MCP-1) concentrations. These cytokines were selected a priori because they each index the nuclear factor-κappa B signaling pathway (Liu et al., 2017) and are previously reported as elevated in individuals with neurodevelopmental and psychiatric disorders (Donfrancesco et al., 2016; Müller and Ackenheil, 1998). Sensitivity analyses considered whether the cytokine-ADHD association was robust to three of the most common potential confounds: child externalizing symptoms, concurrent child mood, and familial ADHD status. We secondarily checked whether similar patterns emerge when considering available teacher-report of child symptoms.
2. Method
2.1. Procedure
Data came from a previously described longitudinal cohort (Gustafsson et al., 2018; Sullivan et al., 2015). Pregnant women were recruited from an urban hospital-based outpatient clinic in their 2nd trimester. Women who reported a family history of ADHD on a routine clinic health screener were over-selected to maximize the range of offspring ADHD symptoms and thus statistical power. See Online Supplement A for additional information about participant recruitment and enrollment. We followed 68 children from 62 women (five sibling pairs and one sibling trio, relatedness handled statistically) from the 2nd trimester of pregnancy until the child was 48–72 months old. Exclusion criteria included high-risk or medically complicated pregnancy, extreme life circumstances (specifically, homelessness), being <18 years old, and active substance use (including alcohol, tobacco, marijuana, opioids, cocaine).
At 24- and 37-weeks of gestation, women completed questionnaire measures of their mood, subjective stress, and demographics and provided a blood sample. Outcome data were obtained when children were 48, 60, and/or 72 months old by parent-report and teacher-report, when available. All procedures were approved by the local Institutional Review Board and was carried out in accordance with the Code of Ethics of the World Medical Association. Parents provided written informed consent.
2.2. Measures
Mothers completed interviews and questionnaires that are widely used to measure prenatal distress and child behavior. The measures are noted in 2.2.1 and 2.2.2 and are summarized in Table S1. To maximize statistical power, latent variables and composite variables were formed when possible (Hoyle, 1995). Figure S1 provides model fit details for the latent variables described in 2.2.1 and 2.2.2.
2.2.1. Prenatal measures
2.2.1.1. Maternal distress
At the 2nd and 3rd trimester visits, mothers completed the Center for Epidemiologic Studies-Depression Scale (CES-D) (Radloff, 1977) and the Perceived Stress Scale (PSS) (Ezzati et al., 2014). CES-D and PSS scores did not change significantly over these two time points (t=0.37, p=.071 and t=1.34, p=0.19, respectively) and averaged CES-D and PSS scores were highly correlated (r=0.81). Thus, despite their partial conceptual distinction, they were standardized and averaged to create a composite prenatal distress score (α=0.83).
2.2.1.2. Maternal omega-3 fatty acid concentrations
Maternal circulating omega-3 fatty acids (concentrations of eicosapentaenoic, docosahexaenoic, and alpha-linolenic acid, all in nmol/ml) were assessed using blood samples collected in the 3rd trimester. Blood was drawn by venipuncture, centrifuged, and plasma was separated, aliquoted, and frozen at −80°C until assayed. Plasma FAs were analyzed by direct transesterification using a Trace GC coupled to a DSQ mass spectrometer (ThermoElectron) as described previously (Lagerstedt et al., 2001). Maternal concentrations of eicosapentaenoic acid, docosahexaenoic acid, and alpha-linolenic acid (all in nmol/ml) were summed to create a total omega-3 fatty acid score. For interpretive simplicity, omega-3 fatty acid values were reverse scored prior to analyses, such that higher values were expected to be correlated with higher levels of ADHD symptoms.
2.2.1.3. Maternal adiposity
Participants’ medical records were reviewed for maternal pre-pregnancy body mass index (BMI) (kg/m2) which was recorded.
2.2.1.4. Maternal inflammation
Maternal inflammation was assessed using plasma concentrations of IL-6, TNF-α and MCP-1.Blood was drawn by one-time venipuncture into K2 EDTA tubes (BD Vacutainer Systems, Franklin Lakes, NJ). The blood was then centrifuged at 2800 rpm for 15 min at 5° C. Plasma was separated, collected, and immediately aliquoted into 2 ml polypropylene tubes (United Laboratory Plastics, St. Louis, MO) and frozen at −80° C until assayed. To minimize participant burden, study visits and blood draws were scheduled around participant’s regular prenatal medical appointments. Blood samples, therefore, were not necessarily able to be drawn at the same time of day for all participants.
Plasma concentrations of IL-6 were measured by enzyme-linked immunosorbent assays (Human IL-6 quantikine HS ELISA kits (HS600B; assay range: 0.2 – 10 pg/ml, sensitivity: 0.11 pg/ml), R&D Systems, Inc., Minneapolis, MN) according to the manufacturer’s instructions. All standards and samples were run in duplicate. Briefly, plasma samples were initially diluted 1:2 using Assay Diluent RD1–75 and incubated for 2 h at room temperature on a horizontal orbital microplate shaker. Following standard wash procedures, human IL-6 HS Conjugate was added to each well, and plates were incubated as described above. Plates were then washed and incubated with the Substrate Solution (60 min), Amplifier Solution (30 min), and Stop Solution. Plates were read within 30 min of adding the Stop Solution using a microplate spectrophotometer (Benchmark Plus microplate, Bio-Rad Laboratories, Inc., Hercules, CA).
Plasma concentrations of TNF-α (assay range: 5.3 – 3,900 pg/ml, sensitivity: 1.5 pg/ml) and MCP-1 (assay range: 32.0–23,500 pg/ml, sensitivity: 0.47 pg/ml) were assayed using Luminex polystyrene bead-based multiplex immunoassays (customized Luminex Performance Human Obesity Panel, FCST08–05; R&D Systems) according to the manufacturer’s instructions. All standards and samples were run in duplicate. Plasma samples were initially diluted 1:2 using the matrix solution provided, and samples were incubated overnight at 4 °C with color-coded beads that were pre-coated with cytokine-specific capture antibodies. The plates were then washed by vacuum filtration, incubated with biotinylated detection antibodies (1 h, room temperature), washed, and incubated with phycoerythrin-conjugated streptavidin (30 min, room temperature). Plates were read on the dual-laser, flow-based Luminex 100 Analyzer (Luminex, Austin, TX). For both the ELISAs and multiplex assays, sample values were determined based on standard curves calculated using computer software to generate four- and five-parameter curve-fits, respectively (Prism 7 for Windows, GraphPad Software, Inc., La Jolla, CA).
Cytokine values were log transformed to correct for non-normality and combined into a latent variable as described in Figure S1.
2.2.2. Postnatal measures
When children were 48, 60, and/or 72 months old, mothers completed questionnaires and an interview as indicated in 2.2.2.1 and 2.2.2.2. For each measure, scores were standardized within each time point and averaged to create a composite score. When possible (i.e., if there were three or more measures of the same construct), we created latent variables. When that was not feasible (i.e., fewer than three measures) we standardized scores within each time point and averaged multiple measures of the same construct to create reliable single aggregate scores for each construct.
2.2.2.1. Child ADHD symptoms
At one time between 48–72 months of child age (M=62.5 months, SD=10.2 months), a parent completed the early childhood version of the Kiddie Schedule for Affective Disorders and Schizophrenia (KSADS-EC) (Gaffrey and Luby, 2012). At 48, 60, and/or 72 months mothers completed the Strengths and Difficulties Questionnaire (SDQ) (Goodman, 2001), the ADHD Rating Scale (ADHD-RS) (DuPaul et al., 1998), and the Strengths and Weaknesses of ADHD Symptoms and Normal Behavior Rating Scale (SWAN) (Lakes et al., 2012). A dimensional latent variable for ADHD symptoms was created from KSADS-EC ADHD total, SDQ Hyperactivity scale, ADHD-RS Total score, and the Total score on the SWAN.
2.2.2.2. Covariates and secondary measures
Concurrent child externalizing behaviors were evaluated at 48–72 months with a latent variable that combined oppositional defiant disorder symptoms from the KSADS-EC, SDQ Conduct Problems (administered at 48, 60, and 72 months), and the Child Behavior Checklist (at 48 and 60 months) (Achenbach and Ruffle, 2000) Aggression scales. Concurrent child negative mood was evaluated using the Early Childhood Behavior Questionnaire Negative Affect Score (the mean of Anger/Frustration, Discomfort, Fear, Sadness, and reverse scored Soothability) (Rothbart et al., 2001). Mothers, and fathers when available, rated their own and their spouse’s ADHD symptoms and history at intake. To be conservative, familial ADHD status was defined generously as the mother or father either (a) reporting a past or current diagnosis of ADHD by a clinician or (b) scoring >= 80th percentile on the Barkley Adult ADHD Rating Scale-IV (BAARS-IV) Quick Screen (Barkley, 2011). Child sex was not associated with maternal prenatal distress, pre-pregnancy BMI, omega-3 fatty acid concentration, cytokine levels, child symptoms of ADHD, externalizing problems, or negative affect (all ps>0.20), and thus was not included as a covariate. Age was removed as an effect by within-time point standardization of all scores.
2.2.3. Teacher ratings
Teachers rated child behavior as listed in Table S1 in a subset of cases (n=23) (not all children had teachers due to the young ages). In brief, teachers completed the ADHD-RS and the SDQ when children were 48, 60, and/or 72 months old. A checklist of ODD symptoms was added to the ADHD-RS. Within time point standardization and data aggregation paralleled the treatment of the parent-report data. Teacher report of child ADHD symptoms was assessed using the ADHD-RS Total Score and the SDQ Hyperactivity scales. Child externalizing symptoms were captured using the SDQ Conduct Problems scale and the ADHD-RS ODD checklist. Child negative affect was assessed using the SDQ Emotional Symptoms subscale. Because of the young age and limited school enrollment, teacher ratings were not able to serve as primary outcome measures, but were nonetheless treated as sensitivity analyses to estimate dependence on maternal ratings for observed effect sizes.
2.2.4. Data Analysis
Research questions were tested using Structural Equation Modeling (SEM) (Hoyle, 1995; Schumacker and Lomax, 2012) using the Mplus 7.4 software package (Muthén and Muthén, 1998–2017) and the robust maximum likelihood estimator. We report the comparative fit index (CFI) and root mean squared error of the approximation (RMSEA) (Hu and Bentler, 1999). However, because the RMSEA often exceeds cutoffs in models with few degrees of freedom (even when the model is correctly specified; Kenny et al., 2015) it was de-emphasized in the interpretation. Non-independent observations (i.e., siblings nested within families) was handled using the Mplus cluster command. Mediation was tested using the model indirect command in combination with 95% confidence intervals (CI).
Auxiliary variables were included in the computation of missing data to maximize precision of the missing data matrix (Graham, 2003). These auxiliary variables were maternal ADHD status (defined later in this paragraph), paternal ADHD status, infant sex, and the highest parental BAARS-IV score. As described in 2.2.2.2., ADHD status was defined as the mother or father a) carrying a current diagnosis of ADHD or a childhood history of ADHD diagnosis by self or spouse report; or b) endorsing a clinically borderline or higher number of current ADHD symptoms at study enrollment. Of the 68 children in this sample, 46 children (67.6%) had positive family history by these limited criteria; 36 children (52.9%) had a mother with a diagnosis or elevated symptoms.
Of the N=68, 42 completed the 48–72 month assessment. Data from all N=68 participants were included in analyses; missing data were handled using full information maximum likelihood. Individuals who did not complete the 48–72-month postpartum assessment, on average had lower Omega-3 fatty acid concentrations during the third trimester (p=.04), but otherwise did not differ from the rest of the sample on any of the variables included in the current study.
2.2.4.1. Primary model
The parent-report of child ADHD symptoms latent variable was first regressed on the maternal inflammation latent variable. To test whether maternal distress, omega-3 fatty acid levels, and BMI were associated with maternal inflammation, the child ADHD symptoms latent variable was regressed on the maternal inflammation latent variable, and the maternal inflammation latent variable was regressed on maternal distress, fatty acids, and BMI. Maternal distress, fatty acids, and BMI were allowed to covary. Direct paths from maternal distress, fatty acids, and BMI to child ADHD symptoms were not of theoretical interest in this model (given that our interest was in testing whether these prenatal factors were associated with maternal inflammation during pregnancy, rather than in explaining the mechanism through which these factors influence child ADHD), and thus were not estimated in order to preserve degrees of freedom and power, given the small sample size. However, in Figures S3–5, we present the results of mediation models (where each predictor of inflammation is considered in its own mediation model) for readers who are interested in these effects. Sensitivity analyses considered whether the maternal cytokine to child ADHD symptoms effect survived controlling for familial ADHD status (child ADHD symptoms were regressed on familial ADHD status).
2.2.4.2. Sensitivity analyses
SEMs were tested sequentially to evaluate covariates (included one at a time to preserve power and interpretability): the child ADHD symptoms variable was regressed on the maternal prenatal inflammation latent variable with (a) no covariates, (b) child externalizing symptoms (the covariance between the externalizing symptoms latent variable and ADHD was estimated and a path from prenatal inflammation to externalizing symptoms was estimated), (c) child negative affect (the covariance between negative affect and ADHD was estimated and a path from prenatal inflammation to negative affect was estimated), and (d) familial ADHD status (familial ADHD status was covaried with prenatal inflammation and a path estimated from familial ADHD status to child ADHD). We also tested whether effects were similar using the more limited teacher ratings.
3. Results
3.1. Sample description
Table 1 provides sample description, including levels of physiological measures. The offspring were 55% male, 80% White/Caucasian, and 3% Hispanic/Latino. Parental education ranged from high-school equivalent to graduate degree with a median of a bachelor’s degree; 73% of the offspring had at least one parent meeting our criteria for ADHD-positive family.
Table 1.
Sample Descriptive Statistics
| Mean(SD) or % | Range | |
|---|---|---|
| Maternal Age | 30.4(5.1) | 18–41 |
| Paternal Age | 33.4(6.4) | 23–50 |
| Maternal Educationa | 6.7(1.2) | 5–9 |
| Paternal Educationa | 6.6(1.5) | 3–9 |
| Maternal Prenatal Distress | ||
| PSS | 15.4(7.4) | 3–31 |
| CES-D | 15.2(11.1) | 0–53 |
| Maternal Pre-Pregnancy BMI (kg/m2) | 26.9(6.8) | 17–49 |
| Maternal Omega-3 Fatty Acids (nmol/ml) | 168.7(60.4) | 68–286 |
| Maternal 3rd Trimester Cytokines (pg/ml) | ||
| Interleukin-6 | 1.7(.8) | .4–3.6 |
| Tumor Necrosis Factor-α | 11.4(3.5) | 5–19 |
| Monocyte Chemoattractant Protein-1 | 94.2(28.3) | 26–172 |
| Parent and Family ADHD Symptoms | ||
| % Family History of ADHD | 73% | |
| Maternal BAARS-IV Quick Screen | 10.0(4.2) | 5–20 |
| Paternal BAARS-IV Quick Screen | 7.8(3.2) | 5–17 |
Note:
1=Grade School, 2=Some High School, 3=High School Equivalent, 4=High School degree, 5=Some College but no degree, 6=Associates degree, 7=Bachelor’s degree, 8=Masters, Law, 2–3 years degree, 9=Doctorate, PhD, Medical degree. PSS=Perceived Stress Scale (scores of 0–13 indicate low perceived stress, 14–26 moderate perceived stress, and 27–40 indicate high perceived stress). CES-D=Center for Epidemiological Studies-Depression Scale (scores of 16–27 indicate mild to moderate depression; 28 and above indicate moderate to severe depression). BMI=Body Mass Index. BAARS-IV=Barkley Adult ADHD Rating Scale-IV Quick Screen (scores >8 indicate that the individual exceeds the 80th percentile based on national norms).
3.2. Primary model
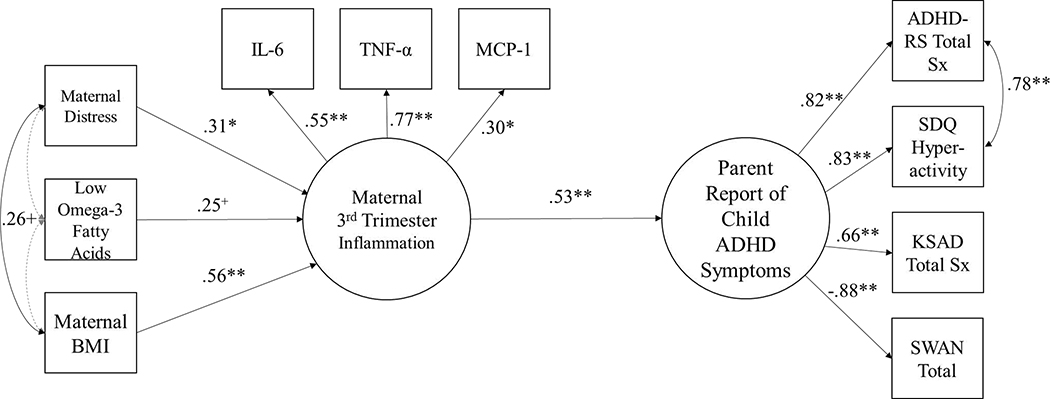
Figure 1 displays the primary model results. Maternal inflammation predicted child ADHD symptoms (β=0.53, 95% CI=0.154, 0.905, p=0.006). Maternal distress (β=0.31, 95% CI=0.024, 0.605, p=0.03) and BMI (β=0.56, 95% CI=0.307, 0.806, p=0.0001) were associated with increased maternal inflammation in the 3rd trimester but the effect of low omega-3 fatty acids on maternal inflammation was marginal (β=0.25, 95% CI=0.036, 0.530, p=0.087). Note that direct paths from maternal distress, fatty acids, and BMI to child ADHD symptoms, not of primary theoretical interest in this model, were not estimated in order to preserve degrees of freedom and power, given the small sample size. See Figures S2–S4 for the results of individual mediation models for readers who are interested in these effects.
Figure 1.
Model Relating Maternal 3rd Trimester Cytokines with Child ADHD Symptoms at 4–6 Years.
Note: +p<0.10,*p<0.05,**p<0.01. χ2 (df=30)=46.70, p=0.03, CFI=0.91, RMSEA=0.09. The gray dashed lines indicate paths that were estimated but were not statistically significant. When considered in separate models (where only one prenatal exposure was considered at a time), maternal distress (β=0.47, 95% CI=0.16, 0.77, p=0.003), low omega-3 fatty acids (β=0.33, 95% CI=0.02, 0.63, p=0.038), and pre-pregnancy BMI (β=0.68, 95% CI=0.45, 0.91, p<0.001) were each significantly associated with increased maternal inflammation. The maternal inflammation➔ADHD effect remained statistically significant when controlling for familial ADHD status, (β=0.46, 95% CI=0.45, 0.91, p=0.017). BMI=Body Mass Index. IL-6=interleukin-6, TNF-α=tumor necrosis factor-alpha, MCP-1=monocyte chemoattractant protein-1. ADHD-RS=ADHD rating scale. SDQ=Strengths and Difficulties Questionnaire.
KSADS=Kiddie schedule for affective disorders and schizophrenia for early childhood. SWAN=Strengths and Weaknesses of ADHD symptoms and Normative Behavior Scale. Sx=Symptoms.
Figures S2–S4 show that maternal cytokines mediated the effect of maternal distress on child ADHD symptoms (βindirect effect=0.21, 95% CI=0.007, 0.419, p=0.04) while indirect effects of maternal pre-pregnancy BMI and omega-3 fatty acids on child ADHD symptoms (via maternal inflammation) were just shy of significance (βindirect effect=0.83, 95% CI=−0.007, 1.67, p=0.052 and βindirect effect=0.20, 95% CI=−0.009, 0.041, p=0.06, respectively).
3.3. Sensitivity analyses
The cytokine➔ADHD effect remained statistically significant when family ADHD status (βinflammation➔ADHD=0.51, 95% CI=0.110, 0.899, p=0.012), child negative affect (βinflammation➔ADHD= 0.60, 95% CI=0.285, 0.904, p=0.0002) or child externalizing behavior were in the model (βinflammation➔ADHD=0.51, 95% CI=0.111, .913, p=.01) (Table S2).
Maternal inflammation predicted teacher-report of child ADHD symptoms (βinflammation➔ADHD=0.46, 95% CI=0.036, 0.889, p=.03), confirming the primary result. When covariates were added to check on confounders, effect sizes were preserved and remained comparable with those for the parent-report models. However, p values fell shy of significance in some models (Table S2). The similarity of effect sizes suggested general reproducibility across reporter (e.g., βinflammation➔ADHD=.51 in both models that control for family ADHD status).
4. Discussion
Evaluation of candidates for early detection of ADHD risk is critical to identify targets for future study to discover mechanisms and set the stage for more effective early intervention programs. Importance is high because ADHD is so costly and its course difficult to alter even by the late preschool period. Proof-of-concept is essential to evaluate merits of the costly prospective studies needed. Here, we examine a novel candidate marker of risk, maternal prenatal cytokine concentrations. Although we do not expect elevated cytokine concentrations to be associated only with future ADHD, an association to ADHD would still be highly informative.
This is the first study to examine in humans whether maternal prenatal inflammation, a purported pathway for early risk, could prospectively predict child symptoms of psychopathology. Results indicate that maternal cytokine concentrations in the 3rd trimester of pregnancy are associated with increased symptoms of ADHD at 4–6 years, as reported by both parents and teachers. The primary finding is thus entirely novel. If these results are confirmed in larger and more diverse studies, they would hold promise that maternal prenatal cytokine concentrations may be an easily obtained, low-cost marker of ADHD risk in offspring. With regard to etiological models, the results are consistent with the idea that maternal systemic inflammation may be one common pathway for multiple upstream etiological inputs that perturb neurodevelopment and contribute to ADHD and other conditions (e.g., maternal adiposity, dietary intake, psychological distress) (Bolton and Bilbo, 2014; Dunn et al., 2019).
Although we would not expect cytokine levels to be specific only to ADHD risk, results from sensitivity analyses did suggest some degree of specificity in this selected sample: maternal cytokine levels predicted child ADHD symptoms, independent of concurrent child negative affect or disruptive-aggressive behaviors. The effect also was not explained by parental ADHD status. Although the children were too young to have fully emerged into academic problems (few were even in first grade), the effect sizes reproduced almost exactly in the limited available teacher ratings, albeit with marginal p-values given lower power once covariates were introduced.
Why might maternal inflammation predict child ADHD? Biologically, several mechanisms may account for this association: maternal cytokine levels (a) can cross the placenta, resulting in elevated cytokine concentrations in amniotic fluid and in the fetal brain (Urakubo et al., 2001; Zaretsky et al., 2004); (b) can induce epigenetic changes in the placenta in ways that increase placental cytokine expression (Hsiao and Patterson, 2011; Urakubo et al., 2001); (c) can activate resident immune cells in the decidua, thereby prompting cytokine release at the maternal-fetal interface; and (d) increased cytokines in the intrauterine milieu can prompt a fetal inflammatory response, which can further contribute to the inflammatory profile of the fetal brain (Schaafsma et al., 2017). Increased cytokines in the intrauterine environment and fetal brain can shape or perturb brain development in ways that alter behavior early in life (Bilbo and Schwarz, 2009; Wadhwa, 2005).
Although of secondary emphasis, our finding that cytokine levels mediated effects of maternal risk factors is, while preliminary, also novel and of interest to theories examining mechanisms of those risks. Results support the hypothesis that cytokines are one pathway through which maternal prenatal distress (and maternal BMI and omega-3 fatty acids, at the margin) influence child behavioral risk. It is notable that whereas previous work has shown an association between prenatal maternal distress with child ADHD (Grizenko et al., 2012; Ronald et al., 2011), this is the first study to provide prospective human evidence that inflammation is a mechanism mediating that effect. Maternal immune activation during pregnancy is often hypothesized to be a mechanism through which maternal prenatal stress impacts child development (Glover, 2015), yet this is only the second study to demonstrate that maternal prenatal cytokine concentrations mediate the effects of prenatal stress on child outcomes (see (Gustafsson et al., 2017) for the other which used this same sample in their first year of life).
Our study had a number of methodological strengths, including the rich multi-method, multi-informant longitudinal data that were collected as part of this study. Our use of measurement aggregation and latent variables may have been helpful in obtaining sufficient sensitivity to detect these effects—that approach increased the reliability of the measurement of our key constructs, removed measure-specific variance, and by extension, maximized our power to detect effects. This is consistent with prior reports that etiological effects are detected more powerfully by measure aggregation than single measures (Friedman et al., 2008; Nigg et al., 2018).
Although this study sample is small and therefore to some extent preliminary, results suggest a path forward in identifying how to signal early risk for ADHD (and likely other dysregulatory disorders in childhood). If confirmed, these results would begin to set the stage for trials to reduce risk in susceptible individuals. For example, if replicated, this finding may suggest that anti-inflammatory interventions that can be administered to expectant mothers prenatally (e.g., omega-3 supplementation) may ameliorate child risk for ADHD and possibly for other forms of psychopathology, for which more study would be needed. Anti-inflammatory therapy modified the effect of prenatal stress-associated inflammation’s effect on offspring hyperactivity in a mouse model (Bronson and Bale, 2014) but have not yet been tested in humans.
Aside from the small sample and the need for more ambitious validation studies, which this study now justifies, other cautions are important. The current study considered three maternal risks that may influence inflammation as a proof-of-concept. Although these accounted for over half the variance in inflammation in this small sample (R2=.60), it would be a mistake to assume that these are the only, or even primary, influences on prenatal inflammation (e.g., maternal infection and toxicant exposure are two other obvious candidates). Further, we examined only three cytokines (from a single signaling pathway) at one time during pregnancy; study of a wider range of inflammatory markers at different times in gestation might be better suited for tests of trimester-specific or cytokine-specific effects. Similarly, inflammation is not the only biological mechanism through which these factors are related to offspring outcomes (glucocorticoid function is of importance, for example; Monk et al. (2019)). Although we controlled for parental ADHD, genetic influences on inflammation were not examined. Additionally, although the current study examined whether the cytokine to ADHD effect was independent of several important confounds, these represent only a few of the dimensions of child development that may be influenced by maternal prenatal inflammation and potentially would be important to control for here; future research should examine others to better understand the bounds and specificity of the effects presented. Participant visits were scheduled around the women’s prenatal medical appoints, and thus study visits and the associated venipuncture did not occur at the same time of day for all participants. Though this helped to reduce participant burden, there is evidence of diurnal variation in some cytokine concentrations (Petrovsky et al., 1998). Due to the small sample, we did not have power to evaluate interactions among the risk factors, another direction for future study. Finally, although we saw robust associations with ADHD symptom levels, few of the offspring would have actually met clinical range severity for ADHD, which has a population prevalence of about 5% and in this deliberately enriched sample was present in about 14%. However, substantial literature suggests that findings for ADHD dimensional variation are etiologically informative. Following literature focused on the role of prenatal maternal distress (Doyle et al., 2015; Monk et al., 2016), we combined a measure of depression and stress, which were highly correlated in this sample. However, it may be useful to further isolate maternal mood and stress (Rallis et al., 2014).
4.1. Conclusion
In conclusion, maternal prenatal inflammation appears to be a promising marker of risk for child ADHD symptoms. This study provides the first evidence that maternal cytokines during pregnancy predict ADHD in early childhood, and that maternal prenatal inflammation is one mechanism through which prenatal risks such as maternal distress influence child psychopathology.
Supplementary Material
Highlights.
Maternal 3rd trimester cytokines predicted child ADHD symptoms at 4–6 years of age
Maternal cytokines mediated the effect of maternal prenatal distress on child ADHD
This is the first study to link maternal pregnancy cytokines and offspring ADHD
Maternal prenatal cytokine levels may be a marker of ADHD risk
Acknowledgements
Research reported in this publication was supported by the Abracadabra Foundation, the Moore Institute for Nutrition and Wellness, and by the National Institutes of Health under National Institute of Mental Health award numbers R3759105 (Nigg), R01MH117177 (Sullivan and Nigg) and K01MH120507 (Gustafsson), and the National Center for Advancing Translational Sciences award number TL1TR002371 (Gustafsson). The funding sources had no role in the study design, collection, analysis and interpretation of data, writing of the report, or the decision to submit the article for publication. This material is the result of work supported with resources and the use of facilities at the VA Portland Health Care System, Portland, OR. Dr. Loftis is an employee of the U.S. Department of Veterans Affairs. Contents do not necessarily represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Footnotes
The authors declare no conflicts of interest relevant to this publication.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achenbach TM, Ruffle TM, 2000. The Child Behavior Checklist and related forms for assessing behavioral/emotional problems and competencies. Pediatrics in Review 21, 265–271. [DOI] [PubMed] [Google Scholar]
- Barkley RA, 2011. Barkley Adult ADHD Rating Scale-IV (BAARS-IV). Guilford Press, New York, NY. [Google Scholar]
- Bilbo SD, Schwarz JM, 2009. Early-life programming of later-life brain and behavior: a critical role for the immune system. Frontiers in Behavioral Neuroscience 3, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton JL, Bilbo SD, 2014. Developmental programming of brain and behavior by perinatal diet: focus on inflammatory mechanisms. Dialogues in Clinical Neuroscience 16, 307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronson SL, Bale TL, 2014. Prenatal stress-induced increases in placental inflammation and offspring hyperactivity are male-specific and ameliorated by maternal antiinflammatory treatment. Endocrinology 155, 2635–2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JC, Nugent BM, Bale TL, 2017. Parental advisory: maternal and paternal stress can impact offspring neurodevelopment. Biological Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deverman BE, Patterson PH, 2009. Cytokines and CNS development. Neuron 64, 61–78. [DOI] [PubMed] [Google Scholar]
- Donfrancesco R, Nativio P, Borrelli E, Giua E, Andriola E, Villa M, DI MT, 2016. Serum cytokines in paediatric neuropsychiatric syndromes: focus on Attention Deficit Hyperactivity Disorder. Minerva pediatrica. [DOI] [PubMed] [Google Scholar]
- Doyle C, Werner E, Feng T, Lee S, Altemus M, Isler JR, Monk C, 2015. Pregnancy distress gets under fetal skin: Maternal ambulatory assessment & sex differences in prenatal development. Developmental psychobiology 57, 607–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn GA, Nigg JT, Sullivan EL, 2019. Neuroinflammation as a risk factor for attention deficit hyperactivity disorder. Pharmacology Biochemistry and Behavior 182, 22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPaul GJ, Power TJ, Anastopoulos AD, Reid R, 1998. ADHD Rating Scale—IV: Checklists, norms, and clinical interpretation. Guilford Press, New York, NY. [Google Scholar]
- Ezzati A, Jiang J, Katz MJ, Sliwinski MJ, Zimmerman ME, Lipton RB, 2014. Validation of the Perceived Stress Scale in a community sample of older adults. International Journal of Geriatric Psychiatry 29, 645–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman NP, Miyake A, Young SE, DeFries JC, Corley RP, Hewitt JK, 2008. Individual differences in executive functions are almost entirely genetic in origin. Journal of Experimental Psychology: General 137, 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffrey M, Luby J, 2012. Schedule for Affective Disorders and Schizophrenia for Early Childhood (K-SADS-EC). St. Louis: Washington University School of Medicine. [Google Scholar]
- Glover V, 2015. Prenatal stress and its effects on the fetus and the child: possible underlying biological mechanisms Perinatal programming of neurodevelopment. Springer, pp. 269–283. [DOI] [PubMed] [Google Scholar]
- Goodman R, 2001. Psychometric properties of the strengths and difficulties questionnaire. Journal of the American Academy of Child & Adolescent Psychiatry 40, 1337–1345. [DOI] [PubMed] [Google Scholar]
- Graham AM, Rasmussen JM, Rudolph MD, Heim CM, Gilmore JH, Styner M, Potkin SG, Entringer S, Wadhwa PD, Fair DA, 2018. Maternal systemic interleukin-6 during pregnancy is associated with newborn amygdala phenotypes and subsequent behavior at 2 years of age. Biological Psychiatry 83, 109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham JW, 2003. Adding missing-data-relevant variables to FIML-based structural equation models. Structural Equation Modeling 10, 80–100. [Google Scholar]
- Grizenko N, Fortier M-E, Zadorozny C, Thakur G, Schmitz N, Duval R, Joober R, 2012. Maternal stress during pregnancy, ADHD symptomatology in children and genotype: gene-environment interaction. Journal of the Canadian Academy of Child and Adolescent Psychiatry 21, 9. [PMC free article] [PubMed] [Google Scholar]
- Gustafsson H, Sullivan E, Nigg J, Loftis J, 2017. Maternal prenatal depression predicts infant negative affect via maternal inflammatory cytokine levels. Brain, Behavior, and Immunity 66, e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson HC, Sullivan EL, Nousen EK, Sullivan CA, Huang E, Rincon M, Nigg JT, Loftis JM, 2018. Maternal prenatal depression predicts infant negative affect via maternal inflammatory cytokine levels. Brain, Behavior, and Immunity 73, 470–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyle RH, 1995. Structural equation modeling: Concepts, issues, and applications. Sage, Thousand Oaks, CA. [Google Scholar]
- Hsiao EY, Patterson PH, 2011. Activation of the maternal immune system induces endocrine changes in the placenta via IL-6. Brain, Behavior, and Immunity 25, 604–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L.t., Bentler PM, 1999. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal 6, 1–55. [Google Scholar]
- Kenny DA, Kaniskan B, McCoach DB, 2015. The performance of RMSEA in models with small degrees of freedom. Sociological Methods & Research 44, 486–507. [Google Scholar]
- Lagerstedt SA, Hinrichs DR, Batt SM, Magera MJ, Rinaldo P, McConnell JP, 2001. Quantitative determination of plasma c8-c26 total fatty acids for the biochemical diagnosis of nutritional and metabolic disorders. Mol Genet Metab 73, 38–45. [DOI] [PubMed] [Google Scholar]
- Lakes KD, Swanson JM, Riggs M, 2012. The reliability and validity of the English and Spanish Strengths and Weaknesses of ADHD and Normal behavior rating scales in a preschool sample: continuum measures of hyperactivity and inattention. Journal of attention disorders 16, 510–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Zhang L, Joo D, Sun S-C, 2017. NF-κB signaling in inflammation. Signal Transduction and Targeted Therapy 2, e17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehler MF, Kessler JA, 1998. Cytokines in brain development and function. Advances in Protein Chemistry 52, 223–251. [DOI] [PubMed] [Google Scholar]
- Monk C, Feng T, Lee S, Krupska I, Champagne FA, Tycko B, 2016. Distress during pregnancy: epigenetic regulation of placenta glucocorticoid-related genes and fetal neurobehavior. American Journal of Psychiatry 173, 705–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk C, Lugo-Candelas C, Trumpff C, 2019. Prenatal developmental origins of future psychopathology: Mechanisms and pathways. Annual Review of Clinical Psychology 15, 317–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller N, Ackenheil M, 1998. Psychoneuroimmunology and the cytokine action in the CNS: implications for psychiatric disorders. Progress in Neuro-Psychopharmacology and Biological Psychiatry 22, 1–33. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO, 1998–2017. Mplus User’s guide. Muthén & Muthén; Los Angeles, CA. [Google Scholar]
- Nigg J, Nikolas M, Burt SA, 2010. Measured gene-by-environment interaction in relation to attention-deficit/hyperactivity disorder. Journal of the American Academy of Child & Adolescent Psychiatry 49, 863–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT, Gustafsson HC, Karalunas SL, Ryabinin P, McWeeney SK, Faraone SV, Mooney MA, Fair DA, Wilmot B, 2018. Working memory and vigilance as multivariate endophenotypes related to common genetic risk for attention-deficit/hyperactivity disorder. Journal of the American Academy of Child & Adolescent Psychiatry 57, 175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne S, Biaggi A, Chua T, Du Preez A, Hazelgrove K, Nikkheslat N, Previti G, Zunszain P, Conroy S, Pariante C, 2018. Antenatal depression programs cortisol stress reactivity in offspring through increased maternal inflammation and cortisol in pregnancy: The Psychiatry Research and Motherhood–Depression (PRAM-D) Study. Psychoneuroendocrinology 98, 211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariante CM, 2017. Why are depressed patients inflamed? A reflection on 20 years of research on depression, glucocorticoid resistance and inflammation. European neuropsychopharmacology 27, 554–559. [DOI] [PubMed] [Google Scholar]
- Petrovsky N, McNair P, Harrison LC, 1998. Diurnal rhythms of pro-inflammatory cytokines: regulation by plasma cortisol and therapeutic implications. Cytokine 10, 307–312. [DOI] [PubMed] [Google Scholar]
- Radloff LS, 1977. The CES-D scale a self-report depression scale for research in the general population. Applied Psychological Measurement 1, 385–401. [Google Scholar]
- Rallis S, Skouteris H, McCabe M, Milgrom J, 2014. The transition to motherhood: towards a broader understanding of perinatal distress. Women and Birth 27, 68–71. [DOI] [PubMed] [Google Scholar]
- Rees S, Harding R, 2004. Brain development during fetal life: influences of the intra-uterine environment. Neuroscience Letters 361, 111–114. [DOI] [PubMed] [Google Scholar]
- Rodriguez A, 2010. Maternal pre-pregnancy obesity and risk for inattention and negative emotionality in children. Journal of Child Psychology and Psychiatry 51, 134–143. [DOI] [PubMed] [Google Scholar]
- Ronald A, Pennell CE, Whitehouse AJ, 2011. Prenatal maternal stress associated with ADHD and autistic traits in early childhood. Frontiers in Psychology 1, 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbart MK, Ahadi SA, Hershey KL, Fisher P, 2001. Investigations of temperament at three to seven years: The Children’s Behavior Questionnaire. Child Development 72, 1394–1408. [DOI] [PubMed] [Google Scholar]
- Rudolph MD, Graham AM, Feczko E, Miranda-Dominguez O, Rasmussen JM, Nardos R, Entringer S, Wadhwa PD, Buss C, Fair DA, 2018. Maternal IL-6 during pregnancy can be estimated from newborn brain connectivity and predicts future working memory in offspring. Nature Neuroscience 21, 765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaafsma W, Basterra LB, Jacobs S, Brouwer N, Meerlo P, Schaafsma A, Boddeke EW, Eggen BJ, 2017. Maternal inflammation induces immune activation of fetal microglia and leads to disrupted microglia immune responses, behavior, and learning performance in adulthood. Neurobiology of Disease 106, 291–300. [DOI] [PubMed] [Google Scholar]
- Schumacker RE, Lomax RG, 2012. A beginner’s guide to structural equation modeling. Routledge, New York, NY. [Google Scholar]
- Sullivan EL, Holton KF, Nousen EK, Barling AN, Sullivan CA, Propper CB, Nigg JT, 2015. Early identification of ADHD risk via infant temperament and emotion regulation: A pilot study. Journal of Child Psychology and Psychiatry 56, 949–957. [DOI] [PubMed] [Google Scholar]
- Urakubo A, Jarskog LF, Lieberman JA, Gilmore JH, 2001. Prenatal exposure to maternal infection alters cytokine expression in the placenta, amniotic fluid, and fetal brain. Schizophrenia Research 47, 27–36. [DOI] [PubMed] [Google Scholar]
- Wadhwa PD, 2005. Psychoneuroendocrine processes in human pregnancy influence fetal development and health. Psychoneuroendocrinology 30, 724–743. [DOI] [PubMed] [Google Scholar]
- Yuan T-F, Li A, Sun X, Ouyang H, Campos C, Rocha NB, Arias-Carrión O, Machado S, Hou G, So KF, 2016. Transgenerational inheritance of paternal neurobehavioral phenotypes: stress, addiction, ageing and metabolism. Molecular Neurobiology 53, 6367–6376. [DOI] [PubMed] [Google Scholar]
- Zaretsky MV, Alexander JM, Byrd W, Bawdon RE, 2004. Transfer of inflammatory cytokines across the placenta. Obstetrics & Gynecology 103, 546–550. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.