Abstract
Stem cells use a variety of mechanisms to help maintain their pluripotency, promote self-renewal, as well as at the appropriate time, to differentiate into specialized cells. One such mechanism that is attracting significant attention from the stem cell, development, and regenerative medicine research communities involves a form of intercellular communication, specifically, the ability of cells to form and release non-traditional membrane-enclosed structures, referred to as extracellular vesicles (EVs). There are two major classes of EVs, microvesicles (MVs), which are generated through the outward budding and fission of the plasma membrane, and exosomes which are formed as multivesicular bodies (MVBs) in the endo-lysosomal pathway that fuse with the cell surface to release their contents. While they differ in how they are formed, both MVs and exosomes have been shown to contain a diverse array of bioactive cargo, such as proteins, RNA transcripts, microRNAs, and even DNA, which can be transferred to other cells and promote phenotypic changes. Here, we will describe what is currently known regarding EVs and the roles they play in stem cell biology and different aspects of early development. We will also highlight how the EVs produced by stem cells are being aggressively pursued for clinical applications, including their potential use as therapeutic delivery systems and for their regenerative capabilities.
Graphical Abstract
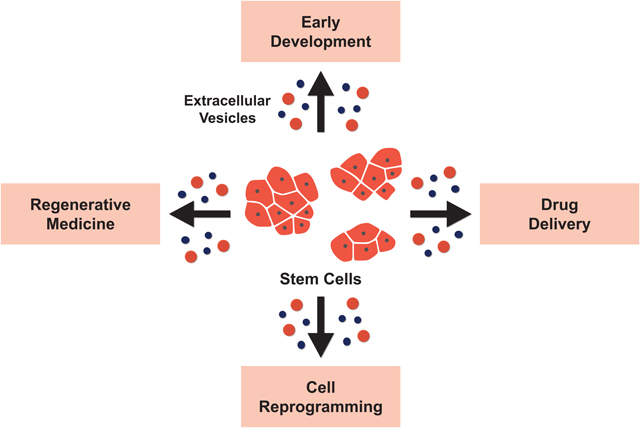
Stem cells generate different classes of extracellular vesicles containing bio-active cargo. These vesicles are transferred to other cells and promote phenotypic changes. Stem cell extracellular vesicles have been shown to play important roles in development and cellular reprogramming. They are also being pursued for clinical applications, including their potential use as a drug delivery system and for their regenerative capabilities.
Introduction
Extracellular vesicles (EVs) represent a mechanism of intercellular communication that involves the ability of cells to form and release multiple distinct types of non-classical vesicles. Due to the diversity of their contents, as well as their ability to impact an ever-increasing number of biological and pathological processes, EVs are attracting a great deal of attention from both basic and translational researchers, as well as the pharmaceutical industry [1,2]. Generally speaking, EVs can be divided into one of two major classes, based on their size and the mechanism underlying their biogenesis. One class of EVs is most often referred to as microvesicles (MVs), although in some cases, MVs have also been called ectosomes, shedding vesicles, large EVs, and when generated by cancer cells, oncosomes. These vesicles generally range from 0.2 μm up to 0.5 μm in diameter and are generated by the outward budding and fission of the plasma membrane. Exosomes make up the second major class of EVs. These EVs are typically 50–150 nm in diameter and are derived from the endo-lysosomal pathway. In particular, multivesicular bodies (MVBs) containing intraluminal vesicles (ILVs) within this pathway are typically trafficked to the lysosome where they are degraded. However, some MVBs escape this fate and are instead trafficked to the cell surface. The MVBs then fuse with the plasma membrane and release their contents which includes exosomes into the extracellular environment [3–5]. While the specific contents of MVs and exosomes can differ greatly depending on their cell of origin, some of the more common types of EV cargo includes cell surface and cytosolic signaling proteins, extracellular matrix proteins, growth factors, transcription factors, metabolic enzymes, DNA and RNA-binding proteins, as well as RNA transcripts, microRNAs and genomic DNA [6,7].
As with any young and rapidly developing field, there are currently far more questions about EVs than answers. Among the most pervasive of these questions concerns the mechanisms that control MV and exosome formation and release, as well as how are they loaded with cargo, whether they can be targeted to specific tissues, and how generally important are they in biology. However, with every passing month, this form of intercellular communication is being implicated in another physiological or pathological process. Thus, efforts are now being made to develop better approaches to isolate MVs and exosomes from biological solutions (i.e., blood). This has also led the International Society of Extracellular Vesicles (ISEV) to publish the Minimal Information for Studies of Extracellular Vesicles (MISEV 2018), which provides guidelines for researchers to follow when studying EVs [8].
It was not long ago that EVs were thought to be generated primarily by diseased or stressed cells, especially cancer cells, where they have been shown to promote many aspects of cancer progression. There are several good reviews that focus on this aspect of EV biology [3,9,10]. However, more recent findings have led to the appreciation that most, if not all, types of cells are capable of producing EVs, where they have been shown to regulate various physiological processes [11,12]. The EVs produced by stem cells have been suggested to be important for embryonic development, however, they also are being examined for their potential clinical applications. In this review, we will highlight some of the roles played by EVs in early development and their possible implications in regenerative medicine and cellular reprogramming.
Roles of Stem Cell-derived EVs in Early Development
EVs and Implantation
Embryo development is a tightly regulated process. Following the fertilization of an egg, the embryo begins to undergo a series of rapid cell divisions until it reaches the blastocyst stage (Figure 1). It is at this point that the embryo undergoes its first of many differentiation processes, forming two distinct cell types. Trophoblasts are one of the cell types formed, and are essential for adhering and invading into the uterine wall of the mother, a process referred to as implantation. The “implanted” trophoblasts go on to develop into the fetal membrane of the placenta. The trophoblasts surround the second cell type that makes up the blastocyst, namely embryonic stem cells (ESCs). These cells reside in a structure referred to as the inner cell mass (ICM) and will go on to give rise to the entire organism. Any errors that occur during this sequence of events can have detrimental consequences on the pregnancy, such as the preimplantation death of the embryo or preeclampsia [13,14].
Figure 1.
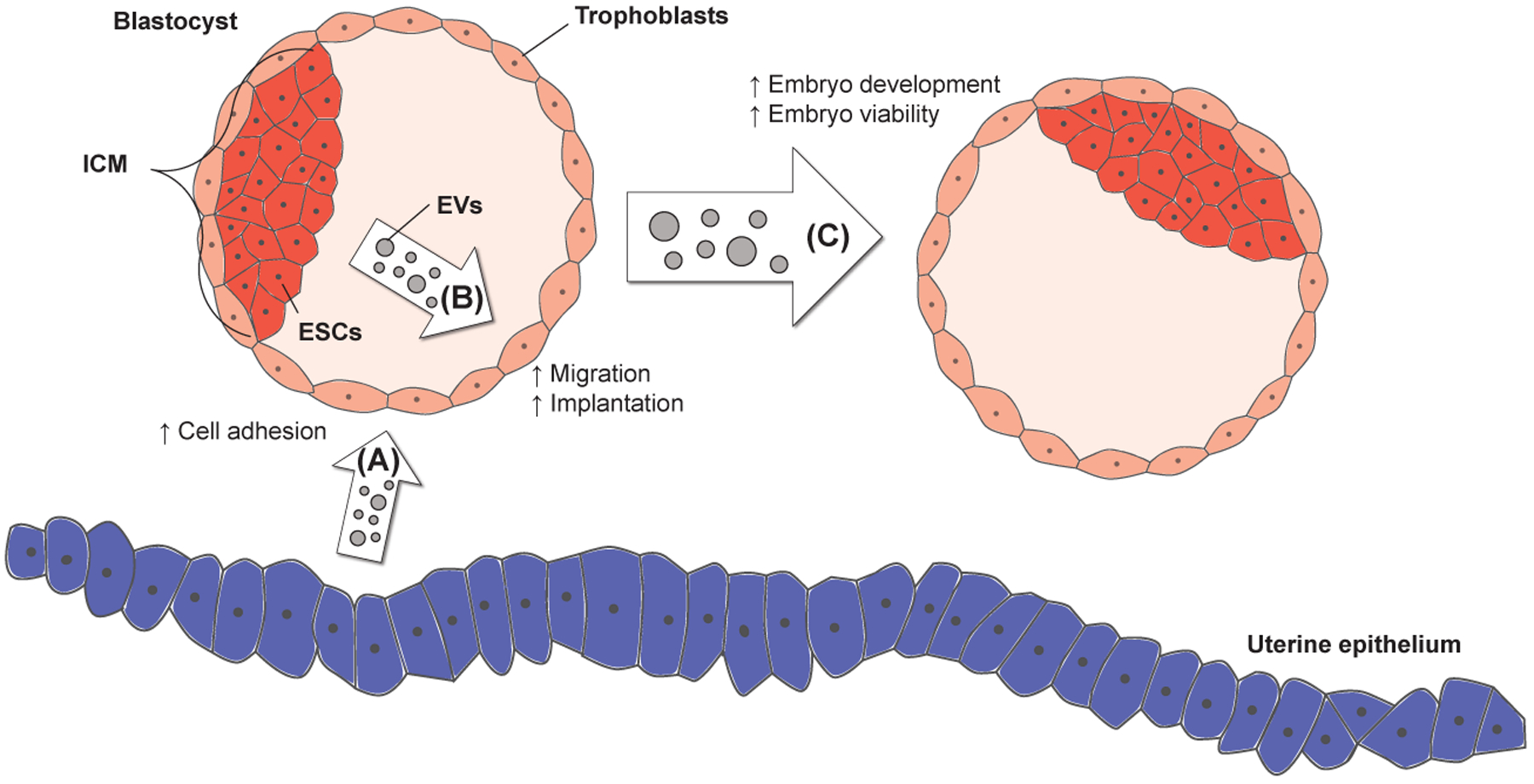
EVs are produced by various embryonic and maternal tissues. (A) The uterine epithelium of the mother generates EVs that promote the attachment of blastocyst-stage embryos to the uterus. (B) The EVs formed by ESCs in the inner cell mass (ICM) of blastocysts have been shown to be taken up by the surrounding layer of trophoblasts. The ability of the trophoblasts to migrate and invade into the uterine lining (i.e., a process referred to as implantation) is enhanced by the EVs. (C) The co-culturing of embryos has been shown to increase embryo development and viability, processes that are mediated by EVs.
Considered the first major physical embryo-maternal interaction, successful implantation requires synchronized communication between an embryo that has developed to the blastocyst stage and a receptive uterus [15]. Such coordination is thought to be regulated primarily by soluble factors secreted by maternal tissues. For example, transforming growth factor-beta 1 (TGF-β1) produced and secreted by the endometrium was shown to stimulate fibronectin production in trophoblasts and enhance their ability to adhere to the endometrium during implantation [16]. The expression of the cytokine leukemia inhibitory factor (LIF) is similarly increased in the mouse endometrial epithelium during early pregnancy, and the absence of sufficient amounts of LIF during this time significantly inhibited the rates of successful implantations [17,18]. In addition to soluble factors mediating communication between the mother and embryo during implantation, the EVs produced by maternal organs also potentially contribute to this process (Figure 1A). For example, it has been shown that endometrial epithelial cells treated with the hormones estrogen and progesterone begin to generate EVs. When these vesicles were isolated and then used to treat cultures of trophoblasts, the cell adhesion protein focal adhesion kinase (FAK) became activated and the adhesive capacity of the trophoblasts was increased, suggesting that EVs generated by maternal tissues may play a role during the implantation process [19,20].
Recent evidence from our laboratory suggests that EVs produced by embryonic tissues can play roles in promoting implantation as well. We showed that ESCs shed large quantities of MVs, the class of EVs generated by the outward budding and fission of the cell surface. These MVs were shown to be efficiently transferred to trophoblasts, where they increased the activation of FAK and c-Jun N-terminal kinase (JNK), and enhanced their ability to migrate (Figure 1B). The effects of the MVs were shown to be mediated by the large amounts of two extracellular matrix proteins, laminin and fibronectin, that were found decorating the surfaces of the MVs. The MV-associated fibronectin and laminin activated integrins expressed on the surfaces of trophoblasts. This led to the signaling changes (i.e., increased JNK and FAK) necessary for promoting cell migration. Importantly, we took our findings one step further by showing that injecting MVs isolated from ESCs into blastocysts was sufficient to increase their rates of implantation [12].
EVs and Embryo Development
There have been indications that embryos generate signals to communicate with each other. For example, it has been well-documented that the viability and successful development of an embryo in culture is significantly increased when placed together with other embryos, as opposed to being cultured individually [21,22]. Even though the factors secreted by embryos that are responsible for this effect have not yet been identified, it is intriguing to consider that EVs may be involved. One line of evidence supporting this idea comes from a study aimed at developing approaches to increase the viability of cultured embryos, in order to better the chances of having a successful pregnancy. Using bovine somatic cell nuclear transfer (SCNT) embryos as a model, it was found that culturing the embryos without replacing their culture medium significantly improved their overall health, enhanced blastocyst formation, and increased the probability of having a successful pregnancy following embryo transfer, compared to embryos that had their medium replaced regularly. While investigating how these effects were mediated, the conditioned medium obtained from the SCNT embryos was shown to contain exosome sized vesicles that were positive for CD9, a classic EV marker. Interestingly, when these exosomes were added to embryos that were having their growth medium regularly replaced, a noticeable improvement in the health and development of the embryos was observed [23].
A role for embryo-derived EVs in promoting embryo viability and development was suggested in another study. Here, the authors were trying to determine why co-culturing cloned embryos with parthenogenetic embryos (i.e., embryos from an unfertilized egg cell) improved the development of cloned embryos. They discovered that this effect was, at least in part, potentially mediated by EVs generated by the parthenogenetic embryos, showing the presence of particles expressing the exosomal marker CD9 in the conditioned medium collected from cultures of these embryos. What made these findings particularly interesting was the fact that the embryo-derived EVs contained RNA transcripts that encoded several pluripotency genes, including Oct3/4, Sox2, Klf4, c-Myc, and Nanog. The internalization of EVs by the recipient embryos, and the accompanying increase in the mRNA levels of pluripotency genes, when co-cultured with the conditioned medium from parthenogenetic embryos, led the authors to suggest that EVs represented a mechanism by which communication occurred between embryos [24].
The Therapeutic Potential of EVs Generated by Stem Cells
EVs in Regenerative Medicine
In addition to the roles being played by EVs in different aspects of development, the EVs generated by stem/progenitor cells are also being aggressively pursued for their potential use in therapeutic applications. The concept underlying regenerative medicine involves repairing or replacing damaged and diseased tissues, such that they regain as much of their original functions as possible. Mesenchymal stem cells (MSCs) are the most widely used cell type for cell transplantation-based treatments, because of their ability to differentiate into multiple lineages, as well as the ease in which they can be isolated from different tissue sources and propagated [25,26]. MSCs derived from bone marrow, adipose tissue, muscles, and ESCs [25–39] are currently being used to treat various diseases and conditions, such as myocardial ischemia, stroke, and musculoskeletal disease, with the idea being that the transplantation of MSCs near the sites of damaged tissue results in their differentiation and reconstitution of the tissue, such that it regains functionality. For example, percutaneous injection of bone marrow-derived MSCs into the damaged myocardium in a pig model of myocardial ischemia reduced the extent of necrosis, promoted the regeneration of contractile myocardium, and improved overall cardiac function [27].
However, despite the therapeutic benefits of engrafting MSCs near diseased/damaged tissues, the MSCs were rarely observed infiltrating the tissue, as would be expected if they promoted recovery by reconstituting the diseased/damaged tissues. Moreover, changes in the damaged site were often detected after only a few hours of MSC transplantation, which was not sufficient time for the MSCs to have undergone differentiation [28]. These findings suggest that the MSCs were not directly responsible for their therapeutic benefits, but rather that these effects might be mediated by factors secreted by the stem cells. Consistent with this idea, several groups showed that treatment of damaged tissues with the conditioned medium collected from cultures of MSCs was sufficient to recapitulate the effects of transplanting the cells. With regard to acute myocardial infarction, Gnecchi et al. showed that culturing adult rat ventricular cardiomyocytes with the conditioned medium from rat bone marrow-derived MSCs, that were engineered to overexpress the growth and survival-promoting kinase Akt1, protected the cardiomyocytes from hypoxia-induced apoptosis. The same conditioned medium was also shown to limit infarct size and improve ventricular function in rat models of myocardial infarction to a similar extent as injecting MSCs [29,30]. Similar benefits were achieved when using the conditioned medium from MSCs to treat other disease models, such as lung ischemia-reperfusion injury [31], inflammatory arthritis [32], and renal tubulointerstitial fibrosis [33].
Efforts were then undertaken to identify the components in the MSC conditioned medium that were responsible for these effects. Using a porcine model of ischemia and reperfusion injury, it was discovered that the cardioprotective effects of the medium were primarily mediated by a fraction that contained proteins and/or structures with a molecular weight of at least 1000 kDa, and 50 nm or greater in diameter [34]. Interestingly, both MVs and exosomes would be expected to be contained in this fraction. Indeed, a follow-up study that built upon these initial findings found that the >1000 kDa fraction of the conditioned medium contained 50–100 nm sized particles that were positive for the EV markers, CD9, CD81, and Alix [35]. Likewise, the EVs generated by MSCs were also shown by different groups to be able to recapitulate the therapeutic benefits of the conditioned medium using a variety of disease models (Figure 2, left side), such as renal ischemia-reperfusion injury [36], stroke [37], and liver fibrosis [38].
Figure 2.
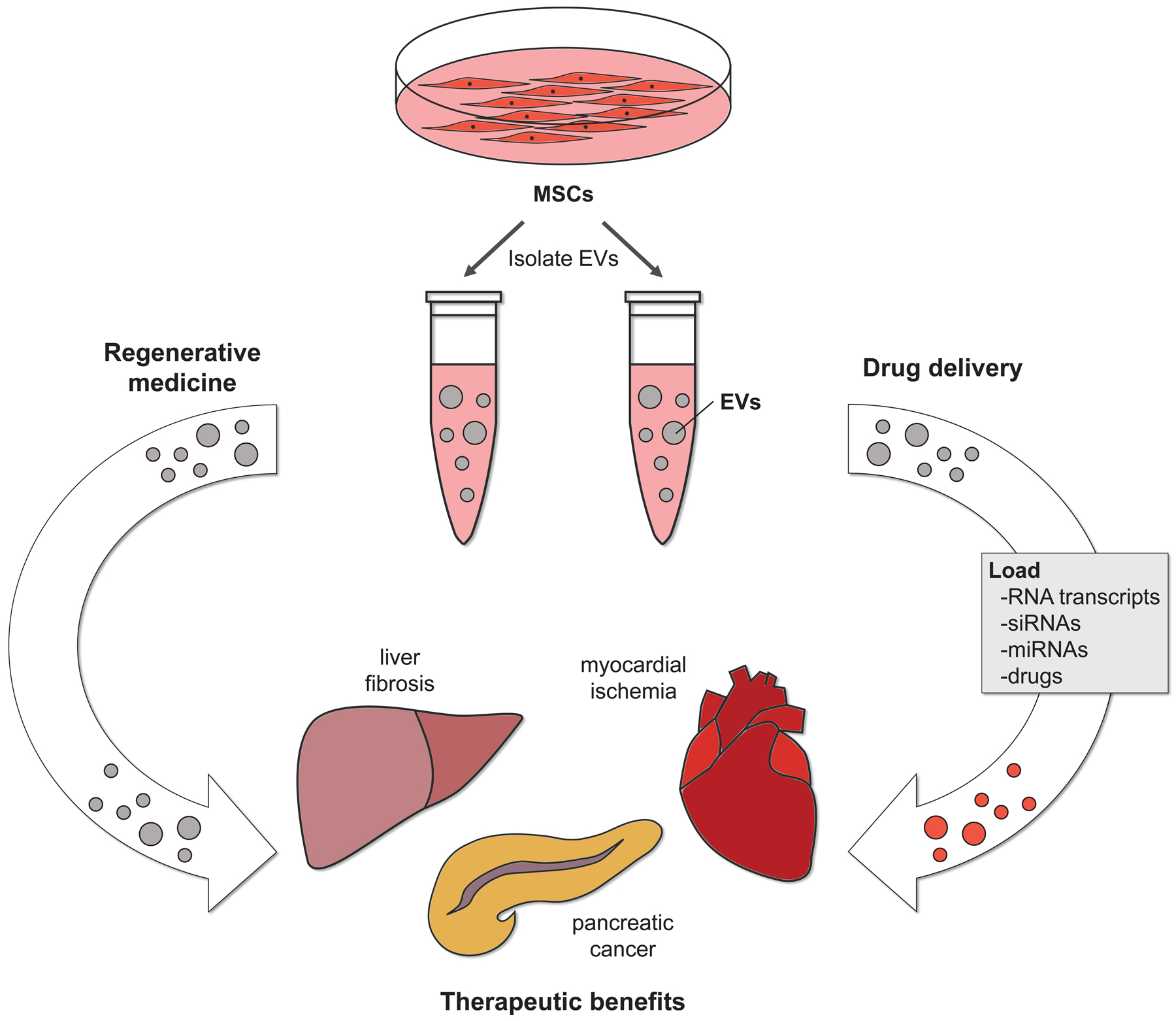
The therapeutic potential of EVs produced by stem cells. The EVs isolated from mesenchymal stem cells (MSCs) have been used to treat various mouse models of disease. These vesicles have been shown to help repair damaged tissues, such that they regain at least a portion of their original function (left side). EVs from stem cells can also be used as a drug delivery system. In this case, the EVs from MSCs are loaded with various types of therapeutic cargo before being administered to the diseased animals. The EV-mediated delivery of therapeutic cargo promotes recovery (right side).
Given these findings, efforts are now turning towards identifying the cargo associated with EVs that is responsible for their regenerative properties. MicroRNAs (miRNAs) are becoming increasingly recognized as bio-active cargo likely to play an important role. In the context of a renal ischemia-reperfusion injury model, it was shown that EVs derived from MSCs mediated the transfer of miR-30 to renal tubular epithelial cells. The increase in the cellular level of miR-30 altered the expression of dynamin-related protein 1 (DRP1), a key regulator of mitochondrial fission, and inhibited the characteristic mitochondrial fragmentation and apoptosis associated with this type of injury [36]. The transfer of miRNAs from EVs to recipient cells was also shown to be important for helping alleviate the complications associated with certain types of liver disease. Specifically, a rodent model of CCl4-induced liver fibrosis has been developed and is characterized by the formation of large collagen deposits, which leads to structural and functional disruption of the liver [39]. However, when these animals were treated with EVs derived from MSCs, the extent of liver fibrosis was reduced. The underlying mechanism responsible for this effect was identified and involved the transfer of miR-122 from the EVs to the hepatic stellate cells in the liver. The presence of miR-122 in the cells was shown to inhibit the expression of genes that are known to promote collagen synthesis, such as insulin-like growth factor receptor 1 (IGFR1), cyclin G1, and prolyl-4-hydroxylase alpha 1. The corresponding reduction in collagen expression resulted in a loss in the collagen deposits and helped preserve liver function [38].
EVs as a Therapeutic Delivery System
EVs are also being examined for their potential as therapeutic vehicles (Figure 2, right side). The EVs generated by stem cells, particularly MSCs, have been isolated and loaded with a variety of therapeutic cargo including RNA transcripts, siRNAs, miRNAs, and drugs. Two of the more commonly used approaches to load cargo into isolated EVs are passive diffusion and electroporation. The loaded EVs are then being administered to patients with various conditions. In this context, EVs have been shown to be taken up by diseased or damaged cells, resulting in their delivering a dose of the therapeutic cargo [40–42].
EVs offer several potential advantages as a therapy delivery system over the more conventional synthetic liposome-based approaches being developed. One such advantage is that EVs are more efficiently taken up by diseased cells, compared to synthetic liposomes. This is thought to be due to the surface composition of the EVs, which includes the presence of various adhesion proteins, i.e. tetraspanins, integrins, and extracellular matrix proteins [1,12,43]. These proteins are thought to help EVs accumulate in specific tissues, raising the possibility that they can be used for targeted therapy approaches. Indeed, efforts to modify the surface composition of EVs to target them to specific locations within the body are currently being undertaken [43]. Being derived from natural sources of the body, another advantage of using EVs to deliver specific cargo is reduced immunogenicity and toxicity [44]. Moreover, EVs are able to cross biological barriers, such as the blood-brain barrier (BBB), and to have a longer half-life in the circulation by avoiding first-pass metabolism in the liver [45].
Recently, MSC-derived EVs have been indeed shown to efficiently deliver siRNAs for the treatment of an animal model of pancreatic cancer. Exosomes from MSCs were electroporated with siRNAs targeting the oncogenic KRas (G12D) mutants. Addition of the MSC-derived engineered exosomes to a human pancreatic cancer cell, Panc-1, induced cell apoptosis. Moreover, upon introducing these exosomes into a patient-derived xenograft model expressing oncogenic KRas (G12D), they increased the survival of the mice and reduced their metastatic burden [46].
One of the limitations of using EVs as a therapy system is the inability to isolate large quantities of vesicles, which would be necessary to treat patients in a reproducible manner. However, MSCs have been shown to generate more EVs, compared to other cell types, making them an attractive source of vesicles for therapeutic applications [46]. There has been a huge effort directed at developing new approaches to efficiently improve the yield of EVs on a large scale. This includes changing the composition of the culturing medium or the culturing condition, such as using the Integra CELLine culture system. This system allows for a constant collection of EVs from the medium, while continuously replenishing nutrients to the cells [47]. If the challenge of isolating large amounts of EVs is met through these approaches, it will accelerate the therapeutic applications of EVs as a drug delivery system.
EVs and Cellular Reprogramming
Another aspect of EV research that has been gaining momentum is their potential use in cellular reprogramming (Figure 3). This idea is largely based on the findings that EVs derived from cancer cells often cause recipient cells to acquire several of the characteristics of transformed cells, including promoting their ability to survive and grow under anchorage independent conditions [3]. Thus, it was logical to consider that EVs isolated from stem/progenitor cells might cause recipient cells to maintain or acquire at least some of the characteristics of stem cells, i.e. express stemness promoting proteins, by transferring factors that are involved in promoting pluripotency, such as RNA transcripts that encode stem cell markers, including the master stemness regulator Oct3/4 [48,49].
Figure 3.
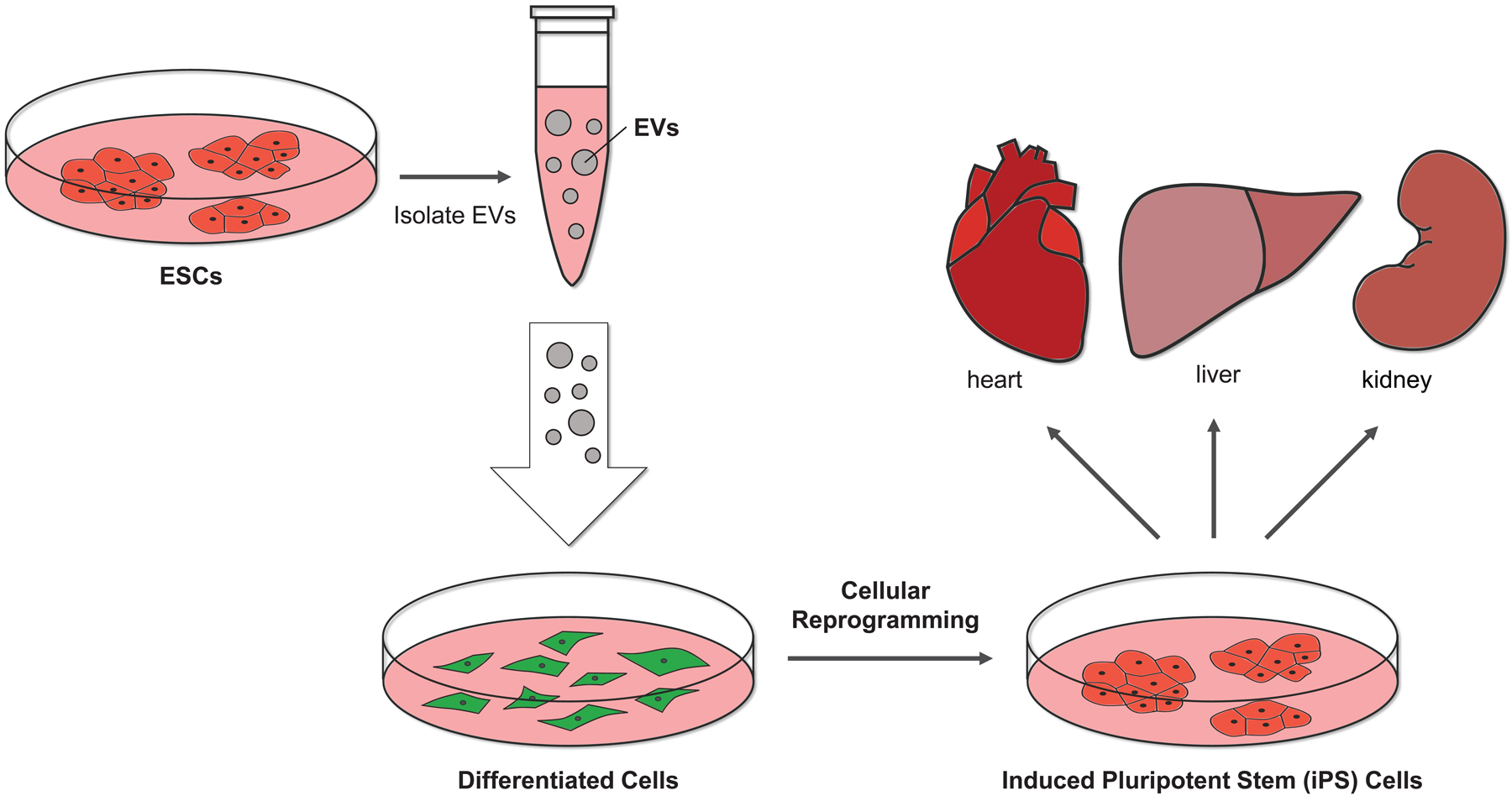
The EVs from ESCs can potentially induce pluripotency. The EVs isolated from ESCs have been shown to contain several different pluripotent markers, including the master regulator of pluripotency, Oct3/4. Thus, it is possible that the EVs generated by stem cells could convert differentiated cells into stem cells (i.e., inducing pluripotency), providing a novel way of generating stem cells for regenerative approaches.
The ability of EVs to mediate the transfer of stemness-promoting cargo to recipient cells was first demonstrated by Ratajczak et al. in 2006. In this study, the EVs produced by both mouse and human ESC lines were shown to contain pluripotent proteins and/or RNA transcripts that encoded pluripotent proteins, including Oct3/4, Wnt-3, Rex-1, Nanog, SCL, and GATA-2. The treatment of murine hematopoietic progenitor SKL cells with these EVs resulted in the activation of mitogen-activated protein kinase (MAPK) p42/44 and Akt protein kinases, and increased the expression of Oct3/4 in the SKL cells. The EVs were also shown to enhance the survival and self-renewal capabilities of these cells [48].
These findings were reinforced by a study showing that EVs derived from ESCs contained the RNA transcripts encoding Oct3/4 and Sox2, as well as miRNAs of the 290 cluster which are known to regulate the cell cycle. When these vesicles were used to treat Müller cells, a type of retinal progenitor cells, their cargo was detected in the cells. Moreover, microarray analysis performed on the EV treated cells showed that the expression levels of genes and miRNAs involved in the maintenance of pluripotency and promotion of cell growth, such as Lin28 and LIF, were upregulated, while the expression of genes and miRNAs involved in promoting differentiation and cell cycle arrest (i.e., GATA4 and miR-let-7 cluster) were downregulated [49].
Although there are several lines of evidence that point to EVs being able to help enhance stemness-related phenotypes in progenitor cells, what continues to be unclear is whether EVs from stem cells can actually cause a differentiated cell to become a stem cell, a process referred to as induced pluripotency. The ability to convert a fully differentiated cell type into a bona fide stem cell was first achieved by Yamanaka and colleagues. They discovered that introducing four pluripotency-related transcription factors, Oct3/4, Sox2, Klf4, and c-Myc, into fibroblasts by viral infection reprogrammed the cells to a pluripotent state. In this case, the reprogrammed cells showed all of the genetic and epigenetic changes typically associated with stem cells, including increased expression of the pluripotent stem cell marker Oct3/4, and changes in the acetylation and methylation status of specific promoter regions. Importantly, these stem cells could be re-differentiated into various cell lineages, proving that this approach can be used to generate patient-specific cells with a low risk of generating an immune response [50].
Since EVs derived from ESCs have been shown to contain proteins that promote stemness or RNA transcripts that encode markers of pluripotency, we and others are becoming interested in determining whether the EVs generated by ESCs can cause fully differentiated cells to undergo cellular reprogramming and become stem cells (Figure 3). If so, this approach would have several advantages over the conventional method for inducing pluripotency, including a decreased risk of chromosomal instability, since neither lentivirus nor retrovirus mediated expression would be used. Therefore, the use of EVs to induce pluripotency raises new and exciting possibilities for regenerative medicine.
Conclusion
The field of EV biology has undergone a major transformation over the last decade. Not long ago, the formation and release of MVs and exosomes from cells were considered specialized processes carried out by a subset of highly aggressive cancer cells. However, it is now recognized that EVs are released by virtually all cells as a mechanism for communicating with their surroundings. Because EVs have been implicated in several different physiological and pathological processes, researchers from diverse fields are now considering how EVs might play important roles in diverse areas of biology. One such field where EVs are just beginning to be appreciated is stem cell biology. The mounting evidence suggests that EVs generated by stem cells contribute to different aspects of early development, and their unique characteristics are being taken advantage of for potential applications in regenerative medicine and cellular reprogramming.
However, there are still many questions to be answered. Foremost among them is whether the distinct classes of EVs generated by stem cells (i.e., MVs versus exosomes) have distinct roles. Several groups have shown that MVs and exosomes are often enriched with specific cargo, suggesting that each class of EV may influence recipient cells in a distinctive manner. Moreover, there are suggestions that sub-classes of both MVs and exosomes exist [51], and how these EVs are formed and function will warrant significant investigation in the upcoming years.
Another important area of future study will be to determine the mechanism used by stem cells to generate MVs and exosomes. Recently, it was discovered that the NAD+-dependent lysine deacetylase sirtuin 1 (Sirt1) is an important regulator of exosome biogenesis in breast cancer cells. Downregulation of Sirt1 expression impaired lysosomal pH and its activity, and as a consequence, resulted in more MVBs fusing with the plasma membrane and releasing exosomes, as opposed to fusing with lysosomes where their exosomal contents are degraded [52].
Interestingly, several groups found that Sirt1 is involved in early development as well. For example, in the absence of Sirt1, trophoblast stem cells showed defects in their ability to properly undergo differentiation, being trapped in a trophoblast progenitor state. Moreover, placental tissue derived from Sirt1-null mice showed morphologic abnormalities [53]. As these findings demonstrate the essential role of Sirt1 in early development, where stem cell-derived EVs have been shown to be involved, they might suggest the potential implication of Sirt1 in the biogenesis of stem cell EVs as well as cancer cell EVs. Answers to these questions will pave the way toward using stem cell derived-EVs in a variety of future research and therapeutic applications.
Significance Statement.
The ability of cells to communicate with their surroundings by producing different classes of extracellular vesicles is known to play important roles in physiological and pathological processes. Here, we will highlight what is known about this form of cell communication in the context of stem cell biology.
Acknowledgments
We would like to thank Cindy Westmiller for her help in preparing this manuscript. This work was supported by NIH grants (GM122575, CA210184) to RAC.
Footnotes
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
Reference
- 1.El Andaloussi S, Mäger I, Breakefield XO, et al. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov 2013; 12: 347–357. [DOI] [PubMed] [Google Scholar]
- 2.Xu R, Rai A, Chen M, et al. Extracellular vesicles in cancer - implications for future improvements in cancer care. Nat Rev Clin Oncol 2018; 15: 617–638. [DOI] [PubMed] [Google Scholar]
- 3.Desrochers LM, Antonyak MA, Cerione RA. Extracellular vesicles: satellites of information transfer in cancer and stem cell biology. Dev Cell 2016; 37: 301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maas SLN, Breakefield XO, Weaver AM. Extracellular vesicles: unique intercellular delivery vehicles. Trends Cell Biol 2017; 27: 172–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Latifkar A, Hur YH, Sanchez JC, et al. New insights into extracellular vesicle biogenesis and function. J Cell Sci 2019; 32: jcs222406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valadi H, Ekström K, Bossios A, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 2007; 9: 654–659. [DOI] [PubMed] [Google Scholar]
- 7.Choi DS, Kim DK, Kim YK, et al. Proteomics, transcriptomics and lipidomics of exosomes and ectosomes. Proteomics 2013; 13: 1554–1571. [DOI] [PubMed] [Google Scholar]
- 8.Théry C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 2018; 7: 1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mathieu M, Martin-Jaular L, Lavieu G, et al. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol 2019; 21: 9–17. [DOI] [PubMed] [Google Scholar]
- 10.Van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol 2018; 19: 213–228. [DOI] [PubMed] [Google Scholar]
- 11.Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol 2014; 14: 195–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desrochers LM, Bordeleau F, Reinhart-King CA, et al. Microvesicles provide a mechanism for intercellular communication by embryonic stem cells during embryo implantation. Nat Commun 2016; 7: 11958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niakan KK, Han J, Pedersen RA, et al. Human pre-implantation embryo development. Development 2012; 139: 829–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sircar M, Thadhani R, Karumanchi SA. Pathogenesis of preeclampsia. Curr Opin Nephrol Hypertens 2015; 24: 131–138. [DOI] [PubMed] [Google Scholar]
- 15.Kelleher AM, Milano-Foster J, Behura SK, et al. Uterine glands coordinate on-time embryo implantation and impact endometrial decidualization for pregnancy success. Nat Commun 2018; 9: 2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feinberg RF, Kliman HJ, Wang CL. Transforming growth factor-β stimulates trophoblast oncofetal fibronectin synthesis in vitro: Implications for trophoblast implantation in vivo. J Clin Endocrinol Metab 1994; 78: 1241–1248. [DOI] [PubMed] [Google Scholar]
- 17.Arici A, Engin O, Attar E, et al. Modulation of leukemia inhibitory factor gene expression and protein biosynthesis in human endometrium. J Clin Endocrinol Metab 1995; 80: 1908–1915. [DOI] [PubMed] [Google Scholar]
- 18.Kojima K, Kanzaki H, Iwai M, et al. Expression of leukemia inhibitory factor in human endometrium and placenta1. Biol Reprod 1994; 50: 882–887. [DOI] [PubMed] [Google Scholar]
- 19.Greening DW, Nguyen HPT, Elgass K, et al. Human endometrial exosomes contain hormone-specific cargo modulating trophoblast adhesive capacity: insights into endometrial-embryo interactions. Biol Reprod 2016; 94: 38. [DOI] [PubMed] [Google Scholar]
- 20.Kurian NK, Modi D. Extracellular vesicle mediated embryo-endometrial cross talk during implantation and in pregnancy. J Assist Reprod Genet 2019; 36: 189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ebner T, Shebl O, Moser M, et al. Group culture of human zygotes is superior to individual culture in terms of blastulation, implantation and life birth. Reprod Biomed Online 2010; 21: 762–768. [DOI] [PubMed] [Google Scholar]
- 22.Stokes PJ, Abeydeera LR, Leese HJ. Development of porcine embryos in vivo and in vitro; evidence for embryo “cross talk” in vitro. Dev Biol 2005; 284: 62–71. [DOI] [PubMed] [Google Scholar]
- 23.Qu P, Qing S, Liu R, et al. Effects of embryo-derived exosomes on the development of bovine cloned embryos. PLoS One 2017; 12: e0174535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saadeldin IM, Kim SJ, Choi YB, et al. Improvement of cloned embryos development by co-culturing with parthenotes: a possible role of exosomes/microvesicles for embryos paracrine communication. Cell Reprogram 2014; 16: 223–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caplan AI. Mesenchymal stem cells. J Orthop Res 1991; 9: 641–650. [DOI] [PubMed] [Google Scholar]
- 26.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science 1999; 284: 143–147. [DOI] [PubMed] [Google Scholar]
- 27.Amado LC, Saliaris AP, Schuleri KH, et al. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc Natl Acad Sci USA 2005; 102: 11474–11479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kurtz A Mesenchymal stem cell delivery routes and fate. Int J Stem Cells 2008; 1: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gnecchi M, He H, Liang OD, et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med 2005; 11: 367–368. [DOI] [PubMed] [Google Scholar]
- 30.Gnecchi M, He H, Noiseux N, et al. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J 2006; 20: 661–669. [DOI] [PubMed] [Google Scholar]
- 31.Shologu N, Scully M, Laffey JG, et al. Human mesenchymal stem cell secretome from bone marrow or adipose-derived tissue sources for treatment of hypoxia-induced pulmonary epithelial injury. Int J Mol Sci 2018; 19: E2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kay AG, Long G, Tyler G, et al. Mesenchymal stem cell-conditioned medium reduces disease severity and immune responses in inflammatory arthritis. Sci Rep 2017; 7: 18019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng J, Wang Q, Leng W, et al. Bone marrow-derived mesenchymal stem cell-conditioned medium attenuates tubulointerstitial fibrosis by inhibiting monocyte mobilization in an irreversible model of unilateral ureteral obstruction. Mol Med Rep 2018; 17: 7701–7707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Timmers L, Lim SK, Arslan F, et al. Reduction of myocardial infarct size by human mesenchymal stem cell conditioned medium. Stem Cell Res 2007; 1: 129–137. [DOI] [PubMed] [Google Scholar]
- 35.Lai RC, Arslan F, Lee MM, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res 2010; 4: 214–222. [DOI] [PubMed] [Google Scholar]
- 36.Gu D, Zou X, Ju G, et al. Mesenchymal stromal cells derived extracellular vesicles ameliorate acute renal ischemia reperfusion injury by inhibition of mitochondrial fission through miR-30. Stem Cells Int 2016; 2016: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xin H, Li Y, Buller B, et al. Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells 2012; 30: 1556–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lou G, Yang Y, Liu F, et al. MiR-122 modification enhances the therapeutic efficacy of adipose tissue-derived mesenchymal stem cells against liver fibrosis. J Cell Mol Med 2017; 21: 2963–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee UE, Friedman SL. Mechanisms of hepatic fibrogenesis. Best Pract Res Clin Gastroenterol 2011; 24: 195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alvarez-Erviti L, Seow Y, Yin H, et al. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol 2011; 29: 341–345. [DOI] [PubMed] [Google Scholar]
- 41.Tian Y, Li S, Song J, et al. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials 2014; 35: 2383–2390. [DOI] [PubMed] [Google Scholar]
- 42.Shimbo K, Miyaki S, Ishitobi H, et al. Exosome-formed synthetic microRNA-143 is transferred to osteosarcoma cells and inhibits their migration. Biochem Biophys Res Commun 2014; 445: 381–387. [DOI] [PubMed] [Google Scholar]
- 43.Johnsen KB, Gudbergsson JM, Skov MN, et al. A comprehensive overview of exosomes as drug delivery vehicles - Endogenous nanocarriers for targeted cancer therapy. Biochim Biophys Acta 2014; 1846: 75–87. [DOI] [PubMed] [Google Scholar]
- 44.El Andaloussi S, Lakhal S, Mäger I, et al. Exosomes for targeted siRNA delivery across biological barriers. Adv Drug Deliv Rev 2013; 65: 391–397. [DOI] [PubMed] [Google Scholar]
- 45.Chen CC, Liu L, Ma F, et al. Elucidation of exosome migration across the blood–brain barrier model in vitro. Cell Mol Bioeng 2016; 9: 509–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kamerkar S, Lebleu VS, Sugimoto H, et al. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 2017; 546: 498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mitchell JP, Court J, Mason MD, et al. Increased exosome production from tumour cell cultures using the Integra CELLine Culture System. J Immunol Methods 2008; 335: 98–105. [DOI] [PubMed] [Google Scholar]
- 48.Ratajczak J, Miekus K, Kucia M, et al. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: Evidence for horizontal transfer of mRNA and protein delivery. Leukemia 2006; 20: 847–856. [DOI] [PubMed] [Google Scholar]
- 49.Katsman D, Stackpole EJ, Domin DR, et al. Embryonic stem cell-derived microvesicles induce gene expression changes in Müller cells of the retina. PLoS One 2012; 7: e50417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006; 126: 663–676. [DOI] [PubMed] [Google Scholar]
- 51.Jeppesen DK, Fenix AM, Franklin JL, et al. Reassessment of exosome composition. Cell 2019; 177: 428–445.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Latifkar A, Ling L, Hingorani A, et al. Loss of sirtuin 1 alters the secretome of breast cancer cells by impairing lysosomal integrity. Dev Cell 2019; 49: 393–408.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arul Nambi Rajan K, Khater M, Soncin F, et al. Sirtuin1 is required for proper trophoblast differentiation and placental development in mice. Placenta 2018; 62: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


