Abstract
In a randomized, cross-over study in healthy volunteers (N=14), 2 g probenecid (PRO)-boosted pharmacokinetics of single dose 600 mg tenofovir disoproxil fumarate (TDF) / 400 mg emtricitabine (FTC) (Treatment, T +PRO) was compared with the current On-Demand HIV Pre-Exposure Prophylaxis (PrEP) from the IPERGAY study (a 600 mg TDF/400 mg FTC on day 1 and 300 mg TDF/200 mg FTC on days 2 and 3) (Control, C IPERGAY). PRO increased mean single dose plasma AUC0−∞ of TFV and FTC by 64% and 62%, respectively. The 24-hour TFV-diphosphate (TFV-DP) concentrations in peripheral blood mononuclear cells (PBMC) were significantly higher (~30%) in T +PRO compared to C IPERGAY and remained nearly unchanged (on average over 20 fmol/106cells) for 72 hours, suggesting prolonged exposure by PRO. The interaction between FTC and PRO was unexpected and novel. Although further study is needed, PRO-boosted TDF/FTC may allow simpler dosing and improved adherence to HIV PrEP.
Keywords: HIV, Pre-exposure prophylaxis, probenecid, tenofovir, emtricitabine
Introduction
The prevention of human immunodeficiency virus (HIV) transmission is a worldwide imperative. Men who have sex with men (MSM) and transgender women (TGW) are 24 and 49 times more likely to acquire HIV than the general population, respectively (1). The mainstay for pre-exposure prophylaxis (PrEP) in HIV-negative individuals is the use of a combination pill containing 300 mg of tenofovir disoproxil fumarate (TDF) and 200 mg of emtricitabine (FTC), originally marketed as Truvada® (2). While many new drugs or formulations are under development for HIV PrEP, Truvada® is the only agent currently FDA-approved for HIV PrEP. It has currently 4 approved therapeutic equivalents or generics that may increase the accessibility and affordability of TDF/FTC for HIV PrEP worldwide. The availability and use of TDF/FTC for HIV PrEP therapy is linked to a significant reduction in new HIV infections in the US (3).
Traditionally, once daily oral dosing of 300 mg TDF/200 mg FTC demonstrated a 92% reduction of new HIV infections in MSM and TGW at high risk for infection when coupled with detectable drug levels reflecting compliance (4). The IPERGAY study, an “On-Demand” dosing regimen of 2 tablets of 300 mg TDF/200 mg FTC 2–24 hours prior to HIV exposure and 1 tablet of 300 mg TDF/200 mg FTC every 24 hours after sexual activity for 2 doses resulted in an 86% reduction in new HIV infections in MSM and TGW (5). Thus, On-Demand dosing provided nearly an equivalent reduction in HIV transmissions compared to traditional daily HIV PrEP with the benefit of significantly less drug dosing requirements. The promise of effective HIV prophylaxis with traditional or On-Demand HIV PrEP regimens is highly dependent on the population and adherence (6).
Medication adherence is a major concern in individuals at high risk for acquiring HIV and crucial for effective protection from HIV acquisition (4,6). Daily or fixed-duration dosing regimens can be burdensome, complex, and expensive for patients. As a result, a single oral dose of TDF/FTC for HIV PrEP could be more advantageous for patient adherence, particularly as an On-Demand HIV PrEP.
Our evaluation of the pharmacokinetic interaction with probenecid (PRO) seeks to evaluate a “boosting strategy” for TDF/FTC using PRO as a booster for the single-dose TDF/FTC kinetics for On-Demand HIV PrEP. Historically, PRO was used to minimize renal secretion of other anti-infective agents via organic anion transporters (OAT1 or OAT3) inhibition (7). Inhibition of renal elimination with PRO increases or prolongs exposure of several anti-infectives (i.e. penicillin, cefazolin) (7). Tenofovir (TFV), the TDF moiety found in circulation, exhibits similarly active renal secretion mediated via OAT1 and OAT3 transporters, making TFV an attractive target for the inhibitory effect of PRO (8). The PRO “boosting” TDF drug-drug interaction (DDI) may provide clinical benefit, simplified dosing and improved adherence, and offer a cost-effective and accessible single-dose alternative for On-Demand HIV PrEP as per the IPERGAY regimen. These potential advantages could improve widespread uptake of PrEP in both resource-replete and resource-limited settings. Thus, we studied the pharmacokinetics of TDF and FTC in combination with PRO and compared it to the current 3-day TDF/FTC IPERGAY HIV PrEP regimen. A prospective, randomized, open-label, two-phase, active-control, pivotal, cross-over, pharmacokinetic study was conducted in healthy volunteers to compare the plasma, urine, and peripheral blood mononuclear cells (PBMC) pharmacokinetics of single-dose PRO with TDF/FTC to the current 3-day IPERGAY HIV PrEP regimen.
Results
Participant Demographics
The enrolled healthy male volunteers (n=15) were self-identified Caucasian (n=10), Asian (n=2), Black (n=2), and bi-racial (n=1). The median age of participants was 26 years (range: 18–47 years) with a median height of 69 inches (range: 64–74) and weight of 75 kg (range: 59–86). All participants had a baseline CrCl >90mL/min with a median SCr of 1 mg/dL (range: 0.72–1.23 mg/dL).
Plasma and Urine Pharmacokinetics of TFV and FTC
The plasma concentration-time profiles of the T +PRO (2 g) “boosted” single-dose TFV/FTC treatment compared to the C IPERGAY HIV PrEP treatment in 14 healthy volunteers are shown in Figure 2. The single-dose TFV (600 mg) and FTC (400 mg) pharmacokinetic parameters (derived using the sampling time of 24 hours after dosing) in both arms are listed in Table 1. PRO significantly increased the single-dose exposure (AUC0–24h) of both TFV and FTC by approximately 60% in the plasma. TFV and FTC apparent oral clearance, CL/F, significantly decreased by nearly 40% in the presence of PRO. A similar trend was observed for the apparent volume of distribution, Vz/F, of both TFV and FTC. Renal clearances of single dose TFV and FTC were significantly decreased in the presence of PRO. There was no difference in the estimated t1/2 of TFV and FTC between treatments. However, the terminal elimination t1/2 of TFV and FTC could not be accurately estimated from the short sampling time (0–24 hours). TFV and FTC demonstrate biphasic elimination. Thus, the single-dose data appears to primarily capture the distribution phase of TFV and FTC. Based on the concentration-time data over 72 hours in the T +PRO treatment, the true terminal t1/2 is approximately 18 h for TFV and 10 h for FTC, which corresponds well with the literature (14). PRO did not significantly change maximum plasma concentration (Cmax) or time to reach maximum plasma concentration (Tmax) of TFV. There was a 20% increase in FTC Cmax for the T +PRO treatment.
Figure 2.
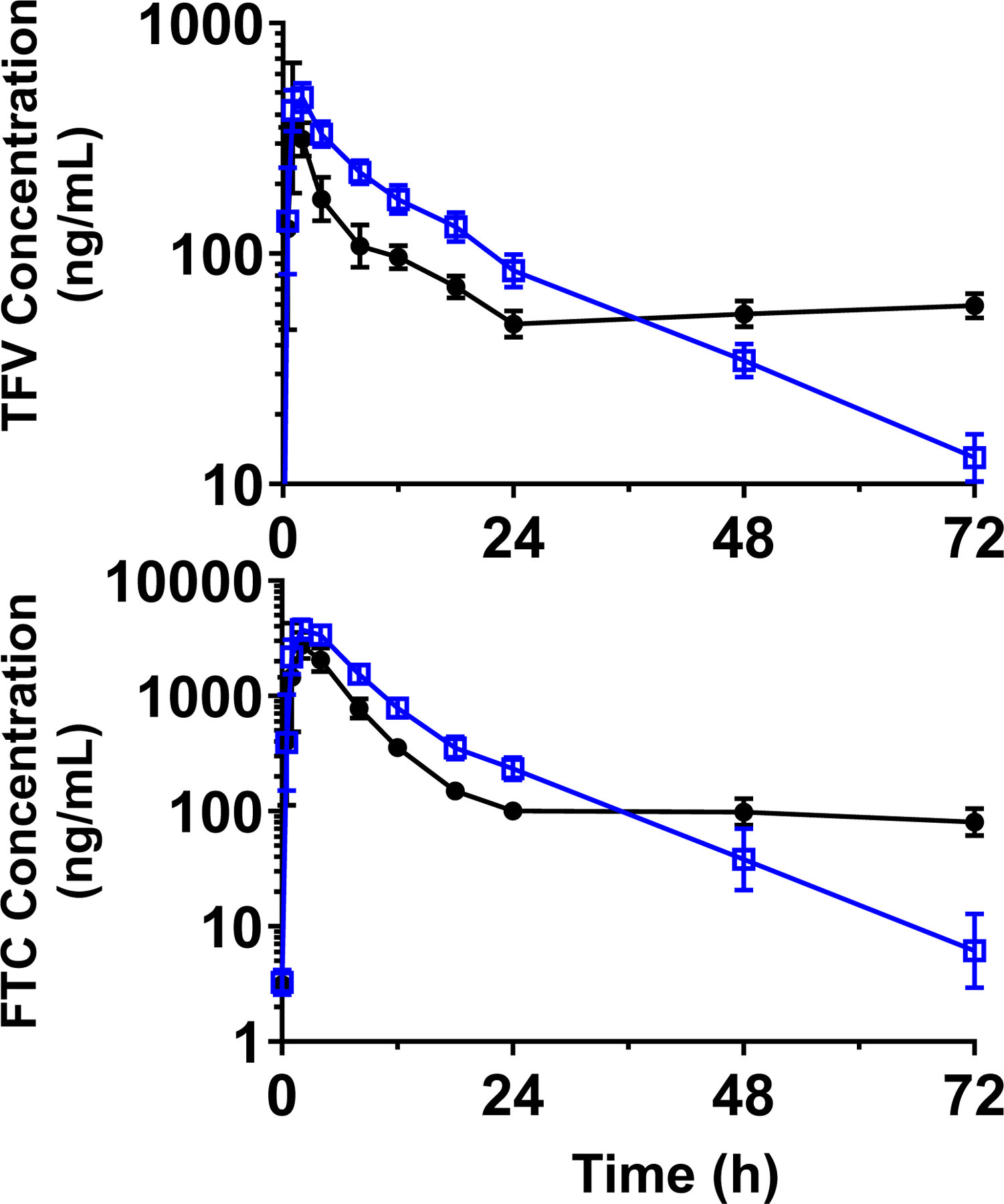
GM and 95% CI for plasma concentration-time profiles for TFV (top) and FTC (bottom) concentrations for the T +PRO (open blue squares) and C IPERGAY (closed black circles) treatments.
Table 1.
Single-dose pharmacokinetics of TFV and FTC in the test (T +PRO) and control (C -PRO) treatments represented as geometric mean (GM) or geometric mean ratio (GMR, T/C) and 90% confidence intervals (90% CI).
| Tenofovir (TFV) | Emtricitabine (FTC) | |||||
|---|---|---|---|---|---|---|
| PK Parameters | C | T | GMR (T/C) | C | T | GMR (T/C) |
| Cmax (ng/mL) | 529 | 538 | 1 | 3426 | 4108 | 1.2 |
| (464–602) | (502–577) | (0.91–1.1) | (3137–4806) | (3720–4535) | (1.0–1.4) | |
| Tmax (h)* | 1 | 1.5 | 2 | 2 | ||
| (1–4) | (1–2) | (1–4) | (1–4) | |||
| AUC0-INF (ng*h/mL) | 3958 | 6444 | 1.6 | 20635 | 34030 | 1.6 |
| (3618–4331) | (5827–7127) | (1.5–1.8) | (18984–22431) | (30886–37494) | (1.5–1.9) | |
| AUC0–24 (ng*h/mL) | 3026 | 4963 | 1.6 | 19488 | 31482 | 1.6 |
| (2799–3271) | (4576–5385) | (1.5–1.8) | (17830–21300) | (28856–34346) | (1.4–1.8) | |
| CL/F (L/h) | 152 | 93 | 0.61 | 19 | 12 | 0.61 |
| (139–166) | (84–103) | (0.56–0.67) | (18–21) | (11–13) | (0.54–0.69) | |
| kel (1/h) | 0.055 | 0.059 | 0.1 | 0.099 | ||
| (0.050–0.60) | (0.054–0.064) | (0.087–0.12) | (0.090–0.11) | |||
| t1/2 (h)a | 13 | 12 | 6.9 | 7 | ||
| (12–14) | (11–13) | (5.9–7.9) | (6.3–7.7) | |||
| t1/2 (h)b | 18 | 10 | ||||
| (16–19) | (8–12) | |||||
| Vz/F (L) | 2778 | 1587 | 0.57 | 192 | 119 | 0.62 |
| (2534–3044) | (1455–1731) | (0.51–0.64) | (158–231) | (107–132) | (0.53–0.73) | |
| % dose in urine | 7.8 | 7.8 | 62 | 68 | ||
| (6.4–9.4) | (6.6–9.3) | (53–72) | (57–80) | |||
| Clrenal (mL/min) | 257 | 158 | 0.60 | 210 | 153 | 0.7 |
| (212–310) | (131–190) | (0.52–0.73) | (175–252) | (124–190) | (0.54–0.89) | |
represents median values;
is recovered up to 24 hour data;
72 is recovered up to 24 hour data.
Intracellular Exposure of TFV-DP and FTC-TP
The intracellular concentration-time profiles and outcomes of TFV-DP and FTC-TP between the T +PRO and C IPERGAY treatments are shown in Figure 3 and Table 2. At 24 hours, TFV-DP and FTC-TP concentrations in the T +PRO treatment were on average 30% and 10% higher than the C IPERGAY treatment, respectively. The two additional doses of TDF in the C IPERGAY treatment resulted in nonlinear accumulation of TFV-DP. In the C IPERGAY treatment, TFV-DP intracellular concentrations doubled after the second dose (C48h). FTC-TP concentrations did not appear to increase with the additional doses and demonstrated wide variability suggesting accumulation of FTC-TP is unlikely. In the T +PRO treatment, TFV-DP concentrations remained consistent over the 72 hours following single-dose administration, but FTC-TP concentrations steadily decreased over 72 hours. At both 48 hours and 72 hours, TFV-DP and FTC-TP concentrations were significantly higher in the C IPERGAY treatment compared to T +PRO treatment.
Figure 3.
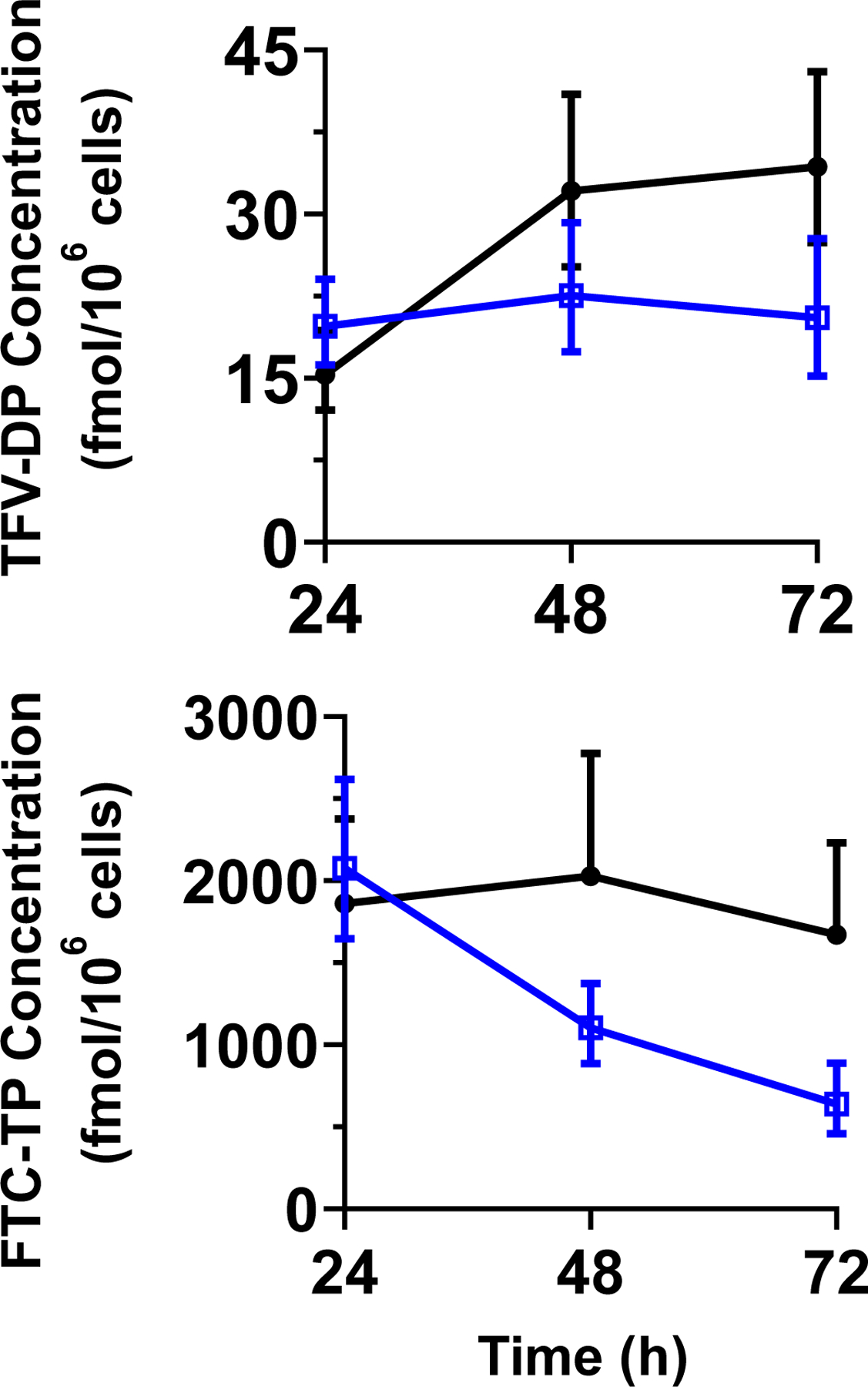
GM and 95% CI intracellular concentration-time profiles of TFV-DP (top) and FTC-TP (bottom) concentrations for the T +PRO (open blue squares) and C IPERGAY (closed black circles) treatments.
Table 2.
Geometric mean (GM) intracellular TFV-DP and FTC-TP concentrations in the T +PRO and Control IPERGAY treatments and geometric mean ratio (GMR, T/C) and 90% confidence intervals (90% CI).
| PBMC Concentration | Tenofovir-DP | Emtricitabine-TP | ||||
|---|---|---|---|---|---|---|
| C | T | GMR (T/C) | C | T | GMR (T/C) | |
| C24h (fmol/106 cells) | 15 | 20 | 1.3 | 1861 | 2077 | 1.1 |
| (13–19) | (17–23) | (1.0–1.6) | (1523–2274) | (1716–2512) | (0.88–1.4) | |
| C48h (fmol/106 cells) | 32 | 23 | 0.7 | 2029 | 1103 | 0.54 |
| (26–19) | (18–28) | (0.52–0.94) | (1569–2624) | (922–1320) | (0.40–0.73) | |
| C72h (fmol/106 cells) | 34 | 21 | 0.6 | 1671 | 639 | 0.38 |
| (29–41) | (16–26) | (0.42–0.86) | (1318–2118) | (488–838) | (0.26–0.56) | |
Bioequivalence test for the primary endpoint
The plasma AUC0–72h and AUC0–24h ratio of the T +PRO treatment compared to C IPERGAY treatment for TFV and FTC are shown in Table 3. Specifically, plasma AUC0–72h for the C IPERGAY treatment was calculated using nonparametric superposition to accommodate the multiple-dose regimen. The concentration-time profiles for multiple-dose TFV and FTC are shown as Supplemental Figure 1. The primary endpoint, AUC0–72h GMR, of 0.86 was 14% (90% CI = 2–24%) lower in the T +PRO treatment for both TFV and FTC. T +PRO treatment did not achieve equivalent AUC0–72h exposure compared to C IPERGAY HIV PrEP. The single-dose plasma AUC0–24h was also compared to further assess the drug-drug interaction potential of PRO with TDF and FTC. The comparison of the AUC0–24h showed that the T +PRO boosted the AUC0–24h exposure by 60% (TFV GMR = 1.64, 90% CI = 1.54–1.75; FTC GMR = 1.62, 90% CI = 1.43–1.82).
Table 3.
Geometric least square mean AUC equivalence test for TFV/TFV-DP and FTC/FTC-TP in the test (T, +PRO) compared to control (C, IPERGAY) treatments and GMR (T/C) with 90% confidence intervals (90% CI).
| PK Parameter | Tenofovir | Emtricitabine | ||||||
|---|---|---|---|---|---|---|---|---|
| C | T | GMR | 90% CI | C | T | GMR | 90% CI | |
| PBMC AUC0–72h | 1.60 | 1.24 | 0.774 | (0.614–0.976) | 117 | 83.4 | 0.712 | (0.561–0.904) |
| Plasma AUC0–72h | 8.05 | 6.97 | 0.865 | (0.764–0.980) | 41.1 | 35.5 | 0.864 | (0.759–0.984) |
| Plasma AUC0–24h | 3.03 | 4.96 | 1.64 | (1.54–1.75) | 19.5 | 31.5 | 1.62 | (1.43–1.82) |
The PBMC AUC0–72h GMR of the T +PRO treatment compared to C IPERGAY treatment was 23% and 29% lower in the T +PRO treatment for TFV-DP and FTC-TP, respectively. Therefore, the overall intracellular exposures of TFV-DP and FTC-TP of the T + PRO treatment were not equivalent to the C IPERGAY treatment.
Safety
All healthy volunteers enrolled were included in the safety analysis. The observed AEs are summarized in Table 4. Overall, the reported AEs were considered low grade in severity (grade 1 and 2). Eighteen adverse events occurred during the entire study with 8 of the 15 subjects enrolled experiencing at least one adverse event. Subjects in the T +PRO treatment experienced more adverse events than the C IPERGAY treatment. The most common adverse event was nausea followed by headache. One subject withdrew from the study due to gastroesophageal reflux disease (GERD) which occurred 2 weeks after T +PRO treatment was completed during the washout period and required medical intervention. This incident was determined probable (and not definitely) related to the study intervention since it occurred during the washout period. Of note, the subject reported prior history of gastric reflux.
Table 4.
Adverse events reported in healthy volunteers in the test (T, +PRO) and control (C, IPERGAY) treatments.
| Adverse Event | C IPERGAY | T +PRO | ||
|---|---|---|---|---|
| # of Subjects (% of 14 total) | Severity grade | # of Subjects (% of 15 total) | Severity grade | |
| Headache | 2 (14) | 1 | 4 (27) | 1 |
| Nausea | 2 (14) | 1 | 6 (40) | 1 |
| Vomiting | 1 (7) | 2 | ||
| Loss of appetite | 1 (7) | 2 | ||
| Nose bleed | 1 (7) | 1 | ||
| GERD | 1 (7) | 2 | ||
| Total AE | 4 | 14 | ||
- Severity grade based on NCI Common Toxicity Criteria.
- Grade 1 = mild; Grade 2 = moderate
In the T +PRO treatment, serum creatinine significantly increased from baseline at 24 and 72 hours with mean differences of 0.14 mg/dL and 0.07 mg/dL, respectively.
Discussion
This work is the first to report 1) the clinical pharmacokinetic outcomes of TDF/FTC in the presence of PRO and 2) the multiple-dose plasma TFV/FTC and intracellular TFV-DP/FTC-TP disposition profiles for the IPERGAY HIV PrEP regimen.
In the present study, PRO significantly increased TFV and FTC plasma exposure to similar extent (by approximately 60%) and decreased total and renal clearances. At 24 hours after dosing, a modest but statistically significant increase in intracellular TFV-DP concentrations (~30%; p=0.024) were noted while FTC-TP concentrations were not different between T +PRO and C IPERGAY treatments. TFV and FTC are predominantly cleared by the kidney via glomerular filtration and active secretion. The glomerular filtration of these drugs is not affected by PRO because the renal clearances exceed the GFR (>90 mL/min) in the presence of PRO. It is well established that OAT-mediated (OAT1 and OAT3) active uptake in to the proximal tubule as well as MRP-mediated (MRP4) efflux to the tubular lumen are responsible for the renal secretion of TFV (8). In vitro studies indicate that PRO inhibits OAT1 and OAT3 at therapeutically relevant concentrations. Indeed, PRO is known to alter the pharmacokinetics of several OAT1/OAT3 substrates. Although PRO-mediated clinical drug interactions with “substrates” of MRP4 have been reported, there is no clear evidence in vitro that PRO is an inhibitor of MRP4 (9,10). We noted that PRO also increased serum creatinine, a known substrate of OAT1 and OAT3. It follows that the mechanism by which PRO increased TFV plasma exposure in the present study could be attributed to inhibition of OAT1/OAT3 by PRO. Of note, the effect of PRO on TFV exposure was relatively small (~60% increase) compare to its effects on other substrates of OAT1 and OAT3 such as cefoxitin and furosemide, which were over 2-fold (11,12). This could be in part due to differences in the contribution of active tubular secretion to the total renal excretion; the contribution of tubular secretion (20–30%) to the overall excretion of TFV renal excretion is relatively small. In contrast to TFV, the specific renal transporters involved in FTC secretion have not been unequivocally identified. Based on in vitro data, FTC renal secretion is generally believed to be mediated through OCT transporters on the basolateral membrane (8,13). In this context, the significant increase in FTC exposure by PRO observed in the present study was unexpected. OAT3 was also implicated in the uptake transport of FTC in vitro, although the primary data were not available to evaluate this claim (14). The specific rate-limiting active transporter responsible for FTC renal secretion remains to be established. Although PRO is generally believed to be selective inhibitor of OATs, the possibility that it may also inhibit OCTs cannot be ruled out. Additional studies are needed to confirm the specific mechanisms underlying this observed drug-drug interaction.
Intracellular concentrations of TFV-DP and FTC-TP from single dose 600 mg TDF / 400 mg FTC at 24h were similar to published observations (15). After single dose TDF and PRO, TFV-DP concentration remains unchanged over 72 hours demonstrating a long intracellular half-life. After multiple doses, our data shows TFV-DP demonstrates a non-linear accumulation in PBMC. It is reported that TFV-DP has a t1/2 of 2 days after a single dose and 4–7 days at steady state in PBMC (15–19). Our data suggests that when TFV is given with PRO, TFV-DP t1/2 is longer than 3 days. In contrast, after single dose FTC and PRO, there is a decline in FTC-TP concentration between 24h and 72h in a linear and first-order fashion suggesting our sampling times captured the elimination phase of FTC. This would be supported by the literature suggesting FTC-TP t1/2 is 17–33 hours (18–19). FTC-TP intracellular accumulation is unclear after multiple doses, with a mild increase at 48h and decrease at 72h. FTC-TP was reported to have a 1.7-fold accumulation in PBMC from single dose to steady state (~3 days) (18). It is difficult to draw conclusions for FTC-TP concentrations in the control arm due to differences in sampling time. PBMC sampling at an earlier time (i.e. 2–4 h after dose) may better capture maximum FTC-TP concentrations (18).
The overall probenecid-boosted TFV and FTC plasma and PBMC AUC0–72h did not meet the pre-defined equivalence endpoints when compared to the IPERGAY HIV PrEP regimen. Given a 4 to 12 hour half-life of probenecid (20), it is not surprising that the effects of probenecid on TFV and FTC would be much less evident during the 2nd or 3rd days of the study. An important limitation of our study is that estimation of the true elimination phase in the C IPERGAY treatment was not accurately captured due to the study design. We used each participant’s kel from the T +PRO treatment for nonparametric superposition of the 2nd and 3rd dose in the C IPERGAY treatment to estimate AUC0–72h. The t1/2 recovered up to 72 hours from the T +PRO treatment corresponded well with literature data (14) for TFV (~ 18 h) and FTC (~ 10 h) without PRO, suggesting PRO did not impact the overall t1/2. Therefore, we believe that this estimation is reasonable for the primary endpoint.
The required concentrations of TFV-DP and FTC-TP in PBMCs for On-demand PrEP to prevent HIV infection is not well established making it difficult to predict the clinical relevance of single-dose PRO-boosted TDF. Anderson et al. established previously TFV-DP concentrations above 16 fmol/106 cells (95% CI: 3–28) and FTC-TP above 3.7 (95% CI: 1.2–6.1) pmol/106 cells in PBMCs were associated with a 90% reduction in the risk of acquiring HIV-1 infection for daily TDF/FTC HIV PrEP (21). However, these intracellular efficacy “cut-off” concentrations are difficult to confidently apply outside of this study by Anderson et al. due to unknown drug degradation parameters from prolonged storage of their PBMC samples whereas in our study PBMC intracellular concentrations were measured relatively quickly. Rectal mucosal tissue is one of the first sites susceptible for HIV transmission in MSM and TGW. Active drug exposure at initial sites vulnerable for HIV acquisition is crucial for non-daily HIV PrEP (22). Literature suggests the TFV-DP rectal mononuclear cell to intracellular (PBMC) concentrations are more than 10 times greater and reach steady state within 5 days (18,22), while FTC-TP rectal concentrations are negligible but highest in PBMC cells reaching maximum concentrations within 2–4 hours (15,18). Due to financial and practical considerations, rectal biopsies were not obtained in this study but would undoubtedly contribute to interpretation of HIV PrEP pharmacokinetic analysis. This may suggest TDF is the moiety in TDF/FTC that provides prolonged rectal HIV protection while FTC may provide rapid PBMC protection in non-daily HIV PrEP regimens. In this context, the clinical relevance of our findings require further investigation.
In summary, we have shown for the first time that PRO reduces the renal secretion of TFV via inhibition of OAT1 and OAT3. We also observed that PRO increased plasma exposure of FTC. This interaction was unexpected and mechanistic studies are warranted. As nondaily HIV PrEP with TDF/FTC becomes more widely accepted, therapy optimized for patient adherence, reduce pill burden, and cost may provide the foundation for HIV PrEP worldwide, particularly in countries with resource- and healthcare- limited settings. Our findings provide proof-of-concept that inhibition of renal transporters alter the disposition of TFV/FTC. However, whether these data would be adequate to recommend PRO-boosted TFV/FTC regimen as simplified and cost-effective PrEP needs further study, particularly to confirm the required intracellular concentrations of these agents for On-demand HIV PrEP including in other target tissues (e.g., rectal).
Methods
Study population
Inclusion.
Non-smoker male participants (18–55 years old) within 32% of their ideal body weight and determined to be healthy through pre-enrollment medical assessment (medical history, laboratory blood and urine tests) were enrolled. The study was limited to males to minimize inter-subject variance due to known sex-based differences in TFV disposition (23). Eligible subjects agreed to refrain from taking medications and herbal/dietary supplements that may interact with TDF, FTC, or PRO for at least two weeks prior to the start of the study and through study completion. The protocol was approved, in advance, by the Indiana University School of Medicine Institutional Review Board (IRB). All volunteers provided written informed consent that was approved by the Indiana University IRB. The study was performed in accordance with ethical standards in the Declaration of Helsinki. The study is registered in ClinicalTrials.gov under NCT03202511.
Exclusion.
Subjects were excluded if they were female or had insufficient renal function (CrCl ≤ 95 mL/min), current major or chronic illness (i.e. hypertension, HIV antibody positive, hepatitis B surface antigen positive), blood donations within the past two months, or conditions that placed the subject at a higher risk for contracting HIV during the study period (i.e. drug abuse, history of sexually transmitted infections, ≥1 sexual partner in the past 6 months).
Study Design
A prospective, randomized, open-label, two-treatment, active-control, pivotal, cross-over, pharmacokinetic study was conducted in healthy volunteers to compare the plasma, urine, and peripheral blood mononuclear cells (PBMC) pharmacokinetics of single-dose PRO with TDF/FTC to the current 3-day IPERGAY HIV PrEP regimen. Participants were randomized (SAS, PROC RANDOM, v. 9.4) by blocks of 4 to initially receive one of two sequences of treatments: (1) the test (“T”) treatment (2g PRO and 600mg TDF / 400mg FTC on day 1 only) and (2) the control (“C”) treatment IPERGAY (600mg TDF / 400mg FTC followed by day 1, 300mg TDF / 200mg FTC on days 2 and 3) (Figure 1). The wash out was a period of at least 6 weeks before participants were assigned to the other study treatment. All medications were purchased, stored, and dispensed by the Indiana University Investigational Drug Services (IDS) Pharmacy (TDF/FTC NDC#:61958-0701-01; PRO NDC#:00378-0156-01 / 68084-0945-25). All medications were administered by trained personnel in the Indiana University Clinical Research Center (CRC).
Figure 1.
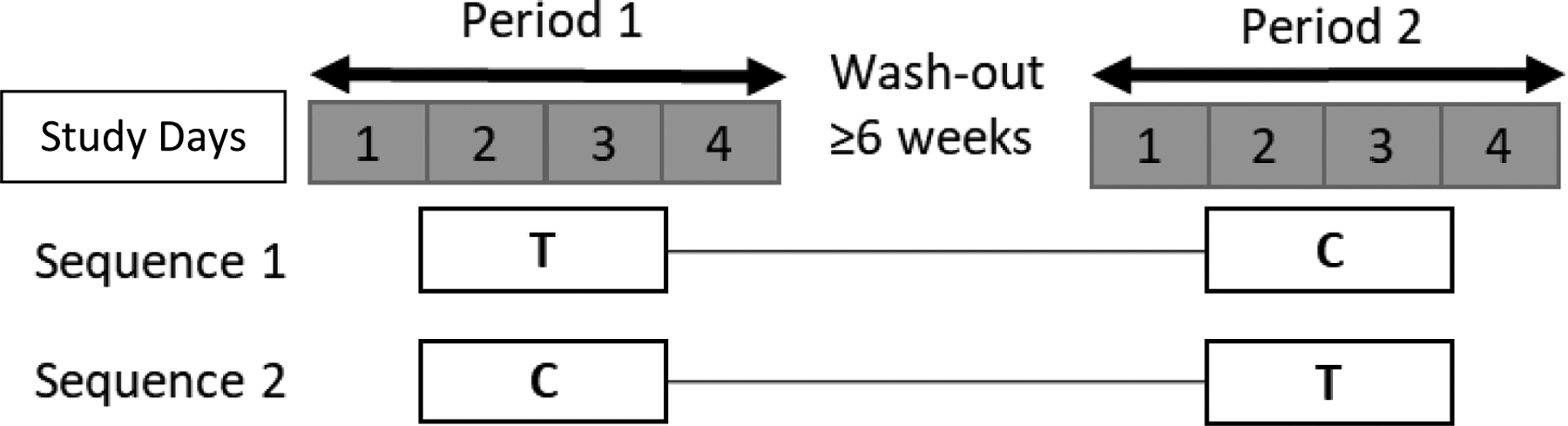
Study Design. Study periods are shown in days 1–4. Study interventions are (1) “T” test +PRO Treatment = 2 g PRO + 600 mg TDF/ 400 mg FTC at t=0 h or (2) “C” control IPERGAY Treatment = 600 mg TDF/ 400 mg FTC at t=0 h; 300 mg TDF/ 200 mg FTC at t= 24, 48 h. Treatment regimens were separated by ≥ 6 weeks wash-out period.
Procedures and Visits.
All study procedures and visits were conducted at the CRC and identical for both test and control treatments with the exception of drug administration regimens. Participants arrived at the CRC for drug administration, blood and urine collection, and safety evaluation on days 1–4 of the study. The first dose of drug was administered on Day 1 at time (t) 0 h after an overnight fast. Subjects were served a full meal 4 hours after dosing on Day 1. All doses were administered with ~250 mL water. Participants stayed overnight in the CRC days 1 through 2 (0–24 hours), followed by ~1 hour outpatient visits on days 3 (48 hour) and 4 (72 hour). On day 4, subjects returned their urine jugs and the final blood draw was collected before completion of the treatment period.
Sample Collections.
Samples collected included (1) blood (plasma, isolated PBMCs) and (2) urine. Blood samples were drawn (~10mL) at 0, 0.5, 1, 2, 4, 8, 12, 18, 24, 48, and 72 hours post-dose. Plasma was harvested for TFV and FRC bioanalytical and pharmacokinetic analysis. Peripheral blood mononuclear cell (PBMC) samples were obtained at 0, 24, 48, and 72 hours. PBMC samples were used for TFV-DP & FTC-TP bioanalytical and pharmacokinetic analysis. Blood samples for the first 24 hours were collected through a sterile indwelling catheter and by peripheral needle sticks for the 48- and 72-hours samples. A urine sample was collected at baseline (spot collection) and all voided urine was collected for the entire 72 hours following dosing. Voided urine was collected for 12-hours intervals for the first 24 hours followed by 24-hours intervals for 24–48 and 48–72 hours. Urine from each time interval was collected in a different container. After the first 24 hours, urine void was collected at home and subjects were instructed to return urine containers at the following visit. Two 10-ml urine aliquots were saved from each time interval after the total urine volume is recorded. All blood samples were processed immediately following collection and stored by the Indiana University Clinical and Translational Science Laboratory (CTSL). Plasma was separated by centrifugation at 1200g for 10 min. PBMCs were freshly lysed following ficol gradient utilizing lymphocyte separation medium (LSM), phosphate-buffered saline (PBS) solution, centrifugation at 1800g for 20 min and collection of the buffy coat layer containing the lymphocytes for counting and analysis (24). Plasma, urine and PBMC samples were immediately stored at −80°C pending analysis.
Safety Evaluation.
Subjects were asked to report adverse events (AEs) to study investigators and CRC personnel. Participants who received at least one dose of TDF/FTC were included in the safety analysis. AEs were documented and reported to the Indiana University IRB. AEs were assessed by NCI Common Toxicity Criteria guidelines and internal safety reviews.
Objectives and Endpoints
Primary.
The primary objective was to determine if the plasma and intracellular concentrations of TFV/TFV-DP from a single PRO-boosted TDF regimen (“T” test treatment) were comparable to the current ‘IPERGAY’ unboosted TDF dosing regimen (“C” control treatment). The primary endpoints were pre-specified as the geometric mean ratio (GMR) of plasma and PBMC AUC0–72h in the T versus C treatment arms.
Secondary.
The secondary outcome was to compare the short-term safety and tolerability of the T versus C treatments.
Quantification of drugs and active metabolites.
Plasma.
TFV and FTC were simultaneously quantified from plasma samples utilizing a validated liquid chromatography-tandem mass spectrometry (LC-MS/MS) assay previously published for TFV and FTC (25). Briefly, analytes chromatographic separation were done using a Synergi Polar-RP column (100 × 2.0 mm i.d.; 2.5μm particle size; Phenomenex, Torrance, CA) using a mobile phase gradient consisting of acetonitrile containing 0.1% formic acid and water containing 0.1% formic acid, flow rate of 0.3 mL/min and injection volume of 5μL. Then, QTRAP 6500+ MS fitted with electrospray ionization was performed for analyte quantification. The total run time was 5 min. The lower limit of quantification (LLOQ) for TFV and FTC was 0.586 and 2.93 ng/mL, respectively. The inter- and intra-day precision and accuracy for the assay was ± 10% and ± 2%, respectively.
Peripheral Blood Mononuclear Cells.
Tenofovir diphosphate (TFV-DP) and FTC triphosphate (FTC-TP) were quantified at the Colorado Antiviral Pharmacology Laboratory (de-identified) University of Colorado, using validated and routinely performed LC-MS/MS assays (24). The lower limit of quantification (LLOQ) for TFV-DP and FTC-TP was 5 to 10 or 100 fmol/sample, respectively.
Urine.
TFV and FTC were quantified simultaneously in urine by the LC-MS/MS assay method analogous to the method for plasma (25). Urine samples were diluted 100-fold prior to analysis. The LLOQ for TFV and FTC were the same as mentioned above. The inter- and intra-day precision and accuracy for the assay was ± 18% and ± 2%, respectively.
Pharmacokinetic
Single-dose pharmacokinetic parameters were estimated using plasma and urine concentration data collected through 24 hours post-dose using conventional non-compartmental analysis (NCA) performed using Phoenix® WinNonlin® (version 6.4; Certara, Princeton, NJ). Pharmacokinetic outcomes included elimination rate constant (kel), maximum plasma concentration (Cmax), time of maximum plasma concentration (tmax), single-dose area under the plasma concentration-time curve from zero extrapolated to infinity (AUC0−∞), single-dose area under the plasma concentration curve from time zero to 24 hours (AUC0–24h), apparent volume of distribution (Vz/F), and apparent oral clearance (CL/F). For the primary endpoint, a single-dose area under the plasma concentration-time curve from zero to 72 hours (AUC0–72h) was calculated for the T treatment using the observed plasma concentration data. Calculation of AUC0–72h for the C treatment was done through the sum of the plasma AUC0–24h for all three doses. Multiple-dose analysis of the observed plasma concentration data was combined with predicted plasma concentration data obtained via nonparametric superposition performed using Phoenix® WinNonlin® (Supplemental Figure 1). AUC estimates were determined using the trapezoidal rule with linear-up/log-down interpolation. The terminal half-life (t1/2) was calculated as t1/2=0.693/kel. Urinary excretion was calculated using Phoenix® WinNonlin®. Urine concentrations were multiplied by total urine volume collected during the interval and summed, Ae0–24h to calculate amount excreted in the urine (Ae). The percentage of the dose in urine was calculated by: % dose in urine = Ae(mg)/600mg for TFV or Ae(mg)/400mg for FTC. Renal clearance was calculated by: Clrenal = Ae/AUC0–24h.
Pharmacokinetic outcomes are reported as geometric mean (GM), geometric mean ratio (GMR), and 90% confidence interval (90% CI).
Statistical
Prior to performing the study, power and sample size were determined using intra-subject variability estimates from a previous study of daily TDF/FTC in healthy volunteers (26). To demonstrate bioequivalence, a total of 14 subjects with complete data were projected to provide 90% power to detect a mean difference of no more than 25% in the primary endpoint assuming a type I error rate of 0.05 and intra-subject variability as previously published (26). If the 90% CI of the plasma and PBMC AUC0–72h GMR were contained entirely within the pre-defined boundaries of 0.8–1.25, then the probenecid “boosting” strategy would be considered pharmacokinetically bioequivalent to the IPERGAY regimen.
Supplementary Material
Study Highlights.
What is the current knowledge on the topic?
The combination pill containing 300 mg TDF / 200 mg FTC is used to prevent HIV infections. Tenofovir (TFV), the major circulating TDF metabolite, undergoes renal secretion via OAT 1 and 3. Probenecid inhibits OAT 1 and 3.
What question did the study address?
We hypothesized that probenecid inhibition of OAT 1 and OAT 3 increases TFV plasma and intracellular concentrations of TFV-diphosphate, providing clinical benefit to allow a simpler and cheaper single-dose HIV prevention regimen of TDF/FTC.
What does this study add to our knowledge?
Probenecid boosted plasma exposure of TFV and early, but not late, intracellular exposure of TFV-diphosphate. Formal bioequivalence was not demonstrated.
How might this change clinical pharmacology or translational science?
Probenecid boosted TFV, but not sufficiently to show TFV-diphosphate bioequivalence. Additional investigation will need to determine the clinical utility of probenecid-boosted TDF/FTC for HIV prevention.
Funding Information:
This work was supported by the Campbell Foundation for AIDS Research. In addition, this project was supported, in part, from the Indiana Clinical and Translational Sciences Institute funded, in part by Award Number UL1TR002529 from the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. SNL and BTG were supported by the National Institute of General Medical Sciences [Grant T32 GM008425]. BTG was also supported by the Indiana Clinical and Translational Sciences Institute Young Investigator Award [Grant UL1 TR001108].
Abbreviations:
- PrEP
pre-exposure prophylaxis
- HIV
human immunodeficiency virus
- CrCl
creatinine clearance
- STIs
sexually transmitted infections
- TDF
tenofovir disoproxil fumarate
- TFV
tenofovir
- FTC
emtricitabine
- TFV-DP
tenofovir diphosphate
- FTC-TP
emtricitabine triphosphate
- PBMCs
peripheral blood mononuclear cells
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
- AE
adverse event
- PRO
probenecid
Footnotes
Conflict of Interest Statement:
SNL, BTG, LRB, RFB and ZD declares no competing interests for this work.
SKG is the site Principal Investigator for ongoing studies supported by Gilead Sciences, Inc. and GlaxoSmithKline/ViiV. He has also has received advisory fees and travel support from Gilead Sciences, Inc. and GlaxoSmithKline/ViiV.
PLA receives contract and research support from Gilead Sciences and paid to his institution.
References
- 1.Sidebottom D, Ekström AM, & Strömdahl S A systematic review of adherence to oral pre-exposure prophylaxis for HIV - how can we improve uptake and adherence? BMC infectious diseases 18, 581 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention: US Public Health Service: Preexposure prophylaxis for the prevention of HIV infection in the United States—2017 Update: a clinical practice guideline. https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2017.pdf (2018). Accessed on 1 April 2019.
- 3.Sullivan PS et al. The impact of pre-exposure prophylaxis with TDF/FTC on HIV diagnoses, 2012–2016, United States AIDS 2018 Poster Abstract https://programme.aids2018.org/Abstract/Print/?abstractid=13004 (2018). [Google Scholar]
- 4.Grant RM et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 363, 2587–2599 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Molina JM et al. On-Demand Preexposure Prophylaxis in Men at High Risk for HIV-1 Infection. N Engl J Med 373, 2237–46 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Grant RM et al. Daily and Nondaily Oral Preexposure Prophylaxis in Men and Transgender Women Who Have Sex With Men: The Human Immunodeficiency Virus Prevention Trials Network 067/ADAPT Study. Clinical Infectious Diseases 66, 1712–1721 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robbins N, Koch SE, Tranter M, Rubinstein J The History and Future of Probenecid. Cardiovasc Toxicol 12, 1–9 (2012). [DOI] [PubMed] [Google Scholar]
- 8.Anderson PL, Kiser JJ, Gardner EM, Rower JE, Meditz A, Grant RM Pharmacological considerations for tenofovir and emtricitabine to prevent HIV infection. J Antimicrob Chemother 66, 240–250 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reid G et al. Characterization of the transport of nucleoside analog drugs by the human multidrug resistance proteins MRP4 and MRP5, Mol Pharmacol 63, 1094–103 (2003). [DOI] [PubMed] [Google Scholar]
- 10.Van Aubel RM, Smeets PH, Peters JG, Bindels RJ, Russel FG The MRP4/ABCC4 gene encodes a novel apical organic anion transporter in human kidney tubules: putative efflux pump for urinary cAMP and cGMP. J Am Soc Nephrol 13, 595–603 (2002). [DOI] [PubMed] [Google Scholar]
- 11.Vlasses PH, Holbrook AM, Schrogie JJ, Roger JD, Ferguson RK, Abrams WB Effect of orally administered probenecid on the pharmacokinetics of cefoxitin. Antimicrob Agents Chemother 17, 847–55 (1980). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vree TB, Van Der Biggelaar-Martea M, Verwey-van Wissen CP Probenecid inhibits the renal clearance of frusemide and its acyl glucuronide. Br J Clin Pharmacol 39, 692–5 (1995). [PMC free article] [PubMed] [Google Scholar]
- 13.Nakatani-Freshwater T, Taft DR Renal excretion of emtricitabine I: effects of organic anion, organic cation, and nucleoside transport inhibitors on emtricitabine excretion. J Pharm Sci 97, 5401–10 (2008). [DOI] [PubMed] [Google Scholar]
- 14.Gilead Sciences, Inc. Genvoya Clinical Pharmacology and Biopharmaceutics Review(s). https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/207561Orig1s000ClinPharmR.pdf (2014). Accessed on 1 April 2019.
- 15.Fonsart J et al. Single-dose pharmacokinetics and pharmacodynamics of oral tenofovir and emtricitabine in blood, saliva and rectal tissue: a sub-study of the ANRS IPERGAY trial, Journal of Antimicrobial Chemotherapy 72, 478–485 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Louissaint NA et al. Single dose pharmacokinetics of oral tenofovir in plasma, peripheral blood mononuclear cells, colonic tissue, and vaginal tissue. AIDS Res Hum Retroviruses 29, 1443–1450 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seifert SM et al. Dose response for starting and stopping HIV preexposure prophylaxis for men who have sex with men. Clin Infect Dis 60, 804–810 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seifert SM et al. Intracellular Tenofovir and Emtricitabine Anabolites in Genital, Rectal, and Blood Compartments from First Dose to Steady State. AIDS Res Hum Retroviruses 32, 981–991 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen X, Seifert SM, Castillo-Mancilla JR, Bushman LR, Zheng JH, et al. Model Linking Plasma and Intracellular Tenofovir/Emtricitabine with Deoxynucleoside Triphosphates. PLOS ONE 11, e0165505 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cummingham RF, Israili ZH, Dayton PG Clinical Pharmacokinetics of Probenecid. Clin Pharmacokinet 6, 135–51 (1981). [DOI] [PubMed] [Google Scholar]
- 21.Anderson PL et al. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med 4, 151ra125 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson PL, García-Lerma JG, Heneine W. Nondaily preexposure prophylaxis for HIV prevention. Curr Opin HIV AIDS 11, 94–101 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cottrell ML et al. A translational Pharmacology Approach to Predicting Outcomes of Preexposure Prophylaxis Against HIV in Men and Women Using Tenofovir Disoproxil Fumarate With or Without Emtricitabine. J Infect Dis 214, 55–64 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Delahunty T, Bushman L, Robbins B, Fletcher CV. The simultaneous assay of tenofovir and emtricitabine in plasma using LC/MS/MS and isotopically labeled internal standards. J Chromatogr B Analyt Technol Biomed Life Sci 87, 1907–1914 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bushman LR, Kiser JJ, Rower JE, Klein B, Zheng JH, Ray ML, Anderson PL*. Determination of nucleoside analog mono-, di-, and tri-phosphates in cellular matrix by solid phase extraction and ultra-sensitive LC-MS/MS detection. J Pharm Biomed Anal 56, 390–401 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baheti G, Kiser JJ, Havens PL, Fletcher CV Plasma and intracellular population pharmacokinetic analysis of tenofovir in HIV-1-infected patients. Antimicrob Agents Chemother 55, 5294–5299 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


