Abstract
Background.
We previously reported a significant volume–outcome relationship in mortality rates after gastrectomies for gastric cancer patients in Texas (1999–2001). We aimed to identify whether changes in the volume distribution of gastrectomies occurred, whether volume–outcome relationships persisted, and potential changes in the factors influencing volume–outcome relationships.
Methods.
We performed a population-based study using the Texas Inpatient Public Use Data File between 2010 and 2015. Hospitals were classified as high-volume centers (HVCs, > 15 cases per year), intermediate-volume centers (IVCs, 3–15 cases per year), and low-volume centers (LVCs, < 3 cases per year). We conducted multivariate analyses to evaluate factors associated with inpatient mortality and adverse events.
Results.
We identified 2733 gastric cancer patients who underwent gastrectomy at 193 hospitals. Fewer hospitals performed gastrectomy than previously (193 vs. 214). There were more HVCs (5 vs. 2) and LVCs (142 vs. 134), but fewer IVCs (46 vs. 78). The proportion of patients who underwent gastrectomy at HVCs and LVCs increased, while the proportion at IVCs decreased. HVCs maintained lower in-hospital mortality rates than IVCs or LVCs, although mortality rates decreased in both LVCs and IVCs. In adjusted multivariate analyses, treatment at HVCs remained a strong predictor for lower rates of mortality (odds ratio [OR] 0.39, p = 0.019) and adverse events (OR 0.56, p = 0.013).
Conclusion.
Despite improvements, patient morbidity and mortality at LVCs and IVCs remain higher than at HVCs, demonstrating that volume–outcome relationships still exist for gastrectomy and that opportunities for improvement remain.
Volume–outcome relationships have been well-reported in many types of surgical interventions, including high-risk resections of organ-specific cancers, in which mortality rates are lower among patients treated by high-volume surgeons or in high-volume institutions.1–9 Such reports led the National Cancer Policy Board of the Institute of Medicine to recommended in 1999 that technically difficult, high-risk procedures be performed at high-volume, experienced centers.10 Subsequently, minimum volume standards for several surgical procedures were established by private payers and US professional organizations as part of a value-based purchasing initiative.11,12 These efforts and recommendations have resulted in centralization or changed referral patterns of pancreatectomies and esophagectomies in the US.4,11 However, gastrectomies were not a primary focus of these efforts, and the effect of the volume–outcome relationship on centralization of gastrectomies in the US has not been well-studied.
Commonly used national databases of cancer patients, such as the National Cancer Database and the Surveillance, Epidemiology, and End Results (SEER) database, do not contain detailed hospital characteristics and inpatient information; therefore, analyses of the volume–outcome relationship using those databases, particularly in factors associated with short-term outcomes after organ-specific oncological resections, are limited. Our previous study using inpatient data revealed a significant volume–outcome relationship in gastrectomies for gastric cancer patients in Texas (1999–2001).13 We observed that high-volume hospitals had significantly lower complication and mortality rates and were associated with better hospital quality indicators, such as higher number of critical care beds and a higher registered nurse:bed ratio. We also found that 21% of gastrectomies were performed at low-volume hospitals, a finding that implied limited regionalization of, or barriers to, access to high-quality gastric cancer care.
The purpose of this study was to investigate whether changes occurred in the volume distribution of gastrectomies, whether volume–outcome relationships persist, and potential changes in the factors influencing volume–outcome relationships using more contemporary data.
METHODS
Study Design
After receiving Institutional Review Board approval, we obtained data from the Texas Inpatient Public Use Data File, which contained patient-level information discharged from non-rural Texas hospitals.13 In a similar fashion to our previous study,13 we identified gastric cancer patients who underwent gastrectomy between 1 July 2010, and 30 June 2015. We ascertained our cohort using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) procedure codes for gastrectomy and concurrent diagnosis codes indicating gastric cancer. The Charlson comorbidity score was estimated using ICD-9 diagnosis codes to identify chronic conditions.14 To construct neighborhood socioeconomic status, hospital discharge data were linked to the 2015 American Community Survey 5-year estimates by zip-code level for education, income, and primary language-speaking status.15 We then dichotomized around the national medians (86.7% high-school graduates, $53,899 median household income, and 80.0% primary English speaking). Hospital-level characteristics were obtained from the Texas Annual Survey of Hospitals.
Outcomes
Two primary outcome measures obtained from the Texas Hospital discharge data were in-hospital mortality and adverse event rates.13 Adverse events were identified by searching the 26 diagnosis fields for specific ICD-9 codes.16 We chose the same 13 adverse event diagnoses for inclusion in the analysis as we did in our previous report, in which these diagnoses were chosen because they have high sensitivity and specificity relative to data abstracted directly from medical records.13,17–20
Statistical Analysis
To categorize hospitals by gastrectomy volumes and to facilitate comparisons, cut-points from our previous manuscript were retained in this analysis.13 Hospitals were stratified as high-volume centers (HVCs, > 15 cases per year), intermediate-volume centers (IVCs, 3–15 cases per year), and low-volume centers (LVCs, < 3 cases per year). Treatment outcomes were compared among hospital volume categories and stratified by the presence of comorbidities.
Multivariate analyses for each outcome defined risk, with the patient as the unit of analysis, in a similar manner as in our previous report.13 Random-effect linear probability regression (in univariate analyses) or logistic regression (in multivariate analyses) was used to adjust for clustering of patients within hospitals. A -p value < 0.05 was considered significant. Statistical analyses were performed using Stata software (StataCorp LLC, College Station, TX, USA).
RESULTS
Trends in Number and Characteristics of Patients by Hospital Volume
We identified 2733 gastric cancer patients who underwent gastrectomy and who were discharged between 2010 and 2015 (Table 1). Of these, 785 underwent gastrectomy at HVCs, 1321 at IVCs, and 627 at LVCs. Compared with the previous study (n = 1864), the proportion of patients who underwent gastrectomy at HVCs increased from 12.4 to 28.7%, while the proportion at IVCs decreased from 66.9 to 48.3%. The proportion treated at LVCs did not change substantially (20.7–22.9%).
TABLE 1.
Trends in characteristics of patients (N = 2733) with gastric cancer who underwent gastrectomy, by hospital volume and study period
| Characteristic | Hospital volume | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Low | Intermediate | High | |||||||
| 1999–2001 (n = 386), % | 2010–2015 (n = 627), % |
p value | 1999–2001 (n = 1247), % | 2010–2015 (n = 1321), % | p value | 1999–2001 (n = 231), % | 2010–2015 (n = 785), % |
p value | |
| Male | 58 | 57 | 0.733 | 62 | 58 | 0.039 | 70 | 66 | 0.239 |
| Age ≥ 75 years | 36 | 31 | 0.095 | 28 | 25 | 0.084 | 15 | 14 | 0.664 |
| NH White | 48 | 39 | 0.006 | 47 | 38 | < 0.001 | 74 | 61 | < 0.001 |
| NH African American | 15 | 15 | 0.988 | 13 | 13 | 0.982 | 9 | 12 | 0.224 |
| Hispanic | 29 | 37 | 0.009 | 34 | 39 | 0.009 | 9 | 12 | 0.224 |
| Charlson comorbidity score ≥ 1 | 35 | 60 | < 0.001 | 33 | 54 | < 0.001 | 23 | 44 | < 0.001 |
| High-school graduate | 73 | 79 | 0.031 | 70 | 78 | < 0.001 | 76 | 83 | 0.018 |
| Primarily English speaking | 72 | 62 | 0.001 | 61 | 60 | 0.606 | 77 | 68 | 0.008 |
| Below median income | 72 | 71 | 0.72 | 75 | 66 | < 0.001 | 70 | 59 | 0.002 |
| Living within 100 miles of a high-volume hospital | 64 | 44 | < 0.001 | 62 | 45 | < 0.001 | 38 | 59 | < 0.001 |
| Metastasis at presentation | 23 | 11 | < 0.001 | 23 | 12 | < 0.001 | 19 | 9 | < 0.001 |
| Total gastrectomy | 26 | 26 | 0.975 | 37 | 35 | 0.292 | 54 | 50 | 0.272 |
| Node dissection | 5.4 | 8 | 0.125 | 6.5 | 22 | < 0.001 | 38 | 59 | < 0.001 |
| Splenectomy | 10 | 4 | < 0.001 | 12 | 8 | 0.001 | 9.1 | 5 | 0.019 |
| Emergency admission | 18 | 25 | 0.008 | 15 | 15 | 0.996 | 6.5 | 5 | 0.364 |
p values compare proportions between 1999 and 2001 and 2010 and 2015
NH non-Hispanic
Higher proportions of patients treated at HVCs were male, younger than 75 years of age, of non-Hispanic White race, without comorbidities, living in an area with a higher proportion of high-school graduates, primarily English speakers, and a median or higher household income status compared with those treated at LVCs and IVCs (all p < 0.001). Total gastrectomy and lymph node disection were more frequently performed at HVCs (p < 0.001). Emergency admission was significantly higher at LVCs and IVCs than at HVCs (25, 15, and 5%, respectively; p < 0.001).
Our results showed signs of selective shift of gastrectomy patients toward HVCs. The proportion of patients living within 100 miles of HVCs increased from 38% to 59%, but decreased at LVCs (64–44%) and IVCs (62–45%; all p < 0.001). The proportion of patients living in areas with below median income decreased at HVCs (70–59%; p = 0.002), but did not change at LVCs (72–71%; p = 0.72). The proportion of patients who had emergency admission before gastrectomy changed little at HVCs (6.5–5%; p = 0.364), but increased at LVCs (18–25%; p = 0.008).
Trends in Number and Characteristics of Hospitals by Gastrectomy Volume
Table 2 presents the trends and characteristics of hospital-specific factors and gastrectomy volume between the two study periods. Even with a longer study period in this study (2010–2015 vs. 1999–2001), fewer hospitals performed gastrectomy (193 vs. 214). There were increases in the number of HVCs (5 vs. 2) and LVCs (142 vs. 134), while there was a remarkable decrease in IVCs (46 vs. 78). Notably, 117 LVCs (82%) performed one or fewer gastrectomies per year, and, as a result, the median hospital volume was one gastrectomy per year. The number of gastrectomies performed at the five HVCs were 78, 24, 22, 18, and 16 per year, respectively, showing a wide variation in volume even among HVCs.
TABLE 2.
Trends in hospital characteristics
| Characteristic | Hospital volume | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Low | Intermediate | High | |||||||
| 1999–2001 (n = 134) | 2010–2015 (n = 142) |
p value | 1999–2001 (n = 78) |
2010–2015 (n = 46) | p value | 1999–2001 (n = 2) | 2010–2015 (n = 5) | p value | |
| Teaching hospital, % | 22 | 9 | 0.0040 | 53 | 43 | 0.3280 | 100 | 100 | 0.257 |
| Non-critical beds, n | 169 | 166 | 0.8606 | 399 | 348 | 0.1779 | 696 | 625 | 0.7611 |
| Critical beds, n | 47 | 53 | 0.0011 | 61 | 67 | < 0.0001 | 69 | 74 | 0.2056 |
| Bed occupancy, % | 17 | 20 | 0.1716 | 34 | 51 | 0.0241 | 87 | 86 | 0.9871 |
| Annual surgical procedures, n | 1643 | 2235 | 0.0012 | 5698 | 5640 | 0.9221 | 12,295 | 8923 | 0.6547 |
| FTE RT-occupied bed ratio | 0.07 | 0.21 | < 0.0001 | 0.08 | 0.22 | 0.0011 | 0.07 | 0.15 | 0.2774 |
| FTE RN-occupied bed ratio | 1.61 | 2.8 | < 0.0001 | 1.74 | 2.48 | < 0.0001 | 2.63 | 3.41 | 0.4910 |
| FTE LVN-occupied bed ratio | 0.3 | 0.3 | 1.000 | 0.28 | 0.17 | < 0.0001 | 0.2 | 0.03 | 0.5122 |
FTE full-time equivalent, LVN licensed vocational nurse, RN registered nurse, RT respiratory therapist
All five HVCs were teaching hospitals, had more critical and noncritical care beds, had a higher occupancy rate, and performed more surgical procedures annually compared with IVCs and LVCs (all p < 0.001). While the characteristics of HVCs did not change over time, there were significant changes at LVCs and IVCs: numbers of critical beds and ratios of full-time equivalent respiratory therapists and registered nurses to beds increased (all p ≤ 0.001), although the proportion of teaching hospitals dropped from 22 to 9% at LVCs.
The geographic distribution of gastrectomy hospitals in Texas by category of hospital volume is illustrated in Fig. 1, showing that the vast majority of Texas is more than 100 miles from HVCs, although the majority of patients lived within 100 miles of one of the HVCs.
FIG. 1.
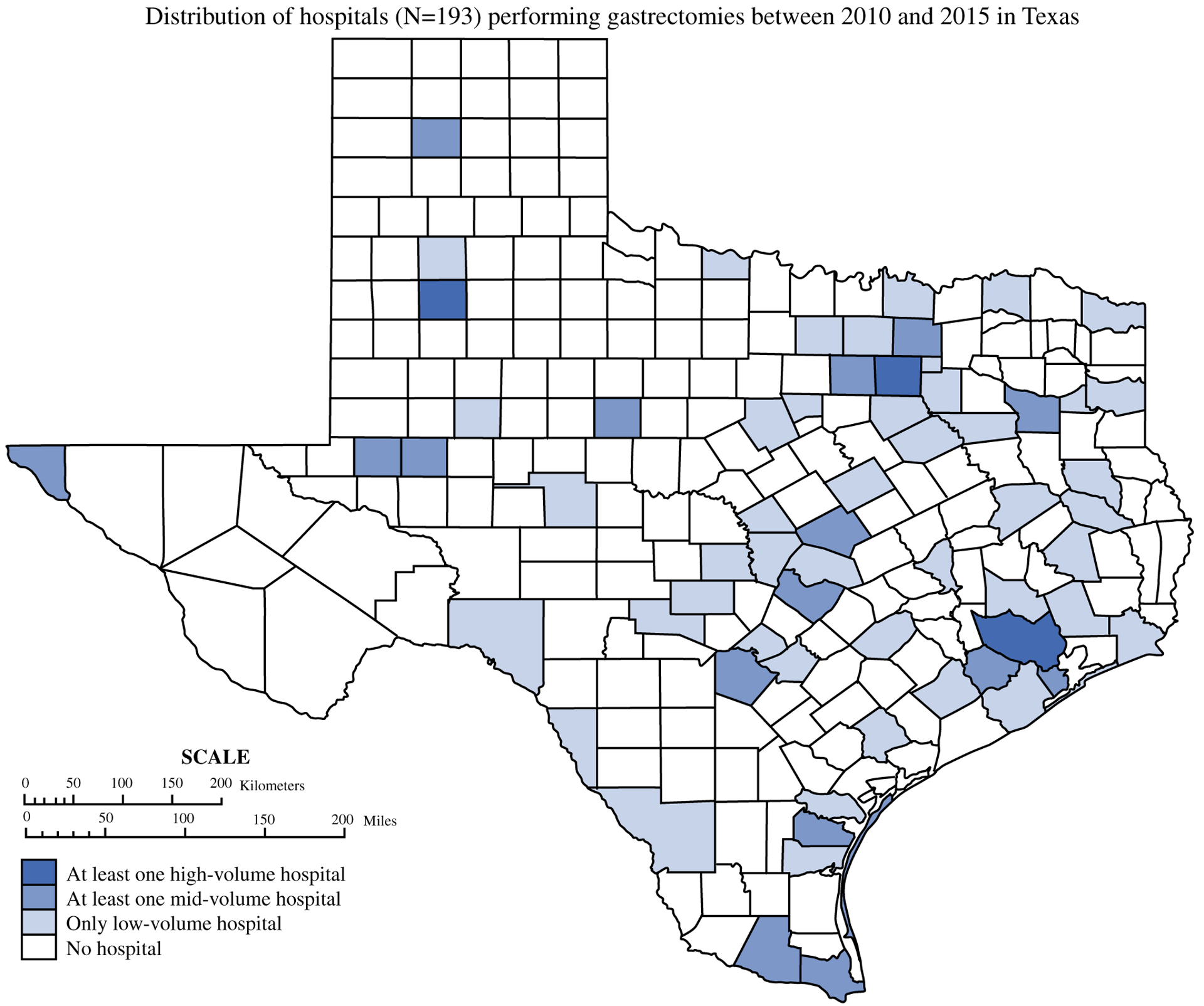
Distribution of hospitals (n = 193) performing gastrectomies between 2010 and 2015 in Texas
Outcomes
HVCs attained lower in-hospital mortality rates (0.9% and 2.6% in patients without and with comorbidities, respectively) than IVCs (2.3% and 5.2%) and LVCs (2.4% and 5.6%) (Table 3). Similarly, HVCs had lower adverse event rates (7.3% and 18.0%) than IVCs (13.7% and 28.1%) and LVCs (17.9% and 31.7%).
TABLE 3.
Outcomes of gastrectomy by hospital volume
| Outcome | No comorbidities (n = 1298) | Comorbidities (n = 1435) | ||||
|---|---|---|---|---|---|---|
| Low volume [n = 252], % (95% CI) | Intermediate volume [n = 606], % (95% CI) | High volume [n = 440], % (95% CI) | Low volume [n = 375], % (95% CI) | Intermediate volume [n = 715], % (95% CI) | High volume [n = 345], % (95% CI) | |
| Primary outcomes | ||||||
| Inpatient mortalitya | 2.38 (2.36–2.40) | 2.31 (2.30–2.32) | 0.91 (0.90–0.92) | 5.60 (5.58–5.62) | 5.17 (5.16–5.19) | 2.61 (2.59–2.63) |
| Complicationsa | 17.86 (17.81–17.90) | 13.70 (13.67–13.72) | 7.27 (7.25–7.30) | 31.73 (31.69–31.78) | 28.11 (28.08–28.14) | 17.97 (17.93–18.01) |
| Other outcomes | ||||||
| Mean length of stay, days | 12.00 (10.8–13.20) | 11.80 (11.1–12.6) | 9.80 (9.30–10.20) | 14.20 (13.10–15.30) | 13.90 (13.1–14.7) | 12.30 (11.3–13.4) |
| Receiving postdischarge carea | 32.14 (32.09–32.20) | 32.34 (32.31–32.38) | 21.82 (21.78–21.86) | 48.00 (47.95–48.05) | 43.64 (43.6–43.67) | 35.36 (35.31–35.41) |
| Other hospitala | 7.54 (7.51–7.57) | 5.78 (5.76–5.79) | 1.82 (1.81–1.83) | 16.80 (16.76–16.84) | 10.49 (10.47–10.51) | 8.70 (8.67–8.73) |
| Skilled nursing facilitya | 4.76 (4.74–4.79) | 3.14 (3.12–3.15) | 1.82 (1.81–1.83) | 10.67 (10.64–10.70) | 9.37 (9.35–9.39) | 5.22 (5.19–5.24) |
| Home health carea | 19.84 (19.79–19.89) | 23.43 (23.4–23.47) | 18.18 (18.15–18.22) | 20.53 (20.49–20.57) | 23.78 (23.74–23.81) | 21.45 (21.41–21.49) |
CI confidence interval
Adjusted for clustering within hospitals by random-effect linear probability regression models
Compared with our previous study, remarkable drops in mortality rates were observed at LVCs (4.1–2.4% and 6.4–5.6% among patients without and with comorbidities, respectively) and at IVCs (4.1–2.3% and 9.5–5.2%, respectively). Similarly, complication rates for most hospital volume categories decreased from the previous study (HVCs: 19.2–7.3% and 22.2–18.0% in patients without and with comorbidities, respectively; IVCs: 25.2–13.7% and 29.2–28.1% in patients without and with comorbidities, respectively; LVCs: 19.1% to 17.9% in patients without comorbidities), with the exception of patients with comorbidities at LVCs, in which the rate increased from 29.2 to 31.7%.
In multivariate analyses, treatment at HVCs remained a strong predictor for lower inpatient mortality (odds ratio [OR] 0.39, 95% confidence interval [CI] 0.18–0.86; p = 0.019) and adverse events (OR 0.56, 95% CI 0.36–0.88; p = 0.013), after adjustment for other factors, including age, comorbidities, emergency admission, and type of gastrectomy (Table 4). High number of critical beds, high occupancy rate, and high nurse-occupied bed ratio were not associated with lower mortality or adverse event rates in this study.
TABLE 4.
Results of multivariate models for factors associated with inpatient mortality and adverse events
| Factors | Inpatient mortality | Adverse events | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p value | OR | 95% CI | p value | |
| Patient-level | ||||||
| Male | 1.13 | 0.72–1.77 | 0.601 | 1.05 | 0.85–1.29 | 0.647 |
| Age ≥ 75 years | 1.72 | 1.08–2.74 | 0.022 | 1.32 | 1.05–1.67 | 0.017 |
| Race | ||||||
| Non-Hispanic White | Ref | Ref | ||||
| Hispanic | 0.81 | 0.46–1.43 | 0.468 | 0.73 | 0.56–0.95 | 0.018 |
| Non-Hispanic African American | 1.06 | 0.55–2.06 | 0.860 | 0.71 | 0.50–1.00 | 0.051 |
| Others | 1.02 | 0.49–2.14 | 0.953 | 1.36 | 0.98–1.89 | 0.069 |
| Low income | 1.28 | 0.68–2.41 | 0.436 | 1.19 | 0.88–1.60 | 0.259 |
| English not the primary language | 1.29 | 0.73–2.29 | 0.383 | 0.96 | 0.73–1.25 | 0.742 |
| Low education level | 0.68 | 0.36–1.29 | 0.238 | 1.02 | 0.75–1.39 | 0.899 |
| Live within 100 miles | 1.25 | 0.77–2.02 | 0.370 | 0.94 | 0.74–1.20 | 0.618 |
| Emergency admission | 1.79 | 1.07–2.98 | 0.026 | 2.13 | 1.64–2.77 | < 0.001 |
| Comorbid | 2.23 | 1.36–3.65 | 0.001 | 2.38 | 1.92–2.96 | < 0.001 |
| Metastasis | 2.59 | 1.53–4.39 | < 0.001 | 1.38 | 1.02–1.87 | 0.039 |
| Total gastrectomy | 1.99 | 1.26–3.13 | 0.003 | 1.92 | 1.55–2.39 | < 0.001 |
| Hospital-level | ||||||
| High volume | 0.39 | 0.18–0.86 | 0.019 | 0.56 | 0.36–0.88 | 0.013 |
| Teaching | 0.99 | 0.56–1.75 | 0.975 | 1.06 | 0.78–1.42 | 0.719 |
| Many critical beds | 1.30 | 0.72–2.33 | 0.378 | 0.90 | 0.65–1.24 | 0.514 |
| High occupancy rate | 0.91 | 0.55–1.51 | 0.716 | 1.27 | 0.97–1.66 | 0.085 |
| High FTE RT-occupied rate | 0.92 | 0.58–1.45 | 0.716 | 1.24 | 0.97–1.59 | 0.084 |
| High FTE RN-occupied rate | 1.23 | 0.74–2.05 | 0.416 | 0.91 | 0.70–1.19 | 0.490 |
| High FTE LVN-occupied rate | 1.16 | 0.65–2.09 | 0.613 | 1.22 | 0.89–1.67 | 0.219 |
| Year | ||||||
| 2010–11 | Ref | Ref | ||||
| 2011–12 | 1.25 | 0.63–2.46 | 0.522 | 0.77 | 0.56–1.04 | 0.086 |
| 2012–13 | 0.82 | 0.38–1.77 | 0.608 | 0.69 | 0.50–0.95 | 0.022 |
| 2013–14 | 1.66 | 0.87–3.13 | 0.122 | 0.78 | 0.58–1.05 | 0.106 |
| 2014–15 | 0.85 | 0.40–1.81 | 0.673 | 0.71 | 0.52–0.98 | 0.035 |
OR odds ratio, CI confidence interval, FTE full-time equivalent, RN registered nurse, RT respiratory therapist, LVN licensed vocational nurse
DISCUSSION
Our results show that significant volume–outcome relationships in short-term outcomes after gastrectomy for gastric cancer patients continued to exist in Texas. At LVCs and IVCs, we observed improved outcomes compared with our previous study, likely from universally improved quality of care represented by improved hospital characteristics such as number of critical beds and registered nurse-to-bed ratios. We also observed evidence of centralization of gastrectomy in Texas over the past 15 years, with a substantial increase in the proportion of patients undergoing gastrectomy at HVCs; however, such a shift toward HVCs appeared to occur selectively and more likely occurred among patients living within 100 miles of HVCs, those residing in areas with better-than-average income, and those who did not require emergency admission; younger patients, non-Hispanic White patients, and those without comorbidities continued to have better access to HVCs compared with other patients.
Although volume–outcome relationships among high-risk surgical procedures have been extensively studied over the past 2 decades, studies investigating changes in practice characteristics and outcomes are relatively limited. Using data from the Nationwide Inpatient Sample, Learn et al. conducted a study on 93,108 adult cancer patients who underwent pancreatectomy, esophagectomy, gastrectomy, or major lung resection from 1997 to 2006.4 Throughout that study period, the mortality gap between high- and low-volume institutions persisted for all procedures. Finks et al. used national Medicare data to study trends in hospital volume and operative mortality for four cancer resections (lung, esophagus, pancreas, and bladder) and reported that the median hospital volumes for those procedures rose substantially during the study period (1999–2008).11 Rising hospital volumes were driven not only by an overall increase in the total procedure volume nationwide but also by a higher concentration of procedures in a smaller number of hospitals; hundreds of US hospitals stopped performing major cancer resection during the study period.11 Similarly, Gasper et al. reported that the proportion of patients who underwent high-risk oncological resections (liver, pancreas, and esophagus) at high-volume institutions remarkably increased in California during the study period (1990–2004).21 Our current study provides additional evidence of regionalization of high-risk oncological surgery. In addition, our unique and detailed analyses showed that such regionalization was limited to specific populations, implicating existent barriers in access to high-quality cancer care in the US.
Despite growing evidence supporting potential benefits of centralization of high-risk surgical procedures, policies that mandated regionalization or restriction of performance of specific high-risk surgeries have never been established in the US, and whether policy should enforce regionalization is controversial.1,22,23 Disadvantages of enforced regionalization may include limited or delayed access to HVCs, potential degradation of quality care if volume further increases at HVCs, and increased health care costs.3,23–26 Modrall et al. used the Nationwide Inpatient Sample (2003–2009) to study the relationship between surgeon volume and outcomes after esophagectomy.27 They reported that surgeons with low esophagectomy volume could achieve low mortality rates after esophagectomy if their surrogate volume (defined as the aggregate annual volume per surgeon of upper gastrointestinal operations, including gastrectomy and other esophageal operations) was high; the researchers concluded that operation-specific volume should not limit surgeon or hospital privileges. Since gastric cancer is a rare cancer type in the US, hospital categorization only by the number of gastrectomies may not be practical, particularly in Texas, which has a large land area with sparse distribution of HVCs (as shown in Fig. 1), and the aggregate annual volume of upper gastrointestinal surgeries may be a better surrogate if hospital certification will be considered to limit institutions for gastrectomy.
On the other hand, more proactive nationwide centralization of high-risk oncological resections has occurred in The Netherlands, driven by the Dutch Healthcare Inspectorate program, volume standards for complex surgical procedures defined by the Association of Surgeons of The Netherlands, and agreement among physicians to centralize high-risk surgeries to select institutions.28–31 As a result, a significant increase in the proportion of patients who underwent pancreatoduodenectomy at high-volume institutions (> 10 cases per year) was reported, from 53 to 91%, and nationwide mortality from the procedure decreased from 10 to 5% during the study period.28 In addition, the proportion of patients who underwent esophagectomy at high-volume institutions (≥ 21 per year) increased from 7 to 64%. Furthermore, nationwide improvement of the 6-month mortality rate (15–7%) and 3-year survival rate (41% to 52%) was observed in this patient population.5 Similarly, the proportion of gastric cancer resections performed at high-volume hospitals (annual composite volume of upper gastrointestinal cancer resections ≥ 40) in The Netherlands increased from 6 to 80%, and the treatment at those high-volume hospitals was associated with a lower mortality rate, higher lymph node yield, and longer survival.31 Medical and insurance systems are different between the US and most other developed countries, therefore continued consideration and discussions of whether and how regionalization of complex surgical care should be conducted are needed to optimize treatment outcomes in the US, especially in the context of our national dialogue regarding the cost of medical care.
The limitations of this study include its retrospective nature and limited patient information. The use of Texas data limits the generalizability of our findings to other states. As discussed in our previous report,13 a lack of data regarding long-term oncological outcomes is a major limitation of this study. However, with mounting evidence of an association between impaired oncological outcomes and postoperative complications,32 short-term outcomes after gastrectomy are important predictors for long-term outcomes. In addition, the fact that a higher proportion of patients underwent lymph node dissection at HVCs indicates that the oncological quality of gastrectomy is improved at HVCs, and oncological quality would likely be correlated with better long-term outcomes.
The strengths of this study include the large number of patients retrieved from the Texas Hospital Discharge Public Use Data File, which enabled us to conduct detailed analyses of volume–outcome relationships. Our updated analyses to evaluate changes in factors associated with outcomes stratified by hospital volume were unique. This study is the first reporting evidence of ongoing regionalization of gastrectomy practice in Texas, although such regionalization appeared limited and selective.
CONCLUSIONS
Despite improvement in patient morbidity and mortality rates at LVCs and IVCs, these rates remained higher than those at HVCs, demonstrating that volume–outcome relationships still exist for gastrectomy in Texas. Although the proportion of patients undergoing gastrectomy at HVCs has increased substantially, most patients still undergo gastrectomy at lower-volume centers. Race and financial status appeared to affect access to HVCs. The benefits and challenges of centralizing technically demanding surgical oncology procedures such as gastrectomy require continued careful consideration.
ACKNOWLEDGMENT
The authors thank the Department of Scientific Publications, MD Anderson Cancer Center, for editorial assistance.
FUNDING This work was supported in part by the National Institutes of Health under Cancer Center Support Grants P30CA016672 and R01CA207216; the Clinical Trials Support Resource was used. Funding from Sun and Do Lee Research and Patient Care Fund, and the Robert F. Fly Professorship, was also used to support this study.
Footnotes
DISCLOSURES Naruhiko Ikoma, Bumyang Kim, Linda S. Elting, Ya-Chen Tina Shih, Brian D. Badgwell, and Paul Mansfield have no conflicts of interest to disclose.
This manuscript was selected as one of five top-scoring posters and was presented at the Poster Grand Rounds session at the Society of Surgical Oncology Annual Cancer Symposium, San Diego, CA, USA, 28 March 2019.
REFERENCES
- 1.Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346(15):1128–37. [DOI] [PubMed] [Google Scholar]
- 2.Gordon TA, Burleyson GP, Tielsch JM, Cameron JL. The effects of regionalization on cost and outcome for one general high-risk surgical procedure. Ann Surg. 1995;221(1):43–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birkmeyer JD, Dimick JB. Potential benefits of the new Leapfrog standards: effect of process and outcomes measures. Surgery. 2004;135(6):569–75. [DOI] [PubMed] [Google Scholar]
- 4.Learn PA, Bach PB. A decade of mortality reductions in major oncologic surgery: the impact of centralization and quality improvement. Med Care. 2010;48(12):1041–9. [DOI] [PubMed] [Google Scholar]
- 5.Dikken JL, Dassen AE, Lemmens VE, et al. Effect of hospital volume on postoperative mortality and survival after oesophageal and gastric cancer surgery in the Netherlands between 1989 and 2009. Eur J Cancer. 2012;48(7):1004–13. [DOI] [PubMed] [Google Scholar]
- 6.Enzinger PC, Benedetti JK, Meyerhardt JA, et al. Impact of hospital volume on recurrence and survival after surgery for gastric cancer. Ann Surg. 2007;245(3):426–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Geest LG, van Rijssen LB, Molenaar IQ, et al. Volume-outcome relationships in pancreatoduodenectomy for cancer. HPB (Oxford). 2016;18(4):317–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finlayson EV, Goodney PP, Birkmeyer JD. Hospital volume and operative mortality in cancer surgery: a national study. Arch Surg. 2003;138(7):721–5 (discussion 726). [DOI] [PubMed] [Google Scholar]
- 9.Begg CB, Cramer LD, Hoskins WJ, Brennan MF. Impact of hospital volume on operative mortality for major cancer surgery. JAMA. 1998;280(20):1747–51. [DOI] [PubMed] [Google Scholar]
- 10.Hewitt M, Simone JV, editors. Ensuring quality cancer care. Washington, DC: National Academy Press; 1999. [PubMed] [Google Scholar]
- 11.Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high-risk surgery. N Engl J Med. 2011;364(22):2128–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Milstein A, Galvin RS, Delbanco SF, Salber P, Buck CR Jr. Improving the safety of health care: the leapfrog initiative. Eff Clin Pract. 2000;3(6):313–6. [PubMed] [Google Scholar]
- 13.Smith DL, Elting LS, Learn PA, Raut CP, Mansfield PF. Factors influencing the volume-outcome relationship in gastrectomies: a population-based study. Ann Surg Oncol. 2007;14(6):1846–52. [DOI] [PubMed] [Google Scholar]
- 14.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–9. [DOI] [PubMed] [Google Scholar]
- 15.US Census Bureau; American Community Survey. 2015. American Community Survey 5-year estimates. http://factfinder.census.gov. Accessed 14 May 2018.
- 16.Iezzoni LI, Daley J, Heeren T, et al. Identifying complications of care using administrative data. Med Care. 1994;32(7):700–15. [DOI] [PubMed] [Google Scholar]
- 17.Rosen AK, Geraci JM, Ash AS, McNiff KJ, Moskowitz MA. Postoperative adverse events of common surgical procedures in the Medicare population. Med Care. 1992;30(9):753–65. [DOI] [PubMed] [Google Scholar]
- 18.McCarthy EP, Iezzoni LI, Davis RB, et al. Does clinical evidence support ICD-9-CM diagnosis coding of complications? Med Care. 2000;38(8):868–76. [DOI] [PubMed] [Google Scholar]
- 19.Lawthers AG, McCarthy EP, Davis RB, Peterson LE, Palmer RH, Iezzoni LI. Identification of in-hospital complications from claims data. Is it valid? Med Care. 2000;38(8):785–95. [DOI] [PubMed] [Google Scholar]
- 20.Romano PS, Chan BK, Schembri ME, Rainwater JA. Can administrative data be used to compare postoperative complication rates across hospitals? Med Care. 2002;40(10):856–67. [DOI] [PubMed] [Google Scholar]
- 21.Gasper WJ, Glidden DV, Jin C, Way LW, Patti MG. Has recognition of the relationship between mortality rates and hospital volume for major cancer surgery in California made a difference?: a follow-up analysis of another decade. Ann Surg. 2009;250(3):472–83. [DOI] [PubMed] [Google Scholar]
- 22.Birkmeyer JD, Lucas FL, Wennberg DE. Potential benefits of regionalizing major surgery in Medicare patients. Eff Clin Pract. 1999;2(6):277–83. [PubMed] [Google Scholar]
- 23.Birkmeyer JD. Should we regionalize major surgery? Potential benefits and policy considerations. J Am Coll Surgeons. 2000;190(3):341–9. [DOI] [PubMed] [Google Scholar]
- 24.Dudley RA, Johansen KL, Brand R, Rennie DJ, Milstein A. Selective referral to high-volume hospitals: estimating potentially avoidable deaths. JAMA. 2000;283(9):1159–66. [DOI] [PubMed] [Google Scholar]
- 25.Finlayson SR, Birkmeyer JD, Tosteson AN, Nease RF Jr. Patient preferences for location of care: implications for regionalization. Med Care. 1999;37(2):204–9. [DOI] [PubMed] [Google Scholar]
- 26.Stitzenberg KB, Sigurdson ER, Egleston BL, Starkey RB, Meropol NJ. Centralization of cancer surgery: implications for patient access to optimal care. J Clin Oncol. 2009;27(28):4671–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Modrall JG, Minter RM, Minhajuddin A, et al. The Surgeon Volume-outcome Relationship: Not Yet Ready for Policy. Ann Surg. 2018;267(5):863–7. [DOI] [PubMed] [Google Scholar]
- 28.de Wilde RF, Besselink MG, van der Tweel I, et al. Impact of nationwide centralization of pancreaticoduodenectomy on hospital mortality. Br J Surg. 2012;99(3):404–10. [DOI] [PubMed] [Google Scholar]
- 29.Gooiker GA, Lemmens VE, Besselink MG, et al. Impact of centralization of pancreatic cancer surgery on resection rates and survival. Br J Surg. 2014;101(8):1000–05. [DOI] [PubMed] [Google Scholar]
- 30.Gouma DJ, De Wit LT, Van Berge Henegouwen MI, Van Gulik TH, Obertop H. Hospital experience and hospital mortality following partial pancreaticoduodenectomy in The Netherlands [in Dutch]. Ned Tijdschr Geneeskd. 1997;141(36):1738–41. [PubMed] [Google Scholar]
- 31.Busweiler LAD, Dikken JL, Henneman D, et al. The influence of a composite hospital volume on outcomes for gastric cancer surgery: a Dutch population-based study. J Surg Oncol. 2017;115(6):738–45. [DOI] [PubMed] [Google Scholar]
- 32.Vicente D, Ikoma N, Chiang YJ, et al. Preoperative therapy for gastric adenocarcinoma is protective for poor oncologic outcomes in patients with complications after gastrectomy. Ann Surg Oncol. 2018;25(9):2720–30. [DOI] [PubMed] [Google Scholar]


