Abstract
Objective:
We sought to identify potential radiologic and serologic markers of pancreatic tumor response to therapy, using pathologic major response (pMR) as the objective endpoint.
Background:
We previously demonstrated that a pMR to preoperative therapy, defined as detection of <5% viable cancer cells in the surgical specimen on histopathological analysis, is an important prognostic factor for patients with pancreatic ductal adenocarcinoma (PDAC).
Methods:
Pretreatment and posttreatment computed tomography scans of consecutive patients who received preoperative chemotherapy and/or (chemo)radiation before pancreatectomy for PDAC between January 2010 and December 2018 were rereviewed. Response per Response Evaluation Criteria in Solid Tumors (RECIST) 1.1, other radiographic changes in tumor size and anatomic extent, and posttreatment CA 19–9 levels were compared between patients who did and did not have a pMR on final histopathologic analysis of their surgical specimens.
Results:
A total of 290 patients with localized PDAC underwent pancreatectomy between 2010 and 2018 after receiving preoperative chemotherapy (n = 36; 12%), (chemo)radiation (n = 87; 30%), or both (n = 167; 58%). Among them, 28 (10%) experienced pMR, including 9 (3.1%) who experienced pathologic complete response. On multivariable logistic regression, low posttreatment CA 19–9 level, RECIST partial response, and reduction in tumor volume were confirmed to be independently associated with pMR (P < 0.01).
Conclusions:
We identified serologic and radiographic indicators of pMR that could help inform the delivery of preoperative therapy to patients with PDAC.
Keywords: neoadjuvant therapy, pancreatic cancer, pathologic response, radiographic predictor, RECIST
Pancreatic ductal adenocarcinoma (PDAC) is anticipated to emerge as the second leading cause of cancer-related death in the United States by 2030.1 For patients with localized PDAC, resection of the primary tumor and regional lymph nodes represents the cornerstone of potentially curative therapy. Systemic therapy after resection improves survival outcomes relative to surgery alone,2 but over recent years, multidisciplinary teams have increasingly treated patients who have localized PDAC with chemotherapy and/or (chemo)radiation before, instead of following, pancreatectomy. In the preoperative setting, these regimens are used primarily in an attempt to reduce the size and/or anatomic extent of the cancer, to identify patients exhibiting a “locally dominant phenotype” for whom resection may be most beneficial, and to maximize the likelihood of a microscopically complete (R0) resection. Practice guidelines now recognize the administration of preoperative therapy as the preferred strategy for patients with borderline resectable PDAC3 and an acceptable option for patients with potentially resectable PDAC.4
Tumor response to preoperative therapies may be measured histologically by the extent of residual viable cancer in the resected specimen, and this metric has important prognostic implications for patients who have undergone resection of PDAC.5,6,7 We previously showed that patients who experience either pathologic complete response (pCR; no viable cancer cells) or pathologic major response (pMR; <5% of residual viable cancer cells) to preoperative therapy live significantly longer than patients who have 5% to 100% viable residual cancer cells in their specimen. 6,7 More recently, we identified 77 (13.2%) patients who experienced pMR among 583 patients who had received preoperative therapy for PDAC, including 23 (3.9%) who experienced pCR. Patients who experienced pMR had a median overall survival of 73.4 months, compared with 32.2 months among patients who did not (P <0.01). On multivariable logistic regression, baseline factors including young age, pretreatment cancer antigen (CA) 19–9 level, and use of gemcitabine as a radiosensitizer were associated with pMR.8
Currently, preoperative regimens are selected on the basis of baseline data such as radiographic stage and serum CA 19–9 level and then are typically administered, in the absence of disease progression, for a prespecified duration as long as 6 months or more.9 The decision to proceed with resection after preoperative therapy likewise rests on the absence of disease progression, not evidence for tumor response.10 Clinical data that could reliably indicate response in real time might allow dynamic modulation of the type and duration of preoperative therapies. It might also allow identification of patients unlikely to benefit from surgery, for whom alternative strategies may be preferable. Unfortunately, the extent to which traditional metrics of therapeutic response reliably indicate response in this setting is unclear. For example, standard Response Evaluation Criteria in Solid Tumors (RECIST, version 1.1)11 have been shown to be insufficient to accurately predict “resectability” in the setting of preoperative therapy for PDAC.12,13 And, although CA 19–9 level is associated with postoperative survival, its association with pathologic response is unknown.14,15 This is an important consideration, as survival may be modulated by multiple factors independent of the effects of preoperative therapy.
The purpose of this study was to estimate the associations between pMR and potential serologic and radiographic measures of response in a large cohort of patients who underwent pancreatectomy after preoperative therapy for PDAC at The University of Texas MD Anderson Cancer Center.
METHODS
The institutional review board of MD Anderson Cancer Center approved this study (IRB #PA 19–0065). Individual informed consent was waived. We used MD Anderson’s prospectively maintained pancreatic tumor database to identify 333 consecutive patients who received preoperative chemotherapy and/or (chemo)radiation before pancreatectomy for PDAC between January 2010 and December 2018.16 Forty-three patients were excluded from analysis: 13 patients who received fewer than 3 cycles of preoperative chemotherapy, 8 patients for whom histopathologic analysis of treatment effect was not recorded, 7 patients with a final diagnosis of PDAC arising in an intraductal papillary mucinous neoplasm, 7 patients with a baseline computed tomography (CT) showing severe acute pancreatitis or no visible mass, and 8 patients for whom CT images taken at baseline or the time of surgery were not available for rereview or who were imaged using a CT scanning protocol other than that described below.
Radiographic Review
Before the initiation and after completion of preoperative therapy, anatomic disease staging was accomplished with multi-detector CT using a 64-detector row scanner and a standard protocol optimized for imaging pancreatic tumors.17 Multiplanar reconstructions were used as necessary to visualize vascular anatomy. Per standardized criteria, tumors were radiographically staged as potentially resectable, borderline resectable, or locally advanced.18 The baseline and preoperative CT images of all patients were rereviewed for this study by a surgeon (GP) blinded to treatment and outcome.
The examiner measured the tumor size using the longest (L) and shortest (W) axial diameter and the craniocaudal diameter (H). The volume of each tumor was calculated according to the formula for a typical ellipsoid (Volume = π/6×L×W×H).19 The radiographic interface between the tumor and each mesenteric vascular structure (TVI) was characterized as either no contact, abutment (≤180° of the circumference), encasement (>180° of the circumference), or occlusion.20 To measure average attenuation in Hounsfield units, a circular region of interest encompassing one-half to two-thirds of the tumor’s area was drawn in the center of the tumor on the section with the largest surface area in portal venous phase CT images. To characterize radiographic changes associated with preoperative therapy, the volume of the primary tumor, TVIs, and tumor attenuation on pretreatment images were compared with those in posttreatment images for each patient. The change in tumor volume after preoperative treatment (%Δvol) was calculated as a percentage of the baseline volume. Changes were also described using modified RECIST (version 1.1).11 Progressive disease (PD) was defined as either the development of metastatic lesions or an increase of ≥20% in the primary tumor’s largest dimension (with a minimum increase of 5 mm). A partial response (PR) was defined as a decrease of ≥30% in the primary tumor’s largest dimension. Stable disease (SD) was defined as insufficient increase or decrease in tumor size to qualify as PD or PR, respectively. A complete response (CR) was defined as total disappearance of the primary tumor.
CA 19–9 level
Serum CA 19–9 (normal range 0–37 U/mL) was measured before and after treatment. Patients in whom CA 19–9 was measured as <1 U/mL both before and after treatment were defined as nonproducers.
Preoperative Therapy and Surgery
Preoperative therapy was administered as part of a clinical trial protocol in some patients, and all treatment decisions were made by a multidisciplinary team. Several preoperative treatment regimens were used. Patients received external-beam radiation therapy (total dose, 50.4 Gy over 6 wk or 30 Gy over 2 wk) with concurrent 5-fluorouracil, capecitabine, or gemcitabine, or stereotactic body radiation therapy over 5 days without a radiosensitizer. When both systemic chemotherapy and (chemo)radiation were used, chemotherapy was administered first. Within 4 to 8 weeks of completing preoperative therapy, patients were clinically and radiographically restaged. Patients with no evidence of PD and with adequate performance status were considered for surgical resection and underwent pancreatoduodenectomy, distal pancreatectomy, or total pancreatectomy, which were performed using standardized techniques.21
Histopathologic Analysis
Gastrointestinal pathologists used a standardized protocol to evaluate all surgical specimens.22 R1 margin status was defined as evidence of cancer cells at the inked bile duct or pancreatic parenchymal margin, or within 1 mm of the superior mesenteric artery margin. Histopathologic response to preoperative therapy was measured as the percentage of residual viable cancer cells within the treated tumor bed or entire resected pancreas if no tumor bed was grossly identified.6,23 A pMR was defined as <5% residual viable cancer cells, a definition that included pCR.6 The pretreatment cytopathology specimen of each patient who experienced pCR was rereviewed.
Postoperative Therapy and Follow-up
After resection, individual patient and tumor characteristics determined the need for and type of postoperative therapy. Patients were typically evaluated every 3 to 4 months at first and then every 6 months with cross-sectional imaging, physical examination, and CA 19–9 analysis.24
Statistical Analysis
Clinical, demographic, and pathologic variables were compared between patients who did and did not experience pMR. Continuous data were expressed as median and range, whereas categorical data were expressed as frequencies and percentages. Continuous variables were compared using a t test if normally distributed and a nonparametric Mann–Whitney U test if not. Categorical variables were compared using Pearson χ2 (or Fisher exact test when appropriate). Possible associations between demographic and clinical factors and pMR were evaluated using univariable and multivariable logistic regression modeling. Due to possible collinearity between PR (as defined by RECIST 1.1) and %Δvol, we developed 2 multivariable models and included each radiographic metric independently as a covariate. Clinical factors that had a P < 0.2 on univariable analysis and those that had potential clinical importance (including radiotherapy) were included in each model. A receiver-operating characteristic curve was constructed for %Δvol and pMR, and the area under the curve was calculated. Overall survival was calculated from the date of diagnosis to the date of death or last follow-up using the Kaplan–Meier method, whereas disease-free survival was calculated from the date of surgery to the date of disease recurrence or last follow-up. Both were compared using the Mantel–Cox log-rank test between patients who did and did not experience pMR. Statistical analyses were performed using SPSS, version 24.0 (SPSS Inc), and P values <0.05 were considered significant. P values were 2-sided.
RESULTS
A total of 290 patients were included in the analysis. Among them, 28 (10%) patients experienced pMR, including 9 (3.1%) patients who experienced pCR. The remaining 262 (90%) patients had ≥5% viable cells in the surgical specimen. Within a mean follow-up period of 34 months, the median disease-free and overall survival durations of patients who experienced pMR were longer than that of patients who did not (disease-free: not reached vs 15 mo, P < 0.01; overall: not reached vs 38 mo, P < 0.01; Fig. 1). The median overall survival duration of patients who experienced pMR, but not pCR, was likewise longer than that of patients who had ≥5% residual cancer cells in their surgical specimen (P < 0.01).
FIGURE 1.
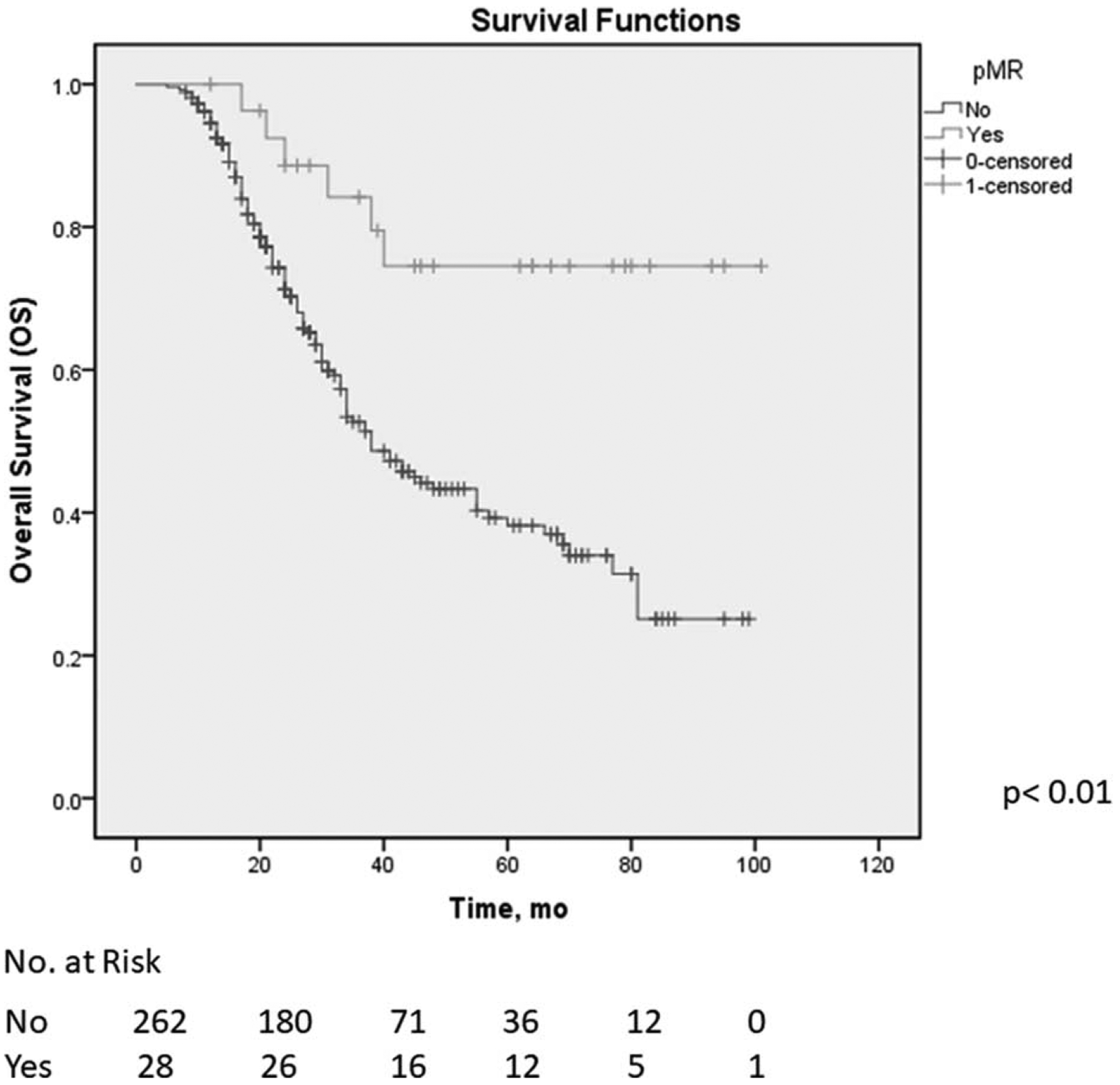
Overall survival of patients with pancreatic ductal adenocarcinoma who underwent preoperative therapy, stratified by pathologic major response (pMR).
Clinical characteristics of the study population are reported in Table 1. Patients received chemotherapy alone (n = 36; 12%), (chemo)radiation alone (n = 87; 30%), or both (n = 167; 58%) before resection. The majority (n = 155; 76%) of patients who received systemic chemotherapy were treated with 5-fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFIRINOX) and/or gemcitabine plus nanoparticle albumin-bound paclitaxel. Patients who experienced pMR were similar to those who did not in terms of sex, age, body mass index, type and duration of preoperative therapy, tumor site, radiographic stage, and type of pancreatectomy. Among histopathologic variables, patients who experienced pMR had a smaller median tumor diameter (0.5 vs 2.9 cm; P < 0.01) and a lower rate of positive lymph nodes (7% vs 55%, P < 0.01) than patients who did not.
TABLE 1.
Demographic, Clinical, and Pathologic Profile of Patients Who Experienced a Major Pathologic Response (pMR, <5% Residual Cancer Cells) and Those Who Did Not (≥5% Residual Cancer Cells) After Preoperative Therapy for PDAC
| pMR, No. (%) | ||||
|---|---|---|---|---|
| Characteristic | Total, No. (%) (N = 290) | Yes = 28 (10) | No = 262 (90) | P |
| Sex | ||||
| Female | 132 (54) | 17 (61) | 115 (44) | 0.08 |
| Male | 158 (46) | 11 (39) | 147 (56) | |
| Age at diagnosis, median (range) | 64 (35–88) | 62 (38–79) | 65 (35–88) | 0.09 |
| BMI, median (range) | 27 (18–46) | 27 (19–34) | 27 (18–46) | 0.5 |
| Preoperative therapy type | ||||
| Chemo + RT | 167 (58) | 18 (64) | 149 (57) | 0.6 |
| Chemo only | 36 (12) | 2 (7) | 34 (13) | |
| RT only | 87 (30) | 8 (29) | 79 (30) | |
| Chemotherapy type | ||||
| FOLFIRINOX | 96 (47) | 12 (60) | 84 (46) | 0.1 |
| FOLFIRINOX + GemAb | 11 (6) | 0 | 11 (6) | |
| GemAb | 48 (23) | 2 (10) | 46 (25) | |
| GemCis | 35 (17) | 6 (30) | 29 (16) | |
| Other | 13 (7) | 0 | 13 (7) | |
| No. of cycles, median (range) | 5 (3–13) | 6 (4–12) | 5 (3–13) | 0.3 |
| Radiation dose | ||||
| 30 Gy/10 fractions | 65 (22) | 4 (14) | 61 (23) | 0.3 |
| 50 Gy/28 fractions | 169 (58) | 21 (75) | 148 (57) | |
| SBRT | 20 (7) | 1 (4) | 19 (7) | |
| None | 36 (13) | 2 (7) | 34 (13) | |
| Radiographic stage | ||||
| Resectable | 173 (60) | 16 (57) | 157 (60) | 0.5 |
| Borderline resectable | 94 (32) | 11 (39) | 83 (32) | |
| Locally advanced | 23 (8) | 1 (4) | 22 (8) | |
| Tumor site | ||||
| Head | 240 (83) | 20 (71) | 220 (84) | 0.09 |
| Body/tail | 50 (17) | 8 (29) | 42 (16) | |
| Type of pancreatectomy | ||||
| PD | 235 (81) | 22 (79) | 213 (81) | 0.5 |
| DP | 48 (16) | 6 (21) | 42 (16) | |
| TP | 7 (3) | 0 | 7 (3) | |
| LN status | ||||
| Negative | 144 (50) | 26 (93) | 118 (45) | <0.01 |
| Positive | 146 (50) | 2 (7) | 144 (55) | |
| LNs examined, median (range) | 27 (1–65) | 27 (6–44) | 27 (1–65) | 0.8 |
| Tumor size, median (range), cm | 2.8 (0–10.5) | 0.5 (0–8) | 2.9 (0–10.5) | <0.01 |
| R Status | ||||
| Negative | 196 (68) | 23 (82) | 173 (66) | <0.01 |
| Positive | 64 (22) | 0 | 64 (24) | |
| N/R | 30 (10) | 5 (18) | 25 (10) | |
BMI indicates body mass index; Chemo, chemotherapy; RT, radiotherapy; FOLFIRINOX, 5-fluorouracil, leucovorin, oxaliplatin, and irinotecan; GemAb, gemcitabine and nabpaclitaxel; GemCis, gemcitabine and cisplatin; SBRT, stereotactic body radiotherapy; PD, pancreatoduodenectomy; DP, distal pancreatectomy; TP, total pancreatectomy; LN, lymph node; N/R, not reported.
RECIST
No patient experienced RECIST CR. Among 63 patients with RECIST PR, 216 patients with RECIST SD, and 11 patients with RECIST PD, respectively, 17 (27%), 11 (5%), and 0 (0%) experienced pMR (P < 0.01). Notably, no patient who experienced pMR had RECIST PD, although 11 (39%) had RECIST SD.
Tumor Volume
Among 251 (87%) patients whose tumor volume decreased after preoperative therapy, 27 (11%) experienced pMR. Among 39 (13%) whose tumor remained stable or increased in volume, only 1 (3%) experienced pMR (Table 2). The median %Δvol among patients who experienced pMR was significantly higher (indicating a greater reduction in volume) than that of patients who did not (68% vs 34%; P < 0.01).
TABLE 2.
Radiographic and Serologic Possible Metrics of Major Pathologic Response (pMR, <5% Residual Cancer Cells) After Preoperative Therapy for PDAC
| pMR, No. (%) | ||||
|---|---|---|---|---|
| Metric | Total, No. (%) (N = 290) | Yes = 28 (10) | No = 262 (90) | P |
| Radiographic metrics | ||||
| RECIST 1.1 | ||||
| CR | 0 | 0 | 0 | <0.01 |
| PR | 63 (22) | 17 (61) | 46 (18) | |
| SD | 216 (74) | 11 (39) | 205 (78) | |
| PD | 11 (4) | 0 | 11 (4) | |
| Increase of attenuation median (range), HU | 0 (−39 to +89) | 5 (−39 to +89) | 0 (−85 to +73) | 0.1 |
| Decreased tumor volume | ||||
| No | 39 (13) | 1 (4) | 38 (14) | 0.1 |
| Yes | 251 (87) | 27 (96) | 224 (86) | |
| %Dvol, median (range) | 38% (−245% to +98%) | 68% (0 to +98%) | 34% (−245% to +98%) | <0.01 |
| ≥55% decreased volume | ||||
| No | 200 (69) | 6 (21) | 194 (74) | <0.01 |
| Yes | 90 (31) | 22 (79) | 68 (26) | |
| Tumor-vessel interface* | ||||
| SMV/PV interface | ||||
| Progression | 18 (6) | 0 | 18 (7) | <0.01 |
| No change | 246 (85) | 19 (68) | 227 (87) | |
| Improvement | 26 (9) | 9 (32) | 17 (6) | |
| SMA/CHA/CA interface | ||||
| Progression | 8 (3) | 0 | 8 (3) | 0.8 |
| No change | 270 (93) | 26 (93) | 244 (93) | |
| Improvement | 12(4) | 2 (7) | 10 (4) | |
| Serologic metric | ||||
| Posttreatment CA 19–9, median (range), U/mL | 29 (1–1,152) | 17 (1–50) | 30 (1–1,152) | <0.01 |
| Posttreatment CA 19–9 level | ||||
| Elevated (>37 U/mL) | 110 (38) | 1 (4) | 109 (42) | <0.01 |
| Normal (≤37 U/mL) | 167 (58) | 25 (89) | 142 (54) | |
| Not expressed | 13 (4) | 2 (7) | 11 (4) | |
| Change in CA19–9 level | ||||
| Elevated to normal | 105 (36) | 20 (71) | 85 (32) | <0.01 |
| Elevated to elevated | 109 (38) | 1 (4) | 108 (41) | |
| Normal to normal | 61 (21) | 5 (18) | 56 (22) | |
| Normal to elevated | 2 (1) | 0 | 2 (1) | |
| Not expressed | 13 (4) | 2 (7) | 11 (4) | |
%Δvol indicates change in tumor volume; CA, celiac artery; CA 19–9, cancer antigen 19–9; CHA, common hepatic artery; CR, complete response; PD, progressive disease; PR, partial response; PV, portal vein; SD, stable disease; SMA, superior mesenteric artery; SMV, superior mesenteric vein.
Classifications for tumor-vessel interface calculation: no contact, abutment (≤180 degrees of the circumference), encasement (>180 degrees of the circumference), occlusion.
Progression: any migration from lower classification to higher classification; improvement: any migration from higher classification to lower classification.
The receiver-operating characteristic curve relating %Δvol and pMR is illustrated in Figure 2. The area under the curve was 0.815. A %Δvol cutoff of 55% simultaneously maximized sensitivity (79%) and specificity (75%) for pMR. A pMR was observed in 22 (24%) of 90 patients who had a %Δvol of 55% or more and 6 (3%) of 200 patients who did not (P < 0.01; Table 2).
FIGURE 2.
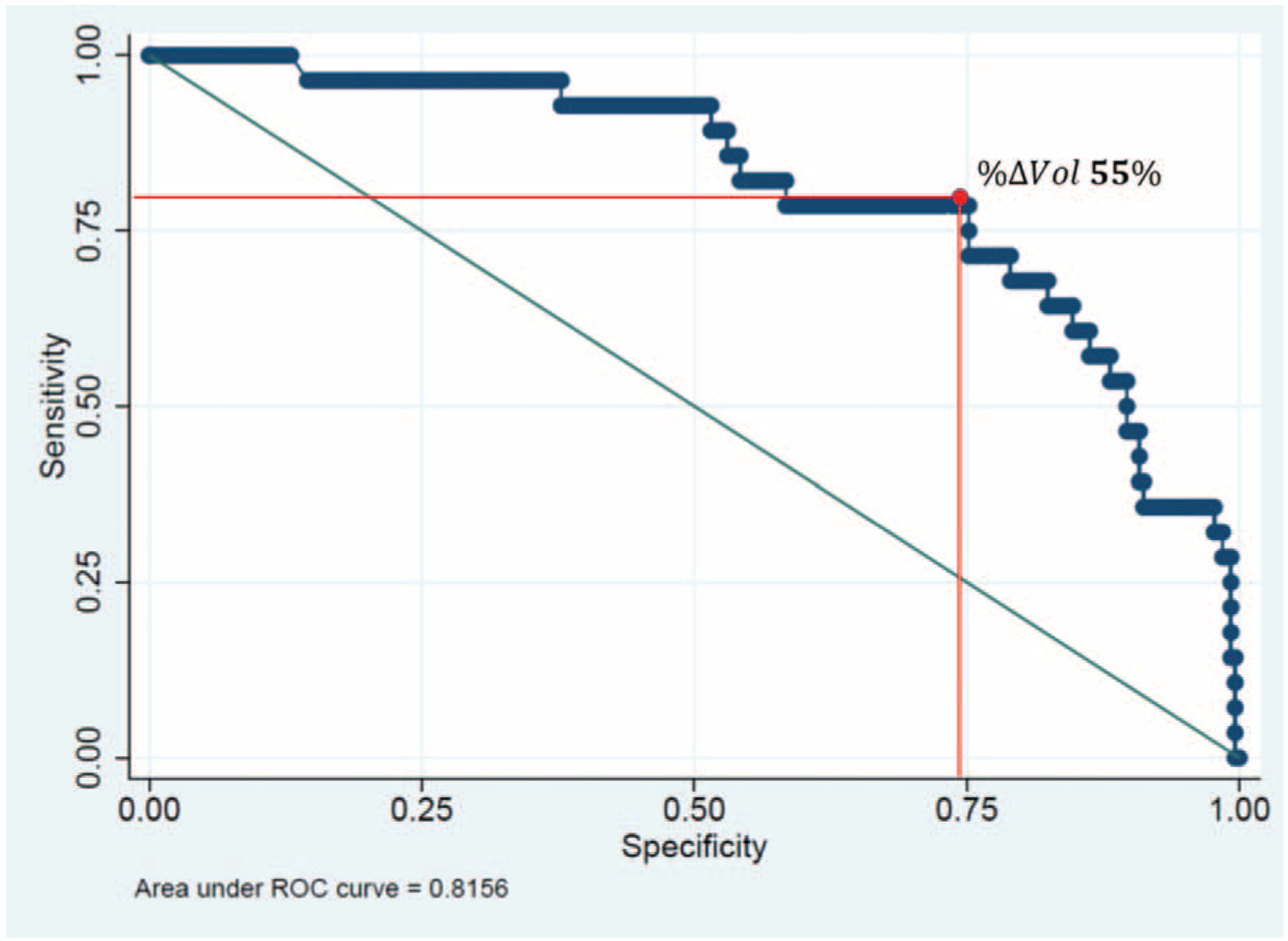
Area under the receiver operating characteristic (ROC) curve (AUC) relating the change in tumor volume after preoperative treatment (%Δvol) and pathologic major response (AUC = 0.81). The optimal cutoff for change in tumor volume (%Δvol) indicating a pathologic major response was a decrease of 55% (sensitivity = 78%, specificity = 75%).
Tumor Attenuation
No difference between patients who did and did not experience pMR was observed in the median change in attenuation that occurred in association with preoperative therapy (P = 0.1; Table 2).
Tumor–Vessel Interface
Only 26 (9%) patients had an improvement in the superior mesenteric vein/portal vein interface and 12 (4%) in the superior mesenteric artery/celiac artery/common hepatic artery interfaces; among these, 9 (35%) and 2 (17%) experienced pMR, respectively (Table 2). Notably, no patient who had progression in either venous or arterial TVI experienced pMR.
CA 19–9 Level
The median posttreatment CA 19–9 level of patients who experienced pMR was significantly lower than that of patients who did not (17 vs 30 U/mL; P < 0.01; Table 2).
Twenty-one (75%) of 28 patients who experienced pMR had an elevated pretreatment CA 19–9 level (median 135, range 38–8947 U/mL). Among them, CA 19–9 declined to within the normal range in 20 (95%); the remaining patient had a CA 19–9 level 50 U/mL after treatment. Of the 7 (25%) patients who experienced pMR and had a CA 19–9 level within the normal range before treatment (2 of whom were nonproducers), all had a CA 19–9 level that remained normal after treatment.
Sixteen (33%) of 49 patients and 21 (32%) of 66 patients who had a RECIST PR or %Δvol of ≥55%, respectively, and also had a posttreatment CA 19–9 within the normal range experienced pMR.
Univariable and Multivariable Analysis of Potential Serologic and Radiographic Predictors of pMR
On univariable and multivariable logistic regression, posttreatment CA 19–9 level (OR 0.97, 95% CI, 0.95–0.99; P < 0.01) and RECIST 1.1 PR (OR 5.78, 95% CI, 2.46–13.59; P < 0.01) were independently associated with pMR (Table 3). In an alternate model constructed to address concerns of collinearity between tumor volume and RECIST response, %Δvol was also independently associated with pMR (OR 1.05, 95% CI, 1.03–1.08; P < 0.01; Table 4).
TABLE 3.
Univariable and Multivariable Logistic Regression Analysis of Preoperative Predictors of Major Pathologic Response
| Predictors | Univariable, Odds Ratio (95% CI) | P | Multivariable, Odds Ratio (95% CI) | P |
|---|---|---|---|---|
| Male sex | 0.50 (0.23–1.12) | 0.09 | NA | 0.2 |
| Age at diagnosis | 0.96 (0.92–1.00) | 0.06 | NA | 0.3 |
| BMI | 0.96 (0.89–1.04) | 0.3 | ||
| Radiotherapy | 1.93 (0.44–8.53) | 0.3 | NA | 0.4 |
| Number of cycles | 1.03 (0.91–1.17) | 0.6 | ||
| Radiographic stage | ||||
| Resectable | 1 [Reference] | |||
| Borderline resectable | 1.30 (0.57–2.93) | 0.5 | ||
| Locally advanced | 0.44 (0.05–3.53) | 0.4 | ||
| Posttreatment CA 19–9 | 0.97 (0.95–0.99) | <0.01 | 0.97 (0.95–0.99) | 0.02 |
| RECIST partial response | 7.25 (3.18–16.51) | <0.01 | 5.78 (2.46–13.59) | <0.01 |
| Increase in attenuation | 1.64 (0.66–3.64) | 0.2 | ||
| %Δvol | 1.05 (1.03–1.07) | <0.01 |
%Δvol indicates change in tumor volume; BMI, body mass index; CA 19–9, cancer antigen 19–9; NA, not applicable.
TABLE 4.
Univariable and Multivariable Logistic Regression Analysis of Preoperative Predictors of Major Pathologic Response; Considering Volume (Instead of RECIST) as a Predictor in the Multivariable Analysis
| Predictors | Univariable, Odds Ratio (95% CI) | P | Multivariable, Odds Ratio (95% CI) | P |
|---|---|---|---|---|
| Male sex | 0.50 (0.23–1.12) | 0.09 | NA | 0.3 |
| Age at diagnosis | 0.96 (0.92–1.00) | 0.06 | NA | 0.2 |
| BMI | 0.96 (0.89–1.04) | 0.3 | ||
| Radiotherapy | 1.93 (0.44–8.53) | 0.3 | NA | 0.4 |
| Number of cycles | 1.03 (0.91–1.17) | 0.6 | ||
| Radiographic stage | ||||
| Resectable | 1 [Reference] | |||
| Borderline resectable | 1.30 (0.57–2.93) | 0.5 | ||
| Locally advanced | 0.44 (0.05–3.53) | 0.4 | ||
| Posttreatment CA 19–9 | 0.97 (0.95–0.99) | <0.01 | 0.97 (0.95–0.99) | 0.02 |
| RECIST partial response | 7.25 (3.18–16.51) | <0.01 | ||
| Increase in attenuation | 1.64 (0.66–3.64) | 0.2 | ||
| %Δvol | 1.05 (1.03–1.07) | <0.01 | 1.05 (1.03–1.08) | <0.01 |
%Δvol indicates change in tumor volume; BMI, body mass index; CA 19–9, cancer antigen 19.9; NA, not applicable.
DISCUSSION
We previously showed that a pMR to preoperative chemotherapy and/or (chemo)radiation, defined as <5% residual viable tumor cells in the pancreatectomy specimen, is associated with prolonged disease-free and overall survival after pancreatectomy.6,25 In this study, we evaluated the associations between various potential clinical measures of therapeutic response to preoperative therapy for PDAC and the objective and clinically relevant endpoint pMR. We found that the posttreatment CA 19–9 level was negatively associated with the likelihood of pMR, and RECIST PR and a reduction in tumor volume were both positively associated with the likelihood of pMR. Approximately one-third of patients who had both a posttreatment CA 19–9 level within the normal range and either RECIST PR or a reduction in tumor volume of 55% experienced pMR. Conversely, pMR was extraordinarily unlikely in the absence of a posttreatment CA 19–9 within the normal range or a reduction in tumor volume with therapy. These findings suggest the potential for routine clinical metrics to be used in real time to help guide the delivery of preoperative therapies according to the likelihood of a meaningful therapeutic response.
Although RECIST reflects therapeutic efficacy (or lack thereof) in other cancer treatment settings, the utility of these criteria in the preoperative setting for PDAC has been challenged. Specifically, several studies have shown that radiographic response does not accurately portray whether or not pancreatic tumors will be amenable to resection after the administration of preoperative therapy.12,13,26 In this setting, radiologic assessment may lead to underestimation of the possibility of resecting a tumor to negative margins. This lack of accuracy may be due to the peculiar nature of the tumor stroma in PDAC, which is associated with a persistence of fibrotic tissue—even after the destruction of cancer cells—that prevents the tumor from shrinking.27 We previously reported that among 129 patients treated with preoperative therapy for borderline resectable PDAC, only 15 (12%) experienced RECIST PR and the tumor of only 1 (0.8%) patient was downstaged to resectable; nevertheless, 85 patients (66%) underwent resection, 81 of whom had negative margins.12 For this reason, therapeutic decision making has historically focused on the detection of disease progression—typically expressed by the interval identification of radiographically evident metastases—as opposed to disease regression in response to effective therapy. As a consequence, patients who do not show signs of overt progression are at risk for receiving prolonged courses of ineffective and therefore unnecessary treatments, thereby delaying resection. Conversely, patients are also at risk for being inappropriately labeled as “well-selected” for aggressive, albeit technically possible, surgical procedures unlikely to lead to long-term survival—merely due to absence of overt disease progression during treatment.
A clinically significant pathologic response to preoperative therapy for PDAC is associated with a median survival duration well beyond 5 years, but a major limitation of this metric is that it can only be measured after the completion of resection.8 One small study previously explored the potential relationship between radiographic parameters and pathologic response in the setting of PDAC.28 Among 38 evaluated patients, no correlation between radiographic and pathologic response could be established. However, 7 (26%) of 27 patients who did not experience RECIST PR or RECIST CR had an Evans grade IIB or greater pathologic response. That a grade IIB response (in contrast to pMR) does not appseem to have prognostic significance notwithstanding,25 these results led the authors to conclude that surgery should not be withheld in the absence of RECIST response.
Our data clearly show that the overall base rate of a clinically meaningful pathologic response after preoperative therapy is only 10%. And, in the absence of a radiographic or serologic response, the likelihood of a pMR is extraordinarily unlikely: only 1 patient whose tumor volume did not decrease with preoperative therapy experienced pMR, and only 1 patient who had an elevated CA 19–9 after treatment (a patient whose tumor volume did decrease by 91%) experienced pMR. Absence of a clinical response should not necessarily preclude resection in an otherwise appropriate candidate, as absence of pMR does not exclude the possibility of prolonged survival. However, it may justify a more conservative approach in some patients, such as those in whom the risk: benefit profile associated with surgery is skewed toward risk. Surgery might be less appropriate, for example, for a frail or otherwise “high risk” patient whose tumor volume did not change at all during preoperative therapy (likelihood of pMR: 3% in this series) than one who experienced both RECIST PR and had a normal posttreatment CA 19–9 level (likelihood of pMR: 33%).
That radiographic and serologic metrics may serve, at least to some degree, as a readout of pathologic response also suggests the potential for such data to help inform the delivery of preoperative therapy. Systemic chemotherapy, for example, is routinely delivered in the absence of overt disease progression and, given increasing interest in “total neoadjuvant therapy,” may be delivered continuously for a duration as long as 6 months or more.29 Our data support the conduct of interval, sequential disease staging that could allow for earlier modifications of the preoperative strategy to be at least considered.
In our study, we focused on metrics that are easily measured in the course of routine clinical practice, including CA 19–9 and radiographic changes in tumor volume, tumor attenuation,30 and TVI20. Importantly, however, novel radiomic and serologic predictors of response are also actively being investigated. For example, Amer et al recently evaluated 4 cohorts of patients and showed that in each the change in the radiographic interface between tumor and adjacent pancreatic parenchyma that often occurred in association with chemoradiation was associated with outcome.31 Moreover, in 1 of the cohorts, patients who met criteria for a radiomic response had a greater likelihood of achieving a pMR or pCR (21% vs 0%, P = 0.01). In another study of a potential serologic marker, Bernard et al calculated the fraction of mutant KRAS in circulating exosomal DNA and found that an increase was associated with disease progression (P = 0.003).32 These novel biomarkers reveal the high potential for longitudinal monitoring and the use of real-time radiographic or circulating biomarkers to direct therapy.
The primary limitations of this study are related to its retrospective and single-institution design of patients who all underwent resection. For example, 88% of the patients were treated with (chemo)radiation before surgery, but the role of radiation in the preoperative setting for pancreatic cancer is undefined, and so it may not be administered to all patients in all centers. We included radiotherapy in the multivariable models to control for the potential effects of its use. Also, the mean follow-up in this study was relatively short as a consequence of our decision to include more patients treated with chemotherapy regimens that are now routinely prescribed in the preoperative setting. However, it is interesting to note that in this series of patients who underwent resection between 2010 and 2018 and of whom 76% received FOLFIRINOX or gemcitabine plus nanoparticle albumin-bound paclitaxel, only 10% of patients experienced pMR and only 3.5% experienced pCR. Contrary to what might be expected, these rates are strikingly similar to the rates calculated in our previous analysis of patients treated between 1990 and 2015 (13.2% pMR and 3.9% pCR), among whom only 12% of patients received those drug regimens.8 It is, however, interesting that the pCR rate calculated herein is somewhat lower than the 10% rate observed in a recent study of 186 patients with borderline resectable or locally advanced PDAC treated with preoperative therapy,33 a study in which the median overall survival duration of patients who experienced a “near complete pathologic response” was not found to differ from that of patients in whom pathologic response was less robust. The latter of these differences may relate to a difference in definition; in that study, a “near complete pathologic response” was assigned to any primary tumor that measured <1 cm and was not associated with nodal metastases. Finally, here we evaluated RECIST, the clinical use of which is not widespread in this setting. However, RECIST is an important, standardized, and reproducible classification system that is used to report and compare response rates to therapy in most prospective clinical trials. Because RECIST is limited as it relies upon 2-dimensional measurement of maximum tumor diameter and uses a fixed cutoff of 30% to discriminate between SD and PR, however, we also evaluated 3-dimensional change in tumor volume as a potential measure of pathologic response.
The aforementioned limitations of the study should be acknowledged in the context of its strengths, which include its large sample size, rereview of all imaging by a single, nonbiased investigator, and use of a validated pathologic endpoint that we previously found to have profound clinical significance—as opposed to survival, which may be influenced by factors independent of the effects of preoperative therapy.7,8
In conclusion, we found an association between both posttreatment CA 19–9 level and radiographic metrics and a clinically significant pathologic response. Future studies should focus on easily measured radiomic and serologic markers to facilitate the individualized treatment and optimize the treatment outcomes of patients with localized PDAC.
ACKNOWLEDGMENTS
The authors thank Bryan F. Tutt and the department of scientific publications at the University of Texas MD Anderson Cancer Center for editorial support.
This study was supported by the NIH/NCI under award number P30CA016672.
Footnotes
The authors report no conflicts of interest.
REFERENCES
- 1.Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. [DOI] [PubMed] [Google Scholar]
- 2.Conroy T, Hammel P, Hebbar M, et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med. 2018;379:2395–2406. [DOI] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Network. NCCN practice guidelines for pancreatic cancer. Available at: http://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf. Accessed March 18, 2019
- 4.Potentially Curable Pancreatic Cancer: American Society of Clinical Oncology Clinical Practice Guideline j Journal of Clinical Oncology. Available at::http://ascopubs.org/doi/10.1200/JCO.2016.67.5553. Accessed March 18, 2019.
- 5.Chuong MD, Frakes JM, Figura N, et al. Histopathologic tumor response after induction chemotherapy and stereotactic body radiation therapy for borderline resectable pancreatic cancer. J Gastrointest Oncol. 2016;7:221–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chatterjee D, Katz MH, Rashid A, et al. Histologic grading of the extent of residual carcinoma following neoadjuvant chemoradiation in pancreatic ductal adenocarcinoma: a predictor for patient outcome. Cancer. 2012;118:3182–3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao Q, Rashid A, Gong Y, et al. Pathologic complete response to neoadjuvant therapy in patients with pancreatic ductal adenocarcinoma is associated with a better prognosis. Ann Diagn Pathol. 2012;16:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cloyd JM, Wang H, Egger ME, et al. Association of clinical factors with a major pathologic response following preoperative therapy for pancreatic ductal adenocarcinoma. JAMA Surg. 2017;152:1048–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abrams RA, Lowy AM, O’Reilly EM, et al. Combined modality treatment of resectable and borderline resectable pancreas cancer: expert consensus statement. Ann Surg Oncol. 2009;16:1751–1756. [DOI] [PubMed] [Google Scholar]
- 10.Katz MHG, Shi Q, Ahmad SA, et al. Preoperative modified FOLFIRINOX treatment followed by capecitabine-based chemoradiation for borderline resectable pancreatic cancer: alliance for clinical trials in oncology trial A021101. JAMA Surg. 2016;151:e161137–e1161137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer Oxf Engl. 19902009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 12.Katz MHG, Fleming JB, Bhosale P, et al. Response of borderline resectable pancreatic cancer to neoadjuvant therapy is not reflected by radiographic indicators. Cancer. 2012;118:5749–5756. [DOI] [PubMed] [Google Scholar]
- 13.Ferrone CR, Marchegiani G, Hong TS, et al. Radiological and surgical implications of neoadjuvant treatment with FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer. Ann Surg. 2015;261:12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katz MHG, Varadhachary GR, Fleming JB, et al. Serum CA 19–9 as a marker of resectability and survival in patients with potentially resectable pancreatic cancer treated with neoadjuvant chemoradiation. Ann Surg Oncol. 2010;17: 1794–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tzeng C-WD, Balachandran A, Ahmad M, et al. Serum carbohydrate antigen 19–9 represents a marker of response to neoadjuvant therapy in patients with borderline resectable pancreatic cancer. HPB. 2014;16:430–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hwang RF, Wang H, Lara A, et al. Development of an integrated biospecimen bank and multidisciplinary clinical database for pancreatic cancer. Ann Surg Oncol. 2008;15:1356–1366. [DOI] [PubMed] [Google Scholar]
- 17.Tamm EP, Balachandran A, Bhosale P, et al. Update on 3D and multiplanar MDCT in the assessment of biliary and pancreatic pathology. Abdom Imaging. 2009;34:64–74. [DOI] [PubMed] [Google Scholar]
- 18.Borderline Resectable Pancreatic Cancer: The Importance of This Emerging Stage of Disease—ScienceDirect. Available at: https://www.sciencedirect.-com/science/article/pii/S1072751507019813. Accessed March 18, 2019. [DOI] [PMC free article] [PubMed]
- 19.Littrup PJ, Williams CR, Egglin TK, et al. Determination of prostate volume with transrectal US for cancer screening. Part II. Accuracy of in vitro and in vivo techniques. Radiology. 1991;179:49–53. [DOI] [PubMed] [Google Scholar]
- 20.Tran Cao HS, Balachandran A, Wang H, et al. Radiographic tumor-vein interface as a predictor of intraoperative, pathologic, and oncologic outcomes in resectable and borderline resectable pancreatic cancer. J Gastrointest Surg Off J Soc Surg Aliment Tract. 2014;18:269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hunt K. Operative Standards for Cancer Surgery. Available at: https://shop.-lww.com/Operative-Standards-for-Cancer-Surgery/p/9781451194753. 2015. Accessed March 25, 2019. [Google Scholar]
- 22.Superior Mesenteric Artery Margin of Posttherapy Pancreaticoduodenectomy and Prognosis in Patients With Pancreatic Ductal Adenocarcinoma | Ovid Available at: https://oce.ovid.com/article/00000478-201510000-00013/HTML. Accessed March 18, 2019. [DOI] [PubMed]
- 23.Validation of a Proposed Tumor Regression Grading Scheme for Pancreatic Ductal Adenocarcinoma After Neoadjuvant Therapy as a Prognostic Indicator for Survival | Ovid. Available at: https://oce.ovid.com/article/00000478-201612000-00010/HTML. Accessed March 18, 2019. [DOI] [PMC free article] [PubMed]
- 24.Tzeng C-WD, Abbott DE, Cantor SB, et al. Frequency and intensity of postoperative surveillance after curative treatment of pancreatic cancer: a cost-effectiveness analysis. Ann Surg Oncol. 2013;20:2197–2203. [DOI] [PubMed] [Google Scholar]
- 25.Lee SM, Katz MHG, Liu L, et al. Validation of a proposed tumor regression grading scheme for pancreatic ductal adenocarcinoma after neoadjuvant therapy as a prognostic indicator for survival. Am J Surg Pathol. 2016;40: 1653–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dholakia AS, Hacker-Prietz A, Wild AT, et al. Resection of borderline resectable pancreatic cancer after neoadjuvant chemoradiation does not depend on improved radiographic appearance of tumor-vessel relationships. J Radiat Oncol. 2013;2:413–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zins M, Matos C, Cassinotto C. Pancreatic adenocarcinoma staging in the era of preoperative chemotherapy and radiation therapy. Radiology. 2018;287: 374–390. [DOI] [PubMed] [Google Scholar]
- 28.Xia BT, Fu B, Wang J, et al. Does radiologic response correlate to pathologic response in patients undergoing neoadjuvant therapy for borderline resectable pancreatic malignancy? J Surg Oncol. 2017;115:376–383. [DOI] [PubMed] [Google Scholar]
- 29.Truty MJ, Kendrick ML, Nagorney DM, et al. Factors predicting response, perioperative outcomes, and survival following total neoadjuvant therapy for borderline/locally advanced pancreatic cancer. Ann Surg. 2019. Published ahead of print. [DOI] [PubMed] [Google Scholar]
- 30.Marchegiani G, Todaro V, Boninsegna E, et al. Surgery after FOLFIRINOX treatment for locally advanced and borderline resectable pancreatic cancer: increase in tumour attenuation on CT correlates with R0 resection. Eur Radiol. 2018;28:4265–4273. [DOI] [PubMed] [Google Scholar]
- 31.Amer AM, Zaid M, Chaudhury B, et al. Imaging-based biomarkers: Changes in the tumor interface of pancreatic ductal adenocarcinoma on computed tomography scans indicate response to cytotoxic therapy. Cancer. 2018;124:1701–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bernard V, Kim DU, San Lucas FA, et al. Circulating nucleic acids are associated with outcomes of patients with pancreatic cancer. Gastroenterology. 2019;156. 108–118.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.He J, Blair AB, Groot VP, et al. Is a pathological complete response following neoadjuvant chemoradiation associated with prolonged survival in patients with pancreatic cancer? Ann Surg. 2018;268:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


