Abstract
Background and Objectives:
Staging laparoscopy is recommended before preoperative therapy in patients with locoregional gastric cancer, but yield of repeated diagnostic laparoscopy at the time of resection is unknown.
Methods:
Retrospective review of a prospective database of patients with gastric adenocarcinoma (1994-2016) who had negative staging laparoscopy followed by preoperative therapy and subsequent attempted resection. Primary outcome was positive exploration (peritoneal or unresectable disease) at the time of resection. Multivariable logistic regression identified factors associated with positive exploration.
Results:
Of the 451 patients with attempted resection, 54 (12.0%) had positive explorations, including 48 with peritoneal disease. Patients with positive explorations were more likely to be female and have poorly differentiated tumors, linitis features, and signet-ring morphology. There was no significant difference by exploration results in age, race, clinical stage, or delayed definitive surgery. Positive explorations were independently associated with poor differentiation (OR 4.6, 95%CI 1.4-15.3; P = 0.01) and linitis (OR 4.2, 95%CI 1.9-9.2; P < 0.001). Positive explorations were seen in 14.0% of patients with poor differentiation, 36.6% of patients with linitis, and 5.8% of patients with neither linitis nor poor differentiation.
Conclusion:
Despite negative pretreatment laparoscopy, post-treatment repeat laparoscopy may prevent non-therapeutic laparotomies. At a minimum, we recommend selective repeat laparoscopy for patients with linitis features.
Keywords: gastric cancer, laparoscopy, neoadjuvant, preoperative therapy, staging
1 |. INTRODUCTION
The 2017 National Comprehensive Cancer Network (NCCN) guidelines recommend that preoperative laparoscopy to detect radiographically occult metastatic disease be considered in all patients with locoregional gastric adenocarcinoma,1 and the literature strongly supports the use of diagnostic laparoscopy in ≥T2 gastric cancers.2,3 These practices are supported by data that suggest that radiographically occult M1 disease is found in as many as 40% of patients with these gastric cancers and that non-therapeutic laparotomy can be avoided in a significant subset of these patients.4–7 NCCN guidelines recommend peritoneal lavage cytology at the time of staging laparoscopy,1,8 and data on gastric cancer patients undergoing staging laparoscopy suggests that 13% of patients will have positive cytology without gross peritoneal disease.9 Despite improvements in imaging technology and high variability in utilization, staging laparoscopy remains an important diagnostic tool in the management of gastric cancer in the Western hemisphere.10
Although the prognosis for patients with gastric cancer treated in the United States is improving, the lack of long-term satisfactory survival—even after maximal surgical management,11 low rates of receipt of adjuvant therapy,12 and delays in receipt of adjuvant therapy13—have prompted investigations into the utility of preoperative therapy. On the basis of multiple clinical trials in gastric cancer, preoperative therapy is an accepted treatment approach for patients with resectable ≥T2 disease and any node-positive disease.14–18 NCCN guidelines recommended that a staging laparoscopy with cytology be considered in all patients before preoperative therapy,1 but there are no recommendations regarding the need for repeat diagnostic laparoscopy (with or without cytology) after preoperative treatment but before attempted resection.
Currently there is no evidence to answer whether repeat diagnostic laparoscopy at the time of resection should be conducted for patients who receive staging laparoscopy followed by preoperative therapy. While repeat staging laparoscopy allows for assessment of treatment response and potentially prevents non-therapeutic laparotomy, repeat laparoscopy at the time of attempted resection may add both time and cost,19,20 and data to support its use are anecdotal. We hypothesized that repeat laparoscopy at the time of definitive resection in patients with gastric cancer who had prior negative staging laparoscopy followed by preoperative therapy would be low yield. To test this hypothesis, we retrospectively evaluated rates of positive explorations at the time of resection in these patients, and we sought to identify factors associated with these positive explorations.
2 |. MATERIALS AND METHODS
After approval by the Institutional Review Board, we abstracted from a prospectively maintained database all adult patients with pathologically proven gastric adenocarcinoma treated at The University of Texas MD Anderson Cancer Center from January 1994 through December 2016. Gastroesophageal tumors involving the gastroesophageal junction were included. In addition, we retrospectively obtained demographic data, pathology and staging data, imaging findings, and operative findings from the electronic medical record. Cancer stage was defined according to the American Joint Committee on Cancer Staging Manual, 8th Edition (2017),21 and positive peritoneal cytology was classified as metastatic disease.22 Clinical preoperative T category was determined mainly by endoscopic ultrasonography findings, with supplemental information from cross-sectional imaging, endoscopy, and staging laparoscopy. Clinical node-positive disease was determined using endoscopic ultrasonography results and cross-sectional imaging findings. Variables collected included: age, sex, race/ethnicity, tumor location, histologic grade, linitis plastica features (defined as diffuse involvement of at least 1/3 stomach, evidenced by at least two diagnostic modalities [endoscopic ultrasound, CT, or laparoscopy]),23 clinical T/N stage, and interval between date of diagnosis and date of attempted/definitive resection. A delay to surgery was defined as an interval greater than 8 months (4th quartile) from diagnosis to attempted/definitive resection.
Staging laparoscopy was performed before initiation of therapy. Staging laparoscopy at MD Anderson routinely includes abdominal exploration, including biopsies of any suspicious lesions as well as lavage cytology, as described previously.9 MD Anderson routinely recommends preoperative chemotherapy or chemoradiation for all advanced gastric cancers (beyond T1aN0 or T1bN0), and the standard practice at the institution is to perform staging laparoscopy before initiation of preoperative therapy.24
The primary outcome was positive findings at abdominal exploration at the time of attempted or definitive resection. Patients who did not get a staging laparoscopy before preoperative therapy (n = 93) and those with metastatic disease on staging laparoscopy (n = 305) were excluded from this study. In addition, patients that had progression on imaging during preoperative therapy (n = 75), had comorbid conditions precluding gastrectomy (n = 17), were lost to follow-up or treated at other institutions (n = 87), or received no preoperative therapy (n = 142) were also excluded (Figure 1). The final cohort included only patients with biopsy-proven gastric cancer who had a negative initial staging laparoscopy followed by preoperative chemotherapy or chemoradiation therapy, and attempted gastrectomy.
FIGURE 1.
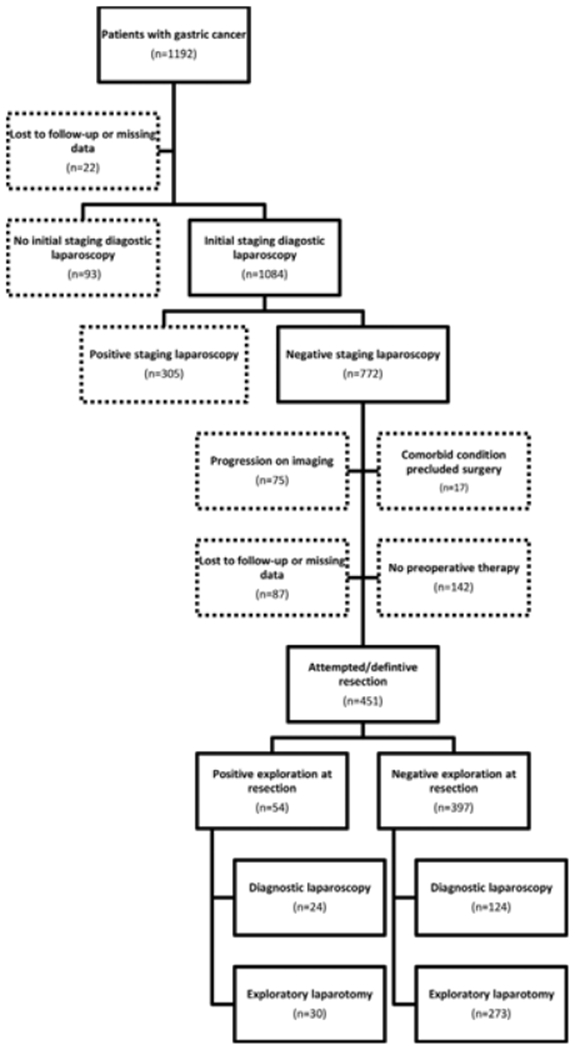
Flow diagram of inclusion criteria
Given that not all patients underwent repeat diagnostic laparoscopy at the time of attempted/definitive resection, data from both laparoscopic and open exploration were included. The decision to perform repeat diagnostic laparoscopy at the time of attempted/ definitive resection was based on surgeon preference and may have been biased by patient risk factors and therefore the outcome of the exploration, regardless of if it was laparoscopic and open, was included as the primary outcome. Positive findings were defined as any metastatic disease (eg, peritoneal disease), locally advanced disease precluding resection (eg, unresectable extension into the liver or pancreas), or other findings precluding safe resection (eg, cirrhosis). The total yield of exploration was defined as the proportion of patients who had positive findings on exploration, regardless of whether the exploration was laparoscopic or open. Although most repeat laparoscopies were done concurrently with the definitive operation, any diagnostic laparoscopy conducted within 7 days before the definitive operation was considered a repeat laparoscopy and included.
Normally distributed data were expressed as mean and standard deviation and compared between groups using the two-tailed Student t-test. Non-normally distributed data were expressed as median and interquartile range and were compared between groups using the Mann-Whitney U-test. The Fisher exact test was used to compare categorical variables. Univariate and multivariable logistic regression were used to identify factors associated with positive exploration. Factors with a P-value of <0.20 in the univariate models were included in the preliminary multivariable model. Stepwise methods with backward elimination with a cutoff of P = 0.10 were used to create the final multivariable model.25 A P-value of <0.05 was considered statistically significant. Statistical analyses were performed using Stata software version 14.1 (StataCorp, College Station, TX).
3 |. RESULTS
We identified 451 patients with gastric cancer who underwent negative staging laparoscopy followed by preoperative chemotherapy or chemoradiation therapy and attempted/definitive resection (Figure 1). One hundred forty-eight (32.8%) patients underwent repeat diagnostic laparoscopy at the time of attempted/definitive resection. Patient demographic characteristics and clinical factors including disease characteristics are shown in Table 1. Of the entire cohort, 12.0% (n = 54) had positive findings at the time of attempted/ definitive resection (n = 24 identified at laparoscopy and n = 30 at laparotomy), while 397 had negative laparoscopic (n = 124) or open (n = 273) explorations at the time of resection. The most common positive findings were biopsy-proven macroscopic peritoneal disease (n = 48; 10.6% of entire cohort). Direct invasion precluding resection was seen in four patients (0.9%), positive lavage cytology was seen in one patient (0.2%), and undiagnosed portal hypertension precluding resection were seen in one patient (0.2%).
TABLE 1.
Patient demographic characteristics and disease characteristics
| Characteristic | Number of patients (n = 451) |
|---|---|
| Age, median (IQR) | 62.1 (53.8-70.1) |
| Male, no. (%) | 285 (63.2) |
| Race/ethnicity, no. (%) | |
| White | 279 (61.9) |
| Asian | 31 (6.9) |
| Black | 29 (6.4) |
| Hispanic | 51 (11.3) |
| Other | 61 (13.5) |
| Tumor location, no. (%) | |
| Gastroesophageal junction or cardia | 211 (46.8) |
| Antrum/body/fundus | 215 (47.7) |
| Entire stomach | 25 (5.5) |
| Grade, no. (%) | |
| Poorly differentiated | 307 (68.1) |
| Moderately differentiated | 105 (23.3) |
| Well differentiated | 2 (0.4) |
| Unknown | 37 (8.2) |
| Linitis, yes | 41 (9.1%) |
| Signet-ring cell morphology, yes | 213 (47.2%) |
| cT stage | |
| T1 | 48 (10.6%) |
| T2 | 8 (1.8%) |
| T3 | 357 (79.2%) |
| T4 | 38 (8.4%) |
| cN stage | |
| N0 | 149 (33.0%) |
| N1+ | 301 (66.7%) |
| Pre-operative therapy type | |
| Chemotherapy and radiation | 391 (86.7) |
| Chemotherapy only | 60 (13.3) |
| Delayed surgery > 8 months | |
| Yes | 98 (21.7%) |
| No | 353 (78.3%) |
IQR, interquartile range.
A comparison of patients who had a negative exploration at the time of resection and those who had a positive exploration at that time is shown in Table 2. Patients with positive explorations were less likely to be male (50.0% vs 65.0% male; P = 0.03), while there was no difference in age or race/ethnicity between the groups. Tumor location varied between the groups: patients with positive explorations were more likely to have the entire stomach involved (18.5% vs 3.8%; P < 0.001) or features consistent with linitis plastica (27.8% vs 6.5%; P < 0.001). Histologically, those with positive explorations were more likely to have poorly differentiated tumors (79.6% vs 66.5%; P = 0.001) or signet-ring cell morphology (64.8% vs 44.8%; P = 0.006). No significant differences were seen in rates of equivocal findings on initial cytology at staging laparoscopy, clinical T category, or rates of clinical node positivity. The proportions of patients with an interval of more than 8 months from diagnosis to attempted/definitive resection also were similar between groups (Table 2).
TABLE 2.
Comparison of patients with positive exploration at the time of definitive operation to those with a negative exploration at the time of attempted/definitive resection
| Characteristic | Negative exploration (n = 397) | Positive exploration (n = 54) | P-value |
|---|---|---|---|
| Age, median (IQR) | 62.2 (54.1-70.5) | 61.2 (50.6-67.9) | 0.28 |
| Male, no. (%) | 258 (65.0) | 27 (50.0) | 0.03 |
| Race/ethnicity, no. (%) | 0.39* | ||
| White | 250 (63.0) | 29 (53.7) | |
| Black | 26 (6.5) | 3 (5.6) | |
| Asian | 28 (7.1) | 3 (5.6) | |
| Hispanic | 41 (10.3) | 10 (18.5) | |
| Other/unknown | 52 (13.1) | 9 (16.7) | |
| Location, no. (%) | <0.001 | ||
| Gastroesophageal junction or cardia | 194 (48.9) | 17 (31.5) | |
| Antrum/body/fundus | 188 (47.4) | 27 (50.0) | |
| Entire stomach | 15 (3.8) | 10 (18.5) | |
| Grade, no. (%) | 0.001 | ||
| Poorly differentiated | 264 (66.5) | 43 (79.6) | |
| Moderately differentiated | 102 (25.7) | 3 (5.6) | |
| Well differentiated | 2 (0.5) | 0 (0.0) | |
| Unknown | 29 (7.3) | 8 (14.8) | |
| Linitis features present, no. (%) | 26 (6.5) | 15 (27.8) | <0.001* |
| Signet-ring cell morphology present, no. (%) | 178 (44.8) | 35 (64.8) | 0.006 |
| Equivocal cytology at presentation, no. (%) | 9 (2.3) | 2 (3.7) | 0.35* |
| cT category, no. (%) | 0.84* | ||
| T1 | 44 (11.1) | 4 (7.4) | |
| T2 | 7 (1.8) | 1 (1.9) | |
| T3 | 313 (78.8) | 44 (81.5) | |
| T4 | 33 (8.3) | 5 (9.3) | |
| cN category, no. (%) | 0.55 | ||
| N0 | 134 (33.8) | 16 (29.6) | |
| N1+ | 263 (66.2%) | 38 (70.4) | |
| Pre-operative therapy Type | 1.0 | ||
| Chemotherapy and radiation | 344 (86.6) | 47 (87.0) | |
| Chemotherapy only | 53 (13.4) | 7 (13.0) | |
| Surgery delayed by >8 months, no. (%) | 0.66 | ||
| Yes | 85 (21.4) | 13 (24.1) | |
| No | 312 (78.6) | 41 (75.9) |
IQR, interquartile range.
Fisher exact test.
On the final multivariable logistic regression (Table 3), only poorly differentiated pathology (odds ratio [OR] 4.58, 95% confidence interval [CI] 1.37-15.27; P = 0.01) and linitis plastica features (OR 4.18, 95%CI 1.90-9.24; P < 0.001) were associated with positive explorations. Of all the patients with linitis features (n = 41), 15 (36.6%) had positive explorations, whereas only 9.5% of patients without linitis features had positive explorations. Positive explorations were seen in 43 (14.0%) of the 307 patients with poorly differentiated tumors but only 11 (7.6%) patients with tumors that were not poorly differentiated (n = 144). Positive explorations were seen in 35 (16.4%) of the patients with signet-ring cell morphology but only 19 (8.0%) patients with other morphology (n = 238). In patients with neither linitis plastica features nor poorly differentiated tumors (n = 127), the rate of positive exploration was 5.8% (n = 8).
TABLE 3.
Univariate and multivariable analyses demonstrate factors associated with positive exploration at the time of attempted/definitive resection
| Factor | Univariate |
Multivariable |
||||||
|---|---|---|---|---|---|---|---|---|
| OR | 95%CI | P-value | OR | 95%CI | P-value | |||
| Age, <60 vs ≥60 years | 0.99 | 0.56 | 1.76 | 0.97 | ||||
| Sex, female vs male | 1.86 | 1.05 | 3.29 | 0.03 | ||||
| Race/ethnicity, white vs other | 0.68 | 0.38 | 1.21 | 0.19 | ||||
| Grade, poorly differentiated vs other | 5.65 | 1.71 | 18.60 | 0.004 | 4.58 | 1.37 | 15.27 | 0.01 |
| Linitis, yes vs no | 5.49 | 2.68 | 11.23 | <0.001 | 4.18 | 1.90 | 9.24 | <0.001 |
| Signet-ring cell morphology, yes vs no | 2.27 | 1.25 | 4.10 | 0.007 | ||||
| cT category, cT3/4 vs cT1/2 | 1.44 | 0.55 | 3.80 | 0.46 | ||||
| cN status, positive vs negative | 1.21 | 0.65 | 2.25 | 0.55 | ||||
| Surgery delayed >8 months, yes vs no | 1.16 | 0.60 | 2.27 | 0.66 | ||||
Metastatic and clinically relevant findings are shown according to the modality that yielded the positive findings (laparoscopic or open) in Table 4. Of the 54 patients who had positive explorations, 24 had a laparoscopic exploration at the time of attempted/definitive resection, of whom 11 went on to open exploration. The reasons for open exploration in these cases were inability to complete the operation laparoscopically (n = 3), negative laparoscopic exploration followed by identification of peritoneal disease on laparotomy (n = 5), and a biopsy of a suspicious lesion that was negative on frozen pathology but positive on final pathology after definitive resection (n = 3). The median length of stay in these 24 patients with positive disease on laparoscopy was 4 days (range 0-36), as some patients underwent palliative gastrectomy for palliation of current, or anticipated future, symptoms. The remaining 30 patients with positive explorations had laparotomies without an associated diagnostic laparoscopy. The operation was subsequently aborted in 21 of these patients, while two of the patients underwent palliative bypass. The median length of stay in the 30 patients with positive disease on laparotomy was 9 days (range 4-19). In 7 patients, the frozen pathology results of peritoneal biopsy initially were negative but were subsequently found to be positive on final pathology after resection.
TABLE 4.
Metastatic or clinically relevant findings at the time of attempted/definitive resection according to the modality yielding positive findings
| Finding | No. (%) | ||
|---|---|---|---|
| Diagnostic laparoscopy (n = 148) | Exploratory laparotomy (n = 303) | Total (n = 451) | |
| Biopsy positive macroscopic peritoneal disease | 22 (14.9%) | 26 (8.6%) | 48 (10.6%) |
| Direct invasion precluding resection | 0 (0.0%) | 4 (1.3%) | 4 (0.9%) |
| Positive lavage cytology | 1 (0.7%) | 0 (0.0%) | 1 (0.2%) |
| Other factors precluding resection | 1 (0.7%)a | 0 (0.0%) | 1 (0.2%) |
| Total yield of diagnostic exploration | |||
| Positive | 24 (16.2%) | 30 (9.9%) | 54 (12.0%) |
| Negative | 124 (83.8%) | 273 (90.1%) | 397 (88.0%) |
Cirrhosis and portal hypertension.
4 |. DISCUSSION
Repeat staging laparoscopy at the time of attempted/definitive resection in patients with gastric cancer treated with preoperative chemoradiation therapy was found to prevent non-therapeutic laparotomy in a small, but significant, cohort of patients despite previous negative staging laparoscopy. These patients—12% of those studied—had a positive exploration at the time of definitive resection, and peritoneal disease was the most common finding among them. Poorly differentiated tumors and linitis features were independently associated with positive explorations; patients with these disease features should be considered for repeat laparoscopy.
As expected, given the selection bias inherent in investigating only those treated with preoperative therapy whose disease did not progress on imaging, the rate of positive exploration in the total cohort was significantly lower than the 36% rate of positive initial staging laparoscopy that we previously reported in pretreatment gastric cancer patients.9 However, the positive exploration rate was considerably greater than the overall rate of 12% for certain subsets of patients, who would likely benefit from repeated laparoscopic exploration at the time of definitive surgery. Given that the positive exploration rate was 37% in patients with linitis features on imaging despite negative initial staging laparoscopy, we recommend repeat laparoscopy in these patients. These findings are confirmed by a previous analysis that suggests that linitis features are associated with positive results on initial staging laparoscopy.9 In addition, our data suggest that repeat staging laparoscopy for patients with poorly differentiated tumors may be warranted since 15% of these patients had a positive exploration. Nassour et al26 also suggested that patients with poorly differentiated tumors are at increased risk of positive exploration, as are those with signet-ring cell morphology and advanced T category.
Although there are no national data on the use of repeat diagnostic laparoscopy after preoperative therapy, only 33% of our patients received repeat staging laparoscopy. We suspect, on the basis of the low rates of use of initial staging laparoscopy nationwide, that the practice of repeat diagnostic laparoscopy is not common.27 A study by Nassour et al26 included a subset of 50 patients who underwent staging laparoscopy after receipt of preoperative therapy; although they reported no difference in rates of positive staging laparoscopy according to whether that laparoscopy preceded or followed preoperative therapy, they did demonstrate that 28% of patients treated with preoperative therapy had M1 disease at the time of planned resection. However, these patients did not undergo staging laparoscopy before therapy; therefore, their reported rate of positive exploration was much higher than that demonstrated in the current study.
A total of 11 patients with positive laparoscopic exploration went on to have a laparotomy in this study. Of these, three patients had the laparotomy to facilitate feeding tube placement or biopsy of lesions on the bowel, and five patients had initially negative laparoscopic explorations followed by the identification of lesions on laparotomy. In multiple patients the missed pathology was found to be at the site of the feeding jejunostomy tube and careful exploration of this area on diagnostic laparoscopy may be beneficial. In addition, three who converted from laparoscopic to open exploration, and seven patients who had open exploration without conversion had lesions identified that appeared benign on frozen section analysis but later were found to be metastatic cancer on final pathology. These events were equally distributed over the duration of the study period. However, these patients almost all had signet ring features and poorly differentiated tumors, and the majority had linitis features. This suggests a possible benefit to conducting the diagnostic laparoscopy in advance of the planned definitive surgery in select high risk patients. In addition, two patients in the open subset underwent palliative bypasses. Excluding the 10 patients who went on to resection owing to false-negative explorations results in a decrease in the overall diagnostic yield of exploration to 9.7%. Although this decision to proceed with resection in the face of equivocal findings must be made on a case-by-case basis, previous studies suggest that very few patients with M1 disease will require a surgical intervention.28,29 However, both at staging laparoscopy9 and at repeat laparoscopy, peritoneal metastasis appears to be the most common positive finding, and it typically can be identified at laparoscopic exploration.
Our study has a number of limitations. As staging laparoscopy and preoperative therapy is only used in select cohorts of high-risk patients in East Asia, differences in presentation, tumor biology, and treatment sequences limit the generalizability of these findings beyond Western countries.30,31 Our analysis is unable to account for the cost of repeat laparoscopy. However, in addition to the cost implications, avoiding a nontherapeutic celiotomy has the potential to reduce length of stay and time to initiation of chemotherapy.32 However, further cost/benefit analysis is warranted. The increasing use of minimally invasive gastrectomy may impact the utility of these findings, however, minimally invasive gastrectomy was utilized in only 12.6% of cases in submitted to the National Cancer Database in 2010-2011.33 Also, since difference in preoperative therapy regimen may affect the yield of repeat laparoscopy, care must be taken when generalizing this study results to other facilities where the different treatment regimens are applied. However, given the increasing use of preoperative treatment sequences and the lack of available data on the utility of repeat diagnostic laparoscopy, we believe that analysis of this highly selected cohort has clinical utility.
5 |. CONCLUSION
Routine diagnostic laparoscopy at the time of definitive resection in patients with gastric cancer given preoperative therapy may prevent non-therapeutic laparotomy in at least 12% of all patients and may provide even greater benefit to high-risk patients, with poorly differentiated tumors or linitis plastic features.
ACKNOWLEDGMENTS
This publication was supported in part by the National Institutes of Health/National Cancer Institute under award number P30CA016672 (using the Clinical Trials Support Resource). This work has not previously or concurrently been submitted for publication and has not previously been presented.
Funding information
National Institutes of Health/National Cancer Institute, Grant number: P30CA016672
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicts of interest.
REFERENCES
- 1.The Gastric Cancer Clinical Practice Guidelines in Oncology (Version 4. 2017). © 2017. National Comprehensive Cancer Network, Inc; Available at: http://www.nccn.org. Accessed [1/7/2017]. [Google Scholar]
- 2.Lowy AM, Mansfield PF, Leach SD, et al. Laparoscopic staging for gastric cancer. Surgery. 1996;119:611–614. [DOI] [PubMed] [Google Scholar]
- 3.Ajani JA, Bentrem DJ, Besh S, et al. Gastric cancer, version 2.2013: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw. 2013;11:531–546. [DOI] [PubMed] [Google Scholar]
- 4.Leake P- A, Cardoso R, Seevaratnam R, et al. A systematic review of the accuracy and indications for diagnostic laparoscopy prior to curative-intent resection of gastric cancer. Gastric Cancer. 2012;15:38–47. [DOI] [PubMed] [Google Scholar]
- 5.Possik RA, Franco EL, Pires DR, et al. Sensitivity, specificity, and predictive value of laparoscopy for the staging of gastric cancer and for the detection of liver metastases. Cancer. 1986;58:1–6. [DOI] [PubMed] [Google Scholar]
- 6.Asencio F, Aguiló J, Salvador JL, et al. Video-laparoscopic staging of gastric cancer. A prospective multicenter comparison with noninvasive techniques. Surg Endosc. 1997;11:1153–1158. [DOI] [PubMed] [Google Scholar]
- 7.Sarela AI, Lefkowitz R, Brennan MF, et al. Selection of patients with gastric adenocarcinoma for laparoscopic staging. Am J Surg. 2006;191: 134–138. [DOI] [PubMed] [Google Scholar]
- 8.Jamel S, Markar SR, Malietzis G, et al. Prognostic significance of peritoneal lavage cytology in staging gastric cancer: systematic review and meta-analysis. Gastric Cancer. 2018;21:10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ikoma N, Blum M, Chiang Y- J, et al. Yield of staging laparoscopy and lavage cytology for radiologically occult peritoneal carcinomatosis of gastric cancer. Ann Surg Oncol. 2016;23:4332–4337. [DOI] [PubMed] [Google Scholar]
- 10.Machairas N,Charalampoudis P, Molmenti EP,et al. The value of staging laparoscopy in gastric cancer. Ann Gastroenterol. 2017;30:287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arsoniadis EG, Marmor S, Diep GK, et al. Survival rates for patients with resected gastric adenocarcinoma finally have increased in the United States. Ann Surg Oncol. 2017;24:3361–3367. [DOI] [PubMed] [Google Scholar]
- 12.Jin LX, Sanford DE, Squires MH, et al. Interaction of postoperative morbidity and receipt of adjuvant therapy on long-term survival after resection for gastric adenocarcinoma: results from the U.S. gastric cancer collaborative. Ann Surg Oncol. 2016;23:2398–2408. [DOI] [PubMed] [Google Scholar]
- 13.Park HS, Jung M, Kim HS, et al. Proper timing of adjuvant chemotherapy affects survival in patients with stage 2 and 3 gastric cancer. Ann Surg Oncol. 2015;22:224–231. [DOI] [PubMed] [Google Scholar]
- 14.Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. [DOI] [PubMed] [Google Scholar]
- 15.Ajani JA, Mansfield PF, Crane CH, et al. Paclitaxel-based chemoradiotherapy in localized gastric carcinoma: degree of pathologic response and not clinical parameters dictated patient outcome. J Clin Oncol. 2005;23:1237–1244. [DOI] [PubMed] [Google Scholar]
- 16.Ajani JA, Mansfield PF, Janjan N, et al. Multi-institutional trial of preoperative chemoradiotherapy in patients with potentially resectable gastric carcinoma. J Clin Oncol. 2004;22:2774–2780. [DOI] [PubMed] [Google Scholar]
- 17.Rivera F, Galán M, Tabernero J, et al. Phase II trial of preoperative irinotecan-cisplatin followed by concurrent irinotecan-cisplatin and radiotherapy for resectable locally advanced gastric and esophagogastric junction adenocarcinoma. Int J Radiat Oncol. 2009;75:1430–1436. [DOI] [PubMed] [Google Scholar]
- 18.Walsh TN, Noonan N, Hollywood D, et al. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med. 1996;335:462–467. [DOI] [PubMed] [Google Scholar]
- 19.Karuna ST, Thirlby R, Biehl T, et al. Cost-effectiveness of laparoscopy versus laparotomy for initial surgical evaluation and treatment of potentially resectable hepatic colorectal metastases: a decision analysis. J Surg Oncol. 2008;97:396–403. [DOI] [PubMed] [Google Scholar]
- 20.Li K, Cannon JGD, Jiang SY, et al. Diagnostic staging laparoscopy in gastric cancer treatment: a cost-effectiveness analysis. J Surg Oncol. 2017. 10.1002/jso.24942 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 21.American Joint Committee on Cancer Staging Manual (8th Edition, 2017). American Joint Committee on Cancer. Available at: https://cancerstaging.org. Accessed [1/7/2017].
- 22.Sano T, Coit DG, Kim HH, et al. Proposal of a new stage grouping of gastric cancer for TNM classification: international Gastric Cancer Association staging project. Gastric Cancer. 2017;20:217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agnes A, Estrella JS, Badgwell B. The significance of a nineteenth century definition in the era of genomics: linitis plastica. World J Surg Oncol. 2017;15:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Badgwell B, Das P, Ajani J. Treatment of localized gastric and gastroesophageal adenocarcinoma: the role of accurate staging and preoperative therapy. J Hematol Oncol. 2017;10:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hosmer DW, Lemeshow S, Sturdivant RX. Applied Logistic Regression. Hoboken: Wiley; 2013. [Google Scholar]
- 26.Nassour I, Fullington H, Hynan LS, et al. The yield of staging laparoscopy in gastric cancer is affected by racial and ethnic differences in disease presentation. Ann Surg Oncol. 2017;24:1787–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karanicolas PJ, Elkin EB, Jacks LM, et al. Staging laparoscopy in the management of gastric cancer: a population-based analysis. J Am Coll Surg. 2011;213:644–651. [DOI] [PubMed] [Google Scholar]
- 28.Sarela AI, Miner TJ, Karpeh MS, et al. Clinical outcomes with laparoscopic stage M1, unresected gastric adenocarcinoma. Ann Surg. 2006;243:189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fujitani K, Yang H-K, Mizusawa J, et al. Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single non-curable factor (REGATTA): a phase 3, randomized controlled trial. Lancet Oncol. 2016;17:309–318. [DOI] [PubMed] [Google Scholar]
- 30.Irino T, Sano T, Hiki N, et al. Diagnostic staging laparoscopy in gastric cancer: a prospective cohort at a cancer institute in Japan. Surg Endosc 2017. 10.1007/s00464-017-5673-z [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 31.Russo A, Li P, Strong VE. Differences in the multimodal treatment of gastric cancer: east versus west. J Surg Oncol. 2017;115: 603–614. [DOI] [PubMed] [Google Scholar]
- 32.Grobmyer SR, Fong Y, D’Angelica M, et al. Diagnostic laparoscopy prior to planned hepatic resection for colorectal metastases. Arch Surg. 2004;139:1326–1330. [DOI] [PubMed] [Google Scholar]
- 33.Ecker BL, Datta J, McMillan MT, et al. Minimally invasive gastrectomy for gastric adenocarcinoma in the United States: utilization and short-term oncologic outcomes. J Surg Oncol. 2015;112:616–621. [DOI] [PubMed] [Google Scholar]


