Abstract
The post-translational modification of serine and threonine residues of nuclear, cytosolic, and mitochondrial proteins by O-linked β-N-acetyl glucosamine (O-GlcNAc) has long been seen as an important regulatory mechanism in the cardiovascular system. O-GlcNAcylation of cardiac proteins has been shown to contribute to the regulation of transcription, metabolism, mitochondrial function, protein quality control and turnover, autophagy, and calcium handling. In the heart, acute increases in O-GlcNAc have been associated with cardioprotection, such as those observed during ischemia/reperfusion. Conversely, chronic increases in O-GlcNAc, often associated with diabetes and nutrient excess, have been shown to contribute to cardiac dysfunction. Traditionally, many studies have linked changes in O-GlcNAc with nutrient availability and as such O-GlcNAcylation is often seen as a nutrient driven process. However, emerging evidence suggests that O-GlcNAcylation may also be regulated by non-nutrient dependent mechanisms, such as transcriptional and post-translational regulation. Therefore, the goals of this review are to provide an overview of the impact of O-GlcNAcylation in the cardiovascular system and how this is regulated and to discuss the emergence of regulatory mechanisms other than nutrient availability.
Keywords: O-GlcNAc, OGT, OGA, heart, nutrient, regulation, transcriptional, post-translational
Introduction
The post-translational modification (PTM) of serine and threonine residues of nuclear, cytosolic, and mitochondrial proteins by O-linked β-N-acetyl glucosamine (O-GlcNAc) is a highly dynamic and reversible, nutrient-driven process, and is an important regulator of cell function[1, 2]. Protein O-GlcNAcylation contributes to the regulation of the cell cycle, transcription and translation, metabolism, mitochondrial function, protein synthesis, protein quality control and turnover, autophagy, epigenetic signaling, and Ca2+ handling[3, 4].
A major emphasis on the regulation of O-GlcNAc levels has been as a nutrient-driven process, and especially dependent on changes in the availability of glucose. A small portion of glucose that enters the cell is metabolized by the hexosamine biosynthesis pathway (HBP) to uridine diphosphate-GlcNAc (UDP-GlcNAc), the end product of the HBP, and the substrate for protein O-GlcNAcylation and other forms of protein glycosylation. Glucose flux through the HBP is regulated by the activity of L-Glutamine: D-fructose-6-phosphate aminotransferase (GFAT), which catalyzes the generation of glucosamine 6-phosphate from fructose 6-phosphate using glutamine as the amine donor. UDP-GlcNAc integrates multiple metabolic pathways providing feedback on overall cellular energy levels and the metabolism of fatty acids, glucose, nitrogen, and nucleotides [5, 6]. In addition to flux through the HBP, O-GlcNAc levels are regulated by O-GlcNAc transferase (OGT), which uses UDP-GlcNAc as its substrate to add the sugar moiety to proteins, and O-GlcNAcase (OGA), which is responsible for its removal. Furthermore, OGT activity is very sensitive to changes in UDP-GlcNAc; consequently, O-GlcNAc levels can also be affected by changes in the availability of UDP-GlcNAc.
It is a common assumption that 2–3% of glucose is metabolized via the HBP; however, this originates from a single study on adipocytes, where the amount of glucose entering the HBP was not directly measured but was estimated based on other measurements[7]. Importantly, this value is presented as a percentage; consequently, given that the rate of glucose utilization varies widely, between for example, cells in culture and the beating heart, it is misleading as to the actual rate of glucose utilization via the HBP. Using 13C-labeled glucose in neonatal cardiomyocytes it was concluded that glucose metabolism via the HBP could be much higher than previously thought[8]. However, this was an inference from other measurements and not a direct measure of glucose flux via the HBP. In contrast, Olson and colleagues using 13C-labeled glucose in an isolated perfused heart combined with liquid chromatography-mass spectrometry, measured an HBP flux of ~2.5 nmole/gram heart protein/minute[9]. Of note, the HBP flux measured in this study was not affected by changes in glucose availability and represented only 0.003–0.006% of glycolytic flux[9]. This is the first report of absolute glucose flux through the HBP in the heart and future studies using this technique will provide valuable insights into the regulation of HBP metabolism under physiological or pathophysiological conditions.
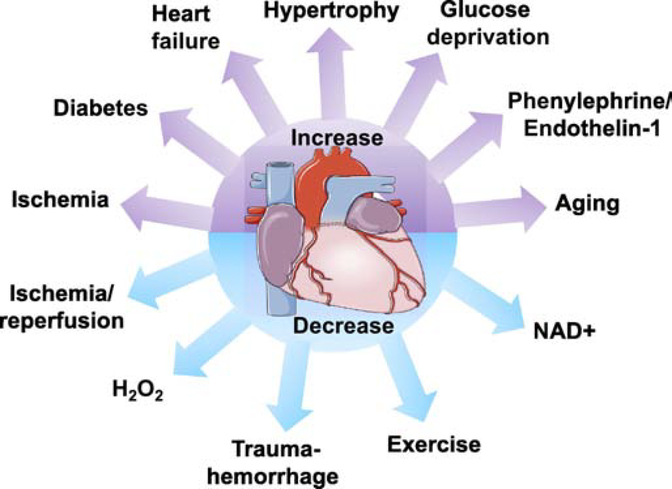
The first report indicating that cardiac proteins could be O-GlcNAcylated was the observation that α,B crystallin was a target for O-GlcNAc-modification in the rat heart[10]. It was later shown that OGT activity was higher in the rat heart compared to other tissues examined and the authors suggested that increased O-GlcNAcylation of proteins could be a mechanism mediating glucose toxicity[11]. In endothelial cells the transcription factor, specificity factor 1 (Sp1), was also shown to be a target for O-GlcNAc modification, which was increased in response to hyperglycemia and was linked to higher mitochondrial superoxide[12]. It was concluded that this could be linked to glucose toxicity and diabetic complications. A subsequent study by the same group linked O-GlcNAcylation of endothelial nitric oxide synthase (eNOS) to impaired vascular function in diabetes[13]. While many studies have linked chronic increases in O-GlcNAc associated with diabetes and nutrient excess to the adverse cardiac dysfunction of diabetes[14–21], there are also reports demonstrating that acute pharmacological increases in O-GlcNAc are cardioprotective, in ischemia/reperfusion (I/R)[22] and septic shock[23]. In other cases, O-GlcNAc levels are decreased in the heart following I/R[24] and during trauma-hemorrhage[25], with increased tissue injury associated with lower O-GlcNAc levels. Interestingly, both acute and chronic exercise have been shown to reduce O-GlcNAc levels in the heart[26]. Clearly, understanding the regulation of O-GlcNAc levels in the heart under normal and pathological conditions is essential to better understand the consequences of these changes on the heart. The alterations in cardiac O-GlcNAc levels in response to different stimuli are summarized in Figure 1.
Figure 1:
Illustration of the wide range of factors that result in increases or decreases in O-GlcNAc levels in cardiomyocytes or the heart, which are discussed in more detail in the text.
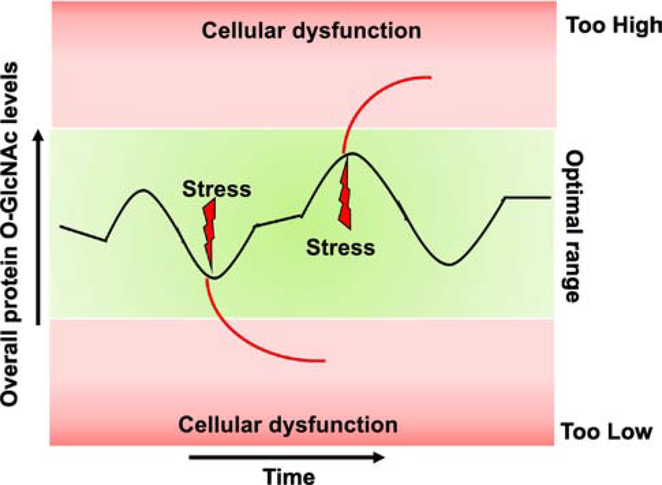
An emerging consensus, as described by Yang and Qian[27], is that there is a limited range of O-GlcNAc levels needed to ensure normal cellular homeostasis and that O-GlcNAcylation is maintained in this range by a balance between OGT/OGA activity and nutrient availability. Consequently, when this homeostatic mechanism fails such that there is to too little or too much O-GlcNAc modification there is cellular dysfunction and pathophysiology[27]. It is of note however, that this concept, typically emphasizes increasing nutrients and stress resulting in higher O-GlcNAc levels, however, as noted above, some stresses result in lower O-GlcNAc levels. In addition, this model does not account for the paradoxical increase in O-GlcNAc levels that occurs in response to glucose deprivation, as we and others have reported[28, 29].
In addition to diabetes, increases in cardiac O-GlcNAc levels have been associated with pressure-overload induced hypertrophy[20, 30–34], heart failure[31], ischemia[3, 24, 35], aging[16, 36–38], as well as in response to agonists such as phenylephrine (PE)[30, 39] and endothelin-1 (ET-1)[40, 41]. Such changes in O-GlcNAc levels do not fit neatly with the concept that nutrient availability is the primary regulator of O-GlcNAc levels. Consequently, it is important to better understand the mechanisms that regulate O-GlcNAcylation in the heart. There have been many recent detailed reviews on O-GlcNAcylation in various contexts including the heart[3, 4, 32, 37, 42, 43]; therefore, here we focus on our understanding of how O-GlcNAc levels are regulated in the heart. In addition to traditional nutrient-driven mechanisms, we will also consider other emerging mechanisms regulating O-GlcNAcylation and their importance in understanding the role of O-GlcNAc in cardiomyocyte function.
Substrate availability and metabolism
In the 1980s it was shown that high glucose in the presence of insulin for 24 hours, induced insulin resistance in cultured adipocytes, and further that this was dependent on the presence of glutamine[7]. Overexpression of GFAT also induced insulin resistance[44] and subsequent studies showed that increasing O-GlcNAc by inhibiting OGA blunted insulin-stimulated glucose uptake[45]; however, some reports indicated that increasing O-GlcNAc levels alone was not sufficient to induce insulin resistance[46, 47]. Nevertheless, additional studies found that several components of the insulin signaling pathway including Insulin receptor 1/2 (IRS1/2), Phosphoinositide dependent protein kinase 1 (PDPK1), Protein kinase B (PKB/Akt), and Glycogen synthase kinase 3β (GSK3β) were all O-GlcNAcylated and that in each case, increased O-GlcNAc levels attenuated their activity[48–50]. In addition, protein tyrosine phosphatase 1B (PTP1B), which dephosphorylates the insulin receptor is also O-GlcNAcylated, increasing its activity, providing another possible link between O-GlcNAc levels and impaired insulin signaling[51]. These observations along with the concept that glucose toxicity was mediated, at least in part, by increased O-GlcNAcylation of specific proteins, resulted in an early focus on the contribution of increased O-GlcNAc levels to diabetic complications seen in the heart. The role of O-GlcNAc in regulating insulin signaling in the heart has not been studied in detail; however, in diabetes increased O-GlcNAc levels on proteins involved in Ca2+ handling (i.e., Sarcoplasmic/endoplasmic reticulum Ca2+ ATPase (SERCA), Phospholamban (PLB), Ca2+/calmodulin dependent kinase II (CaMKII)[17, 52, 53]), contractile proteins (i.e., α-actin, troponin I)[54, 55], and mitochondrial proteins (i.e, VDAC1/2, NDUFA9, ATP synthase A/B)[56–58] have all been linked to the adverse effects of diabetes on cardiac function. The sustained increase in O-GlcNAc in the heart in response to diabetes is typically attributed to increased nutrient availability; however, it raises the question as to why the normal homeostatic mechanisms are unable to compensate. It is possible, therefore, that regulation at the level of OGT and OGA function is likely impaired, resulting in a new higher steady state level of O-GlcNAcylation. Similar mechanisms could also contribute to the chronic increase in cardiac O-GlcNAc levels seen in response to hypertrophy and aging, which are not conditions of nutrient excess.
Protein O-GlcNAcylation has also been implicated in regulating cardiac metabolism. Low concentrations of glucosamine were found to increase fatty acid oxidation in the perfused heart, possibly as a result of increased plasma membrane levels of the fatty acid transporter, CD36[59]. In the heart, glutamine is often considered to be an anaplerotic substrate, feeding directly into the TCA cycle via its metabolism to glutamate and α-ketoglutarate. However, a study in hearts perfused with 13C-labeled glutamine found very low levels of 13C-enrichment in TCA cycle intermediates, suggesting that this was not the primary pathway for glutamine metabolism in the normoxic heart; on the other hand, they found that similar to glucosamine, glutamine increased fatty acid oxidation[60]. Additional studies lead to the conclusion that glutamine was being metabolized by the HBP and increasing fatty acid oxidation in a CD36-dependent manner[60]. These studies were consistent with earlier reports in adipocytes demonstrating increased HBP flux, O-GlcNAcylation, and increased fatty acid oxidation[61]. Additional evidence of a link between O-GlcNAcylation and lipid metabolism was the observation that a splice variant of OGA was associated with lipid droplets[62].
A growing number of proteins that regulate glucose metabolism have been identified as targets for O-GlcNAcylation, including Glucose transporter 4 (GLUT4), hexokinase, glycogen synthase, phosphofructokinase, and pyruvate kinase [63]. Studies on the role of O-GlcNAc regulation of glucose metabolism is most advanced in the setting of cancer; however, it is reasonable to expect that similar types of regulation also occur in the heart, particularly in light of the parallels in metabolic remodeling between cancer and the stressed heart[64, 65]. In addition, proteins that play key roles in regulating metabolism such as AMP-activated protein kinase (AMPK), Akt, GSK3β, are also modified and regulated by O-GlcNAcylation[48, 61]. There is also extensive evidence that O-GlcNAcylation plays an important role in mediating the transcriptional regulation of metabolism via modification of transcription factors such as Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α), forkhead transcription factor (FoxO), and cAMP-response element binding protein (CREB)[66–69]. Interestingly, AMPK is O-GlcNAcylated by OGT increasing its activity[70], and GFAT1 is known to be phosphorylated by AMPK[71, 72]. In cardiomyocytes, AMPK has been reported to decrease GFAT1 regulation[39], suggesting a possible feedback mechanism between the regulation of O-GlcNAc levels via metabolism and O-GlcNAc regulation of metabolism.
Transcriptional regulation
In 2004, it was demonstrated that O-GlcNAc levels in cells increased in response to a number of stressors, including heat shock, hydrogen peroxide (H2O2), and osmotic shock[73]. It was also shown that this was an endogenous stress response that increased cell survival. The beneficial effects of pharmacologically increasing O-GlcNAc in heart was shown in a number of in vivo and in vitro studies[24, 74]. In recent years, some of the mechanisms underlying the changes in O-GlcNAcylation during ischemia/reperfusion (I/R) have been studied. Myocardial I/R in vivo in the mouse, resulted in a marked increase in O-GlcNAc in the ischemic but not the border zone[75]. This was paralleled by significant increases in GFAT1 mRNA and protein, as well as increases in several other enzymes in the HBP that contribute to the regulation of UDP-GlcNAc levels. These findings are consistent with a transcriptionally mediated upregulation of the HBP as a contributing factor in the increase in O-GlcNAc levels in the ischemic region. The study found that spliced X-box protein 1 (Xbp1s) levels, a transcription factor regulating unfolded protein response (UPR) gene expression, was markedly upregulated in the ischemic zone, and this was responsible for the upregulation of several proteins in the HBP, including GFAT [75]. Moreover, they also demonstrated that both Xbp1s and GFAT1 were required for the I/R-induced increase in O-GlcNAc and that the Xbp1s-UPR-HBP/GFAT signaling axis was essential for protection in I/R injury[75]. Of note, OGT and OGA were not regulated by Xbp1s, which supports the concept that it was increased availability of UDP-GlcNAc that was the driver of the increase in O-GlcNAc in this instance. However, as the effects of I/R on OGT or OGA activities were not determined, they could also be contributing factors. GFAT has also been shown to be regulated by activating transcription factor 4 (ATF4)[76], another component of the UPR; however, whether this occurs in the heart is not known.
Changes in GFAT, OGT, and OGA protein and mRNA levels in the heart also occur in chronic settings including diabetes, cardiac hypertrophy, and aging. In hearts from diabetic mice OGT mRNA and protein were both significantly increased[53]. In humans and mice with cardiac hypertrophy, OGT mRNA and protein were also increased in addition to increases in GFAT and OGA[31]. Furthermore, aging increases cardiac O-GlcNAc levels and in the heart this increase in O-GlcNAc was associated with a paradoxical decrease in OGT levels but also with increased UDP-GlcNAc levels and increased GFAT mRNA[36]. While OGA protein levels were not determined, OGA mRNA was significantly increased in response to aging, whereas OGT mRNA was unchanged. Consequently, whether in response to acute stress such as I/R or under more sustained cardiac stresses, GFAT, OGT and OGA are all subject to alterations at both the mRNA and protein levels.
Little is known about the transcriptional regulation of either OGT or OGA in the heart; however, it is clear that changes in protein levels do occur. For example, cardiac O-GlcNAc levels have been shown to exhibit time-of-day dependent changes that occur in parallel with changes in OGT mRNA and protein[77]. While these changes are dependent on the cardiomyocyte circadian clock, the specific mechanisms that regulate cardiac OGT levels are not known. Interestingly, a recent study in liver, showed that REV-Erbα, a core component of the circadian clock, binds to and stabilizes OGT protein levels[78]. Since protein levels of REV-Erbα exhibit time-of-day dependent changes, this could contribute to rhythmic changes in OGT and O-GlcNAc observed in the heart. There is also evidence to suggest that O-GlcNAc levels contribute to the regulation of OGT and OGA expression. For example, low O-GlcNAc levels are associated with increased OGT transcription, whereas pharmacologically increasing O-GlcNAc levels increased OGA protein levels[79, 80]. Other studies, using luciferase reporter assays have shown that knockdown of OGA decreased the level of OGT protein, whereas increased OGA expression led to increased OGT transcription [81]. The transcription factor E2f1 has been reported to repress both OGT and OGA[82] and E2f1 is O-GlcNAcylated and this could regulate its transcriptional activity[83]. Although this relationship is likely to be more complicated given that Dassanayaka et al. have recently shown that deletion of E2f1 did not impact either OGT or OGA[84]. A recent study has demonstrated that hepatocyte nuclear factor 1 (HNF1) represses OGT transcription, which is enhanced by O-GlcNAcylation of HNF1, indicative of a feedback inhibition mechanism, which provides some insight into potential mechanisms regulating O-GlcNAc homeostasis[85]. In a mouse model of heart failure, increased O-GlcNAcylation is associated with lower OGA protein levels and an upregulation of miRNA-539[86]. In neonatal cardiomyocytes, miRNA-539 was found to decrease OGA protein levels and this was associated with an increase in O-GlcNAcylation[86]. OGT protein levels also decreased in response to miR-539 overexpression even though it does not contain miR-539 binding sites[86]; this would be consistent with the concept of reciprocal regulation of OGT and OGA. It remains to be determined whether miR-539 represents a physiological mechanism for regulating OGA and O-GlcNAc levels or whether it is primarily a pathogenic mechanism.
Post-translational modification
The three enzymes that play a major role in regulating O-GlcNAc levels, GFAT, OGT, and OGA are all themselves subject to several different post-translational modifications, including phosphorylation, acetylation, and O-GlcNAcylation. GFAT1, the predominant isoform in the heart can be phosphorylated by protein kinase A (PKA), AMPK, and Ca2+/calmodulin-dependent protein kinase (CaMK) II. PKA phosphorylates Ser205 and Ser235 on GFAT1 leading to a reduction in its activity[87–89]; however, to our knowledge the role of PKA phosphorylation on HBP flux or O-GlcNAc levels in the heart has yet to be evaluated. GFAT1 is also phosphorylated at Ser243, by AMPK, CaMKII, and mechanistic target of Rapamycin (mTOR)[90]. Since its original description as a target for phosphorylation and activation by CaMKII, little additional information has emerged on the role of CaMKII on regulating GFAT activity[72]. However, there have been more extensive studies on the role of AMPK in regulating GFAT1 activity. Initial reports indicated that AMPK phosphorylation increased GFAT1 activity[71, 72]; however, recent studies in the heart, reported that AMPK activation decreases O-GlcNAc levels via its inhibition of GFAT1[39]. In response to nutrient limitations, mTOR was found to increase GFAT1 activity by phosphorylating Ser243, but whether this occurs in the heart is unclear. AMPK also phosphorylates OGT (hOGT Thr454; rOGT Thr444), however, rather than altering its activity AMPK-mediated phosphorylation increased OGT translocation to the nucleus, resulting in higher nuclear O-GlcNAcylation and histone acetylation[39].
OGT is extensively phosphorylated (http://www.phosphosite.org)[91], however the kinases involved have only been characterized for a limited number of phosphorylation sites. GSK3β phosphorylates OGT (Ser3 or 4), resulting in an increase in activity[92] and insulin-mediated activation of IRS1 leads to tyrosine phosphorylation of OGT (Y976) resulting in both increased activity and O-GlcNAcylation of key components of the insulin signaling pathway[93], such as Akt and GSK3β. In the liver, IP3-mediated activation of CaMKII resulted in OGT phosphorylation (Ser20), increasing its activity and contributed to the regulation of liver autophagy[94]. As discussed below, in the heart CaMKII activity has been shown to be regulated by O-GlcNAcylation, however, its role in regulating cardiac O-GlcNAcylation is not known. Checkpoint kinase 1 (Chk1) stabilizes OGT in cell culture by phosphorylating Ser20[95], which could be of relevance in the heart given the potential role of Chk1 in contributing to the regulation of apoptosis in cardiomyocytes[96]. OGT also O-GlcNAcylates itself[92], including the same sites phosphorylated by GSK3β as well as additional sites in the TPR domain and C-terminus, but the effects of these modifications on OGT function/activity remain unclear. OGT is also acetylated[91], but what impact this has on its function are not known. OGA also has multiple phosphorylation sites; however, the specific regulatory kinases have not been identified. OGA also has an O-GlcNAc modification site (Ser405), which is in the region that is believed to interact with OGT; however, how changes in O-GlcNAcylation of this site affects this interaction remain unknown. It is clear that O-GlcNAc levels can be directly regulated via PTM-mediated changes in activity of the proteins responsible for its regulation. Several of the kinases identified to date, namely AMPK, CaMKII, and GSK3β have well characterized roles in the heart; however, our understanding of their impact on regulating protein O-GlcNAcylation in the heart, remains poorly understood.
Cross talk between Ca2+ and O-GlcNAc signaling
As discussed above, Ca2+/calmodulin-dependent protein kinase (CaMK) II and IV phosphorylate GFAT1 and OGT, in the case of OGT this is reported to increase its activity[97]. Consequently, it is perhaps surprising that Ca2+-dependent regulation of cellular O-GlcNAc levels remains under explored. In 2004, Kneass and Marchase demonstrated that treatment of neutrophils with fMLP (N-formyl-Met-Leu-Phe) resulted in approximately a 5-fold increase in O-GlcNAc levels within 30 seconds, gradually returning to basal levels over the next few minutes[98]. While this study did not assess Ca2+ levels, fMLP is recognized as mobilizing intracellular Ca2+ stores thereby activating calmodulin and calcineurin, and the observed temporal changes in O-GlcNAc levels were consistent with Ca2+-dependent activation. Song et al., subsequently reported that depolarization of neuroblastoma cells rapidly increased OGT activity reaching a peak within 1 minute resulting in increased O-GlcNAc levels[97]. They also demonstrated that extracellular Ca2+ was required for the increase in O-GlcNAc and that the increase in O-GlcNAc levels was blocked by CaMKIV inhibition[97]. Studies in other cell types including neonatal cardiomyocytes demonstrated that stress-induced increases in O-GlcNAc levels were also dependent on extracellular Ca2+ and could be attenuated by inhibition of CaMKII[29]. Leptin[99] and ET-1 [40, 41] have both been reported to increase cellular O-GlcNAc levels, and both have been shown to result in activation of CaMKII.
In the heart and isolated cardiomyocytes, PE, which activates the canonical hypertrophic signaling pathway, increases GFAT1 phosphorylation and O-GlcNAcylation[30, 39]. The increase in O-GlcNAc occurred as early as 2-hours following treatment and was associated with increases in GFAT protein and cell size over a similar period of time[39]. PE also leads to the activation of the Ca2+-dependent phosphatase calcineurin, resulting in the nuclear translocation of nuclear factor of activated T-cells (NFAT). Hyperglycemia has been shown to inhibit this response in an O-GlcNAc-dependent manner and in neonatal cardiomyocytes increased O-GlcNAc levels attenuated angiotensin II-induced increase in cytosolic Ca2+[100, 101]. On the other hand, Facundo et al., found that increasing O-GlcNAc levels through overexpression of OGT was sufficient to initiate hypertrophic signaling in cardiomyocytes[30], possibly via Ca2+/calcineurin mediated pathway. Also, of note, AMPK, which as discussed above regulates both GFAT1 and OGT, can also be activated in a CaMKII-dependent manner. Overall there is strong evidence that OGT activity and O-GlcNAc levels can be increased via activation of canonical Ca2+ signaling pathways.
The relationship between O-GlcNAcylation and Ca2+ signaling is complicated by the fact that Ca2+-activated proteins such as CaMK are themselves regulated by O-GlcNAc levels. For example, CaMKIV has been shown to have at least 5 individual O-GlcNAc modification sites and that during activation the interaction with OGA increased and O-GlcNAc levels quickly decreased[102]. Conversely, during inactivation O-GlcNAcylation of CaMKIV returned to normal, suggesting increased interaction with OGT[102]. Mutation of one of the O-GlcNAc sites, S189 to alanine to prevent O-GlcNAcylation, markedly reduced overall CaMKIV O-GlcNAcylation and increased basal kinase activity. In the heart, CaMKII has been shown to be O-GlcNAcylated at T286 and S279 and hyperglycemia increased CaMKII activity via increased O-GlcNAcylation of S279[17]. The hyperglycemia-mediated increase in O-GlcNAc levels was linked to an increased susceptibility to arrhythmias in diabetes. While excessive O-GlcNAc levels result in dysregulation of CaMKII, it does not preclude the possibility of O-GlcNAcylation playing a role in the normal regulation of CaMKII activity in a similar fashion as CaMKIV. The observations by Facundo et al., that increasing O-GlcNAc was sufficient to initiate NFAT translocation[30], which is activated by the Ca2+ sensitive phosphatase calcineurin, raises the possibility of other proteins involved in Ca2+ signaling also being regulated by O-GlcNAcylation. In addition, it has recently been shown that OGT overexpression inhibited NFAT activation via increased O-GlcNAcylation of GSK3β [103]. Moreover, STIM1, a protein that regulates store-operated calcium signaling in neonatal cardiomyocytes is O-GlcNAcylated, which was shown to reduce its function[104]. Other Ca2+ handling proteins have been reported to be O-GlcNAcylated including the inositol triphosphate receptor (IP3R)[105], PLB[52], and SERCA)[58] and a recent study also showed that although SERCA was unaffected that phosphorylation of PLB and troponin I were reduced in OGT KO mice[106].
Down regulation of O-GlcNAc levels
While there is considerable attention to mechanisms underlying either acute or chronic increases in O-GlcNAc levels, factors that might reduce O-GlcNAc levels are less well known (Figure 1). Ngoh et al., reported that treatment of cardiomyocytes with H2O2 decreased overall O-GlcNAcylation and this could be attenuated by inhibiting OGA with PUGNAc[107]. In the setting of hemorrhagic shock or I/R, low O-GlcNAc levels at the end of resuscitation or reperfusion correlated with increased indices of tissue injury in the heart and other tissues[108, 109]. In both cases inhibition of OGA at the time of resuscitation/reperfusion attenuated the loss of O-GlcNAc as well as reduced the level of tissue injury. The specific mechanism underlying the loss of O-GlcNAc levels is unclear; however, Laczy et al., showed that both nuclear and cytosolic levels of OGT were significantly decreased following I/R and this was accompanied by high molecular weight OGT immunoreactive bands, possibly reflecting the accumulation of inactive aggregates of OGT[24]. It is possible, therefore, that OGT is susceptible to damage induced by oxidative stress leading to decreased O-GlcNAc levels, and increased susceptibility to cellular injury. Inhibition of OGA appears to be protective in these circumstances, preventing the loss of O-GlcNAc levels and improving cell survival, however, the mechanisms by which this occurs are not well understood.
The above decreases in O-GlcNAc levels were all associated with pathophysiological conditions. Several recent studies have also suggested that exercise may result in decreased O-GlcNAc levels in the heart. Interestingly, in the mouse heart a single short bout of exercise lead to decrease in cytosolic O-GlcNAc and an increase in O-GlcNAc levels of lower molecular weight proteins in the nucleus[18]. There were no differences in OGT protein expression between sedentary and exercise groups, but O-GlcNAcylation of OGT was modestly reduced in response to exercise[18]. There was no evaluation of the effects of exercise on GFAT or OGA levels; however, the differential responses of cytosolic and nuclear O-GlcNAc levels highlights the importance of looking at subcellular distribution of O-GlcNAcylation in response to specific stimuli. Endurance training also resulted in a marked overall decrease in O-GlcNAc levels in the heart, which was associated with lower OGT protein levels and reduced mRNA levels of OGT, OGA, and GFAT2[26]. The authors concluded that reduced O-GlcNAcylation may contribute to physiological hypertrophy[26].
Recently, we have shown that treatment of adult and neonatal cardiomyocytes with exogenous NAD+ resulted in a time- and dose-dependent decrease in cellular O-GlcNAc levels, which occurred in the absence of changes in OGT or OGA protein levels[77]; however, GFAT was not examined. In a later study, we showed that glucose deprivation induced increases in cardiac O-GlcNAc levels and that NAD+ treatment prevented this response to glucose deprivation[110]. Lee et al., have also shown that NAD+ treatment reduces O-GlcNAc levels, specifically on Sp1, which resulted in its degradation[111]. Interestingly, NAD+ and its precursors have been shown to be cardioprotective in the setting of I/R injury[112, 113], which would seem counterintuitive given that lower O-GlcNAc levels have been associated with greater injury in similar settings[24]. However, the reduction in O-GlcNAc induced by NAD+ is modest and likely within normal homeostatic levels and thus unlikely to have adverse effects. NAD+ is increasingly recognized as playing an important role in cell signaling, well beyond its classical role as a redox carrier. Consequently, the influence of NAD+ on O-GlcNAc levels requires further studies to better understand the crosstalk between these two signaling pathways.
Conclusions
The regulation of O-GlcNAc in the heart, as well as other systems, has primarily focused on the role of increased nutrient availability, such as in the setting of hyperglycemia and diabetes, with an emphasis on the adverse effects of chronically elevated O-GlcNAc levels. There are however several studies that have shown that physiological and pathological stimuli can increase O-GlcNAc levels via transcriptional mechanisms as well as post-translational modifications. In addition, there is accumulating evidence of significant crosstalk between O-GlcNAc and Ca2+ signaling. There are a growing number of physiological agonists such as insulin, leptin, glucagon, ghrelin[114], and vasopressin[115] that have been shown to increase O-GlcNAc levels in range of different biological systems, highlighting the need to better understand how such agonists regulate cardiac O-GlcNAc levels. Consequently, it is increasingly apparent that cardiac O-GlcNAcylation is subject to dynamic regulation by a diverse range of physiological stimuli in addition to nutrient availability and that changes in O-GlcNAc levels can occur over varying time periods (Figure 2). Different stresses can increase or decrease O -GlcNAc levels to levels beyond the optimal zone, potentially leading to cardiomyocyte dysfunction, depending of the duration of this response. Furthermore, an additional layer of complexity exists due to the known associations (or putative associations) and cross talk between the regulation of OGT, OGA, GFAT, and O-GlcNAcylation and interactions with metabolic kinases and calcium signaling proteins (Figure 3). Future studies focusing on shorter time-dependent changes in cardiac O-GlcNAc levels and smaller transient changes, are also important to help better integrate O-GlcNAcylation as a key component of the cardiomyocyte signaling network.
Figure 2: Regulation of protein O-GlcNAc homeostasis:
Black line indicates changes in protein O-GlcNAc levels over an arbitrary period of time. In healthy cardiomyocytes, overall cellular O-GlcNAc levels vary within an optimal range over time periods of minutes, hours, or longer in response to different physiological stimuli due to changes in nutrient availability, regulation of GFAT, OGT, and OGA activities or alterations in the levels of these proteins. External stressors can lead to changes in O-GlcNAc levels beyond the optimal zone, either too high or too low, which can result in cellular dysfunction and potentially cell death depending on the duration of these excursions; however, brief pharmacologically induced increases in O-GlcNAc levels may be cardioprotective in the setting of ischemia/reperfusion or oxidative stress. This schematic illustrates overall changes in cellular O-GlcNAc levels and does not reflect the fact that O-GlcNAc levels can exhibit differential changes on individual proteins in response to both physiological and pathological stimuli; it also does not include the time of day dependent changes in O-GlcNAc that also occur in the heart.
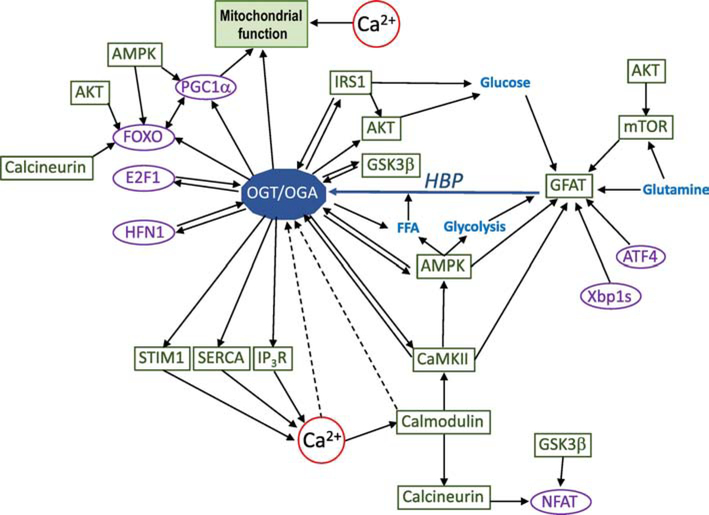
Figure 3: Illustration of the complex interactions associated with the regulation by and of protein O-GlcNAcylation:
Solid lines indicate established interactions, although not necessarily fully confirmed in the heart. Dotted lines indicate possible interactions that have yet to be established. There are no assumptions as to whether the interactions shown are positive or negative as this will likely be dependent on the specific stimulus and conditions (i.e., physiological or pathological). This is not a comprehensive illustration of all known interactions, but rather is focused on interactions discussed in the text with an emphasis on regulatory crosstalk known to be associated with O-GlcNAcylation or its regulatory enzymes (i.e. OGT, OGA, and GFAT). For example, both AMPK and CaMKII are known to regulate both OGT and GFAT and both are regulated by OGT; moreover, AMPK directly regulates glucose and fatty acid metabolism, which in turn influences HBP flux. In addition, AMPK contributes to transcriptional regulation of metabolism via its action on FoxO and PGC-1α, which in turn are both targets for O-GlcNAcylation. All relevant citations and abbreviations are included in the main body of the manuscript. OGT/OGA represents the O-GlcNAc cycle and the interactions shown could be influenced by either one or both of proteins. Note: Blue = metabolites/metabolic pathways; Purple = transcription factors; Green = all other proteins.
Highlights.
Modification of proteins by O-linked β-N-acetyl glucosamine (O-GlcNAc) have diverse effects on cardiac function.
Changes in O-GlcNAc levels are frequently associated with alterations in nutrient availability.
New studies indicate that O-GlcNAc levels are also regulated by nutrient-independent mechanisms.
We provide an overview of the role of O-GlcNAc in the cardiovascular system and how it is regulated.
Acknowledgments
We would like to thank all members of the Chatham laboratory and members of the UAB O-GlcNAc interest group for valued discussions. Many thanks to Dr. Adam Wende for his comments on Figure 3.
Funding
This work has been supported by a National Heart, Lung, and Blood Institute Grant (HL110366; JCC HL133011, HL142216), a UAB AMC21 reload multi-investigator grant to JCC and an American Diabetes Association Postdoctoral Fellowship (1-16-PDF-024; HEC).
Abbreviations
- AMPK
AMP-activated protein kinase
- CAMKII
Calcium/ calmodulin-dependent protein kinase II
- CAMKIV
Calcium/ calmodulin-dependent protein kinase type IV
- CREB
cAMP response element binding protein
- eNOS
Endothelial nitric oxide synthase
- ET-1
Endothelin 1
- FoxO
Forkhead transcription factors
- GD
glucose deprivation
- GFAT
Glutamine-fructose-6-phosphate aminotransferase
- GSK3β
Glycogen synthase kinase 3β
- HBP
Hexosamine biosynthesis pathway
- H202
Hydrogen peroxide
- IRS1/2
Insulin receptor 1/2
- I/R
ischemia/reperfusion
- mTOR
mechanistic target of rapamycin
- NAD+
Nicotinamide adenine dinucleotide
- NFAT
nuclear factor of activated T-cells
- O-GlcNAc
O-linked N-acetyl glucosamine
- OGT
O-GlcNAc transferase
- OGA
O-GlcNAcase
- PGC-1α
Peroxisome proliferator-activated receptor gamma coactivator 1-alpha
- PE
Phenylephrine
- PDPK1
Phosphoinositide dependent protein kinase 1
- PTM
Post-translational modification
- PKA
Protein Kinase A
- SERCA
Sarcoplasmic/endoplasmic reticulum Ca2+ ATPase
- Sp1
Specificity factor 1
- TET
Ten-eleven translocation
- UDP-GlcNAc
Uridine disphosphate-GlcNAc
- Xbp1s
X-box binding protein 1s
Footnotes
Disclosures
The authors do not have any conflicts of interest and/ or disclosures to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Torres CR, Hart GW, Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes. Evidence for O-linked GlcNAc, The Journal of biological chemistry, 259 (1984) 3308–3317. [PubMed] [Google Scholar]
- [2].Wang Z, Gucek M, Hart GW, Cross-talk between GlcNAcylation and phosphorylation: site-specific phosphorylation dynamics in response to globally elevated O-GlcNAc, Proceedings of the National Academy of Sciences of the United States of America, 105 (2008) 13793–13798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Marsh SA, Collins HE, Chatham JC, Protein O-GlcNAcylation and Cardiovascular (Patho)physiology, The Journal of biological chemistry, 289 (2014) 34449–34456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wright JN, Collins HE, Wende AR, Chatham JC, O-GlcNAcylation and cardiovascular disease, Biochemical Society transactions, 45 (2017) 545–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zachara NE, Hart GW, O-GlcNAc a sensor of cellular state: the role of nucleocytoplasmic glycosylation in modulating cellular function in response to nutrition and stress, Biochimica et biophysica acta, 1673 (2004) 13–28. [DOI] [PubMed] [Google Scholar]
- [6].Hart GW, Slawson C, Ramirez-Correa G, Lagerlof O, Cross talk between O-GlcNAcylation and phosphorylation: roles in signaling, transcription, and chronic disease, Annual review of biochemistry, 80 (2011) 825–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Marshall S, Bacote V, Traxinger RR, Discovery of a metabolic pathway mediating glucose-induced desensitization of the glucose transport system. Role of hexosamine biosynthesis in the induction of insulin resistance, The Journal of biological chemistry, 266 (1991) 4706–4712. [PubMed] [Google Scholar]
- [8].Gibb AA, Lorkiewicz PK, Zheng YT, Zhang X, Bhatnagar A, Jones SP, Hill BG, Integration of flux measurements to resolve changes in anabolic and catabolic metabolism in cardiac myocytes, The Biochemical journal, 474 (2017) 2785–2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Olson AK, Bouchard B, Zhu WZ, Chatham JC, Des Rosiers C, First characterization of glucose flux through the hexosamine biosynthesis pathway (HBP) in ex vivo mouse heart, The Journal of biological chemistry, (2020). doi: 10.1074/jbc.RA119.010565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Roquemore EP, Chevrier MR, Cotter RJ, Hart GW, Dynamic O-GlcNAcylation of the small heat shock protein alpha B-crystallin, Biochemistry, 35 (1996) 3578–3586. [DOI] [PubMed] [Google Scholar]
- [11].Yki-Jarvinen H, Vogt C, Iozzo P, Pipek R, Daniels MC, Virkamaki A, Makimattila S, Mandarino L, DeFronzo RA, McClain D, Gottschalk WK, UDP-N-acetylglucosamine transferase and glutamine: fructose 6-phosphate amidotransferase activities in insulin-sensitive tissues, Diabetologia, 40 (1997) 76–81. [DOI] [PubMed] [Google Scholar]
- [12].Du XL, Edelstein D, Rossetti L, Fantus IG, Goldberg H, Ziyadeh F, Wu J, Brownlee M, Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation, Proceedings of the National Academy of Sciences of the United States of America, 97 (2000) 12222–12226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Du XL, Edelstein D, Dimmeler S, Ju Q, Sui C, Brownlee M, Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the Akt site, The Journal of clinical investigation, 108 (2001) 1341–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Fricovsky ES, Suarez J, Ihm SH, Scott BT, Suarez-Ramirez JA, Banerjee I, Torres-Gonzalez M, Wang H, Ellrott I, Maya-Ramos L, Villarreal F, Dillmann WH, Excess protein O-GlcNAcylation and the progression of diabetic cardiomyopathy, American journal of physiology. Regulatory, integrative and comparative physiology, 303 (2012) R689–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Clark RJ, McDonough PM, Swanson E, Trost SU, Suzuki M, Fukuda M, Dillmann WH, Diabetes and the accompanying hyperglycemia impairs cardiomyocyte cycling through increased nuclear O-GlcNAcylation, J. Biol. Chem., 278 (2003) 44230–44237. [DOI] [PubMed] [Google Scholar]
- [16].Fulop N, Mason MM, Dutta K, Wang P, Davidoff AJ, Marchase RB, Chatham JC, The impact of Type-2 diabetes and aging on cardiomyocyte function and O-Linked N-acetylglucosamine levels in the heart, American journal of physiology. Cell physiology, 292 (2007) C1370–1378. [DOI] [PubMed] [Google Scholar]
- [17].Erickson JR, Pereira L, Wang L, Han G, Ferguson A, Dao K, Copeland RJ, Despa F, Hart GW, Ripplinger CM, Bers DM, Diabetic hyperglycaemia activates CaMKII and arrhythmias by O-linked glycosylation, Nature, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Cox EJ, Marsh SA, Exercise and diabetes have opposite effects on the assembly and O-GlcNAc modification of the mSin3A/HDAC1/2 complex in the heart, Cardiovascular diabetology, 12 (2013) 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bennett CE, Johnsen VL, Shearer J, Belke DD, Exercise training mitigates aberrant cardiac protein O-GlcNAcylation in streptozotocin-induced diabetic mice, Life sciences, 92 (2013) 657–663. [DOI] [PubMed] [Google Scholar]
- [20].Marsh SA, Dell’Italia LJ, Chatham JC, Activation of the hexosamine biosynthesis pathway and protein O-GlcNAcylation modulate hypertrophic and cell signaling pathways in cardiomyocytes from diabetic mice, Amino Acids, 40 (2011) 819–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Marsh SA, Powell PC, Dell’italia LJ, Chatham JC, Cardiac O-GlcNAcylation blunts autophagic signaling in the diabetic heart, Life sciences, 92 (2013) 648–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Champattanachai V, Marchase RB, Chatham JC, Glucosamine protects neonatal cardiomyocytes from ischemia-reperfusion injury via increased protein-associated O-GlcNAc, American journal of physiology. Cell physiology, 292 (2007) C178–187. [DOI] [PubMed] [Google Scholar]
- [23].Ferron M, Cadiet J, Persello A, Prat V, Denis M, Erraud A, Aillerie V, Mevel M, Bigot E, Chatham JC, Gauthier C, Rozec B, Lauzier B, O-GlcNAc stimulation: A new metabolic approach to treat septic shock, Scientific reports, 9 (2019) 18751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Laczy B, Marsh SA, Brocks CA, Wittmann I, Chatham JC, Inhibition of O-GlcNAcase in perfused rat hearts by NAG-thiazolines at the time of reperfusion is cardioprotective in an O-GlcNAc-dependent manner, American journal of physiology. Heart and circulatory physiology, 299 (2010) H1715–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zou L, Yang S, Bounelis P, Chatham JC, Chaudry IH, Marchase RB, Glucosamine improves recovery following trauma hemorrhage in rat, FASEB J., 19 (2005) A1224. [Google Scholar]
- [26].Belke DD, Swim-exercised mice show a decreased level of protein O-GlcNAcylation and expression of O-GlcNAc transferase in heart, J Appl Physiol (1985), 111 (2011) 157–162. [DOI] [PubMed] [Google Scholar]
- [27].Yang X, Qian K, Protein O-GlcNAcylation: emerging mechanisms and functions, Nat Rev Mol Cell Biol, 18 (2017) 452–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Taylor RP, Geisler TS, Chambers JH, McClain DA, Up-regulation of O-GlcNAc transferase with glucose deprivation in HepG2 cells is mediated by decreased hexosamine pathway flux, The Journal of biological chemistry, 284 (2009) 3425–3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zou L, Zhu-Mauldin X, Marchase RB, Paterson AJ, Liu J, Yang Q, Chatham JC, Glucose Deprivation-induced Increase in Protein O-GlcNAcylation in Cardiomyocytes Is Calcium-dependent, The Journal of biological chemistry, 287 (2012) 34419–34431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Facundo HT, Brainard RE, Watson LJ, Ngoh GA, Hamid T, Prabhu SD, Jones SP, O-GlcNAc Signaling is Essential for NFAT-Mediated Transcriptional Reprogramming During Cardiomyocyte Hypertrophy, American journal of physiology. Heart and circulatory physiology, (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Lunde IG, Aronsen JM, Kvaloy H, Qvigstad E, Sjaastad I, Tonnessen T, Christensen G, Gronning-Wang LM, Carlson CR, Cardiac O-GlcNAc signaling is increased in hypertrophy and heart failure, Physiological genomics, 44 (2012) 162–172. [DOI] [PubMed] [Google Scholar]
- [32].Mailleux F, Gelinas R, Beauloye C, Horman S, Bertrand L, O-GlcNAcylation, enemy or ally during cardiac hypertrophy development?, Biochimica et biophysica acta, 1862 (2016) 2232–2243. [DOI] [PubMed] [Google Scholar]
- [33].Ledee D, Smith L, Bruce M, Kajimoto M, Isern N, Portman MA, Olson AK, c-Myc Alters Substrate Utilization and O-GlcNAc Protein Posttranslational Modifications without Altering Cardiac Function during Early Aortic Constriction, PloS one, 10 (2015) e0135262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Cannon MV, Sillje HH, Sijbesma JW, Vreeswijk-Baudoin I, Ciapaite J, van der Sluis B, van Deursen J, Silva GJ, de Windt LJ, Gustafsson JA, van der Harst P, van Gilst WH, de Boer RA, Cardiac LXRalpha protects against pathological cardiac hypertrophy and dysfunction by enhancing glucose uptake and utilization, EMBO Mol Med, 7 (2015) 1229–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Chatham JC, Marchase RB, The role of protein O-linked beta-N-acetylglucosamine in mediating cardiac stress responses, Biochimica et biophysica acta, 1800 (2010) 57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Fulop N, Feng W, Xing D, He K, Not LG, Brocks CA, Marchase RB, Miller AP, Chatham JC, Aging leads to increased levels of protein O-linked N-acetylglucosamine in heart, aorta, brain and skeletal muscle in Brown-Norway rats, Biogerontology, 9 (2008) 139–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Banerjee PS, Lagerlof O, Hart GW, Roles of O-GlcNAc in chronic diseases of aging, Molecular aspects of medicine, 51 (2016) 1–15. [DOI] [PubMed] [Google Scholar]
- [38].Zhao L, Feng Z, Zou X, Cao K, Xu J, Liu J, Aging leads to elevation of O-GlcNAcylation and disruption of mitochondrial homeostasis in retina, Oxid Med Cell Longev, 2014 (2014) 425705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Gelinas R, Mailleux F, Dontaine J, Bultot L, Demeulder B, Ginion A, Daskalopoulos EP, Esfahani H, Dubois-Deruy E, Lauzier B, Gauthier C, Olson AK, Bouchard B, Des Rosiers C, Viollet B, Sakamoto K, Balligand JL, Vanoverschelde JL, Beauloye C, Horman S, Bertrand L, AMPK activation counteracts cardiac hypertrophy by reducing O-GlcNAcylation, Nat Commun, 9 (2018) 374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Lima VV, Giachini FR, Carneiro FS, Carvalho MH, Fortes ZB, Webb RC, Tostes RC, O-GlcNAcylation contributes to the vascular effects of ET-1 via activation of the RhoA/Rho-kinase pathway, Cardiovascular research, 89 (2011) 614–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Lima VV, Giachini FR, Carneiro FS, Carneiro ZN, Saleh MA, Pollock DM, Fortes ZB, Carvalho MH, Ergul A, Webb RC, Tostes RC, O-GlcNAcylation contributes to augmented vascular reactivity induced by endothelin 1, Hypertension, 55 (2010) 180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Wende AR, Post-translational modifications of the cardiac proteome in diabetes and heart failure, Proteomics. Clinical applications, 10 (2016) 25–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Dassanayaka S, Jones SP, O-GlcNAc and the cardiovascular system, Pharmacol Ther, 142 (2014) 62–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Hebert LF, Daniels MC, Zhou J, Crook ED, Turner RL, Simmons ST, Neidigh JL, Zhu JS, Baron AD, McClain DA, Overexpression of glutamine:fructose-6-phosphate amidotransferase in transgenic mice leads to insulin resistance, J. Clin. Invest., 98 (1996) 930–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Vosseller K, Wells L, Lane MD, Hart GW, Elevated nucleocytoplasmic glycosylation by O-GlcNAc results in insulin resistance associated with defects in Akt activation in 3T3-L1 adipocytes, Proceedings of the National Academy of Sciences of the United States of America, 99 (2002) 5313–5318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Macauley MS, Shan X, Yuzwa SA, Gloster TM, Vocadlo DJ, Elevation of Global O-GlcNAc in rodents using a selective O-GlcNAcase inhibitor does not cause insulin resistance or perturb glucohomeostasis, Chem Biol, 17 (2010) 949–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Macauley MS, Bubb AK, Martinez-Fleites C, Davies GJ, Vocadlo DJ, Elevation of global O-GlcNAc levels in 3T3-L1 adipocytes by selective inhibition of O-GlcNAcase does not induce insulin resistance, The Journal of biological chemistry, 283 (2008) 34687–34695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Patti ME, Virkamaki A, Landaker EJ, Kahn CR, Yki-Jarvinen H, Activation of the hexosamine pathway by glucosamine in vivo induces insulin resistance of early postreceptor insulin signaling events in skeletal muscle, Diabetes, 48 (1999) 1562–1571. [DOI] [PubMed] [Google Scholar]
- [49].Copeland RJ, Bullen JW, Hart GW, Cross-talk between GlcNAcylation and phosphorylation: roles in insulin resistance and glucose toxicity, American journal of physiology. Endocrinology and metabolism, 295 (2008) E17–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Issad T, Masson E, Pagesy P, O-GlcNAc modification, insulin signaling and diabetic complications, Diabetes & metabolism, 36 (2010) 423–435. [DOI] [PubMed] [Google Scholar]
- [51].Zhao Y, Tang Z, Shen A, Tao T, Wan C, Zhu X, Huang J, Zhang W, Xia N, Wang S, Cui S, Zhang D, The Role of PTP1B O-GlcNAcylation in Hepatic Insulin Resistance, Int J Mol Sci, 16 (2015) 22856–22869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Yokoe S, Asahi M, Takeda T, Otsu K, Taniguchi N, Miyoshi E, Suzuki K, Inhibition of phospholamban phosphorylation by O-GlcNAcylation: implications for diabetic cardiomyopathy, Glycobiology, 20 (2010) 1217–1226. [DOI] [PubMed] [Google Scholar]
- [53].Hu Y, Belke D, Suarez J, Swanson E, Clark R, Hoshijima M, Dillmann WH, Adenovirus-mediated overexpression of O-GlcNAcase improves contractile function in the diabetic heart, Circulation research, 96 (2005) 1006–1013. [DOI] [PubMed] [Google Scholar]
- [54].Ramirez-Correa GA, Jin W, Wang Z, Zhong X, Gao WD, Dias WB, Vecoli C, Hart GW, Murphy AM, O-linked GlcNAc modification of cardiac myofilament proteins: a novel regulator of myocardial contractile function, Circulation research, 103 (2008) 1354–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Ramirez-Correa GA, Ma J, Slawson C, Zeidan Q, Lugo-Fagundo NS, Xu M, Shen X, Gao WD, Caceres V, Chakir K, DeVine L, Cole RN, Marchionni L, Paolocci N, Hart GW, Murphy AM, Removal of Abnormal Myofilament O-GlcNAcylation Restores Ca2+ Sensitivity in Diabetic Cardiac Muscle, Diabetes, 64 (2015) 3573–3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Banerjee PS, Ma J, Hart GW, Diabetes-associated dysregulation of O-GlcNAcylation in rat cardiac mitochondria, Proceedings of the National Academy of Sciences of the United States of America, 112 (2015) 6050–6055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Hu Y, Suarez J, Fricovsky E, Wang H, Scott BT, Trauger SA, Han W, Oyeleye MO, Dillmann WH, Increased enzymatic O-GlcNAcylation of mitochondrial proteins impairs mitochondrial function in cardiac myocytes exposed to high glucose, The Journal of biological chemistry, 284 (2009) 547–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Johnsen VL, Belke DD, Hughey CC, Hittel DS, Hepple RT, Koch LG, Britton SL, Shearer J, Enhanced cardiac protein glycosylation (O-GlcNAc) of selected mitochondrial proteins in rats artificially selected for low running capacity, Physiol Genomics, 45 (2013) 17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Laczy B, Fulop N, Onay-Besikci A, Des Rosiers C, Chatham JC, Acute regulation of cardiac metabolism by the hexosamine biosynthesis pathway and protein O-GlcNAcylation, PloS one, 6 (2011) e18417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Lauzier B, Vaillant F, Merlen C, Gelinas R, Bouchard B, Rivard ME, Labarthe F, Dolinsky VW, Dyck JR, Allen BG, Chatham JC, Des Rosiers C, Metabolic effects of glutamine on the heart: anaplerosis versus the hexosamine biosynthetic pathway, Journal of molecular and cellular cardiology, 55 (2013) 92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Luo B, Parker GJ, Cooksey RC, Soesanto Y, Evans M, Jones D, McClain DA, Chronic hexosamine flux stimulates fatty acid oxidation by activating AMP-activated protein kinase in adipocytes, The Journal of biological chemistry, 282 (2007) 7172–7180. [DOI] [PubMed] [Google Scholar]
- [62].Keembiyehetty CN, Krzeslak A, Love DC, Hanover JA, A lipid-droplet-targeted O-GlcNAcase isoform is a key regulator of the proteasome, Journal of cell science, 124 (2011) 2851–2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Bacigalupa ZA, Bhadiadra CH, Reginato MJ, O-GlcNAcylation: key regulator of glycolytic pathways, J Bioenerg Biomembr, 50 (2018) 189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Taegtmeyer H, Karlstaedt A, Rees ML, Davogustto G, Oncometabolic Tracks in the Heart, Circulation research, 120 (2017) 267–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Karlstaedt A, Schiffer W, Taegtmeyer H, Actionable Metabolic Pathways in Heart Failure and Cancer-Lessons From Cancer Cell Metabolism, Front Cardiovasc Med, 5 (2018) 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Housley MP, Rodgers JT, Udeshi ND, Kelly TJ, Shabanowitz J, Hunt DF, Puigserver P, Hart GW, O-GlcNAc regulates FoxO activation in response to glucose, The Journal of biological chemistry, 283 (2008) 16283–16292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Housley MP, Udeshi ND, Rodgers JT, Shabanowitz J, Puigserver P, Hunt DF, Hart GW, A PGC-1alpha-O-GlcNAc transferase complex regulates FoxO transcription factor activity in response to glucose, The Journal of biological chemistry, 284 (2009) 5148–5157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Rexach JE, Clark PM, Mason DE, Neve RL, Peters EC, Hsieh-Wilson LC, Dynamic O-GlcNAc modification regulates CREB-mediated gene expression and memory formation, Nat Chem Biol, 8 (2012) 253–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Jin N, Ma D, Gu J, Shi J, Xu X, Iqbal K, Gong CX, Liu F, Chu D, O-GlcNAcylation modulates PKA-CREB signaling in a manner specific to PKA catalytic subunit isoforms, Biochem Biophys Res Commun, 497 (2018) 194–199. [DOI] [PubMed] [Google Scholar]
- [70].Bullen JW, Balsbaugh JL, Chanda D, Shabanowitz J, Hunt DF, Neumann D, Hart GW, Cross-talk between two essential nutrient-sensitive enzymes: O-GlcNAc transferase (OGT) and AMP-activated protein kinase (AMPK), The Journal of biological chemistry, 289 (2014) 10592–10606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Eguchi S, Oshiro N, Miyamoto T, Yoshino K, Okamoto S, Ono T, Kikkawa U, Yonezawa K, AMP-activated protein kinase phosphorylates glutamine : fructose-6-phosphate amidotransferase 1 at Ser243 to modulate its enzymatic activity, Genes Cells, 14 (2009) 179–189. [DOI] [PubMed] [Google Scholar]
- [72].Li Y, Roux C, Lazereg S, LeCaer JP, Laprevote O, Badet B, Badet-Denisot MA, Identification of a novel serine phosphorylation site in human glutamine:fructose-6-phosphate amidotransferase isoform 1, Biochemistry, 46 (2007) 13163–13169. [DOI] [PubMed] [Google Scholar]
- [73].Zachara NE, O’Donnell N, Cheung WD, Mercer JJ, Marth JD, Hart GW, Dynamic O-GlcNAc modification of nucleocytoplasmic proteins in response to stress. A survival response of mammalian cells, The Journal of biological chemistry, 279 (2004) 30133–30142. [DOI] [PubMed] [Google Scholar]
- [74].Jones SP, Zachara NE, Ngoh GA, Hill BG, Teshima Y, Bhatnagar A, Hart GW, Marban E, Cardioprotection by N-acetylglucosamine linkage to cellular proteins, Circulation, 117 (2008) 1172–1182. [DOI] [PubMed] [Google Scholar]
- [75].Wang ZV, Deng Y, Gao N, Pedrozo Z, Li DL, Morales CR, Criollo A, Luo X, Tan W, Jiang N, Lehrman MA, Rothermel BA, Lee AH, Lavandero S, Mammen PP, Ferdous A, Gillette TG, Scherer PE, Hill JA, Spliced X-box binding protein 1 couples the unfolded protein response to hexosamine biosynthetic pathway, Cell, 156 (2014) 1179–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Chaveroux C, Sarcinelli C, Barbet V, Belfeki S, Barthelaix A, Ferraro-Peyret C, Lebecque S, Renno T, Bruhat A, Fafournoux P, Manie SN, Nutrient shortage triggers the hexosamine biosynthetic pathway via the GCN2-ATF4 signalling pathway, Scientific reports, 6 (2016) 27278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Durgan DJ, Pat BM, Laczy B, Bradley JA, Tsai JY, Grenett MH, Ratcliffe WF, Brewer RA, Nagendran J, Villegas-Montoya C, Zou C, Zou L, Johnson RL Jr., Dyck JR, Bray MS, Gamble KL, Chatham JC, Young ME, O-GlcNAcylation, novel post-translational modification linking myocardial metabolism and cardiomyocyte circadian clock, The Journal of biological chemistry, 286 (2011) 44606–44619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Berthier A, Vinod M, Porez G, Steenackers A, Alexandre J, Yamakawa N, Gheeraert C, Ploton M, Marechal X, Dubois-Chevalier J, Hovasse A, Schaeffer-Reiss C, Cianferani S, Rolando C, Bray F, Duez H, Eeckhoute J, Lefebvre T, Staels B, Lefebvre P, Combinatorial regulation of hepatic cytoplasmic signaling and nuclear transcriptional events by the OGT/REV-ERBalpha complex, Proceedings of the National Academy of Sciences of the United States of America, 115 (2018) E11033–E11042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Zhang Z, Tan EP, VandenHull NJ, Peterson KR, Slawson C, O-GlcNAcase Expression is Sensitive to Changes in O-GlcNAc Homeostasis, Front Endocrinol (Lausanne), 5 (2014) 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Park SK, Zhou X, Pendleton KE, Hunter OV, Kohler JJ, O’Donnell KA, Conrad NK, A Conserved Splicing Silencer Dynamically Regulates O-GlcNAc Transferase Intron Retention and O-GlcNAc Homeostasis, Cell Rep, 20 (2017) 1088–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Qian K, Wang S, Fu M, Zhou J, Singh JP, Li MD, Yang Y, Zhang K, Wu J, Nie Y, Ruan HB, Yang X, Transcriptional regulation of O-GlcNAc homeostasis is disrupted in pancreatic cancer, The Journal of biological chemistry, 293 (2018) 13989–14000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Muthusamy S, Hong KU, Dassanayaka S, Hamid T, Jones SP, E2F1 Transcription Factor Regulates O-linked N-acetylglucosamine (O-GlcNAc) Transferase and O-GlcNAcase Expression, The Journal of biological chemistry, 290 (2015) 31013–31024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Wells L, Slawson C, Hart GW, The E2F-1 associated retinoblastoma-susceptibility gene product is modified by O-GlcNAc, Amino Acids, 40 (2011) 877–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Dassanayaka S, Brittian KR, Jurkovic A, Higgins LA, Audam TN, Long BW, Harrison LT, Militello G, Riggs DW, Chitre MG, Uchida S, Muthusamy S, Gumpert AM, Jones SP, E2f1 deletion attenuates infarct-induced ventricular remodeling without affecting O-GlcNAcylation, Basic Res Cardiol, 114 (2019) 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Zhang C, Xie F, Li L, Zhang C, Zhang Y, Ying W, Liu L, Yan X, Yin F, Zhang L, Hepatocyte nuclear factor 1 alpha (HNF1A) regulates transcription of O-GlcNAc transferase in a negative feedback mechanism, FEBS letters, (2019). [DOI] [PubMed] [Google Scholar]
- [86].Muthusamy S, DeMartino AM, Watson LJ, Brittian KR, Zafir A, Dassanayaka S, Hong KU, Jones SP, MicroRNA-539 is up-regulated in failing heart, and suppresses O-GlcNAcase expression, The Journal of biological chemistry, 289 (2014) 29665–29676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Milewski S, Glucosamine-6-phosphate synthase - the multi-facets enzyme, Biochimica et biophysica acta, 1597 (2002) 173–192. [DOI] [PubMed] [Google Scholar]
- [88].Hu Y, Riesland L, Paterson AJ, Kudlow JE, Phosphorylation of mouse glutamine-fructose-6-phosphate amidotransferase 2 (GFAT2) by cAMP-dependent protein kinase increases the enzyme activity, The Journal of biological chemistry, 279 (2004) 29988–29993. [DOI] [PubMed] [Google Scholar]
- [89].Chang Q, Su K, Baker JR, Yang X, Paterson AJ, Kudlow JE, Phosphorylation of human glutamine:fructose-6-phosphate amidotransferase by cAMP-dependent protein kinase at serine 205 blocks the enzyme activity, The Journal of biological chemistry, 275 (2000) 21981–21987. [DOI] [PubMed] [Google Scholar]
- [90].Moloughney JG, Vega-Cotto NM, Liu S, Patel C, Kim PK, Wu CC, Albaciete D, Magaway C, Chang A, Rajput S, Su X, Werlen G, Jacinto E, mTORC2 modulates the amplitude and duration of GFAT1 Ser-243 phosphorylation to maintain flux through the hexosamine pathway during starvation, The Journal of biological chemistry, 293 (2018) 16464–16478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Hornbeck PV, Zhang B, Murray B, Kornhauser JM, Latham V, Skrzypek E, PhosphoSitePlus, 2014: mutations, PTMs and recalibrations, Nucleic Acids Res, 43 (2015) D512–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Kaasik K, Kivimae S, Allen JJ, Chalkley RJ, Huang Y, Baer K, Kissel H, Burlingame AL, Shokat KM, Ptacek LJ, Fu YH, Glucose sensor O-GlcNAcylation coordinates with phosphorylation to regulate circadian clock, Cell metabolism, 17 (2013) 291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Whelan SA, Lane MD, Hart GW, Regulation of the O-linked beta-Nacetylglucosamine transferase by insulin signaling, The Journal of biological chemistry, 283 (2008) 21411–21417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Ruan HB, Ma Y, Torres S, Zhang B, Feriod C, Heck RM, Qian K, Fu M, Li X, Nathanson MH, Bennett AM, Nie Y, Ehrlich BE, Yang X, Calcium-dependent O-GlcNAc signaling drives liver autophagy in adaptation to starvation, Genes Dev, 31 (2017) 1655–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Hu X, Li Z, Ding Y, Geng Q, Xiahou Z, Ru H, Dong MQ, Xu X, Li J, Chk1 modulates the interaction between myosin phosphatase targeting protein 1 (MYPT1) and protein phosphatase 1cbeta (PP1cbeta), Cell Cycle, 17 (2018) 421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Xie L, Pi X, Wang Z, He J, Willis MS, Patterson C, Depletion of PHD3 protects heart from ischemia/reperfusion injury by inhibiting cardiomyocyte apoptosis, Journal of molecular and cellular cardiology, 80 (2015) 156–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Song M, Kim HS, Park JM, Kim SH, Kim IH, Ryu SH, Suh PG, o-GlcNAc transferase is activated by CaMKIV-dependent phosphorylation under potassium chloride-induced depolarization in NG-108–15 cells, Cell Signal, 20 (2008) 94–104. [DOI] [PubMed] [Google Scholar]
- [98].Kneass ZT, Marchase RB, Neutrophils exhibit rapid agonist-induced increases in protein-associated O-GlcNAc, The Journal of biological chemistry, 279 (2004) 45759–45765. [DOI] [PubMed] [Google Scholar]
- [99].Zimmerman AD, Harris RB, In vivo and in vitro evidence that chronic activation of the hexosamine biosynthetic pathway interferes with leptin-dependent STAT3 phosphorylation, American journal of physiology. Regulatory, integrative and comparative physiology, 308 (2015) R543–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Hunton DL, Lucchesi PA, Pang Y, Cheng X, Dell’Italia LJ, Marchase RB, Capacitative calcium entry contributes to nuclear factor of activated T-cells nuclear translocation and hypertrophy in cardiomyocytes, The Journal of biological chemistry, 277 (2002) 14266–14273. [DOI] [PubMed] [Google Scholar]
- [101].Pang Y, Hunton DL, Bounelis P, Marchase RB, Hyperglycemia inhibits capacitative calcium entry and hypertrophy in neonatal cardiomyocytes, Diabetes, 51 (2002) 3461–3467. [DOI] [PubMed] [Google Scholar]
- [102].Dias WB, Cheung WD, Wang Z, Hart GW, Regulation of calcium/calmodulin-dependent kinase IV by O-GlcNAc modification, The Journal of biological chemistry, 284 (2009) 21327–21337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Nakagawa T, Furukawa Y, Hayashi T, Nomura A, Yokoe S, Moriwaki K, Kato R, Ijiri Y, Yamaguchi T, Izumi Y, Yoshiyama M, Asahi M, Augmented O-GlcNAcylation attenuates intermittent hypoxia-induced cardiac remodeling through the suppression of NFAT and NF-kappaB activities in mice, Hypertens Res, 42 (2019) 1858–1871. [DOI] [PubMed] [Google Scholar]
- [104].Zhu-Mauldin X, Marsh SA, Zou L, Marchase RB, Chatham JC, Modification of STIM1 by O-linked N-acetylglucosamine (O-GlcNAc) attenuates store-operated calcium entry in neonatal cardiomyocytes, The Journal of biological chemistry, 287 (2012) 39094–39106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Rengifo J, Gibson CJ, Winkler E, Collin T, Ehrlich BE, Regulation of the inositol 1,4,5-trisphosphate receptor type I by O-GlcNAc glycosylation, J Neurosci, 27 (2007) 13813–13821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Zhu WZ, El-Nachef D, Yang X, Ledee D, Olson AK, O-GlcNAc Transferase Promotes Compensated Cardiac Function and Protein Kinase A O-GlcNAcylation During Early and Established Pathological Hypertrophy From Pressure Overload, J Am Heart Assoc, 8 (2019) e011260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Ngoh GA, Watson LJ, Facundo HT, Jones SP, Augmented O-GlcNAc signaling attenuates oxidative stress and calcium overload in cardiomyocytes, Amino Acids, 40 (2011) 895–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Not LG, Marchase RB, Fulop N, Brocks CA, Chatham JC, Glucosamine administration improves survival rate after severe hemorrhagic shock combined with trauma in rats, Shock, 28 (2007) 345–352. [DOI] [PubMed] [Google Scholar]
- [109].Zou LY, Yang S, Chaudry IH, Marchase RB, Chatham JC, PUGNAc administration during resuscitation improves organ function following trauma-hemorrhage, Shock, 27 (2007) 402–408. [DOI] [PubMed] [Google Scholar]
- [110].Zou L, Marchase RB, Chatham JC, The effects of NAD on glucose deprivation induced activation of protein O-GlcNAcylation, ER stress and autophagy mediated by CD38 and cADPR, Faseb journal, 29.1_supplement.798.4 (2015). [Google Scholar]
- [111].Lee HI, Cho HJ, Han JA, Jang SY, Wang KM, Kang HT, Hwan ES, Transient downregulation of protein O-N-acetylglucosaminylation by treatment of high-dose nicotinamide in human cells, Exp Mol Med, 40 (2008) 246–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Yamamoto T, Byun J, Zhai P, Ikeda Y, Oka S, Sadoshima J, Nicotinamide mononucleotide, an intermediate of NAD+ synthesis, protects the heart from ischemia and reperfusion, PloS one, 9 (2014) e98972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Nadtochiy SM, Wang YT, Nehrke K, Munger J, Brookes PS, Cardioprotection by nicotinamide mononucleotide (NMN): Involvement of glycolysis and acidic pH, Journal of molecular and cellular cardiology, 121 (2018) 155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Ruan HB, Dietrich MO, Liu ZW, Zimmer MR, Li MD, Singh JP, Zhang K, Yin R, Wu J, Horvath TL, Yang X, O-GlcNAc transferase enables AgRP neurons to suppress browning of white fat, Cell, 159 (2014) 306–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Hoffert JD, Pisitkun T, Saeed F, Song JH, Chou CL, Knepper MA, Dynamics of the G protein-coupled vasopressin V2 receptor signaling network revealed by quantitative phosphoproteomics, Molecular & cellular proteomics : MCP, 11 (2012) M111 014613. [DOI] [PMC free article] [PubMed] [Google Scholar]