Abstract
Background
The American Joint Committee on Cancer (AJCC) recently released its 8th edition staging system, which created a separate staging system for gastric cancer patients who have undergone preoperative therapy (ypStage). The objective of this retrospective study was to apply the new ypStage to patients who have undergone preoperative therapy and potentially curative gastrectomy.
Methods
We collected data from a prospectively maintained institutional database of gastric cancer patients who underwent potentially curative gastrectomy after preoperative therapy (1995–2015). Kaplan–Meier survival estimations and log-rank tests were performed to compare survival. Univariable and multivariable analyses were performed to determine risk factors for overall survival.
Results
A total of 354 patients met our criteria. Most patients completed planned preoperative therapy (94%; 332/354) and received chemoradiation therapy (75%; 265/354). Although clinical stage (cStage) provided a poor discrimination of survival, postneoadjuvant pathological stage (ypStage) identified significant variation in survival (p < 0.001). Multivariable analysis showed the following factors were associated with survival after adjustment for ypStage: Asian race (HR 0.52; p = 0.028), linitis plastica (HR 1.66; p = 0.037), and R1 resection (HR 1.91; p = 0.016). Survival was not longer in ypT0N0 patients than in ypStage I patients (HR 1.29; p = 0.377).
Conclusions
The AJCC 8th edition staging system for gastric cancer demonstrated reasonable survival prediction by ypStage, but not cStage, in patients who had undergone preoperative therapy. ypT0N0 patients, although not defined in the 8th edition, may be considered for inclusion in the ypStage I group.
Keywords: Gastric cancer, AJCC 8th edition staging system, Preoperative therapy, Surgery, Survival
Introduction
Recently, the American Joint Committee on Cancer (AJCC) published the 8th edition of its cancer staging system [1]. The most notable change in the gastric cancer section is that the new edition has three discrete staging systems: clinical stage (cTNM or cStage), pathological stage (pTNM or pStage), and postneoadjuvant pathological stage (ypTNM or ypStage). The concept of creating ypStage is novel, based on the assumption that survival predictions for a specific stage (e.g., stage II) may be different between patients who have undergone upfront surgery (pStage II) and those who have undergone preoperative therapy (ypStage II). ypStage represents the combination of tumor status and its response to preoperative therapy; pStage does not incorporate tumor treatment response. However, this new ypStage system was created based on the National Cancer Database (NCDB), which included a relatively small number of patients treated with preoperative therapy (<700) with a short median follow-up (23 months); therefore, additional studies from US cancer centers that utilize preoperative therapy are needed.
A further question regarding the new staging system relates to cStage. A preoperative cStage is needed to determine appropriate preoperative treatment and stage patients at diagnosis. However, the cStage system may be limited in utility because of the reported difficulty in accurately staging tumor thickness and nodal status [2–4]; cStage grouping is defined by only three cT categories (cT1/2, cT3/4a, and cT4b) and two cN categories (cN0 and cN-positive). Further, because clinical stage information in the NCDB is inadequate, the cStage was created by using data for 4091 gastric cancer patients from Shizuoka Cancer Center in Japan. The use of this population may be problematic because the tumor biology of gastric cancer and clinical staging accuracy are likely different between Japan and the United States [5]. Moreover, use of preoperative therapy has been increasing in the United States, and it is not known whether survival predictions based on clinical stage are valid in patients who undergo preoperative therapy.
To clarify these aspects of the new AJCC staging system, the purpose of this study was to evaluate survival predictions by ypStage and cStage among patients who underwent preoperative therapy followed by surgical resection for gastric adenocarcinoma.
Methods
After receiving institutional review board approval, we collected data from a prospectively maintained database of patients with primary gastric adenocarcinoma who were diagnosed with clinically M0 disease (no distant metastasis) and underwent a potentially curative surgical resection at our institution (1995–2015). We identified patients with pathologically confirmed gastric or Siewert type III gastroesophageal junction (GEJ) adenocarcinoma who received preoperative chemotherapy or chemoradiation therapy or both. cStage was determined by the combination of upper gastrointestinal endoscopy, endoscopic ultrasonography (EUS), computed tomography (CT) scan, and positron emission tomography (PET) scan. In general, EUS was used to define cT stage, and cN stage was considered positive if either EUS, CT, or PET identified suspicious lymph nodes (e.g., short-axis diameter ≥6 mm on CT scan, suspicious shape with enlarged size on EUS; pathological confirmation with fine-needle aspiration was not required, or suspicious uptake at lymph nodes on PET). Variables recorded included age, sex, race/ethnicity, date of diagnosis, tumor location, clinical stage information, date and type of surgery, pathological stage information, histological grade, presence of signet-ring cells, and number of lymph nodes examined in pathological analysis.
Statistical methods
The differences in categorical variables were compared using Fisher’s exact test or chi-square tests, as appropriate. Overall survival (OS) was defined as the time interval between the dates of diagnosis and death and was censored at the last follow-up date for patients who were alive. The probabilities of 3- and 5-year OS were estimated using the Kaplan–Meier method, and the differences in OS between subgroups of patients were assessed using two-sided log-rank tests. Patients with ypT0 status (pathological complete response in the primary tumor site) are not defined in the new AJCC staging system; therefore, we analyzed ypT0N0 and ypT0N1 groups separately in this study. Univariable and multivariable Cox proportional hazards regression models were used to assess the associations between patient characteristics and OS. Patient characteristics that were significant in the univariable models at the 0.20 level were included in the multivariable model. A p value less than 0.05 was considered statistically significant. Statistical analyses were performed using Stata software version 14.1 (StataCorp LP, College Station, TX, USA).
Results
We identified a total of 354 patients who met inclusion criteria. Patient and tumor demographics are summarized in Table 1; 142 (40%) were older than 65 years, 213 (60%) were male, and 196 (55%) were white. Seventy-eight (22%) patients had GEJ tumors, and the majority of patients had poorly differentiated histological grade (77%, 251/354). All patients had upper gastrointestinal endoscopy and CT scans, and the majority (93%, 329/354) of patients underwent EUS. In 284 (80%) patients, staging laparoscopy was performed before the initiation of preoperative therapy to exclude cM1 disease using a previously described procedure [6]. The majority of study patients completed planned preoperative therapy (94%; 332/354) and received chemoradiation therapy [75%, 265/354; generally 45 Gy with concurrent 5-fluorouracil (5-FU) or capecitabine] as a part of preoperative therapy, commonly after induction chemotherapy with a 5-FU-based regimen. One hundred ninety-three (54%) patients underwent total gastrectomy, 58 (16%) required a concomitant organ resection, and 304 (86%) underwent extended (D1+/D2) lymph node dissection. Eighteen (5%) patients were found to have ypM1 disease at the time of surgery; all 18 patients were found to have peritoneal carcinomatosis at either immediate or final pathological assessment. R0 resection was performed in 94% (316/336) of ypM0 patients.
Table 1.
Patient and tumor characteristics (n = 354)
| Variable | No. of patients (%) (n = 354) | |
|---|---|---|
| Age ≥65 years | 142 (40) | |
| Sex | Female | 141 (40) |
| Male | 213 (60) | |
| Race/ethnicity | White | 196 (55) |
| Black | 34 (10) | |
| Asian | 39 (11) | |
| Hispanic/Latino | 85 (24) | |
| Surgery year | 1995–2005 | 134 (38) |
| 2006–2015 | 220 (62) | |
| Histology grade (n = 328) | Well differentiated | 1 (0.3) |
| Moderately differentiated | 76 (23) | |
| Poorly differentiated | 251 (77) | |
| Signet-ring cells | Yes | 180 (51) |
| Linitis plastica | Yes | 21 (6) |
| Suspicious | 14(4) | |
| Location | GEJ | 78 (22) |
| Cardia/fundus | 36 (10) | |
| Body | 173 (49) | |
| Antrum | 67 (19) | |
| Type of resection | Total gastrectomy | 193 (54) |
| Subtotal gastrectomy | 144 (41) | |
| Proximal gastrectomy | 17 (5) | |
| Preoperative therapy | Yes | 354 (100) |
| Chemoradiation | 265 (75) | |
| Postoperative therapy | Yes | 56 (16) |
| Concomitant organ resection | Yes | 58 (16) |
| Pancreatectomy | 19 (5) | |
| Splenectomy | 20 (6) | |
| Hepatectomy | 25 (7) | |
| Colon/small bowel | 10 (3) | |
| Extent of lymph node (LN) dissection = D1+/D2 | Yes | 304 (86) |
| Number of LNs examined ≥16 | Yes | 259 (73) |
| Postoperative mortality <90 days | Yes | 9 (2.5) |
| cT category | 1a/1b | 0 (0) |
| 2 | 53 (15) | |
| 3/4a | 274 (77) | |
| 4b | 27 (8) | |
| cN category | Negative | 159 (45) |
| Positive | 195 (55) | |
| ypT category | 0 | 56 (16) |
| 1a/1b | 57 (16) | |
| 2 | 67 (19) | |
| 3 | 124 (35) | |
| 4a | 36 (10) | |
| 4b | 14 (4) | |
| ypN category | 0 | 202 (57) |
| 1 | 79 (22) | |
| 2 | 36 (10) | |
| 3 | 37 (10) | |
| ypM category | 0 | 336 (95) |
| 1 | 18 (5) | |
| Residual tumor | R0 | 316 (94) |
| [n = 336 (ypM0)] | R1 | 20 (6) |
Survival estimates by cStage and ypStage
The median follow-up duration was 2.9 years, and 167 (47%) patients had died at the last follow-up. Survival estimates by cStage and ypStage as determined by the Kaplan–Meier method are shown in Fig. 1a, b, respectively. Survival comparison by cStage was statistically significant as an entire model (p = 0.01), but differences in survival between cStages I and IIA (p = 0.505) and among cStages IIB, III, and IVA (p = 0.183) were not statistically significant (Fig. 1a, Table 2).
Fig. 1.
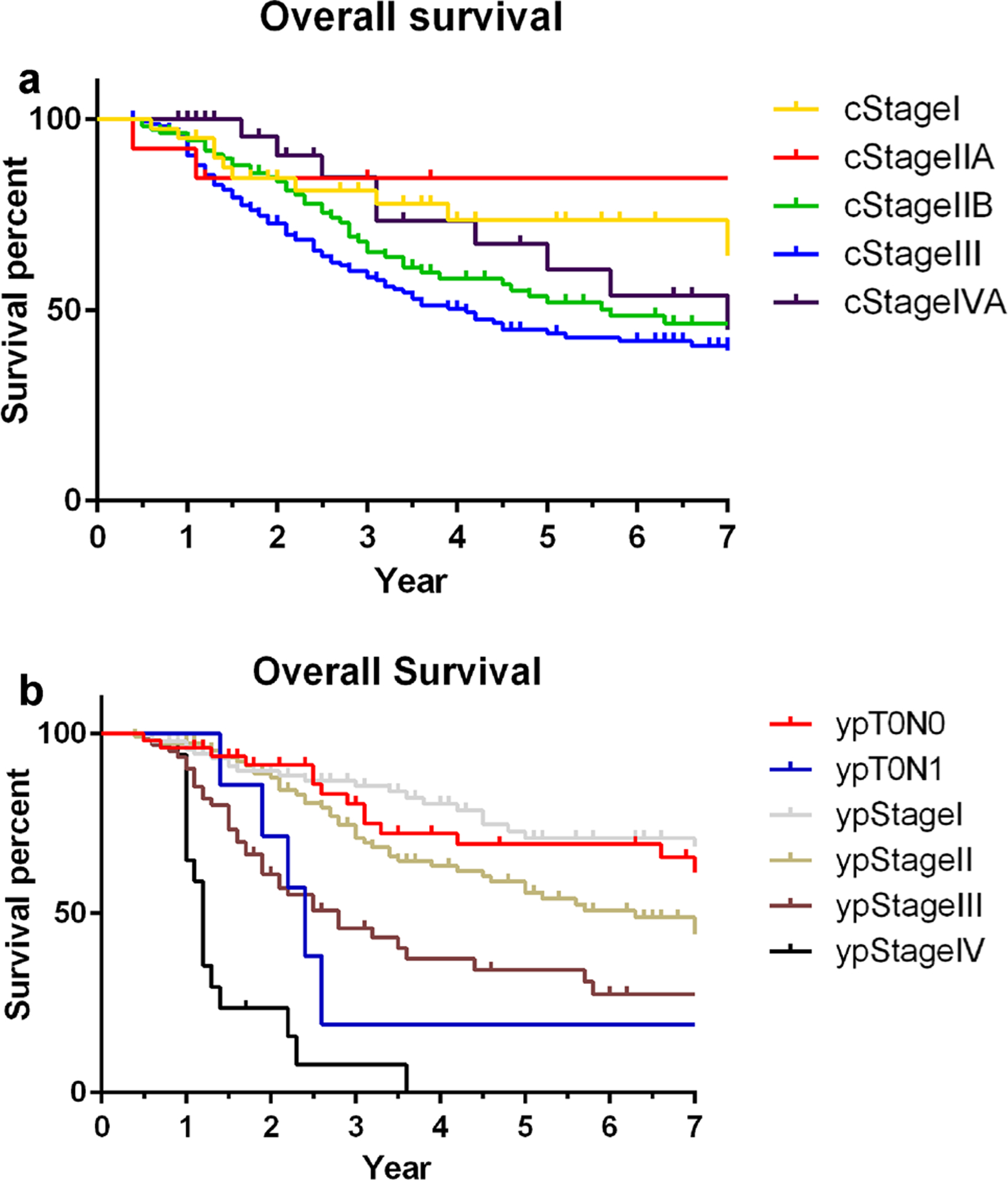
Kaplan–Meier estimates for overall survival (OS) by cTNM stage group (a) and ypTNM stage group (b)
Table 2.
Overall survival (OS) from diagnosis by cTNM stage group
| cStage | cT | cN | Number of patients (%) | Median OS, years | 3-year OS (%) | 5-year OS (%) | p value* |
|---|---|---|---|---|---|---|---|
| I | T2 | N0 | 40 (11) | 10.0 | 81 | 72 | |
| IIA | T2 | N+ | 13 (4) | Undefined | 84 | 82 | 0.505 |
| IIB | T3/4a | N0 | 112 (32) | 5.7 | 68 | 57 | |
| III | T3/4a | N+ | 162 (46) | 4.1 | 60 | 45 | |
| IVA | T4b | Any N | 27 (8) | 7.1 | 85 | 72 | 0.183 |
p values shown in the table are for comparison of survival between cStage I and IIA, and between cStage IIB, III, and IVA
Survival estimations by ypStage showed reasonable discrimination (p < 0.001; Fig. 1b, Table 3). Fifty-six (16%) patients had complete response in the primary tumor (ypT0). Although ypT0N0 patients (n = 49) had survival outcome as good as ypStage 0 patients, ypT0N1 patients (n = 7) had a substantially shorter survival (3-year survival, 23%; p value comparing survival of ypT0N0 and ypT0N1, 0.005). Patients who had ypM0 disease and underwent R1 resection (n = 20; 8 ypStage III, 10 ypStage II, and 2 ypStage I patients) had a short survival duration (median survival duration, 2.8 years), which was similar to or slightly worse than that of ypStage III patients, and ypM1 patients had a very short survival (median survival duration, 1.2 years).
Table 3.
Overall survival from diagnosis by ypTNM stage group
| ypStage | ypT | ypN | Patients, n | Median OS, years | 3-year OS | 5-year OS | p value* |
|---|---|---|---|---|---|---|---|
| 0 | Total | 56 (16%) | 10.0 | 73% | 64% | ||
| T0 | N0 | 49 | 10.1 | 82% | 70% | ||
| N1 | 7 | 2.4 | 23% | 23% | 0.005 | ||
| I | Total | 90 (25%) | 12.4 | 86% | 74% | ||
| T1 | N0 | 44 | 12.4 | 84% | 74% | ||
| N1 | 8 | 13.1 | 100% | 100% | |||
| T2 | N0 | 38 | 13.0 | 86% | 68% | 0.653 | |
| II | Total | 109 (31%) | 6.3 | 75% | 62% | ||
| T1 | N2 | 2 | Undefined | (100%) | (100%) | ||
| N3 | 2 | Undefined | (100%) | (100%) | |||
| T2 | N1 | 20 | 5.2 | 78% | 59% | ||
| N2 | 6 | Undefined | 80% | 80% | |||
| T3 | N0 | 44 | 7.8 | 82% | 81% | ||
| N1 | 29 | 3.1 | 61% | 36% | |||
| T4a | N0 | 6 | 3.4 | 82% | 27% | 0.007 | |
| III | Total | 61 (17%) | 2.8 | 46% | 35% | ||
| T2 | N3 | 1 | Undefined | (0%) | (0%) | ||
| T3 | N2 | 20 | 2.8 | 43% | 39% | ||
| N3 | 15 | 2.2 | 31% | 25% | |||
| T4a | N1 | 5 | Undefined | 80% | 80% | ||
| N2 | 4 | 3.2 | (71%) | (14%) | |||
| N3 | 7 | 1.7 | 43% | 29% | |||
| T4b | N0 | 6 | 1.6 | 40% | 40% | ||
| N1 | 2 | 2.8 | (33%) | (33%) | |||
| N2 | 0 | NA | NA | NA | |||
| N3 | 1 | Undefined | (0%) | (0%) | 0.032 | ||
| IV (M1) | Any T | Any N | 18 (5%) | 1.2 | 8% | 0% | |
| M0 R1 | Any T | Any N | 20 (6%) | 2.8 | 50% | 22% |
Survival percentages enclosed in parentheses indicate the numbers were estimated based on more than five cases. p values <0.05 are in bold font
p values shown in the table are for comparison of survival within each ypStage group
Analyses of prognostic factors for overall survival
Results of univariable and multivariable analyses of prognostic factors for overall survival are summarized in Table 4. The following factors were associated with overall survival by multivariable analysis after adjustment for ypStage: Asian race [hazard ratio (HR), 0.52, 95% confidence interval (CI), 0.30–0.93; p = 0.028], linitis plastica (HR, 1.66, 95% CI, 1.03–2.69; p = 0.037), and R1 resection (HR, 1.91, 95% CI, 1.13–3.23; p = 0.016). Concomitant organ resection (HR, 1.45, 95% CI, 0.99–2.11; p = 0.057) and examination of fewer than 16 lymph nodes (HR, 1.38, 95% CI, 0.98–1.95; p = 0.061) showed a trend toward shorter survival. Survival for patients with ypT0N0 did not differ from that of ypStage I patients (HR, 1.29, 95% CI, 0.73–2.28; p = 0.377), whereas survival for patients with y pT0N1 was significantly shorter than that of ypStage I patients (HR, 4.34, 95% CI, 1.63–11.6; p = 0.003).
Table 4.
Univariable and multivariable Cox analysis of overall survival (OS)
| Variable | Univariable | Multivariable | |||
|---|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | ||
| Age at diagnosis | ≥65 vs. <65 years | 1.35 (0.99–1.85) | 0.058 | 1.30 (0.93–1.84) | 0.127 |
| Sex | Female vs. male | 0.82 (0.60–1.13) | 0.221 | ||
| Race/ethnicity | White (ref) | ||||
| Black | 1.24 (0.73–2.10) | 0.430 | 1.31 (0.75–2.28) | 0.341 | |
| Asian | 0.57 (0.33–1.00) | 0.052 | 0.52 (0.30–0.93) | 0.028 | |
| Hispanic | 0.89 (0.61–1.29) | 0.537 | 0.86 (0.58–1.27) | 0.444 | |
| Location | GEJ vs. other | 1.20 (0.85–1.69) | 0.307 | ||
| Histology grade | Poorly vs. well/moderately differentiated | 1.08 (0.73–1.61) | 0.691 | ||
| Signet-ring cell | Yes vs. no | 1.10 (0.81–1.49) | 0.548 | ||
| Linitis | Yes or suspicious | 2.27 (1.47–3.51) | <0.001 | 1.66 (1.03–2.69) | 0.037 |
| ypStage | T0N0 | 1.24 (0.71–2.18) | 0.452 | 1.29 (0.73–2.28) | 0.377 |
| T0N1 | 4.08 (1.57–10.6) | 0.004 | 4.34 (1.63–11.6) | 0.003 | |
| 1 (ref) | |||||
| 2 | 1.71 (1.09–2.67) | 0.019 | 1.78 (1.11–2.83) | 0.016 | |
| 3 | 3.36 (2.10–5.38) | <0.001 | 3.42 (2.08–5.60) | <0.001 | |
| 4 | 13.83 (7.30–26.2) | <0.001 | 13.11 (6.60–26.0) | <0.001 | |
| Concomitant organ resection | Yes vs. no | 1.63 (1.13–2.35) | 0.009 | 1.45 (0.99–2.11) | 0.057 |
| R1 resection | Yes vs. no | 3.05 (1.90–4.89) | <0.001 | 1.91 (1.13–3.23) | 0.016 |
| Preoperative XRT | Yes vs. no | 0.95 (0.66–1.37) | 0.801 | ||
| Adjuvant therapy | Yes vs. no | 0.90 (0.58–1.40) | 0.648 | ||
| Number of LNs examined <16 | Yes vs. no | 1.25 (0.91–1.72) | 0.169 | 1.38 (0.98–1.95) | 0.061 |
p values <0.05 are in bold font
Survival in ypT0N1
Because ypT0N1 patients had diminished survival as compared to ypStage I patients by multivariable Cox regression analysis, additional assessments were conducted to compare tumor characteristics among ypT0N0, ypT0N1, and ypStage I groups (Table 5). ypT0N1 patients had higher rates of concomitant organ resection (57%; p = 0.008), cN-positive disease (86%; p = 0.035), and use of preoperative chemoradiation therapy (100%; p = 0.019) than other groups; these results indicated that ypT0N1 patients had more advanced tumors at presentation. Of note, ypT1N1 patients (n = 8) in ypStage I group had excellent survival.
Table 5.
Tumor characteristics in ypT0N0, ypT0N1, and ypStage I patients
| Variable | ypT0N0 | ypT0N1 | ypStage I | p value* |
|---|---|---|---|---|
| n = 49 | n = 7 | n = 90 | ||
| N (%) | N (%) | N (%) | ||
| Age ≥65 | 24 (49) | 2 (29) | 31 (34) | 0.177 |
| GEJ tumor | 12 (24) | 3 (43) | 21 (23) | 0.502 |
| Linitis, yes or suspicious | 2 (4) | 0 (0) | 3 (3) | |
| cN positive | 29 (59) | 6 (86) | 40 (43) | 0.035 |
| cT stage | 0.612 | |||
| cT2 | 6 (12) | 1 (14) | 18 (20) | |
| cT3/4a | 37 (76) | 6 (86) | 68 (74) | |
| cT4b | 6(12) | 0 (0) | 6 (7) | |
| Preoperative chemoradiation | 47 (96) | 7 (100) | 73 (79) | 0.019 |
| Concomitant organ resection | 6(12) | 4 (57) | 9 (10) | 0.008 |
| Lymph nodes examined ≥16 | 35 (71) | 2 (29) | 61 (66) | 0.084 |
p values <0.05 are in bold font
GEJ gastroesophageal junction
Fisher’s exact test
Discussion
In this single-institutional retrospective analysis of gastric cancer patients who underwent preoperative therapy and attempted curative-intent surgery, ypStage effectively predicted survival whereas cStage did not. Our findings suggest that ypT0N0 should be considered for inclusion in the ypStage I group, but the shorter survival observed in ypT0N1 patients needs further study to define the best category for staging. This study also showed important findings for prognostic factors for survival; patients of Asian race had a remarkably improved survival that was associated with a shorter survival after adjustment for ypStage, and if fewer than 16 lymph nodes were examined, a trend was seen toward shorter survival.
After a few large randomized controlled studies showed the survival benefit of preoperative therapy for gastric cancer [7, 8], its use has increased; it was used in more than 50% of gastric cancer patients who were treated at US academic centers in 2012 [9]. Although the best regimen is still under investigation [10, 11], this trend of increased use of preoperative treatment raised the question of whether we can apply the same pathological staging system to patients who do or do not have preoperative therapy. Although further modification may be necessary, the concept of ypStage is novel in the AJCC 8th edition staging system for gastric cancer. This study revealed that the new ypStage effectively predicted survival of patients who underwent preoperative therapy and remained significant after adjustment for other prognostic factors. There was some heterogeneity in survival among the ypTNM groups within each ypStage, which may need to be improved in future updates to the system. For example, ypT3N0 patients likely have better survival than do ypT3N1 patients (median survival in this study, 7.8 vs. 3.1 years, respectively; 3-year OS, 82% vs. 61%), although both ypT3N0 and ypT3N1 are included in ypStage II (Table 3). The AJCC 8th edition staging system also is incomplete in that stages for patients who had a complete pathological response in the primary tumor (ypT0) are not defined. In a previous report from a multi-institutional phase II trial, pathological complete response was found in 26% of patients with localized gastric cancer following induction chemotherapy and chemoradiation therapy [12]. In the current study, ypT0N0 patients did not have better survival than ypStage I patients. The challenge of the results of this study is how we can interpret the poor survival observed in ypT0N1 patients. Theoretically, ypT0N1 cannot be categorized higher than ypStage I, because ypT1N1 is defined as the ypStage I group and showed excellent survival in the current study despite the small number of patients (n = 8). Based on comparisons of tumor characteristics (Table 5), the poor survival in the ypT0N1 group was thought to be mainly derived from advanced tumor status at presentation, which frequently required concomitant organ resections. A higher rate of chemoradiation therapy use may have contributed to the enhanced treatment effect in the primary tumor in the ypT0N1 group.
In esophageal cancer, where preoperative chemoradiation therapy is more commonly used, there are several reports indicating diminished survival associated with persistent ypN-positive status after preoperative therapy. Kim et al. reported that survival of ypT0N1 patients was similar to that of stage II patients rather than that of stage I patients [13]. Zanoni et al. reported that cN-positive status was associated with diminished survival among patients with ypN0 status, indicating that downstaged ypStage does not guarantee survival similar to that of ypStage without downstaging [14]. Verlato et al. also reported that persistent ypN-positive status was associated with poor survival, regardless of pathological response in the primary tumor (tumor regression grade) [15]. These esophageal cancer data highlight the fact that the 8th edition of the AJCC esophageal cancer staging manual categorizes T0–T2N1 diseases in ypStage IIIA group [16]. In the current study, the impact of ypN1 status did not appear as robust as reported in the aforementioned reports of esophageal cancer. When comparing 3-year OS of ypT2N0 versus ypT2N1 (86% vs. 78%) and ypT3N0 vs. ypT3N1 (82% vs. 61%), the impact of ypN1 status was modest, and ypT stage seemed equally or more important in survival prediction in gastric cancer. However, the impact of persistent ypN-positive status may be different in ypT0–1 patients. Further accumulation of survival data with accurate ypStage information is necessary to validate and improve the current ypStaging system for gastric cancer. Based on the results of the current study, it is important to not underestimate the survival risk of ypT0N1 (persistent nodal disease) patients, particularly in patients who have advanced cStage tumors.
In contrast to reasonable survival discrimination by ypStage, we observed poor survival discrimination by cStage. Two main factors contribute to the difficulty of survival estimation before preoperative therapy. First, the accuracy of clinical staging is suboptimal, according to reports from Western countries. EUS is the mainstay method to estimate cT stage in Western countries; however, reports of EUS staging accuracy have shown significant heterogeneity [17–20]. The reported accuracy of EUS is worse in Western reports, particularly in those from the United States [2–4], than in Eastern reports [21]. Accuracy of cN staging is even less accurate [19]. Second, and more importantly, survival of gastric cancer patients is significantly affected by response to preoperative therapy [22–24], and we cannot effectively predict response to therapy at the time of diagnosis [24]. In the current study, 88% of patients (49/56) among those who had pathological complete response in the primary tumor (ypT0) had cT3–4b disease and 63% (35/56) had cN-positive disease. Clinical staging should be used for patient selection for preoperative therapy with consideration of the inaccuracy of staging methods, and physicians should be aware of the uncertainty of cStage for survival prediction to avoid providing misleading information to their patients.
Prognostic factors after preoperative therapy in gastric cancer patients have only been described in a limited number of reports [24, 25]. In our previous report of a smaller number of patients (n = 192), we did not include pStage grouping in the final model of multivariable analysis [26]. In the current study, we confirmed that known prognostic factors remained significant, or very close to statistical significance, after preoperative therapy (i.e., linitis plastica, concomitant organ resection, and R1 resection), whereas other factors were found not to be risk factors after preoperative therapy (i.e., histological grade, signet-ring cells, and tumor location at the GEJ) after adjustment for ypStage. The retrospective nature of this study and the inherent selection bias indicate that it is not appropriate to assess the benefit of adding preoperative radiation therapy and adjuvant chemotherapy, which were not statistically significant for survival in this study. Inclusion of Siewert type III GEJ tumors in the gastric cancer staging system seems to be reasonable among patients who undergo preoperative therapy, because there was no difference in survival by tumor location (GEJ vs. other) in this study. The absence of survival impact from histological grade and signet-ring cells in this study supports the well-accepted approach of using preoperative therapy for more aggressive tumors. Another notable finding of this study was that Asian patients had a better survival than white patients after adjustment for other prognostic factors. Although preoperative therapy is not commonly used in Asian countries, where upfront gastrectomy and selective use of adjuvant therapy constitute the standard approach [27], this finding indicates a potential role for preoperative therapy in Asian countries as well. These risk factors associated with diminished survival observed in this study should be considered in conjunction with ypStage.
The number of lymph nodes examined, if fewer than 16, showed a trend toward shorter survival after adjustment for ypStage. This finding supports the recommendation by AJCC that at least 16 regional nodes be removed/assessed pathologically. Although most of the patients in the current study underwent D1+/D2 lymph node dissection, for only 85% of them (259/304) were 16 or more lymph nodes examined. The number of lymph nodes examined in pathological analysis is affected by multiple factors, including patient factor (the number of lymph nodes patients actually have), disease factor (advanced or inflamed disease causes lymph node enlargement), preoperative treatment factor (treatment response may cause shrinkage of lymph nodes), surgical factor (how extensively lymph nodes are dissected), and pathological assessment factor (how carefully pathologists examine the specimen). Among these factors, the pathological assessment factor may be the most responsible for the small number of lymph nodes examined in Western countries [28]. Because location of positive lymph nodes does not contribute to pStage of gastric cancer with recent AJCC staging systems (since its 5th edition) [29], gastric cancer lymph node specimens are typically sent to pathology en bloc in the majority of US institutions, and pathologists or their assistants identify the lymph nodes, generally after formalin fixation. A recent report from Memorial Sloan Kettering Cancer Center showed improvement in number of lymph nodes examined by conducting “ex vivo lymphadenectomy,” which partially employed the Japanese or Korean style back table procedure that divided lymph node specimens into stations before sending them to pathology [30]. At MD Anderson, we are currently conducting a quality improvement project to improve the quality of pathological assessment of gastric cancer specimens, and continued work is warranted to improve gastric cancer staging in the Western countries. The actual survival benefit of D2 lymph node dissection remains unclear, particularly if performed after modern preoperative chemoradiation therapy, but D2 lymph node dissection with thorough assessment of the resected specimen is recommended for accurate staging and better survival prediction.
This study has some limitations. The retrospective nature of the study has an inherent selection bias for treatment; for example, patients with advanced tumors may have received more aggressive postoperative therapy, which may have affected survival. Although this was the largest series of gastric cancer patients who underwent preoperative therapy, some of the ypTNM groups had small numbers of patients, which made it difficult to evaluate the validity of ypStage for those groups. Particularly in ypT0N1 patients who had poor survival and ypT1N1 patients who had excellent survival, careful interpretation of those results is necessary. The results of cStage evaluation are limited by the accuracy of EUS and CT [3, 17–20], although the patients in our cohort are rigorously evaluated with high-quality imaging and EUS performed by experienced endoscopists. ypM1 patients in this study represent those who were found to have peritoneal carcinomatosis upon surgery, which may not represent more heterogeneous ypStage IV patients. Some heterogeneity in preoperative therapy regimen (e.g., difference in chemotherapy, use and type of radiation therapy, treating institution) may have affected the results. The strengths of this study include the large number of patients and consistent data from a single large cancer center where the treatment and surgery are standardized. This study is the first study to examine prognostic factors of gastric cancer patients after preoperative therapy with the current AJCC staging system (cStage and ypStage), and we believe this report will contribute to future improvement of postneoadjuvant therapy staging for gastric cancer patients. In addition, improved survival in Asian patients highlights an area of active controversy regarding the use of preoperative therapy for Asian patients.
In conclusion, the AJCC 8th edition staging system for gastric cancer demonstrated a reasonable survival prediction by ypStage, although cStage did not effectively predict survival in this single-institutional retrospective study of gastric cancer patients who were treated with preoperative therapy. ypT0 patients, which category is not defined in the AJCC 8th edition, did not have better survival than ypStage I patients; therefore, physicians should not underestimate the survival risk of ypT0 patients, particularly in ypT0N1 patients. These risk factors associated with diminished survival observed in this study should be considered in conjunction with ypStage. Finally, thorough lymph node assessment, even after preoperative therapy, remains important for accurate survival prediction.
Funding
Supported in part by the NIH/NCI under award number P30CA016672 and used the Clinical Trials Support Resource.
Footnotes
Conflict of interest The authors have no conflicts of interest to disclose.
Ethical standards All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- 1.Amin MB, Edge SB, Greene FL, et al. , editors. AJCC cancer staging manual. 8th ed. New York: Springer; 2017. [Google Scholar]
- 2.Spolverato G, Ejaz A, Kim Y, Squires MH, Poultsides GA, Fields RC, et al. Use of endoscopic ultrasound in the preoperative staging of gastric cancer: a multi-institutional study of the US gastric cancer collaborative. J Am Coll Surg. 2015;220(1):48–56. doi: 10.1016/j.jamcollsurg.2014.06.023. [DOI] [PubMed] [Google Scholar]
- 3.Fairweather M, Jajoo K, Sainani N, Bertagnolli MM, Wang J. Accuracy of EUS and CT imaging in preoperative gastric cancer staging. J Surg Oncol. 2015;111(8):1016–20. doi: 10.1002/jso.23919. [DOI] [PubMed] [Google Scholar]
- 4.Bentrem D, Gerdes H, Tang L, Brennan M, Coit D. Clinical correlation of endoscopic ultrasonography with pathologic stage and outcome in patients undergoing curative resection for gastric cancer. Ann Surg Oncol. 2007;14(6):1853–9. doi: 10.1245/s10434-006-9037-5. [DOI] [PubMed] [Google Scholar]
- 5.Badgwell B Multimodality therapy of localized gastric adenocarcinoma. J Natl Compr Cancer Netw. 2016;14(10):1321–7. [DOI] [PubMed] [Google Scholar]
- 6.Ikoma N, Blum M, Chiang YJ, Estrella JS, Roy-Chowdhuri S, Fournier K, et al. Yield of staging laparoscopy and lavage cytology for radiologically occult peritoneal carcinomatosis of gastric cancer. Ann Surg Oncol. 2016;23(13):4332–7. doi: 10.1245/s10434-016-5409-7. [DOI] [PubMed] [Google Scholar]
- 7.Ychou M, Boige V, Pignon JP, Conroy T, Bouche O, Lebreton G, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29(13):1715–21. doi: 10.1200/JCO.2010.33.0597. [DOI] [PubMed] [Google Scholar]
- 8.Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355(1):11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 9.Greenleaf EK, Hollenbeak CS, Wong J. Trends in the use and impact of neoadjuvant chemotherapy on perioperative outcomes for resected gastric cancer: evidence from the American College of Surgeons National Cancer Database. Surgery (St. Louis) 2016;159(4):1099–112. doi: 10.1016/j.surg.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 10.Leong T, Smithers BM, Michael M, Gebski V, Boussioutas A, Miller D, et al. TOPGEAR: a randomised phase III trial of perioperative ECF chemotherapy versus preoperative chemoradiation plus perioperative ECF chemotherapy for resectable gastric cancer (an international, intergroup trial of the AGITG/TROG/EORTC/NCIC CTG). BMC Cancer. 2015;15:532. doi: 10.1186/s12885-015-1529-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dikken JL, van Sandick JW, Swellengrebel HAM, Lind PA, Putter H, Jansen EP, et al. Neo-adjuvant chemotherapy followed by surgery and chemotherapy or by surgery and chemoradiotherapy for patients with resectable gastric cancer (CRITICS). BMC Cancer. 2011;11:329. doi: 10.1186/1471-2407-11-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ajani JA, Winter K, Okawara GS, Donohue JH, Pisters PW, Crane CH, et al. Phase II trial of preoperative chemoradiation in patients with localized gastric adenocarcinoma (RTOG 9904): quality of combined modality therapy and pathologic response. J Clin Oncol. 2006;24(24):3953–8. doi: 10.1200/JCO.2006.06.4840. [DOI] [PubMed] [Google Scholar]
- 13.Kim MP, Correa AM, Lee J, Rice DC, Roth JA, Mehran RJ, et al. Pathologic T0N1 esophageal cancer after neoadjuvant therapy and surgery: an orphan status. Ann Thorac Surg. 2010;90(3):884–90. doi: 10.1016/j.athoracsur.2010.03.116 (discussion 90–91). [DOI] [PubMed] [Google Scholar]
- 14.Zanoni A, Verlato G, Giacopuzzi S, Motton M, Casella F, Weindelmayer J, et al. ypN0: does it matter how you get there? Nodal downstaging in esophageal cancer. Ann Surg Oncol. 2016;23(suppl 5):998–1004. doi: 10.1245/s10434-016-5440-8. [DOI] [PubMed] [Google Scholar]
- 15.Verlato G, Zanoni A, Tomezzoli A, Minicozzi A, Giacopuzzi S, Di Cosmo M, et al. Response to induction therapy in oesophageal and cardia carcinoma using Mandard tumour regression grade or size of residual foci. Br J Surg. 2010;97(5):719–25. doi: 10.1002/bjs.6949. [DOI] [PubMed] [Google Scholar]
- 16.Rice TW, Ishwaran H, Ferguson MK, Blackstone EH, Goldstraw P. Cancer of the esophagus and esophagogastric junction: an eighth edition staging primer. J Thorac Oncol. 2017;12(1):36–42. doi: 10.1016/j.jtho.2016.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mocellin S, Marchet A, Nitti D. EUS for the staging of gastric cancer: a meta-analysis. Gastrointest Endosc. 2011;73(6):1122–34. doi: 10.1016/j.gie.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 18.Kwee RM, Kwee TC. The accuracy of endoscopic ultrasonography in differentiating mucosal from deeper gastric cancer. Am J Gastroenterol. 2008;103(7):1801–9. doi: 10.1111/j.1572-0241.2008.01923.x. [DOI] [PubMed] [Google Scholar]
- 19.Kwee RM, Kwee TC. Imaging in local staging of gastric cancer: a systematic review. J Clin Oncol. 2007;25(15):2107–16. doi: 10.1200/JCO.2006.09.5224. [DOI] [PubMed] [Google Scholar]
- 20.Cardoso R, Coburn N, Seevaratnam R, Sutradhar R, Lourenco LG, Mahar A, et al. A systematic review and meta-analysis of the utility of EUS for preoperative staging for gastric cancer. Gastric Cancer. 2012;15(suppl 1):S19–26. doi: 10.1007/s10120-011-0115-4. [DOI] [PubMed] [Google Scholar]
- 21.Mocellin S, Pasquali S. Diagnostic accuracy of endoscopic ultrasonography (EUS) for the preoperative locoregional staging of primary gastric cancer. Cochrane Database Syst Rev. 2015;2:CD009944. doi: 10.1002/14651858.CD009944.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lowy AM, Mansfield PF, Leach SD, Pazdur R, Dumas P, Ajani JA. Response to neoadjuvant chemotherapy best predicts survival after curative resection of gastric cancer. Ann Surg. 1999;229(3):303–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Becker K, Mueller JD, Schulmacher C, Ott K, Fink U, Busch R, et al. Histomorphology and grading of regression in gastric carcinoma treated with neoadjuvant chemotherapy. Cancer (Phila). 2003;98(7):1521–30. doi: 10.1002/cncr.11660. [DOI] [PubMed] [Google Scholar]
- 24.Blackham AU, Greenleaf E, Yamamoto M, Hollenbeak C, Gusani N, Coppola D, et al. Tumor regression grade in gastric cancer: predictors and impact on outcome. J Surg Oncol. 2016;114(4):434–9. doi: 10.1002/jso.24307. [DOI] [PubMed] [Google Scholar]
- 25.Mansour JC, Tang L, Shah M, Bentrem D, Klimstra DS, Gonen M, et al. Does graded histologic response after neoadjuvant chemotherapy predict survival for completely resected gastric cancer? Ann Surg Oncol. 2007;14(12):3412–8. doi: 10.1245/s10434-007-9574-6. [DOI] [PubMed] [Google Scholar]
- 26.Badgwell B, Blum M, Estrella J, Chiang YJ, Das P, Matamoros A, et al. Predictors of survival in patients with resectable gastric cancer treated with preoperative chemoradiation therapy and gastrectomy. J Am Coll Surg. 2015;221(1):83–90. doi: 10.1016/j.jamcollsurg.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 27.Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357(18):1810–20. doi: 10.1056/NEJMoa072252. [DOI] [PubMed] [Google Scholar]
- 28.Noguchi M, Stamey TA, McNeal JE, Yemoto CE. Assessment of morphometric measurements of prostate carcinoma volume. Cancer (Phila). 2000;89(5):1056–64. [DOI] [PubMed] [Google Scholar]
- 29.Fleming IDCJ Henson DE. Manual for staging of cancer American Joint Committee on Cancer (AJCC). 5th ed. Philadelphia: Lippincott; 1997. [Google Scholar]
- 30.Afaneh C, Levy A, Selby L, Ku G, Tang L, Yoon SS, et al. Ex vivo lymphadenectomy during gastrectomy for adenocarcinoma optimizes lymph node yield. J Gastrointest Surg. 2016;20(1):165–71. doi: 10.1007/s11605-015-2948-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


