Abstract
Marine actinomycetes were the main origin of marine natural products in the past forty years. This review was to present the sources, structures and antimicrobial activities of 313 new natural products from marine actinomycetes reported from 1976 to 2019.
Keywords: Marine actinomycetes, Marine natural products, Chemical structures, Antimicrobial bioactivities
Introduction
Marine actinomycetes were the major resource of marine natural products owing to their chemical structures and diverse bioactivities. According to a statistic analysis of marine microbial natural products from 2010 to 2013, marine-derived actinomycetes accounted for 28% (= 253/895) of new marine natural products isolated from microbial origin (Zhao et al. 2013). This review covered the sources, structures and antimicrobial activities of 313 compounds derived from marine actinomycetes reported from 1976 to 2019. These new antimicrobial compounds have diverse chemical structures including polyketides, nitrogen-containing compounds, sterols and terpenoids. Majority of these compounds were antibacterial natural products, which consisted of 87% of the new marine natural products from marine-derived actinomycetes.
Antimicrobial compounds from Streptomyces species
Antimicrobial compounds from Streptomyces sp. associated with sponges
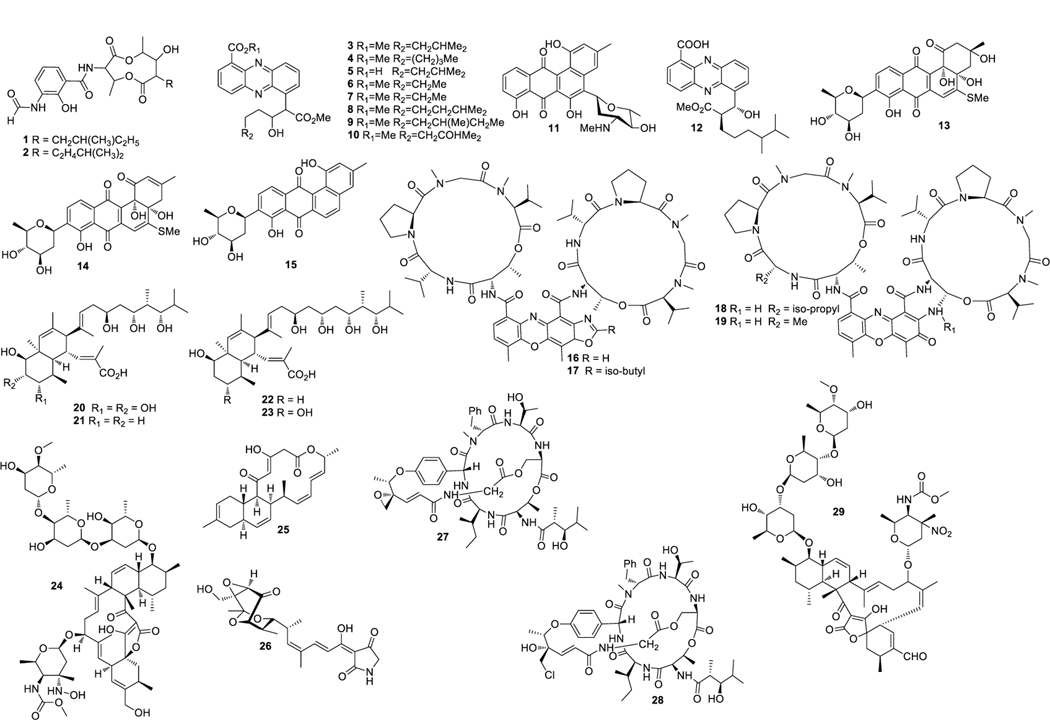
Urauchimycins A and B (1 and 2) (Fig. 1) were isolated from Streptomyces sp. Ni-80. Compounds 1 and 2 exhibited antifungal activity against Candida albicans at 10 μg/mL (Imamura et al. 1993). Eight new antibacterial streptophenazines A–H (3‒10) were obtained from Streptomyces sp. HB202 (Mitova et al. 2008). These compounds showed broad spectrum of inibitory activity against bacterial strains with MIC values ranging from 15.6 to 62.5 μg/mL (Mitova et al. 2008). Mayamycin (11) exhibited antibacterial activity with MIC values ranging from 2.5 to 8.4 μg/mL (Schneemann et al. 2010). Streptophenazine K (12) was isolated from Streptomyces HB202, which showed antibacterial activity against B. subtilis and S. epidermidis with MIC values of 21.6 and 14.5 μM, respectively (Kunz et al. 2014). Streptomyces sp. BCC45596 yielded urdamycinone E (13), urdamycinone G (14), and dehydroxyaquayamycin (15), which were active against M. tuberculosis with MIC values of 3.13, 12.50 and 6.25 μg/mL, respectively (Supong et al. 2012). Jiao et al. isolated four new compounds from Streptomyces sp. LHW52447, namely actinomycins D1−D4 (16–19), which displayed inhibitory activity against S. aureus (MRSA) with MIC values ranging from 0.125 to 1.0 μg/mL (Jiao et al. 2018).
Fig. 1.
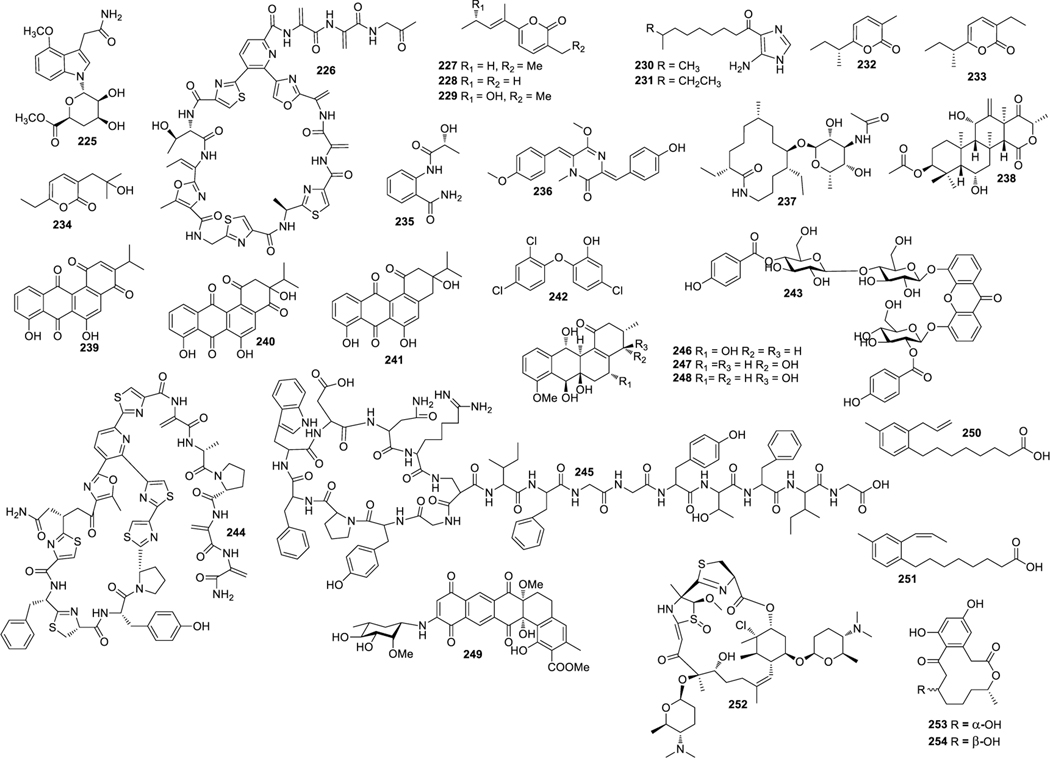
Structures of compounds 1–29
Antimicrobial compounds from Streptomyces sp. associated with corals
Four nahuoic acids B–E (20–23) were isolated from Streptomyces sp. SCSGAA 0027, which exhibited weak antibiofilm activity against Shewanella onedensis MR-1 biofilm (Nong et al. 2016). Streptomyces sp. M-207 produced lobophorin K (24), which inhibited S. aureus EPI167 (MRSA) with an MIC90 value in the range of 40−80 μg/mL (Braña et al. 2017a). Anthracimycin B (25) was obtained from a culture of Streptomyces cyaneofuscatus M-169, which displayed antimicrobial activity against S. aureus MRSA (MB5393), S. aureus MSSA (ATCC 29213), E. faecium VANS (CL144754) and E. faecalis VANS (CL144492) with MICs below the lowest concentration tested at 0.03 μg/mL and inhibited M. tuberculosis (H37Ra) with an MIC value of 1−2 μg/mL (Rodríguez et al. 2018). Isotirandamycin B (26) was isolated from a culture of Streptomyces sp. SCSIO 41399, which displayed antimicrobial activity against Streptococcus agalactiae with an MIC value of 11.5 μM (Cong et al. 2019).
Antimicrobial compounds from Streptomyces sp. associated with other marine animals
S. hygroscopicus yielded salinamides A (27) and B (28). Both compounds were active against S. pneumoniae with an equal MIC value of 4 μg/mL. Both compounds were also active against S. pyrogenes with MIC values of 4 and 2 μg/mL, respectively (Trischman et al. 1994). Streptomyces sp. 1053U.I.1a.3b produced lobophorin I (29), which exhibited inhibitory activity against M. tuberculosis and B. subtilis with MIC values of 2.6 and 10.6 μM, respectively (Lin et al. 2014). Salinamide F (30) (Fig. 2) obtained from Streptomyces sp. CNB091, had a broad spectrum of antibacterial activity (Hassan et al. 2015). Streptoseomycin (31) was isolated from Streptomyces seoulensis A01, which exhibited inhibitory activityagainst H. pylori, Lactobacillus acidophilus, Bifidobacterium bifidum, Eubacterium brachy, Propionibacterium acnes, S. aureus, Micrococcus luteus and B. subtilis with MIC values ranging from 2 to 64 μg/mL (Zhang et al. 2018a).
Fig. 2.
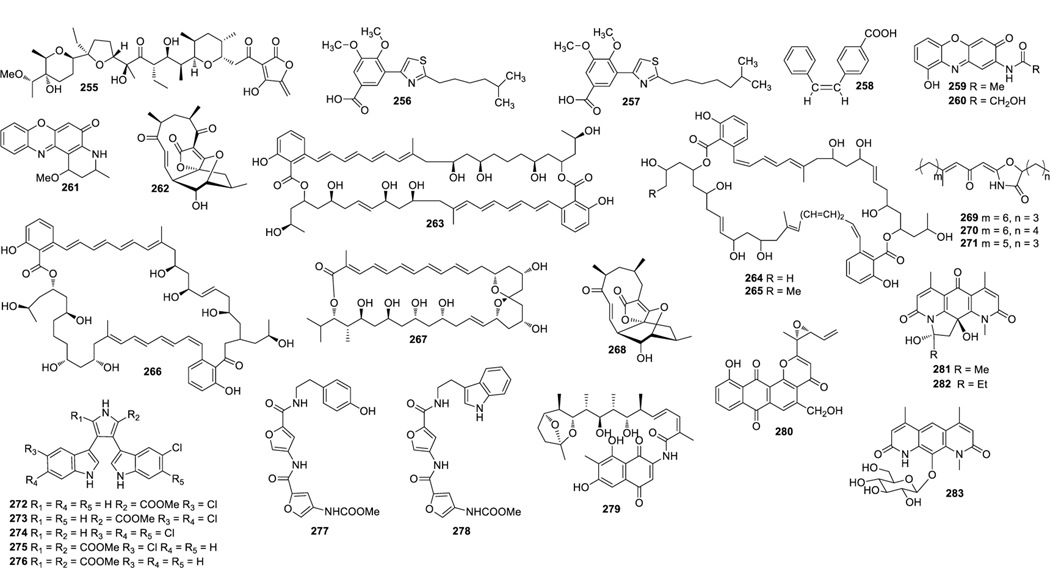
Structures of compounds 30–57
Antimicrobial compounds from Streptomyces sp. associated with marine algae
Bisanthraquinone derivatives A−C (32‒34), were isolated from Streptomyces sp. N1–78-1, which displayed antimicrobial activity against MRSA with IC50 values of 0.15, 0.36 and 31 μM, respectively (Socha et al. 2006). 2-Hydroxy-5-((6-hydroxy-4-oxo-4H-pyran-2-yl) methyl)-2-propylchroman-4-one (35) was obtained from Streptomyces sp. WR1L1S8, which showed antibacterial activity against E. coli ATCC 25922 and MRSA ATCC 43300 with MIC values of 16 and 2 μM, respectively (Djinni et al. 2013). Braña et al. isolated desertomycin G (36) from Streptomyces althioticus MSM3, which exhibited inhibitory activity against a wide spectrum of bacterial strains, with MIC values ranging from 4 to 64 μg/mL (Braña et al. 2019).
Antimicrobial compounds from Streptomyces sp. associated with mangrove
Divergolides A–D (37–40), were isolated from a culture of Streptomyces sp. HKI0576, which displayed antimicrobial activity against B. subtilis, Mycobacterium vaccae and MRSA with inhibition zone diameters of 10−20 mm (Ding et al. 2011a). Xiamycin B (41), indosespene (42), and sespenine (43) were obtained from Streptomyces sp. HKI0595, which exhibited antibacterial activity against MRSA (Ding et al. 2011b). Kandenols A–E (44‒48) were isolated from Streptomyces sp. HKI0595, which showed weak antimicrobial activity against B. subtilis ATCC 6633 and Mycobacterium vaccae IMET 10670 (Ding et al. 2012). Antimycin B2 (49) was discovered from S. lusitanus XM52, which displayed antibacterial activity against S. aureus and L. hongkongensis with MIC values of 32 and 8 μg/mL, respectively (Han et al. 2012).
Antimicrobial compounds from Streptomyces sp. associated with other plants
Streptomyces sp. MA-12 yielded 7,3’-di-(γ, γ-dimethylallyloxy)-5-hydroxy-4’-methoxyflavone (50). Compound 50 was active against C. musae, G. zeae (Schweinitz) Petch, and P. citrinum at 0.25 mM with inhibition zone diameters of 12.7, 13.00 and 12.17 mm, respectively (Ding et al. 2013). Juanlimycin A (51) was isolated from a culture of Streptomyces sp. LC6, which showed moderate inhibition on the secretion of Salmonella Pathogenicity Island-1 effectors, SipA/B/C/D (Zhang et al. 2014).
Antimicrobial compounds from Streptomyces sp. from marine sediments
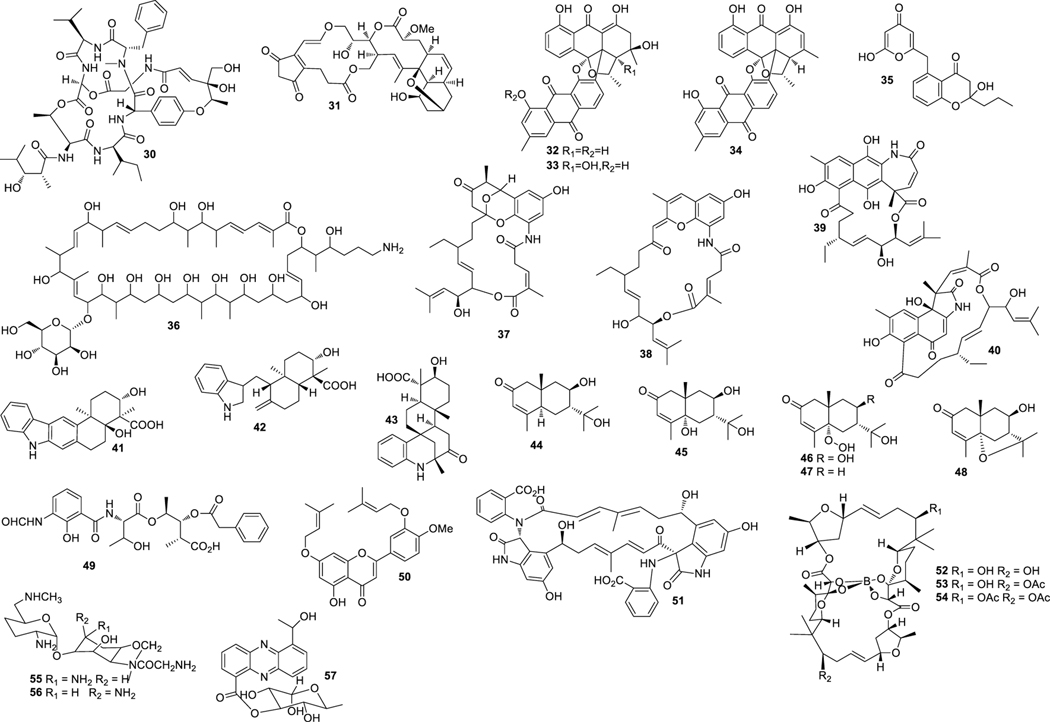
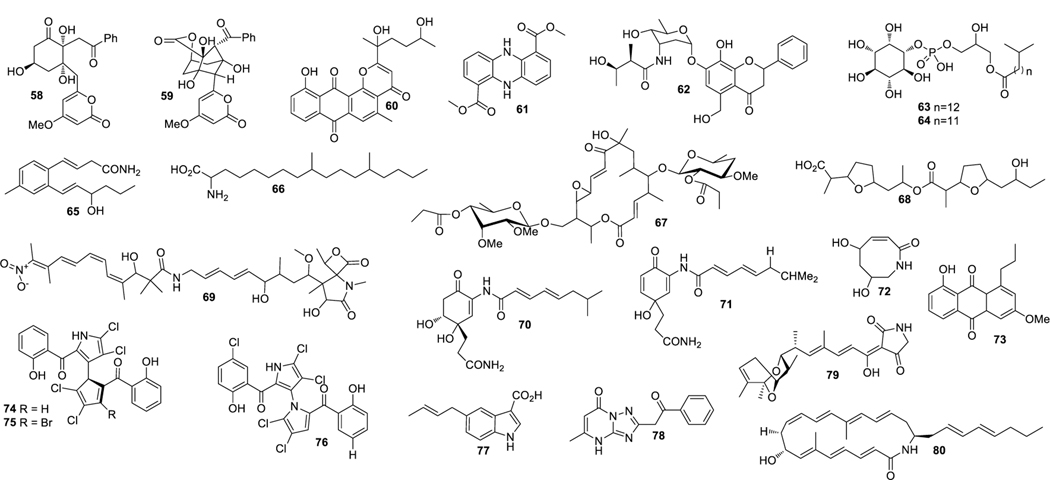
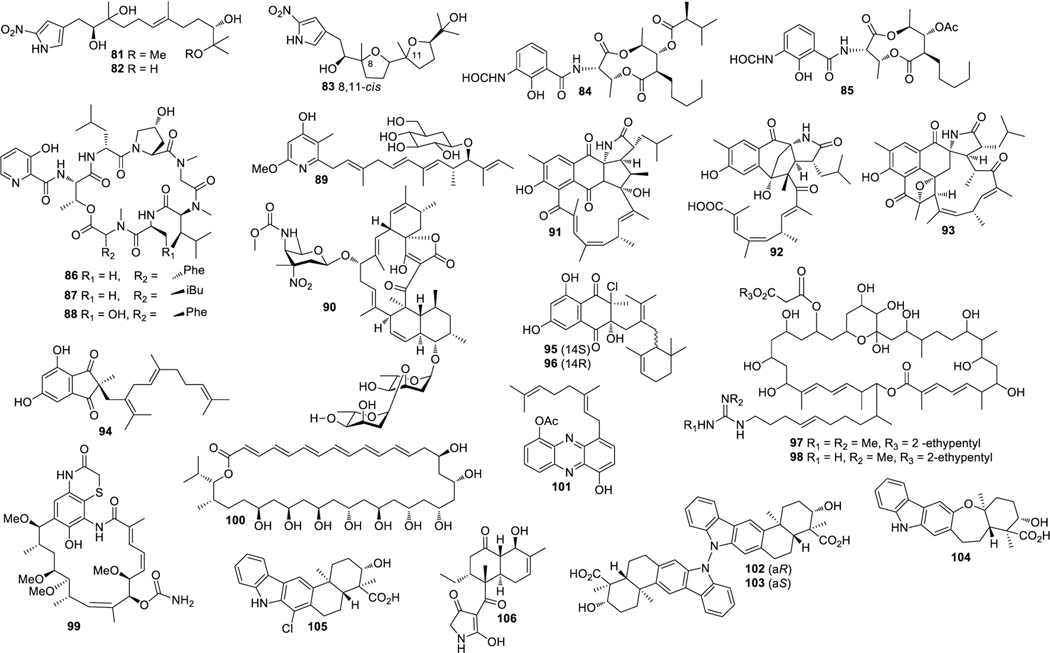
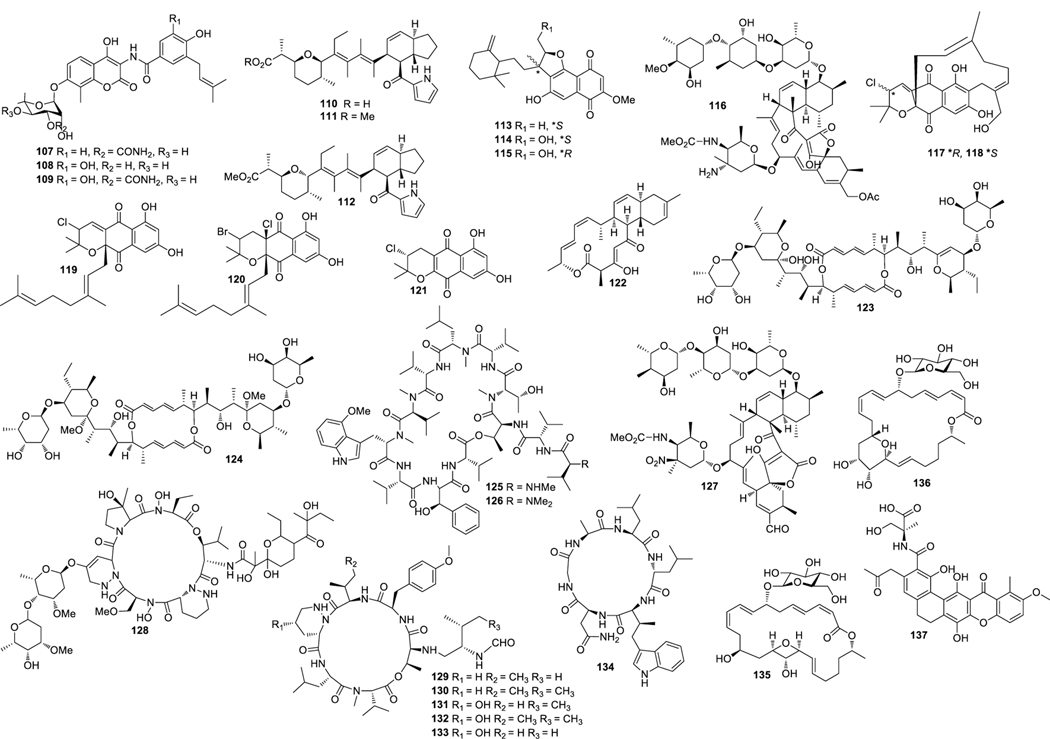
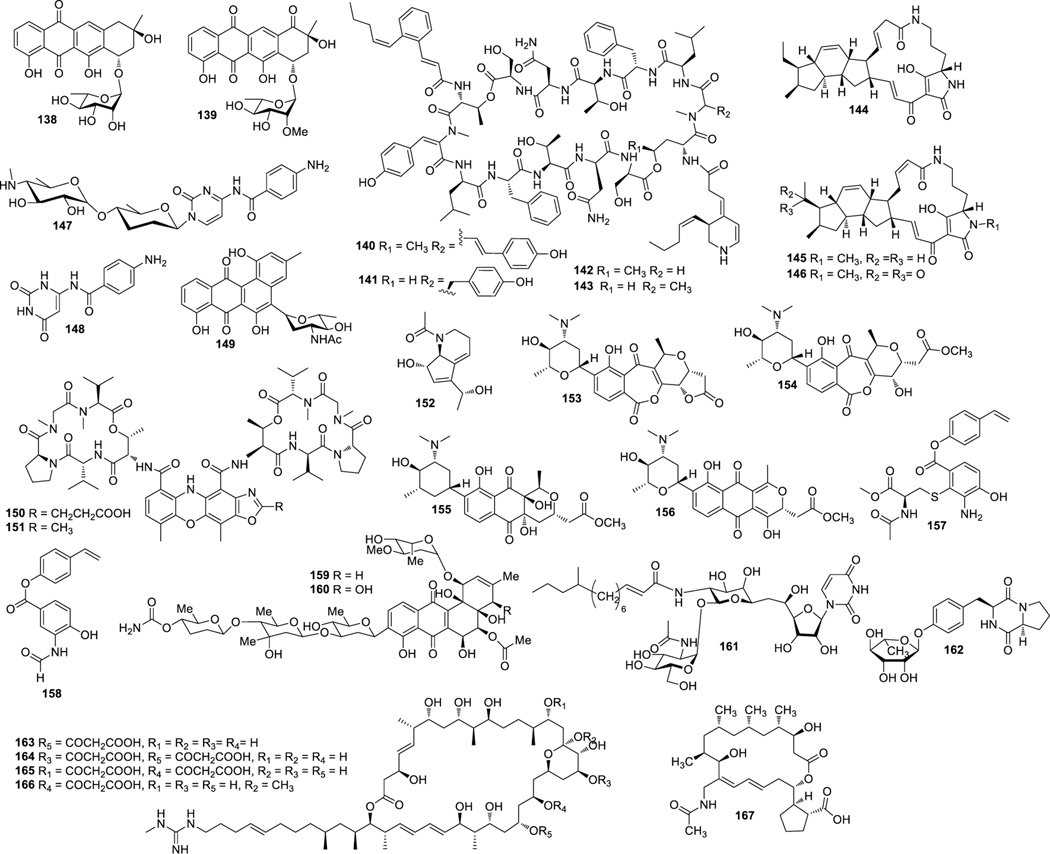
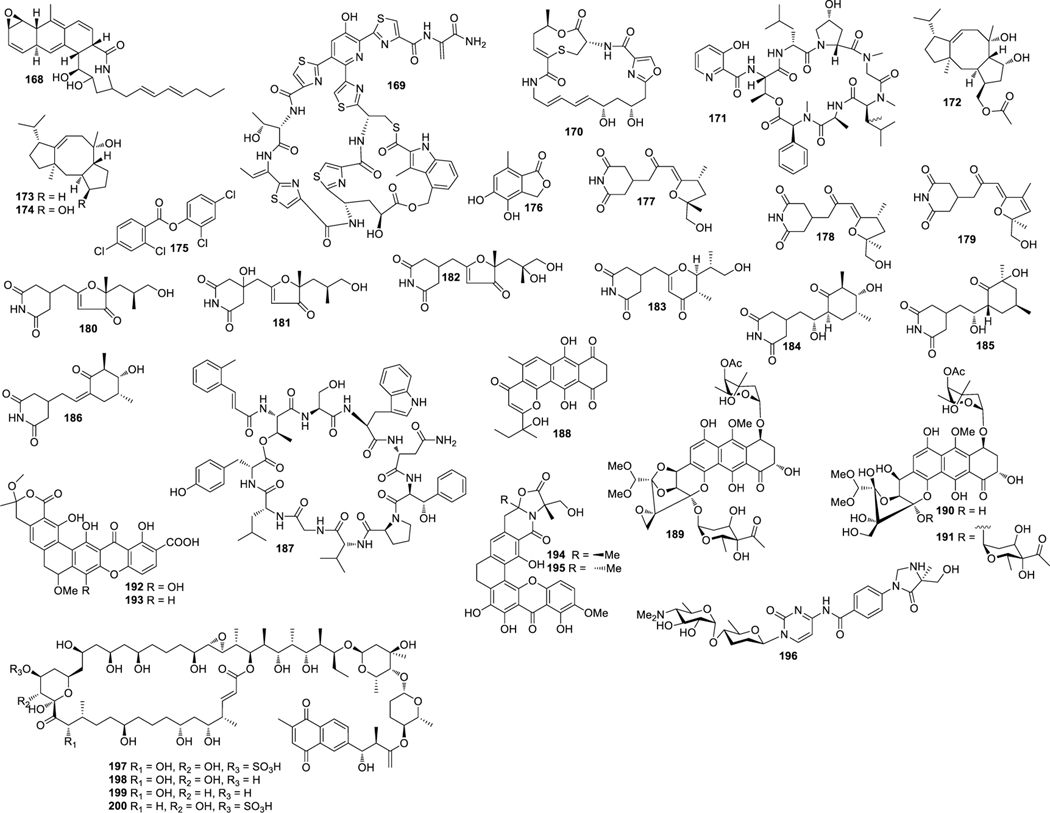
Aplasmomycins A−C (52‒54) were isolated from S. griseus SS-20, which inhibited the growth of Gram-positive bacteria (Okami et al. 1976; Sato et al. 1978). Istamycins A and B (55 and 56) were purified from S. tenjimariensis SS-939, which showed inhibition against Gram-positive and Gram-negative bacteria (Okami et al. 1979). Phenazine alkaloid (57) was obtained from a culture of Streptomyces sp. CNB-253, which displayed antimicrobial activity against Hemophilus influenza and Clostridium perfringens with MIC values of 1 and 4 μg/mL, respectively (Pathirana et al. 1992). Wailupemycin A (58) (Fig. 3) and 3-epideoxyenterocin (59) were isolated from Streptomyces sp. BD-26T(20) (Sitachitta et al. 1996). Compound 58 showed antibacterial activity against S. aureus with an inhibition zone diameter of 18 mm at 1 mg/6 mm disk. Compound 59 showed antibacterial activity against E. coli with an inhibition zone diameter of 15 mm at 0.1 mg/6 mm disk. δ-Indomycinone (60) was obtained from Streptomyces sp. B 8300, which showed antibacterial activity against B. subtilis with an MIC value of 100 μg/mL (Biabani et al. 1997). Streptomyces sp. CNB-689 produced actinoflavoside (61), which exhibited wide antibacterial activity against S. pneumonia, S. pyrogenes, S. aureus and M. luteusat with an equal MIC value of 64 μg/mL (Jiang et al. 1997). Dimethyl 5, 10-dihydrophenazine-l,6-dicarboxylate (5, 10-Dihydrophencomycin methyl ester) (62) was isolated from Streptomyces sp. B 8251, which displayed weak antimicrobial activity against E. coli and B. subtilis (Pusecker et al. 1997). Lysophosphatidyl inositols A and B (63 and 64) were isolated from Streptomyces sp. M428, and both compounds showed antifungal activities against C. albican with MIC values of 5.0 and 2.5 μg/mL, respectively (Cho et al. 1999). Lornemide A (65) was discovered from Streptomyces sp. MSTMA190, which demonstrated inhibitory activity against B. subtilitis with a LD99 value of 50 μg/mL (Capon et al. 2000). 2-Amino-9,13-dimethyl heptadecanoic acid (66) was produced by Streptomyces sp. 1010, which showed inhibitory activity against M. luteus and B. subtilis with MIC values of 15 and 50 μg/mL, respectively (Ivanova et al. 2001). A study of Streptomyces sp. B7064 led to the identification of chalcomycin B (67), which displayed antibacterial activity against S. aureus, E. coli and B. subtilis with inhibition zone diameters of 23, 28, and 21 mm at 10 μg/disk, respectively (Asolkar et al. 2002). Bonactin (68) was isolated from Streptomyces sp. BD21–2 and was active against S. aureus, B. megaterium and S. cerevisiae with the inhibition zone diameters of 7.0, 8.0 and 7.5 mm at 1 mg/mL, respectively (Schumacher et al. 2003). Lajollamycin (69) was discovered from S. nodosus NPS007994, which displayed antibacterial activity against S. pneumonia and S. aureus with MIC values of 1.5 and 5 μg/mL, respectively (Manam et al. 2005). Daryamides A and B (70 and 71) was obtained from Streptomyces sp. CNQ-085, which exhibited antifungal activity against C. albicans with MIC values of 62.5 and 125 μg/mL, respectively (Asolkar et al. 2006). 5,7-Dihydroxy-5,6,7,8-tetrahydroazocin-2(1H)-one (72) obtained from Streptomyces sp. QD518 showed inhibitory activity against S. aureus at 40 μg/disc with an inhibition zone diameter of 11 mm (Wu et al. 2006). Streptomyces sp. B8000 yielded 8-hydroxy-3-methoxy-1-propylanthraquinone (73), which was active against S. aureus and Streptomyces viridochromogenes at 40 μg/disc with inhibition zone diameters of 14 and 12 mm, respectively (Poumale et al. 2006). Marinopyrroles A (74) and B (75) were isolated from a culture of Streptomyces sp. CNQ-418, which demonstrated antimicrobial activity against MRSA with MICs of 0.61 and 1.10 μM, respectively (Hughes et al. 2008). Marinopyrrole C (76) displayed antimicrobial activity against MRSA with an MIC value less than 1 μg/mL (Hughes et al. 2010). Streptomyces sp. MS239 produced 77, which showed weak antibacterial activity against B. subtilis ATCC6633 (Motohashi K et al. 2008). Essramycin (78) was obtained from Streptomyces sp. Merv8102, which displayed antibacterial activity against E. coli (ATCC 10536), P .aeruginosa (ATCC 10145), B. subtilis (ATCC6051), S. aureus (ATCC 6538), and M. luteus (ATCC 9341) with the MIC values of 8.0, 3.5, 1.0, 1.0 and 1.5 μg/mL, respectively (El-Gendy et al. 2008). Tirandamycins C (79) was isolated from a culture of Streptomyces sp. 307–9, which demonstrated antimicrobial activity against vancomycin-resistant E. faecalis with an MIC value of 110 μM (Carlson et al. 2009). 8-Deoxyheronamide C (80) was isolated from Streptomyces sp. CMB-M0406, which exhibited inhibitory activity against wild-type fission yeast with an MIC value of 5.8 μM (Sugiyama et al. 2014). Heronapyrroles A−C (81‒83) (Fig. 4) were isolated from Streptomyces sp. CMB-M0423, which inhibited the growth of Gram-positive bacteria with MIC values ranging from 0.6 to 6.5 μM (Raju et al. 2010). Antimycins A19 and A20 (84 and 85) were discovered from S. antibioticus H74–18, which displayed antifungal activity against C. albicans with MIC values of 5 and 10 μg/mL, respectively (Xu et al. 2011). Fijimycins A‒C (86‒88) were obtained from Streptomyces sp. CNS-575, which inhibited the growth of MRSA (ATCC33591, Sanger 252, UAMS1182) with MIC values ranging from 4 to 16 μg/mL (Sun et al. 2011). Glucopiericidin C (89) isolated from Streptomyces sp. B8112 was active against Mucor miehei (Shaaban et al. 2011). Lobophorin F (90) was produced by Streptomyces sp. SCSIO 01127, which demonstrated inhibitory activity against S. aureus ATCC 29213 and E. faecalis ATCC 29212 with an equal MIC value of 8 μg/mL (Niu et al. 2011). Ansalactams B−D (91‒93) were purified from Streptomyces sp. CNH-189, which exhibited inhibitory activities against MRSA with MIC values of 31.2, 31.2 and 62.5 μg/mL, respectively (Wilson et al. 2011). Three compounds meroindenon (94), merochlorins E (95) and F (96) were produced by Streptomyces sp. CNH-189. Compound 94 displayed antibacterial activity against B. subtilis, K. rhizophila and S. aureus with MIC values of 16, 64 and 128 μg/mL, respectively. Compounds 95 and 96 displayed antibacterial activities against B. subtilis, K. rhizophila and S. aureus with MIC values in the range of 1−2 μg/mL (Ryu et al. 2019). Coumpounds 97 and 98 identified from Streptomyces sp. 211726 were active against C. albicans with MIC values of 2.34 and 12.50 μg/mL, respectively (Yuan et al. 2011). Heronamycin A (99) was produced by Streptomyces sp. CMB-M0392, which displayed inhibition against B. subtilis ATCC6052 and ATCC6633 with MIC values of 8 and 14 μg/mL, respectively (Raju et al. 2012). Bahamaolide A (100) was produced by Streptomyces sp. CNQ343, which showed inhibition against C. albicans and various pathogenic fungi (Kim et al. 2012). Geranylphenazinediol (101) was isolated from Streptomyces sp. LB173, which exhibited weak antibacterial activity (Ohlendorf et al. 2012). Dixiamycins A (102) and B (103), oxiamycin (104) and chloroxiamycin (105) were purified from Streptomyces sp. SCSIO 02999, which demonstrated inhibitory activity against E. coli ATCC 25922 with MIC values of 8, 8, 16 and 4 μg/mL, respectively (Zhang et al. 2012). Compounds 102‒105 also exhibited inhibitory activity against S. aureus ATCC29 213 with MIC values of 8, 16, 16 and 8 μg/mL, respectively. Compounds 102, 103 and 105 displayed inhibitory activity against B. subtilis SCSIO BS01 with MIC values of 64, 128 and 64 μg/mL, respectively. Compounds 102 and 103 showed inhibitory activity against B. thuringiensis SCSIO BT01 with MIC values of 64 and 64 μg/mL, respectively. Streptosetin A (106) was obtained from Streptomyces sp. CP13–10, and it displayed antifungal activity against yeast Sir2p with an MIC value of 2.5 mM (Amagata et al. 2012). Streptomyces sp. RJA2961 was reported to produce novobiocin (107) (Fig. 5), desmethylnovobiocin (108) and 5-hydroxynovobiocin (109), which displayed antibacterial activity against MRSA (ATCC 33591) with MIC values of 0.25, 16 and 8 μg/mL, respectively (Dalisay et al. 2013). Iso-16-deethylindanomycin (110), 16-deethylindanomycin methyl ester (111) and iso-16-deethylindanomycin methyl ester (112) were isolated from a culture of S. antibioticus PTZ0016, which showed antimicrobial activity against S. aureus ATCC6538 with MIC values of 6.0, 6.0 and 8.0 μg/mL, respectively (Lian et al. 2013). Three compounds marfuraquinocins A (113), C and D (114 and 115) were produced by S. niveus SCSIO 3406, and they displayed antibacterial activities against S. aureus ATCC 29213 with an equal MIC value of 8 μg/mL. Compounds 114 and 115 showed antibacterial activities against methicillin-resistant Staphylococcus epidermidis shhs-E1 with an equal MIC value of 8 μg/mL (Song Y et al. 2013). Streptomyces sp. MS100061 yielded lobophorin G1 (116), which inhibited the growth of B. subtilis and M. tuberculosis H37Rv with MIC values of 3.1 and 32 μg/mL, respectively (Chen et al. 2013). Napyradiomycins A and B (117 and 118) were produced by Streptomyces sp. CNQ-329, which possessed inhibitory activity against MRSA with MIC values of 16 and 64 μg/mL, respectively (Cheng et al. 2013). Designated 4-dehydro-4a-dechlorona pyradiomycin A1 (119), 3-dechloro-3-bromonapyradiomycin A1 (120), and 3-chloro-6,8-dihydroxy-8-α-lapachone (121) from Streptomyces sp. SCSIO 10428 exhibited antibacterial activity against B. thuringensis SCSIO BT01 with MIC values of 8, 1 and 16 μg/mL, respectively. They exhibited antibacterial activity against B. subtilis SCSIOBS01 with MIC values of 4, 1 and 8 μg/mL, respectively (Wu et al. 2013a). Compounds 119 and 120 showed antibacterial activity against S. aureus ATCC 29213 with MIC values of 4.0 and 0.5 μg/mL, respectively. Streptomyces sp. CNH365 afforded anthracimycin (122), which exhibited antibacterial activity against B. anthracis UM23C1–1, S. aureus ATCC, E. faecalis ATCC 29212, S. pneumoniae ATCC 51916 and H. influenzae ATCC 31517 with MIC values of 0.03125, 0.0625, 0.125, 0.25 and 4 μg/mL, respectively (Jang et al. 2013). 11′,12′-Dehydroelaiophylin (123) and 11,11′-O-dimethyl-14′-deethyl-14′-methylelaiophylin (124) were isolated from Streptomyces sp. 7–145, which displayed good inhibitory activity against MRSA and vancomycin-resistant enterococci pathogens (Wu et al. 2013b). Two new compounds ohmyungsamycins A (125) and B (126) were isolated from Streptomyces sp. SNJ042. Compound 125 exhibited inhibitory activity against B. subtilis ATCC6633, K. rhizophila NBRC12708 and P. hauseri NBRC3851 with MIC values of 4.28, 1.07 and 2.14 μM, respectively, while compound 126 was active against K. rhizophila NBRC12708 with an MIC value of 8.5 μM (Um et al. 2013). Lobophorin H (127) was discovered from Streptomyces sp. 12A35, which displayed inhibitory activity against S. aureus ATCC29213 and B. subtilis CMCC63501 with MIC values of 50 and 1.57 μg/mL, respectively (Pan et al. 2013). Mollemycin A (128) was identified from Streptomyces sp. CMBM0244 and it was active against S. aureus ATCC 25293 and ATCC 9144, S. epidermidis ATCC 12228, B. subtilis ATCC 6051 and ATCC 6633, E. coli ATCC 25922, P. aeruginosa ATCC 27853 and Mycobacterium bovis (BCG) with MIC values of 50, 10, 50, 10, 10, 10, 50 and 3200 nM, respectively (Raju et al. 2014). Marformycins A−E (129‒133) exhibited inhibitory activities against M. luteus with MIC values of 0.25, 4.0, 0.25, 0.063 and 4.00 μg/mL, respectively (Zhou et al. 2014). Desotamide B (134) was obtained from a culture of S. scopuliridis SCSIO ZJ46, which demonstrated antimicrobial activity against S. aureus ATCC29213, S. pnuemoniae NCTC 7466 and MRSA with MIC values of 16.0, 12.5 and 32.0 μg/mL, respectively (Song et al. 2014). Glycosylated macrolactins A1 (135) and B1 (136) were isolated from Streptomyces sp. 06CH80, which displayed antibacterial activities against B. subtilis, E. coli, P. aeruginosa, S. aureus and S. cerevisiae with MIC values in the range of 0.027 to 0.22 μM/mL (Mondol et al. 2014). Buanmycin (137) was isolated from Streptomyces sp. SNR69, and compound 137 exhibited antibacterial activity against five bacterial strains with MIC values ranging from 0.7 to 21.1 μg/mL (Moon et al. 2015). Chemical investigation of a culture extract of Streptomyces sp. CMB-M0150 led to the discovery of aranciamycins I (138) (Fig. 6) and J (139). 138 and 139 showed inhibitory activity against M. tuberculosissurrogate with MIC values in the range of 0.7 to 1.7 μM, respectively (Khalil et al. 2015). A fermentation broth of Streptomyces sp. SNM5 yielded mohangamides A (140) and B (141), which exhibited inhibitory activity against C. albicans ICL with IC50 values of 4.4 and 20.5 μM, respectively (Bae et al. 2015a). Hormaomycins B (142) and C (143) from Streptomyces sp. SNM5 displayed broad antibacterial activities with MIC values ranging from 0.23 to 114 μM (Bae et al. 2015b). Streptomyces zhaozhouensis CA-185989 yielded isoikarugamycin (144), 28-N-methylikarugamycin (145), and 30-oxo-28-N-methylikarugamycin (146). 144– 146 were active against MRSA with MIC values of 1–4, 1–4, 32–64 μg/mL, respectively. Compound 144 was active against C. albicans and A. fumigatus with MIC values of 2–4 and 4–8 μg/mL, respectively, and 145 was active against C. albicans and A. fumigatus with MIC values of 4 and 4–8 μg/mL, respectively (Lacret et al. 2015). S. rochei 06CM016 yielded compounds 147 and 148. 147 showed antimicrobial activity against E. coli O157:H7 RSKK 234, MRSA DSM 11729 and C. albicans DSM 5817 with MIC values of 16, 8 and 4 μg/mL, respectively (Aksoy et al. 2016). 148 exhibited antimicrobial activity against E. coli O157:H7 RSKK 234, MRSA DSM 11729 and C. albicans DSM 5817 with MIC values of 16, 16 and 8 μg/mL, respectively (Aksoy et al. 2016). N-acetyl-N-demethylmayamycin (149) was obtained from Streptomyces sp. 182SMLY, which was active against MRSA with an MIC of 20.0 μM (Liang et al. 2016). Neo-actinomycins A (150) and B (151) were discovered from Streptomyces sp. IMB094, which displayed antibacterial activity against MRSA and vancomycin-resistant Enterococci with MIC values in the range of 16 to 64 μg/mL (Wang et al. 2017). Strepchazolin A (152) was obtained from Streptomyces chartreusis NA02069, which showed antibacterial activity against B. subtilis with an MIC value of 64 μM (Yang et al. 2017). Jiang et al. isolated four new naphthoquinone derivatives from Streptomyces sp. XMA39, namely strepoxepinmycins A–D (153–156), which displayed inhibitory activity against a wide spectrum of strains with MIC values ranging from 6.0 to 10.0 μg/mL (Jiang et al. 2018). Bagremycins F (157) and G (158) were obtained from Streptomyces sp. ZZ745 and they showed inhibitory activities against E. coli with MIC values of 41.8 and 67.1 μM, respectively (Zhang et al. 2018b). Streptomyces Pratensis NA-ZhouS1 yielded stremycins A (159) and B (160). 159 and 160 were active against P. aeruginosa, MRSA, K. pneumonia and E. coli with the same MIC value of 16 μg/mL. Both were also active against B. subtilis with MIC values from 8 to 16 μg/mL (Akhter et al. 2018). Tunicamycin E (161) was obtained from Streptomyces xinghaiensis SCSIO S15077, which exhibited inhibitory activity against B. thuringiensis BT01, B. thuringiensis, C. albicans (ATCC 96901) and C. albicans CMCC (F) 98001 with MIC values of 2.0, 0.5, 32 and 8 μg/mL, respectively (Zhang et al. 2018c). A fermentation broth of Streptomyces sp. ZZ446 yielded a new compound maculosin-O-α-L-rhamnopyranoside (162), which showed antimicrobial activity against MRSA, E. coli and C. albicans with MIC values of 37.0, 28.0 and 26.0 μg/mL, respectively (Chen et al. 2018a). Niphimycins C−E (163−165) and 17-O-methylniphimycin (166) were isolated from a culture of Streptomyces sp. IMB7–145, which displayed antimicrobial activity against C. albican with MIC values of 8−32 μg/mL (Hu et al. 2018). Compound 163 showed anti-bacterial activity against MRSE, MRSA and M. tuberculosis with MIC values ranging from 4 to 64 μg/mL. Streptomyces mutabilis sp. MII yielded N-acetylborrelidin B (167), which was active against B. subtilis, B. cereus and S. aureus with inhibition zone diameters of 8−11 mm. Compound 167 was also active against S. warneri with an inhibition zone diameter of 18 mm (Hamed et al. 2018a). Nivelactam B (168) (Fig. 7), a new biphenyl derivative, was obtained from S. varsoviensis HF-11225, which exhibited inhibitory activity against Sclerotinia sclerotiorum with an inhibition zone diameter of 9 mm at 100 μg per 7 mm paper disks (Chen et al. 2018b). Nosiheptide (169), griseoviridin (170) and etamycin (171) were produced by Streptomyces sp. OPMA 1245. Compound 169 displayed antibacterial activity against M. avium JCM15430, M. intracellulare JCM6384 and M. bovis BCG Pasteur with MIC values of 0.024, 0.024 and 0.012 μg/mL, respectively. Compound 170 showed antibacterial activity against M. avium JCM15430, M. intracellulare JCM6384 and M. bovis BCG Pasteur with MIC values of 1.56, 1.56 and 6.25 μg/mL, respectively. Compound 171 was active against M. avium JCM15430, M. intracellulare JCM6384 and M. bovis BCG Pasteur with MIC values of 0.097, 0.190 and 0.780 μg/mL, respectively (Hosoda et al. 2019). Streptomyces sp. ZZ820 yielded diterpenoids 18-acetyl-cyclooctatin (172), 5,18-dedihydroxy-cyclooctatin (173) and 5-dehydroxy-cyclooctatin (174), which inhibited the growth of MRSA and E. coli with MIC values ranging from 24.11 to 55.12 μM (Yi et al. 2019). Streptomyces sp. G212 produced 2,4-dichlorophenyl 2,4-dichloro benzoate (175) and 4,5-dihydroxy-7-methylphthalide (176). Compound 175 exhibited inhibitory activity against C. albicans with an MIC value of 64 μg/mL, and compound 176 inhibited E. faecalis with the same MIC value of 64 μg/mL (Cao et al. 2019). Streptoglutarimides A–J (177–186) were obtained from Streptomyces sp. ZZ741. 177–186 showed antifungal activity against C. albicans with MIC values in the range of 8−20 μg/mL. They showed inhibitory activity against MRSA with MIC values ranging from 9−11 μg/mL, and against E. coli with MIC values in the range of 8−12 μg/mL (Zhang et al. 2019a). Atratumycin (187) was produced by Streptomyces atratus SCSIOZH16, which displayed inhibition against M. tuberculosis H37Ra and H37Rv with MIC values of 3.8 and 14.6 μM, respectively (Sun et al. 2019).
Fig. 3.
Structures of compounds 58–80
Fig. 4.
Structures of compounds 81–106
Fig. 5.
Structures of compounds 107–137
Fig. 6.
Structures of compounds 138–167
Fig. 7.
Structures of compounds 168–200
Antimicrobial compounds from Streptomyces sp. from marine seawater
Parimycin (188) and trioxacarcins D–F (189–191) obtained from Streptomyces sp. B8652 had a broad spectrum of antibacterial activity (Maskey et al. 2002; Maskey et al. 2004). Streptomyces caelestis afforded new antibacterial citreamicins A (192), B (193), citreaglycon A (194) and dehydrocitreaglycon A (195). 192–195 showed broad spectrum of antibacterial activity against bacterial strains (Liu et al. 2012). Streptcytosine A (196) was discovered from Streptomyces sp. TPU1236A, and it exhibited antibacterial activity against M. smegmatis with an MIC value of 32 μg/mL (Bu et al. 2014).
Antimicrobial compounds from Streptomyces sp. from other marine sources
Streptomyces caniferus CA-271066 afforded caniferolides A–D (197‒200). They showed a broad spectrum of antifungal activity against A. fumigatus ATCC46645 and C. albicans MY1055 with MIC values ranging from 0.5 to 8.0 μg/mL (Pérez-Victoria et al. 2019).
Antimicrobial compounds from Micromonospora species
Antimicrobial compounds from Micromonospora sp. associated with ascidians
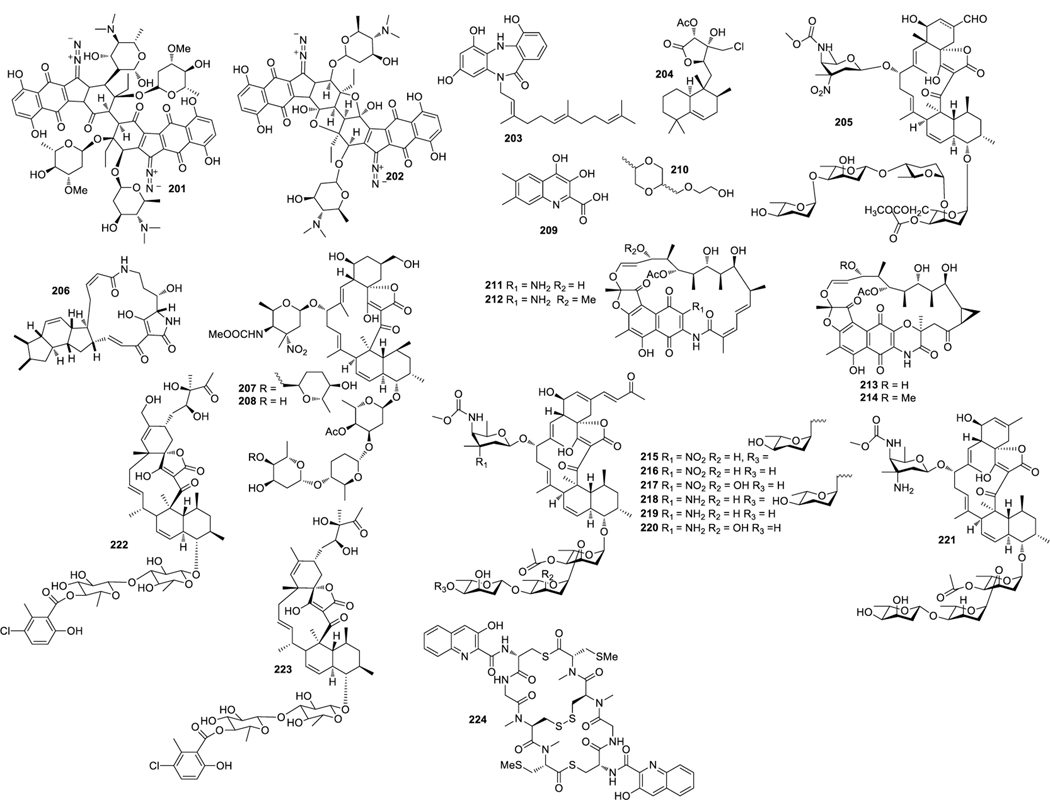
Lomaiviticins A (201) (Fig. 8) and B (202) were isolated from Micromonospora lomaivitiensis LL-37I366 and showed inhibitory activities against S. aureus and E. faecium with MIC values in the range of 6 to 25 ng/spot (He et al. 2001). Diazepinomicin (203) was obtained from Micromonospora sp. DPJ12, which exhibited antibacterial activity against Gram-positive bacteria with MICs of about 32 μg/mL (Charan et al. 2004). Micromonohalimane B (204) was isolated from Micromonospora sp. WMMC-218, and 204 inhibited MRSA with an MIC value of 40 μg/mL (Zhang et al. 2016a).
Fig. 8.
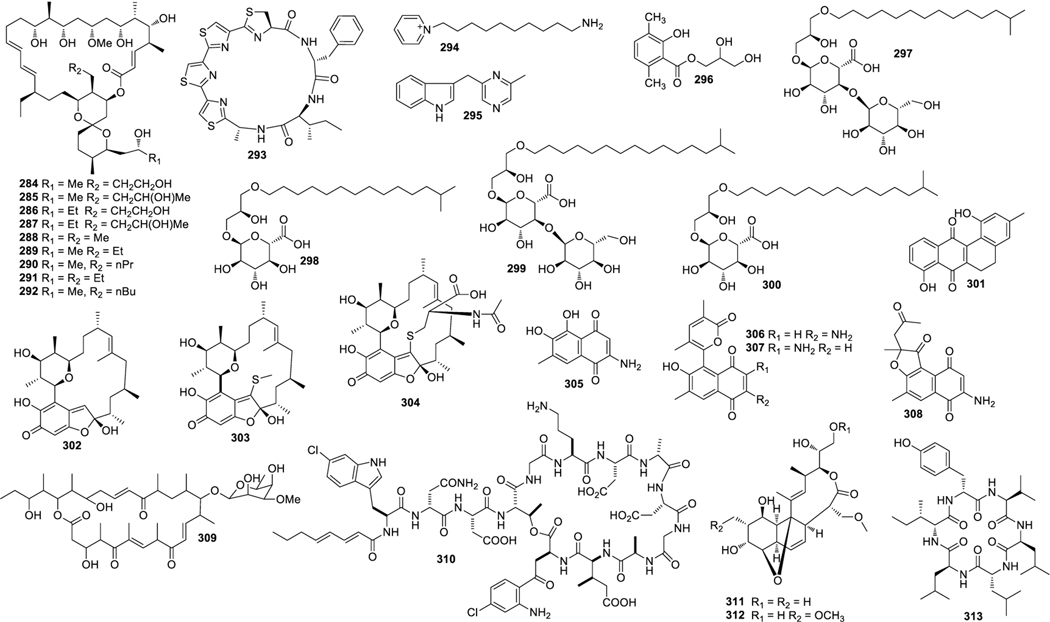
Structures of compounds 201–224
Antimicrobial compounds from Micromonospora sp. associated with sponges
Tetrocarcin Q (205) was discovered from Micromonospora carbonacea LS276, which displayed antibacterial activity against B. subitlis ATCC 63501 with an MIC value of 12.5 μM (Gong et al. 2018).
Antimicrobial compounds from Micromonospora sp. from marine sediments
Butremycin (206) was isolated from Micromonospora sp. K310, which exhibited weak antibacterial activity against S. aureus ATCC 25923, E. coli ATCC 2592 and MRSA (Kyeremeh K et al. 2014). Chemical investigation of a culture extract of Micromonospora sp.5–297 led to the discovery of two glycosidic spirotetronates tetrocarcins N (207) and O (208). 207 and 208 showed inhibitory activity against B. subtilis with MIC values of 2 and 64 μg/mL, respectively (Tan et al. 2016). 3,4-Dihydroxy-6,7-dimethyl-quinoline-2-carboxylic acid (209) were isolated from Micromonospora sp. G019, which demonstrated inhibitory activity against E. coli, S. enterica and E. faecalis with the MIC values of 48, 96 and 128 μg/mL, respectively (Thi et al. 2016a). 2-[(5-Methyl-1,4-dioxan-2-yl)methoxy]ethanol (210) showed inhibitory activity against E. faecalis and C. albican with MIC values of 32 and 64 μg/mL, respectively (Thi et al. 2016a). 3-amino-27-demethoxy-27-hydroxyrifamycin S (211), 3-amino-rifamycin S (212), sporalactams A (213) and B (214) were produced by Micromonospora sp. RJA4480. Compounds 211‒214 displayed antibacterial activities against MRSA, E. coli and M. tuberculosis with MIC values of 0.0009, 0.0003 and 0.0009; 0.0008, 0.0001 and 0.0008; 7.0, 1.8 and 0.8; and 1.80, 0.40 and 0.06 μg/mL, respectively (Williams et al. 2017). Microsporanates A–F (215‒220) and tetrocarcin P (221) obtained from Micromonospora harpali SCSIO GJ089 displayed a wide range of antibacterial activities (Gui et al. 2017). Phocoenamicins B (222) and C (223) were isolated from Micromonospora sp. CA-214671, both compounds showed a broad spectrum of antibacterial activities with MIC values ranging from 2 to 64 μg/mL (Pérez-Bonilla et al. 2018).
Antimicrobial compounds from Micromonospora sp. from other marine sources
Thiocoraline (224) was isolated from Micromonospora sp. L-13-ACM2–092, which inhibits the growth of Gram-positive bacteria (Perez et al. 1997).
Antimicrobial compounds from Nocardiopsis species
Antimicrobial compounds from Nocardiopsis sp. from marine sediments
Nocardiopsis dassonvillei produced kahakamide A (225) (Fig. 9), which showed weak antibacterial activity against B. subtilis (Schumacher et al. 2001). Thiopeptide TP-1161 (226) from Nocardiopsis sp. TFS65–07 displayed broad antibacterial activity with MIC values ranging from 0.25 to 1.0 μg/mL (Engelhardt et al. 2010). Nocapyrones E−G (227‒229), were isolated from Nocardiopsis dassonvillei HR10–5, which exhibited inhibitory activities against B. subtilis with MIC values of 26, 14 and 12 μM, respectively (Fu et al. 2011). Nocarimidazoles A (230) and B (231), were produced by Nocardiopsis sp. CNQ115. They displayed antimicrobial activities against B. subtilis with an equal MIC value of 64 μg/mL. Compound 231 displayed antimicrobial activity against S. epidermidis with an MIC value of 64 μg/mL (Leutou et al. 2015). Three α-pyrones 4-deoxyphomapyrone C (232), 4-deoxy-11-methylphomapyrone C (233) and 10-hydroxymucidone (234) were produced by Nocardiopsis sp. SCSIO 10419. Compound 232 displayed antibacterial activity against B. subtilis SCSIO BS01 with an MIC value of 64 μg/mL. Compound 233 and 234 displayed antibacterial activities against M. luteus with the same MIC value of 64 μg/mL (Zhang et al. 2016b). 2-[(2R-Hydroxypropanoyl)amino] benzamide (235) was isolated from Nocardiopsis sp. G057, which displayed inhibitory activity against E. coli with an MIC value of 16 μg/mL (Thi et al. 2016b). Nocazine G (236) was produced by Nocardiopsis sp. YIM M13066, which possessed inhibitory activity against B. subtilis ATCC 6051 with an MIC value of 25.8 μM (Sun et al. 2017). Fluvirucin B6 (237) was isolated from Nocardiopsis sp. CNQ-115, which exhibited inhibitory activity against B. subtilis, K. rhizophila and S. aureus with MIC values of 64, 32 and 32 μg/L, respectively (Leutou et al. 2018). Terretonin N (238) obtained from Nocardiopsis sp. LGO5 had a broad spectrum of antibacterial activity against bacteria (Hamed et al. 2018b).
Fig. 9.
Structures of compounds 225–254
Antimicrobial compounds from Nocardiopsis sp. associated with sponges
Nocardiopsistins A−C (239‒241), were isolated from Nocardiopsis sp. HB-J378, which showed antibacterial activity against MRSA with MIC values ranging from 3.12 to 12.5 μg/mL (Xu et al. 2018).
Antimicrobial compounds from other marine actinomycetes
Antimicrobial compounds from other actinomycetes associated with sponges
2,4,4’-Trichloro-28-hydroxydiphenylether (242) was isolated from Micrococcus luteus, which showed a broad spectrum of antibacterial activity with MIC values ranging from 16 to 64 μg/mL (Bultel-Poncé et al. 1998). Microluside A (243) was obtained from a culture of Micrococcus sp. EG45, which displayed antimicrobial activity against E. faecalis JH212 and S. aureus NCTC 8325 with MIC values of 10 and 13 μM, respectively (Eltamany et al. 2014). PM18110448 (Kocurin) (244) discovered from Kocuria palustris demonstrated a broad spectrum of antibacterial activity (Martín et al. 2013). A study of Actinokineospora spheciospongiae DSM45935T led to the identification of actinokineosin (245), which exhibited antibacterial activity against M. luteus with an inhibition zone diameter of 8.0 mm at 50 μg/disk (Takasaka et al. 2017).
Antimicrobial compounds from other actinomycetes associated with other marine animals
Saccharothrix espanaensis An 113 produced saccharothrixins A−C (246‒248), which showed modest antibacterial activity (Kalinovskaya et al. 2008). Arenjimycin (249) from Salinispora arenicola CNR-647 displayed broad antibacterial activity (Asolkar et al. 2010). Solwaraspora sp. WMMB329 yielded solwaric acids A and B (250 and 251). Both compounds were active against E. coli, MRSA, MSSA and P. aeruginosa with MIC values of 128, 32, 64, 128 μM and 128, 32, 64, 128 μM, respectively (Ellis et al. 2014). Forazoline A (252) was isolated from Actinomadura sp. WMMB-499, which exhibited inhibitory activity against C. albicans with an MIC value of 16 μg/mL (Wyche et al. 2014). (11S,15R)-11-Hydroxycurvularin (253) and (11R,15R)-11-hydroxycurvularin (254) were obtained from Pseudonocardia sp. HS7. They showed antibacterial activity against E. coli with an equal MIC value of 20 μg/mL (Ye et al. 2016). Actinomadura sp. WMMB499 yielded ecteinamycin (255) (Fig. 10), which showed antibacterial activity against E. coli, S. aureus (MRSA and MSSA), and P. aeruginosa with MIC values of 16, 0.125 and 8 μg/mL, respectively. Compound 255 exhibited inhibition against C. difficile with an MIC value of 0.059−0.117μg/mL (Wyche et al. 2017).
Fig. 10.
Structures of compounds 255–283
Antimicrobial compounds from other actinomycetes associated with mangroves and algae
Lechevalieria aerocolonigenes K10–0216 afforded pyrizomicins A and B (256, 257). They showed broad spectrum of antimicrobial activity (Kimura et al. 2018). Kocuria marina CMG S2 afforded kocumarin (258), which showed activity against MRSA with an MIC value of 10−15 μg/mL (Uzair et al. 2018).
Antimicrobial compounds from other actinomycetes associated with marine sediments
Cultivation of Actinomadura sp. M045 produced three new phenoxazin-3-one antibiotics chandrananimycins A–C (259–261). Compounds 259 and 260 exhibited inhibitory activity against Mucor meihei with inhibition zone diameters of 11 and 12 mm at 20 μg/platelet, respectively. Compound 261 showed activity at 20 μg/platelet against B. subtilis, Mucor meihei and S. aureus with inhibition zone diameters of 23, 27 and 22 mm, respectively (Maskey et al. 2003). Abyssomicin C (262) was obtained from Verrucosispora sp. AB-18–032, which exhibited antibacterial activity against S. aureus N315 and S. aureus Mu50s with MIC values of 4 and 13 μg/mL, respectively (Bister et al. 2004). Chemical investigation of a culture extract of Marinispora sp. CNQ-140 led to the discovery of marinomycins A−D (263−266). These compounds showed inhibitory activity against MRSA with MIC90 values of 0.13, 0.25, 0.25 and 0.25 μM, respectively. Compound 264 showed inhibitory activity against VRFE and C. albicans with MIC90 values of 0.13 and 7.8 μM, respectively (Kwon et al. 2006). Marinispora sp. CNQ-140 produced marinisporolide A (267). 267 displayed antifungal activity against C. albicans with an MIC value of 22 μg/mL (Kwon et al. 2009). Atropabyssomicin C (268) was obtained from Verrucosispora sp. AB-18–032, which showed antibacterial activity against MRSA N315 with an MIC value of 2.67 μg/mL (Keller et al. 2007). Marinispora NPS008920 yielded lipoxazolidinones A‒C (269‒271). These three compounds were active against S. aureus ATCC 29213 (MSSA) and E. faecalis ATCC 29212 (VSE) with MIC values of 0.9, 6.0 and 4.0; and 1.0, 3.0 and 2 μg/mL, respectively. Compound 269 was also active against H. influenza with an MIC value of 12 μg/mL (Macherla et al. 2007). Lynamicins A–E (272–276) were isolated from Marinispora sp. NPS12745, which exhibited inhibitory activity against MRSA and vancomycin-resistant E. faecium with MIC values ranging from 1.8 to 57.0 μg/mL (McArthur et al. 2008). Cultivation of Verrucosispora maris AB-18–032 produced proximicins B and C (277 and 278). Compound 277 showed antibacterial activity against Brevibacillus brevis DSM with an inhibition zone diameter of 12 mm at 0.3 mg/mL, Compound 278 exhibited a slight inhibition against Brevibaccillus brevis DSM30 (Fiedler et al. 2008). Salinisporamycin (279) was isolated from a culture of Salinispora arenicora YM23–082, which displayed antimicrobial activity against B. subtilis IFO 3134 and Salinispora aureus IFO12732 with MIC values of 4.1 and 0.46 μg/mL, respectively (Matsuda et al. 2009). Culture of Salinispora arenicola yielded saliniquinone A (280), which showed weak activity against MRSA (Murphy et al. 2010). Pseudonocardians A–C (281–283) were ontained from Pseudonocardia sp. SCSIO 01299, which exhibited inhibitory activities against S. aureus ATCC 29213, E. faecalis ATCC 29212 and B. thuringensis SCSIO BT01 with MIC values ranging from 1 to 4 μg/mL (Li et al. 2011). Actinoalloteichus sp. NPS702 afforded neomaclafungins A–I (284–292) (Fig. 11). These compounds showed antifungal activity against Trichophyton mentagrophytes (ATCC 9533) with MIC values ranging from 1 to 3 μg /mL (Sato et al. 2012). Marthiapeptide A (293) was isolated from Marinactinospora thermotolerans SCSIO 00652, which inhibited the growth of Gram-positive bacteria with MIC values ranging from 2 to 8 μg/mL (Zhou et al. 2012). 1-(10-aminodecyl) pyridinium salt antibiotic (294) was purified from Amycolatopsis alba var. nov. DVR D4, which demonstrated inhibitory activity against Gram-positive and Gram-negative bacteria with MIC values ranging from 70 to 160 μg/mL (Dasari et al. 2012). 3-[(6-Methylpyrazin-2-yl)methyl]-1H-indole (295) was obtained from Serinicoccus profundi sp. nov., which displayed weak antibacterial activity against S. aureus ATCC 25923 with an MIC value of 96 μg/mL (Yang et al. 2013). Glycerol 1-hydroxy-2,5-dimethyl benzoate (296) was isolated from Verrucosispora sp. MS100047, which exhibited inhibitory activity against MRSA with an MIC value of 12.5 μg/mL (Huang et al. 2016). Kribellosides A‒D (297‒300) were discovered from Kribbella sp. MI481–42F6 and they inhibited S. cerevisiae with MICs ranging from 3.12 to 100 μg/mL (Igarashi et al. 2017). 5,6-Dihydro-1,8-dihydroxy-3-methylbenz[a]anthracene-7,12-quinone (301) was separated from Actinomadura sp. DS-MS-114, which was active against S. aureus NBRC12732 with an inhibition zone diameter of 12.7 mm at 100 μg/mL (Kurata et al. 2017). Kendomycins B–D (302−304) obtained from Verrucosispora sp. SCSIO 07399 had a broad spectrum of antibacterial activity against S. aureus ATCC 29213, S. aureus 745524, MRSA shhs-A1, E. faecalis ATCC 29212, B. subtilis BS01 and B. thuringiensis BT01 with MIC values ranging from 0.5 to 8.0 μg/mL (Zhang et al. 2019b). Salinaphthoquinones A–D (305−308) were isolated from Salinispora arenicola BRA-213, they showed antibacterial activities against S. aureus and E. faecalis with MIC values ranging from 16 to 125 μg/mL (da Silva et al. 2019).
Fig. 11.
Structures of compounds 284–313
Antimicrobial compounds from other actinomycetes from other marine sources
Maduralide (309) was obtained from an unidentified marine bacterium of the order Actinomycetales, which displayed weak antibacterial activity against B. subtilis (Pathirana et al. 1991). Taromycin A (310) was isolated from Saccharomonospora sp. CNQ-490, which exhibited inhibitory activity against MRSA and Enterococcus faecalis 613D with MIC values ranging from 6 to 100 μM (Yamanaka et al. 2014). Pseudonocardia carboxydivorans M-227 afforded branimycins B (311) and C (312). They showed a broad spectrum of antibacterial activities (Braña et al. 2017b). Thermoactinoamide A (313) was discovered from Thermoactinomyces vulgaris ISCAR 2354 and was active against S. aureus ATCC 6538 with an MIC value of 35 μM (Teta et al. 2017).
Conclusion
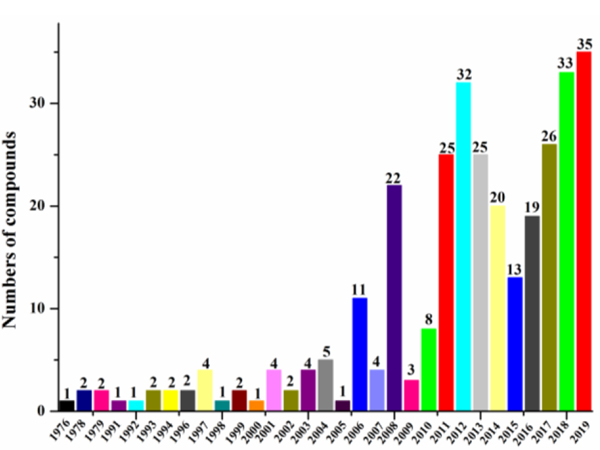
According to the statistic results (Table 1, Fig. 12), the investigation of antimicrobial compounds from marine-derived actinomycetes could be dated back to 1976 when aplasmomycin A (52) was isolated from Streptomyces griseus SS-20 (Table 2) (Okami et al. 1976). Until the end of 2019, 313 new antimicrobial compounds derived from marine actinomycetes have been reported. Since 2016, more secondary metabolites have been isolated from marine actinomyces than ever before except 2007 and 2009.
Table 1.
Antimicrobial compounds isolated from marine actinomycetes (1976‒2019)
| Compound | Producing strain | Environmental source | Bioactivity | Ref. |
|---|---|---|---|---|
| 1–2 | Streptomyces sp. Ni-80 | Unidentified sponge, Urauchicove, Iriomote, Japan | Antifungal activity | Imamura et al.1993. |
| 3–12 | Streptomyces sp. HB202 | Halichondria panicea sponge, Baltic Sea (Germany) | Antibacterial activity | Mitova et al.2008; Schneemann et al. 2010; Kunz et al. 2014. |
| 13–15 | Streptomyces sp. BCC45596 | Xestospongia sp. sponge, Thailand | Antibacterial activity | Supong et al.2012. |
| 16–19 | Streptomyces sp. LHW52447 | Phyllospongia foliascens sponge, Xisha Islands, South China Sea | Antibacterial activity | Jiao et al. 2018 |
| 20–23 | Streptomyces sp. SCSGAA 0027 | gorgonian coral Melitodes squamata,, the South China Sea. | Antibacterial activity | Nong et al. 2016 |
| 24 | Streptomyces sp. M-207 | coral Lophelia pertusa, submarine canyon | Antibacterial activity | Braña et al. 2017a |
| 25 | Streptomyces cyaneofuscatus M-169 | gorgonian coral (Order Gorgonacea), Avilés submarine Canyon | Antibacterial activity | Rodríguez et al. 2018 |
| 26 | Streptomyces sp. SCSIO 41399 | Porites sp. coral, Wenchang, Hainan, C | Antibacterial activity | Cong et al 2019 |
| 27–28 | Streptomyces hygroscopicus | Jellyfish Cassiopeia xamachana, Florida Keys | Antibacterial activity | Trischman et al. 1994 |
| 29 | Streptomyces sp. 1053U.I.1a.3b | L. totopotens, Mactan Island, Cebu, Philippines | Antibacterial activity | Lin et al. 2014 |
| 30 | Streptomyces sp. CNB091 | a jellyfish (C. xamachana), Florida Keys | Antibacterial activity | Hassan, et al. 2015 |
| 31 | Streptomyces seoulensis A01 | marine prawn, Yellow Sea, in China | Antibacterial activity | Zhang et al.2018a |
| 32–34 | Streptomyces sp. # N1–78-1 | Unidentified green algae, Rhode Island | Antibacterial activity | Socha et al 2006 |
| 35 | Streptomyces sp. WR1L1S8 | the brown marine algae Fucus sp., Bejaia coastline | Antibacterial activity | Djinni et al 2013 |
| 36 | Streptomyces althioticus MSM3 | Seaweed Ulva sp., Cantabrian Sea (Northeast Atlantic Ocean) | Antibacterial activity | Braña et al 2019 |
| 37–40 | Streptomyces sp. HKI0576 | mangrove tree Bruguiera gymnorrhiza | Antibacterial activity | Ding et al 2011a |
| 41–48 | Streptomyces sp. HKI0595 | mangrove tree Kandelia candel, Xiamen, China | Antibacterial activity | Ding et al. 2011a; Ding et al. 2012 |
| 49 | S. lusitanus XM52 | Mangrove root, Fujian, China | Antibacterial activity | Han et al. 2012 |
| 50 | Streptomyces sp. MA-12. | Myoporum root, Leizhou Peninsula | Antibacterial and antifungal activity | Ding et al 2013 |
| 51 | Streptomyces sp. LC6 | Leaves of Kandelia candel, Longhai, Fujian, China | Antibacterial activity | Zhang et al 2014 |
| 52–54 | Streptomyces griseus SS-20 | Shallow sea sediment, Sagami Bay | Antibacterial activity | Okami et al 1976; Sato al. 1978 |
| 55–56 | S.tenjimariensis SS-939 | sea mud sample, Tenjin-island, Sagami-Bay | Antibacterial activity | Okami et al 1979 |
| 57 | Streptomyces sp. CNB-253 | Sediment, Bodega Bay, CA | Antibacterial activity | Pathirana et al. 1992 |
| 58–59 | Streptomyces sp. BD-26T(20) | Sediment, Hawaii | Antibacterial activity | Sitachitta et al.1996 |
| 60 | Streptomyces sp. B 8300 | Sediment, Gulf of Mexico | Antibacterial activity | Biabani et al. 1997 |
| 61 | Streptomyces sp. CNB-689 | Sediment, Christchurch, New Zealand | Antibacterial activity | Jiang et al 1997 |
| 62 | Streptomyces sp. strain B 8251 | Sediment, Gulf of Mexico | Antibacterial activity | Pusecker et al.1997 |
| 63–64 | Streptomyces sp. M428 | Sediment, Geomun island | Antifungal activity | Cho et al. 1999 |
| 65 | StreptomycesMSTMA190 | Sediment, Victorian | Antibacterial activity | Capon et al. 2000 |
| 66 | Streptomyces sp. 1010 | Sediment, Livingston | Antibacterial activity | Ivanova et al. 2001 |
| 67 | Streptomyces sp. B7064 | Sediment, Hawaii | Antibacterial activity | Asolkar et al 2002. |
| 68 | Streptomyces sp. BD21–2 | Sediment,Kailua Beach, Oahu, Hawaii | Antibacterial and antifungal activity | Schumacher et al. 2003 |
| 69 | S. nodosus NPS007994 | Sediment, Scripps Canyons, La Jolla | Antibacterial activity | Manam et al. 2005 |
| 70–71 | Streptomyces sp.CNQ-085 | Sediment, San Diego, CA. | Antifungal activity | Asolkar et al. 2006 |
| 72 | Streptomyces sp.QD518 | Sediment, Jiaozhou Bay, China | Antibacterial activity | Wu et al. 2006 |
| 73 | Streptomyces sp.B8000 | Sediment, Gulf of Mexico | Antibacterial activity | Poumale et al. 2006 |
| 74–76 | Streptomyces sp CNQ-418 | Sediment, La Jolla, CA | Antibacterial activity | Hughes et al. 2008; Hughes et al. 2010 |
| 77 | Streptomyces sp. MS239 | Sediment, Tokushima, Japan. | Antibacterial activity | Motohashi K et al. 2008 |
| 78 | Streptomyces sp. Merv8102 | Sediment, Mediterranean Sea, Egypt | Antibacterial activity | El-Gendy et al. 2008 |
| 79 | Streptomyces sp. 307–9 | Sediment,Salt Cay, U.S. Virgin Islands | Antibacterial activity | Carlson et al. 2009 |
| 80 | Streptomyces sp. CMB- M0406 | Sediment, Heron island, Australai | Antifungal activity | Sugiyama et al. 2014 |
| 81–83 | Streptomyces sp. CMB- M0423 | Sediment Heron Island, Queensland | Antibacterial activity | Raju et al. 2012 |
| 84–85 | S. antibioticus H74–18 | Sediment, South China Sea | Antifungal activity | Xu et al. 2011 |
| 86–88 | Streptomyces sp. CNS-575 | Sediment, Figi island | Antibacterial activity | Sun et al. 2011 |
| 89 | Streptomyces species B8112 | Sediment, Gulf of Mexico | Antifungal activity | Shaaban et al. 2011 |
| 90 | Streptomyces sp. SCSIO 01127 | Sediment, South China Sea | Antibacterial activity | Niu et al. 2011 |
| 91–96 | Streptomyces sp. CNH-189 | marine sediments, retrieved off shore of Oceanside, California. | Antibacterial activity | Wilson et al. 2011; Ryu et al. 2019 |
| 97–98 | Streptomyces sp. 211726 | rhizosphere soil of Heritiera globose, Wenchang, China | Antifungal activity | Yuan et al. 2011 |
| 99 | Streptomyces sp. CMB- M0392 | Sediment, Heron Island, Queensland | Antibacterial activity | Raju et al. 2012 |
| 100 | Streptomyces sp. CNQ343 | Sediment, North Cat Cay, Bahamas | Antifungal activity | Kim et al. 2012 |
| 101 | Streptomyces sp. LB173 | Sediment, Baltic Sea, Germany | Antibacterial activity | Ohlendorf etal. 2012 |
| 102–105 | Streptomyces sp. SCSIO 02999 | Sediment, South China Sea | Antibacterial activity | Zhang et al.,2012 |
| 106 | Streptomyces sp. CP13–10 | Sediment, SanFrancisco Bay, CA | antifungal activity | Amagata et al. 2012 |
| 107–109 | Streptomyces sp. RJA2961 | Sediment, British Columbia coast | Antibacterial activity | Dalisay et al. 2013 |
| 110–112 | S. antibioticus PTZ0016 | Sediment, Unknown place | Antibacterial activity | Lian et al. 2013 |
| 113–115 | S. niveus SCSIO 3406 | Sediment, South China Sea | Antibacterial activity | Song et al., 2013 |
| 116 | Streptomyces sp. MS100061 | Sediment, South China Sea | Antibacterial activity | Chen et al. 2013 |
| 117–118 | Streptomyces sp.CNQ-329 | Sediment, San Diego, CA. | Antibacterial activity | Cheng et al. 2013 |
| 119–121 | Streptomyces sp. SCSIO 10428 | Sediment, Beihai, Guangxi, China | Antibacterial activity | Wu et al. al.,2013a |
| 122 | Streptomyces sp. CNH365 | Sediment, Santa Barbara, CA | Antibacterial activity | Jang et al. 2013 |
| 123–124 | Streptomyces sp. 7–145 | Sediment, Heishijiao Bay, China, | Antibacterial activity | Wu et al. 2013b |
| 125–126 | Streptomyces sp. SNJ042 | Sediment, jeju Island | Antibacterial activity | Um et al. 2013 |
| 127 | Streptomyces sp. 12A35 | Sediment, South China Sea | Antibacterial activity | Pan et al. 2013 |
| 128 | Streptomyces sp. CMBM0244 | Sediment, Molle Island, Queensland | Antibacterial activity | Raju et al.,2014 |
| 129–133 | S . drozdowiczii SCSIO 10141 | Sediment, South China Sea | Antibacterial activity | Zhou al.,2014 |
| 133 | S . scopuliridis SCSIO ZJ46 | Sediment, South China Sea | Antibacterial activity | Song et al. 2014 |
| 135–136 | Streptomyces sp. 06CH80 | Sediment, Chuuk, Federated States of Micronesia and Ieodo, Korea | Antibacterial activity | Mondol et al. 2014 |
| 137 | Streptomyces sp. SNR69 | tidal mudflat in Buan, Korea | Antibacterial activity | Moon et al. 2015 |
| 138–139 | Streptomyces sp. CMB- M0150 | sediment collected off the Sunshine Coast, Queensland, Australia | Antibacterial activity | Khalil et al. 2015 |
| 140–143 | Streptomyces sp. SNM5 | intertidal zone mudflat, Mohang, Korea | Antibacterial:142–143 antifungal activity: 140–141 | Bae et al. 2015a and b |
| 144–146 | Streptomyces zhaozhouensis CA-185989 | Sediment, Utonde, Equatorial Guinea. | Antibacterial:144–146 antifungal activity: 144–145 | Lacret et al. 2015 |
| 147–148 | S . rochei 06CM016 | sediment sample, Kaş, Turkey | Antibacterial and antifungal activity | Aksoy et al. 2016 |
| 149 | Streptomyces sp. 182SMLY 06CM016 | Sediment, East China Sea | Antibacterial | Liang et al. 2016 |
| 150–151 | Streptomyces sp. IMB094 | marine sediment, Heishijiao Bay, Dalian, China. | Antibacterial activity: | Wang et al. 2017 |
| 152 | Streptomyces chartreusis NA02069 | marine sediment, Hainan Island, Dalian, China. | Antibacterial activity: | Yang et al. 2017 |
| 153–156 | Streptomyces chartreusis XMA39 | marine sediment, Xiamen Island, Fujian, China. | Antibacterial and antifungal activity | Jiang et al.2018 |
| 157–158 | Streptomyces sp. ZZ745 | marine sediment, Zhejiang, China. | Antibacterial activity | Zhang et al. 2018b |
| 159–160 | Streptomyces Pratensis NA-ZhouS1 | Marine sediment, Zhoushan, China. | Antibacterial activity | Akhter et al. 2018 |
| 161 | Streptomyces xinghaiensis SCSIO S15077 | Marine sediment, South China Sea, China. | Antibacterial and antifungal activity | Zhang et al. 2018c |
| 162 | Streptomyces sp. ZZ446 | coastal soil | Antibacterial and antifungal activity | Chen et al. 2018a |
| 163–166 | Streptomyces sp. IMB7– 145 | Marine sediment, Daliang, China. | Antibacterial: 163 antifungal activity :163–166 | Hu et al. 2018 |
| 167 | Streptomyces mutabilis sp. MII | Marine sediment, Red Sea, Hurghada Coast | Antibacterial activity | Hamed et al. 2018a |
| 168 | S . varsoviensis HF-11225 | Marine sediment, East Sea, Hurghada Coast | Antifungal activity | Chen et al. 2018b |
| 169–171 | Streptomyces sp. OPMA 1245 | Marine sediment, Okinawa prefecture, Japan | Antibacterial activity | Hosoda et al. 2019 |
| 172–174 | Streptomyces sp. ZZ820 | coastal soil | Antibacterial activity | Yi et al. 2019 |
| 175–176 | Streptomyces sp. G212 | Sediment, Quang Binh- Vietnam | Antibacterial:176 Antifugal: 175 | Cao et al. 2019 |
| 177–186 | Streptomyces sp. ZZ741 | marine mud, the coastal area of Jintang Island, Zhoushan, China | Antibacterial and Antifungal activity | Zhang et al. 2019a |
| 187 | Streptomyces atratus SCSIOZH16 | sediment sample | Antibacterial activity | Sun et al. 2019 |
| 188–191 | Streptomyces sp. B8652 | Sediment, Laguna de Terminos, Gulf of Mexico | Antibacterial activity | Maskey et al. 2002; Maskey et al. 2004 |
| 192–195 | Streptomyces caelestis | coastal water of the Red Sea, near Jeddah | Antibacterial activity | Liu et al. 2012 |
| 196 | Streptomyces sp. TPU1236A | Seawater, Okinawa, Japan | Antibacterial activity | Bu et al. 2014 |
| 197–200 | Streptomyces caniferus CA-271066 | Unknown source | Antifugal activity | Pérez-Victoria et al. 2019 |
| 201–202 | Micromonospora lomaivitiensis LL-37I366 | ascidian | Antibacterial activity | He et al. 2001 |
| 203 | Micromonospora sp. DPJ12 | Didemnum proliferum Kott, Japan | Antibacterial activity | Charan et al. 2004 |
| 204 | Micromonospora sp.WMMC-218 | Ascidian, Florida | Antibacterial activity | Zhang et al. 2016a |
| 205 | Micromonospora carbonacea LS276 | Sponge, Hainan, China | Antibacterial activity | Gong et al. 2018 |
| 206 | Micromonospora sp. K310 | sediment, Ghanaian | Antibacterial activity | Kyeremeh K et al. 2014 |
| 207–208 | Micromonospora sp.5–297 | sediment, Dalian, China | Antibacterial activity | Tan et al. 2016 |
| 209–210 | Micromonospora sp. G019 | sediment, Viet Nam | Antibacterial activity:209–210 Antifugal : 210 | Thi et al. 2016a |
| 211–214 | Micromonospora sp. RJA4480 | Marine sediment BarkleySound, British Columbia | Antibacterial activity | Williams et al. 2017 |
| 215–221 | Micromonospora harpali SCSIO GJ089 | marine sediment, South China Sea | Antibacterial activity | Gui et al. 2017 |
| 222–223 | Micromonospora sp. CA-214671 | Marine sediment, Canary Island | Antibacterial activity | Pérez-Bonilla et al. 2018 |
| 224 | Micromonospora sp. L-13- ACM2–092 | Unknown source | Antibacterial activity | Perez Baz et al. 1997 |
| 225 | Nocardiopsis dassonvillei | sediment sample, island of Kauai, Hawaii. | Antibacterial activity | Schumacher et al. 2001 |
| 226 | Nocardiopsis sp. TFS65– 07 | sediment sample, Trondheim Fjord, Norway | Antibacterial activity | Engelhardt et al. 2010 |
| 227–229 | Nocardiopsis dassonvillei HR10–5 | marine sediment, Yellow River. | Antibacterial activity | Fu et al. 2011 |
| 230–231 | Nocardiopsis sp. CNQ115 | marine sediment, the coast of southern California | Antibacterial activity | Leutou et al. 2015 |
| 232–234 | Nocardiopsis sp. SCSIO 10419 | marine sediment, Xieyang Island, Beihai, Guangxi, China | Antibacterial activity | Zhang et al. 2016b |
| 235 | Nocardiopsis sp. G057 | marine sediment, Cô Tô- Quảng Ninh in Vietnam | Antibacterial activity | Thi et al. 2016b |
| 236 | Nocardiopsis sp YIM M13066 | marine sediment, Cô Tô- Quảng Ninh in Vietnam | Antibacterial activity | Sun et al. 2017 |
| 237 | Nocardiopsis sp. CNQ-115 | marine sediment, Southern California | Antibacterial activity | Leutou et al. 2018 |
| 238 | Nocardiopsis sp. LGO5 | Marine sediment, Helwan, Egypt | Antibacterial activity | Hamed et al. 2018b |
| 239–241 | Nocardiopsis sp. HB-J378 | marine sponge Theonella sp. | Antibacterial activity | Xu et al. 2018 |
| 242 | Micrococcus luteus | sponge Xestospongia sp., New Caledonia | Antibacterial activity | Bultel-Poncé et al. 1998 |
| 243 | Micrococcus sp. EG45 | Red Sea sponge Spheciospongia vagabunda | Antibacterial activity | Eltamany et al. 2014 |
| 244 | Kocuria Palustris | Sponge, Florida Keys | Antibacterial activity | Martín et al. 2013 |
| 245 | Kocuria Palustris | Sponge | Antibacterial activity | Takasaka et al. 2017 |
| 246–248 | Saccharothrix espanaensis An 113 | a marine mollusc the Great Bay, Sea of Japan, Russia | Antibacterial activity | Kalinovskaya et al. 2008 |
| 249 | Salinispora arenicola CNR-647 | ascidian Ecteinascidia turbinata, Sweetings Cay, Grand Bahama Island | Antibacterial activity | Asolkar et al. 2010 |
| 250–251 | Solwaraspora sp. WMMB329 | ascidian Trididemnum orbiculatum | Antibacterial activity | Ellis et al. 2014 |
| 252 | Actinomadura sp. WMMB-499 | ascidian Ecteinascidia turbinata | Antifungal activity | Wyche et al. 2014 |
| 253–254 | Pseudonocardia sp HS7. | the cloacal aperture of sea cucumber Holothuria moebii. | Antibacterial activity | Ye et al. 2016 |
| 255 | Actinomadura sp. | ascidian Ecteinascidia turbinata | Antibacterial activity | Wyche et al. 2017 |
| 256–257 | Lechevalieria aerocolonigenes K10–0216 | Mangrove, Iriomote island | Antibacterial and antifungal activity | Kimura et al. 2018 |
| 258 | Lechevalieria aerocolonigenes K10–0216 | brown seaweed Pelvetia canaliculata (Linnaeus), the rocks of Sonmiani Beach (Karachi, Pakistan) | Antibacterial activity | Uzair et al. 2018 |
| 259–261 | Actinomadura sp. M045 | Sediment, Jiaozhou Bay. | Antifugal :259–261 Antibacterial activity:261 | Maskey et al. 2003 |
| 262 | Verrucosispora sp. AB-18– 032 | sediment | Antibacterial activity | Bister et al. 2004 |
| 263–267 | Marinispora sp. CNQ-140 | sediment, La Jolla, California | Antibacterial activity:263–266 Antifugal:263 and 267 | Kwon et al. 2006; Kwon et al. 2009 |
| 268 | Verrucosispora sp. AB-18– 032 | Sediment, Sea of Japan | Antibacterial activity | Keller et al. 2007 |
| 269–271 | Marinispora NPS008920 | sediment, Cocos Lagoon, Guam | Antibacterial activity | Macherla et al. 2007 |
| 272–276 | Marinispora sp. NPS12745 | sediment, the coast of San Diego, California | Antibacterial activity | McArthur et al. 2008 |
| 277–278 | Verrucosispora maris AB-18–032 | sediment, Raune Fjord, Norway | Antibacterial activity | Fiedler et al. 2008 |
| 279 | Salinispora arenicora YM23–082 | sediment, Yap, Micronesia | Antibacterial activity | Matsuda et al. 2009 |
| 280 | Salinispora arenicola | sediment, Palau | Antibacterial activity | Murphy et al. 2010 |
| 281–283 | Pseudonocardia sp. SCSIO 01299 | sediment, the South China Sea | Antibacterial activity | Li et al. 2011 |
| 284–292 | Actinoalloteichus sp. NPS702 | sediment, Usa Bay, Kochi Prefecture, Japan | Antifungal activity | Sato et al. 2012 |
| 293 | Marinactinospora thermotolerans SCSIO 00652 | sediment, the South China Sea | Antibacterial activity | Zhou et al. 2012 |
| 294 | Amycolatopsis alba var. nov. DVR D4 | sediments from, Bay of Bengal | Antibacterial activity | Dasari et al. 2012 |
| 295 | Serinicoccus profundi sp. nov. | a deep-sea sediment, Indian Ocean | Antibacterial activity | Yang et al. 2013 |
| 296 | Verrucosispora sp. MS100047 | sediment, the South China Sea | Antibacterial activity | Huang et al. 2016 |
| 297–300 | Kribbella sp. MI481–42F6 | sediment, Japna | Antifungal activity | Igarashi et al. 2017 |
| 301 | Actinomadura sp. DS-MS-114 | sediment, Sagami Bay | Antibacterial activity | Kurata et al. 2017 |
| 302–304 | Verrucosispora sp. SCSIO 07399 | sediment, the South China Sea | Antibacterial activity | Zhang et al. 2019b |
| 305–308 | Salinispora arenicola BRA-213 | sediment, St.Peter and St. Paul Archipelago, Brazil | Antibacterial activity | da Silva et al. 2019 |
| 309 | unidentified marine bacterium of the order Actinomycetales | the shallow waters of Bodega Bay | Antibacterial activity | Pathirana et al. 1991 |
| 310 | Saccharomonospora sp. CNQ-490 | Unknown source | Antibacterial activity | Yamanaka et al. 2014 |
| 311–312 | Pseudonocardia carboxydivorans M-227 | deep seawater of the Aviles submarine Canyon | Antibacterial activity | Braña et al. 2017b |
| 313 | Thermoactinomyces vulgaris ISCAR 2354 | coastal hot spring, Icelandic marine waters | Antibacterial activity | Teta et al. 2017 |
Fig. 12.
Annual numbers of antimicrobial compounds identified (1976‒2019)
Table 2.
The initial research on antimicrobial active compounds from actinomycetes
| Fist Producing Strain | Environment source | Compound. | Time |
|---|---|---|---|
| Streptomyces griseus SS-20 | shallow sea sediment, Sagami Bay | Aplasmomycin A | 1976 |
| Micromonospora sp. L-13-ACM2–09 | Unknown source | Thiocoraline | 1997 |
| Nocardiopsis dassonvillei | sediment sample, island of Kauai, Hawaii | Kahakamide A | 2001 |
| Other actinomycetes (unidentified marine bacterium of the order Actinomycetales) | the shallow waters of Bodega Bay | Maduralide | 1991 |
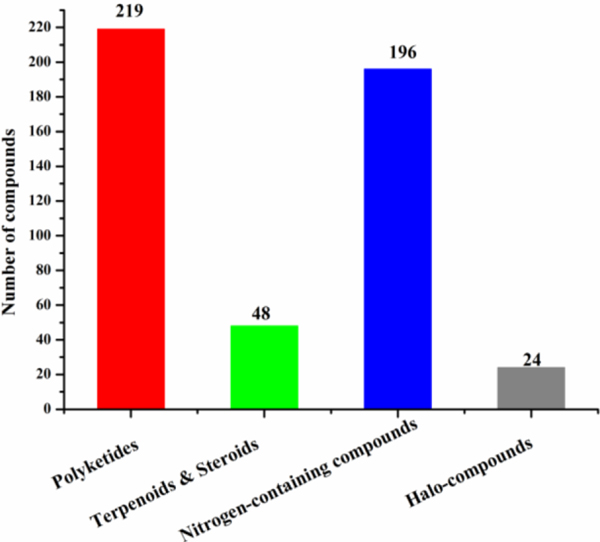
These new marine natural products from actinomycetes have different types of structural skeletons including nitrogen-containing compounds, sterols and terpenoids, polyketides, and others (Fig. 13). Polyketides and nitrogen-containing compounds (e.g., alkaloids and peptides) are the two main classes (Fig. 13). Because of high halogen concentrations in the Ocean when compared with that on Land, marine actinomyces produced more halogen-containing compounds than their terrestrial counterparts. None of the terpenoids and steroids among the 313 compounds cited in this review article showed potent antimicrobial activity when compared with the other classes of compounds. Compounds 74 and 76, halogenated alkaloids each with two pyrrolphenone moieties inhibited MRSA with an MIC value less than 1 μg/mL (Hughes et al. 2008 and Hughes et al. 2010). Compounds 201, 202 and 211‒214 are polyketides-derived 1,4-naphthoquinone alkaloids. Compounds 201 and 202 inhibited S. aureus and E. faecium with MIC values ranging from 6 to 25 ng/spot (He et al. 2001). Compounds 211‒214 inhibited MRSA, E.coli and M. tuberculosis with MIC values in the range of 0.3–0.9, 0.1–0.8, and 60–1800 ng/mL, respectively (Williams et al. 2017). Compounds 16‒19 are bicyclic nitrogen-containing compounds each with a phenoxazine bridge. One cyclic peptide fragment (threonine-valine-proline-glycine-valine) was connected to one aromatic ring through an amide bond, and another cyclic peptide fragment (threonine-valine-proline-glycine-valine) was connected to another aromatic ring also through an amide bond. Compounds 16‒19 inhibited MRSA with MIC values less than 1.0 μg/mL (Jiao et al. 2018). Compound 128, a cyclic peptide, exhibited potent antibacterial activity at nanomolar concentrations (Raju et al. 2014). Cyclic peptides 129‒133 inhibited M. luteus with MIC values in the range of 0.061–4.00 μg/mL (Zhou et al. 2014). Compounds 169‒171 are cyclic peptides with some nonstandard amino acids (169 and 171) or hybrids of polyketide and peptide (170). Compounds169 and 171 strongly inhibited M. avium JCM15430, M. intracellulare JCM6384 and M. bovis BCG Pasteur with MIC values in the range of 12 to 780 ng/mL (Hosoda et al. 2019). Besides 170 (hybrids of polyketide and peptide), 201, 202 and 211‒214 (1,4-naphthoquinone alkaloids derived from polyketides), some other polyketides (for examples, 25, 32‒34, 122, 255, and 263‒266) also demonstrated potent antimicrobial activity. Compounds 32‒34 are polyketide anthraquinone derivatives, among which compounds 32 and 33 inhibited MRSA with IC50 values of 0.15 and 0.36 μM, respectively (Socha et al. 2006). Compound 255 is a polyketide derived polyether. It inhibited MRSA, MSSA and C. difficile with MIC values in the range of 59‒125 ng/mL (Wyche et al. 2017). The macrolides 263‒266 are polyketide polyenes, and they inhibited MRSA with MIC90 values in the range of 0.13‒0.25 μM. Other two macrolides 25 (Rodríguez et al. 2018) and 122 (Jang et al. 2013) also exhibited antibacterial activity at ng/mL level. Glycosylated macrolides 135 and 136 inhibited B. subtilis, E. coli, P. aeruginosa, S. aureus and S. cerevisiae with MIC values in the range of 0.027 to 0.22 μM (Mondol et al. 2014).
Fig. 13.
Structural classes of antimicrobial compounds isolated from marine actinomycetes (1976‒2019)
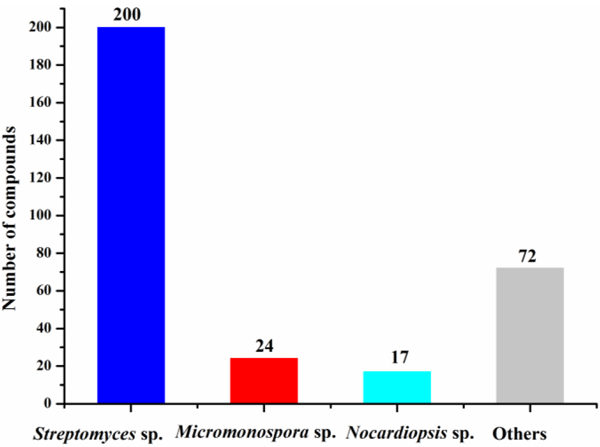
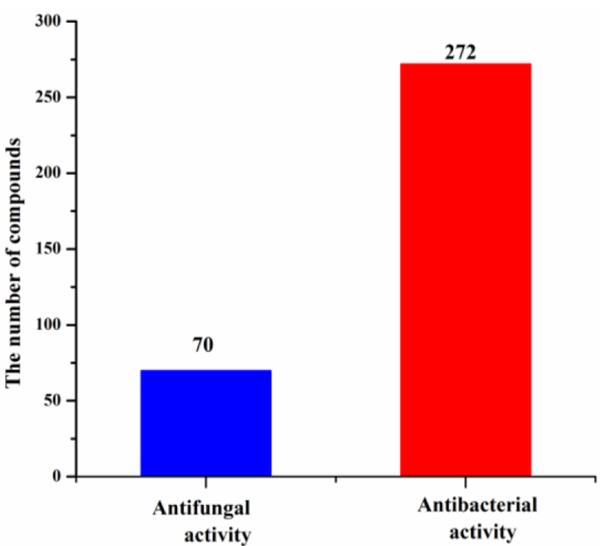
Marine actinomycetes are efficient producers of new secondary metabolites. The numbers of antimicrobial compounds from marine Streptomyces sp., Micromonospora sp., Nocardiopsis sp. and the other actinomycetes except Streptomyces sp., Micromonospora sp., and Nocardiopsis sp. were 200, 24, 17 and 72, respectively (Fig. 14), among which about 64% were produced by Streptomyces sp. Other actinomycetes (for examples, Micromonospora, Nocardiopsis, Salinispora and Pseudonocardia) are also prolific producers of secondary metabolites in the marine environment. The numbers of antibacterial and anti-fungal compounds identified from marine actinomycetes are 272 and 70, respectively (Fig. 15).
Fig. 14.
Numbers of antimicrobial compounds from different marine actinomycetes (1976‒2019)
Fig. 15.
Numbers of antibacterial and anti-fungal compounds from marine actinomycetes (1976‒2019)
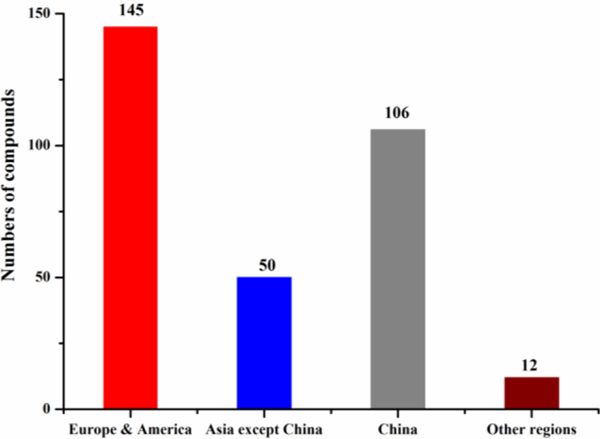
Scholars in Europe and America, China and other Asian countries published 145, 106 and 50 antimicrobial compounds, respectively (Fig. 16). Different from the antimicrobial study of marine fungi in which Chinese scientists are the most productive in recent years, researchers in Europe and America published 156 antimicrobial compounds from marine actinomycetes, slightly more than scholars in Asia who reported 145 antimicrobial compounds.
Fig. 16.
Numbers of antimicrobial marine metabolites by different countries (1976‒2019)
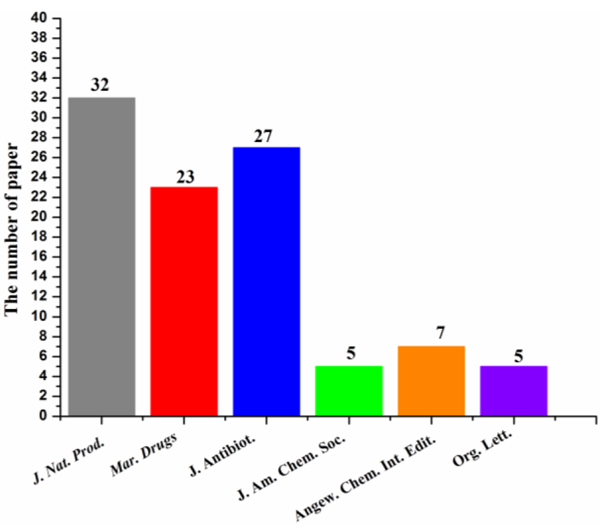
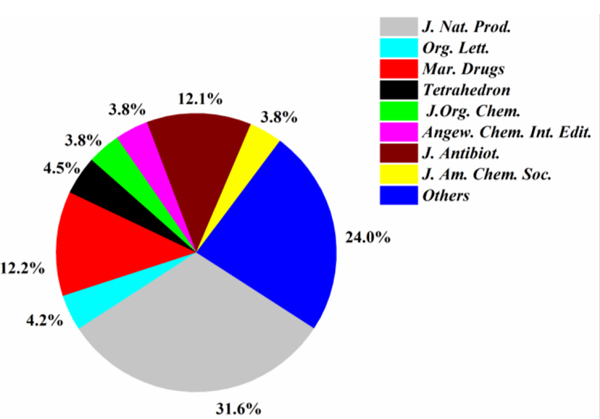
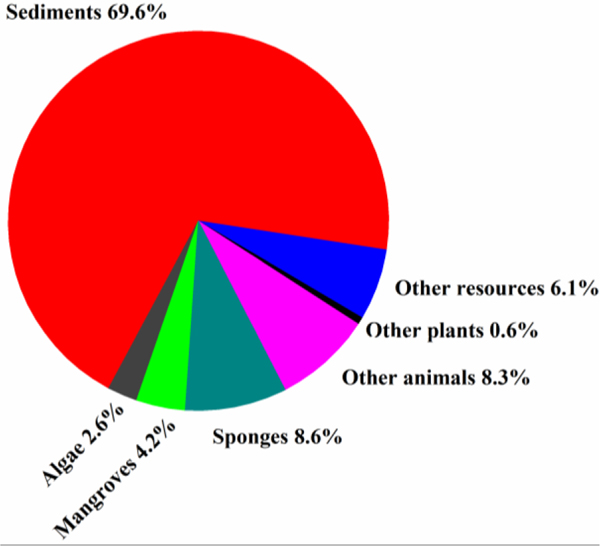
J. Nat. Prod. attracted the most contributions (32 articles), followed by J. Antibiot. (27 articles) and Mar. Drugs (23 articles), which accounts for 83% (= 82/99) of all the published papers (Fig. 17). Nearly one-third (31.6%) of all the new antimicrobial compounds were pubished in J. Nat. Prod. followed by Mar. Drugs (12.2%) and by J. Antibiot. (12.1%) (Fig. 18). The dominant host of actinomycetes was marine sediment with a ratio of 69.6% (Fig. 19). Marine animals were also good hosts for actinomycetes (16.9%). Rare marine actinomycetes (for example, Salinispora sp. from deep-sea sediments) in combination of new screening approach will provide more antimicrobial agents.
Fig. 17.
Journals and numbers of papers that published antimicrobial compounds (1976‒2019)
Fig. 18.
Percentages of antimicrobial compounds published in different journals (1976‒2019)
Fig. 19.
Percentages of antimicrobial compounds on the basis of the hosts of actinomycetes (1976‒2019)
Acknowledgements
This work was financially supported mainly by Seed Grants from University of Hawaii at Hilo (UHH), start-up funding from University of Hawaii Cancer Center and Daniel K. Inouye College of Pharmacy (DKICP), and the Victoria S. and Bradley L. Geist Foundation (15ADVC-74420 and 17CON-86295) (to SC). Funding for this work was also supported by Hawaii IDeA Network for Biomedical Research Excellence III and IV (INBRE-III and INBRE-IV) project: NIGMS Grant 5P20GM103466.
Footnotes
Compliance with ethical standards
Conflict of interest The authors declare that they have no conflict of interest.
References
- Akhter N, Liu YQ, Auckloo B N, Shi YT, Wang KW, Chen JJ, Wu XD, Wu B (2018) Stress–driven discovery of new angucycline–type antibiotics from a marine Streptomyces pratensis NA–ZhouS1. Mar Drugs 16:331 DOI: 10.3390/md16090331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksoy SC, Uzel A, Bedir E (2016) Cytosine–type nucleosides from marine–derived Streptomyces rochei 06CM016. J Antibiot (Tokyo) 69:51–56. DOI: 10.1038/ja.2015.72 [DOI] [PubMed] [Google Scholar]
- Amagata T, Xiao J, Chen YP, Holsopple N, Oliver AG, Gokey T, Guliaev AB, Minoura K (2012) Creation of an HDAC–based yeast screening method for evaluation of marine–derived actinomycetes: discovery of streptosetin A. J Nat Prod 75:2193–2199. DOI: 10.1021/np300640g [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asolkar RN, Jensen PR, Kauffman CA, Fenical W (2006) Daryamides A–C, weakly cytotoxic polyketides from a marine–derived actinomycete of the genus Streptomyces Strain CNQ–085. J Nat Prod 69:1756–1759. DOI: 10.1021/np0603828 [DOI] [PubMed] [Google Scholar]
- Asolkar RN, Kirkland TN, Jensen PR, Fenical W (2010) Arenimycin, an antibiotic effective against rifampin– and methicillin–resistant Staphylococcus aureus from the marine actinomycete Salinispora arenicola. J Antibiot 63:37–39. DOI: 10.1038/ja.2009.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asolkar RN, Maskey RP, Helmke E, Laatsch H (2002) Chalcomycin B, a new macrolide antibiotic from the marine isolate Streptomyces sp. B7064. J Antibiot (Tokyo), 55:893–898. DOI: 10.7164/antibiotics.55.893 [DOI] [PubMed] [Google Scholar]
- Bae M, Chung B, Oh KB, Shin J, Oh DC (2015b) Hormaomycins B and C: new antibiotic cyclic depsipeptides from a marine mudflat–derived Streptomyces sp. Mar Drugs 13:5187–5200. DOI: 10.3390/md13085187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae M, Kim H, Moon K, Nam SJ, Shin J, Oh KB, Oh DC. (2015a) Mohangamides A and B, new dilactone-tethered pseudo-dimeric peptides inhibiting Candida albicans isocitrate lyase. Org Lett 17:712–715. DOI: 10.1021/ol5037248 [DOI] [PubMed] [Google Scholar]
- Biabani MAF, Laatsh H, Helmke E, Weyland H (1997) δ-Indomycinone: a new member of pluramycin class of antibiotics isolated from marine Streptomyces sp. J Antibiot (Tokyo), 50:874–877. DOI: 10.7164/antibiotics.50.874 [DOI] [PubMed] [Google Scholar]
- Bister B, Bischoff D, Ströbele M, Riedlinger J, Reicke A, Wolter FE, Bull AT, Zahner H, Fiedler H, Sussmuth RD (2004) Abyssomicin C–A Polycyclic Antibiotic from a Marine Verrucosispora Strain as an Inhibitor of the p-Aminobenzoic Acid/Tetrahydrofolate Biosynthesis Pathway. Angew Chem Int Edit 43:2574–2576. DOI: 10.1002/anie.200353160 [DOI] [PubMed] [Google Scholar]
- Braña AF, Sarmiento-Vizcaíno A, Osset M, Pérez-Victoria I, Martín J, De Pedro N, Díaz C, Vicente F, Reyes F, García LA, Blanco G (2017a) Lobophorin K, a new natural product with cytotoxic activity produced by Streptomyces sp. M-207 associated with the deep–sea coral Lophelia pertusa. Mar Drugs 15:144 DOI: 10.3390/md15050144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braña AF, Sarmiento-Vizcaíno A, Pérez-Victoria I, Martín J, Otero L, Palacios-Gutiérrez JJ, Fernández J, Mohamedi Y, Fontanil T, Salmón M, Cal S, Reyes F, García LA, Blanco G (2019) Desertomycin G, a new antibiotic with activity against Mycobacterium tuberculosis and human breast tumor cell lines produced by Streptomyces althioticus MSM3, isolated from the Cantabrian Sea Intertidal Macroalgae Ulva sp. Mar Drugs 17:114 DOI: 10.3390/md17020114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braña AF, Sarmiento-Vizcaíno A, Pérez-Victoria I, Otero L, Femandez J, Palacios J, Martin J, Ceuz MDL, Diaz C, Vicente F, Reyes F, Garcia LA, Blanco G (2017b) Branimycins B and C, antibiotics produced by the abyssal actinobacterium Pseudonocardia carboxydivorans M-227. J Nat Prod 80:569–573. DOI: 10.1021/acs.jnatprod.6b01107 [DOI] [PubMed] [Google Scholar]
- Bultel-Poncé V, Debitus C, Berge JP, Cerceau C, Guyot M (1998) Metabolites from the sponge-associated bacterium Micrococcus luteus. J Mar Biotechnol 6:233–236. [PubMed] [Google Scholar]
- Bu YY, Yamazaki H, Ukai K, Namikoshi M (2014). Anti-mycobacterial nucleoside antibiotics from a marine-derived Streptomyces sp. TPU1236A. Mar Drugs 12:6102–6112. DOI: 10.3390/md12126102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao DT, Nguyen TL, Tran VH, Doan-Thi-Mai H, Vu-thi Q, Nguyen MA, Le-Thi H, Chau VM, Pham VC (2019) Synthesis, structure and antimicrobial activity of novel metabolites from a marine actinomycete in Vietnam’s East Sea. Nat Prod Commun 14:121–124. DOI: 10.1177/2F1934578X1901400132 [DOI] [Google Scholar]
- Capon RJ, Skene C, Lacey E, Gill JH, Wicker J, Heiland K, Friedel T (2000) Lorneamides A and B: two new aromatic amides from a southern Australian marine actinomycete. J Nat Prod 63(12):1682–1683. DOI: 10.1021/np000241k [DOI] [PubMed] [Google Scholar]
- Carlson JC, Li SY, Burr DA, Sherman DH (2009) Isolation and characterization of tirandamycins from a marine-derived Streptomyces sp. J Nat Prod 72:2076–2079. DOI: 10.1021/np9005597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charan RD, Schlingmann G, Janso JE, Bernan VS, Feng XD, Carter GT (2004) Diazepinomicin, a new antimicrobial alkaloid from a marine Micromonospora sp. J Nat Prod 67:1431–1433. DOI: 10.1021/np040042r [DOI] [PubMed] [Google Scholar]
- Chen H, Cai K, Yao R (2018b) A new macrolactam derivative from the marine actinomycete HF-11225. J Antibiot 71:477–479. DOI: 10.1038/s41429-017-0021-z [DOI] [PubMed] [Google Scholar]
- Chen S, Zhang D, Chen M, Zhang Z, Lian XY (2018a) A rare diketopiperazine glycoside from marine-sourced Streptomyces sp. ZZ446. Nat Prod Res 1–5. DOI: 10.1080/14786419.2018.1544978 [DOI] [PubMed] [Google Scholar]
- Chen CX, Wang J, Guo H, Hou W, Yang, Ren B, Liu M, Dai H, Liu X, Song F, Zhang L (2013) Three antimycobacterial metabolites identified from a marine–derived Streptomyces sp. MS100061. Appl Microbiol Biotechnol 97:3885–3892. DOI: 10.1007/s00253-012-4681-0 [DOI] [PubMed] [Google Scholar]
- Cheng YB, Jensen PR, Fenical W (2013) Cytotoxic and antimicrobial napyradiomycins from two marine-derived, MAR4 Streptomyces strains. European J Org Chem 2013: 3751–3757 DOI: 10.1002/ejoc.201300349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho KW, Seo YW, Yoon TM, Shin JH (1999) Purification and structure determination of antifungal phospholipids from a marine Streptomyces. J Microbiol Biotechn 9:709–715 [Google Scholar]
- Cong Z, Huang X, Liu Y, Liu Y, Wang P, Liao S, Wang B, Zhou X, Huang D, Wang J (2019) Cytotoxic anthracycline and antibacterial tirandamycin analogues from a marine-derived Streptomyces sp. SCSIO 41399. J Antibiot 72:45–49. DOI: 10.1038/s41429-018-0103-6 [DOI] [PubMed] [Google Scholar]
- Da Silva AB, Silveira ER, Wilke DV, Ferreira EG, Costalotufo LV, Torres MCM, Ayala AP, Costa WS, Canuto KM, Araujonobre ARD, Araujo AJ, Filho JDBM, Pessoa ODL (2019) Antibacterial salinaphthoquinones from a strain of the bacterium Salinispora arenicola recovered from the marine sediments of St. Peter and St. Paul Archipelago, Brazil. J Nat Prod 82:1831–1838. DOI: 10.1021/acs.jnatprod.9b00062 [DOI] [PubMed] [Google Scholar]
- Dalisay DS, Williams DE, Wang XL, Centko R, Chen J, Andersen RJ (2013) Marine sediment-derived Streptomyces bacteria from british columbia, canada are a promising microbiota resource for the discovery of antimicrobial natural products. PLoS ONE 8:1–14 DOI:https://dx.doi.org/10.1371%2Fjournal.pone.0077078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasari VRRK Muthyala MKK, Nikkuf MY Donthireddy SRR (2012) Novel Pyridinium compound from marine actinomycete, Amycolatopsis alba var. nov. DVR D4 showing antimicrobial and cytotoxic activities in vitro. Microbiol Res 167:346–351. DOI: 10.1016/j.micres.2011.12.003 [DOI] [PubMed] [Google Scholar]
- Ding L, Maier A, Fiebig HH, Görls H, Lin WH, Peschel G, Hertweck C (2011a) Divergolides A–D from a Mangrove Endophyte Reveal an Unparalleled Plasticity in ansa–Macrolide Biosynthesis. Angew Chem Int Ed 50:1630–1634. DOI: 10.1002/anie.201006165 [DOI] [PubMed] [Google Scholar]
- Ding L, Maier A, Fiebig HH, Lin WH, Hertweck C (2011b) A family of multicyclic indolosesquiterpenes from a bacterial endophyte. Org Biomol Chem 9:4029–4031. DOI: 10.1039/C1OB05283G [DOI] [PubMed] [Google Scholar]
- Ding L, Maier A, Fiebig HH, Lin WH, Peschel G, Hertweck C (2012) Kandenols A−E, eudesmenes from an endophytic Streptomyces sp. of the mangrove tree Kandelia candel. J Nat Prod 75:2223–2227. DOI: 10.1021/np300387n [DOI] [PubMed] [Google Scholar]
- Ding WJ, Zhang SQ, Wang JH, Lin YX, Liang QX, Zhao WJ, Li CY (2013) A new di-O-prenylated flavone from an actinomycete Streptomyces sp. MA-12. J Asian Nat Prod Res 15:209–214. DOI: 10.1080/10286020.2012.751979 [DOI] [PubMed] [Google Scholar]
- Djinni I, Defant A, Kecha M, Mancini I (2013) Antibacterial polyketides from the marine alga–derived endophitic Streptomyces sundarbansensis: a study on hydroxypyrone tautomerism. Mar Drugs 11:124–135. DOI: 10.3390/md11010124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El–Gendy MMA, Shaaban M, Shaaban KA, El-Bondkly AM, Laatsch H (2008) Essramycin: a first triazolopyrimidine antibiotic isolated from nature. J Antibiot (Tokyo) 61:149–157 DOI: 10.1038/ja.2008.124 [DOI] [PubMed] [Google Scholar]
- Ellis GA, Wyche TP, Fry CG, Braun DR, Bugni TS (2014) Solwaric acids A and B, antibacterial aromatic acids from a marine Solwaraspora sp. Mar Drugs 12:1013–1022. DOI: 10.3390/md12021013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltamany EE, Abdelmohsen UR, Ibrahim AK, Hassanean HA, Hentschel U, Ahmed SA (2014) New antibacterial xanthone from the marine sponge–derived Micrococcus sp. EG45. Bioorg Med Chem Lett 24:4939–4942. DOI: 10.1016/j.bmcl.2014.09.040 [DOI] [PubMed] [Google Scholar]
- Engelhardt K, Degnes KF, Kemmler M, Bredholt H, Fjaervik E, Klinkenberg G, Sletta H, Ellingsen TE, Zotchev SB (2010) Production of a new thiopeptide antibiotic, TP-1161, by a marine Nocardiopsis species. Appl Environ Microb 76:4969–4976. DOI: 10.1128/AEM.00741-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler HP, Bruntner C, Riedlinger J, Bull AT, Knutsen G, Goodfellow M, Jones A, Maldonado LA, Pathomaree W, Beil W, Schneider K, Keller S, Sussmuth RD (2008) Proximicin A, B and C, novel aminofuran antibiotic and anticancer compounds isolated from marine strains of the actinomycete Verrucosispora. J Antibiot 61:158–163. DOI: 10.1038/ja.2008.125 [DOI] [PubMed] [Google Scholar]
- Fu P, Liu PP, Qu HJ, Wang Y, Chen DF, Wang H, Li J, Zhu WM (2011) α-Pyrones and Diketopiperazine Derivatives from the Marine-Derived Actinomycete Nocardiopsis dassonvillei HR10–5. J Nat Prod 74:2219–2223. DOI: 10.1021/np200597m [DOI] [PubMed] [Google Scholar]
- Gong T, Zhen X, Li XL, Chen JJ, Chen TJ, Yang JL, Zhu P (2018) Tetrocarcin Q, a new spirotetronate with a unique glycosyl group from a marine–derived actinomycete Micromonospora carbonacea LS276. Mar Drugs 16:74 DOI: 10.3390/md16020074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui C, Zhang SW, Zhu XC, Ding WJ, Huang HB, Gu YC, Duan YW, Ju JH (2017) Antimicrobial spirotetronate metabolites from marine-derived Micromonospora harpali SCSIO GJ089. J Nat Prod 80:1594–1603. DOI: 10.1021/acs.jnatprod.7b00176 [DOI] [PubMed] [Google Scholar]
- Hamed A, Abdel–Razek AS, Frese M, Stammler H, Elhaddad AF, Ibrahim TM, Sewald N, Shaaban M (2018b) Terretonin N: a new meroterpenoid from Nocardiopsis sp. Molecules 23:299 DOI: 10.3390/molecules23020299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamed A, Abdel-Razek A S, Frese M, Wibberg D, Elhaddad A F, Ibrahim TM, Kalinowski J, Sewald N, Shaaban M (2018a) N-Acetylborrelidin B: a new bioactive metabolite from Streptomyces mutabilis sp. MII. Z Naturforsch C 73:49–57. DOI: 10.1515/znc-2017-0140 [DOI] [PubMed] [Google Scholar]
- Han Z, Xu Y, McConnell O, Liu L, Li Y, Qi S, Huang X, Qian P (2012) Two antimycin A analogues from marine-derived actinomycete Streptomyces lusitanus. Mar Drugs 10:668–676. DOI: 10.3390/md10030668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan HM, Degen D, Jang KH, Ebright RH, Fenical W (2015) Salinamide F, new depsipeptide antibiotic and inhibitor of bacterial RNA polymerase from a marine-derived Streptomyces sp. J Antibiot 68:206–209. DOI: 10.1038/ja.2014.122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He HY, Ding WD, Bernan VS, Richardson AD, Ireland CM, Greenstein M, Ellestad GA, Carter GT (2001) Lomaiviticins A and B, Potent antitumor antibiotics from Micromonospora lomaivitiensis. J Am Chem Soc 123:5362–5363. DOI: 10.1021/ja010129o [DOI] [PubMed] [Google Scholar]
- Hosoda K, Koyama N, Kanamoto A, Tomoda H (2019) Discovery of nosiheptide, griseoviridin, and etamycin as potent anti-mycobacterial agents against Mycobacterium avium complex. Molecules 24:1495 DOI: 10.3390/molecules24081495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Wang M, Wu C, Tan Y, Li J, Hao X, Duan Y, Guan Y, Shang X, Wang Y, Xiao C, Gan M (2018) Identification and proposed relative and absolute configurations of niphimycins C–E from the marine-derived Streptomyces sp. IMB7–145 by genomic analysis. J Nat Prod 81:178–187. DOI: 10.1021/acs.jnatprod.7b00859 [DOI] [PubMed] [Google Scholar]
- Huang P, Xie F, Ren B, Wang Q, Wang J, Wang Q, Abodeimageed WM, Liu MM, Han JY, Oyeleye A, Shen JZ, Song FH, Dai HQ, Liu XT, Zhang LX (2016) Anti-MRSA and anti-TB metabolites from marine-derived Verrucosispora sp. MS100047. Appl Microbiol Biot 100:7437–7447. DOI: 10.1002/anie.200353160 [DOI] [PubMed] [Google Scholar]
- Hughes CC, Kauffman CA, Jensen PR, Fenical W (2010) Structures, reactivities, and antibiotic properties of the marinopyrroles A–F. J Org Chem 75:3240–3250. DOI: 10.1021/jo1002054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes CC, Prieto–Davo A, Jensen PR, Fenical W (2008) The marinopyrroles, antibiotics of an unprecedented structure class from a marine Streptomyces sp. Org Lett 10:629–631. DOI: 10.1021/ol702952n [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi M, Sawa R, Yamasaki M, Hayashi C, Umekita M, Hatano M, FuJiwara T, Mizumoto K, Nomoto A (2017) Kribellosides, novel RNA 5′–triphosphatase inhibitors from the rare actinomycete Kribbella sp. MI481–42F6. J Antibiot 70:582–589. DOI: 10.1038/ja.2016.161 [DOI] [PubMed] [Google Scholar]
- Imamura N, Nishijima M, Adachi K, Sano H (1993) Novel antimycin antibiotics, urauchimycins A and B, produced by marine actinomycete. J Antibiot (Tokyo) 46:241–246. DOI: 10.7164/antibiotics.46.241 [DOI] [PubMed] [Google Scholar]
- Ivanova V, Oriol M, Montes MJ, García A, Guinea J (2001) Secondary metabolites from a Streptomyces strain isolated from Livingston Island, Antarctica. Z Naturforsch C 5:1–5. DOI: 10.1515/znc-2001-1201 [DOI] [PubMed] [Google Scholar]
- Jang KH, Nam SJ, Locke JB, Kauffman CA, Beatty DS, Paul LA, Fenical W (2013) Anthracimycin, a potent anthrax antibiotic from a marine–derived actinomycete. Angew Chem Int Ed 52:7822–78241. DOI: 10.1002/anie.201302749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang YJ, Zhang DS, Zhang HJ, Li JQ, Ding WJ, Xu CD, Ma ZJ (2018) Medermycin-type naphthoquinones from the marine–derived Streptomyces sp. XMA39. J Nat Prod 81:2120–2124. DOI: 10.1021/acs.jnatprod.8b00544 [DOI] [PubMed] [Google Scholar]
- Jiang ZD, Jensen PR, Fenical W (1997) Actinoflavoside, a novel flavonoid-like glycoside produced by a marine bacterium of the genus Streptomyces. Tetrahedron Lett 38:5065–5068. DOI: 10.1016/S0040-4039(97)01127-1 [DOI] [Google Scholar]
- Jiao WH, Yuan W, Li ZY, Li J, Li L, Sun JB, Gui YH, Wang J, Ye BP, Lin HW (2018) Anti-MRSA actinomycins D1–D4 from the marine sponge-associated Streptomyces sp. LHW52447. Tetrahedron 74:5914–5919. DOI: 10.1016/j.tet.2018.08.023 [DOI] [Google Scholar]
- Kalinovskaya NI, Kalinovsky AI, Romanenko LA, Pushilin MA, Dmitrenok PS, Kuznetsova TA (2008) New angucyclinones from the marine mollusk associated actinomycete Saccharothrix espanaensis An 113. Nat Prod Commun 3:10 DOI: [DOI] [PubMed] [Google Scholar]
- Keller S, Nicholson G, Drahl C, Sorensen EJ, Fiedler H, Sussmuth RD (2007) Abyssomicins G and H and atrop–abyssomicin C from the marine Verrucosispora strain AB-18–032. J Antibiot 60:391–394. DOI: 10.1038/ja.2007.54 [DOI] [PubMed] [Google Scholar]
- Khalil ZG, Raju R, Piggott AM, Salim AA, Blumenthal A, Capon RJ (2015) Aranciamycins I and J, Antimycobacterial Anthracyclines from an Australian marine-derived Streptomyces sp. J Nat Prod 78:949–952. DOI: 10.1021/acs.jnatprod.5b00095 [DOI] [PubMed] [Google Scholar]
- Kim DG, Moon K, Kim SH, Park SH, Lee SK, Oh KB, Shin J, Oh DC (2012). Bahamaolides A and B, antifungal polyene polyol macrolides from the marine actinomycete Streptomyces sp. J Nat Prod 75:959–967. DOI: 10.1021/np3001915 [DOI] [PubMed] [Google Scholar]
- Kimura T, Inahashi Y, Matsuo H, Suga T, Iwatsuki M, Shiomi K, Takahashi Y, Omura S, Nakashima T (2018) Pyrizomicins A and B: Structure and bioactivity of new thiazolyl pyridines from Lechevalieria aerocolonigenes K10–0216. J Antibiot 71:606–608. DOI: 10.1038/s41429-018-0038-y [DOI] [PubMed] [Google Scholar]
- Kunz AL, Labes A, Wiese J, Bruhn T, Bringmann G, Imhoff JF (2014) Nature’s lab for derivatization: new and revised structures of a variety of streptophenazines produced by a sponge-derived Streptomyces strain. Mar Drugs 12:1699–1714. DOI: 10.3390/md12041699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurata A, Sugiura M, Kokoda K, Tsujimoto H, Numata T, Kato C, Nakasone K, Kishimoto N (2017) Taxonomy of actinomycetes in the deep–sea Calyptogena communities and characterization of the antibacterial compound produced by Actinomadura sp. DS-MS-114. Biotechnol Biotec Eq 31:1000–1006. DOI: 10.1080/13102818.2017.1342563 [DOI] [Google Scholar]
- Kwon HC, Kauffman CA, Jensen PR, Fenical W (2009) Marinisporolides, polyene-polyol macrolides from a marine actinomycete of the new genus Marinispora. J Org Chem 74:675–684. DOI: 10.1002/chin.200920193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon HC, Kauffman CA, Jensen PR, Fenical W (2006) Marinomycins A−D, antitumor–antibiotics of a new structure class from a marine actinomycete of the recently discovered genus “Marinispora”. J Am Chem Soc 128:1622–1632. DOI: 10.1002/chin.200624214 [DOI] [PubMed] [Google Scholar]
- Kyeremeh K, Acquah KS, Sazak A, Houssen WE, Tabudravu JN, Deng H, Jaspars M (2014) Butremycin, the 3–hydroxyl derivative of ikarugamycin and a protonated aromatic tautomer of 5′-methylthioinosine from a Ghanaian Micromonospora sp. K310. Mar Drugs 12:999–1012. DOI: 10.3390/md12020999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacret R, Oves-Costales D, Gomez C, Gómez C, Diaz C, La Cruz MD, Perezvictoria I, Vicente F, Genilloud O, Reyes F (2015) New ikarugamycin derivatives with antifungal and antibacterial properties from Streptomyces zhaozhouensis. Mar Drugs 13:128–140. DOI: 10.3390/md13010128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutou AS, Yang I, Kang H, Seo EK, Nam S, Fenical W (2015) Nocarimidazoles A and B from a marine-derived actinomycete of the genus Nocardiopsis. J Nat Prod 78:2846–2849. DOI: 10.1021/acs.jnatprod.5b00746 [DOI] [PubMed] [Google Scholar]
- Leutou AS, Yang I, Le TC, Hahn D, Lim K, Nam S, Fenical W (2018) Fluvirucin B6, a new macrolactam isolated from a marine-derived actinomycete of the genus Nocardiopsis. J Antibiot 71:609–611. DOI: 10.1038/s41429-018-0033-3 [DOI] [PubMed] [Google Scholar]
- Li S, Tian X, Niu S, Zhang WJ, Chen YC, Zhang HB, Yang XB, Zhang WM, Li WJ, Zhang S, Ju JH, Zhang CS (2011) Pseudonocardians A–C, new diazaanthraquinone derivatives from a deap-sea actinomycete Pseudonocardia sp. SCSIO 01299. Mar Drugs 9:1428–1439. DOI: 10.3390/md9081428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian XY, Zhang ZZ (2013) Indanomycin–related antibiotics from marine Streptomyces antibioticus PTZ0016. Nat Prod Res 27:2161–2167. DOI: 10.1080/14786419.2013.793688 [DOI] [PubMed] [Google Scholar]
- Liang Y, Xie X, Chen L, Yan SL, Ye XW, Anjum KA, Huang HC, Lian XY, Zhang ZZ (2016) Bioactive polycyclic quinones from marine Streptomyces sp. 182SMLY. Mar Drugs 14:1–11. DOI: 10.3390/md14010010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin ZJ, Koch M, Pond CD, Mabeza G, Seronay RA, Concepcion GP, Barrows LR, Olivera BM, Schmidt EW (2014) Structure and activity of lobophorins from a turrid mollusk-associated Streptomyces sp. J Antibiot (Tokyo) 67:121–126. DOI: 10.1038/ja.2013.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu LL, Xu Y, Han Z, Li YX, Lu L, Lai PY, Zhong JL, Guo XR, Zhang XX, Qian PY (2012) Four New Antibacterial Xanthones from the Marine-Derived Actinomycetes Streptomyces caelestis. Mar Drugs 10:2571–2583. DOI: 10.3390/md10112571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macherla VR, Liu J, Sunga M, White DJ, Grodberg J, Teisan S, Lam KS, Potts BCM (2007) Lipoxazolidinones A, B, and C: antibacterial 4-oxazolidinones from a marine actinomycete isolated from a Guam marine sediment. J Nat Prod 70:1454–1457. DOI: 10.1021/np0702032 [DOI] [PubMed] [Google Scholar]
- Manam RR, Teisan S, White DJ, Nicholson B, Grodberg J, NeuteboomKin STC, Lam KS, Mosca DA, LloydBarbara GK, Potts BCM (2005) Lajollamycin, a Nitro-tetraene Spiro-β-lactone-γ-lactam antibiotic from the marine actinomycete Streptomyces nodosus. J Nat Prod 68:240–243. DOI: 10.1021/np049725x [DOI] [PubMed] [Google Scholar]
- Martín J, Sousa TS, Crespo G, Palomo S, Gonzalez I, Tormo JR, Cruz MDL, Anderson MA, Hill RT, Vicente F, Genilloud O, Reyes F (2013) Kocurin, the true structure of PM181104, an anti-methicillin-resistant Staphylococcus aureus (MRSA) thiazolyl peptide from the marine-derived bacterium Kocuria palustris. Mar Drugs 11:387–398. DOI: 10.3390/md11020387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maskey RP, Helmke E, Fiebig HH, Laatsch H (2002) Parimycin isolation and structure elucidation of a novel cytotoxic 2,3-dihydroquinizarin analogue of γ-indomycinone from a marine Streptomycete. J Antibiot (Tokyo) 55:1031–1035. DOI: 10.7164/antibiotics.55.1031 [DOI] [PubMed] [Google Scholar]
- Maskey RP, Li FC, Qin S, Fiebig HH, Laatsch H (2003) Chandrananimycins A-C: production of novel anticancer antibiotics from a marine Actinomadura sp. isolate M048 by variation of medium composition and growth conditions. J Antibiot 56:622–629. DOI: 10.1002/chin.200352223 [DOI] [PubMed] [Google Scholar]
- Maskey RP, Sevvana M, Uson I, Helmke E, Laatsch H. (2004) Gutingimycin: a highly complex metabolite from a marine streptomycete. Angew Chem Int Ed 43:1281–1283. DOI: 10.1002/anie.200352312 [DOI] [PubMed] [Google Scholar]
- Matsuda S, Adachi K, Matsuo Y, Nukina M, Shizuri Y (2009) Salinisporamycin, a novel metabolite from Salinispora arenicora. J Antibiot 62:519–526. DOI: 10.1038/ja.2009.75 [DOI] [PubMed] [Google Scholar]
- McArthur KA, Mitchell SS, Tsueng G, Rheingold AL, White DJ, Grodberg J, Lam KS, Potts BCM (2008) Lynamicins A–E, chlorinated bisindole pyrrole antibiotics from a novel marine actinomycete. J Nat Prod 71:1732–1737. DOI: 10.1021/np800286d [DOI] [PubMed] [Google Scholar]
- Mitova MI, Lang G, Wiese J, Imhoff JF (2008) Subinhibitory concentrations of antibiotics induce phenazine production in a marine Streptomyces sp. J Nat Prod 71:824–827. DOI: 10.1021/np800032a [DOI] [PubMed] [Google Scholar]
- Mondol MAM, Shin HJ (2014) Antibacterial and antiyeast compounds from marine-derived bacteria. Mar Drugs 12:2913–2921. DOI: 10.3390/md12052913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon K, Chung B, Shin Y, Rheingold AL, Moore CE, Park SJ, Park S, Lee SK, Oh K, Shin J, Oh D (2015) Pentacyclic antibiotics from a tidal mud flat–derived actinomycete. J Nat Prod 78:524–529. DOI: 10.1021/np500736b [DOI] [PubMed] [Google Scholar]
- Motohashi K, Irie K, Toda T, Matsuo Y, Kasai H, Sue M, Furihata K, Seto H (2008) Studies on Terpenoids Produced by Actinomycetes. J Antibiot 61:75–80 DOI: 10.1038/ja.2008.113 [DOI] [PubMed] [Google Scholar]
- Murphy BT, Narender T, Kauffman CA, Woolery M, Jersen PR, Fenical W (2010) Saliniquinones A–F, new members of the highly cytotoxic anthraquinone–γ–pyrones from the marine actinomycete Salinispora arenicola. Aust J Chem 63:929–934. DOI: 10.1071/CH10068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu SW, Li SM, Chen YC, Li S, Chen Y, Tian X, Zhang H, Zhang G, Zhang W, Yang X, Zhang S, Ju J, Zhang C (2011) Lobophorins E and F, new spirotetronate antibiotics from a South China Sea–derived Streptomyces sp SCSIO 01127. J Antibiot 64:711–716. DOI: 10.1038/ja.2011.78 [DOI] [PubMed] [Google Scholar]
- Nong XH, Zhang XY, Xu XY, Wang J, Qi SH (2016) Nahuoic acids B–E, polyhydroxy polyketides from the marine-derived Streptomyces sp. SCSGAA 0027. J Nat Prod 79:141–148. DOI: 10.1021/acs.jnatprod.5b00805 [DOI] [PubMed] [Google Scholar]
- Ohlendorf B, Schulz D, Erhard A, Nagel K, Imhoff JF (2012) Geranylphenazinediol, an acetylcholinesterase inhibitor produced by a Streptomyces species. J Nat Prod 75:1400–1404. DOI: 10.1021/np2009626 [DOI] [PubMed] [Google Scholar]
- Okami Y, Hotta K, Yoshida M, Ikeda D, Kondo S, Umezawa H (1979) New aminoglycoside antibiotics, istamycins A and B. J Antibiot (Tokyo) 32:964–966. DOI: 10.7164/antibiotics.32.964 [DOI] [PubMed] [Google Scholar]
- Okami Y, Okazaki T, Kitahara T, Umezawa H (1976) Studies on marine microorganisms. A new antibiotic, Aplasmomycin, produced by a streptomycete isolated from shallow sea mud. J Antibiot (Tokyo), 29:1019–1025. DOI: 10.7164/antibiotics.29.1019 [DOI] [PubMed] [Google Scholar]
- Pan HQ, Zhang SY, Wang N, Li ZL, Hua HM, Hu JC, Wang SJ (2013) New spirotetronate antibiotics, lobophorins H and I, from a South China Sea-derived Streptomyces sp. 12A35. Mar Drugs 11:3891–3901. DOI: 10.3390/md11103891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathirana C, Jensen PR, Dwight R, Fenical W (1992) Rare Phenazine L-Quinovose Esters from a Marine Actinomycete. J Org Chem 57:740–742. DOI: 10.1021/jo00028a060 [DOI] [Google Scholar]
- Pathirana C, Tapiolas D, Jensen PR, Dwight RH, Fenical W (1991) Structure determination of maduralide: a new 24-membered ring macrolide glycoside produced by a marine bacterium (Actinomycetales). Tetrahedron Lett 32:2323–2326. DOI: 10.1016/S0040-4039(00)79914-X [DOI] [Google Scholar]
- Perez BJ, Canedo LM, Fernandez PJL, Silva EMV (1997) Thiocoraline, a novel depsipeptide with antitumor activity produced by a marine Micromonospora: II. Physico-chemical properties and structure determination. J Antibiot 50:738–741. DOI: 10.7164/antibiotics.50.738 [DOI] [PubMed] [Google Scholar]
- Perez-Bonilla M, Ovescostales D, Cruz MDL, Kokkini M, Martin J, Vicente F, Genilloud O, Reyes F (2018) Phocoenamicins B and C, New Antibacterial Spirotetronates Isolated from a Marine Micromonospora sp. Mar Drugs 16:95 DOI: 10.3390/md16030095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Victoria I, Oves-Costales D, Lacret R, Martín J, Sánchez-Hidalgo M, Díaz C, Cautain B, Vicente F, Genilloud O, Reyes F (2019) Structure elucidation and biosynthetic gene cluster analysis of caniferolides A–D, new bioactive 36-membered macrolides from the marine-derived Streptomyces caniferus CA-271066. Org Biomol Chem 17:2954–2971. DOI: 10.1039/C8OB03115K [DOI] [PubMed] [Google Scholar]
- Poumale HMP, Ngadjui BT, Helmke E, Laatscha H (2006) New anthraquinones from a marine Streptomyces sp. – isolation, structure determination and biological activities. Z Naturforsch B 61:1450–1454. DOI: 10.1515/znb-2006-1122 [DOI] [Google Scholar]
- Pusecker K, Laatsch H, Helmke E, Weyland H (1997) Dihydrophencomycin methyl ester, a new phenazine derivative from a marine Streptomycete. J Antibiot (Tokyo), 50:479–483. DOI: 10.7164/antibiotics.50.479 [DOI] [PubMed] [Google Scholar]
- Raju R, Khalil ZG, Piggott AM, Blumenthal A, Gardiner DL, Skinneradams TS, Capon RJ (2014) Mollemycin A: an antimalarial and antibacterial glycol-hexadepsipeptide-polyketide from an Australian marine-derived Streptomyces sp. (CMB–M0244). Org Lett 16:1716–1719. DOI: 10.1021/ol5003913 [DOI] [PubMed] [Google Scholar]
- Raju R, Piggott AM, Conte MM, Capon RJ (2010) Heronamides A–C, new polyketide macrolactams from an Australian marine-derived Streptomyces sp. A biosynthetic case for synchronized tandem electrocyclization. Org Biomol Chem 8:4682–4689. DOI: 10.1039/C0OB00267D [DOI] [PubMed] [Google Scholar]
- Raju R, Piggott AM, Khalil Z, Bernhardt PV. Capona RJ (2012) Heronamycin A : a new benzothiazine ansamycin from an Australian. Tetrahedron Lett 53:2063–1065. DOI: 10.1016/j.tetlet.2011.12.064 [DOI] [Google Scholar]
- Rodríguez V, Martín J, Sarmiento–Vizcaíno A, De la Cruz M, García LA, Blanco G, Fernando F (2018) Anthracimycin B, a potent antibiotic against gram-positive bacteria isolated from cultures of the deep-sea actinomycete Streptomyces cyaneofuscatus M–169. Mar Drugs 16:406 DOI: 10.3390/md16110406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu MJ, Hwang S, Kim S, Yang I, Oh D, Nam S, Fenical W (2019) Meroindenon and merochlorins E and F, antibacterial meroterpenoids from a marine-derived sediment bacterium of the Genus Streptomyces. Org Lett 21:5779–5783. DOI: 10.1021/acs.orglett.9b01440 [DOI] [PubMed] [Google Scholar]
- Sato K, Okazak T, Maeda K, Okami Y (1978) New antibiotics, aplasmomycins B and C. J Antibiot 31:632–635. DOI: 10.7164/antibiotics.31.632 [DOI] [PubMed] [Google Scholar]
- Sato S, Iwata F, Yamada S, Katayama M (2012) Neomaclafungins A–I: Oligomycin-Class Macrolides from a Marine-Derived Actinomycete. J Nat Prod 75:1974–1982. DOI: 10.1021/np300719g [DOI] [PubMed] [Google Scholar]
- Schneemann I, Kajahn I, Ohlendorf B, Zinecker H, Erhard A, Nagel K, Wiese J, Imhoff JF (2010) Mayamycin, a cytotoxic polyketide from a Streptomyces strain isolated from the marine sponge Halichondria panicea. J Nat Prod 73:1309–1312. DOI: 10.1021/np100135b [DOI] [PubMed] [Google Scholar]
- Schumacher RW, Harrigan BL, Davidson BS (2001) Kahakamides A and B, new neosidomycin metabolites from a marine-derived actinomycete. Tetrahedron Lett 42:5133–5135. DOI: 10.1016/S0040-4039(01)00979-0 [DOI] [Google Scholar]
- Shaaban K A, Helmke E, Kelter G, Fiebig HH, Laatsch H (2011) Glucopiericidin C: a cytotoxic piericidin glucoside antibiotic produced by a marine Streptomyces isolate. J Antibiot (Tokyo) 64:205–209. DOI: 10.1038/ja.2010.125 [DOI] [PubMed] [Google Scholar]
- Schumacher RW, Talmage SC, Miller SA, Sarris KE, Davidson BS, Goldberg (2003) Isolation and structure determination of an antimicrobial ester from a marine sediment-derived bacterium. J Nat Prod 66:1291–1293. DOI: 10.1021/np020594e [DOI] [PubMed] [Google Scholar]
- Sitachitta N, Gadepalli M, Davidson BS (1996) New a-pyrone-containing metabolites from a marine-derived actinomycete. Tetrahedron 52:8073–8080. DOI: 10.1016/0040-4020(96)00391-2 [DOI] [Google Scholar]
- Socha AM, Garcia D, Sheffer R, Rowley DC (2006) Antibiotic bisanthraquinones produced by a Streptomycete isolated from a Cyanobacterium. J Nat Prod 69:1070–1073. DOI: 10.1021/np050449b [DOI] [PubMed] [Google Scholar]
- Song YX, Li QL, Liu X, Chen YC, Zhang Y, Sun AJ, Zhang WM, Zhang JR, Ju JH (2014) Cyclic hexapeptides from the deep south china sea-derived Streptomyces scopuliridis SCSIO ZJ46 active against pathogenic gram–positive bacteria. J Nat Prod 77:1937–1941. DOI: 10.1021/np500399v [DOI] [PubMed] [Google Scholar]
- Song YX, Huang HB, Chen YC, Ding J, Zhang Y, Sun A, Zhang W, Ju J (2013) Cytotoxic and antibacterial marfuraquinocins from the deep south china sea–derived Streptomyces niveus SCSIO 3406. J Nat Prod 76:2263–2268. DOI: 10.1021/np4006025 [DOI] [PubMed] [Google Scholar]
- Sugiyama R, Nishimura S, Matsumori N, Tsunematsu Y, Hattori A, Kakeya H (2014) Structure and biological activity of 8-deoxyheronamide C from a marine-derived Streptomyces sp.: heronamides target saturated hydrocarbon chains in lipid membranes. J Am Chem Soc 136(14):5209–5212. DOI: 10.1021/ja500128u [DOI] [PubMed] [Google Scholar]
- Sun CL, Yang ZJ, Zhang CY, Liu ZY, He JQ, Liu Q, Zhang TY, Ma JY (2019) Genome Mining of Streptomyces atratus SCSIO ZH16: Discovery of Atratumycin and Identification of Its Biosynthetic Gene Cluster. Org Lett 21:1453–1457. DOI: 10.1021/acs.orglett.9b00208 [DOI] [PubMed] [Google Scholar]
- Sun P, Maloney KN, Nam SJ, Haste NM, Raju R, Aalbersberg W, Jensen PR, Nizet V, Hensler M, Fenical W (2011) Fijimycins A–C, three antibacterial etamycin–class depsipeptides from a marine-derived Streptomyces sp. Bioorg Med Chem 19:6557–6562. DOI: 10.1016/j.bmc.2011.06.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun MW, Chen XT, Li WJ, Lu CH, Shen YM (2017) New diketopiperazine derivatives with cytotoxicity from Nocardiopsis sp. YIM M13066. J Antibiot 70:795–797. DOI: 10.1038/ja.2017.46 [DOI] [PubMed] [Google Scholar]
- Supong K, Thawai C, Suwanborirux K, Choowong W, Supothina S, Pittayakhajonwut P (2012) Antimalarial and antitubercular C–glycosylated benz[a]anthraquinones from the marine-derived Streptomyces sp. BCC45596. Phytochem Lett 5:651–656. DOI: 10.1016/j.phytol.2012.06.015 [DOI] [Google Scholar]
- Takasaka N, Kaweewan I, Ohnishi–Kameyama M, Kodani S (2017) Isolation of a new antibacterial peptide actinokineosin from Actinokineospora spheciospongiae based on genome mining. Lett Appl Microbiol 64:150–157. DOI: 10.1111/lam.12693 [DOI] [PubMed] [Google Scholar]
- Tan Y, Hu YY, Wang Q, Zhou HX, Wang YG, Gan ML (2016) Tetrocarcins N and O, glycosidic spirotetronates from a marine-derived Micromonospora sp. identified by PCR-based screening. Rsc Adv 6:91773–91778. DOI: 10.1039/C6RA17026A [DOI] [Google Scholar]
- Teta R, Marteinsson VT, Longeon A, Klonowski AM, Groben R, Bourguetkondracki M, Costantion V, Mangori A (2017) Thermoactinoamide A, an Antibiotic Lipophilic Cyclopeptide from the Icelandic Thermophilic Bacterium Thermoactinomyces vulgaris. J Nat Prod 80:2530–2535. DOI: 10.1021/acs.jnatprod.7b00560 [DOI] [PubMed] [Google Scholar]
- Thi QV, Tran VH, Mai HD, Le CV, Hong MLE, Murphy BT, Chau VM, Pham VC (2016b) Secondary metabolites from an Actinomycete from Vietnam’s East Sea. Nat Prod Commun 11:401–404. DOI: [DOI] [PubMed] [Google Scholar]
- Thi QV, Tran VH, Mai HD, Le CV, Hong MLY, Murphy BT, Chau VM, Pham VC (2016a) Antimicrobial metabolites from a marine–derived Actinomycete in Vietnam’s East Sea. Nat Prod Commun 11:49–51. DOI: 10.1080/14786419.2018.1468331 [DOI] [PubMed] [Google Scholar]
- Trischman JA, Tapiolas DM, Jensen PR, Dwight R, Fenical W, McKee TC, Ireland CM, Stout TJ, Clardy J (1994) Salinamides A and B: anti–inflammatory depsipeptides from a marine Streptomycete. J Am Chem Soc 116:757–758. DOI: 10.1021/ja00081a042 [DOI] [Google Scholar]
- Um S, Choi TJ, Kim H, Kim BY, Kim SH, Lee SK, Oh K, Shin JS, Oh D (2013) Ohmyungsamycins A and B: cytotoxic and antimicrobial cyclic peptides produced by Streptomyces sp. from a volcanic island. J Org Chem 78:12321–12329. DOI: 10.1021/jo401974g [DOI] [PubMed] [Google Scholar]
- Uzair B, Menaa F, Khan BA, Mohammad FV, Ahmad VU, Djeribi R, Menaa B (2018) Isolation, purification, structural elucidation and antimicrobial activities of kocumarin, a novel antibiotic isolated from actinobacterium Kocuria marina CMG S2 associated with the brown seaweed Pelvetia canaliculata. Microbiol Res 206:186–197. DOI: 10.1016/j.micres.2017.10.007 [DOI] [PubMed] [Google Scholar]
- Wang Q, Zhang YX, Wang M, Tan Y, Hu XX, He HW, Xiao CL, You XF, Wang YG, Gan ML (2017) Neo–actinomycins A and B, natural actinomycins bearing the 5 H-oxazolo [4, 5-b] phenoxazine chromophore, from the marine-derived Streptomyces sp. IMB094. SCI REP-UK 7:1–8. DOI: 10.1038/s41598-01703769-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DE, Dalisay DS, Chen J, Polishchuck EA, Patrick BO, Narula G, Ko M, Avgay Y, Li HX, Magarvey NA, Andersen RJ (2017) Aminorifamycins and sporalactams produced in culture by a Micromonospora sp. isolated from a Northeastern-Pacific marine sediment are potent antibiotics. Org Lett 19:766–769. DOI: 10.1021/acs.orglett.6b03619 [DOI] [PubMed] [Google Scholar]
- Wilson MC, Nam SJ, Gulder TA, Kauffman CA, Jensen PR, Fenical W, Moore BS (2011) Structure and biosynthesis of the marine Streptomycete ansamycin ansalactam A and its distinctive branched chain polyketide extender unit. J Am Chem Soc 133:1971–1977. DOI: 10.1021/ja109226s [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CY, Tan Y, Gan ML, Wang YG, Guan Y, Hu XX, Zhou HX, Shang XY, You XF, Yang ZY, Xiao CL (2013b) Identification of elaiophylin derivatives from the marine–derived actinomycete Streptomyces sp. 7–145 using pcr-based screening. J Nat Prod 76:2153–2157. DOI: 10.1021/np4006794 [DOI] [PubMed] [Google Scholar]
- Wu SJ, Fotso S, Li FC, Qin S, Kelter G, Fiebig HH, Laatsch H (2006) N-carboxamido-staurosporine and Selina-4(14),7(11)-diene-8,9-diol, new metabolites from a marine Streptomyces sp. J Antibiot (Tokyo) 59:331–337 DOI: 10.1038/ja.2006.46 [DOI] [PubMed] [Google Scholar]
- Wu ZC, Li SM, Li J, Chen Y, Saurav K, Zhang Q, Zhang H, Zhang W, Zhang W, Zhang S, Zhang C (2013a) Antibacterial and cytotoxic new napyradiomycins from the marine-derived Streptomyces sp. SCSIO 10428. Mar Drugs 11:2113–2125. DOI: 10.3390/md11062113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyche TP, Alvarenga RFR, Piotrowski JS, Duster M, Warrack S, Comilescu G, Wolfe TJD, Hou YP, Braun DR, Ellis GA, Simokins SW, Nelson J, Myers CL, Steele JL, Mon H, Safdar N, Markley JL, Rajski SR, Bugni TS (2017) Chemical genomics, structure elucidation, and in vivo studies of the marine-derived anticlostridial ecteinamycin. Acs Chem Biol 12:2287–2295. DOI: 10.1021/acschembio.7b00388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyche TP, Piotrowski JS, Hou Y, Braun DR, Deshpande R, Mcilwain S, Ong IM, Myers CL, Guzei IA, Westler WM, Andes DR, Bugni TS (2014) Forazoline a: marine-derived polyketide with antifungal in vivo efficacy. Angew Chem Int Edit 53:11583–11586. DOI: 10.1002/ange.201405990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu DB, Nepal KK, Chen J, Harmody D, Zhu HN, Mccarthy PJ, Wright AE, Wang GJ (2018) Nocardiopsistins AC: New angucyclines with anti–MRSA activity isolated from a marine sponge-derived Nocardiopsis sp. HB–J378. Synth Syst Biotechnol 3:246–251. DOI: 10.1016/j.synbio.2018.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu LY, Quan XS, Wang C, Wang C, Sheng HF, Zhou GX, Lin BR, Jiang RW, Yao XS (2011) Antimycins A19 and A20, two new antimycins produced by marine actinomycete Streptomyces antibioticus H74–18. J Antibiot (Tokyo) 64:661–665. DOI: 10.1038/ja.2011.65 [DOI] [PubMed] [Google Scholar]
- Yamanaka K, Reynolds KA, Kersten RD, Ryan KS, Gonzalez DJ, Nizet V, Dorrestein PC, Moore BS (2014) Direct cloning and refactoring of a silent lipopeptide biosynthetic gene cluster yields the antibiotic taromycin A. P Natl Acad Sci 111:1957–1962. DOI: 10.1073/pnas.1319584111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CL, Wang YS, Liu CL, Zeng YJ, Cheng P, Jiao RH, Bao SX, Huang HQ, Tan RX, Ge HM (2017) Strepchazolins A and B: two new alkaloids from a marine Streptomyces chartreusis NA02069. Mar Drugs 15:244 DOI: 10.3390/md15080244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XW, Zhang GY, Ying JX, Yang B, Zhou XF, Steinmetz A, Liu YH, Wang N (2013) Isolation, characterization, and bioactivity evaluation of 3-((6-methylpyrazin-2-yl) methyl)–1H–indole, a new alkaloid from a deep–sea–derived actinomycete Serinicoccus profundi sp. nov. Mar Drugs 11:33–39. DOI: 10.3390/md11010033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Anjum K, Song TF, Wang WL, Yu SR, Huang HC, Lian XY, Zhang ZZ (2016) A new curvularin glycoside and its cytotoxic and antibacterial analogues from marine actinomycete Pseudonocardia sp. HS7. Nat Prod Res 30:1156–1161. DOI: 10.1080/14786419.2015.1047775 [DOI] [PubMed] [Google Scholar]
- Yi WW, Li Q, Song TF, Chen L, Li XC, Zhang ZZ, Lian XY (2019) Isolation, structure elucidation, and antibacterial evaluation of the metabolites produced by the marine-sourced Streptomyces sp. ZZ820. Tetrahedron 75:1186–1193. DOI: 10.1016/j.tet.2019.01.025 [DOI] [Google Scholar]
- Yuan GJ, Lin H, Wang C, Hong K, Liu Y, Li J (2011) 1H and 13C assignments of two new macrocyclic lactones isolated from Streptomyces sp. 211726 and revised assignments of Azalomycins F3a, F4a and F5a. Magn Reson Chem 49:30–37. DOI: 10.1002/mrc.2697 [DOI] [PubMed] [Google Scholar]
- Zhang B, Wang KB, Wang W, Bi SF, Mei YN, Deng XZ, Jiao RH, Tan RX, Ge HM (2018a) Discovery, biosynthesis, and heterologous production of streptoseomycin, an anti–microaerophilic bacteria macrodilactone. Org Lett 20:2967–2971. DOI: 10.1021/acs.orglett.8b01006 [DOI] [PubMed] [Google Scholar]
- Zhang D, Shu CY, Lian XY, Zhang ZZ (2018b) New antibacterial bagremycins F and G from the marine-derived Streptomyces sp. ZZ745. Mar Drugs 16:330 DOI: 10.3390/md16090330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Yi WW, Ge HJ, Zhang ZZ, Wu B (2019a) Bioactive Streptoglutarimides A–J from the Marine-Derived Streptomyces sp. ZZ741. J Nat Prod 82:2800–2808. DOI: 10.1021/acs.jnatprod.9b00481 [DOI] [PubMed] [Google Scholar]
- Zhang HB, Saurav K, Yu ZQ, Mandi A, Kurtan T, Li J, Tian XP, Zhang QB, Zhang WJ, Zhang CS (2016b) α-Pyrones with diverse hydroxy substitutions from three marine–derived Nocardiopsis Strains. J Nat Prod 79:1610–1618. DOI: 10.1021/acs.jnatprod.6b00175 [DOI] [PubMed] [Google Scholar]
- Zhang J, Qian Z, Wu X, Ding J, Lu C, Shen Y (2014) Juanlimycins A and B, ansamycin macrodilactams from Streptomyces sp. Org Lett 16:2752–2755. DOI: 10.1021/ol501072t [DOI] [PubMed] [Google Scholar]
- Zhang S, Xie Q, Sun C, Tian XP, Gui C, Win XJ, Zhang H, Ju JH (2019b) Cytotoxic Kendomycins Containing the Carbacylic Ansa Scaffold from the Marine–Derived Verrucosispora sp. SCSIO 07399. J Nat Prod 82:3366–3371. DOI: 10.1021/acs.jnatprod.9b00654 [DOI] [PubMed] [Google Scholar]
- Zhang Q, Mándi Z, Li S, Chen Y, Zhang W, Tian X, Zhang H, Li H, Zhang W, Zhang S, Ju J, Kurtán T, Zhang C (2012) N–N–Coupled indolo–sesquiterpene atropo-diastereomers from a marine-derived actinomycete. Eur J Org Chem 16:2752–2755. DOI: 10.1002/ejoc.201200599 [DOI] [Google Scholar]
- Zhang SW, Gui C, Shao MW, Kumar PS, Huang HB, Ju JH (2018c) Antimicrobial tunicamycin derivatives from the deep sea-derived Streptomyces xinghaiensis SCSIO S15077. Nat Prod Res 1–6. DOI: 10.1080/14786419.2018.1493736 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Adnani N, Braun DR, Ellis GA, Barns KJ, Parkernance S, Guzei IA, Bugni TS (2016a) Micromonohalimanes A and B: Antibacterial halimane-type diterpenoids from a marine Micromonospora species. J Nat Prod 79:2968–2972. DOI: 10.1021/acs.jnatprod.6b00555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Zhu T, Zhu W (2013) New marine natural products of microbial origin from 2010 to 2013. Chinese J Org Chem 33:1195–1234. DOI:http://sioc-journal.cn/Jwk_yjhx/CN/10.6023/cjoc201304039 [Google Scholar]
- Zhou X, Huang H, Chen Y, Tan JH, Song YX, Zou JH, Tian XP, Hua Y, Ju JH (2012) Marthiapeptide A, an anti–infective and cytotoxic polythiazole cyclopeptide from a 60 L scale fermentation of the deep sea-derived Marinactinospora thermotolerans SCSIO 00652. J Nat Prod 75:2251–2255. DOI: 10.1021/np300554f [DOI] [PubMed] [Google Scholar]
- Zhou X, Huang HB, Li J, Song YX, Jiang RW, Liu J, Zhang S, Hua Y, Ju JH (2014) New anti–infective cycloheptadepsipeptide congeners and absolute stereochemistry from the deep sea-derived Streptomyces drozdowiczii SCSIO 10141. Tetrahedron 70:7795–7801. DOI: 10.1016/j.tet.2014.02.007 [DOI] [Google Scholar]