Abstract
Introduction
To explore the ethnobiological wisdom of the tribals of three western districts of West Bengal, India against poisonous and non-poisonous bites and stings, a quantitative approach was adopted. These age-old yet unexplored knowledge can be utilized in finding lead-molecules against poisonous and non-poisonous animal-bites. Further, an evidence-based approach is needed to assess the venom-neutralization ability of plants by experimental studies.
Materials and methods
During 2008–2009 and 2012–2017, 11 ethnomedicinal surveys were carried out to explore the use of medicinal flora and fauna via conducting open semi-structured interviews with 47 traditional healers (THs) or informants. The retrieved dataset was statistically evaluated using seven quantitative-indexes: use-value (UV), informants'-consensus-factor (ICF), fidelity-level (FL), relative-importance (RI), cultural importance-index (CI), index of agreement on remedies (IAR) and cultural agreement-index (CAI). Anti-phospholipaseA2 (PLA2) properties of selected plant extracts were also examined. In addition, the cytotoxicity and genotoxicity of the water extract of the plants showing high FL as well as significant PLA2 inhibitory potential were investigated using Allium cepa root tip assay.
Results
A total of 41 traditional-formulations (TFs) containing 40 plant species (of 39 genera from 28 families) and 3 animal species were prescribed by the THs. Fabaceae exhibited most number of medicinal plants. Piper nigrum (1.78) and Apis cerana indica and Crossopriza lyoni (both 0.21) exhibited the highest UV among the plants and the animals respectively. Stinging of centipede and dog/cat/hyena bite displayed highest ICF (1.00 each). Among the plants, the maximum RI (0.91) and CI (4.98) values were observed for Aristolochia indica. IAR (1.00) was recorded maximum for Achyranthes aspera, Gloriosa superba, Lycopodium cernuum, Smilax zeylanica and Streblus asper. Maximum CAI value was noted for Piper nigrum (5.5096). Among the animals, Apis cerana indica (0.31) and Crossopriza lyoni (1.52) displayed the highest RI and CI values respectively. Crossopriza lyoni (0.99) and Apis cerana indica (1.3871) exhibited maximum IAR and CAI values respectively. Plants showing higher FL exhibited higher anti-PLA2 activity via selective inhibition of human-group PLA2. In addition, Allium cepa root tip assay has indicated the safety and/or toxicity of the plant parts prescribed by the THs. Root water extracts of Aristolochia indica and Gloriosa superba exhibited significant genotoxicity and cytotoxicity.
Conclusions
Three western districts of West Bengal is the natural abode for many tribal and non-tribal communities. A noteworthy correlation was established between the plants used against poisonous-bites and their anti-PLA2 activity. A few plant parts used by the THs also exhibited high toxicity. Such alternative medical practices serve as the only option in these underprivileged and backward areas during medical-exigencies.
Introduction
Snake envenomation is considered as a major problem worldwide especially in the tropical and subtropical countries including South East Asia [1–4]. There are more than 440,000 snake envenoming and 20,000 deaths each year [2]. Antivenom is considered as an effective treatment against snakebite; however snakebite-associated mortality remains significantly high due to unavailability, limited therapeutic efficacy and safety concerns of conventional antivenins [5]. India presents a very high number of snakebite incidences compared to other countries. Insufficient hospital-based reports indicated the total number of snakebite mortality to be ranging over 1,300 to 50,000 in India [6]. Besides snakebites, scorpion stings also cause severe venom-related injury [7]. Scorpion stings result in severe consequences in human which may lead to even mortality due to neurotoxins present in the venom [8]. Centipede bites have reportedly caused local pain, erythema and edema, nausea and vomiting, headache, lymphadenopathy, rhabdomyolysis, myocardial infarction, hypotension etc. in humans due to myocardial toxic effects of the venom and anaphylaxis [9, 10]. Bee stings are also known to cause heart blocks, syncope and cardiac arrest [11]. Even the stings of the members of Hymenoptera such as bees, hornets, and wasps can be fatal [12]. Animal bites are reported as a serious global health concern and are responsible for almost 1–2% of all visits to the hospital emergency. Most of the animal bites are imposed by dogs (80–90%) and cats (5–15%) and the most common complication following animal bite is wound infection [by Pasteurella multocida, Capnocytophaga canimorsus, Eikenella corrodens and Rhabdovirus (rabies only) etc.] which may lead to sepsis and death especially in the immunocompromised victims [13, 14].
There has always been a philosophical conflict between the mainstream Western medicine and the traditional and complementary medicine or TCM with their possible coexistence and prevalence in the global context [15]. World Health Organization (WHO) has advocated herbal medicines as a valid alternative therapy against many human ailments. According to the WHO, almost 80% of the world’s population rely on TCM for primary healthcare [16]. In India, other alternative medication strategies such as Ayurveda, Siddha, Unani, Tibbi and Homoeopathy have also been popularly used [17]. Purulia, one of the remote and backward districts of India is rich in aboriginals with their age old ethnomedicinal treatments. Remoteness from the nearby towns, unfavorable topography, sparse healthcare facilities and poverty have persuaded the aboriginals to use ethnomedicines especially during serious medical exigencies such as poisonous envenomations.
Phospholipase A2 (PLA2) enzymes, commonly present in the venoms of the snakes from the families Viperidae, Hydrophidae and Elaphidae are implicated to the venom-induced toxic effects [18–20]. Many natural compounds from plants, marine organisms, serum plasma etc. have been tested for their anti-PLA2 properties on snake venoms and/or isolated toxins [20]. Higher PLA2 inhibitory properties of the plant extracts indicate their possible snake venom neutralization ability. Earlier, in-vitro, in-vivo and in-silico studies performed on the anti-PLA2 properties of the natural compounds indicated the tremendous potential of the compounds as novel inhibitors of ophidian toxins [21–23].
Standardized Allium cepa root tip test is commonly used as a fast and reliable method to assess cytotoxicity and genotoxicity of plant extracts, isolated compounds, synthetic derivatives, nano-materials, environmental pollutants, pesticides, industrial effluents etc. mostly on the basis of mitotic anomalies and chromosomal aberrations studies [24–28]. Medicinal plants, although used for their therapeutic properties, also exhibit dose dependent toxic effects. Allium cepa root tip meristematic cells assay easily determines dose dependent cytotoxicity and genotoxicity of medicinal plant extracts and plant-derived compounds [29–32].
The primary aim of the present study is to enumerate the indigenous use of phyto- and zoo-therapeutics prescribed by the traditional healers (THs) practicing in the three westernmost districts of West Bengal, India, against poisonous and non-poisonous animal bites and insect stings. The present work is also intended to elucidate the preparations and applications of traditional formulations (TFs) and the statistical interpretation of the retrieved ethnobiological information. In the present study, anti-PLA2 properties of selected plant extracts were also evaluated against human group PLA2 and porcine group PLA2 to find out any selective inhibition of the said extracts on pro-inflammatory human group PLA2 without or minimally inhibiting the porcine group digestive PLA2. In addition, Allium cepa root tip meristematic cells were employed to assess the genotoxic and cytotoxic potential of selected antivenin plants showing higher fidelity level (FL) as well as superior PLA2 inhibitory potential.
Materials and methods
Ethics statement
The permission (ethnomedicinal survey, sanctioned in 2012) was granted by the West Bengal Biodiversity Board [OM No. 040/3/K(Bio)−1/2012], Govt. of West Bengal, India. West Bengal Biodiversity Board is a Govt. of the State of West Bengal who issues necessary permission for ethnobiological surveys. Earlier, preliminary information on the area and the people were gathered by an approval granted by West Bengal Biodiversity Board (1.1.2008–22.5.2009) [Memo No. 5k(bio)-2/2007]. This Govt. body has approved this work which compiles with the guidelines. In addition, written and verbal consent were taken from the local people during the survey which also compiles to the ethical guidelines as provided by the aforesaid Govt. body.
Selection of informants and data collection
During 2008–2009, initial surveys were conducted to explore the people and their practices in different villages of the three districts (Fig 1). Later, during 2012–2017, a total number of 11 ethnomedicinal surveys were conducted to explore the use of medicinal flora and fauna to treat poisonous and non-poisonous animal bites and insect stings. A total number of 47 THs (also known as the informants) were interviewed on the basis of their ethnomedicinal knowledge as well as their reputation and social acceptability as practitioners. A semi-structured questionnaire (S1 and S2 Figs) was supplied to each TH to explain the use (against particular ailments) and composition of each monoherbal or polyherbal ethnomedicinal preparations, their local names, plant/animal parts used, detection of disease symptoms, method of composing formulations, route of administration, addition of other ingredients etc. Before the start of the questionnaire session, prior consent was sought from the informants (S3 Fig). Besides, name, gender, age, aboriginal group etc. of the THs were also noted. Habit, habitat, taxonomic and identification features of the ethnobiologicals as well as date, time, season and site of collection were also documented. Herbarium sheets were prepared using medicinal plants preferably in their flowering phases and specific voucher numbers were assigned to each. Plants, plant parts and methods of preparations were also photographed for documentation. Literatures such as Prain (1903) [33] and Sanyal (1994) [34] were used to identify local flora and Tropicos plant database from Missouri Botanical Garden (www.tropicos.org) was consulted to verify names, synonyms and authors’ citations. Economic Botany Data Collections Standards (EBDCS) proposed by Cook (1995) [35] was used to present the use categories mentioned by the informants (Table 1). Herbarium specimens with respective voucher numbers were preserved at the Department of Zoology, Sidho-Kanho-Birsha University, Purulia, West Bengal. Ethnozoologicals were initially documented during the field visits and were subsequently identified by the first author of this article. Animal samples were photographed and common samples were preserved for future reference.
Fig 1. Map of the study area (maps not to scale) (Map was created using the editor tools of the ArcGIS 10.3.1 software.).
Table 1. Traditional Formulations (TFs) used against poisonous and non-poisonous animal bites and insect stings.
| Disease/ Disorder1 1: Scorpion sting (20090400) | ||
|---|---|---|
| symptoms: pain and burning sensation around stinging area and full organ | ||
| Traditional Formulations (TFs) | Composition3 | Modes of preparation and methods of administration2 |
| TF1 | Bisalya karani (Barleria lupulina) bark: 50 g | Bark is grinded to make a paste; the paste is used as ointment (1070000) on the wound. |
| TF2 | Khakra change/Bandarlej (Setaria glauca) roots: 10 g | Roots are grinded to make a paste; the paste is applied on the wound. |
| Tar genda (Tridax procumbens) roots: 10 g | ||
| Bhin Kambal (Premna herbacea) roots: 10 g | ||
| TF3 | Rasun (Allium sativum) bulbs: 5 in no. | Roots and Rasun bulbs are grinded to make a paste; the paste is applied on the wound with a pinch of salt. |
| Nirbisi (Cyperus kyllingia) root: 20 g | ||
| Common salt (NaCl): a pinch | ||
| TF4 | Seowra (Streblus asper) leaves: 4–5 in no. | Leaves are taken and rubbed by hand to extract juice and the juice is given on the stinging area. |
| TF5 | Alkushi (Mucuna pruriens) seeds: 2–3 in no. | A powder is made from the seeds; the powder is applied over the stinging areas. |
| TF6 | Harin singha (Euphorbia tirucalli) latex: 3–4 drops | Applied on the stinging area. |
| TF7 | Gave verenda (Jatropha gossypifolia) bark | Bark paste is directly applied on the affected area. |
| TF8 | Scorpion (Buthus sp.) | Internal material/ digestive system of scorpion by which the person was attacked is to rub on stinging area. |
| TF9 | Ol (Amorphophallus paeoniifolius) stem | The stem is heated over the flame and the affected area is covered with this heated stem. |
| TF10 | Kend (Diospyros melanoxylon) bark | Bark juice is applied on the stinging area. |
| TF11 | Kalojira (Nigella sativa) seeds | Grinded seeds are applied on the stinging area. |
| TF12 | Liyaya ful/ Bishlanguli (Gloriosa superba) roots | Fresh root is rubbed on the affected area. |
| TF13 | Chatpati ghash (Ruellia tuberosa) leaves: 10 g | Paste is applied on the affected area. |
| Tarnd mula (Premna herbacea) roots: 10 g | ||
| TF14 | Narkol (Cocos nucifera) oil: 5 to 10 ml | Both the ingredients are mixed well; the mixture is used as an ointment on the stinging area. |
| Naphthalene: 1 ball | ||
| TF15 | Sada dhurba ghash (Cynodon dactylon) leaves: 10 g | Both the ingredients are crushed to make a paste; the paste with kalo saban is applied on the stinging area. |
| Boka benri (Ipomoea carnea) leaves: 10 g | ||
| Kapor kachar kalo saban (local black soap for washing clothes): 5 g | ||
| Disease/ Disorder 2: Stinging of honey bee or wasp or hornet (20090200 and 20090300) | ||
| Symptoms: Swelling of affected area and severe burning sensation | ||
| TF16 | Hetal (Crateva adansonii) bark | Bark of Hetal is grinded to make paste. Bark paste is applied on the wound. |
| TF17 | Lal Tulsi (Ocimum sanctum) leaves: 50 g | Leaf paste is smeared over the body to keep away bees. |
| TF18 | Narkol (Cocos nucifera) oil: 5 to 10 ml | Both the ingredients are mixed well. Mixture is used like ointment on stinging area. |
| Naphthalene: 1 ball | ||
| TF19 | Common salt (NaCl): ½ teaspoonful | Application of a lotion (1050000) prepared from the salt and the kerosene oil just after stinging is helpful to relieve pain. |
| Kerosene oil: 1 teaspoonful | ||
| TF20 | Petrol: 5–10 ml | After removing the sting, cotton is soaked with petrol and it is rubbed on the stinging area until the burning sensation is gone. Then juice of Piyaj is then applied on the affected area. |
| Cotton/cotton cloth: 1 in no. | ||
| Piyaj (Allium cepa) bulb: 50 g | ||
| TF21 | Liyaya ful/Bishlanguli (Gloriosa superba) roots | Fresh root is rubbed on the affected area. |
| TF22 | Baichi (Flacourtia indica) stem and bark: 10 g | A paste of stem and bark is applied on the wound. |
| TF23 | Rangani/Shiyal kanta (Argemone mexicana) seeds: 10 g | The seeds are crushed well to make a paste and the paste is applied on the stinging area. |
| Disease/ Disorder 3: Stinging of centipede | ||
| TF24 | Ol (Amorphophallus paeoniifolius) stem | The stem is heated over the flame and the affected area is covered with this heated stem. |
| Disease/ Disorder 4: Dog/cat/hyena bite (12020000) | ||
| TF25 | Chitchiti (Achyranthes aspera) roots: 20 g | Root paste is applied on the wound and molasses is taken orally. |
| Molasses: 20 g | ||
| TF26 | Crossopriza lyoni (Blackwall, 1867) (tailed cellar spiders): 1–2 in no. | Ingredients are properly mixed and taken orally to reduce the effect of the poison. |
| Chanchhi (The deposits at the bottom of a pot after the milk is boiled): 50 g | ||
| TF27 | Tentul (Tamarindus indica) tender twigs | Freshly taken twigs are heated over the flame and to cover the wound. |
| Disease/ Disorder 5: Snake bite (20090600) | ||
| TF28 | Bisalya karani (Barleria lupulina) bark: 50 g | Bark is grinded to make a paste. The paste is used as ointment at the wound. |
| TF29 | Mrita sanjiboni (Lycopodium cernuum) leaves: 50 g | All the ingredients are grinded to make a paste; the paste is applied on the wound. |
| Asthi sancharini (Scindapsus officinalis) leaves: 50 g | ||
| Bisalya karani (Barleria lupulina) leaves: 50 g | ||
| TF30 | Chotopard (Cissampelos pareira) roots: 10 g | Root is grinded to make a paste; the paste is fed to the victim to prevent the spread of the poison. |
| TF31 | Bamun hati/Ram datum (Smilax zeylanica) roots: 1 inch | Root of Ram datum and Iswarmul along with Rabing seeds are grinded to form a paste; the paste is applied on the wound. |
| Iswarmul (Aristolochia indica) roots: 1 inch | ||
| If patient becomes senseless, powder of dry leaves and roots of Kanch mala is given to the victim as snuff. | ||
| Black pepper or Rabing (Piper nigrum) seeds: 2½ in no. | ||
| Kanch mala (Abrus precatorius) dry leaves: 5 in no. | ||
| Kanch mala roots: 2 g | ||
| TF32 | Dusatin lata (Gloriosa superba) bulb | Dusatin lata is purified with bovine urine for seven days. The purified Dusatin lata is taken with dust of black pepper. |
| Black pepper or Rabing (Piper nigrum) seeds | ||
| Bovine urine | ||
| TF33 | White Kuch fall/Kukurmala (Abrus precatorius) roots: 2 ½ g | Roots of Kuch fall and black pepper are grinded together and taken orally. |
| Black pepper or Rabing (Piper nigrum) seeds: 10 in no. | ||
| TF34 | Ishwarmul (Aristolochia indica) roots: 1½ inch | Paste is made and given to the patient orally. |
| Rangani (Solanum surattense) roots: 1½ inch | ||
| Bagh nakhi (Martynia annua) roots: 1½ inch | ||
| Piyal (Buchanania lanzan) bark: 1½ inch | ||
| Black pepper or Golki/Rabing (Piper nigrum) seeds: 12 in no. | ||
| TF35 | Ishwarmul (Aristolochia indica) bark and roots: 5 g | Juice of bark and root is drunk twice daily. |
| TF36 | Barachadar (Rauvolfia canescens) roots: 10 g | 10 ml of root juice is prescribed orally and is also applied on the wound. |
| TF37 | Anantamul/Analsing (Hemidesmus indicus) roots: 10 g | Root paste is applied on the wound. |
| Apis cerana indica Fabricius (Indian honeybee) fresh honey from hives: 50 ml | ||
| TF38 | Ishermul/Bhedi-Janete (Aristolochia indica) roots | Root paste with a paste of ten peppers is given as antidote. |
| Black pepper or Golki/Rabing (Piper nigrum) seeds: 10 in no. | ||
| TF39 | Chotopar/Chotkipar/Tijumala (Cissampelos pareira) roots | Root paste with a paste of ten peppers is given as antidote. |
| Black pepper or Golki/ Rabing (Piper nigrum) seeds: 10 in no. | ||
| TF40 | Mahadevjata/Ishwarjata (Uraria picta) leaves | Leaf paste is given as an antidote |
| TF 41 | Swet akanda (Calotropis gigantea) leaves | A paste is prepared with all the ingredients and is offered to the patient to drink. |
| Bishaynandi (Glossogyne bidens) whole plant | ||
| Krishnajata (Nardostachys jatamansi) roots | ||
1, 2 The numbers used in parenthesis after the diseases/disorders and methods of administration are according to the recommendations given by Cook, 1995 as Economic botany data collection standard (EBDCS), plant parts, body parts and processes, disorders/effects, medicinal applications and non-vertebrate organisms (Master lists of states for Level 3 descriptors) (Economic Botany Data Standard; https://www.kew.org/tdwguses/rptMasterListMain.htm).
3In composition, in no. used after the numbers stands for in number i.e. the number of that plant part used.
Analyses of ethnobiological data
Use Value (UV)
Use value (UV) is a quantitative analysis applied to enumerate the relative importance of local ethnobiologicals [36]. The equation is:
(where U denotes the number of citations per ethnobiological and n denotes the informant numbers who were interviewed for a given ethnobiological)
Informant Consensus Factor (ICF)
The informant consensus factor (ICF) was calculated to evaluate user variability of ethnobiologicals [37]. The ICF is estimated by the following equation:
(where nur is the used citation number in each category and nt is the number of ethnobiologicals reported)
Fidelity Level (FL)
The fidelity level (FL) is determined as the percentage of informants reporting the application of specific ethnobiological for similar purpose [38]. It was calculated as:
(where Np is the informant numbers separately reporting an application of an ethnobiological to treat a specific disease whereas N is the total number of informants citing the use of an ethnobiological to treat any specific ailment)
Relative Importance (RI)
The relative importance (RI) of an ethnobiological depicts the most used ethnobiologicals with the most notable number of ethnomedicinal applications [39, 40]. The RI is represented by the equation:
(where PP is the total number of curative properties ascribed to an ethnobiological divided by the highest number of similar activities ascribed to the widely used ethnobiologicals. AC represents the number of ailment categories reported to be cured by a specific ethnobiological divided by the highest number of ailment categories treated by the most prolifically used ethnobiologicals).
Cultural Importance index (CI)
The cultural importance index (CI) is calculated by the total number of informants citing the use of each ethnobiological [41]. The CI is represented by:
(where UR represents the use report, N represents the gross number of informants whereas from u1 to uNC represent UR of each use-category [41, 42]).
Index of Agreement on Remedies (IAR)
The index of agreement on remedies (IAR) depicts the significance of each ethnobotanicals [43]. IAR is calculated as:
(where nr is the summation of the reports on the use of an ethnobiological and na is the number of ailment categories against which the ethnobiological is administered [44]).
Cultural Agreement Index (CAI)
The cultural agreement index (CAI) [45] is calculated as:
(where CII represents the cultural importance index and IAR represents the index of agreement on remedies)
Enzymes and reagents
Human group PLA2 and porcine group PLA2 were used as PLA2. Lecithin (egg yolk phosphatidylcholine), red phenol, sodium taurodeoxycholate (NaTDC) and ethylmethanesulfonate (EMS) were procured from Sigma, India. A UV-VIS spectrophotometer was used for the measurements using the visible spectra.
PLA2 inhibitory activity
PLA2 inhibitory activity of medicinal plants were tested only with the plants showing high FL. A protocol by De Aranjo and Radvany (1987) was followed to assess the PLA2 inhibitory activity of the plant extracts [46]. In this study, the inhibitory effects of the water extracts were assessed on the pro-inflammatory human group PLA2. As a control set, porcine group PLA2 was used. The substrate was composed of egg yolk phosphatidylcholine or lecithin (3.5 mM) in a mixture containing NaTDC (3 mM), NaCl (100 mM), CaCl2 (10 mM) and red phenol (0.055 mM) employed as colorimetric indicator dissolved in H2O (100 ml). The pH of the reaction mixture was calibrated to 7.6. The two PLA2 were solubilized in acetonitrile (10%) at 0.02 and 0.002 μg/μl concentrations respectively. Each plant extract (10 ml) was incubated with PLA2 solution (10 μl) at room temperature for 20 min. Following this, PLA2 substrate (1 ml) was added, and following hydrolysis in next 5 min, the OD was read at 558 nm. The inhibition percentage was determined by comparing with the control (absence of plant extract) and the IC50 values were obtained from the curve.
Allium cepa root tip meristem genotoxicity test
A protocol by Aşkin Çelik and Aslantürk (2010) with minor modifications was employed to assess the genotoxicity and cytotoxicity of medicinal plants with high FL as well as superior human-group PLA2 inhibitory properties [47]. Tap water was used as negative control whereas ethyl methanesulfonate (EMS, 2 X 10−2 M) was employed as positive control. After 24 h exposure to the control sets and the aqueous extract (2.5, 5 and 10 mg/ml) of the plants, Allium cepa root tips were cut from the bulbs to fix them in ethanol: glacial acetic acid: ethanol (1:3 v/v) overnight at 4 ˚C. Following fixation, the roots were put in 70% (v/v) aqueous ethanol and were stored in a fridge. The root tips were then hydrolyzed using hydrochloric acid (HCl) (1N) for 3 min and were subsequently squashed on microscope slides with 45% acetic acid after staining with aceto-orcein [2% (w/v)]. Slides were visualized under a Carl Zeiss compound microscope with 40X10 magnification (n = 5/set). The cytotoxic and genotoxic features visualized were i. mitotic-index (MI) which is the ratio of the total number of mitotically dividing cells and the total number of cells present in a microscopic field expressed in percentage; ii. micronuclei (MNC) formation during interphase cells per 1000 cells (‰MNC) and iii. chromosomal anomalies viz. chromosomal fragments, anaphase bridges, multipolarity, laggard chromosomes and ghost cells.
Results
Demographic profile of the THs
Among the 47 THs interviewed, 41 were men and 6 were women aged between 32 to 85 years. Thirty three male THs were having primary or parallel professions like agriculture, animal husbandry or other services whereas the women were all housewives. Remaining eight male THs practiced traditional medicine as their primary profession. District-wise, among 47 THs, 33 represented Purulia and 8 and 6 THs represented Bankura and West Midnapore districts respectively. Most THs have been practicing traditional medicine for years which they acquired from their ancestry.
Traditional therapeutics using ethnobiologicals
A total number of 41 TFs were prescribed by the THs against poisonous and non-poisonous animal bites and insect stings (Table 1). They have reported 40 plant species (of 39 genera from 28 families) and 3 animal species (of 3 genera from 3 families) as direct ingredients in TFs against 5 poisonous and non-poisonous animal bites and insect stings. Among plant families, Fabaceae exhibited most number of medicinal plants (4 species) (Fig 2). A total number of 3 animal species were reported (Table 1), parts of which were used as direct ingredients or as additives in TFs. Salt, soap, kerosene oil, petrol, molasses, black pepper, milk, bovine urine and honey were added as additives, as taste enhancers and/or to enhance the efficacy of formulations applied topically. The plant and animal species with their local names, families and part(s) used are presented in Table 2 indicating their use in respective TFs and against the particular type of bite. Plant voucher numbers and habit types are also tabulated in Table 2. Herbs (20 records) exhibited the most common plant habit (Fig 3) whereas roots (21 uses) were reported as the most commonly used plant part (Fig 4).
Fig 2. Distribution of plant families.
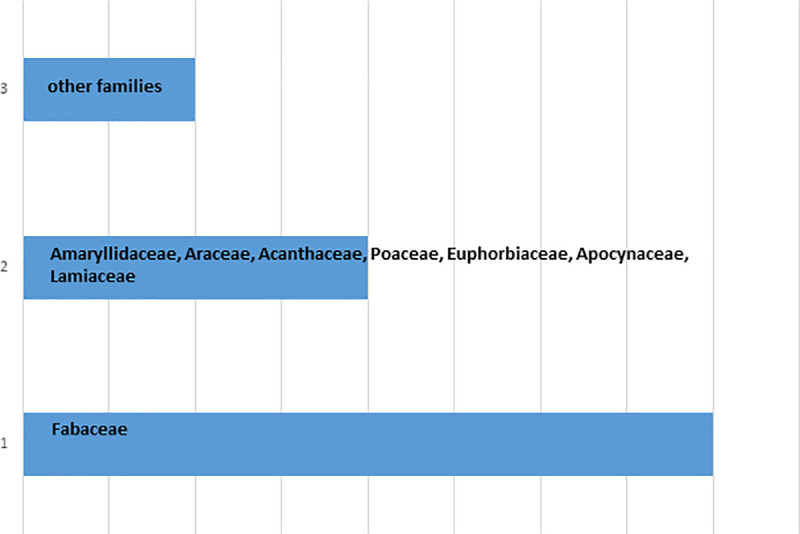
Table 2. Details of plants and animals as ingredients in Traditional Formulations (TFs) against poisonous and non-poisonous animal bites and insect stings.
| Botanical/Zoological binomials | Voucher No.1 | Family | Vernacular names | Habit | Part(s) used2 | Used in TFs3 | Ailment/disorder treated4 | UV | RI | CI | IAR | CAI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abrus precatorius L. | PB001 | Fabaceae | Kuch fall/ Kukurmala/Kanch Mala | climber | root (12040000) | 31, 33 | 5 | 0.22 | 0.31 | 1.43 | 0.98 | 1.4014 |
| Achyranthes aspera L. | PB002 | Amaranthaceae | Chitchiti | herb | root | 25 | 4 | 0.23 | 0.34 | 1.54 | 1.00 | 1.54 |
| Argemone mexicana L. | PB036 | Papavaveraceae | Rangani/ Shiyal kanta | herb | seed (11000000) | 23 | 2 | 0.24 | 0.39 | 1.59 | 0.99 | 1.5741 |
| Allium cepa L. | PB003 | Amaryllidaceae | Piyaj | herb | bulb (12020000) | 20 | 2 | 0.27 | 0.37 | 1.57 | 0.98 | 1.5386 |
| Allium sativum L. | PB004 | Amaryllidaceae | Rasun | herb | bulb | 3 | 1 | 0.31 | 0.37 | 2.66 | 0.98 | 2.6068 |
| Amorphophallus paeoniifolius (Dennst.) Nicolson | PB005 | Araceae | Ol | herb | stem (6000000) | 9, 24 | 1, 3 | 0.48 | 0.55 | 3.71 | 0.99 | 3.6729 |
| Apis cerana indica Fabricus (8031100) | - | Apidae | Moumachhi | - | honey | 37 | 5 | 0.21 | 0.31 | 1.43 | 0.97 | 1.3871 |
| Aristolochia indica L. | PB006 | Aristolochiaceae | Iswarmul/Ishermul/Bhedi-Janete | herb | root, bark (7000000) | 31, 34, 35, 38 | 5 | 1.59 | 0.91 | 4.98 | 0.99 | 4.9302 |
| Barleria lupulina Lindl. | PB007 | Acanthaceae | Bisalya karani | herb | bark | 1, 28, 29 | 1, 5 | 1.38 | 0.89 | 3.87 | 0.98 | 3.7926 |
| Buchanania lanzan Spreng. | PB008 | Anacardiaceae | Piyal | tree | bark | 34 | 5 | 0.20 | 0.26 | 1.55 | 0.98 | 1.519 |
| Buthus sp. Leach, 1815 (8010100) | - | Buthidae | Kankra bichha | - | digestive system (6000000) | 8 | 1 | 0.19 | 0.25 | 1.44 | 0.97 | 1.3968 |
| Cissampelos pareira L. | PB009 | Menispermaceae | Chokipar/Tijumala/ Chotopard | climber | root | 30, 39 | 5 | 1.11 | 0.79 | 3.65 | 0.98 | 3.577 |
| Cocos nucifera L. | PB010 | Arecaceae | Narcol | tree | oil | 14, 18 | 1, 2 | 0.98 | 0.77 | 3.60 | 0.98 | 3.528 |
| Crateva adansonii DC. | PB011 | Capparaceae | Hetal | tree | bark | 16 | 2 | 0.20 | 0.30 | 1.49 | 0.97 | 1.4453 |
| Crossopriza lyoni (Blackwall, 1867) (8010200) | - | Pholcidae | Makadsa | - | whole body (11040000) | 26 | 4 | 0.21 | 0.28 | 1.52 | 0.99 | 1.5048 |
| Cynodon dactylon (L.) Pers. | PB037 | Poaceae | Sada dhurba ghash | herb | leaf (8000000) | 15 | 1 | 0.20 | 0.29 | 1.49 | 0.97 | 1.4453 |
| Cyperus kyllingia Endl. | PB012 | Cyperaceae | Nirbishi | herb | root | 3 | 1 | 0.21 | 0.30 | 1.52 | 0.99 | 1.5048 |
| Diospyros melanoxylon Roxb. | PB013 | Ebenaceae | Kend | tree | bark | 10 | 1 | 0.23 | 0.29 | 1.57 | 0.97 | 1.5229 |
| Euphorbia tirucalli L. | PB014 | Euphorbiaceae | Harin singha | tree | latex (13020000) | 6 | 1 | 0.24 | 0.32 | 1.57 | 0.99 | 1.5543 |
| Flacourtia indica (Burm. f.) Merr. | PB015 | Salicaceae | Baichi | tree | stem, bark | 22 | 2 | 0.22 | 0.32 | 1.53 | 0.98 | 1.4994 |
| Gloriosa superba L. | PB016 | Colchicaceae | Liyaya ful/Bishlanguli/Dusatin lata | herb | root | 12, 21, 32 | 1, 2, 5 | 1.25 | 0.88 | 3.43 | 1.00 | 3.43 |
| Glossogyne bidens (Retz.) Alston | PB039 | Asteraceae | Bishaynandi | herb | whole plant (2000000) | 41 | 5 | 0.21 | 0.28 | 1.47 | 0.99 | 1.4553 |
| Hemidesmus indicus (L.) R. Br. ex Schult. | PB017 | Apocynaceae | Anantamul/Analsing | herb | root | 37 | 5 | 0.29 | 0.37 | 1.59 | 0.98 | 1.5582 |
| Ipomoea carnea Jacq. | PB038 | Convolvulaceae | Boka benri | shrub | leaf | 15 | 1 | 0.28 | 0.38 | 1.55 | 0.98 | 1.519 |
| Jatropha gossypifolia L. | PB018 | Euphorbiaceae | Gave verenda | shrub | bark | 7 | 1 | 0.22 | 0.33 | 1.49 | 0.97 | 1.4453 |
| Lycopodium cernuum L. | PB019 | Lycopodiaceae | Mrita sanjibani | herb | leaf | 29 | 5 | 0.23 | 0.33 | 1.50 | 1.00 | 1.5 |
| Martynia annua L. | PB020 | Martyniaceae | Bagh nakhi | herb | root | 34 | 5 | 0.23 | 0.29 | 1.51 | 0.98 | 1.4798 |
| Mucuna pruriens Scop. | PB021 | Fabaceae | Alkushi | shrub | seed | 5 | 1 | 0.31 | 0.28 | 1.77 | 0.97 | 1.7169 |
| Nardostachys jatamansi (D. Don) DC. | PB040 | Caprifoliaceae | Krishnajata | herb | root | 41 | 5 | 0.21 | 0.29 | 1.41 | 0.98 | 1.3818 |
| Nigella sativa L. | PB022 | Ranunculaceae | Kalojira | herb | seed | 11 | 1 | 0.29 | 0.35 | 1.75 | 0.99 | 1.7325 |
| Ocimum sanctum L. | PB023 | Lamiaceae | Lal tulsi | herb | leaf | 17 | 2 | 0.30 | 0.34 | 1.86 | 0.99 | 1.8414 |
| Piper nigrum L. | PB024 | Piperaceae | Rabing/Golki | vine | seed | 31, 32, 33, 34, 38, 39 | 5 | 1.78 | 0.80 | 5.68 | 0.97 | 5.5096 |
| Premna herbacea Roxb. | PB025 | Lamiaceae | Tarnd mula | herb | root | 2 | 1 | 0.25 | 0.33 | 1.49 | 0.98 | 1.4602 |
| Rauvolfia canescens L. | PB026 | Apocynaceae | Barachadar | shrub | root | 36 | 5 | 0.25 | 0.31 | 1.54 | 0.98 | 1.5092 |
| Ruella tuberosa L. | PB027 | Acanthaceae | Chatpati ghash | herb | leaf | 13 | 1 | 0.24 | 0.30 | 1.65 | 0.99 | 1.6335 |
| Scindapsus officinalis (Roxb.) Schott | PB028 | Araceae | Asthi sancharini | climber | leaf | 29 | 5 | 0.20 | 0.29 | 1.49 | 0.97 | 1.4453 |
| Setaria glauca (L.) P. Beauv. | PB029 | Poaceae | Khakra change/Bandarlej | herb | root | 2 | 1 | 0.21 | 0.29 | 1.50 | 0.98 | 1.47 |
| Smilax zeylanica L. | PB030 | Smilacaceae | Bamunhati/Ramdatun | shrub | root | 31 | 5 | 0.25 | 0.29 | 1.55 | 1.00 | 1.55 |
| Solanum surattense Burm. f. | PB031 | Solanaceae | Rangani | herb | root | 34 | 5 | 0.19 | 0.25 | 1.46 | 0.99 | 1.4454 |
| Streblus asper Lour. | PB032 | Moraceae | Seowra | tree | leaf | 4 | 1 | 0.22 | 0.31 | 1.49 | 1.00 | 1.49 |
| Tamarindus indica L. | PB033 | Fabaceae | Tentul | tree | twig | 27 | 4 | 0.27 | 0.31 | 1.60 | 0.97 | 1.552 |
| Tridax procumbens L. | PB034 | Asteraceae | Tar genda | herb | root | 2 | 1 | 0.26 | 0.32 | 1.58 | 0.98 | 1.5484 |
| Uraria picta (Jacq.) Desv. | PB035 | Fabaceae | Mahadevjata/Ishwarjata | herb | leaf | 40 | 5 | 0.22 | 0.32 | 1.49 | 0.99 | 1.4751 |
1Voucher no. indicates the herbarium sheet number deposited for future reference.
2The numbers used in parenthesis after the parts used are according to the recommendations given by Cook, 1995 as Economic botany data collection standard (EBDCS), plant parts, body parts and processes, disorders/effects, medicinal applications and non-vertebrate organisms (Master lists of states for Level 3 descriptors) (Economic Botany Data Standard; https://www.kew.org/tdwguses/rptMasterListMain.htm).
3Used in TFs indicates the plants used in traditional formulations given in Table 1. UV: use value; RI: Relative importance; CI: Cultural importance index; IAR: Index of agreement on remedies; CAI: cultural agreement index.
4The numbers use in this column are in accordance to the numbers used for different disease/ disorders in Table 1.
Fig 3. Distribution of plant habit types.

Fig 4. Distribution of plant and animal parts used by the THs.

Preparations and applications of TFs
Paste or ointment (23 uses) was recorded as the most used form of preparations (Fig 5) and topical administration was the most common mode of application (Fig 6). Among the animal parts, whole body, digestive system and honey were used. In most of the cases, plant and/or animal parts were ground to powder to make an ointment and were applied topically. In a few cases, the plant parts were heated over the flame and the affected area was covered with this heated part. TF19 was found to be applied as a lotion prepared from the salt and the kerosene oil and was applied on the stinging area to relieve pain just after an insect-sting. Most of the preparations were used either orally or topically; however, TF36 was used both orally as well as topically on the wound. In another interesting study, in TF8, the internal material/ digestive system of scorpion (Buthus sp.) by which the person was stung was rubbed on the stinging area. In TF26, Crossopriza lyoni (tailed cellar spiders) was used with plant part and milk and was taken orally to reduce the effect of the poison.
Fig 5. Distribution of drug preparations used by the THs.

Fig 6. Distribution of modes of drug administration prescribed by the THs.

Quantitative ethnobiology: UV, ICF, FL, RI, CI, IAR and CAI
Piper nigrum (1.78) and Apis cerana indica and Crossopriza lyoni (both 0.21) exhibited the highest UV among the plants and the animals respectively (Table 2). Stinging of centipede and dog/cat/hyena bite displayed highest ICF (ICF = 1.00 each) (Table 3). The ethnobotanicals/ethnozoologicals exhibiting high (70–100%), moderate (50–70%) and low (<50%) FL are presented in Table 4.
Table 3. Sting/bite category and corresponding Informant Consensus Factor (ICF) depicted from the interviews with the THs.
| Sting/bite category | nur = number of use citations in each category | nt = number of species used against a particular ailment by all informants | Informant consensus factor (ICF) nur−nt/nur−1 |
|---|---|---|---|
| Scorpion sting | 28 | 22 | 0.22 |
| Stinging of honey bee or wasp or hornet | 16 | 9 | 0.47 |
| Stinging of centipede | 4 | 1 | 1 |
| Dog/cat/hyena bite | 7 | 1 | 1 |
| Snake bite | 30 | 25 | 0.17 |
Table 4. Fidelity Level (FL) of the ethnobiologicals used depicted from the interviews with the THs.
| Disease/ailment category | FL (%) category | Most favored plant/animal used against particular bite/sting |
|---|---|---|
| Scorpion sting | High FL (70–100%) | Allium sativum |
| Barleria lupulina | ||
| Cynodon dactylon | ||
| Cyperus kyllingia | ||
| Gloriosa superba | ||
| Ipomoea carnea | ||
| Mucuna pruriens | ||
| Nigella sativa | ||
| Premna herbacea | ||
| Setaria glauca | ||
| Moderate FL (50–70%) | Diospyros melanoxylon | |
| Euphorbia tirucalli | ||
| Jatropha gossypifolia | ||
| Streblus asper | ||
| Low FL (<50%) | Amorphophallus paeoniifolius | |
| Buthus sp. | ||
| Cocos nucifera | ||
| Ruellia tuberosa | ||
| Stinging of honey bee or wasp or hornet | High FL (70–100%) | Allium cepa |
| Cocos nucifera | ||
| Ocimum sanctum | ||
| Moderate FL (50–70%) | Argemone Mexicana | |
| Flacourtia indica | ||
| Gloriosa superba | ||
| Low FL (<50%) | Crateva adansonii | |
| Stinging of centipede | Moderate FL (50–70%) | Amorphophallus paeoniifolius |
| Dog/cat/hyena bite | Moderate FL (50–70%) | Achyranthes aspera |
| Crossopriza lyoni | ||
| Tamarindus indica | ||
| Snake bite | High FL (70–100%) | Abrus precatorius |
| Apis cerana indica | ||
| Aristolochia indica | ||
| Cissampelos pareira | ||
| Gloriosa superba | ||
| Hemidesmus indicus | ||
| Piper nigrum | ||
| Rauvolfia canescens | ||
| Smilax zeylanica | ||
| Moderate FL (50–70%) | Barleria lupulina | |
| Buchanania lanzan | ||
| Glossogyne bidens | ||
| Martynia annua | ||
| Nardostachys jatamansi | ||
| Scindapsus officinalis | ||
| Low FL (<50%) | Calotropis gigantea | |
| Lycopodium cernuum | ||
| Solanum surattense | ||
| Uraria picta |
FL: fidelity level.
Other quantitative indices viz. RI, CI, IAR and CAI were also calculated for the plants and animals used as ethnomedicine (Table 2). Among the plants, maximum RI value (0.91) as well as CI value (4.98) were observed for Aristolochia indica. IAR which was calculated on the basis of importance of each species, was recorded maximum (1.00) for 5 plant species such as Achyranthes aspera, Gloriosa superba, Lycopodium cernuum, Smilax zeylanica and Streblus asper. Maximum CAI value was noted for the plant species Piper nigrum (5.5096). Among the animals, Apis cerana indica (0.31) displayed the highest RI value and Crossopriza lyoni (1.52) exhibited maximum CI value. Crossopriza lyoni also exhibited maximum IAR value (0.99) whereas Apis cerana indica (1.3871) was recorded for the maximum CAI value.
Use of ethnobotanicals: Toxicity aspects and conservation status
The use of the whole body of tailed cellar spider Crossopriza lyoni and the digestive system of the scorpion Buthus sp. may be implicated to possible toxicity and adverse effects in the recipients of oral or topical mode of administration. However, no such toxicity was reported by the THs. One of the prime conservation strategies adopted by the tribal people was to protect the plants by worshipping them in sacred groves. The authors found that plants such as Tamarindus indica, Cissampelos pareira, Streblus asper, Calotropis gigantea, Abrus precatorius, Ocimum sanctum, Achyranthes aspera and Aristolochia indica etc. were conserved in sacred groves.
Determination of PLA2 inhibition
The objective here was to find out plant extracts showing selective inhibition against the pro-inflammatory human group PLA2 without or minimally suppressing the porcine group digestive PLA2. Initially, three extracts [water, methanol and chloroform-ethanol (1:1)] exhibited promising outcome regarding PLA2 inhibition. However, only water extract was analyzed further since most of the ethnomedicines were prepared using water. The water extract of Aristolochia indica roots demonstrated the most significant inhibition of the enzyme human PLA2 with an IC50 value of 0.73 mg/ml. Following Aristolochia indica, water extracts of other plant parts showing promising PLA2 inhibition were Mucuna pruriens seeds (IC50 value = 0.79 mg/ml), Allium cepa bulbs, Gloriosa superba roots (both with IC50 value = 0.81 mg/ml), Hemidesmus indicus roots (IC50 value = 0.83 mg/ml), Nardostachys jatamansi roots (IC50 value = 0.88 mg/ml), Rauvolfia canescens roots (IC50 value = 0.90 mg/ml) and Piper nigrum seeds (IC50 value = 0.97 mg/ml) (Table 5). These findings indicated a selective inhibition of the extracts against the two PLA2.
Table 5. PLA2 inhibitory activities of the water extracts of the ethnobotanicals with high FL.
| Ethnobotanicals and parts | IC50 values (mg/ml) on human group PLA2 | IC50 values (mg/ml) on porcine group PLA2 | Inhibition specificity (IC50 porcine group PLA2/IC50 human group PLA2) |
|---|---|---|---|
| Abrus precatorius L. root | 1.76 | >5 | >2.84 |
| Achyranthes aspera L. root | 1.89 | >5 | >2.64 |
| Argemone mexicana L. seed | 1.67 | >5 | >2.99 |
| Allium cepa L. bulb | 0.81 | 3.2 | 3.95 |
| Allium sativum L. bulb | 1.98 | >5 | >2.52 |
| Amorphophallus paeoniifolius (Dennst.) Nicolson stem | 2.20 | >5 | >2.27 |
| Aristolochia indica L. root | 0.73 | 3.1 | 4.24 |
| Barleria lupulina Lindl. bark | 2.34 | >5 | >2.13 |
| Buchanania lanzan Spreng. bark | 3.34 | >5 | >1.49 |
| Cissampelos pareira L. root | 3.76 | >5 | >1.33 |
| Cocos nucifera L. oil | 3.67 | >5 | >1.36 |
| Crateva adansonii DC. bark | 2.89 | >5 | >1.73 |
| Cynodon dactylon (L.) Pers. leaf | 2.67 | >5 | >1.87 |
| Cyperus kyllingia Endl. root | 3.45 | >5 | >1.45 |
| Diospyros melanoxylon Roxb. bark | 4.54 | >5 | >1.10 |
| Euphorbia tirucalli L. latex | 3.09 | >5 | >1.62 |
| Flacourtia indica (Burm. f.) Merr. stem | 4.12 | >5 | >1.21 |
| Gloriosa superba L. root | 0.81 | 3.3 | 4.07 |
| Glossogyne bidens (Retz.) Alston whole plant | 2.98 | >5 | 1.67 |
| Hemidesmus indicus (L.) R. Br. ex Schult. root | 0.83 | 3.4 | 4.09 |
| Ipomoea carnea Jacq. leaf | 3.67 | >5 | >1.36 |
| Jatropha gossypifolia L. bark | 3.09 | >5 | >1.61 |
| Lycopodium cernuum L. leaf | 4.86 | >5 | >1.03 |
| Martynia annua L. root | 4.71 | >5 | >1.06 |
| Mucuna pruriens Scop. seed | 0.79 | 3.2 | 4.05 |
| Nardostachys jatamansi (D. Don) DC. root | 0.88 | 2.9 | 3.29 |
| Nigella sativa L. seed | 3.22 | >5 | >1.55 |
| Ocimum sanctum L. leaf | 3.33 | >5 | >1.50 |
| Piper nigrum L. seed | 0.97 | 3.6 | 3.71 |
| Premna herbacea Roxb. root | 4.60 | >5 | >1.08 |
| Rauvolfia canescens L. root | 0.90 | >5 | >5.55 |
| Ruella tuberosa L. leaf | 4.23 | >5 | >1.18 |
| Scindapsus officinalis (Roxb.) Schott leaf | 3.49 | >5 | >1.43 |
| Setaria glauca (L.) P. Beauv. root | 3.32 | >5 | >1.50 |
| Smilax zeylanica L. | 3.48 | >5 | >1.43 |
| Solanum surattense Burm. f. root | 4.04 | >5 | >1.23 |
| Streblus asper Lour. root | 3.19 | >5 | >1.56 |
| Tamarindus indica L. leaf | 4.04 | >5 | >1.23 |
| Tridax procumbens L. twig | 4.54 | >5 | >1.10 |
| Uraria picta (Jacq.) Desv. leaf | 2.78 | >5 | >1.79 |
IC50: The half maximal inhibitory concentration; PLA2: phospholipases A2.
Assessment of cytotoxicity and genotoxicity by Allium cepa root tip assay
Aqueous extracts of Allium cepa bulb, Hemidesmus indicus root, Nardostachys jatamansi root and Piper nigrum seed did not affect MI significantly since they demonstrated almost similar results as shown by the negative control. Mucuna pruriens seed and Rauvolfia canescens root water extracts exhibited slight inhibition of MI whereas the roots of Aristolochia indica and Gloriosa superba exhibited significant inhibition of MI especially at higher concentrations. Although the positive control EMS (2 × 10−2 M) showed maximum inhibition of MI (%) (1.58), Aristolochia indica root extracts at 10 mg/ml concentration showed potent antimitotic activity in Allium cepa root meristems with an MI (%) of 1.77. In addition, with increasing concentration of the water extracts of the used plant parts, a gradual decline in the MI was noted which was statistically comparable with both the controls (Fig 7). A few plant part extracts, at higher concentration caused chromosomal aberrations such as chromosome breaks, anaphase bridges, multipolarity, laggard chromosomes and ghost cells. However, the chromosomal aberrations were most profound in root tips treated with the positive control EMS. Genotoxicity of the root extracts was also recorded in the interphase MNC formation which was expressed as per 1000 cells (‰MNC). The root water extracts of Aristolochia indica and Gloriosa superba exhibited maximum genotoxic and cytotoxic potential which was comparable to the EMS. MNC formation was also found to be the highest when root tip cells were treated with the root water extracts of Aristolochia indica and Gloriosa superba (Table 6).
Fig 7. Mitotic Index (MI) in the root meristematic cells of Allium cepa in control and treatment concentrations of water extract of ethnobotanicals with high Fidelity Level (FL).

Table 6. Chromosomal aberrations and micronuclei formation in the root meristematic cells of Allium cepa in control and treatment concentrations of water extract of ethnobotanicals showing high FL and high PLA2 inhibition.
| Treatment groups | Concentrations | Chromosomal fragments ±SD | Multipolarity ±SD | Anaphase bridge ±SD | Laggard chromosome ±SD | Ghost cells ±SD | MNC (‰)±SD |
|---|---|---|---|---|---|---|---|
| negative control (tap water) | - | - | - | - | - | - | - |
| positive control (EMS) | 2 × 10−2 M | - | 9.61±4.49 | 4.02±3.56 | 3.39±2.45 | 13.90±3.32 | 0.48±0.19 |
| Allium cepa L. bulb, water extract | 2.5 mg/ml | - | - | - | - | - | - |
| 5 mg/ml | - | - | - | - | - | - | |
| 10 mg/ml | - | - | - | - | - | - | |
| Aristolochia indica L. root, water extract | 2.5 mg/ml | 6.65±1.19 | 7.85±2.21 | 1.91±2.22 | 2.46±3.33 | 10.59±2.87 | 0.54±0.23 |
| 5 mg/ml | 5.76±0.46 | 7.04±1.09 | 2.08±1.87 | 1.50±2.09 | 11.38±3.90 | 0.49±0.22 | |
| 10 mg/ml | 6.09±0.44 | 6.28±2.24 | 2.23±1.89 | 1.70±3.45 | 11.10±4.05 | 0.44±0.45 | |
| Gloriosa superba L. root, water extract | 2.5 mg/ml | 4.66±0.37 | 6.84±1.76 | 2.66±0.71 | 1.02±0.67 | 12.76±3.67 | 0.39±0.17 |
| 5 mg/ml | 5.54±1.45 | 5.50±0.89 | 1.89±0.98 | 2.81±0.98 | 12.06±4.56 | 0.59±0.14 | |
| 10 mg/ml | 4.81±0.67 | 6.93±0.69 | 1.94±0.91 | 1.19±0.20 | 10.90±2.68 | 0.60±0.09 | |
| Hemidesmus indicus (L.) R. Br. ex Schult. root, water extract | 2.5 mg/ml | 1.67±0.76 | - | - | - | 1.27±0.22 | - |
| 5 mg/ml | 2.02±0.34 | - | - | - | 2.32±1.48 | - | |
| 10 mg/ml | 1.88±1.73 | - | - | 0.56±2.831.75 | 2.01±1.09 | - | |
| Mucuna pruriens Scop. seed, water extract | 2.5 mg/ml | 2.02±1.77 | 4.77±0.73 | 0.67±2.56 | 0.98±1.79 | 7.70±2.67 | - |
| 5 mg/ml | 1.72±0.80 | 5.07±0.66 | 1.04±1.29 | 0.68±1.20 | 8.93±2.90 | 0.12±0.08 | |
| 10 mg/ml | 1.90±0.47 | 4.17±1.39 | 1.87±1.62 | 1.10±1.11 | 8.88±1.96 | 0.11±0.17 | |
| Nardostachys jatamansi (D. Don) DC. root, water extract | 2.5 mg/ml | 1.05±0.33 | 2.21±1.98 | - | - | 2.76±2.69 | - |
| 5 mg/ml | 0.66±0.21 | 1.65±1.54 | - | - | 2.90±2.34 | - | |
| 10 mg/ml | 1.14±0.20 | 2.94±2.02 | - | - | 1.98±2.93 | - | |
| Piper nigrum L. seed, water extract | 2.5 mg/ml | - | - | - | - | - | - |
| 5 mg/ml | - | - | - | - | - | - | |
| 10 mg/ml | - | - | - | - | - | - | |
| Rauvolfia canescens L. root, water extract | 2.5 mg/ml | 3.45±0.22 | 5.09±1.45 | 1.56±1.98 | 1.92±2.34 | 9.09±2.82 | 0.29±0.18 |
| 5 mg/ml | 4.98±0.29 | 4.96±0.46 | 2.20±2.74 | 2.02±2.67 | 11.10±2.78 | 0.32±0.23 | |
| 10 mg/ml | 4.02±33 | 5.01±0.48 | 1.85±2.89 | 2.22±3.03 | 10.63±3.22 | 0.17±0.25 |
Discussion
Study area and the aboriginals
The three western districts of the state of West Bengal, India are Purulia, Bankura and West Midnapore. This area is an extension of the Chota Nagpur Plateau (22°-25° 30'N and 83°47'-87° 50'E). Santhalis, Oraons, Mundas, Bhumijs, Birhors, Gonds, Kharias, Mal Pahariyas and Hos are the major tribal groups residing in these three districts. Austro-Asiatic and Dravidian languages are common among the tribal people. Natural tropical forests are prevalent in the westernmost district i.e. Purulia; however the forests have been constantly decreasing due to human exploitation. In this area, forests have supported the livelihood of its inhabitants via providing food, fodder, clothing, building materials, timber and so on. Undulated topography and extreme climate also counted for their overdependence on forest resources instead of opting for conventional agricultural practices. However the two districts Bankura and West Midnapore have cultivable lands and the presence of tribal groups is significantly lower in the two districts compared to Purulia. Over the past few years, irrigation and social forestry have improved the agricultural practices and productivity as well as forest coverage in the aforesaid areas. Once, Purulia was mentioned as the one of the 250 most backward districts (out of 640) of India, for which it received fund from the Backward Regions Grant und Program (BRGF) [48]. Non-availability of mainstream medication and remoteness of hospitals contribute to their over-reliance on age-old traditional medicines prescribed by THs. However, recent technological and communicational advancement alongside medical and backward development facilities have lifted the standard of living of the tribal populations. This elevation in social and economic conditions in the tribal populations subsequently declined the use of traditional medication that also led to the indifference and ignorance among the younger generations in taking up traditional healing as professions. Hence, the present documentation is also a timely representation of rapidly vanishing medicinal folklore of the local ethnic groups.
Traditional therapeutics using ethnobiologicals
Earlier, a number of ethnobiological surveys were conducted globally to explore the use of plants and animals against poisonous bites in northwest Colombia [49], Ethiopia [50], Hainan Island, China [51] and so on. Moreover, plants used against snakebites in Santarém, western Pará, Brazil were validated scientifically against hemorrhagic activity caused by Bothrops jararaca venom [52]. Ethnobotanical use of antivenin plants used in Uganda were also evidenced by pharmacological analysis [53]. Neutralization of lethal, enzymatic and hemorrhagic effects of Bothrops atrox venom by medicinal plants from Colombia was documented in a series of studies [49, 54, 55].
Some plants viz. Hemidesmus indicus [56], Gloriosa superba [57], Tamarindus indica [58], Aristolochia indica [59–61] etc. depicted in the present study have been investigated previously in vitro and/or in vivo for evaluating the anti-venom properties of their extracts and phytochemicals. The present study also reveals a few animals or animal products used in the folkloric medicine in polyherbal formulations active against poisonous or non-poisonous bites. The authors have also noted the inseparable existence of magical beliefs and prescribed medicine. For example, in TF8, the scorpion envenomation victim is given the internal material/ digestive system of the same scorpion by which the person was stung.
In India, up to one million snake bites are recorded in a single year of which as many as 50,000 are recorded as initial death. In fact, roughly the number of people died of snakebites in India, is almost equal to the total deaths due to snakebite in the rest of the world. And yet most of these deaths could have been prevented if necessary medical care was taken. Till date, the snake anti-venom is the only treatment for poisonous snakebites, which is produced from the snake venom itself. Most of the snake bites are either dry bites or from non-poisonous snakes. Still death occurs. There are many instances where patients die only because of fear and psychological shock. THs provide at least first aid and mental support to both patient and his/her families, where there is no other option [62, 63].
Preparations and applications of TFs
Mode of administration of TFs against poisonous and non-poisonous bites seemed to have played a crucial role since few of the plant or animal materials would have caused toxicity if applied orally instead of topical application. Authors have noted that most of these medicines were applied on the bitten or stinging area as paste or ointment or lotion, which was obviously meant to relieve the pain instantly or to alleviate symptoms of poisonous bites at some later stages. Among the TFs, only TF26, TF30, TF32, TF33, TF35 and TF41 were taken orally. Rest of the TFs were applied topically. In case of TF31, if patient becomes senseless, powder of dry leaves and roots of Kanch mala is given to the victim as snuff. As a prophylactic measure, Lal Tulsi (Ocimum sanctum) leaves were smeared over the body to keep away bees. In TF25, Chitchiti (Achyranthes aspera) root paste and molasses were prescribed topically and orally respectively against dog/cat/hyena bite.
Quantitative ethnobiology
Relative importance of the plants and animals used in the ethnomedicines is indicated by UV and among the plants Piper nigrum exhibited maximum UV (1.78) because of its widespread use in TFs to attenuate bitter taste of the formulations. Aristolochia indica (1.59), Barleria lupulina (1.38) and Gloriosa superba (1.25) were recorded among the other botanicals with high UVs which reflects their widespread acceptance as ethnomedicine. Similarly, Aristolochia indica (0.91), Barleria lupulina (0.89) and Gloriosa superba (0.88) also exhibited high RI values. The highest CI and CAI values were displayed by Piper nigrum which were 5.68 and 5.95 respectively. Highest IAR (1.00) was exhibited by four plants such as Achyranthes aspera, Gloriosa superba, Lycopodium cernuum and Streblus asper. In comparison to the plants, very less number of animals were present in the TFs which is reflected in their low UVs. High/moderate/low FL is displayed by various plant or animal species since they have been differentially accepted by the THs in their formulations.
Use of plants and animals as drugs: Toxicity and conservation aspects
Despite being used since time immemorial, use of traditional medicine must consider safety and toxicity issues. Animal samples such as digestive system of scorpion, whole body of spider and animal excreta such as bovine urine may possess toxicity and pathogens causing adverse effects in the recipients. Topical application of naphthalene, kerosene and petrol is also needed to be assessed for toxicity and side effects. THs have reported Aristolochia indica as a potent anti-venom herb. However, it has also been reported to cause aristolochic acid nephropathy (AAN) in different parts of the globe which has led to the discontinuation of the drug in different herbal products containing aristolochic acid [64]. Among the ethnozoologicals, Apis cerana indica, Buthus sp. and Crossopriza lyoni have not yet been assessed by the International Union for Conservation of Nature and Natural Resources (IUCN) Red List [source: The IUCN Red List of Threatened SpeciesTM (http://www.iucnredlist.org/search))].
PLA2 inhibitory activity of the medicinal plants
Interestingly, most of the plants (Aristolochia indica, Mucuna pruriens, Allium cepa, Gloriosa superba, Hemidesmus indicus, Nardostachys jatamansi, Rauvolfia canescens and Piper nigrum) showing high FL (70–100%) exhibited high PLA2 inhibitory activity against human group PLA2 without or minimally inhibiting the digestive porcine group PLA2. Similar observations were noted while elucidating the anti-PLA2 activity of Aloe vera leaf skin extracts. Earlier, selective inhibition of pro-inflammatory PLA2 group IIA was attributed to the catechin tannins present in the water extract of Aloe vera. It was further suggested that the compounds present in the extracts exhibiting anti-PLA2 properties were different than those displaying antioxidant properties [65]. High PLA2 inhibitory potential of the plants indicates their possible effectiveness against snake venom. In exploration of alternative antivenin, especially in rural India where lack of conventional antivenin against poisonous snakebites causes a number of mortality and morbidity every year. Available literature also supports the traditional use of Aristolochia indica, Gloriosa superba, Hemidesmus indicus [66] and Nardostachys jatamansi [67] against snakebite. Snake venom neutralization ability of Hemidesmus indicus [55], Aristolochia indica [58] and Gloriosa superba [68] has also been assessed via in vitro and in vivo studies. A number of phytochemicals such as 2-hydroxy-4-methoxy benzoic acid and lupeol acetate from Hemidesmus indicus [69, 70] and aristolochic acid from Aristolochia indica [59, 60] also exhibited potent antivenin as well as anti-PLA2 activities.
Aristolochic acid [8-methoxy-6-nitrophenanthro(3,4-d)-1,3-dioxole-5-carboxylic acid], an uncompetitive inhibitor with a Ki of 9.9 × 10−4 M (with phosphatidylcholine as substrate), was found to interact with the major basic PLA2 from Vipera russelli venom. Administration of aristolochic acid inhibited edema-inducing activity of Vipera russelli PLA2. Suppression of edema-induction by aristolochic acid was manifested when it reached the site; however, it did not aid in recovery. Aristolochic acid also failed to suppress other pathological properties of PLA2 [60]. In another study, aristolochic acid inhibited human synovial fluid (HSF)-PLA2, porcine pancreatic PLA2, Naja naja PLA2, and human platelet derived PLA2 dose dependently with sensitivity of these PLA2s to aristolochic acid varied significantly: HSF-PLA2> Naja naja PLA2> human platelet PLA2> porcine pancreatic PLA2. In addition, it was indicated that inhibition of HSF-PLA2 was possibly mediated via direct interaction with the enzyme [70]. The compound 2-hydroxy-4-methoxy benzoic acid from Hemidesmus indicus exhibited adjuvant efficacy and antiserum potentiation in rabbits immunized with Vipera russellii venom demonstrating potent venom neutralization ability (lethal and hemorrhage) [69]. Hemidesmus indicus root extract-derived lupeol acetate remarkably neutralized Daboia russellii venom-induced edema, haemorrhage, defibrinogenation, PLA2 activity and lethality in male albino mice. Furthermore, Naja kaouthia venom-induced neurotoxicity, cardiotoxicity, respiratory modulations and lethality in the animals were also neutralized by the compound. In addition, venom-induced alterations in super oxide dismutase (SOD) activity and lipid peroxidation were also antagonized by lupeol acetate [70]. Therefore, ethnobiological use of a few medicinal plants reported in the present study is being supported by scientific literature describing their in vitro and in vivo efficacy as potent antivenin.
Genotoxic and cytotoxic effects of plant extracts on Allium cepa root tip meristems
The plant parts used by the THs were extracted in water and the roots of Aristolochia indica and Gloriosa superba demonstrated significant antimitotic and genotoxic potential in Allim cepa root tip assay. Therefore, oral administration of such extracts are discouraged also keeping in mind the worldwide occurrence of aristolochic acid nephropathy (AAN) due to the consumption of Aristolochia preparations in traditional medicine and in health supplements [71]. Earlier, Allium cepa root tip meristems were used to assess the genotoxic and cytotoxic effects of Aristolochia birostris water and alcoholic extracts [72]. Hemidesmus indicus root extract was evaluated in cultured lymphocytes for its genotoxic and antigenotoxic effects [73]. Species of Gloriosa were also evaluated for their antimitotic effects on onion roots [74]. The present study evaluated the mutagenic and genotoxic properties of the plant extracts in a dose dependent manner and further suggested to take precautions before using few plant extracts in human.
Conclusions
The three western districts of the state of West Bengal are the natural dwelling place for many indigenous communities surviving the climatic and economic hardships and exercising their time tested medical practices mostly based upon the uses of ethnobiologicals. These traditional and alternative treatments serve as the only option in these underprivileged and geographically remote areas during medical-exigencies like snake envenomation. The present study depicts a quantitative ethnobiological analysis among the aboriginals from the area against poisonous and non-poisonous animal bites and insect stings. However, the efficacy of the antivenin ethnobiologicals are needed to be validated scientifically. In addition, bioactivity guided isolation of phyto-constituents may lead to the templates for synthesis of novel antivenins. In addition, plants with higher FL displayed superior anti-PLA2 properties via selective inhibition of human-group PLA2. In addition, Allium cepa root tip assay revealed significant genotoxic and cytotoxic properties of some plant extracts. Therefore, concomitant studies on toxicology and safety of the plant extracts are also needed for safe efficacious application of botanical-derived antivenin.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(PPTX)
Acknowledgments
Local people of the study area are thankfully acknowledged for sharing ethnomedicinal information. We would also like to thank Mr Mrinal Mondal, Assistant Professor, Department of Geography, Sidho-Kanho-Birsha University, Purulia for constructing the map of the study area.
Data Availability
All relevant data are within the manuscript and its Supporting Informations files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Allen GE, Brown SG, Buckley NA, O’Leary MA, Page CB, Currie BJ, et al. Clinical effects and antivenom dosing in brown snake (Pseudonaja spp.) envenoming—Australian snakebite project (ASP-14). PLoS One. 2012;7(12):e53188 10.1371/journal.pone.0053188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kasturiratne A, Wickremasinghe AR, de Silva N, Gunawardena NK, Pathmeswaran A, Premaratna R, et al. The global burden of snakebite: a literature analysis and modelling based on regional estimates of envenoming and deaths. PLoS Med. 200;5(11):e218 10.1371/journal.pmed.0050218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Warrell DA. Snake bite. Lancet, 2010;375(9708):77–88. 10.1016/S0140-6736(09)61754-2 [DOI] [PubMed] [Google Scholar]
- 4.Gupta YK, Peshin SS. Do herbal medicines have potential for managing snake bite envenomation? Toxicol Int. 2012;19(2):89 10.4103/0971-6580.97194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harrison RA, Cook DA, Renjifo C, Casewell NR, Currier RB, Wagstaff SC. Research strategies to improve snakebite treatment: Challenges and progress. J Proteomics. 2011;74(9):1768–80. 10.1016/j.jprot.2011.06.019 [DOI] [PubMed] [Google Scholar]
- 6.Mohapatra B, Warrell DA, Suraweera W, Bhatia P, Dhingra N, Jotkar RM, et al. , Million Death Study Collaborators. Snakebite mortality in India: a nationally representative mortality survey. PLoS Negl Trop Dis. 2011;5(4):e1018 10.1371/journal.pntd.0001018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cupo P. Clinical update on scorpion envenoming. Revista da Sociedade Brasileira de Medicina Tropical. 2015;48(6):642–9. 10.1590/0037-8682-0237-2015 [DOI] [PubMed] [Google Scholar]
- 8.Ortiz E, Gurrola GB, Schwartz EF, Possani LD. Scorpion venom components as potential candidates for drug development. Toxicon. 2015;93:125–35. 10.1016/j.toxicon.2014.11.233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yildiz A, Biceroglu S, Yakut N, Bilir C, Akdemir R, Akilli A. Acute myocardial infarction in a young man caused by centipede sting. Emer Med J. 2006;23(4):e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Üreyen ÇM, Arslan Ş, Baş CY. Cardiovascular collapse after myocardial infarction due to centipede bite. Wien Klin Wochenschr. 2015;127(13–14):577–9. 10.1007/s00508-015-0801-z [DOI] [PubMed] [Google Scholar]
- 11.Gupta PN, Kumar BK, Velappan P, Sudheer MD. Possible complication of bee stings and a review of the cardiac effects of bee stings. Case Reports. 2016;2016:bcr2015213974. 10.1136/bcr-2015-213974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kularatne SA, Raveendran S, Edirisinghe J, Karunaratne I, Weerakoon K. First reported case of fatal stinging by the large carpenter bee Xylocopa tranquebarica. Wilderness. Env Med. 2016;27(2):262–5. 10.1016/j.wem.2015.12.018 [DOI] [PubMed] [Google Scholar]
- 13.Griego RD, Rosen T, Orengo IF, Wolf JE. Dog, cat, and human bites: a review. J Am Acad Dermatol. 1995;33(6):1019–29. 10.1016/0190-9622(95)90296-1 [DOI] [PubMed] [Google Scholar]
- 14.Szczypa K, Hryniewicz W. Epidemiology, microbiology and diagnostics of dog and cat bites related infections. Pol Merkur Lekarski. 2015;39(232):199–204. [PubMed] [Google Scholar]
- 15.Lee JK, Tan RB, Chung E. Erectile dysfunction treatment and traditional medicine—can East and West medicine coexist? Transl Androl Urol. 2017;6(1):91 10.21037/tau.2016.11.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Azaizeh H, Fulder S, Khalil K, Said O. Ethnobotanical knowledge of local Arab practitioners in the Middle Eastern region. Fitoterapia. 2003;74(1–2):98–108. 10.1016/s0367-326x(02)00285-x [DOI] [PubMed] [Google Scholar]
- 17.Dey A, De JN. Ethnomedicinal plants used by the tribals of Purulia district, West Bengal, India against gastrointestinal disorders. J Ethnopharmacol. 2012;143(1):68–80. 10.1016/j.jep.2012.05.064 [DOI] [PubMed] [Google Scholar]
- 18.Gutiérrez J, Lomonte B. Phospholipase A2 myotoxins from Bothrops snake venoms. Toxicon. 1995;33(11):1405–24. 10.1016/0041-0101(95)00085-z [DOI] [PubMed] [Google Scholar]
- 19.Kini RM. Excitement ahead: structure, function and mechanism of snake venom phospholipase A2 enzymes. Toxicon. 2003;42(8):827–40. 10.1016/j.toxicon.2003.11.002 [DOI] [PubMed] [Google Scholar]
- 20.Marcussi S, Sant'Ana CD, Oliveira CZ, Quintero Rueda A, Menaldo DL, Beleboni RO, et al. Snake venom phospholipase A2 inhibitors: medicinal chemistry and therapeutic potential. Current topics in medicinal chemistry. 2007;7(8):743–56. 10.2174/156802607780487614 [DOI] [PubMed] [Google Scholar]
- 21.Cardoso FF, Borges RJ, Dreyer TR, Salvador GH, Cavalcante WL, Dal Pai M, et al. Structural basis of phospholipase A2-like myotoxin inhibition by chicoric acid, a novel potent inhibitor of ophidian toxins. Biochimica et Biophysica Acta (BBA)-General Subjects. 2018;1862(12):2728–37. 10.1016/j.bbagen.2018.08.002 [DOI] [PubMed] [Google Scholar]
- 22.Rodrigues CF, Ferreira MJ, Belchor MN, Costa CR, Novaes DP, dos Santos Junior AB, et al. Evaluation of the Inhibitory Potential of Casuarictin, an Ellagitannin Isolated from White Mangrove (Laguncularia racemosa) Leaves, on Snake Venom Secretory Phospholipase A2. Marine drugs. 2019;17(7):403 10.3390/md17070403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cesar PH, Trento MV, Sales TA, Simão AA, Ramalho TC, Marcussi S. Vanillic acid as phospholipase A2 and proteases inhibitor: In vitro and computational analyses. Biotechnology and Applied Biochemistry. 2020. [DOI] [PubMed] [Google Scholar]
- 24.Leme DM, Marin-Morales MA. Allium cepa test in environmental monitoring: a review on its application. Mutation Research/Reviews in Mutation Research. 2009;682(1):71–81. 10.1016/j.mrrev.2009.06.002 [DOI] [PubMed] [Google Scholar]
- 25.Rahman MM, Rahman MF, Nasirujjaman K. A study on genotoxicity of textile dyeing industry effluents from Rajshahi, Bangladesh, by the Allium cepa test. Chemistry and Ecology. 2017;33(5):434–46. [Google Scholar]
- 26.Bonciu E, Firbas P, Fontanetti CS, Wusheng J, Karaismailoğlu MC, Liu D, et al. An evaluation for the standardization of the Allium cepa test as cytotoxicity and genotoxicity assay. Caryologia. 2018;71(3):191–209. [Google Scholar]
- 27.Datta S, Singh J, Singh J, Singh S, Singh S. Assessment of genotoxic effects of pesticide and vermicompost treated soil with Allium cepa test. Sustainable Environment Research. 2018;28(4):171–8. [Google Scholar]
- 28.Debnath P, Mondal A, Sen K, Mishra D, Mondal NK. Genotoxicity study of nano Al2O3, TiO2 and ZnO along with UV-B exposure: An Allium cepa root tip assay. Science of The Total Environment. 2020;713:136592 10.1016/j.scitotenv.2020.136592 [DOI] [PubMed] [Google Scholar]
- 29.Prajitha V, Thoppil JE. Genotoxic and antigenotoxic potential of the aqueous leaf extracts of Amaranthus spinosus Linn. using Allium cepa assay. South African Journal of Botany. 2016;102:18–25. [Google Scholar]
- 30.Pesnya DS, Romanovsky AV, Serov DA, Poddubnaya NY. Genotoxic effects of Heracleum sosnowskyi in the Allium cepa test. Caryologia. 2017;70(1):55–61. [Google Scholar]
- 31.Liman R, Ciğerci İH, Gökçe S. Cytogenetic and genotoxic effects of Rosmaniric Acid on Allium cepa L. root meristem cells. Food and Chemical Toxicology. 2018;121:444–9. 10.1016/j.fct.2018.09.022 [DOI] [PubMed] [Google Scholar]
- 32.Akwu NA, Naidoo Y, Singh M. Cytogenotoxic and biological evaluation of the aqueous extracts of Grewia lasiocarpa: An Allium cepa assay. South African Journal of Botany. 2019;125:371–80. [Google Scholar]
- 33.Prain D. Bengal Plants 1–2. 1903. Botanical Survey of India, Calcutta. [Google Scholar]
- 34.Sanyal MN. Flora of Bankura District, West Bengal. 1994. Bishen Singh Mahendra Pal Singh, New Delhi.
- 35.Cook FE. Economic botany data collection standard. Royal Botanic Gardens (Kew). 1995. [Google Scholar]
- 36.Phillips O, Gentry AH, Reynel C, Wilkin P, Gálvez‐Durand B C. Quantitative ethnobotany and Amazonian conservation. Conserv Biol. 1994;8(1):225–48. [Google Scholar]
- 37.Heinrich M, Ankli A, Frei B, Weimann C, Sticher O. Medicinal plants in Mexico: Healers' consensus and cultural importance. Soc Sci Med. 1998;47(11):1859–71. 10.1016/s0277-9536(98)00181-6 [DOI] [PubMed] [Google Scholar]
- 38.Friedman J, Yaniv Z, Dafni A, Palewitch D. A preliminary classification of the healing potential of medicinal plants, based on a rational analysis of an ethnopharmacological field survey among Bedouins in the Negev Desert, Israel. J Ethnopharmacol. 1986;16(2–3):275–87. 10.1016/0378-8741(86)90094-2 [DOI] [PubMed] [Google Scholar]
- 39.Oliveira ES, Torres DF, Brooks SE, Alves RR. The medicinal animal markets in the metropolitan region of Natal City, Northeastern Brazil. J Ethnopharmacol. 2010;130(1):54–60. 10.1016/j.jep.2010.04.010 [DOI] [PubMed] [Google Scholar]
- 40.Kadir MF, Sayeed MS, Mia MM. Ethnopharmacological survey of medicinal plants used by indigenous and tribal people in Rangamati, Bangladesh. J Ethnopharmacol. 2012;144(3):627–37. 10.1016/j.jep.2012.10.003 [DOI] [PubMed] [Google Scholar]
- 41.Tardío J, Pardo-de-Santayana M. Cultural importance indices: a comparative analysis based on the useful wild plants of Southern Cantabria (Northern Spain). Econ Bot. 2008;62(1):24–39. [Google Scholar]
- 42.Kufer J, Heinrich M, Förther H, Pöll E. Historical and modern medicinal plant uses—the example of the Ch'orti ‘Maya and Ladinos in Eastern Guatemala. J Pharm Pharmacol. 2005;57(9):1127–52. 10.1211/jpp.57.9.0008 [DOI] [PubMed] [Google Scholar]
- 43.Trotter RT, Logan MH. Informant consensus: a new approach for identifying potentially effective medicinal plants In: Etkin N.L. (Ed.), Plants in Indigenous Medicine and Diet, Behavioural Approaches. Redgrave Publishing Company, Bredford Hills, New York, 1986, pp. 91–112. [Google Scholar]
- 44.Thomas E, Vandebroek I, Sanca S, Van Damme P. Cultural significance of medicinal plant families and species among Quechua farmers in Apillapampa, Bolivia. J Ethnopharmacol. 2009;122(1):60–7. 10.1016/j.jep.2008.11.021 [DOI] [PubMed] [Google Scholar]
- 45.Bruschi P., Morganti M., Mancini M., Signorini M.A. Traditional healers and lay people: a qualitative and quantitative approach to local knowledge on medicinal plants in Muda (Mozambique). J. Ethnopharmacol. 2011; 138:543–63. 10.1016/j.jep.2011.09.055 [DOI] [PubMed] [Google Scholar]
- 46.De Araújo A.L., Radvanyi F. Determination of phospholypase A2 activity by a colorimetric assay using a pH indicator. Toxicon 1987, 25(11):1181–8. 10.1016/0041-0101(87)90136-x [DOI] [PubMed] [Google Scholar]
- 47.Aşkin Çelik T, Aslantürk ÖS. Evaluation of cytotoxicity and genotoxicity of Inula viscosa leaf extracts with Allium test. Journal of BioMed Research. 2010;2010 10.1155/2010/189252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anonymous. A Note on the Backward Regions Grant Fund Programme. National Institute of Rural Development. Ministry of Panchayati Raj, India: 2009. http://www.nird.org.in/brgf/doc/brgf_BackgroundNote.pdf. [Google Scholar]
- 49.Otero R, Fonnegra R, Jiménez SL, Núñez V, Evans N, Alzate SP, et al. Snakebites and ethnobotany in the northwest region of Colombia: Part I: traditional use of plants. J Ethnopharmacol. 2000;71(3):493–504. 10.1016/s0378-8741(00)00243-9 [DOI] [PubMed] [Google Scholar]
- 50.Giday M, Asfaw Z, Woldu Z. Medicinal plants of the Meinit ethnic group of Ethiopia: an ethnobotanical study. J Ethnopharmacol. 2009;124(3):513–21. 10.1016/j.jep.2009.05.009 [DOI] [PubMed] [Google Scholar]
- 51.Zheng XL, Xing FW. Ethnobotanical study on medicinal plants around Mt. Yinggeling, Hainan Island, China. J Ethnopharmacol. 2009;124(2):197–210. 10.1016/j.jep.2009.04.042 [DOI] [PubMed] [Google Scholar]
- 52.de Moura VM, de Sousa LA, Dos-Santos MC, Raposo JD, Lima AE, de Oliveira RB, et al. Plants used to treat snakebites in Santarém, western Pará, Brazil: an assessment of their effectiveness in inhibiting hemorrhagic activity induced by Bothrops jararaca venom. J Ethnopharmacol. 2015;161:224–32. 10.1016/j.jep.2014.12.020 [DOI] [PubMed] [Google Scholar]
- 53.Omara T, Kagoya S, Openy A, Omute T, Ssebulime S, Kiplagat KM, et al. Antivenin plants used for treatment of snakebites in Uganda: ethnobotanical reports and pharmacological evidences. Trop Med Health 2020;48(1):6 10.1186/s41182-019-0187-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Otero R, Núñez V, Jiménez SL, Fonnegra R, Osorio RG, Garcıa ME, et al. Snakebites and ethnobotany in the northwest region of Colombia: Part II: neutralization of lethal and enzymatic effects of Bothrops atrox venom. J Ethnopharmacol. 2000;71(3):505–11. 10.1016/s0378-8741(99)00197-x [DOI] [PubMed] [Google Scholar]
- 55.Otero R, Núñez V, Barona J, Fonnegra R, Jiménez SL, Osorio RG, et al. Snakebites and ethnobotany in the northwest region of Colombia: Part III: Neutralization of the haemorrhagic effect of Bothrops atrox venom. J Ethnopharmacol. 2000;73(1–2):233–41. 10.1016/s0378-8741(00)00321-4 [DOI] [PubMed] [Google Scholar]
- 56.Alam MI, Auddy B, Gomes A. Viper venom neutralization by Indian medicinal plant (Hemidesmus indicus and Pluchea indica) root extracts. Phytother Res. 1996;10(1):58–61. [Google Scholar]
- 57.Mors WB, Do Nascimento MC, Pereira BM, Pereira NA. Plant natural products active against snake bite—the molecular approach. Phytochemistry. 2000;55(6):627–42. 10.1016/s0031-9422(00)00229-6 [DOI] [PubMed] [Google Scholar]
- 58.Ushanandini S, Nagaraju S, Harish Kumar K, Vedavathi M, Machiah DK, Kemparaju K, et al. The anti‐snake venom properties of Tamarindus indica (leguminosae) seed extract. Phytother Res. 2006;20(10):851–8. 10.1002/ptr.1951 [DOI] [PubMed] [Google Scholar]
- 59.Bhattacharjee P, Bhattacharyya D. Characterization of the aqueous extract of the root of Aristolochia indica: evaluation of its traditional use as an antidote for snake bites. J Ethnopharmacol. 2013;145(1):220–6. 10.1016/j.jep.2012.10.056 [DOI] [PubMed] [Google Scholar]
- 60.Vishwanath BS, Gowda TV. Interaction of aristolochic acid with Vipera russelli phospholipase A2: its effect on enzymatic and pathological activities. Toxicon, 1987;25(9):929–937. 10.1016/0041-0101(87)90155-3 [DOI] [PubMed] [Google Scholar]
- 61.Vishwanath BS, Fawzy AA, Franson RC. Edema-inducing activity of phospholipase A 2 purified from human synovial fluid and inhibition by aristolochic acid. Inflammation. 1988;12(6):549–61. 10.1007/BF00914317 [DOI] [PubMed] [Google Scholar]
- 62.Modak BK. An overview of fast declining snake charmers in India. Indian J Landscape Systems Eco Stud. 2009;32:67–72. [Google Scholar]
- 63.Konar AK, Modak BK. Socialising Snake Society: An Indian Instance. Social Change 2010;40 (2):157–174. [Google Scholar]
- 64.Jadot I, Declèves AE, Nortier J, Caron N. An integrated view of aristolochic acid nephropathy: update of the literature. 18(2):297. Int J Mol Sci. 2017;18(2):297 10.3390/ijms18020297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kammoun M, Miladi S, Ali YB, Damak M, Gargouri Y, Bezzine S. In vitro study of the PLA2 inhibition and antioxidant activities of Aloe vera leaf skin extracts. Lipids in health and disease. 2011;10(1):30 10.1186/1476-511X-10-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Samy RP, Thwin MM, Gopalakrishnakone P, Ignacimuthu S. Ethnobotanical survey of folk plants for the treatment of snakebites in Southern part of Tamilnadu, India. J Ethnopharmacol. 2008;115(2):302–12. 10.1016/j.jep.2007.10.006 [DOI] [PubMed] [Google Scholar]
- 67.Kala CP. Herbal treatment for snakebites in Uttarakhand state of India. Indian J Trad Knowl. 2015;6(1):56–61. [Google Scholar]
- 68.Khan H, Ali Khan M, Hussan I. Enzyme inhibition activities of the extracts from rhizomes of Gloriosa superba Linn (Colchicaceae). J Enz Inhib Med Chem. 2007;22(6):722–5. [DOI] [PubMed] [Google Scholar]
- 69.Alam MI, Gomes A. Adjuvant effects and antiserum action potentiation by a (herbal) compound 2-hydroxy-4-methoxy benzoic acid isolated from the root extract of the Indian medicinal plantsarsaparilla'(Hemidesmus indicus R. Br.). Toxicon. 1998;36(10):1423–31. 10.1016/s0041-0101(98)00076-2 [DOI] [PubMed] [Google Scholar]
- 70.Chatterjee I, Chakravarty AK, Gomes A. Daboia russellii and Naja kaouthia venom neutralization by lupeol acetate isolated from the root extract of Indian sarsaparilla Hemidesmus indicus R. Br. Journal of ethnopharmacology. 2006;106(1):38–43. 10.1016/j.jep.2005.11.031 [DOI] [PubMed] [Google Scholar]
- 71.Michl J, Jennings HM, Kite GC, Ingrouille MJ, Simmonds MS, Heinrich M. Is aristolochic acid nephropathy a widespread problem in developing countries?: A case study of Aristolochia indica L. in Bangladesh using an ethnobotanical–phytochemical approach. Journal of ethnopharmacology. 2013;149(1):235–44. 10.1016/j.jep.2013.06.028 [DOI] [PubMed] [Google Scholar]
- 72.Mendes SS, Andrade JA, Xavier MA, Secundo Junior JA, Pantaleão SM, Estevam CS, et al. Genotoxicity test of Maytenus rigida and Aristolochia birostris in the radicular meristem of the onion, Allium cepa. Revista Brasileira de Farmacognosia. 2012;22(1):76–81. [Google Scholar]
- 73.Ananthi R, Chandra N, Santhiya ST, Ramesh A. Genotoxic and antigenotoxic effects of Hemidesmus indicus R. Br. root extract in cultured lymphocytes. Journal of Ethnopharmacology. 2010;127(2):558–60. 10.1016/j.jep.2009.10.034 [DOI] [PubMed] [Google Scholar]
- 74.Bharathi P, Philomina D, Chakkaravarthi S. Antimitotic effect of colchicine from six different species of Gloriosa in onion roots (Allium cepa). J. Med. Sci. 2006;6(3):420–5. [Google Scholar]



