Abstract
Background
Early and accurate prognosis prediction of the patients was urgently warranted due to the widespread popularity of COVID-19. We performed a meta-analysis aimed at comprehensively summarizing the clinical characteristics and laboratory abnormalities correlated with increased risk of mortality in COVID-19 patients.
Methods
PubMed, Scopus, Web of Science, and Embase were systematically searched for studies considering the relationship between COVID-19 and mortality up to 4 June 2020. Data were extracted including clinical characteristics and laboratory examination.
Results
Thirty-one studies involving 9407 COVID-19 patients were included. Dyspnea (OR = 4.52, 95%CI [3.15, 6.48], P < 0.001), chest tightness (OR = 2.50, 95%CI [1.78, 3.52], P<0.001), hemoptysis (OR = 2.00, 95%CI [1.02, 3.93], P = 0.045), expectoration (OR = 1.52, 95%CI [1.17, 1.97], P = 0.002) and fatigue (OR = 1.27, 95%CI [1.09, 1.48], P = 0.003) were significantly related to increased risk of mortality in COVID-19 patients. Furthermore, increased pretreatment absolute leukocyte count (OR = 11.11, 95%CI [6.85,18.03], P<0.001) and decreased pretreatment absolute lymphocyte count (OR = 9.83, 95%CI [6.72, 14.38], P<0.001) were also associated with increased mortality of COVID-19. We also compared the mean value of them between survivors and non-survivors, and found that non-survivors showed significantly raise in pretreatment absolute leukocyte count (WMD: 3.27×109/L, 95%CI [2.34, 4.21], P<0.001) and reduction in pretreatment absolute lymphocyte count (WMD = -0.39×109/L, 95%CI [-0.46, -0.33], P<0.001) compared with survivors. The results of pretreatment lactate dehydrogenase (LDH), procalcitonin (PCT), D-Dimer and ferritin showed the similar trend with pretreatment absolute leukocyte count.
Conclusions
Among the common symptoms of COVID-19 infections, fatigue, expectoration, hemoptysis, dyspnea and chest tightness were independent predictors of death. As for laboratory examinations, significantly increased pretreatment absolute leukocytosis count, LDH, PCT, D-Dimer and ferritin, and decreased pretreatment absolute lymphocyte count were found in non-survivors, which also have an unbeneficial impact on mortality among COVID-19 patients. Motoring these indicators during the hospitalization plays a very important role in predicting the prognosis of patients.
Introduction
Since December 2019, the pandemic of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pneumonia has caused more than 8 million infections, with 440,290 deaths worldwide until June 17th, 2020 [1]. Increasing evidence is investigating the clinical features and laboratory abnormalities in patients with COVID-19 infection. Considering the widespread of COVID-19, early and accurate prognosis prediction is urgently warranted. However, the specific symptoms and laboratory biomarkers which may help predict the poor prognosis of COVID-19 patients were unclear. Therefore, we aimed to perform a systematic meta-analysis to summarize the clinical characteristics and laboratory test before treatment among COVID-19 patients and identify the possible risk factors for mortality.
Materials and methods
Search strategy
We conducted a systematic search in PubMed, Scopus, Web of Science and Embase to identify studies in patients with COVID-19 infection up to 4 June 2020. The following keywords were used: “2019 novel coronavirus disease”, “severe acute respiratory syndrome coronavirus 2”, “COVID-19”, “2019-nCoV”, “SARS-CoV-2” and “clinical”, “laboratory”, “risk factor”, and “mortality”, “mortal”, “fatality”, “fatal”, “lethality” or “death”. No restrictions on publication status were imposed. Only studies published in English and Chinese were retrieved for this meta-analysis. In addition, reference lists of relevant records were manually screened for further potentially eligible articles.
Inclusion criteria and exclusion criteria
Two researchers reviewed all articles independently based on titles and abstracts. The inclusion criteria were as follows: 1) all patients were confirmed with COVID-19; 2) studies reported the clinical characteristics, hematological and serological abnormalities both in survivors and non-survivors.
The exclusion criteria were as follows: 1) reviews, letters, case reports, conference abstracts and duplicated publications; 2) insufficient data were provided for extrapolating the mean±SD for hematologic parameters.
Data extraction and assessment of risk of bias
Studies that met the inclusion and exclusion criteria underwent full-text rescreening. Data extraction was performed by two investigators independently. The following data were collected: the name of first author, publication year, region of studies, number of the patients with COVID-19, clinical characteristics together with of the laboratory examination in each group. Continuous data were extracted as mean ± standard deviation (SD). While data were expressed as median, range and/or interquartile range (IQR), mean and SD were extrapolated according to Wan et al. [2]. Any disagreements were resolved via discussion and consensus. The risk of bias of each included study was assessed by utilizing the MINORS score [3].
Statistical analysis
ORs together with the weighted mean difference (WMD) and the 95% confidence interval (CI) were merged and we assessed heterogeneity by using Cochran’s Q statistic test and the I2 statistic. When p-values for heterogeneity were no greater than 0.05 or I2 value exceeded 50%, random-model was applied. Otherwise, the fixed-effects model was adopted. We explored the publication bias by the Egger’s regression test and the funnel plot. All statistical analyses were conducted by Review Manager (version 5.3), and R (version 3.6.1). Two-tailed P values ≤0.05 were considered statistically significant.
Results
Literature search and assessment of risk of bias
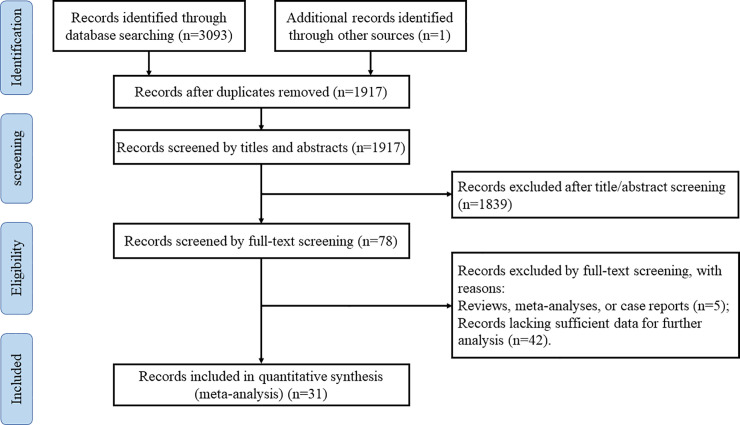
A total of 3093 potentially relevant publications were yielded according to our search strategy from PubMed, Scopus, Web of Science and Embase up to 4 June 2020. One additional relevant study was identified from the reference list of included articles. We discarded 1177 articles as duplicates. Two researchers reviewed 1917 articles based on titles and abstracts. After 1839 irrelevant records were excluded, we screened the full text versions of the remaining 78 articles. The following studies were eliminated: reviews, meta-analyses or case reports and studies lacking sufficient data for further analysis. Ultimately, thirty-one qualified articles [4–34] were included in this meta-analysis. The detailed process of the literature search was presented in Fig 1. All included studies were non-randomized. The MINORS scores varied between 18 and 21, suggesting a low risk of bias overall (Table 1 and S1 Table).
Fig 1. Flow chart of the literature search.
Table 1. Characteristics of all included studies.
| Author | Year | Country | City | MINORS score | Total S NS |
Age S NS |
Male (%) S NS |
Hypertension (%) S NS |
Diabetes (%) S NS |
Malignancy (%) S NS |
CCD (%) S NS |
CB (%) S NS |
CRD (%) S NS |
COPD (%) S NS |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cao JL | 2020 | China | Wuhan | 18 | 85 | 17 | 53 (47, 66) | 72 (63, 81) | 47 | 77 | 20 | 65 | 6 | 35 | 4 | 6 | 2 | 18 | 4 | 18 | 1 | 18 | NA | NA |
| Chen RC | 2020 | China | Guangzhou | 19 | 445 | 103 | 53.5 (13.9) | 66.9 (12.1) | 55 | 67 | 23 | 44 | 9 | 19 | 3 | 3 | NA | NA | 2 | 8 | 3 | 2 | 0 | 5 |
| Chen RC (2) | 2020 | China | Guangzhou | 21 | 1540 | 50 | 48 (1–94) a | 69 (51–86) a | 57 | 78 | 16 | 56 | 8 | 26 | 1 | 6 | NA | NA | 2 | 12 | 1 | 10 | 1 | 12 |
| Chen T | 2020 | China | Wuhan | 21 | 161 | 113 | 51 (37, 66) | 68 (62, 77) | 55 | 74 | 24 | 48 | 14 | 21 | 1 | 4 | 4 | 14 | 0 | 4 | 1 | 4 | NA | NA |
| Deng Y | 2020 | China | Wuhan | 18 | 116 | 109 | 40 (33, 57) | 69 (62, 74) | 44 | 67 | 16 | 37 | 8 | 16 | 2 | 6 | 3 | 12 | NA | NA | NA | NA | NA | NA |
| Du RH | 2020 | China | Wuhan | 19 | 158 | 21 | 56 (13.5) | 70.2 (7.7) | 55 | 48 | 29 | 62 | 17 | 29 | 2 | 5 | NA | NA | NA | NA | NA | NA | NA | NA |
| Fan H * | 2020 | China | Wuhan | 19 | 26 | 47 | 46.2 (12) | 65.5 (9.7) | 65 | 68 | 12 | 45 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Goicoechea M | 2020 | Spain | Madrid | 18 | 25 | 11 | 69 (14) | 75 (6) | 68 | 55 | 100 | 91 | 68 | 55 | NA | NA | NA | NA | NA | NA | NA | NA | 32 | 9 |
| Giacomelli A | 2020 | Italy | Milan | 19 | 185 | 48 | NA | NA | 34 | 19 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Huang J | 2020 | China | Yichang | 18 | 283 | 16 | 52.5 (16.6) | 69.2 (9.7) | 53 | 69 | 22 | 69 | 11 | 25 | 2 | 25 | NA | NA | 4 | 13 | NA | NA | 2 | 19 |
| Javanian M | 2020 | Iran | Babol | 18 | 81 | 19 | 57.7 (13.6) | 69.3 (11.1) | 49 | 57 | 25 | 63 | 33 | 53 | 1 | 16 | 15 | 42 | 1 | 11 | 9 | 26 | 9 | 26 |
| Li LL | 2020 | China | Wuhan | 19 | 68 | 25 | 43.7 (13.1) | 69 (10.5) | 38 | 60 | 0 | 20 | 9 | 20 | 4 | 4 | 0 | 16 | NA | NA | NA | NA | 9 | 8 |
| Nowak B | 2020 | Poland | Warsaw | 18 | 123 | 46 | 59.3 (20.1) | 75.3 (11.9) | 46 | 65 | 43 | 59 | 13 | 35 | 16 | 33 | 29 | 48 | NA | NA | 22 | 17 | 11 | 20 |
| Ruan QR | 2020 | China | Wuhan | 19 | 82 | 68 | 50 (44, 81) | 67 (15, 81) | 65 | 72 | 28 | 43 | 16 | 18 | 1 | 3 | 0 | 19 | 6 | 10 | 0 | 3 | 1 | 3 |
| Shi Q | 2020 | China | Wuhan | 21 | 259 | 47 | NA | NA | 47 | 60 | 38 | 68 | NA | NA | 4 | 9 | 12 | 36 | 3 | 15 | 3 | 11 | NA | NA |
| Shi SB * | 2020 | China | Shanghai | 21 | 609 | 62 | 61 (49, 70) | 74 (66, 81) | 47 | 57 | 27 | 60 | 13 | 27 | 3 | 7 | NA | NA | 2 | 13 | 3 | 19 | 3 | 3 |
| Sun H | 2020 | China | Wuhan | 18 | 123 | 121 | 67 (64, 72) | 72 (66, 78) | 42 | 68 | 50 | 63 | 20 | 23 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Tang N | 2020 | China | Wuhan | 19 | 162 | 21 | 52.4 (15.6) | 64 (20.7) | 51 | 76 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Wang DW | 2020 | China | Wuhan | 19 | 88 | 19 | 44.5 (35, 58.8) | 73 (64, 81) | 47 | 84 | 18 | 53 | 7 | 26 | NA | NA | 7 | 37 | 3 | 16 | 2 | 5 | 2 | 5 |
| Wang K | 2020 | China | Wuhan | 21 | 470 | 78 | 58 (46–67) a | 67 (61.8–78) a | 48 | 71 | 27 | 49 | 14 | 24 | 5 | 4 | NA | NA | NA | NA | 1 | 6 | 2 | 9 |
| Wang L (1) 1 | 2020 | China | Wuhan | 18 | 274 | 65 | 68 (64, 74) | 76 (70, 83) | 46 | 60 | 39 | 50 | 16 | 17 | 4 | 5 | 12 | 33 | 4 | 16 | 3 | 6 | 4 | 17 |
| Wang L (2) | 2020 | China | Wuhan | 19 | 169 | 33 | 61 (49, 67) | 74 (65, 84) | 39 | 70 | 26 | 49 | 11 | 12 | 4 | 6 | 7 | 15 | 2 | 21 | 3 | 12 | 2 | 15 |
| Wang Y * | 2020 | China | Nanjing | 19 | 211 | 133 | 57 (47–69) | 70 (62–77) | 50 | 56 | 34 | 52 | 16 | 23 | NA | NA | 9 | 17 | NA | NA | NA | NA | 1 | 10 |
| Wu CM * | 2020 | China | Wuhan | 19 | 40 | 44 | 50 (40.3, 56.8) | 68.5 (59.3, 75) | 78 | 66 | 18 | 36 | 13 | 25 | NA | NA | 10 | 9 | NA | NA | NA | NA | NA | NA |
| Xu PP | 2020 | China | MC | 21 | 659 | 33 | 45 (14.6) | 64.7 (13.4) | 53 | 73 | 14 | 52 | 7 | 36 | 1 | 3 | 3 | 36 | NA | NA | 1 | 9 | 1 | 12 |
| Xu B | 2020 | China | Wuhan | 21 | 117 | 28 | 56 (43, 66) | 73 (68, 77.3) | 50 | 61 | 18 | 36 | NA | NA | NA | NA | NA | NA | NA | NA | 2 | 7 | NA | NA |
| Yang XB * | 2020 | China | Wuhan | 19 | 20 | 32 | 51.9 (12.9) | 64.6 (11.2) | 70 | 66 | NA | NA | 10 | 22 | 5 | 3 | 10 | 9 | 0 | 22 | NA | NA | NA | NA |
| Yang KY | 2020 | China | MCr | 21 | 165 | 40 | 62 (57, 69) | 63 (53, 75) | 41 | 73 | 34 | 28 | 12 | 5 | NA | NA | NA | NA | NA | NA | NA | NA | 3 | 0 |
| Yan XS | 2020 | China | Wuhan | 19 | 964 | 40 | 62 (50, 70) | 68 (58, 79) | 48 | 68 | 22 | 50 | 10 | 25 | 1 | 3 | NA | NA | 2 | 23 | NA | NA | 1 | 0 |
| Zhang J * | 2020 | China | Wuhan | 18 | 11 | 8 | 68 (38, 87) | 77 (66, 91) | 55 | 63 | 55 | 63 | 9 | 38 | NA | NA | 0 | 38 | 9 | 25 | NA | NA | NA | NA |
| Zhou F | 2020 | China | Wuhan | 21 | 137 | 54 | 52 (45, 58) | 69 (63, 76) | 59 | 70 | 23 | 48 | 14 | 32 | 2 | 0 | NA | NA | NA | NA | 0 | 4 | 2 | 7 |
Abbreviation: COVID-19, coronavirus disease 2019; S: survivors; NS: non-survivors; CCD: Chronic cardiac disease; CB: Cerebrovascular disease; CRD: Chronic renal disease; COPD: Chronic obstructive pulmonary disease; MINORS: Methodological Index for Non-Randomized Studies; NA, Not available; MC: Multi-center.
a: Reported as median (range). Other studies were reported as median (IQR) or mean (SD).
*: All patients with ARDS or severe/critically ill patients.
1: All patients were over 60 years old.
Characteristics of included studies
As shown in Table 1, the included studies were carried out in China (n = 27), Spain (n = 1), Italy (n = 1), Iran (n = 1) and Poland (n = 1). In total, 9407 confirmed COVID-19 patients were included, of which 7856 were survivors and 1551 were non-survivors. The mean or median age of survivors varied from 40 to 69 years, and that of the non-survivors ranged between 63 to 75.3 years. The proportions of male patients in survivors and non-survivors were 52% and 65%, respectively. For comorbidities, similar to the findings of Ielapi N et al. [35], a history of hypertension was more common among non-survivors (52%) than among survivors (29%). Similar to hypertension, non-survivors were more likely to report having diabetes, malignancy, chronic obstructive pulmonary disease, chronic cardiac disease, cerebrovascular disease and chronic renal disease (Table 1). Clinical characteristics included fever, cough, dyspnea, fatigue, diarrhea, myalgia, expectoration, headache, emesis, pharyngalgia, anorexia, abdominal pain, dizziness, hemoptysis, nausea, chest pain, chest tightness and shiver. As for laboratory test, we focused on leukocytes, lymphocytes, procalcitonin (PCT), D-Dimer, lactate dehydrogenase (LDH) and ferritin. Of these studies, nineteen studies provided the clinical characteristics and the laboratory findings of COVID-19 patients [6–9, 11–13, 15, 17, 20, 22, 24, 26–28, 31–34], seven studies only targeted the clinical characteristics [4, 5, 14, 16, 18, 23, 29], and another five studies only focused on the laboratory findings [10, 19, 21, 25, 30].
Meta-analysis results of clinical characteristics
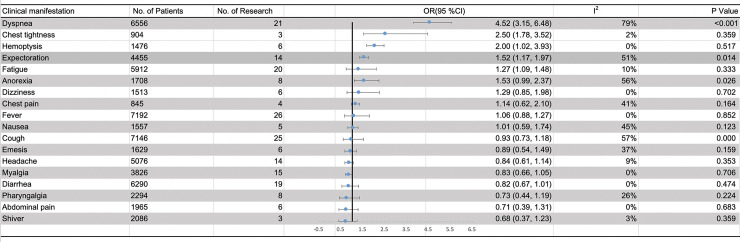
Twenty-six studies involving 7274 COVID-19 patients (5926 survivors and 1348 non-survivors) provided data regarding clinical characteristics (S2 Table). The association between various clinical characteristics and the risk of mortality in COVID-19 patients were shown in Fig 2. Compared with survivors, non-survivors were more likely to present with dyspnea (66% vs. 34%), chest tightness (46% vs. 30%), hemoptysis (4% vs. 3%), expectoration (42% vs. 32%) and fatigue (50% vs. 44%) (S2 Table). In addition, dyspnea, chest tightness, hemoptysis, expectoration and fatigue were observed as significant poor risk factors of mortality (dyspnea: OR = 4.52, 95%CI [3.15, 6.48], P<0.001; chest tightness: OR = 2.50, 95%CI [1.78, 3.52], P<0.001; hemoptysis: OR = 2.00, 95%CI [1.02, 3.93], P = 0.045; expectoration: OR = 1.52, 95%CI [1.17, 1.97], P = 0.002; and fatigue: OR = 1.27, 95%CI [1.09, 1.48], P = 0.003). The heterogeneity test results of dyspnea, chest tightness, hemoptysis, expectoration and fatigue evaluated by I2 were 79%, 2%, 0%, 51% and 10%, respectively. However, no significant relationships were found between mortality and fever, cough, diarrhea, headache, abdominal pain, dizziness, nausea, chest pain and so on (Fig 2).
Fig 2. Meta-analysis results of the relationship between clinical manifestation and the increasing risk of mortality in COVID-19 patients.
Abbreviation: OR, odds ratio; CI, confidence interval.
Meta-analysis results of laboratory findings
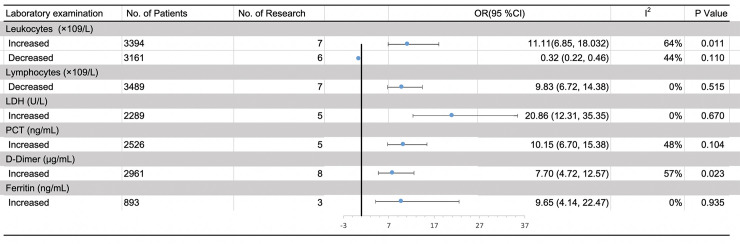
A total of twenty-four studies consisting of 5900 cases (4639 survivors and 1261 non-survivors) reported laboratory findings of COVID-19 patients (S3 Table). We compared the pretreatment absolute leukocytes count, absolute leukocytes count, LDH, D-Dimer, PCT and ferritin between survivors and non-survivors. Compared with survivors, significant increases were found in non-survivors in pretreatment absolute leukocytes count (WMD = 3.27×109/L, 95% CI [2.34, 4.21], P<0.001) (Table 2, S1 Fig) and we further observed significant negative correlation between the risk of mortality and decreased pretreatment absolute leukocytes count (OR = 0.32, 95%CI [0.22, 0.46], P<0.001; I2 = 44%, P = 0.11) (Fig 3). The mean value of pretreatment absolute lymphocytes count was significantly decreased in non-survivors with a WMD of -0.39×109/L, 95% CI [-0.46, -0.33]; P<0.001) compared with survivors (Table 2, S1 Fig) and the reduction of pretreatment absolute lymphocytes count was also significantly related to the increased risk of mortality (OR = 9.83, 95%CI [6.72, 14.38], P<0.001). No pronounced heterogeneity was observed by the heterogeneity test (I2 = 0%, P = 0.515) (Fig 3). What’s more, LDH, D-Dimer, PCT and ferritin were also found to be elevated in non-survivors (Table 2, S1 Fig) and the increased indicators mentioned above were also associated with increased risk of mortality (Fig 3).
Table 2. Meta-analysis results of comparing laboratory abnormalities between survivor and non-survivor COVID-19 patients.
| Laboratory findings | No. of the studies | No. of the patients | WMD | P | Test of heterogeneity I2 (%) P |
|
|---|---|---|---|---|---|---|
| Leukocytes (×109/L) | 19 | 5408 | 3.27 (2.34, 4.21) | <0.001 | 90 | <0.001 |
| Lymphocytes (×109/L) | 20 | 4825 | -0.39 (-0.46, -0.33) | <0.001 | 83 | <0.001 |
| Lactate dehydrogenase (LDH) (U/L) | 13 | 3336 | 211.60 (148.63, 274.57) | <0.001 | 68 | 0.008 |
| Procalcitonin (ng/mL) | 11 | 3330 | 0.31 (0.20, 0.42) | <0.001 | 88 | <0.001 |
| D-Dimer (μg/mL) | 17 | 3108 | 4.97 (3.55, 6.39) | <0.001 | 90 | <0.001 |
| Ferritin (ng/mL) | 6 | 1500 | 770.05 (530.34, 1009.76) | <0.001 | 86 | <0.001 |
Abbreviation: WMD, weighted mean difference; LDH, lactate dehydrogenase.
Fig 3. Meta-analysis results of the relationship between laboratory abnormalities and the increasing risk of mortality in COVID-19 patients.
Abbreviation: OR, odds ratio; CI, confidence interval.
Publication bias
The funnel plots and Egger’s tests showed that there was no evidence of publication bias either in any clinical characteristic analysis or in any laboratory test analysis (S2 and S3 Figs).
Discussion
In this article, we summarized the incidence of some common symptoms of COVID-19 infections and found that dyspnea, chest tightness, hemoptysis, expectoration and fatigue were significantly associated with poor prognosis in COVID-19 patients. For laboratory tests, our study indicated significant increased pretreatment absolute leukocytes count and decreased pretreatment absolute lymphocytes count were observed in non-survivors and they were also associated with the increased risk of mortality in COVID-19 patients.
As an emerging infectious disease, the rapid global rise of COVID-19 pneumonia infections and deaths has attracted significant attention. To foresee the prognosis of COVID-19 infected individuals, it is essential to ascertain the risk factors for death fast and reliably. A large number of clinical studies have explored the clinical characteristics and laboratory examinations of severe and critical COVID-19 patients. Zheng et al. [36] reported that the fever, shortness of breath or dyspnea indicated the disease deterioration. Our results were consistent with the findings of Shi et al. that the presence of dyspnea was risk factors for death, rather than fever [37]. Another recent retrospective study of 179 patients with confirmed COVID-19 found that fatigue and expectoration were more frequently observed in non-survivors than survivors, which were associated with increased risk of mortality [9]. Hemoptysis was an uncommon symptom in COVID-19 patients [38]. In several studies, the incidence of hemoptysis was higher in survivors [9, 14], while many others reported that hemoptysis occurred more often in non-survivors [6, 7, 17], consistent with our observations. More researches on the role of hemoptysis in predicting the prognosis of COVID-19 patients was required.
For laboratory tests, in addition to pretreatment absolute leukocytes and lymphocytes count, increased LDH, PCT, and ferritin were also observed in non-survivors. Further analyses showed them were all associated with the mortality of patients.
Concerning lymphocyte, some studies found no significant correlation between lymphocyte counts and the severity of the disease [39, 40], whereas other research concluded that lymphopenia was a good predictor of disease progression [41, 42]. The present study is the first meta-analysis, which identified the correlation between lymphopenia and mortality in COVID-19 patients. Regardless of the baseline disease severity, lymphocyte was significantly lower on admission and maintained a lower level during hospitalization in non-survivors, while it increased after treatment in survivors [6–8, 43, 44]. The lymphopenia may result from destruction of lymphocytes (particularly T lymphocytes) and suppression of the proliferation of lymphocytes caused by virus invasion, and recovered lymphocyte could be a predictor of gradual recovery [45].
The present study had some limitations that should be acknowledged. First, all included studies were retrospective. Secondly, subgroup analyses were not performed due to the limited data we can draw from the enrolled studies. Additionally, due to the limitations of language, we included the studies written in English and Chinese only.
Conclusions
To sum up, we found that dyspnea, chest tightness, hemoptysis, expectoration and fatigue were predictors of increased risk of mortality. Besides, significantly increased pretreatment absolute leukocyte count, PCT, D-Dimer, LDH and ferritin, and decreased pretreatment absolute lymphocyte count were identified in non-survivors, which were all related to increased risk of mortality. Motoring these indicators during the hospitalization of patients plays a very important role in predicting the prognosis of patients. Collectively, our results are helpful in clinical practice, which should be verified by additional large-sample or multi-center studies.
Supporting information
Forest plot of the laboratory abnormalities (A) leukocytes, (B) lymphocytes, (C) lactate dehydrogenase (LDH), (D) procalcitonin, (E) D-Dimer, (F) ferritin levels in survivors versus non-survivors.
(TIF)
The publication bias of the clinical characteristics (A. dyspnea; B. chest tightness; C. hemoptysis; D. expectoration; E. fatigue; F. anorexia; G. dizziness; H. chest pain; I. fever; J. nausea; K. cough; L. emesis; M. headache; N. myalgia; O. diarrhea; P. pharyngalgia; Q. abdominal pain; R. shiver) between survivors and non-survivors.
(TIF)
The publication bias of the laboratory abnormalities (A) increased leukocytes, (B) decreased leukocytes, (C) decreased lymphocytes, (D) increased lactate dehydrogenase (LDH), (E) increased procalcitonin (PCT), (F) increased D-Dimer, (G) increased ferritin between survivors and non-survivors.
(TIF)
(XLSX)
Abbreviation: CI, confidence interval; NA, not available.
(XLSX)
Abbreviation: CI, confidence interval; SD, standard deviation.
(XLSX)
(DOC)
(DOCX)
Abbreviations
- COVID-19
coronavirus disease 2019
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- IQR
interquartile range
- SARS
severe acute respiratory syndrome
- CI
confidence interval
- LDH
lactate dehydrogenase
- PCT
procalcitonin
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was supported by National Nature Science Foundation of China [grant numbers 91859203 and 81871890] and Major Science and Technology Innovation Project of Chengdu City [grant number 2020-YF08-00080-GX].
References
- 1.Guo YR, Cao QD, Hong ZS, Tan YY, Chen SD, Jin HJ, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak—an update on the status. Military Medical Research. 2020;7(1):11 10.1186/s40779-020-00240-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135–. 10.1186/1471-2288-14-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (MINORS): development and validation of a new instrument. ANZ Journal of Surgery. 2003;73(9):712–6. 10.1046/j.1445-2197.2003.02748.x [DOI] [PubMed] [Google Scholar]
- 4.Cao J, Tu WJ, Cheng W, Yu L, Liu YK, Hu X, et al. Clinical Features and Short-term Outcomes of 102 Patients with Corona Virus Disease 2019 in Wuhan, China. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen R, Liang W, Jiang M, Guan W, Zhan C, Wang T, et al. Risk Factors of Fatal Outcome in Hospitalized Subjects With Coronavirus Disease 2019 From a Nationwide Analysis in China. Chest. 2020. 10.1016/j.chest.2020.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen R, Sang L, Jiang M, Yang Z, Jia N, Fu W, et al. Longitudinal hematologic and immunologic variations associated with the progression of COVID-19 patients in China. The Journal of allergy and clinical immunology. 2020. 10.1016/j.jaci.2020.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ (Clinical research ed). 2020;368:m1091 10.1136/bmj.m1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deng Y, Liu W, Liu K, Fang YY, Shang J, Zhou L, et al. Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 (COVID-19) in Wuhan, China: a retrospective study. Chinese medical journal. 2020. 10.1097/CM9.0000000000000824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du RH, Liang LR, Yang CQ, Wang W, Cao TZ, Li M, et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. The European respiratory journal. 2020;55(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fan H, Zhang L, Huang B, Zhu M, Zhou Y, Zhang H, et al. Cardiac injuries in patients with coronavirus disease 2019: Not to be ignored. International journal of infectious diseases: IJID: official publication of the International Society for Infectious Diseases. 2020;96:294–7. 10.1016/j.ijid.2020.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giacomelli A, Ridolfo AL, Milazzo L, Oreni L, Bernacchia D, Siano M, et al. 30-day mortality in patients hospitalized with COVID-19 during the first wave of the Italian epidemic: a prospective cohort study. Pharmacological research. 2020:104931 10.1016/j.phrs.2020.104931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goicoechea M, Sánchez Cámara LA, Macías N, Muñoz de Morales A, González Rojas Á, Bascuñana A, et al. COVID-19: Clinical course and outcomes of 36 maintenance hemodialysis patients from a single center in Spain. Kidney international. 2020. 10.1016/j.kint.2020.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang J, Cheng A, Kumar R, Fang Y, Chen G, Zhu Y, et al. Hypoalbuminemia predicts the outcome of COVID-19 independent of age and co-morbidity. Journal of medical virology. 2020. 10.1002/jmv.26003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Javanian M, Bayani M, Shokri M, Sadeghi-Haddad-Zavareh M, Babazadeh A, Yeganeh B, et al. Clinical and laboratory findings from patients with COVID-19 pneumonia in Babol North of Iran: a retrospective cohort study. Romanian journal of internal medicine = Revue roumaine de medecine interne. 2020. 10.2478/rjim-2020-0013 [DOI] [PubMed] [Google Scholar]
- 15.Li L, Yang L, Gui S, Pan F, Ye T, Liang B, et al. Association of clinical and radiographic findings with the outcomes of 93 patients with COVID-19 in Wuhan, China. Theranostics. 2020;10(14):6113–21. 10.7150/thno.46569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nowak B, Szymański P, Pańkowski I, Szarowska A, Życińska K, Rogowski W, et al. Clinical characteristics and short-term outcomes of patients with coronavirus disease 2019: a retrospective single-center experience of a designated hospital in Poland. Polish archives of internal medicine. 2020;130(5):407–11. 10.20452/pamw.15361 [DOI] [PubMed] [Google Scholar]
- 17.Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive care medicine. 2020;46(5):846–8. 10.1007/s00134-020-05991-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi Q, Zhang X, Jiang F, Zhang X, Hu N, Bimu C, et al. Clinical Characteristics and Risk Factors for Mortality of COVID-19 Patients With Diabetes in Wuhan, China: A Two-Center, Retrospective Study. Diabetes care. 2020. 10.2337/dc20-0598 [DOI] [PubMed] [Google Scholar]
- 19.Shi S, Qin M, Cai Y, Liu T, Shen B, Yang F, et al. Characteristics and clinical significance of myocardial injury in patients with severe coronavirus disease 2019. European heart journal. 2020. 10.1093/eurheartj/ehaa408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun H, Ning R, Tao Y, Yu C, Deng X, Zhao C, et al. Risk Factors for Mortality in 244 Older Adults With COVID-19 in Wuhan, China: A Retrospective Study. Journal of the American Geriatrics Society. 2020. 10.1111/jgs.16533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. Journal of thrombosis and haemostasis: JTH. 2020;18(4):844–7. 10.1111/jth.14768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang D, Yin Y, Hu C, Liu X, Zhang X, Zhou S, et al. Clinical course and outcome of 107 patients infected with the novel coronavirus, SARS-CoV-2, discharged from two hospitals in Wuhan, China. Critical care (London, England). 2020;24(1):188 10.1186/s13054-020-02895-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang K, Zhang Z, Yu M, Tao Y, Xie M. 15-day mortality and associated risk factors for hospitalized patients with COVID-19 in Wuhan, China: an ambispective observational cohort study. Intensive care medicine. 2020:1–3. 10.1007/s00134-020-06047-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang L, He W, Yu X, Hu D, Bao M, Liu H, et al. Coronavirus disease 2019 in elderly patients: Characteristics and prognostic factors based on 4-week follow-up. The Journal of infection. 2020;80(6):639–45. 10.1016/j.jinf.2020.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang L, He WB, Yu XM, Liu HF, Zhou WJ, Jiang H. Prognostic value of myocardial injury in patients with COVID-19. [Zhonghua yan ke za zhi] Chinese journal of ophthalmology. 2020;56(0):E009 10.3760/cma.j.cn112148-20200313-00202 [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, Lu X, Li Y, Chen H, Chen T, Su N, et al. Clinical Course and Outcomes of 344 Intensive Care Patients with COVID-19. American journal of respiratory and critical care medicine. 2020;201(11):1430–4. 10.1164/rccm.202003-0736LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA internal medicine. 2020. 10.1001/jamainternmed.2020.0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu B, Fan CY, Wang AL, Zou YL, Yu YH, He C, et al. Suppressed T cell-mediated immunity in patients with COVID-19: A clinical retrospective study in Wuhan, China. The Journal of infection. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu PP, Tian RH, Luo S, Zu ZY, Fan B, Wang XM, et al. Risk factors for adverse clinical outcomes with COVID-19 in China: a multicenter, retrospective, observational study. Theranostics. 2020;10(14):6372–83. 10.7150/thno.46833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yan X, Li F, Wang X, Yan J, Zhu F, Tang S, et al. Neutrophil to Lymphocyte Ratio as Prognostic and Predictive Factor in Patients with Coronavirus Disease 2019: A Retrospective Cross-sectional Study. Journal of medical virology. 2020. 10.1002/jmv.26061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang K, Sheng Y, Huang C, Jin Y, Xiong N, Jiang K, et al. Clinical characteristics, outcomes, and risk factors for mortality in patients with cancer and COVID-19 in Hubei, China: a multicentre, retrospective, cohort study. The Lancet Oncology. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. The Lancet Respiratory medicine. 2020;8(5):475–81. 10.1016/S2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang J, Liu P, Wang M, Wang J, Chen J, Yuan W, et al. The clinical data from 19 critically ill patients with coronavirus disease 2019: a single-centered, retrospective, observational study. Zeitschrift fur Gesundheitswissenschaften = Journal of public health. 2020:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet (London, England). 2020;395(10229):1054–62. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ielapi N, Licastro N, Provenzano M, Andreucci M, Franciscis Sd, Serra R. Cardiovascular disease as a biomarker for an increased risk of COVID-19 infection and related poor prognosis. Biomark Med. 2020;14(9):713–6. 10.2217/bmm-2020-0201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zheng Z, Peng F, Xu B, Zhao J, Liu H, Peng J, et al. Risk factors of critical & mortal COVID-19 cases: A systematic literature review and meta-analysis. The Journal of infection. 2020:S0163–4453(20)30234-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shi L, Wang Y, Wang Y, Duan G, Yang H. Dyspnea rather than fever is a risk factor for predicting mortality in patients with COVID-19. The Journal of infection. 2020. 10.1016/j.jinf.2020.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. The New England journal of medicine. 2020;382(18):1708–20. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fu L, Wang B, Yuan T, Chen X, Ao Y, Fitzpatrick T, et al. Clinical characteristics of coronavirus disease 2019 (COVID-19) in China: A systematic review and meta-analysis. The Journal of infection. 2020;80(6):656–65. 10.1016/j.jinf.2020.03.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen L, Liu HG, Liu W, Liu J, Liu K, Shang J, et al. Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia. Zhonghua jie he he hu xi za zhi = Zhonghua jiehe he huxi zazhi = Chinese journal of tuberculosis and respiratory diseases. 2020;43(0):E005 10.3760/cma.j.issn.1001-0939.2020.0005 [DOI] [PubMed] [Google Scholar]
- 41.Zhao Q, Meng M, Kumar R, Wu Y, Huang J, Deng Y, et al. Lymphopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: A systemic review and meta-analysis. International journal of infectious diseases: IJID: official publication of the International Society for Infectious Diseases. 2020;96:131–5. 10.1016/j.ijid.2020.04.086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang I, Pranata R. Lymphopenia in severe coronavirus disease-2019 (COVID-19): systematic review and meta-analysis. Journal of intensive care. 2020;8:36–. 10.1186/s40560-020-00453-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet (London, England). 2020;395(10223):507–13. 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guan WJ, Liang WH, Zhao Y, Liang HR, Chen ZS, Li YM, et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. The European respiratory journal. 2020;55(5). 10.1183/13993003.00547-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gu J, Gong E, Zhang B, Zheng J, Gao Z, Zhong Y, et al. Multiple organ infection and the pathogenesis of SARS. The Journal of experimental medicine. 2005;202(3):415–24. 10.1084/jem.20050828 [DOI] [PMC free article] [PubMed] [Google Scholar]