Summary
Combinatorial action of transcription factors (TFs) with partially overlapping expression is a widespread strategy to generate novel gene-expression patterns and, thus, cellular diversity. Known mechanisms underlying combinatorial activity require co-expression of TFs within the same cell. Here, we describe the mechanism by which two TFs that are never co-expressed generate a new, intersectional expression pattern in C. elegans embryos: lineage-specific priming of a gene by a transiently expressed TF generates a unique intersection with a second TF acting on the same gene four cell divisions later; the second TF is expressed in multiple cells but only activates transcription in those where priming occurred. Early induction of active transcription is necessary and sufficient to establish a competent state, maintained by broadly expressed regulators in the absence of the initial trigger. We uncover additional cells diversified through this mechanism. Our findings define a mechanism for combinatorial TF activity with important implications for generation of cell-type diversity.
Keywords: competence, priming, transcription factors, combinatorial transcription, neuronal asymmetry, neuron diversification, C. elegans, miRNA, epigenetics, T-box
Graphical Abstract
Highlights
-
•
Lineage-specific priming enables asymmetric gene expression in L/R neuron pairs
-
•
Transient, lineage-specific TFs prime a locus for later activation by a bilateral TF
-
•
An early active transcriptional state is necessary and sufficient for priming
-
•
Maintenance of asymmetric primed state occurs in a symmetric regulatory environment
Studying neuronal specification in C. elegans, Charest et al. reveal that lineage-specific priming by a transiently expressed transcription factor allows activation of the primed gene by another transcription factor acting four cell divisions later. The findings suggest that the intersection of temporally segregated transcription factors contributes to cell-specific gene expression and cell diversification.
Introduction
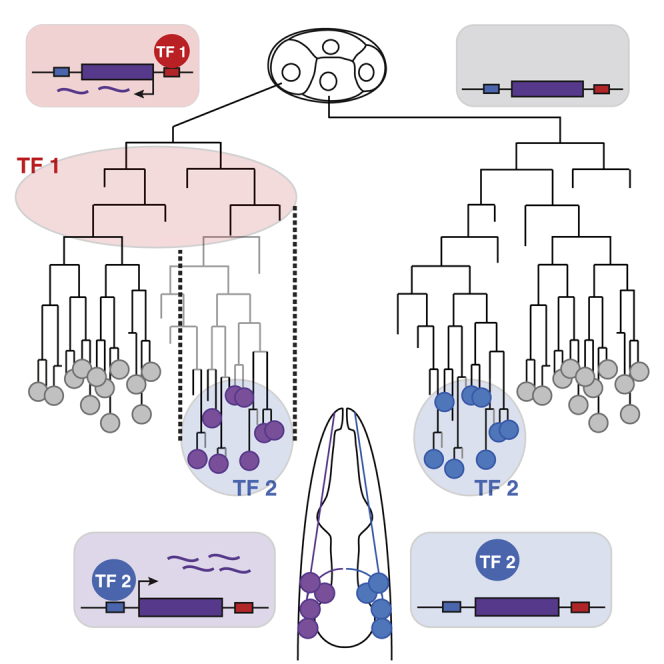
Transcription of developmentally regulated genes typically requires combinatorial activity of multiple transcription factors (TFs). Combinatorial use of TFs enables the generation of novel, specific gene-expression patterns that exceed the number of available TFs, through creation of intersectional gene-expression domains (Figure 1A, left) (Allan and Thor, 2015; Reiter et al., 2017; Spitz and Furlong, 2012). In fact, different cell types are largely determined by the differential expression of combinations of TFs that activate transcription of sets of effector genes, giving each cell type its unique structural and physiological properties (Hobert, 2008). The specification of individual cell identities by combinations of TFs enables integration of signaling events and intrinsic transcriptional programs; thus, combinatorial action of TFs is a basis for cellular diversification during development (Allan and Thor, 2015; Hobert, 2016).
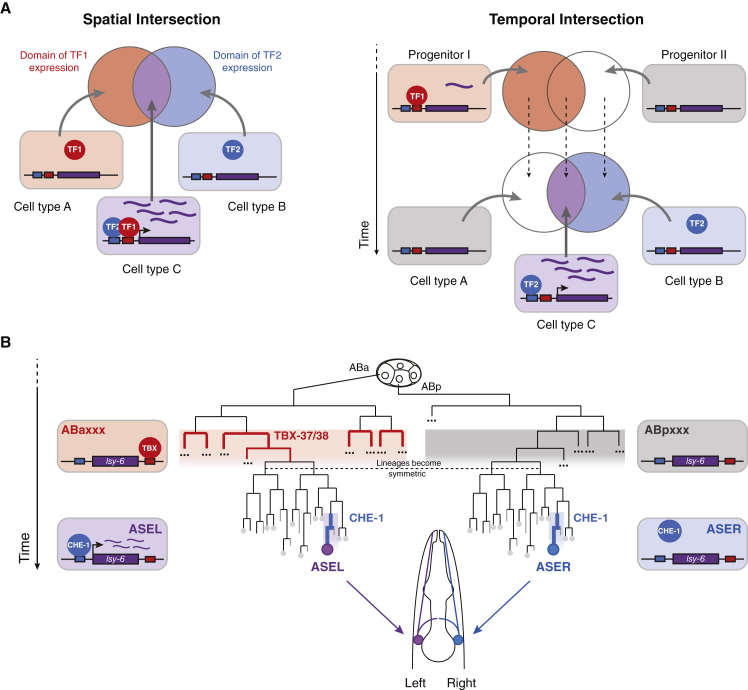
Figure 1.
Integration of Transcriptional Inputs over Time as a Mechanism for Cell Diversification
(A) Left. Schematic of the classic mode of combinatorial TF action based on spatial intersection of two TFs. Circles denote the partially overlapping domains of expression of two TFs. Boxes represent cells within those domains. Expression of TF1 or TF2 alone is not sufficient to activate a gene relevant for cell identity (in purple) but action of both TFs together is. Right. Schematic of the concept of temporal intersection in which two TFs are required for transcriptional activation, but separated in time. TF1 is expressed transiently in a subset of progenitor cells, while TF2 is expressed in a subset of cells undergoing terminal differentiation. Only a cell expressing TF2 that derived from a progenitor that expressed TF1 will activate the purple gene. Therefore, the outcome of the presence of TF2 in a cell depends on the transcriptional history of that cell, and this mechanism can contribute to cellular diversification.
(B) Schematic of the lineage histories of the left and right ASE neurons (ASEL and ASER), which derive from the ABa and ABp blastomeres, respectively, at the 4-cell stage of embryogenesis. Both neurons express the terminal TF CHE-1 at the endpoint. CHE-1 is necessary for lsy-6 expression, but only ASEL expresses lsy-6. Asymmetric expression of lsy-6 is genetically dependent on the transiently expressed TBX-37/38, which have a predicted binding site in the lsy-6 locus.
Different molecular mechanisms for combinatorial activity of two or more TFs have been described (Lee et al., 2008; Long et al., 2016; Reiter et al., 2017; Wenick and Hobert, 2004; Zaret and Carroll, 2011; Zaret and Mango, 2016), most of which are based on TFs simultaneously present within the same cell. Consistently, known cases of combinations of TFs to generate cell-type diversity during development rely on spatial intersection of TFs with partially overlapping expression patterns (Figure 1A, left) (Alaynick et al., 2011; Wenick and Hobert, 2004; Xue et al., 1993; Zhang et al., 2014).
The C. elegans microRNA (miRNA) lsy-6 is transcribed exclusively in one of two left/right morphologically symmetric neurons, the ASE sensory-neuron pair (Cochella and Hobert, 2012; Johnston and Hobert, 2003). Specific transcription of lsy-6 in the left ASE neuron (ASEL) defines two alternative sensory-neuron fates with different molecular and functional properties (Hobert, 2014) that are necessary for animal behavior (Pierce-Shimomura et al., 2001; Suzuki et al., 2008) and represent the only currently known directed asymmetry in the worm nervous system. Intriguingly, asymmetric lsy-6 transcription requires direct action of the ASE terminal selector TF CHE-1 on a well-characterized binding site, even though CHE-1 is present in both ASE neurons where it activates hundreds of symmetric genes (Etchberger et al., 2007, 2009; Leyva-Díaz and Hobert, 2019; Uchida et al., 2003). Thus, ASEL-specific expression of lsy-6 cannot be determined solely by CHE-1: it depends instead on an intersectional strategy defined by the different lineage histories of the two ASE neurons, which diverge at the 4-cell stage of embryogenesis, eight cell divisions prior to the birth of the ASEs (Figure 1B) (Cochella and Hobert, 2012; Poole and Hobert, 2006; Sulston et al., 1983).
The two lineage branches that give rise to the ASE neurons are distinguished by exclusive expression of two redundant T-box TFs, TBX-37/38, in the ASEL precursor lineage, ABa (Figure 1B) (Good et al., 2004). Early expression of TBX-37/38 is necessary for lsy-6 transcription in ASEL four cell divisions later (Cochella and Hobert, 2012). In addition, a cis-regulatory element in the lsy-6 locus, required for lsy-6 transcription, contains two putative TBX-37/38 binding sites (Cochella and Hobert, 2012). These findings implied that TBX-37/38 act combinatorially with CHE-1 to activate lsy-6 exclusively in ASEL. However, TBX-37/38 were reported to be transiently expressed four cell divisions before the onset of CHE-1 expression (Figure 1B) (Good et al., 2004), suggesting a model in which TBX-37/38 would prime the lsy-6 locus at an early time point, in a lineage-specific manner, to enable subsequent activation by CHE-1 in ASEL (Cochella and Hobert, 2012). This model raised a number of mechanistic questions about how these two transcriptional inputs are integrated to achieve lsy-6 expression.
Here, we took advantage of several recently available methods to experimentally test and dissect the mechanism of priming by TBX-37/38 and its temporal separation from the action of CHE-1. We demonstrate that TBX-37/38 act directly on lsy-6 and that this activity is indeed only transiently required, providing experimental support for the combinatorial activity of TBX-37/38 and CHE-1 on the lsy-6 locus. Because these two TFs act on the same locus but have non-overlapping temporal expression and only partially overlapping spatial expression, we refer to this as “temporal intersection,” in analogy to “spatial intersection” mechanisms (Figure 1A, right). We also show that the transient action of TBX-37/38 establishes a transcriptionally active state, which is necessary and sufficient to prime lsy-6. The asymmetric active transcriptional state of lsy-6 can be maintained in the absence of the asymmetric trigger in a symmetric trans-acting factor environment, indicating that this is an epigenetic phenomenon. Finally, we provide evidence for the idea that, akin to spatial integration of TFs, temporal integration may be a general mechanism for the generation of cellular diversity during development. Our findings define a mechanistic framework for a mode of combinatorial TF activity with important implications for understanding development and for current efforts to generate defined cell types in vitro.
Results
Direct, Early Binding of TBX-37/38 Is Necessary and Sufficient to Prime lsy-6
To begin to dissect the mechanism by which TBX-37/38 contribute to the specific expression of lsy-6 in the post-mitotic ASEL, we explored the possibility that these TFs bind directly to the lsy-6 locus already at the 28-cell stage, when they are first detected. We generated gfp-tagged alleles of endogenous tbx-37 or -38 using CRISPR-Cas9 (Figure S1A). Because these TFs are almost identical in sequence and fully redundant (Good et al., 2004), we tagged each TF in the background of a deletion of its paralog, such that the only source of TBX factor is tagged with GFP. N-terminal fusion of GFP did not cause defects in TBX activity, as animals developed normally, and were, thus, used for chromatin immunoprecipitation sequencing (ChIP-seq) from embryos staged at the peak of TBX-37/38 expression (90 min post 2-cell). De-novo motif discovery analysis of the TBX-37/38 binding sites revealed a highly enriched sequence motif practically identical to the known TBX-38 binding sequence (Figure 2A) (Narasimhan et al., 2015). Focusing on the lsy-6 locus, we observed that both TBX-37/38 bound downstream of lsy-6, overlapping with the cis-regulatory element that had been genetically defined (Cochella and Hobert, 2012) (Figure 2A).
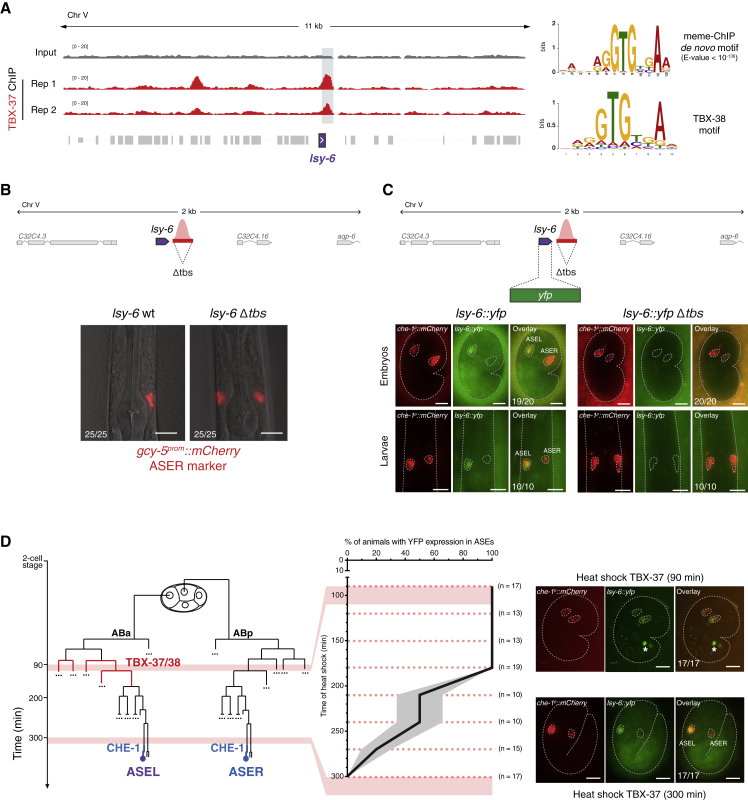
Figure 2.
Early, Direct Binding of TBX-37/38 to lsy-6 Is Necessary and Sufficient to Establish Competence for Transcription in the ASE Neurons
(A) GFP-TBX-37 ChIP-seq signal over the lsy-6 locus and flanking regions obtained from embryos staged at the peak of TBX-37/38 expression (90 min post 2-cell); two biological replicates. A peak of GFP-TBX-37 slightly downstream of lsy-6 (gray bar) was detected in all individual replicates (p value < 10−5 in each replicate). De-novo motif discovery within TBX-37/38 binding sites, retrieved a highly enriched motif that matches the binding site for TBX-38 derived from in vitro binding studies (Narasimhan et al., 2015). The peak in the lsy-6 locus overlaps two such motifs.
(B) Deletion of a 150-bp region containing the TBX-37/38 binding site (Δtbs) using CRISPR-Cas9, from the wild-type lsy-6 locus, caused fully penetrant transformation of ASEL into ASER, monitored by expression of the ASER-specific marker gcy-5prom::mCherry (n = 25 for each genotype).
(C) A yfp reporter inserted in the endogenous lsy-6 locus via CRISPR-Cas9 resulted in exclusive YFP expression in ASEL in embryos and larvae (n = 20 and n = 10 for each genotype, respectively). Deletion of the TBX-37/38 binding site (Δtbs) in this context completely abolished expression of YFP at every stage. ASEs are marked by a genome-integrated che-1prom::mCherry reporter. Representative images are shown.
(D) Embryos carrying the engineered lsy-6::yfp endogenous locus (C) and a heat-shockprom::tbx-37 transgene, were subjected to heat shock at the indicated times (dashed lines) (n ≥ 10 for each time point). Ectopic TBX-37 expression in the whole embryo at early time points induced additional lsy-6::yfp expression in ASER (∗heat-shock treatment alone caused variable expression in two cells in the tail). Beyond the 180-min time point, TBX-37 progressively lost ability to activate lsy-6::yfp expression in ASER or any other cell. Plot shows proportion and standard error of proportion (SEP). Representative images are shown for the 90- and 300-min time points. All scale bars represent 10 μm.
To functionally test the contribution of this binding site to lsy-6 expression, we used CRISPR-Cas9 to delete a 150-bp region spanning two predicted TBX-37/38 binding sequence motifs (Δtbs). This deletion abolished transcription of endogenous lsy-6 miRNA, whose activity we followed via expression of an ASER reporter: wild-type animals express gcy-5 exclusively in ASER, but in animals lacking lsy-6, ASEL adopts the ASER fate and expresses gcy-5 as well (Johnston and Hobert, 2003). Deletion of the tbs resulted in complete lsy-6 loss of function, with all animals expressing the ASER marker in both ASE neurons (Figure 2B). Moreover, deletion of the tbs completely eliminated expression of a yfp reporter inserted in place of the lsy-6 miRNA, also using CRISPR-Cas9 (Figure 2C). These data indicate that the requirement for TBX-37/38 is mediated through a binding site downstream of the lsy-6 miRNA sequence.
It was previously shown that early expression of TBX-37/38 could prime a lsy-6 reporter transgene for subsequent activation by CHE-1, but expression of TBX-37/38 after the birth of the ASE neurons was unable to do so (Cochella and Hobert, 2012). To define the ability of the TBXs to prime endogenous lsy-6 over time, we ectopically expressed TBX-37 in all cells, at different time points (using a heat-shock promoter) and followed expression of the endogenously labeled lsy-6::yfp. If heat-shock-induced TBX-37 is sufficient to prime lsy-6, we expect YFP expression in ASER, in addition to ASEL. Heat-shock-activated TBX-37 was sufficient to prime lsy-6 for expression in ASER up to the 180-min time point, resulting in embryos with YFP in both ASEs (Figure 2D). However, beyond this time point, TBX-37 progressively lost its ability to prime lsy-6 in both neuron precursors and was ultimately unable to activate transcription of the lsy-6 locus (Figure 2D). This defined a window during which priming needs to occur, beyond which lsy-6 becomes refractory to transcriptional activation. Moreover, although TBX-37 was expressed throughout the embryo, YFP was expressed exclusively in the ASE neurons, supporting that lsy-6 specificity is generated by the intersection with the ASE-specific CHE-1.
Together, these data show that early, direct action of TBX-37/38 on the lsy-6 locus is necessary and sufficient to prime lsy-6 for subsequent activation by CHE-1 in the ASEs.
TBX-37/38 Are Transiently Required to Prime lsy-6
Previous work suggested that TBX-37/38 expression is temporally separated from that of CHE-1, but it has remained unclear whether low levels of TBX-37/38 persist and continue to act on lsy-6 at later time points. The onset of CHE-1 expression is in the mother of the ASE neurons, based on single-molecule fluorescence in situ hybridization (smFISH) and imaging of endogenously tagged CHE-1 (Cochella and Hobert, 2012; Leyva-Díaz and Hobert, 2019). TBX-37/38 were reported, based on immunostaining, to be expressed at the 8 ABa stage (when the ABa branch has produced 8 descendants) and at lower level one cell division later, at the 16 ABa stage (Good et al., 2004) (Figure 3A). We set out to further rigorously examine the temporal separation of these TFs.
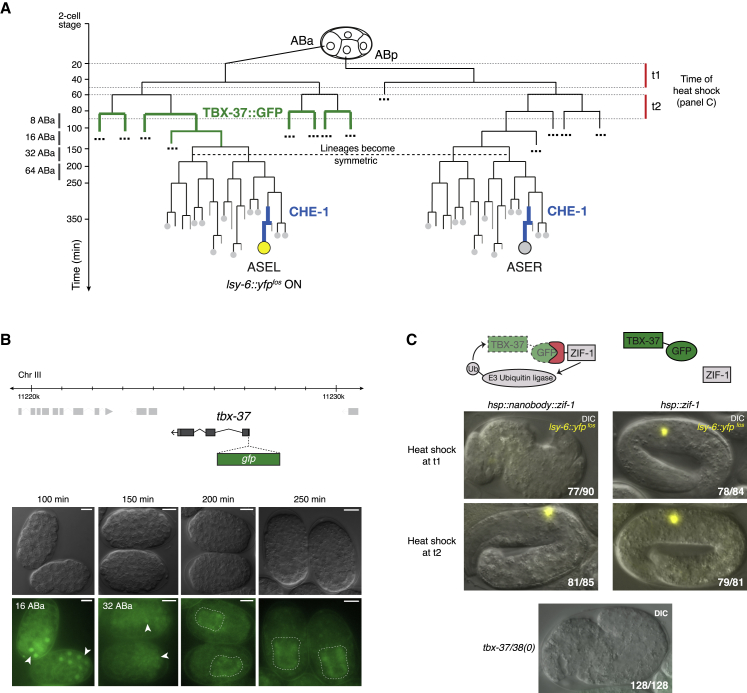
Figure 3.
TBX-37/38 Are Not Continuously Required for lsy-6 Expression
(A) Developmental lineage of ASEL and ASER with relevant timing information for (B) and (C).
(B) Expression of N-terminally tagged, endogenous TBX-37 (or TBX-38, Figure S1) was followed by GFP fluorescence over time. Representative images are shown. Fluorescence was clearly visible at 16 ABa stage (arrowheads) and was dim in a few nuclei at 32 ABa stage but no signal was visible beyond this time point (also Figures S2A and S2B). Autofluorescence from the developing gut is outlined with a dashed line. DIC (differential interference contrast) and fluorescence are shown. Scale bars represent 10 μm.
(C) Degradation of GFP-TBX-37 (in a tbx-38(0) background) was induced using an anti-GFP nanobody fused to the ubiquitin-ligase adaptor ZIF-1, expressed under control of a heat-shock promoter. Representative images show embryos heat shocked at two different times (see A): t1 targeted the peak of TBX-37/38 expression (Figure S2) and caused 77/90 embryos to phenocopy tbx-37/38(0) morphological defects and fail to express lsy-6::yfpfosmid; t2 (onset of degradation 40 min later, Figure S2) caused no defects in morphology or lsy-6 expression in 81/85 embryos. Heat shock of animals expressing ZIF-1 without the targeting nanobody had no effect at either time. Comparison of control and nanobody treatments was done using a chi-squared test: p valuet1 < 0.0001, p valuet2 = 0.297 (Table S1). DIC and fluorescence were overlaid in the same image.
First, we used the endogenous TBX-GFP fusions to follow expression of TBX-37/38 by GFP fluorescence and smFISH to visualize tbx-37/38 transcripts. Our data largely confirmed the published spatio-temporal restriction of TBX-37/38 (Figures 3B and S1). We did, however, observe dim GFP fluorescence also at the 32 ABa stage, which was not reported by immunostaining. This suggests that either the GFP fusion provides a more sensitive read out or, alternatively, that it could stabilize TBX-37/38. Nevertheless, we did not detect fluorescence above background at later stages (Figures S1A and S1B). Given the onset of expression of CHE-1 in the mother of the ASE neurons, these data suggest that its action on the lsy-6 locus is separated from TBX-37/38 by at least three, most likely four cell divisions, out of a total of 11 cell divisions from fertilized zygote to post-mitotic ASE (Sulston et al., 1983).
Second, to investigate whether TBX-37/38 might be continuously required even if these factors are expressed below the limit of detection, we designed a timed, forced degradation experiment. We targeted GFP-TBX-37 for degradation using an anti-GFP nanobody fused to the ubiquitin-ligase adaptor ZIF-1 (Wang et al., 2017b). The nanobody-ZIF-1 fusion was expressed from a single-copy transgene (Frøkjaer-Jensen et al., 2012) under the heat-shock promoter, enabling inducible degradation of endogenous GFP-TBX-37, in a tbx-38 deletion background. Upon induction at different time points, we assessed expression of a lsy-6::yfp fosmid reporter with 40 kb of genomic context (Cochella and Hobert, 2012), as well as the embryo morphology at the end of embryogenesis. Degradation of GFP-TBX-37 at 8–16 ABa (t1) caused penetrant loss of lsy-6 expression and phenocopied complete TBX-37/38 loss of function, with embryos lacking the anterior part of the pharynx and showing severely impaired morphogenesis (Good et al., 2004) (Figures 3C and S2). However, if the onset of GFP-TBX-37 degradation was 40 min later (t2), roughly the duration of a cell cycle, there was no effect on either lsy-6 expression or morphogenesis (Figures 3C and S2). We further confirmed that the degradation system works efficiently at both time points by also monitoring degradation of an abundant GFP-tagged TF, PHA-4, which is expressed in many cells from the ABa lineage (Gaudet and Mango, 2002) (Figure S2). Moreover, a control strain expressing ZIF-1 without the anti-GFP nanobody did not display defects when subjected to heat shock at either time point.
Together, these data strongly support the early, transient requirement of TBX-37/38 for later expression of lsy-6.
TBX-37/38 Establish a Lineage-Specific Accessible State of the lsy-6 Locus
We hypothesized that transient action of TBX-37/38 would establish a lineage-specific, competent chromatin state of lsy-6. Decompaction of a lsy-6 transgene in ABa grand-daughters was previously reported to correlate with TBX-37/38 expression, using a lac-operator-tagging strategy (Cochella and Hobert, 2012). However, this technique did not allow to follow decompaction at later time points and lacked resolution to distinguish between different cis-regulatory elements within the lsy-6 locus. We, thus, set out to specifically isolate cells derived from the two ASE-originating lineages (ABa and ABp) for analysis by ATAC-seq (Corces et al., 2017). We generated a transgenic strain carrying lineage-specific reporters (Figures 4A, S3A, and S3B), synchronized and dissociated embryos, and sorted viable cells from ABa or ABp based on fluorescence, at different time points (Figure S3C).
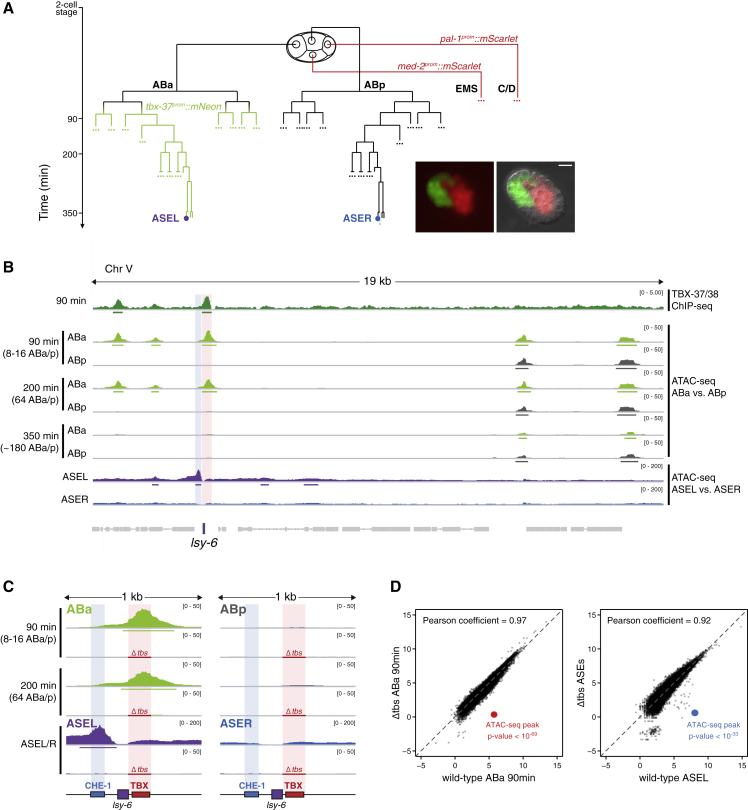
Figure 4.
TBX-37/38 Establish a Differentially Accessible State of the lsy-6 Locus
(A) Schematic of the embryo-labeling strategy for ABa and ABp isolation. The indicated time points (90, 200, and 350 min) were used for the experiments in (B) and (C). Representative image of an embryo at the 8 ABa stage carrying the three-reporter combination (Figures S3A–S3C).
(B) (Top) Aggregated GFP-TBX-37/38 ChIP-seq signal over the lsy-6 locus and flanking sequences. (Middle) ATAC-seq signal in ABa- and ABp-derived cells at three different time points showing ABa-specific accessibility of lsy-6, overlapping with the TBX-37/38 binding site (red shading); two biological replicates were analyzed per condition. For reference, downstream locus shows equal accessibility in both lineages (also Figure S3D). Number of ABa/ABp descendants at the different time points are shown. (Bottom) ATAC-seq signal from sorted ASEL and ASER shows accessibility in ASEL upstream of lsy-6, overlapping with a CHE-1 binding site (blue shading). Peaks called by MACS2 are marked with a bar.
(C) Close-up view of the lsy-6 locus and its ATAC-seq signal in ABa, ABp, ASEL, and ASER isolated from wild-type or Δtbs embryos (deletion marked with a red line); two biological replicates were analyzed per condition. Loss of TBX-37/38 binding sites causes loss of accessibility of the CHE-1 binding site in mature ASEs. For Δtbs embryos, ASEL and ASER cannot be distinguished as lsy-6 is not expressed and the cells become symmetric (Figure 2B); the ASE ATAC-seq in this case was done on che-1prom::mCherry-expressing cells, and the same track is shown in duplicate under ASEL and ASER.
(D) Correlation analysis between ATAC-seq data from wild-type or Δtbs embryos shows that datasets are highly similar and differ almost exclusively in their signal over the lsy-6 locus. Plotted are signals in log2 (cpm) for all peaks called by MACS2 in at least one ATAC-seq sample. The p values for the called lsy-6 peaks in ABa (overlapping with TBX-binding sites, red) and ASEL (overlapping with CHE-1 binding site, blue) are shown.
The earliest time point at which we could separate cells from the different lineages was at the onset of TBX-37/38 expression in the ABa lineage (∼90 min post 2-cell). At this time point, genome-wide accessibility patterns of ABa- and ABp-derived cells are extremely similar (Pearson correlation coefficient = 0.95; Figure S3D). However, the lsy-6 locus was clearly accessible only in ABa-derived cells, in a region overlapping with the TBX-37/38 binding site defined by ChIP-seq (Figures 4B and 4C). TBX-37/38 are likely the main determinants of differences in accessibility between ABa and ABp at this time, as de-novo motif discovery among ABa-specific accessible regions recovered the known TBX-38 motif as a clear top hit (Figure S3E). We also performed ATAC-seq at the 200-min time point and found that the specific difference in accessibility of the the lsy-6 locus remains (Figures 4B, 4C, and S3D), although TBX-37/38 are no longer present at that time point. At this time, there are 64 descendants of each ABa or ABp. The substantial signal observed at the lsy-6 locus in ABa descendants suggests that it must be accessible in a large fraction of ABa descendants.
At the time point when the ASEs are born (350 min), ABa and ABp have each produced ∼180 descendants. When we sampled all of them, the lsy-6 locus appeared equally inaccessible in both lineages (Figure 4B), even though these datasets were of high quality and globally highly correlated to the 200 min datasets (Pearson correlation coefficient = 0.85 for ABa and 0.87 for ABp). However, at this time most cells are differentiated, and we could not exclude that lsy-6 remained accessible in the one ASEL neuron among the ∼180 ABa descendants. To directly assess lsy-6 accessibility in ASEs, we used fluorescence-activated cell sorting (FACS) to isolate the left and right neurons from embryos expressing cell-specific labels. The genome-wide patterns of accessibility between ASEL and ASER neurons are highly similar (Pearson correlation coefficient = 0.94) (Figure S3D). Both are largely determined by CHE-1, consistent with its known expression and function (Etchberger et al., 2007; Leyva-Díaz and Hobert, 2019; Uchida et al., 2003), as de-novo motif discovery among ASE-specific accessible regions recovered the known CHE-1 motif in the top five hits, both in ASEL and ASER (Figure S3E). However, ATAC-seq analysis revealed an accessible region upstream of lsy-6 in ASEL, which was inaccessible in ASER (Figures 4B and 4C). The peak of accessibility in ASEL overlaps with a CHE-1 binding site that has been well-characterized genetically and biochemically (Etchberger et al., 2007, 2009). This suggests that TBX-37/38 binding during early development impacts later accessibility of the CHE-1 binding site. Further supporting this, accessibility of the CHE-1 binding region was fully lost upon deletion of the TBX-37/38 binding sites (Δtbs) (Figures 4C and 4D).
Together, these data indicate that TBX-37/38 establish an early asymmetry in lsy-6 accessibility that impacts later ability of CHE-1 to bind and promote lsy-6 transcription.
TBX-37/38 Promotes Bidirectional Transcription of lsy-6, which Is Necessary to Establish the Competent State
We envisioned two possible mechanisms for establishment of the lsy-6 competent state by TBX-37/38. In the first, binding by TBX-37/38 is sufficient to induce accessibility, which is then relayed by other intermediate TFs that could keep the locus open for CHE-1, as it has been proposed for many pioneer TFs (Zaret and Carroll, 2011). In the second, TBX-37/38-activated transcription of lsy-6 establishes the competent state and is required for later CHE-1-mediated boosting. Here, we set out to distinguish between these two possibilities.
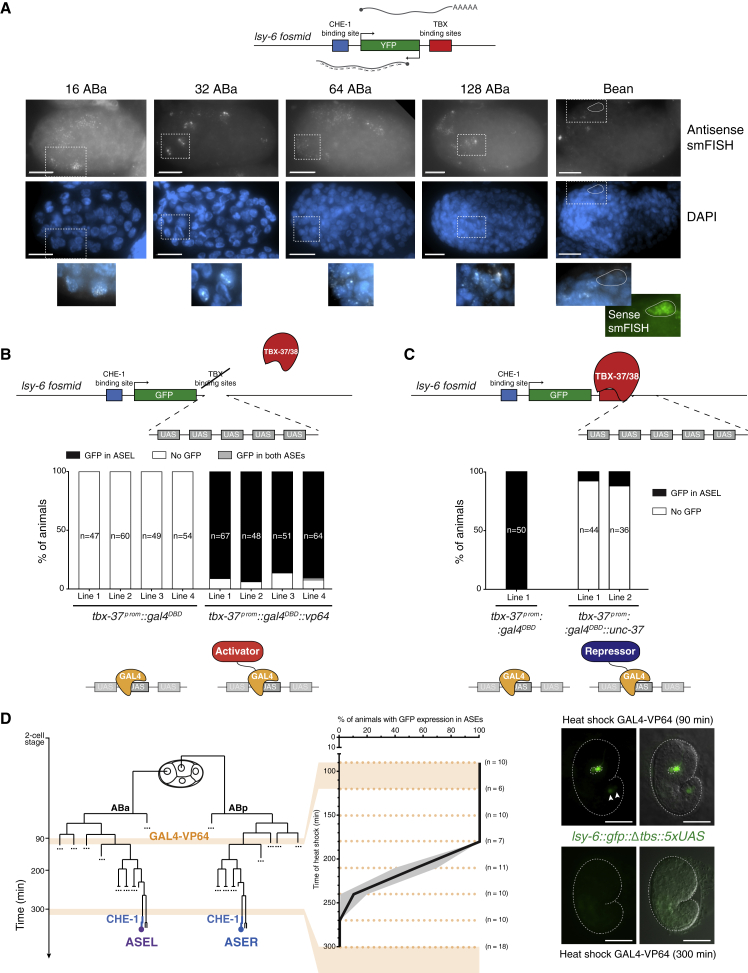
Although robust transcription of lsy-6 is only observed upon onset of CHE-1, previous smFISH of the lsy-6::yfp fosmid transgene revealed a low level of transcription from the lsy-6 locus starting shortly after onset of TBX-37/38 expression (Cochella and Hobert, 2012). Given that TBX-37/38 bind downstream of lsy-6, we asked whether the locus was also transcribed in the antisense orientation. Indeed, many cells in the anterior part of the embryo displayed antisense transcription of the lsy-6::yfp fosmid transgene, at levels considerably higher than those of the sense transcription (Figures 5A and S4A). Both sense and antisense signals were fully dependent on TBX-37/38 and the tbs-containing region (Figure S4B). In wild-type embryos, antisense transcription persisted until the birth of ASEL, as determined by the presence of high-intensity nuclear foci (Figures 5A and S4A). At this stage, sense transcription became restricted to ASEL and dramatically increased in level, consistent with the onset of expression and known activity of CHE-1 (Etchberger et al., 2007, 2009), whereas antisense transcription remained at relatively low levels and in a few cells (Figure 5A).
Figure 5.
Transcription over the lsy-6 Locus Occurs Bidirectionally and Is Required for Priming
(A) smFISH on embryos carrying the lsy-6::yfpfosmid showed robust antisense transcription of the lsy-6 locus (see Figure S4 for sense transcript and dependence on TBX-37/38 and their binding sites). Representative images are shown (n ≥ 10). Dashed boxes indicate zoomed regions showing bright nuclear foci. At the bean stage, the mature ASEL neuron is outlined as determined by strong signal against the sense yfp transcript. Scale bars represent 10 μm.
(B) The TBX-37/38 binding sites were replaced by five UAS sites in the context of the lsy-6::gfp fosmid reporter. YFP expression was restored exclusively in ASEL by expression of the GAL4DBD-VP64 transcriptional activator under the tbx-37prom (Table S2). Four independent, extrachromosomal transgenic lines were scored for each condition. Number of animals scored per line are shown.
(C) Five UAS sites were inserted downstream of the TBX-37/38 binding sites in the context of the lsy-6::gfp fosmid reporter. Expression of GAL4DBD-UNC-37 under the tbx-37prom prevented later expression in ASEL (Table S2). Two independent, extrachromosomal transgenic lines were scored.
(D) Embryos carrying the integrated lsy-6::gfp::Δtbs::5xUAS reporter and a transgene containing GAL4DBD-VP64 under a heat-shock promoter were subjected to heat shock at the indicated times (dashed lines). GAL4DBD-VP64 expression in the whole embryo at early time points induced lsy-6::gfp::Δtbs::5xUAS expression in both ASEs. Beyond the 180-min time point, GAL4DBD-VP64 progressively lost ability to activate lsy-6::gfp::Δtbs::5xUAS expression. Plot shows proportion and SEP (n ≥ 6 per time point). Representative images are shown for the 90- and 300-min time points. Arrowheads point to sporadic ectopic expression in two cells in the tail upon heat-shock treatment (independently of GAL4-VP64 expression). Scale bars represent 20 μm.
To examine the function of early transcription at the lsy-6 locus, we first attempted to bypass the requirement for TBX-37/38 with a general transcriptional activator. To this end, we replaced the region containing the two TBX-binding sites in the lsy-6::gfp fosmid reporter with five upstream activation sequence (UAS) binding sites, which recruit the S. kudriavzevii TF GAL4 (Wang et al., 2017a) (lsy-6::gfp::Δtbs::5xUAS) (Figure 5B). This reporter did not drive the GFP expression as expected, given the lack of the TBX-37/38 binding sites (Table S1). We then used the tbx-37 promoter to drive expression of the GAL4 DNA binding domain alone (GAL4DBD) or fused to the VP64 transcriptional activator (GAL4DBD-VP64), consisting of four copies of the VP16 minimal activation domain (Beerli et al., 1998). GAL4DBD-VP64 restored the exclusive expression of GFP in ASEL, whereas the GAL4DBD alone did not (Figure 5B). These data suggest that early transcriptional activation of the lsy-6 locus is sufficient for the establishment of the competent state, and further suggest that TBX-37/38 are not uniquely required to prime the lsy-6 locus, as their function can be replaced by a heterologous, unrelated transcriptional activator.
We further validated that this heterologous system replicates the expression pattern of lsy-6 through the combinatorial action of an early primer and later activation by CHE-1. To this aim, we asked if ectopic expression of GAL4DBD-VP64 using a heat-shock promoter would replicate the time window for priming observed for TBX-37 (Figure 2D). Almost indistinguishably from TBX-37, GAL4DBD-VP64 was sufficient to prime lsy-6::gfp::Δtbs::5xUAS during the first ∼180 min of development but not beyond this time point (Figure 5D). Priming by GAL4DBD-VP64 also recapitulated the specificity of lsy-6 activation: even though this activator is expressed in all embryonic cells upon heat shock, we observed GFP expression exclusively in the ASE neurons suggesting that specificity in this heterologous system is also ultimately determined by CHE-1 (Figure 5D). Consistent with this, expression of the reporter fails to be boosted in absence of CHE-1 (Figures S4C and S4D).
Based on the finding that early transcriptional activation seems sufficient for priming, we hypothesized that blocking this early transcription, via the recruitment of a transcriptional repressor, would impair it. Briefly, we inserted five UAS sites downstream of the intact TBX-37/38 binding sites in the lsy-6::gfp fosmid reporter (lsy-6::gfp::5xUAS) and used the tbx-37 promoter to drive expression of GAL4DBD or GAL4DBD fused to the transcriptional repressor Groucho/UNC-37 (GAL4DBD-UNC-37) (Chambers et al., 2017; Kaul et al., 2014) (Figure 5C). Absence of tethering, or tethering of GAL4DBD alone, did not affect lsy-6 expression (Table S1; Figure 5C). However, early recruitment of GAL4DBD-UNC-37 repressor to the UAS sites abolished GFP expression in ASEL (Figure 5C). We conclude that early adoption of a transcriptionally active state is necessary and sufficient to prime lsy-6 for later robust activation and explains the requirement for TBX-37/38.
The Activity of TBX-37/38 Is Not Relayed by Other T-Box Transcription Factors
Given that TBX-37/38 bind the lsy-6 locus early and are not continuously required, we set out to investigate how this competent state of lsy-6 is maintained. Based on the continuous transcription over the lsy-6 locus, we hypothesized that the competent state would be maintained by intermediate TFs, likely accompanied by a specific chromatin state. We envisioned that other T-box TFs could relay the initial activation by TBX-37/38, based on observations that sequentially expressed Sox TFs can bind to the same enhancer at different times during neurogenesis (Bergsland et al., 2011). The C. elegans genome encodes 22 T-box TFs, and many of them share similar binding sites (Narasimhan et al., 2015; Okkema, 2017). Theoretically, other T-box factors could use the same TBX-37/38 binding sites at later time points. Moreover, we found TBX-37/38 binding sites in the vicinity of six tbx genes (including tbx-37 and tbx-38), which could be putative targets of TBX-37/38 activation and, thus, be expressed in the ABa lineage (Figure S5A). To determine whether other T-box TFs propagate the competent state of lsy-6 over time, we followed expression of reporters for most T-box TFs through C. elegans development. For factors expressed before the ASE neurons are born, we traced expression throughout the whole lineage, with single-cell resolution (Figure S5B). We found seven T-box TFs expressed in the ABalpp lineage branch that gives rise to the ASEL neuron. To examine their potential role in lsy-6 transcription, we analyzed lsy-6 expression or function upon depletion of T-box factors either by RNA interference (RNAi) or by crossing to mutant alleles. Because some of these T-box factors occur in recently duplicated pairs, we also included conditions where two factors were ablated at the same time. None of the tested manipulations for individual or pairs of T-box TFs resulted in loss of lsy-6 expression (Figures S5C and S5D). These results strongly argue that the asymmetric active state of lsy-6 is not maintained by a cascade of T-box TFs.
The Asymmetric Competence of lsy-6 Is Maintained in a Symmetric Trans-Acting Factor Environment
The competent state of lsy-6 could be maintained by two distinct classes of factors. On one hand, it could be maintained by other TFs (or regulatory factors) that are induced by TBX-37/38 and are, thus, asymmetrically expressed across ABa and ABp. These factors (or their targets) could ultimately be present in ASEL (but not ASER) and act together with CHE-1 to activate lsy-6 in a more conventional, cooperative manner (Figure 1A). In this model, TBX-37/38 would play both a direct role in establishing the lsy-6 active state and an indirect role in maintaining this state through the activity of one or more of their transcriptional targets. Alternatively, TBX-37/38 could provide an asymmetric trigger that could be propagated by symmetrically expressed factors in cells that develop through paths that share extensive gene expression similarity: the two branches that will give rise to ASEL and ASER (ABalppp and ABpraaa) become symmetric around the 32 AB stage, share common gene-expression profiles over time (Poole et al., 2011; Sarin et al., 2009), and eventually give rise to the same set of 13 cells on each side of the head. The lsy-6 competent state could, thus, be maintained by TFs and other regulators present in both lineage branches, but only if the locus was previously primed.
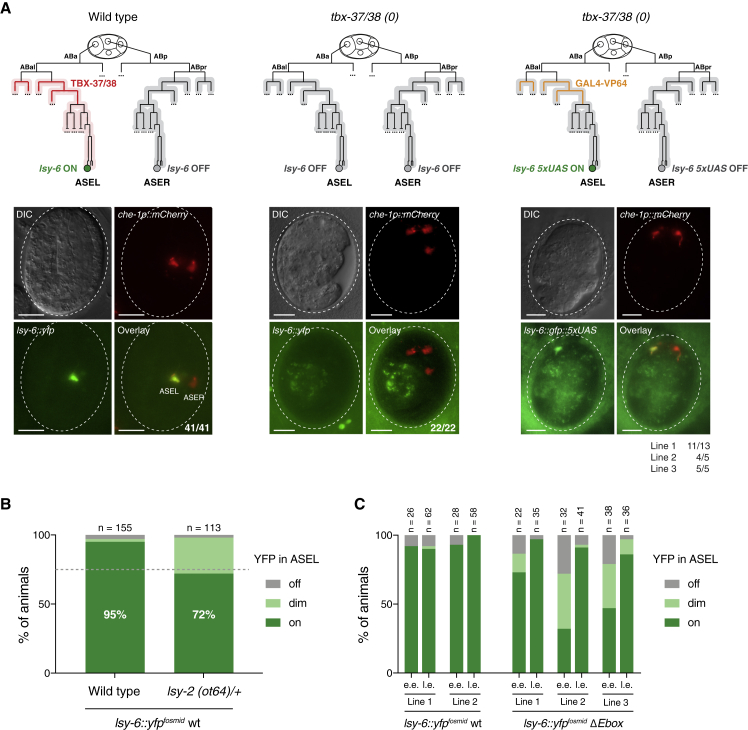
To distinguish between these two possibilities, we asked whether asymmetrically primed lsy-6 could retain competence in complete absence of TBX-37/38, which ensures the two branches develop symmetrically from an early time point and eliminates any possible indirect effect of TBX-37/38. To do this, we took advantage of the heterologous priming of the lsy-6::gfp::Δtbs::5xUAS reporter by the tbx-37prom-driven GAL4DBD-VP64 (Figure 5B) and asked whether ABa-specific priming can be maintained in a tbx-37/38 double-mutant background. In embryos carrying tbx-37/38 deletions, ABa adopts an ABp identity, and its descendants produce one or sometimes two ASE neurons that express ASER markers (Poole and Hobert, 2006) and never express lsy-6 (Figure 6A). We found that tbx-37prom-driven GAL4DBD-VP64 activated the lsy-6::gfp::Δ150::5xUAS reporter in one (or sometimes two out of three ASE neurons) in the tbx-37/38 null background, indicating that asymmetric priming of lsy-6 could be maintained in a symmetric trans-acting factor environment (Figure 6A). These results indicate that other TBX-37/38 targets are not necessary for maintenance of lsy-6 competence in ABa descendants, and if additional factors are indeed required, they must be symmetrically expressed across ABa and ABp.
Figure 6.
Asymmetric lsy-6 Competence Is Maintained in a Symmetric Trans-acting Factor Environment
(A) Schematics of the developmental lineage and representative images of embryos of the indicated genotypes. The ASE neurons are marked by che-1prom::mCherry. (Left) 41/41 wild-type embryos express the lsy-6::yfpfosmid reporter in ASEL. Light-red highlight of the lineage indicates potential downstream asymmetries triggered by TBX-37/38. (Middle) Deletion of tbx-37/38 resulted in 22/22 embryos without lsy-6::yfpfosmid expression. (Right) Heterologous, asymmetric priming using the lsy-6::gfp::Δtbs::5xUAS + tbx-37prom::gal4::vp64 system (Figure 5B) was sufficient to activate lsy-6::yfp expression in only one ASE neuron in tbx-37/38(0) animals. Three independent transgenic lines were scored, numbers of embryos are shown. All scale bars represent 10 μm.
(B) The ubiquitous TF lsy-2 is necessary for robust lsy-6::yfpfosmid expression in ASEL (Table S3). Progeny of lsy-2 heterozygous mothers were scored as larvae for YFP expression and compared with wild type. 25% of larvae are expected to be homozygous for lsy-2, setting the maximum possible effect. Animals scored as “dim” had a barely visible signal. n ≥ 113 per genotype.
(C) Deletion of an E-box in the lsy-6 locus causes a delay in onset of lsy-6::yfp expression. Expression was scored in embryos from comma to 2-fold stage (early embryos, e.e.) or at the 3-fold stage (late embryos, l.e.). Three independent extrachromosomal lines show a delay in onset of YFP expression, n ≥ 22 per condition (Table S3).
These findings provide an explanation for the observation that genetic screens recovered mutations in a number of symmetric trans-acting factors that cause loss of ASE neuron asymmetry (Sarin et al., 2007). For example, LSY-2 is a zinc finger TF that is ubiquitously expressed yet necessary specifically for ASEL identity (Johnston and Hobert, 2005). Given that lsy-2-deficient animals are sterile, we examined the progeny of heterozygous mothers and found that they failed to robustly express the lsy-6::yfp fosmid reporter (Figure 6B). Moreover, a consensus binding motif for a bHLH TF is necessary for early onset of lsy-6::yfp expression (Figure 6C), suggesting that a TF of this family may also be necessary to efficiently relay the competent state. A good candidate is the symmetrically expressed HLH-14, which is necessary to specify the neuronal identity of the ASEs (Poole et al., 2011). Unfortunately, we could not test the role of HLH-14 in lsy-6 expression, as in hlh-14 mutant animals the ASE grandmother cells fail to adopt their neuroblast identity and instead become epidermal cells (Poole et al., 2011). In addition, two ubiquitously expressed chromatin modifying complexes: the Set1/COMPASS complex, which acts as a histone H3K4 methyltransferase, and a complex containing a MYST histone acetyltransferase are necessary for lsy-6 transcription (O'Meara et al., 2010; Poole et al., 2011). Together, these are good candidates for propagation of the lsy-6 competent state until the onset of CHE-1 expression.
TBX-37/38 Regulates Left/Right Asymmetric Gene Expression in Additional Neuron Pairs
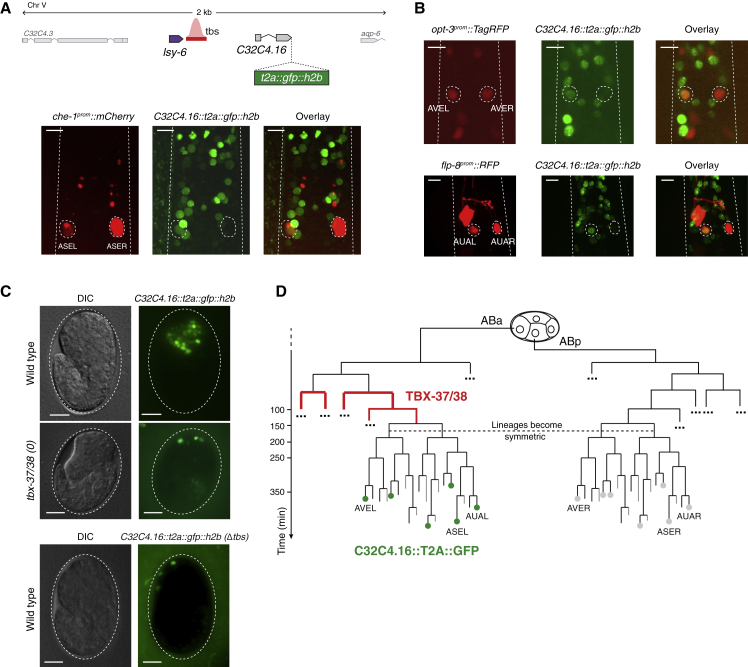
TBX-37/38 and CHE-1 activities are integrated on the lsy-6 locus, resulting in ASEL-specific transcription. We postulated that TBX-37/38 might prime other loci to achieve asymmetric gene expression in other neuron pairs derived from the ABa and ABp lineages (Figure 7). To assess this, we examined the expression pattern of the gene downstream of lsy-6, C32C4.16 (Figure 7A), which shares the TBX-37/38 binding sites with lsy-6 and is expressed in multiple neurons. A fosmid-based reporter for C32C4.16 was combined with a red fluorescent ASE marker (che-1prom::mCherry) that enables the distiction between the left and right sides of the head based on axon morphology. Indeed, we found that C32C4.16 was not only asymmetrically expressed across the ASE neurons but also showed expression in a number of additional nuclei on the left side of the head of the worm, without obvious counterparts on the right side (Figure 7A).
Figure 7.
TBX-37/38 Determine Left-Right Molecular Asymmetry across Multiple Bilateral Neuron Pairs with ABa/ABp Lineage Origin
(A) Schematic of the reporter used to monitor expression of C32C4.16 (in the context of a fosmid clone containing ~40 kb of flanking genomic sequence), producing nuclear GFP separated from the C32C4.16 protein product through a 2A peptide (Ahier and Jarriault, 2014). Representative images show a larval head with left-specific expression across ASE neurons (labeled with che-1prom::mCherry) and potentially other neurons on the left side.
(B) C32C4.16 expression is also lateralized across AVE and AUA neuron pairs (labeled with opt-3prom::TagRFP and flp-8prom:: RFP, respectively) (n = 10 per genotype).
(C) Expression of C32C4.16 requires TBX-37/38 and their binding sites for the majority of its expression pattern (n = 20 per genotype). All scale bars represent 10 μm.
(D) Schematic of the lineage origin of the AVE, AUA, and other neuron pairs that were also identified as having asymmetric C32C4.16 expression (Figure S6).
To identify the precise neurons in which C32C4.16 is expressed, we took advantage of the NeuroPal system, which uses a combination of >40 neuron-specific markers in three different colors, making neurons easily identifiable by their unique color combination and position (Yemini et al., 2019). We identified an additional five neuron pairs with dual-lineage origin that display C32C4.16 expression in the left, but not the right member of the pair: AUAL, AWBL, AVEL, RMDL, and OLLL (Figure S6). Notably, none of these neuron pairs were previously known to display any molecular asymmetry, but they are all specified by bilaterally expressed terminal selectors akin to CHE-1 in the ASE neurons (Pereira et al., 2015; Serrano-Saiz et al., 2013). We confirmed the asymmetric expression across the AVE and AUA pairs by combining the C32C4.16 reporter with red fluorescent reporters expressed bilaterally in these neurons (Figure 7B).
Consistent with TBX-37/38 dependence, neuron pairs in which both members arise from ABa showed symmetric expression of C32C4.16 (e.g., AVJL/R, SMDVL/R). We further tested this dependence by crossing the C32C4.16 fosmid reporter into the tbx-37/38(0) background and by generating a fosmid reporter in which we deleted the TBX-37/38 binding sites. In both cases, expression of the reporter was lost in most cells (Figure 7C). Together, these observations suggest that priming by TBX-37/38 is integrated with other, yet to be identified TFs to achieve directed molecular asymmetries in the C. elegans nervous system (Figure 7D).
To define additional candidate loci that could be regulated in a manner similar to lsy-6 and C32C4.16, we further mined the GFP-TBX ChIP-seq and ABa and ABp ATAC-seq datasets described above. We identified 86 genomic regions bound by TBX-37/38 and accessible in ABa, but not in ABp, at the 90-min time point. We assigned these to the nearest promoter, defining 86 genes, including protein-coding genes but also, intriguingly, two additional miRNAs. These genes form a set of compelling candidate loci that could be similarly primed by TBX-37/38 and could contribute to additional asymmetries among these neurons, but also among other ABa and ABp-derived lineage branches (Figure S6C).
Discussion
A Molecular Mechanism for Temporal Integration of Transcriptional Inputs
The use of TF combinations for gene activation is a widespread phenomenon that has been long recognized to enable generation of cell-type-specific gene-expression patterns and, in turn, cell-type diversity. Here, we revealed mechanistic insight into how two temporally segregated TFs can act combinatorially to achieve cell-type specific transcription of a locus that controls neuronal identity. We also provide evidence that temporal integration of TFs may be a more general mechanism for cellular diversification.
The transiently expressed, lineage-specific TFs, TBX-37/38, bind directly to the lsy-6 locus and promote an accessible state in cells that derive from the ABa blastomere. In contrast, in ABp descendants that do not express TBX-37/38, the lsy-6 locus remains inaccessible throughout embryogenesis. Binding of TBX-37/38 promotes a transcriptionally active state, which primes lsy-6 for later activation by CHE-1. Early transcription is primarily in the antisense orientation and does not produce active lsy-6. TBX-37/38 are not expressed or functionally required beyond the priming event, and no other downstream targets of TBX-37/38 are needed to establish or maintain the competence of the lsy-6 locus. CHE-1 expression in the mother of both ASE neurons boosts lsy-6 expression, specifically in ASEL. The locus remains inaccessible and refractory to activation in ASER despite the presence of CHE-1, which is otherwise able to promote the transcription of hundreds of other genes in this neuron (Etchberger et al., 2007). We propose that the transcriptionally active state in the ASEL precursors prevents lsy-6 from adopting a repressed, inaccessible state that remains molecularly undefined.
The role of TBX-37/38 may seem similar to that of pioneer TFs, which are defined by their ability to bind nucleosomal DNA and unmask binding sites for other TFs (Zaret and Carroll, 2011; Zaret and Mango, 2016). However, we argue that there are important mechanistic distinctions between the two; unlike pioneer TFs, which initiate chromatin remodeling within inactive chromatin, TBX-37/38 must act during a permissive time window, before the inactive state sets in. Supporting this, a recent large-scale screen for TFs that can bind nucleosomal DNA suggested that TFs of the immunoglobulin superfamily, such as T-box factors, lack the molecular features that would enable pioneer function (Fernandez Garcia et al., 2019). A different study, however, showed some T-box factors can bind nucleosomes when reconstituted with DNA containing two binding sites on adjacent DNA gyres (Zhu et al., 2018). Nevertheless, our data indicate that TBX-37/38 fail to activate the lsy-6 transcription once the locus becomes restricted and, thus, do not seem to act as pioneer TFs.
The ability of pioneer TFs to bind within inactive chromatin may explain why many of them do not need to activate transcription to establish accessibility (Sartorelli and Puri, 2018; Zaret and Carroll, 2011; Zaret and Mango, 2016). In fact, the best-studied pioneer TFs have been shown to bind at least hours before the onset of transcription, which is typically triggered by the additional binding of other TFs to their exposed sites (e.g., Charney et al., 2017; Gualdi et al., 1996). The pioneer TF PHA-4, the C. elegans homolog of FoxA, does recruit RNA polymerase II (RNA pol II). However, this remains poised at the TSS and transcription occurs only at a later time point (Hsu et al., 2015). In contrast, our work suggests that binding of TBX-37/38 alone may not be sufficient, but that immediate transcriptional activation is necessary to impart competence to the lsy-6 locus.
An important consequence of this distinction between the priming we describe and the action of pioneer TFs becomes evident in the context of efforts to program and reprogram different cell types in vitro. Whereas pioneer TFs are able to remodel the chromatin state of the cells to be reprogrammed (e.g., the pioneer activity of the Yamanaka factors has been studied in depth in vivo and in vitro; Iwafuchi-Doi and Zaret, 2016; Soufi et al., 2015), priming events that need to occur under specific chromatin contexts may not be fully recapitulated by protocols in which cells do not undergo the transcriptional histories they normally experience during development.
Transcriptional Activation as an Integral Player for Establishing Competence
How could establishing an active transcriptional state determine later competence for robust transcription? A role for transcription in establishing a competent epigenetic state was described for Zdbf2 in mouse embryos (Greenberg et al., 2017), which requires early embryonic transcription from an alternative, upstream promoter to become competent for transcription during adulthood. The early transcription promotes DNA methylation (on cytosines) and this in turn evicts H3K27me3 marks that cover the adult promoter and prevents re-establishment of this mark by Polycomb. In absence of early transcription, the adult promoter remains silenced by Polycomb (Greenberg et al., 2017). The system we describe here cannot rely on cytosine methylation as this mark does not occur in C. elegans. Adenosine methylation has been shown to occur in C. elegans but at levels at least an order of magnitude lower than cytosine methylation in mammals (0.01%–0.4% of adenosines) (Greer et al., 2015). Relying on DNA methylation enables maintenance of the active state across many cell divisions, from the embryo to the adult. In contrast, we suggest that the competent state that is established on lsy-6 may require a more active maintenance mechanism, for example, through continuous, low-level transcription.
The recruitment and action of RNA pol II and associated factors during transcription initiation and elongation could change the chromatin landscape of the lsy-6 locus either directly, by displacing nucleosomes (Venkatesh and Workman, 2015), or indirectly, through recruitment of histone modifiers. Elongating RNA pol II can recruit the Set1/COMPASS complex, which methylates histone H3 at position K4 (Ng et al., 2003). This modification has been shown to promote acetylation of histones on multiple different residues (Ginsburg et al., 2014; Noma and Grewal, 2002; Taverna et al., 2006) and inhibits methylation on H3K9 (Greenstein et al., 2020). Together, this can result in the destabilization of nucleosomes and prevent spreading of repressive chromatin onto transcriptionally active genes, all of which would favor active transcription (Greenstein et al., 2020). All components of the Set1/COMPASS complex were identified in a genetic screen as being necessary for robust lsy-6 expression and establishment of ASE asymmetry (Poole et al., 2011). Our work provides a framework to further address the potential contribution of Set1/COMPASS to establishing and/or maintaining developmental competence.
The transcriptionally competent state of lsy-6 needs to be maintained through three to four cell divisions. Our smFISH analysis revealed high-intensity nuclear foci that typically mark the site of active transcription, even at time points when TBX-37/38 are no longer active. We could discard the idea that continuous transcription is mediated by other targets of TBX-37/38 that may be asymmetrically expressed between ABa and ABp descendants. This indicates that if other TFs are necessary for maintenance of the active state, they are likely symmetrically expressed across both lineages. Broadly acting TFs could promote low levels of transcription of the lsy-6 locus that, together with the active chromatin marks, may propagate the competent state until the onset of CHE-1.
Temporal Integration of Transcriptional Inputs as a General Mechanism for Cell Diversification
Priming of enhancers prior to the time point when robust transcription is required is a broadly observed phenomenon that can be caused by different mechanisms, e.g., pre-establishment of contact between promoter and distal enhancers (Ghavi-Helm et al., 2014; Ng et al., 2018; Rubin et al., 2017), action of pioneer TFs (Charney et al., 2017; Gualdi et al., 1996; Hsu et al., 2015; Kueh et al., 2016), acquisition of chromatin modifications (Creyghton et al., 2010; Rada-Iglesias et al., 2011), or as shown here by promoting early antisense transcription. However, this pre-activation step is most often studied and interpreted in the context of achieving robust and/or timely gene activation, by reducing the rate-limiting step of chromatin remodeling. For example, multiple enhancers carry histone modifications associated with activity (e.g., H3K4me1) in progenitor cells, even though they are not associated with robust transcription at that stage, but this is predictive for future transcription upon differentiation into specific cell types (Creyghton et al., 2010; Rada-Iglesias et al., 2011). In Drosophila embryos, priming of Notch-responsive enhancers enables a deterministic and sustained transcriptional response, whereas in the absence of priming, transcription is stochastic and “bursty” (Falo-Sanjuan et al., 2019).
Our work places these priming mechanisms under a different light. Lineage-specific priming can be used to generate unique temporal combinations of TFs with later-acting TFs that act both within and outside the lineage where priming occurred. Thus, we suggest that temporal integration of TFs, much like spatial integration, may be a more general mechanism for creating intersectional gene-expression patterns and, therefore, cellular diversity. One scenario in which this mechanism could be used in a manner analogous to what we describe for the ASE neurons is to diversify cells that develop through different trajectories but then acquire the same terminal identity, i.e., convergent cell types. Priming events would exploit transient differences in developmental trajectories, which if maintained through development could result in distinct outcomes from the execution of an otherwise identical terminal differentiation program.
The C. elegans lineage reveals multiple cases of cells that seemingly adopt the same terminal fate, through expression of the same terminal TFs, but do so through developmental trajectories with distinct transcriptional histories (Sulston et al., 1983). Instances of such convergence are also known in vertebrate systems, e.g., the gut endoderm of the mouse is formed by intercalation of cells from embryonic and extraembryonic lineages (Kwon et al., 2008), and multiple cell types can originate both from the neural crest or from mesodermal lineages (Dupin et al., 2018). Cases of convergence were until recently limited to specific systems studied in depth using classical lineage tracing; recent single-cell studies combining transcriptome and lineage analysis have revealed that cell-fate convergence is widespread during animal development (Chan et al., 2019; Konstantinides et al., 2018; Liu et al., 2019; Raj et al., 2018; Wagner et al., 2018). These cases provide a rich substrate to further explore the contribution of temporal integration of TFs to diversifying cell types.
Our work shows that priming does not require a unique activity of the transient TF, but the general ability to induce an active transcriptional state. Moreover, our data indicate that other terminal selectors, in addition to CHE-1, can “read out” the priming event. These observations make this mechanism highly versatile, and we, therefore, suggest this may be a more general, yet, unexplored source of cellular diversification.
STAR★Methods
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit polyclonal anti-GFP | Abcam | Cat# ab290; RRID: AB_303395 |
| Bacterial and Virus Strains | ||
| Escherichia coli: HB101 Strain | CGC | HB101 |
| Escherichia coli: HT115 Strain | CGC | HT115 |
| Escherichia coli: OP50 Strain | CGC | OP50 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Chitinase | Sigma | Cat# C6137 |
| cOmplete™, Mini Protease Inhibitor Cocktail | Roche | Cat# 11836153001 |
| Dextran sulfate sodium salt | Sigma | Cat# D6001 |
| EvaGreen® Dye | Biotium | Cat# 31000 |
| Formaldehyde solution | Sigma | Cat# F8775 |
| Formamide (Deionized) | Ambion | Cat# AM9342 |
| Leibovitz;s L-15 Medium, no phenol red | Gibco | Cat# 21083027 |
| Penicillin-Streptomycin | Sigma | Cat# P4458 |
| ProLong™ Gold Antifade reagent with DAPI | Invitrogen | Cat# P36935 |
| Pronase | Sigma | Cat# P6911 |
| SYBR™ Green I Nucleic Acid Gel Stain | ThermoFisher | Cat# S7563 |
| SYTOX™ AADvanced Dead Cell Stain Kit | Invitrogen | Cat# S10349 |
| Critical Commercial Assays | ||
| AMPure XP Beads | Beckman Coulter | Cat# A63882 |
| DNA Clean & Concentrator Kit-5 | Zymo Research | Cat# D4013 |
| Dynabeads™ Protein A Immunoprecipitation Kit | ThermoFisher | Cat# 100006D |
| NEBNext® High Fidelity 2x PCR Master Mix | New England BioLabs | Cat# M0541L |
| NEBNext® Ultra II DNA Library Prep Kit for Illumina® | New England BioLabs | Cat# E7645S |
| Nextera DNA Library Prep Kit | Illumina | Cat# 15028212 |
| Nextera i7 and i5 adapters | Illumina | Cat# 20027213 |
| Protein Assay Dye Reagent Concentrate | Bio-Rad | Cat# 5000006 |
| Quasar 670-conjugated RNA FISH Probe Sets | Biosearch Technologies | N/A |
| Deposited Data | ||
| ATAC-seq & ChIP-seq data | This Study | GEO: GSE155392 |
| Experimental Models: Organisms/Strains | ||
| Please see Table S4 for a complete list of strains used in this study | ||
| Oligonucleotides | ||
| crRNA targeting che-1 #1: TAAAGAGGGTGGAGCTTCAG |
IDT | N/A |
| crRNA targeting che-1 #2: CACAGAGTGGGAACTTGCAT |
IDT | N/A |
| crRNA targeting lsy-6 #1: ATGAGACGCATTTCGATGAC |
IDT | N/A |
| crRNA targeting tbx-37 #1: GCTCAAAATTACAATAATTT |
IDT | N/A |
| crRNA targeting tbx-37 #2: GAGCAGCAAACTGTGGCTGG |
IDT | N/A |
| crRNA targeting tbx-38 #1: ATTGTACTGCACTGATCTCC |
IDT | N/A |
| crRNA targeting tbx-38 #2: TACCTATTGCCTTTCTCCCC |
IDT | N/A |
| crRNA targeting tbx-11 #1: AACAGGAAAAATACACCCGG |
IDT | N/A |
| crRNA targeting tbx-11 #2: CCGCCCAACACGTGGCAAGG |
IDT | N/A |
| crRNA targeting tbx-43 #1: ATTTTTCCATAAGCGCCACG |
IDT | N/A |
| crRNA targeting tbx-43 #2: GGCCGAGAACTCCTCCAACG |
IDT | N/A |
| Recombinant DNA | ||
| Please see Table S5 for a complete list of Recombinant DNA used in this study | ||
| Software and Algorithms | ||
| bowtie2 (v2.2.4) | Langmead and Salzberg, 2012 | N/A |
| ChIPseeker (v1.22.1) | Yu et al., 2015 | N/A |
| Cutadapt (v1.18) | Martin, 2011 | N/A |
| GenomicRanges (v1.38.0) | Lawrence et al., 2013 | N/A |
| ImageJ (Fiji) | NIH | N/A |
| kent-uscs (v2.79) | Kent et al., 2010 | N/A |
| macs2 (v2.1.0) | Zhang et al., 2008 | N/A |
| MEME Suite (v5.1.1) | Machanick & Bailey, 2011 | N/A |
| MetaMorph | Molecular Devices | N/A |
| rtracklayer (v1.46.0) | Lawrence et al., 2009 | N/A |
| Picard-tools (v2.18.27) | Broad Institute | N/A |
| Prism 7 | GraphPad Prism | N/A |
| Rsubread (v2.0.1) | Liao et al., 2019 | N/A |
| SAMtools (v0.1.18) | Li et al., 2009 | N/A |
| SciPy | Virtanen et al., 2020 | N/A |
| SIMI BioCell | Schnabel et al., 1997 | N/A |
| Time to Live | Caenotec | N/A |
| Zen | Zeiss | N/A |
| Other | ||
| Axio Imager.Z2 | Zeiss | N/A |
| Axio Observer Confocal with Airyscan | Zeiss | Cat# LSM880 |
| Bioruptor® Plus sonication device | Diagenode | Cat# B01020001 |
| HiSeq 2500 Sequencing System | Illumina | N/A |
| NanoDrop™ 3300 Fluorospectrometer | ThermoFisher | Cat# ND-3300 |
| pluriStrainer® 5 μm cell strainer | pluriSelect | Cat# 43-10005-40 |
| SH800S Cell Sorter | Sony | N/A |
| Sorting Chip 100 μm | Sony | Cat# LE-C3210 |
| Visiscope Spinning Disc Confocal | Visitron Systems GmbH | N/A |
Resource Availability
Lead Contact
Further information and requests concerning resources and reagents should be directly addressed to Luisa Cochella (cochella@imp.ac.at).
Materials Availability
All C. elegans strains generated in this study will be made available through the CGC. Plasmid and fosmid-based reporters generated in this study are available from the corresponding author upon request.
Data and Code Availability
All datasets (ChIP-seq and ATAC-seq) generated in this study are available under GEO accession number GSE155392. This study did not generate code.
Experimental Model and Subject Details
Strains
All Caenorhabditis elegans strains were maintained on nematode growth media (NGM) plates seeded with OP50 bacteria (Brenner, 1974), at 20°C unless otherwise indicated. A full list of strains used in this study can be found in Table S4.
Method Details
Strain Generation
Standard microinjection procedures were used to generate transgenic worms with extra chromosomal arrays (Mello and Fire, 1995). Briefly, DNA was injected as complex arrays in the gonads of young adults. Injection mixes contained 1-5 ng/μL of the plasmid of interest and a similar amount of a co-injection marker (typically che-1prom::mCherry, ttx-3prom::mCherry or unc-122prom::mCherry), in addition to 100 ng/μL of sonicated genomic DNA from E. coli OP50. Some extrachromosomal arrays were then integrated into the genome by random integration stimulated by gamma irradiation (14 minutes; 4000 rads). All integrants were outcrossed 3-6 times with the wild type strain N2.
CRISPR-Cas9 Directed Homology Repair
Homology-directed genome editing using in vitro assembled Cas9, CRISPR RNA (crRNA) and trans-activating crRNA (tracrRNA) ribonucleoprotein complexes was performed as previously described (Paix et al., 2015). Briefly, purified Cas9 and synthetic RNAs were pre-incubated to enable complex formation and injected into the gonad of young adults. Alt-R® CRISPR-Cas9 crRNA targeting the DNA sequences available in the Key Resources Table were obtained from Integrated DNA Technologies (IDT). che-1(luc174) was created by deleting 3,384 bp of the che-1 locus, including most of its coding sequence. lsy-6(luc157) was created by replacing the lsy-6 hairpin sequence with the yfp sequence. lsy-6(luc160) was created by deleting 150 bp downstream of lsy-6, overlapping with the TBX-37/38 binding sites. lsy-6(luc156) was created by replacing the lsy-6 hairpin sequence with yfp and simultaneously introducing the same 150 bp deletion downstream. tbx-37(luc41) and tbx-38(luc54) were created by insertion of a gfp with a flexible linker (4x GGGGS) at the N terminus of tbx-37 or tbx-38. tbx-11(luc144) was created by deleting 1303 bp of the tbx-11 locus, including the whole coding sequence. tbx-43(luc131) was created by deleting 952 bp of the tbx-43 promoter region and part of the first exon.
Mos1-Mediated Single-Copy Insertions (mosSCI)
The published procedure (Frøkjaer-Jensen et al., 2012) was used to generate transgenic worms with targeted single-copy insertions of the hsp-16.41p::vhhGFP4::zif-1::SL2::mCherry::his-11::tbb-2 3’UTR transgene (lucSi100) or hsp-16.41p::zif-1::SL2::mCherry::his-11::tbb-2 3’UTR (lucSi102) transgene at the Chr II landing site ttTi5605 (www.wormbuilder.org). Briefly, a defined Mos1 transposon insertion in Chr II (in an unc-119 mutant background which is paralyzed) is mobilized by transgenic expression of the transposase and the desired insert is provided flanked by homology arms to the excision site to promote homology-directed repair. The inserted cassette also contains a rescuing unc-119 minigene and thus selection of animals with the desired insertion is done based on rescue of the paralysis phenotype. Counterselection for the formation of extrachromosomal arrays is performed using heat-shock inducible peel-1 toxin.
Fosmid Recombineering
Fosmid-based reporters have been generated as previously described (Tursun et al., 2009). Fosmid clones carrying 35-45 kb of genomic sequence (containing the gene of interest) in the pCC1Fos backbone were transformed into SW105 E. coli. A GFP or T2A::GFP::H2B cassette containing a galK minigene for positive selection was amplified while adding 50 bp of homology to the desired insertion site. The linear PCR products were transformed into SW105 E. coli carrying the fosmid of interest and induced for expression of Red recombinase. Bacteria carrying the desired insertion were selected on minimal medium with galactose as sole carbon source. Positive clones were further isolated according to (Tursun et al., 2009) and verified by Sanger sequencing. Primer sequences used to generate all fosmid constructs in this work, as well as sequences, are available upon request. A full list of fosmid-based reporters used in this study can be found in Table S5.
Plasmid Construction
All constructs were generated by standard molecular cloning procedures with restriction digest, PCR, and Gibson assembly or T4 ligation. The coding sequences in the constructs were verified by Sanger sequencing. Plasmid maps and sequences are available upon request. A full list of plasmids used in this study can be found in the Table S5.
Chromatin Immunoprecipitation Sequencing (ChIP-Seq)
ChIP-seq experiments were performed as previously described in (Askjaer et al., 2014) with minor modifications as described below. We performed two biological replicates for TBX-37 and one for TBX-38.
Worm Synchronization and Eggs Extraction
N2, MLC813 (endogenous GFP-TBX-37 and tbx-38 deleted) and MLC893 (endogenous GFP-TBX-38 and tbx-37 deleted) worms were cultivated on at least 10 x 10 cm peptone enriched plates seeded with concentrated HB101 E. coli bacteria. The resulting adult worms were bleached using hypochlorite solution (1% NaOCl; 1 M NaOH) and isolated eggs were washed and incubated overnight in M9 buffer (22 mM KH2PO4; 42 mM Na2HPO4; 86 mM NaCl; 1 mM MgSO4) at 20°C under agitation. Up to 90000 synchronized L1 larvae were incubated at 18°C for ∼71 h on 16 cm peptone enriched plates with concentrated HB101 to obtain gravid adult worms about to lay their first eggs. Worms were collected, washed 3 times with M9 buffer, and bleached with hypochlorite solution to release synchronized eggs at 1–2-cell stage. These embryos were allowed to develop for 90 min at 20°C and then used for the ChIP assay.
Chromatin Immunoprecipitation
Eggs were washed 3 times with M9 and resuspended in 45 mL of M9 and fixed with 2% formaldehyde solution [Sigma; cat #F8775] for 30 min at room temperature under agitation. Fixation was stopped by adding glycine to 125 mM final concentration for 5 min at room temperature, under agitation. Fixed embryos were then washed 2 times with M9 buffer, resuspended in 1 mL PBS, 1 mM PMSF and protease inhibitor solution [Roche; cat #11836153001], transferred to microfuge tube and pelleted at 6000 g for 1 min at room temperature and washed one more time in 1 mL PBS, 1 mM PMSF with protease inhibitor solution. About 100 μL of packed embryos were washed once with 1 mL of FA buffer (50 mM HEPES/KOH pH 7,5; 1 mM EDTA; 1% Triton™ X-100; 0,1% sodium deoxycholate; 150 mM NaCl; 1mM PMSF; 0,1% sarkosyl and protease inhibitor). Embryos were dounced on ice for at least 30 strokes using a glass dounce homogenizer pestle type B. Resulting embryos were transferred to polystyrene 15 mL tubes and sonicated at 4°C for 15 min using a Bioruptor® [Diagenode; cat #B01020001] with the following settings: High, 30 seconds ON, 30 seconds OFF. Chromatin was cleared by centrifugation at top speed (13000-17000 g) for 15 min at 4°C. Cleared chromatin was transferred into a new microcentrifuge tube and 25 μL of the sample was saved to determine quality of DNA shearing. Protein concentration of the cleared chromatin was then determined using Bradford assay [Bio-rad; cat #5000006]; 4 mg of total chromatin was used for each assay. The reaction volume was brought up to 1 mL in FA buffer + 1 mM PMSF, 0,1% sarkosyl and protease inhibitor. 5% of the reaction volume was saved as Input fraction. 1 μg of Anti-GFP antibody [Abcam; cat #ab290] was added to the reaction and incubated at 4°C overnight under rotation. The next day 40 μL of magnetic protein A beads [Thermofisher; cat #10006D] equilibrated with FA buffer were added to the reaction for 2h at 4°C. Beads were subsequently washed 2 times with FA buffer for 5 min, 1 time with FA Buffer + 1 M NaCl for 5 min, transferred to a new microfuge tube and washed with FA-500 mM NaCl (50 mM HEPES/KOH pH 7,5; 1 mM EDTA; 1% Triton X-100; 0.1% sodium deoxycholate; 500 mM NaCl) for 10 min, 1 time with TEL buffer (0.25 M LiCl, 1% NP-40, 1% sodium deoxycholate, 1 mM EDTA, 10 mM Tris-HCl, pH 8.0) for 10 min and finally 2 times with TE for 5 min (10 mM Tris-HCl, 1 mM EDTA).
To elute, 125 μL of ChIP elution buffer (1% SDS; 250 mM NaCl; 10 mM Tris pH 8,0; 1 mM EDTA) was added to the beads and incubated at 65°C for 15 min with vortexing every 5 min. Beads were precipitated at 6000 g for 1 min, the supernatant was kept in a new microfuge tube and elution was repeated a second time before pooling the supernatants together. Input samples were eluted by adding 200 μL of ChIP elution buffer + 2 μg proteinase K and incubated for 1h at 50°C. Samples were reverse-crosslinked by incubation at 65°C overnight. The resulting DNA was purified using DNA Clean and Concentration kit [Zymo research; ref #D4013]. ChIP DNA was eluted in 32 μL and Input DNA in 50 μL.
Library Preparation
DNA concentration was assessed using SYBR™ Green I [ThermoFisher; cat #S7563] and a Fluorospectrophotometer [ThermoFisher; cat #ND-3300]. Libraries were prepared using NEBNext® Ultra™ II DNA Library Prep Kit [New England Biolabs; cat #E7645S] according to the instructions of the manufacturer. The ChIP-seq libraries were sequenced on Hiseq 2500 Sequencing System (Illumina) with single-end 50 nt parameter.
ChIP-seq Data Processing and Analysis
The single-end raw reads of ChIP-seq dataset were mapped to the C. elegans genome (ce11) with bowtie2 (v2.2.4) (Langmead and Salzberg, 2012). Uniquely mapped reads were retained and duplicate reads were marked and discarded using SAMtools (v0.1.18) (Li et al., 2009). For visualization, the coverage tracks (i.e. bigWig files) were generated from bam files using kent-ucsc (v2.79) (Kent et al., 2010). Shaped-base identification was used to call the peaks with MACS2 (v2.1.0) against the input sample with default parameters (Zhang et al., 2008). The identified peaks conserved in two out of three TBX-37 and TBX-38 ChIP-seq replicates were retained. De novo motif discovery was performed using the MEME Suite (v5.1.1) (Machanick and Bailey, 2011). ChIP-seq datasets were deposited in GEO under accession number GSE155392.
Genetically Induced Ectopic TBX-37 Expression
MLC2203 strain, carrying the endogenously tagged lsy-6::yfp allele, a red fluorescent ASE marker (che-1prom::mCherry) and an extrachromosomal transgene for expression of the tbx-37 cDNA under a heat-shock promoter, was grown at 20°C prior to heat-shock experiments. 2-cells embryos were picked with a capillary mouth pipette, following dissection of gravid mothers, and transferred to a plate without food. Embryos were allowed to develop at 20°C for 90 min (first time point – 8 ABa; TBX-37/38 peak of expression), 120 min (16 ABa), 150 min (32 ABa), 180 min (64 ABa), 210 min (128 ABa), 240 min, 270 min or 300 min (final time point – bean stage; prior the birth of the ASEs). The embryos were then heat shocked for 20 min at 30°C to induce ectopic TBX-37 expression and put back at 20°C to continue development. Embryos were mounted after 400 min on a 5% agarose pad on a glass slide and examined for YFP expression in both ASEs using a Zeiss Axio Imager.Z2 with sCMOS camera, Sola SM2 solid state white light excitation system and 63x/1.4 plan-apochromat Oil DIC objective.
Single Molecule Fluorescence In Situ Hybridisation (smFISH)
smFISH was performed as previously described in (Raj et al., 2008) with minor modifications as described in detail below. Probe sets targeting the GFP transcript (GFP sense) and its reverse complement (GFP antisense) were designed using the Stellaris RNA FISH probe designer. The purified Quasar 670-conjugated probes were obtained from Biosearch Technologies. The sequences used for the probe sets design are available here:
GFP Sense
atgagtaaaggagaagaacttttcactggagttgtcccaattcttgttgaattagatggtgatgttaatgggcacaaattttctgtcagtggagagggtgaaggtgatgcaacatacggaaaacttacccttaaatttatttgcactactggaaaactacctgttccatggccaacacttgtcactactttctgttatggtgttcaatgcttctcgagatacccagatcatatgaaacggcatgactttttcaagagtgccatgcccgaaggttatgtacaggaaagaactatatttttcaaagatgacgggaactacaagacacgtgctgaagtcaagtttgaaggtgatacccttgttaatagaatcgagttaaaaggtattgattttaaagaagatggaaacattcttggacacaaattggaatacaactataactcacacaatgtatacatcatggcagacaaacaaaagaatggaatcaaagttaacttcaaaattagacacaacattgaagatggaagcgttcaactagcagaccattatcaacaaaatactccaattggcgatggccctgtccttttaccagacaaccattacctgtccacacaatctgccctttcgaaagatcccaacgaaaagagagaccacatggtccttcttgagtttgtaacagctgctgggattacacatggcatggatgaactatacaaa
GFP Antisense
tttgtatagttcatccatgccatgtgtaatcccagcagctgttacaaactcaagaaggaccatgtggtctctcttttcgttgggatctttcgaaagggcagattgtgtggacaggtaatggttgtctggtaaaaggacagggccatcgccaattggagtattttgttgataatggtctgctagttgaacgcttccatcttcaatgttgtgtctaattttgaagttaactttgattccattcttttgtttgtctgccatgatgtatacattgtgtgagttatagttgtattccaatttgtgtccaagaatgtttccatcttctttaaaatcaataccttttaactcgattctattaacaagggtatcaccttcaaacttgacttcagcacgtgtcttgtagttcccgtcatctttgaaaaatatagttctttcctgtacataaccttcgggcatggcactcttgaaaaagtcatgccgtttcatatgatctgggtatctcgagaagcattgaacaccataacagaaagtagtgacaagtgttggccatggaacaggtagttttccagtagtgcaaataaatttaagggtaagttttccgtatgttgcatcaccttcaccctctccactgacagaaaatttgtgcccattaacatcaccatctaattcaacaagaattgggacaactccagtgaaaagttcttctcctttactcat
Egg Extraction and Fixation
MLC813 [tbx-37(luc41[GFP::flex::TBX-37]) tbx-38(tm581) III], MLC893 [tbx-37(tm314) tbx-38(luc54[GFP::flex::TBX-38]) III], OH8993 [otIs252[lsy-6::YFP (fosmid); rol-6(su1006)] II], OH11115 [otIs386[lsy-6::GFP::Δ150 (fosmid); ttx-3p::mCherry]] and MLC954 [otIs252 II; tbx-37(tm314) tbx-38(tm581) III/qC1[dpy-19(e1259) glp-1(q339) qIs26[lag-2::GFP; rol-6(su1006)]] worms were cultivated on 5 x 10 cm peptone enriched plates seeded with concentrated HB101 E. coli bacteria. Adult worms were bleached using hypochlorite solution and isolated eggs were washed three times in M9 buffer. Eggs were resuspended in 1 mL of fixation buffer (3,7% formaldehyde; 1X PBS), incubated 15 minu at room temperature, vortexed and then immediately submerged in liquid nitrogen for 1 min to freeze crack the eggshell. Eggs were then thawed at room temperature in water, vortexed and fixed on ice for an additional 20 min. Eggs were washed twice in 1 mL of 1X PBS, resuspended in 1 mL of 70% ethanol and permeabilized at 4°C for 48 h.
Hybridization
Following permeabilization, the eggs were washed in 1 mL of Wash Buffer (2X SSC; 5% formamide [Ambion; cat #AM9342]), incubated at room temperature for 5 min before resuspension in 100 μL of Hybridization Buffer (2X SSC; 10% formamide; 10% dextran sulfate [Sigma; cat #D6001]) containing 100 nM of fluorescently labeled RNA probes. Samples were incubated overnight at 37°C, in the dark. The next day, the samples were washed twice in 1 mL of Wash Buffer for 30 min each time (in the dark). The Wash Buffer was then removed and ProLong™ Gold Antifade reagent with DAPI [Invitrogen; cat #P36935] added to the samples.
Genetically Induced Protein Degradation
MLC1219 [lucSi102[hsp-16.41p::zif-1::SL2::mCherry::his-11::tbb-2 3’UTR] otIs252[lsy-6::YFP (fosmid); rol-6(su1006)] II; tbx-37(luc41[GFP::flex::tbx-37]) tbx-38(tm581) III], MLC1300 [lucSi100[hsp-16.41p::vhhGFP4::zif-1::SL2::mCherry::his-11::tbb-2 3’UTR] otIs252 II; tbx-37(luc41) tbx-38(tm581) III], MLC1466 [lucSi100 II; wgIs37[pha-4::TY1::EGFP::3xFLAG; unc-119]] and MLC1467 [lucSi102 II; wgIs37] strains were grown at 25°C prior to heat-shock experiments. 1- or 2-cells embryos were picked with a mouth pipette, following dissection of gravid mothers, and transferred on a plate without food, at 25°C, for 20 min (first time point (t1) – TBX-37/38 expression peak) or 60 min (second time point (t2) – following TBX-37/38 peak of expression). The embryos were heat-shocked at 30°C for 30 min to induce zif-1 or vhhGFP4::zif-1 expression. The plates were kept at 20°C for 8h and embryos were scored for lsy-6::YFP expression in ASEL, as well as for the tbx-37/38(0) phenotype, using a Zeiss Axio Imager.Z2 with sCMOS camera, Sola SM2 solid state white light excitation system and 40x/1.3 EC plan-neofluar Oil DIC objective.
Assay for Transposase-Accessible Chromatin Using Sequencing (ATAC-seq)
ATAC-seq libraries were prepared as described in (Corces et al., 2017) with minor modifications as described below:
Strain Design
To enable the sorting of cells from the ABa and ABp lineage, we generated an integrated array (lucIs39) which allows for the labeling and fluorescence-activated cell sorting (FACS) of the ABa descendants using tbx-37prom::mNeon (Hostettler et al., 2017; Shaner et al., 2013), the EMS lineage by med-2prom::mScarlet-I and the C and D lineages by pal-1prom::mScarlet-I (Bindels et al., 2017). The choice of promoters used for the design of lucIs398 is based on previously reported time-resolved transcriptomic data (Boeck et al., 2016). This leaves cells from the ABp lineage, as well cells from the P lineage (Z2, Z3), unlabeled.
Worm Synchronization and Egg Extraction
MLC1480 [lucIs39[tbx-37p::mNeonGreen::2xNLS::tbx-37 3’UTR; pal-1p::mScarlet-I::2xNLS::tbb-2 3’UTR; med-2p::mScarlet-I::2xNLS::tbb-2 3’UTR]], MLC2239 [otIs252[lsy-6::YFP (fosmid); rol-6(su1006)] II; otIs235[che-1p::mChopti; rol-6(su1006)] V], MLC2309 [lucIs39; lsy-6(luc160[lsy-6::d150]) V] and MLC2310 [otIs232[che-1p::mChopti; rol-6] II; lsy-6(luc160) V] strains were synchronized through two cycles of bleaching as described above. On the first round, worms were cultivated on 150 mm peptone enriched plate seeded with concentrated HB101 E. coli bacteria. Adult worms were bleached using hypochlorite solution and isolated eggs were washed three times in M9 buffer and hatched for 14 h in M9 buffer at 20°C, with gentle agitation. The next day, 75000 starved L1s were plated on 150 mm peptone enriched plates with concentrated HB101 and incubated at 21°C for ∼52h. As the worms reached the stage of young adults, the population was closely monitored and worms were collected when the first 2-4 eggs could be observed in the gonads of ∼30% of the population. Collected worms were washed with ice-cold M9 buffer to remove residual bacteria and bleached with hypochlorite solution to release the eggs used for the ATAC-seq. Collected embryos were washed in egg buffer (25 mM HEPES pH 7,3; 118 mM NaCl; 48 mM KCl; 48 mM KCl; 2 mM CaCl2; 2 mM MgCl2) to remove residual M9 buffer and incubated at 21°C for 90, 200 and 350 min (ABa/ABp experiments) or 350 min (ASEL/R experiment). The developmental stages of embryos were monitored using a Zeiss Axio Imager.Z2 with sCMOS camera, Sola SM2 solid state white light excitation system and 40x/1.3 EC plan-neofluar Oil DIC objective.
Embryo Dissociation and Cell Sorting
The embryo suspension was concentrated to 0,5 mL by centrifugation at 1200 rcf for 1minute. To dissociate the eggshells, 0,5 mL of 2 mg/mL chitinase solution [Sigma; cat #C6137] was added to the embryo suspension and incubated for 25-30 min on ice with periodical shaking. 100 μL of 15 mg/mL pronase solution [Sigma; cat #P6911] was then added to the sample and a 2,5 mL syringe fitted with a 21 g needle was used to dissociate the embryos. The suspension was repeatedly passed through the needle until ∼80% of embryos were dissociated. The digestion reaction was stopped by adding complete L-15 medium (L-15 no phenol red [Gibco; cat #21083027]; 10% FBS; 50 U/mL penicillin; 50 μg/mL streptomycin [Sigma; cat #P4458]) and cell suspension was filtered on 5 μm cell strainer [pluriStrainer; cat #43-10005-40] to remove undissociated embryos or cell aggregates. To label the dead cells, SYTOX AADvanced [Invitrogen; cat #S10349] was added to the sample to a final concentration of 1 μL/mL and the sample was incubated for 5 min, protected from light, and Fluorescent Activated Cell Sorting (FACS) was performed on a Sony SH800, equipped with 488 and 561 nm lasers. Sorting was performed using 100 μm microfluidic chips [Sony, cat #LE-C3210] with standard settings.
Library Preparation
For ATAC-seq library preparation, 15000-25000 cells (for isolated ABa/ABp descendants) or 1000-2500 cells (for sorted ASEL/ASER) were sorted in 300 μl of complete L-15 media and resuspended in cytosol extraction buffer (15 mM Tris-HCl pH 7,5; 5 mM MgCl2; 60 mM KCl; 0,5 mM DTT; 15 mM NaCl; 30 mM sucrose and 1% IGEPAL CA-630). Nuclei were precipitated by centrifugation and washed in ATAC-Resuspension Buffer (10 mM Tris-HCl pH 7,4; 10 mM NaCl, 3 mM MgCl2). DNA was tagmented using the Nextera DNA Library Prep Kit [Illumina; cat #15028212] and purified using the DNA Clean & Concentrator-5 [Zymo Research; cat #D4004]. The libraries were amplified using the NEBNext High Fidelity 2x PCR Master Mix [New England Biolabs; cat #M0541L] and Nextera i7 and i5 adapters [Nextera; cat #20027213] using the following thermocycling protocol: 72°C for 5 min, 98°C for 30 seconds, and several cycles of 98°C for 10 seconds, 63°C for 30 seconds and 1 min at 72°C. The number of PCR cycles required for amplification of individual libraries was determined empirically by real-time monitoring of the amplification profiles of the samples via their relative fluorescence units (RFUs) using EvaGreen [Biotium; cat #31000]. Typically, 10-18 cycles were needed. Libraries were cleaned-up using AMPure XP Beads [Beckman Coulter; cat #A63882].
Sequencing
Sequencing was performed by the Next Generation Sequencing Facility (VBCF) on a HiSeq 2500 system (Illumina) generating paired-end 50 bp reads. Barcoded libraries were pooled based on their measured concentration and sequenced on one lane (∼ 20-40 million reads per sample). Two biological replicates were obtained for each time point.
Data Processing and Analysis
The paired-end raw reads of ATAC-seq data were trimmed with Cutadapt (v1.18) (Martin, 2011) and mapped to the C. elegans genome (ce11) with bowtie2 (v2.2.4) (Langmead and Salzberg, 2012). For all samples, multi-mapped reads were filtered with SAMtools (v0.1.20) (Li et al., 2009) and duplicate reads were marked and discarded with Picard-tools (v2.18.27; Broad Institute). For visualization, the normalized coverage tracks (i.e. bigWig files) were generated from bam files with the R packages GenomicRanges (v1.38.0) (Lawrence et al., 2013) and rtracklayer (v1.46.0) with paired-end option (Lawrence et al., 2009). Shape-based identification was used to call the peaks with MACS2 (v2.1.0) with default parameters (Zhang et al., 2008). The read counts for the pooled peaks were quantified using the Rsubread (v2.0.1) package (Liao et al., 2019) and the read counts per million (cpm) values were calculated as normalized peak signal. The R package ChIPseeker (v1.22.1) was used to assign the peaks to the closest genes (Yu et al., 2015). De novo motif discovery was performed using the MEME Suite (v5.1.1) (Machanick and Bailey, 2011) to uncover motifs specific to the ABa lineage (using the sequences under the peaks detected in the ABa lineage, non-overlapping with peaks from the ABp lineage at 90 min), ASEL-specific motifs (using ASEL peaks non-overlapping with ABa lineage peaks at 350 min) and ASER-specific motifs (using ASER peaks non-overlapping with ABp lineage peaks at 350 min).
ATAC-seq datasets were deposited in GEO under accession number GSE155392.
Heterologous Gal4-UAS Tethering Assays
Strain Design
To test the requirement for transcriptional activation in lsy-6 priming, we generated reporters in which the 150 bp region containing the TBX-37/38 binding sites were replaced by five repeats of the Upstream Activation Sequence (UAS) (lsy-6::gfp::Δtbs::5xUAS reporter), or in which the 5xUAS repeat was inserted downstream of the 150 bp element (lsy-6::gfp::5xUAS), in the context of the lsy-6::GFP fosmid. We then used the tbx-37 promoter to dive expression of the S. kudriavzevii GAL4 DNA binding domain alone (GAL4DBD), fused to four repeats of the VP16 transcriptional activation domain (GAL4DBD-VP64) or fused to the transcriptional repressor Groucho/UNC-37 (GAL4DBD-UNC-37) (Beerli et al., 1998; Chambers et al., 2017; Kaul et al., 2014; Wang et al., 2017a). The GAL4DBD-VP64 fusion was also cloned downstream of a heat-shock promoter for the time-controlled activation experiments.
GAL4DBD-VP64 Heat-Shock Time-Course
MLC2242 [syIs400[hsp-16.42p::2xNLS::GAL4SK::VP64::let-858 3’UTR; unc-122p::RFP] V; lucIs42[lsy-6::GFP::d150::5xUAS; ttx-3p::mCherry]] animals were grown at 20°C prior to heat-shock experiments. 2-cells embryos were collected following dissection of gravid mothers, using a mouth pipette, and transferred on a plate without food. Embryos were allowed to develop at 20°C for 90 min (first time point – 8 ABa; TBX-37/38 peak of expression), 120 min (16 ABa), 150 min (32 ABa), 180 min (64 ABa), 210 min (128 ABa), 240 min, 270 min or 300 min (final time point – bean stage; prior the birth of the ASEs). The embryos were then heat-shocked for 30 min at 30°C to induce GAL4DBD-VP64 expression and put back at 20°C to continue development. The following day, L1 animals were mounted on a glass slide covered with a 5% agarose pad and examined for GFP expression in ASEs using a Zeiss Axio Imager.Z2 with sCMOS camera, Sola SM2 solid state white light excitation system and 40x/1.3 EC plan-neofluar Oil DIC objective.
RNA Interference (RNAi)
RNA interference assays were performed by feeding worms with bacteria expressing dsRNA as described in (Conte et al., 2015). All feeding RNAi experiments with OH8996 were performed at 20° C. E. coli HT115 clones expressing either dsRNA of target transcripts or empty vector control (pL4440) were fed to L4 animals on NGM agar plates containing 25 μg/mL carbenicillin and 1 mM IPTG. Progenies were scored 3 days later for loss of lsy-6 using a Zeiss Axio Imager.Z2 with sCMOS camera with 40x objective by assessing gcy-5p::GFP expression in 1 or 2 ASEs. The majority of the bacterial clones used in the study were obtained for the Ahringer library (Kamath and Ahringer, 2003). A full list of dsRNA expressing clones used in this study is available in the Key Resources Table.
Differential Interference Contrast and Fluorescence Microscopy
A Zeiss Axio Imager.Z2 fitted for DIC optics and with motorized stage, with sCMOS camera and Sola SM2 solid state white light excitation system was used for all scoring and acquisition of single timepoint z-stacks. Images were acquired using ZEN software from Zeiss.
4D Microscropy and Lineage Analysis
2-cell embryos were mounted in water, on microscopy slides with a thin 5% bacto-agar pad. Time-lapsed images were acquired using a Zeiss Axioplan 2 with Normaski/DIC and fluorescence optics. A LED emitting at 470 nm was used for GFP excitation and images were collected with Time to Live software (Caenotec). Lineage analysis was aided by SIMI BioCell software (Schnabel et al., 1997). Blastomere identities, positions, and divisions were followed through embryogenesis to precisely assess spatial and temporal expression onset of GFP reporters.
Confocal Microscopy
Neuropal
NeuroPal images were acquired and analyzed as described in (Yemini et al., 2019). Animals were mounted on 5% bacto-agar pad and immobilized in 50 mM NaN3. Images were acquired using a LSM880 inverted point laser scanning confocal microscope equipped with Airyscan GaSaP detector, plan-apochromat 63x/1.4 oil objective and 405 nm 25 mW, argon 458/488/514 nm 30 mW, DPSS 561 nm 15 mW & HeNe 633 5mW lasers.
Spinning Disk Confocal Microscopy
Animals were mounted on 5% bacto-agar pad and immobilized in 50mM NaN3. Images were acquired using a Visiscope Spinning Disc Confocal (Visitron Systems GmbH, Puching, Germany) with PCO Edge 4.2m sCMOS camera, CFI plan Apo lambda 100x/1.45 oil and 100% power of lasers 488 nm 200 mW and 561 nm 150 mW (300 ms exposure time for both). Z-stack space 0.5 um.
Quantification and Statistical Analysis
Statistical analyses were performed using the SciPy library for Python 3.0 (Virtanen et al., 2020). Differences in proportions were determined using the chi-squared test. Statistical details of the experiments can be found in the figure legends. All data are expressed as the proportion ± standard error of proportion (SEP).
Acknowledgments
We thank Alexander Stark, Oliver Hobert, Angela Andersen (Life Science Editors), and the members of the Cochella Lab for feedback. Also, Sevinç Ercan for ChIP protocols, Paul Sternberg’s lab for GAL4-UAS reagents, Manuel Zimmer’s lab for neuronal markers, and WormBase. Some strains were provided by the CGC, funded by NIH Office of Research Infrastructure Programs (P40 OD010440).
Funding: This work was supported by an FP7/2007-2013 grant from the European Research Council to L.C. (ERC-StG-337161) and grants from the Austrian Science Fund (SFB-F43-23 and P 32636-B). J.C. received a scholarship from the Fonds de Recherche du Québec–Santé (35049). Basic research at IMP is supported by Boehringer Ingelheim GmbH.
Author Contributions
J.C., T.D., and L.C. designed experiments, wrote the manuscript, and made figures with contributions from other authors. J.C. and T.D. conducted most of the experiments. A.M. and A.B. did the ATAC-seq experiments. J.W. did the bioinformatic analyses. M.A. generated strains and performed NeuroPal imaging. J.R. generated strains and conducted time course experiments. P.G.-P. carried out confocal microscopy.
Declaration of Interests
The authors declare no competing interests.
Published: September 30, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.devcel.2020.09.002.
Supplemental Information
References
- Ahier A., Jarriault S. Simultaneous expression of multiple proteins under a single promoter in Caenorhabditis elegans via a versatile 2A-based toolkit. Genetics. 2014;196:605–613. doi: 10.1534/genetics.113.160846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alaynick W.A., Jessell T.M., Pfaff S.L. SnapShot: spinal cord development. Cell. 2011;146:178–178.e1. doi: 10.1016/j.cell.2011.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan D.W., Thor S. Transcriptional selectors, masters, and combinatorial codes: regulatory principles of neural subtype specification. Wiley Interdiscip. Rev. Dev. Biol. 2015;4:505–528. doi: 10.1002/wdev.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askjaer P., Ercan S., Meister P. WormBook: the online review of C. elegans biology. 2014. Modern techniques for the analysis of chromatin and nuclear organization in C. elegans; pp. 1–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beerli R.R., Segal D.J., Dreier B., Barbas C.F. Toward controlling gene expression at will: specific regulation of the erbB-2/HER-2 promoter by using polydactyl zinc finger proteins constructed from modular building blocks. Proc. Natl. Acad. Sci. USA. 1998;95:14628–14633. doi: 10.1073/pnas.95.25.14628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsland M., Ramsköld D., Zaouter C., Klum S., Sandberg R., Muhr J. Sequentially acting Sox transcription factors in neural lineage development. Genes Dev. 2011;25:2453–2464. doi: 10.1101/gad.176008.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindels D.S., Haarbosch L., van Weeren L., Postma M., Wiese K.E., Mastop M., Aumonier S., Gotthard G., Royant A., Hink M.A., Gadella T.W.J., Jr. mScarlet: a bright monomeric red fluorescent protein for cellular imaging. Nat Methods. 2017;14:53–56. doi: 10.1038/nmeth.4074. [DOI] [PubMed] [Google Scholar]
- Boeck M.E., Huynh C., Gevirtzman L., Thompson O.A., Wang G., Kasper D.M., Reinke V., Hillier L.W., Waterston R.H. The time-resolved transcriptome of C. elegans. Genome Res. 2016;26:1441–1450. doi: 10.1101/gr.202663.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers M., Turki-Judeh W., Kim M.W., Chen K., Gallaher S.D., Courey A.J. Mechanisms of Groucho-mediated repression revealed by genome-wide analysis of Groucho binding and activity. BMC Genomics. 2017;18:215. doi: 10.1186/s12864-017-3589-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan M.M., Smith Z.D., Grosswendt S., Kretzmer H., Norman T.M., Adamson B., Jost M., Quinn J.J., Yang D., Jones M.G. Molecular recording of mammalian embryogenesis. Nature. 2019;570:77–82. doi: 10.1038/s41586-019-1184-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charney R.M., Forouzmand E., Cho J.S., Cheung J., Paraiso K.D., Yasuoka Y., Takahashi S., Taira M., Blitz I.L., Xie X. Foxh1 occupies cis-regulatory modules prior to dynamic transcription factor interactions controlling the mesendoderm gene program. Dev. Cell. 2017;40:595–607.e4. doi: 10.1016/j.devcel.2017.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochella L., Hobert O. Embryonic priming of a miRNA locus predetermines postmitotic neuronal left/right asymmetry in C. elegans. Cell. 2012;151:1229–1242. doi: 10.1016/j.cell.2012.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte D., Jr., MacNeil L.T., Walhout A.J.M., Mello C.C. RNA interference in Caenorhabditis elegans. Curr. Protoc. Mol. Biol. 2015;109:26.3.1–26.3.30. doi: 10.1002/0471142727.mb2603s109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corces M.R., Trevino A.E., Hamilton E.G., Greenside P.G., Sinnott-Armstrong N.A., Vesuna S., Satpathy A.T., Rubin A.J., Montine K.S., Wu B. An improved ATAC-seq protocol reduces background and enables interrogation of frozen tissues. Nat. Methods. 2017;14:959–962. doi: 10.1038/nmeth.4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creyghton M.P., Cheng A.W., Welstead G.G., Kooistra T., Carey B.W., Steine E.J., Hanna J., Lodato M.A., Frampton G.M., Sharp P.A. Histone H3K27ac separates active from poised enhancers and predicts developmental state. Proc. Natl. Acad. Sci. USA. 2010;107:21931–21936. doi: 10.1073/pnas.1016071107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupin E., Calloni G.W., Coelho-Aguiar J.M., Le Douarin N.M. The issue of the multipotency of the neural crest cells. Dev. Biol. 2018;444:S47–S59. doi: 10.1016/j.ydbio.2018.03.024. [DOI] [PubMed] [Google Scholar]
- Etchberger J.F., Flowers E.B., Poole R.J., Bashllari E., Hobert O. Cis-regulatory mechanisms of left/right asymmetric neuron-subtype specification in C. elegans. Development. 2009;136:147–160. doi: 10.1242/dev.030064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchberger J.F., Lorch A., Sleumer M.C., Zapf R., Jones S.J., Marra M.A., Holt R.A., Moerman D.G., Hobert O. The molecular signature and cis-regulatory architecture of a C. elegans gustatory neuron. Genes Dev. 2007;21:1653–1674. doi: 10.1101/gad.1560107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falo-Sanjuan J., Lammers N.C., Garcia H.G., Bray S.J. Enhancer priming enables fast and sustained transcriptional responses to Notch signaling. Dev. Cell. 2019;50:411–425.e8. doi: 10.1016/j.devcel.2019.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez Garcia M., Moore C.D., Schulz K.N., Alberto O., Donague G., Harrison M.M., Zhu H., Zaret K.S. Structural features of transcription factors associating with nucleosome binding. Mol. Cell. 2019;75:921–932.e6. doi: 10.1016/j.molcel.2019.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frøkjaer-Jensen C., Davis M.W., Ailion M., Jorgensen E.M. Improved Mos1-mediated transgenesis in C. elegans. Nat. Methods. 2012;9:117–118. doi: 10.1038/nmeth.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudet J., Mango S.E. Regulation of organogenesis by the Caenorhabditis elegans FoxA protein PHA-4. Science. 2002;295:821–825. doi: 10.1126/science.1065175. [DOI] [PubMed] [Google Scholar]
- Ghavi-Helm Y., Klein F.A., Pakozdi T., Ciglar L., Noordermeer D., Huber W., Furlong E.E.M. Enhancer loops appear stable during development and are associated with paused polymerase. Nature. 2014;512:96–100. doi: 10.1038/nature13417. [DOI] [PubMed] [Google Scholar]
- Ginsburg D.S., Anlembom T.E., Wang J., Patel S.R., Li B., Hinnebusch A.G. NuA4 links methylation of histone H3 lysines 4 and 36 to acetylation of histones H4 and H3. J. Biol. Chem. 2014;289:32656–32670. doi: 10.1074/jbc.M114.585588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good K., Ciosk R., Nance J., Neves A., Hill R.J., Priess J.R. The T-box transcription factors TBX-37 and TBX-38 link GLP-1/Notch signaling to mesoderm induction in C. elegans embryos. Development. 2004;131:1967–1978. doi: 10.1242/dev.01088. [DOI] [PubMed] [Google Scholar]
- Greenberg M.V.C., Glaser J., Borsos M., Marjou F.E., Walter M., Teissandier A., Bourc'his D. Transient transcription in the early embryo sets an epigenetic state that programs postnatal growth. Nat. Genet. 2017;49:110–118. doi: 10.1038/ng.3718. [DOI] [PubMed] [Google Scholar]
- Greenstein R.A., Barrales R.R., Sanchez N.A., Bisanz J.E., Braun S., Al-Sady B. Set1/COMPASS repels heterochromatin invasion at euchromatic sites by disrupting Suv39/Clr4 activity and nucleosome stability. Genes Dev. 2020;34:99–117. doi: 10.1101/gad.328468.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer E.L., Blanco M.A., Gu L., Sendinc E., Liu J., Aristizábal-Corrales D., Hsu C.H., Aravind L., He C., Shi Y. DNA methylation on N6-adenine in C. elegans. Cell. 2015;161:868–878. doi: 10.1016/j.cell.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gualdi R., Bossard P., Zheng M., Hamada Y., Coleman J.R., Zaret K.S. Hepatic specification of the gut endoderm in vitro: cell signaling and transcriptional control. Genes Dev. 1996;10:1670–1682. doi: 10.1101/gad.10.13.1670. [DOI] [PubMed] [Google Scholar]
- Hobert O. Regulatory logic of neuronal diversity: terminal selector genes and selector motifs. Proc. Natl. Acad. Sci. USA. 2008;105:20067–20071. doi: 10.1073/pnas.0806070105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobert O. Development of left/right asymmetry in the Caenorhabditis elegans nervous system: from zygote to postmitotic neuron. Genesis. 2014;52:528–543. doi: 10.1002/dvg.22747. [DOI] [PubMed] [Google Scholar]
- Hobert O. Terminal selectors of neuronal identity. Curr. Top. Dev. Biol. 2016;116:455–475. doi: 10.1016/bs.ctdb.2015.12.007. [DOI] [PubMed] [Google Scholar]
- Hostettler L., Grundy L., Käser-Pébernard S., Wicky C., Schafer W.R., Glauser D.A. The bright fluorescent protein mNeonGreen facilitates protein expression analysis in vivo. G3 (Bethesda) 2017;7:607–615. doi: 10.1534/g3.116.038133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu H.T., Chen H.M., Yang Z., Wang J., Lee N.K., Burger A., Zaret K., Liu T., Levine E., Mango S.E. Transcription. Recruitment of RNA polymerase II by the pioneer transcription factor PHA-4. Science. 2015;348:1372–1376. doi: 10.1126/science.aab1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwafuchi-Doi M., Zaret K.S. Cell fate control by pioneer transcription factors. Development. 2016;143:1833–1837. doi: 10.1242/dev.133900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R.J., Hobert O. A novel C. elegans zinc finger transcription factor, lsy-2, required for the cell type-specific expression of the lsy-6 microRNA. Development. 2005;132:5451–5460. doi: 10.1242/dev.02163. [DOI] [PubMed] [Google Scholar]
- Johnston R.J., Jr., Hobert O. A microRNA controlling left/right neuronal asymmetry in Caenorhabditis elegans. Nature. 2003;426:845–849. doi: 10.1038/nature02255. [DOI] [PubMed] [Google Scholar]
- Kamath R.S., Ahringer J. Genome-wide RNAi screening in Caenorhabditis elegans. Methods. 2003;30:313–321. doi: 10.1016/s1046-2023(03)00050-1. [DOI] [PubMed] [Google Scholar]
- Kaul A., Schuster E., Jennings B.H. The Groucho co-repressor is primarily recruited to local target sites in active chromatin to attenuate transcription. PLoS Genet. 2014;10:e1004595. doi: 10.1371/journal.pgen.1004595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent D.M., Rothwell P.M., Ioannidis J.P., Altman D.G., Hayward R.A. Assessing and reporting heterogeneity in treatment effects in clinical trials: a proposal. Trials. 2010;11:85. doi: 10.1186/1745-6215-11-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstantinides N., Kapuralin K., Fadil C., Barboza L., Satija R., Desplan C. Phenotypic convergence: distinct transcription factors regulate common terminal features. Cell. 2018;174:622–635.e13. doi: 10.1016/j.cell.2018.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kueh H.Y., Yui M.A., Ng K.K.H., Pease S.S., Zhang J.A., Damle S.S., Freedman G., Siu S., Bernstein I.D., Elowitz M.B., Rothenberg E.V. Asynchronous combinatorial action of four regulatory factors activates Bcl11b for T cell commitment. Nat. Immunol. 2016;17:956–965. doi: 10.1038/ni.3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon G.S., Viotti M., Hadjantonakis A.K. The endoderm of the mouse embryo arises by dynamic widespread intercalation of embryonic and extraembryonic lineages. Dev. Cell. 2008;15:509–520. doi: 10.1016/j.devcel.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B., Salzberg S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence M., Huber W., Pagès H., Aboyoun P., Carlson M., Gentleman R., Morgan M.T., Carey V.J. Software for computing and annotating genomic ranges. PLoS Comput. Biol. 2013;9:e1003118. doi: 10.1371/journal.pcbi.1003118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence M.F., Gentleman R., Carey V.J. rtracklayer: an R package for interfacing with genome browsers. Bioinformatics. 2009;25:1841–1842. doi: 10.1093/bioinformatics/btp328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Lee B., Joshi K., Pfaff S.L., Lee J.W., Lee S.K. A regulatory network to segregate the identity of neuronal subtypes. Dev. Cell. 2008;14:877–889. doi: 10.1016/j.devcel.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyva-Díaz E., Hobert O. Transcription factor autoregulation is required for acquisition and maintenance of neuronal identity. Development. 2019;146:dev177378. doi: 10.1242/dev.177378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Handsaker B., Wysoker A., Fennell T., Ruan J., Homer N., Marth G., Abecasis G., Durbin R., 1000 Genome Project Data Processing Subgroup The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y., Smyth G.K., Shi W. The R package Rsubread is easier, faster, cheaper and better for alignment and quantification of RNA sequencing reads. Nucleic Acids Res. 2019;47:e47. doi: 10.1093/nar/gkz114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Chen W., Li W., Li Y., Priest J.R., Zhou B., Wang J., Zhou Z. Single-cell RNA-seq of the developing cardiac outflow tract reveals convergent development of the vascular smooth muscle cells. Cell Rep. 2019;28:1346–1361.e4. doi: 10.1016/j.celrep.2019.06.092. [DOI] [PubMed] [Google Scholar]
- Long H.K., Prescott S.L., Wysocka J. Ever-changing landscapes: transcriptional enhancers in development and evolution. Cell. 2016;167:1170–1187. doi: 10.1016/j.cell.2016.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machanick P., Bailey T.L. MEME-ChIP: motif analysis of large DNA datasets. Bioinformatics. 2011;27:1696–1697. doi: 10.1093/bioinformatics/btr189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011;17:10–12. [Google Scholar]
- Mello C., Fire A. DNA transformation. Methods Cell Biol. 1995;48:451–482. [PubMed] [Google Scholar]
- Narasimhan K., Lambert S.A., Yang A.W.H., Riddell J., Mnaimneh S., Zheng H., Albu M., Najafabadi H.S., Reece-Hoyes J.S., Fuxman Bass J.I. Mapping and analysis of Caenorhabditis elegans transcription factor sequence specificities. eLife. 2015;4:e06967. doi: 10.7554/eLife.06967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng H.H., Robert F., Young R.A., Struhl K. Targeted recruitment of Set1 histone methylase by elongating Pol II provides a localized mark and memory of recent transcriptional activity. Mol. Cell. 2003;11:709–719. doi: 10.1016/s1097-2765(03)00092-3. [DOI] [PubMed] [Google Scholar]
- Ng K.K., Yui M.A., Mehta A., Siu S., Irwin B., Pease S., Hirose S., Elowitz M.B., Rothenberg E.V., Kueh H.Y. A stochastic epigenetic switch controls the dynamics of T-cell lineage commitment. eLife. 2018;7:e37851. doi: 10.7554/eLife.37851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma K.-I., Grewal S.I.S. Histone H3 lysine 4 methylation is mediated by Set1 and promotes maintenance of active chromatin states in fission yeast. Proc. Natl. Acad. Sci. USA. 2002;99:16438–16445. doi: 10.1073/pnas.182436399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okkema P.G. The remarkably diverse family of T-box factors in Caenorhabditis elegans. Curr. Top. Dev. Biol. 2017;122:27–54. doi: 10.1016/bs.ctdb.2016.08.005. [DOI] [PubMed] [Google Scholar]
- O'Meara M.M., Zhang F., Hobert O. Maintenance of neuronal laterality in Caenorhabditis elegans through MYST histone acetyltransferase complex components LSY-12, LSY-13 and LIN-49. Genetics. 2010;186:1497–1502. doi: 10.1534/genetics.110.123661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paix A., Folkmann A., Rasoloson D., Seydoux G. High efficiency, homology-directed genome editing in Caenorhabditis elegans using CRISPR-Cas9 ribonucleoprotein complexes. Genetics. 2015;201:47–54. doi: 10.1534/genetics.115.179382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira L., Kratsios P.P., Serrano-Saiz E., Sheftel H., Mayo A.E., Hall D.H., White J.G., LeBoeuf B., Garcia L.R., Alon U., Hobert O. A cellular and regulatory map of the cholinergic nervous system of C. elegans. eLife. 2015;4:e12432. doi: 10.7554/eLife.12432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce-Shimomura J.T., Faumont S., Gaston M.R., Pearson B.J., Lockery S.R. The homeobox gene lim-6 is required for distinct chemosensory representations in C. elegans. Nature. 2001;410:694–698. doi: 10.1038/35070575. [DOI] [PubMed] [Google Scholar]
- Poole R.J., Bashllari E., Cochella L., Flowers E.B., Hobert O. A genome-wide RNAi screen for factors involved in neuronal specification in Caenorhabditis elegans. PLoS Genet. 2011;7:e1002109. doi: 10.1371/journal.pgen.1002109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole R.J., Hobert O. Early embryonic programming of neuronal left/right asymmetry in C. elegans. Curr. Biol. 2006;16:2279–2292. doi: 10.1016/j.cub.2006.09.041. [DOI] [PubMed] [Google Scholar]
- Rada-Iglesias A., Bajpai R., Swigut T., Brugmann S.A., Flynn R.A., Wysocka J. A unique chromatin signature uncovers early developmental enhancers in humans. Nature. 2011;470:279–283. doi: 10.1038/nature09692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj A., van den Bogaard P., Rifkin S.A., van Oudenaarden A., Tyagi S. Imaging individual mRNA molecules using multiple singly labeled probes. Nat. Methods. 2008;5:877–879. doi: 10.1038/nmeth.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj B., Wagner D.E., McKenna A., Pandey S., Klein A.M., Shendure J., Gagnon J.A., Schier A.F. Simultaneous single-cell profiling of lineages and cell types in the vertebrate brain. Nat. Biotechnol. 2018;36:442–450. doi: 10.1038/nbt.4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter F., Wienerroither S., Stark A. Combinatorial function of transcription factors and cofactors. Curr. Opin. Genet. Dev. 2017;43:73–81. doi: 10.1016/j.gde.2016.12.007. [DOI] [PubMed] [Google Scholar]
- Rubin A.J., Barajas B.C., Furlan-Magaril M., Lopez-Pajares V., Mumbach M.R., Howard I., Kim D.S., Boxer L.D., Cairns J., Spivakov M. Lineage-specific dynamic and pre-established enhancer-promoter contacts cooperate in terminal differentiation. Nat. Genet. 2017;49:1522–1528. doi: 10.1038/ng.3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarin S., Antonio C., Tursun B., Hobert O. The C. elegans Tailless/TLX transcription factor nhr-67 controls neuronal identity and left/right asymmetric fate diversification. Development. 2009;136:2933–2944. doi: 10.1242/dev.040204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarin S., O'Meara M.M., Flowers E.B., Antonio C., Poole R.J., Didiano D., Johnston R.J., Chang S., Narula S., Hobert O. Genetic screens for Caenorhabditis elegans mutants defective in left/right asymmetric neuronal fate specification. Genetics. 2007;176:2109–2130. doi: 10.1534/genetics.107.075648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartorelli V., Puri P.L. Shaping gene expression by landscaping chromatin architecture: lessons from a master. Mol. Cell. 2018;71:375–388. doi: 10.1016/j.molcel.2018.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnabel R., Hutter H., Moerman D., Schnabel H. Assessing normal embryogenesis in Caenorhabditis elegans using a 4D microscope: variability of development and regional specification. Dev. Biol. 1997;184:234–265. doi: 10.1006/dbio.1997.8509. [DOI] [PubMed] [Google Scholar]
- Serrano-Saiz E., Poole R.J., Felton T., Zhang F., De La Cruz E.D., Hobert O. Modular control of glutamatergic neuronal identity in C. elegans by distinct homeodomain proteins. Cell. 2013;155:659–673. doi: 10.1016/j.cell.2013.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaner N.C., Lambert G.G., Chammas A., Ni Y., Cranfill P.J., Baird M.A., Sell B.R., Allen J.R., Day R.N., Israelsson M. A bright monomeric green fluorescent protein derived from Branchiostoma lanceolatum. Nat. Methods. 2013;10:407–409. doi: 10.1038/nmeth.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soufi A., Garcia M.F., Jaroszewicz A., Osman N., Pellegrini M., Zaret K.S. Pioneer transcription factors target partial DNA motifs on nucleosomes to initiate reprogramming. Cell. 2015;161:555–568. doi: 10.1016/j.cell.2015.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitz F., Furlong E.E.M. Transcription factors: from enhancer binding to developmental control. Nat. Rev. Genet. 2012;13:613–626. doi: 10.1038/nrg3207. [DOI] [PubMed] [Google Scholar]
- Sulston J.E., Schierenberg E., White J.G., Thomson J.N. The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev. Biol. 1983;100:64–119. doi: 10.1016/0012-1606(83)90201-4. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Thiele T.R., Faumont S., Ezcurra M., Lockery S.R., Schafer W.R. Functional asymmetry in Caenorhabditis elegans taste neurons and its computational role in chemotaxis. Nature. 2008;454:114–117. doi: 10.1038/nature06927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taverna S.D., Ilin S., Rogers R.S., Tanny J.C., Lavender H., Li H., Baker L., Boyle J., Blair L.P., Chait B.T. Yng1 PHD finger binding to H3 trimethylated at K4 promotes NuA3 HAT activity at K14 of H3 and transcription at a subset of targeted ORFs. Mol. Cell. 2006;24:785–796. doi: 10.1016/j.molcel.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tursun B., Cochella L., Carrera I., Hobert O. A toolkit and robust pipeline for the generation of fosmid-based reporter genes in C. elegans. PLoS One. 2009;4:e4625. doi: 10.1371/journal.pone.0004625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida O., Nakano H., Koga M., Ohshima Y. The C. elegans che-1 gene encodes a zinc finger transcription factor required for specification of the ASE chemosensory neurons. Development. 2003;130:1215–1224. doi: 10.1242/dev.00341. [DOI] [PubMed] [Google Scholar]
- Venkatesh S., Workman J.L. Histone exchange, chromatin structure and the regulation of transcription. Nat. Rev. Mol. Cell Biol. 2015;16:178–189. doi: 10.1038/nrm3941. [DOI] [PubMed] [Google Scholar]
- Virtanen P., Gommers R., Oliphant T.E., Haberland M., Reddy T., Cournapeau D., Burovski E., Peterson P., Weckesser W., Bright J. SciPy 1.0: fundamental algorithms for scientific computing in Python. Nat. Methods. 2020;17:261–272. doi: 10.1038/s41592-019-0686-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D.E., Weinreb C., Collins Z.M., Briggs J.A., Megason S.G., Klein A.M. Single-cell mapping of gene expression landscapes and lineage in the zebrafish embryo. Science. 2018;360:981–987. doi: 10.1126/science.aar4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Liu J., Gharib S., Chai C.M., Schwarz E.M., Pokala N., Sternberg P.W. cGAL, a temperature-robust GAL4-UAS system for Caenorhabditis elegans. Nat. Methods. 2017;14:145–148. doi: 10.1038/nmeth.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Tang N.H., Lara-Gonzalez P., Zhao Z., Cheerambathur D.K., Prevo B., Chisholm A.D., Desai A., Oegema K. A toolkit for GFP-mediated tissue-specific protein degradation in C. elegans. Development. 2017;144:2694–2701. doi: 10.1242/dev.150094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenick A.S., Hobert O. Genomic cis-regulatory architecture and trans-acting regulators of a single interneuron-specific gene battery in C. elegans. Dev. Cell. 2004;6:757–770. doi: 10.1016/j.devcel.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Xue D., Tu Y., Chalfie M. Cooperative interactions between the Caenorhabditis elegans homeoproteins UNC-86 and MEC-3. Science. 1993;261:1324–1328. doi: 10.1126/science.8103239. [DOI] [PubMed] [Google Scholar]
- Yemini E., Lin A., Nejatbakhsh A., Varol E., Sun R., Mena G.E., Samuel A.D.T., Paninski L., Venkatachalam V., Hobert O. NeuroPAL: a neuronal polychromatic atlas of landmarks for whole-brain imaging in C. elegans. bioRxiv. 2019 doi: 10.1101/676312. [DOI] [Google Scholar]
- Yu G., Wang L.G., He Q.Y. ChIPseeker: an R/Bioconductor package for ChIP peak annotation, comparison and visualization. Bioinformatics. 2015;31:2382–2383. doi: 10.1093/bioinformatics/btv145. [DOI] [PubMed] [Google Scholar]
- Zaret K.S., Carroll J.S. Pioneer transcription factors: establishing competence for gene expression. Genes Dev. 2011;25:2227–2241. doi: 10.1101/gad.176826.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaret K.S., Mango S.E. Pioneer transcription factors, chromatin dynamics, and cell fate control. Curr. Opin. Genet. Dev. 2016;37:76–81. doi: 10.1016/j.gde.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F., Bhattacharya A., Nelson J.C., Abe N., Gordon P., Lloret-Fernandez C., Maicas M., Flames N., Mann R.S., Colón-Ramos D.A., Hobert O. The LIM and POU homeobox genes ttx-3 and unc-86 act as terminal selectors in distinct cholinergic and serotonergic neuron types. Development. 2014;141:422–435. doi: 10.1242/dev.099721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Liu T., Meyer C.A., Eeckhoute J., Johnson D.S., Bernstein B.E., Nusbaum C., Myers R.M., Brown M., Li W., Liu X.S. Model-based analysis of ChIP-seq (MACS) Genome Biol. 2008;9:R137. doi: 10.1186/gb-2008-9-9-r137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu F., Farnung L., Kaasinen E., Sahu B., Yin Y., Wei B., Dodonova S.O., Nitta K.R., Morgunova E., Taipale M. The interaction landscape between transcription factors and the nucleosome. Nature. 2018;562:76–81. doi: 10.1038/s41586-018-0549-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All datasets (ChIP-seq and ATAC-seq) generated in this study are available under GEO accession number GSE155392. This study did not generate code.