Summary.
Therapy with fresh frozen plasma (FFP) confers serious risks, such as contraction of bloodborne viruses, allergic reaction, volume overload and development of alloantibodies. The aim of this study was to apply principles of pharmacokinetic (PK) modelling to individual factor content of FFP to optimize individualized dosing, while minimizing potential risks of therapy. We used PK modelling to successfully target individual factor replacement in an 8-month-old patient receiving FFP for treatment of a severe congenital factor V (FV) deficiency. The model fit for the FV activity vs. time data was excellent (r = 0.98) and the model accurately predicted FV activity during the intraoperative and postoperative period. Accurate PK modelling of individual factor activity in FFP has the potential to provide better targeted therapy, enabling clinicians to more precisely dose patients requiring coagulation products, while avoiding wasteful and expensive product overtreatment, minimizing potentially life-threatening complications due to undertreatment and limiting harmful product-associated risks.
Keywords: factor V, FFP, pharmacokinetic modelling, pk
Introduction
Inherited deficiencies in proteins involved in the coagulation cascade lead to lifelong bleeding disorders, the severity of which is usually inversely proportional to the factor level [1]. The prevention of life-threatening bleeding episodes depends on administration or replacement of the deficient factor or protein, as individual factor concentrate (such as factor VIII and IX for Haemophilia A and B respectively), fresh frozen plasma (FFP) or cryoprecipitate [2].
Over the last decade, the introduction of recombinant concentrates of factor VIII and factor IX, has significantly improved treatment options for haemophilia A and B; however, guidelines for treatment and factor replacement for bleeding episodes associated with rare inherited coagulation disorders (i.e. deficiencies in coagulation factors other than FVIII and FIX), are not well established [1,2]. Furthermore, specific replacement products, or single-factor concentrates, are currently not licensed or commercially unavailable in the US for many of the rare coagulation disorders. Accordingly, FFP remains the mainstay of treatment for most rare inherited coagulation disorders [3–5].
Rare inherited coagulation disorders (RICDs), are a collection of recessively inherited abnormalities in haemostasis that account for up to 15% of all inherited bleeding disorders [2], and include deficiencies in fibrinogen, prothrombin and factors V, VII, V + VIII, X, XI and XIII [1,2,6]. The prevalence of RICDs in the general population varies between 1 in 500 000 to 1 in 2 000 000 [1–3]. In contrast with the more prevalent bleeding disorders, such as classic haemophilia A and B, little progress has been made in the treatment of RICDs, partly due to the lack of pharmacokinetic (PK) studies [3,7]. Although some PK data are available for concentrates of FVIII and FIX [8–11], to our knowledge, the PKs of other coagulation factors contained in FFP have not been thoroughly investigated. Use of targeted PK dosing would enable clinicians to more precisely dose patients requiring coagulation products, while avoiding wasteful and expensive overtreatment, minimizing potentially life-threatening undertreatment and limiting harmful product-associated risks.
Congenital FV deficiency is an example of a rare inherited coagulation disorder. Since factor V plays a key role in haemostasis, yet factor V concentrates are not available, treatment of FV deficiency relies on factor repletion with FFP [12]. Unfortunately, FFP therapy confers serious risks and complications, including allergic reaction, development of alloantibodies, volume overload and contraction of bloodborne viruses [3,12]. Accurate PK modelling of individual factor activity in FFP has the potential to provide better targeted therapy and to significantly reduce these risks.
Methods
Pharmacokinetic modelling was used to target individual factor replacement in an 8-month-old male who was receiving FFP for treatment of a severe congenital FV deficiency [FV activity <1% (IU dL−1)], and who had had two intracranial haemorrhages, including one while on recombinant factor VIIa prophylactic dosing. The PK was done as part of patient care; therefore, consent for the levels was not obtained. Consent was subsequently obtained to report the findings.
The patient was listed to undergo orthotopic liver transplantation to correct deficient hepatic production of FV, and FFP was being used both for prophylaxis to prevent recurrent haemorrhage, and to control bleeding during transplant. After a single 15 mg kg−1 dose of FFP, given over 25 min by a constant rate intravenous infusion, plasma factor V activity was assessed at 0.25, 0.5, 1, 2, 4, 6, 12, 18, 24, 36 and 48 h after the conclusion of the FFP infusion. Factor V activity was measured using both the Factor V Activity assay (Siemens BCS coagulation analyzer, Siemens Thromboplastin C reagent; available from the Blood Center of Wisconsin, Milwaukee, WI, USA; www.bcw.edu) and the Factor V Assay (Stago STA-R Evolution XP coagulation analyzer, Stago Deficient V plasma reagent; available from the Children’s Mercy Hospital, Kansas City, MO, USA; www.childrensmercy.org/lab). The limits of quantitation were 1 to 1000 IU dL and 7% to 98% activity level respectively. Samples were run in duplicate in two different laboratories to ensure accuracy. Factor V activity vs. time data from a single laboratory (the Blood Center of Wisconsin) was used for PK modelling, as this assay provided more accurate information at the lower limits of factor activity.
The information obtained from the PK study, completed prior to transplant, was used to determine FFP dosing during the transplant. Plasma factor V activity vs. time data were curve fit using a one compartment open model with reciprocal (1 y−2) weighting, using algorithms nested in a validated PK analysis software package (WinNonlin 5.2.1, Pharsight Corp., Mountain View, CA, USA). Polyexponential parameter estimates required for simulation of dosing regimens were selected based on established goodness-of-fit criteria (e.g. sum of squared residuals, Akaike Information Criteria). Simulation, likewise, utilized a one compartment open model to explore dosing regimens that would achieve FV target activity levels of ≥20% (IU dL−1).
In performing FV PK modelling, a number of assumptions were made. These included linearity in the dose –response relationship over a dynamic range of FV activity values [i.e. 1–30% (IU dL−1)] and that polyexponential parameter estimates derived from the experimental data are first order. As well, we assumed that the FV activity profile observed on the patient visit used to determine PKs is reflective of what would be anticipated for subsequent factor V administrations.
Results
Results of the PK modelling for FV activity in FFP (Table 1) revealed a peak plasma concentration (Cmax) of 16% (IU dL−1), a plasma clearance (CL) of 0.05 ml kg−1 h−1 and an apparent half-life (t½) of 13.5 h for off-set in FV activity, a value consistent with ranges previously reported in the literature [2,4,13].
Table 1.
Pharmacokinetic values obtained for FV activity following a single bolus dose of 15 mg kg−1 of FFP.
| Parameter | Value ± SE |
|---|---|
| AUC (IU dL−1) | 310 ± 22.6 |
| t½ (h) | 13.5 ± 1.18 |
| Cmax (IU dL−1) | 16 ± 0.78 |
| CL (mL kg−1 h−1) | 0.05 ± 0.003 |
| AUMC (IU dL−1) | 6039 ± 926 |
| MRT (h) | 19.5 ± 1.7 |
| Vss (mL kg−1) | 0.9 ± 0.05 |
AUC, area under the curve; Cmax, maximum plasma concentration; CL, clearance; AUMC, area under the first moment curve; MRT, mean residence time; Vss, volume of distribution at steady state.
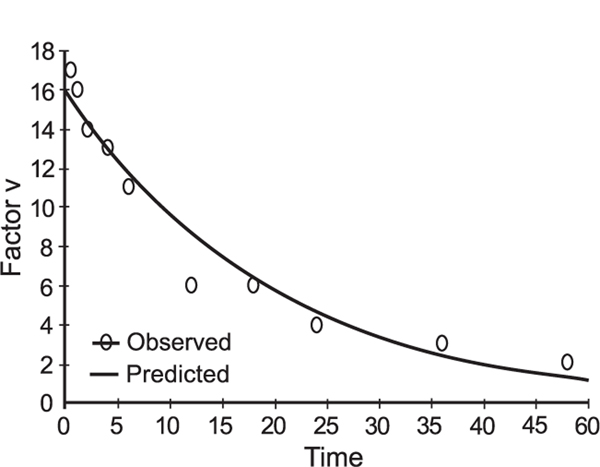
The information obtained from the PK study completed prior to transplant was used to determine FFP doing during the transplant. The observed values for FV (Table 2) are during the transplant, such that some values may be influenced by the activity of the liver allograft, even before hour 20. These data also suggested that administration of a 20 mL kg−1 bolus dose immediately prior to surgery, followed by a continuous infusion of 1.5 mL h−1 kg−1 over a 48-h perioperative period, would be required to achieve target factor V activity levels (Table 2). The fit of the FV activity vs. time data was excellent (r = 0.98; Fig. 1). It is important to note that the activity-time profile contains several early time points where the observed activity of FV was higher than the predicted (i.e. activity of 17% (IU dL−1) at 0.5 h and 16% (IU dL−1) at 1 h) and that later time points may slightly underestimate the actual observed values. This was considered to be acceptable, as a major concern was bleeding due to inadequate FV activity. As denoted previously [14], mean FV level in FFP is 80% (IU dL−1), with a minimum of 70% (IU dL−1). Given the variability of content in coagulation factors between units of FFP, and the fact that we did not measure FV concentrations in the units administered to our patient, we cannot adequately comment on the percent of recovery of FV in this instance.
Table 2.
Predicted and observed FV activity over 48 h, after administration of a loading dose of 20 mL kg−1, followed by continuous infusion of 1.5 mL kg−1 h−1. Target goal FV activity to prevent bleeding complications was set at ≥20% (IU dL−1). Normal FV activity ranges from 50 to 150% (IU dL−1 ).
| Time (h) | Predicted FV Activity (IU dL−1 or%) | Observed FV Activity (IU dL−1 or%) |
|---|---|---|
| 0 | 22 | 17 |
| 3 | 23 | 32 |
| 12 | 27 | 28 (at hour 13) |
| 15 | 27 | 21 (at hour 16.5) |
| 18 | 28 | 20 |
| 20 | 28 | 53* |
| 36 | 30 | 108* (at hour 33) |
| 48 | 31 | 127* (at hour 42) |
Indicates transplanted orthotopic liver function.
Fig. 1.
Activity time profile for FV after FFP bolus of 15 mL kg−1, where time is in hours and FV activity is in % (IU dL−1). R = 0.98.
Finally, as illustrated by the data contained in Table 2, our model predicted FV activity levels within desired target ranges (e.g. 15% to >20% (IU dL−1) with considerable accuracy.
Discussion
Use of PK modelling to achieve individual coagulation factor activity levels
Exposure–response studies of coagulation products differ from traditional PK studies of drugs in that bioassays of coagulation activity (as opposed to plasma concentrations) are used as the biomarker to assess the effect [8,15]. In this report, we have shown that factor V activity can be modelled using traditional PK approaches, similar to those used by Shapiro et al. [8] to model activity for factors VIII and IX. According to the literature, a range of FV activity levels in the plasma required for effective replacement is 20–25% (IU dL−1), with a threshold of 15% (IU dL−1) [4,6]. Given the risk of bleeding associated with undertreatment, the safety profile for factor V, and the variability in factor V content in given batches of FFP, we chose to model replacement doses of FFP that overestimated factor V activity early on in the course of therapy.
Bolus dose administration and plasma collection times
Pharmacokinetic analysis is optimally performed when a single standardized bolus dose of coagulation product is administered over a specific period of time [8]. For desired factor V activity of >15% (IU dL−1), it is recommended that a bolus dose of 15–20 mL kg−1 of FFP be administered [6]. This dose produces a dynamic range of factor V activity levels over time, so as to permit repeated assessment needed to characterize the PKs of effect.
Ideally, for assessing the kinetics of all coagulation products, sampling should be performed at frequent intervals, and extended over a period of at least 2–2.5 times the half-life (t½) of the factor under study [8]. Half-life values for FV, reported in the literature, range from 12 to 36 h [4,12,13]. Given the interpatient variability of the t½ of other coagulation factors (FVIII and VIX), it is recommended that the t½ of the factor in question be determined in the individual clinical scenario [8]. As such, we chose to sample over a 48-h postinfusion period, to assess the kinetics of factor V activity.
Calculation of PK values from raw data
A minimalist approach to assessing the PKs of a coagulation factor might be to use an approach ranging from semi-quantitative description (e.g. activity levels exceeded 15% (IU dL−1) for a certain number of hours postdose) to a graphical or simple estimation of the apparent half-life associated with offset of activity. In contrast, a more informative and accurate approach resides with the application of a curve-fitting algorithm, which, traditionally, has been used to support PK data analysis. These modelling approaches enable estimation of all polyexponential parameters (i.e. those described with onset and offset of activity) from the plasma activity vs. time profile necessary to completely characterize the disposition profile of the factor of interest and also, to simulate dosing regimens associated with the attainment of desired ‘target’ activity levels with therapeutic factor administration. It is important to note that in the modelling of treatment of bleeding disorders, activity underestimation early in the treatment course can be readily remedied with additional factor replacement, avoiding severe bleeding complications. If supplemental bolus doses are used, the resultant activity vs. time profile must be taken into account when initial data are curve fit, so as to insure that polyexponential parameter estimates used for subsequent dose projection are appropriately corrected.
Model limitations
In contrast with classical plasma concentration vs. time data, where various distribution volumes can be identified and clearance rates estimated, interpretation of the factor activity vs. time data is limited within the constructs of our model. The most important parameter estimated is that associated with time-dependent reduction in factor activity. Initially, the observed ‘decay’ profile may represent an initial rate of decline in activity for protein that is in excess of that required to saturate the binding sites on the vascular endothelium and in other tissue compartments. The subsequent decline in activity of protein likely represents dissociation from these binding sites, re-equilibration with the central compartment and subsequent catabolism of the protein.
Significance
The World Health Organization, the World Federation of Haemophilia and the International Society of Thrombosis and Haemostasis all agree that treatment with coagulation products needs to be optimized and that the application of PK principles to this process is fundamental to its success [8]. It has been suggested that for optimal clinical outcome, dosing decisions should be made on an individual patient basis, taking into account the clinical situation, the severity of the factor deficiency and the location and extent of the haemorrhage [8]. Keeping in mind that previously conducted individual PK studies in patients receiving factors VIII and IX have shown significant interpatient variability in the PKs of coagulation products with differences in in vivo recovery [16–22], apparent terminal half-life [11,16–22] and clearance [10,11,23], individualized approaches to dosing, driven by PK estimates obtained within a given patient, are superior to those which might use ‘population-derived’ mean data. The need for individual PK studies is further emphasized by recent evidence that age and weight may play a significant role in the PKs of coagulation factor preparations, including recombinant factor VIII [11].
Conclusion
Currently, considerable variability exists in treatment practices of RICDs [2,3,6]. Due to paucity of evidence-based guidelines, many important questions, such as optimal dosing and duration of factor replacement therapy, particularly during bleeding episodes or at times of surgical procedures, remain unanswered [1,3,14]. We demonstrate that successful PK-based dosing of FV in FFP is both possible and clinically relevant in the treatment of RICDs. Furthermore, we believe that similar PK-based strategies can be applied to the dosing of other individual coagulation factors contained in FFP, whose activity in plasma can be reliably measured. Such individual PK studies may prove to be of particular benefit in other diseases requiring FFP treatment, especially at the onset of therapy, in cases of inadequate treatment response, in circumstances of alloantibody development, in situations of prophylactic dosing, and in special clinical populations, such as children and obese patients.
Targeted PK-based dosing also has the potential to decrease the risks that accompany frequent or repeat FFP administration, minimizing exposure to bloodborne pathogens, allergic reactions, volume overload and development of alloantibodies to individual factor content of FFP [3,12]. In turn, optimization of FFP treatment may reduce the cost of replacement therapy in RICDs, as efficient use of FFP would be less expensive, and more readily available, than treatment with individual factor concentrates [3,5].
Acknowledgements
The authors would like to thank Judith Kauffman, RN, PNP, for coordination of the reported study.
Footnotes
Disclosures
B. Wicklund has acted as a paid consultant to Novo Nordisk and Bayer Healthcare. The other authors have no disclosures to declare.
References
- 1.Peyvandi F, Duga S, Akhavan S, Mannucci PM. Rare coagulation deficiencies. Haemophilia 2002; 8: 308–21. [DOI] [PubMed] [Google Scholar]
- 2.Di Paola J, Nugent D, Young G. Current therapy for rare factor deficiencies. Haemophilia 2001; 7(S1): 16–22. [DOI] [PubMed] [Google Scholar]
- 3.Mannucci PM, Duga S, Peyvandi F. Recessively inherited coagulation disorders. Blood 2004; 104: 1243–52. [DOI] [PubMed] [Google Scholar]
- 4.Huang JN, Koerper MA. Factor V deficiency: a concise review. Haemophilia 2008; 14: 1164–9. [DOI] [PubMed] [Google Scholar]
- 5.Shapiro AD, Soucie JM, Peyvandi F, Aschman DJ, DiMichele DM. Knowledge and therapeutic gaps: a public health problem in the rare coagulation disorders population. Am J Prev Med 2011; 41(6S4): S324–31. [DOI] [PubMed] [Google Scholar]
- 6.Bolton-Maggs PHB, Perry DJ, Chalmers EA et al. The rare coagulation disorders-review with guidelines for management from the United Kingdom Haemophilia Centre Doctor’s Organisation. Haemophilia 2004; 10: 593–628. [DOI] [PubMed] [Google Scholar]
- 7.Bergman G. Progress in the treatment of bleeding disorders. Thromb Res 2010; 127: S3–5. [DOI] [PubMed] [Google Scholar]
- 8.Shapiro AD, Korth-Bradley J, Poon MC. Use of pharmacokinetics in the coagulation factor treatment of patients with haemophilia. Haemophilia 2005; 11: 571–82. [DOI] [PubMed] [Google Scholar]
- 9.Björkman S, Blanchette VS, Fischer K et al. Comparative pharmacokinetics of plasma- and albumin-free recombinant factor VIII in children and adults: the influence of blood sampling schedule on observed age-related differences and implications for dose tailoring. J Thromb Haemost 2010; 8: 730–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Björkman S A commentary on the differences in pharmacokinetics between recombinant and plasma-derived factor IX and their implications for dosing. Haemophilia 2011; 17: 179–84. [DOI] [PubMed] [Google Scholar]
- 11.Björkman S, Oh M, Spotts G et al. Population pharmacokinetics of recombinant factor VIII: the relationships of pharmacokinetics to age and body weight. Blood 2012; 119: 612–8. [DOI] [PubMed] [Google Scholar]
- 12.Asselta R, Peyvandi F. Factor V deficiency. Semin Thromb Hemost 2009; 35: 382–9. [DOI] [PubMed] [Google Scholar]
- 13.Chingale A, Eisenhut M, Gadiraju A, Liesner R. A neonatal presentation of factor V deficiency: a case report. BMC Pediatrics 2007; 7: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bolton-Maggs PHB, Stobart K, Smyth RL. Evidence-based treatment of haemophilia. Haemophilia 2004; 10(S4): 20–4. [DOI] [PubMed] [Google Scholar]
- 15.Björkman S, Berntrop E. Pharmacokinetics of coagulation factors: clinical relevance for patients with haemophilia. Clin Pharmacokinet 2001; 40: 815–32. [DOI] [PubMed] [Google Scholar]
- 16.White GC, Courter S, Bray GL, Lee Ml, Gomperts ED The recombinate previously treated patient study group: a multicenter study of recombinant factor VIII (Recombinate™) in previously treated patients with hemophilia A. Thromb Haemost 1997; 77: 433–5. [PubMed] [Google Scholar]
- 17.White GC, Shapiro AD, Ragni M, Garzone P, Goodfellow J, Tubridy K. Clinical evaluation of recombinant factor IX. Semin Hematol 1998; 35 (S2): 33–8. [PubMed] [Google Scholar]
- 18.Morfini M, Longo G, Messori A, Lee M, White G, Mannucci P. Pharmacokinetic properties of recombinant factor VIII compared with a monoclonally purified concentrate (Hemofil® M). Thromb Haemost 1992; 68: 433–5. [PubMed] [Google Scholar]
- 19.Fukui H, Yoshioka A, Shima M et al. Clinical evaluation of recombinant human factor VIII (BAY w 6240) in the treatment of hemophilia A. Int J Hematol 1991; 54: 419–27. [PubMed] [Google Scholar]
- 20.Finjnvandraat K, Berbtorp E, ten Cate JW et al. Recombinant, B-domain deleted factor VIII (r-Viii SQ): pharmacokinetics and initial safety aspects in hemophilia A patients. Thromb Haemost 1997; 77: 298–302. [PubMed] [Google Scholar]
- 21.Poon MC, Lillicrap D, Hensman C, Card R, Scully MF. Recombinant factor IX recovery and inhibitor safety: a Canadian post-licensure surveillance study. Thromb Haemost 2002; 87: 431–4. [PubMed] [Google Scholar]
- 22.Ewenstein BM, Joist JH, Shapiro AD et al. Pharmacokinetic analysis of plasma-derived and recombinant FIX concentrates in previously treated patients with moderate or severe hemophilia B. Transfusion 2002; 42: 190–7. [DOI] [PubMed] [Google Scholar]
- 23.Barnes C, Lillicrap D, Pazmino-Canizares J et al. Pharmacokinetics of recombinant factor VIII (Kogenate-FS®) in children and causes of inter-patient pharmacokinetic variability. Haemophilia 2006; 12(S4): 40–9. [Google Scholar]