Abstract
Distressful symptoms associated with gastroesophageal reflux constitute a clinical description of gastroesophageal reflux disease, a pathologic condition commonly treated with proton pump inhibitors (PPIs). Despite their pervasive use in neonates and infants, PPIs have not been approved for gastroesophageal reflux disease treatment in this population. This creates a therapeutic conundrum: namely, whether to institute PPI treatment based on knowledge and experience or to withhold it consequent to absence of labeling for young infants.
“The only time you don’t fail is the last time you try anything—and it works.”
–William Strong
DEVELOPMENT OF GASTROESOPHAGEAL REFLUX DISEASE
Neonatal/infantile GERD is a multidimensional disorder in that it has a “mechanical” basis that may or may not be linked to acid-induced tissue injury. This facet of GERD is the target for the use of acid-modifying drugs such as the proton pump inhibitors (PPIs).1–3 The antecedent to gastroesophageal reflux disease (GERD) is gastroesophageal reflux (GER), the retrograde passage of gastric contents into the esophagus. Normally, the lower esophageal sphincter contracts as the stomach fills, creating an anatomic barrier between acidic gastric contents and the esophagus.4 The integrity of this barrier has a clear developmental dependence, which produces an increased susceptibility to GER in neonates and young infants. Additional unique features of the infant gastrointestinal tract include (i) a short, narrow esophagus that allows easier passive regurgitation of gastric contents; (ii) cephalad positioning of the lower esophageal sphincter above rather than below the diaphragm, which creates a less effective barrier against the retrograde passage of gastric content into the esophagus; (iii) delayed gastric emptying, which increases the residence time of feedings; and (iv) frequent liquid feedings, which can distend the stomach and thus predispose the infant to regurgitation.4 These developmental physiologic features create the propensity for GER to transition to GERD in neonates and young infants.1
As anyone who has cared for a neonate or young infant knows from experience, GER is a very common event. Gastroesophageal reflux occurs daily in up to 65% of infants.4 Symptoms associated with GERD include frequent regurgitation, vomiting, wheezing, back arching, inconsolable crying, feeding difficulty, failure to thrive, irritability, sleep disturbance, cough, apnea, and aspiration.5–7 It should be noted that these symptoms are not merely troublesome to the patient (or parent) but may, in some cases, produce serious morbidity (e.g., aspiration).
THE PROTON PUMP INHIBITORS
Successful use of PPIs to treat GERD resides with the fact that their pharmacodynamics is inextricably linked to the physiology of the enzyme responsible for gastric acid secretion, H+-K+-ATPase. As recently described by Ward and Kearns,8 ligand binding (e.g., histamine, gastrin, or acetylcholine) to this acid proton pump creates a 1 million–fold gradient in H+ concentration from inside the parietal cell to the gastric lumen, in return for inward transport of K+. Activation of H+-K+-ATPase is the common final pathway for gastric acid secretion in humans of all ages,9–11 as reflected by the fact that neonates of 24 weeks gestational age can secrete enough acid to lower the intragastric pH to <4.12 It is also the pathway that is the mechanistic “target” for PPIs. Following their protonation (i.e., bioactivation step), PPIs bind to the cysteines of the H+-K+-ATPase complex. The degree of reversibility of drug action depends on the site of cysteine binding within the enzyme. For example, binding by omeprazole and rabeprazole to more superficial cysteines is reversible, whereas binding by pantoprazole and lansoprazole to cysteines that lie deeper within the ATPase produces irreversible, permanent inactivation of the proton pump.10
As with the predisposition of neonates and young infants to GERD, inhibition of gastric acid production by PPIs must be viewed in a developmental context. When the currently used doses of PPIs clinically presumed to be effective in neonates and infants are compared with those used in adults, the “effective” weight-normalized PPI dose used to treat neonates is several times higher.8 This discrepancy in apparent dose requirement between neonates and adults appears to be dependent, in part, on developmental differences in drug disposition. In particular, the activities of both CYP2C19 and CYP3A4, the enzymes primarily responsible for the biotransformation of the PPIs,8 have distinct, developmental profiles associated with markedly diminished activity in the first few months of life.13
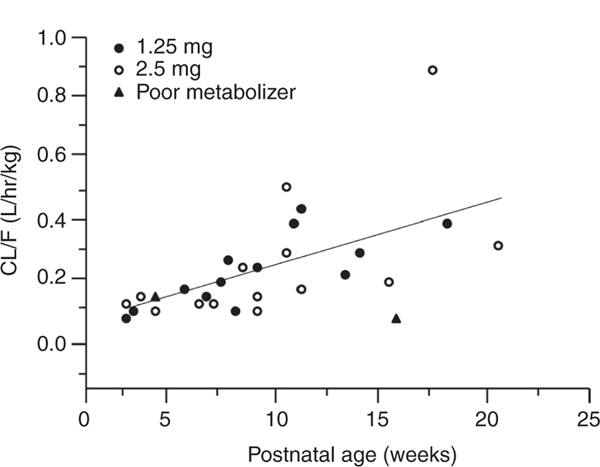
A consistent finding from pharmacokinetic studies of PPIs in neonates and infants is a significantly reduced plasma drug clearance in the first month of life, which is associated with a reciprocal increase in systemic exposure (as measured by dose-normalized area under the plasma concentration vs. time curve (AUC)).14–16 Also, as reflected by composite data for apparent pantoprazole clearance over the first 5 months of postnatal life (Figure 1), there is considerable variability in the pharmacokinetics of the drug produced by development and, potentially, CYP2C19 polymorphism. Recent data on pantoprazole17 suggest less developmental variability in PPI pharmacodynamics with a pH response to treatment being similar between preterm and term infants. Despite the information on PPI clinical pharmacology during early life, a clear and predictable pharmacokinetic–pharmacodynamic relationship for these drugs in neonates and young infants remains to be characterized. It is therefore possible that variability in PPI pharmacokinetics in early life contributes to the observed pharmacodynamic variability of these drugs in the treatment of GERD in neonates and infants.
Figure 1.
Association between the apparent oral plasma clearance (CL/F) for pantoprazole and postnatal age (PNA) in a cohort of neonates and young infants given either a 1.25-mg (filled circles) or 2.5-mg (open circles) single oral dose of pantoprazole. A significant linear association between CL/F and PNA (P < 0.01) was observed. Of note is that two of the infants (filled triangles) had a CYP2C19 genotype predictive of a slow-metabolizer phenotype whereas all other study subjects had a CYP2C19 genotype predictive of an extensive-metabolizer phenotype. These data also demonstrate apparent genotype–phenotype discordance during the first month of life.16
TREATMENT CONUNDRUM
If persistent acid reflux has been shown to cause GERD in infants and children older than 12 months of age, it is quite reasonable that the same pathophysiology applies to neonates and infants <12 months of age. The challenge is separating the irritable baby who cries with any emesis, regardless of the absence of erosive esophagitis, from the infant with acid-peptic disease. Clinicians are familiar with the infant who refuses oral feeds and exhibits clear signs of Sandifer’s syndrome, to the point of failing to thrive. These infants often show dramatic improvements in feeding and growing with PPI therapy, despite the lack of labeling for this indication. Nonetheless, treatment with PPIs requires a thoughtful balance of the potential therapeutic benefit associated with raising intragastric pH against the potential risks associated with the drug itself and the real potential for gastric refluxate to produce esophageal tissue injury. It is this latter concern that, for many clinicians, provides a developmental and pharmacologic construct to initiate PPI therapy as treatment for GERD.
As is the case with most drugs, the known adverse-event profile of PPIs is generally better characterized in adults than children, and it is particularly less understood in neonates and young infants. Recent data profiling the adverse events associated with long-term PPI use, primarily in adults, have reported Clostridium difficile–related diarrhea,18 hypomagnesemia,19 increased risk of respiratory infections in children with asthma,20 increased risk of hip fracture in women,21 and increased risk of community-acquired pneumonia.22 Additional concerns over the theoretical risks of long-term PPI use in infancy include the development of prolonged hypergastrinemia (which in later years may lead to enterochromaffin-like cell hyperplasia), carcinoid formation, vitamin B12 deficiency, osteoporosis, atrophic gastritis, and altered native gastrointestinal flora and subsequent potential for an increase in bacterial infections.23 It is important to note that none of these potential risks were identified in the US Food and Drug Administration’s (FDA’s) 2010 safety review of the reported cases of serious adverse events associated with the use of esomeprazole, lansoprazole, omeprazole, or rabeprazole in children.24
As recently reviewed by Ward and Kearns,8 the pharmacologic evidence available on PPIs would portend their beneficial effects as acid-modifying agents in the treatment of GERD in neonates and young infants. Nonetheless, the few randomized controlled trials performed to date have not shown efficacy of the PPIs in the treatment of GERD in this population.25 Invariably, the pharmacokinetic and pharmacodynamic data collected from these trials demonstrated significant suppression of gastric acid production, as measured by intragastric pH. By contrast, efficacy studies uniformly failed to demonstrate PPI efficacy when defined by reduction in subjective clinical symptoms associated with GERD.
Chen et al.25 recently reviewed four randomized controlled trials examining the efficacy of PPIs in the treatment of infantile GERD that were conducted by pharmaceutical companies under formal written requests issued by the FDA. A primary goal of these studies was to expand the labeled indication for the treatment of GERD to infants.25 Data from these studies, three of which were double-blind, placebo-controlled randomized controlled trials, are summarized in Table 1. All studies were limited by small sample size, symptom-based diagnoses, and subjective caregiver assessment of efficacy.
Table 1.
Summary of the four RCTs examining the efficacy of PPI therapy in infants with GERD
| Parameter | Esomeprazole28 | Lansoprazole29 | Pantoprazole30 | Omeprazole31 |
|---|---|---|---|---|
| Control group | Placebo | Placebo | Placebo | Dosing range |
| Blinding | Double | Double | Double | Single |
| Length of randomized phase in weeks | 4 | 4 | 4 | 8 |
| Open-label phase to identify PPI responders | Yes (2 weeks) | No | Yes (4 weeks) | No |
| Age in months | 1–12 | 1–12 | 1–12 | 0–24 |
| N | 40 | 80 | 50 | 35 |
| GERD symptoms definition | Vomiting Regurgitation Irritability Supraesophageal disturbance Respiratory disturbance Feeding difficulty |
Crying Fussiness Irritability |
Vomiting Regurgitation Spitting up Irritability Fussiness Feeding refusal Choking Gagging |
Vomiting Regurgitation |
| Primary end point | Time from randomization to discontinuation due to symptom worsening, perceived by parent and physician | Proportion of infants with ≥50% reduction in Physician Global Assessment of GERD-related symptoms | Proportion of infants who withdrew due to “lack of efficacy,” perceived by physician and/or worsening esophagitis on endoscopy | Change from baseline in daily symptoms based on Physician Global Assessment and parent perception |
| Primary end point efficacy result | Hazard ratio = 0.69 95% CI [0.35–1.35] P = 0.275 |
PPI: 54% Placebo: 54% P = 1.000 |
PPI: 12% Placebo: 11% P = 1.000 |
P > 0.50 in all dosing-group comparisons |
On the basis of these data, the FDA advisory committee charged with the evaluation of PPIs for treatment of GERD in neonates and infants published the following statement: “Use of PPIs should be reserved for infants with an endoscopically documented acid-induced condition such as erosive esophagitis. The risk/benefit relation of administration of PPIs in infants with GER or GERD without documented acid-induced conditions is not favorable because no benefit can be attributed to the PPI.”25 Thus, a labeled indication for GERD in neonates and young infants was not granted. In our experience, conventional clinical thinking does not uniformly support this regulatory conclusion.
With regard to the aforementioned FDA decision, several questions remain. Was the apparent failure of the PPIs to achieve efficacy end points in the multiple labeling trials due to an ineffective drug or, alternatively, to study end points that were inappropriate or not sufficiently sensitive or specific to detect drug effect? These inherent problems in study design may explain the discordance between observed achievement of drug effect, such as change in gastric pH, and lack of statistically significant subjective improvement in clinical symptoms such as crying and irritability. Also, it is possible that the symptoms we have traditionally attributed to GERD (e.g., crying, irritability, back arching) are not caused by GERD or acid reflux. Rather, they may be related to the dysmotility component of GER and the recurrent pathologic retrograde passage of gastric content into the esophagus, as suggested by Condino et al.26 Simply, it is impossible to assess the efficacy of treatment when one cannot reliably and accurately diagnose the disease in a given patient population and objectively measure efficacy in the face of drug effect. Clearly, better study end points are essential for evaluating drugs used to treat GERD in infants.
PRESCRIPTION AND DIAGNOSIS TRENDS
Despite the lack labeling of PPIs for treating infantile GERD, the use of these drugs in infants continues to increase, as evidenced by more than 400,000 prescriptions dispensed to 145,000 patients under the age of 12 months nationwide in 2010, an 11-fold increase as compared with 2002 (ref. 25). Although anecdotal evidence and prescription trends would indicate that infantile GERD is on the rise, the frequency of GERD in infancy has not been widely studied. A recent cohort study by Nelson et al.6 demonstrated an apparent 262% increase in the incidence of the diagnosis of GERD in the infant population across the United States between 2000 and 2005. It is not clear whether this increase represents a greater frequency of the disorder or an increase in its diagnosis, driven, in part, by readily available treatments.
Despite numerous technological advances in the care and treatment of neonates and young infants, the diagnosis of infantile GERD still relies on parent-reported symptoms of distress. To date, there is no convenient, noninvasive clinical test or biologic marker to diagnose GERD in infancy or to predict its response to therapy.1 Until such a diagnostic tool becomes available, clinicians must rely on subjective clinical symptoms to establish the diagnosis of GERD. This state of affairs produces a difficult decision for the clinician faced with a sick neonate or young infant with presumed GERD: (i) to either institute or withhold acid-modifying treatment intended to raise intragastric pH, thereby minimizing the likelihood that GER will progress to GERD; (ii) if acid-modifying therapy is felt to be clinically indicated, to choose between two classes of drugs, namely, H2-receptor antagonists or PPIs, both of which have been studied in neonates and young infants but the latter lacking approved product labeling in such patients; or (iii) to institute therapy with drugs that have prokinetic activity (e.g., erythromycin or metoclopramide). Bolstered by the known clinical pharmacology profile of PPIs in pediatric patients8 and current standards of medical practice, the decision often made is to institute treatment with PPIs with the assumption and hope that the potential benefits of treating infantile GERD will outweigh the known risks of the drug selected.
CONCLUSION
By virtue of their ability to decrease gastric acid production in neonates and young infants, the therapeutic goal of using PPIs to treat gastroesophageal reflux in such patients is targeted at taking the D (“disease”) out of GERD by preventing mucosal damage, allowing mucosal healing, and eliminating distressing symptoms. In this regard, the selection of a PPI to achieve these goals may represent drug use that, although being “off-label,” is, conversely, “on-knowledge,” as suggested by the available evidence.
Acknowledgments
CONFLICT OF INTEREST
G.L.K.’s institution has previously received support from pharmaceutical companies to conduct pediatric clinical trials of omeprazole (AstraZeneca Pharmaceuticals) and pantoprazole (Wyeth Laboratories). R.M.W.’s institution has likewise received funding to support pediatric clinical trials of pantoprazole (Wyeth Laboratories). Both G.L.K. and R.M.W. have served as consultants to the US Food and Drug Administration through participation in advisory committee meetings concerning the treatment of gastroesophageal reflux in infants and children. V.S. declared no conflict of interest.
References
- 1.Vandenplas Y et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN). J. Pediatr. Gastroenterol. Nutr. 49, 498–547 (2009). [DOI] [PubMed] [Google Scholar]
- 2.Tafuri G, Trotta F, Leufkens HG, Martini N, Sagliocca L & Traversa G Off-label use of medicines in children: can available evidence avoid useless paediatric trials? The case of proton pump inhibitors for the treatment of gastroesophageal reflux disease. Eur. J. Clin. Pharmacol. 65, 209–216 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Romano C, Chiaro A, Comito D, Loddo I & Ferrau V Proton pump inhibitors in pediatrics: evaluation of efficacy in GERD therapy. Curr. Clin. Pharmacol. 6, 41–47 (2011). [DOI] [PubMed] [Google Scholar]
- 4.Henry SM Discerning differences: gastroesophageal reflux and gastroesophageal reflux disease in infants. Adv. Neonatal Care 4, 235–247 (2004). [DOI] [PubMed] [Google Scholar]
- 5.Higginbotham TW Effectiveness and safety of proton pump inhibitors in infantile gastroesophageal reflux disease. Ann. Pharmacother. 44, 572–576 (2010). [DOI] [PubMed] [Google Scholar]
- 6.Nelson SP, Kothari S, Wu EQ, Beaulieu N, McHale JM & Dabbous OH Pediatric gastroesophageal reflux disease and acid-related conditions: trends in incidence of diagnosis and acid suppression therapy. J. Med. Econ. 12, 348–355 (2009). [DOI] [PubMed] [Google Scholar]
- 7.Barron JJ, Tan H, Spalding J, Bakst AW & Singer J Proton pump inhibitor utilization patterns in infants. J. Pediatr. Gastroenterol. Nutr. 45, 421–427 (2007). [DOI] [PubMed] [Google Scholar]
- 8.Ward RM & Kearns GL Proton pump inhibitors in pediatrics: mechanism of action, pharmacokinetics, pharmacogenetics, and pharmacodynamics. Paediatr. Drugs (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Litalien C, Théorêt Y & Faure C Pharmacokinetics of proton pump inhibitors in children. Clin. Pharmacokinet. 44, 441–466 (2005). [DOI] [PubMed] [Google Scholar]
- 10.Gibbons TE & Gold BD The use of proton pump inhibitors in children: a comprehensive review. Paediatr. Drugs 5, 25–40 (2003). [DOI] [PubMed] [Google Scholar]
- 11.Roche VF The chemically elegant proton pump inhibitors. Am. J. Pharm. Educ. 70, 101 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boyle JT et al. Do children with gastroesophageal reflux become adults with gastroesophageal reflux? What is the role of Acid suppression in children? J. Pediatr. Gastroenterol. Nutr. 37 (suppl. 1), S65–S68 (2003). [DOI] [PubMed] [Google Scholar]
- 13.Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS & Kauffman RE Developmental pharmacology–drug disposition, action, and therapy in infants and children. N. Engl. J. Med. 349, 1157–1167 (2003). [DOI] [PubMed] [Google Scholar]
- 14.Omari T et al. Pharmacodynamics and systemic exposure of esomeprazole in preterm infants and term neonates with gastroesophageal reflux disease. J. Pediatr. 155, 222–228 (2009). [DOI] [PubMed] [Google Scholar]
- 15.Zhang W, Kukulka M, Witt G, Sutkowski-Markmann D, North J & Atkinson S Age-dependent pharmacokinetics of lansoprazole in neonates and infants. Paediatr. Drugs 10, 265–274 (2008). [DOI] [PubMed] [Google Scholar]
- 16.Ward RM et al. Single-dose, multiple-dose, and population pharmacokinetics of pantoprazole in neonates and preterm infants with a clinical diagnosis of gastroesophageal reflux disease (GERD). Eur. J. Clin. Pharmacol. 66, 555–561 (2010). [DOI] [PubMed] [Google Scholar]
- 17.Kierkus J et al. Pharmacodynamics and safety of pantoprazole in neonates, preterm infants, and infants aged 1 through 11 months with a clinical diagnosis of gastroesophageal reflux disease. Dig. Dis. Sci. 56, 425–434 (2011). [DOI] [PubMed] [Google Scholar]
- 18.Kuehn BM Reflux drugs linked to C. difficile-related diarrhea. JAMA 307, 1014 (2012). [DOI] [PubMed] [Google Scholar]
- 19.Sumukadas D, McMurdo ME & Habicht D Proton pump inhibitors are associated with lower magnesium levels in older people with chronic kidney disease. J. Am. Geriatr. Soc. 60, 392–393 (2012). [DOI] [PubMed] [Google Scholar]
- 20.Holbrook JT et al. ; Writing Committee for the American Lung Association Asthma Clinical Research Centers. Lansoprazole for children with poorly controlled asthma: a randomized controlled trial. JAMA 307, 373–381 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khalili H, Huang ES, Jacobson BC, Camargo CA Jr, Feskanich D & Chan AT Use of proton pump inhibitors and risk of hip fracture in relation to dietary and lifestyle factors: a prospective cohort study. BMJ 344, e372 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Desilets AR, Asal NJ & Dunican KC Considerations for the use of proton-pump inhibitors in older adults. Consult. Pharm. 27, 114–120 (2012). [DOI] [PubMed] [Google Scholar]
- 23.Tolia V & Boyer K Long-term proton pump inhibitor use in children: a retrospective review of safety. Dig. Dis. Sci. 53, 385–393 (2008). [DOI] [PubMed] [Google Scholar]
- 24.US Food and Drug Administration. Pediatric Focused Safety Review: Proton Pump Inhibitors: Esomeprazole, Lansoprazole, Omeprazole, Rabeprazole: Pediatric Advisory Committee Meeting <http://www.fda.gov/downloads/advisorycommittees/committeesmeetingmaterials/pediatricadvisingcommittee/ucm216303.pdf> (2010).
- 25.Chen IL et al. Proton pump inhibitor use in infants: FDA reviewer experience. J. Pediatr. Gastroenterol. Nutr. 54, 8–14 (2012). [DOI] [PubMed] [Google Scholar]
- 26.Condino AA, Sondheimer J, Pan Z, Gralla J, Perry D & O’Connor JA Evaluation of infantile acid and nonacid gastroesophageal reflux using combined pH monitoring and impedance measurement. J. Pediatr. Gastroenterol. Nutr. 42, 16–21 (2006). [DOI] [PubMed] [Google Scholar]
- 27.US Food and Drug Administration. Gastrointestinal Drugs Advisory Committee <http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/GastrointestinalDrugsAdvisoryCommittee/UCM232026.pdf> (2010).
- 28.US Food and Drug Administration. Briefing Document for the Gastrointestinal Drugs Advisory Committee Meeting November 5, 2010: Clinical Experience Related to the Esomeprazole Clinical Studies in Patients <1 Year of Age with Gastroesophageal Reflux Disease <http://www.fda.gov/downloads/advisorycommittees/committeesmeetingmaterials/pediatricadvisingcommittee/ucm232029.pdf> (2010).
- 29.US Food and Drug Administration. Division of Gastroenterology Products Medical Officer Review (for Lansoprazole) <http://www.fda.gov/downloads/advisorycommittees/committeesmeetingmaterials/pediatricadvisorycommittee/ucm214709.pdf> (2008).
- 30.US Food and Drug Administration. Office of Clinical Pharmacology Review (for pantoprazole sodium). <http://www.fda.gov/downloads/drugs/developmentapprovalprocess/developmentresources/ucm195511.pdf> (2008–2009).
- 31.US Food and Drug Administration <http://www.accessdata.fda.gov/drugsatfda_docs/nda/2008/022056s000medr.pdf> (2008).