Abstract
Objective
The overall survival of patients with recurrent glioblastoma (rGBM) is quite different, so clinical outcome prediction is necessary to guide personalized clinical treatment for patients with rGBM. The expression level of lncRNA FAM225B was analyzed to determine its prognostic value in rGBMs.
Methods
We collected 109 samples of Chinese Glioma Genome Atlas (CGGA) RNA sequencing dataset and divided into training set and validation set. Then, we analyzed the expression of FAM225B, clinical characteristics, and overall survival (OS) information. Kaplan-Meier survival analysis was used to estimate the OS distributions. The prognostic value of FAM225B in rGBMs was tested by univariate and multivariate Cox regression analyses. Moreover, we analyzed the biological processes and signaling pathways of FAM225B.
Results
We found that FAM225B was upregulated in rGBMs (P = 0.0009). The expression of FAM225B increased with the grades of gliomas (P < 0.0001). The OS of rGBMs in the low-expression group was significantly longer than that in the high-expression group (P = 0.0041). Similar result was found in the training set (P = 0.0340) and verified in the validation set (P = 0.0292). In multivariate Cox regression analysis, FAM225B was identified to be an independent prognostic factor for rGBMs (P = 0.003). Biological process and KEGG pathway analyses implied FAM225B mainly played a functional role on transcription, regulation of transcription, cell migration, focal adhesion, etc.
Conclusions
FAM225B is expected to be as a new prognostic biomarker for the identification of rGBM patients with poor outcome. And our study provided a potential therapeutic target for rGBMs.
1. Introduction
As the most common intracranial malignant tumor in adults [1, 2], glioblastoma (GBM) has a median overall survival (OS) of only 14.6 months [3]. Most GBMs will relapse after standard treatment such as surgical resection, radiotherapy, and chemotherapy [4–6]. In recurrent glioblastoma (rGBM) cases, the patients may lose the opportunity for surgery because of the invasion to the functional area by tumors [7]. And rGBMs are less sensitive to chemotherapy or radiotherapy than primary ones as well [8–10]. So progress in prognostic biomarker identification is required for personalized treatment of patients with rGBM [11]. Further understanding the molecular factors of rGBMs will provide novel insights for the potential biological characteristics and elucidate the possible therapeutic targets.
Long noncoding RNAs (lncRNAs) refer to noncoding RNAs more than 200 nucleotides in length [12, 13] and are associated with a series of cellular process [14–16], such as epigenetic regulation and transcriptional regulation. The dysregulated lncRNAs can be used as prognostic factors for patients [17–20].
In this study, we collected 109 rGBM samples from the Chinese Glioma Genome Atlas (CGGA, http://www.cgga.org.cn/) RNA sequencing dataset. We identified a new prognostic lncRNA FAM225B in rGBMs. We investigated the expression patterns of FAM225B and evaluated its prognostic value. The expression of FAM225B increased with the glioma grades, and it indicated the poor prognosis in rGBM patients. Therefore, FAM225B could be a prognostic indicator and a potential therapeutic target for rGBMs.
2. Materials and Methods
2.1. Patients and Datasets
Our study obtained 109 rGBM samples from the CGGA RNA sequencing dataset with expression data and clinical information [21, 22]. All samples were initially diagnosed with primary glioblastoma (pGBM) and relapsed after standard treatment. The cases were randomly divided into training set and validation set without bias [23]. And the median relative expression of FAM225B was used as the cut-off value to divide the samples into a low-expression group and high-expression group. All these samples were diagnosed histologically by 2 neuropathologists according to the 2016 WHO classification guideline of nervous system tumors. Our study was approved by the Ethics Committee of Beijing Tiantan Hospital.
2.2. Statistical and Bioinformatic Analysis
SPSS software (version 22; SPSS Inc., Chicago, IL, USA) and R programming language (version 3.2.3) were used for statistical analyses. Survival distributions were estimated by using Kaplan-Meier survival analysis (GraphPad Software Inc., La Jolla, CA, USA). Univariate and multivariate Cox regression analyses evaluated the hazard ratios (HRs) of different prognostic factors and were used to identify the independent prognostic factor. A two-sided P value less than 0.05 was set to be statistically significant. Biological process and Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis were conducted by using DAVID (the Database for Annotation, Visualization and Integrated Discovery, https://david-d.ncifcrf.gov/home.jsp) [24, 25].
3. Results
3.1. FAM225B Was Dysregulated in rGBMs
We first investigated the expression pattern of FAM225B. The expression of FAM225B showed significant difference between the pGBMs and rGBMs (P = 0.0009, Figure 1(a)). Moreover, the expression of FAM225B increased with grades of gliomas (P = 0.0436; P < 0.0001; P < 0.0001, Figure 1(a)). Then, we compared the FAM225B expression level in primary and recurrent gliomas of grade II (P = 0.0568) and grade III (P = 0.0629, Figure 1(a)).
Figure 1.
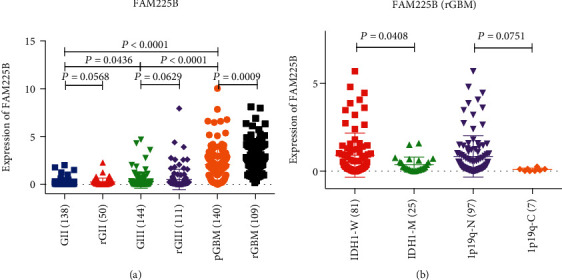
The expression pattern of FAM225B in gliomas: (a) FAM225B expression in different grades (grade II to grade IV) and types (primary or recurrent); (b) FAM225B expression in different IDH1 status and 1p/19q status.
The IDH1 and 1p/19q status is related to the prognosis of gliomas [26–29]. Therefore, we explored the expression pattern of FAM225B in the subtypes of rGBMs. The result showed that the expression of FAM225B enhanced in the IDH1-wild-type subtype (P = 0.0408, Figure 1(b)). However, no statistically significant differences were observed in the expression of FAM225B in rGBM patients with 1p/19q-codeletion or 1p/19q-noncodeletion (P = 0.0751, Figure 1(b)).
3.2. FAM225B Predicted Poorer Overall Survival in rGBMs
All 109 rGBM samples were randomly divided into training set and validation set with no significant data bias. By using the median relative expression of FAM225B as the cut-off value, samples were divided into two categories including the low-expression group and high-expression group. Kaplan-Meier analysis was used to investigate the correlation between FAM225B expression and OS of rGBM patients.
We found that the OS of patients in the low-expression group were significantly longer (P = 0.0041, Figure 2(a)). In the training group, the OS of patients in the high-expression group were shorter than those in the low-expression group (P = 0.0340, Figure 2(b)). And similar result was observed in the validation set (P = 0.0292, Figure 2(c)).
Figure 2.
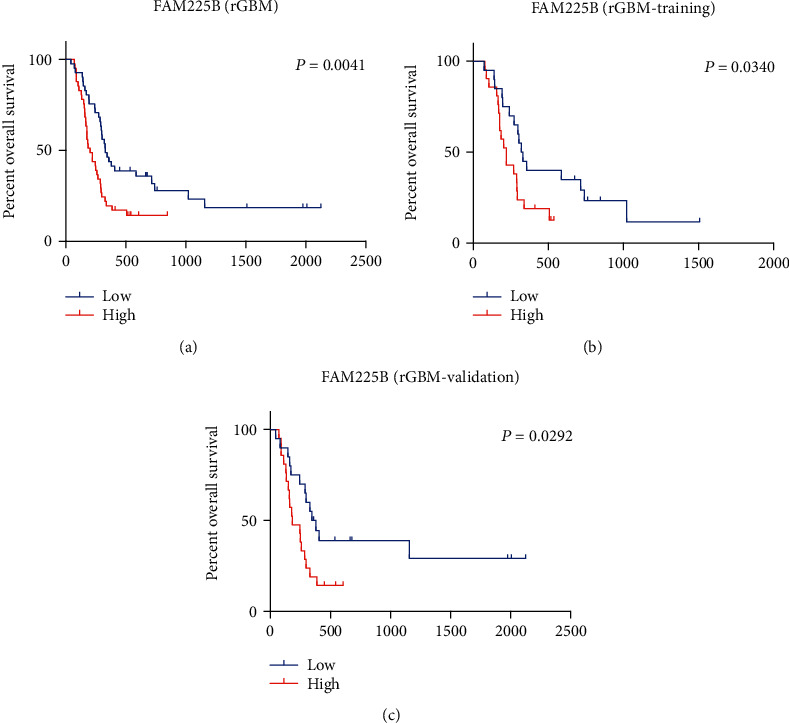
Kaplan-Meier curves of OS among rGBM patients from different groups stratified by the expression of FAM225B: (a) Kaplan-Meier curves of OS among rGBMs with different FAM225B-expressing levels (high-expression group and low-expression group); (b) Kaplan-Meier curves of OS among rGBMs with different FAM225B-expressing levels in the training set (high-expression group and low-expression group); (c). Kaplan-Meier curves of OS among rGBMs with different FAM225B-expressing levels in the validation set (high-expression group and low-expression group).
3.3. Associations between FAM225B and Clinicopathologic Features
We analyzed the relationship between FAM225B expression and clinicopathologic features (Figure 3). The results were obtained by the univariate and multivariate Cox regression analyses (Table 1). The univariate Cox regression analysis found that chemotherapy (P = 0.003) and FAM225B (P = 0.007) were potential survival predictors. On the multivariate Cox regression analysis, it showed that FAM225B (P = 0.003) was an independent prognostic factor. FAM225B was significantly related to the prognosis of rGBMs.
Figure 3.
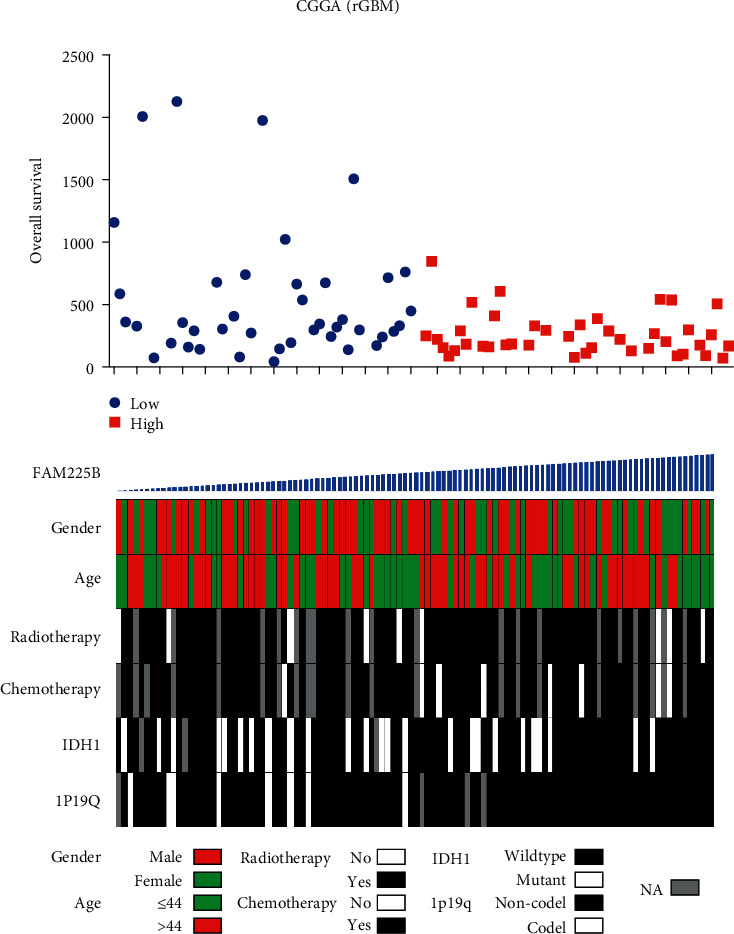
Distribution of clinicopathologic features according to the expression of FAM225B. Rows represent corresponding genes, while columns indicate corresponding patients.
Table 1.
Univariate and multivariate Cox regression analysis of survival in rGBMs.
| Items | Univariate Cox | Multivariate Cox | ||
|---|---|---|---|---|
| p value | HR | p value | HR | |
| Gender | 0.415 | 0.811 | ||
| Age | 0.286 | 1.010 | ||
| IDH1 | 0.083 | 0.645 | ||
| 1p/19q | 0.100 | 0.375 | ||
| Radiotherapy | 0.102 | 1.805 | ||
| Chemotherapy | 0.003 | 0.256 | 0.003 | 0.257 |
| FAM225B | 0.007 | 1.279 | 0.003 | 1.313 |
Gender: male, female; IDH1 status: wild type, mutant; 1p/19q status: noncodeletion, codeletion; radiotherapy: no, yes; chemotherapy: no, yes.
3.4. Functional Annotation of FAM225B
To explain the different prognoses of rGBMs divided by FAM225B, we extracted 902 related genes using Pearson correlation analysis (correlation value 0.4; P < 0.001). The biological process and KEGG pathway analyses of FAM225B were performed using DAVID. It showed that major biological processes were enriched in transcription, regulation of transcription, cell division, positive regulation of migration, cell migration, etc. (Figure 4(a)). KEGG pathway analysis showed that selected genes were enriched in ubiquitin-mediated proteolysis, Wnt signaling pathway, cell cycle, focal adhesion, regulation of actin cytoskeleton, etc. (Figure 4(b)).
Figure 4.
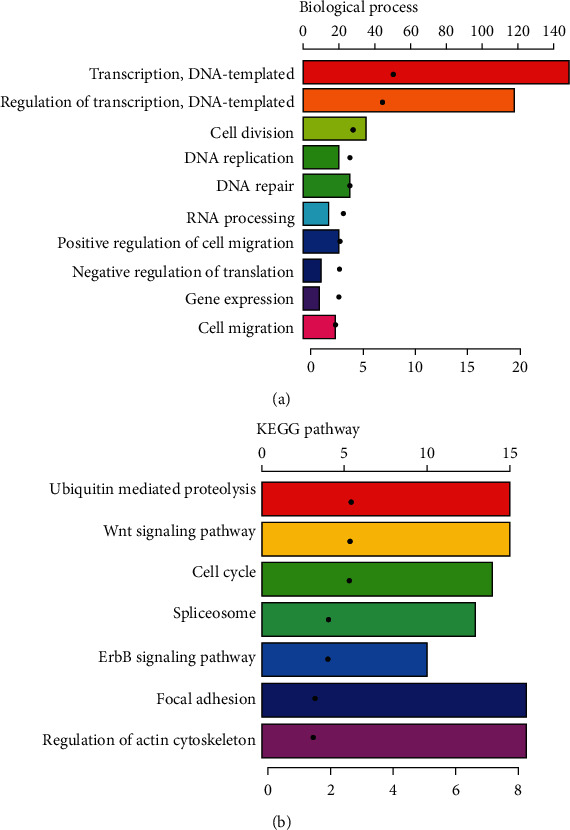
Functional annotation of FAM225B in rGBM patients: (a) biological process analysis of the related genes; (b) KEGG pathway analysis of the related genes.
4. Discussion
Despite great efforts on the multimodal diagnosis and treatment of rGBMs, the clinical prognosis for patients remains poor due to the proliferation potential and antiapoptosis characteristics of this malignant tumor [30–32]. Therefore, understanding the expression of key factors may significantly influence the development and clinical outcome of rGBM patients. Recent studies have found that the dysregulated lncRNAs can provide insights for glioma diagnosis, prognosis, and treatment strategy [33–37].
In this study, we established a dataset of 109 rGBMs collected from the CGGA RNA sequencing dataset to explore the effect of lncRNAs on the prognosis of rGBM patients. By comparing the expression level of FAM225B between pGBMs and rGBMs, we found that FAM225B expression enhanced in rGBMs (P = 0.0009). It indicated that FAM225B may play a key role in the recurrence process of GBMs. Then, we analyzed the expression of FAM225B in different grades of glioma, including grade II, grade III, and GBM (P < 0.0001). The result showed that the expression of FAM225B was positively correlated with the grade of gliomas. This indicated that FAM225B was associated with glioma malignancy and may serve as a potential indicator for glioma grade. In addition, we explored that the expression of FAM225B enhanced in IDH1-wild type (P = 0.0408). Based on the data analysis above, we inferred that FAM225B can be used as a specific diagnostic marker for GBM recurrence.
We explored the correlation between FAM225B expression and survival distribution of rGBM patients. It showed that the OS decreased with the enhancement of FAM225B expression (P = 0.0041). FAM225B enhancement may predict the poor prognosis in rGBMs. We analyzed the relationship between FAM225B expression and clinicopathologic features. It indicated that FAM225B expression could be a significantly independent prognostic maker in rGBMs.
In our studies, functional annotation analysis showed a strong correlation between FAM225B and transcription. It suggested that FAM225B may play an important role in rGBM through transcription and its regulation. By using the starBase website (https://starbase.sysu.edu.cn/), we found the candidate miRNAs (miR-1-3p, miR-206, and miR-205-5p) which may be correlated to FAM225B. FAM225B could competitively bind with miR-1-3p and miR-206, inducing the upregulation of AXL. It may lead to the enhancement of drug resistance and cell proliferation in glioma [38]. Furthermore, it has reported that miR-205-5p is involved in Wnt signaling, cell cycle, and focal adhesion [39], which is consistent with our KEGG findings. These processes may be associated with the recurrence of GBM. However, there are a few limitations in our study. The sample size of our dataset is still limited. Moreover, this is a retrospective study, and different treatments may have an impact on patient survival outcomes. And these findings were based on bioinformatic analysis. Prospective study is needed to clarify the mechanism of FAM225B and to further validate the clinical application in patients with rGBMs.
5. Conclusion
Our study found that FAM225B enhanced in rGBMs and its upregulation was related to the poor prognosis of rGBM patients. Therefore, our study provides a new perspective for FAM225B, as a prognosis biomarker and a potential therapeutic target for rGBMs. Further study will investigate the mechanisms of FAM225B in rGBMs.
Acknowledgments
This work was supported by grants from Beijing Municipal Administration of Hospitals' Mission Plan (SML20150501); “13th Five-Year Plan” National Science and Technology supporting plan (2015BAI09B04); and Foundation of Beijing Tiantan Hospital (No. 2018-YQN-6).
Contributor Information
Wen Wang, Email: wangwenttyy@126.com.
Jizong Zhao, Email: zhaojz205@163.com.
Data Availability
All CGGA data used in this study was available from the CGGA website (http://www.cgga.org.cn).
Ethical Approval
This study was approved by the Ethics Committee in Beijing Tiantan Hospital.
Consent
Patient informed consents were waived due to the retrospective nature of the study.
Conflicts of Interest
The authors have declared that no competing interests exist.
Authors' Contributions
Junsheng Li analyzed the results and wrote the manuscript. Qian Zhang and Peicong Ge made the statistical comparison. Chaofan Zeng and Fa Lin revised the manuscript. Wen Wang performed the gene analysis and the functional annotation analysis. Jizong Zhao designed the study. All authors have read and approved the final manuscript.
References
- 1.Zeng T., Li L., Zhou Y., Gao L. Exploring Long noncoding RNAs in glioblastoma: regulatory mechanisms and clinical potentials. International journal of genomics. 2018;2018:13. doi: 10.1155/2018/2895958.2895958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Geraldo L., Garcia C., da Fonseca A., et al. Glioblastoma therapy in the age of molecular medicine. Trends in cancer. 2019;5(1):46–65. doi: 10.1016/j.trecan.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Tully P., Gogos A., Love C., Liew D., Drummond K., Morokoff A. Reoperation for recurrent glioblastoma and its association with survival benefit. Neurosurgery. 2016;79(5):678–689. doi: 10.1227/NEU.0000000000001338. [DOI] [PubMed] [Google Scholar]
- 4.Steffens R., Semrau S., Lahmer G., et al. Recurrent glioblastoma: who receives tumor specific treatment and how often? Journal of Neuro-Oncology. 2016;128(1):85–92. doi: 10.1007/s11060-016-2079-z. [DOI] [PubMed] [Google Scholar]
- 5.Krivoshapkin A., Gaytan A., Salim N., et al. Repeat resection and intraoperative radiotherapy for malignant gliomas of the brain: a history and review of current techniques. World Neurosurgery. 2019;132:356–362. doi: 10.1016/j.wneu.2019.09.037. [DOI] [PubMed] [Google Scholar]
- 6.Weller M., le Rhun E., Preusser M., Tonn J., Roth P. How we treat glioblastoma. ESMO open. 2020;4, article e000520(Suppl 2) doi: 10.1136/esmoopen-2019-000520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Botros D., Dux H., Price C., Khalafallah A., Mukherjee D. Assessing the efficacy of repeat resections in recurrent glioblastoma: a systematic review. Neurosurgical Review. 2020 doi: 10.1007/s10143-020-01331-1. [DOI] [PubMed] [Google Scholar]
- 8.Huang J., Chaudhary R., Cohen A. L., et al. A multicenter phase II study of temozolomide plus disulfiram and copper for recurrent temozolomide-resistant glioblastoma. Journal of Neuro-Oncology. 2019;142(3):537–544. doi: 10.1007/s11060-019-03125-y. [DOI] [PubMed] [Google Scholar]
- 9.Kazmi F., Soon Y. Y., Leong Y. H., Koh W. Y., Vellayappan B. Re-irradiation for recurrent glioblastoma (GBM): a systematic review and meta-analysis. Journal of Neuro-Oncology. 2019;142(1):79–90. doi: 10.1007/s11060-018-03064-0. [DOI] [PubMed] [Google Scholar]
- 10.Campos B., Olsen L. R., Urup T., Poulsen H. S. A comprehensive profile of recurrent glioblastoma. Oncogene. 2016;35(45):5819–5825. doi: 10.1038/onc.2016.85. [DOI] [PubMed] [Google Scholar]
- 11.Sasmita A. O., Wong Y. P., Ling A. P. K. Biomarkers and therapeutic advances in glioblastoma multiforme. Asia-Pacific Journal of Clinical Oncology. 2018;14(1):40–51. doi: 10.1111/ajco.12756. [DOI] [PubMed] [Google Scholar]
- 12.Ulitsky I., Bartel D. P. lincRNAs: genomics, evolution, and mechanisms. Cell. 2013;154(1):26–46. doi: 10.1016/j.cell.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J., Meng H., Bai Y., Wang K. Regulation of lncRNA and its role in cancer metastasis. Oncology Research. 2016;23(5):205–217. doi: 10.3727/096504016X14549667334007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Batista P. J., Chang H. Y. Long noncoding RNAs: cellular address codes in development and disease. Cell. 2013;152(6):1298–1307. doi: 10.1016/j.cell.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noh J. H., Kim K. M., McClusky W. G., Abdelmohsen K., Gorospe M. Cytoplasmic functions of long noncoding RNAs. Wiley interdisciplinary reviews RNA. 2018;9(3, article e1471) doi: 10.1002/wrna.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmitz S. U., Grote P., Herrmann B. G. Mechanisms of long noncoding RNA function in development and disease. Cellular and molecular life sciences. 2016;73(13):2491–2509. doi: 10.1007/s00018-016-2174-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou M., Zhang Z., Zhao H., Bao S., Cheng L., Sun J. An immune-related six-lncRNA signature to improve prognosis prediction of glioblastoma multiforme. Molecular Neurobiology. 2017;55(5):3684–3697. doi: 10.1007/s12035-017-0572-9. [DOI] [PubMed] [Google Scholar]
- 18.Liu S., Mitra R., Zhao M. M., et al. The potential roles of long noncoding RNAs (lncRNA) in glioblastoma development. Molecular Cancer Therapeutics. 2016;15(12):2977–2986. doi: 10.1158/1535-7163.MCT-16-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li M., Long S., Hu J., Wang Z., Geng C., Ou S. Systematic identification of lncRNA-based prognostic biomarkers for glioblastoma. Aging. 2019;11(21):9405–9423. doi: 10.18632/aging.102393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peng W. X., Koirala P., Mo Y. Y. LncRNA-mediated regulation of cell signaling in cancer. Oncogene. 2017;36(41):5661–5667. doi: 10.1038/onc.2017.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng W., Ren X., Zhang C., et al. Bioinformatic profiling identifies an immune-related risk signature for glioblastoma. Neurology. 2016;86(24):2226–2234. doi: 10.1212/WNL.0000000000002770. [DOI] [PubMed] [Google Scholar]
- 22.Wang W., Zhao Z., Wu F., et al. Bioinformatic analysis of gene expression and methylation regulation in glioblastoma. Journal of Neuro-Oncology. 2018;136(3):495–503. doi: 10.1007/s11060-017-2688-1. [DOI] [PubMed] [Google Scholar]
- 23.Zhang J. X., Han L., Bao Z. S., et al. HOTAIR, a cell cycle-associated long noncoding RNA and a strong predictor of survival, is preferentially expressed in classical and mesenchymal glioma. Neuro-Oncology. 2013;15(12):1595–1603. doi: 10.1093/neuonc/not131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen L., Zhang Y. H., Lu G., Huang T., Cai Y. D. Analysis of cancer-related lncRNAs using gene ontology and KEGG pathways. Artificial Intelligence in Medicine. 2017;76:27–36. doi: 10.1016/j.artmed.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 25.Chen L., Zhang Y. H., Wang S., Zhang Y., Huang T., Cai Y. D. Prediction and analysis of essential genes using the enrichments of gene ontology and KEGG pathways. PLoS One. 2017;12(9, article e0184129) doi: 10.1371/journal.pone.0184129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Szopa W., Burley T. A., Kramer-Marek G., Kaspera W. Diagnostic and therapeutic biomarkers in glioblastoma: current status and future perspectives. BioMed Research International. 2017;2017:13. doi: 10.1155/2017/8013575.8013575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ludwig K., Kornblum H. I. Molecular markers in glioma. Journal of Neuro-Oncology. 2017;134(3):505–512. doi: 10.1007/s11060-017-2379-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rahman M., Kresak J., Yang C., et al. Analysis of immunobiologic markers in primary and recurrent glioblastoma. Journal of Neuro-Oncology. 2018;137(2):249–257. doi: 10.1007/s11060-017-2732-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aldape K., Zadeh G., Mansouri S., Reifenberger G., von Deimling A. Glioblastoma: pathology, molecular mechanisms and markers. Acta Neuropathologica. 2015;129(6):829–848. doi: 10.1007/s00401-015-1432-1. [DOI] [PubMed] [Google Scholar]
- 30.Seystahl K., Wick W., Weller M. Therapeutic options in recurrent glioblastoma--an update. Critical Reviews in Oncology/Hematology. 2016;99:389–408. doi: 10.1016/j.critrevonc.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 31.Shah J. L., Li G., Shaffer J. L., et al. Stereotactic radiosurgery and hypofractionated radiotherapy for glioblastoma. Neurosurgery. 2018;82(1):24–34. doi: 10.1093/neuros/nyx115. [DOI] [PubMed] [Google Scholar]
- 32.Frischer J. M., Marosi C., Woehrer A., et al. Gamma knife radiosurgery in recurrent glioblastoma. Stereotactic and Functional Neurosurgery. 2016;94(4):265–272. doi: 10.1159/000448924. [DOI] [PubMed] [Google Scholar]
- 33.Wu F., Zhang C., Cai J., et al. Upregulation of long noncoding RNA HOXA-AS3 promotes tumor progression and predicts poor prognosis in glioma. Oncotarget. 2017;8(32):53110–53123. doi: 10.18632/oncotarget.18162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao W., Sun C., Cui Z. A long noncoding RNA UCA1 promotes proliferation and predicts poor prognosis in glioma. Clinical and Translational Oncology. 2017;19(6):735–741. doi: 10.1007/s12094-016-1597-7. [DOI] [PubMed] [Google Scholar]
- 35.Dong C. Y., Cui J., Li D. H., Li Q., Hong X. Y. HOXA10-AS: a novel oncogenic long non-coding RNA in glioma. Oncology Reports. 2018;40(5):2573–2583. doi: 10.3892/or.2018.6662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiao H., Ding N., Liao H., et al. Prediction of relapse and prognosis by expression levels of long noncoding RNA PEG10 in glioma patients. Medicine. 2019;98(45, article e17583) doi: 10.1097/MD.0000000000017583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ma K. X., Wang H. J., Li X. R., et al. Long noncoding RNA MALAT1 associates with the malignant status and poor prognosis in glioma. Tumor Biology. 2015;36(5):3355–3359. doi: 10.1007/s13277-014-2969-7. [DOI] [PubMed] [Google Scholar]
- 38.Ma Y., Zhou G., Li M., et al. Long noncoding RNA DANCR mediates cisplatin resistance in glioma cells via activating AXL/PI3K/Akt/NF-κB signaling pathway. Neurochemistry International. 2018;118:233–241. doi: 10.1016/j.neuint.2018.03.011. [DOI] [PubMed] [Google Scholar]
- 39.Zhang B. L., Dong F. L., Guo T. W., Gu X. H., Huang L. Y., Gao D. S. MiRNAs mediate GDNF-induced proliferation and migration of glioma cells. Cellular Physiology and Biochemistry. 2018;44(5):1923–1938. doi: 10.1159/000485883. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All CGGA data used in this study was available from the CGGA website (http://www.cgga.org.cn).


