Abstract
Lin28a has diverse functions including regulation of cancer, reprogramming and regeneration, but whether it promotes injury or is a protective reaction to renal injury is unknown. We studied how Lin28a acts in unilateral ureteral obstruction (UUO)-induced renal fibrosis following unilateral ureteral obstruction, in a mouse model. We further defined the role of Lin28a in transforming growth factor (TGF)-signaling pathways in renal fibrosis through in vitro study using human tubular epithelium-like HK-2 cells. In the mouse unilateral ureteral obstruction model, obstruction markedly decreased the expression of Lin28a, increased the expression of renal fibrotic markers such as type I collagen, α-SMA, vimentin and fibronectin. In TGF-β-stimulated HK-2 cells, the expression of Lin28a was reduced and the expression of renal fibrotic markers such as type I collagen, α-SMA, vimentin and fibronectin was increased. Adenovirus-mediated overexpression of Lin28a inhibited the expression of TGF-β-stimulated type I collagen, α-SMA, vimentin and fibronectin. Lin28a inhibited TGF-β-stimulated SMAD3 activity, via inhibition of SMAD3 phos-phorylation, but not the MAPK pathway ERK, JNK or p38. Lin28a attenuates renal fibrosis in obstructive nephropathy, making its mechanism a possible therapeutic target for chronic kidney disease.
Keywords: Lin28a, Renal fibrosis, Renal tubular epithelial cell, SMAD3, TGF-beta signaling
INTRODUCTION
Renal fibrosis is a key feature of the final, common pathway of chronic kidney disease (CKD), and is characterized by extracellular matrix (ECM) deposition. Renal function is progressively lost and ultimately leads to end-stage renal disease (ESRD), requiring dialysis or kidney transplantation (1-4). During CKD, excessive and pathological deposition of ECM in the kidneys disrupts organ architecture leading to decreased blood supply and organ dysfunction. Progressive renal fibrosis is accompanied by reduced ability to tissue repair and eventually causes kidney failure (5).
Transforming growth factor-β (TGF-β) is an essential fibrotic factor that plays a crucial role in the development of renal fibrosis and regulates the renal fibrotic process (6). It stimulates collagen expression and deposition in the ECM, stabilizes ECM proteins and thereby inhibits their degradation (7). TGF-β binds to TGF-β receptors and phosphorylates downstream receptor-associated transcription factors called SMAD proteins, the so-called regulated SMADs (R-SMADs) such as SMAD2 and SMAD3 (8). The common mediator SMAD (SMAD4) binds to the activated SMAD2/3 to form a oligomeric complex that translocate into the nucleus to regulate the transcription of target genes in interaction with various co-activators and co-repressors (9). Additionally, TGF-β1 activates several SMAD-independent pathways such as p38 MAP kinase, extracellular-signal-regulated kinase (ERK), and c-Jun N-terminal kinase (JNK). Elevated TGF-β expression was observed in many types of experimental and human kidney disease, including diabetic nephropathy, glomerulonephritis (GN) and tubulointerstitial nephritis (10).
Unilateral ureteral obstruction (UUO) is the most usually used method for experimentally-induced renal fibrosis in animal models and is thought to mimic human chronic obstructive nephropathy (11-13). In this study, therefore we used UUO-induced kidney and TGF-β-treated HK-2 cells to determine the mechanisms of renal fibrosis and to explore the putative role of Lin28a in ameliorating renal fibrosis.
Lin28 is an RNA-binding protein that consists of two homologs, Lin28a and Lin28b, which have similar structural and functional characteristics (14). Lin28 has been shown to selectively repress the expression of microRNAs and is involved in cell proliferation and differentiation in embryonic cells, stem cells, cancer, skeletal myogenesis, neurogliogenesis, lymphopoiesis and glucose metabolism (15-20). Two recent studies have suggested that Lin28b/let-7b plays an important role in the repression of diabetic nephro-pathy (21-23). Park et al. showed that the micro RNA, let-7b, upregulates collagen expression by transforming growth factor-1-induced Lin28b in glomerular mesangial cells, under diabetic conditions (21). However, the role of Lin28a in CKD is currently unknown. Therefore, we examined whether Lin28a exerts a protective or a causative effect in the development of renal fibrosis.
In this study, we found that Lin28a expression is decreased in UUO kidney and, that TGF-β inhibited Lin28a expression in HK-2 cell and induced renal fibrotic factors, collagen type I, fibronectin, vimentin, and α-SMA. However, adenovirus-mediated overexpression of Lin28a inhibited TGF-β-induced renal fibrotic factors by inhibiting the phosphorylation of SMAD3. Contrary to some previous reports (21-23), these observations suggest that Lin28a is an ameliorating factor, rather than a causative one, for TGF-β-induced renal fibrosis.
RESULTS
Lin28a expression is decreased in the tubule area of kidney in a UUO-induced renal fibrosis model
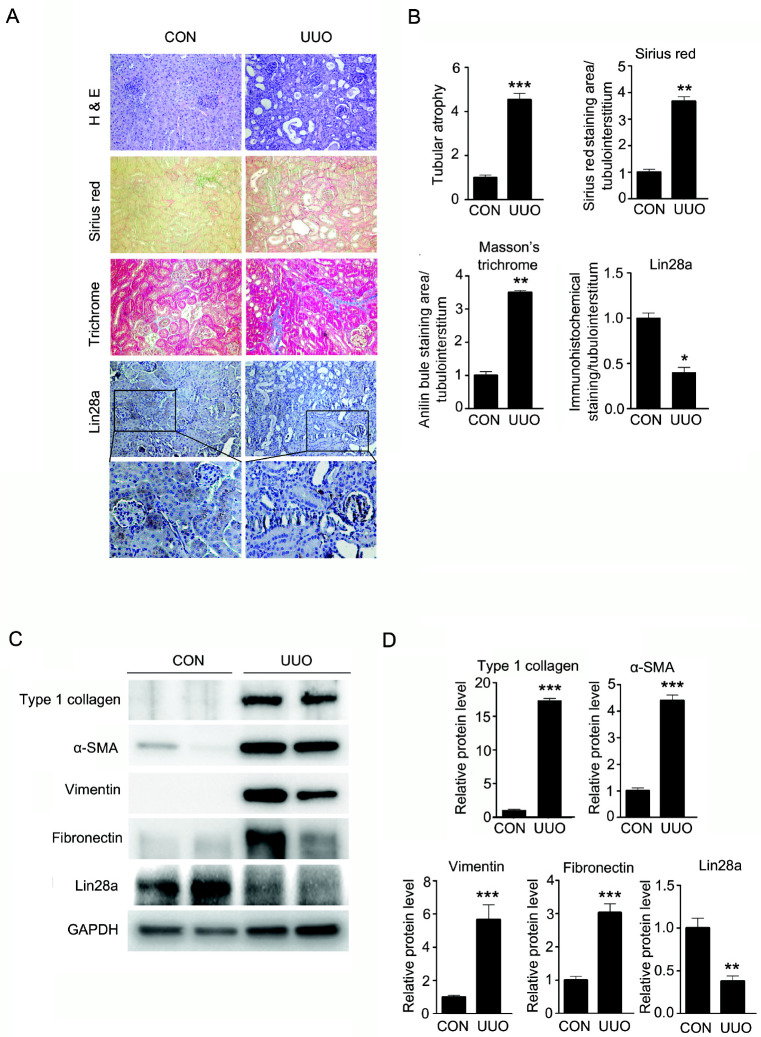
First, we examined whether the expression levels of Lin28a in kidney were altered by UUO, as a model for renal fibrosis. Hematoxylin and eosin (H&E) staining showed that tubular atrophy and renal damage were significantly increased in UUO kidneys. Additionally, Sirius Red and Masson Trichrome staining presented that renal tubulointerstitial damage and fibrosis were markedly elevated in UUO kidneys. Immunohistochemical staining showed that Lin28a was expressed in the renal tubule area of the control kidney. However, the expression of Lin28a in the renal tubule area was decreased in UUO-induced fibrotic kidneys (Fig. 1A, B). In addition, western blot analysis showed that the protein levels of type 1 collagen, α-SMA, vimentin and fibronectin in the UUO kidney were increased, compared with those in the control kidney. Similarly, according to immunohistochemical staining, Lin28a protein expression was decreased in UUO kidneys (Fig. 1C, D). Taken together, these data suggest that the down-regulation of Lin28a is related to the upregulation of renal fibrotic factors in obstructive nephropathy.
Fig. 1.
Effect of UUO-induced renal fibrosis on Lin28a expression and relative protein levels of type I collagen, α-SMA, vimentin, fibro-nectin and Lin28a in kidneys of mice with UUO. C57BL/6 mice were sacrificed 14 days after UUO. (A) Representative kidney tissue sections stained with hematoxylin and eosin (H&E), Sirius Red and Masson’s trichrome stain, and immunostained with antibody targeting Lin28a (Magnification, ×200). The number of atrophic tubules was determined by measuring the abnormal irregular and dilated tubular basement membranes in (H&E) stained kidney sections in five random fields under high-power magnification. Renal fibrosis area was assessed by Sirius Red and Masson’s trichrome staining. Lin28a expression in the tubulointerstitium of kidneys was measured by immunostaining. (B) Areas of positive staining with Sirius Red, Masson’s Trichrome and Lin28a in the UUO kidneys were quantitated by computer-based morphometric analysis and normalized to the control (=1) were expressed as the fold increase relative to the control in all bar graphs. Data are the mean ± SEM of five independent measurements (n = 5 in each group). *P < 0.05, **P < 0.01 and ***P < 0.001 compared with control mice. (C) Western blot analysis of protein levels of type I collagen, α-SMA, vimentin, fibronectin and Lin28a in the control and UUO kidneys. (D) Quantification of western blot analysis results expressed as the mean ± SEM of three independent measurements. GAPDH levels were analyzed as an internal control. **P < 0.01 and ***P < 0.001 compared with control mice.
TGF-β induced the upregulation of fibrotic gene expression and the downregulation of Lin28a expression in HK-2 cells
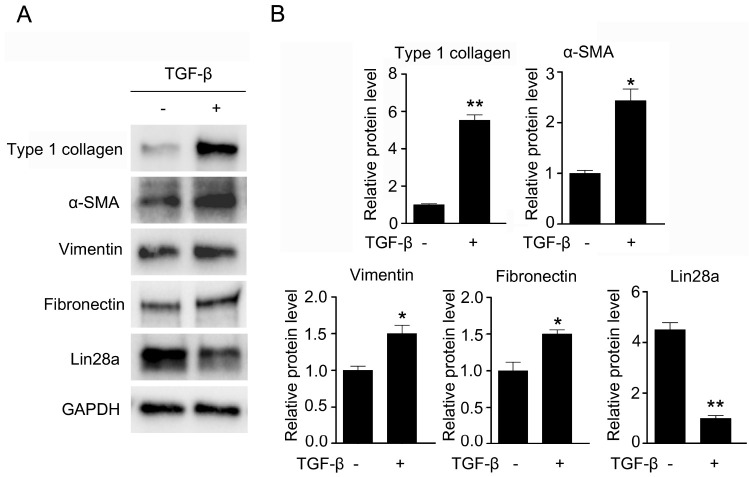
TGF-β is central to the development of renal fibrosis through its stimulating effect on renal fibrotic factors (6). An increase in TGF-β expression is a key feature of the UUO kidney, and induces its target fibrotic genes, including type I collagen, fibronectin and vimentin in UUO kidneys (24, 25). We investigated whether TGF-β affects fibrotic target gene expression in cultured human kidney cortex/proximal tubule (HK-2) cells. As expected, the expression of TGF-β target fibrotic factors, including type I collagen, α-SMA, vimentin and fibronectin, were increased in TGF-β-treated HK-2 cells compared to untreated HK-2 cells. Moreover, we examined whether TGF-β affects the expression levels of Lin28a in HK-2 cells with a similar trend to the downregulation of Lin28a induced by UUO. Interestingly, Lin28a expression was high in the untreated cells, and its expression was markedly decreased in TGF-β-treated HK-2 cells (Fig. 2).
Fig. 2.
Effect of TGF-β on protein expression of Lin28a and renal fibrotic factors. (A) Western blot analysis of type I collagen, α-SMA, vimentin, fibronectin and Lin28a in TGF-β-treated HK-2 cells. Cells were treated with TGF-β (5 ng/ml) for 24 h. (B) Quantification of western blot analysis results expressed as the mean ± SEM of three independent measurements. GAPDH levels were analyzed as an internal control. *P < 0.05, **P < 0.01 and ***P < 0.001 compared with TGF-β (−).
Adenovirus-mediated overexpression of Lin28a inhibits TGF-β-stimulated fibrotic factors
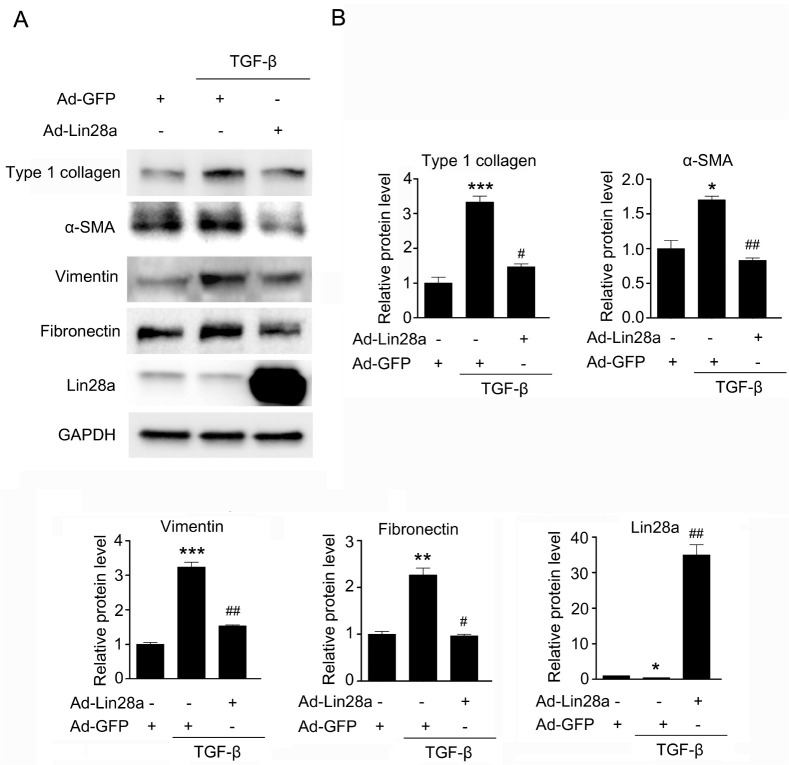
To examine the possible beneficial effects of Lin28a in renal fibrosis, we examined whether Lin28a inhibits TGF-β-stimulated fibrotic factors in HK-2 cells. As shown in Fig. 3, adenovirus (Ad)-mediated overexpression of Lin28a in HK-2 cells inhibited TGF-β-stimulated type 1 collagen, α-SMA, vimentin and fibronectin protein expression. These data suggest that Lin28a inhibits TGF-β-induced renal fibrotic factors expression.
Fig. 3.
Effect of Lin28a on TGF-β-stimulated renal fibrotic factors, type I collagen, α-SMA, vimentin and fibronectin in HK-2 cells. (A) Western blot analysis of type I collagen, α-SMA, vimentin, fibronectin and Lin28a in TGF-β-stimulated HK-2 cells with or without adeno-Lin28a infection. Cells were infected with 20 moi of Ad-Lin28a or Ad-GFP and then incubated with TGF-β (5 ng/ml) for 24 h. (B) Quantification of western blot analysis results expressed as the mean ± SEM of three independent measurements. GAPDH levels were analyzed as an internal control. *P < 0.05, **P < 0.01 and ***P < 0.001 compared with control, #P < 0.01 and ##P < 0.001 compared with TGF-β alone.
Lin28a inhibits TGF-β-induced phosphorylation of SAMD3
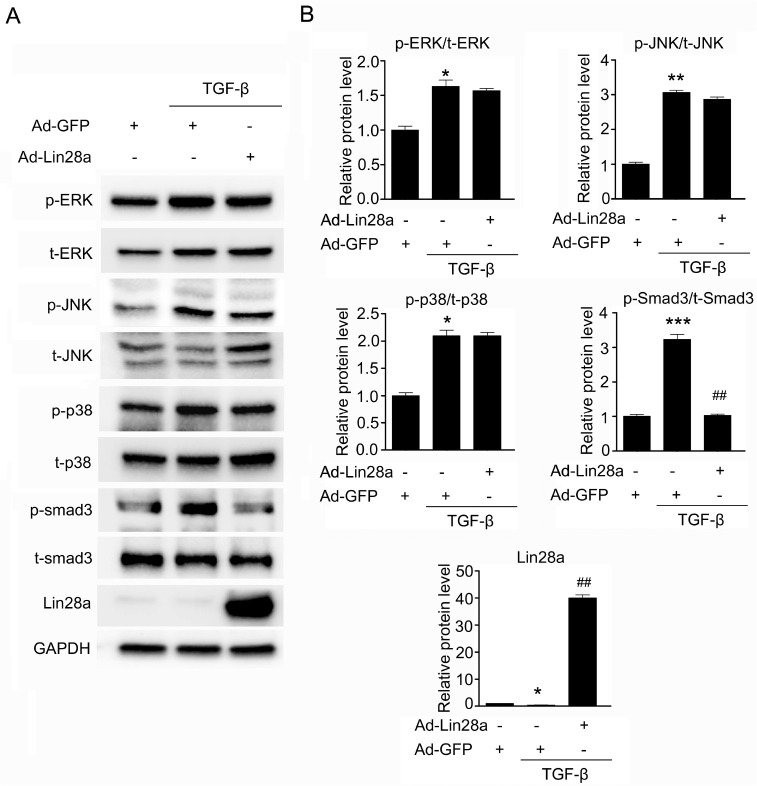
TGF-β stimulates the expression of many ECM proteins in renal cells through the SMAD dependent or independent pathways (7, 8). To determine the mechanism of Lin28a suppression on renal fibrotic factors, we examined whether Lin28a inhibits SMAD3 activity or MAPK activity. TGF-β stimulated the phosphorylation of MAPK, ERK, JNK, and p38 as well as the phosphorylation of SMAD3 in HK-2 cells (Fig. 4). Although TGF-β activated both SMAD3-dependent and -independent pathways, phosphorylation of SMAD3 in HK-2 cells that had been treated with TGF-β was only inhibited by Ad-mediated overexpression of Lin28a (Fig. 4). These data suggest that Lin28a inhibits TGF-β-renal fibrotic factors via the inhibition of SMAD3 phosphorylation.
Fig. 4.
Effect of Lin28a on the TGF-β/SMAD3 signaling pathway. (A) Western blot analysis of the expression of p-ERK, p-JNK, p-p38 and p-smad3 in TGF-β-stimulated HK-2 cells. Cells were infected with 20 moi of Ad-Lin28a or Ad-GFP and then incubated with TGF-β (5 ng/ml) for 24 h. (B) Quantification of western blot analysis results expressed as the mean ± SEM of three independent measurements. GAPDH levels were analyzed as an internal control. *P < 0.05, **P < 0.01 and ***P < 0.001 compared with control, ##P < 0.001 compared with TGF-β alone.
DISCUSSION
In this study, we gained novel insights into the role of Lin28a in the pathogenesis of renal fibrosis, during obstructive nephropathy. We found that the expression of Lin28a was markedly reduced in human tubular epithelium-like HK-2 cells, when treated with TGF-β, and in mouse kidneys with complete ureteral obstruction. The expression of fibrotic proteins including type 1 collagen, α-SMA, vimentin and fibronectin was significantly increased in these cells and in kidney tissue, which induced renal fibrosis, following UUO. Furthermore, adenovirus-mediated overexpression of Lin28a prevented the expression of TGF-β-induced Type 1 collagen, α-SMA, vimentin and fibronectin protein. Lin28a appears to downregulate the expression of renal fibrotic factors via downregulation of the TGF-β/SMAD pathway.
Lin28a and Lin28b are highly conserved RNA-binding proteins with similar structures and functions (14). Numerous reports have indicated that they play critical roles in embryonic development, tumorigenesis, and pluripotency, and also participate in the progression of organ damage and fibrosis in metabolic diseases such as diabetes (15, 16, 20, 25, 26). Although Lin28a and Lin28b share similarities in many characters and functions in cancer development and progression (16), there are still some differences in their expression and functions. Lin28b has been studied in various types of human cancer (27-31) and in the regulation of fibrosis in several organs including the kidney, liver, and lung (21, 27, 32). Lin28a, has been reported to be differentially localized within cells, and to function through distinct mechanisms (14), yet this has remained largely unexplored. The present study generated some intriguing results, Lin28a expression was markedly decreased in UUO kidneys and TGF-β-treated HK-2 cells. These data contradict some previous reports in which Lin28b expression was induced by TGF-β and resulted in the suppression of let-7 and the upregulation of collagen expression in glomerular mesangial cells under diabetic conditions (21). Nevertheless, we focused on the pattern of Lin28a expression in renal fibrotic conditions and examined the effect of regulation of Lin28a on renal fibrosis. Adenovirus-mediated overexpression of Lin28a inhibited TGF-β-induced renal fibrotic factors. Therefore, we examined the effect of Lin28a on the TGF-β signaling pathway to elucidate the mechanism by which Lin28a seems to inhibit the protein expression of renal fibrotic factors such as type 1 collagen, α-SMA, vimentin and fibronectin.
ECM deposition in glomerulus and tubulointerstitium is involved in CKD progression during renal fibrosis (33). UUO-induced renal fibrosis is a well-established experimental model that mimics the pathological changes in chronic obstructive nephropathy found in CKD patients (34). Increased TGF-β in experimental renal fibrotic UUO models promotes accumulation of ECM proteins which is a major mediator of diabetic nephropathy and tubulointerstitial (35-38). In UUO kidneys, TGF-β expression is increased in several renal cells, including renal tubular epithelial cells (39-42). We found that Lin28a expression was significantly downregulated in renal epithelial tubules of UUO kidneys which highly express renal fibrotic factors and show chronic interstitial nephritis (Fig. 1). These results suggested that UUO-induced TGF-β expression in renal tubules affected the decrease in Lin 28a expression. In addition, we found that the Lin28a protein was highly expressed in TGF-β-untreated HK-2 cells, but was less expressed in TGF-β-treated HK-2 cells. Renal fibrotic factors, including type 1 collagen, α-SMA, vimentin and fibronectin, were significantly increased in TGF-β-treated HK-2 cells (Fig. 2). To determine whether the regulation of Lin28a plays an important role in renal fibrosis, we examined the effects of adenovirus-mediated Lin28a overexpression on renal fibrotic factors, in TGF-β-treated HK-2 cells, and found that they were significantly inhibited (Fig. 3).
We investigated whether Lin28a overexpression decreases renal fibrotic factors through TGF-β signaling. TGF-β mainly activates SMAD signaling, controls the expression of renal fibrotic proteins, and subsequently contributes to tubulointerstitial fibrosis (8, 37). In addition, TGF-β stimulates non-SMAD signaling, including Ras and mitogen-activated protein kinase signaling pathways such as ERK, JNK, and p38 MAPK (10, 38, 43). In this study, we found that Lin28a effectively repressed TGF-β-stimulated SMAD3 phosphorylation but did not affect TGF-β-stimulated non-SMAD signaling and, MAP kinase signaling pathways (Fig. 4). Previous reports have shown that TGF-β induces Lin28b expression via SMAD2/3 activation in glomerular mesangial cells (21). Although our results are contradictory to previous reports, we suggest that Lin28a inhibits renal fibrotic factors through inhibition of SMAD3 phosphorylation. Expression of Lin28a was markedly reduced in TGF-β-treated HK-2 cells and in the renal tubule area of UUO kidneys. Phosphorylation of SMAD3 in HK-2 cells treated with TGF-β was inhibited by adenovirus-mediated over-expression of Lin28a.
CONCLUSION
Our results suggest that the downregulation of Lin28a expression in kidneys after UUO, and in TGF-β-treated renal tubulointerstitial cells, plays an important role in the pathogenesis of fibrotic renal disease. The upregulation of Lin28a prevents renal fibrotic factor expression in TGF-β-treated renal tubulointerstitial cells. This effect was associated with the downregulation of p-SMAD3. To elucidate the role of Lin28a in renal fibrosis, it would be useful to confirm the effect of Lin28a overexpression in UUO kidneys or to generate animals in which Lin28a expression in the kidney is conditionally overexpressed or knocked out. Our findings suggest that the significant anti-fibrotic effect of Lin28a may be a promising therapeutic target in fibrotic renal disease.
MATERIALS AND METHODS
Experimental UUO animal model
Animal experiments were performed with male, 8-week-old C57BL/6J mice provided by The Koatech Technology Corporation (Korea) and have been approved by The animal Care and Use Committee of DGIST (DGIST-IACUC-19052105-01). The surgical procedure for unilateral ureteral obstruction (UUO) was modified from a previously described method (44). To induce tubulointerstitial fibrosis, anesthetized mice underwent a two-part ligation of left ureter using sterilized 5-0 silk, via the flank, and were then sutured, to observed for 2 weeks. Then mice were sacrificed and both kidneys collected; the right kidney was used as control. The kidneys were rinsed with PBS and frozen in liquid nitrogen or fixed in 10% formalin solution (Sigma, USA) and then embedded in paraffin.
Cell culture
Human kidney cortex/proximal tubule cell line HK-2 cells were purchased from American Type Culture Collection (ATCC, USA). The HK-2 cells were cultured in keratinocyte serum-free media (Gibco, USA) supplemented with 50 µg/ml of bovine pituitary extract, 5 ng/ml of human recombinant epidermal growth factor, 100 U/ml penicillin, and 100 µg/ml streptomycin (Welgene, Korea) in 5% CO2 at 37°C. The cells were treated with 5 ng/ml of recombinant human TGF-β1 (R&D Systems, USA) for 24 h.
Generation of recombinant adenovirus
The recombinant adenovirus was produced by following the method from Professor In-Kyu Lee (Department of Internal Medicine, Kyungpook National University School of Medicine, Korea). The cDNA encoding full-length mouse Lin28a was ligated as an XhoI/ BglII site of the pAdTrack-CMV shuttle vector. To prepare a recombinant adenovirus, the resulting vector was electroporated into BJ5138 cells containing the AdEasy adenoviral vector. The recombinants were amplified in AD293 cells and infected cell lysates were purified using CsCl (sigma) gradient ultracentrifugation. The titer of adenovirus was measured using an AdEasy Viral Titer Kit (Agilent Technologies, USA).
Western blot analysis
Proteins were obtained from HK-2 cell lysates and animal tissue, using RIPA buffer (Thermo, USA) containing Complete, Mini Protease Inhibitor Cocktail (Roche, USA) and Halt Phosphatase Inhibitor Cocktail (Thermo, USA). To examine the expression of proteins, separated protein in SDS/PAGE (gradient gels) were transferred onto polyvinylidene difluoride membranes (PVDF) (Bio-rad, USA), and then incubated with 5% BSA (sigma) for blocking. The membranes were incubated with anti-GAPDH antibody (Santacruz, USA), anti-type 1 collagen antibody (abcam, UK), anti-fibronectin antibody (abcam), anti-α-SMA antibody, anti-Vimentin antibody, anti-Lin28a antibody, anti-phospho-ERK antibody, anti-ERK antibody, anti-phospho-JNK antibody, anti-JNK antibody, anti-phospho-p38 antibody, anti-p38 antibody, anti-phospho-smad3 antibody and anti-smad3 antibody (Cell signaling, USA). After incubating with HRP-linked antibody (Cell signaling), the protein expression on blots was detected by ChemiDocTMXRS+ (Bio-rad) and the bands were quantified using the Image LabTM Software (Bio-rad).
Histological analysis
Paraffin sections (4 µm thick) were cut using a microtome and subjected to immunohistochemical staining. Histochemical staining was conducted using hematoxylin and eosin (H&E), Sirius Red (Sigma-Aldrich, Missouri, USA) and Masson’s trichrome staining (Sigma-Aldrich) according to the manufacturer’s instructions. Immuno-histochemical staining was performed using an anti-Lin28a anti-body (1:250) (Cell Signaling Technology) and DAB staining kit (Roche, Basel, Switzerland). The number of atrophic tubules was determined by measuring abnormal irregular and dilated tubular basement membranes in the fields of five random H&E-stained sections from each kidney of five different animals under high-power magnification. The amount of interstitial collagen deposition was evaluated by Sirius Red or Masson’s trichrome staining. For Sirius Red staining, slides were immersed for 18 hours in saturated picric acid with 0.1% Sirius red F3BA (Aldrich Chemicals). Slides were then washed in 0.01 N hydrochloric acid for 2 min and rapidly dehydrated through graded alcohol concentrations, starting at 70%. The slides were transferred to xylene, and the coverslips were mounted with Permount (Fisher Scientific, Edmonton, Alberta, Canada). For Masson’s trichrome staining, after mordanting 1 h in Bouin’s solution, kidney sections were treated sequentially with hematoxylin for 10 min, Biebrich scarlet-acid fuchsin for 5 min, phosphotungstic acid/phosphomolybdic acid for 10 min, and aniline blue for 15 min. Tissue was de-stained in 1% acetic acid for 5 min, dehydrated through graded ethanol to xylene, and finally mounted on glass slides for examination by light micros-copy. The renal fibrotic area was subjected to morphometric analysis using a light microscope equipped with an imaging system comprising a Leica Microscope (Leica Microsystems, Germany) and Leica Application Suite V3.8 software (Leica Microsystems, Germany). Quantification of aniline-blue-positive areas (collagen, blue), and positive areas of Sirius red (collagen fiber, red) and immunostaining for Lin28a antibody (brown color) were evaluated by computer-based morphometric analysis.
Statistical analyses
Analysis of variance was used to determine significant differences in multiple comparisons and was performed by Student’s t-test. All results are represented as the mean ± SEM for independent experiments performed at least three times. Values of P < 0.05 were considered statistically significant.
ACKNOWLEDGEMENTS
This work was supported by the DGIST project 20-BT-06 and 2020010077, and NRF-2018R1C1B6008955 from the Ministry of Science and ICT of the Republic of Korea.
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting interests.
REFERENCES
- 1.Norman JT, Orphanides C, Garcia P, et al. Hypoxia-nduced changes in extracellular matrix metabolism in renal cells. Exp Nephrol. 1999;7:463–493. doi: 10.1159/000020625. [DOI] [PubMed] [Google Scholar]
- 2.Zeisberg M, Kalluri R. Experimental strategies to reverse chronic renal disease. Blood Purif. 2004;22:440–445. doi: 10.1159/000080790. [DOI] [PubMed] [Google Scholar]
- 3.Harris RC, Neilson EG. Toward a unified theory of renal progression. Annu Rev Med. 2006;57:365–380. doi: 10.1146/annurev.med.57.121304.131342. [DOI] [PubMed] [Google Scholar]
- 4.De Vecchi AF, Dratwa M, Wiedemann ME. Healthcare systems and end-stage renal disease (ESRD) therapies-an international review: costs and reimbursement/funding of ESRD therapies. Nephrol Dial Transplant. 1999;14(Suppl 6):31–41. doi: 10.1093/ndt/14.suppl_6.31. [DOI] [PubMed] [Google Scholar]
- 5.Remuzzi G, Benigni A, Remuzzi A. Mechanisms of progression and regression of renal lesions of chronic nephro-pathies and diabetes. J Clin Invest. 2006;116:288–296. doi: 10.1172/JCI27699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Border WA, Noble NA. Transforming growth factor-β in tissue fibrosis. N Engl J Med. 1994;331:1286–1292. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- 7.Nakamura T, Miller D, Ruoslahti E, et al. Production of extracellular matrix by glomerular epithelial cells is regulated by transforming growth factor-β. Kidney Int. 1992;41:1213–1221. doi: 10.1038/ki.1992.183. [DOI] [PubMed] [Google Scholar]
- 8.Wrana JL, Attisano L, Wieser R, et al. Mechanism of activation of the TGF-β receptor. Nature. 1994;370:341–347. doi: 10.1038/370341a0. [DOI] [PubMed] [Google Scholar]
- 9.Massague J, Wotton D. Transcriptional control by the TGF-beta Smad signaling system. EMBO J. 2000;19:1745–1754. doi: 10.1093/emboj/19.8.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamamoto T, Noble NA, Cohen AH, et al. Expression of transforming growth factor-beta isoforms in human glomerular disease. Kidney Int. 1996;49:461–469. doi: 10.1038/ki.1996.65. [DOI] [PubMed] [Google Scholar]
- 11.Klahr S. New insights into the consequences and mechanisms of renal impairment in obstructive nephrophaty. Am J Kidney Dis. 1991;18:689–699. doi: 10.1016/S0272-6386(12)80611-1. [DOI] [PubMed] [Google Scholar]
- 12.Klahr S, Purkerson ML. The pathophysilolgy of obstructive nephrophaty: The role of vasoactive compounds in the hemo-dynamic and structural abnormalities of the obstructed kidney. Am J Kidney Dis. 1994;23:219–223. doi: 10.1016/S0272-6386(12)80975-9. [DOI] [PubMed] [Google Scholar]
- 13.Klahr S, Morrissey J. Obstructive nephrophathy and renal fibrosis. Am J Physiol. 2002;283:F861–875. doi: 10.1152/ajprenal.00362.2001. [DOI] [PubMed] [Google Scholar]
- 14.Piskounova E, Polytarchou C, Thornton JE, et al. Lin28A and Lin28B inhibit let-7 microRNA biogenesis by distinct mechanisms. Cell. 2011;147:1066–1079. doi: 10.1016/j.cell.2011.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu B, Huang Y. Lin28 modulates cell growth and associates with a subset of cell cycle regulator mRNAs in mouse embryonic stem cells. RNA. 2009;15:357–361. doi: 10.1261/rna.1368009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balzeau J, Menezes MR, Cao S, et al. The LIN28/let-7 pathway in Cancer. Front Genet. 2017;8:31. doi: 10.3389/fgene.2017.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Polesskaya A, Cuvellier S, Naguibneva I, et al. Lin-28 binds IGF-2 mRNA and participates in skeletal myogenesis by increasing translation efficiency. Genes Dev. 2007;21:1125–1138. doi: 10.1101/gad.415007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cimadamore F, Amador A. SOX2-Lin28/let-7 pathway regulates proliferation and neurogenesis in neural precursors. Proc Natl Acad Sci U S A. 2013;110:E3017–3026. doi: 10.1073/pnas.1220176110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yuan J, Nguyen CK, Liu X, et al. Lin28b reprograms adult bone marrow hematopoietic progenitors to mediate feta-like lymphopoiesis. Science. 2012;335:1195–1200. doi: 10.1126/science.1216557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu H, Shyh-Chang N, Segrè AV, et al. The Lin28/let-7 axis regulates glucose metabolism. Cell. 2011;147:81–94. doi: 10.1016/j.cell.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park JT, Kato M, Lanting L, et al. Repression of let-7 by transforming growth factor-β1-induced Lin28 upregulates collagen expression in glomerular mesangial cells under diabetic conditions. Am J Physiol Renal Physiol. 2014;307:F1390–1403. doi: 10.1152/ajprenal.00458.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang B, Jha JC, Hagiwara S, et al. Transforming growth factor-β1-mediated renal fibrosis is dependent on the regulation of transforming growth factor receptor 1 expression by let-7b. Kidney Int. 2014;85:352–361. doi: 10.1038/ki.2013.372. [DOI] [PubMed] [Google Scholar]
- 23.Li N, Wang LJ, Xu WL, et al. MicroRNA-379-5p suppresses renal fibrosis by regulating the LIN28/let-7 axis in diabetic nephropathy. Int J Mol Med. 2019;44:1619–1628. doi: 10.3892/ijmm.2019.4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chevalier RL, Goyal S, Wolstenholme JT, et al. Obstructive nephropathy in the neonatal rat is attenuated by epidermal growth factor. Kidney Int. 1998;54:38–47. doi: 10.1046/j.1523-1755.1998.00966.x. [DOI] [PubMed] [Google Scholar]
- 25.Chevalier RL, Forbes MS, Thornhill BA. Ureteral obstruction as a model of renal interstitial fibrosis and obstructive nephropathy. Kidney Int. 2009;75:1145–1152. doi: 10.1038/ki.2009.86. [DOI] [PubMed] [Google Scholar]
- 26.Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 27.McDaniel K, Huang L, Sato K, et al. The let-7/Lin28 axis regulates activation of hepatic stellate cells in alcoholic liver injury. J Biol Chem. 2017;292:11336–11347. doi: 10.1074/jbc.M116.773291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madison BB, Liu Q, Zhong X, et al. Lin28B promotes growth and tumorigenesis of the intestinal epithelium via Let-7. Genes Dev. 2013;27:2233–2245. doi: 10.1101/gad.224659.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Molenaar JJ, Domingo-Fernández R, Ebus ME, et al. LIN28B induces neuroblastoma and enhances MYCN levels via let-7 suppression. Nat Genet. 2012;44:1199–1206. doi: 10.1038/ng.2436. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen LH, Robinton DA, Seligson MT, et al. Lin28b is sufficient to drive liver cancer and necessary for its maintenance in murin models. Cancer Cell. 2014;26:248–261. doi: 10.1016/j.ccr.2014.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Viswanathan SR, Powers JT, Einhorn W, et al. Lin28 promotes transformation and is associated with advanced human malignancies. Nat Genet. 2009;41:843–848. doi: 10.1038/ng.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liang H, Liu S, Chen Y, et al. mi-R-26a suppresses EMT by disrupting the lin28B/let-7d axis: potential cross-talks among miRNA in IPE. J Mol Med (Berl) 2016;94:655–665. doi: 10.1007/s00109-016-1381-8. [DOI] [PubMed] [Google Scholar]
- 33.Strutz F, Zeisberg M. Renal fibroblasts and myofibroblasts in chronic kidney disease. J Am Soc Nephrol. 2006;17:2292–2298. doi: 10.1681/ASN.2006050420. [DOI] [PubMed] [Google Scholar]
- 34.Verrecchia F, Mauviel AJ. Transforming growth factoreta signaling through the Smad pathway: Role in extracellular matrix gene expression and regulation. J Invest Dermatol. 2002;118:211–215. doi: 10.1046/j.1523-1747.2002.01641.x. [DOI] [PubMed] [Google Scholar]
- 35.Yamamoto T, Nakamura T, Noble NA, et al. Expression of transforming growth factor beta is elevated in human and experimental diabetic nephropathy. Proc Natl Acad Sci U S A. 1993;90:1814–1818. doi: 10.1073/pnas.90.5.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakamura T, Ebihara I, Fukui M, et al. Messenger RNA expression for growth factors in glomeruli from focal glome-rular sclerosis. Clin Immunol Immunopathol. 1993;66:33–42. doi: 10.1006/clin.1993.1005. [DOI] [PubMed] [Google Scholar]
- 37.Yoshioka K, Takemura T, Murakami K, et al. Transforming growth factor-beta protein and mRNA in glomeruli in normal and diseased human kidneys. Lab Invest. 1993;68:154–163. [PubMed] [Google Scholar]
- 38.Cheng J, Grand JP. Transforming growth factor-beta signal transduction and progressive renal disease. Exp Biol Med. 2002;227:943–956. doi: 10.1177/153537020222701102. [DOI] [PubMed] [Google Scholar]
- 39.Li JH, Zhu HJ, Huang XR, et al. Smad7 inhibits fibrotic effect of TGF-Beta on renal tubular epithelial cells by blocking Smad2 activation. J Am Soc Nephrol. 2002;13:1464–1472. doi: 10.1097/01.ASN.0000014252.37680.E4. [DOI] [PubMed] [Google Scholar]
- 40.Deng B, Yang X, Liu J, et al. Focal adhesion kinase mediates TGF-beta1-induced renal tubular epithelial-to-mesenchymal transition in vitro. Mol Cell Biochem. 2010;340:21–29. doi: 10.1007/s11010-010-0396-7. [DOI] [PubMed] [Google Scholar]
- 41.OH CJ, Kim JY, Choi YK, et al. Dimethylfumarate attenuates renal fibrosis via NF-E2-related factor 2-mediated inhibition of transforming growth factor-β/Smad signaling. PLoS One. 2012;7:e45870. doi: 10.1371/journal.pone.0045870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou X, Zhang J, Xu C, et al. Curcumin ameliorates renal fibrosis by inhibiting local fibroblast proliferation and extracellular matrix deposition. J Pharmacol Sci. 2014;126:344–350. doi: 10.1254/jphs.14173FP. [DOI] [PubMed] [Google Scholar]
- 43.Zhang YE. Non-smad pathways in TGF-β signaling. Cell Res Res. 2009;19:128–139. doi: 10.1038/cr.2008.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamashita S, Maeshima A, Kojima I, et al. Activin A is a potent activator of renal interstitial fibroblasts. J Am Soc Nephrol. 2004;15:91–101. doi: 10.1097/01.ASN.0000103225.68136.E6. [DOI] [PubMed] [Google Scholar]