Abstract
Macrophages are re-educated and polarized in response to myocardial infarction (MI). The M2 anti-inflammatory phenotype is a known dominator of late stage MI. Mesenchymal stem cells (MSCs) represent a promising tool for cell therapy, particularly heart related diseases. In general, MSCs induce alteration of the macrophage subtype from M1 to M2, both in vitro and in vivo. We conjectured that hypoxic conditions can promote secretome productivity of MSCs. Hypoxia induces TGF-β1 expression, and TGF-β1 mediates M2 macrophage polarization for anti-inflammation and angiogenesis in infarcted areas. We hypothesized that macrophages undergo advanced M2 polarization after exposure to MSCs in hypoxia. Treatment of MSCs derived hypoxic conditioned medium (hypo-CM) promoted M2 phenotype and neovascularization through the TGF-β1/Smad3 pathway. In addition, hypo-CM derived from MSCs improved restoration of ischemic heart, such as attenuating cell apoptosis and fibrosis, and ameliorating microvessel density. Based on our results, we propose a new therapeutic method for effective MI treatment using regulation of macrophage polarization.
Keywords: Hypoxic conditioned medium, M2 macrophage, Macrophage polarization, Mesenchymal stem cells, Myocardial infarction therapy
INTRODUCTION
Despite current advances in pharmacological and biomedical techniques, myocardial infarction (MI) remains a major cause of morbidity and mortality worldwide (1, 2). In infarcted regions, insufficient oxygen supply results in inflammatory responses and necrosis of cardiac cells (3, 4). Following MI, macrophages are identified as the key components that control innate immunity and tissue homeostasis (5, 6). They are heterogeneous and plastic cells which polarize into pro-inflammatory and classically activated (M1) or anti-inflammatory and alternatively activated (M2) subtypes in the post-MI physiological and pathological environment (5, 7-9). Lipoproteins, cytokines, chemokines of activated neighboring cells, cell debris and microbial products are known to affect macrophage reprogramming (9, 10). Especially, M2 activation is induced by the transforming growth factor (TGF)-β1, indoleamine 2,3-dioxygenase (IDO), prostaglandin E2 (PGE2), interleukin (IL)-4 and IL-13. (4, 9, 11). In response to MI, macrophages regulate multiple aspects of the injured area including inflammation, scar formation and angiogenesis (12). Previous studies report that M1 macrophages undergo conversion to M2 in the post-MI inflammation stage (10, 13).
Mesenchymal stem cells (MSCs) provide immunosuppressive effects that have great potential in providing treatment for immunological and inflammatory disorders (14). MSCs have anti-inflammatory properties that affect the bone marrow-derived macrophage (BMDM) infiltration and regulation of their activated state (15). Recent studies indicate that MSCs instruct the reprogramming of macrophages into alternative phenotypes, thereby producing immunoregulatory factors such as IDO, IL-10, or TGF-β1 (16, 17). Conditioned medium (CM) derived from MSCs contains various bioactive molecules including cytokines, growth factors and RNA fragments, which play a prominent role in MSC-mediated therapies (14, 16). These therapeutic developments of MSCs have engaged the attention of researchers for investigating suitable applications for optimizing environmental factors which enhance the bioactive action of MSCs (18). One of the factors, O2 concentration affects various MSC properties including cell proliferation, plasticity and engraftment, and immunomodulatory ability (18). Hypoxia affects alterations in the gene expression, secretion of paracrine factors of MSCs (19, 20).
Given the significantly therapeutic properties of M2 macrophages and MSCs in MI, the current study demonstrates the effects of CM derived from MSCs under hypoxic culture condition, on the polarization and function of BMDMs. We investigated how hypoxic CM (hypo-CM) derived from MSCs regulates the M2 polarization, and affects the cardioprotective effects.
RESULTS
Expression of hypoxia related molecules induced under hypoxic conditions in MSCs
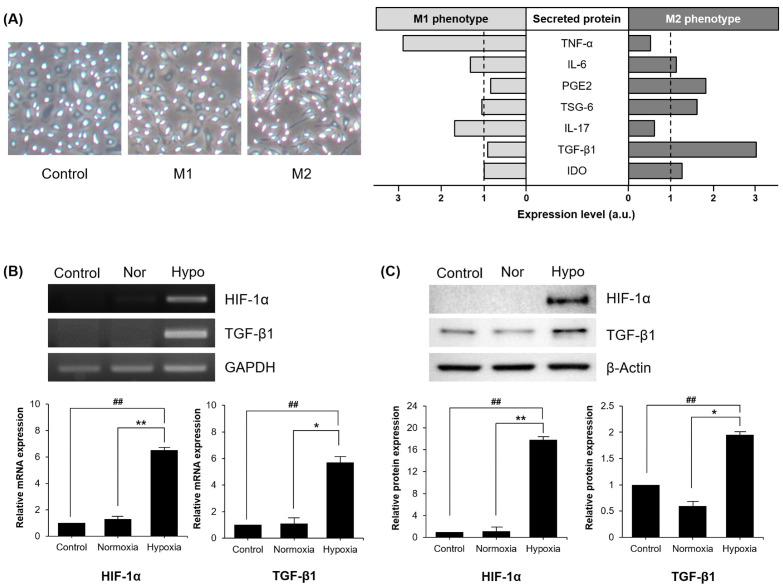
In the process for rescuing infarcted regions, MSCs secrete various paracrine factors that facilitate macrophage transition. We analyzed secretomes from MSCs for macrophage polarization (Fig. 1A). Macrophage polarization toward alternative phenotype was induced by PGE2, TNF-stimulated gene 6 protein (TSG-6), IDO, TGF-β1 from MSCs. Conversely, transition from naïve to the classical phenotype was advanced by TNF-α and IL-17 from MSCs. Hypoxia is determined to have an effect on the therapeutic effects of stem cells (21). MSCs treated under hypoxic condition is reported to generate extensive secretion of diverse cytokines and growth factors (22, 23). Assuming that hypoxia promotes paracrine products derived from MSCs, we examined alterations in the expression of secretomes in MSCs treated under hypoxic condition (Fig. 1B, C). As expected, mRNA expression of hypoxia inducible factor (HIF)-1α, a transcription factor that accumulates under low-oxygen conditions, was detected in MSCs exposed to hypoxia. Increase of mRNA and protein expression of TGF-β1, a factor related to immunosuppressive response and M2 polarization, was also detected in hypoxic MSCs. Our results indicate that a low oxygen environment significantly increases the expression level of TGF-β1, subsequent to inducement of HIF-1α expression.
Fig. 1.
Hypoxia induced TGFβ-1 expression on MSCs. (A) MSCs secrete various proteins involved in macrophage polarization. (B, C) mRNA and protein expression levels of HIF-1α and TGF-β1 are presented in MSCs treated with hypoxia. Control, MSCs treated with 10% FBS medium in a humidified atmosphere containing 5% CO2; Nor, normoxia, MSCs treated with SF medium in a humidified atmosphere containing 5% CO2; Hypo, hypoxia. Each experiment was repeated three times. Columns, mean; bars, SE. **P <0.01 and *P <0.05, and ##P < 0.001.
Enhancement of M2 macrophage polarization after exposure to hypoxic conditioned medium derived from MSCs
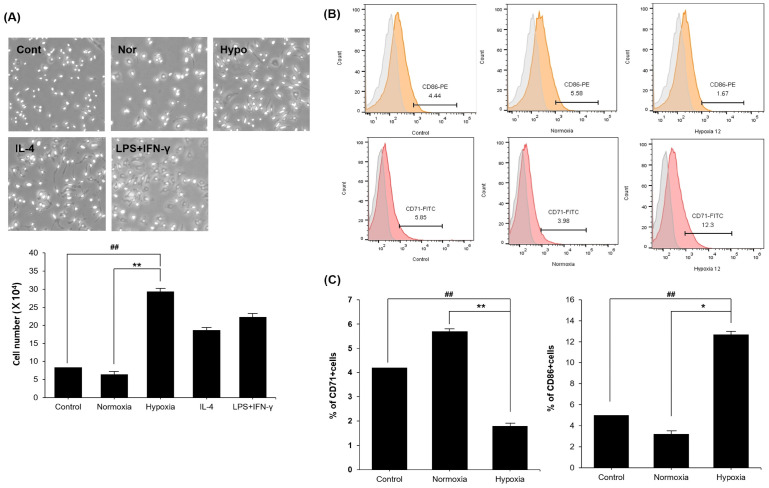
TGF-β1 induces the transition of non-activated macrophages into an alternatively activated phenotype (24, 25). We investigated whether hypo-CM derived from MSCs affects the progress of BMDMs for specialization toward the M2 subtype. BMDMs were divided into 5 groups cultured in different media. Morphological alterations verified that BMDMs exposed to IL-4 were more elongated, in comparison with the lipopolysaccharides (LPS) and interferon (IFN)-γ exposed group. Treatment with LPS and IFN-γ stimulated the M1 macrophage, while treatment with IL-4 resulted in a transition to the M2 macrophage (25). BMDMs treated with the hypo-CM derived from MSCs had similar morphology as the IL-4 exposed group rather than LPS and IFN-γ group. Quantitative analysis of polarized BMDMs are presented in Fig. 2A. To detect polarized macrophages, we selected CD86 antibody as an M1 marker, and CD71 antibody as an M2 marker. FITC-CD71 signals were detected in 5.85% control cells, 3.98% cells exposed to nor-CM derived from MSCs, and 13.2% cells exposed to hypo-CM derived from MSCs (Fig. 2B). Taken together, these data indicate that the hypo-CM derived from MSCs has capability to increase M2 polarization.
Fig. 2.
MSCs derived hypoxic conditioned medium promotes M2 macrophage polarization. (A) The morphology of polarized BMDMs treated with hypo-CM derived from MSCs were similar to IL-4. (B, C) De-crease of M1 polarization is presented through treatment of hypo-CM derived from MSCs in vitro. Treatment of hypo-CM derived from MSCs in vitro resulted in increased M2 polarization. Control, BMDMs treated with 10% FBS medium; Nor, BMDMs treated with SF medium; Hypo, hypoxia, BMDMs treated with hypo-CM derived from MSCs. All groups were cultured at 37°C in a humidified atmosphere containing 5% CO2. Each experiment was repeated three times. Columns, mean; bars, SE. **P <0.01 and *P <0.05, and ##P < 0.001.
Activation of TGF-β1/Smad3 signaling pathway and neovascularization of polarized macrophages by hypoxic conditioned medium derived from MSCs
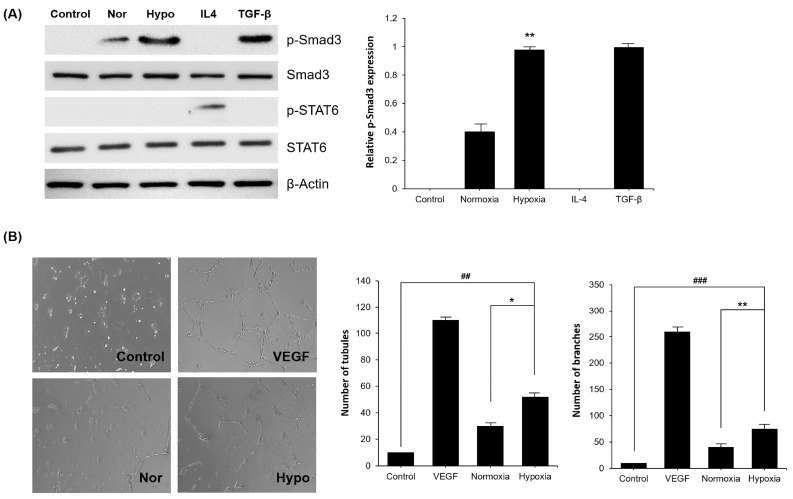
Polarization of M2 macrophages is achieved through several signaling pathways, including TGF-β1/Smad3, IL-4/STAT6, and IL-10/STAT3 (26). In the present study, we examined whether TGF-β1/Smad3 pathway is related to M2 polarization after exposure to hypo-CM derived from MSCs. The results show that expression level of p-Smad3 was higher in the group exposed to hypo-CM derived from MSCs, than normoxic CM (nor-CM) derived from MSCs. However, p-STAT6 was detected only in the IL-4 treated group. This indicates that hypo-CM derived from MSCs promotes M2 polarization through the TGF-β1/Smad3 pathway (Fig. 3A). To confirm the angiogenesis capacity of polarized macrophages induced by hypo-CM derived from MSCs, human umbilical vein endothelial cells (HUVECs) were co-cultured with BMDMs exposed to different CM. The results indicate that BMDMs cultured with hypo-CM derived from MSCs enhances branchial and tubular structure formation, as compared to nor-CM derived from MSCs (Fig. 3B). Taken together, these results indicate that hypo-CM derived from MSCs promotes M2 polarization and neovascularization by inducing the TGF-β1/Smad3 signaling pathway.
Fig. 3.
Activation of Smad pathway in polarized M2 macrophages by hypo-CM derived from MSCs. (A) The expression levels of p-Smad3/Smad3 and p-STAT6/STAT6 were assessed in polarized macrophages. Relative expression of p-Smad3 was increased in the hypo-CM derived from MSCs treated group. (B) Formation of branchial and tubular structures of HUVECs co-cultured with macrophages treated under different conditions. Treatment of hypo-CM derived from MSCs promotes the angiogenic capacity of polarized macrophages in vitro. Control, BMDMs treated with 10% FBS medium; Nor, BMDMs treated with SF medium; Hypo, hypoxia, BMDMs treated with hypo-CM derived from MSCs. All groups were cultured at 37°C in a humidified atmosphere containing 5% CO2. Each experiment was repeated three times. Columns, mean; bars, SE. **P <0.01 and *P <0.05, and ##P < 0.001.
Recovery of ischemic heart by MSCs exposed to hypoxia in vivo
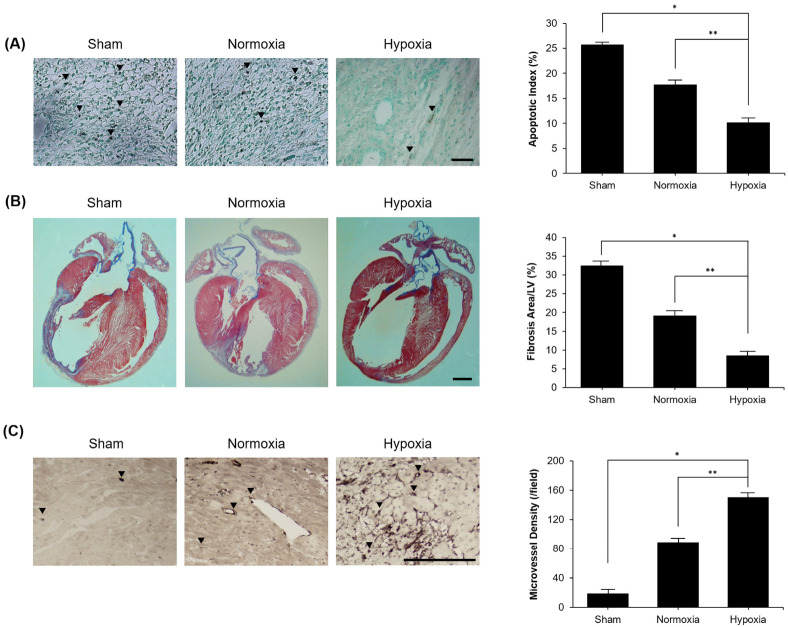
Based on our in vitro data, we anticipated that hypoxia encourages therapeutic effects of hypo-CM derived from MSCs through activation of M2 polarization in infarcted regions. Analysis of apoptotic cell death in infarcted heart was identified by the TUNEL assay. Normal cells were stained green and TUNEL-positive cells were stained brown. We observed that TUNEL-positive myocardial cells were significantly reduced in the hypo-CM derived from MSCs, as compared to control group (Fig. 4A). Masson’s trichrome staining was performed for determining area of cardiac fibrosis, where fibrotic tissue stains purple. Fibrosis was significantly decreased in the hypo-CM derived from MSCs, and remained noticeably unchanged in the nor-CM derived from MSCs (Fig. 4B). We next measured the microvessel density. Quantitative analysis of stained brown CD31-positive cells showed significant increase in hypo-CM derived from MSCs as compared to the control group (Fig. 4C).
Fig. 4.
Hypo-CM derived from MSCs improved ischemic heart. (A) TUNEL-positive myocardial cells were significantly reduced in group treated with hypo-CM derived from MSCs, as compared to sham group (scale bar = 100 µm, magnification: 200×). (B) Fibrotic area confirmed by Masson’s trichrome staining was significantly decreased in treatment group of hypo-CM derived from MSCs (scale bar = 2 mm). (C) Quantitative analysis was performed to ascertain positive microvessel density. Stained cells were significantly increased in group exposed to hypo-CM derived from MSCs, as compared to control group (scale bar = 200 µm, magnification: 200×). Each experiment was repeated five times. Columns, mean; bars, SE. *P < 0.001, **P < 0.01.
DISCUSSION
Post-MI, the continuous deterioration in infarcted regions require supplementary therapeutic methods. It has previously been reported that macrophage polarization plays a significant role in cardiac remodeling, overcoming clinical challenges in the progression of MI (24, 27). In response to MI, monocytes in BM and spleen proceed to the infarcted heart and infiltrate the ischemic area (4, 28). These cells are heterogeneous, and are polarized to M1 and M2 subtypes for providing cardioprotective functions (4, 24). In the mice model, M1 macrophages are predominant at 1-3 days after MI, whereas M2 macrophages are the major component at 5-7 days after MI (28). We focused on the M2 phenotype as a therapeutic target for post-MI rebuilding and restoration. Our study aimed to promote polarization of the M2 macrophages which result in decreased cardiac cell apoptosis and fibrosis, and increased microvessel construction (29).
Numerous studies in stem cell research have focused on the repair and remodeling of the infarcted LV post-MI (30). Recent studies of stem cell therapy present cooperative work with macrophage polarization. MSCs are a promising source of stem cells for application to cardiac disease treatments, and have supported the salutary effects of M2 macrophages for resolution of inflammation during healing in ischemic region (30). Transplantation of MSCs increases the number of M2 macrophages after MI (31). Hence, we endeavored to propose a way to enhance the therapeutic effect of MSCs. Hypoxic conditions induce TGF-β1 production from MSCs (32). We considered that hypoxia would promote the various potentials of MSCs, including regulation of immune modulatory properties associated with macrophages. Moreover, TGF-β1/Smad3 signaling in macrophages is known for regulating restoration and remodeling in the infarcted myocardium (33).
In the current study, we hypothesized that MSCs exposed to hypoxia would accelerate BMDM polarization to the M2 phenotype. We observed that hypoxia affects the expression levels of TGF-β1 and HIF-1α in MSCs. HIF-1α leads to TGF-β1 expression through regulation of TGF-β1 promoter activity in hypoxic condition (34). We further demonstrated the effects of hypo-CM derived from MSCs to switch the BM-derived macrophages phenotype, increasing the frequency of alternatively activated anti-inflammatory macrophages. Macrophage-specific TGF-β1/Smad3 signaling pathway are activated at the site of injury (33). Our results indicate that TGF-β1/Smad3 signaling pathway is the probable mechanism for M2 macrophage polarization, thereby establishing the mechanism of M2 polarization TGF-β1/Smad3 signaling pathway. Treatment of hypo-CM derived from MSCs also encouraged M2 macrophage polarization through increase of p-Smad3/Smad3 expression. Neovascularization is one of the healing processes that presents after MI. Macrophages treated with hypo-CM derived from MSCs increase the formation of tubular and branch-like forms in vitro, thereby ascertaining that hypo-CM derived from MSCs promotes macrophage polarization to M2 subtype through TGF-β1/Smad3 signaling pathway by increasing the neovascularization process. In addition, we demonstrated that MSCs treated with hypoxia improves states of ischemic stroke, by reducing apoptotic cells and fibrosis, and increasing neovascularization in the infarcted area. When macrophages are exposed to MSCs treated with hypoxic condition, they can alleviate cardiac fibrosis and apoptosis, and reinforce microvessel density after MI injury.
Hypoxia exerts a synergistic potential which increases the therapeutic effects of MSCs by increasing BMDM polarization toward M2 phenotype. After careful deliberation of our data, we propose that this approach provides a new advanced stem cell therapy for treatment of acute infarcted heart.
MATERIALS AND METHODS
See supplementary information for Materials and Methods.
Supplemental Materials
ACKNOWLEDGEMENTS
This research was supported by a 2-Year Research Grant of Pusan National University.
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting interests.
REFERENCES
- 1.Weinberger T, Schulz C. Myocardial infarction: a critical role of macrophages in cardiac remodeling. Front Physiol. 2015;6:107. doi: 10.3389/fphys.2015.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duncan SE, Gao S, Sarhene M, et al. Macrophage Activities in Myocardial Infarction and Heart Failure. Cardiol Res Pract. 2020;2020:4375127. doi: 10.1155/2020/4375127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lambert JM, Lopez EF, Lindsey ML. Macrophage roles following myocardial infarction. Int J Cardiol. 2008;130:147–158. doi: 10.1016/j.ijcard.2008.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gombozhapova A, Rogovskaya Y, Shurupov V, et al. Macrophage activation and polarization in post-infarction cardiac remodeling. J Biomed Sci. 2017;24:13. doi: 10.1186/s12929-017-0322-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y, Smith W, Hao D, He B, Kong L. M1 and M2 macrophage polarization and potentially therapeutic naturally occurring compounds. Int Immunopharmacol. 2019;70:459–466. doi: 10.1016/j.intimp.2019.02.050. [DOI] [PubMed] [Google Scholar]
- 6.Dick SA, Macklin JA, Nejat S, et al. Self-renewing resident cardiac macrophages limit adverse remodeling following myocardial infarction. Nat Immunol. 2019;20:29–39. doi: 10.1038/s41590-018-0272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Orecchioni M, Ghosheh Y, Pramod AB, Ley K. Macrophage polarization: different gene signatures in M1 (LPS+) vs. classically and M2(LPS−) vs. alternatively activated macrophages. Front Immunol. 2019;10:1084. doi: 10.3389/fimmu.2019.01084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parisi L, Gini E, Baci D, et al. Macrophage Polarization in Chronic Inflammatory Diseases: Killers or Builders? J Immunol Res. 2018;2018:8917804. doi: 10.1155/2018/8917804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gong M, Zhuo X, Ma A. STAT6 Upregulation promotes M2 macrophage polarization to suppress atherosclerosis. Med Sci Monit Basic Res. 2017;23:240–249. doi: 10.12659/MSMBR.904014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horckmans M, Ring L, Duchene J, et al. Neutrophils orchestrate post-myocardial infarction healing by polarizing macrophages towards a reparative phenotype. Eur Heart J. 2017;38:187–197. doi: 10.1093/eurheartj/ehw002. [DOI] [PubMed] [Google Scholar]
- 11.Thapa B, Lee K. Metabolic influence on macro-phage polarization and pathogenesis. BMB Rep. 2019;52:360–372. doi: 10.5483/BMBRep.2019.52.6.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mouton AJ, DeLeon-Pennell KY, Rivera Gonzalez OJ, et al. Mapping macrophage polarization over the myocardial infarction time continuum. Basic Res Cardiol. 2018;113:26. doi: 10.1007/s00395-018-0686-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nahrendorf M, Swirski FK. Monocyte and macro-phage heterogeneity in the heart. Circ Res. 2013;112:1624–1633. doi: 10.1161/CIRCRESAHA.113.300890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin L, Deng Z, Zhang J, et al. Mesenchymal stem cells promote type 2 macrophage polarization to ameliorate the myocardial injury caused by diabetic cardiomyopathy. J Transl Med. 2019;17:251. doi: 10.1186/s12967-019-1999-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dayan V, Yannarelli G, Billia F, et al. Mesenchymal stromal cells mediate a switch to alternatively activated monocytes/macrophages after acute myocardial infarction. Basic Res Cardiol. 2011;106:1299–1310. doi: 10.1007/s00395-011-0221-9. [DOI] [PubMed] [Google Scholar]
- 16.Zhou YZ, Cheng Z, Wu Y, et al. Mesenchymal stem cell-derived conditioned medium attenuate angiotensin II-induced aortic aneurysm growth by modulating macrophage polarization. J Cell Mol Med. 2019;23:8233–8245. doi: 10.1111/jcmm.14694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim SH, Oh KW, Jin HK, Bae JS. Immune inflammatory modulation as a potential therapeutic strategy of stem cell therapy for ALS and neurodegenerative diseases. BMB Rep. 2018;51:545–546. doi: 10.5483/BMBRep.2018.51.11.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noronha NC, Mizukami A, Caliári-Oliveira C, et al. Priming approaches to improve the efficacy of mesenchymal stromal cell-based therapies. Stem Cell Res Ther. 2019;10:131. doi: 10.1186/s13287-019-1224-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ejtehadifar M, Shamsasenjan K, Movassaghpour A, et al. The effect of hypoxia on mesenchymal stem cell biology. Adv Pharm Bull. 2015;5:141–149. doi: 10.15171/apb.2015.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yabluchanskiy A, Ma Y, DeLeon-Pennell KY, et al. Myocardial infarction superimposed on aging: MMP-9 deletion promotes M2 macrophage polarization. J Gerontol A Biol Sci Med Sci. 2016;71:475–483. doi: 10.1093/gerona/glv034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwon SY, Chun SY, Ha YS, et al. Hypoxia enhances cell properties of human mesenchymal stem cells. Tissue Eng Regen Med. 2017;14:595–604. doi: 10.1007/s13770-017-0068-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kinnaird T, Stabile E, Burnett MS, et al. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res. 2004;94:678–685. doi: 10.1161/01.RES.0000118601.37875.AC. [DOI] [PubMed] [Google Scholar]
- 23.Almeria C, Weiss R, Roy M, et al. Hypoxia conditioned mesenchymal stem cell-derived extracellular vesicles induce increased vascular tube formation in vitro. Front Bioeng Biotechnol. 2019;7:292. doi: 10.3389/fbioe.2019.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang F, Wang H, Wang X, et al. TGF-β induces M2-like macrophage polarization via SNAIL-mediated suppression of a pro-inflammatory phenotype. Oncotarget. 2016;7:52294–52306. doi: 10.18632/oncotarget.10561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gong D, Shi W, Yi SJ, Chen H, et al. TGFβ signaling plays a critical role in promoting alternative macrophage activation. BMC Immunol. 2012;13:31. doi: 10.1186/1471-2172-13-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson HM. SOCS proteins in macrophage polarization and function. Front Immunol. 2014;5:357. doi: 10.3389/fimmu.2014.00357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peet C, Ivetic A, Bromage DI, Shah AM. Cardiac monocytes and macrophages after myocardial infarction. Cardiovasc Res. 2020;116:1101–1112. doi: 10.1093/cvr/cvz336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma Y, Mouton AJ, Lindsey ML. Cardiac macrophage biology in the steady-state heart, the aging heart, and following myocardial infarction. Transl Res. 2018;191:15–28. doi: 10.1016/j.trsl.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu J, Niu J, Li X, et al. TGF-β1 induces senescence of bone marrow mesenchymal stem cells via increase of mitochondrial ROS production. BMC Dev Biol. 2014;14:21. doi: 10.1186/1471-213X-14-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swirski FK, Nahrendorf M. Macrophage-stem cell crosstalk after myocardial infarction. J Am Coll Cardiol. 2013;62:1902–1904. doi: 10.1016/j.jacc.2013.07.058. [DOI] [PubMed] [Google Scholar]
- 31.Ben-Mordechai T, Holbova R, Landa-Rouben N, et al. Macrophage subpopulations are essential for infarct repair with and without stem cell therapy. J Am Coll Cardiol. 2013;62:1890–1901. doi: 10.1016/j.jacc.2013.07.057. [DOI] [PubMed] [Google Scholar]
- 32.Jiang Y, Dai A, Li Q, Hu R. Hypoxia induces transforming growth factor-beta1 gene expression in the pulmonary artery of rats via hypoxia-inducible factor-1alpha. Acta Biochim Biophys Sin (Shanghai) 2006;(Shanghai):73–80. doi: 10.1111/j.1745-7270.2007.00249.x. [DOI] [PubMed] [Google Scholar]
- 33.Chen B, Huang S, Su Y, et al. Macrophage Smad3 protects the infarcted heart, stimulating phagocytosis and regulating inflammation. Circ Res. 2019;125:55–70. doi: 10.1161/CIRCRESAHA.119.315069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hung SP, Yang MH, Tseng KF, Lee OK. Hypoxia-induced secretion of TGF-β1 in mesenchymal stem cell promotes breast cancer cell progression. Cell Transplant. 2013;22:1869–1882. doi: 10.3727/096368912X657954. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.