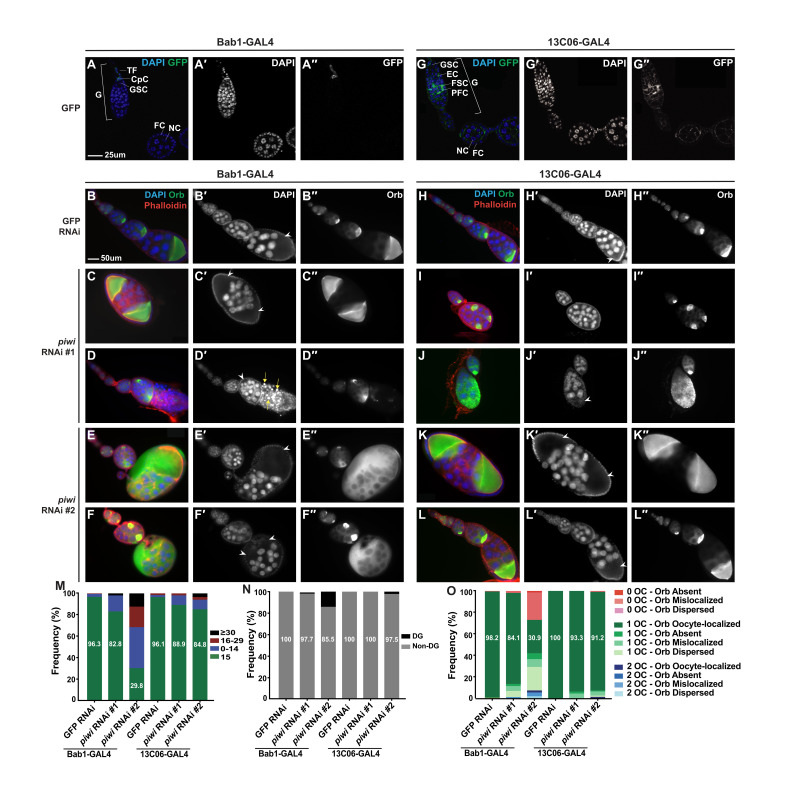
Figure 1. Knockdown of piwi expression in terminal filament and cap cells, but not posterior escort cells, results in GSC proliferation and differentiation defects.
(A – A′′) Bab1-GAL4 driving UASp-GFP demonstrates that the driver is expressed in terminal filament and cap cells. (B – F′′) Bab1-GAL4 RNAi experiments show that piwi depletion in terminal filament and cap cells causes dramatic morphological defects. DAPI marks nuclei, Orb is an oocyte-specific protein, and Phalloidin marks F-actin structures, including ring canals. Presumptive oocytes, identified by DAPI and Phalloidin staining, are indicated with white arrow heads. Degenerate nurse cells, identified by pyknotic nuclei, are indicated with yellow arrows. (G – G′′) 13C06-GAL4 driving UASp-GFP demonstrates that the driver is expressed in posterior escort cells, follicle stem cells, and prefollicle cells. (H – L′′) 13C06-GAL4 RNAi experiments show that piwi depletion in posterior escort cells, follicle stem cells, and prefollicle cells causes some morphological defects. DAPI staining marks nuclei, Orb is an oocyte-specific protein, and Phalloidin marks F-actin structures, including ring canals. Presumptive oocytes, identified by DAPI and Phalloidin staining, are indicated with white arrow heads. (M) The frequency of egg chambers with 15, 0-14, 16-29, and a tumorous (≥ 30) number of non-degenerate nurse cells per egg chamber for each genotype. Piwi-bab1KD#2 resulted in a decrease in the number of egg chambers with the expected 15 nurse cells. Piwi-13C06KDresulted in relatively few egg chambers with defects in nurse cell number. (N) The frequency of egg chambers that had non-degenerate and degenerate nurse cells. Both piwi-bab1KDand piwi-13C06KDresulted in relatively few egg chambers with degenerate nurse cells. (O) The frequency of egg chambers with zero, one, two, or three presumptive oocytes (OC) and with oocyte-localized, absent, mislocalized, or dispersed Orb staining. Piwi-bab1KD#2 resulted in a decrease in the number of egg chambers with only one presumptive oocyte and oocyte-localized Orb staining, while piwi-13C06KDresulted in relatively few egg chambers with defects in oocyte specification or Orb localization. The number of egg chambers counted for each genotype is as follows: bab1- GAL4>GFP RNAi = 109; bab1-GAL4>piwi RNAi #1 = 88; bab1-GAL4>piwi RNAi #2 = 94; 13C06-GAL4>GFP RNAi = 1210; 13C06-GAL4>piwi RNAi #1 = 90; 13C06-GAL4>piwi RNAi #2 = 79. CpC = cap cell, DG = degenerate, EC = escort cell, FC = follicle cell, FSC = follicle stem cell, G = germarium, GSC = germline stem cell, NC = nurse cell, OC = oocyte, PFC = prefollicle cell, TF = terminal filament.
Description
In the Drosophila ovary, the proliferation of germline cells and differentiation of the oocyte is informed by cues from a variety of ovarian somatic cell (OSC) types. In particular, the stem cell niche, composed of cap cells, terminal filament cells, and escort cells, directly signals to germline stem cells (GSCs) to promote their self-renewal and differentiation into cystoblasts. Cap cells express Decapentaplegic (Dpp), which signals to the germline to promote proliferation and maintenance of GSCs (Xie and Spradling 1998, Chen and McKearin 2003, Song et al. 2004). Subsequently, the differentiation of cystoblasts and their four synchronous divisions with incomplete cytokinesis to form 16-cell cysts depends on the repression of Dpp by Wnt, EGF, and Hedgehog signaling from escort cells (Liu et al. 2010, Luo et al. 2015, Mottier-Pavie et al. 2016, Huang et al. 2017). The germline cyst then becomes encased in somatic follicle cells to form an egg chamber, in which one germline cell is the oocyte and the other 15 are polyploid nurse cells. Follicle cells impact multiple aspects of germline differentiation throughout the rest of oogenesis, including polarization of the oocyte guided by JAK/STAT and Notch signaling (reviewed in Riechmann and Ephrussi 2001).
Early studies aimed at identifying genes involved in the balance of GSC maintenance and differentiation uncovered piwi (Cox et al. 1998, Cox et al. 2000). Clonal analysis showed that piwi expression in somatic, but not germline, cells is required for GSC maintenance (Cox et al. 1998). This led to the suggestion that Piwi mainly functions on GSCs through the stem cell niche. Mechanistic studies into Piwi and its associated small noncoding Piwi-interacting RNAs (piRNA), which guide Piwi to target sequences, have since focused on their role in transposon suppression. There is also a growing body of evidence that Piwi regulates some non-transposon gene expression programs during development (reviewed in Ozata et al. 2019).
Tissue-specific knockdown using the UAS/GAL4 system now provides an opportunity to dissect the function of Piwi in specific cell types of the ovary. Early studies that used this approach focused on the proliferation and differentiation of germline cells within the germarium, and revealed that depletion of piwi in escort cells results in an over-proliferation of undifferentiated GSCs into “GSC tumors” (Jin et al. 2013, Ma et al. 2014). In our study focusing on mid-stage egg chambers, after depleting piwi in all OSCs using traffic jam-GAL4 (“piwi-sKD”), we observed defects in nurse cell number, oocyte number, and oocyte specification (Gonzalez et al. 2020). Because the aberrant egg chambers often contained improper numbers of nurse cells (but rarely in multiples of 15) and could contain from 0 to 3 “oocytes” (determined by morphology and localization of the oocyte marker protein Orb), we concluded that this phenotype was more likely due to improper germline proliferation and differentiation than improper encapsulation of the germline by follicle cells. Here, we further investigate whether these defects are due to loss of Piwi activity in terminal filament and cap cells (using bab1-GAL4, Figure 1A-A′′), and/or in escort cells (using 13C06-GAL4, Figure 1G-G′′).
Depletion of piwi in terminal filament and cap cells via bab1-GAL4 (herein referred to as piwi–bab1KD, Figure 1B-1F′′) resulted in egg chambers with defects in nurse cell number and oocyte specification, partially phenocopying piwi-sKD driven by tj-GAL4, which depletes piwi in all OSCs. 96.3% of the GFP-bab1KD egg chambers contained the expected 15 nurse cells, while only 82.8% and 29.8% of egg chambers in piwi–bab1KD #1 and piwi-bab1KD #2, respectively, contained exactly 15 nurse cells (Figure 1M). However, unlike depletion of piwi in all somatic cells, very few egg chambers contained either tumorous (Figure 1M) or degenerate (Figure 1N) germline cells. Thus, piwi depletion in terminal filament and cap cells explains some, but not all, of the nurse cell number defects caused by piwi-sKD.
We also observed defects in oocyte specification upon piwi-bab1KD (Figure 1B-1F′′). Only 84.1% and 30.9% of piwi– bab1KD #1 and piwi–bab1KD #2 egg chambers, respectively, contained egg chambers with a single oocyte at the posterior of the egg chamber and displaying Orb accumulation (Figure 1O). Within egg chambers that contained one presumptive oocyte (96.6% and 65.8% of egg chambers in piwi–bab1KD #1 and piwi-bab1KD #2, respectively), the most common abnormality in oocyte phenotype was a dispersal of Orb throughout the egg chamber (Figure 1O), indicating that oocyte specification is impaired upon piwi–bab1KD.
Notably, piwi-bab1KD #1 had a milder effect on both nurse cell number and oocyte specification than piwi–bab1KD #2. We had previously shown that piwi-RNAi #2 more strongly depletes piwi mRNA levels compared to piwi-RNAi #1 when driven in all somatic cells (Gonzalez et al. 2020), so this may reflect that a threshold level of piwi is necessary in the stem cell niche for this function.
We then investigated the effect of depleting piwi in posterior escort cells, follicle stem cells, and pre-follicle cells using 13C06-GAL4 (Figure 1G-1L′′). Previous studies had shown a major role for Piwi in escort cells in regulating cystocyte differentiation (Jin et al. 2013, Ma et al. 2014), so we were surprised to observe relatively few egg chambers with defects in nurse cell number and/or oocyte specification following piwi-13C06KD (Figure 1M-1O). Our result indicates that Piwi activity in posterior escort cells and follicle stem cells may not regulate nurse cell number and oocyte specification, and suggests that the GSC tumors described by previous studies may not be linked to the mid-stage germline defects we have described. However, we depleted piwi using 13C06-GAL4, which is expressed weakly in anterior escort cells but strongly in posterior escort cells (Sahai-Hernandez and Nystul 2013), while previous studies used c587-GAL4, which expresses more strongly in anterior escort cells (Song et al. 2004), so it is possible that Piwi’s escort cell function is primarily in anterior escort cells.
Altogether, these results indicate that Piwi functions within the stem cell niche, and perhaps mostly within terminal filament and cap cells, to regulate germline proliferation and differentiation within the germarium, and that this has long-lasting developmental effects on egg chambers throughout oogenesis. The proliferation of germline cells and specification of the oocyte are temporally and spatially separate from the GSC-niche interaction, so the observation that gene expression in terminal filament and cap cells can influence these processes is striking. It suggests that the niche not only regulates the self-renewal of GSCs and their immediate differentiation into cystoblasts, but also modulates their developmental potential for processes that occur later in oogenesis. Alternatively, because bab1-GAL4 is also active in somatic cells of the larval gonad (Cho et al. 2018), our results may reflect a requirement for somatic piwi during gonadogenesis for the subsequent organization, proliferation, and specification of germline cells in the adult ovary.
Piwi regulates gene expression at both the transcriptional level (Brower-Toland et al. 2007, Wang & Elgin 2011, Sienski et al. 2012) and the post-transcriptional level (Robine et al. 2009, Saito et al. 2009, Klein et al. 2016), so further investigations should seek to understand which gene expression programs Piwi directly regulates to influence these oogenic processes. The observation that piwi depletion in somatic cells impacts the proliferation and specification of germline cells suggests that Piwi regulates the expression of genes involved in soma-to-germline signaling. Piwi has previously been shown to mediate Dpp signaling to regulate GSC maintenance (Jin et al. 2013, Ma et al. 2014), and the piRNA biogenesis factor Yb has been suggested to interact with the Notch pathway to regulate very similar nurse cell and oocyte phenotypes to those we have described upon piwi-sKD (Johnson et al. 1995). Future studies should investigate whether Piwi in the stem cell niche regulates the expression of genes in the Dpp, Notch, or other signaling pathways. These new findings would add to the growing evidence that the biological function of the PIWI/piRNA pathway extends well beyond the suppression of transposons.
Methods
Drosophila husbandry and genetics
All Drosophila stocks were raised on standard agar/molasses medium and raised at 25°C for experiments. Bab1-GAL4 (BDSC stock #6802) was used to express UASpconstructs in all cap cells and terminal filament cells throughout development, and 13C06-GAL4 (BDSC stock #47860) was used to express UASp constructs in posterior escort cells, follicle stem cells, and prefollicle cells. UASp-GFP (BDSC #1521) was used to verify the expression pattern of GAL4 lines. Two anti-Piwi RNAi lines (“piwi RNAi #1” and “piwi-RNAi #2” in this study) were used to knock down piwi expression. Piwi-siRNA #1 targets exon 2 of the piwi mRNA and is BDSC stock #37483; piwi siRNA #2 targets exon 3 of the piwi mRNA and was a gift from T. Xie, Stowers Institute For Medical Research, Kansas City, MO. GFP-RNAi (BDSC stock #41550) was used as a negative control. To generate GAL4/UASp flies for analysis, two males carrying the GAL4 driver were crossed with three virgin females carrying the UASp construct. GAL4/UASp females were aged for two to three days in a ratio of 2:1 with w1118 males prior to ovary dissection.
Immunostaining
Ovaries from 2-3 day old females were dissected in 1X PBS and fixed with 200 μL of the following fixing solution (v/v%): PBS (89.5%), 10% Nonidet P-40 (5%), 37% formaldehyde (5.5%). The fixed ovaries were then washed 3 times for 15 minutes each in PBST (PBS and 0.2% Triton X-100). Ovaries were then blocked overnight in 5% NGS at 4oC, followed by incubation overnight at 4oC in primary antibody diluted in PBST + 2% NGS. Samples were washed three times in PBST and incubated in secondary antibodies overnight at 4oC. Samples were washed three times with PBST, stained with DAPI (1:500) and Phalloidin (1:200, ThermoFisher, #R415) for 15 minutes and mounted in Vectashield mounting media (Vector Labs, #H1000).
We used mouse anti-Orb 4H8 (1:300, DSHB) to visualize Orb localization. The following conjugated secondary antibodies were used, all at 1:500 dilutions: The Alexa 488-conjugated goat anti-mouse antibody, the Alexa 555- conjugated goat anti-mouse. Orb, Phalloidin, and DAPI were used qualitatively and quantitatively characterize the observed phenotypes of the egg chambers from all crosses.
Microscopy and phenotypic characterization
To observe the GAL4 expression pattern, confocal images of DAPI stained bab1-GAL4>UASp-GFP and 13C06-GAL4>UASp-GFP ovaries were taken using Leica TCS SP5 Confocal Laser Scanning Microscope. Piwi-knockdown samples were analyzed on the ZEISS Axio Imager2 for quantitative and qualitative characterizations.
DAPI and Phalloidin staining was used to identify nurse cells and oocytes. To more accurately quantify the effect of piwi– bab1KD and piwi-13C06KD on the number of nurse cells, we created four categories to describe how many nurse cells were in an egg chamber: 15 normal nurse cells; 0-14 normal nurse cells; 16-29 normal nurse cells; and ≥30 normal nurse cells, which we referred to as a tumorous number. Degenerate nurse cells were identified by the presence of bright pyknotic nuclei. Any large area of cytoplasm without a polyploid nucleus was considered a presumptive oocyte. We quantified how many egg chambers had zero, one, two, or three presumptive oocytes. We also characterized the localization of the Orb staining based on its relative position with the presumptive oocyte. We created four categories of Orb’s localization pattern: absent, oocyte-localized, mislocalized, and dispersed. If the egg chamber had no trace of Orb staining, the pattern was considered absent. If the Orb staining localized with the presumptive oocyte(s) it was designated oocyte-localized; but if the Orb staining was not localized to the presumptive oocyte and formed discrete patches, it was considered mislocalized. If the Orb staining filled the entire egg chamber, its pattern was considered dispersed.
Acknowledgments
Acknowledgments
We acknowledge the Developmental Studies Hybridoma Bank for providing antibodies, the Bloomington Drosophila Stock Center and Ting Xie for providing fly stocks, and members of the H. Lin Lab for discussion and technical support.
Funding
This work was funded by a gift from Luye Life Sciences to H.L. Gina Zhu was supported by The Yale College Dean’s Research in the Sciences Fellowship, The John E. Linck and Alanne Headland Linck Fellowship, and the Gary Stein Fellowship. Lauren E Gonzalez was supported by NSF Graduate Research Fellowship Program (DGE1752134) and the Training Program in Genetics (NIH T32 GM007499).
References
- Brower-Toland B, Findley SD, Jiang L, Liu L, Yin H, Dus M, Zhou P, Elgin SC, Lin H. Drosophila PIWI associates with chromatin and interacts directly with HP1a. Genes Dev. 2007 Sep 15;21(18):2300–2311. doi: 10.1101/gad.1564307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, McKearin D. Dpp signaling silences bam transcription directly to establish asymmetric divisions of germline stem cells. Curr Biol. 2003 Oct 14;13(20):1786–1791. doi: 10.1016/j.cub.2003.09.033. [DOI] [PubMed] [Google Scholar]
- Cho Y, Lai CM, Lin KY, Hsu HJ. A Targeted RNAi Screen Reveals Drosophila Female-Sterile Genes That Control the Size of Germline Stem Cell Niche During Development. G3 (Bethesda) 2018 Jul 01;8(7):2345–2354. doi: 10.1534/g3.118.200355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox DN, Chao A, Baker J, Chang L, Qiao D, Lin H. A novel class of evolutionarily conserved genes defined by piwi are essential for stem cell self-renewal. Genes Dev. 1998 Dec 01;12(23):3715–3727. doi: 10.1101/gad.12.23.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox DN, Chao A, Lin H. piwi encodes a nucleoplasmic factor whose activity modulates the number and division rate of germline stem cells. Development. 2000 Feb 01;127(3):503–514. [Google Scholar]
- Gonzalez LE, Zhu G, Lin H. 2020. Ovarian somatic Piwi regulates nurse cell proliferation and oocyte specification in Drosophila. MiroPublication Biology. [DOI] [PMC free article] [PubMed]
- Huang J, Reilein A, Kalderon D. Yorkie and Hedgehog independently restrict BMP production in escort cells to permit germline differentiation in the Drosophila ovary. Development. 2017 Jun 15;144(14):2584–2594. doi: 10.1242/dev.147702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Z, Flynt AS, Lai EC. Drosophila piwi mutants exhibit germline stem cell tumors that are sustained by elevated Dpp signaling. Curr Biol. 2013 Jul 25;23(15):1442–1448. doi: 10.1016/j.cub.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E, Wayne S, Nagoshi R. fs (1) Yb is required for ovary follicle cell differentiation in Drosophila melanogaster and has genetic interactions with the Notch group of neurogenic genes. Genetics. 1995 May 01;140(1):207–217. doi: 10.1093/genetics/140.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein JD, Qu C, Yang X, Fan Y, Tang C, Peng JC. c-Fos Repression by Piwi Regulates Drosophila Ovarian Germline Formation and Tissue Morphogenesis. PLoS Genet. 2016 Sep 13;12(9):e1006281–e1006281. doi: 10.1371/journal.pgen.1006281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Lim TM, Cai Y. The Drosophila female germline stem cell lineage acts to spatially restrict DPP function within the niche. Sci Signal. 2010 Jul 27;3(132):ra57–ra57. doi: 10.1126/scisignal.2000740. [DOI] [PubMed] [Google Scholar]
- Luo L, Wang H, Fan C, Liu S, Cai Y. Wnt ligands regulate Tkv expression to constrain Dpp activity in the Drosophila ovarian stem cell niche. J Cell Biol. 2015 May 25;209(4):595–608. doi: 10.1083/jcb.201409142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Wang S, Do T, Song X, Inaba M, Nishimoto Y, Liu LP, Gao Y, Mao Y, Li H, McDowell W, Park J, Malanowski K, Peak A, Perera A, Li H, Gaudenz K, Haug J, Yamashita Y, Lin H, Ni JQ, Xie T. Piwi is required in multiple cell types to control germline stem cell lineage development in the Drosophila ovary. PLoS One. 2014 Mar 21;9(3):e90267–e90267. doi: 10.1371/journal.pone.0090267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottier-Pavie VI, Palacios V, Eliazer S, Scoggin S, Buszczak M. The Wnt pathway limits BMP signaling outside of the germline stem cell niche in Drosophila ovaries. Dev Biol. 2016 Jun 27;417(1):50–62. doi: 10.1016/j.ydbio.2016.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozata DM, Gainetdinov I, Zoch A, O'Carroll D, Zamore PD. PIWI-interacting RNAs: small RNAs with big functions. Nat Rev Genet. 2019 Feb 01;20(2):89–8108. doi: 10.1038/s41576-018-0073-3. [DOI] [PubMed] [Google Scholar]
- Riechmann V, Ephrussi A. Axis formation during Drosophila oogenesis. Curr Opin Genet Dev. 2001 Aug 01;11(4):374–383. doi: 10.1016/s0959-437x(00)00207-0. [DOI] [PubMed] [Google Scholar]
- Robine N, Lau NC, Balla S, Jin Z, Okamura K, Kuramochi-Miyagawa S, Blower MD, Lai EC. A broadly conserved pathway generates 3'UTR-directed primary piRNAs. Curr Biol. 2009 Dec 29;19(24):2066–2076. doi: 10.1016/j.cub.2009.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahai-Hernandez P, Nystul TG. A dynamic population of stromal cells contributes to the follicle stem cell niche in the Drosophila ovary. Development. 2013 Oct 16;140(22):4490–4498. doi: 10.1242/dev.098558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K, Inagaki S, Mituyama T, Kawamura Y, Ono Y, Sakota E, Kotani H, Asai K, Siomi H, Siomi MC. A regulatory circuit for piwi by the large Maf gene traffic jam in Drosophila. Nature. 2009 Oct 01;461(7268):1296–1299. doi: 10.1038/nature08501. [DOI] [PubMed] [Google Scholar]
- Sienski G, Dönertas D, Brennecke J. Transcriptional silencing of transposons by Piwi and maelstrom and its impact on chromatin state and gene expression. Cell. 2012 Nov 15;151(5):964–980. doi: 10.1016/j.cell.2012.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Wong MD, Kawase E, Xi R, Ding BC, McCarthy JJ, Xie T. Bmp signals from niche cells directly repress transcription of a differentiation-promoting gene, bag of marbles, in germline stem cells in the Drosophila ovary. Development. 2004 Feb 18;131(6):1353–1364. doi: 10.1242/dev.01026. [DOI] [PubMed] [Google Scholar]
- Wang SH, Elgin SC. Drosophila Piwi functions downstream of piRNA production mediating a chromatin-based transposon silencing mechanism in female germ line. Proc Natl Acad Sci U S A. 2011 Dec 12;108(52):21164–21169. doi: 10.1073/pnas.1107892109. [DOI] [PMC free article] [PubMed] [Google Scholar]