Abstract:
Salmonella enterica is the most important foodborne pathogen, and it is often associated with the contamination of poultry products. Annually, Salmonella causes around 93 million cases of gastroenteritis and 155,000 deaths worldwide. Antimicrobial therapy is the first choice of treatment for this bacterial infection; however, antimicrobial resistance has become a problem due to the misuse of antibiotics both in human medicine and animal production. It has been predicted that by 2050, antibiotic-resistant pathogens will cause around 10 million deaths worldwide, and the WHO has suggested the need to usher in the post-antibiotic era. The purpose of this review is to discuss and update the status of Salmonella antibiotic resistance, in particular, its prevalence, serotypes, and antibiotic resistance patterns in response to critical antimicrobials used in human medicine and the poultry industry. Based on our review, the median prevalence values of Salmonella in broiler chickens, raw chicken meat, and in eggs and egg-laying hens were 40.5% ( interquartile range [IQR] 11.5-58.2%), 30% (IQR 20-43.5%), and 40% (IQR 14.2-51.5%), respectively. The most common serotype was Salmonella Enteritidis, followed by Salmonella Typhimurium. The highest antibiotic resistance levels within the poultry production chain were found for nalidixic acid and ampicillin. These findings highlight the need for government entities, poultry researchers, and producers to find ways to reduce the impact of antibiotic use in poultry, focusing especially on active surveillance and finding alternatives to antibiotics.
Keywords: antimicrobial, poultry, resistance, Salmonella
Introduction
Salmonella enterica subsp. enterica is one of the most important foodborne pathogens worldwide and remains the leading cause of infectious gastroenteritis. Cases are often related to the consumption of food of animal origin, mainly poultry products, such as eggs and raw chicken [1-3]. Globally, non-typhoidal Salmonella causes around 93 million cases of gastroenteritis and 155 000 deaths each year [4-6]. The disease manifestation depends on the serotype involved, virulence factors, infective dose, and host immunity. Immunocompromised patients, children, and elderly people tend to be more susceptible and suffer more serious clinical symptoms, including sepsis [7-11]. In other cases, the infection can cause a chronic state of asymptomatic carriage in the host [7]. The serotypes Salmonella Enteritidis and Salmonella Typhimurium are the most frequent causes of salmonellosis in humans [12-14]. However, emerging serotypes such as Salmonella Heidelberg, Salmonella Javiana, Salmonella Infantis, and Salmonella Thompson have been reported to infect humans in the United States and are becoming more prevalent in certain segments of the poultry production chain [2,15].
Meanwhile, antimicrobial resistance is another global threat in animal and human medicine. Its dangers lie mainly in the failure to successfully treat patients infected with antibiotic-resistant pathogens and in the high risk of transmission of such resistant pathogens [16]. The development of this resistance is related to the misuse of antibiotics, including their use in animal production systems as growth promoters and their excessive use in clinical treatments [16,17]. This is a great concern because much of the antibiotic-resistant Salmonella have been acquired through the consumption of contaminated food of animal origin, resulting in health risk to humans and increasing the cost of health care [4,18,19]. Some authors have predicted that antimicrobial-resistant pathogens will be responsible for 10 million deaths worldwide by the year 2050 [20,21]. In addition, the use of generic antimicrobials in veterinary and human medicine poses a risk because certain strains of bacteria with β- lactamases and/or AmpC β-lactamases have been isolated from food animal products. In addition, extended-spectrum cephalosporin-resistant Salmonella has been isolated from poultry. These bacteria have been responsible for failure in human treatments, resulting in a demand for a second line of antibiotics to control infections [22,23]. Different government agencies have encouraged the prudent use of antibiotics in veterinary and human medicine. For example, antimicrobials such as carbapenems, glycopeptides, tigecycline, and third- and fourth-generation cephalosporins have been restricted in their use. Antibiotic resistance in Salmonella strains and virulent clones can compromise infection treatment in humans, making it difficult to control the disease, and poses a severe risk to global public health [24]. Thus, the World Health Organization has defined Salmonella as a “priority pathogen” and aims to guide and promote research and development into new antibiotics for its treatment [11,25].
This review discusses the worldwide prevalence, serotypes, and antibiotic resistance patterns of Salmonella isolates from different segments of the poultry production chain.
Antibiotic Resistance of Salmonella Isolates
Several multidrug-resistant (MDR) Salmonella strains have been isolated in beef, pork, and poultry products, and each of these has the potential to spread and generate a global emergency [22,26,27]. In the case of poultry products, the diverse sources of contamination include: Infection at the site of primary production (e.g., parent stock, incubator, and farm); cross-contamination in the handling of food or byproducts; and the consumption of undercooked poultry meat, eggs, or egg products. All of these sources have been related to infection by Salmonella in humans [28-33]. Cases of salmonellosis have been caused by antibiotic-resistant strains of Salmonella, which are selected for by the indiscriminate use of antibiotics in human and veterinary medicine and in animal production [18].
Historically, antimicrobials have been used as growth promoters since 1950, when it was discovered that small, sub-therapeutic quantities of antibiotics such as penicillin and tetracycline delivered to animals in feed, could enhance the weight of poultry, swine, and beef cattle [34]. The first report of a chloramphenicol-resistant S. Typhi occurred 2 years after the introduction of chloramphenicol in the market (1948), and 13 years later, the first plasmid-mediated transfer of antibiotic resistance in S. Typhimurium was reported in Germany. Since then, MDR strains have been isolated around the world (Figure-1) [35-50] . During this time, many serotypes of Salmonella spp. started to show resistance to antibiotics such as quinolones and cephalosporin, which have been the first choice antibiotics for the treatment of humans [51].
Figure-1.
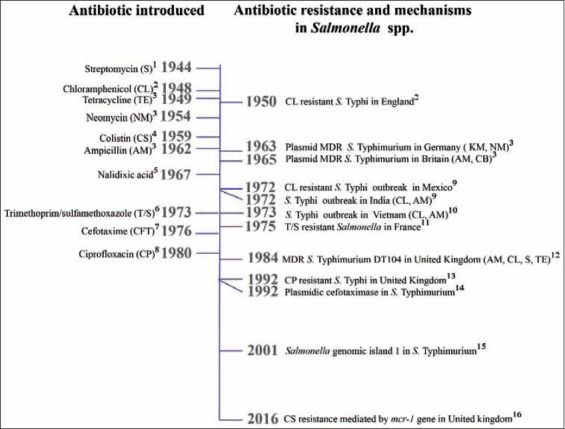
Timeline representing both antibiotic deployment and antibiotic resistance and mechanisms in Salmonella spp. Source: The authors. Murray et al. [35]1, Colquhoun and Weetch [36]2, Summers [43]3, Ross et al. [44]4, Jacoby et al. [45]5, Smith and Sensakovic [46]6, Batabyal [47]7, Ugboko and De [48]8, Anderson [49]9, Butler [50]10, Zaki and Karande [37]11, Crump et al. [38]12, Umasankar et al. [39]13, Bauernfeind et al. [40]14, Boyd et al. [41]15, Doumith et al. [42]16.
Transmission of Antibiotic Resistance in Salmonella
Through billions of years of evolution, Salmonella has accumulated a large number of metabolic and protective mechanisms that can be mobilized in response to different external aggressions, including antibiotics [52]. Antibiotic resistance can be achieved by mutations in different chromosomal loci that are a part of a core set of genes, such as genomic islands. In addition, antibiotic resistance can be acquired through exogenous resistance genes carried by mobile genetic elements that can be disseminated horizontally between bacteria [48,53,54].
Genomic islands are conserved zones found in accessory regions in the Salmonella genome. They are considered fundamental to the evolution of this genus, as they have conferred a number of fitness advantages on their host through virulence and multidrug resistance (MD) genes [55]. Genomic island 1 (Salmonella genomic island 1 [SGI-1]) harbors genes associated with MD to streptomycin, spectinomycin, sulfonamides, chloramphenicol, florfenicol, tetracyclines, and β- lactam antibiotics. In addition, these genes can be carried in mobile elements such as Class 1 integrons found in the antibiotic-resistance cluster located at the 3’end of the island [56,57]. SGI-1 has been associated with the MDR strain DT 104, widely isolated from humans and food-producing animals in most parts of the world since 1980 [58].
Furthermore, Salmonella can acquire resistance through mobile elements such as plasmids that account for the high rates of transfer of genes that are beneficial to the survival of the host bacteria [59]. These plasmids typically encode both virulence factors and MD traits similar to those on genomic islands. However, plasmids can be transferred horizontally and may carry other mobile elements such as transposons and integrons. Therefore, they increase phenotypic diversity and confer fitness advantages during times of environmental changes, thus providing the host with opportunities for niche expansion [57,60]. Plasmids are classified according to incompatibility (Inc) types that are based on the degree of relatedness between plasmids and that control the replication of different types of plasmids within the same bacteria [61]. As a result, certain replicon types such as IncC and IncA are associated with MD and disseminate, among others, the extended-spectrum β-lactamase trait from foodborne Salmonella that has been responsible for past disease outbreaks [56,61,62].
Integrons are natural recombination systems that constitute one of the most efficient mechanisms for accumulating antimicrobial resistance. Their structure incorporates several open reading frames in the form of gene cassettes that code for traits related to antimicrobial resistance [57,63]. These mobile elements are composed of three key parts: A gene encoding an integrase (intI), a primary recombination site (attI), and a promoter for the transcription of captured genes (Pc) [64]. Integrons are important mainly because they are responsible for MD; some antibiotic resistance determinants are preferentially associated with integrons and include those responsible for resistance to streptomycin, trimethoprim, sulfafurazole, and some aminoglycosides [60]. Class 1 integrons have been found extensively both clinically and in the food of animal origin, and they have been associated significantly with the presence of MD. While integrons themselves are not mobile, they can be associated with a mobile element called a transposon that is capable of moving from one carrier replicon to another. Transposons are generally located on plasmids, further enhancing the spread of gene cassettes [64]. The association of integrons with mobile elements and resistance genes has led to their rapid dispersal among various bacteria found in environments exposed to antibiotics [65].
Mechanisms of Resistance in Salmonella
Antimicrobial resistance in Salmonella is media- ted by several mechanisms (Figure-2) [45,48,57,63,66,67]; that include drug inactivation, which is the most common cause of resistance. In this mechanism, antimicrobial agents are destroyed or inactivated through chemical modification using enzymes that catalyze reactions such as acetylation, phosphorylation, and adenylation [63]. For example, aminoglycoside-modifying acetyltransferase AAC(6’)-Ib-cr harbors two amino acid substitutions at Trp102Arg and Asp179Tyr, which confers the ability to acetylate the unsubstituted nitrogen of the C7 piperazine ring present in several quinolones [68]. In addition, enzymes such as penicillinase and chloramphenicol acetyltransferase are able to acetylate the ß-lactam ring of penicillin and some cephalosporins and the two hydroxyl groups of chloramphenicol, respectively [63,69].
Figure-2.
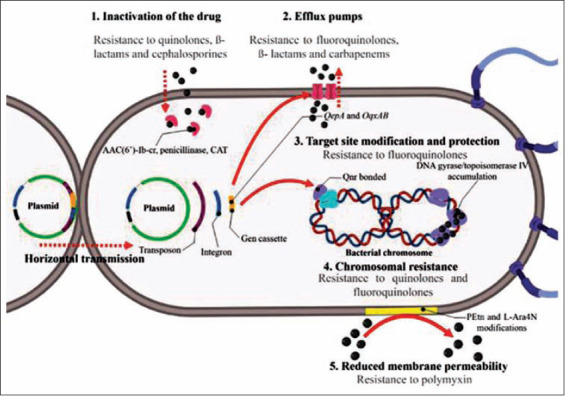
Antibiotic resistance and transmission mechanisms in Salmonella spp. and the respective antimicrobials which become ineffective. Source: The authors. Based on Jacoby et al. [45], Ugboko and De [48], Silva et al. [57], Munita and Arias [63], Rodríguez-Martínez et al. [67], Davies and Davies [66].
Salmonella can acquire antibiotic resistance by protecting the target site of the antibiotic, which can either be an enzyme or a specific cell structure. For example, the plasmid-encoded quinolone resistance protein (Qnr) confers resistance to quinolones by acting as a DNA homolog that competes for the binding of DNA gyrase and topoisomerase IV [63]. This reduces the possibility of the quinolone molecule binding to DNA gyrase, thus protecting the bacteria from the lethal effects of the antibiotic [45,67]. In addition, Salmonella can modify the antibiotic target site to avoid it from binding. For example, the resistance of rifampicin is based on single-step point mutations that result in amino acid substitutions in the rpoB gene. These substitutions decrease the affinity of the drug for DNA-dependent RNA polymerase, thus allowing the transcription of rpoB to continue [63,70].
Another mechanism of resistance in Salmonella is through the reduction in its membrane permeability, thus preventing drug entry [48]. The alteration occurs when membrane proteins undergo changes through new genetic information that alters the membrane transport system pores and thus prevents the passage of antibiotics. In the case of polymyxin resistance, a modification in the lipid A moiety of the lipopolysaccharide structure consisting of phosphoethanolamine and 4-amino-4-deoxy-L-arabinose results in a reduction in the net negative charge of the membrane, which reduces its affinity for polymyxin [71,72].
Salmonella has also developed the ability to pump out a drug after it has gained entry into the system, using efflux pumps or MD pumps. This mechanism is relatively nonspecific and can pump out many different drugs, including fluoroquinolones, ß- lactams, and carbapenems [63]. Many of these efflux pumps are encoded by genes within mobile elements such as plasmids. For example, genes such as QepA and oqxAB confer resistance to several fluoroquinolones [68].
In Salmonella as well as in other bacteria, resistance to antibiotics may be mediated to a lesser extent by chromosomal mechanisms based on mutations in genes that code for either the target of the drug or the membrane transport system that controls the uptake of the drug [48]. For example, chromosomal-mediated quinolone resistance may result from selective pressure on the bacterial population due to the uncontrolled use of the drug. Under normal conditions, quinolones enter bacteria through porins and then bind to the gyrase/topoisomerase IV–DNA complex. The resulting bound complex is prevented from replicating, thus explaining the drug’s bacteriostatic action. Salmonella has developed single point mutations in the quinolone resistance determining region of the topoisomerase genes parC and parE and the DNA gyrase genes gyrA and gyrB. As a result, the lethal action of some fluoroquinolones and quinolones is blocked by the accumulation of these genes and by the structural change in the protein that leads to a reduced affinity for the drug [37,73].
Antibiotic Resistance by the Family in Poultry and Poultry Products
Our literature search used the search terms “Salmonella” in combination with “chicken,” “broiler,” “raw,” “poultry,” “eggs,” and “antibiotic resistance.” We selected a total of 112 papers published between 2003 and September 2019.
We developed resistance profiles from production segments worldwide consisting of broiler chickens, chicken at processing plants, markets, eggs, and egg-laying hens. The choice of antimicrobials is based on reports of the WHO, namely, the List of Critically Important Antimicrobials from Human Medicine [11] and the List of Essential Medicines [25]. We took into account five of the most important antibiotic families consisting of aminoglycosides, β- lactams, quinolones and fluoroquinolones, cephalosporins, and carbapenems and monobactam. Antibiotic resistance prevalence data were summarized according to the antimicrobial, using the median and IQR across studies. The data were plotted for comparative purposes in heat maps generated by GraphPad Prism software v.7 (La Jolla, California). The prevalence of resistance against specific antimicrobials in individual studies was compiled in tabular form and antibiotic resistance was classified as either high (>50%), medium (21-50%), or low (0-20%). We considered isolates from the studies as MDR if they showed resistance to three or more antimicrobials.
Antibiotic Resistance in Poultry Farms Worldwide
A total of 45 publications investigated antibiotic resistance in 7033 isolates from raw chicken meat from the Americas (11 studies), Africa (13 studies), Europe (3 studies), and Asia (18 studies) (Figure-3) [2,13,74-115]. The median prevalence of Salmonella in broiler chickens was 40.5% (IQR 11.5-58.2%), and the most prevalent serotypes were S. Enteritidis (13 studies) and S. Typhimurium (4 studies), while the presence of S. Heidelberg (3 studies) and S. Infantis (3 studies) was also found. Thirteen of the articles did not report Salmonella serotypes.
Figure-3.
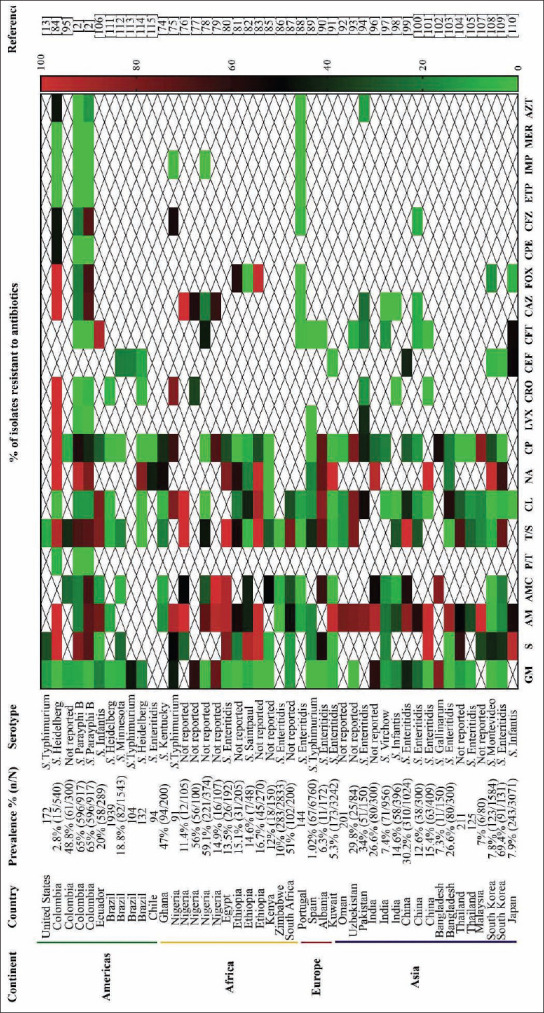
Antibiotic resistance patterns in Salmonella spp. isolated from poultry farms in the world. 1Aminoglycosides: Gentamycin (GM), streptomycin (S); 2β-lactams: Ampicillin (AM), amoxicillin/clavulanic acid (AMC), piperacillin/tazobactam (P/T); 3folate antagonist: Trimethoprim/sulfamethoxazole (T/S); 4phenicol: Chloramphenicol (CL); 5quinolones and fluoroquinolones: Nalidixic acid (NA), ciprofloxacin (CP), levofloxacin (LVX); 6cephalosporines: Ceftriaxone (CRO), ceftiofur (CEF), cefotaxime (CFT), ceftazidime (CAZ), cefoxitin (FOX), cefepime (CPE), cefazolin (CFZ); 7carbapenems: Ertapenem (ETP), imipenem (IMP), meropenem (MER); 8monobactams: Aztreonam (AZT) [2,13,74-115].
MD was found in 91.1% (41/45) of the articles, representing research conducted worldwide. High levels of antibiotic resistance (80.3% and 64.8%, respectively) were found in Salmonella isolates from broiler chickens treated by commonly used antibiotics in poultry production, such as nalidixic acid (fluoroquinolone family; IQR 43.6-97.6%) and ampicillin (β-lactam family; IQR 17.7-92.1%). Medium levels of resistance (33%, 29.4%, and 39.3%, respectively) were found to antibiotics such as streptomycin (aminoglycosides; IQR 16.4-80.8%), amoxicillin/clavulanic acid (β-lactam family; IQR 9-56.7%), and trimethoprim/sulfamethoxazole (folate antagonist; IQR 7.9-76.5%). Surprisingly, resistance levels were low (6%) to gentamycin (IQR 0-17.1%) although it is widely used in day-old chickens [116]. Similarly, resistance levels were low (19%) to ciprofloxacin (IQR 0.6-40.1%), an antibiotic broadly used in poultry production. Resistance levels were also low (13.6%) against chloramphenicol (IQR 0.3-38.2%), which is probably due to the low usage of this antibiotic in recent years due to its various toxic and carcinogenic effects in humans [117].
In the cephalosporin family, antibiotic resistance levels were low (4.1%, 7.9%, 18.8%, 0%, and 18.6%, respectively) for ceftriaxone (IQR 0-28.9%), cefotaxime (IQR 0-20%), ceftazidime (IQR 1.3-77.2%), cefepime (IQR 0-46%), and cefazolin (IQR 5.3-84.8%); while the resistance level was medium (38.3%) for cefoxitin (IQR 4.2-90%). It should be mentioned that ceftiofur resistance was low (9.8%), despite being used in day-old chickens in poultry production (IQR 2.2-20%) [116]. In addition, no resistance was detected for the carbapenem family (ertapenem, imipenem, and meropenem).
Levels of antibiotic-resistant Salmonella in European countries and the US tended to be low, which is related to the establishment of control and monitoring programs such as the European Food Safety Authority and the European Centre for Disease Prevention and Control, and the National Antibiotic Resistance Monitoring System in the US [118].
Antibiotic Resistance in Raw Chicken Meat at Processing Plants and Markets
A total of 46 publication investigated antibiotic resistance in 3301 isolates from raw chicken meat in study sites in the Americas (12 studies), Africa (6 studies), Europe (11 studies), and Asia (17 studies) (Figure-4) [17,26,27,119-161]. It should be mentioned that most of the studies focused on antibiotic resistance at markets (37 studies) rather than processing plants (9 studies). This is probably because markets are the last segment in the poultry production chain and are thus widely linked to human infections.
Figure-4.
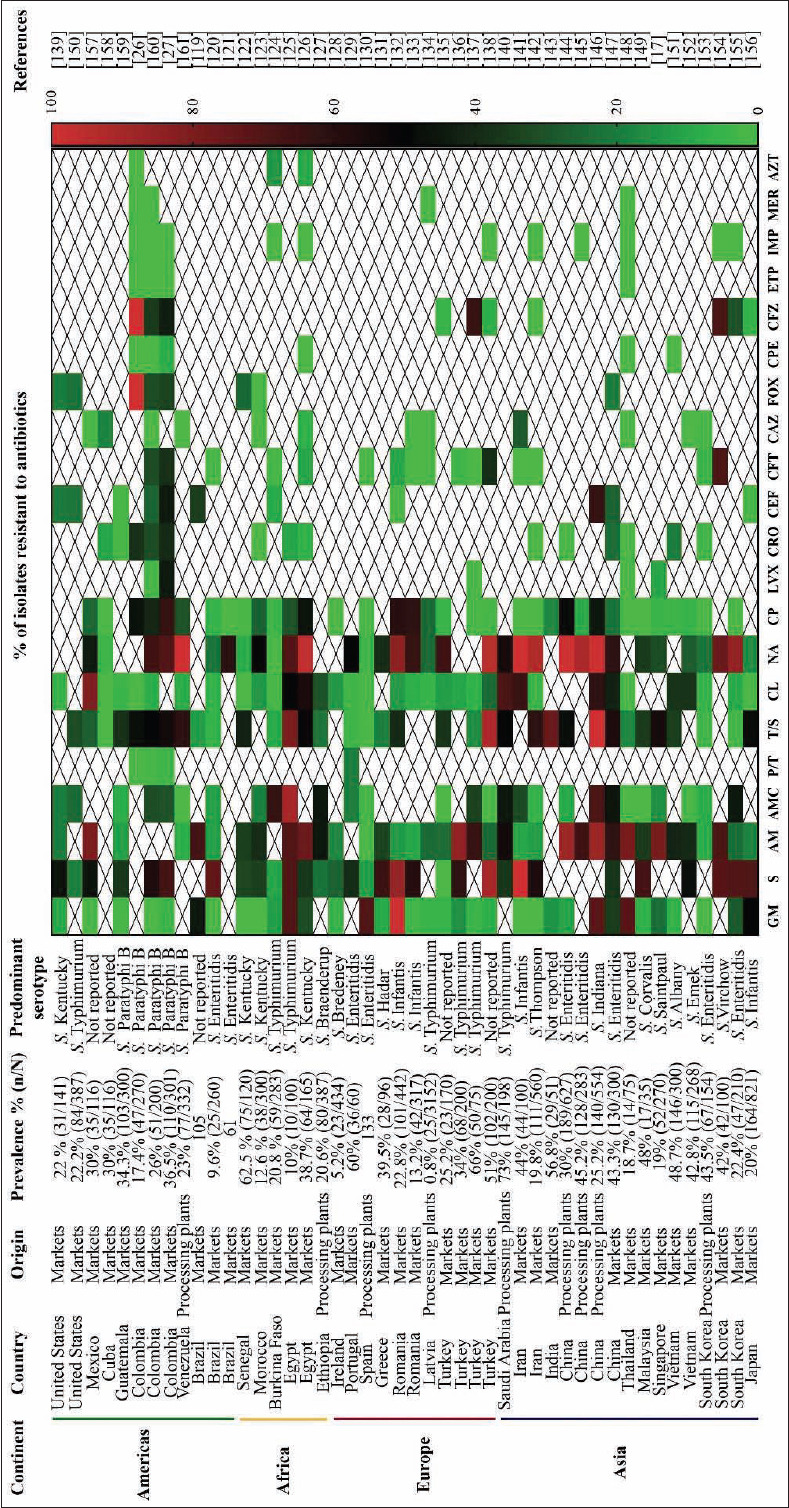
Antibiotic resistance patterns in Salmonella spp. isolated from chickens in processing plants and at market in the world. 1Aminoglycosides: Gentamycin (GM), streptomycin (S); 2β-lactams: Ampicillin (AM), amoxicillin/ clavulanic acid (AMC), piperacillin/tazobactam (P/T); 3folate antagonist: Trimethoprim/sulfamethoxazole (T/S); 4phenicol: Chloramphenicol (CL); 5quinolones and fluoroquinolones: Nalidixic acid (NA), ciprofloxacin (CP), levofloxacin (LVX); 6cephalosporines: Ceftriaxone (CRO), ceftiofur (CEF), cefotaxime (CFT), ceftazidime (CAZ), cefoxitin (FOX), cefepime (CPE), cefazolin (CFZ); 7carbapenems: Ertapenem (ETP), imipenem (IMP), meropenem (MER); 8monobactams: Aztreonam (AZT) [17,26,27,119-161].
The median prevalence of Salmonella in raw chicken meat was 30% (IQR 20-43.5%), and the most prevalent serotypes were S. Enteritidis (9 studies) and S. Typhimurium (7 studies). These trends are similar to those reported in broilers, eggs, and egg-laying hens. In addition, the serotypes S. Paratyphi B (5 studies), S. Infantis, and S. Kentucky (4 studies) have also been reported. Only seven articles did not report the most prevalent serotype in their studies.
MD was present in 97.8% (45/46) of the articles included in this study. A high level of antibiotic resistance (60.7%) in Salmonella isolates was associated with commonly used antibiotics in poultry production, such as nalidixic acid (fluoroquinolone family; IQR 26.8-86.6%). Medium levels of antibiotic resistance (47.3%, 35.5%, and 37.9%, respectively) were associated with antibiotics such as streptomycin (aminoglycoside family; IQR 34.2-69.2%), ampicillin (β-lactam family; IQR 14.9-68%), and trimethoprim/sulfamethoxazole (folate antagonist family; IQR 16-54.2%).
Low levels of resistance (7%, 5%, and 8%, respectively) were associated with gentamycin (IQR 1.1-31%), ciprofloxacin (IQR 0-30%), and chloramphenicol (IQR 3.6-37.3%), findings that are similar to corresponding antibiotics in broiler chickens (above). It should be mentioned that antibiotics commonly used in human medicine are poorly represented in the studies included in this review. However, in most cases, Salmonella isolates showed low levels of resistance to these antibiotics. Isolates from raw chicken meat showed medium levels of resistance (21.4%, 23.5%, and 32.6%, respectively) to antibiotics from the cephalosporin family such as ceftiofur (IQR 2-35%), cefoxitin (IQR 19.1-32.7%), and cefazolin (IQR 5.5-65.2%), while low levels of resistance (0%, 8%, 2.2%, and 0%, respectively) were found for ceftazidime (IQR 0-4.7%), ceftriaxone (IQR 0-25.4%), cefotaxime (IQR 0-26%), and cefepime (IQR 0-1.9%). This family is considered critically important for clinical treatment because it has an extended spectrum of effectivity and can be safely administered to pregnant women and children [162]. No resistance has been detected against the carbapenem family of antibiotics such as ertapenem, imipenem and meropenem, which is consistent among broiler chickens, eggs, and egg-laying hens.
Antibiotic Resistance in Eggs and Egg-laying Hens in the World
A total of 21 publications investigated antibiotic resistance in 1009 isolates from egg-laying hens farms and eggs from Asia (9 studies), Africa (7 studies), Europe (3 studies), and the Americas (2 studies) (Figure-5) [32,74,96,163-180]. Most of the studies report the presence of Salmonella on eggshells (11 studies), followed by egg-laying hens (10 studies). The median prevalence of Salmonella in eggs and egg-laying hens was 40% (IQR 14.2-51.5%), and the most prevalent serotype was S. Enteritidis (4 studies), followed by S. Typhimurium (2 studies). Eight studies did not report serotypes. The high prevalence of S. Enteritidis in eggs and egg-laying hens is due to the ability of this serotype to disseminate in reproductive tissues such as the ovary and oviduct in infected hens [181]. As a result, this serotype is often reported to infect humans through the consumption of eggs, which is an important transmission source [182].
Figure-5.
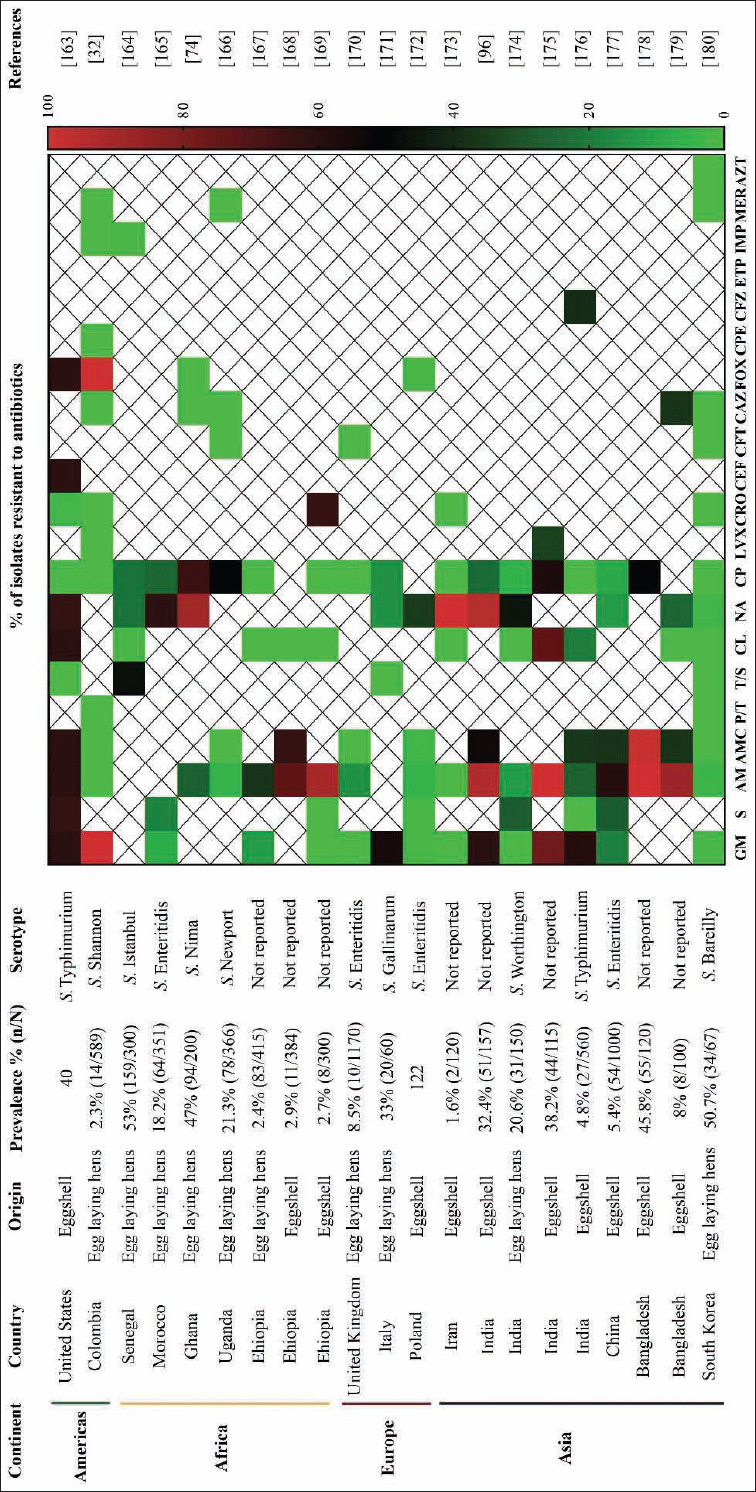
Antibiotic resistance patterns in Salmonella spp. isolated from egg laying hen farms and eggs in different parts of the world. 1Aminoglycosides: Gentamycin (GM), streptomycin (S); 2β-lactams: Ampicillin (AM), amoxicillin/clavulanic acid (AMC), piperacillin/tazobactam (P/T); 3folate antagonist: Trimethoprim/sulfamethoxazole (T/S); 4phenicol: Chloramphenicol (CL); 5quinolones and fluoroquinolones: Nalidixic acid (NA), ciprofloxacin (CP), levofloxacin (LVX); 6cephalosporines: Ceftriaxone (CRO), ceftiofur (CEF), cefotaxime (CFT), ceftazidime (CAZ), cefoxitin (FOX), cefepime (CPE), cefazolin (CFZ); 7carbapenems: Ertapenem (ETP), imipenem (IMP), meropenem (MER); 8monobactams: Aztreonam (AZT) [32,74,96,163-180].
Medium levels of antibiotic resistance (31.9%, 36.9%, and 40.3%, respectively) were found in Salmonella strains isolated from eggs and laying hens for ampicillin (β-lactam family; IQR 5-88.1%), amoxicillin/clavulanic acid (β-lactam family; IQR 0-58.9%), and nalidixic acid (fluoroquinolone family; IQR 167-82.9%). Low levels of antibiotic resistance (12.5%, 18%, and 7.6%, respectively) were found for aminoglycoside antibiotics such as gentamycin (IQR 0-60.7%) and streptomycin (IQR 0-27.7%) and for the fluoroquinolone ciprofloxacin (IQR 0-31%).
No resistance has been detected to antibiotics commonly used in human medicine that is of “highest priority critically important antimicrobials” according to the WHO [11]. These include cephalosporins such as ceftriaxone, cefotaxime, ceftazidime, and cefepime; and carbapenems such as imipenem, meropenem, and aztreonam.
Low levels of antibiotic resistance (12.5%, 18%, 0%, 7.6%, and 0%, respectively) were found for aminoglycosides such as gentamycin (IQR 0-60.7%) and streptomycin (IQR 0-27.7%); and for chloramphenicol (IQR 0-19.9%), ciprofloxacin (IQR 0-31%), and trimethoprim/sulfamethoxazole (IQR 0-35.2%). In the case of trimethoprim/sulfamethoxazole, the low level of resistance is probably because sulfonamides are not commonly used in egg-laying hens, in contrast to broiler chickens [116].
Conclusion
Antibiotic resistance is a global health threat that impacts the poultry industry, as MDR Salmonella strains have been reported mainly originating from poultry sources. This study found a worldwide median prevalence values of Salmonella in broiler chickens of 40.5%, in raw chicken meat of 30% and in eggs and egg-laying hens of 40.5% as well as a higher number of MDR isolates from poultry farms (91.1%) and from raw chicken meat at processing plants and markets (97.8%) worldwide. In addition, the highest antibiotic resistance levels within the poultry production chain were found for nalidixic acid and ampicillin. This situation demands a better understanding of the bacterial resistance in animal and human isolates, to enable the establishment of control strategies that reduce the risk of antibiotic-resistant pathogens. Government entities, researchers, and poultry producers have the responsibility of managing antibiotic resistance by reducing the use of antibiotics, conducting active surveillance of MDR strains, and searching for alternatives to control and prevent infectious disease outbreaks.
Authors’ Contributions
REC, MPH, RR, and ISR conceptualized the review. REC, MPH, and RR collected the literature. REC made the figures. REC and MPH made the statistical analysis. All authors were involved in the writing, analysis of the data, and reviewed the manuscript, and they approved the final manuscript.
Acknowledgments
The authors acknowledges at the Laboratory of Immunology and Molecular Biology – LIBM of the University of Tolima, for providing facilities to publish the manuscript. The authors did not receive any funds for this study.
Competing Interests
The authors declare that they have no competing interests.
Publisher’s Note
Veterinary World remains neutral with regard to jurisdictional claims in published institutional affiliation.
References
- 1.Braden C.R. Salmonella enterica Serotype Enteritidis and Eggs:A National Epidemic in the United States. Clin. Infect. Dis. 2006;43(4):512–517. doi: 10.1086/505973. [DOI] [PubMed] [Google Scholar]
- 2.Donado-Godoy P, Gardner I, Byrne B.A, Leon M, Perez-Gutierrez E, Ovalle M.V, Tafur M.A, Miller W. Prevalence, Risk Factors, and Antimicrobial Resistance Profiles of Salmonella from Commercial Broiler Farms in Two Important Poultry-Producing Regions of Colombia. J. Food. Prot. 2012;75(5):874–883. doi: 10.4315/0362-028X.JFP-11-458. [DOI] [PubMed] [Google Scholar]
- 3.Heng Y, Peterson H.H, Li X. Consumer attitudes toward farm-animal welfare:The case of laying hens. J. Agric. Resour. Econ. 2013;38(3):418–434. [Google Scholar]
- 4.CDC. (2013) Antibiotic resistance threats in the United States. 2013. [Retrieved on 20-01-y2019]. Available from:https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf .
- 5.Cosby D.E, Cox N.A, Harrison M.A, Wilson J.L, Jeff-Buhr R, Fedorka-Cray P.J. Salmonella and antimicrobial resistance in broilers:A review. J. Appl. Poult. Res. 2015;24(3):408–426. [Google Scholar]
- 6.Majowicz S.E, Musto J, Scallan E, Angulo F.J, Kirk M, O'Brien S.J, Jones T.F, Fazil A, Hoekstra R.M. The Global Burden of Nontyphoidal Salmonella Gastroenteritis. Clin. Infect. Dis. 2010;50(6):882–889. doi: 10.1086/650733. [DOI] [PubMed] [Google Scholar]
- 7.Coburn B, Grassl G.A, Finlay B.B. Salmonella the host and disease:A brief review. Immunol. Cell. Biol. 2007;85(2):112–118. doi: 10.1038/sj.icb.7100007. [DOI] [PubMed] [Google Scholar]
- 8.Fierer J, Guiney D. Diverse virulence traits underlying different clinical outcomes of Salmonella infection. Bact. Polymorphisms. 2001;107(7):492–493. doi: 10.1172/JCI12561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kothary M, Babu U. Infective Dose of Foodborne Pathogens in Volunteers:a Review. J. Food. Saf. 2001;21(1):49–68. [Google Scholar]
- 10.Luquez J.L. Detection of Salmonella spp. in chicken meat in outlets in the city of Valledupar, UNAD. 2017. [Retrieved on 16-03-2019]. Available from:https://repository.unad.edu.co/bitstream/handle/10596/11474/77182668.pdf?sequence=1&isAllowed=y .
- 11.WHO. List of Critically Important Antimicrobials for Human Medicine. 2017. [Retrieved on 16-03-2019]. Available from:https://apps.who.int/iris/bitstream/handle/10665/255027/9789241512220-eng.pdf?sequence=1 .
- 12.Gantois I, Ducatelle R, Pasmans F, Haesebrouck F, Gast R, Humphrey T.J, Immerseel F.V. Mechanisms of egg contamination by Salmonella Enteritidis. FEMS. Microbiol. Rev. 2009;33(4):718–738. doi: 10.1111/j.1574-6976.2008.00161.x. [DOI] [PubMed] [Google Scholar]
- 13.Liljebjelke K.A, Hofacre C.L, White D.G, Ayers S, Lee M.D, Maurer J.J. Diversity of Antimicrobial Resistance Phenotypes in Salmonella Isolated from Commercial Poultry Farms. Front. Vet. Sci. 2017;23(4):1–9. doi: 10.3389/fvets.2017.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rajan K, Shi Z, Ricke S.C. Current aspects of Salmonella contamination in the US poultry production chain and the potential application of risk strategies in understanding emerging hazards. Crit. Rev. Microbiol. 2017;43(3):370–392. doi: 10.1080/1040841X.2016.1223600. [DOI] [PubMed] [Google Scholar]
- 15.Foley S.L, Nayak R, Hanning I.B, Johnson T.J, Han J, Ricke S.C. Population dynamics of Salmonella enterica serotypes in commercial egg and poultry production. Appl. Environ. Microbiol. 2011;77(13):4273–4279. doi: 10.1128/AEM.00598-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roca I, Akova M, Baquero F, Carlet J, Cavaleri M, Coenen S, Cohen J, Findlay D, Gyssens I, Heure O.E. The global threat of antimicrobial resistance:science for intervention. New. Microbes. New. Infect. 2015;6:22–29. doi: 10.1016/j.nmni.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zwe Y.H, Yen-Tang V.C, Aung K.T, Gutiérrez R.A, Ng L.C, Yuk H.G. Prevalence, sequence types, antibiotic resistance and gyrA mutations of Salmonella isolated from retail fresh chicken meat in Singapore. Food Control. 2018;90(3):233–240. [Google Scholar]
- 18.Barreto M, Castillo-Ruiz M, Retamal P. Salmonella enterica a review or the trilogy agent, host and environment and its importance in Chile. Rev. Chil. Infectología. 2016;33(5):547–557. doi: 10.4067/S0716-10182016000500010. [DOI] [PubMed] [Google Scholar]
- 19.Eng S.K, Pusparajah P, Ab-Mutalib N.S, Ser H.L, Chan K.G, Lee L.H. Salmonella:A review on pathogenesis, epidemiology and antibiotic resistance. Front. Life. Sci. 2015;8(3):284–293. [Google Scholar]
- 20.FAO. Drivers, Dynamics and Epidemiology of Antimicrobial Resistance in Animal Production, Food and Agriculture Organization of the United Nations. 2016. [Retrieved on 01-02-2019]. Available from:http://www.fao.org/3/a-i6209e.pdf.
- 21.Marquardt R.R, Li S. Antimicrobial resistance in livestock :advances and alternatives to antibiotics. Anim. Front. 2018;8(2):30–37. doi: 10.1093/af/vfy001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castellanos L.R, van der Graaf-van Bloois L, Donado-Godoy P, León M, Clavijo V, Arévalo A, Bernal J.F, Mevius D.J, Wagenaar J.A, Zomer A, Hordijk J. Genomic Characterization of Extended-Spectrum Cephalosporin-Resistant Salmonella enterica in the Colombian Poultry Chain. Front. Microbiol. 2018;9(oct):1–11. doi: 10.3389/fmicb.2018.02431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.CDC. [Retrieved on 01-02-2019];Salmonella page Salmonella. 2017 [Google Scholar]
- 24.Pan H, Paudyal N, Li X, Fang W, Yue M. Multiple food-animal-borne route in transmission of antibiotic-resistant Salmonella Newport to humans. Front. Microbiol. 2018;9(jan):1–10. doi: 10.3389/fmicb.2018.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.WHO. Model List of Essential Medicines. 2017. [Retrieved on 01-02-2019]. Available from:https://www.who.int/medicines/publications/essentialmedicines/20th_EML2017.pdf?ua=1 .
- 26.Cortés D, Rodríguez V, Verjan N. Phenotypic and genotypic antibiotic resistance of Salmonella from chicken carcasses marketed at Ibague, Colombia. Rev. Bras. Ciéncia Avícola. 2017;19(2):347–354. [Google Scholar]
- 27.Donado-Godoy P, Clavijo V, León M, Arevalo A, Castellanos R, Bernal J, Tafur M, Ovalle M.V, Alali W.Q, Hume M, Romero-Zuñiga J.J, Walls I, Doyle M.P. Counts, Serovars, and Antimicrobial Resistance Phenotypes of Salmonella on Raw Chicken Meat at Retail in Colombia. J. Food. Prot. 2014;77(2):227–235. doi: 10.4315/0362-028X.JFP-13-276. [DOI] [PubMed] [Google Scholar]
- 28.Chai S.J, Cole D, Nisler A, Mahon B.E. Poultry:The most common food in outbreaks with known pathogens, United States 1998-2012. Epidemiol. Infect. 2017;145(2):316–325. doi: 10.1017/S0950268816002375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De-Sousa C.P. The impact of food manufacturing practices on food borne diseases. Brazilian. Arch. Biol. Technol. 2008;51(4):815–823. [Google Scholar]
- 30.Fandiño L.C, Verjan N. A common Salmonella Enteritidis sequence type from poultry and human gastroenteritis in Ibagué, Colombia. Biomédica. 2019;39(1):50–62. doi: 10.7705/biomedica.v39i1.4155. [DOI] [PubMed] [Google Scholar]
- 31.Mogollon C, Rodriguez V, Verjan N. Serotyping and molecular typing of Salmonella isolated from commercial eggs at Ibague, Colombia. Rev. Salud. Anim. 2016;38(3):1–10. [Google Scholar]
- 32.Rodríguez R, Fandiño C, Donado P, Guzmán L, Verjan N. Characterization of Salmonella from Commercial Egg-Laying Hen Farms in a Central Region of Colombia. Avian Dis. 2015;59(1):57–63. doi: 10.1637/10873-052714-reg. [DOI] [PubMed] [Google Scholar]
- 33.Rodriguez J, Rondón I, Verjan N. Serotypes of Salmonella in Broiler Carcasses Marketed at Ibague, Colombia. Rev. Bras. Ciência Avícola. 2015;17(4):545–552. [Google Scholar]
- 34.Marshall B.M, Levy S.B. Food animals and antimicrobials:Impacts on human health. Clin. Microbiol. Rev. 2011;24(4):718–733. doi: 10.1128/CMR.00002-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murray J.F, Schraufnagel D.E, Hopewell P.C. Treatment of Tuberculosis A Historical Perspective. Ann. Am. Thorac. Soc. 2015;12(12):1749–1759. doi: 10.1513/AnnalsATS.201509-632PS. [DOI] [PubMed] [Google Scholar]
- 36.Colquhoun J, Weetch R. Resistance to chloramphenicol developing during treatment of typhoid fever. Lancet Infect. Dis. 1950;256(2):621–623. doi: 10.1016/s0140-6736(50)91585-6. [DOI] [PubMed] [Google Scholar]
- 37.Zaki S.A, Karande S. Multidrug-resistant typhoid fever:A review. J. Infect. Dev. Ctries. 2011;5(5):324–337. doi: 10.3855/jidc.1405. [DOI] [PubMed] [Google Scholar]
- 38.Crump J.A, Sjölund M, Gordon M.A, Parry C.M. Salmonella Infections. Clin. Microbiol. Rev. 2015;28(4):901–937. doi: 10.1128/CMR.00002-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Umasankar S, Wall R, Berger J. A case of ciprofloxacin-resistant typhoid fever. Commun. Dis. Rep. 1992;2(12):139–140. [PubMed] [Google Scholar]
- 40.Bauernfeind A, Holley M, Jungwirth R, Mangold P, Röhnisch T, Schweighart S, Wilhelm R, Casellas J.M, Goldberg M. A new plasmidic cefotaximase from patients infected with Salmonella Typhimurium. Infection. 1992;20(3):158–163. doi: 10.1007/BF01704610. [DOI] [PubMed] [Google Scholar]
- 41.Boyd D, Peters G.A, Cloeckaert A, Boumedine K.S, Chaslus-dancla E, Imberechts H, Mulvey M.R, Al B. Complete Nucleotide Sequence of a 43-Kilobase Genomic Island Associated with the Multidrug Resistance Region of Salmonella enterica Serovar Typhimurium DT104 and Its Identification in Phage Type DT120 and Serovar Agona. J Bacteriol. 2001;183(19):5725–5732. doi: 10.1128/JB.183.19.5725-5732.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Doumith M, Godbole G, Ashton P, Larkin L, Dallman T, Day M, Day M, Muller-pebody B, Ellington M.J, Pinna D, Johnson A.P, Hopkins K.L, Woodford N. Detection of the plasmid-mediated mcr-1 gene conferring colistin resistance in human and food isolates of Salmonella enterica and Escherichia coli in England and Wales. J Antimicrob Chemother. 2016;71(8):2300–2305. doi: 10.1093/jac/dkw093. [DOI] [PubMed] [Google Scholar]
- 43.Summers D. The clinical and veterinary importance of the plasmids. 1st ed. England: Wiley; 1996. [Google Scholar]
- 44.Ross S, Puig J, Zaremba E. Colistin:some preliminary laboratory and clinical observations in specific gastroenteritis in infants and children. Antibiot Ann. 1960;7(1):89–100. [PubMed] [Google Scholar]
- 45.Jacoby G.A, Strahilevitz J, Hooper D. Plasmid-mediated quinolone resistance. Microbiol Spectr. 2014;2(5):1–10. doi: 10.1128/microbiolspec.PLAS-0006-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith L.G, Sensakovic J. Trimethoprim-Sulfamethoxazole. Med Clin North Am. 1982;66(1):143–156. doi: 10.1016/s0025-7125(16)31448-1. [DOI] [PubMed] [Google Scholar]
- 47.Batabyal B. Prevalence of urinary tract pathogens and antimicrobial resistance patterns in children aged 1 to 12 Years. Austin Publ Gr. 2018;5(2):11. [Google Scholar]
- 48.Ugboko H, De N. Review Article Mechanisms of Antibiotic resistance in Salmonella Typhi. Int. J. Curr. Microbiol. Appl. Sci. 2014;3(12):461–476. [Google Scholar]
- 49.Anderson E, Smith H. Chloramphenicol Resistance in the Typhoid Bacillus. J hyg. 1972;74(3):289. doi: 10.1136/bmj.3.5822.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Butler T, Arnold K, Linh N, Pollack M. Chloramphenicol-resistant Typhoid fever in Vietnam associated with R factor. Lancet. Infect. Dis. 1973;302(7836):983–985. doi: 10.1016/s0140-6736(73)91086-6. [DOI] [PubMed] [Google Scholar]
- 51.Karon A.E, Archer J.R, Sotir M.J, Monson T.A, Kazmierczak J.J. Human multidrug-resistant Salmonella Newport infections, Wisconsin 2003-2005. Emerg. Infect. Dis. 2007;13(11):1777–1780. doi: 10.3201/eid1311.061138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aminov R. A brief history of the antibiotic era:lessons learned and challenges for the future. Front Microbiol. 2010;1(dec):1–7. doi: 10.3389/fmicb.2010.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Canal N, Meneghetti K.L, De-Almeida C.P, Da-Rosa M, Otton L.M, Corção G. Characterization of the variable region in the class 1 integron of antimicrobial-resistant Escherichia coli isolated from surface water. Brazilian J. Microbiol. 2016;47(2):337–344. doi: 10.1016/j.bjm.2016.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ferri M, Ranucci E, Romagnoli P, Giaccone V. Antimicrobial resistance:A global emerging threat to public health systems. Crit. Rev. Food. Sci. Nutr. 2017;57(13):2857–2876. doi: 10.1080/10408398.2015.1077192. [DOI] [PubMed] [Google Scholar]
- 55.Seth-smith H.M, Fookes M, Okoro C.K, Baker S, Harris S.R, Scott P, Pickard D, Quail M.A, Churcher C, Sanders M, Harmse J, Dougan G, Parkhill J, Thomson N.R. Structure , Diversity , and Mobility of the Salmonella Pathogenicity Island 7 Family of Integrative and Conjugative Elements within. J. Bacteriol. 2012;194(6):1494–1504. doi: 10.1128/JB.06403-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Doudard G, Karine P, Axel C, Doublet B. The Salmonella genomic island 1 is specifically mobilized in trans by the IncA/C multidrug resistance plasmid family. PLoS One. 2010;5(12):1–8. doi: 10.1371/journal.pone.0015302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Silva C, Wiesner M, Calva E. The Importance of Mobile Genetic Elements in the Evolution of Salmonella:Pathogenesis, Antibiotic Resistance and Host Adaptation. InTech. Rijeka. 2012. [Retrieved on 20-06-2019]. Available from:https://www.intechopen.com/books/salmonella-a-diversified-superbug/the-importance-of-mobile-genetic-elements-in-the-evolution-of-salmonella-pathogenesis-antibiotic-res .
- 58.Hello S, Weill F, Guibert V, Praud K, Cloeckaert A, Doublet B. Early Strains of Multidrug-Resistant Salmonella enterica Serovar Kentucky Sequence Type 198 from Southeast Asia Harbor Salmonella Genomic Island 1-J Variants with a Novel Insertion Sequence. Antimicrob Agents Chemother. 2012;56(10):5096–5102. doi: 10.1128/AAC.00732-12. doi 5096–5102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Heuer H, Abdo Z, Smalla K. Patchy distribution of flexible genetic elements in bacterial populations mediates robustness to environmental uncertainty. FEMS Microbiol Ecol. 2008;65(3):361–371. doi: 10.1111/j.1574-6941.2008.00539.x. [DOI] [PubMed] [Google Scholar]
- 60.Rodríguez I, Rodicio R, Guerra B, Hopkins K. Potential international Spread of Multidrug-Resistant Invasive Salmonella enterica Serovar Enteritidis. Emerg. Infect. Dis. 2012;18(7):1173–1176. doi: 10.3201/eid1807.120063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lindsey R.L, Fedorka-cray P.J, Frye J.G, Meinersmann R. IncA/C Plasmids Are Prevalent in Multidrug-Resistant Salmonella enterica Isolates. Appl. Environ. Microbiol. 2009;75(7):1908–1915. doi: 10.1128/AEM.02228-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Harmer C.J, Hall R.M. The A to Z of A/C Plasmids. Plasmids. 2015;80(3):63–82. doi: 10.1016/j.plasmid.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 63.Munita J.M, Arias C.A. Mechanisms of Antibiotic Resistance. Microbiol. Spectr. 2016;4(2):1–37. doi: 10.1128/microbiolspec.VMBF-0016-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gillings M.R. Integrons :Past , Present , and Future Structure of Integrons. Microbiol. Mol. Biol. Rev. 2014;78(2):257–277. doi: 10.1128/MMBR.00056-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cambray G, Guerout A, Mazel D. Integrons. Annu. Rev. 2010;44(1):141–166. doi: 10.1146/annurev-genet-102209-163504. doi:10.1146/annurevgenet-102209-163504. [DOI] [PubMed] [Google Scholar]
- 66.Davies J, Davies D. Origins and Evolution of Antibiotic Resistance. Microbiol. Mol. Biol. Rev. 2010;74(3):417–433. doi: 10.1128/MMBR.00016-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rodríguez-Martínez J.M, Eliecer M, Velasco C, Martínez-Martínez L, Pascual Á. Plasmid-mediated quinolone resistance:an update. J. Infect. Chemother. 2011;17(2):149–182. doi: 10.1007/s10156-010-0120-2. [DOI] [PubMed] [Google Scholar]
- 68.Correia S, Poeta P, Hébraud M, Capelo J.L, Igrejas G. Mechanisms of quinolone action and resistance:where do we stand? J. Med. Microbiol. 2017;66(5):551–559. doi: 10.1099/jmm.0.000475. [DOI] [PubMed] [Google Scholar]
- 69.Willey J, Sherwood L, Wolverton C. Prescott s Microbiology. 9th edition. New York: McGraw-Hill; 2013. [Google Scholar]
- 70.Floss H.G, Yu T. Rifamycins Mode of Action, Resistance , and Biosynthesis. Chem. Rev. 2005;105(2):621–632. doi: 10.1021/cr030112j. [DOI] [PubMed] [Google Scholar]
- 71.Liu Y.Y, Wang Y, Walsh T.R, Yi L.X, Zhang R, Spencer J, Doi Y, Tian G, Dong B, Huang X, Yu L.F, Gu D, Ren H, Chen X, Lv L, He D, Zhou H, Liang Z, Liu J.H, Shen J. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China:A microbiological and molecular biological study. Lancet Infect. Dis. 2016;16(2):161–168. doi: 10.1016/S1473-3099(15)00424-7. [DOI] [PubMed] [Google Scholar]
- 72.Olaitan A.O, Morand S, Rolain J.M. Mechanisms of polymyxin resistance:acquired and intrinsic resistance in bacteria Abiola. Front. Microbiol. 2014;5(1):643. doi: 10.3389/fmicb.2014.00643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nordmann P, Poirel L. Emergence of plasmid-mediated resistance to quinolones in Enterobacteriaceae. J. Antimicrob. Chemother. 2005;56(3):463–469. doi: 10.1093/jac/dki245. [DOI] [PubMed] [Google Scholar]
- 74.Andoh L.A, Dalsgaard A, Newman M.J. Prevalence and antimicrobial resistance of Salmonella serovars isolated from poultry in Ghana. Epidemiol. Infect. 2016;144(15):3288–3299. doi: 10.1017/S0950268816001126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ibrahim T, Ngwai Y.B, Pennap G.I, Ishaleku D, Tsaku P.A, Abimiku R.H, Nkene I.H, Bassey E.B. Antimicrobial Resistance Profile of Salmonella Typhimurium Isolated from Commercial Poultry and Poultry Farm Handlers in Nasarawa State, Nigeria. Microbiol. Res. J. Int. 2019;28(4):1–12. [Google Scholar]
- 76.Ejeh F, Lawan F, Abdulsalam H, Mamman P, Kwanashie C. Multiple antimicrobial resistance of Escherichia coli and Salmonella species isolated from broilers and local chickens retailed along the roadside in Zaria, Nigeria. Sokoto J. Vet. Sci. 2017;15(3):45–53. [Google Scholar]
- 77.Ugwu M.C, Omanukwue C, Chimezie C, Okezie U, Ejikeugwu C.P, Nnnabuife-iloh E, Esimone C.O. Poultry Farm and Poultry Products as Sources of Multiple Antimicrobial-Resistant Salmonella and S. aureus. J. Trop. Dis. 2019;7(3):1–23. [Google Scholar]
- 78.Yangkam-Yhiler N, Enya B. Antimicrobial Susceptibility Patterns of Salmonella Species from Sources in Poultry Production Settings in Calabar, Cross River State, Nigeria. Am. J. Heal. Res. 2015;3(2):76–81. [Google Scholar]
- 79.Atere V. Multidrug resistant Salmonella sp. isolated from chicken. Int. J. Biol. Res. 2016;4(1):64–66. [Google Scholar]
- 80.Elkenany R.M, Eladl A.H, El-Shafei R.A. Genetic characterisation of class 1 integrons among multidrug-resistant Salmonella serotypes in broiler chicken farms. J. Glob. Antimicrob. Resist. 2018;14(sept):202–208. doi: 10.1016/j.jgar.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 81.Abunna F, Bedasa M, Beyene T, Ayana D, Mamo B, Duguma R. Salmonella:isolation and antimicrobial susceptibility tests on isolates collected from poultry farms in and around Modjo, Central Oromia, and Ethiopia. J. Anim. Poult. Sci. 2016;5(2):21–35. [Google Scholar]
- 82.Eguale T. Non-typhoidal Salmonella serovars in poultry farms in central Ethiopia:Prevalence and antimicrobial resistance. BMC Vet. Res. 2018;14(1):1–8. doi: 10.1186/s12917-018-1539-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Abdi R.D, Mengstie F, Beyi A.F, Beyene T, Waktole H, Mammo B, Ayana D, Abunna F. Determination of the sources and antimicrobial resistance patterns of Salmonella isolated from the poultry industry in Southern Ethiopia. BMC Infect. Dis. 2017;17(1):1–12. doi: 10.1186/s12879-017-2437-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Castro-Vargas R, Fandiño-de-Rubio L.C, Vega A, Rondón-Barragán I. Phenotypic and Genotypic Resistance of Salmonella Heidelberg Isolated from One of the Largest Poultry Production Region from Colombia. Int. J. Poult. Sci. 2019;18(12):610–617. [Google Scholar]
- 85.Langata L.M, Maingi J.M, Musonye H.A, Kiiru J, Nyamache A.K. Antimicrobial resistance genes in Salmonella and Escherichia coli isolates from chicken droppings in Nairobi, Kenya. BMC Res. Notes. 2019;12(1):1–6. doi: 10.1186/s13104-019-4068-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Makaya P.V, Matope G, Pfukenyi D.M. Distribution of Salmonella serovars and antimicrobial susceptibility of Salmonella Enteritidis from poultry in Zimbabwe. Avian Pathol. 2012;41(2):221–226. doi: 10.1080/03079457.2012.667558. [DOI] [PubMed] [Google Scholar]
- 87.Zishiri O, Mkhize N, Mukaratirwa S. Prevalence of virulence and antimicrobial resistance genes in Salmonella spp . isolated from commercial chickens and human clinical isolates from South Africa and Brazil. Onderstepoort J. Vet. Res. 2016;83(1):1–11. doi: 10.4102/ojvr.v83i1.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Figueiredo R, Henriques A, Sereno R, Mendoza N, da-Silva G. Antimicrobial resistance and extended-spectrum ?-lactamases of Salmonella enterica serotypes isolated from livestock and processed food in Portugal:an update. Foodborne Pathog. Dis. 2015;12(2):110–117. doi: 10.1089/fpd.2014.1836. [DOI] [PubMed] [Google Scholar]
- 89.Lamas A, Miranda J.M, Regal P, Vázquez B, Franco C.M, Cepeda A. A comprehensive review of non- enterica subspecies of Salmonella enterica. Microbiol. Res. 2018;206(sept):60–73. doi: 10.1016/j.micres.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 90.Alcaine S.D, Molla L, Nugen S.R, Kruse H. Results of a pilot antibiotic resistance survey of Albanian poultry farms. J. Glob. Antimicrob. Resist. 2016;4(1):60–64. doi: 10.1016/j.jgar.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 91.Al-Zenki S, Al-Nasser A, Al-Safar A, Alomirah H, Al-Haddad A, Hendriksen R.S, Aarestrup F.M. Prevalence and Antibiotic Resistance of Salmonella Isolated from a Poultry Farm and Processing Plant Environment in the State of Kuwait. Foodborne Pathog. Dis. 2007;4(3):367–373. doi: 10.1089/fpd.2007.0017. [DOI] [PubMed] [Google Scholar]
- 92.Al-Bahry S.N, Elshafie A.E, Al-Busaidy S, Al-Hinai J, Al-Shidi I. Antibiotic-resistant Salmonella spp. from human and non-human sources in Oman. East Mediterr. Heal J. 2007;13(1):49–55. [PubMed] [Google Scholar]
- 93.Abdukhalilova G, Kaftyreva L, Wagenaar J.A, Tangyarikov B, Bektimirov A. Occurence and Antimicrobial Resistance of Salmonella and Campylobacter in Humans and Broiler Chicken in Uzbekistan. Public. Heal. Panor. 2016;2(3):340–347. [Google Scholar]
- 94.Asif M, Rahman H, Qasim M, Khan T.A, Ullah W, Jie Y. Molecular detection and antimicrobial resistance profile of zoonotic Salmonella Enteritidis isolated from broiler chickens in Kohat, Pakistan. J. Chinese Med. Assoc. 2017;80(5):303–306. doi: 10.1016/j.jcma.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 95.Duarte A, Hoyos K. Evaluation of susceptibility to antibiotics of Salmonella spp., Isolated from samples of poultry production that entered the Servet laboratory. 2018. [Retrieved on 20-01-2019]. Available from:https://repository.udca.edu.co/bitstream/11158/973/1/TESIS%20FINAL.pdf .
- 96.Bhuvaneswa M, Shanmughap S, Natarajase K Prevalence of Multidrug-Resistant (MDR) Salmonella Enteritidis in Poultry and Backyard Chicken from Tiruchirappalli. India Microbiol. J. 2015;5(2):28–35. [Google Scholar]
- 97.Waghamare R.N, Paturkar A.M, Vaidya V.M, Zende R.J, Dubal Z.N, Dwivedi A, Gaikwad R.V. Phenotypic and genotypic drug resistance profile of Salmonella serovars isolated from poultry farm and processing units located in and around Mumbai city. India. Vet. World. 2018;11(12):1682–1688. doi: 10.14202/vetworld.2018.1682-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rodney S, Umakanth S, Chowdhury G, Saha R.N, Mukhopadhyay A.K, Ballal M. Poultry:A receptacle for non-typhoidal salmonellae and antimicrobial resistance. Iran J. Microbiol. 2019;11(1):31–38. [PMC free article] [PubMed] [Google Scholar]
- 99.Lu Y, Zhao H, Sun J, Liu Y, Zhou X, Beier R.C, Wu G, Hou X. Characterization of multidrug-resistant Salmonella enterica serovars Indiana and enteritidis from chickens in Eastern China. PLoS One. 2014;9(5):1–6. doi: 10.1371/journal.pone.0096050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhao X, Gao Y, Ye C, Yang L, Wang T, Chang W. Prevalence and Characteristics of Salmonella Isolated from Free-Range Chickens in Shandong Province, China. Biomed. Res. Int. 2016;6(1):1–6. doi: 10.1155/2016/8183931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yang J, Gao S, Chang Y, Su M, Xie Y, Sun S. Occurrence and Characterization of Salmonella Isolated from Large-Scale Breeder Farms in Shandong Province, China. Biomed. Res. Int. 2019;2019(apr):1–9. doi: 10.1155/2019/8159567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Parvej M.S, Nazmul-Hussain H.M, Bahanur-Rahman M, Jahan M, Rahman-Khan M.F, Rahman M. Prevalence and characterization of multi-drug resistant Salmonella enterica serovar Gallinarum biovar Pullorum and Gallinarum from chicken. Vet. World. 2016;9(1):65–70. doi: 10.14202/vetworld.2016.65-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Akond M.A, Shirin M, Alam S, Hassan S, Rahman M.M, Hoq M. Frequency of drug resistant Salmonella spp. isolated from poultry samples in Bangladesh. Stamford J. Microbiol. 2013;2(1):15–19. [Google Scholar]
- 104.Khemtong S, Chuanchuen R. Class 1 integrons and Salmonella genomic island 1 among Salmonella enterica isolated from poultry and swine. Microb. Drug. Resist. 2008;14(1):65–70. doi: 10.1089/mdr.2008.0807. [DOI] [PubMed] [Google Scholar]
- 105.Chuanchuen R, Pathanasophon P, Khemtong S, Wannaprasat W, Padungtod P. Susceptibilities to Antimicrobials and Disinfectants in Salmonella Isolates Obtained from Poultry and Swine in Thailand. J. Vet. Med. Sci. 2008;70(6):595–601. doi: 10.1292/jvms.70.595. [DOI] [PubMed] [Google Scholar]
- 106.Villagómez S, Logacho M, Vinueza C. Presence and Resistance to Antimicrobials of Salmonella enterica serovars isolated in an integrated poultry company in Ecuador. Rev. Ecuat. Med. Cienc. Biol. 2017;38(1):11–24. [Google Scholar]
- 107.Geidam Y.A, Zakaria Z, Aziz S.A, Bejo S.K, Abu J, Omar S. High prevalence of multi-drug resistant bacteria in selected poultry farms in Selangor, Malaysia. Asian J. Anim. Vet. Adv. 2012;7(9):891–897. [Google Scholar]
- 108.Shang K, Wei B, Kang M. Distribution and dissemination of antimicrobial-resistant Salmonella in broiler farms with or without enrofloxacin use. BMC Vet. Res. 2018;14(1):1–14. doi: 10.1186/s12917-018-1590-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rayamajhi N, Jung B.Y, Cha S.B, Shin M.K, Kim A, Kang M.S, Lee K.M, Yoo H.S. Antibiotic Resistance Patterns and Detection of bla DHA-1 in Salmonella Species Isolates from Chicken Farms in South Korea. Appl. Environ. Microbiol. 2010;76(14):4760–4764. doi: 10.1128/AEM.02536-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Duc V.M, Nakamoto Y, Fujiwara A, Toyofuku H, Obi T, Chuma T. Prevalence of Salmonella in broiler chickens in Kagoshima, Japan in 2009 to 2012 and the relationship between serovars changing and antimicrobial resistance. BMC Vet. Res. 2019;15(1):1–8. doi: 10.1186/s12917-019-1836-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fitch F.M, Carmo-rodrigues M.S, Sales V, Gaspari M, dos-Santos A, Barros-de-Freitas J, Pignatar A. B-Lactam Resistance Genes:Characterization, Epidemiology, and First Detection of bla CTX-M-1 and bla CTX-M-14 in Salmonella spp. Isolated from Poultry in Brazil—Brazil Ministry of Agriculture's Pathogen Reduction Program. Microb. Drug. Resist. 2015;22(2):164–171. doi: 10.1089/mdr.2015.0143. [DOI] [PubMed] [Google Scholar]
- 112.Voss-Rech D, Vaz C.L, Alves L, Coldebella A, Leao J.A, Rodrigues D.P, Back A. A temporal study of Salmonella enterica serotypes from broiler farms in Brazil. Poult. Sci. 2015;94(3):433–441. doi: 10.3382/ps/peu081. [DOI] [PubMed] [Google Scholar]
- 113.Biffi C.P, Stefani L.M, Miletti L.C, Matiello C, Backes R.G, Almeida J.M, Neves G.B. Phenotypic and Genotypic resistance Profile of Salmonella Typhimurium to Antimicrobials Commonly Used in Poultry. Rev. Bras. Cienc. Avic. 2014;16(2):93–96. [Google Scholar]
- 114.das-Neves G.B, Stefani L.M, Pick E, Araujo D.N, Giuriatti J, Percio C, Brisola M.C. Salmonella Heidelberg Isolated from Poultry Shows a Novel Resistance Profile. Acta Sci. Vet. 2016;44(1):1–6. [Google Scholar]
- 115.San-Martín B, Lapierre L, Toro C, Bravo V, Cornejo J, Hormazabal J.C, Borie C. Isolation and molecular characterization of quinolone resistant Salmonella spp. from poultry farms. Vet. Microbiol. 2005;110(3–4):239–244. doi: 10.1016/j.vetmic.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 116.Singer R.S, Hofacre C.L. Potential Impacts of Antibiotic Use in Poultry Production. Avian Dis. 2006;50(2):161–172. doi: 10.1637/7569-033106R.1. [DOI] [PubMed] [Google Scholar]
- 117.Shukla P, Bansode F.W, Singh R.K. Chloramphenicol Toxicity :A Review. J. Med. Med. Sci. 2011;2(13):1313–1316. [Google Scholar]
- 118.Roth N, Käsbohrer A, Mayrhofer S, Zitz U, Hofacre C, Domig K.J. The application of antibiotics in broiler production and the resulting antibiotic resistance in Escherichia coli:A global overview. Poult. Sci. 2019;98(4):1791–1804. doi: 10.3382/ps/pey539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Maciel J, Machado G, Avancini C.A. Investigation of resistance of Salmonella spp. isolated from products and raw material of animal origin (swine and poultry) to antibiotics and disinfectants. Rev. Bras. Saúde. Prod. Anim. 2019;20(2):1–13. [Google Scholar]
- 120.Duarte D, Moliterno A, Vasconcelos A, Santos S, Silva J, Andrade P, Falcão L. Occurrence of Salmonella spp. in broiler chicken carcasses and their susceptibility to antimicrobial agents. Brazilian J. Microbiol. 2009;40(3):569–573. doi: 10.1590/S1517-838220090003000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ribeiro A.R, Kellermann A, Ruschel L, Bessa M.C, Pinheiro V. Salmonella spp. in raw broiler parts:occurrence, antimicrobial resistance profile and phage typing of the Salmonella Enteritidis isolates. Brazilian J. Microbiol. 2007;38(2):296–299. [Google Scholar]
- 122.Bada-alambedji R, Fofana A, Seydi M, Akakpo A.J. Antimicrobial resistance of Salmonella isolated from poultry carcasses in Dakar (Senegal) Brazilian J. Microbiol. 2006;37(4):510–515. [Google Scholar]
- 123.Khallaf M, Ameur N, Terta M, Lakranbi M, Senouci S, Ennaji M.M. Prevalence and antibiotic-resistance of Salmonella isolated from chicken meat marketed in Rabat, Morocco. Int. J. Innov. Appl. Stud. 2014;6(4):1123–1128. [Google Scholar]
- 124.Bouda S.C, Kagambèga A, Bonifait L, Gall F.L, Ibrahim H.B, Bako E, Bagre T.S, Zongo C, N A.W, Traore S.A, Chemaly M, Salvat G, Barro N. Prevalence and Antimicrobial Resistance of Salmonella enterica Isolated from Chicken and Guinea Fowl in Burkina Faso. Int. J. Microbiol. Biotechnol. 2019;4(3):64–71. [Google Scholar]
- 125.Gharieb R.M, Tartor Y.H, Khedr M.H. Non-Typhoidal Salmonella in poultry meat and diarrhoeic patients:Prevalence, antibiogram, virulotyping, molecular detection and sequencing of class I integrons in multidrug resistant strains. Gut Pathog. 2015;7(1):1–11. doi: 10.1186/s13099-015-0081-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Abdel-Maksoud M, Abdel-Khalek R, El-Gendy A, Gamal R.F, Abdelhady H.M, House B.L. Genetic characterisation of multidrug-resistant Salmonella enterica serotypes isolated from poultry in Cairo, Egypt. Afr. J. Lab. Med. 2015;4(1):1–7. doi: 10.4102/ajlm.v4i1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Molla B, Mesfin A, Alemayehu D. Multiple antimicrobial-resistant Salmonella serotypes isolated from chicken carcass and giblets in Debre Zeit and Addis Ababa, Ethiopia. Ethiop. J. Heal. Dev. 2004;17(2) [Google Scholar]
- 128.Wilson I.G. Antimicrobial resistance of Salmonella in raw retail chickens, imported chicken portions, and human clinical specimens. J. Food. Prot. 2004;67(6) doi: 10.4315/0362-028x-67.6.1220. [DOI] [PubMed] [Google Scholar]
- 129.Antunes P, Réu C, Sousa J, Peixe L, Pestana N. Incidence of Salmonella from poultry products and their susceptibility to antimicrobial agents. Int. J. Food. Microbiol. 2003;82:97–103. doi: 10.1016/s0168-1605(02)00251-9. [DOI] [PubMed] [Google Scholar]
- 130.Carramiñana J.J, Rota C, Agustín I, Herrera A. High prevalence of multiple resistance to antibiotics in Salmonella serovars isolated from a poultry slaughterhouse in Spain. Vet. Microbiol. 2004;104(1–2):133–139. doi: 10.1016/j.vetmic.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 131.Zdragas A, Mazaraki K, Vafeas G, Giantzi V, Papadopoulos T, Ekateriniadou L. Prevalence, seasonal occurrence and antimicrobial resistance of Salmonella in poultry retail products in Greece. Lett. Appl. Microbiol. 2012;55(4):308–313. doi: 10.1111/j.1472-765X.2012.03298.x. [DOI] [PubMed] [Google Scholar]
- 132.Mihaiu L, Lapusan A, Tanasuica R, Sobolu R, Mihaiu R, Oniga O, Mihaiu M. First study of Salmonella in meat in Romania. J. Infect. Dev. Ctries. 2014;8(1):50–58. doi: 10.3855/jidc.3715. [DOI] [PubMed] [Google Scholar]
- 133.Tîrziu E, Laz?r R, Sala C, Nichita I, Morar A, Şereş M, Imre K. Salmonella in raw chicken meat from the Romanian seaside:Frequency of isolation and antibiotic resistance. J. Food Prot. 2015;78(5):1003–1006. doi: 10.4315/0362-028X.JFP-14-460. [DOI] [PubMed] [Google Scholar]
- 134.Terentjeva M, Avsejenko J, Streikiša M, Utināne A, Kovaļenko K, Bērziņš A. Prevalence and antimicrobial resistance of Salmonella in meat and meat products in Latvia. Ann. Agric. Environ. Med. 2017;24(2):317–321. doi: 10.5604/12321966.1235180. [DOI] [PubMed] [Google Scholar]
- 135.Ezgi-Telli A, Biçer Y, Ahu-Kahraman H, Telli N, Doğruer Y. Presence and antibiotic resistance of Salmonella spp. isolated from chicken meat and giblets consumed in Konya, Turkey. Eurasian J. Vet. Sci. 2018;34(3):164–170. [Google Scholar]
- 136.Yildirim Y, Gonulalan Z, Pamuk S, Ertas N. Incidence and antibiotic resistance of Salmonella spp. on raw chicken carcasses. Food Res. Int. 2011;44(3):725–728. [Google Scholar]
- 137.Arslan S, Eyi A. Occurrence and antimicrobial resistance profiles of Salmonella species in retail meat products. J. Food Prot. 2010;73(9):1613–1617. doi: 10.4315/0362-028x-73.9.1613. [DOI] [PubMed] [Google Scholar]
- 138.Bilge N, Vatansever L, Sezer Ç. PiliçKanatlarından İzole Edilen Salmonella spp.'nin Antibiyotik Direnci. Kafkas Univ. Vet. Fak. Derg. 2018;24(3):431–435. [Google Scholar]
- 139.Lestari S.I, Han F, Wang F, Ge B. Prevalence and antimicrobial Resistance of Salmonella serovars in conventional and organic chickens from Louisiana retail stores. J. Food Prot. 2009;72(6):1165–1172. doi: 10.4315/0362-028x-72.6.1165. [DOI] [PubMed] [Google Scholar]
- 140.Abdullah-Badahdah S, Aldagal M.M. Antibiotic Resistance in Salmonella spp. Isolated from Local Chickens in Saudi Arabia. Int. J. Food. Sci. Nutr. Eng. 2018;8(5):127–130. [Google Scholar]
- 141.Fallah S.H, Asgharpour F, Naderian Z, Moulana Z. Isolation and Determination of Antibiotic Resistance Patterns in Non-typhoid Salmonella spp isolated from chicken. Int. J. Enteric. Pathog. 2015;1(1):17–21. [Google Scholar]
- 142.Sodagari H.R, Mashak Z, Ghadimianazar A. Prevalence and antimicrobial resistance of Salmonella serotypes isolated from retail chicken meat and giblets in Iran. J. Infect. Dev. Ctries. 2015;9(5):463–469. doi: 10.3855/jidc.5945. [DOI] [PubMed] [Google Scholar]
- 143.Rahman M, Rahman A, Islam M.A, Alam M.M. Detection of multi–drug resistant Salmonella from milk and meat in Bangladesh. Bangladesh J. Vet. Med. 2018;16(1):115–120. [Google Scholar]
- 144.Zhu Y, Lai H, Zou L, Yin S, Wang C, Han X, Xia X, Hu K, He L, Zhou K, Chen S, Ao X, Liu S. Antimicrobial resistance and resistance genes in Salmonella strains isolated from broiler chickens along the slaughtering process in China. Int. J. Food Microbiol. 2017;259(aug):43–51. doi: 10.1016/j.ijfoodmicro.2017.07.023. [DOI] [PubMed] [Google Scholar]
- 145.Bai L, Lan R, Zhang X, Cui S, Xu J, Guo Y, Li F, Zhang D. Prevalence of Salmonella Isolates from Chicken and Pig Slaughterhouses and Emergence of Ciprofloxacin and Cefotaxime Co-Resistant S. enterica Serovar Indiana in Henan, China. PLoS One. 2015;. 10(12):1–14. doi: 10.1371/journal.pone.0144532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lu Y, Wu C.M, Wu G.J, Zhao H.Y, He T, Cao X.Y, Dai L, Xia L.N, Qin S.S, Shen J.Z. Prevalence of Antimicrobial Resistance Among Salmonella Isolates from Chicken in China. Foodborne Pathog. Dis. 2011;8(1):45–53. doi: 10.1089/fpd.2010.0605. [DOI] [PubMed] [Google Scholar]
- 147.Yang B, Cui Y, Shi C, Wang J, Xia X, Xi M, Wang X, Meng J, Alali W.Q, Walls I, Doyle M.P. Counts, serotypes, and antimicrobial resistance of Salmonella isolates on retail raw poultry in the People's Republic of China. J. Food Prot. 2014;77(6):894–902. doi: 10.4315/0362-028X.JFP-13-439. [DOI] [PubMed] [Google Scholar]
- 148.Chaisatit C, Tribuddharat C, Dejsirilert S, Pulsrikarn C. Molecular characterization of antibiotic resistant bacteria isolated from chicken meats sold at supermarkets in Bangkok, Thailand. Int. J. Infect. Dis. 2012;16(1):e342. doi: 10.7883/yoken.65.527. [DOI] [PubMed] [Google Scholar]
- 149.Goni A.M, Effarizah M.E, Rusul G. Prevalence, antimicrobial resistance, resistance genes and class 1 integrons of Salmonella serovars in leafy vegetables, chicken carcasses and related processing environments in Malaysian fresh food markets. Food Control. 2018;91(3):170–180. [Google Scholar]
- 150.M'ikanatha N.M, Sandt C.H, Localio A.R, Tewari D, Rankin S.C, Whichard J.M, Altekruse S.F, Lautenbach E, Folster J.P, Russo A, Chiller T.M, Reynolds S.M, McDermott P.F. Multidrug-Resistant Salmonella Isolates from Retail Chicken Meat Compared with Human Clinical Isolates. Foodborne Pathog. Dis. 2010;7(8):929–934. doi: 10.1089/fpd.2009.0499. [DOI] [PubMed] [Google Scholar]
- 151.Ta Y.T, Nguyen T.T, To P.B, Pham D.X, Le T.H, Thi G.N, Alali W.Q, Walls I, Doyle M.P. Quantification, serovars, and antibiotic resistance of Salmonella isolated from retail raw chicken meat in Vietnam. J. Food Prot. 2014;77(1):57–66. doi: 10.4315/0362-028X.JFP-13-221. [DOI] [PubMed] [Google Scholar]
- 152.Ha T, Hirai T, Thi N, Yamaguchi R. Antibiotic resistance profiles of Salmonella serovars isolated from retail pork and chicken meat in North Vietnam. Int. J. Food Microbiol. 2012;156(2):147–151. doi: 10.1016/j.ijfoodmicro.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 153.Kidie D.H, Bae D.H, Lee Y.J. Prevalence and antimicrobial resistance of Salmonella isolated from poultry slaughterhouses in Korea. Jpn. J. Vet. Res. 2013;61(4):129–136. [PubMed] [Google Scholar]
- 154.Choi D, Chon J.W, Kim H.S, Kim D.H, Lim J.S, Yim J.H, Seo K.H. Incidence, antimicrobial resistance, and molecular characteristics of nontyphoidal Salmonella including extended-spectrum ?-lactamase producers in retail chicken meat. J. Food Prot. 2015;78(11):1932–1937. doi: 10.4315/0362-028X.JFP-15-145. [DOI] [PubMed] [Google Scholar]
- 155.Kim M.S, Lim T.H, Jang J.H, Lee D.H, Kim B.Y, Kwon J.H, Choi S.W, Noh J.Y, Hong Y.H, Lee S.B, Yang S.Y, Lee H.J, Lee J.B, Park S.Y, Choi I.S, Song C.S. Prevalence and antimicrobial resistance of Salmonella species isolated from chicken meats produced by different integrated broiler operations in Korea. Poult. Sci. 2012;91(9):2370–2375. doi: 10.3382/ps.2012-02357. [DOI] [PubMed] [Google Scholar]
- 156.Iwabuchi E, Yamamoto S, Endo Y, Ochiai T, Hirai K. Prevalence of Salmonella Isolates and Antimicrobial Resistance Patterns in Chicken Meat throughout Japan. J. Food Prot. 2011;74(2):270–273. doi: 10.4315/0362-028X.JFP-10-215. [DOI] [PubMed] [Google Scholar]
- 157.Miranda J.M, Mondrago A.C, Martinez B, Guarddon M, Rodriguez J.A. Prevalence and Antimicrobial Resistance Patterns of Salmonella from Different Raw Foods in Mexico. J. Food Prot. 2009;72(5):966–971. doi: 10.4315/0362-028x-72.5.966. [DOI] [PubMed] [Google Scholar]
- 158.Sonali M, Corona R, Granda A.E, Felipe L, Bonachea H. Antimicrobial resistance in strains of Salmonella enterica subsp. enterica isolated in imported poultry meat Rev. Salud Anim. 2012;34(2):120–126. [Google Scholar]
- 159.Jarquin C, Alvarez D, Morales O, Morales A.J, López B, Donado P, Valencia M.F, Arévalo A, Muñoz F, Walls I, Doyle M.P, Alali W.Q. Salmonella on Raw Poultry in Retail Markets in Guatemala:Levels, Antibiotic Susceptibility, and Serovar Distribution. J. Food Prot. 2015;78(9):1642–1650. doi: 10.4315/0362-028X.JFP-15-117. [DOI] [PubMed] [Google Scholar]
- 160.Donado-Godoy P, Byrne B.A, Leon M, Castellanos R, Vanegas C, Coral A, Arevalo A, Clavuo V, Vargas M, Zuniga J.R, Tafur M, Perez-gutierrez E. Prevalence , Resistance Patterns , and Risk Factors for Antimicrobial Resistance in Bacteria from Retail Chicken Meat in Colombia. J. Food Prot. 2015;78(4):751–759. doi: 10.4315/0362-028X.JFP-14-349. [DOI] [PubMed] [Google Scholar]
- 161.Boscan-Duque L.A, Arzalluz-Fisher A.M, Ugarte C, Sanchez D, Wittum T.E, Hoet A.E. Reduced susceptibility to quinolones among Salmonella serotypes isolated from poultry at slaughter in Venezuela. J. Food Prot. 2007;70(9):2030–2035. doi: 10.4315/0362-028x-70.9.2030. [DOI] [PubMed] [Google Scholar]
- 162.Dunne E.F, Fey P.D, Kludt P, Shillam P, Wicklund J, Miller C, Holland B, Stamey K, Barrett T.J, Rasheed J.K, Tenover F.C. Emergence of Domestically Acquired Ceftriaxone-Resistant Salmonella Infections Associated with AmpC B-Lactamase. JAMA. 2000;284(24):3151–3156. doi: 10.1001/jama.284.24.3151. [DOI] [PubMed] [Google Scholar]
- 163.Snow L.C, Davies R.H, Christiansen K.H, Carrique-Mas J.J, Wales A.D, O'Connor J.L, Cook A.J, Evans S.J. Survey of the prevalence of Salmonella species on commercial laying farms in the United Kingdom. Vet. Rec. 2007;161(14):471–476. doi: 10.1136/vr.161.14.471. [DOI] [PubMed] [Google Scholar]
- 164.Dipineto L, Scarpetta C, Calabria M, Sensale M, Baiano A, Menna L.F, Fioretti A. Antimicrobial susceptibility of Salmonella spp. strains isolated from layer hens in Campania Region from 2000 to 2003. Ital. J. Anim. Sci. 2005;4(3):279–281. [Google Scholar]
- 165.Mąka Ł, Maćkiw E, Ścieżyńska H, Popowska M. Occurrence and antimicrobial resistance of Salmonella spp. Isolated from food other than meat in Poland. Ann Agric. Environ. Med. 2015;22(3):403–408. doi: 10.5604/12321966.1167701. [DOI] [PubMed] [Google Scholar]
- 166.Karimiazar F, Soltanpour M.S, Aminzare M, Hassanzadazar H. Prevalence, genotyping, serotyping, and antibiotic resistance of isolated Salmonella strains from industrial and local eggs in Iran. J. Food Saf. 2019;39(1):1–7. [Google Scholar]
- 167.Harsha H. Prevalence and antibiotic resistance of Salmonella from the eggs of commercial samples. J. Microbiol. Infect. Dis. 2011;1(3):93–100. [Google Scholar]
- 168.Bandyopadhyay M, Jha V, Ajitkumar B.S, Jhangiani A.R. Prevalence Study and Resistance Profiles of Multi-Drug Resistant Salmonella Obtained from Poultry Across Mumbai Region. Acta Sci. Microbiol. 2019;2(5):2581–3226. [Google Scholar]
- 169.Singh S, Yadav A.S, Singh S.M, Bharti P. Prevalence of Salmonella in chicken eggs collected from poultry farms and marketing channels and their antimicrobial resistance. Food Res. Int. 2010;43(8):2027–2030. [Google Scholar]
- 170.Xie T, Wu G, He X, Lai Z, Zhang H, Zhao J. Antimicrobial resistance and genetic diversity of Salmonella enterica from eggs. Food Sci. Nutr. 2019;7(9):2847–2853. doi: 10.1002/fsn3.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mahmud T, Hassan M.M, Alam M, Khan M.M, Bari M.S, Islam A. Prevalence and multidrug-resistant pattern of Salmonella from the eggs and egg-storing trays of retail markets of Bangladesh. Int. J. One Heal. 2016;2(1):7–11. [Google Scholar]
- 172.Ahmed M.M, Rahman M.M, Mahbub K.R, Wahiduzzaman M. Characterization of Antibiotic Resistant Salmonella spp Isolated from Chicken Eggs of Dhaka City. J Sci. Res. 2010;3(1):191–196. [Google Scholar]
- 173.Im M.C, Jeong S.J, Kwon Y.K, Jeong O.M, Kang M.S, Lee Y.J. Prevalence and characteristics of Salmonella spp. isolated from commercial layer farms in Korea. Poult. Sci. 2015;94(7):1691–1698. doi: 10.3382/ps/pev137. [DOI] [PubMed] [Google Scholar]
- 174.Musgrove M.T, Jones D.R, Northcutt J.K, Cox N.A, Harrison M.A, Fedorka-Cray P.J, Ladely S.R. Antimicrobial resistance in Salmonella and Escherichia coli isolated from commercial shell eggs. Poult. Sci. 2006;85(9):1665–1669. doi: 10.1093/ps/85.9.1665. [DOI] [PubMed] [Google Scholar]
- 175.Fall-Niang N.K, Sambe-Ba B, Seck A, Deme S.N, Wane A.A, Bercion R, Alambedji-Bada R, Gassama-Sow A. Antimicrobial Resistance Profile of Salmonella Isolates in Chicken Carcasses in Dakar, Senegal. Foodborne Pathog. Dis. 2019;16(2):130–136. doi: 10.1089/fpd.2018.2459. [DOI] [PubMed] [Google Scholar]
- 176.Ziyate N, Karraouan B, Kadiri A, Darkaoui S, Soulaymani A, Bouchrif B. Prevalence and antimicrobial resistance of Salmonella isolates in Moroccan laying hens farms. J. Appl. Poult. Res. 2016;25(4):539–546. [Google Scholar]
- 177.Odoch T, Sekse C, L'abee-Lund T.M, Hansen C.H, Kankya C, Wasteson Y. Diversity and antimicrobial resistance genotypes in non-typhoidal Salmonella isolates from poultry farms in Uganda. Int. J. Environ. Res. Public Health. 2018;15(2):324. doi: 10.3390/ijerph15020324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kemal J, Sibhat B, Menkir S, Beyene D. Prevalence, assessment, and antimicrobial resistance patterns of Salmonella from raw chicken eggs in Haramaya, Ethiopia. J. Infect. Dev. Ctries. 2016;10(11):1230–1235. doi: 10.3855/jidc.7885. [DOI] [PubMed] [Google Scholar]
- 179.Tessema K, Bedu H, Ejo M, Hiko A. Prevalence and Antibiotic Resistance of Salmonella Species Isolated from Chicken Eggs by Standard Bacteriological Method. J. Vet. Sci. Technol. 2017;08(1):1–5. [Google Scholar]
- 180.Tadesse G, Mitiku H, Teklemariam Z, Marami D. Salmonella and Shigella Among Asymptomatic Street Food Vendors in the Dire Dawa city, Eastern Ethiopia:Prevalence, Antimicrobial Susceptibility Pattern, and Associated Factors. Environ. Health Insights. 2019;13(1):1–8. doi: 10.1177/1178630219853581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Gast R.K, Guraya R, Jones D.R, Anderson K.E. Persistence of fecal shedding of Salmonella Enteritidis by experimentally infected laying hens housed in conventional or enriched cages. Poult. Sci. 2015;94(7):1650–1656. doi: 10.3382/ps/pev113. [DOI] [PubMed] [Google Scholar]
- 182.De-Buck J, Van-Immerseel F, Haesebrouck F, Ducatelle R. Protection of laying hens against Salmonella Enteritidis by immunization with type 1 fimbriae. Vet. Microbiol. 2005;105(2):93–101. doi: 10.1016/j.vetmic.2004.10.008. [DOI] [PubMed] [Google Scholar]


