Abstract
Background
Australia, although a high income economy, carries a significant burden of rheumatic heart disease (RHD). Acute rheumatic fever (ARF) and RHD are endemic in the Indigenous population. Immigrants from low/lower-income countries (‘non-Indigenous high-risk’) are also at increased risk compared with ‘non-Indigenous low-risk’ Australians. This study describes the utilisation of surgical and percutaneous procedures for RHD-related valve disease among patients aged less than 50 years, from 2002 to 2017.
Methods
A descriptive study using data from the ‘End RHD in Australia: Study of Epidemiology (ERASE) Project’ linking RHD Registers and hospital inpatient data from five states/territories, and two surgical databases. Trends across three-year periods were determined and post-procedural all-cause 30-day mortality calculated.
Results
A total of 3900 valves interventions were undertaken in 3028 procedural episodes among 2487 patients. Over 50% of patients were in the 35–49 years group, and 64% were female. Over 60% of procedures for 3-24 year-olds were for Indigenous patients. There were few significant changes across the study period other than downward trends in the number and proportion of procedures for young Indigenous patients (3–24 years) and ‘non-Indigenous/low risk’ patients aged ≥35 years. Mitral valve procedures predominated, and multi-valve interventions increased, including on the tricuspid valve. The majority of replacement prostheses were mechanical, although bioprosthetic valve use increased overall, being highest among females <35 years and Indigenous Australians. All-cause mortality (n = 42) at 30-days was 1.4% overall (range 1.1–1.7), but 2.0% for Indigenous patients.
Conclusions
The frequency of cardiac valve procedures, and 30-day mortality remained steady across 15 years. Some changes in the distribution of procedures in population groups were evident. Replacement procedures, the use of bioprosthetic valves, and multiple-valve interventions increased. The challenge for Australian public health officials is to reduce the incidence, and improve the early detection and management of ARF/RHD in high-risk populations within Australia.
Keywords: Rheumatic heart disease, RHD, Valve surgery, Bioprosthesis, Indigenous health
Abbreviations: ANZSCTS, Australian & New Zealand Society of Cardiac & Thoracic Surgeons national cardiac surgery database; CABG, coronary artery bypass surgery; SA, South Australia; NT, Northern Territory
Highlights
-
•
Epidemic RHD in Indigenous Australians drives RHD-related cardiac valve procedures.
-
•
30-day mortality post-procedural is low in those under 50 years.
-
•
Bioprosthetic valve replacements higher in young women, and increasing in older patients.
1. Introduction
Australia is one of few high-income countries with a significant burden of rheumatic heart disease (RHD) among its population. While very uncommon in the non-Indigenous population, acute rheumatic fever (ARF) and RHD occur at endemic rates among Aboriginal and Torres Strait Islander peoples (Indigenous Australians) [1], who comprised 3.3% of the 24 million total population in mid-2016 [2]. It is also a health issue for immigrants to Australia from populations at higher risk, including Māori and Pacific Islanders [3] and immigrants from low- and lower-middle-income countries (ILICs) [4].
The estimated annual incidence of ARF in Australia rose from 3/100,000 in 2013 to 6/100,000 in 2017 overall, females being 56%, and highest in those aged 5–14 years at diagnosis. For Indigenous Australians the annual incidence of ARF was 85/100,000 while that for new diagnoses of RHD was 50/100,000. Of prevalent cases of RHD, 87% were among Indigenous people [1].
The estimates of incidence and prevalence are derived from information recorded on ARF/RHD registers in five of the eight Australian States and Territories. The registers were established between 1997 and 2015 in the Northern Territory (NT), Queensland (Qld), Western Australia (WA), South Australia (SA), and New South Wales (NSW) and include those with the largest Aboriginal populations (NSW and Qld), and that with the highest proportion of Aboriginal people (NT).
There are, however, recognised inconsistencies in the collecting, recording and reporting of information for the registers, including that on procedures [5]. To overcome this limitation and facilitate epidemiological study of ARF and RHD in Australia the ‘End RHD in Australia: Study of Epidemiology (ERASE)’ Project was developed.
There is little information on the use of surgery and catheter-based procedures in well-documented populations, with the majority of reports coming from single centres. ERASE is the first study to publish population-based data using linked information from multiple sources [6].
After evaluation and validation, an ERASE ‘analysis cohort’ was developed using data from the registers, hospital administrative data from each of the five jurisdictions, and two cardiothoracic surgical databases. The additional sources of ARF/RHD cases more than doubled the numbers aged <60 years (n = 12, 907) compared to registry data alone (5, 049). The majority (91%) were recorded as being Aboriginal or Torres Strait Islander people, 5% were Māori or other Pacific Islander people or other ILICs, and 4% were non-Indigenous Australians [6].
Australia has a well-resourced universal healthcare system with no structural limitations to the frequency or type of procedures available to those with RHD-related valve disease. An examination of the use of procedures in this population-based cohort provides insights into the trends in care against which future Australian cohorts, and other jurisdictions, can measure the services that they provide. The aim of this study was to describe the use of surgical and percutaneous valvular procedures to manage RHD in an Australian population from 2002 to 2017.
2. Methods
This is a descriptive, cross-sectional study to identify trends over time in the use of valve-related interventions for RHD. It was confined to those aged <50 years to limit the risk for inclusion of cases whose diagnosis of RHD-related valve disease, based on hospital morbidity data alone, was less certain [34]. Procedures recorded among patients diagnosed with RHD before, or at the time of intervention, in five jurisdictions in Australia (NSW, Qld, SA, WA and the NT) between July 1, 2002 and June 30, 2017 were included. These five jurisdictions carry the greatest burden of ARF/RHD, and are home to 86% of Aboriginal and Torres Strait Islander peoples (at June 30, 2016) [2].
2.1. Data sources
2.1.1. The ERASE database
Details of the ERASE database have been published elsewhere [6]. In summary, this database comprises a cohort drawn from ARF/RHD registers, in-patient RHD-coded hospital admission/discharge data, and RHD-coded death records. Records from these sources, and the surgical/procedural data, were probabilistically linked, providing a de-identified data set including encrypted unique person identifiers (Appendix Table A).
The ERASE database was used to identify patient characteristics, including date of birth, sex, date of first recorded diagnosis of RHD, population status (defined below), and, where appropriate, date of death.
Identification as a RHD case was based on (1) a record of a confirmed RHD diagnosis in the ARF/RHD register AND/OR (2) RHD recorded on a surgical database AND/OR (3) a diagnosis of RHD in hospital inpatient data by a statistical prediction model developed to address shortcomings in ICD codes for RHD [34] that risk high false positive rates [8]. Cases with congenital heart disease as the principal diagnosis were excluded.
2.1.2. Valvular intervention dataset
Data on valvular procedures for the RHD cohort were obtained from, and harmonised across, four sources:
-
1.
The Australian and New Zealand Society of Cardio-thoracic Surgeons (ANZCTS) National Cardiac Surgery Database provides detailed surgical information but does not include percutaneous procedures, nor do all hospitals performing cardiac surgery contribute to the database [9].
-
2.
The paediatric surgery dataset from the Royal Children's Hospital in Melbourne, Victoria. This provided surgical information for cases transferred from SA and NT (no paediatric cardiothoracic surgery service available).
-
3.
Inpatient hospital data; the most comprehensive source of data regarding surgical and non-surgical procedures, with common variables and standardised diagnostic and procedure codes using the International Classification of Diseases and related Health Problems, Tenth Revision, Australian Modification (ICD-10-AM) and the Australian Classification of Health Interventions (ICD-10 ACHI). The ICD-10 codes used to identify valvular procedures are listed Appendix Table B.
-
4.
RHD registers provided basic data about the procedures.
2.2. Study variables
To allow for variations in the recorded procedure date across the integrated databases, any procedure performed within the same 14-day period for a patient was considered part of a single ‘procedural episode’.
The following information was collected: demographic data, type of surgical or percutaneous procedure, valve(s) treated, number of procedures per patient, type of prosthetic valve implanted (bioprosthesis, mechanical, homograft), concomitant coronary artery bypass graft, and death within 30 days from a procedure.
The cohort was grouped according to population/risk as developed for ERASE; ‘Indigenous Australian’ (Aboriginal and Torres Strait Islander peoples), ‘non-Indigenous/high-risk’ (ILICs, immigrants from New Zealand), and ‘non-Indigenous/low-risk’ (non-Indigenous Australians, being the majority, and immigrants from high-income countries) [6]. Population category was assigned from the RHD registers, if available, or from country of birth recorded in the hospital data.
Procedures were classified as either surgical valve repair or valve replacement, or percutaneous balloon valvuloplasty (PBV). Only one trans-catheter aortic valve implantation (TAVI) was coded in this young population. Procedures were classified as ‘other’ if assigned specific ICD-10 procedure codes (i.e. 38456-05 ‘Other intrathoracic procedure on mitral valve with cardiopulmonary bypass’) and there was a small number with an ‘unknown’ procedure where the registry entry lacked sufficient detail and was not matched with an admission (probably cross-jurisdictional). Newer catheter-based and trans-apical procedures, if used, could not be identified, as specific ICD-10 codes were not established at the time. The number and types of procedures for each ‘procedural episode’, the type of prosthetic valve(s) and concomitant coronary artery bypass graft (CABG) surgery were identified for each episode. If multiple valves were repaired and/or replaced during an episode, we collected information for each procedure.
2.3. Data analysis
The mid-2002 to mid-2017 study period was divided into five three-year periods from mid-year. Age group at procedure was 3–14 years, 15–24 years, 25–34 years or 35–49 years. With low numbers, children, adolescents and young adults were compressed into one group (3–24 years) for most analyses. As patients may have undergone multiple procedures within a period, or in more than one period, patient characteristics were recorded at the first procedure-related admission in the period.
Trends in the frequency of procedures on the mitral valve (MV), aortic valve (AV) and tricuspid valve (TV) (when numbers were sufficient), by age group, sex and population category were examined.
All-cause mortality within 30 days of a procedure was calculated from the last recorded procedure, to exclude double counting. Descriptive results are presented as means with 95% confidence interval (CI), median plus interquartile range (IQR), or proportions (%). Quantitative data were compared using ANOVA or the Kruskal-Wallis test, and categorical variables were compared using the chi-square test. Temporal trends of proportions within groups were examined using the Cochran-Armitage trend test. All data manipulations and statistical analyses were conducted using SAS version 9.4 (SAS institute, Cary, North Carolina, U.S.). A statistical significance level was set at 0.05.
2.4. Ethics
The ERASE Project has been approved by Human Research Ethics Committees of the Health Departments in the relevant states and territories, the Aboriginal Ethics Committees in WA, SA, NT and NSW and, and from supporting institutions [6]. The ERASE study is registered with the Australian New Zealand Clinical Trials Registry and has been reported in line with the STROCSS criteria [10]. The ERASE data are not publicly available due to privacy and ethical restrictions.
3. Results
There were 3028 procedural episodes among 2487 patients during the study period, representing 33.6% of all RHD cases aged <50 years identified for the ERASE analysis cohort in the study period (n = 7, 403).
The majority of patients had a prior diagnosis of RHD (or ARF), but for 566 patients (23%), RHD was first recorded at the index procedure.
The median age was 37.2 years (IQR 26.7–44.8), with >50% of cases aged 35–49 years. Females were 64% overall, with a median age of 37.3 years (IQR 27.4–44.8); males (36.9 years, IQR 24.0–44.8).
Indigenous Australians were 38% of all cases and were younger, with a median age of 31.3 years (IQR 20.4–40.6), than non-Indigenous/low risk (38.5, 28.6–45.2 years) and non-Indigenous/high-risk Australians (40.8, 32.4–46.5 years).
Other than a significant decrease in the number and proportion of non-Indigenous/low risk patients aged 35–49 years, there was little change in the characteristics of patients across the study period (Table 1).
Table 1.
Characteristics of patients undergoing a RHD-related cardiac valve procedure by 3-year period, 2002–2017.
| 2002–2005 | 2005–2008 | 2008–2011 | 2011–2014 | 2014–2017 | Total 2002-17 | ‘p’ | ||
|---|---|---|---|---|---|---|---|---|
| Procedural episodes N | 596 | 601 | 640 | 600 | 591 | 3028 | 0.89 | |
| Patients N | 556 | 557 | 588 | 551 | 552 | 2487 | 0.82 | |
| Mean episodes/patient | 1.07 | 1.08 | 1.09 | 1.09 | 1.07 | 1.22 | ||
| Single episode in period | 519 (93.3) | 514 (92.3) | 540 (91.8) | 506 (91.8) | 515 (93.3) | 2, 042 | 0.88 | |
| ≥2 episodes in period | 37 (6.7) | 43 (7.7) | 48 (8.2) | 45 (8.2) | 37 (6.7) | 445 | ||
| Median age (IQR) | 37.6 (25.4–45.4) | 37.1 (26.7–44.3) | 37.3 (26.5–44.6) | 35.1 (25.6–43.8) | 36.7 (26.6–44.4) | 37.2 (26.7–44.8) | 0.46 | |
| Female* n (%) | 363 (65.4) | 349 (62.7) | 373 (63.7) | 357 (65.0) | 353 (65.1) | 1581 (64.0) | 0.74 | |
| Age group at first episode in study period n (%) | ||||||||
| 3–14 years | 32 (5.8) | 40 (7.2) | 36 (6.1) | 49 (8.9) | 42 (7.6) | 183 (7.4) | 0.12 | |
| 15–24 | 102 (18.3) | 85 (15.3) | 101 (17.2) | 86 (15.6) | 79 (14.3) | 374 (15.0) | 0.12 | |
| 25–34 | 119 (21.4) | 111 (19.9) | 107 (18.2) | 134 (24.3) | 133 (24.1) | 525 (21.1) | 0.08 | |
| 35–49 | 303 (54.5) | 321 (57.6) | 344 (58.5) | 282 (51.2) | 298 (54.0) | 1405 (56.5) | 0.27 | |
| Population group **n (%) | ||||||||
| Indigenous | 215 (38.7) | 209 (37.5) | 232 (39.5) | 210 (38.1) | 240 (43.5) | 934 (37.9) | 0.10 | |
| Non-Indigenous/high-risk | 122 (21.9) | 128 (23.0) | 119 (20.2) | 141 (25.6) | 129 (23.4) | 598 (24.3) | 0.30 | |
| Non-Indigenous/low-risk | 211 (37.9) | 220 (39.5) | 235 (40.0) | 198 (35.9) | 172 (31.2) | 932 (37.8) | 0.01 | |
NB. Patients may be counted more than once in a period, and in more than one period *Information on gender missing for 15 patients **Information on population group missing for 23 patients.
In the young age group (3–24 years) 63% of procedures were among the Indigenous population. The decrease in the number and proportion of procedures for young non-Indigenous high-risk patients, and increase for non-Indigenous/high-risk patients, were not statistically significant (Table 2).
Table 2.
Number of cardiac valve procedures in each 3-year period by population group and age group, 2002–2017.
| 2002–2005 | 2005–2008 | 2008–2011 | 2011–2014 | 2014–2017 | Total 2002-17 | |
|---|---|---|---|---|---|---|
| Age 3–24 years - N | 153 | 145 | 155 | 151 | 128 | 732 |
| Indigenous n (%) | 102 (66.7) | 95 (65.5) | 98 (63.0) | 88 (58.3) | 78 (60.9) | 461 (63.0) |
| Non-indigenous – high-risk | 21 (13.7) | 26 (17.4) | 26 (16.9) | 36 (23.8) | 29 (22.7) | 138 (18.9) |
| Non-indigenous – low-risk | 30 (19.6) | 24 (16.6) | 31 (20.0) | 27 (17.9) | 21 (16.4) | 133 (18.2) |
| Age 25–34 years - N | 125 | 117 | 120 | 141 | 135 | 638 |
| Indigenous n (%) | 63 (50.4) | 42 (35.9) | 60 (50.0) | 63 (44.7) | 65 (48.1) | 293 (45.9) |
| Non-indigenous - high-risk | 22 (17.6) | 30 (25.6) | 22 (18.3) | 33 (23.4) | 38 (28.1) | 145 (22.7) |
| Non-indigenous - low-risk | 40 (32.0) | 45 (38.5) | 38 (31.7) | 45 (31.9) | 32 (23.7) | 200 (31.3) |
| Age 35–49 years - N | 309 | 339 | 363 | 306 | 317 | 1, 634 |
| Indigenous n (%) | 77 (24.9) | 96 (28.3) | 103 (28.4) | 84 (27.5) | 114 (36.0) | 474 (29.0) |
| Non-indigenous - high-risk | 82 (26.5) | 83 (24.5) | 81 (22.3) | 82 (26.8) | 76 (24.0) | 404 (24.7) |
| Non-indigenous - low-risk | 150 (48.5) | 160 (47.2) | 179 (49.3) | 140 (45.8) | 127 (40.1) | 756 (46.3)* |
Notes: N of procedures = 3004 (‘population group’ information missing for 24 procedures). Cases may have multiple procedures within a period or within the study period. * trend p < 0.05.
Among adults (25–34years) 46% of procedures were in the Indigenous group. Trends to decreasing incidence in the non-Indigenous low-risk group and to higher incidence in the non-Indigenous high-risk group, did not reach statistical significance.
In the older adult group (35–49 years) less than 30% of procedures were among Indigenous patients, with over 45% in the non-Indigenous low-risk group. This pattern may have been changing; with a trend to decreasing numbers of procedures among non-Indigenous low-risk patients, while the last time period showing a 37% increase in procedures for Indigenous patients.
The mean number of procedures per person across the study period was 1.22 overall (Table 1). It was highest among the younger patients, being 1.45 for those aged 3–14 years and 1.40 for those 15–24 years, falling to 1.13 for those aged 35–49 years. Among those <15 years 23% had more than one procedure as a child, and 38% had more than procedure in the study period. For patients aged 35–49 years 15% had >1 procedure in the study period, but may have undergone valve interventions prior to the study period.
3.1. Valve procedures
Of the 3, 900 valves treated, MV procedures were the most frequent (65.6%). Isolated MV surgery decreased proportionally over the study period as procedures on multiple valves increased significantly; MV + AV being most frequent (Table 3). AV procedures, 23.2% overall, increased significantly, surgical replacement being the most frequent procedure, ≈90% throughout. TV procedures, mostly repairs, rose from 6% to 14.5% of total procedures, and were performed in 52% of the multiple valve procedures performed in 2014–2017. The small numbers of isolated procedures and replacements over the 15-year period indicate the low incidence of organic TV disease.
Table 3.
Surgical and percutaneous procedures undertaken for RHD-related cardiac valve disease by 3-year period. Valve not specified in 4 cases *Including isolated and multiple valve procedures.
| 2002–2005 | 2005–2008 | 2008–2011 | 2011–2014 | 2014–2017 | Total 2002-17 | ‘p’ trend | |
|---|---|---|---|---|---|---|---|
| Total procedural episodes | 596 | 601 | 640 | 600 | 591 | 3, 028 | |
| Total valves treated* | 720 | 747 | 817 | 794 | 822 | 3, 900 | |
| Valve treated n (% of all valves treated) | |||||||
| Mitral | 500 (69.4) | 501 (67.1) | 549 (67.2) | 505 (63.6) | 502 (61.1) | 2557 (65.6) | 0.54 |
| Aortic | 171 (23.8) | 169 (22.6) | 178 (21.8) | 190 (23.9) | 198 (24.1) | 906 (23.2) | 0.03 |
| Tricuspid | 46 (6.4) | 75 (10.0) | 88 (10.8) | 98 (12.3) | 119 (14.5) | 426 (10.9) | <0.001 |
| Pulmonary | <5 (0.4) | <5 (0.3) | <5 (0.2) | <5 (0.1) | <5 (0.4) | 11 (0.3) | – |
| Type of procedure n (%) | |||||||
| MV - Surgical replacement | 278 (55.6) | 267 (53.3) | 332 (60.5) | 305 (60.4) | 330 (65.7) | 1512 (59.1) | <0.001 |
| Surgical repair | 109 (21.8) | 137 (27.3) | 109 (21.7) | 109 (21.6) | 96 (19.1) | 560 (21.9) | 0.04 |
| PBMV | 111 (22.2) | 94 (18.8) | 97 (19.3) | 79 (15.6) | 68 (13.5) | 449 (17.6) | <0.001 |
| Other** | <5 (0.4) | <5 (0.2) | 8 (1.6) | 12 (2.4) | 8 (1.6) | 31 (1.1) | – |
| Unknown | 0 | <5 (0.4) | <5 (0.6) | 0 | 0 | 5 (0.2) | – |
| AV - Surgical replacement | 152 (88.9) | 151 (89.3) | 159 (89.3) | 175 (92.1) | 175 (88.4) | 812 (89.6) | 0.83 |
| Surgical repair | 13 (7.6) | 15 (8.9) | 15 (8.4) | 12 (6.3) | 15 (7.6) | 70 (7.7) | 0.68 |
| Percutaneous and ‘other’** | 6 (3.5) | <5 (1.8) | <5 (2.2) | <5 (1.6) | 8 (4.0) | 24 (2.7) | – |
| TV - Surgical replacement | <5 (8.7) | 15 (20.0) | 8 (9.1) | 18 (18.4) | 18 (15.1) | 63 (14.8) | – |
| Surgical repair | 41 (89.1) | 58 (77.3) | 79 (89.8) | 78 (79.6) | 98 (82.4) | 354 (83.1) | 0.54 |
| Other** | <5 (2.2) | <5 (2.7) | <5 (1.1) | <5 (2.0) | <5 (2.5) | 9 (2.1) | – |
| Combinations of valves n (%) | |||||||
| MV isolated | 393 (65.9) | 379 (63.2) | 396 (62.1) | 339 (56.5) | 313 (56.5) | 1820 (60.2) | <0.001 |
| AV isolated | 83 (13.9) | 76 (12.7) | 68 (10.7) | 74 (12.3) | 66 (11.2) | 367 (12.1) | 0.17 |
| AV + MV | 71 (11.9) | 69 (11.5) | 84 (13.2) | 88 (14.7) | 90 (15.3) | 402 (13.3) | 0.03 |
| MV + TV | 22 (3.7) | 35 (5.8) | 46 (7.2) | 51 (8.5) | 63 (10.7) | 217 (7.2) | <0.001 |
| AV + MV + TV | 14 (2.3) | 18 (3.0) | 23 (3.6) | 26 (4.3) | 35 (5.9) | 116 (3.8) | <0.01 |
| TV isolated | 8 (1.3) | 15 (2.5) | 16 (2.5) | 19 (3.2) | 15 (2.5) | 73 (2.4) | 0.12 |
| Other** | 5 (0.8) | 8 (1.3) | 5 (0.8) | <5 (0.5) | 8 (1.4) | 29 (2.4) | 0.88 |
| Concomitant CABG | 26 (4.4) | 20 (3.3) | 18 (2.8) | 27 (4.5) | 29 (4.9) | 120 (4.0) | 0.37 |
**ICD-10 coded as ‘Other’ e.g. ICD-10 procedure code 38,456–16 ‘Other intrathoracic procedure on mitral valve without cardiopulmonary bypass’ See Appendix Table B.
MV procedures also predominated in each age group, with MV replacement increasing with age as repair procedures fell (Table 4). PBV of the MV (PBMV) use declined across the study period in each age group. Surgical AV repair (with or without additional procedures) was most frequent in younger patients, being 47% among those aged 8–14 years and 27% for 15–24 years of the total of 70 procedures. TV repairs increased in all age groups.
Table 4.
Surgical valve replacement and repair and percutaneous procedures by 3-year period and age group.
| Procedure | 2002–2005 | 2005–2008 | 2008–2011 | 2011–2014 | 2014–2017 | Total | ‘p’ trend | |||
|---|---|---|---|---|---|---|---|---|---|---|
| 3–24 years n (%) | ||||||||||
| Total valves | 190 | 184 | 201 | 204 | 183 | 962 | ||||
| MV replacement | 59 (31.0) | 50 (27.2) | 75 (37.3) | 58 (28.4) | 50 (27.3) | 292 (30.4) | 0.66 | |||
| MV repair | 58 (30.5) | 61 (33.2) | 43 (21.4) | 58 (28.4) | 49 (26.8) | 269 (28.0) | 0.82 | |||
| MV ‘PBMV’ | 14 (7.4) | 14 (7.6) | 14 (7.0) | 10 (4.9) | 7 (3.8) | 59 (6.1) | 0.16 | |||
| AV replacement | 33 (17.4) | 33 (17.9) | 34 (16.9) | 50 (24.5) | 48 (26.2) | 198 (20.6) | <0.01 | |||
| AV repair | 13 (6.8) | 11 (6.0) | 12 (6.0) | 8 (3.9) | 8 (4.4) | 52 (5.4) | 0.06 | |||
| TV repair | 11 (5.8) | 13 (7.1) | 19 (9.4) | 18 (8.8) | 18 (9.8) | 79 (8.2) | 0.04 | |||
| TV replacement | <5 (1.0) | <5 (1.1) | <5 (2.0) | <5 (0.1) | <5 (1.6) | 13 (1.4) | – | |||
| 25–34 years n (%) | ||||||||||
| Total valves | 145 | 139 | 142 | 175 | 185 | 789* | ||||
| MV replacement | 53 (36.6) | 40 (28.8) | 59 (41.5) | 65 (37.1) | 81 (43.8) | 298 (37.8) | <0.01 | |||
| MV repair | 17 (11.7) | 29 (20.9) | 15 (10.6) | 22 (12.6) | 11 (5.9) | 94 (11.9) | 0.03 | |||
| MV ‘PBMV’ | 35 (24.1) | 25 (18.0) | 29 (20.4) | 29 (16.6) | 25 (13.5) | 143 (18.1) | 0.06 | |||
| AV replacement | 30 (20.7) | 26 (18.7) | 28 (19.7) | 35 (20.0) | 43 (23.2) | 162 (20.5) | 0.19 | |||
| TV repair | 8 (5.5) | 14 (10.1) | 9 (6.3) | 20 (11.4) | 22 (11.9) | 73 (9.2) | 0.02 | |||
| TV replacement | <5 (1.4) | 5 (3.6) | <5 (1.4) | <5 (2.3) | <5 (1.6) | 16 (2.0) | – | |||
| 35–49 years n (%) | ||||||||||
| Total valves | 373 | 410 | 453 | 393 | 425 | 2, 069** | ||||
| MV replacement | 166 (44.5) | 177 (43.2) | 198 (43.7) | 182 (46.3) | 199 (46.8) | 922 (44.6) | <0.01 | |||
| MV repair | 34 (9.1) | 47 (11.5) | 51 (11.3) | 29 (7.4) | 36 (8.5) | 197 (9.5) | 0.54 | |||
| MV ‘PBMV’ | 62 (16.6) | 55 (13.4) | 54 (11.9) | 40 (10.2) | 36 (8.5) | 247 (11.9) | <0.01 | |||
| AV replacement | 89 (23.9) | 92 (22.4) | 97 (21.4) | 90 (22.9) | 84 (19.8) | 452 (21.8) | 0.81 | |||
| TV repair | 22 (6.0) | 31 (7.6) | 51 (11.3) | 40 (10.2) | 58 (13.6) | 202 (9.8) | <0.001 | |||
| TV replacement | – | 8 (2.0) | <5 (<1.0) | 12 (3.0) | 12 (2.8) | 34 (1.7) | – | |||
Note: Excluding those with ‘unknown’ procedures, pulmonary valves (n = 11) *Total includes AV repair n = 3 **AV repair n = 15 (<1.0%).
Among 2, 313 AV and MV replacements with a known prosthesis type, mechanical prostheses (n = 1623, 70.1%) were more frequent than bioprostheses (n = 690, 29.8%).
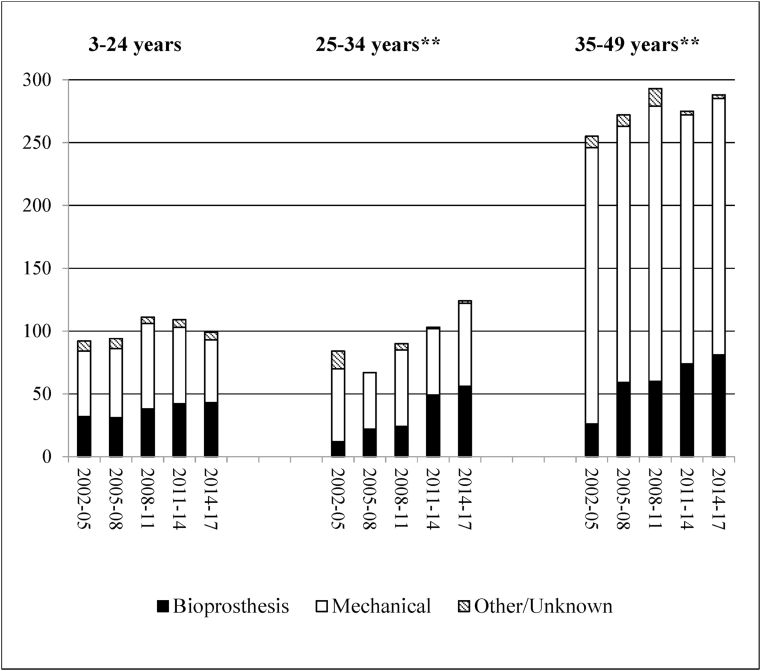
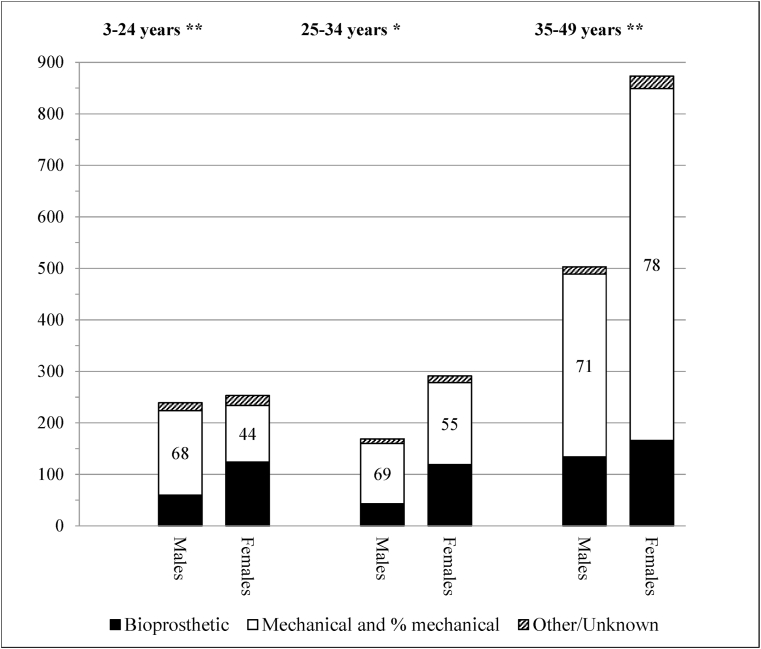
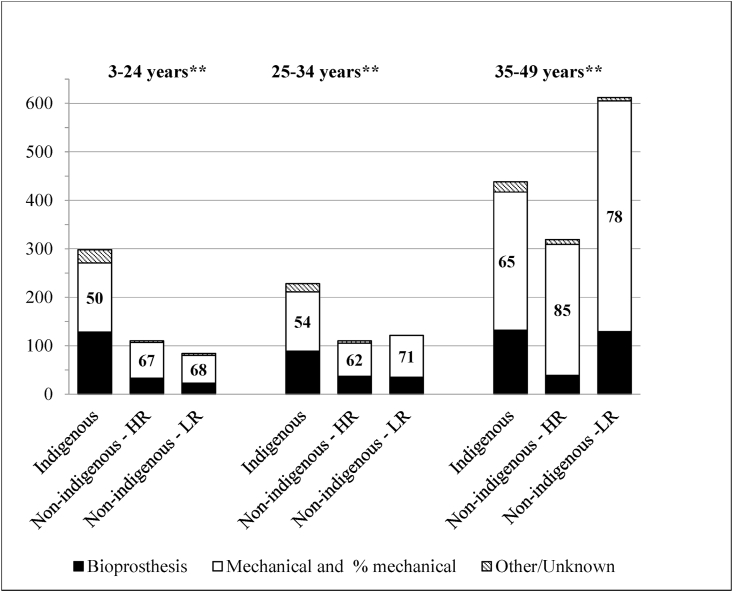
Mechanical replacements declined across the study period, while bioprosthetic valves became more frequent, notably in the older age-groups (Fig. 1). Bioprosthetic valves were implanted more frequently in girls and women aged less than 35 years, being over 40%, but <20% in older women and <30% for males overall (Fig. 2). Bioprosthetic valve replacements were higher among the Indigenous population in each age group, especially in the 3–24 years age group (Fig. 3). For MV replacement bioprostheses were 47% for Indigenous children and young adults versus <30% for both other risk groups. For AV replacement, however, the proportion of bioprosthetic replacements exceeded 40% for both Indigenous and non-Indigenous low-risk young Australians.
Fig. 1.
Type of prosthesis used in the replacement of 2, 347 cardiac valves (mitral and aortic) by 3-year period and age group.
Fig. 2.
Type of prosthesis used in the replacement of 2, 328 cardiac valves (mitral and aortic) by sex and age group.
Fig. 3.
Type of prosthesis used in the replacement of 2, 320 cardiac valves (mitral and aortic) by population and age group.
3.1.1. 30-Day mortality
There were 42 deaths within 30 days of the 3028 procedures (1.7%) with no significant difference across the study period (range 1.4–2.1, χ2 0.85, p = 0.93). Mortality was higher among Indigenous patients (2.0%, n = 25) than in the non-Indigenous/high-risk (0.7%, n = 5) and non-Indigenous low-risk groups (1.1%, 5 patients) (χ2 6.6, p = 0.04), but did not differ significantly by age group. Mortality among those undergoing a single procedure in the study period (n = 21) was 1.1%, 2.2% for those undergoing a second procedure (n = 16) and 2.1% for those having ≥3 procedures (n = 5) (χ2 5.9, p = 0.52).
4. Discussion
While Australia is a high-income country with a universal health care system, away from the major cities and larger regional centres access is poorer, and there is less infrastructure for primary care, and reduced access to specialist services [11]. The cases on the RHD registers are from the largest states, with widely dispersed populations but centralised tertiary health services, and home to the majority of Aboriginal and Torres Strait Islander peoples. It is in this setting that surgical services are delivered. The capacity to provide more accurate estimates of the incidence and prevalence of RHD in Australia using linked data has only recently been realised. With the burden greater than previously estimated, and falling disproportionately among Indigenous Australians, calls have been made for urgent government action to address the evident health inequaity.
The procedures undertaken between 2002 and 2017 are related to interactions between population group, age, and time period. While Indigenous people (3.3% of the Australian population) were 56% of the 12,097 ARF/RHD cases aged <60 years identified for the ERASE project [6], they represented 38% of surgical cases aged <50 years. This relatively small proportion is related to the unequal distribution of cases within age groups. The proportion from the Indigenous population was highest in those aged <15 years (63%) the 183 cases in that age group making up 7.4% of total cases. Conversely the proportion of Indigenous cases was lowest (29%) in those aged 35–49 years (n = 1405.56.5% of total cases).
The greater need for surgery among young Indigenous people is underpinned by the persistently high incidence of ARF/RHD in this population group, contrasting with the near-eradication of RHD among non-Indigenous children since the mid-20th century [12]. The relatively poor management of ARF/RHD among Indigenous children, while showing some improvement over previous decades [13], probably drives the steady need for procedures in surviving older people. The reduction in procedures in the later periods among older non-Indigenous low-risk patients may represent the tail end of the waning ARF epidemic among non-Indigenous Australians [12].
How population growth and the management of ARF/RHD have contributed to the steady numbers of procedures across the study period is difficult to unravel. There has been relative growth in the Indigenous population, with the growth rate more than doubling compared with that for Australia overall since the mid-20th century [14,15]. This relative growth, however, is not easy to quantify as the Australian Bureau of Statistics reports that approximately 30% of the changes in population numbers from one census to the next cannot be explained [16]. The changing definition of ‘Aboriginality’ over time is but one factor that may affect the decision to identify as Aboriginal [15,16]. Furthermore, the growth in the Indigenous population is mostly in the larger cities [16] while the majority of Indigenous cases are from regional and remote areas of northern Australia [2].
Better diagnosis and follow-up of RHD with the introduction of RHD Registers and the associated increases in hospital admissions is a likely driver of the ongoing need for surgical and catheter-based procedures, while better treatment and management of ARF may contribute to reduced need over time. Overall, possible trends, such as to fewer procedures among the young Indigenous population aged <25 years, and an increase for non-Indigenous high-risk Australians, cannot be confirmed as the low numbers provide insufficient statistical power.
The divergent trends in the number of procedures across the population groups resulted in little change over time to the median age at intervention, and to sex distribution.
The low numbers of procedures among some population and age groups may have also obscured changes in the surgical management of RHD. Significant procedural trends included a reduction in the proportion of MV repair in favour of replacement. The relationship between age and type of procedure was evident, with MV repair most frequent in those aged <25 years, less so among 25-34 years-old and least in those aged 35–49 years. Initial surgical repair of the MV is associated with better long-term outcomes for children and young adults [17,18] and is recommended where technically feasible [19]. This approach was evident in this study. Increasing use of MV replacement and reduction in PBMV may be related to changing preferences in the timing and type of intervention [20] and to the demographics of the cohort, the majority of patients being aged 25–49 years at the first recorded procedure. Isolated MV surgery also fell as interventions on multiple valves increased.
Mechanical MV replacement was the procedure of choice for adults <50 years, with, as expected, bioprosthetic valve predominant for girls and women of child-bearing age [21].
While mechanical prostheses were more frequent than bioprostheses overall, accounting for 70% of MV and 66% of AV replacements, the relatively greater proportion of bioprosthetic replacements in the Indigenous population (32–49%) probably reflects concerns regarding patients being lost to follow-up, and inadequate management of anti-coagulation in regional and remote areas [22], as well as the risks for young women who become pregnant while on anticoagulants [23]. This greater use of bioprosthetic valves was also evident in the ANZSCTS data for a slightly earlier period [24].
The increasing use of bioprostheses (MV 18%–34% and AV 16%–39%), mainly among patients older than 25 years, reflects a trend worldwide for all valve replacements, as confidence grows in the longevity of bioprostheses [25]. Bioprosthetic replacements also increased among older patients, some of whom may have undergone valve repair or PBV prior to the study period.
The preponderance of AV replacement reflects the difficulty of repair of this valve (8% of AV procedures), the majority among those <25 years of age. The increasing use of bioprosthetic valves was also evident for the AV. Percutaneous procedures were rare, but TAVI for RHD-related AV disease is being explored [26].
While only 14% of procedures involved the TV overall, the significant increase of TV repair in combination with other valve surgery reflects a shift in the management of valve diseases; with concomitant TV repair to maintain function associated with improved clinical outcomes and acceptable results for otherwise incurable RHD-related valve disease [27,28].
4.1.4 Mortality within 30 days of a procedure was low and did not significantly change, being around 1.4% in this young population, compared to 3.8% at 30-days among all-comers on the ANZSCTS database, whose mean age was 59.7 years [29]. While early mortality among Aboriginal and Torres Strait Islander people was higher than for non-Indigenous patients, the number of deaths was small; two previous studies did not find Indigenous status to be associated with early death post valve surgery for RHD [29,30].
The estimate of the proportion of patients with RHD undergoing a procedure (33.6%) was based on the accumulated cases on the ERASE database. As the majority of RHD cases in the ERASE cohort were garnered from hospital data, the overall risk for surgery for those with RHD is probably over-estimated. The ERASE patients likely represent those with moderate to severe disease requiring hospital admission. Among young Indigenous people from the NT with an initial diagnosis of severe RHD (1999–2012), the risk for surgery within two years of diagnosis of severe RHD was 50%, and that proportion persisted with for those progressing to severe disease [31]. The capacity to provide more accurate estimates of the incidence and prevalence of RHD in Australia using linked data has only recently been realised [32]. With the burden greater than previously estimated, and falling disproportionately among Indigenous Australians, urgent government action has been called for to address the evident health inequality and support a RHD elimination strategy [33]. Contemporary Incidence and Prevalence of Rheumatic Fever and Rheumatic Heart Disease in Australia Using Linked Data: The Case for Policy Change J Am
4.1. Strengths
The use of state population-based administrative data (hospital inpatient records) and ARF/RHD registries, combined with the available surgical databases, provides the largest and most complete study of the use of invasive procedures to manage RHD-related valve disorders in Australia. We were able to examine the use of percutaneous valve interventions in addition to surgical procedures, adding to the information available from the surgical databases.
4.2. Limitations
The hospital morbidity records for states, other than WA, have insufficient years of lookback to obtain the patients’ medical and surgical history. This limited the estimation of risk for mortality at 30-days, as contributing factors such as prior surgery could not be adjusted for.
As the State and Territory registers commenced recording cases at intervals across 18 years, using information from varying sources, they cannot provide a reliable denominator of RHD cases. The hospital administrative data and surgical databases are the more reliable source of surgical and percutaneous interventions, but only capture those admitted for treatment. Lacking reliable denominators no attempt was made to estimate the rates of valve procedures among Australians with RHD. Without data from Victoria, Tasmania and the Australian Capital Territory our findings are not a complete representation of procedures for RHD in Australia.
5. Conclusions
The frequency of heart valve procedures, and 30-day mortality post-procedure remained steady between 2002 and 2017. The use of procedures reflects current surgical practice in developed countries, procedural mortality was low. There are indications that the volume of RHD-related valve surgery is decreasing, rather than increasing, in the young Indigenous population, perhaps reflecting improvements in early detection and treatment.
The burden of RHD falls disproportionately on Indigenous Australians; only by diligent multidisciplinary efforts in prevention, early diagnosis, effective management and ongoing care can this imbalance be reduced. These efforts also should be extended to those who have come to Australia from high-risk countries.
While high quality surgical care for prevalent cases of RHD is available within a publicly-funded health service, the challenge for Australian public health officials is to reduce the incidence of ARF in the high-risk populations within Australia, and to effectively intervene to stop the progression to RHD.
Acknowledgements
The authors value the support/endorsement provided to the project by the following peak bodies representing the Aboriginal Community Controlled Health sector: Aboriginal Medical Services Alliance Northern Territory, Kimberley Aboriginal Medical Service (regional Western Australian peak body) and Aboriginal Health Council of South Australia, Aboriginal Health and Medical Research Council (NSW). We also received support from the Aboriginal divisions of QLD and WA Health Departments. We are committed to providing feedback to the said organisations and ensuring that the findings are accessible and provide the evidence needed for policy that can reduce the burden of ARF and RHD in Australia.
We acknowledge that figures and other statistics represent the loss of health and human life with profound impact and sadness for people, families, community and culture. We hope that the ‘numbers story’ emanating from this project can augment the ‘lived stories’ that reflect the voices of people with RHD and their families, thus jointly contributing to evidence to erase suffering from ARF and RHD in Australia.
The authors also wish to thank the staff of the data linkage units of the State and Territory governments (WA, SA-NT, NSW, QLD) for the linkage of the data. We thank the State and Territory Registries of Births, Deaths and Marriages, the State and Territory Coroners, and the National Coronial Information System for enabling Cause of Death Unit Record File data to be used for this project.
Further, we thank the data custodians and data managers for the provision of the following data:
• Inpatient hospital data (5 States and Territories)
• Emergency Department data (5 States and Territories)
• RHD registers (5 States and Territories)
• ANZ Society of Cardiac & Thoracic Surgeons data base (single data source from 5 States and Territories)
• Royal Melbourne Children's Hospital Paediatric Cardiac Surgery data base (single data source for RHD paediatric patients from SA and NT receiving surgery in Melbourne)
• Primary health care data from NT Department of Health.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amsu.2020.11.055.
Funding
This work was supported by the National Health and Medical Research Council of Australia [grant number 114625]; the Heart Foundation of Australia [grant number 102043]; and HeartKids [Grant-in-Aid 2017].
Conflict of interest
None.
**p < 0.01 Includes isolated and multiple valves replaced in one or more episodes within the study period, and within 3-year periods. Not shown: TV n = 63 (55% bioprosthetic, and pulmonary valves n = 8, 5 homograft).
*p < 0.05 **p < 0.01 Patient sex missing for 19 procedures.
**p < 0.01 Non-Indigenous HR = high risk, LR = low risk. Population status missing for 27 procedures.
Author contribution
Please specify the contribution of each author to the paper, e.g. study design, data collections, data analysis, writing. Others, who have contributed in other ways should be listed as contributors.
Pamela Bradshaw - lead author, data analysis.
Hideo Tohira – data manager and data analysis, writing.
James Marangou (Cardiologist) – clinical advisor, study design, editing and review.
Mark Newman (Cardiothoracic surgeon)- clinical advisor, study design, editing and review.
Bo Reményi – RHD specialist, data collections, review.
Vicki Wade – Indigenous cultural advisor, review and edit.
Christopher Reid – Study design, editing and review.
Judith M Katzenellenbogen - Principal Investigator, funding, study design, review.
Appendix. ASupplementary data
The following is the Supplementary data to this article:
References
- 1.Australian Institute of Health and Welfare . AIHW; Canberra: 2019. Acute Rheumatic Fever and Rheumatic Heart Disease in Australia.https://www.aihw.gov.au/reports/indigenous-australians/acute-rheumatic-fever-and-rheumatic-heart-disease/contents/table-of-contents Cat. no: CVD 87. [Google Scholar]
- 2.Australian Bureau of Statistics . June 2016. Canberra. Estimates of Aboriginal and Torres Strait Islander Australians.https://www.abs.gov.au/ausstats/abs@.nsf/mf/3238.0.55.001 [Google Scholar]
- 3.Gurney J.K., Stanley J., Baker M.G., Wilson N.J., Sarfati D. Estimating the risk of acute rheumatic fever in New Zealand by age, ethnicity and deprivation. Epidemiol. Infect. 2016;144:3058–3067. doi: 10.1017/S0950268816001291. Epub 2016 Jun 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Curry C., Zuhlke L., Mocumbi A. Acquired heart disease in low-income and middle-income countries. Arch. Dis. Child. 2018;103:73–77. doi: 10.1136/archdischild-2016-31252. [DOI] [PubMed] [Google Scholar]
- 5.Health Policy Analysis 2017, Evaluation of the commonwealth rheumatic fever strategy – final report. Canberra: Primary Healthcare Branch, Commonwealth Department of Health.
- 6.Katzenellenbogen J.M., Bond-Smith D., Cunneen R., Dempsey K., Greenland M., Cannon J. The end rheumatic heart disease in Australia study of Epidemiology (ERASE) project: data sources, case ascertainment and cohort profile. Clin. Epidemiol. 2019;11:1–14. doi: 10.2147/CLEP.S224621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katzenellenbogen J.M., Nedkoff L., Canon J., Kruger D., Pretty F., Carapetis J.R., Dempsey K.E., de Dassel J., Anderson M., de Klerk N., Hung J. Low positive predictive value of ICD-10 codes in relation to rheumatic heart disease: a challenge for global surveillance. Int. Med. J. 2019;49(3):400–403. doi: 10.1111/imj.14221. [DOI] [PubMed] [Google Scholar]
- 9.Shardey G, Williams-Spence J, Solman N, McLaren J, Brennan A, Baker R, Newcomb A, Reid C. reportOn Behalf of the ANZSCTS Database. The Australian and New Zealand Society of Cardiac and Thoracic Surgeons Cardiac Surgery Database Program National Annual Report 2017. Monash University, DEPM, October2018. Report No 11.
- 10.Agha R., Abdall-Razak A., Crossley E., Dowlut N., Iosifidis C., Mathew G., for the STROCSS Group The STROCSS 2019 guideline: strengthening the reporting of cohort studies in surgery. Int. J. Surg. 2019;72:156–165. doi: 10.1016/j.ijsu.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Rural & remote health Web report. https://www.aihw.gov.au/reports/rural-remote-australians/rural-remote-health/contents/summary Last updated: 22 Oct 2019, Australian Institute of Health and Welfare.
- 12.Steer A. Historical aspects of rheumatic fever. J. Paediatr. Child Health. 2015;51:21–27. doi: 10.1111/jpc.12808. [DOI] [PubMed] [Google Scholar]
- 13.Ralph A.P., Fittock M., Schultz R. Improvement in rheumatic fever and rheumatic heart disease management and prevention using a health-based continuous quality improvement approach. BMC Health Serv. Res. 2013;13:525. doi: 10.1186/1472-6963-13-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Australian Bureau of Statistics 3101 . Dec 2016. 0 - Australian Demographic Statistics.https://www.abs.gov.au/Ausstats/ABS@.nsf/7d12b0f6763c78caca257061001cc588/40269d5a7e7bd7a1ca2581470023db40!OpenDocument Canberra. [Google Scholar]
- 15.Australian Bureau of Statistics 3126 . 2001. 0 - Demography Working Paper 2001/4 - Issues in Estimating the Indigenous Population.https://www.abs.gov.au/ausstats/abs@.NSF/525a1b9402141235ca25682000146abc/5a0ec5f1f21faad6ca256888001daede!OpenDocument Canberra. [Google Scholar]
- 16.ABS 2077.0 - Census of Population and Housing: Understanding the Increase in Aboriginal and Torres Strait Islander Counts. 2016. https://www.abs.gov.au/ausstats/abs@.nsf/Lookup/by%20Subject/2077.0~2006-2011~Main%20Features~Contents~1 [Google Scholar]
- 17.Chauvaud S., Fuzellier J.F., Berrebi A., Deloche A., Fabiani J.N., Carpentier A. Long-term (29 years) results of reconstructive surgery in rheumatic mitral valve insufficiency. Circulation. 2001;1041:I12–I15. doi: 10.1161/hc37t1.094707. [DOI] [PubMed] [Google Scholar]
- 18.Remenyi B., Webb R., Gentles T., Russell P., Finucane K., Lee M. Improved long-term survival for rheumatic mitral valve repair compared to replacement in the young. World J Pediatr Congenit Heart Surg. 2013;4(2):155–164. doi: 10.1177/2150135112474024. [DOI] [PubMed] [Google Scholar]
- 19.Krishna Moorthy P.S., Sivalingam S., Dillon J., Kong P.K., Yakub M.A. Is it worth repairing rheumatic mitral valve disease in children? Long-term outcomes of an aggressive approach to rheumatic mitral valve repair compared to replacement in young patients. Interact. Cardiovasc. Thorac. Surg. 2019;28:191–198. doi: 10.1093/icvts/ivy234. [DOI] [PubMed] [Google Scholar]
- 20.Palacios I.F. Percutaneous mitral balloon valvuloplasty: worldwide trends. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.119.012898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirsch R. Should we offer a bioprosthetic valve to women of child-bearing age who need valve replacement? Intervent Cardiol. 2014;6:425–431. [Google Scholar]
- 22.Matebele M.P., Meda B., Rohde S., Clarke A., Fraser J.F. Cardiac surgery in indigenous Australians: early onset cardiac disease with follow-up challenges. Heart. Lung Circ. 2014;23:566–571. doi: 10.1016/j.hlc.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 23.Sadler L., McCowan L., White H., Stewart A., Bracken M., North R. Pregnancy outcomes and cardiac complications in women with mechanical, bioprosthetic and homograft valves. BJOG. 2000;107(2):245–253. doi: 10.1111/j.1471-0528.2000.tb11696.x. [DOI] [PubMed] [Google Scholar]
- 24.Russell E.A., Tran L., Baker R.A., Bennetts J.S., Brown A., Reid C.M. A review of valve surgery for rheumatic heart disease in Australia. BMC Cardiovasc. Disord. 2014;14(1):134. doi: 10.1186/1471-2261-14-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bartus K., Sadowski J., Litwinowicz R., Filip G., Jasinski M., Deja M., Kusmierczyk M., Pawlak S., Jemielity M., Jagielak D., Hendzel P., Suwalski P., Tobota Z., Maruszewski B., Kapelak B. Changing trends in aortic valve procedures over the past ten years—from mechanical prosthesis via stented bioprosthesis to TAVI procedures—analysis of 50,846 aortic valve cases based on a Polish National Cardiac Surgery Database. J. Thorac. Dis. 2019;11(6):2340–2349. doi: 10.21037/jtd.2019.06.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nsethke M., Scherman J. TAVI for rheumatic aortic stenosis – the next frontier? Int. J. Cardiol. 2019 Apr 1;280:51–52. doi: 10.1016/j.ijcard.2019.01.015. Epub 2019 Jan 7. [DOI] [PubMed] [Google Scholar]
- 27.Kim J.B., Yoo D.G., Kim G.S., Song H., Jung S.H., Choo S.J. Mild-to-moderate functional tricuspid regurgitation in patients undergoing valve replacement for rheumatic mitral disease: the influence of tricuspid valve repair on clinical and echocardiographic outcomes. Heart. 2012;98(1):24–30. doi: 10.1136/heartjnl-2011-300403. [DOI] [PubMed] [Google Scholar]
- 28.Tornos Mas P., Rodríguez-Palomares J.F., Antunes M.J. Secondary tricuspid valve regurgitation: a forgotten entity. Heart. 2015;101:1840–1848. doi: 10.1136/heartjnl-2014-307252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Russell E.A., Tran L., Baker R.A., Bennetts J.S., Brown A., Reid C.M., Tam R., Walsh W.F., Maguire G.P. A review of outcome following valve surgery for rheumatic heart disease in Australia. BMC Cardiovasc. Disord. 2015;15:103. doi: 10.1186/s12872-015-0094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alizzi A.M., Knight J.L., Tully P.J. Surgical challenges in rheumatic heart disease in the Australian indigenous population. Heart Lung Circ. 2010;19:295–298. doi: 10.1016/j.hlc.2010.02.010. Epub 2010 Mar 30. [DOI] [PubMed] [Google Scholar]
- 31.Cannon J., Roberts K., Milne C., Carapetis J.R. Rheumatic heart disease severity, progression and outcomes: a multi-state model. J Am Heart Assoc. 2017 Mar;6(3) doi: 10.1161/JAHA.116.003498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Katzenellenbogen Judith, Seth Rebecca, Dempsey Karen. Contemporary incidence and prevalence of Rheumatic Fever and Rheumatic Heart Disease in Australia using linked data: the case for policy change. J. Am. Heart Assoc. 2020 Oct 20;9(19) doi: 10.1161/JAHA.120.016851. In this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wyber Rosemary, Noonan Katharine, Halkon Katherine, Cannon Jeffrey, Haynes Emma, END RHD CRE Investigators and Collaborators Ending rheumatic heart disease in Australia: the evidence for a new approach. Med. J. Aust. 2020;213 (10)(S3):S1–S31. doi: 10.5694/mja2.50853. In this issue. [DOI] [PubMed] [Google Scholar]
- 34.Bond-Smith Daniela, Seth Rebecca, De Klerk Nicholas. Development and evaluation of a prediction model for ascertaining Rheumatic Heart Disease status in administrative data. Clin. Epidemiol. 2020;12:717–730. doi: 10.2147/CLEP.S241588. In this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.