Abstract
目的
探讨miR-324-5p调控Syk/Ras/c-fos信号通路对大鼠肾小球系膜(HBZY-1)细胞增殖能力的影响。
方法
体外培养HBZY-1细胞;设计并合成miR-324-5p mimics,miR-324-5p mimics-NC片段;采用Lipo3000试剂盒瞬时转染miR-324-5p mimics,miR-324-5p mimics-NC片段至脂多糖(LPS)诱导的HBZY-1细胞内,RT-qPCR验证转染效率;将HBZY-1细胞分为对照组(生理盐水),LPS组(LPS 0.5 μg/mL),LPS+mimics组(LPS 0.5 μg/mL+miR-324-mimics),LPS+mimics-NC组(LPS 0.5 μg/mL+miR-324-mimics-NC);MTT法检测各组HBZY-1细胞增殖活性;RT-qPCR法检测各组细胞miR-324-5p及Syk、Ras、MEK1/2、ERK1/2、c-fos mRNA的表达;Western blot和免疫荧光法检测各组细胞p-Syk、Ras、p-MEK1/2、p-ERK1/2、c-fos蛋白的表达。
结果
MTT结果显示,LPS组细胞增殖水平较正常组显著升高,与LPS组及LPS+mimics-NC组相比,LPS+mimics组细胞增殖能力降低;RT-qPCR结果显示,LPS+mimics组中miR-324-5p表达明显高于LPS组及LPS+mimics-NC组,且LPS+mimics组中Syk、Ras、MEK1/2、ERK1/2、c-fos mRNA的表达显著低于LPS组及LPS+mimics-NC组, 差异具有统计学意义(P < 0.05);Western Blot结果显示,与LPS组及LPS+mimics-NC组相比LPS+mimics组中p-Syk、Ras、p-MEK1/2、p-ERK1/2、c-fos蛋白表达显著降低,差异具有统计学意义(P < 0.05);免疫荧光结果显示,与LPS组及LPS+mimics-NC组相比,LPS+mimics组p-Syk、Ras、p-MEK1/2、p-ERK1/2、c-fos蛋白标记细胞数目明显减少。
结论
miR-324-5p可通过抑制Syk/Ras/c-fos信号通路降低LPS诱导的慢性肾小球肾炎细胞的增殖活性,miR-324-5p有望成为慢性肾小球肾炎诊断和治疗的潜在靶标。
Keywords: 慢性肾小球肾炎, miR-324-5p, Syk/Ras/c-fos信号通路, 细胞增殖
Abstract
Objective
To investigate the effect of miR-324-5p on the proliferation of rat glomerular mesangial (HBZY-1) cells and the role of Syk/Ras/c-fos signaling pathway in mediating this effect.
Methods
HBZY-1 cells cultured in vitro were transiently transfected with miR-324-5p mimics or miR-324-5p-mimics-NC followed by treatment with lipopolysaccharide (LPS). MTT assay was used to detect the proliferation activity of HBZY-1 cells, and RT-qPCR was used to detect the expressions of miR-324-5p and the mRNA expressions of Syk, Ras, MEK1/2, ERK1/2 and c-fos mRNA. The protein expressions of p-Syk, Ras, p-MEK1/2, p-ERK1/2 and c-Fos were detected by Western blotting and immunofluorescence assay.
Results
MTT assay showed that exposure to LPS significantly enhanced the proliferative activity of HBZY-1 cells. Compared with the cells treated with LPS and LPS + mimics NC, the cells transfected with miR-324-5p mimics prior to LPS exposure exhibited significantly lowered proliferative activity. Transfection with miR-324-5p mimics significantly lowered the mRNA expressions of Syk, Ras, MEK1/2, ERK1/2 and c-fos and the protein expressions of p-Syk, Ras, MEK1/2, ERK1/2 and c-Fos (P < 0.05), and reduced numbers of cells positive for p-Syk, Ras, p-MEK1/2, p-ERK1/2 and c-Fos proteins following LPS exposure.
Conclusion
miR-324-5p can inhibit the proliferation of rat chronic glomerulonephritis cells induced by LPS by inhibiting Syk/Ras/c-fos signaling pathway and may potentially serve as a diagnostic indicator and a therapeutic target for chronic glomerulonephritis.
Keywords: Chronic glomerulonephritis, miR-324-5p, Syk/Ras/c-fos signaling pathway, cell proliferation
慢性肾小球肾炎(CGN)是一种肾内科常见病[1],其发生发展的确切分子学机制目前尚不清楚[2]。临床对其治疗手段多集中于对症治疗,缺乏特异性的药物以及治疗手段,更有许多患者存在预后不佳的情况,因此研究新的生物标志物,探寻CGN产生发展的确切分子学机制是当今亟需解决的难题[3]。肾小球系膜细胞(GMC)参与了CGN的病变过程,可被多种刺激激活后异常增殖并构成了CGN的病理改变[4]。因此,CGN中系膜细胞的增殖活性研究具有重要意义,因此本实验采用体外培养的大鼠肾小球系膜细胞系HBZY-1建立体外CGN模型进行后续研究。
MicroRNA(miRNA)是长度为19~23个核苷酸的内源非编码小RNA。它通过直接与mRNA 3'-UTRs结合,特异性识别目标基因的mRNA,并在转录后水平上调控目标基因的表达[5],从而参与细胞增殖[6],分化[7]和凋亡[8]的调控,与许多疾病的发生密切相关,故miRNA已成为新的研究焦点。本课题组前期通过查阅文献筛选出系列肾炎相关miRNA,进一步通过RNAhybrid、targetscan和miRanda分析预测miR-324-5p种子序列与课题组前期验证的肾炎中心基因Syk可能存在互补序列。目前已发现miR-324-5p参与调控癌症细胞的增殖迁移侵袭[9-10],小鼠心肌细胞的再生过程[11],以及抑制棕榈酸诱导的脂肪细胞凋亡作用[12]。然而其在肾脏组织中的表达及其对肾小球系膜细胞增殖的作用尚未见报道,miR-324-5p在慢性肾小球肾炎中的作用机制是值得关注的研究方向。
脾酪氨酸激酶(Syk)是大小为72 000的一种非受体酪氨酸激酶,在许多细胞表面受体如Fc受体,补体受体和整联蛋白的下游信号的传导过程中起着重要作用[13]。有报道表明,Syk通过调控下游信号通路来发挥免疫性和非免疫性功能[14],本课题组前期研究也证明了抑制Syk/Ras/c-fos信号通路可以减轻CGN大鼠的炎性反应[15],但其在CGN中表达的上游调控机制尚未明确。因而本实验采用LPS诱导的HBZY-1细胞作为研究对象,通过在HBZY-1细胞系中过表达miR-324-5p,观察过表达的miR-324-5p对HBZY-1细胞增殖能力的影响是否与Syk/Ras/c-fos相关,以Syk基因的上游miRNA为切入点进行分析,为CGN发病的分子学机制提供了新的靶标与思路。
1. 材料和方法
1.1. 主要试剂与仪器
大鼠肾小球系膜细胞系HBZY-1(Procell,批号:CL0092),EZ-10 Total RNA Mini-Preps Kit(Sangon Biotech,批号:F702KA2320),ABScript Ⅱ RT Mix for qPCR with gDNA Remover(ABclonal,批号:9619580410),Universal SYBR Green Fast qPCR Mix(ABclonal,批号:9619030419),超净工作台(苏州苏净仪器自控设备有限公司),台式高速冷冻离心机(eppendorf),分光光度计(上海菁华科技仪器有限公司),PCR仪(Biorad),real-time PCR仪(BioRad)。
1.2. 实验方法
1.2.1. 细胞的培养与分组
HBZY-1按常规方法培养[16],并合成miR-324-5p模拟物序列及其NC序列。采用lipo3000试剂盒瞬时转染序列到HBZY-1细胞,并将HBZY-1细胞分组:(1)对照组:HBZY-1细胞仅用生理盐水处理;(2)LPS组:HBZY-1细胞加入浓度为0.5 μg/mL的脂多糖刺激24 h;(3)LPS+mimics组:HBZY-1细胞转入miR-324-mimics片段后加入浓度为0.5 μg/mL的脂多糖刺激24h;(4)LPS+mimics-NC组:HBZY-1细胞转入miR-324-mimics-NC片段后加入浓度为0.5 μg/mL的脂多糖刺激24 h。培养24 h后离心细胞,收集细胞用于各项实验。
1.2.2. MTT法测定细胞增殖活性
按1.2.1项下将HBZY-1细胞分组。用2.5 g/L胰蛋白酶-0.2 g/L乙二胺四乙酸消化各组HBZY-1细胞并用含100 mL/L胎牛血清的DMEM培养基配制单细胞悬液,以1×104/孔接种于96孔板中,在培养24、48、72 h时每孔加入20 μL MTT溶液(5 mg/mL),继续孵育4 h,终止培养,吸尽各孔液体,每孔加200 μL二甲亚砜,室温振荡溶解10 min后,置酶标仪490 nm处测吸光度,每组设6个复孔。
1.2.3. RT-qPCR法检测miR-324-5p表达
实验分组同1.2.1项下分组。EZ-10 Total RNAMini-Preps Kit提取总RNA,紫外分光光度计测定RNA浓度和纯度。取2 μL总RNA,采用miRNA第一链cDNA合成(加尾法)(Sangon Biotech)合成miR-324-5p cDNA后进行RT-qPCR,反应总体系20 μL。miR-324-5p上游引物序列为F:5'-ATCG CATCCCCTAGGGCATTGGTGTAAT-3',采用试剂盒提供的下游通用引物以及内参U6进行定量PCR分析。
1.2.4. RT-qPCR法检测Syk/Ras/c-fos信号轴相关mRNA表达
按1.2.1项下分组HBZY-1细胞并接种于6孔板中孵育。EZ-10 Total RNA Mini-Preps Kit用于抽提总RNA并测定浓度和纯度。反应总体系20 μL。根据逆转录反应试剂盒说明书逆转录合成通路相关基因cDNA后进行RT-qPCR。Gene Bank获取相关基因序列,Primer 5.0软件设计引物,β-actin作为内参进行定量PCR分析,PCR引物如表 1所示。
1.
RT-qPCR引物信息
Sequences and expected product lengths of the primers for RT-qPCR
| Targeted gene | Forward sequence and reverse sequence | Product length (bp) |
| Syk | F: 5'-TGGGTGGTTTTGCTTTGT-3' R: 5'-TCCTGGGAGTGGTAATGG-3' |
142 |
| c-fos | F: 5'-CCTACCCCGAGGCTGAC-3' R: 5'-CCTGCCTTCTCTGACTGCT-3' |
131 |
| Ras | F: 5'-TGGCTGGAAGTAGGAGGTG-3' R: 5'-GCAGGCAGAAGAGAAGGG-3' |
147 |
| MEK1 | F: 5'-GCGGGCAGCTAATTGAC-3' R: 5'-CCCCATGCTCCAGATGT-3' |
116 |
| MEK2 | F: 5'-CGCTCACCATCAACCCTAC-3' R: 5'-CCGCTTCCTCTGCTGTTC-3' |
128 |
| ERK-1 | F: 5'-GAACCCCACCCCATTTTC-3' R: 5'-TCCACATCCAATCACCCA-3' |
148 |
| ERK-2 | F: 5'-CGGCGGTTAGTTCTCTCTT-3' R: 5'-GACTTGTGCTGCGCTTC-3' |
109 |
| β-actin | F: 5'-CCTCACTGTCCACCTTCCA-3' R: 5'-GGGTGTAAAACGCAGCTCA-3' |
120 |
1.2.5. Western blot法检测Syk/Ras/c-fos信号轴相关蛋白表达
收集各分组的HBZY-1细胞,按说明书方法操作提取总蛋白,取20 μg提取的细胞蛋白溶液,SDSPAGE电泳分离蛋白,并转移至PVDF膜上,5%脱脂牛奶室温封闭2 h。封闭完成后加入适宜浓度Syk(1: 1000)、p-Syk(1:500)、Ras(1:2000)、MEK1/2(1:1000)、p-MEK1/2(1:800)、ERK1/2(1:1000)、p-ERK1/2(1: 800)、c-fos(1:500)一抗,4 ℃过夜,加入二抗(1:10 000)室温孵育1 h后显影分析。
1.2.6. 免疫荧光共标染色鉴定Syk/Ras/c-fos信号轴相关蛋白表达
取细胞生长状态良好,密度适中的HBZY-1爬片放入6孔板内;弃上清液,PBS洗3次,4%多聚甲醛室温固定15 min,PBS洗3次,4 ℃、0.1% Triton-X破膜30 min,0.5% BSA封闭,加入Syk(1:100)、p-Syk(1: 100)、Ras(1:250)、MEK1/2(1:25)、p-MEK1/2(1:50)、ERK1/2(1:50)、p-ERK1/2(1:50)、c-fos(1:100)一抗4 ℃孵育过夜,PBS洗3次,避光滴加二抗:Ig G-FITC和IgG-Cy3(1:200),37 ℃孵育1 h,DAPI(1:100)室温染核10 min,荧光封片剂封片,荧光显微镜观察、拍照。
1.3. 统计学分析
将数据输入SPSS22.0软件进行统计处理,结果表示为均数±标准差。随机区组设计的方差分析用于分析多个组之间的差异,P < 0.05时认为差异具有统计学意义。
2. 结果
2.1. MTT法测定HBZY-1细胞增殖活性
与对照组相比,LPS处理后的HBZY-1细胞增殖能力增强;在转染miR-324-5p-mimics片段后,LPS刺激引起的细胞增殖活性增强可被抑制(图 1)。与24 h和72 h相比,在LPS处理48 h后HBZY-1细胞增殖水平最高,48 h为LPS为本实验的最佳作用时间,并取处理48 h的HBZY-1细胞进行后续实验。
1.
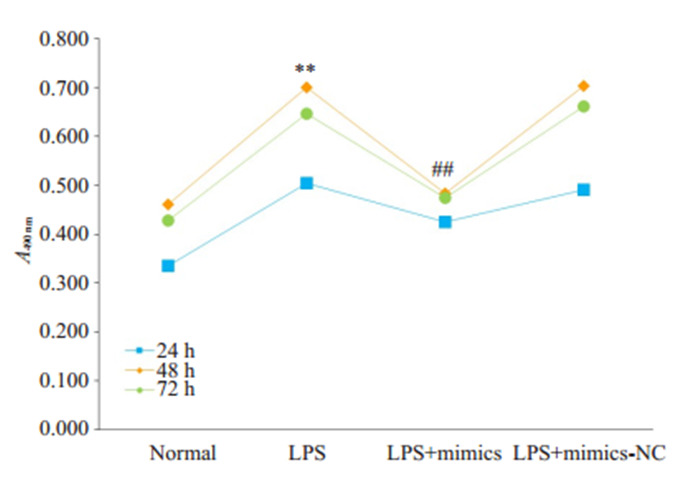
各组HBZY-1细胞增殖活性
Proliferative activity of HBZY-1 cells in different groups. **P < 0.01 vs normal group after 48 h of treatment. ##P < 0.01 vs LPS group after 48 h of treatment.
2.2. RT-qPCR检测miR-324-5p表达水平
LPS诱导的HBZY-1细胞中miR-324-5p表达水平明显低于对照组表达水平,组间差异具有统计学意义(P < 0.01)。此外,LPS+mimics组与LPS组相比,miR-324-5p表达显著升高,差异有统计学意义(P < 0.01,图 2)。
2.
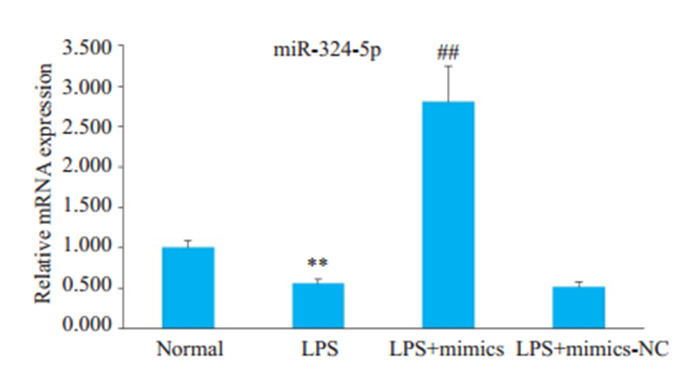
miR-324-5p在各组HBZY-1细胞中的表达水平
Expression level of miR-324-5p in HBZY-1 cells in different groups. **P < 0.01 vs normal group. ##P < 0.01 vs LPS group.
2.3. 过表达miR-324-5p对Syk表达的影响
与对照组相比,LPS组HBZY-1细胞中Syk mRNA表达水平升高;而与LPS组相比,LPS + mimics组HBZY-1细胞中Syk mRNA表达水平降低。Western blot和免疫荧光结果显示,LPS组中p-Syk蛋白的表达明显高于对照组,而与LPS组相比,LPS+mimics组pSyk蛋白的表达显著降低(图 3,P < 0.05)。
3.
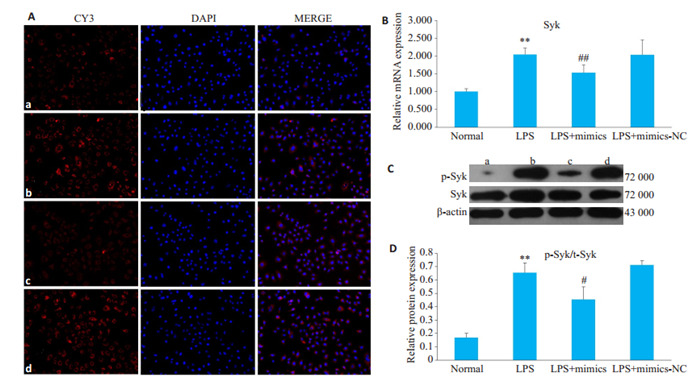
过表达miR-324-5p对LPS诱导的HBZY-1细胞中Syk表达的影响
Effect of overexpression of miR- 324-5p on Syk expression in LPS- induced HBZY-1 cells. A: Immunofluorescence assay of p-Syk protein in HBZY-1 cells (Original magnification: ×200). a: Normal group; b: LPS group; c: LPS+mimics group; d: LPS+mimics-NC group. B: RT-qPCR for detecting mRNA changes of Syk in HBZY-1 cells. C: Western blotting for detecting protein expression of p-Syk. D: Semi-quantitative analysis of p-Syk protein expression. **P < 0.01 vs normal group. ##P < 0.01 vs LPS group; #P < 0.05 vs LPS group.
2.4. 过表达miR-324-5p对Ras表达的影响
LPS组HBZY-1细胞中Ras的mRNA和蛋白表达水平较对照组明显升高,而与LPS组相比,LPS+mimics组HBZY-1细胞中Ras的mRNA和蛋白表达水平明显降低(图 4,P < 0.01)。
4.
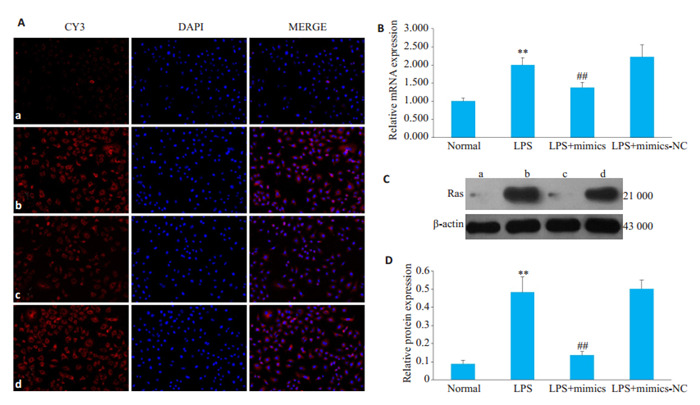
过表达miR-324-5p对LPS诱导的HBZY-1细胞中Ras表达的影响
Effect of overexpression of miR-324-5p on Ras expression in LPS-induced HBZY-1 cells. A: Immunofluorescence method was used to observe the changes of Ras in HBZY-1 (×200). a: Normal group, b: LPS group, c: LPS+mimics group, d: LPS+mimics-NC group; B: RTqPCR was used to detect the mRNA changes of Ras in HBZY-1; C: Western Blot was used to detect the protein expression of Ras; D: Semi quantitative analysis of Ras **P < 0.01 vs normal group. ##P < 0.01 vs LPS group.
2.5. 过表达miR-324-5p对MEK1/2表达的影响
LPS组中MEK1,MEK2 mRNA表达显著高于对照组,而与LPS组相比,LPS+mimics组中MEK1,MEK2 mRNA表达明显降低;Western blot和免疫荧光的结果显示,LPS组中p-MEK1/2蛋白的表达明显高于对照组,而与LPS组相比,LPS+mimics组p-MEK1/2蛋白的表达显著降低(图 5,P < 0.01)。
5.
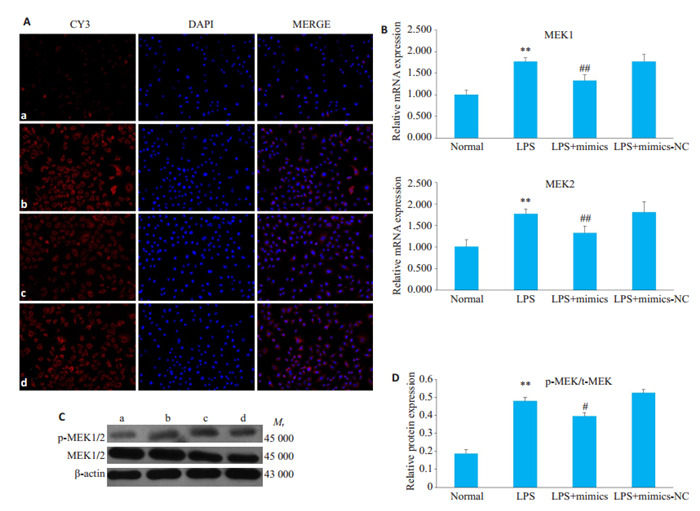
过表达miR-324-5p对LPS诱导的HBZY-1细胞中MEK1/2表达的影响
Effect of overexpression of miR-324-5p on MEK1/2 expression in HBZY-1 cells induced by LPS. A: Immunofluorescence method was used to observe the changes of MEK1/2 in HBZY-1 (×200). a: Normal group, b: LPS group, c: LPS+mimics group, d: LPS+ mimicsNC group; B: RT-qPCR was used to detect the mRNA changes of MEK1/2 in HBZY-1 cells; C: Western blotting was used to detect the protein expression of MEK1/2; D: Semi quantitative analysis of p-MEK expression. **P < 0.01 vs normal group. ##P < 0.01 vs LPS group.
2.6. 过表达miR-324-5p对ERK1/2表达的影响
LPS组中ERK1,ERK2 mRNA表达明显高于对照组,而与LPS组相比,LPS+mimics组中ERK1,ERK2 mRNA表达显著降低;Western Blot和免疫荧光结果显示,LPS组中p-ERK1/2蛋白的表达明显高于对照组,而与LPS组相比,LPS+mimics组p-ERK1/2蛋白的表达显著降低(图 6,P < 0.01)。
6.
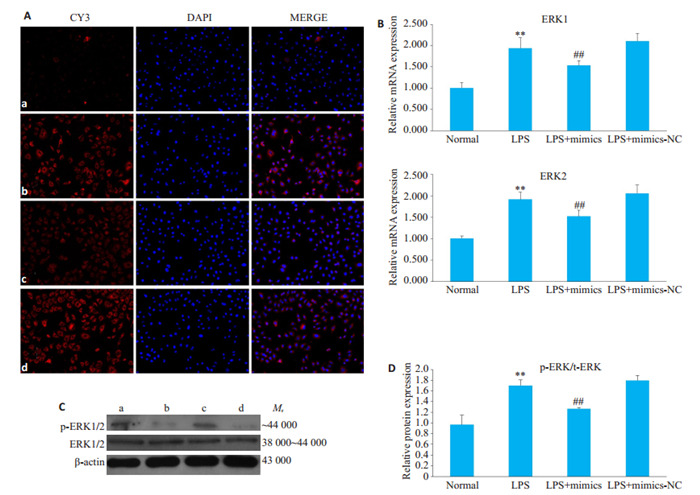
过表达miR-324-5p对LPS诱导的HBZY-1细胞中ERK1/2表达的影响
Effect of overexpression of miR-324-5p on ERK1/2 expression in HBZY-1 cells induced by LPS. A: Immunofluorescence method was used to observe the changes of ERK1/2 in HBZY-1 (×200). a: Normal group, b: LPS group, c: LPS+mimics group, d: LPS+mimics-NC group; B: RT-qPCR was used to detect the mRNA changes of ERK1/2 in HBZY-1 cells; C: Western blotting was used to detect the protein expression of ERK1/2; D: Semi-quantitative analysis of p-ERK expression. **P < 0.01 vs normal group. ##P < 0.01 vs LPS group.
2.7. 过表达miR-324-5p对c-fos表达的影响
LPS组HBZY-1细胞中c-fos mRNA和蛋白表达水平明显高于对照组,而与LPS组HBZY-1细胞相比,LPS+mimics组中c-fos mRNA和蛋白表达水平显著降低(图 7,P < 0.01)。
7.
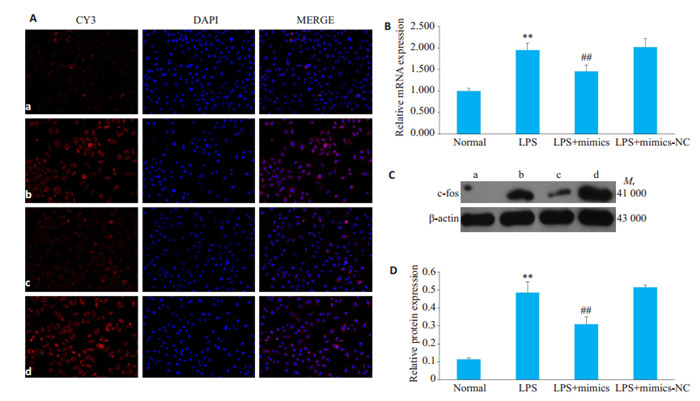
过表达miR-324-5p对LPS诱导的HBZY-1细胞中c-fos表达的影响
Effect of overexpression of miR-324-5p on c-fos expression in LPS-induced HBZY-1 cells. A: Immunofluorescence method was used to observe the changes of c-fos in HBZY-1 (×200). a: Normal group, b: LPS group, c: LPS+mimics group, d: LPS+ mimics-NC group; B: RT-qPCR was used to detect the mRNA changes of c-fos in HBZY-1 cells; C: Western blotting was used to detect the protein expression of c-fos; D: Semi-quantitative analysis of c-fos. **P < 0.01 vs normal group. ##P < 0.01 vs LPS group.
3. 讨论
GMC是一种固有的散布在肾小球系膜基质中的细胞类型,在肾脏中参与许多重要的生物学功能,而GMC的增殖和扩大是许多慢性肾病产生的前兆[17]。LPS是一种促细胞因子释放,趋化炎症细胞的内毒素,研究证明,LPS具有诱导肾小球系膜细胞增殖的作用[18]。因此,本实验采用LPS诱导的HBZY-1细胞建立慢性肾小球肾炎细胞增殖模型。
近年来,许多研究从基因层面探索慢性肾小球肾炎的发病机制[19]。miRNA可通过调节一个途径或者通过多个串扰途径的基因的能力,对复杂的调控网络及最终的生理过程和疾病的产生有着重大影响,同时对表观遗传学[20],转录[21],翻译[22]和蛋白质修饰[23]具有广泛调节作用,并与许多疾病的进展密切相关[24]。miR-324-5p是miR-324家族的一种多功能小分子RNA,在人类癌症中发挥着独特的生物学功能[25]。研究表明,过表达miR-324-5p在神经胶质瘤[10]和大肠癌[26]中均降低了细胞增殖活性,因此我们推断miR-324-5p在CGN中发挥分子学机制可能与抑制细胞增殖有关。本研究中MTT结果显示,转染miR-324-5p模拟物可以有效逆转LPS诱导的HBZY-1细胞增殖情况,与以往的研究结果一致。
Syk是一种胞内酪氨酸蛋白激酶,属于ZAP70蛋白激酶家族中的成员,对机体免疫反应和炎症反应过程、过敏性疾病、某些癌症和感染疾病的发病具有重要意义[27]。Ras是Syk下游的一个重要信号分子[28],Ras单体GTPases是细胞内信号分子,可将上游信息引导至参与细胞生长和分化的下游效应子,也是进行性纤维化驱动因素的大多数细胞因子下游的信号级联反应中的汇聚点[29]。它在控制增殖,分化和细胞死亡中起主要作用,将活化的RTK和GPCR连接到效应子途径Raf /促分裂原活化蛋白激酶(MAPK)/细胞外信号调节激酶1和2(ERK1/2)和磷脂酰肌醇3激酶(PI3K)-Akt[30]。研究证明,K-Ras是控制人肾成纤维细胞增殖的关键Ras亚型[31],MEK/ERK是MAPK级联通路Ras基因激活后的下游信号通路[32]。Huang等[33]的实验表明在糖尿病肾病大鼠模型中MEK/ERK途径被激活,增加转化生长因子-β(TGF-β),FN和胶原蛋白Ⅰ和Ⅳ在肾小球系膜细胞中的表达,从而导致肾纤维化的产生。Liang等人的研究还表明,黄连素对肾脏损伤和肾小球系膜细胞增殖的改善作用可能是通过减少属于MEK/ERK依赖性转录因子的c-fos表达来调节生长因子的活化和细胞周期的再进入过程[34]。c-fos是即早基因(IEGs)家族中的一员,参与调控细胞的增殖,分化,凋亡,炎症因子的产生以及组织纤维化的进程,在多种肾病的病理过程中发挥作用[35]。有研究表明[36],在糖尿病肾病导致的肾小球系膜细胞肥大与增殖的细胞株中,c-fos含量明显上调,证明了c-fos在GMC增殖中起着重要的调控作用。本研究中RT-qPCR,Western blot和免疫荧光的结果共同显示,转染miR-324-5p模拟物可有效逆转LPS建立的慢性肾炎模型中Syk/Ras/c-fos通路相关基因和蛋白表达上调。证明过表达miR-324-5p对慢性肾炎中肾小球系膜细胞增殖能力有显著的抑制作用,其可能的分子学机制是通过抑制Syk/Ras/c-fos通路相关基因的表达来发挥作用的。
综上所述,本实验通过MTT比色法,RT-qPCR,Western blot和免疫荧光实验共同验证了上调的miR- 324-5p调控Syk/Ras/c-fos信号通路并影响通路相关基因Syk、Ras、MEK1/2、ERK1/2、c-fos的表达,从而降低了LPS诱导的HBZY-1细胞的增殖活性。为我们进一步探索CGN发病的分子学机制提供了新的思路,亦为CGN的临床治疗提供了新的靶点。
Biography
王晶,硕士,E-mail: wangjing7@stu.ahtcm.edu.cn
Funding Statement
国家自然科学基金(81973546);安徽省自然科学基金(1808085MH276)
Supported by National Natural Science Foundation of China (81973546)
Contributor Information
王 晶 (Jing WANG), Email: wangjing7@stu.ahtcm.edu.cn.
高 家荣 (Jiarong GAO), Email: zyfygjr2006@163.com.
References
- 1.杨 倩春, 杨 霓芝. 急性肾小球肾炎病因病机探讨. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=lnzyxyxb200301006. 辽宁中医学院学报. 2003;1:13. [杨倩春, 杨霓芝.急性肾小球肾炎病因病机探讨[J].辽宁中医学院学报, 2003, 1: 13.] [Google Scholar]
- 2.卢 作奎, 许 为佳, 史 秀岩. 基于WNT/β-catenin信号通路研究DMBT1调控慢性肾小球肾炎发生发展的作用机制. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=lcszbzz202004007. 临床肾脏病杂志. 2020;20(4):297–302. [卢作奎, 许为佳, 史秀岩.基于WNT/β-catenin信号通路研究DMBT1调控慢性肾小球肾炎发生发展的作用机制[J].临床肾脏病杂志, 2020, 20(4): 297-302.] [Google Scholar]
- 3.Decleves A, Sharma K. Novel targets of antifibrotic and antiinflammatory treatment in CKD. Nat Rev Nephrol. 2014;10(5):257–67. doi: 10.1038/nrneph.2014.31. [Decleves A, Sharma K. Novel targets of antifibrotic and antiinflammatory treatment in CKD[J]. Nat Rev Nephrol, 2014, 10(5): 257-67.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.杨莉.福辛普利对肾小球系膜细胞增殖的影响[D].中国医科大学, 2007.
- 5.Dweep H, Sticht C, Pandey P, et al. miRWalk-Database: Prediction of possible miRNA binding sites by walking the genes of three genomes. http://dl.acm.org/citation.cfm?id=2304897. J Biomed Informatics. 2011;44(5):839–47. doi: 10.1016/j.jbi.2011.05.002. [Dweep H, Sticht C, Pandey P, et al. miRWalk-Database: Prediction of possible miRNA binding sites by walking the genes of three genomes [J]. J Biomed Informatics, 2011, 44(5): 839-47.] [DOI] [PubMed] [Google Scholar]
- 6.Liu N, Bezprozvannaya S, Williams AH, et al. microRNA-133a regulates cardiomyocyte proliferation and suppresses smooth muscle gene expression in the heart. Genes Dev. 2008;22(23):3242–54. doi: 10.1101/gad.1738708. [Liu N, Bezprozvannaya S, Williams AH, et al. microRNA-133a regulates cardiomyocyte proliferation and suppresses smooth muscle gene expression in the heart[J]. Genes Dev, 2008, 22(23): 3242-54.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suh JS, Lee JY, Choi YS, Chung CP, Park YJ. Peptide-mediated intracellular delivery of miRNA-29b for osteogenic stem cell differentiation. Biomaterials. 2013;34(17):4347–59. doi: 10.1016/j.biomaterials.2013.02.039. [Suh JS, Lee JY, Choi YS, Chung CP, Park YJ. Peptide-mediated intracellular delivery of miRNA-29b for osteogenic stem cell differentiation[J]. Biomaterials, 2013, 34(17): 4347-59.] [DOI] [PubMed] [Google Scholar]
- 8.Salim H, Akbar NS, Zong D, et al. miRNA-214 modulates radiotherapy response of non-small cell lung cancer cells through regulation of p38MAPK, apoptosis and senescence. Br J Cancer. 2012;107(8):1361–73. doi: 10.1038/bjc.2012.382. [Salim H, Akbar NS, Zong D, et al. miRNA-214 modulates radiotherapy response of non-small cell lung cancer cells through regulation of p38MAPK, apoptosis and senescence[J]. Br J Cancer, 2012, 107(8): 1361-73.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song LQ, Liu D, Zhao Y, et al. Sinomenine inhibits breast cancer cell invasion and migration by suppressing NF-κB activation mediated by IL-4/miR-324-5p/CUEDC2 Axis. http://smartsearch.nstl.gov.cn/paper_detail.html?id=87d1b9ff3b4b77cf201bed0b91d77be4. Biochem Biophys Res Commun. 2015;464(3):705–10. doi: 10.1016/j.bbrc.2015.07.004. [Song LQ, Liu D, Zhao Y, et al. Sinomenine inhibits breast cancer cell invasion and migration by suppressing NF-κB activation mediated by IL-4/miR-324-5p/CUEDC2 Axis[J]. Biochem Biophys Res Commun, 2015, 464(3): 705-10.] [DOI] [PubMed] [Google Scholar]
- 10.Xu H, Zong H, Shang M, et al. MiR-324-5p inhibits proliferation of glioma by target regulation of GLI1. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=2706c0d2b32eafecf6671d58a13fa472. Eur Rev Med Pharmacol Sci. 2014;18(6):828. [Xu H, Zong H, Shang M, et al. MiR-324-5p inhibits proliferation of glioma by target regulation of GLI1[J]. Eur Rev Med Pharmacol Sci, 2014, 18(6): 828.] [PubMed] [Google Scholar]
- 11.Liu HL, Zhu JG, Liu YQ, et al. Identification of the microRNA expression profile in the regenerative neonatal mouse heart by deep sequencing. Cell Biochem Biophys. 2014;70(1):635–42. doi: 10.1007/s12013-014-9967-7. [Liu HL, Zhu JG, Liu YQ, et al. Identification of the microRNA expression profile in the regenerative neonatal mouse heart by deep sequencing[J]. Cell Biochem Biophys, 2014, 70(1): 635-42.] [DOI] [PubMed] [Google Scholar]
- 12.Sun CX, Zhu L, Ma RJ, et al. Astrocytic miR-324-5p is essential for synaptic formation by suppressing the secretion of CCL5 from astrocytes. Cell Death Dis. 2019;10(2):141. doi: 10.1038/s41419-019-1329-3. [Sun CX, Zhu L, Ma RJ, et al. Astrocytic miR-324-5p is essential for synaptic formation by suppressing the secretion of CCL5 from astrocytes[J]. Cell Death Dis, 2019, 10(2): 141.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bartaula-Brevik S, Lindstad Brattås MK, Tvedt THA, et al. Splenic tyrosine kinase (SYK) inhibitors and their possible use in acute myeloid leukemia. Expert Opin Investig Drugs. 2018;27(4):377–87. doi: 10.1080/13543784.2018.1459562. [Bartaula-Brevik S, Lindstad Brattås MK, Tvedt THA, et al. Splenic tyrosine kinase (SYK) inhibitors and their possible use in acute myeloid leukemia[J]. Expert Opin Investig Drugs, 2018, 27(4): 377- 87.] [DOI] [PubMed] [Google Scholar]
- 14.Mocsai A, Ruland J, Tybulewicz VLJ. The SYK tyrosine kinase: a crucial player in diverse biological functions. Nat Rev Immunol. 2010;10(6):387–402. doi: 10.1038/nri2765. [Mocsai A, Ruland J, Tybulewicz VLJ. The SYK tyrosine kinase: a crucial player in diverse biological functions[J]. Nat Rev Immunol, 2010, 10(6): 387-402.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.高 家荣, 吴 溪, 宋 俊梅, et al. 芪藤消浊颗粒对慢性肾小球肾炎大鼠Syk/Ras/c-Fos信号通路的调控作用. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zyylylc201705038. 中药药理与临床. 2017;33(5):143–9. [高家荣, 吴溪, 宋俊梅, 等.芪藤消浊颗粒对慢性肾小球肾炎大鼠Syk/Ras/c-Fos信号通路的调控作用[J].中药药理与临床, 2017, 33 (5): 143-9.] [Google Scholar]
- 16.Gao JR, Wei LB, Song JM, et al. In-vitro and in-vivo study of the expression of the Syk/Ras/c-Fos pathway in chronic glomerulonephritis. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=5db74b3cea8d849b1e17d68f38492931. Mol Med Report. 2018;18(4):3683–90. doi: 10.3892/mmr.2018.9355. [Gao JR, Wei LB, Song JM, et al. In-vitro and in-vivo study of the expression of the Syk/Ras/c-Fos pathway in chronic glomerulonephritis[J]. Mol Med Report, 2018, 18(4): 3683-90.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.López-Hernández FJ, López-Novoa JM. Role of TGF-β in chronic kidney disease: an integration of tubular, glomerular and vascular effects. http://europepmc.org/abstract/MED/22105921. Cell Tissue Res. 2012;347(1):141–54. doi: 10.1007/s00441-011-1275-6. [López-Hernández FJ, López-Novoa JM. Role of TGF-β in chronic kidney disease: an integration of tubular, glomerular and vascular effects[J]. Cell Tissue Res, 2012, 347(1): 141-54.] [DOI] [PubMed] [Google Scholar]
- 18.Li ZY, Zhang M, Li XF, et al. Wnt/β-catenin pathway involved in the regulation of rat mesangial cell proliferation by adipose-derived mesenchymal stem cells. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=xbyfzmyxzz201611010. Chin J Cell Mol Immunol. 2016;32(11):1486. [Li ZY, Zhang M, Li XF, et al. Wnt/β-catenin pathway involved in the regulation of rat mesangial cell proliferation by adipose-derived mesenchymal stem cells[J]. Chin J Cell Mol Immunol, 2016, 32(11): 1486.] [PubMed] [Google Scholar]
- 19.Hnatyszyn A, Wielgus K, Kaczmarek-Rys M, et al. Interleukin-1 gene polymorphisms in chronic gastritis patients infected with Helicobacter pylori as risk factors of gastric cancer development. Arch Immunol Ther Exp. 2013;61(6):503–12. doi: 10.1007/s00005-013-0245-y. [Hnatyszyn A, Wielgus K, Kaczmarek-Rys M, et al. Interleukin-1 gene polymorphisms in chronic gastritis patients infected with Helicobacter pylori as risk factors of gastric cancer development[J]. Arch Immunol Ther Exp, 2013, 61(6): 503-12.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szulwach KE, Li XK, Smrt RD, et al. Cross talk between microRNA and epigenetic regulation in adult neurogenesis. J Cell Biol. 2010;189(1):127–41. doi: 10.1083/jcb.200908151. [Szulwach KE, Li XK, Smrt RD, et al. Cross talk between microRNA and epigenetic regulation in adult neurogenesis[J]. J Cell Biol, 2010, 189(1): 127-41.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amici C, la Frazia S, Brunelli C, et al. Inhibition of viral protein translation by indomethacin in vesicular stomatitis virus infection: role of eIF2α kinase PKR. Cell Microbiol. 2015;17(9):1391–404. doi: 10.1111/cmi.12446. [Amici C, la Frazia S, Brunelli C, et al. Inhibition of viral protein translation by indomethacin in vesicular stomatitis virus infection: role of eIF2α kinase PKR[J]. Cell Microbiol, 2015, 17(9): 1391-404.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henke JI, Goergen D, Zheng JF, et al. microRNA-122 stimulates translation of hepatitis C virus RNA. EMBO J. 2008;27(24):3300–10. doi: 10.1038/emboj.2008.244. [Henke JI, Goergen D, Zheng JF, et al. microRNA-122 stimulates translation of hepatitis C virus RNA[J]. EMBO J, 2008, 27(24): 3300-10.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nozaki T, Sasaki Y, Fukuda I, et al. Next-generation sequencingbased miRNA expression analysis in Parp1-deficient embryonic stem cell-derived exosomes. Biochem Biophys Res Commun. 2018;499(3):410–5. doi: 10.1016/j.bbrc.2018.03.073. [Nozaki T, Sasaki Y, Fukuda I, et al. Next-generation sequencingbased miRNA expression analysis in Parp1-deficient embryonic stem cell-derived exosomes[J]. Biochem Biophys Res Commun, 2018, 499(3): 410-5.] [DOI] [PubMed] [Google Scholar]
- 24.Adlakha YK, Saini N. Brain microRNAs and insights into biological functions and therapeutic potential of brain enriched miRNA-128. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=10.1097/HTR.0000000000000568. Mol Cancer. 2014;13(1):33. doi: 10.1186/1476-4598-13-33. [Adlakha YK, Saini N. Brain microRNAs and insights into biological functions and therapeutic potential of brain enriched miRNA-128 [J]. Mol Cancer, 2014, 13(1): 33.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuo WT, Yu SY, Li S, et al. MicroRNA-324 in human cancer: miR- 324-5p and miR-324-3p have distinct biological functions in human cancer. Anticancer Res. 2016;36(10):5189–96. doi: 10.21873/anticanres.11089. [Kuo WT, Yu SY, Li S, et al. MicroRNA-324 in human cancer: miR- 324-5p and miR-324-3p have distinct biological functions in human cancer[J].Anticancer Res, 2016, 36(10): 5189-96.] [DOI] [PubMed] [Google Scholar]
- 26.Gu CJ, Zhang MY, Sun WL, et al. Upregulation of miR-324-5p inhibits proliferation and invasion of colorectal cancer cells by targeting ELAVL1. Oncol Res. 2019;27(5):515–24. doi: 10.3727/096504018X15166183598572. [Gu CJ, Zhang MY, Sun WL, et al. Upregulation of miR-324-5p inhibits proliferation and invasion of colorectal cancer cells by targeting ELAVL1[J]. Oncol Res, 2019, 27(5): 515-24.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao H, Caflisch A. Discovery of dual ZAP70 and Syk kinases inhibitors by docking into a rare C-helix-out conformation of Syk. Bioorg Med Chem Lett. 2014;24(6):1523–7. doi: 10.1016/j.bmcl.2014.01.083. [Zhao H, Caflisch A. Discovery of dual ZAP70 and Syk kinases inhibitors by docking into a rare C-helix-out conformation of Syk [J]. Bioorg Med Chem Lett, 2014, 24(6):1523-7.] [DOI] [PubMed] [Google Scholar]
- 28.Kawakami Y, Kitaura J, Yao LB, et al. A Ras activation pathway dependent on Syk phosphorylation of protein kinase C. Proc Natl Acad Sci USA. 2003;100(16):9470–5. doi: 10.1073/pnas.1633695100. [Kawakami Y, Kitaura J, Yao LB, et al. A Ras activation pathway dependent on Syk phosphorylation of protein kinase C[J]. Proc Natl Acad Sci USA, 2003, 100(16): 9470-5.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Newbury LJ, Wang JH, Hung G, et al. Inhibition of Kirsten-Ras reduces fibrosis and protects against renal dysfunction in a mouse model of chronic folic acid nephropathy. Sci Rep. 2019;9:14010. doi: 10.1038/s41598-019-50422-7. [Newbury LJ, Wang JH, Hung G, et al. Inhibition of Kirsten-Ras reduces fibrosis and protects against renal dysfunction in a mouse model of chronic folic acid nephropathy[J]. Sci Rep, 2019, 9: 14010.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodríguez-Peña AB, Fuentes-Calvo I, Docherty NG, et al. Effect of angiotensin Ⅱ and small GTPase ras signaling pathway inhibition on early renal changes in a murine model of obstructive nephropathy. http://pubmedcentralcanada.ca/pmcc/articles/pmid/25101263. Biomed Res Int. 2014;2014:1–14. doi: 10.1155/2014/124902. [Rodríguez-Peña AB, Fuentes-Calvo I, Docherty NG, et al. Effect of angiotensin Ⅱ and small GTPase ras signaling pathway inhibition on early renal changes in a murine model of obstructive nephropathy [J]. Biomed Res Int, 2014, 2014: 1-14.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang JH, Newbury LJ, Knisely AS, et al. Antisense knockdown of Kras inhibits fibrosis in a rat model of unilateral ureteric obstruction. Am J Pathol. 2012;180(1):82–90. doi: 10.1016/j.ajpath.2011.09.036. [Wang JH, Newbury LJ, Knisely AS, et al. Antisense knockdown of Kras inhibits fibrosis in a rat model of unilateral ureteric obstruction [J].Am J Pathol, 2012, 180(1): 82-90.] [DOI] [PubMed] [Google Scholar]
- 32.Shimizu T, Tolcher AW, Papadopoulos KP, et al. The clinical effect of the dual-targeting strategy involving PI3K/AKT/mTOR and RAS/ MEK/ERK pathways in patients with advanced cancer. Clin Cancer Res. 2012;18(8):2316–25. doi: 10.1158/1078-0432.CCR-11-2381. [Shimizu T, Tolcher AW, Papadopoulos KP, et al. The clinical effect of the dual-targeting strategy involving PI3K/AKT/mTOR and RAS/ MEK/ERK pathways in patients with advanced cancer[J]. Clin Cancer Res, 2012, 18(8): 2316-25.] [DOI] [PubMed] [Google Scholar]
- 33.Huang HQ, Ni HF, Ma KL, et al. ANGPTL2 regulates autophagy through the MEK/ERK/Nrf-1 pathway and affects the progression of renal fibrosis in diabetic nephropathy. Am J Transl Res. 2019;11(9):5472. [Huang HQ, Ni HF, Ma KL, et al. ANGPTL2 regulates autophagy through the MEK/ERK/Nrf-1 pathway and affects the progression of renal fibrosis in diabetic nephropathy[J]. Am J Transl Res, 2019, 11 (9): 5472.] [PMC free article] [PubMed] [Google Scholar]
- 34.Liang K, Ting C, Yin SC, et al. Berberine suppresses MEK/ERKdependent Egr-1 signaling pathway and inhibits vascular smooth muscle cell regrowth after in vitro mechanical injury. Biochem Pharmacol. 2006;71(6):806–17. doi: 10.1016/j.bcp.2005.12.028. [Liang K, Ting C, Yin SC, et al. Berberine suppresses MEK/ERKdependent Egr-1 signaling pathway and inhibits vascular smooth muscle cell regrowth after in vitro mechanical injury[J]. Biochem Pharmacol, 2006, 71(6): 806-17.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.王 晶, 姜 辉, 高 家荣, et al. 原癌基因c-fos与肾脏疾病关系研究进展. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=syzdyzlzz201912025. 中华实用诊断与治疗杂志. 2019;33(12):1236–8. [王晶, 姜辉, 高家荣, 等.原癌基因c-fos与肾脏疾病关系研究进展[J].中华实用诊断与治疗杂志, 2019, 33(12): 1236-8.] [Google Scholar]
- 36.王 志程, 刘 铜华, 黄 粤. 复方中药糖耐康对高糖诱导大鼠系膜细胞ERK信号途径的影响. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zgzxyjhsbzz201112007. 中国中西医结合肾病杂志. 2011;12(12):1056–8. [王志程, 刘铜华, 黄粤.复方中药糖耐康对高糖诱导大鼠系膜细胞ERK信号途径的影响[J].中国中西医结合肾病杂志, 2011, 12(12): 1056-8.] [Google Scholar]


