Highlights
-
2•
Our results showed that this new chip had an important clinical value of patients with metastatic breast cancer.
-
2•
CTCs count showed good prospects in monitoring cancer prognosis and guiding future individualized treatment.
-
2•
We have manufactured a new microfluidic chip to isolate and identify CTCs and CTCs clusters with high throughput.
Keywords: Circulating tumor cells, Microfluidic chip, CTCs clusters, Breast cancer
Abstract
Background
Circulating tumor cells (CTCs) existing in peripheral blood can be used to predict the prognosis and survival of cancer patients. The study was designed to detect circulating tumor cells and circulating tumor single cell genes by applying microfluidic chip technology. It was used to explore the clinical application value in breast cancer.
Methods
We have developed a size-based CTCs sorting microfluidic chip, which contains a hexagonal array and a micro-pipe channel array to isolate and confirm both single CTCs and CTCs clusters. The sorting performance of the as-fabricated chip was tested by analyzing the clinical samples collected from 129 breast cancer patients and 50 healthy persons.
Results
In this study, the chip can detect different immunophenotypes of CTCs in breast cancer patients. It was found that the new microfluidic device had high sensitivity (73.6%) and specificity (82.0%) in detecting CTCs. By detecting the blood samples of 129 breast cancer patients and 50 healthy blood donors, it was found that the number of CTCs was not associated with clinical factors such as age, gender, pathological type, and tumor size of breast cancer patients (P > 0.05), but was associated with TNM staging of breast cancer, with or without metastasis (P < 0.005). There was a statistically significant difference in the number of CTCs between luminal A (ER+/PR+/HER2-) and HER-2+ (ER-/PR-/HER2+) (P < 0.05). The best cut-off level distinguished by CTC between the breast cancer patients and the healthy persons was 3.5 cells/mL, with 0.845 for AUC-ROC, 0.790–0.901 for 95% CI, 73.6% for sensitivity, and 82% for specificity (P = 0.000). The combination of CTC, CEA, CA125 and CA153 can provide more effective breast cancer screening.
Conclusions
The CTCs analysis method presented here doesn't rely on the specific antibody, such as anti-EpCAM, which would avoid the missed inspection caused by antibody-relied methods and offer more comprehensive biological information for clinical breast cancer diagnosis and treatment.
Introduction
Breast cancer is one of the most common malignancies among female patients and the main cause of cancer-related deaths [1]. Metastasis is the leading cause of death in most breast cancer patients. Epithelial metastasis is thought to involve a series of sequential steps: primary tumor formation and growth, epithelial to mesenchymal transition (EMT) and intravasation, hematogenous spread, extravasation and secondary tumor formation, where mesenchymal to epithelial transition (MET) culminates as an epithelial metastatic deposit of cell proliferation [2,3]. Circulating tumor cells (CTCs) are tumor cells circulated in the peripheral blood that have been shed or migrated into the vasculature from the primary or metastatic lesions. They can lead to metastasis from primary or other lesions (seeding hypothesis) in distant organs, which is considered to be the primary responsibility of cancer-related deaths [4]. Therefore, detection of CTCs could be used for early diagnosis of tumor metastasis, planning of individualized treatment, and prognostic judgment, which has the advantages of repeatability, simple sampling, and non-invasiveness [5,6]. Compared with single CTCs, CTCs clusters are even rare in the circulation, but they are more valuable for predicting metastasis than single CTCs [7]. However, rarity and heterogeneity of CTCs make them extremely difficult to be detected and utilized as a biomarker. Therefore, it is imminent to develop a highly efficient and highly sensitive method to analyze CTCs.
Although the detection and analysis of CTCs has important clinical value, the diversity of rapidly evolving detection methods increases the difficulty of practical application. As the detection method for CTCs approved by the USA FDA for clinical application, CellSearch system is difficult to identify CTCs lacking Ep-CAM epithelial markers, which may lead to a certain degree of false negatives [8]. The CTCs detection technology has emerged as a research hotspot for clinical application. The microfluidic chip has the advantages of fast analysis, portability, low reagent consumption, which has been widely used in CTCs sorting and enrichment [9], [10], [11], [12]. In recent years, a large number of platforms for detecting CTCs have emerged [13], [14], [15], [16], but most of them are in the early stage of research and lack of large-scale clinical trials. The operation steps are tedious, the preparation is complex, additional surface modification is needed, separation efficiency is low, and blockage is easy. It needs to rely on other expensive and complex experimental equipment, such as laser detection.
Size-based microfluidic chip has the advantages of low cost, simple operation and high throughput, which greatly improves the capture efficiency of cancer cells and has higher development potential [17], [18], [19]. The PDMS chip includes an inlet, a mass filter area, a single cell filtration area, and an outlet. In the present study, we designed a microfluidic chip by integrating hexagonal micro-columns to capture both CTCs and CTCs clusters from breast cancer patients with high sensitivity and selectivity, which could be potentially developed for CTCs-based early diagnosis, disease progression, prognostic prediction, and the efficacy of breast cancer treatment. Because the clinical treatment response and survival of different molecular sub-types of breast cancer are quite different, the molecular typing of breast cancer has also been highly valued. The presented method could also be employed in the study of the molecular markers and molecular typing of breast cancer as the clinical treatment response and survival of different molecular subtypes of breast cancer vary a lot, which is promising in clinical cancer treatment and prognosis.
Materials and methods
Experimental Materials and Instruments was showed in Supporting Information, Table S1.
Blood processing
Blood cells were pre-processed by centrifugation using Ficoll-Paque PLUS (GE). The details in Supporting Information, Fig. S1.
Patients
There were 129 breast cancer patients and 50 healthy people, who received assessment of their disease by CTCs analysis from March 2017 to May 2018. Clinical data of patients, including Age, gender, pathological diagnosis and radiotherapy and chemotherapy. The Ethics Review Committee of the Affiliated Hospital of Nantong University approved the research protocol. The number is 2014–049.
Chip design and fabrication
The device was made of a PDMS chip containing micro channels bonded to glass slide. Si master with a microchannel structure was manufactured by photolithography, wet etching and deep reactive ion etching (DRIE). The Si master was exposed to trichloro-silane (1H, 1H, 1H, 2H-perfliuorooctyl) vapor under vacuum overnight, before PDMS molding for the PDMS layer's release. After being degassed, the PDMS was poured on the Si mold and cured in an oven at 75 °C for about 1 h. After curing, the PDMS was peeled from the Si mold. Finally, the layers of microfluidic chips were obtained by fixing PDMS on clean glass slide by plasma treatment. The specific production process is shown in Fig. S2.
Cell culture and spiking
Human breast cancer SKBR3, MDAMB-231 and MCF-7 cell lines were obtained from the Cell Bank of the Chinese Academy of Sciences (shanghai, China). MCF-7 cells were cultured in RPMI medium 1640 containing 10% (v/v) FBS. MDA-MB-231 and Sk-Br-3 cells were cultured in DMEM containing 10% (v/v) FBS. Using 0.25% trypsin containing EDTA, prior to testing, cells of known concentration was added to PBS or healthy donors’ samples. Use a Count star automated cell counter to measure cell concentration and diameter and the experiment were repeated at least three times.
Use microfluidic chip for CTC detection and identification
After the blood sample was processed, tumor cells were detected and identified by immunofluorescence staining. All the processes were done directly in the chip. Firstly, after the processed blood sample entered the chip, the chip was washed with 200 μL of PBS containing 0.05% Tween. Next, added 20 μl 0.2% Triton X-100 PBS to the chip and incubated for 10 min. After that, the chip was rinsed with 200 μL of wash buffer. Then treated with 20 μL of PBS containing 1% BSA for 10 min, then added phycoerythrin-conjugated anti-CD45 antibody, and isothiocyanate-conjugated anti-CK antibody to immunostain the cells and 4′−6-di mid −2-Phenylindole was incubated at 37 °C for 40 min. Finally, a washing buffer was added to the chip to remove the unbound antibody. The inverted microscope (IX51; Olympus) was connected to the image analysis software (DP controller; Olympus). CTCs were identified by positive staining for both phycoerythrin-conjugated cytokeratin (CK-8, 18, and 19) and double stranded DNA (DAPI), and CD45 negative staining (CK+/DAPI+/CD45-). The process of cell capture in the microfluidic chip was showed in Fig. S3.
Statistical analysis
Use GraphPad Prism 5 for statistical analysis. Regression analysis was carried out to evaluate the accuracy. Mann Whitney test was used in two independent samples. All tests were double-sided and performed at a 5% level of significance. The receiver operating characteristic (ROC) curve of the receiver is introduced to analyze the sensitivity and feasibility of CTC through SPSS software (version 20.0, SPSS Inc., Chicago, IL).
Results and discussion
Design and profile of the size-based microfluidic chip
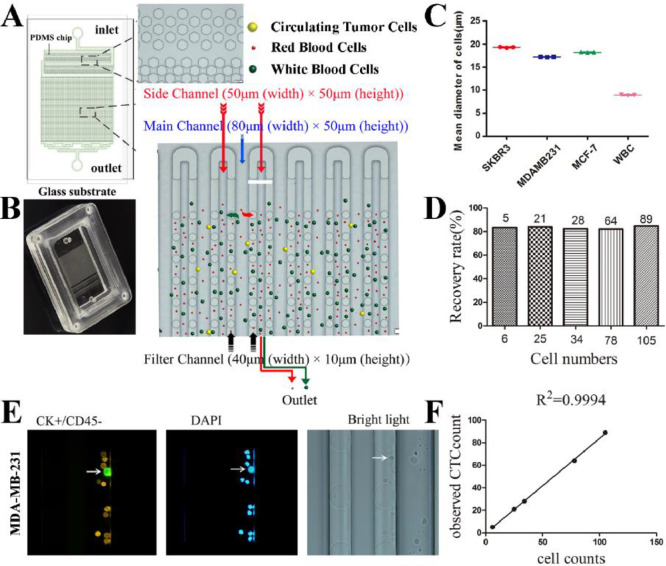
As shown in (Fig. 1A), the chip contained inlet, cell intercept area, and outlet. The inlet and the outlet were connected by pipeline that are branched step by step, the solution can be evenly distributed in the chip. The filtration microchannels contained two layers of hexagonal columns that filtered impurities and cell clusters. The width of the first and second narrow channel is 50 μm and 20 μm, respectively. The single cell filtration area contains 30 main channels and 31 side channels. The separation distance and diameter of each continuous cylinder are 100 μ m and 40 μ m respectively.
Fig. 1.
Design and operation of the size-based microfluidic device and Cell lines staining and test for capture rates. (A) Diagrammatic sketch of the microfluidic chip: the inlet, the filtration area, the Side channel, PDMS support pillars, and the outlet. The illustration shows the structure of the bulk filtration area and single cell filtration area under a microscope. The isolation strategy of the microfluidic chip (lower right). (B) Physical map of the micro-fluidic chip. (C) Three repeated gauges for the mean diameter of SKBR3, MDA-MB-231, MCF-7 and normal lymphocytes. (D) Plot of MDA-MB-231 cell recovery with the flow rate of 10 mL/h. (E) staining of MDA-MB-231 cells and leukocytes. (F) Each point represents the ratio of observed tumor cells to the expected number of cells.
The dimensions of the device are: Main microchannel 80 μm (width) × 50 μm (height), side microchannel 50 μm (width) × 50 μm (height), filter microchannel 40 μm (width) × 10 μm (height). The principle of microfluidic chip capturing cells is shown in Fig. 1A (lower right). As the sample flowed into the chip under vacuum, impurities and cell clusters were intercepted in the filtering area, where larger tumor cells were captured in the chip under the lateral pressure of fluid in the main channels, while smaller cells such as leukocytes was discharged into the waste liquid pool from the outlet through the side channel. The image of the microfluidic chip is shown in Fig. 1B.
Chip optimization
The diameter of three breast cancer cell strains (SKBR3, MCF-7 and MDAMB231) and leukocytes was measured by Countstar Automated Cell Counter, and their mean diameter was 19.26 ± 0.31, 18.19 ± 0.28, 17.13 ± 0.26 and 8.93 ± 0.19 μm, respectively (Fig. 1C). Cytokeratin (CK) and DAPI were used as the confirming reagents. Images of different cell lines were collected under a fluorescent microscope (Fig. S4). We spiked the smallest cells (MDA-MB-231) (6, 25, 34, 78, 105 cells/mL) into samples from healthy persons and captured them using the size-based microfluidic chip. The average values of each concentration were measured three times. The cells were stained with specific antibodies to identify breast cancer cells and white blood cells. As shown in Fig. 1E, the immuno-phenotype of MDA-MB-231 cells was CK+/CD45-/DAPI+, and the leukocytes was CK-/CD45+/DAPI+. The recovery rate (define it) of cancer cells was calculated by using 10 mL/h flow rate according to the results of staining labeling, and the standard curve was made. As shown in Fig. 1D, the recovery rate of the chip is above 82% for different number of cancer cells. The linear regression curve between the number of tumor cells added and the number of tumor cells detected by the chip shows a good linear relationship (R2 = 0.9994) (Fig. 1F). which showed a potential clinical utility.
Identification of CTCs from breast cancer patients using the microfluidic device
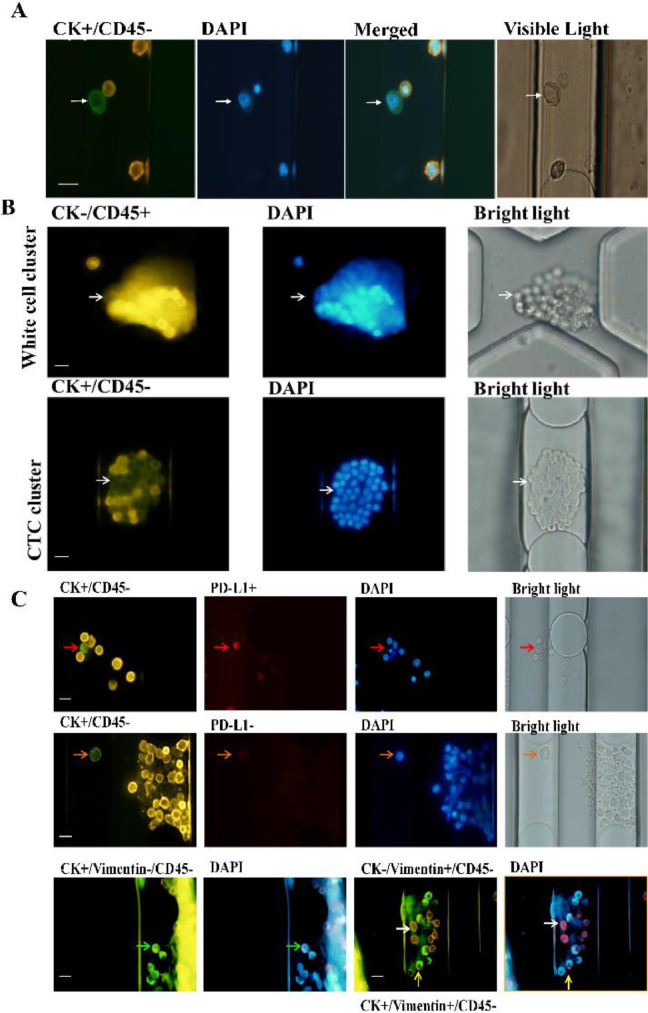
The size-based microfluidic chip can not only catch CTCs but also CTCs clusters. It also enables simultaneous isolation and confirmation of tumor cells in the same microfluidic device. The confirmation of the presence of CTCs clusters in blood suggests that this method paves a new way in CTCs evaluation to illustrate the diagnostic value of CTCs clusters [20], as studies have found that CTCs clusters may have greater metastatic potential than individual CTCs, and cells within the clusters may evade immunocyte attack and can survive longer [21]. Additionally, this may have a potential clinical relevance to predicting the likelihood of long-term recurrence. The chip was further applied to capture various types of cells from the samples of the breast cancer patients, using CK-FITC, DAPI and leukocyte marker (CD45-PE) as the confirmed reagents. CTCs in the blood samples of the breast cancer patients were marked as CK+/DAPI+/CD45- (Fig. 2A). A few CD45+ cell clusters and CTCs clusters were also catched in the bulk filtration area of the chip (Fig. 2B). In the next work, our team will be sequencing individual and aggregated CTCs from a team of breast cancer patients, analyze their different metastatic potentials, and further investigate the effects of individual and aggregated CTCs on tumor survival.
Fig. 2.
CTCs staining results of breast cancer patients. (A) CTCs in the blood samples of the breast cancer patients were marked as CK+/DAPI+/CD45-. (B) Representative images of white cell cluster (up) staining with CK- (green)/ CD45+ (orange) /DAPI+ (nuclei, blue); CTCs cluster (down) staining with CK+ (green) /CD45- (orange) /DAPI+ (nuclei, blue), isolated by the chip. Scale bars 20 μm. (C) Typical images of different types of cells tested include CK+/PDL-1+/CD45- (red arrow), CK+/PDL-1-/CD45- (orange arrow), CK+/Vimentin-/CD45- (green arrow), CK-/Vimentin+/CD45- (white arrow), CK+/Vimentin+/CD45- (yellow arrow). Scale bars 20 μm. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Because of the heterogeneity of CTCs and EMT, some epithelial markers will be down-regulated or disappeared in this process. It has been reported that the beneficiary can be screened by detecting the expression of PD-L1 on CTCs. Therefore, the capture of CTCs with different phenotypes was validated by using vimentin and PD-L1. Typical images of different types of cells detected by breast cancer patients include CK+/PDL-1+/CD45- (red arrow), CK+/PDL-1-/CD45-(orange arrow), CK+/Vimentin-/CD45- (green arrow), CK-/Vimentin+/CD45-(white arrow), CK+/Vimentin+/CD45- (yellow arrow). As showed in Fig. 2C, the chip can detect different immunophenotypes of CTCs in breast cancer patients. Detection of CTCs by multiple markers has great significance to the classification and identification of CTCs. Further analysis of its practical clinical significance is needed to expand clinical samples.
CTC has been widely used in the study of breast cancer and is considered as a prognostic biomarker of breast cancer metastasis. CTCs can be detected in a dynamic real-time manner without being affected by tumor heterogeneity, thus providing more comprehensive information. Analysis of CTCs has achieved rapid development in early diagnosis, efficacy evaluation, prognosis judgment, individualized treatment and disease monitoring of breast cancer. Thus, the urgent task at present is to establish a reliable strategy to analyze CTCs [22].
In this work, we continue to study the prognostic value of breast cancer CTC. Detected the blood samples of 129 breast cancer patients and 50 healthy blood donors, the capability of size-based microfluidic device for capturing CTC in breast cancer patients was evaluated. Clinical data of patients was shown in Table S2. It was found that the number of CTCs was not associated with clinical factors such as age, gender, pathological type, and tumor size of breast cancer patients (P > 0.05), but was associated with TNM staging of breast cancer, with or without metastasis (P < 0.005).
Application of microfluidic chips in breast cancer patients
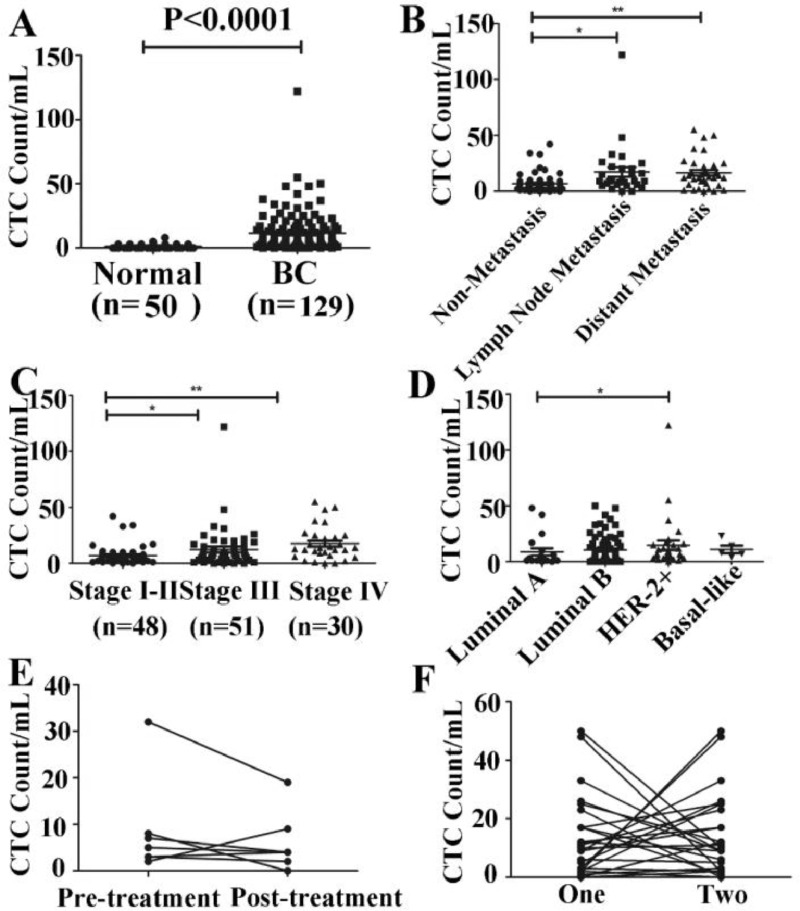
Further analysis of the relationship between CTCs levels and the clinic-pathological features in breast cancer patients showed that the CTCs level in the breast cancer patients was significantly higher than that in the control group (11.61±15.21/mL vs 1.14±1.62/mL, P < 0.0001) (Fig. 3A). Metastasis is one of the prime reasons of death in tumor patients. Statistical analysis of the impact of metastasis on the number of CTCs showed that the number of CTCs in the peripheral blood of patients with lymph node and distant metastases was significantly higher than that in patients without metastasis (P < 0.005) (Fig. 3B). With respect to TNM stages, the statistical results showed that the CTCs level was 1.50±7.78/mL in stage I, 9.00±11.60/mL in stage II, 10.00±7.86/mL in stage III, and 21.00±25.73/mL in stage IV, showing statistically significant differences between breast cancer patients with different stages (P < 0.05) (Fig. 3C). The CTCs level in patients with stage IV breast cancer was significantly higher than that in stage I-II (P < 0.0005). The result of our study showed that the increased CTCs number had a predictive value for tumor metastasis, and that the number of CTCs was positively correlated with the TNM stage. The number of CTCs was the highest in the fourth stage and the least in the first stage. This may mean that the number of CTCs can be used to assess the severity of breast cancer. Study has also shown that the higher the positive ratio of CTCs in peripheral blood of patients, the greater the possibility of tumor metastasis recurrence [23]. Traditional tumor pathological staging (such as TNM staging, including the tumor appearance, infiltration depth, lymph node metastasis) cannot be underestimated for predicting tumor recurrence and metastasis, and is a clinically mature risk assessment index. However, because breast cancer is a heterogeneous tumor, there are great differences in histomorphology, immunophenotype, biological behavior and therapeutic response.
Fig. 3.
Correlations between CTCs count and clinical features of breast cancer patients. (A) The distribution of CTC in 129 breast cancer patients and 50 healthy was detected by microfluidic chip. (B) The quantity of CTCS in three transfer states (**) indicates P < 0.05. (C) The pathological type distribution of CTC in breast cancer patients. Each data point represents the participant's CTC count, while a black horizontal line represents the group median. (*, ** means P < 0.05). (D) CTCs enumeration was associated with the molecular subtyping of breast cancer patients. (E) Changes in the number of CTCs before and after surgery. (F) Changes in the number of CTCs during two treatment.
Based on the expression change of ER, PR, HER-2 and Ki-67, breast cancer can be classified into four molecular subtypes. Luminal A (ER+/PR+/HER2-), luminal B (ER+/PR+/HER2+), HER-2+ (ER-/PR-/HER2+) and Basal-like (ER-/PR-/HER2-). To measure the correlation between CTCs and molecular type in breast cancer, we performed four types of statistical analysis on 129 breast cancer patients. As shown in Fig. 3D, there was a statistically significant difference in the number of CTCs between luminal A (ER+/PR+/HER2-) and HER-2+ (ER-/PR-/HER2+) (P<0.05). Molecular typing of breast cancer in our study showed that HER2-positive patients had more CTCs than luminal A patients, indicating that the prognosis of HER2 positive patients is poor. This is consistent with the report, and the study have shown that, for breast cancer patients with HER2-positive, regardless of the treatment, CTCs counts have prognostic value [24]. As shown in Table S2, CTCs count had no significant correlation with different expression levels of ER, PR, HER2 and Ki-67 and the molecular type.
Further study the effect of treatment on the number of CTCs, the number of CTCs was observed in 6 preoperative and postoperative breast cancer patients. It was found that the number of CTCs was decreased after surgery (Fig. 3E). At the same time, the number of CTCs in 18 breast cancer patients was observed in two consecutive treatment (Fig. 3F). The results showed that the number of CTCs decreased during continuous treatment, suggesting that the treatment is effective, and patients with elevated CTCs suggest that the risk of recurrence and metastasis is increased or has occurred.
Analysis of the ROC, determination of the critical value and evaluation of the diagnostic efficacy
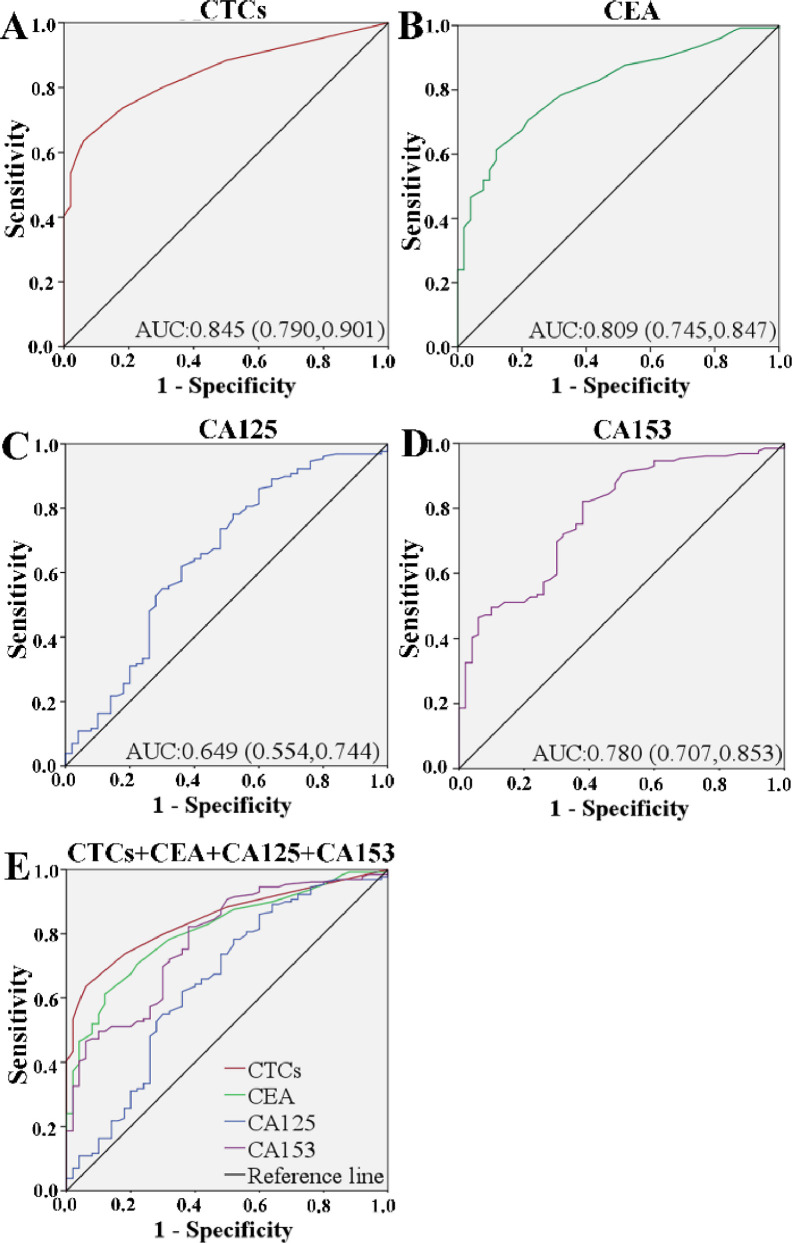
In order to study the characteristics of CTC as a potential biomarker for breast cancer, data on 129 breast cancer patients and 50 healthy controls were analyzed by receiver operating characteristic (ROC) curve and area under the ROC curve (AUC). The best cut-off level distinguished by CTC between the breast cancer patients and the healthy persons was 3.5 cells/mL, with 0.845 for AUC-ROC, 0.790–0.901 for 95% CI, 73.6% for sensitivity, and 82% for specificity (P = 0.0001) (Fig. 4A). Therefore, the cutoff value was defined as 3.5 cells/mL (AUC = 0.845), CTC positive patients were defined as CTC≥3.5 in 1 mL of peripheral venous blood. Compared with the traditional clinical indicators of CEA (Fig. 4B), CA125 (Fig. 4C) and CA153 (Fig. 4D), the diagnostic value of CTCs was significant (Fig. 4E). Interestingly, there is increasing evidence that combined testing can improve diagnostic accuracy. In this study, we investigated whether the combination of CTC, CEA, CA125 and CA153 can provide more effective breast cancer screening. As shown in Table S3, the sensitivity (73.6%) and specificity (82.0%) of CTCs is higher than the other three. The sensitivity of CTCs was 73.6%, at a higher level than CEA (70.5%) and CA125 (62.0%). More importantly, combination of these four indicators increased the sensitivity to 99.5%, suggestion that CTCs combined with CEA and CA125 and CA153 could be used to enhance the diagnostic efficiency.
Fig. 4.
The ROC curve of CTCs counts (A), CEA (B), CA125 (C), CA153 (D) and CTC + CEA + CA125 + CA153 (E) to discriminate breast cancer patients from healthy persons.
The correlations between the number of CTCs and traditional tumor markers
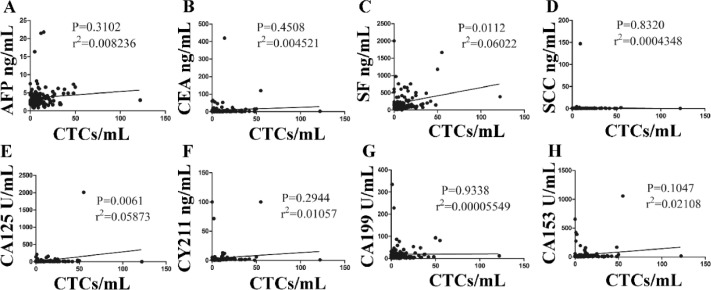
CEA, CA125 and CA153 are commonly used to assist in the diagnosis of breast cancer. However, they are not highly sensitive and specific, especially in early diagnosis of breast cancer. In the research, we investigated whether the combination of CTCs, CEA, CA125 and CA153 can provide more effective screening for breast cancer. Compared with the CEA, CA125 and CA153, the diagnostic performance of CTCs was outstanding (Fig. 5). We found that CTCs count was not significantly correlated with the level of traditionally used markers of AFP, CEA, SCC, CA153, CA199, CY211 and CA152 (P >0.05) (Fig. 5A, B, D–F, H). Only SF and CA125 levels were correlated with CTCs counts (P <0.05) (Fig. 5C and G).
Fig. 5.
Correlations between CTCs counts and the traditional tumor markers. The interrelation between CTCs and AFP, CEA, SF, CA199, CY211, CA125, CA153 (A-H).
Conclusions
In this work, we demonstrated a novel microfluidic chip for separation of CTCs and CTCs clusters from patient peripheral blood. The microfluidic device does not rely on antibody capture, can capture many types of CTCs, and provide more comprehensive medical information. As long as the target is larger than blood cells, the device can be extended to separate CTCs from other cancers and separate other liquid biopsy sources. In addition, some other large cell tumors, such as lung cancer and liver cancer, can also be detected by liquid biopsy of CTCs in our equipment. A potentially application of our technology is in the separation of CTCs clusters. CTCs clusters are more aggressive than single CTCs. Our results showed that this new chip had an important clinical value in the diagnosis, treatment and prognostic prediction of patients with metastatic breast cancer with remarkable specificity and sensitivity. CTCs count showed good prospects in monitoring cancer prognosis and guiding future individualized treatment.
Associated content
Additional file: Fig. S1. Compared the two blood pre-processing methods of Ficoll gradient and Red Blood Cell Lysates. Fig. S2. Schematic illustration of microfluidic chip fabrication. Fig. S3. The process of cell capture using microfluidic chip. Fig. S4. Images of different cell lines were collected under a fluorescent microscope. Scale bars 20 μm. Epithelial marker (CK-FITC) and DAPI were used as the confirming reagents. Images of different cell lines were collected under a fluorescent microscope. Table S1. The materials in Supporting Information. Table S2. Basic characteristics of the cancer patients subjected to CTCs analysis. Table S3. ROC curves of a combination of CTCs, CEA, CA125 and CA153 to discriminate breast cancer.
Funding
This work was supported by the National Natural Science Foundation of China (Program No. 81472751); Jiangsu Provincial Funds for Six Categories of Top Talents (Program No.WS-066); The Technology Project of Nantong (No. MS12017008-1).
Authors contributions
Chunping Jia and Hui Cong designed the study and obtained funding for the study. Xiaofen Zhang was responsible for experimental research, collected data, analyzes and drafted the manuscript. Wanlei Gao andYanmin Wang designed and facture of microchip. All authors contributed to data interpretation and preparation of the final manuscript. All authors approved the final manuscript.
Availability of data and materials
The datasets generated during and/or analysed during the present study are not publicly available, owing to confidentiality reasons, but anonymized versions may be available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The study was approved by the Affiliated Hospital of Nantong University Ethics Committee (reference 2014-049).
Consent for publication
Not applicable.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2020.100959.
Appendix. Supplementary materials
References
- 1.Chen W., Zheng R., Baade P.D., Zhang S., Zeng H., Bray F., He J. Cancer statistics in China. CA Cancer J. Clin. 2015;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 2.Lee A., Park J., Lim M., Sunkara V., Kim S.Y., Kim G.H., Kim M.H., Cho Y.K. All-in-one centrifugal microfluidic device for size-selective circulating tumor cell isolation with high purity. Anal. Chem. 2014;86(22):11349–11356. doi: 10.1021/ac5035049. [DOI] [PubMed] [Google Scholar]
- 3.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Haber D.A., Velculescu V.E. Blood-based analyses of cancer: circulating tumor cells and circulating tumor DNA. Cancer Discov. 2014;4(6):650–661. doi: 10.1158/2159-8290.CD-13-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harb W., Fan A., Tran T., Danila D.C., Keys D., Schwartz M., Zanetti C.L. Mutational analysis of circulating tumor cells using a novel microfluidic collection device and qPCR assay. Transl. Oncol. 2013;6(5):528–538. doi: 10.1593/tlo.13367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Attard G., Swennenhuis J.F., Olmos D., Reid A.H.M., Bono J.S.D. Characterization of ERG, AR and PTEN gene status in circulating tumor cells from patients with castration-resistant prostate cancer. Cancer Res. 2009;69(7):2912–2918. doi: 10.1158/0008-5472.CAN-08-3667. [DOI] [PubMed] [Google Scholar]
- 7.Aceto N., Bardia A., Miya-moto D.T., Donaldson M.C., Wittner B.S., Spencer J.A. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell. 2014;158(5):1110–1122. doi: 10.1016/j.cell.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farace F., Massard C., Vimond N., Drusch F., Jacques N., Billiot F., Laplanche A., Chauchereau A., Lacroix L., Planchard D., Le Moulec S., André F., Fizazi K., Soria J.C., PBrJ Vielh. A direct comparison of cellsearch and ISET for circulating tumour-cell detection in patients with metastatic carcinomas. Br. J. Cancer. 2011;105(6):847–853. doi: 10.1038/bjc.2011.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whitesides G.M. The origins and the future of microfluidics. Nature. 2006;442(7101):368–373. doi: 10.1038/nature05058. [DOI] [PubMed] [Google Scholar]
- 10.Todenhöfer T., Park E.S., Duffy S., Deng X., Jin C., Abdi H., Ma H., Black P.C. Microfluidic enrichment of circulating tumor cells in patients with clinically localized prostate cancer. Urol. Oncol. 2016;34:483.e9–483.e16. doi: 10.1016/j.urolonc.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Danova M., Torchio M., Mazzini G. Isolation of rare circulating tumor cells in cancer patients: technical aspects and clinical implications. Expert. Rev. Mol. Diagn. 2011;11:473–485. doi: 10.1586/erm.11.33. [DOI] [PubMed] [Google Scholar]
- 12.Fan X., Jia C., Yang J., Li G., Mao H., Jin Q., Zhao J. A microfluidic chip integrated with a high-density PDMS-based microfiltration membrane for rapid isolation and detection of circulating tumor cells. Biosens. Bioelectron. 2015;71:380–386. doi: 10.1016/j.bios.2015.04.080. [DOI] [PubMed] [Google Scholar]
- 13.Nagrath S., Sequist L.V., Maheswaran S., Bell D.W., Irimia D., Ulkus L., Smith M.R., Kwak E.L., Digumarthy S., Muzikansky A., Ryan P., Balis U.J., Tompkins R.G., Haber D.A, Toner M. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature. 2007;450:1235–1239. doi: 10.1038/nature06385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schiro P.G., Zhao M., Kuo J.S., Schneider T., Koehler K.M., Sabath D.E., Chiu D.T. Sensitive and high-throughput isolation of rare cells from peripheral blood with ensemble-decision aliquot ranking. Angew. Chem. Int. Ed. 2012;51:4618–4622. doi: 10.1002/anie.201108695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murlidhar V., Zeinali M., Grabauskiene S., Ghannadrezaie M., Wicha M.S., Simeone D.M., Ramnath N., Reddy R.M., Nagrath S. A radial flow microfluidic device for ultra-high-throughput affinity-based isolation of circulating tumor cells. Small. 2015;10:4895–4904. doi: 10.1002/smll.201400719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson E.S., Anand R.K., Chiu D.T. Improved detection by ensemble-decision aliquot ranking of circulating tumor cells with low numbers of a targeted surface antigen. Anal. Chem. 2015;87:9389–9395. doi: 10.1021/acs.analchem.5b02241. [DOI] [PubMed] [Google Scholar]
- 17.Gao W., Yuan H., Jing F., Wu S., Zhou H., Mao H., Jin Q, Zhao J., Cong H., Jia C. Analysis of circulating tumor cells from lung cancer patients with multiple biomarkers using high-performance size-based microfluidic chip. Oncotarget. 2016;8:12917–12928. doi: 10.18632/oncotarget.14203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou J., Kulasinghe A., Bogseth A., O'Byrne K., Punyadeera C., Papautsky I. Isolation of circulating tumor cells in non-small-cell-lung-cancer patients using a multi-flow microfluidic channel. Microsyst Nanoeng. 2019;5:8. doi: 10.1038/s41378-019-0045-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khamenehfar A., Beischlag T.V., Russell P.J. Label-free isolation of a prostate cancer cell among blood cells and the single-cell measurement of drug accumulation using an integrated microfluidic chip. Biomicrofluidics. 2015;9(6) doi: 10.1063/1.4934715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gkountela S., Castro-Giner F., Szczerba B.M, Vetter M., Landin J., Scherrer R., Krol I., Scheidmann M.C., Beisel C., Stirnimann C.C., Kurzeder C., Schwarz V.H., Rochlitz C., We-ber W.P., Aceto N. Circulating tumor cell clustering shapes DNA methylation to enable metastasis seeding. Cell. 2019;176:98–112. doi: 10.1016/j.cell.2018.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheung K.J., Padmanaban V., Silvestri V., Schipper K., Cohen J.D., Fairchild A.N., Gorin M.A., Verdone J.E., Pienta K.J., Bader J.S., Ewald A.J. Polyclonal breast cancer metastases arise from collective dissemination of keratin 14-expressing tumor cell clusters. Proc. Natl. Acad. Sci. U.S.A. 2016;113:E854–E863. doi: 10.1073/pnas.1508541113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tseng J.Y., Yang C.Y., Liang S.C., Liu R.S., Jiang J.K., Lin C.H. Dynamic changes in numbers and properties of circulating tumor cells and their potential applications. Cancers Basel. 2014;6:2369–2386. doi: 10.3390/cancers6042369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee S.H., Lee J.K., Ahn M.J., Kim D.W., Sun J.M., Keam B., Kim T.M., Heo D.S., Ahn J.S., Choi Y.L., Min H.S., Jeon Y.K., Park K. Vandetanib in pretreated patients with advanced non-small cell lung cancer-harboring RET rearrangement: a phase II clinical trial. Ann. Oncol. 2017;28:292. doi: 10.1093/annonc/mdw559. [DOI] [PubMed] [Google Scholar]
- 24.Jiang Z.F., Cristofanilli M., Shao Z.M., Tong Z.S., Song E.W., Wang X.J., Liao N., Hu X.C., Liu Y., Wang Y., Zeng L., Zhang M. Circulating tumor cells predict progression-free and overall survival in Chinese patients with metastatic breast cancer, HER2-positive or triple-negative (CBCSG004): a multicenter, double-blind, prospective trial. Ann. Oncol. 2013;24:2766–2772. doi: 10.1093/annonc/mdt246. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the present study are not publicly available, owing to confidentiality reasons, but anonymized versions may be available from the corresponding author on reasonable request.