Highlights
-
•
Down-regulated expressions of PDCD4-AS1 and IQGAP2were observed in TNBC.
-
•
Over-expressed miR-10b-5p was detected in TNBC.
-
•
PDCD4-AS1/IQGAP2 inhibits proliferation, migration and invasion of TNBC cells.
-
•
miR-10b-5p increases proliferation, migration and invasion of TNBC cells.
-
•
PDCD4-AS1 inhibits TNBC via acting as a ceRNA for miR-10b-5p.
Keywords: Triple-negative breast cancer, PDCD4-AS1, miR-10b-5p, IQGAP2, Proliferation, Invasion, Migration, Apoptosis
Abstract
Objective
Mounting evidence demonstrates that long non-coding RNA (lncRNA) is dysregulated in breast cancers. This study was designed to detect the influences and regulatory mechanism of lncRNA PDCD4-AS1 in triple-negative breast cancer (TNBC).
Methods
qRT-PCR and Western blot were utilized to investigate the expression levels of PDCD4-AS1, miR-10b-5p and IQGAP2 in TNBC tissues and cells. Online software and luciferase reporter gene system were employed to testify the interactions among these molecules. Loss and gain of function of PDCD4-AS1, miR-10b-5p or IQGAP2 were performed before MTT and colony formation assay, TUNEL staining in addition to Transwell and scratch assays were applied to measure the cell biological functions.
Results
In this work, PDCD4-AS1 and IQGAP2 were lowly expressed while miR-10b-5p was strongly expressed in TNBC tissues and cells. PDCD4-AS1 or IQGAP2 overexpression effectively attenuated TNBC cell proliferation, migration and invasion, and increased the apoptosis rate, while this effect was abandoned in response to miR-10b-5p mimics transfection. miR-10b-5p bound to IQGAP2 and acted as a downstream target of PDCD4-AS1.
Conclusion
Our findings identified lncRNA PDCD4-AS1 as a tumor suppressor in TNBC by regulating IQGAP2 expression via miR-10b-5p, giving a novel insight into the regulatory mechanism of PDCD4-AS1 in the pathogenesis of TNBC.
Introduction
Breast cancer persists as the most frequently diagnosed malignancy and the leading cause of cancer death among females globally [1]. Incident rates of breast cancer have been rising over the last decades mainly in those countries undergoing demographic and epidemiologic transitions [2]. At present, breast cancer has been recognized as a heterogeneous disease which can be categorized into different pathological subtypes according to the status of estrogen receptor, human epidermal growth factor receptor 2 and progesterone receptor [3,4]. Triple-negative breast cancer (TNBC) is the most aggressive subtype of breast cancer, characterized by deficiencies of estrogen receptor, human epidermal growth factor receptor 2 and progesterone receptor, and high recurrence and mortality rates [5], [6], [7]. Investigation into the molecular mechanisms involved in TNBC progression is helpful for developing endocrine therapies to improve the survival of TNBC patients.
Long non-coding RNAs (lncRNAs), a heterogeneous class of transcripts with a minimum length of 200 bases and limited protein-coding potential, regulate biological processes by diverse mechanisms [8]. LncRNAs can drive cancer phenotypes through interactions with DNA, chromatin, signaling, regulatory proteins, and a variety of cellular RNA species [9]. Recently, the competing endogenous RNA (ceRNA) network, consisting of transcripts that cross-regulate each other by competing for shared microRNAs (miRNAs), is suggested to be a widespread form of post-transcriptional regulation in cancers [10]. MiRNAs are short non-coding RNAs of 18 ∼ 25 nucleotides that can bind to mRNA and inhibit mRNA function either by cleavage and degradation of the target mRNA or by repressing protein translation [11]. The emerging role of lncRNAs has been demonstrated in breast cancers [12], [13], [14], but lncRNA-mediated ceRNA networks that can predict the outcome of breast cancer are still lacking [15]. The regulatory mechanisms of lncRNAs in the specific subtype of breast cancer, TNBC, also remain largely unknown.
A previous study has identified lncRNA PDCD4-AS1 as a tumor suppressor gene in TNBC without elucidating its regulatory mechanism [16]. In the present work, we found that PDCD4-AS1 hindered the proliferation, migration and invasion of TNBC cells. Further mechanistic investigations indicated that PDCD4-AS1 upregulated IQGAP2 expression by competitively binding to miR-10b-5p, which finally led to the inhibition on TNBC progression. IQGAP2 is a member of the IQ motif containing GTPase activating protein family, and the reduced expression of IQGAP2 is correlated with poor prognosis of cancers including lung, breast, gastric, liver, kidney and colorectal cancers [17]. An integrated genomic profiling study suggests miR-92a as a regulator of IQGAP2 in locally advanced rectal cancer [18]. miR-29a-3p negatively regulated the expression of IQGAP2 through an unknown pathway in colorectal cancer cells [19]. However, little is known about the regulators of IQGAP2 in TNBC. This study validated the regulation of PDCD4-AS1 on IQGAP2 in TNBC, providing novel targets for breast cancer therapy.
Materials and methods
Tissue samples
A total of 42 fresh tissue samples of TNBC and adjacent normal tissues were obtained from the First People's Hospital of Jingzhou City. This work was ethically licensed by the ethics committee of the First People's Hospital of Jingzhou City with informed consent of all subjects. The ethical approval code of this study is K20170501. The tissue samples were immediately frozen in liquid nitrogen, and stored at −80 °C.
Cell culture and transfection
Human mammary epithelial cell line (MCF-12F) and human TNBC cell lines (HCC-1937 and MDA-MB-231) were obtained from the American Type Culture Collection (ATCC; Manassas, CA, USA). MCF-12F and HCC-1937 cells were cultured in 90% RPMI-1640 + 10% FBS and MDA-MB-231 cells were maintained in 90% DMEM + 10% FBS. pcDNA3.1-PDCD4-AS1, pcDNA3.1-IQGAP2, si-IQGAP2, miR-10b-5p mimic, miR-10b-5p inhibitor, blank pcDNA3.1 and their negative controls were purchased from Shanghai GenePharma Co., Ltd. (Shanghai, China) and were transfected into TNBC cells via Lipofectamine 2000 (Thermo Fisher Scientific, MA, USA) according to the manufacturer's directions. Correspondingly, TNBC cells were grouped into pcDNA3.1-PDCD4-AS1 group, pcDNA3.1-IQGAP2 group, si-IQGAP2 group, miR-10b-5p mimic group, miR-10b-5p inhibitor group, pcDNA3.1 group, si-NC group, NC-mimic group, NC inhibitor group and Control group.
Quantitative reverse transcription polymerase chain reaction (qRT-PCR)
Cells were lysed in 1 mL of TRIzol reagent (Thermo Fisher Scientific, MA, USA) before the total RNA was extracted in compliance with the instructions. After quantification, the RNA was reversely transcribed into cDNA. The expressions of target genes were analyzed using the real-time PCR instrument LightCycler 480 (Roche, Indianapolis, IN, USA) and the reaction conditions were in accordance to the instructions of Fast Start SYBR Green Mix (Roche Diagnostics, Indianapolis, IN). The specific reaction conditions were as follows: pre-denaturation at 95 °C for 10 min, denaturation at 95 °C for 10 s, annealing at 60 °C for 20 s and extension at 72 °C for 34 s for totally 40 cycles. Additionally, all PCR primers are displayed in Table 1. The internal reference of mRNA was GAPDH and the internal reference of miRNA was U6. The data analysis was conducted by 2−ΔΔCt method according to the following formula: ΔΔCt = [Ct(target gene) - Ct(reference gene)] experimental group - [Ct(target gene) - Ct(reference gene)] control group.
Table 1.
Sequences of the primers for quantitative reverse transcription polymerase chain reaction to determine the expression levels of PDCD4-AS1, miR-10b-5p, IQGAP2, U6 and GAPDH.
| Name | Primer |
|---|---|
| PDCD4-AS1 | F: 5′-TTAGAAACGCAGCAGACAGC-3′ |
| R: 5′-CAGAACCAAGGCCGATCACC-3′ | |
| miR-10b-5p | F: 5′-GTGTTTAAGCCAAGATGTCCCAT-3′ |
| R: 5′-GCAGGGTCCGAGGTATTC-3′ | |
| IQGAP2 | F: 5′-TTAGAAACGCAGCAGACAGC-3′ |
| R: 5′-CAGAACCAAGGCCGATCACC-3′ | |
| U6 | F: 5′-CTCGCTTCGGCAGCACA-3′ |
| R: 5′-AACGCTTCACGAATTTGCGT-3′ | |
| GAPDH | F: 5′-TCTTGTGCAGTGCCAGCCT-3′ |
| R: 5′-TGAGGTCAATGAAGGGGTCG-3′ |
Notes: F, forward; R, reverse.
Western blot
The collected cells were washed with pre-cooled PBS buffer for three times, followed by incubation in RIPA lysis buffer on ice for 30 min. Subsequently, under the condition of 12,000 rpm at 4 °C, the cell lysates were centrifuged for 10 min. The supernatant was collected and a BCA protein assay kit (Beyotime Biotechnology lnc., Shanghai, China) was used for protein quantification. SDS-PAGE was performed to isolate proteins. Then the proteins were transferred to membranes in pre-cooled transfer buffer (4 °C) for 1.5 h. The membranes were blocked with TBST containing 5% non-fat milk for 1 h and added with primary antibodies (abcam, Cambridge, USA) against GAPDH (1:10,000, ab181602) and IQGAP2 (1:1000, ab187153) at 4 °C overnight. After TBST wash for three times, the membranes were added with secondary goat anti-rabbit IgG antibody (1:5000, Beijing ComWin Biotech Co., Ltd) for 30 min at room temperature, followed by TBST wash for four times. The electrogenerated chemiluminescence (ECL) system (GE Healthcare, Beijing, China) was used for observation of the blots.
MTT
Cells (1 × 106/mL) were inoculated in a 96-well plate (100 µL/well) and cultured at 37 °C in 5% CO2. Then, the cells were incubated with 10 µL of MTT solution (5 mg/mL) at 37 °C for 4 h. The MTT solution was discarded and the reaction was terminated by 20% SDS (100 µL/well) at 37 °C with 5% CO2 for another 4 h. Finally, the absorbance of each well was determined at 570 nm using a microplate reader (Sectramax 190, Molecular Devices Corporation, Sunnyvale, CA).
Colony formation assay
Cells of each group after 24-h transfection were collected and trypsinized, after which the cells were resuspended in complete medium. The cells (200 per well) were then cultured in 10 ml of 37 °C complete medium at 37 °C with 5% CO2 for 2 to 3 weeks. When cell colonies were visible to naked eyes, the culture was terminated and the medium was discarded. The cells were washed twice in PBS and then fixed for 15 min with formaldehyde solution (5 ml). The formaldehyde solution was then discarded, and the cells were stained with Giemsa solution (1 ml) away from light. About 20 min later, the Giemsa solution was slowly washed away with running water. The culture dishes were air-dried and the numbers of cell colonies were counted by naked eyes or under an optical microscope at low magnification.
TUNEL assay
Cells were fixed by 4% paraformaldehyde for 30 min, followed by fixation in 70% cooled alcohol for 15 min. The cells were incubated for 5 min with PBS containing 0.3% Triton X-100 at room temperature, and then treated with TUNEL solution (Beyotime Biotechnology, Shanghai, China) for 60 min at 37 °C in the dark. The cells were mounted with anti-fluorescence quenching agent and observed under a fluorescence microscope. Cell nuclei were stained with Hoechst.
Cell scratch test
Firstly, the cells were placed into 6-well plates. When they grew to 90% confluence, a pipette (100 µl) tip was used to scratch the single layer of cells. After that, the cells were washed with PBS and cultured in serum-free medium. The cells were cultured for 24 h and then the relative distance of cell migration was measured under a low-magnification phase-contrast microscope (Olympus MK, Tokyo, Japan). Migration rate = (0 h scratch distance – 24 h scratch distance)/0 h scratch distance.
Transwell assay
A total of 5 × 104 TNBC cells, suspended in serum-free DMEM medium, were placed into a transwell chamber which was equipped with a Matrigel-coated membrane (BD Biosciences, Bedford, MA, USA). Subsequently, 600 µL of culture medium with 10% FBS was added into the lower chamber. The cells were cultured for 48 h at 37 °C in a humid atmosphere with 5% CO2. Cells that invaded through the membrane were fixed with 100% methanol and stained with 0.1% crystal violet, and the non-invasive cells were removed using a cotton swab. The stained cells were imaged and counted in 5 randomly chosen fields under a microscope (Olympus Corp., Tokyo, Japan).
Dual-luciferase reporter gene assay
The target sites for binding of miR-10b-5p and PDCD4-AS1, miR-10b-5p and IQGAP2 were predicted by online software TargetScan and starBase. According to the prediction, mutant and wild sequences of the binding sites of PDCD4-AS1 and IQGAP2 were designed. The mutant or wild sequence fragments were cloned and conjugated to Promega vectors, designated mut-PDCD4-AS1, wt-PDCD4-AS1, mut-IQGAP2 and wt-IQGAP2, respectively. The vectors were transfected with NC mimic or miR-10b-5p mimic into HEK-293T cells. After 48 h, fluorescence intensity of each group was measured by a dual-luciferase reporter assay system (Promega, WI, USA).
Statistical analysis
Statistical analysis was conducted in GraphPad Prism 7. Measurement data were presented as mean ± standard deviation (SD). Statistical difference between two sets of data was determined by T-test, while One-way analysis of variance (ANOVA) was used to test the differences among multiple sets of groups. Dunnett's multiple comparisons test was applied for multiple comparisons after ANOVA. The correlation analysis of gene expressions was performed using Pearson's test. Kaplan–Meier analysis was utilized to analyze the association of PDCD4-AS1 expression with the overall survival of TNBC patients. The relation of PDCD4-AS1 expression to the pathological grade and karnofsky performance scale (KPS) score of TNBC patients was determined by chi-square test, and T-test was used to detect the relation of PDCD4-AS1 expression to the patient age at diagnosis. P < 0.05 was considered statistically significant.
Results
PDCD4-AS1 expression is related with clinical-pathological characteristics of TNBC patients
We firstly examined the relative expression level of PDCD4AS1 in tumor and adjacent normal tissues from 42 TNBC patients. Compared with Normal group, the expression level of PDCD4-AS1 was markedly decreased in Tumor group (Fig. 1A, P < 0.01). In addition, we collected the clinical information of the TNBC patients, including age, size and grade of tumor, KPS score and distant metastasis. Patients with PDCD4-AS1 expression below average was classified into low PDCD4-AS1 group (n = 25) while the others were into high PDCD4-AS1 group (n = 17). Chi-square test and T-test revealed that PDCD4-AS1 expression had no statistically significant association with patient age (P = 0.4787), but was significantly associated with tumor size (P = 0.0016), pathological grade (P = 0.0043), KPS score (P = 0.0109) and distant metastasis (P = 0.0040). Patients in low PDCD4-AS1 group had a larger tumor size, higher pathological grade and higher risk of tumor distant metastasis coupled with lower KPS score than in high PDCD4-AS1 group (Table 2). Further, we followed up the survival of the TNBC patients (n = 42) within 120 months. Kaplan–Meier analysis showed that the median survival of patients in high PDCD4-AS1 group was 105 months while that in low PDCD4-AS1 group was 72 months (Fig. 1B, P < 0.05). The above results suggest that underexpression of PDCD4-AS1 in TNBC is positively related to the disease progression while negatively associated with the postoperative survival of TNBC patients.
Fig. 1.
PDCD4-AS1 expression is associated with overall survival of TNBC patients
Note: qRT-PCR was used to detect the expression level of PDCD4-AS1 in TNBC tissues and adjacent normal tissues; T-test compared the difference between two groups, ⁎⁎P < 0.01 (A). Association between the expression of PDCD4-AS1 and overall survival of patients with TNBC was analyzed by Kaplan–Meier analysis; log-rank test compared the curve difference, P = 0.0486 (B). Data were presented as mean ± standard deviation; TNBC, triple-negative breast cancer.
Table 2.
The association of PDCD4-AS1 expression with the clinical pathological characteristics of TNBC patients.
| Pathological characteristics | PDCD4-AS1 low | PDCD4-AS1 high | P-values |
|---|---|---|---|
| Age (years) | 51.29 ± 10.67 | 48.83 ± 11.34 | 0.4787 |
| Tumor size (≤ 2 cm/> 2 cm) | 5/20 | 12/5 | 0.0016 |
| Grade (1–2/3) | 6/19 | 12/5 | 0.0043 |
| KPS score (≥ 70/< 70) | 8/17 | 13/4 | 0.0109 |
| Distant metastasis (Absence/Presence) | 7/18 | 13/4 | 0.0040 |
Notes: TNBC, triple-negative breast cancer; KPS score, karnofsky performance scale score (< 70, relative severe disease progression; ≥ 70, relative moderate disease progression).
PDCD4-AS1 blocks TNBC progression
We detected the expression levels of PDCD4-AS1 in human mammary epithelial cell line (MCF-12F) and two human TNBC cell lines (HCC-1937 and MDA-MB-231), and found that the expression level of PDCD4-AS1 in HCC-1937 and MDA-MB-231 cells was significantly lower than in MCF-12F cells (Fig. 2A, P < 0.01). To investigate the role of PDCD4-AS1 in TNBC cells, we overexpressed PDCD4-AS1 in TNBC cell lines. qRT-PCR showed that the expression of PDCD4-AS1 in pcDNA3.1-PDCD4-AS1 group was successfully elevated (vs. Control group) (Supplementary Fig. 1A, P < 0.001), whilst the expression level of PDCD4-AS1 in pcDNA3.1 group showed no detectable change compared with that in Control group (Supplementary Fig. 1A, P > 0.05). The above data indicate effective transfection of pcDNA3.1-PDCD4-AS1 in TNBC cells.
Fig. 2.
PDCD4-AS1 inhibits the proliferation, migration and invasion of TNBC cells
Note: qRT-PCR was applied to measure the expression levels of PDCD4-AS1 in human mammary epithelial cell line (MCF-12F) and two human TNBC cell lines (HCC-1937 and MDA-MB-231) (A). After overexpression of PDCD4-AS1 in TNBC cells, the proliferation, apoptosis, invasion and migration of TNBC cells were measured by MTT and colony formation assays (B-C), TUNEL staining (D), Transwell (E) and cell scratch assay (F), respectively. Data were presented as mean ± standard deviation; T-test compared the differences between two groups and One-way analysis of variance compared the differences among multiple sets of groups, *P < 0.05, ⁎⁎P < 0.01, ⁎⁎⁎P < 0.001; TNBC, triple-negative breast cancer.
Next, we explored the effect of PDCD4-AS1 on proliferation of TNBC cells. MTT and colony formation assays showed that the cell proliferation was inhibited in pcDNA3.1-PDCD4-AS1 group (Fig. 2B-C, P < 0.05) while remained unaffected in pcDNA3.1 group in contrast to Control group (Fig. 2B-C, P > 0.05).
Moreover, TUNEL staining showed that the cell apoptosis in pcDNA3.1-PDCD4-AS1 group was markedly accelerated (vs. Control group), and the apoptosis level of pcDNA3.1 group was similar to that of Control group (Fig. 2D, Supplementary Fig. 2A).
Transwell and cell scratch assays were performed to detect the cell invasion and migration, respectively. It was found that the invasion and migration capacities of TNBC cells were weakened in pcDNA3.1-PDCD4-AS1 group while remained unchanged in pcDNA3.1 group in contrast to Control group (Fig. 2E-F). The aforementioned results indicate that PDCD4-AS1 can inhibit the proliferation, invasion and migration of TNBC cells.
IQGAP2 represses TNBC progression
The IQGAP family comprises three members, among which IQGAP2 is suggested to be a tumor suppressor [20]. Therefore, we detected the expression levels of IQGAP2 in collected clinical samples. qRT-PCR and Western blot showed underexpression of IQGAP2 in TNBC tissues in contrast to normal tissues (Fig. 3A-B, P < 0.01). Additionally, we detected that the expression levels of IQGAP2 in HCC-1937 and MDA-MB-231 cells were lower than in MCF-12F cells (Fig. 3C-D, P < 0.01). To investigate the role of IQGAP2 in TNBC cells, we overexpressed IQGAP2 in HCC-1937 and MDA-MB-231 cells. qRT-PCR and Western blot showed that the expression of IQGAP2 in pcDNA3.1-IQGAP2 group was remarkably enhanced (Supplementary Fig. 3A-B, P < 0.001), while there were no detectable change in pcDNA3.1 group in contrast to Control group (Supplementary Fig. 3A-B, P > 0.05). The above data indicate successful transfection of pcDNA3.1-IQGAP2 in TNBC cells.
Fig. 3.
IQGAP2 suppresses the proliferation, migration and invasion of TNBC cells
Note: qRT-PCR and Western blot detected the mRNA and protein levels of IQGAP2 in TNBC tissues (A-B) and TNBC cell lines (C-D). After transfection with pcDNA3.1-IQGAP2 in TNBC cells, the cell proliferation, apoptosis, invasion and migration were measured by MTT and colony formation assay (E-F), TUNEL staining (G), Transwell assay (H) and cell scratch assay (I), respectively. Data were presented as mean ± standard deviation; T-test compared the differences between two groups and One-way analysis of variance compared the differences among multiple sets of groups, *P < 0.05, ⁎⁎P < 0.01, ⁎⁎⁎P < 0.001; TNBC, triple-negative breast cancer.
Next, we investigated the effect of IQGAP2 on the proliferation of TNBC cells. MTT and colony formation assays showed that the proliferation ability of TNBC cells in pcDNA3.1-IQGAP2 group was restrained (vs. Control group) (Fig. 3E-F, P < 0.05), and pcDNA3.1 group and Control group showed no significant difference in the cell proliferation (Fig. 3E-F, P > 0.05).
Moreover, TUNEL staining showed that the apoptosis in pcDNA3.1-IQGAP2 group was distinctly accelerated, and the apoptosis level of pcDNA3.1 group was similar to that of Control group (Fig. 3G, Supplementary Fig. 2B).
Transwell and cell scratch assays showed that the cell invasion and migration were weakened in pcDNA3.1-IQGAP2 group while remained unchanged in pcDNA3.1 group in contrast to Control group (Fig. 3H-I). The aforementioned results indicate that IQGAP2 can inhibit the proliferation, invasion and migration of TNBC cells.
miR-10b-5p targets IQGAP2 to facilitate TNBC progression
The expression of miR-10b-5p has been reported to be dysregulated in breast cancer and associated with the prognosis [21]. In this step, the role of miR-10b-5p in TNBC was surveyed. The miR-10b-5p expression was augmented in TNBC tissues in contrast to adjacent normal tissues (Fig. 4A, P < 0.01). Meanwhile, we found that the expression of miR-10b-5p in HCC-1937 and MDA-MB-231 cells was much higher than in the MCF-12F cells (Fig. 4B, P < 0.01). Furthermore, correlation analysis revealed that the expressions of IQGAP2 and miR-10b-5p were negatively correlated in TNBC (Fig. 4C, P < 0.001). Also, online database analysis and dual-luciferase report assay confirmed the binding sites of IQGAP2 and miR-10b-5p (Fig. 4D-E, P < 0.01). These findings indicate that miR-10b-5p negatively regulates the expression of IQGAP2 by binding to it.
Fig. 4.
miR-10b-5p targets IQGAP2
Note: qRT-PCR detected the expression level of miR-10b-5p in TNBC tissues (A) and TNBC cell lines (B). The correlation between the expressions of IQGAP2 and miR-10b-5p was analyzed by Pearson's test (C). The binding sites of IQGAP2 and miR-10b-5p were predicted by online database (D). Dual-luciferase reporter assay was used to verify the interaction between IQGAP2 and miR-10b-5p (E). Data were presented as mean ± standard deviation; T-test compared the differences between two groups and One-way analysis of variance compared the differences among multiple sets of groups, ⁎⁎P < 0.01, ⁎⁎⁎P < 0.001; TNBC, triple-negative breast cancer.
Subsequently, HCC-1937 and MDA-MB-231 cells were transfected with miR-10b-5p mimic, miR-10b-5p inhibitor or pcDNA3.1-IQGAP2 + miR-10b-5p mimic. qRT-PCR showed that the expression level of miR-10b-5p in miR-10b-5p mimic group was elevated, whilst a reversed expression pattern of miR-10b-5p was found in miR-10b-5p inhibitor group (vs. Control group) (Supplementary Fig. 4A, P < 0.001), which indicated successful overexpression or inhibition of miR-10b-5p in TNBC cells. qRT-PCR and Western blot showed that the expression level of IQGAP2 was reduced in miR-10b-5p mimic group but increased in miR-10b-5p inhibitor group (vs. Control group) (Fig. 5A-B, P < 0.001). Notably, IQGAP2 was strongly expressed in pcDNA3.1-IQGAP2 + miR-10b-5p mimic group in contrast to miR-10b-5p mimic group (Fig. 5A-B, P < 0.01).
Fig. 5.
miR-10b-5p expedites TNBC progression by regulating IQGAP2
Note: After transfection with miR-10b-5p mimic, miR-10b-5p inhibitor and miR-10b-5p mimic + pcDNA3.1-PDCD4-AS1 in TNBC cell lines, qRT-PCR (A) and Western blot (B) detected the mRNA and protein levels of IQGAP2. The cell proliferation, apoptosis, invasion and migration abilities were measured by MTT and colony formation assays (C-D), TUNEL staining (E), Transwell assay (F) and cell scratch assays (G), respectively. Data were presented as mean ± standard deviation; T-test compared the differences between two groups and One-way analysis of variance compared the differences among multiple sets of groups, *P < 0.05, ⁎⁎P < 0.01, ⁎⁎⁎P < 0.001; TNBC, triple-negative breast cancer.
The effect of miR-10b-5p on TNBC cell proliferation was assessed by MTT and colony formation assays. The detection showed that the proliferation ability of TNBC cells was enhanced in miR-10b-5p mimic group while refrained in miR-10b-5p inhibitor group compared with Control group (Fig. 5C-D, P < 0.05). Notably, the proliferation ability of the cells in pcDNA3.1-IQGAP2 + miR-10b-5p mimic group was much lower than in miR-10b-5p mimic group (Fig. 5C-D, P < 0.05). Moreover, TUNEL showed that the cell apoptosis was ameliorated in miR-10b-5p mimic group while promoted in miR-10b-5p inhibitor group (vs. Control group); cells in pcDNA3.1-IQGAP2 + miR-10b-5p group had an expedited cell apoptosis rate than miR-10b-5p mimic group (Fig. 5E, Supplementary Fig. 2C, P < 0.05). Transwell and cell scratch assays showed that compared to Control group, the invasion and migration of TNBC cells were enhanced in miR-10b-5p mimic group and suppressed in miR-10b-5p inhibitor group (Fig. 5F-G, P < 0.05). However, the invasion and migration abilities of TNBC cells were weakened in pcDNA3.1 IQGAP2 + miR-10b-5p mimic group compared to miR-10b-5p mimic group (Fig. 5F-G, P < 0.05). Taken together, miR-10b-5p facilitates the proliferation, invasion and migration of TNBC cells via negatively regulating IQGAP2.
PDCD4-AS1 sponges miR-10b-5p to hinder TNBC progression
Correlation analysis showed that the expressions of PDCD4-AS1 and miR-10b-5p were negatively correlated in TNBC tissues (Fig. 6A, P < 0.001). Online database predicted the binding sites of PDCD4-AS1 and miR-10b-5p (Fig. 6B), which were verified by dual-luciferase reporter assay (Fig. 6C, P < 0.01). These results indicate that PDCD4-AS1 negatively regulates the expression of miR-10b-5p by binding to it. HCC-1937 and MDA-MB-231 cells were transfected with pcDNA3.1-PDCD4-AS1, miR-10b-5p mimic or pcDNA3.1-PDCD4-AS1 + miR-10b-5p mimic. qRT-PCR showed that the expression level of miR-10b-5p in pcDNA3.1-PDCD4-AS1 + miR-10b-5p mimic group was significantly decreased (vs. miR-10b-5p mimic group) (Fig. 6D, P < 0.01). MTT and colony formation assays showed decreased proliferation ability of the cells in pcDNA3.1-PDCD4-AS1 + miR-10b-5p mimic group when compared with miR-10b-5p mimic group (Fig. 6E-F, P < 0.05). TUNEL showed that cells in pcDNA3.1-PDCD4-AS1 + miR-10b-5p mimic group had an expedited cell apoptosis rate when compared to miR-10b-5p mimic group (Fig. 6G, Supplementary Fig. 2D). The results of Transwell and cell scratch assays showed that when compared to miR-10b-5p mimic group, the invasion and migration abilities of TNBC cells were significantly weakened in pcDNA3.1-PDCD4-AS1 + miR-10b-5p mimic group (Fig. 6H-I, P < 0.05). Taken together, PDCD4-AS1 represses the proliferation, invasion and migration of TNBC cells via sponging miR-10b-5p.
Fig. 6.
PDCD4-AS1 hinders TNBC progression by downregulating miR-10b-5p
Note: The correlation of PDCD4-AS1 and miR-10b-5p expressions was determined by Pearson's test (A). The binding sites of PDCD4-AS1 and miR-10b-5p were predicted by online database (B). Dual-luciferase reporter assay verified the interaction between PDCD4-AS1 and miR-10b-5p (C). After transfection with pcDNA3.1-PDCD4-AS1, miR-10b-5p mimic or pcDNA3.1-PDCD4-AS1 + miR-10b-5p mimic in TNBC cell lines, qRT-PCR was applied to measure the expression of miR-10b-5p (D). The cell proliferation, apoptosis, invasion and migration abilities were measured by MTT and colony formation assay (E-F), TUNEL staining (G), Transwell assay (H) and cell scratch assay (I), respectively. Data were presented as mean ± standard deviation; T-test compared the differences between two groups and One-way analysis of variance compared the differences among multiple sets of groups, *P < 0.05, ⁎⁎P < 0.01, ⁎⁎⁎P < 0.001; TNBC, Triple-negative breast cancer.
PDCD4-AS1 serves as a ceRNA to up-regulate IQGAP2 in TNBC cells
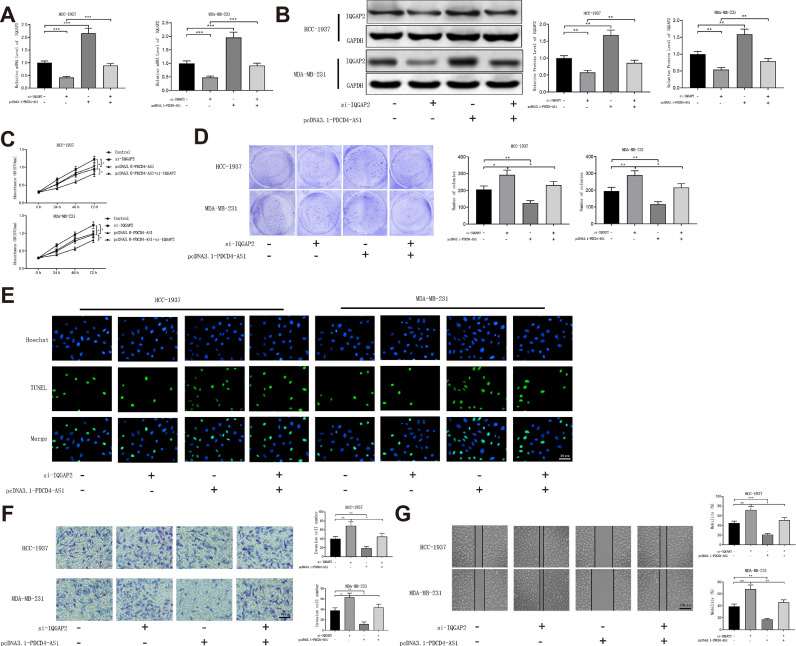
HCC-1937 and MDA-MB-231 cell lines were transfected with pcDNA3.1-PDCD4-AS1, si-IQGAP2 or pcDNA3.1-PDCD4-AS1 + si-IQGAP2. qRT-PCR and Western blot showed that the expression level of IQGAP2 was increased in pcDNA3.1-PDCD4-AS1 + si-IQGAP2 group (vs. si-IQGAP2 group) (Fig. 7A-B, P < 0.01). To determine the involvement of miR-10b-5p in the regulation of PDCD4-AS1 on IQGAP2, we transfected HCC-1937 and MDA-MB-231 cells with pcDNA3.1-PDCD4-AS1 or pcDNA3.1-PDCD4-AS1 + miR-10b-5p mimic. The expression of IQGAP2 was significantly decreased in pcDNA3.1-PDCD4-AS1 + miR-10b-5p mimic group compared with pcDNA3.1-PDCD4-AS1 group (Fig. 8A-B, P < 0.05). The results of MTT and colony formation assays showed that the proliferation ability of the cells in pcDNA3.1-PDCD4-AS1 + si-IQGAP2 group was decreased, when compared with si-IQGAP2 group (Fig. 7C-D, P < 0.05). TUNEL showed that cells in pcDNA3.1-PDCD4-AS1 + si-IQGAP2 group had an expedited cell apoptosis rate in contrast to si-IQGAP2 group (Fig. 7E, Supplementary Fig. 2E). Transwell and cell scratch assays showed that the invasion and migration abilities of TNBC cells were weakened in pcDNA3.1-PDCD4-AS1 + si-IQGAP2 group (vs. si-IQGAP2 group) (Fig. 7F-G, P < 0.01). The aforementioned results showed that PDCD4-AS1 acts as a ceRNA of miR-10b-5p to promote IQGAP2 expression, thereby suppressing TNBC progression.
Fig. 7.
PDCD4-AS1 blocks TNBC progression by upregulating IQGAP2
Note: After transfection with pcDNA3.1-PDCD4-AS1, si-IQGAP2 and pcDNA3.1-PDCD4-AS1 + si-IQGAP2 in TNBC cell lines, qRT-PCR (A) and Western blot (B) detected the mRNA and protein levels of IQGAP2, respectively. The cell proliferation, apoptosis, invasion and migration abilities were measured by MTT and colony formation assay (C-D), TUNEL staining (E), Transwell assay (F) and cell scratch assay (G), respectively. Data were presented as mean ± standard deviation; T-test compared the differences between two groups and One-way analysis of variance compared the differences among multiple sets of groups, *P < 0.05, ⁎⁎P < 0.01, ⁎⁎⁎P < 0.001; TNBC, Triple-negative breast cancer.
Fig. 8.
PDCD4-AS1 upregulates the expression of IQGAP2 by targeting miR-10b-5p
Note: TNBC cell lines were transfected with pcDNA3.1-PDCD4-AS1, miR-10b-5p mimic or pcDNA3.1-PDCD4-AS1 + miR-10b-5p mimic. qRT-PCR (A) and Western blot (B) detected the mRNA and protein levels of IQGAP2. Data were presented as mean ± standard deviation; T-test compared the differences between two groups and One-way analysis of variance compared the differences among multiple sets of groups, *P < 0.05, ⁎⁎P < 0.01, ⁎⁎⁎P < 0.001.
Discussion
TNBC is a subtype of breast cancer, accounting for 15% of all breast cancers with no effective target therapies available [22]. Therefore, exploring effective methods for TNBC treatment is needed to improve the survival of TNBC patients. In the present research, we uncovered the role and regulatory mechanism of lncRNA PDCD4-AS1 in TNBC cells.
LncRNAs are involved in many cancer-related mechanisms. A plenty of lncRNAs have been proved to get involved in the progression of TNBC, such as lncRNA LINK-A [23], lncRNA PVT1 [24], lncRNA MIR100HG [25] and lncRNA LINC01638 [26]. What's more, existing studies have also reported that lncRNA PDCD4-AS1 is a tumor suppressor gene which positively regulates the expression and activity of PDCD4 in TNBC [27]. To determine the role of PDCD4-AS1 in TNBC, we firstly detected the expression of PDCD4-AS1 in TNBC tissues. PDCD4-AS1 was downregulated in TNBC tissues, and underexpression of PDCD4-AS1 was positively related to the disease progression while negatively associated with the postoperative survival of TNBC patients, suggesting that PDCD4-AS1 may act as a prognostic biomarker for TNBC. Though high PDCD4-AS1 expression did not significantly improve the patient survival, the low expression group had an even worse outcome. Moreover, functional experiments revealed that PDCD4-AS1 repressed the proliferation, invasion, migration and promoted apoptosis in TNBC cells. This study then focused on the mechanism of PDCD4-AS1 in TNBC.
One important functionary mechanism of lncRNA is acting as a ceRNA or a molecular sponge of miRNA [28,29]. LncRNAs recognize both RNA and DNA targets through simple one-to-one base pairing interactions, and more importantly, lncRNAs can fold into intricate three-dimensional structures that expand the target scope of molecules that lncRNAs can bind with high affinity and specificity [30]. Moreover, lncRNAs can influence the function of target mRNAs at any time point during the mRNA life cycle in both normal and pathological cellular processes. Therefore, lncRNAs show great potential as therapeutic targets in control of disease progression. In this study, we demonstrated that PDCD4-AS1 negatively regulated the expression of miR-10b-5p by binding to it. Several studies have reported that miR-10b-5p functions as an oncogene or tumor suppressor in various cancers. For example, miR-10b-5p was upregulated in glioma and related to poor outcome [31]. However, miR-10b-5p was downregulated significantly in renal cell carcinoma [32]. In this work, we provided the evidence that miR-10b-5p promoted the growth, migration and invasion of TNBC cells. Interestingly, overexpressed PDCD4-AS1 could reverse the unfavorable effects of miR-10b-5p on TNBC progression. Therefore, PDCD4-AS1 functions as a sponge of miR-10b-5p in TNBC, displaying antagonisms to the function of miR-10b-5p in TNBC progression.
Furthermore, we identified IQGAP2 as a downstream target of miR-10b-5p in TNBC cells. Recently, IQGAP2 has been reported as a tumor suppressor in many cancers including prostate cancer [33], gastric cancer [34] and ovarian cancer [35]. Consistently, our findings showed that IQGAP2 was lowly expressed in TNBC cells and upregulated expression of IQGAP2 suppressed the malignant behaviors of TNBC cells. Additionally, overexpression of PDCD4-AS1 could suppress the proliferation, migration and invasion induced by IQGAP2 inhibition in TNBC cells. Taken together, PDCD4-AS1 increased the expression of IQGAP2 by acting as a ceRNA for miR-10b-5p.
In conclusion, PDCD4-AS1 expression is of predictive value for the prognosis of TNBC patients, and PDCD4-AS1 promotes the proliferation, invasion and migration of TNBC cells by upregulating the expression of IQGAP2 via miR-10b-5p. Despite the absence of in vivo experiments to further support the results of in vitro experiments, this study unravels a novel molecular mechanism that is regulated by PDCD4-AS1 in TNBC, giving insights into the role of PDCD4-AS1 in cancers. Although PDCD4-AS1 regulates the expression of IQGAP2 via miR-10b-5p, the therapeutic benefit of miR10b-5p seems to be limited, which might be attributed to the dual role of miR10b-5p in cancer development. Further investigation into the function of miR10b-5p in TNBC is warranted.
Declaration of Competing Interest
The authors declare that they have no competing interests.
Acknowledgments
Acknowledgement
Not applicable.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors contributions
WDL and SSR conceived the ideas. WDL designed the experiments. WZ performed the experiments. ZLJ analyzed the data. SSR and WDL provided critical materials. WDL and WZ wrote the manuscript. SSR supervised the study. All the authors have read and approved the final version for publication.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2020.100958.
Appendix. Supplementary materials
References
- 1.Gooding A.J., Zhang B., Gunawardane L., Beard A., Valadkhan S., Schiemann W.P. The lncRNA BORG facilitates the survival and chemoresistance of triple-negative breast cancers. Oncogene. 2019;38:2020–2041. doi: 10.1038/s41388-018-0586-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferlay J., Soerjomataram I., Dikshit R., Eser S., Mathers C., Rebelo M., Parkin D.M., Forman D., Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 3.Harbeck N., Gnant M. Breast cancer. Lancet. 2017;389:1134–1150. doi: 10.1016/S0140-6736(16)31891-8. [DOI] [PubMed] [Google Scholar]
- 4.O'Sullivan C.C., Loprinzi C.L., Haddad T.C. Updates in the evaluation and management of breast cancer. Mayo Clin. Proc. 2018;93:794–807. doi: 10.1016/j.mayocp.2018.03.025. [DOI] [PubMed] [Google Scholar]
- 5.Lebert J.M., Lester R., Powell E., Seal M., McCarthy J. Advances in the systemic treatment of triple-negative breast cancer. Curr. Oncol. 2018;25:S142–SS50. doi: 10.3747/co.25.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Telli M.L., Sledge G.W. The future of breast cancer systemic therapy: the next 10 years. J. Mol. Med. Berl. 2015;93:119–125. doi: 10.1007/s00109-014-1238-y. [DOI] [PubMed] [Google Scholar]
- 7.Wang O., Yang F., Liu Y., Lv L., Ma R., Chen C., Wang J., Tan Q., Cheng Y., Xia E. C-MYC-induced upregulation of lncRNA SNHG12 regulates cell proliferation, apoptosis and migration in triple-negative breast cancer. Am. J. Transl. Res. 2017;9:533–545. [PMC free article] [PubMed] [Google Scholar]
- 8.Beermann J., Piccoli M.T., Viereck J., Thum T. Non-coding RNAs in development and disease: background, mechanisms, and therapeutic approaches. Physiol. Rev. 2016;96:1297–1325. doi: 10.1152/physrev.00041.2015. [DOI] [PubMed] [Google Scholar]
- 9.Schmitt A.M., Chang H.Y. Long noncoding RNAs in cancer pathways. Cancer Cell. 2016;29:452–463. doi: 10.1016/j.ccell.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qi X., Zhang D.H., Wu N., Xiao J.H., Wang X., Ma W. ceRNA in cancer: possible functions and clinical implications. J. Med. Genet. 2015;52:710–718. doi: 10.1136/jmedgenet-2015-103334. [DOI] [PubMed] [Google Scholar]
- 11.Simonson B., Das S. MicroRNA therapeutics: the next magic bullet? Mini Rev. Med. Chem. 2015;15:467–474. doi: 10.2174/1389557515666150324123208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin C., Zhang S., Wang Y., Wang Y., Nice E., Guo C., Zhang E., Yu L., Li M., Liu C. Functional role of a novel long noncoding RNA TTN-AS1 in esophageal squamous cell carcinoma progression and metastasis. Clin. Cancer Res. 2018;24:486–498. doi: 10.1158/1078-0432.CCR-17-1851. [DOI] [PubMed] [Google Scholar]
- 13.Sas-Chen A., Aure M.R., Leibovich L., Carvalho S., Enuka Y., Korner C., Polycarpou-Schwarz M., Lavi S., Nevo N., Kuznetsov Y. LIMT is a novel metastasis inhibiting lncRNA suppressed by EGF and downregulated in aggressive breast cancer. EMBO Mol. Med. 2016;8:1052–1064. doi: 10.15252/emmm.201606198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu S., Kong D., Chen Q., Ping Y., Pang D. Oncogenic long noncoding RNA landscape in breast cancer. Mol. Cancer. 2017;16:129. doi: 10.1186/s12943-017-0696-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fan C.N., Ma L., Liu N. Systematic analysis of lncRNA-miRNA-mRNA competing endogenous RNA network identifies four-lncRNA signature as a prognostic biomarker for breast cancer. J. Transl. Med. 2018;16:264. doi: 10.1186/s12967-018-1640-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jadaliha M., Gholamalamdari O., Tang W., Zhang Y., Petracovici A., Hao Q., Tariq A., Kim T.G., Holton S.E., Singh D.K. A natural antisense lncRNA controls breast cancer progression by promoting tumor suppressor gene mRNA stability. PLoS Genet. 2018;14 doi: 10.1371/journal.pgen.1007802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar D., Hassan M.K., Pattnaik N., Mohapatra N., Dixit M. Reduced expression of IQGAP2 and higher expression of IQGAP3 correlates with poor prognosis in cancers. PLoS ONE. 2017;12 doi: 10.1371/journal.pone.0186977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pelossof R., Chow O.S., Fairchild L., Smith J.J., Setty M., Chen C.T., Chen Z., Egawa F., Avila K., Leslie C.S. Integrated genomic profiling identifies microRNA-92a regulation of IQGAP2 in locally advanced rectal cancer. Genes Chromosom. Cancer. 2016;55:311–321. doi: 10.1002/gcc.22329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He P.Y., Yip W.K., Chai B.L., Chai B.Y., Jabar M.F., Dusa N., Mohtarrudin N., Seow H.F. Inhibition of cell migration and invasion by miR29a3p in a colorectal cancer cell line through suppression of CDC42BPA mRNA expression. Oncol. Rep. 2017;38:3554–3566. doi: 10.3892/or.2017.6037. [DOI] [PubMed] [Google Scholar]
- 20.White C.D., Brown M.D., Sacks D.B. IQGAPs in cancer: a family of scaffold proteins underlying tumorigenesis. FEBS Lett. 2009;583:1817–1824. doi: 10.1016/j.febslet.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang J., Yan Y., Zhang Z., Li Y. Role of miR-10b-5p in the prognosis of breast cancer. PeerJ. 2019;7:e7728. doi: 10.7717/peerj.7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo B., Wu S., Zhu X., Zhang L., Deng J., Li F., Wang Y., Zhang S., Wu R., Lu J. Micropeptide CIP2A-BP encoded by LINC00665 inhibits triple-negative breast cancer progression. EMBO J. 2019 doi: 10.15252/embj.2019102190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin A., Li C., Xing Z., Hu Q., Liang K., Han L., Wang C., Hawke D.H., Wang S., Zhang Y. The LINK-A lncRNA activates normoxic HIF1alpha signalling in triple-negative breast cancer. Nat. Cell Biol. 2016;18:213–224. doi: 10.1038/ncb3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang J., Li Y., Sang Y., Yu B., Lv D., Zhang W., Feng H. LncRNA PVT1 regulates triple-negative breast cancer through KLF5/beta-catenin signaling. Oncogene. 2018;37:4723–4734. doi: 10.1038/s41388-018-0310-4. [DOI] [PubMed] [Google Scholar]
- 25.Wang S., Ke H., Zhang H., Ma Y., Ao L., Zou L., Yang Q., Zhu H., Nie J., Wu C. LncRNA MIR100HG promotes cell proliferation in triple-negative breast cancer through triplex formation with p27 loci. Cell Death Dis. 2018;9:805. doi: 10.1038/s41419-018-0869-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luo L., Tang H., Ling L., Li N., Jia X., Zhang Z., Wang X., Shi L., Yin J., Qiu N. LINC01638 lncRNA activates MTDH-Twist1 signaling by preventing SPOP-mediated c-Myc degradation in triple-negative breast cancer. Oncogene. 2018;37:6166–6179. doi: 10.1038/s41388-018-0396-8. [DOI] [PubMed] [Google Scholar]
- 27.M J. Role of long non-coding RNAs in breast cancer progression. 2018, 1–112.
- 28.Paraskevopoulou M.D., Hatzigeorgiou A.G. Analyzing MiRNA-LncRNA interactions. Methods Mol. Biol. 2016;1402:271–286. doi: 10.1007/978-1-4939-3378-5_21. [DOI] [PubMed] [Google Scholar]
- 29.Sahu A., Singhal U., Chinnaiyan A.M. Long noncoding RNAs in cancer: from function to translation. Trends Cancer. 2015;1:93–109. doi: 10.1016/j.trecan.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geisler S., Coller J. RNA in unexpected places: long non-coding RNA functions in diverse cellular contexts. Nat. Rev. Mol. Cell Biol. 2013;14:699–712. doi: 10.1038/nrm3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Z X., M J., X G., Al E. Identification of the clinical diagnostic value of miR-10b-5p and the key targets and pathways in glioma, a study based on meta-analysis and bioinformatics. Int. J. Clin. Exp. Med. 2018;11:6532–6546. [Google Scholar]
- 32.Li Y., Chen D., Li Y., Jin L., Liu J., Su Z., Qi Z., Shi M., Jiang Z., Ni L. Oncogenic cAMP responsive element binding protein 1 is overexpressed upon loss of tumor suppressive miR-10b-5p and miR-363-3p in renal cancer. Oncol. Rep. 2016;35:1967–1978. doi: 10.3892/or.2016.4579. [DOI] [PubMed] [Google Scholar]
- 33.Xie Y., Zheng L., Tao L. Downregulation of IQGAP2 correlates with prostate cancer recurrence and metastasis. Transl. Oncol. 2019;12:236–244. doi: 10.1016/j.tranon.2018.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu L., Shao Y., Ren L., Liu X., Li Y., Xu J., Ye Y. IQGAP2 inhibits migration and invasion of gastric cancer cells via elevating SHIP2 phosphatase activity. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21061968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deng Z., Wang L., Hou H., Zhou J., Li X. Epigenetic regulation of IQGAP2 promotes ovarian cancer progression via activating Wnt/beta-catenin signaling. Int. J. Oncol. 2016;48:153–160. doi: 10.3892/ijo.2015.3228. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.