Abstract
Enteric fever is an invasive bacterial infection mostly caused by Salmonella enterica serovar Typhi, which is a common agent of enteric fever. This illness has been a major public health issue, as it affects a large number of individuals globally. The box-plot analytic method is involved in exploratory data analysis using statistical techniques to identify patterns that may be hidden in a group of numbers used to visually summarize and compare groups of data. We evaluted the effect of enteric fever on various haematologic parameters using the box-plot distribution model. Samples were obtained from 400 volunteer patients as well as healthy subjects (controls). Assay for typhoid fever was carried out using obtained serum samples to detect specific O and H antigens. Antibody titres of 1:80 and higher for anti-TO and 1:160 and higher for anti-TH antibodies were taken as cutoff values to indicate recent infection of typhoid fever. The haematologic parameters were evaluated using an automated haematology analyser. A statistically significant decrease was observed in packed cell volume, white blood cell count, erythrocyte sedimentation rate and haemoglobin concentration, while a statistically insignificant difference was observed in the neutrophils, lymphocytes and monocytes seen in the box-plot distribution analysis. Typhoid fever causes significant haematologic changes which could be helpful in diagnosis. The box-and-whisker plots compared the distributions of the haematologic parameters, spread and overall ranges. Awareness of these parameters could be useful in providing accurate diagnosis and therapy, particularly in underresourced endemic regions in developing countries.
Keywords: Box-plot distribution, diagnostics evaluation, enteric fever, haematological indices, salmonella spp
Introduction
Enteric fever is a systemic disease characterized by fever and abdominal pain caused by dissemination of Salmonella enterica serovar Typhi or serovar Paratyphi type A, B or C. Enteric fever remains a main public health issue globally, as various studies have demonstrated that this illness affects a large number of people annually, especially in developing and underdeveloped countries. Enteric fever is an acute systemic febrile illness which is sometimes life-threatening caused by Salmonella Typhi and Salmonella Paratyphi A, B or C [1,2].
Although the incidence of this fever has greatly decreased in developed countries in recent years, this may be attributed to the development and effectiveness of vaccines, particularly typhoid conjugate vaccines. This illness particularly affects areas with poor hygiene and poor water quality, with an estimated incidence of 200<thinsp>000 deaths every year and about 25 million new cases [3].
This illness affects vital organs of the body if left untreated or if complications occur [4]. Typhoid fever is a multisystem disease which affects bone marrow, resulting in a decrease in packed cell volume and neutrophils but increased lymphocytes [5,6]. Lack of proper awareness regarding the severity of enteric fever has resulted in an increased rate of infection among both adults and children. Further complications of the resulting lesions on the intestinal system results in haemorrhage and intestinal perforation. Carrier state is common among chronically ill patients, with bacilli found in the blood, faeces and urine of such individuals as a result of massive multiplication of bacilli in the bloodstream [7].
We sought to evaluate enteric fever in study subjects using various haematologic parameters and to establish the relationship between typhoid fever and haematologic disorders using the box-plot distribution method. A box-and-whisker plot is a way to summarize a data set measured using an interval scale.
Methods
Population and sample size
The study was conducted at the Landmark University Medical Centre, Omu-Aran, Kwara state, Nigeria. The study population comprised volunteer subjects and patients attending the outpatient department of the health facility. The age range of the study and control groups was 18 to 60 years. Four hundred patients were recruited for the study after oral and documented consent was provided, comprising 100 each of typhoid-positive female, typhoid-negative female (control), typhoid-positive male and typhoid-negative male (control) subjects. We compared data of typhoid-positive (study) and -negative (normal control) subjects in order to assess the changes from normal (decrease or increase) of various parameters.
Sample size formulas
We used a single population proportion formula for the determination of sample size,
| N = Z2P(1 − P)/W2, |
where N is the number of suspected enteric fever patients, Z = Level of confidence according to the standard normal distribution (for a level of confidence of 95%, Z= 1.96)., P is the prevalence of enteric infection (9.9%) and W is the margin of error (taken as 5%). Accordingly, a total of 400 serum samples were collected from patients with suspected enteric fever.
Ethical permit
The project proposal was submitted to and approved by the ethical review board of the Landmark University Medical Center (approval LMC/2019/02/29). This research was conducted following established standards for reporting diagnostic accuracy and guidelines.
Consent
Only participants who provided consent were documented and enrolled onto the study. Demographic data (including sex and age) of each participant were collected using a standardized questionnaire.
Inclusion criteria
All patients diagnosed with typhoid fever were included in the study. Patients with complaints of fever of more than 1 week's duration were investigated for typhoid infection. Only patients who tested positive for typhoid fever were recruited for the study arms.
Exclusion criteria
Patients who had initiated antibiotic treatment before seeking care at the health facility were excluded. Patients with other major systemic illnesses, such as history of liver disease, renal disease, haematologic disorders, malaria, recent history of drug intake that could alter blood-profile parameters and active alcoholics were excluded from the study. Detailed clinical histories were obtained to rule out the confounding illnesses listed above.
Sample collection
Samples were collected with 5 mL disposable syringes. Venous blood samples (3–5 mL) were obtained from each subject for complete blood count (CBC) and Widal test. Samples were used for blood culture and haematologic investigations as indicated. All blood samples were obtained using standard procedures. Collected samples were placed into EDTA-containing universal containers, while serum samples were used for Widal test assay. Blood (plasma) from subjects was used to determine the CBC.
Sample processing
Assay of the collected blood samples for CBC to determine haematologic parameters were performed according to standard procedures. The Widal test was performed by using serum separated from sample in plain vials. Salmonella Typhi O and H agglutination titres >1:80 and > 1:160 were considered to be significant and were included in the study as Widal-positive cases. Diagnosis was confirmed by positive blood culture or Widal test. CBC evaluation was done in the following order.
Haemoglobin estimation test
Haemoglobin estimation testing was carried out using the Drabkin method. A total of 0.02 mL of blood was poured into 5 mL of Drabkin solution using a haemoglobin pipette, mixed and left for 5 minutes. The absorbance was then measured at 540 nm with a spectrophotometer.
Packed cell volume
Oxalated blood was thoroughly mixed using the repeated inversion method. It was then centrifuged for 30 minutes at 2300 rpm; the volume of the packed cells was then measured [4].
Platelet estimation
A small amount of platelet-diluting solution was aspirated into the red blood cell pipette and the fluid expelled. Using the diluting solution, serial dilution of blood (1/200) was performed. The counting chamber was placed in a petri dish containing wet cotton wool. After the platelets settled, they were then counted using a microscope with a high-power objective lens.
White blood cell count estimation
Blood was drawn to the 0.5 point on the white blood cell (WBC) pipette and the counting chamber was filled. Thereafter, the cells were left to settle for 3 minutes and then counted. The other parameters were tested using an autoanalyser.
Statistical analysis
Statistical analysis was carried out on the results. Data obtained via independent-sample t test were used to infer the difference between the two groups; mean ± standard deviation was used to describe the data. Box plots were also used to visualize the distribution comparison of the data belonging to the two groups for each parameter. SPSS VERSION 23 software (IBM, Armonk, NY, USA) was used to conduct the statistical analyses.
Results
As Table 1 shows, a statistically significant decrease was observed in packed cell volume, total WBC count and erythrocyte sedimentation rate (p < 0.01). Neutrophils also showed an increase, but this was not statistically significantly different. A decrease was observed in the mean values of lymphocytes and monocytes; this was also not statistically significant (p < 0.05).
Table 1.
Haematologic alterations due to typhoid fever infection
| Sample no. | Parameter | Positive | Control | p | Status |
|---|---|---|---|---|---|
| 1 | Packed cell volume -% | 38.09 ± 5.03 | 41.17 ± 4.15 | 0.000 | S∗∗ |
| 2 | Total white blood cell count -cells/µL | 2.09 | 6.74 ± 2.68 | 0.004 | S∗∗ |
| 3 | Neutrophils- cells/µL | 62.88 ± 16.06 | 61.62 ± 15.62 | 0.427 | N |
| 4 | Lymphocytes - cells/µL | 29.50 ± 15.01 | 30.64 ± 14.42 | 0.440 | N |
| 5 | Monocytes -cells/µL | 7.55 ± 3.05 | 7.65 ± 2.13 | 0.718 | N |
| 6 | Erythrocyte sedimentation rate - mm/h | 209.38 ± 81.19 | 258.51 ± 53.77 | 0.000 | S∗∗ |
| 7 | Haemoglobin concentration -g/dL | 12.58 ± 1.67 | 13.65 ± 1.42 | 0.000 | S∗∗ |
| 8 | Platelets - cells/µL | 11.22 ± 6.89 | 5.64 ± 6.77 | 0.000 | S∗∗ |
Data are presented as mean ± standard deviation. N, not significantly different; S, significantly different.
∗∗p < 0.01.
WBC count
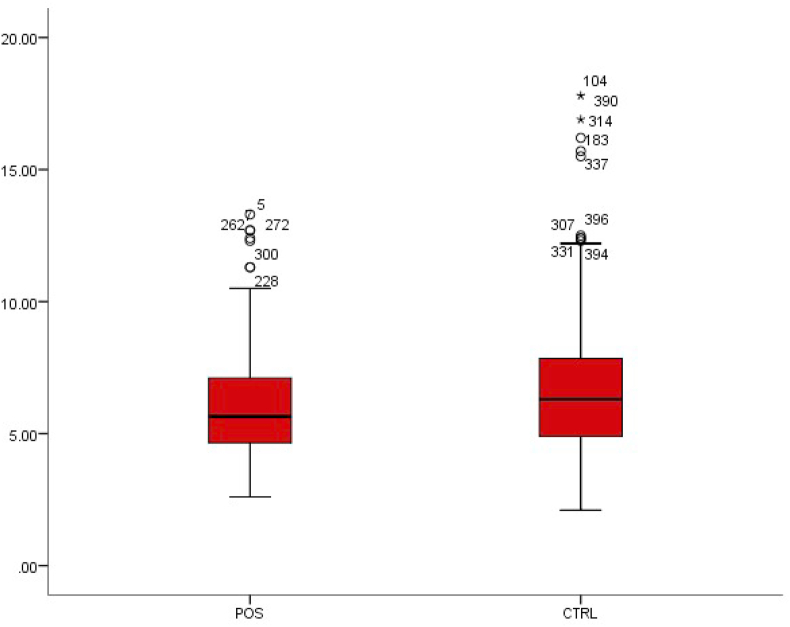
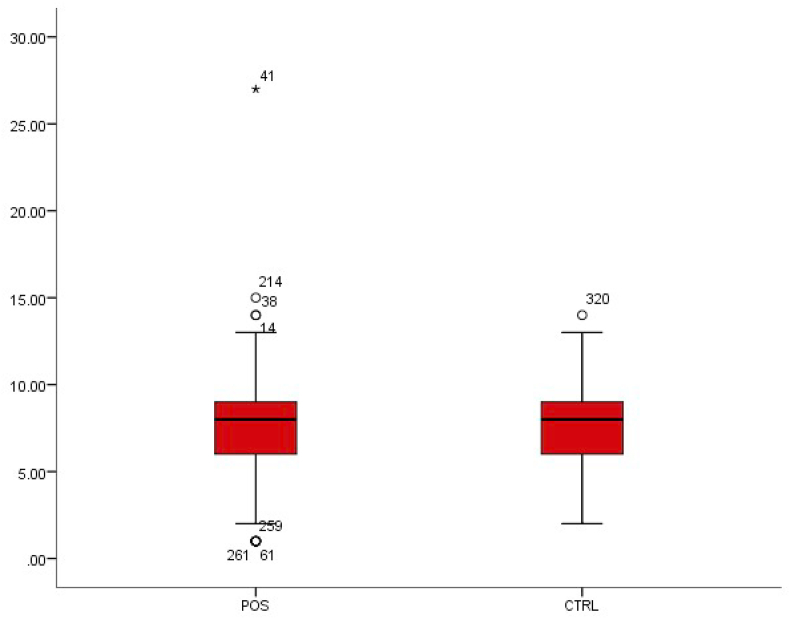
The WBC count in enteric or typhoid fever is often low [5]. The WBC count was 6.03 ± 2.09 in patients with typhoid fever and 6.74 ± 2.68 in patients without typhoid fever (Table 1). This was a statistically significant decrease in WBC (p < 0.01) (Fig. 1).
Fig. 1.
Box-plot distribution of white blood cells.
Packed cell volume
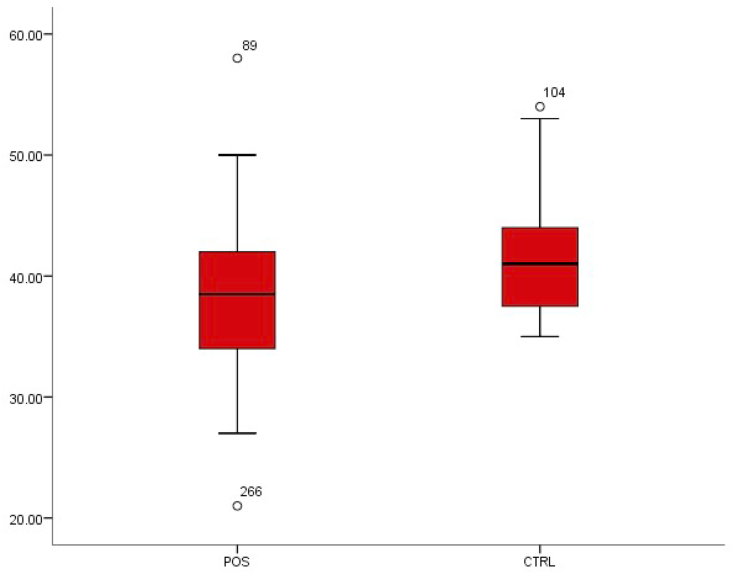
From the comparative values of the packed cell volume of subjects screened, it was observed that there was a statistically significant decrease from 41.17 ± 4.15 in patients without typhoid fever to 38.09 ± 5.03 in patients with typhoid fever (p < 0.01) (Fig. 2).
Fig. 2.
Box-plot distribution of packed cell volume.
Neutrophil level
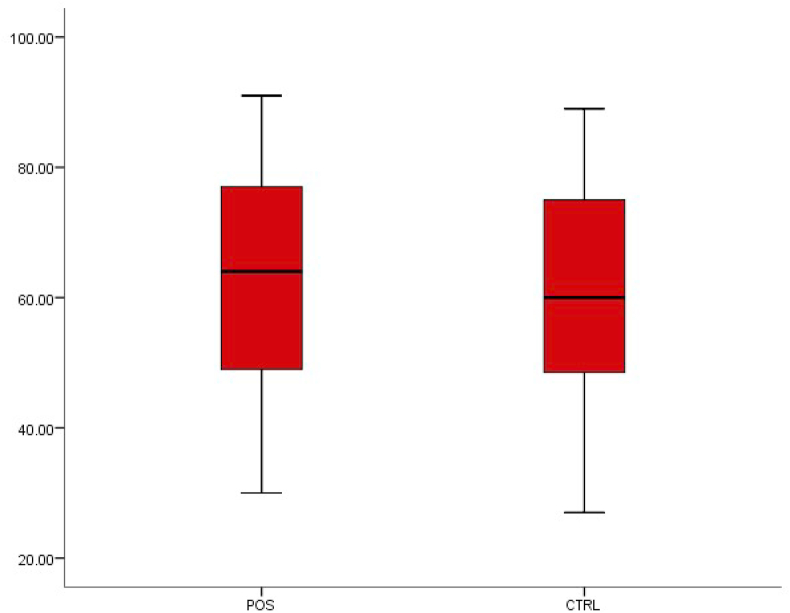
The neutrophil level observed in patients with typhoid fever was 62.88 ± 16.06, while in patients without typhoid fever it was 61.62 ± 15.62. This shows an increase in neutrophil level in patients with typhoid fever, although this difference was not statistically significant (p < 0.05) (Fig. 3).
Fig. 3.
Box-plot distribution of neutrophils.
Lymphocyte and monocyte levels
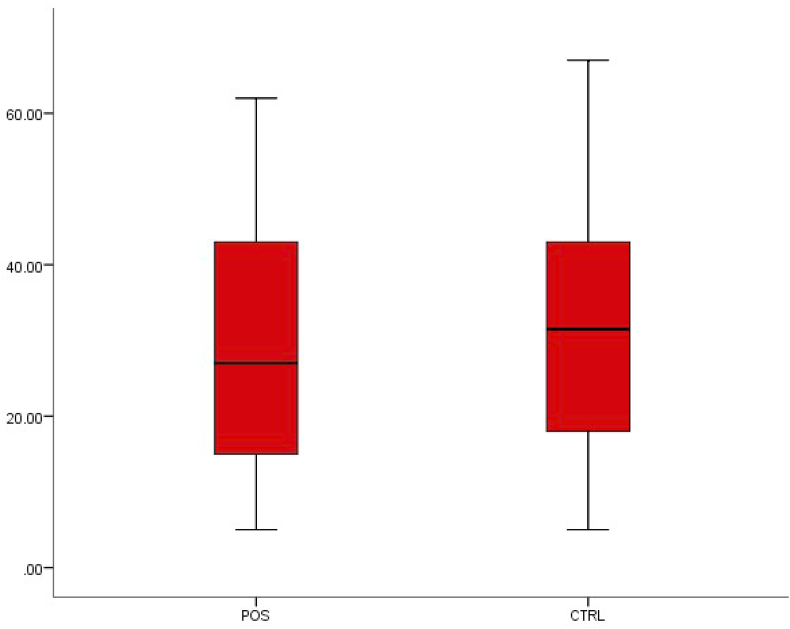
A decrease was observed in lymphocyte concentration from 30.64 ± 14.42 in healthy individuals to 29.50 ± 15.01 in patients with typhoid fever, and in monocytes from 7.65 ± 2.13 to 7.55 ± 3.05, but neither difference was statistically significant (p < 0.05) (Fig. 4, Fig. 5).
Fig. 4.
Box-plot distribution of lymphocytes.
Fig. 5.
Box-plot distribution of monocytes.
Erythrocyte sedimentation rate and haemoglobin concentration
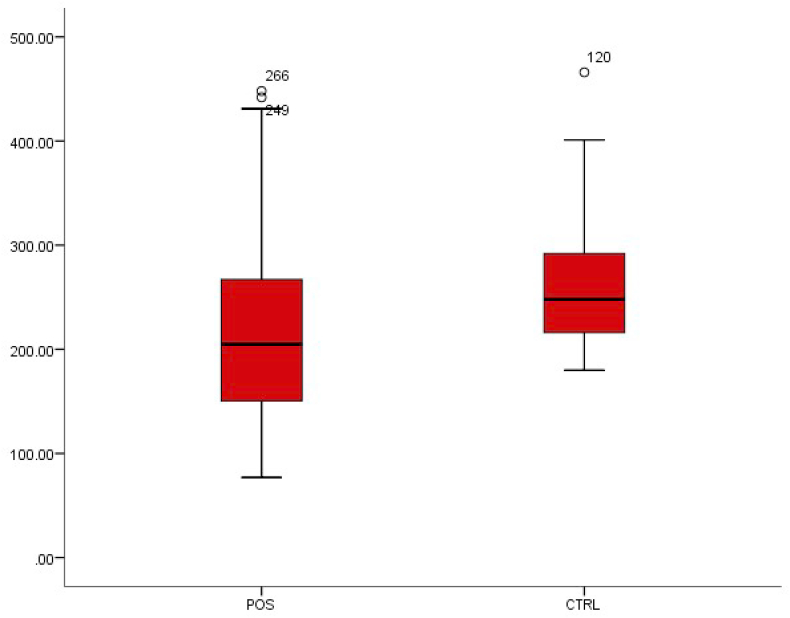
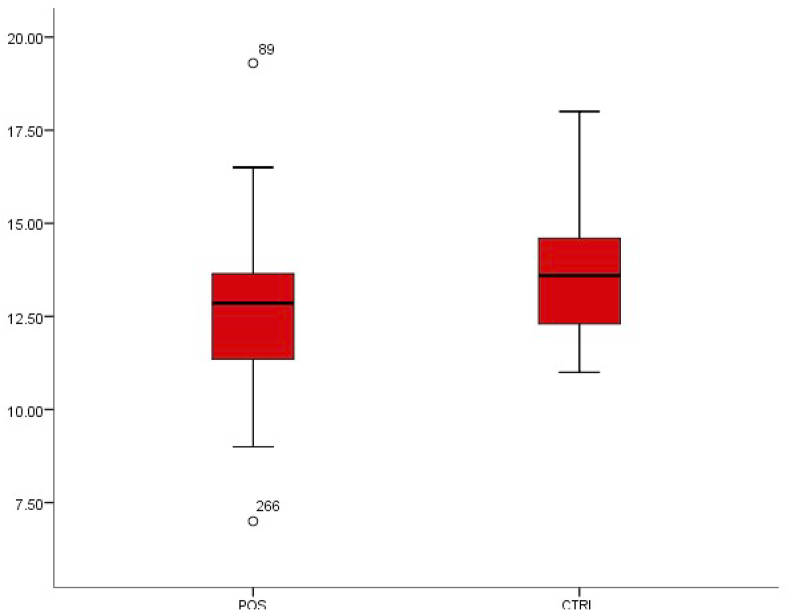
We observed that the mean values of erythrocyte sedimentation rate decreased in patients with typhoid fever from 209.38 ± 81.19 to 258.51 ± 53.77 in patients without typhoid fever. This decrease was also observed in haemoglobin concentration from 13.65 ± 1.42 to 12.58 ± 1.67. Both differences were statistically significant (p < 0.01) (Fig. 6, Fig. 7).
Fig. 6.
Box-plot distribution of erythrocyte sedimentation rate.
Fig. 7.
Box-plot distribution of haemoglobin concentration.
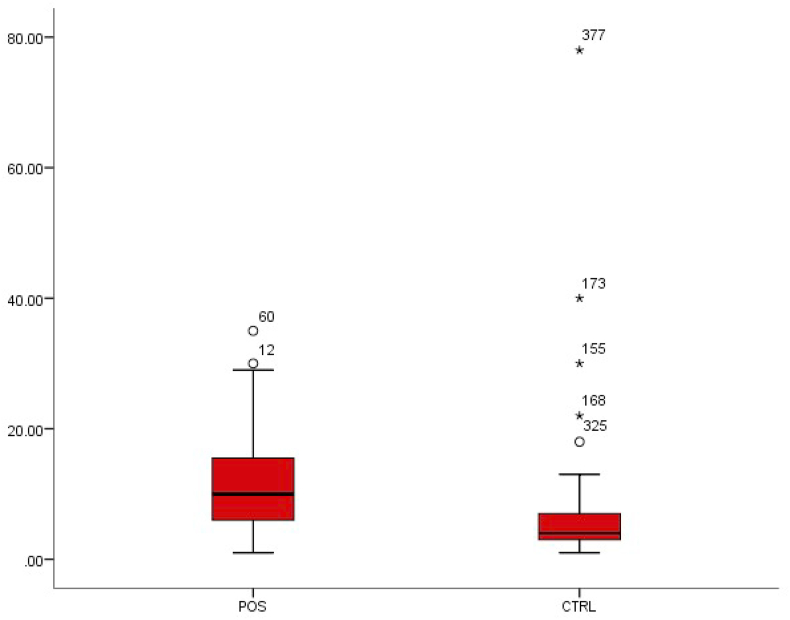
Platelet count
Platelet level was observed to significantly increase from 5.64 ± 6.77 in healthy individuals to 11.22 ± 6.89 in patients with typhoid fever (p < 0.01) (Fig. 8).
Fig. 8.
Box-plot distribution of platelets.
Discussion
From the obtained result, the observed decrease in the value of WBC in patients with typhoid fever can be attributed to the production of toxins in the bone marrow—that is, the site of myelopoiesis during bacterial metabolism. When the body becomes infected, the presence of WBCs is important in aiding the elimination of such infection. Therefore, a low level of WBCs (aka leukopenia) reduces the body's ability to fight infections [6].
Considering packed cell volume, a statistically significant difference was observed, with 41.17 ± 4.15 in patients without typhoid fever and 38.09 ± 5.03 in patients with typhoid fever, indicating a decrease in the percentage of red blood cells involved in blood circulation. A decrease in the production of red blood cells in the body could lead to suppressed bone marrow activity. This is however in contrast to the results of similar work carried out by Dangana et al. [8] in Abuja, which found a statistically insignificant increase in patients with typhoid fever. Moreover, the low packed cell volume observed among patients with typhoid fever suggests that typhoid fever could be a possible cause of anaemia [7]. Bone marrow suppression and haemophagocytosis are considered to be important mechanisms in producing haematologic changes [9].
The neutrophil level observed in patients with typhoid fever was 62.88 ± 16.06 compared to 61.62 ± 15.62 in patients without typhoid fever. Neutrophils are vital in oxygen-dependent toxicity in the elimination of bacteria when fighting infections, and they make up a large number of WBCs in the body. Therefore, there is always an increase in the number of neutrophils in response to the presence of an infection, as observed in typhoid fever. This shows that in this study, typhoid fever had no significant effect on neutrophils.
A decrease was observed in the lymphocyte concentration from 30.64 ± 14.42 in healthy individuals to 29.50 ± 15.01 in patients with typhoid fever, and in monocytes from 7.65 ± 2.13 to 7.55 ± 3.05; however, neither differences was statistically significant (p < 0.05) (Fig. 4, Fig. 5). Lymphocytes are an important part of the immune system; a reduced number of lymphocytes is referred to as lymphocytopenia. Monocytes have similar functions to lymphocytes, and they are produced in the bone marrow. They are vital in the elimination of infections [10].
The mean values of erythrocyte sedimentation rate were lower in patients with typhoid fever, at 209.38 ± 81.19 versus 258.51 ± 53.77 in patients without typhoid fever. This decrease was also observed in haemoglobin concentration, at respectively 13.65 ± 1.42 to 12.58 ± 1.67; both differences were statistically significant (p < 0.01) (Fig. 6, Fig. 7), indicating that a possible chance of developing anaemia exists. This can also lead to intestinal haemorrhage and other complications [7,11].
Increased platelet count, known as thrombocytosis, can lead to anaemia due to iron deficiency. This increase in platelets can also be the result of the presence of abnormal cells in the bone marrow, which is the site for myelopoiesis. Thrombocytosis has been discovered to be an indicator of severity of typhoid fever; its presence indicates that the individual is at risk of various complications [9,12].
The decrease in these haematologic parameters, except for observed increases in neutrophils and platelet counts in patients with typhoid fever, indicates that typhoid fever could have a more depressive effect on bone marrow activity and haematopoiesis than is commonly known. Typhoid fever diagnostic evaluations conducted on hospitalized patients provide little insight into applying diagnostic tests in the community healthcare setting. However, it is at the primary healthcare level where sensitive, specific, rapid, cheap and user-friendly typhoid diagnostic kits are most required [6,10].
To clinically differentiate typhoid fever from the other causes of febrile illness present in endemic regions, it is necessary to determine haemataologic parameters to further validate the health status of typhoid fever–positive subjects [13]. In addition, the high prevalence of anaemia recorded among patients with typhoid confirms a previous report [3]; this finding could be attributed to myeloid maturation arrest and a decrease in the number of erythroblasts. It is worth noting that leukopenia and thrombocytopenia are commonly present in Plasmodium and Salmonella infections [8,14].
Conclusion
From the evaluations conducted in this study, it is evident that the occurrence of anaemia, leucopenia and thrombocytopenia may be attributed to invasion of haematopoietic organs by Salmonella Typhi, causing depression of haematopoiesis. Our findings demonstrate that enteric fever affects most of the vital organs of the body, which can cause marked haematologic changes and which is therefore effective in diagnosing typhoid and paratyphoid fever. In addition, from the association we observed of leucopenia and thrombocytopenia among patients with typhoid fever, thrombocytopenia must be emphasized as one of the main factors to consider in diagnosing typhoid fever. In tropical countries where typhoid fever is endemic, awareness of this relationship is necessary for accurate diagnosis and therapy. Appropriate use of vaccines to prevent the occurrence of this illness is strongly advocated, particularly in highly endemic areas.
Conflict of interest
None declared.
Acknowledgements
The authors acknowledge the management of Landmark University Medical Center LMU, Omu-Aran, for granting approval to use the medical laboratory facility. The efforts of all the medical laboratory scientists are deeply appreciated. We particularly thank O. Olatinsu and S.-L. Jegede for providing editorial and statistical help.
References
- 1.Okafor A. Haematological parameters of Salmonella Typhi and Paratyphi culture positive patients from Kathmandu valley, Nepal. Malays J Microbiol. 2015;37:40–46. [Google Scholar]
- 2.Azmatullah A., Qamar F.N., Thaver D., Zaidi A.K., Bhutta Z.A. Systematic review of the global epidemiology, clinical and laboratory profile of enteric fever. J Glob Health. 2015;5:20407. doi: 10.7189/jogh.05.020407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abro A.H., Gangwani J.L., Ustadi A.M., Younis N.J., Hussaini H.S. Hematological and biochemical changes in typhoid fever. Pak J Med Sci. 2009;25:166–171. [Google Scholar]
- 4.Anusuya B., Sumathi S. Haematological alterations due to typhoid fever in Mayiladuthurai area, Nagapattinam. Int J Res Pharmacol Pharmacother. 2015;4(2) [Google Scholar]
- 5.Kayode O.T., Kayode A.A.A., Awonuga O.O. Status of Selected hematological and biochemical parameters in malaria and malaria–typhoid coinfection. J Biol Sci. 2011;11:367–373. [Google Scholar]
- 6.Ifeanyi O.E. Changes in some haematological parameters in typhoid patients attending university health services department of Michael Okpara university of agriculture, Nigeria. Int J Curr Microbiol Appl Sci. 2014;3:670–674. [Google Scholar]
- 7.Unaiza Q., Javeria A. Haematological changes associated with typhoid fever. Rawal Med J Microbiol. 2013;38(1) [Google Scholar]
- 8.Dangana A., Ajobiewe J., Nuhu A. Haematological changes associated with Salmonella Typhi and Salmonella Paratyphi in humans. Int J Biomed Health Sci. 2010;6(4) [Google Scholar]
- 9.Khosla S.N., Anand A., Singh U., Khosla A. Haematological profile in typhoid fever. Trop Doct. 1995;25:156–158. doi: 10.1177/004947559502500404. [DOI] [PubMed] [Google Scholar]
- 10.Parker T.M. Enteric infections: typhoid and paratyphoid fevers. Topley Wilson Princ Bacteriol Virol Immun. 2000;3:407. [Google Scholar]
- 11.Basten J.P.V., Stockenbrugger R. Typhoid perforation: a review of the literature since 1960. Trop Geogr Med. 1994;46:336–339. [PubMed] [Google Scholar]
- 12.Kawano R.L., Leano S.A., Agdamag D.M. Comparison of serological test kits for diagnosis of typhoid fever in the Philippines. J Clin Microbiol. 2007;45:246–247. doi: 10.1128/JCM.01403-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parry C.M., Wijedoru L., Arjyal A., Baker S. The utility of diagnostic tests for enteric fever in endemic locations. Expert Rev Anti Infect Ther. 2011;9:711–725. doi: 10.1586/eri.11.47. [DOI] [PubMed] [Google Scholar]
- 14.Okafor A.I. Haematological alterations due to typhoid fever in Enugu, urban Nigeria. Malaysian J Microbiol. 2007;3:19–22. [Google Scholar]