Abstract
Background
Interpretation of the increase in certain inflammatory markers in virally suppressed HIV-infected individuals must rely on an appropriate uninfected control group well characterized for non-HIV-related factors that contribute to chronic inflammation, e.g. smoking, alcohol consumption, or being overweight. We compared the inflammatory profiles of HIV-infected participants under long-term antiretroviral therapy (ART) with those of two HIV-uninfected groups with contrasting health behaviours.
Methods
We studied 150 HIV-infected participants (42 women, 108 men) under long-term ART (median, 6 years) followed in the ANRS PRIMO cohort since acute/early HIV-1 infection (AHI) diagnosis. Sex and age-matched controls were sampled from i) the ANRS IPERGAY pre-exposure prophylaxis trial among men at high risk for HIV infection and with high frequencies of non-HIV factors of inflammation ii) the ANRS COHVAC cohort of volunteers in vaccine trials with a low-risk profile for HIV infection. We measured the plasma levels of ten inflammatory markers.
Findings
After adjusting for smoking, alcohol use and body mass index, both HIV-infected men and women had higher levels of sCD14, sCD163, sTNFRII and I-FABP than their high-risk IPERGAY and low-risk COHVAC counterparts. Hierarchical clustering showed a subset of 15 PRIMO participants to have an inflammatory profile similar to that of most HIV-negative participants. These participants already had favourable markers at AHI diagnosis.
Interpretation
Long-term ART, even when initiated at a low level of immunodeficiency, fails to normalize monocyte/macrophage activation and gut epithelial dysfunction. Persistent inflammation under treatment may be related to an increased inflammatory profile since AHI.
Funding
ANRS and Paris-Saclay University.
Keywords: HIV infection, Inflammation, Immune activation, Long-term ART
Research in Context.
Evidence before this study
Many studies have assessed changes in inflammatory biomarkers after antiretroviral therapy (ART) initiation and reported only partial recovery to levels close to those seen in HIV-negative people. We searched PubMed with no language restrictions until September 01, 2020 with the terms: "Inflammation" [tiab] AND ("markers" [tiab] OR "biomarkers" [tiab]) AND ("HIV-negative" OR "HIV-uninfected" [tiab]) AND ("treatment" [tiab] OR "therapy" [tiab] OR "HAART" [tiab]). This search yielded 122 items. Among them, we identified only six studies that reported comparisons between virally-suppressed and HIV-negative individuals adjusted for exposures other that HIV that contribute to systemic immune activation (e.g., smoking, alcohol, substance abuse or coinfections) and/or had enrolled an appropriate group of HIV-negative participants. We also found limited data on women, and on people treated at a low level of immunodeficiency or from acute and early HIV infection (AHI). This is particularly important when considering people diagnosed in AHI , in whom restoration to levels close to HIV-negative subjects may be easier to achieve.
Added value of this study
We studied a large prospective cohort of both men and women diagnosed in acute and early HIV-1 infection and characterized their inflammatory profile under long-term ART (median 6·1 years, range, 3·2 – 17·0 years). Besides, we included two age- and sex-matched HIV-negative control groups, with contrasting profiles, in terms of risk of HIV infection and inflammatory cofactors (e.g. smoking, alcohol and drugs use). The first comparison group, comprising 102 men who have sex with men (MSM) at high risk for HIV infection, from the ANRS IPERGAY trial, can be seen as a sample of the uninfected source population from which most of the HIV-infected MSM enrolled in the PRIMO cohort could have originated. The second comparison group, comprising 141 volunteers for HIV preventive vaccine trials with a very low-risk profile for HIV infection, aimed at providing information on standard levels of inflammation in a population of the same sex and age as the HIV-infected participants in our study. We compared long-term ART-treated participants with these two HIV-uninfected control groups, for the levels of ten biomarkers that we selected to integrate the most described sources of inflammation, i.e. monocyte activation (soluble CD14 [sCD14], sCD163, CXCL10), mucosal inflammation (intestinal fatty-acid binding protein [I-FABP], interleukin 17 [IL-17]), and fibrosis (hyaluronic acid), in addition to standard non-specific markers of inflammation (ultrasensitive C-reactive protein [us-CRP], IL-6, tumor necrosis factor α [TNF-α], soluble TNF receptor II [sTNFRII]). Our virally-suppressed participants showed higher levels of soluble markers associated with monocyte activation and gut epithelial dysfunction, compared with both control groups. The inflammatory profiles of HIV-uninfected IPERGAY and COHVAC men were similar for markers associated with monocyte activation (sCD14, sCD163, CXCL10), but differed on some other markers. IPERGAY men showed higher levels of IL-17, TNF-α, and I-FABP, and lower levels of sTNFRII than COHVAC men. The differences in IL-17 and TNF-α levels persisted after adjusting for age, smoking and alcohol use, while those for sTNFRII or I-FABP levels were explained by these cofactors. Interestingly, we identified a small subset of 15 PRIMO participants with particularly low inflammatory profile under ART and also at the diagnosis of AHI , which was not explained by the HIV- or non-HIV-related factors we measured. This suggests that persistent inflammation under treatment may be related to an increased inflammatory profile since AHI.
Implications of all the available evidence
Monocyte activation and gut epithelial dysfunction appeared to be important drivers of inflammation in HIV-infected individuals. Exploring factors that determine activation levels prior to treatment would be of great interest to the development of therapeutic strategies to reduce persistent immune activation and inflammation in treated people living with HIV.
Alt-text: Unlabelled box
1. Introduction
The introduction of antiretroviral therapy (ART) has substantially improved the life expectancy of people living with HIV (PLHIV). However, overall rates of non-AIDS morbidities and mortalities remain higher in treated PLHIV than in the general population, particularly for those who initiated ART at late disease stages [1]. It is now well demonstrated that abnormally high immune activation persists, despite suppressive ART, and predicts a risk of subsequent non-AIDS related morbidity and mortality [2], [3], [4], [5], [6], [7]. Plasma levels of several markers fail to decrease to those of at-risk but HIV-uninfected controls, even in participants treated within the days after HIV acquisition, suggesting that certain drivers of immune activation are established very early in the course of infection [3,8].
However, non-HIV-related factors may contribute to this apparently abnormal persistent inflammatory state, such as smoking, alcohol consumption, viral infections, or being overweight [6]. Few studies have reported adjusted comparisons for these confounding factors, which are common and sometimes more prevalent in PLHIV than in the general population [6]. Elevated levels of IL-6, D-Dimers, and high-sensitivity CRP were described in virally suppressed participants of the SMART trial compared to those in two North-American general population cohorts, after adjusting for age, ethnicity, gender, body mass index (BMI), and smoking [9]. More recently, the MACS and COBRA cohorts reported higher monocyte activation in treated PLHIV than controls with a similar lifestyle [10,11]. However, these studies comprised almost exclusively men who have sex with men (MSM), whereas some studies reported sex-based disparities in associations between HIV status and inflammatory levels [12,13]. The participants of the COBRA study were also enrolled at an advanced stage of immunodeficiency. We therefore aimed to determine whether results observed in men are also true for women and people diagnosed during acute and early HIV-1 infection (AHI), in whom restoration to levels close to HIV-negative subjects could be easier to achieve.
Here, we characterized the persistent inflammatory state in men and women participants who had been under suppressive ART for years and followed since AHI in the ANRS PRIMO cohort [14]. We compared them with two HIV-uninfected control groups with either low- or high-risk health behaviours, particularly for HIV acquisition.
2. Methods
2.1. Participants
HIV-infected participants were followed in the PRIMO cohort, which enrolls patients with AHI from 95 French hospitals, as described elsewhere [14]. Clinical visit and biological measurements were planned at months 1, 3, and 6 and every 6 months thereafter. Samples of whole blood and plasma were collected and frozen at inclusion, at months 1, 3, 6, 12, and every 12 months thereafter. We selected participants who were treated for ≥ 36 consecutive months (with no interruption > 15 days) and with a sustained virological response (i.e. plasma viral load < 50 copies/mL within 9 months following ART initiation and > 90% of viral load measurements under ART < 50 copies/mL afterwards). The most recent visit with available frozen samples while on-ART was selected for exploration. We did not consider individuals of non-white ethnicity or those who died within the year after the selected sample or had a history of an AIDS-event or with a current B or C hepatitis coinfection (positive HbS antigenemia; positive HCV PCR). Participants were selected regardless of their time of treatment initiation, based on the results of a previous study of our group which did not show any difference in inflammatory levels after more than three years of treatment depending on the time to ART initiation, immediate at AHI diagnosis versus deferred during chronic infection [14]. We pragmatically set at 150 the number of PRIMO participants enrolled in this study. We had previously studied 97 participants who met the criteria mentioned above [14]. Therefore, we selected 53 additional participants, comprising all 27 eligible female participants, to best represent women, and 26 male participants randomly selected from the 244 eligible men. All selected participants were enrolled in the PRIMO cohort between 1997 and 2012, i.e. at a time when ART initiation in AHI was based on presence of symptoms and CD4+ T-cell count [15].
The PRIMO participants were compared with two contrasted HIV-uninfected groups, originating from the ANRS IPERGAY preexposure prophylaxis trial [16] and the ANRS COHVAC postvaccine trial cohort [17]. IPERGAY participants were MSM at high risk for HIV infection, defined as a history of unprotected anal sex with at least two partners during the previous six months, and exhibited high frequencies of non-HIV-related factors of inflammation: at enrolment in the trial, 28% were diagnosed with syphilis, gonorrhea, or chlamydia, 23% reported > 5 alcoholic drinks per day in the month, and 44% the use of recreational drugs in the past 12 months [16]. In contrast, COHVAC participants were volunteers for ANRS HIV preventive vaccine trials who were selected for trials because they were in good health and had a very low-risk profile for HIV infection. At enrolment in the COHVAC cohort, a low percentage (< 10%) of participants reported unprotected sex with partners of unknown HIV status [17].
Cisgender participants from the IPERGAY and COHVAC studies who did not acquire HIV nor HCV or HBV and with available frozen samples during follow-up were eligible to be controls. For IPERGAY participants, the selection was restricted to European individuals, whereas no data on ethnicity or geographical origin was collected in the COHVAC cohort. IPERGAY and COHVAC participants were matched 1:1 with PRIMO participants for sex and age (by five-year strata of current age). The sample size was pragmatically set at 150 HIV-positive PRIMO participants and 1:1 matched IPERGAY and COHVAC controls. No formal sample size calculation was performed. This sample size was considered to be sufficient to allow estimation of parameters with sufficient precision based on previous reports [10,14,18].
2.2. Ethics
The PRIMO and COHVAC cohorts were approved by the ethics committee (Comité de Protection des Personnes Ile-de-France III, n. 1157 and 2522 respectively). The IPERGAY trial protocol was approved by public health authorities and by ethics committees in France and Canada (Comité de Protection des Personnes Ile-de-France IV, Comité d'Ethique de la Recherche de Montreal, n. 2011/26). All participants gave their written informed consent to participate.
2.3. Measures
We centrally measured plasma levels of ten soluble biomarkers of all participants. sTNFRII, sCD14, sCD163, CXCL10, I-FABP, and hyaluronic acid were measured by specific ELISA (Human TNF RII/TNFRSF1B DuoSet ELISA, Human CD14 DuoSet ELISA, Human CD163 DuoSet ELISA, Human CXCL10/IP10 DuoSet ELISA, Human FABP2/I-FABP DuoSet ELISA, and Hyaluronan DuoSet ELISA, R&D Systems). IL-17, IL-6, and TNF-α were measured by single-molecule array (SiMoA) assay (Quanterix), and CRP by immunochemistry (CRP LX HS, Cobas C, integra, Roche Diagnostics). Samples with undetectable levels were attributed half the threshold value.
We measured plasma HIV RNA levels of PRIMO participants, using an ultrasensitive real-time PCR technique (GENERIC HIV, Biocentric, France) and total HIV DNA levels from whole blood samples using a real-time PCR assay (GENERIC Biocentric, France) [19].
The data that support the findings of this study are available on request to the corresponding author. The data are not publicly available due to privacy restrictions.
2.4. Statistics
We compared the levels of each biomarker of the PRIMO, IPERGAY and COHVAC participants using multiple linear regression models stratified by sex. We compared the levels of each biomarker of the PRIMO, IPERGAY and COHVAC participants using multiple linear regression models stratified by sex and adjusted for age, smoking, and alcohol use (Multivariate.1). We additionally adjusted the comparisons for BMI in further models restricted to men from the PRIMO and IPERGAY groups, where information on BMI was available (Multivariate.2). Because of the high percentage of individuals with undetectable CXCL10 levels (from 69 to 87% in each group), we transformed CXCL10 into a binary variable (undetectable versus detectable CXCL10 level) and used logistic regression models for this marker.
We also plotted radar charts to visualize the inflammatory profiles of each group, separately for men and women. To do this, for each marker we divided individuals into low and high-producers, depending on whether they had a level below versus equal or above the overall median level of all groups combined. The radar chart represents the percentage of high-producers in each group for the ten biomarkers. Finally, we performed a principal component analysis (PCA) and ascendant hierarchical clustering to identify individuals with similar inflammatory profiles. PCA allows to visualize proximities between individuals in a two-dimensional map. Hierarchical clustering aggregates similar individuals in clusters. We assessed to what extent participants of the three groups, PRIMO, COHVAC and IPERGAY, clustered together.
Given the very low percentage of missing data, all models were run in participants with complete data. Analysis was conducted in R (R Core Team, 2018).
2.5. Role of funders
This work was supported by the French Agency for Research on AIDS and Viral Hepatitis (ANRS) and a doctoral grant from Paris-Saclay University to S.N.
The funders had no role in the analyses, interpretation of the data, or decision to submit results
The corresponding author has full access to all the data in the study and had final responsibility for the decision to submit for publication.
3. Results
3.1. Participant characteristics
We analysed 150 participants of the PRIMO cohort, 42 women and 108 men, with a median age of 47 years (IQR, 41–53). They had been under ART for a median of 6·1 years (IQR, 5·0–7·8; range, 3·2–17·0). The median CD4+ T-cell count was 733 cells/µL and 40% still had a CD4:CD8 ratio < 1. The ultrasensitive viral load was undetectable for 64% of participants (median threshold: 3 copies/mL). We compared them to 41 women and 100 men of the COHVAC cohort and 102 men of the IPERGAY trial. Despite the matching, the COHVAC men tended to be older than their IPERGAY counterparts, likely due to the width of the 5 year-strata of age chosen for matching with the PRIMO participants. The COHVAC participants particularly differed from the IPERGAY and PRIMO participants in that they were less likely to report tobacco and alcohol use, whereas the PRIMO and IPERGAY individuals were similar in terms of smoking, alcohol use and BMI (Table 1).
Table 1.
Characteristics of the study participants.
| Variables | Men |
Women |
|||||
|---|---|---|---|---|---|---|---|
| PRIMO, n = 108 | COHVAC n = 100 | IPERGAY n = 102 | P-valuea | PRIMO n = 42 | COHVAC n = 41 | P-valuea | |
| Age, years | 47 (41, 52) | 49 (44, 54) | 46 (41, 51) | 0·05 | 49 (42, 59) | 48 (44, 60) | 0·73 |
| BMI, kg/m2 | |||||||
| Normal, (18·5–24·9) | 64·8 (70) | nd | 71·6 (73) | 0·68 | 57·1 (24) | nd | |
| Overweight, (25–29·9) | 25·0 (27) | nd | 20·6 (21) | 23·8 (10) | nd | ||
| Obesity (> 30) | 7·4 (8) | nd | 7·8 (8) | 9·5 (4) | nd | ||
| Missing | 2·8 (3) | nd | 0 (0) | 9·5 (4) | nd | ||
| Current smokerb | 50·0 (54) | 11·0 (11) | 42·2 (43) | < 0·0001 | 50·0 (21) | 17·1 (7) | 0·002 |
| Alcohol consumerb | 77·8 (84) | 25·0 (25) | 75·5 (77) | < 0·0001 | 54·8 (23) | 19·5 (8) | 0·001 |
| Cumulative treatment duration, months | 75 (61, 94) | 70 (59, 94) | |||||
| CD4+ T-cell count, cells/µL | 715 (595, 859) | 779 (630, 980) | |||||
| CD4:CD8 ratio | 1·1 (0·9, 1·5) | 1·4 (1·0, 1·8) | |||||
| Ultrasensitive HIV RNA level | |||||||
| Undetectable | 59·3 (64) | 69·0 (29) | |||||
| Detection threshold, copies/mLc | 3 (2, 3) | 3 (2, 3) | |||||
| Detectable samples, copies/mL | 10 (3, 32) | 15 (11, 46) | |||||
| Total HIV DNA levels, log10 copies/106 PBMCsd | 2·6 (2·1, 2·9) | 2·5 (1·9, 2·9) | |||||
| Current ART regimene | |||||||
| With INSTI | 15·7 (17) | 16·7 (7) | |||||
| With PI | 22·2 (24) | 33·3 (14) | |||||
| With NNRTI | 61·1 (66) | 47·6 (20) | |||||
| Others | 0·9 (1) | 2·4 (1) | |||||
Data are presented as the median (interquartile range) or percentage (No.).
Abbreviations. ART, antiretroviral therapy; BMI, body mass index; nd, not documented; INSTI, integrase stand transfer inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor; PBMCs, peripheral blood mononuclear cells; PI, protease inhibitor
Wilcoxon Mann-Whitney test for continuous variables and χ2 test for categorical variables.
in the past year, except for COHVAC participants, consumption at the enrolment visit.
the detection threshold depended on the available plasma volume.
HIV DNA levels measured within a year before the measurement of inflammatory markers.
classification rule: regimen with INSTI, if not with PI, if not with NNRTI, if not others.
3.2. Impact of HIV-1 infection
In univariate analyses, PRIMO men and women differed from both their IPERGAY and COHVAC counterparts by higher levels of sCD163, sCD14, sTNFRII and I-FABP (Fig. 1, Supplementary Table 1). The differences for these four markers remained statistically significant after adjusting for age, current smoking and alcohol use, and also after a further adjustment for BMI in a model restricted to the PRIMO and IPERGAY men (Table 2).
Fig. 1.
Comparison of inflammatory profiles as shown by radar chart.
Each axis displays the proportion from 0 to 100% of high producers for a given biomarker, i.e. the proportion of individuals who had a level greater than the median level of the three groups combined. The values for each axis can be connected by lines to form the central polygon area, which represents the magnitude of the inflammatory profiles.
Table 2.
Impact of HIV-1 infection on each inflammatory biomarker among men and women
| Univariatea | Multivariate.1b | Multivariate.2 c | ||||
|---|---|---|---|---|---|---|
| Estimate (95%CI) | P-value | Estimate (95%CI) | P-value | Estimate (95%CI) | P-value | |
| sCD14, ng/mL | ||||||
| Men | ||||||
| PRIMO | Ref. | – | Ref. | – | Ref. | – |
| COHVAC | −507 (−622, −392) | < 0·0001 | −499 (−633, −365) | < 0·0001 | ||
| IPERGAY | −544 (−659, −430) | < 0·0001 | −535 (−651, −420) | < 0·0001 | −547 (−672, −422) | < 0·0001 |
| Age, years | 4 (−2, 10) | 0·24 | 4 (−1, 10) | 0·10 | 7 (0, 14) | 0·05 |
| Alcohol consumer | 110 (−1, 221) | 0·05 | −16 (−128, 95) | 0·77 | −20 (−170, 129) | 0·79 |
| Current smoker | 143 (29, 257) | 0·01 | 52 (−56, 159) | 0·34 | 37 (−92, 166) | 0·57 |
| BMI | ||||||
| Normal | Ref. | – | Ref. | – | ||
| Overweight | −97 (−271, 77) | 0·27 | −163 (−317, −9) | 0·04 | ||
| Obesity | −216 (−491, 59) | 0·12 | −239 (−476, −3) | 0·05 | ||
| Women | ||||||
| PRIMO | Ref. | – | Ref. | – | ||
| COHVAC | −647 (−897, −396) | < 0·0001 | −626 (−906, −346) | < 0·0001 | ||
| Age, years | 3 (−8, 14) | 0·57 | 4 (−6, 14) | 0·41 | ||
| Alcohol consumer | 275 (−17, 567) | 0·06 | 6 (−302, 314) | 0·97 | ||
| Current smoker | 271 (−28, 570) | 0·08 | 63 (−257, 384) | 0·70 | ||
| sCD163, ng/mL | ||||||
| Men | ||||||
| PRIMO | Ref. | – | Ref. | – | Ref. | – |
| COHVAC | −91 (−144, −38) | 0·001 | −110 (−171, −48) | 0·001 | ||
| IPERGAY | −71 (−124, −19) | 0·01 | −76 (−129, −23) | 0·01 | −77 (−136, −18) | 0·01 |
| Age, years | 1 (−1, 3) | 0·38 | 1 (−2, 3) | 0·47 | −1 (−4, 3) | 0·77 |
| Alcohol consumer | 33 (−12, 78) | 0·15 | 17 (−34, 68) | 0·52 | 8 (−63, 78) | 0·83 |
| Current smoker | −38 (−85, 8) | 0·11 | −68 (−118, −19) | 0·01 | −59 (−120, 2) | 0·06 |
| BMI | ||||||
| Normal | Ref. | – | Ref. | – | ||
| Overweight | 39 (−32, 109) | 0·28 | 22 (−50, 95) | 0·54 | ||
| Obesity | 70 (−42, 181) | 0·22 | 58 (−53, 170) | 0·30 | ||
| Women | ||||||
| PRIMO | Ref. | – | Ref. | – | ||
| COHVAC | −117 (−195, −38) | 0·004 | −156 (−242, −71) | 0·001 | ||
| Age, years | −1 (−4, 3) | 0·69 | −1 (−4, 2) | 0·56 | ||
| Alcohol consumer | −25 (−110, 61) | 0·57 | −55 (−148, 39) | 0·25 | ||
| Current smoker | −25 (−112, 62) | 0.57 | −63 (−161, 34) | 0.20 | ||
| sTNFRII, pg/mL | ||||||
| Men | ||||||
| PRIMO | Ref. | – | Ref. | – | Ref. | – |
| COHVAC | −1013 (−1387, −639) | < 0·0001 | −1171 (−1609, −733) | < 0·0001 | ||
| IPERGAY | −1358 (−1731, −985) | < 0·0001 | −1341 (−1717, −966) | < 0·0001 | −1331(−1751, −911) | < 0·0001 |
| Age, years | 5 (−13, 23) | 0·58 | 5 (−−12, 23) | 0·53 | 1 (−24, 24) | 0·97 |
| Alcohol consumer | −114 (−455, 228) | 0·51 | −421 (−786, −57) | 0·02 | −463 (−971, 44) | 0·07 |
| Current smoker | 321 (−28, 670) | 0·07 | 199 (−150, 548) | 0·26 | 286 (−146, 718) | 0·19 |
| BMI | ||||||
| Normal | Ref. | – | Ref. | – | ||
| Overweight | 29 (−519, 576) | 0·92 | 22 (−498, 542) | 0·93 | ||
| Obesity | 93 (−765, 950) | 0·83 | 132(−660, 924) | 0·74 | ||
| Women | ||||||
| PRIMO | Ref. | – | Ref. | – | ||
| COHVAC | −1189 (−1727, −651) | < 0·0001 | −1490 (−2064, −915) | < 0·0001 | ||
| Age, years | 9 (−14, 32) | 0·44 | 8 (−13, 29) | 0·45 | ||
| Alcohol consumer | −131 (−753, 490) | 0·68 | −541 (−1175, 93) | 0·09 | ||
| Current smoker | −115 (−752, 523) | 0·72 | −362 (−1020, 297) | 0·28 | ||
| I-FABP, pg/mL | ||||||
| Men | ||||||
| PRIMO | Ref. | – | Ref. | – | Ref. | – |
| COHVAC | −1171 (−1364, −977) | < 0·0001 | −1053 (−1279, −827) | < 0·0001 | ||
| IPERGAY | −934 (−1364, −742) | < 0·0001 | −913 (−1108, −718) | < 0·0001 | −930 (−1137, −723) | < 0·0001 |
| Age, years | −4 (−15, 7) | 0·51 | 0 (−9, 9) | 0,96 | −2 (−14, 10) | 0·79 |
| Alcohol consumer | 478 (281, 674) | < 0·0001 | 135 (−53, 323) | 0,16 | 182 (−68, 433) | 0·15 |
| Current smoker | 407 (203, 610) | < 0·0001 | 108 (−73, 289) | 0,24 | 106 (−108, 321) | 0·33 |
| BMI | ||||||
| Normal | Ref. | – | Ref. | – | ||
| Overweight | 29 (−258, 315) | 0·84 | −30 (−286, 226) | 0·82 | ||
| Obesity | −210 (−659, 238) | 0·36 | −235 (−605, 135) | 0·21 | ||
| Women | ||||||
| PRIMO | Ref. | – | Ref. | – | ||
| COHVAC | −1259 (−1540, −977) | < 0·0001 | −1224 (−1533, −915) | < 0·0001 | ||
| Age, years | 6 (−10, 21) | 0·48 | 7 (−5, 18) | 0·24 | ||
| Alcohol consumer | 489 (84, 893) | 0·02 | 15 (−321, 351) | 0·93 | ||
| Current smoker | 476 (59, 893) | 0·03 | 105 (−248, 459) | 0·55 | ||
Results from linear regression models.
Multivariate 1: results from a multiple linear regression adjusted for age, alcohol, and smoking.
Multivariate 2: results from a multiple linear regression adjusted for age, alcohol, smoking and BMI and restricted to men of the PRIMO and IPERGAY groups.
Abbreviations: BMI, body mass index; 95%CI, 95% confidence interval; Ref., reference
See Supplementary Table 2 for us-CRP, IL-6, TNF-α, IL-17, CXCL10 and hyaluronic acid.
Some other differences between the ART-treated and HIV-uninfected participants depended on the control group. Indeed, PRIMO and COHVAC participants had similar IL-17 levels, both among men and women, whereas the IPERGAY men had higher IL-17 levels. Moreover, PRIMO participants had higher TNF-α levels than their COHVAC counterparts, but similar levels to their IPERGAY counterparts (Supplementary Table 2). The PRIMO and IPERGAY men had similar us-CRP levels, and elevated compared to the COHVAC men. Of note, this difference was no longer statistically significant after adjustment for smoking and alcohol. Finally, there was no difference between groups for hyaluronic acid levels. All these results are summarized in terms of high (versus low) levels for each marker and depicted in Fig. 1.
Certain other differences were sex-specific: IL-6 levels were higher in HIV-infected women than HIV-uninfected women, but not between the men, regardless of the control group. HIV-infected men showed higher CXCL10 levels than HIV-uninfected men, whereas no difference was observed among the women.
We then performed a PCA to visualize correlations between biomarkers and identify participants with similar inflammatory profiles. We removed CXCL10 from the following analyses because 80% of the participants had undetectable levels of this marker. Those with complete data for the nine other markers were 133, 138, and 93 participants from the PRIMO, COHVAC, and IPERGAY groups, respectively. Principal components PC1 and PC2 explained 41·6% of the total variance (Fig. 2a). PC1 was mainly defined by sTNFRII and sCD163, plus us-CRP and TNF-α. PC2 was defined by the opposition between IL-17 and I-FABP. sCD14 contributed to both components. The concentration ellipses (Fig. 2b) showed that the IPERGAY ellipse comprised all the COHVAC participants and only one-third of the PRIMO participants; the remaining two-thirds PRIMO participants were predominantly located in the lower right quadrant and were those who showed higher levels of all inflammatory markers than the other participants.
Fig. 2.
Principal components analysis.
a. Graph of variables. The first principal component (PC) was mainly defined by sTNFRII, us-CRP, TNF-α and sCD163. The second PC was mainly defined by IL-17 and I-FABP. Soluble CD14 contributed to both components. b. Graph of individuals. Near one-third of the PRIMO participants overlapped with most of the COHVAC and IPERGAY participants. The remaining two-thirds were located into the lower right quadrant of the graph. The COHVAC ellipse was completely included in the IPERGAY ellipse, which was vertically wider, due to some individuals with particularly high IL-17 levels who projected into the upper right quadrant of the graph. PC: principal component
Ascendant hierarchical clustering from raw data revealed two clusters. Cluster 1 comprised 74% of COHVAC participants (n = 102), 62% of IPERGAY participants (n = 58) and only 15 PRIMO participants. Most PRIMO participants (89%, n = 118) were clustered in cluster 2, along with one-quarter and one-third of the COHVAC and IPERGAY control groups (26%, n = 36 and 38%, n = 35, respectively). The 15 PRIMO individuals included in cluster 1 showed lower levels of sTNFRII, sCD163, sCD14 and I-FABP than their cluster 2 PRIMO counterparts. These differences in inflammatory marker levels were not explained by age, sex, smoking, alcohol consumption nor BMI. PRIMO participants of the two clusters also had similar levels of residual viral replication, CD4+ T-cell counts and CD4:CD8 ratios on treatment (Table 3). However, cluster 1 PRIMO participants already had higher CD4+ T-cell counts and CD4:CD8 ratios during AHI than their cluster 2 counterparts, and therefore they less often initiated ART in AHI compared to their cluster 2 counterparts.
Table 3.
Comparison of PRIMO participants between the two clusters revealed by hierarchical clustering.
| Characteristics | Cluster 1, n = 15 Low inflammatory levels on ART | Cluster 2, n = 118. Elevated inflammatory levels on ART | P-valuea | ||
|---|---|---|---|---|---|
| Sex, men | 73·3 (11) | 72·0 (85) | 1 | ||
| Age, years | 46·4 (41·8, 53·8) | 48·2 (42·9, 53·7) | 0·67 | ||
| Under ART | |||||
| Current smoker | 46·7 (7) | 49·2 (58) | 0·87 | ||
| Alcohol consumer | 73·3 (11) | 70·3 (83) | 1·00 | ||
| BMI, kg/m² | 25·2 (23·0, 28·5) | 23·8 (21·4, 25·5) | 0·17 | ||
| ART duration, months | 66·9 (59·9, 71·8) | 75·8 (60·3, 94·9) | 0·17 | ||
| CD4+ T-cell count, cells/µL | 776 (637, 844) | 712 (590, 872) | 0·50 | ||
| CD4:CD8 Ratio | 1·3 (1·0, 1·5) | 1·2 (0·9, 1·7) | 0·75 | ||
| Ultrasensitive HIV RNA levels | |||||
| Undetectable | 60·0 (9) | 61·9 (73) | 0·89 | ||
| Detection threshold, copies/mLb | 2 (2, 4) | 3 (2, 3) | 0·89 | ||
| Detectable samples, copies/mL | 46 (14, 32) | 11 (4, 32) | 0·20 | ||
| Total HIV DNA level, log10 copies/106 PBMCsc | 2·8 (2·6, 3·1) | 2·5 (2·1, 2·8) | 0·02 | ||
| us-CRP, mg/mL | 1·47 (0·78, 2·47) | 1·59 (0·83, 3·35) | 0·53 | ||
| IL-6, pg/mL | 1·25 (0·88, 1·86) | 1·23 (0·78, 1·94) | 0·91 | ||
| IL-17, pg/mL | 0·07 (0·05, 0·16) | 0·09 (0·06, 0·17) | 0·78 | ||
| TNF-α, pg/mL | 2·79 (2·29, 3·40) | 3·15 (2·62, 3·98) | 0·08 | ||
| CXCL10 < 37 pg/mL | 86·7 (13) | 68·6 (81) | 0·23 | ||
| sTNFRII, pg/mL | 2595 (2115, 3532) | 3455 (2764, 4232) | 0·02 | ||
| Hyaluronic acid, ng/mL | 16·3 (10·6, 27·3) | 13·4 (8·5, 21·4) | 0·21 | ||
| sCD163, ng/mL | 404 (293, 485) | 476 (379, 643) | 0·02 | ||
| sCD14, ng/mL | 1554 (1401, 1770) | 2205 (1937, 2616) | < 0·0001 | ||
| I-FABP, pg/mL | 1547 (1154, 2212) | 2587 (1999, 2961) | < 0·0001 | ||
| At AHI diagnosis | |||||
| Symptomatic AHI | 73·3 (11) | 89·0 (105) | 0·10 | ||
| CD4+ T-cell count, cells/µL | 636 (428, 852) | 523 (371, 681) | 0·19 | ||
| Plasma HIV RNA levels, log10 copies/mL | 5·1 (3·9, 5·8) | 5·1 (4·5, 5·6) | 0·83 | ||
| CD4:CD8 ratio | 0·9 (0·5, 1·2) | 0·5 (0·3, 0·8) | 0·004 | ||
| Total HIV DNA level, log10 copies/106 PBMCs | 3·3 (2·9, 3·5) | 3·3 (3·0, 3·6) | 0·52 | ||
| Protective HLA I alleled | 33·3 (3) | 25·0 (20) | 0·69 | ||
| ART initiated in the month following AHI diagnosis | 13·3 (2) | 47·5 (56) | 0·01 | ||
Data are presented as the median (interquartile range) or percentage (No.).
Wilcoxon Mann–Whitney test for continuous variables and χ2 test for categorical variables.
detection threshold depended on the available plasma volume.
HIV DNA levels measured within a year before the measurement of inflammatory markers.
HLA class I alleles associated with slow progression of HIV disease [20]: (HLA-A*25, HLA-A*32, HLA-A*74, HLA-B*14, HLA-B*27, HLA-B*51, HLA-B*52, HLA-B*57) Data available for 9 and 80 participants.
Abbreviations: AHI, acute and early HIV infection; ART, antiretroviral therapy; BMI, body mass index; PBMCs, peripheral blood mononuclear cells.
Moreover, they already had lower levels of sCD14 and CCL2 (another marker of monocyte activation) during AHI and tended to have lower levels for other inflammation markers, although the differences were not always statistically significant (Fig. 3).
Fig. 3.
Inflammatory levels measured at AHI diagnosis.
Intergroup comparisons were performed using tobit regression models for left-censored variables with undetectable values, or otherwise linear regression models. P-values are adjusted for age, sex, and time since the estimated date of HIV acquisition.
3.3. Cofactors of inflammation and the IPERGAY/COHVAC comparison
In the PRIMO participants, no inflammatory marker after a median of six years of ART was associated with the duration of treatment, time to ART initiation, or markers of HIV infection (CD4+ T-cell count, CD4/CD8 ratio, ultrasensitive viral load, and HIV DNA levels), except for sTNFRII levels which slightly correlated with the CD4:CD8 ratio (Spearman rho = −0·22, P = 0·01).
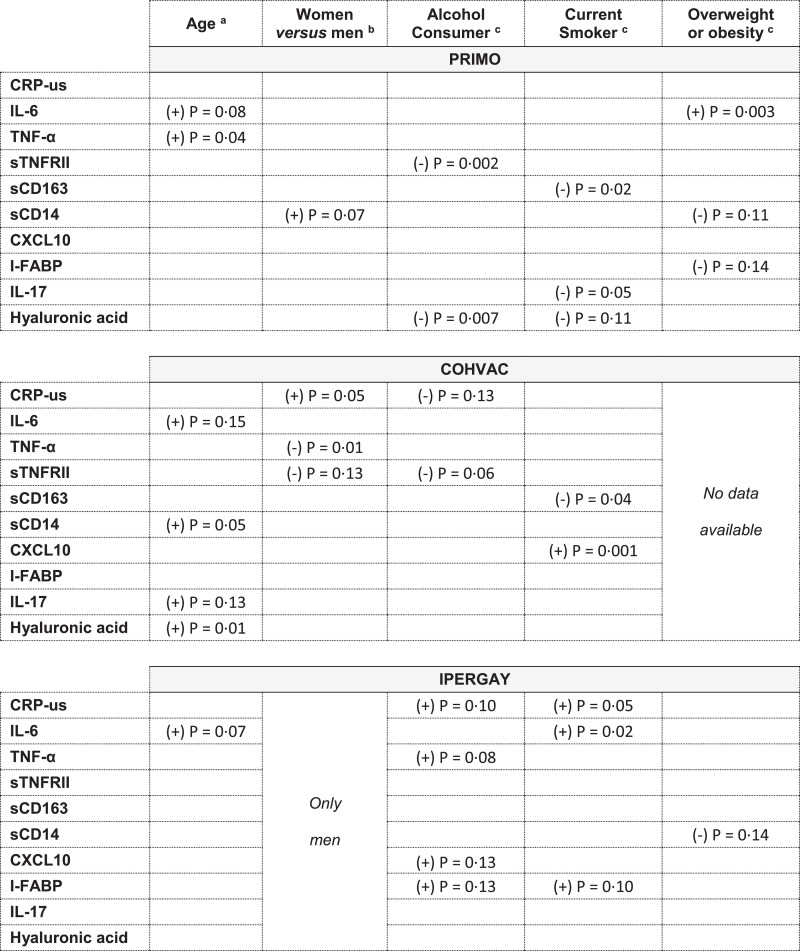
We then evaluated associations of non-HIV related factors (smoking, alcohol consumption and BMI) with inflammatory levels, first in the PRIMO participants only. We ran univariate regression models and then multivariate regression models adjusted for age and sex. Secondly, we looked for similar associations in the group of uninfected COHVAC and IPERGAY participants. We proceeded this way for each of the 10 markers (Fig. 4).
Fig. 4.
Inflammatory levels and associations with non-HIV-related factors. (a) from multivariate linear (or logistic for CXCL10) regression models adjusted for sex. (b) from multivariate linear (or logistic for CXCL10) regression models adjusted for age. (c) from multivariate linear (or logistic for CXCL10) regression models adjusted for age and sex. Summary table of the associations between inflammatory levels and non-HIV related factors, obtained from multiple linear (or logistic for CXCL-10) regression models, by cohort. For each marker, the direction of the association is symbolized by a sign positive (+) or negative (-). P-values are estimated from likelihood-ratio test. Empty box is used to indicate a P-value > 0·15.
In the PRIMO participants, smoking was associated with lower sCD163 and IL-17 levels (likelihood-ratio test, multiple regression, P = 0·02 and 0·05, respectively), alcohol consumption with lower sTNFRII and hyaluronic acid levels (P = 0·002 and P = 0·007, respectively), and obesity with higher IL-6 levels (P = 0·003). Women had higher sCD14 levels than men, although the difference was not statistically significant (+201 ng/mL, P = 0·07). Age was associated with increase in TNF-α levels (P = 0·04).
The associations between sCD163 and smoking and between sTNFRII and alcohol consumption were also found in the COHVAC participants (Fig. 4).
The inflammatory profiles of IPERGAY and COHVAC men were similar for markers associated with monocyte activation (sCD14, sCD163, CXCL10), but not for some other markers. IPERGAY men showed higher levels of IL-17, TNF-α, and I-FABP, and lower levels of sTNFRII than COHVAC men. The differences in IL-17 and TNF-α levels persisted after adjusting for age, smoking and alcohol use, but not those for sTNFRII or I-FABP levels (Fig. 1, Table 4, Supplementary Table 3).
Table 4.
Higher inflammatory levels in IPERGAY men compared to COHVAC men
| Univariate a |
Multivariate b |
|||
|---|---|---|---|---|
| Estimate (95%CI) | P-value | Estimate (95%CI) | P-value | |
| TNF-α, pg/mL | ||||
| COHVAC | Ref. | – | Ref. | – |
| IPERGAY | 0·83 (0·42, 1·24) | < 0·0001 | 0·73 (0·22, 1·23) | 0·01 |
| Age, years | 0·01 (−0·02, 0·03) | 0·56 | 0·02 (−0·01, 0·04) | 0·14 |
| Alcohol consumer | 0·61 (0·19, 1·03) | 0·01 | 0·25 (−0·23, 0·73) | 0·30 |
| Current smoker | 0·38 (−0·10, 0·86) | 0·12 | 0·07 (−0·44, 0·57) | 0·79 |
| sTNFRII, pg/mL | ||||
| COHVAC | Ref. | – | Ref. | – |
| IPERGAY | −345 (−630, −59) | 0·02 | −286 (−640, 68) | 0·11 |
| Age, years | 13 (−3, 29) | 0·12 | 10 (−7, 26) | 0·26 |
| Alcohol consumer | −225 (−515, 64) | 0·13 | −67 (−406, 272) | 0·70 |
| Current smoker | −116 (−445, 213) | 0·49 | 44 (−308, 396) | 0·80 |
| IL-17, pg/mL | ||||
| COHVAC | Ref. | – | Ref. | – |
| IPERGAY | 0·48 (0·21, 0·76) | 0·001 | 0·48 (0·18, 0·80) | 0·001 |
| Age, years | −0·01 (−0·02, 0·01) | 0·43 | −0·00 (−0·02, 0·02) | 0·87 |
| Alcohol consumer | 0·30 (0·02, 0·58) | 0·04 | 0·09 (−0·23, 0·42) | 0·57 |
| Current smoker | 0·04 (−0·28, 0·37) | 0·80 | −0·19 (−0·53, 0·15) | 0·28 |
| I-FABP, pg/mL | ||||
| COHVAC | Ref. | – | Ref. | – |
| IPERGAY | 251 (69, 432) | 0·01 | 154 (−67, 375) | 0·17 |
| Age, years | 1 (−9, 12) | 0·80 | 5 (−5, 16) | 0·34 |
| Alcohol consumer | 230 (48, 413) | 0·01 | 126 (−86, 339) | 0·24 |
| Current smoker | 221 (12, 430) | 0·04 | 136 (−86, 359) | 0·23 |
Results from linear regression models.
Results from a multiple linear regression adjusted for age, alcohol, and smoking
Abbreviations: 95%CI, 95% confidence interval; Ref. Reference
See Supplementary Table 3 for us-CRP, IL-6, sTNFRII, CXCL10, sCD14, sCD163 and hyaluronic acid.
4. Discussion
Here we compared long-term ART-treated participants with two HIV-uninfected control groups, with either a low or high risk of HIV acquisition, for the levels of ten biomarkers that we selected to integrate the most described sources of inflammation, i.e. monocyte activation (sCD14, sCD163, CXCL10), mucosal inflammation (I-FABP, IL-17), and fibrosis (hyaluronic acid), in addition to standard non-specific markers of inflammation (us-CRP, IL-6, TNF-α, sTNFRII). Among these markers, sTNFRII, sCD14, sCD163, and I-FABP were the most discriminant between ART-treated participants and HIV-uninfected controls: they differed between both ART-treated men and women and their HIV-uninfected sex-matched counterparts, and the differences persisted after adjusting for age, smoking, alcohol use, and BMI.
Our findings are consistent with those of previous studies that, in mainly chronically-infected patients, compared treated PLHIV to appropriate controls [11,18,21]. Interestingly, a recent study from the MACS cohort reported abnormally elevated concentrations of certain inflammatory biomarkers including sCD14, sTNFRII, but also CXCL10 and TNF-α, even in MSM who reported 100% ART adherence [22]. sCD14 and sCD163 have been identified several times as the markers that best discriminated between HIV-infected and uninfected individuals [11,18,21]. In an older population, with a median age 59 years, Booiman et al. did not observe any difference in sCD14 according to HIV status, but still reported elevated levels of sCD163 and I-FABP in PLHIV after a median of eight years of ART [10]. Monocyte activation thus appears to be an important driver of inflammation in HIV-infected patients under ART. Increased levels of sCD14 can also be associated with microbial translocation [23]. Similarly, high levels of I-FABP, a marker commonly associated with mucosal homeostasis, may reflect ongoing enterocyte damage, although epithelial cell regeneration under effective ART has also been suggested [24,25]. sTNFRII, sCD14, and I-FABP have been shown to be predictive for morbidity and mortality in ART-treated PLHIV [26], [27], [28], [29], and sCD163 has been related to subclinical atherosclerosis [30]. Further studies are needed to address the long-term clinical relevance of the differences that we and others have reported.
Our cluster analysis revealed a subset of 15 PRIMO participants with a reduced inflammatory profile under ART, comparable to HIV-uninfected participants. Of note, these individuals already had a particularly favourable CD4:CD8 ratio in AHI. Accordingly, most of them had initiated ART at distance of AHI diagnosis. In addition to better immunological characteristics, they also had lower levels of inflammatory markers during AHI than other PRIMO participants. This is consistent with the results of Gandhi et al., who reported that pre-ART levels of several inflammatory markers significantly correlated with on-therapy levels of the same biomarkers, even after years of suppressive ART, and not with markers of HIV persistence [31]. Taken together, these findings suggest that persistent inflammation under treatment may be related to an increased inflammatory profile since AHI; the low inflammatory profile under treatment observed in few participants might be explained by viral and host factors, including genetic ones.
This study had some limitations. First, there were unmeasured factors, including some comorbidities, medications or measurement of waist circumference. These factors could contribute to explain the differences we observed between the PRIMO participants and their IPERGAY and COHVAC HIV-uninfected counterparts. Nevertheless, this study was designed to compare the levels of inflammation in HIV-infected participants with those observed in sex and age-matched HIV-uninfected participants, at either low or high risk for HIV acquisition. The presence of comorbidities, drug treatments and visceral fat, which are often more common in HIV-infected participants than in uninfected subjects [32], would then be more likely to be explanatory factors for the observed differences than confounding factors that should be adjusted for. Secondly, our selection of ten biomarkers did not allow a complete integration of all inflammatory sources, in particular the contribution of metabolic inflammation. Thirdly, we restricted our study population to HCV and HBV uninfected individuals. Our results may therefore not be generalizable to other populations in a different context of the HIV epidemic.
Fourthly, we also probably did not have sufficient statistical power to identify a sex-modifying effect on associations between certain markers of inflammation and non-HIV-related factors.
Among the strengths of this study were the use of multiplex assays that measured biomarker levels, even at low concentrations, the enrolment of both men and women, and the choice of two control groups with contrasting lifestyles and health behaviour profiles, and with information collected on the main cofactors of inflammation, which are age, sex, smoking, alcohol consumption, and BMI. The comparison group of participants from the IPERGAY trial can be seen as a sample of the uninfected source population from which most of the MSM of the PRIMO cohort could have originated, while the second comparison group from the COHVAC cohort provided information on standard levels of inflammation in a population of the same sex and age as the HIV-infected participants in our study. We had hypothesized that these two control groups would have different inflammatory profiles, given their differences in their health behaviours. And in fact, PRIMO men no longer had significantly higher levels of us-CRP than COHVAC men, after adjusting for alcohol and smoking, whereas the differences for the other more specific markers remained significant after adjustment. IPERGAY men had levels of soluble markers associated with monocyte activation (sCD14, sCD163, CXCL10) similar to those of COHVAC men, but higher TNF-α and IL-17 levels. These higher levels of IL-17 in IPERGAY men, even higher than those observed in PRIMO men, suggest different responses to various mucosal/epithelial infections [33] and may be linked to a higher exposure of these participants with high-risk behaviours to diverse infectious agents that trigger the innate immune system. Finally, we identified different inflammatory profiles depending on the sex: PRIMO men, but not women, exhibited higher CXCL10 levels than their uninfected counterparts. Conversely, PRIMO women, but not men, exhibited higher IL-6 levels. The production of CXCL10 and IL-6 being associated with different sources (IFN pathway versus metabolic pathway respectively), it is thus possible that these two pathways make a different contribution in male and female participants.
In conclusion, after many years of suppressive ART, participants followed since AHI in the PRIMO cohort still showed elevated monocyte/macrophage activation and persistent gut epithelial dysfunction, relative to two groups of age- and sex-matched HIV-negative participants with either a low or high prevalence of cofactors of inflammation. Persistent inflammation under treatment may be related to an increased inflammatory profile since AHI. Comparisons of inflammation/activation levels between HIV-infected and uninfected participants should consider exposures other than HIV that contribute to systemic immune activation.
Contributors
C.G. and L.M. were the main investigators of the ANRS PRIMO cohort.
O.L. and J-M. M. were the main investigators of the ANRS COHVAC cohort and the ANRS IPERGAY trial, respectively.
C.B., C.G. and L.M. designed the study. A.E., A.V., C.L., J.R., L.B., and V.A-F contributed the data.
S.N. performed the statistical analyses and L.M. supervised her.
S. N., L. M., C. B. and C. G. contributed to the interpretation of the results.
S.N. wrote the first version of the manuscript.
All authors read the manuscript, provided critical feedback and approved the final manuscript.
Data sharing statement
Data are not owned by the authors. Data of the ANRS PRIMO, COHVAC and IPERGAY studies are owned by the Institut National de la Santé et de la Recherche Médicale (Inserm). French law requires that everyone who wants to share cohort data or clinical study data on humans must ask permission from the French data protection authority, la Commission Nationale de l'Informatique et des Libertés (CNIL). The request for access to the data must be made to the corresponding author and the sponsor.
Declaration of Competing Interest
Dr. Avettand-Fenoël reports grants from ANRS, during the conduct of the study; grants and personal fees from ViiV, grants from Janssen, outside the submitted work. Dr. Reynes reports personal fees and non-financial support from Gilead Science, personal fees and non-financial support from ViiV Healthcare, personal fees and non-financial support from MSD France, personal fees and non-financial support from Janssen, personal fees and non-financial support from Pfizer, outside the submitted work. Dr. Molina reports personal fees from Gilead Sciences, personal fees from Merck, personal fees from ViiV Healthcare, outside the submitted work. Dr. Launay reports grants from Assistance Publique - Hopitaux de Paris (AP-HP), during the conduct of the study. Dr. Novelli, Dr. Lécuroux, Dr. Villemant, Dr. Essat, Dr. Blum , Dr. Bourgeois, Dr. Goujard and Dr. Meyer have nothing to disclose.
Acknowledgements
We thank all the patients and physicians who participated in the ANRS PRIMO cohort, the ANRS COHVAC cohort and the ANRS IPERGAY trial. We also thank Prof. Patrice Thérond and Dr. Claudine Cosson from the Department of Biochemistry of Bicetre Hospital for CRP measurements.
This work was supported by the French Agency for Research on AIDS and Viral Hepatitis (ANRS) and a doctoral grant from Paris-Saclay University to Sophie Novelli.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ebiom.2020.103129.
Appendix. Supplementary materials
References
- 1.Sabin CA. Do people with HIV infection have a normal life expectancy in the era of combination antiretroviral therapy? BMC Med. 2013;11:251. doi: 10.1186/1741-7015-11-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rajasuriar R, Wright E, Lewin SR. Impact of antiretroviral therapy (ART) timing on chronic immune activation/inflammation and end-organ damage. Curr Opin HIV AIDS. 2015;10:35–42. doi: 10.1097/COH.0000000000000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sereti I, Krebs SJ, Phanuphak N, Fletcher JL, Slike B, Pinyakorn S. Persistent, albeit reduced, chronic inflammation in persons starting antiretroviral therapy in acute HIV infection. Clin Infect Dis. 2017;64:124–131. doi: 10.1093/cid/ciw683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vinikoor MJ, Cope A, Gay CL, Ferrari G, McGee KS, Kuruc JD. Antiretroviral therapy initiated during acute HIV infection fails to prevent persistent T-Cell activation. J Acquir Immune Defic Syndr. 2013;62:505–508. doi: 10.1097/QAI.0b013e318285cd33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ananworanich J, Schuetz A, Vandergeeten C, Sereti I, de Souza M, Rerknimitr R. Impact of multi-targeted antiretroviral treatment on gut T cell depletion and HIV reservoir seeding during acute HIV infection. PLoS One. 2012;7:e33948. doi: 10.1371/journal.pone.0033948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hunt PW, Lee SA, Siedner MJ. Immunologic biomarkers, morbidity, and mortality in treated HIV infection. J Infect Dis. 2016;214:S44–S50. doi: 10.1093/infdis/jiw275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Macatangay BJ, Yang M, Sun X, Morton J, Gruttola VD, Little S. Changes in levels of inflammation following antiretroviral treatment during early HIV infection in ACTG A5217. Ournal Acquir Immune Defic Syndr. 2017:1. doi: 10.1097/QAI.0000000000001320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hellmuth J, Slike BM, Sacdalan C, Best J, Kroon E, Phanuphak N. Very early initiation of antiretroviral therapy during acute HIV infection is associated with normalized levels of immune activation markers in cerebrospinal fluid but not in plasma. J Infect Dis. 2019;220:1885–1891. doi: 10.1093/infdis/jiz030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neuhaus J, Jacobs Jr DR, Baker JV, Calmy A, Duprez D, La Rosa A. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis. 2010;201:1788–1795. doi: 10.1086/652749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Booiman T, Wit FW, Maurer I, De Francesco D, Sabin CA, Harskamp AM. High cellular monocyte activation in people living with human immunodeficiency virus on combination antiretroviral therapy and lifestyle-matched controls is associated with greater inflammation in cerebrospinal fluid. Open Forum Infect Dis. 2017;4 doi: 10.1093/ofid/ofx108. ofx108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wada NI, Jacobson LP, Margolick JB, Breen EC, Macatangay B, Penugonda S. The effect of HAART-induced HIV suppression on circulating markers of inflammation and immune activation. AIDS. 2015;29:463–471. doi: 10.1097/QAD.0000000000000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siedner MJ, Zanni M, Tracy RP, Kwon DS, Tsai AC, Kakuhire B. Increased systemic inflammation and gut permeability among women with treated HIV infection in rural Uganda. J Infect Dis. 2018;218:922–926. doi: 10.1093/infdis/jiy244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fitch KV, Srinivasa S, Abbara S, Burdo TH, Williams KC, Eneh P. Noncalcified coronary atherosclerotic plaque and immune activation in HIV-infected women. J Infect Dis. 2013;208:1737–1746. doi: 10.1093/infdis/jit508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Novelli S, Lécuroux C, Avettand-Fenoel V, Seng R, Essat A, Morlat P. Long-term therapeutic impact of the timing of antiretroviral therapy in patients diagnosed with primary human immunodeficiency virus type 1 infection. Clin Infect Dis. 2018;66:1519–1527. doi: 10.1093/cid/cix1068. [DOI] [PubMed] [Google Scholar]
- 15.Krastinova E, Seng R, Yeni P, Viard J-P, Vittecoq D, Lascoux-Combe C. Is clinical practice concordant with the changes in guidelines for antiretroviral therapy initiation during primary and chronic HIV-1 infection? The ANRS PRIMO and COPANA cohorts. PLoS One. 2013;8:e71473. doi: 10.1371/journal.pone.0071473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molina J-M, Capitant C, Spire B, Pialoux G, Cotte L, Charreau I. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N Engl J Med. 2015;373:2237–2246. doi: 10.1056/NEJMoa1506273. [DOI] [PubMed] [Google Scholar]
- 17.Durier C, Desaint C, Launay O. Social and Behavioral Consequences of participation in HIV preventive vaccine trials in the ANRS COHVAC cohort. J Acquir Immune Defic Syndr. 2018;79:S37–S50. doi: 10.1097/QAI.0000000000001807. [DOI] [PubMed] [Google Scholar]
- 18.Williams JC, Zhang X, Karki M, Chi Y-Y, Wallet SM, Rudy BJ. Soluble CD14, CD163, and CD27 biomarkers distinguish ART-suppressed youth living with HIV from healthy controls. J Leukoc Biol. 2018;103:671–680. doi: 10.1002/JLB.3A0717-294RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Avettand-Fènoël V, Chaix M-L, Blanche S, Burgard M, Floch C, Toure K. LTR real-time PCR for HIV-1 DNA quantitation in blood cells for early diagnosis in infants born to seropositive mothers treated in HAART area (ANRS CO 01) J Med Virol. 2009;81:217–223. doi: 10.1002/jmv.21390. [DOI] [PubMed] [Google Scholar]
- 20.Goulder PJR, Walker BD. HIV and HLA Class I: an evolving relationship. Immunity. 2012;37:426–440. doi: 10.1016/j.immuni.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Babu H, Ambikan AT, Gabriel EE, Svensson Akusjärvi S, Palaniappan AN, Sundaraj V. Systemic inflammation and the increased risk of inflamm-aging and age-associated diseases in people living with HIV on long term suppressive antiretroviral therapy. Front Immunol. 2019;10:1965. doi: 10.3389/fimmu.2019.01965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castillo-Mancilla JR, Brown TT, Palella FJ, Macatangay BJC, Breen EC, Jacobson LP. Partial normalization of biomarkers of inflammation and immune activation among virally suppressed men with HIV infection and high ART adherence. Open Forum Infect Dis. 2020;7 doi: 10.1093/ofid/ofaa099. ofaa099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Landmann R, Knopf HP, Link S, Sansano S, Schumann R, Zimmerli W. Human monocyte CD14 is upregulated by lipopolysaccharide. Infect Immun. 1996;64:1762–1769. doi: 10.1128/iai.64.5.1762-1769.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hunt PW. Very early ART and persistent inflammation in treated HIV. Clin Infect Dis. 2017;64:132–133. doi: 10.1093/cid/ciw697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.El Kamari V, Sattar A, Mccomsey GA. Brief report: gut structural damage: an ongoing process in chronically untreated HIV infection. J Acquir Immune Defic Syndr. 2019;80:242–245. doi: 10.1097/QAI.0000000000001910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sandler NG, Wand H, Roque A, Law M, Nason MC, Nixon DE. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis. 2011;203:780–790. doi: 10.1093/infdis/jiq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hunt PW, Sinclair E, Rodriguez B, Shive C, Clagett B, Funderburg N. Gut epithelial barrier dysfunction and innate immune activation predict mortality in treated HIV infection. J Infect Dis. 2014;210:1228–1238. doi: 10.1093/infdis/jiu238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wada NI, Bream JH, Martínez-Maza O, Macatangay B, Galvin SR, Margolick JB. Inflammatory biomarkers and mortality risk among HIV-suppressed men: a multisite prospective cohort study. Clin Infect Dis. 2016;63:984–990. doi: 10.1093/cid/ciw409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tenorio AR, Zheng Y, Bosch RJ, Krishnan S, Rodriguez B, Hunt PW. Soluble markers of inflammation and coagulation but not T-Cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. J Infect Dis. 2014;210:1248–1259. doi: 10.1093/infdis/jiu254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burdo TH, Lo J, Abbara S, Wei J, DeLelys ME, Preffer F. Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J Infect Dis. 2011;204:1227–1236. doi: 10.1093/infdis/jir520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gandhi RT, McMahon DK, Bosch RJ, Lalama CM, Cyktor JC, Macatangay BJ. Levels of HIV-1 persistence on antiretroviral therapy are not associated with markers of inflammation or activation. PLoS Pathog. 2017;13 doi: 10.1371/journal.ppat.1006285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wing EJ. HIV and aging. Int J Infect Dis. 2016;53:61–68. doi: 10.1016/j.ijid.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 33.Guglani L, Khader SA. Th17 cytokines in mucosal immunity and inflammation. Curr Opin HIV AIDS. 2010;5:120–127. doi: 10.1097/COH.0b013e328335c2f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.