Abstract
Background
Patients with locally advanced rectal cancer (LARC) are more likely to suffer local recurrence and distant metastases, contributing to worse prognoses. Considering the provided dramatic reduction of local recurrences, neoadjuvant CRT (nCRT) followed by curative resection with total mesorectal excision (TME) and adjuvant chemotherapy has been established as standard therapy for LARC patients. However, the efficacy of adding bevacizumab in neoadjuvant therapy, especially in induction therapy-containing nCRT for LARC patients remains uncertain.
Materials
PubMed, Embase, and Web of Science were searched to retrieve records on the application of bevacizumab in a neoadjuvant setting for LARC patients. The endpoints of interest were pCR and the rates of patients suffering Grade 3/4 bevacizumab-specific adverse events, namely bleeding, wound healing complications, and gastrointestinal perforation.
Results
29 cohorts covering 1134 subjects were included in this systematic review. The pooled pCR rate for bevacizumab-relevant cohorts was 21% (95% confidence interval (95% CI), 17–25%; I2 = 61.8%), the pooled estimates of Grade 3/4 bleeding, Grade 3/4 wound healing complication, Grade 3/4 gastrointestinal perforation were 1% (95% CI, 0–3%; I2 = 0%), 2% (95% CI, 1–5%; I2 = 4.7%), and 2% (95% CI, 0–5%; I2 = 0%), respectively.
Conclusion
The addition of bevacizumab in the nCRT, especially in the TNT, for LARC patients provides promising efficacy and acceptable safety. However, the results should be interpreted cautiously due to the small amount of relevant data and need further confirmation by future studies.
Keywords: Locally advanced rectal cancer, Neoadjuvant chemoradiotherapy, Induction therapy, VEGF-inhibitor, Bevacizumab
Graphical abstract
Introduction
Rectal cancer, which comprises approximately one-third of all colorectal cancer cases, is among the most diagnosed and lethal malignancies for decades [1]. Patients with locally advanced rectal cancer (LARC) are more likely to suffer local recurrence and distant metastases, contributing to worse prognoses [2]. Neoadjuvant therapy refers to performing chemoradiotherapy (CRT) preoperatively, aiming to achieve tumor downstaging and thus facilitate curative resection and organ preservation, improving the local control, survival outcomes, and quality of life of LARC patients [3, 4]. Considering the provided dramatic reduction of local recurrences, neoadjuvant CRT (nCRT) followed by curative resection with total mesorectal excision (TME) and adjuvant chemotherapy has been established as standard therapy for LARC patients [5]. However, the efficacy of fluoropyrimidine- or capecitabine-based nCRT strategies are far from optimal, considering the insufficient pathologic complete response (pCR) rates reported in the relevant clinical trials [6], [7], [8]. More intensified and more efficacious nCRT regimens are demanded for improving the prognoses of LARC patients.
Substantial work has been made by oncologists to evaluate the efficacy of targeted agents as an addition to nCRT strategies for LARC patients in the past decade. Bevacizumab, a monoclonal antibody against vascular endothelial growth factor (VEGF), has been reported to enhance the activity of radiotherapy (RT), based on phase II trials exploring whether bevacizumab facilitates the tumor downstaging-effect of nCRT [9], [10], [11], [12]. A previous meta-analysis [13] critically reviewed and evaluated the efficacy of adding bevacizumab in the neoadjuvant setting for LARC patients and reported a promising pooled pCR rate of 27% in 23 bevacizumab-relevant cohorts. Of these, four cohorts (analyzed in Dipetrillo et al. [14], Borg et al. [11], Nogue et al. [12], and Xiao et al. [15]) received induction therapy ahead of concurrent CRT before TME and achieved decent pCR rates of 20.0%, 23.8%, 35.6%, and 39.1%, respectively. More recently, several studies have been published focusing on the efficacy of bevacizumab in induction therapy-containing nCRT, referred to as a total neoadjuvant therapy (TNT) approach, along with those not containing induction therapy [5, [16], [17], [18], [19], [20], [21], [22]]. Following these advances, we performed this systematic review and meta-analysis to update the efficacy and safety profile of bevacizumab in nCRT, especially those containing induction therapy, for LARC patients.
Methods
Study selection
This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statements checklist [23].
The pre-defined criteria for inclusion and exclusion were: (1) patients with LARC (clinical T stage 3–4 and/or lymph node metastasis, no distant metastatic diseases observed). (2) Administration of bevacizumab in a neoadjuvant setting. (3) Sample size > 10. (4) Reporting on pCR and Grade 3–4 bevacizumab-specific adverse events such as bleeding, wound healing complication, and gastrointestinal perforation. (5) If research cohorts were overlapping, the more recent and larger studies were chosen for inclusion. (6) Only original studies were included, excluding reviews, systematic reviews, case reports, case series, and letters to editors.
Search strategy
PubMed, Embase, and Web of Science were searched using the following strategy: (rectal OR rectum OR colorectal) AND (tumor OR cancer OR neoplasm OR malignan*) AND (neoadjuvant OR preoperative OR perioperative OR induction) AND (targeted OR vegf OR bevacizumab) for relevant publications up to December 20th, 2019. References of the relevant studies were manually screened for potential candidates for inclusion. No restriction of language was applied.
Data extraction
The primary endpoints were pCR and the rates of patients suffering Grade 3/4 bevacizumab-specific adverse events, namely bleeding, wound healing complications, and gastrointestinal perforation. The baseline characteristics of the included studies were extracted: first author, year of publication, study design, country/district, population, nCRT regimens, median age, and staging at enrollment. The whole data extraction process was independently conducted by two authors and discrepancies were resolved through discussion. The methodological quality of the included studies was evaluated using the Newcastle-Ottawa quality assessment scale (NOS) [24]. Studies scoring five or more were considered moderate-quality, whereas those with seven or more were deemed of high-quality [24].
Statistical analysis
Quantitative syntheses were performed using a random-effect model to provide more conservative estimates [25, 26]. Data concerning Grade 3/4 bevacizumab-specific adverse events were within the range of 0–0.3, thus they were first double arcsine transformed and then synthesized. The Cochrane's Q test and inconsistent index (I2) were performed to detect heterogeneity [27]. Subgroup analyses and sensitivity analysis were performed to detect potential origins of heterogeneity. Small study effects were evaluated using Egger's test when sufficient data were provided (≥10) [28]. All statistical analyses were performed using STATA version 12.0 (STATA, College Station, TX).
Results
Study inclusion and baseline characteristics of eligible studies
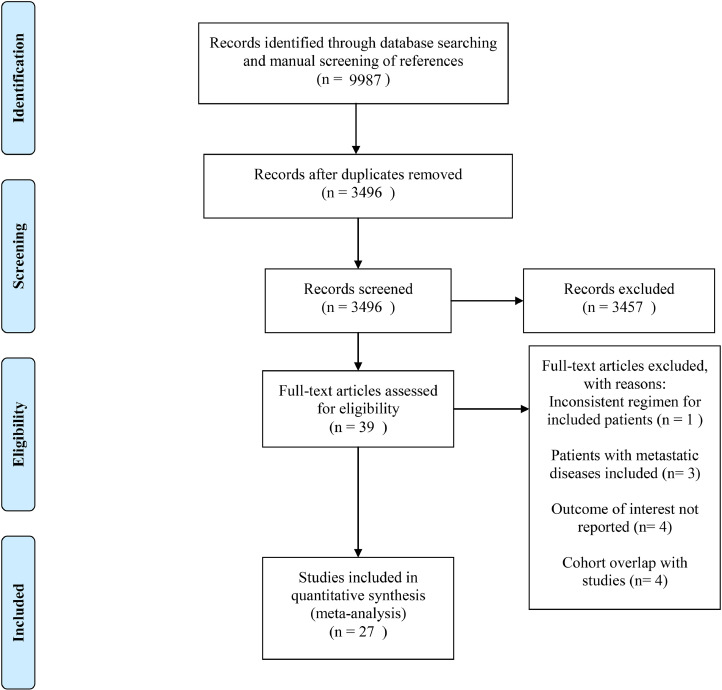
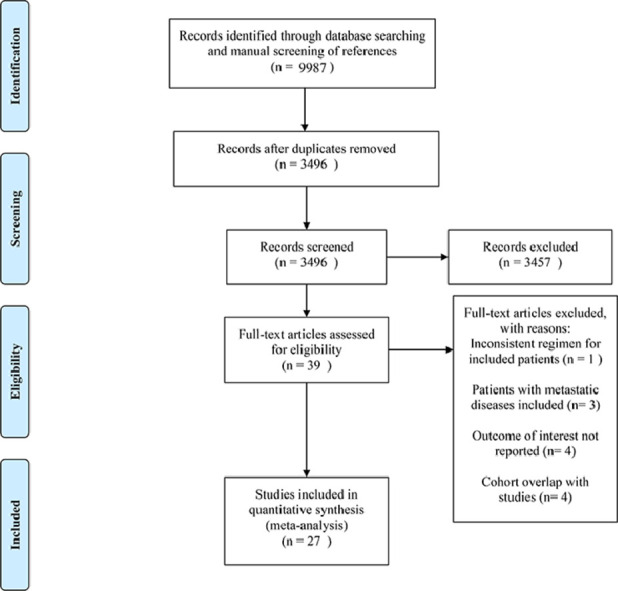
The initial database search and manual screening of references retrieved 9987 records, of which 6491 were removed as duplicates, leaving 3496 records which proceeded on to title and abstract screening. 39 potential candidates underwent full-text review, of which 27 [[10], [11], [12], [14], [15], [16], [17], [18], [19], [20], [21], [22], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43]] were adequate for inclusion, with 12 studies deemed inadequate for various reasons shown in Fig. 1. Of note, among the eligible studies, five [5, 11, 17, 18] explored the role of TNT. Concerning the countries/districts where these trials were conducted, seven [16, 17, 19, 22, 30, 33, 35] were in Japan, five [10, 14, 37, 41, 42] in the United States of America, four in China (two [15], [18] in the mainland, two [21], [32] in Taiwan), four [12], [29], [31], [34] in Spain, two [38,43] in Italy, one [11] in France, one [36] in Germany, one [40] in Greece, one [39] in Slovenia, and one [20] in the United Kingdom. The detailed baseline characteristics and data regarding endpoints of interest from the included studies are shown in Table 1. The detailed methodological quality assessment results of the included studies are shown in Table 2. Four of the 27 studies scored seven points on the NOS analysis and were deemed high-quality, while the remaining 23 scored six points and were considered as moderate-quality studies.
Fig. 1.
Literature search and study selection.
Table 1.
Baseline characteristics of cohort groups of bevacizumab for meta-analysis.
| Study | Study design | Country /District | Enrollment, n | Neoadjuvant therapy | Median age, y | Stage at enrollment, n | Grade3/4 Bevacizumab-specific adverse effects | pCR |
|---|---|---|---|---|---|---|---|---|
| Konishi et al. 2019 | Prospective Phase II | Japan | 43 | Induction therapy: mFOLFOX6+bevacizumab Concurrent chemoradiotherapy:S1+RT | 54.0 | cT3: 31; cT4: 12; cN0: 3; cN1 (lateral node -): 10; cN1 (lateral node +): 30 | NR | 37.2% (16/43) |
| Masi et al. 2019 | Prospective Phase II | Italy | 49 | Induction therapy: FOLFOXIRI+bevacizumab Concurrent chemoradiotherapy: capecitabine +bevacizumab+RT | 53.0 | cT3: 31; cT4: 17; cN0: 8; cN1–2: 40 | NR | 36.4% (16/44) |
| Tomida et al. 2019 | Prospective Phase II | Japan | 32 | Capox+bevacizumab | 62.0 | cT3: 13; cT4a: 9; cT4b: 10; cN0: 6; cN1: 14; cN2: 12 | NR | 13.8% (4/29) |
| Glynne-Jones et al. 2018 | Prospective Phase II | UK | 10 | FOLFOX+bevacizumab | 58.0 | mrT3b: 5; mrT3c: 5; mrN0: 4; mrN1: 2; mrN2: 4 | Grade 3/4 Bleeding: 0; Grade 3/4 Wound healing complication: 0 | 0 |
| 10 | FOLFOXIRI+bevacizumab | 58.0 | mrT3b: 5; mrT3c: 3; mrT3d: 1; mrT4 (peritoneal involvement): 1; mrN0: 0; mrN1: 5; mrN2: 5 | Grade 3/4 Bleeding: 0; Grade 3/4 Wound healing complication: 1/10 (10%) | 20% (2/10) | |||
| Maeda et al. 2018 | Prospective Phase II | Japan | 25 | Capecitabine+ bevacizumab | 65.0 | cT3: 18; cT4: 7; cN0: 9; cN1: 6; cN2: 10 | NR | 25% (4/25) |
| Yu et al. 2018 | Prospective Phase II | China (mainland) | 45 | Induction therapy: Capox+bevacizumab Concurrent chemoradiotherapy: Capox+bevacizumab+RT | 48.0 | cT2: 1; cT3: 18; cT4a: 21; cT4b: 5; cN0: 5; cN1: 15; cN2: 25 | NR | 39.5% (15/38) |
| Hasegawa et al. 2017 | Prospective Phase II | Japan | 20 | mFOLFOX6+ bevacizumab | 63.0 | cT2: 0; cT3: 15; cT4a: 5; cN0: 13; cN1-2: 7 | NR | 15% (3/20) |
| Liang et al. 2017 | Retrospective | China (Taiwan) | 76 | FOLFOX+bevacizumab | NR | cT3N0: 44; cT3N1: 19; cT3N2: 13 | NR | 34.2% (26/76) |
| Garcia et al. 2015 | Prospective Phase II | Spain | 41 | Capecitabine+bevacizumab+ RT | 63.0 | cT3 a: 32; cT3a: 3; cT3b: 1; cT3c: 2; cT4: 2 | NR | 7.5% (3/40) |
| Landry et al. 2015 | Prospective Phase II | USA | 54 | Capox+bevacizumab+RT | 54.0 | cT3: 50; cT4: 4; cNx: 2; cN0: 17; cN1: 30; cN2: 5 | Grade 3/4 Bleeding: 1/54 (1.85%) | 17.0% (9/53) |
| Sadahiro et al. 2015 | Prospective Phase II | Japan | 52 | S-1+bevacizumab+ RT | 59.0 | cT2: 2; cT3: 49; cT4: 1; cN0: 16; cN1: 36 | NR | 19.2% (10/52) |
| Salazar et al. 2015 | Prospective Phase II | Spain | 90 | Capecitabine+ bevacizumab+ RT |
64.0 | Ⅱ A: 6; Ⅱ B: 1; Ⅲ B: 18; Ⅲ C: 19 | NR | 15.9% (7/44) |
| Xiao et al. 2015 | Prospective Phase II | China (mainland) | 25 | Induction therapy: FOLFOX+bevacizumab Concurrent chemoradiotherapy:5-FU+bevacizumab+RT Consolidation therapy: FOLFOX | 45.0 | cT2: 2; cT3: 9; cT4a: 8; cT4b: 6; cN-: 4; cN+: 21 | NR | 39.1% (9/23) |
| Borg et al. 2014 | Prospective Phase II | France | 46 | Induction therapy: FOLFOX4+bevacizumab Concurrent chemoradiotherapy:5-FU+bevacizumab+RT | 60.6 | cT3N0: 10; cT3N1: 31; Tc3N2: 5 | Grade 3/4 Bleeding: 2/46 (4.35%) Grade 3/4 Wound healing complication: 0 Grade 3/4 Gastrointestinal perforation: 1/46 (2.17%) | 23.8% (10/42) |
| 45 | 5-FU+bevacizumab+ RT | 60.1 | cT3N0: 8; cT3N1: 28; cT3N2: 9 | Grade 3/4 Bleeding: 0 Grade 3/4 Wound healing complication:2/45 (4.44%) Grade 3/4 Gastrointestinal perforation: 0 | 11.4% (5/44) | |||
| Fernandez-Martos et al. 2014 | Prospective Phase II | Spain | 46 | Capox+ bevacizumab | NR | cT3: 46 | NR | 19.6% (9/46) |
| Hasegawa et al. 2014 | Prospective Pilot study | Japan | 25 | Capox+bevacizumab | 63.0 | cT4aN0M0: 1; cT4bN0M0: 3; cT2,cT3N2M0: 3; cT3,cT4aN1M0: 10; cT4aN2M0: 1; cT4bN1/N2M0: 7 | NR | 4.3% (1/23) |
| Wang et al. 2014 | Prospective Phase II | China (Taiwan) | 12 | FOLFOX+bevacizumab+ RT/5-FU+bevacizumab+ RT | 52.5 | cT2: 1; cT3: 8; cT4: 3; cN0: 2; cN1: 2; cN2: 8 | NR | 33.3% (4/12) |
| Dellas et al. 2013 | Prospective Phase II | Germany | 69 | Capox+bevacizumab+ RT | 61.0 | cT2Nx: 2; cT3N0: 12; cT3N0+: 44; cT4N0: 3; cT4N+: 4: | Grade 3/4 Wound healing complication: 1/69 (1.45%) | 17.4% (12/69) |
| Uehara et al. 2013 | Prospective Phase II | Japan | 32 | Capox+bevacizumab | 62.0 | cT3: 13; cT4a: 9; cT4b: 10; cN0: 6; cN1: 14; cN2: 12 | Grade 3/4 Gastrointestinal perforation: 1/32 (3.13%) | 13.3% (4/30) |
| Dipetrillo et al. 2012 | Prospective Phase II | USA | 25 | Induction therapy: mFOLFOX6+bevacizumab+ RT Concurrent chemoradiotherapy:5-FU+ oxaliplatin+bevacizumab+RT |
50.0 | T2: 2; T3: 20; T4: 3; N-: 7; N+: 16; Nx: 2 | NR | 20% (5/25) |
| Gasparini et al. 2012 | Prospective Phase II | Italy | 43 | Capecitabine+ bevacizumab+ RT |
64.0 | cT2N1M0: 4; cT3N0M0: 14; cT3N1M0: 20; cT3NxM0: 1; cT4N1M0: 1; cT4N1M0: 1; cT4N2M0: 1; cTxN1M0: 1; cT4N2M1: 1 | Grade 3/4 Bleeding: 0 | 14.0% (6/43) |
| Spigel et al. 2012 | Prospective Phase II | USA | 35 | 5-FU+bevacizumab+ RT | 57.0 | II: 11; III: 24 | Grade 3/4 Wound healing complication: 0 | 28.6% (10/35) |
| Koukourakis et al. 2011 | Prospective Phase II | Greece | 19 | Capecitabine+ amifostine+bevacizumab+ RT |
68.0 | pT3: 19; pT4: 0; pN1: 12 | Grade 3/4 Bleeding: 0 | 36.8% (7/19) |
| Nogue et al. 2011 | Prospective Phase II | Spain | 47 | Induction therapy: Capox+bevacizumab Concurrent chemoradiotherapy: capecitabine +bevacizumab+RT | 58.5 | cT3N0: 5; cT3N1: 22; cT3N2: 14; cT4N0: 2; cT4N1: 2; cT4N2: 2 | Grade 3/4 Bleeding: 0 | 35.6% (16/45) |
| Velenik et al. 2011 | Prospective Phase II | Slovenia | 61 | Capecitabine+ bevacizumab+ RT |
60.0 | cT3N0: 12; cT2N1: 1; cT3N1: 19; cT2N2: 2; cT3N2: 22; cT4N2: 5 | Grade 3/4 Bleeding: 0 | 13.3% (8/60) |
| Crane et al. 2010 | Prospective Phase II | USA | 25 | Capecitabine+ bevacizumab+ RT |
54.0 | cT3N0: 5; cT3N0+: 20 | NR | 32% (8/25) |
| Willett et al. 2010 | Prospective Phase II | USA | 32 | 5-FU+bevacizumab+ RT | 51.0 | cT3: 28; cT4: 4; cN0: 9; cN1-2: 23 | NR | 15.6% (5/32) |
Abbreviations: pCR: pathologic complete response; RT: radiotherapy; 5-FU: fluorouracil; FOLFOXIRI: fluorouracil plus leucovorin plus oxaliplatin plus irinotecan; FOLFOX: fluorouracil plus leucovorin plus oxaliplatin; Capox: capecitabine plus oxaliplatin; S-1: tegafur plus gimeracil plus potassium oxonate; NR: not reported.
It was not specified if the cT3 status was cT3a, cT3b or cT3c.
Table 2.
The NOS quality of included studies.
| Study | Selection | Comparability | Outcome | Total | Quality | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| REC | SNEC | AE | DO | SC | AF | AO | FU | AFU | |||
| Konishi et al. 2019 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 | Moderate |
| Masi et al. 2019 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 | Moderate |
| Tomida et al. 2019 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 | Moderate |
| Glynne-Jones et al. 2018 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 7 | High |
| Maeda et al. 2018 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 | Moderate |
| Yu et al. 2018 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 | Moderate |
| Hasegawa et al. 2017 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 | Moderate |
| Liang et al. 2017 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 7 | High |
| Garcia et al. 2015 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 | Moderate |
| Landry et al. 2015 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 | Moderate |
| Sadahiro et al. 2015 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 | Moderate |
| Salazar et al. 2015 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 7 | High |
| Xiao et al. 2015 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 | Moderate |
| Borg et al. 2014 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 7 | High |
| Fernandez-Martos et al. 2014 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 | Moderate |
| Hasegawa et al. 2014 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 | Moderate |
| Wang et al. 2014 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 | Moderate |
| Dellas et al. 2013 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 | Moderate |
| Uehara et al. 2013 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 | Moderate |
| Dipetrillo et al. 2012 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 | Moderate |
| Gasparini et al. 2012 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 | Moderate |
| Spigel et al. 2012 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 | Moderate |
| Koukourakis et al. 2011 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 | Moderate |
| Nogue et al. 2011 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 | Moderate |
| Velenik et al. 2011 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 | Moderate |
| Crane et al. 2010 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 | Moderate |
| Willett et al. 2010 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 | Moderate |
Abbreviations: REC: representativeness of the exposed cohort; SNEC: selection of the nonexposed cohort; AE: ascertainment of exposure; DO: demonstration that outcome of interest was not present at start of study; SC: study controls for age, sex; AF: study controls for any additional factors; AO: assessment of outcome; FU: follow-up long enough (36 M) for outcomes to occur; AFU: adequacy of follow-up of cohorts (≥90%). “1″ means that the study is satisfied the item and “0″ means the opposite situation.
The efficacy of bevacizumab
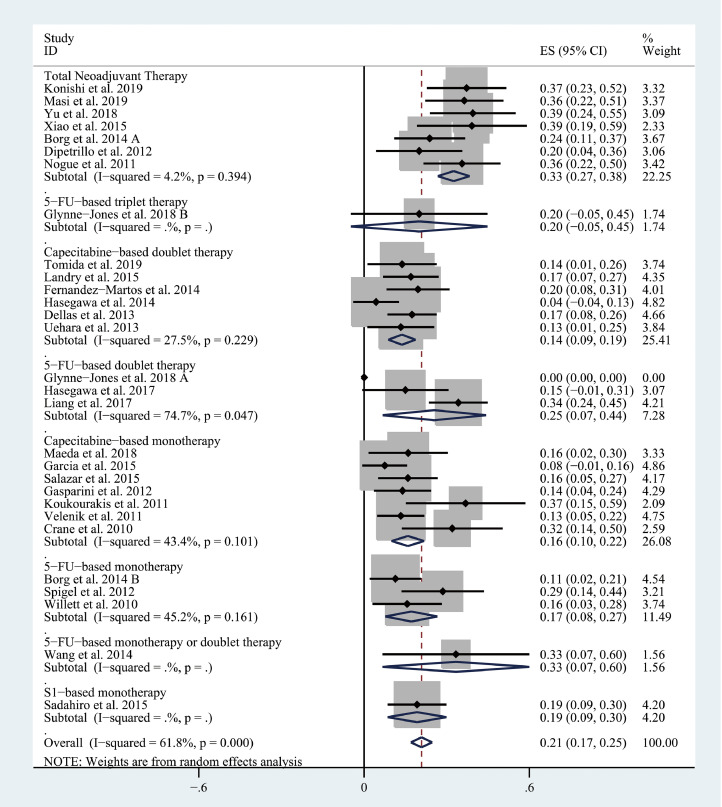
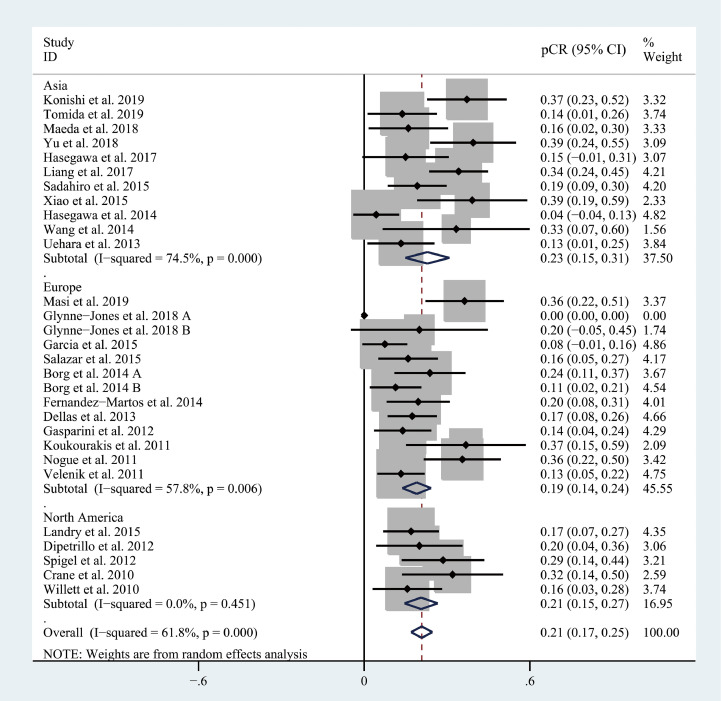
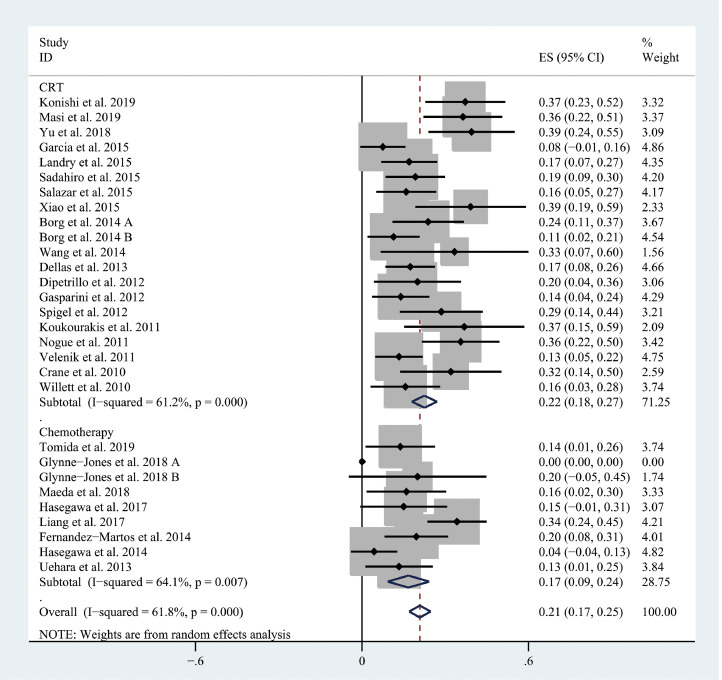
The pooled pCR rate for bevacizumab-relevant cohorts was 21% (95% confidence interval (95% CI), 17–25%; I2 = 61.8%) as shown in Figs. 2 and 3. Subgroup analyses based on the backbone therapy and region found a pooled pCR rate for TNT-relevant cohorts (7) of 33% (95% CI, 27–38%; I2 = 4.2%) while capecitabine-based double therapy-relevant cohorts (6), fluorouracil (5-FU hereafter)-based doublet therapy-relevant cohorts (3), capecitabine-based monotherapy-relevant cohorts (7), and 5-FU-based monotherapy-relevant cohorts (3) had pCR of 14% (95% CI, 9–19%; I2 = 27.5%), 25% (95% CI, 7–44%; I2 = 74.7%), 16% (95% CI, 10–22%; I2 = 43.4%), and 17% (95% CI, 8–27%; I2 = 45.2%), respectively (Fig. 2). The pooled pCR rates for Asian-, European-, and North American-originated cohorts (11, 13, and 5, respectively) were 23% (95% CI, 15–31%, I2 = 74.5%), 19% (95% CI, 14–24%; I2 = 57.8%), and 21% (95% CI, 15–27%; I2 = 0%), respectively (Fig. 3). To explore whether there is any difference in the efficacy of bevacizumab between CRT use and chemotherapy use, another subgroup analysis was performed. As shown in Fig. 4, CRT-relevant cohorts presented better efficacy than chemotherapy-relevant cohorts (22% versus 17%). Significant small study effects were detected, as the P value of Egger's test was < 0.001 (Supporting Information Fig. S1a). The sensitivity analysis indicated that the pooled estimate can be as large as 22% (95% CI, 18–25%) by excluding Hasegawa et al. and as small as 20% (95% CI, 16–24%) by excluding Liang et al. (Supporting Information Fig. S1b).
Fig. 2.
The forest plot of pooled estimate of pCR (sub-grouped by backbone therapy).
Fig. 3.
The forest plot of pooled estimate of pCR (sub-grouped by region).
Fig. 4.
The forest plot of pooled estimate of pCR (sub-grouped by CRT or chemotherapy).
The safety of bevacizumab
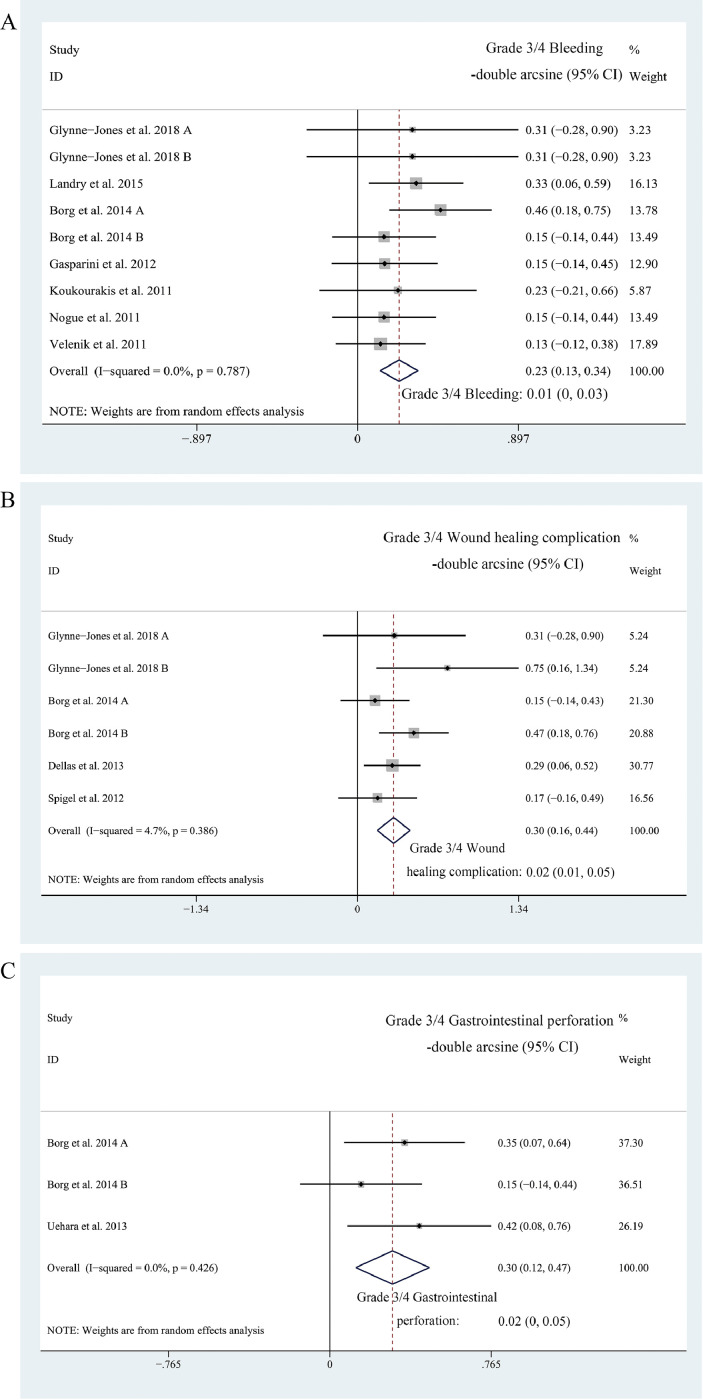
Nine cohorts reported on Grade 3/4 bleeding, six reported on Grade 3/4 wound healing complications, and three reported on Grade 3/4 gastrointestinal perforation. The pooled estimates of Grade 3/4 bleeding, Grade 3/4 wound healing complication, Grade 3/4 gastrointestinal perforation were 1% (95% CI, 0–3%; I2 = 0%, Fig. 5a), 2% (95% CI, 1–5%; I2 = 4.7%, Fig. 5b), and 2% (95% CI, 0–5%; I2 = 0%, Fig. 5c), respectively. Subgroup analyses and Egger's test were not performed due to the insufficient amount of data.
Fig. 5.
A, the forest plot of pooled estimate of Grade 3/4 bleeding; B, the forest plot of pooled estimate of Grade 3/4 wound healing complications; C, the forest plot of pooled estimate of Grade 3/4 gastrointestinal perforation.
Discussion
Main findings and interpretations
The current body of literature concerning the efficacy of neoadjuvant bevacizumab for LARC patients mostly consists of single-arm phase II clinical trials, largely lacking head-to-head data comparing neoadjuvant regimens with or without bevacizumab. In the previous meta-analysis by Zhong et al. [13], a benchmark pCR was set at 17% using relevant data from ten LARC cohorts extracted from the individual patient data-leveled meta-analysis of Maas et al. [44].
In this work, we double arcsine transformed these data and synthesized a pooled pCR rate of 15% (95% CI, 13–17%), as shown in Supplementary Fig. 1. Baseline characteristics of the included cohorts are presented in Supporting Information Table S1. Besides, increasing evidences demonstrate that induction therapy can improve the exposure to chemotherapy without impairing the delivery of neoadjuvant radiotherapy and that induction therapy is safer than adjuvant chemotherapy [43]. Therefore, we paid specific attention to the efficacy of bevacizumab in LARC cohorts receiving TNT in this study.
In this systematic review and meta-analysis, we reached a pooled pCR rate of 21% (95% CI, 17–25%) for all bevacizumab-relevant cohorts, which is superior to the current benchmark set at 15%. Although small study effects were detected, the pooled pCR lies within the range of 20 to 22, according to the results of sensitivity analysis, surpassing the benchmark even when excluding any of the included cohorts. Subgroup analyses were performed based on nCRT regimen or study location. The pooled pCR for cohorts receiving TNT was superior to that for any other subgroup including at least three cohorts. The 5-FU-based doublet therapy-subgroup achieved a higher pooled pCR rate than the capecitabine-based doublet therapy-subgroup. However, the 5-FU-based doublet therapy-subgroup contained only three cohorts containing substantial heterogeneity - no one achieved pCR in the cohort by Glynne-Jones et al. [20] while the cohort by Liang et al. [21] presented a pCR rate of 34.2% in 76 subjects – which weakens the credibility of this result. On the other hand, the pooled pCR for the capecitabine-based monotherapy-subgroup was comparable to that for the 5-FU-based monotherapy-subgroup or the capecitabine-based doublet therapy-subgroup, consistent with relevant conclusions in the NCCN guidelines [5]. For the subgroup analysis based on region, the pooled pCR rates for European-, Asian-, and North American- originated cohorts were largely comparable. As for the subgroup analysis based on CRT or chemotherapy, the CRT-revelant cohorts presented better pooled pCR rate (22%) than chemotherapy-relevant cohorts (17%) did. However, whether this benefit comes from the RT-enhancing activity of bevacizumab or RT itself remains inconclusive.
To evaluate the safety of adding bevacizumab, we synthesized the risk of Grade 3/4 bevacizumab-specific adverse events, namely Grade 3/4 bleeding- 1% (95% CI, 0–3%), Grade 3/4 wound healing complication- 2% (95% CI, 1–5%), and Grade 3/4 gastrointestinal perforation- 2% (95% CI, 0–5%). This safety is more than acceptable, considering that previously published clinical trials reported overall risks of Grade 3/4 toxicity ranging from 13.9% to 27%. However, only nine, six, and three of the 29 included cohorts reported data concerning Grade 3/4 bleeding, Grade 3/4 wound healing complications, and Grade 3/4 gastrointestinal perforation, respectively. More data concerning the safety profile of neoadjuvant bevacizumab are badly needed in the future.
Strengths and limitations
This is the most comprehensive systematic review to date evaluating the efficacy and safety of bevacizumab in the nCRT for LARC patients, and the first to put an emphasis on the role of bevacizumab in TNT. We also used double arcsine transformation to process data to better cope with the feature of all these data lying within the range of 0 to 0.3.
Nonetheless, there are several limitations that must be addressed. First, a lack of head-to-head survival data weakens our analyses; pCR is only a surrogate endpoint for prognosis. Second, more relevant studies are needed to provide more stable and robust results. Third, significant heterogeneity existed among the included studies, confounding factors such as the intensity of backbone nCRT required to be accounted for as the number of eligible trials increases in the future.
Conclusions
In conclusion, the addition of bevacizumab in the nCRT, especially in the TNT, for LARC patients provides promising efficacy and acceptable safety. However, the results should be interpreted cautiously due to the small amount of relevant data and need further confirmation by future studies.
CRediT authorship contribution statement
Yue Zhou: Conceptualization, Methodology, Software, Investigation, Resources, Data curation, Writing - original draft, Writing - review & editing. Zhexu Guo: Methodology, Software, Investigation, Resources, Data curation, Writing - original draft, Writing - review & editing. Zhonghua Wu: Formal analysis, Writing - original draft, Writing - review & editing. Jinxin Shi: Formal analysis, Writing - original draft, Writing - review & editing. Cen Zhou: Formal analysis, Writing - original draft, Writing - review & editing. Jie Sun: Formal analysis, Writing - original draft, Writing - review & editing. Iko Hidasa: Formal analysis, Writing - original draft, Writing - review & editing. Xuefei Lu: Formal analysis, Writing - original draft, Writing - review & editing. Chong Lu: Conceptualization, Methodology, Software, Validation, Writing - review & editing, Visualization, Supervision, Project administration.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
None.
Disclosure
All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2020.100964.
Appendix. Supplementary materials
Reference
- 1.Siegel R.L., Miller K.D., Fedewa S.A. Colorectal cancer statistics, 2017. CA Cancer J. Clin. 2017;67(3):177e93. doi: 10.3322/caac.21395. [DOI] [PubMed] [Google Scholar]
- 2.Schrag D., Weiser M.R., Goodman K.A., Gonen M., Hollywood E., Cercek A. Neoadjuvant chemotherapy without routine use of radiation therapy for patients with locally advanced rectal cancer: a pilot trial. J. Clin. Oncol. 2014;32:513–518. doi: 10.1200/JCO.2013.51.7904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bosset J.F., Collette L., Calais G., Mineur L., Maingon P., Radosevic-Jelic L. Chemotherapy with preoperative radiotherapy in rectal cancer. N. Engl. J. Med. 2006;355:1114–1123. doi: 10.1056/NEJMoa060829. [DOI] [PubMed] [Google Scholar]
- 4.Sauer R., Becker H., Hohenberger W., Rodel C., Wittekind C., Fietkau R. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N. Engl. J. Med. 2004;351:1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 5.Rectal cancer V.2+. 2019. NCCN Clinical Practical Guidelines in Oncology. Available at: http://www.nccn.org/professionals/physician_gls/.
- 6.Gerard J.P., Conroy T., Bonnetain F., Bouche O., Chapet O., Closon-Dejardin M.T. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: results of FFCD 9203. J. Clin. Oncol. 2006;24:4620–4625. doi: 10.1200/JCO.2006.06.7629. [DOI] [PubMed] [Google Scholar]
- 7.Bosset J.F., Calais G., Mineur L., Maingon P., Radosevic-Jelic L., Daban A. Enhanced tumorocidal effect of chemotherapy with preoperative radiotherapy for rectal cancer: preliminary results–EORTC 22921. J. Clin. Oncol. 2005;23:5620–5627. doi: 10.1200/JCO.2005.02.113. [DOI] [PubMed] [Google Scholar]
- 8.Craven I., Crellin A., Cooper R., Melcher A., Byrne P., Sebag-Montefiore D. Preoperative radiotherapy combined with 5 days per week capecitabine chemotherapy in locally advanced rectal cancer. Br. J. Cancer. 2007;97:1333–1337. doi: 10.1038/sj.bjc.6604042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Willett C.G., Kozin S.V., Duda D.G., Tomaso E., Kozak K.R., Boucher Y. Combined vascular endothelial growth factor-targeted therapy and radiotherapy for rectal cancer: theory and clinical practice. Semin. Oncol. 2006;33:S35–S40. doi: 10.1053/j.seminoncol.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Landry J.C., Feng Y., Prabhu R.S., Cohen S.J., Staley C.A., Whittington R. Phase II trial of preoperative radiation with concurrent capecitabine, oxaliplatin, and bevacizumab followed by surgery and postoperative 5-fluorouracil, leucovorin, oxaliplatin (FOLFOX), and bevacizumab in patients with locally advanced rectal cancer: 5-year clinical outcomes ECOG-ACRIN cancer research group E3204. Oncologist. 2015;20:615–616. doi: 10.1634/theoncologist.2015-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borg C., Andre T., Mantion G., Boudghene F., Mornex F., Maingon P. Pathological response and safety of two neoadjuvant strategies with bevacizumab in MRI-defined locally advanced T3 resectable rectal cancer: a randomized, noncomparative phase II study. Ann. Oncol. 2014;25:2205–2210. doi: 10.1093/annonc/mdu377. [DOI] [PubMed] [Google Scholar]
- 12.Nogue M., Salud A., Vicente P., Arrivi A., Roca J.M., Losa F. Addition of bevacizumab to XELOX induction therapy plus concomitant capecitabine-based chemoradiotherapy in magnetic resonance imaging-defined poor-prognosis locally advanced rectal cancer: the AVACROSS study. Oncologist. 2011;16:614–620. doi: 10.1634/theoncologist.2010-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhong X., Wu Z., Gao P., Shi J., Sun J., Guo Z. The efficacy of adding targeted agents to neoadjuvant therapy for locally advanced rectal cancer patients: a meta-analysis. Cancer Med. 2018;7:565–582. doi: 10.1002/cam4.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dipetrillo T., Pricolo V., Lagares-Garcia J., Vrees M., Klipfel A., Cataldo T. Neoadjuvant bevacizumab, oxaliplatin, 5-fluorouracil, and radiation for rectal cancer. Int. J. Radiat. Oncol. 2012;82:124–129. doi: 10.1016/j.ijrobp.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Xiao J., Chen Z., Li W., Yang Z., Huang Y., Zheng J. Sandwich-like neoadjuvant therapy with bevacizumab for locally advanced rectal cancer: a phase II trial. Cancer Chemother. Pharm. 2015;76:21–27. doi: 10.1007/s00280-015-2763-2. [DOI] [PubMed] [Google Scholar]
- 16.Tomida A., Uehara K. Neoadjuvant CAPOX and bevacizumab alone for locally advanced rectal cancer: long-term results from the N-SOG 03 trial. Int. J. Clin. Oncol. 2019;24:403–410. doi: 10.1007/s10147-018-1372-6. [DOI] [PubMed] [Google Scholar]
- 17.Konishi T., Shinozaki E., Murofushi K., Taguchi S., Fukunaga Y., Nagayama S. Phase II trial of neoadjuvant chemotherapy, chemoradiotherapy, and laparoscopic surgery with selective lateral node dissection for poor-risk low rectal cancer. Ann. Surg. Oncol. 2019;26:2507–2513. doi: 10.1245/s10434-019-07342-7. [DOI] [PubMed] [Google Scholar]
- 18.Yu X., Wang Q.X., Xiao W.W., Chang H., Zeng Z.F., Lu Z.H. Neoadjuvant oxaliplatin and capecitabine combined with bevacizumab plus radiotherapy for locally advanced rectal cancer: results of a single-institute phase II study. Cancer Commun. 2018;38:24. doi: 10.1186/s40880-018-0294-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maeda K., Shibutani M., Otani H., Fukuoka T., Iseki Y., Matsutani S. Neoadjuvant radiotherapy with capecitabine plus bevacizumab for locally advanced lower rectal cancer: results of a single-institute phase II study. Anticancer Res. 2018;38:4193–4197. doi: 10.21873/anticanres.12713. [DOI] [PubMed] [Google Scholar]
- 20.Glynne-Jones R., Hall M.R., Lopes A., Pearce S., Goh V., Bosompem S. BACCHUS: a randomised non-comparative phase II study of neoadjuvant chemotherapy (NACT) in patients with locally advanced rectal cancer (LARC) Heliyon. 2018;4:e00804. doi: 10.1016/j.heliyon.2018.e00804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang J.T., Chen T.C., Huang J., Jeng Y.M., Cheng J.C. Treatment outcomes regarding the addition of targeted agents in the therapeutic portfolio for stage II-III rectal cancer undergoing neoadjuvant chemoradiation. Oncotarget. 2017;8:101832–101846. doi: 10.18632/oncotarget.21762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hasegawa S., Goto S., Matsumoto T., Hida K., Kawada K., Matsusue R. A multicenter phase 2 study on the feasibility and efficacy of neoadjuvant chemotherapy without radiotherapy for locally advanced rectal cancer. Ann. Surg. Oncol. 2017;24:3587–3595. doi: 10.1245/s10434-017-5967-3. [DOI] [PubMed] [Google Scholar]
- 23.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 25.Freeman M.F., Tukey J.W. Transformations related to the angular and the square root. Ann. Math. Stat. 1950;21:607–611. [Google Scholar]
- 26.Liebig C., Ayala G., Wilks J., Verstovsek G., Liu H., Agarwal N. Perineural invasion is an independent predictor of outcome in colorectal cancer. J. Clin. Oncol. 2009;27:5131–5137. doi: 10.1200/JCO.2009.22.4949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sterne J.A., Sutton A.J., Ioannidis J.P., Terrin N., Jones D.R., Lau J. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- 29.Salazar R., Capdevila J., Laquente B., Manzano J.L., Pericay C., Villacampa M.M. A randomized phase II study of capecitabine-based chemoradiation with or without bevacizumab in resectable locally advanced rectal cancer: clinical and biological features. BMC Cancer. 2015;15:60. doi: 10.1186/s12885-015-1053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sadahiro S., Suzuki T., Tanaka A., Okada K., Saito G., Kamijo A. Phase II study of preoperative concurrent chemoradiotherapy with S-1 plus bevacizumab for locally advanced resectable rectal adenocarcinoma. Oncology. 2015;88:49–56. doi: 10.1159/000367972. [DOI] [PubMed] [Google Scholar]
- 31.Garcia M., Martinez-Villacampa M., Santos C., Navarro V., Teule A., Losa F. Phase II study of preoperative bevacizumab, capecitabine and radiotherapy for resectable locally-advanced rectal cancer. BMC Cancer. 2015;15:59. doi: 10.1186/s12885-015-1052-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang C.C., Liang J.T., Tsai C.L., Chen Y.H., Lin Y.L., Shun C.T. Neoadjuvant bevacizumab and chemoradiotherapy in locally advanced rectal cancer: early outcome and technical impact on toxicity. World J. Surg. Oncol. 2014;12:329. doi: 10.1186/1477-7819-12-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hasegawa J., Nishimura J., Mizushima T., Miyake Y., Kim H.M., Takemoto H. Neoadjuvant capecitabine and oxaliplatin (XELOX) combined with bevacizumab for high-risk localized rectal cancer. Cancer Chemother. Pharm. 2014;73:1079–1087. doi: 10.1007/s00280-014-2417-9. [DOI] [PubMed] [Google Scholar]
- 34.Fernandez-Martos C., Brown G., Estevan R., Salud A., Montagut C., Maurel J. Preoperative chemotherapy in patients with intermediate-risk rectal adenocarcinoma selected by high-resolution magnetic resonance imaging: the GEMCAD 0801 phase II multicenter trial. Oncologist. 2014;19:1042–1043. doi: 10.1634/theoncologist.2014-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uehara K., Hiramatsu K., Maeda A., Sakamoto E., Inoue M., Kobayashi S. Neoadjuvant oxaliplatin and capecitabine and bevacizumab without radiotherapy for poor-risk rectal cancer: N-SOG 03 phase II trial. Jpn. J. Clin. Oncol. 2013;43:964–971. doi: 10.1093/jjco/hyt115. [DOI] [PubMed] [Google Scholar]
- 36.Dellas K., Hohler T., Reese T., Wurschmidt F., Engel E., Rodel C. Phase II trial of preoperative radiochemotherapy with concurrent bevacizumab, capecitabine and oxaliplatin in patients with locally advanced rectal cancer. Radiat. Oncol. 2013;8:90. doi: 10.1186/1748-717X-8-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spigel D.R., Bendell J.C., McCleod M., Shipley D.L., Arrowsmith E., Barnes E.K. Phase II study of bevacizumab and chemoradiation in the preoperative or adjuvant treatment of patients with stage II/III rectal cancer. Clin. Colorectal Cancer. 2012;11:45–52. doi: 10.1016/j.clcc.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 38.Gasparini G., Torino F., Ueno T., Cascinu S., Troiani T., Ballestrero A. A phase II study of neoadjuvant bevacizumab plus capecitabine and concomitant radiotherapy in patients with locally advanced rectal cancer. Angiogenesis. 2012;15:141–150. doi: 10.1007/s10456-011-9250-0. [DOI] [PubMed] [Google Scholar]
- 39.Velenik V., Ocvirk J., Music M., Bracko M., Anderluh F., Oblak I. Neoadjuvant capecitabine, radiotherapy, and bevacizumab (CRAB) in locally advanced rectal cancer: results of an open-label phase II study. Radiat. Oncol. 2011;6:105. doi: 10.1186/1748-717X-6-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koukourakis M.I., Giatromanolaki A., Tsoutsou P., Lyratzopoulos N., Pitiakoudis M., Kouklakis G. Bevacizumab, capecitabine, amifostine, and preoperative hypofractionated accelerated radiotherapy (HypoArc) for rectal cancer: a phase II study. Int. J. Radiat. Oncol. 2011;80:492–498. doi: 10.1016/j.ijrobp.2010.02.037. [DOI] [PubMed] [Google Scholar]
- 41.Willett C.G., Duda D.G., Ancukiewicz M., Shah M., Czito B.G., Bentley R. A safety and survival analysis of neoadjuvant bevacizumab with standard chemoradiation in a phase I/II study compared with standard chemoradiation in locally advanced rectal cancer. Oncologist. 2010;15:845–851. doi: 10.1634/theoncologist.2010-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crane C.H., Eng C., Feig B.W., Das P., Skibber J.M., Chang G.J. Phase II trial of neoadjuvant bevacizumab, capecitabine, and radiotherapy for locally advanced rectal cancer. Int. J. Radiat. Oncol. 2010;76:824–830. doi: 10.1016/j.ijrobp.2009.02.037. [DOI] [PubMed] [Google Scholar]
- 43.Masi G., Vivaldi C., Fornaro L., Lonardi S., Buccianti P., Sainato A. Total neoadjuvant approach with FOLFOXIRI plus bevacizumab followed by chemoradiotherapy plus bevacizumab in locally advanced rectal cancer: the TRUST trial. Eur. J. Cancer. 2019;110:32–41. doi: 10.1016/j.ejca.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 44.Maas M., Nelemans P.J., Valentini V., Das P., Rodel C., Kuo L.J. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010;11:835–844. doi: 10.1016/S1470-2045(10)70172-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.