Highlights
-
•
A major obstacle for the effective treatment of PDAC is its molecular heterogeneity.
-
•
Stratification of PDAC using markers highly specific, reproducible, sensitive, easily measurable and inexpensive is necessary.
-
•
At the early stages, clinician’s priority lies in rapid diagnosis, so that the patient receives surgery without delay.
-
•
At advanced disease stages, priority is to determine the tumor subtype and select a suitable effective treatment.
Keywords: Pancreatic cancer, Personalized medicine, Patients stratification, Biomarkers, Immunotherapy
Abstract
A major obstacle for the effective treatment of pancreatic ductal adenocarcinoma (PDAC) is its molecular heterogeneity, reflected by the diverse clinical outcomes and responses to therapies that occur. The tumors of patients with PDAC must therefore be closely examined and classified before treatment initiation in order to predict the natural evolution of the disease and the response to therapy. To stratify patients, it is absolutely necessary to identify biological markers that are highly specific and reproducible, and easily measurable by inexpensive sensitive techniques. Several promising strategies to find biomarkers are already available or under development, such as the use of liquid biopsies to detect circulating tumor cells, circulating free DNA, methylated DNA, circulating RNA, and exosomes and extracellular vesicles, as well as immunological markers and molecular markers. Such biomarkers are capable of classifying patients with PDAC and predicting their therapeutic sensitivity. Interestingly, developing chemograms using primary cell lines or organoids and analyzing the resulting high-throughput data via artificial intelligence would be highly beneficial to patients. How can exploiting these biomarkers benefit patients with resectable, borderline resectable, locally advanced, and metastatic PDAC? In fact, the utility of these biomarkers depends on the patient's clinical situation. At the early stages of the disease, the clinician's priority lies in rapid diagnosis, so that the patient receives surgery without delay; at advanced disease stages, where therapeutic possibilities are severely limited, the priority is to determine the PDAC tumor subtype so as to estimate the clinical outcome and select a suitable effective treatment.
Graphical abstract
Pancreatic ductal adenocarcinoma
Pancreatic ductal adenocarcinoma (PDAC) is a common type of pancreatic cancer and one of the most lethal malignancies in humans, due to its highly metastatic characteristics and poor responsiveness to currently available therapeutics [1]. The earliest genetic event in the progression of the normal ductal epithelia to premalignant pancreatic intraepithelial neoplasia (PanIN) is the mutation of the KRAS oncogene [2]. The mutational activation of the KRAS protein triggers diverse downstream effector proteins that sustain proliferation, metabolic reprogramming, anti-apoptosis, evasion of the immune response, and remodeling of the microenvironment. Subsequently, chromosomal rearrangements, genetic inactivation, and mutations in CDKN2A, TP53, SMAD4, and other genes cause the progression from low-grade to high-grade PanIN leading to PDAC [3,4]. A tumor mass is surrounded by a complex microenvironment enriched with cells such as cancer‐associated fibroblasts, T cells, stellate cells, macrophages, regulatory T cells, endothelial cells, and cancer stem cells [5,6]. These cell types play an active role in maintaining a microenvironment favoring cancer cell survival. At a molecular level, the coordination among stromal cells and between tumor and stromal cells is sustained through the exchange of molecules and extracellular vesicles (EVs). Since Meenhard Herlyn, from the Koprowski's laboratory, discovered the CA 19–9 antigen 35 years ago, it has become the only useful blood test in the treatment response monitoring and recurrent disease diagnosis of patients with PDAC [7], an exception to this statement is the recent proposed analysis of the BRCA mutations in PDAC which are particularly senstitive to PARP inhibitors [8]. However, new promising methods, that will be presented and discussed in this review, are under development and will help early diagnosis and the selection of personalized treatments.
PDAC has a poor prognosis; only around 7% of patients survive up to 5 years after diagnosis [9]. Almost all recent phase II and III clinical trials implemented in random PDAC populations revealed no robust survival benefits. This can probably be attributed to the fact that these studies were conducted in unselected PDAC populations that were highly heterogeneous [10], [11], [12]. In fact, a major impediment for curing PDAC is the molecular heterogeneity of the disease, reflected by the diverse clinical response patterns to therapy, where patients’ survival time can range from 2 to 3 months up to more than 5 years after diagnosis, and the strong variability in sensitivity to classical as well as novel drugs. This heterogeneity is controlled by a combination of several aberrations in intracellular signaling pathways, resulting in variable susceptibility to drugs, metastasis development, and, therefore, survival rates [13,14]. Furthermore, “virtual microdissection” of PDAC transcriptomic data has not only allowed the identification of different tumor subtypes, but also revealed two main distinct stromal subtypes [15]. However, to date no proposed treatments have allowed for this heterogeneity. Indeed, in clinical practice it is the general performance status and stage of disease that guides the choice of drugs given to patients with PDAC. Studies thus far have been unable to predict patients’ responsiveness to treatment or provide a prognosis for progression of the disease. As an example, studies have reported objective response rates in gemcitabine-treated patients of 9.4% and in FOLFIRINOX-treated patients of 31.6%, demonstrating that around 90% and 70% of patients, respectively, do not respond to treatment [16,17].
One of the major advantages of examining and classifying patients with PDAC before the onset of treatment is the ability to predict the natural evolution of the disease and the patient's response to therapy using established biological markers that are highly specific and reproducible, and easily measurable by inexpensive sensitive techniques. In this review I will analyze, as critically as possible, the biomarker candidates with the potential to predict clinical outcomes of patients with PDAC.
Markers to classify PDAC
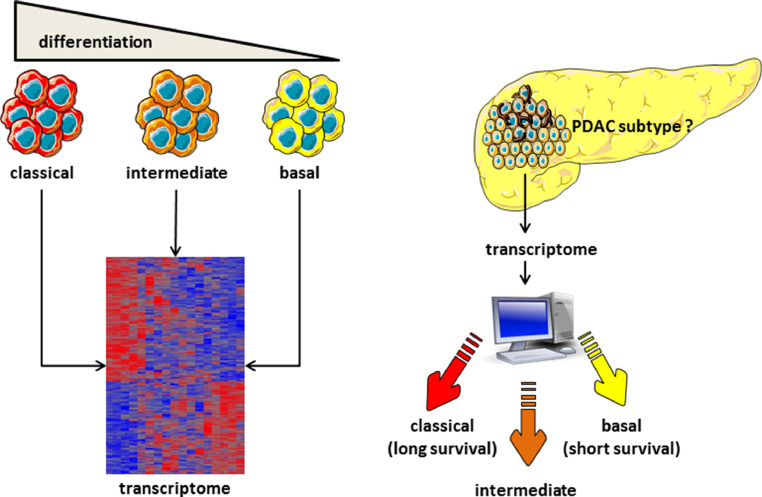
In an attempt to find tools to guide precision medicine, there have been considerable efforts made to classify PDAC molecular hallmarks beyond the known driver mutations. Different molecular subtypes have previously been defined: Collisson et al. defined subtypes as classical, quasi-mesenchymal, and exocrine-like; Moffitt et al. defined subtypes as basal-like, classical, normal, and activated stroma; and the International Cancer Genome Consortium defined squamous or basal-like, pancreatic progenitor or classical, exocrine-like, and immunogenic subtypes [15,18,19]. Using a patient-derived xenograft-based strategy and a complex algorithm, we recently demonstrated that PDACs can be better classified on a continuous gradient and that this molecular gradient correlates well with patient outcomes [20]. The established basal-like/squamous and classical/pancreatic progenitor subtypes have also been described coupled with low stromal signals. Tumors with high stromal content have been classified into three subtypes: desmoplastic, immune classical, and stroma-activated. Patients with tumors classified as classical/pancreatic progenitor have a significantly better prognosis than do those with basal-like/squamous tumors [21]. To date, there have been many challenges that have prevented this transcriptomic characterization process being routinely used in clinical practice, such as the requirement for fresh-frozen tumor tissue, and so it has failed to substantially impact guidance around the treatment of PDAC. Furthermore, molecular classification that is determined only by PDAC tumor tissue is likely to be inadequate, as the stroma and immune cells making up the tumor microenvironment play a pivotal role in progression of disease and resistance to treatment. Our recent analysis revealed five distinct PDAC subtypes based on signatures derived from tumor, stromal, and immune cells [20]. These findings are similar to previous molecular classifications; however, the classifications were expanded by the incorporation of the signals from the tumor microenvironment (Fig. 2). Recently, three consecutive papers report that GATA6 expression can diferentiate between classical and basal-like subtypes, knowing that basal-like subtype is resistant to FOLFIRINOX, therefore GATA6 can be utilized as a marker of sensitivity of PDAC to FOLFIRINOX [22], [23], [24].
Fig. 2.
Heterogeneity of PDAC. Transcriptomic analysis is able to classify PDAC into phenotypic subtypes, from classical (well differentiated) to basal (poorly differentiated) phenotypes. Several RNAs have been defined as specific markers. These phenotypes are associated with very distinct clinical evolutions. The clinical outcome of a new PDAC can be defined by obtaining its transcriptome and analyzing it to classify the PDAC as a classical, intermediate, or basal tumor.
Liquid biopsies
Circulating tumor cells (CTCs)
Recent years have witnessed substantial work investigating CTCs in the context of a variety of cancers. Increasing levels of CTCs have been repeatedly shown to correlate with a worse prognosis in cancer patients [25], [26], [27], [28]. Similarly, in patients with PDAC, elevated CTCs were associated with a poor survival rate [29]. CTCs may also provide useful information regarding the effectiveness of an ongoing treatment, such as through longitudinal monitoring of real-time treatment response. Elucidating the molecular basis of PDAC in individual patients may provide the means to personalize treatments, leading to more effective precision medicine for the treatment of this lethal cancer [30,31]. CTCs are rare cells among the billions of hematopoietic cells circulating in the blood. This rarity means it has been difficult to develop efficient ways to track and isolate CTCs [32,33]. A highly attractive aspect of CTCs is their ability to assist in clinical management throughout all stages of cancer, using only a minimally invasive liquid biopsy as opposed to traditional tissue biopsy [31]. This allows for more frequent and repeated use of CTC measurements to manage patients in a less invasive, lower risk, and cost-effective manner.
Existing CTC technologies principally aim to quantify and detect CTCs based on either their immune-affinity profiles or physical properties. Immunocytostaining is commonly used to detect CTCs [34,35]. However, there are significant variations in the size of CTCs, even in an individual sample from one patient [36]. Isolating CTCs based on cell size may therefore mean that small CTCs are underrepresented or even absent. Basing isolation and enrichment of CTCs on their immune-affinity is another approach, but CTC heterogeneity - even within an individual patient sample - is a limitation for this approach. Studies using different isolation approaches have estimated that the sensitivity of CTCs to detect pancreatic cancer is 38%−100% [37]. The lack of current consensus around the best methods to detect and quantify CTCs may account for this large range [38]. Exciting and promising future research areas in CTC are yet to be explored. In particular, further work is required to standardize methods for the successful culture of CTCs and to allow study of their genetic and molecular properties. This in turn would help to improve and individualize therapy regimens for patients. CTCs are therefore appealing innovative tools for use in precision medicine.
Circulating free DNA (cfDNA)
cfDNA is a short deoxyribonucleic fragment that can be isolated from the plasma or serum by noninvasive procedures. cfDNA is released from apoptotic cells and remains at a consistent level in healthy individuals. However, due to the high cell turnover rate of tumors, the release of cfDNA increases and genetic alterations of cfDNA are also more likely to be present and elevated in cancer patients. When released from tumor cells, cfDNA is also called circulating tumor DNA (ctDNA). In terms of PDAC, cfDNA is considered a promising prognostic factor. Mutations, concentration, and hypermethylation of cfDNA are all associated with cancer progression in and survival of patients with pancreatic cancer [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49]. A recent meta-analysis revealed that ctDNA, either at baseline or a postoperative stage, might be a useful prognostic biomarker for detecting risk of death and recurrence in resectable PDAC [50]. Importantly, pembrolizumab demonstrated an 83% objective response rate in the six pancreatic cancer patients evaluated in one study, suggesting that MSI status can predict the response to anti-PD-1/PD-L1 blockade [51]. Consequently, it is now recommended that clinicians assess the MSI status of all patients with PDAC; this can be feasibly done using cfDNA.
Diagnosing PDAC at an early stage is challenging, as patients typically develop symptoms at advanced stages of disease and these symptoms are often nonspecific. Given the current lack of effective clinical screening at the early disease stage, ctDNA had been pursued as a way to overcome this problem. Unfortunately, to date the low levels of ctDNA in early stage PDAC have presented a barrier to its use in diagnosis. However, advances in ctDNA detection technology mean that even minute amounts of ctDNA can now be accurately detected. Some studies report that ctDNA is detected in around 50% of PDAC patients with localized disease (stage I to III) and in more than 90% of patients with metastatic disease (stage IV) [52], [53], [54]. There are many mutations observed in cfDNA, such as in KRAS, BRCA2, EGFR, KDR, and ERBB2 gene loci. Among them, KRAS mutation is one of the most concerned mutations. However, the prognostic value of a KRAS mutation in cfDNA as a biomarker remains controversial. At present, we may assume that the level of cfDNA could be used for identifying the advanced metastatic disease, but the methods used for this purpose are not sensitive enough for diagnosis at the early stages. Therefore, to diagnose patients at early stages of disease, methods to detect ctDNA need to improve. A recent study was able to detect mutations in cfDNA from conditioned media from PDAC organoids [55], which opens new possibilities for the use of cfDNA as a diagnostic marker.
Methylated DNA
In the entire human genome, approximately 50% to 70% of CpG dinucleotides are methylated. The majority of methylated CpG dinucleotides are found in repetitive genomic sequences. Because of its informative and stable nature, many studies have evaluated DNA methylation as a promising epigenetic cfDNA biomarker. During PDAC development, genetic and epigenetic changes occur. Aberrant DNA methylation is detected in precursor pancreatic lesions [56], [57], [58], which indicates that promoter hypermethylation occurs in the very early stages of carcinogenesis. The prevalence of methylation progressively increases with increased dysplasia, and it has been proposed that aberrant methylation may occur in different genes at different stages of pancreatic neoplastic progression [56,58]. DNA hypermethylation of cell-free DNA in plasma and serum is potentially tumor specific and is undetectable similarly to other blood-based diagnostic markers of pancreatic cancer [59,60]. Data from several studies suggest that assessing the methylation of a small number of genes may allow differentiation between patients with malignant transformations and healthy individuals [61,62]. Evaluating the methylation promoters of BNC1, MESTv2, BMP3, RASSF1A, TFPI2, SFRP1, APC, and SFRP2 in cfDNA has shown high specificity and sensitivity for PDAC [63]. Interestingly, it has been reported that the genes ONECU and GSTM1 are differentially methylated in PDAC tissue of gemcitabine responders and nonresponders [64]. Currently, no epigenetic biomarker has been approved for PDAC diagnosis. However, it is anticipated that the availability of modern techniques for genome-wide analysis means that future studies will validate this important epigenetic marker in large patient cohorts.
Circulating microRNA (miRNA): miRNAs are noncoding RNAs that regulate posttranscriptional gene expression. The increasingly recognized role of miRNAs in oncogenesis and tumor metastasis has been described [65,66]. miRNA profiles specific for PDAC have been found in serum, pancreatic tissue, cyst fluid, and more recently in the whole blood [67], [68], [69]. Among these miRNAs, the plasma level of miR-125b-5p acts as an independent biomarker in predicting overall survival of patients with PDAC [70]. Other miRNAs, including miR-21, miR-155, and miR-196, are upregulated in PDAC tissue samples and are able to distinguish tumors from premalignant lesions [71], [72], [73], [74]. Additionally, three miRNAs (miR-143, miR-223, and miR-30e) can be assessed in urinary and fecal samples and are overexpressed in stage I cancer compared to healthy tissue. Moreover, miR-223 and miR-204 in urine can distinguish early stage cancer from chronic pancreatitis [75]. Although a large number of miRNAs have been implicated as potential biomarkers able to distinguish between healthy individual and PDAC patients, the next steps must involve the clinical validation of such promising markers in larger patient cohorts, especially focusing on those able to discriminate between low-grade and high-grade dysplasia.
Exosomes and extracellular vesicles (EVs)
All types of cells release exosomes, which are 40–150-nm extracellular vesicles. Exosomes are made up of a lipid bilayer and at any particular time can comprise all known molecular cell constituents, including DNA, RNA, and proteins. It is estimated that normal human blood contains around 2 × 1021 exosomes, while blood taken from patients with cancer is estimated to contain around 4 × 1021 exosomes [76]. Exosomes have been depicted as tumor progression promoters [77,78]. As they can be found in liquid biopsies, cancer exosomes can aid the diagnosis of cancers, including pancreatic cancer [79]. Analysis of the systemic circulation of patients with ovarian cancer, breast cancer, and PDAC revealed elevated concentrations of exosomes [79]. Markers that are specifically associated with cancer exosomes could improve their isolation/enrichment from the heterogeneous exosome populations found in body fluid, and thus help in diagnosis. Several in vitro and preclinical studies have increased what we know about the contents of exosomes and the potential utility of this to monitor or detect cancer. Although the lipids and metabolites found in cancer exosomes may offer additional insights into the biology of cancer and for cancer detection, the utility of nucleic acids and proteins found in exosomes is still being researched. DNA found in exosomes could offer information about cancer-specific mutations [80,81]. Whole-genome sequencing revealed that the complete genomic double-stranded DNA was contained within exosomes found in the serum of patients with pancreatic cancer [80]. In addition, analysis of the exosomal DNA revealed driver mutations associated with PDAC [80], [81], [82], [83]. It therefore appears that whole-genome sequencing of exosomal DNA taken from the serum exosomes of patients with cancer may be able to inform diagnosis and predict treatment responses (Fig. 1).
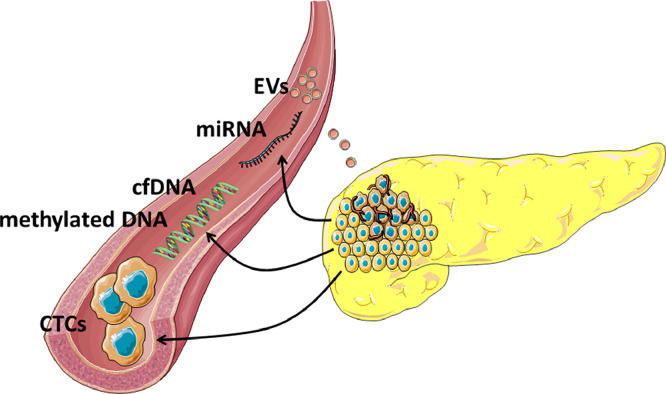
Fig. 1.
Liquid biopsies. Several products, such as CTCs, cfDNA and methylated DNA, miRNA, and EVs, are released from PDAC into the blood and other body fluids. These products reflect tumor biology and their analysis could reveal predicting markers.
Metabolomics
Metabolomics, an omics technique able to provide a dynamic picture of metabolic profiles, is newly emerging in the field of cancer. This approach can be a non-invasive approach that may help in managing patients with a PDAC in terms of diagnosis, prognosis and response to treatments. Metabolomics analysis of plasma taken from individuals at a high risk of developing a PDAC display elevated levels of branched-chain amino, these findings propose the increased whole-body protein breakdown as an early event in development of PDAC this parameter can be potentially used as a screening method early PDAC disease [84]. Another metabolomics study was performed using plasmas from two independent cohorts of PDAC patients and compared to plasma of healthy individuals. Five metabolites (acetylspermidine, diacetylspermine, an indole-derivative, and two lysophosphatidylcholines) were detected as to be significantly discriminative [85]. Early diagnosis of PDAC via a non-invasive approach remains a major challenge for oncologist. Interestingly, metabolomics analysis using blood samples is able to distinguish between patients with PDAC and chronic pancreatitis cohorts. Results showed that glycocholic acid, N-palmitoyl glutamic acid, hexanoylcarnitine, phenylacetylglutamine and chenodeoxyglycocholate were identified as single markers discriminating between PDAC and chronic pancreatitis [86]. In addition, a biomarker signature (nine metabolites and additionally CA19–9) was identified for the differential diagnosis between PDAC and chronic pancreatitis. This study concludes that the clinical use of this newly identified biomarker signature can improve diagnosis and treatment stratification compared to CA19–9 alone in almost one third of patients [87]. Plasma samples from patients with localized, locally advanced, and metastatic PDAC were analyzed with mass spectrometry to assess if some metabolites could delineate different stages of PDAC. Five metabolic components were identified using principal component analysis but each cohort was characterized with a unique combination of components that do not occur in a linear stepwise progression [88]. A preliminary work from Phua et al. reports that a metabolomics profile using plasma can predict response of patients treated with gemcitabine as an adjuvant chemotherapy [89]. These reports are very encouraging, however it still need to be validated in larger cohorts of patients before being transferred to the clinic.
Immunological landscape
The host immune response, represented by infiltrating immune cells, is known to strongly influence cancer progression [90, 91]. There are several scoring systems that assess the association between the survival of patients with cancer and the host immune response [92], [93], [94], [95]. Tumor-infiltrating lymphocytes correlate with survival in gastric, esophageal, and rectal cancers [96], [97], [98]. Recent research into the inflammatory response and tumor microenvironment in PDAC has revealed their significance [5]. Several biomarkers have been assessed for their capacity to act as predictors of prognosis or to guide the therapeutic approach [99]. It is well documented that in PDAC there is an association between the survival of patients with cancer and the immune microenvironment of the tumor [94,[100], [101], [102], [103]]. Studies have proposed various inflammatory biomarkers that could be used to predict prognosis. Among these, the neutrophil to lymphocyte ratio (NLR) is the most accurate. An NLR of >5 seems to indicate a poor prognosis in PDAC [104]. A number of immune markers correlate with a poor prognosis, including CD66b, CD163, CD68, CD204, and FOXP3 [101]; markers that are associated with a better prognosis include CD20, CD4, CD8, and CD3 [94]. One favorable prognostic factor for survival is the presence of a high number of CD4+/CD8+ tumor-infiltrating lymphocytes following resection [105]. In addition, the presence of intratumoral tertiary lymphoid organs (lymphoid follicles) is a marker of long survival [106]. In addition, our analysis of the soluble forms of the BTN3A subfamily (BTN3A1 and pan-BTN3A), PD-L1, PD-1, and BTLA in the plasma of patients with PDAC (using specific antibodies and ad hoc developed ELISAs) revealed that high concentration levels of these immune checkpoint proteins can be used as prognostic factors and also correlate with poor outcome [107]. There is a complex interplay between chronic inflammation and the immune response and so, despite the proposal of various combinations of intratumoral and peritumoral immune cells as prognostic factors in pancreatic cancer tumoral tissue [94], we are yet to understand the situation fully.
Blockade of the PD-1/PD-L1 axis showed encouraging results in phase II/III clinical trials [108], [109], [110] for treatment of several solid tumors. However, for most patients with PDAC, this immunotherapy has not shown significant clinical effectiveness [111]. Early clinical studies investigating PD-1/PDL1 antagonists showed no activity in patients with PDAC, despite remarkable efficacy seen across a wide range of malignancies [112]. Similar findings have been reported with CTLA-4 antagonists [113] and, more recently, when PD-1 blockade was combined with small molecules [114]. The apparent lack of efficacy observed with checkpoint blockade in PDAC has led to the development of combination studies with chemotherapy, based on the premise that chemotherapy can be immunogenic [115,116]. However, to date, efforts to combine immune checkpoint blockade (i.e., anti-PD-1 and anti-CTLA-4 therapies) with chemotherapy have not produced remarkable clinical benefits in human PDAC beyond what is to be expected using chemotherapy alone [17,117,118]. Several new vaccine therapies that aim to induce T-cell responses in order to surpass immune resistance in PDAC are under investigation in clinics, and IL17 expression in the tumor microenvironment is being assessed for use as a biomarker for vaccine-induced antitumor responses (NCT02451982).
Markers to define sensitivity
Transcriptomic analysis is informative about the prognosis of patients with PDAC, but the main drawback is the difficulty of obtaining sufficient useful material for the analysis. For example, surgical samples are only accessible in 15%−20% of patients, and biopsies obtained by endoscopic ultrasound-guided fine-needle aspiration biopsy (EUS-FNA) yield small and contaminated samples that are difficult to exploit. PDAC is a heterogeneous disease that has a variable clinical evolution, exhibits distinct responses to treatments, and is histopathologically characterized by a limited differentiation grades. We [119] and others [19] have reported that the clinical evolution, the response to the treatments, and the degree of differentiation cannot be explained by genetic mutations. On the contrary, we demonstrated that at least the overall survival and histological characteristics of tumors are determined by the epigenetic landscape, including DNA methylation [119] and specific histone marks [120,121] that carries as consequences a variety of PDAC phenotypes with specific clinical outcomes [121]. This is conceptually important, since the epigenetic landscape, in contrast with most genetic mutations, is druggable and can be modified. In addition, it can be identified in small samples of PDAC and therefore used as a potential biomarker if proven to be clinically specific. Given the increasing evidence for the role of epigenetics in cancer, we hypothesize that the DNA methylation landscape and chromatin-associated changes of PDAC tumors are mandatory for the tumor aggressiveness, opening the opportunity to treat PDAC with specific epidrugs in the contex of personalized teratments (Fig. 3).
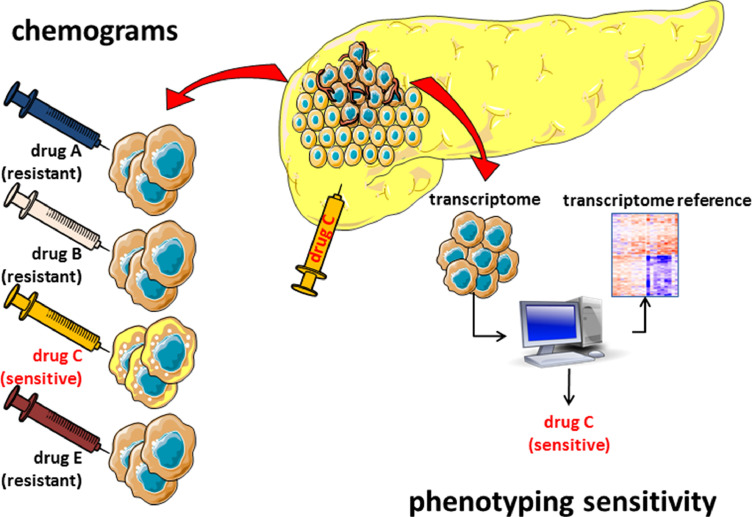
Fig. 3.
Sensitivity of PDAC. In operable patients, cells from PDAC can be obtained and challenged in vitro with different drugs to evaluate their sensitivity, which therefore reflects the sensitivity of the individual patients. In locally advanced or metastatic patients, a small number of cells can be obtained by EUS-FNA; from these cells, an RNA profile can be obtained and compared to the reference profiles previously defined in order to categorize the PDAC as having a sensitive or resistant phenotype to a given drug. Patients should be treated with any of the drugs eliciting a response rather than given a drug at random.
Inactivating mutations in BRCA1, BRCA2 and PALB genes are associated with an increased risk of PDAC. These genes code for proteins involved in homologous recombination repair of DNA double-strand breaks and to be noted is that cells with a deficiency in homologous recombination repair are sensitive to PARP inhibition. Thus, PARP inhibitors cause an accumulation of DNA damage and tumor-cell death [122]. Based on these rational a recent clinical trial on patients with mutated BRCA1 or BRCA2 treated with the PARP inhibitor olaparib showed a longer progression-free survival [8]. These data indicate that detecting these mutations in PDAC patients will be of interest for their specific treatment. No other gene mutations-targeted therapies have been showed to be efficient in patients with PDAC.
Chemograms
Chemograms are in vitro or ex vivo methods of determining the prognosis of an individual with PDAC, or the efficacy of a compound for treating PDAC in each individual, and so are a practical approach toward personalized treatments. Our preliminary study evaluated the sensitivity of PDAC primary cell lines to increasing concentrations (from 0.001 to 1000 µM) of five gold-standard chemotherapeutic agents (5FU, docetaxel, oxaliplatin, gemcitabine, and SN-38, the active metabolite of irinotecan). With personalized chemograms for each patient, we obtained dose-response curves characterizing tumor chemosensitivity. Our study resulted in the production of an individual chemogram profile for each patient-derived cell line, which revealed a large heterogeneity in the chemosensitivity of PDAC [123]. This is relevant for clinical practice; resistance or sensitivity to one agent does not predict resistance or sensitivity to another. It is important to also note that, despite incubation times of 72 h with very high concentrations of some agents (100 µM of SN-38, 1000 µM of docetaxel, oxaliplatin, gemcitabine, or 5FU), some cells survived. This is explained by the fact that PDAC tumors exhibit heterogeneity and so these primary cultures represent the different cell populations present in the tumors [124]. The clinical relevance of this is that if a chemogram can detect the proportion of cells that are resistant or sensitive to a particular drug, it can be helpful tool to guide clinicians in selecting second-line treatments for individual patients. We performed a supervised clustering analysis of the transcriptome to estimate whether a correlation between PDAC phenotype and drug response existed. Surprisingly, some sets of genes were identified as being specifically overexpressed or underexpressed in resistant and sensitive cells, supporting the hypothesis that the tumor phenotype determines the response to a treatment. Importantly, only a very small number of common genes were associated with drug resistance or sensitivity, suggesting that the phenotype of the sensitivity or resistance is specific to each drug. Lastly, another important point to note is that genes associated with sensitivity or resistance to the drugs are not typically genes associated with survival, indicating that outcome and drug sensitivity are regulated by independent mechanisms [123]. This observation was recently confirmed in a larger cohort [20,125]. More recently, similar observations were published using PDAC organoids as models [55,126], indicating that the transcriptome may be used to discriminate sensitive and resistant patients. This is a very important concept, since performing chemograms for each patient requires a substantial amount of material and a long time for amplification, whereas a smaller amount of material is needed for whole transcriptomic analysis. In our lab, we amplify the material obtained by EUS-FNA to yield enough cancer cells and grow them in a 3D extracellular matrix to form organoids within a short period of only a few days. These organoids can then be dissociated, and RNA is collected to be analyzed by RNA sequencing (Fig. 3).
Increasing models with translational relevance
Human xenograft models
Animal models have been used as surrogates of human biology due to the logistical and ethical restrictions of working with cell and tissue samples from human donors. Small animals, especially mice, are widely used as mammalian model systems due to their small size, ease of maintenance and handling, a short reproductive cycle, similarity of genomic and physiological properties with humans and the ability to be easily manipulated genetically. Patient derived xenograft (PDX) tumor models are the most frequently used testing systems for the in vivo evaluation of efficacy of novel anticancer drug candidates. Cell line-derived xenograft tumor models are well established with a long history of model characterization. PDX models are based on the implantation of human tumor cells into immunocompromised mice. Immunodeficient mice are used to avoid graft rejection due to the host immunological reaction against the foreign tumor tissue. PDX can be developed subcutaneously or orthotopically. Although numerous basic biology knowledge has been obtained from mouse studies, there are limitations to this models, since several components of murine biological systems are different than those in humans, particularly their immune system.
Currently, humanized mice have begun to fill this gap and becoming important preclinical tools for biomedical research. Immunodeficient mice with mutations in IL2 receptor common gamma chain (IL2Rγnull) have already been described during the last years [127]. NOD-scid IL2Rγnull mice engrafted with human peripheral blood mononuclear cells have been utilized successfully in xenograft models to evaluate efficacy and pharmacodynamics of immune checkpoint inhibitors targeting epitopes on human T-Lymphocytes. This model offers an advantage over conventional xenografts since functional human T-cells can target human epitopes on a tumor in a mouse with a better clinical relevance. Humanized tumor xenograft mouse models are powerful experimental systems for studying in vivo interactions between human immune cells and human tumors. In recent years, humanized tumor xenograft mouse models have been integrated into checkpoint inhibitor development programs at many pharmaceutical and biotechnology companies as an indispensable part of the drug development process. As these models have improved, they have been shown to support higher levels of human myeloid-derived cells and regulatory T cells, and have been used successfully in recent studies of anti-PD1 drugs in some tumors [128].
Organoids
Organoid is as a group of epithelial cells growing in a 3D structure, with self-renewal and self-organization capacities, which recapitulates the tissue of origin. Organoids can be maintained through indefinite passage and preserve their genetic stability. In 2013, Huch et al. [129] described 3D structures for pancreatic tissue. Later, in 2015, Boj et al. [130] reported the first 3D organoid model from murine and human PDAC by embedding cells in Matrigel with the addition of several growth factors. Human organoids as patient-specific models of PDAC is a promising tool for a new era of personalized medicine. Organoids can provide a platform for drug testing of individual tumors in a short period of time before or even in parallel to clinical treatment of a PDAC patient, thereby offering a safe haven for individualized precision medicine as we recently reported [131,132].
However, current organoid technology still represents an imperfect version. Firstly, organoids only contain epithelial layer without components of tumor microenvironment, such as immune system and nervous system. Secondly, another obstacle is the dependency on the extracellular matrix Matrigel to grow produced from mouse tumor lines and that might be non suitable for human. Thirdly, culture medium needs to be further refined for long-term expansion of some organoids. Fourthly, growth factors or molecular inhibitors in culture medium might have some effects on drug responses of organoids. Fifthly, under present culture conditions the differentiated phenotype is strongly favored. Further efforts should be urgently exerted to surpass these problems.
Organ-on-chip and multiple-organs-on-chip
Organ-on-chip and multiple-organs-on-chip systems represent an innovative in vitro cell culture model that utilizes physiologically accurate tissue and organ modeling for pharmacology studies for testing cancer treatments. These sophisticated platforms result from the combination of tissue engineering, microfluidics and microfabrication techniques. With the developed tissue engineering models, the microengineering techniques allow the integration of multiple sensing units for monitoring of pH, O2, temperature, or molecules secreted by cells and automatic systems for controlling dosing and dilution of multiple drugs in circulating media. In addition, multiple-organs-on-chip represents a complex organism which may includes the diseased tissue with some normal tissues connected one to others through vessels-like structures.
Remarkably, organs-on-chips can be personalized to reflect individual physiology. The personalized nature of such systems, combined with physiologically relevant read-outs, provides new opportunities for patient-specific assessment of drug efficacy, as well as personalized strategies for PDAC treatment, however current personalized organs-on-chips have not yet been used for precision medicine yet. To be noted the organs-on-chip models represents recent devices that are rapidly evolving and will open the possibility for testing the sensitivity to several drugs concomitantly. There are no reports yet about its utilization in PDAC but in a period of a few years it may become one of the most commonly used devices to predict the most efficient drug for each given patient. This device is complementary to PDX animal models since it is less expensive and more rapid. Lastly, the industry of drug development is starting to implement this technology in their own research and development departments.
Single-cell RNA sequencing
In recent years, single-cell RNA sequencing (scRNA-seq) technologies have provided in-depth analysis of cell heterogeneity in PDAC. It is highly effective especially in cell-type-specific molecular identification and detecting interactions between cancer cells and the stromal microenvironment. Transcriptomic analysis of the PDAC epithelial compartment revealed the ability of the inter-tumor heterogeneity to classify into different subtypes such as for example the binary classical and basal-like [15] or the most accurate continuous gradient PAMG (Pancreatic Adenocarcinoma Molecular Gradient) [20] which seems to be more precise in terms of prognosis. However, the intra-tumor heterogeneity particularly in the epithelial compartment is poorly described. Growing evidences suggest that this phenotypic segregation is not very specific and different cancerous cell subtypes may coexist in a single tumor. We recently performed a scRNA-seq analysis exclusively on epithelial cells from several PDAC samples obtained by EUS-FNA. Remarkably, although all these tumors were classified as well diferentiated, one cluster presented in all these samples corresponded to a basal-like phenotype. These results reveal an unanticipated high heterogeneity of PDAC and demonstrate that basal-like cells, which have a highly aggressive phenotype, are more widespread than expected. In this way, Chan-Seng-Yue et al. [133] confirmed the presence of several subpopulations with differential proliferative and migratory potentials in PDAC. In other words, very classical or very basal-like subtypes are mainly composed by pure cells, whereas the intermediate subtype is the consequence of a mix of classical and basal-like subtypes and/or an intermediate phenotype. Clinically relevant is that stratification of PDAC cells, based on a simple RNA signature, could predict the response to mFOLFIRINOX [20,22], Carfilzomib [131] or Gemcitabine [125]. We assume that the intratumoral heterogeneity, in which sensitive cells coexist with resistanes ones, is responsible, at least in part, of the recurrence of the traitements. Therefore, using scRNA-sequencing we can predict the best combinatorial drug regimens to personalize the treatment for each patient.
Artificial intelligence
In the near future, high-throughput data analysis, artificial intelligence, and machine learning are expected to help clinicians and oncologists develop better therapeutic strategies suitable for each patient, particularly for diseases such as PDAC that have high heterogeneity in clinical responses and short survival times after treatment. However, these promising tools are still in their initial stages. The strong intertumoral and intratumoral heterogeneity of PDAC, the unpredictable response to standard treatments, and the variability of survival time after diagnosis are of a singular complexity that only seems to be effectively determined through artificial intelligence approaches. Using artificial intelligence for concomitant analysis of images, biological and molecular data, somatic and germinal genetics, and blood parameters of the same patient is a reality that will be introduced in the next few years in the field of precision medicine [134,135]. Artificial intelligence will help scientists to understand how cancer cells become resistant to anticancer drugs, which can help to improve drug development and adjust the drug use for each patient; and it will aid radiologists to automatically define the plan radiation treatment programs; to oncologists to manage the use of chemotherapy drugs and predict the tolerance to chemotherapies; and more globaly, to adapt beneficial treatment decisions, reduce unnecessary surgeries, and help oncologists improve patients’ cancer treatment plans.
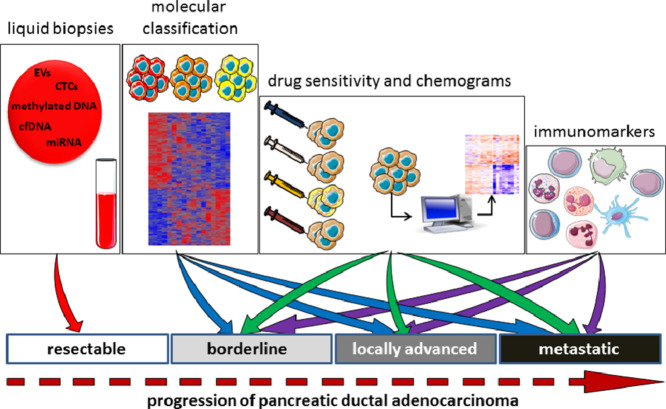
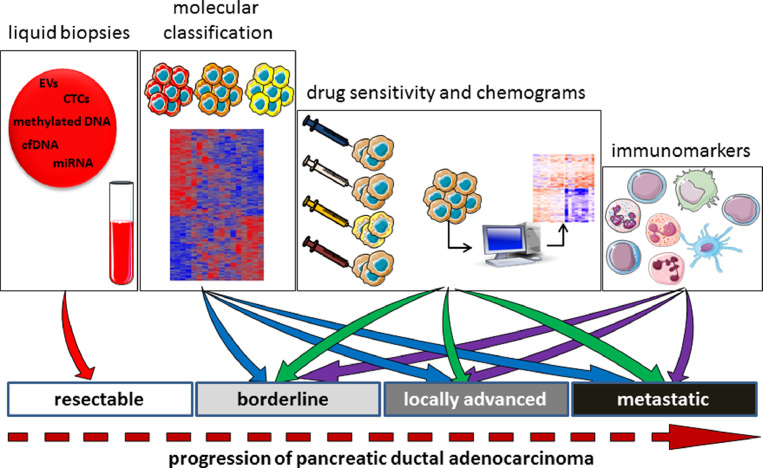
How can biological markers serve patients with resectable, borderline resectable, locally advanced, and metastatic PDAC?
Resectable PDAC tumors are those that can be removed surgically; surgery often occurs rapidly after diagnosis. These tumors may be located solely in the pancreas or may have spread to the area around it, without extending into nearby veins or arteries. Around 15% of patients are diagnosed at this stage of disease. Borderline resectable PDAC tumors are not suitable for surgery after the initial diagnosis, as such tumors are difficult or impossible to remove. However, chemotherapy and/or radiotherapy can reduce the size of the tumor, which can then be removed with negative margins. Locally advanced PDAC tumors are still located in the area around the pancreas; however, these cannot be removed surgically as they have grown close to or into nearby organs, veins, or arteries, and their removal presents a high risk of damaging these structures. No signs of the tumor spreading to any distant parts of the body are present. Around 40% of patients are diagnosed at this stage of disease. Metastatic PDAC involves a tumor that has spread to other organs beyond the pancreatic area, such as the lungs, liver, or other parts of the abdomen. Around 45% of patients are at this stage of disease at diagnosis.
Clearly, each of these disease stages are associated with a different prognosis and therefore require appropriate therapeutic strategies. None of the current classifications take into account the differentiation grade or any molecular characteristics (such as mutations or the transcriptome). Although tumor classification is well implemented in clinical practice, not all patients have tumors that can be easily classified. Current classification takes into account the time course of the disease, in which resectable PDAC occurs before metastasis and so the survival time is longer. However, within the same stage of disease we find heterogeneous behavior, which could be explained by the intrinsic molecular characteristics of the tumor or the host. At the moment, these characteristics are underestimated. This is why it is important to discuss the therapeutic needs at each stage of the disease (Fig. 4).
Fig. 4.
Markers that are needed at each stage of the disease.
How can we improve the treatment of patients with resectable PDAC? This stage of the disease has a better outcome, and the most convenient treatment is surgical resection; median survival time is 2 years. Despite the fact that tumors at this stage are small in size, it is not uncommon that some patients die a few months after resection owing to general dissemination of the tumor. For these patients, it would be interesting to predict the characteristics associated with the poor clinical outcome. Some possibilities that are or will soon be available to predict disease evolution are blood markers such as CTCs, EVs, miRNA, and ctDNA which may be evaluated easily using blood samples. It is noteworthy that liquid biopsies are also explored for predicition of drug responses, disease monitoring and detection of recurrence [136,137]. In addition, samples taken during surgery could be analyzed to identify the PDAC subtype (knowing that basal-like subtype tumors have a poorer prognosis than do classical PDAC subtype tumors) or to define immune markers. Somatic mutations do not seem to be very informative, except in a small number of cases in which targetable mutations are detected [138]. Finding sensitive biomarkers to classify tumors, in addition to identifying new treatments, will greatly improve treatment strategies for PDAC. Furthermore, in patients with resectable PDAC, who are generally asymptomatic and in whom tumors are not easily detected by imaging, it would be important to use liquid biopsies to track circulating markers such as CTC or ctDNA to accelerate the diagnosis and therefore ensure patients receive surgery more quickly. Also, organoids, directly obtained from surgical samples, can be utilized to perform chemosensitivity tests.
What are the needs for patients with borderline resectable PDAC? Since a borderline resectable PDAC tumor cannot be removed surgically upon diagnosis but becomes operable after treatment, it is important to determine the most effective drug for each patient, to predict the PDAC subtype and therefore the outcome, and also to characterize the immunological landscape so that beneficial immunotherapy can be identified in the near future. CTCs, EVs, miRNA, and ctDNA may be evaluated easily in blood of these patients. Importantly, PDAC tumoral samples can be obtained by EUS-FNA and amplified as organoids before to test their chemosensitivity.
How we can improve the survival of patients with locally advanced or metastatic PDAC? Patients with these tumors cannot be operated on and therefore the best therapeutic option is to use the most effective drugs against the disease following a personalized treatment regimen. In rare cases, these patients can receive surgery after treatment, similar to patients with borderline resectable PDAC. Patients with locally advanced or metastatic PDAC, and particularly patients with a basal-like PDAC, need to be treated rapidly due to their short survival time. To improve survival of individual patients at this stage of disease, clinicians need to be able to identify suitable drugs that will be most effective for their disease subtype and their immunological landscape. Most likely, at this stage of the disease the most important thing is to access to the PDAC material for testing the chemosensitivity of the primary and metastasis samples which can be obtained by EUS-FNA and amplified as organoids.
In conclusion, the biological markers needed for patients with PDAC depend on their own clinical situation. At the early stages of disease, the priority is rapid diagnosis so that the patient can quickly receive surgery without delay; at the advanced stages, where the therapeutic possibilities are limited, to the priority is to determine the PDAC subtype in order to estimate the clinical outcome and select the most suitable effective treatments for each patient. Moreover, taking advantage of the distinct immunological landscape of PDAC is critical for the identification of novel immunological markers and development of new immunotherapies able to eradicate this dismal disease.
CRediT authorship contribution statement
Juan Iovanna: Conceptualization, Writing - original draft, Writing - review & editing.
Declaration of Competing Interest
The author has no competing or financial interests.
Acknowledgments
Acknowledgments
I apologize to the many researchers in this field whose work I was unable to cite due to space limitations. My thanks to M Swayden for her helpful comments and criticisms.
Funding
This work was supported by La Ligue Contre le Cancer, Institut National du Cancer INCa (Grant number 2018-079), Canceropole PACA, and INSERM.
References
- 1.Siegel R., Naishadham D., Jemal A. Cancer statistics. CA Cancer J. Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Deramaudt T., Rustgi A.K. Mutant KRAS in the initiation of pancreatic cancer. Biochim. Biophys. Acta. 2005;1756:97–101. doi: 10.1016/j.bbcan.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Feldmann G., Beaty R., Hruban R.H., Maitra A. Molecular genetics of pancreatic intraepithelial neoplasia. J. Hepatobiliary Pancreat. Surg. 2007;14:224–232. doi: 10.1007/s00534-006-1166-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Furukawa T., Sunamura M., Horii A. Molecular mechanisms of pancreatic carcinogenesis. Cancer Sci. 2006;97:1–7. doi: 10.1111/j.1349-7006.2005.00134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pillarisetty V.G. The pancreatic cancer microenvironment: an immunologic battleground. Oncoimmunology. 2014;3 doi: 10.4161/21624011.2014.950171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carr R.M., Fernandez-Zapico M.E. Pancreatic cancer microenvironment, to target or not to target? EMBO Mol. Med. 2016;8:80–82. doi: 10.15252/emmm.201505948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herlyn M., Blaszczyk M., Bennicelli J., Sears H.F., Ernst C., Ross A.H., Koprowski H. Selection of monoclonal antibodies detecting serodiagnostic human tumor markers. J. Immunol. Methods. 1985;80:107–116. doi: 10.1016/0022-1759(85)90169-3. [DOI] [PubMed] [Google Scholar]
- 8.Golan T., Hammel P., Reni M., Van Cutsem E., Macarulla T., Hall M.J., Park J.O., Hochhauser D., Arnold D., Oh D.Y. Maintenance olaparib for germline BRCA-mutated metastatic pancreatic cancer. N. Engl. J. Med. 2019;381:317–327. doi: 10.1056/NEJMoa1903387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siegel R.L., Miller K.D., Jemal A. Cancer statistics. CA Cancer J. Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 10.Kindler H.L., Ioka T., Richel D.J., Bennouna J., Letourneau R., Okusaka T., Funakoshi A., Furuse J., Park Y.S., Ohkawa S. Axitinib plus gemcitabine versus placebo plus gemcitabine in patients with advanced pancreatic adenocarcinoma: a double-blind randomised phase 3 study. Lancet Oncol. 2011;12:256–262. doi: 10.1016/S1470-2045(11)70004-3. [DOI] [PubMed] [Google Scholar]
- 11.Moore M.J., Goldstein D., Hamm J., Figer A., Hecht J.R., Gallinger S., Au H.J., Murawa P., Walde D., Wolff R.A. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada clinical trials group. J. Clin. Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 12.Van Cutsem E., Vervenne W.L., Bennouna J., Humblet Y., Gill S., Van Laethem J.L., Verslype C., Scheithauer W., Shang A., Cosaert J. Phase III trial of bevacizumab in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. J. Clin. Oncol. 2009;27:2231–2237. doi: 10.1200/JCO.2008.20.0238. [DOI] [PubMed] [Google Scholar]
- 13.Costello E., Greenhalf W., Neoptolemos J.P. New biomarkers and targets in pancreatic cancer and their application to treatment. Nat. Rev. Gastroenterol. Hepatol. 2012;9:435–444. doi: 10.1038/nrgastro.2012.119. [DOI] [PubMed] [Google Scholar]
- 14.Iovanna J., Mallmann M.C., Goncalves A., Turrini O., Dagorn J.C. Current knowledge on pancreatic cancer. Front. Oncol. 2012;2:6. doi: 10.3389/fonc.2012.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moffitt R.A., Marayati R., Flate E.L., Volmar K.E., Loeza S.G., Hoadley K.A., Rashid N.U., Williams L.A., Eaton S.C., Chung A.H. Virtual microdissection identifies distinct tumor- and stroma-specific subtypes of pancreatic ductal adenocarcinoma. Nat. Genet. 2015;47:1168–1178. doi: 10.1038/ng.3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burris H.A., 3rd, Moore M.J., Andersen J., Green M.R., Rothenberg M.L., Modiano M.R., Cripps M.C., Portenoy R.K., Storniolo A.M., Tarassoff P. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J. Clin. Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 17.Conroy T., Desseigne F., Ychou M., Bouche O., Guimbaud R., Becouarn Y., Adenis A., Raoul J.L., Gourgou-Bourgade S., de la Fouchardiere C. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 18.Collisson E.A., Sadanandam A., Olson P., Gibb W.J., Truitt M., Gu S., Cooc J., Weinkle J., Kim G.E., Jakkula L. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat. Med. 2011;17:500–503. doi: 10.1038/nm.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bailey P., Chang D.K., Nones K., Johns A.L., Patch A.M., Gingras M.C., Miller D.K., Christ A.N., Bruxner T.J., Quinn M.C. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. 2016;531:47–52. doi: 10.1038/nature16965. [DOI] [PubMed] [Google Scholar]
- 20.Nicolle R., Blum Y., Duconseil P., Vanbrugghe C., Brandone N., Poizat F., Roques J., Bigonnet M., Gayet O., Rubis M. Establishment of a pancreatic adenocarcinoma molecular gradient (PAMG) that predicts the clinical outcome of pancreatic cancer. EBioMedicine. 2020;57 doi: 10.1016/j.ebiom.2020.102858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Puleo F., Nicolle R., Blum Y., Cros J., Marisa L., Demetter P., Quertinmont E., Svrcek M., Elarouci N., Iovanna J. Stratification of pancreatic ductal adenocarcinomas based on tumor and microenvironment features. Gastroenterology. 2018;155:1999–2013. doi: 10.1053/j.gastro.2018.08.033. e1993. [DOI] [PubMed] [Google Scholar]
- 22.Aung K.L., Fischer S.E., Denroche R.E., Jang G.H., Dodd A., Creighton S., Southwood B., Liang S.B., Chadwick D., Zhang A. Genomics-driven precision medicine for advanced pancreatic cancer: early results from the COMPASS trial. Clin. Cancer Res. 2018;24:1344–1354. doi: 10.1158/1078-0432.CCR-17-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Kane G.M., Grunwald B.T., Jang G.H., Masoomian M., Picardo S., Grant R.C., Denroche R.E., Zhang A., Wang Y., Lam B. GATA6 expression distinguishes classical and basal-like subtypes in advanced pancreatic cancer. Clin. Cancer Res. 2020;26:4901–4910. doi: 10.1158/1078-0432.CCR-19-3724. [DOI] [PubMed] [Google Scholar]
- 24.Martinelli P., Carrillo-de Santa Pau E., Cox T., Sainz B., Jr., Dusetti N., Greenhalf W., Rinaldi L., Costello E., Ghaneh P., Malats N. GATA6 regulates EMT and tumour dissemination, and is a marker of response to adjuvant chemotherapy in pancreatic cancer. Gut. 2017;66:1665–1676. doi: 10.1136/gutjnl-2015-311256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cristofanilli M., Hayes D.F., Budd G.T., Ellis M.J., Stopeck A., Reuben J.M., Doyle G.V., Matera J., Allard W.J., Miller M.C. Circulating tumor cells: a novel prognostic factor for newly diagnosed metastatic breast cancer. J. Clin. Oncol. 2005;23:1420–1430. doi: 10.1200/JCO.2005.08.140. [DOI] [PubMed] [Google Scholar]
- 26.Cohen S.J., Punt C.J., Iannotti N., Saidman B.H., Sabbath K.D., Gabrail N.Y., Picus J., Morse M., Mitchell E., Miller M.C. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J. Clin. Oncol. 2008;26:3213–3221. doi: 10.1200/JCO.2007.15.8923. [DOI] [PubMed] [Google Scholar]
- 27.Krebs M.G., Sloane R., Priest L., Lancashire L., Hou J.M., Greystoke A., Ward T.H., Ferraldeschi R., Hughes A., Clack G. Evaluation and prognostic significance of circulating tumor cells in patients with non-small-cell lung cancer. J. Clin. Oncol. 2011;29:1556–1563. doi: 10.1200/JCO.2010.28.7045. [DOI] [PubMed] [Google Scholar]
- 28.Miller M.C., Doyle G.V., Terstappen L.W. Significance of circulating tumor cells detected by the cellsearch system in patients with metastatic breast colorectal and prostate cancer. J. Oncol. 2010;2010 doi: 10.1155/2010/617421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poruk K.E., Blackford A.L., Weiss M.J., Cameron J.L., He J., Goggins M., Rasheed Z.A., Wolfgang C.L., Wood L.D. Circulating tumor cells expressing markers of tumor-initiating cells predict poor survival and cancer recurrence in patients with pancreatic ductal adenocarcinoma. Clin. Cancer Res. 2017;23:2681–2690. doi: 10.1158/1078-0432.CCR-16-1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riethdorf S., Pantel K. Advancing personalized cancer therapy by detection and characterization of circulating carcinoma cells. Ann. N.Y. Acad. Sci. 2010;1210:66–77. doi: 10.1111/j.1749-6632.2010.05779.x. [DOI] [PubMed] [Google Scholar]
- 31.Palmirotta R., Lovero D., Cafforio P., Felici C., Mannavola F., Pelle E., Quaresmini D., Tucci M., Silvestris F. Liquid biopsy of cancer: a multimodal diagnostic tool in clinical oncology. Ther. Adv. Med. Oncol. 2018;10 doi: 10.1177/1758835918794630. 1758835918794630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paoletti C., Hayes D.F. Circulating tumor cells. Adv. Exp. Med. Biol. 2016;882:235–258. doi: 10.1007/978-3-319-22909-6_10. [DOI] [PubMed] [Google Scholar]
- 33.Chinen L.T.D., Abdallah E.A., Braun A.C., Flores B., Corassa M., Sanches S.M., Fanelli M.F. Circulating tumor cells as cancer biomarkers in the clinic. Adv. Exp. Med. Biol. 2017;994:1–41. doi: 10.1007/978-3-319-55947-6_1. [DOI] [PubMed] [Google Scholar]
- 34.Toss A., Mu Z., Fernandez S., Cristofanilli M. CTC enumeration and characterization: moving toward personalized medicine. Ann. Transl. Med. 2014;2:108. doi: 10.3978/j.issn.2305-5839.2014.09.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Riva F., Dronov O.I., Khomenko D.I., Huguet F., Louvet C., Mariani P., Stern M.H., Lantz O., Proudhon C., Pierga J.Y. Clinical applications of circulating tumor DNA and circulating tumor cells in pancreatic cancer. Mol. Oncol. 2016;10:481–493. doi: 10.1016/j.molonc.2016.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Allard W.J., Matera J., Miller M.C., Repollet M., Connelly M.C., Rao C., Tibbe A.G., Uhr J.W., Terstappen L.W. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin. Cancer Res. 2004;10:6897–6904. doi: 10.1158/1078-0432.CCR-04-0378. [DOI] [PubMed] [Google Scholar]
- 37.Court C.M., Ankeny J.S., Hou S., Tseng H.R., Tomlinson J.S. Improving pancreatic cancer diagnosis using circulating tumor cells: prospects for staging and single-cell analysis. Expert Rev. Mol. Diagn. 2015;15:1491–1504. doi: 10.1586/14737159.2015.1091311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lewis A.R., Valle J.W., McNamara M.G. Pancreatic cancer: are "liquid biopsies" ready for prime-time? World J. Gastroenterol. 2016;22:7175–7185. doi: 10.3748/wjg.v22.i32.7175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen H., Tu H., Meng Z.Q., Chen Z., Wang P., Liu L.M. K-ras mutational status predicts poor prognosis in unresectable pancreatic cancer. Eur. J. Surg. Oncol. 2010;36:657–662. doi: 10.1016/j.ejso.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 40.Cheng H., Liu C., Jiang J., Luo G., Lu Y., Jin K., Guo M., Zhang Z., Xu J., Liu L. Analysis of ctDNA to predict prognosis and monitor treatment responses in metastatic pancreatic cancer patients. Int. J. Cancer. 2017;140:2344–2350. doi: 10.1002/ijc.30650. [DOI] [PubMed] [Google Scholar]
- 41.Earl J., Garcia-Nieto S., Martinez-Avila J.C., Montans J., Sanjuanbenito A., Rodriguez-Garrote M., Lisa E., Mendia E., Lobo E., Malats N. Circulating tumor cells (CTC) and KRAS mutant circulating free DNA (cfDNA) detection in peripheral blood as biomarkers in patients diagnosed with exocrine pancreatic cancer. BMC Cancer. 2015;15:797. doi: 10.1186/s12885-015-1779-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hadano N., Murakami Y., Uemura K., Hashimoto Y., Kondo N., Nakagawa N., Sueda T., Hiyama E. Prognostic value of circulating tumour DNA in patients undergoing curative resection for pancreatic cancer. Br. J. Cancer. 2016;115:59–65. doi: 10.1038/bjc.2016.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kinugasa H., Nouso K., Miyahara K., Morimoto Y., Dohi C., Tsutsumi K., Kato H., Matsubara T., Okada H., Yamamoto K. Detection of K-ras gene mutation by liquid biopsy in patients with pancreatic cancer. Cancer. 2015;121:2271–2280. doi: 10.1002/cncr.29364. [DOI] [PubMed] [Google Scholar]
- 44.Tjensvoll K., Lapin M., Buhl T., Oltedal S., Steen-Ottosen Berry K., Gilje B., Soreide J.A., Javle M., Nordgard O., Smaaland R. Clinical relevance of circulating KRAS mutated DNA in plasma from patients with advanced pancreatic cancer. Mol. Oncol. 2016;10:635–643. doi: 10.1016/j.molonc.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pietrasz D., Pecuchet N., Garlan F., Didelot A., Dubreuil O., Doat S., Imbert-Bismut F., Karoui M., Vaillant J.C., Taly V. Plasma circulating tumor DNA in pancreatic cancer patients is a prognostic marker. Clin. Cancer Res. 2017;23:116–123. doi: 10.1158/1078-0432.CCR-16-0806. [DOI] [PubMed] [Google Scholar]
- 46.Semrad T., Barzi A., Lenz H.J., Hutchins I.M., Kim E.J., Gong I.Y., Tanaka M., Beckett L., Holland W., Burich R.A. Pharmacodynamic separation of gemcitabine and erlotinib in locally advanced or metastatic pancreatic cancer: therapeutic and biomarker results. Int. J. Clin. Oncol. 2015;20:518–524. doi: 10.1007/s10147-014-0730-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Castells A., Puig P., Mora J., Boadas J., Boix L., Urgell E., Sole M., Capella G., Lluis F., Fernandez-Cruz L. K-ras mutations in DNA extracted from the plasma of patients with pancreatic carcinoma: diagnostic utility and prognostic significance. J. Clin. Oncol. 1999;17:578–584. doi: 10.1200/JCO.1999.17.2.578. [DOI] [PubMed] [Google Scholar]
- 48.Singh N., Gupta S., Pandey R.M., Chauhan S.S., Saraya A. High levels of cell-free circulating nucleic acids in pancreatic cancer are associated with vascular encasement, metastasis and poor survival. Cancer Invest. 2015;33:78–85. doi: 10.3109/07357907.2014.1001894. [DOI] [PubMed] [Google Scholar]
- 49.Nakano Y., Kitago M., Matsuda S., Nakamura Y., Fujita Y., Imai S., Shinoda M., Yagi H., Abe Y., Hibi T. KRAS mutations in cell-free DNA from preoperative and postoperative sera as a pancreatic cancer marker: a retrospective study. Br. J. Cancer. 2018;118:662–669. doi: 10.1038/bjc.2017.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee J.S., Rhee T.M., Pietrasz D., Bachet J.B., Laurent-Puig P., Kong S.Y., Takai E., Yachida S., Shibata T., Lee J.W. Circulating tumor DNA as a prognostic indicator in resectable pancreatic ductal adenocarcinoma: a systematic review and meta-analysis. Sci. Rep. 2019;9:16971. doi: 10.1038/s41598-019-53271-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Le D.T., Durham J.N., Smith K.N., Wang H., Bartlett B.R., Aulakh L.K., Lu S., Kemberling H., Wilt C., Luber B.S. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409–413. doi: 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bettegowda C., Sausen M., Leary R.J., Kinde I., Wang Y., Agrawal N., Bartlett B.R., Wang H., Luber B., Alani R.M. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci. Transl. Med. 2014;6:224. doi: 10.1126/scitranslmed.3007094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sausen M., Phallen J., Adleff V., Jones S., Leary R.J., Barrett M.T., Anagnostou V., Parpart-Li S., Murphy D., Kay Li Q. Clinical implications of genomic alterations in the tumour and circulation of pancreatic cancer patients. Nat. Commun. 2015;6:7686. doi: 10.1038/ncomms8686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sefrioui D., Blanchard F., Toure E., Basile P., Beaussire L., Dolfus C., Perdrix A., Paresy M., Antonietti M., Iwanicki-Caron I. Diagnostic value of CA19.9, circulating tumour DNA and circulating tumour cells in patients with solid pancreatic tumours. Br. J. Cancer. 2017;117:1017–1025. doi: 10.1038/bjc.2017.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dantes Z., Yen H.Y., Pfarr N., Winter C., Steiger K., Muckenhuber A., Hennig A., Lange S., Engleitner T., Ollinger R. Implementing cell-free DNA of pancreatic cancer patient-derived organoids for personalized oncology. JCI Insight. Aug 6 2020;6;5(15):e137809. doi: 10.1172/jci.insight.137809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sato N., Fukushima N., Hruban R.H., Goggins M. CpG island methylation profile of pancreatic intraepithelial neoplasia. Mod. Pathol. 2008;21:238–244. doi: 10.1038/modpathol.3800991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yi J.M., Guzzetta A.A., Bailey V.J., Downing S.R., Van Neste L., Chiappinelli K.B., Keeley B.P., Stark A., Herrera A., Wolfgang C. Novel methylation biomarker panel for the early detection of pancreatic cancer. Clin. Cancer Res. 2013;19:6544–6555. doi: 10.1158/1078-0432.CCR-12-3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sato N., Ueki T., Fukushima N., Iacobuzio-Donahue C.A., Yeo C.J., Cameron J.L., Hruban R.H., Goggins M. Aberrant methylation of CpG islands in intraductal papillary mucinous neoplasms of the pancreas. Gastroenterology. 2002;123:365–372. doi: 10.1053/gast.2002.34160. [DOI] [PubMed] [Google Scholar]
- 59.Jiao L., Zhu J., Hassan M.M., Evans D.B., Abbruzzese J.L., Li D. K-ras mutation and p16 and preproenkephalin promoter hypermethylation in plasma DNA of pancreatic cancer patients: in relation to cigarette smoking. Pancreas. 2007;34:55–62. doi: 10.1097/01.mpa.0000246665.68869.d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Henriksen S.D., Madsen P.H., Krarup H., Thorlacius-Ussing O. DNA hypermethylation as a blood-based marker for pancreatic cancer: a literature review. Pancreas. 2015;44:1036–1045. doi: 10.1097/MPA.0000000000000487. [DOI] [PubMed] [Google Scholar]
- 61.Kisiel J.B., Raimondo M., Taylor W.R., Yab T.C., Mahoney D.W., Sun Z., Middha S., Baheti S., Zou H., Smyrk T.C. New DNA methylation markers for pancreatic cancer: discovery, tissue validation, and pilot testing in pancreatic juice. Clin. Cancer Res. 2015;21:4473–4481. doi: 10.1158/1078-0432.CCR-14-2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Matsubayashi H., Canto M., Sato N., Klein A., Abe T., Yamashita K., Yeo C.J., Kalloo A., Hruban R., Goggins M. DNA methylation alterations in the pancreatic juice of patients with suspected pancreatic disease. Cancer Res. 2006;66:1208–1217. doi: 10.1158/0008-5472.CAN-05-2664. [DOI] [PubMed] [Google Scholar]
- 63.Henriksen S.D., Madsen P.H., Larsen A.C., Johansen M.B., Pedersen I.S., Krarup H., Thorlacius-Ussing O. Cell-free DNA promoter hypermethylation in plasma as a predictive marker for survival of patients with pancreatic adenocarcinoma. Oncotarget. 2017;8:93942–93956. doi: 10.18632/oncotarget.21397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tan A.C., Jimeno A., Lin S.H., Wheelhouse J., Chan F., Solomon A., Rajeshkumar N.V., Rubio-Viqueira B., Hidalgo M. Characterizing DNA methylation patterns in pancreatic cancer genome. Mol. Oncol. 2009;3:425–438. doi: 10.1016/j.molonc.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Iorio M.V., Croce C.M. MicroRNAs in cancer: small molecules with a huge impact. J. Clin. Oncol. 2009;27:5848–5856. doi: 10.1200/JCO.2009.24.0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Farazi T.A., Spitzer J.I., Morozov P., Tuschl T. miRNAs in human cancer. J. Pathol. 2011;223:102–115. doi: 10.1002/path.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li A., Yu J., Kim H., Wolfgang C.L., Canto M.I., Hruban R.H., Goggins M. MicroRNA array analysis finds elevated serum miR-1290 accurately distinguishes patients with low-stage pancreatic cancer from healthy and disease controls. Clin. Cancer Res. 2013;19:3600–3610. doi: 10.1158/1078-0432.CCR-12-3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schultz N.A., Dehlendorff C., Jensen B.V., Bjerregaard J.K., Nielsen K.R., Bojesen S.E., Calatayud D., Nielsen S.E., Yilmaz M., Hollander N.H. MicroRNA biomarkers in whole blood for detection of pancreatic cancer. JAMA. 2014;311:392–404. doi: 10.1001/jama.2013.284664. [DOI] [PubMed] [Google Scholar]
- 69.Wang J., Paris P.L., Chen J., Ngo V., Yao H., Frazier M.L., Killary A.M., Liu C.G., Liang H., Mathy C. Next generation sequencing of pancreatic cyst fluid microRNAs from low grade-benign and high grade-invasive lesions. Cancer Lett. 2015;356:404–409. doi: 10.1016/j.canlet.2014.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhou X., Lu Z., Wang T., Huang Z., Zhu W., Miao Y. Plasma miRNAs in diagnosis and prognosis of pancreatic cancer: a miRNA expression analysis. Gene. 2018;673:181–193. doi: 10.1016/j.gene.2018.06.037. [DOI] [PubMed] [Google Scholar]
- 71.Bloomston M., Frankel W.L., Petrocca F., Volinia S., Alder H., Hagan J.P., Liu C.G., Bhatt D., Taccioli C., Croce C.M. MicroRNA expression patterns to differentiate pancreatic adenocarcinoma from normal pancreas and chronic pancreatitis. JAMA. 2007;297:1901–1908. doi: 10.1001/jama.297.17.1901. [DOI] [PubMed] [Google Scholar]
- 72.Caponi S., Funel N., Frampton A.E., Mosca F., Santarpia L., Van der Velde A.G., Jiao L.R., De Lio N., Falcone A., Kazemier G. The good, the bad and the ugly: a tale of miR-101, miR-21 and miR-155 in pancreatic intraductal papillary mucinous neoplasms. Ann. Oncol. 2013;24:734–741. doi: 10.1093/annonc/mds513. [DOI] [PubMed] [Google Scholar]
- 73.Tang S., Bonaroti J., Unlu S., Liang X., Tang D., Zeh H.J., Lotze M.T. Sweating the small stuff: microRNAs and genetic changes define pancreatic cancer. Pancreas. 2013;42:740–759. doi: 10.1097/MPA.0b013e3182854ab0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Szafranska A.E., Davison T.S., John J., Cannon T., Sipos B., Maghnouj A., Labourier E., Hahn S.A. MicroRNA expression alterations are linked to tumorigenesis and non-neoplastic processes in pancreatic ductal adenocarcinoma. Oncogene. 2007;26:4442–4452. doi: 10.1038/sj.onc.1210228. [DOI] [PubMed] [Google Scholar]
- 75.Debernardi S., Massat N.J., Radon T.P., Sangaralingam A., Banissi A., Ennis D.P., Dowe T., Chelala C., Pereira S.P., Kocher H.M. Noninvasive urinary miRNA biomarkers for early detection of pancreatic adenocarcinoma. Am. J. Cancer Res. 2015;5:3455–3466. [PMC free article] [PubMed] [Google Scholar]
- 76.Caradec J., Kharmate G., Hosseini-Beheshti E., Adomat H., Gleave M., Guns E. Reproducibility and efficiency of serum-derived exosome extraction methods. Clin. Biochem. 2014;47:1286–1292. doi: 10.1016/j.clinbiochem.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 77.Melo S.A., Sugimoto H., O'Connell J.T., Kato N., Villanueva A., Vidal A., Qiu L., Vitkin E., Perelman L.T., Melo C.A. Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer Cell. 2014;26:707–721. doi: 10.1016/j.ccell.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Costa-Silva B., Aiello N.M., Ocean A.J., Singh S., Zhang H., Thakur B.K., Becker A., Hoshino A., Mark M.T., Molina H. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat. Cell Biol. 2015;17:816–826. doi: 10.1038/ncb3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Melo S.A., Luecke L.B., Kahlert C., Fernandez A.F., Gammon S.T., Kaye J., LeBleu V.S., Mittendorf E.A., Weitz J., Rahbari N. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature. 2015;523:177–182. doi: 10.1038/nature14581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kahlert C., Melo S.A., Protopopov A., Tang J., Seth S., Koch M., Zhang J., Weitz J., Chin L., Futreal A. Identification of double-stranded genomic DNA spanning all chromosomes with mutated KRAS and p53 DNA in the serum exosomes of patients with pancreatic cancer. J. Biol. Chem. 2014;289:3869–3875. doi: 10.1074/jbc.C113.532267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thakur B.K., Zhang H., Becker A., Matei I., Huang Y., Costa-Silva B., Zheng Y., Hoshino A., Brazier H., Xiang J. Double-stranded DNA in exosomes: a novel biomarker in cancer detection. Cell Res. 2014;24:766–769. doi: 10.1038/cr.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.San Lucas F.A., Allenson K., Bernard V., Castillo J., Kim D.U., Ellis K., Ehli E.A., Davies G.E., Petersen J.L., Li D. Minimally invasive genomic and transcriptomic profiling of visceral cancers by next-generation sequencing of circulating exosomes. Ann. Oncol. 2016;27:635–641. doi: 10.1093/annonc/mdv604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee T.H., Chennakrishnaiah S., Audemard E., Montermini L., Meehan B., Rak J. Oncogenic ras-driven cancer cell vesiculation leads to emission of double-stranded DNA capable of interacting with target cells. Biochem. Biophys. Res. Commun. 2014;451:295–301. doi: 10.1016/j.bbrc.2014.07.109. [DOI] [PubMed] [Google Scholar]
- 84.Mayers J.R., Wu C., Clish C.B., Kraft P., Torrence M.E., Fiske B.P., Yuan C., Bao Y., Townsend M.K., Tworoger S.S. Elevation of circulating branched-chain amino acids is an early event in human pancreatic adenocarcinoma development. Nat. Med. 2014;20:1193–1198. doi: 10.1038/nm.3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fahrmann J.F., Bantis L.E., Capello M., Scelo G., Dennison J.B., Patel N., Murage E., Vykoukal J., Kundnani D.L., Foretova L. A plasma-derived protein-metabolite multiplexed panel for early-stage pancreatic cancer. J. Natl. Cancer Inst. 2019;111:372–379. doi: 10.1093/jnci/djy126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lindahl A., Heuchel R., Forshed J., Lehtio J., Lohr M., Nordstrom A. Discrimination of pancreatic cancer and pancreatitis by LC-MS metabolomics. Metabolomics. 2017;13:61. doi: 10.1007/s11306-017-1199-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mayerle J., Kalthoff H., Reszka R., Kamlage B., Peter E., Schniewind B., Gonzalez Maldonado S., Pilarsky C., Heidecke C.D., Schatz P. Metabolic biomarker signature to differentiate pancreatic ductal adenocarcinoma from chronic pancreatitis. Gut. 2018;67:128–137. doi: 10.1136/gutjnl-2016-312432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Moore H.B., Culp-Hill R., Reisz J.A., Lawson P.J., Sauaia A., Schulick R.D., Del Chiaro M., Nydam T.L., Moore E.E., Hansen K.C. The metabolic time line of pancreatic cancer: opportunities to improve early detection of adenocarcinoma. Am. J. Surg. 2019;218:1206–1212. doi: 10.1016/j.amjsurg.2019.08.015. [DOI] [PubMed] [Google Scholar]
- 89.Phua L.C., Goh S., Tai D.W.M., Leow W.Q., Alkaff S.M.F., Chan C.Y., Kam J.H., Lim T.K.H., Chan E.C.Y. Metabolomic prediction of treatment outcome in pancreatic ductal adenocarcinoma patients receiving gemcitabine. Cancer Chemother. Pharmacol. 2018;81:277–289. doi: 10.1007/s00280-017-3475-6. [DOI] [PubMed] [Google Scholar]
- 90.Galon J., Mlecnik B., Bindea G., Angell H.K., Berger A., Lagorce C., Lugli A., Zlobec I., Hartmann A., Bifulco C. Towards the introduction of the 'Immunoscore' in the classification of malignant tumours. J. Pathol. 2014;232:199–209. doi: 10.1002/path.4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Coussens L.M., Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang M. ImmunoScore predicts gastric cancer postsurgical outcome. Lancet Oncol. 2017;18:e68. doi: 10.1016/S1470-2045(17)30008-6. [DOI] [PubMed] [Google Scholar]
- 93.Stotz M., Gerger A., Eisner F., Szkandera J., Loibner H., Ress A.L., Kornprat P., AlZoughbi W., Seggewies F.S., Lackner C. Increased neutrophil-lymphocyte ratio is a poor prognostic factor in patients with primary operable and inoperable pancreatic cancer. Br. J. Cancer. 2013;109:416–421. doi: 10.1038/bjc.2013.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ino Y., Yamazaki-Itoh R., Shimada K., Iwasaki M., Kosuge T., Kanai Y., Hiraoka N. Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. Br. J. Cancer. 2013;108:914–923. doi: 10.1038/bjc.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cai S.W., Yang S.Z., Gao J., Pan K., Chen J.Y., Wang Y.L., Wei L.X., Dong J.H. Prognostic significance of mast cell count following curative resection for pancreatic ductal adenocarcinoma. Surgery. 2011;149:576–584. doi: 10.1016/j.surg.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 96.Anitei M.G., Zeitoun G., Mlecnik B., Marliot F., Haicheur N., Todosi A.M., Kirilovsky A., Lagorce C., Bindea G., Ferariu D. Prognostic and predictive values of the immunoscore in patients with rectal cancer. Clin. Cancer Res. 2014;20:1891–1899. doi: 10.1158/1078-0432.CCR-13-2830. [DOI] [PubMed] [Google Scholar]
- 97.Hatogai K., Kitano S., Fujii S., Kojima T., Daiko H., Nomura S., Yoshino T., Ohtsu A., Takiguchi Y., Doi T. Comprehensive immunohistochemical analysis of tumor microenvironment immune status in esophageal squamous cell carcinoma. Oncotarget. 2016;7:47252–47264. doi: 10.18632/oncotarget.10055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jiang W., Liu K., Guo Q., Cheng J., Shen L., Cao Y., Wu J., Shi J., Cao H., Liu B. Tumor-infiltrating immune cells and prognosis in gastric cancer: a systematic review and meta-analysis. Oncotarget. 2017;8:62312–62329. doi: 10.18632/oncotarget.17602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Philip P.A., Mooney M., Jaffe D., Eckhardt G., Moore M., Meropol N., Emens L., O'Reilly E., Korc M., Ellis L. Consensus report of the national cancer institute clinical trials planning meeting on pancreas cancer treatment. J. Clin. Oncol. 2009;27:5660–5669. doi: 10.1200/JCO.2009.21.9022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tahkola K., Leppanen J., Ahtiainen M., Vayrynen J., Haapasaari K.M., Karttunen T., Kellokumpu I., Helminen O., Bohm J. Immune cell score in pancreatic cancer-comparison of hotspot and whole-section techniques. Virchows Arch. 2019;474:691–699. doi: 10.1007/s00428-019-02549-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tang Y., Xu X., Guo S., Zhang C., Tang Y., Tian Y., Ni B., Lu B., Wang H. An increased abundance of tumor-infiltrating regulatory T cells is correlated with the progression and prognosis of pancreatic ductal adenocarcinoma. PLoS ONE. 2014;9:e91551. doi: 10.1371/journal.pone.0091551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fukunaga A., Miyamoto M., Cho Y., Murakami S., Kawarada Y., Oshikiri T., Kato K., Kurokawa T., Suzuoki M., Nakakubo Y. CD8+ tumor-infiltrating lymphocytes together with CD4+ tumor-infiltrating lymphocytes and dendritic cells improve the prognosis of patients with pancreatic adenocarcinoma. Pancreas. 2004;28:e26–e31. doi: 10.1097/00006676-200401000-00023. [DOI] [PubMed] [Google Scholar]
- 103.Jamieson N.B., Mohamed M., Oien K.A., Foulis A.K., Dickson E.J., Imrie C.W., Carter C.R., McKay C.J., McMillan D.C. The relationship between tumor inflammatory cell infiltrate and outcome in patients with pancreatic ductal adenocarcinoma. Ann. Surg. Oncol. 2012;19:3581–3590. doi: 10.1245/s10434-012-2370-y. [DOI] [PubMed] [Google Scholar]
- 104.Xue P., Kanai M., Mori Y., Nishimura T., Uza N., Kodama Y., Kawaguchi Y., Takaori K., Matsumoto S., Uemoto S. Neutrophil-to-lymphocyte ratio for predicting palliative chemotherapy outcomes in advanced pancreatic cancer patients. Cancer Med. 2014;3:406–415. doi: 10.1002/cam4.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shibuya K.C., Goel V.K., Xiong W., Sham J.G., Pollack S.M., Leahy A.M., Whiting S.H., Yeh M.M., Yee C., Riddell S.R. Pancreatic ductal adenocarcinoma contains an effector and regulatory immune cell infiltrate that is altered by multimodal neoadjuvant treatment. PLoS ONE. 2014;9:e96565. doi: 10.1371/journal.pone.0096565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hiraoka N., Ino Y., Yamazaki-Itoh R., Kanai Y., Kosuge T., Shimada K. Intratumoral tertiary lymphoid organ is a favourable prognosticator in patients with pancreatic cancer. Br. J. Cancer. 2015;112:1782–1790. doi: 10.1038/bjc.2015.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bian B., Fanale D., Dusetti N., Roque J., Pastor S., Chretien A.S., Incorvaia L., Russo A., Olive D., Iovanna J. Prognostic significance of circulating PD-1, PD-L1, pan-BTN3As, BTN3A1 and BTLA in patients with pancreatic adenocarcinoma. Oncoimmunology. 2019;8 doi: 10.1080/2162402X.2018.1561120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tsai K.K., Daud A.I. The role of anti-PD-1/PD-L1 agents in melanoma: progress to date. Drugs. 2015;75:563–575. doi: 10.1007/s40265-015-0376-z. [DOI] [PubMed] [Google Scholar]
- 109.Meng X., Liu Y., Zhang J., Teng F., Xing L., Yu J. PD-1/PD-L1 checkpoint blockades in non-small cell lung cancer: new development and challenges. Cancer Lett. 2017;405:29–37. doi: 10.1016/j.canlet.2017.06.033. [DOI] [PubMed] [Google Scholar]
- 110.Gong J., Chehrazi-Raffle A., Reddi S., Salgia R. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: a comprehensive review of registration trials and future considerations. J. Immunother. Cancer. 2018;6:8. doi: 10.1186/s40425-018-0316-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Beatty G.L., Eghbali S., Kim R. Deploying immunotherapy in pancreatic cancer: defining mechanisms of response and resistance. Am. Soc. Clin. Oncol. Educ. Book. 2017;37:267–278. doi: 10.1200/EDBK_175232. [DOI] [PubMed] [Google Scholar]
- 112.Brahmer J.R., Tykodi S.S., Chow L.Q., Hwu W.J., Topalian S.L., Hwu P., Drake C.G., Camacho L.H., Kauh J., Odunsi K. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Royal R.E., Levy C., Turner K., Mathur A., Hughes M., Kammula U.S., Sherry R.M., Topalian S.L., Yang J.C., Lowy I. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J. Immunother. 2010;33:828–833. doi: 10.1097/CJI.0b013e3181eec14c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chowdhury P.S., Chamoto K., Honjo T. Combination therapy strategies for improving PD-1 blockade efficacy: a new era in cancer immunotherapy. J. Intern. Med. 2018;283:110–120. doi: 10.1111/joim.12708. [DOI] [PubMed] [Google Scholar]
- 115.Zitvogel L., Apetoh L., Ghiringhelli F., Kroemer G. Immunological aspects of cancer chemotherapy. Nat. Rev. Immunol. 2008;8:59–73. doi: 10.1038/nri2216. [DOI] [PubMed] [Google Scholar]
- 116.Pfirschke C., Engblom C., Rickelt S., Cortez-Retamozo V., Garris C., Pucci F., Yamazaki T., Poirier-Colame V., Newton A., Redouane Y. Immunogenic chemotherapy sensitizes tumors to checkpoint blockade therapy. Immunity. 2016;44:343–354. doi: 10.1016/j.immuni.2015.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Aglietta M., Barone C., Sawyer M.B., Moore M.J., Miller W.H., Jr., Bagala C., Colombi F., Cagnazzo C., Gioeni L., Wang E. A phase I dose escalation trial of tremelimumab (CP-675,206) in combination with gemcitabine in chemotherapy-naive patients with metastatic pancreatic cancer. Ann. Oncol. 2014;25:1750–1755. doi: 10.1093/annonc/mdu205. [DOI] [PubMed] [Google Scholar]
- 118.Von Hoff D.D., Ramanathan R.K., Borad M.J., Laheru D.A., Smith L.S., Wood T.E., Korn R.L., Desai N., Trieu V., Iglesias J.L. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J. Clin. Oncol. 2011;29:4548–4554. doi: 10.1200/JCO.2011.36.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nicolle R., Blum Y., Marisa L., Loncle C., Gayet O., Moutardier V., Turrini O., Giovannini M., Bian B., Bigonnet M. Pancreatic adenocarcinoma therapeutic targets revealed by tumor-stroma cross-talk analyses in patient-derived xenografts. Cell Rep. 2017;21:2458–2470. doi: 10.1016/j.celrep.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lomberk G., Blum Y., Nicolle R., Nair A., Gaonkar K.S., Marisa L., Mathison A., Sun Z., Yan H., Elarouci N. Distinct epigenetic landscapes underlie the pathobiology of pancreatic cancer subtypes. Nat. Commun. 2018;9:1978. doi: 10.1038/s41467-018-04383-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lomberk G., Dusetti N., Iovanna J., Urrutia R. Emerging epigenomic landscapes of pancreatic cancer in the era of precision medicine. Nat. Commun. 2019;10:3875. doi: 10.1038/s41467-019-11812-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.O'Connor M.J. Targeting the DNA damage response in cancer. Mol. Cell. 2015;60:547–560. doi: 10.1016/j.molcel.2015.10.040. [DOI] [PubMed] [Google Scholar]
- 123.Duconseil P., Gilabert M., Gayet O., Loncle C., Moutardier V., Turrini O., Calvo E., Ewald J., Giovannini M., Gasmi M. Transcriptomic analysis predicts survival and sensitivity to anticancer drugs of patients with a pancreatic adenocarcinoma. Am. J. Pathol. 2015;185:1022–1032. doi: 10.1016/j.ajpath.2014.11.029. [DOI] [PubMed] [Google Scholar]
- 124.Penchev V.R., Rasheed Z.A., Maitra A., Matsui W. Heterogeneity and targeting of pancreatic cancer stem cells. Clin. Cancer Res. 2012;18:4277–4284. doi: 10.1158/1078-0432.CCR-11-3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nicolle R., Gayet O., Duconseil P., Vanbrugghe C., Roques J., Bigonnet B., Blum Y., Elarouci N., Armenoult L., Ayadi M. A transcriptomic signature to predict adjuvant gemcitabine sensitivity in pancreatic adenocarcinoma. Ann. Oncol. Nov 11 2020 doi: 10.1016/j.annonc.2020.10.601. S0923-7534(20)43131-X Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 126.Tiriac H., Belleau P., Engle D.D., Plenker D., Deschenes A., Somerville T.D.D., Froeling F.E.M., Burkhart R.A., Denroche R.E., Jang G.H. Organoid profiling identifies common responders to chemotherapy in pancreatic cancer. Cancer Discov. 2018;8:1112–1129. doi: 10.1158/2159-8290.CD-18-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pearson T., Greiner D.L., Shultz L.D. Creation of "humanized" mice to study human immunity. Curr. Protoc. Immunol. May 2008 doi: 10.1002/0471142735.im1521s81. Chapter 15:Unit 15.21. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rosato R.R., Davila-Gonzalez D., Choi D.S., Qian W., Chen W., Kozielski A.J., Wong H., Dave B., Chang J.C. Evaluation of anti-PD-1-based therapy against triple-negative breast cancer patient-derived xenograft tumors engrafted in humanized mouse models. Breast Cancer Res. 2018;20:108. doi: 10.1186/s13058-018-1037-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Huch M., Bonfanti P., Boj S.F., Sato T., Loomans C.J., van de Wetering M., Sojoodi M., Li V.S., Schuijers J., Gracanin A. Unlimited in vitro expansion of adult bi-potent pancreas progenitors through the Lgr5/R-spondin axis. EMBO J. 2013;32:2708–2721. doi: 10.1038/emboj.2013.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Boj S.F., Hwang C.I., Baker L.A., Chio I.I., Engle D.D., Corbo V., Jager M., Ponz-Sarvise M., Tiriac H., Spector M.S. Organoid models of human and mouse ductal pancreatic cancer. Cell. 2015;160:324–338. doi: 10.1016/j.cell.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fraunhoffer N.A., Abuelafia A.M., Bigonnet M., Gayet O., Roques J., Telle E., Santofimia-Castano P., Borrello M.T., Chuluyan E., Dusetti N. Evidencing a pancreatic ductal adenocarcinoma subpopulation sensitive to the proteasome inhibitor carfilzomib. Clin. Cancer Res. 2020;26:5506–5519. doi: 10.1158/1078-0432.CCR-20-1232. [DOI] [PubMed] [Google Scholar]
- 132.Iovanna J., Dusetti N. Speeding towards individualized treatment for pancreatic cancer by taking an alternative road. Cancer Lett. 2017;410:63–67. doi: 10.1016/j.canlet.2017.09.016. [DOI] [PubMed] [Google Scholar]
- 133.Chan-Seng-Yue M., Kim J.C., Wilson G.W., Ng K., Figueroa E.F., O'Kane G.M., Connor A.A., Denroche R.E., Grant R.C., McLeod J. Transcription phenotypes of pancreatic cancer are driven by genomic events during tumor evolution. Nat. Genet. 2020;52:231–240. doi: 10.1038/s41588-019-0566-9. [DOI] [PubMed] [Google Scholar]
- 134.Ho D. Artificial intelligence in cancer therapy. Science. 2020;367:982–983. doi: 10.1126/science.aaz3023. [DOI] [PubMed] [Google Scholar]
- 135.Liang G., Fan W., Luo H., Zhu X. The emerging roles of artificial intelligence in cancer drug development and precision therapy. Biomed. Pharmacother. 2020;128 doi: 10.1016/j.biopha.2020.110255. [DOI] [PubMed] [Google Scholar]
- 136.Yin L., Pu N., Thompson E.D., Miao Y., Wolfgang C.L., Yu J. Improved assessment of response status in patients with pancreatic cancer treated with neoadjuvant therapy using somatic mutations and liquid biopsy analysis. Clin. Cancer Res. Oct 20 2020 doi: 10.1158/1078-0432.CCR-20-1746. [DOI] [PubMed] [Google Scholar]
- 137.Groot V.P., Mosier S., Javed A.A., Teinor J.A., Gemenetzis G., Ding D., Haley L.M., Yu J., Burkhart R.A., Hasanain A. Circulating tumor DNA as a clinical test in resected pancreatic cancer. Clin. Cancer Res. 2019;25:4973–4984. doi: 10.1158/1078-0432.CCR-19-0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pishvaian M.J., Blais E.M., Brody J.R., Lyons E., DeArbeloa P., Hendifar A., Mikhail S., Chung V., Sahai V., Sohal D.P.S. Overall survival in patients with pancreatic cancer receiving matched therapies following molecular profiling: a retrospective analysis of the know your tumor registry trial. Lancet Oncol. 2020;21:508–518. doi: 10.1016/S1470-2045(20)30074-7. [DOI] [PMC free article] [PubMed] [Google Scholar]