Abstract
We examined the relationships between sleep and inflammatory biomarkers during late pregnancy. Seventy-four women underwent an overnight sleep assessment by polysomnography. Blood samples were collected before bedtime and again within 1 h upon awakening to measure C-reactive protein (CRP), interleukin (IL)-6, and IL-6 soluble receptor. Sleep parameters included variables characterizing sleep architecture and sleep continuity. The participants were 32.2 (SD = 4.1) years old and the average gestational age was 32.8 (3.5) weeks. Controlling for covariates, evening CRP was negatively associated with N3 sleep (β = −0.30, P = 0.010). N3 sleep was also negatively associated with morning CRP (β = −0.26, P = 0.036), with a higher percentage of N3 sleep associated with a lower level of morning CRP. Contrarily, there was a tendency for a positive association between stage N2 sleep and morning CRP (β = 0.23, P = 0.065). Stage N1 sleep was associated with morning IL-6 (β = 0.28, P = 0.021), with a higher percentage of N1 sleep associated with a higher morning IL-6. No significant associations were found between morning inflammatory biomarkers and sleep continuity parameters. In conclusion, increased light sleep was associated with increased inflammatory biomarkers, whereas more deep sleep was associated with decreased inflammatory biomarkers. These findings further support the interactions between sleep and the immune system during late pregnancy.
Keywords: sleep stage, inflammation, cytokine, polysomnography, pregnancy
Graphical abstract:
We examined the relationships between sleep and inflammatory biomarkers during late pregnancy. Seventy-four women underwent an overnight sleep assessment by polysomnography. No significant associations were found between morning inflammatory biomarkers and sleep continuity parameters. In conclusion, increased light sleep was associated with increased inflammatory biomarkers, whereas more deep sleep was associated with decreased inflammatory biomarkers. These findings further support the interactions between sleep and the immune system during late pregnancy.
Introduction
Maternal sleep during pregnancy can affect many metabolic, neurological, and immune functions that are critical to maintaining a healthy pregnancy and fetal growth. Pregnant women frequently experience various sleep disturbances due to pregnancy-related hormonal changes and frequent nocturia.1 These disturbances include self-reported poor sleep quality and short sleep duration2, 3 Objective assessment by the gold standard of polysomnography (PSG) also supports those findings and suggests that compared with nonpregnant women, pregnant women have lower sleep efficiency and more sleep fragmentation.4 Using PSG for assessing sleep architecture, sleep can be classified into rapid eye movement (REM) sleep and nonrapid eye movement (NREM) stages N1, N2, and N3 sleep. Stage N1 sleep is an intermediate stage between wakefulness and sleep. Stage N2, characterized by the occurrence of spindles and K-complex, progresses into Stage N3 sleep. Both stage N1 and N2 sleep are lighter stages of sleep. Stage N3 sleep, also known as slow-wave sleep (SWS), is an important sleep stage during which the body is restored and immune functions are promoted.5 SWS is characterized by high-voltage slow waves with a frequency range of 1–4 Hz.6 Pregnant women typically experience changes in sleep architecture, including increased superficial sleep (stage N1 and N2 sleep), decreased deep sleep (stage N3 sleep), and REM sleep.1, 4, 7, 8 These changes are more evident during late pregnancy8 and have been related to various maternal-fetal outcomes (e.g., gestational diabetes, altered growth, and gestational length of the fetus),9, 10 which may be mediated by several pathways, including the immune system.11–13
During pregnancy, there are selective pro- and anti-inflammatory conditions depending on the stage of gestation.14 Overall, the altered immunologic profile could maintain a balance between the pro- and anti-inflammatory processes to achieve normal gestation and delivery.13, 15 Compared with nonpregnant women, pregnant women experience greater systemic inflammation, characterized by an increased level of C-reactive protein (CRP).16 CRP has been associated with pregnancy-related outcomes, such as lower birthweight and preeclampsia.17, 18 A recent prospective study19 also reported that increased CRP level in early pregnancy was related to the risk of GDM at 24–28 weeks of gestation. More alarmingly, the risk of GDM in women with the highest level of CRP was three times higher than that of women with the lowest level of CRP. Another important proinflammatory cytokine relevant to pregnancy is interleukin (IL)-6,13 which has been the most investigated cytokines for preterm birth.20 An elevated serum concentration of IL-6 was reported as a risk factor for preterm birth.21 Based on the above evidence, pregnant women experience changes in both sleep and the immune system; however, how changes in sleep interact with the inflammation process in this population remains to be determined. Prior studies have been focused primarily on nonpregnant women. In midlife women, lower sleep efficiency and short sleep have been associated with higher inflammatory biomarkers (e.g., IL-6 and CRP).22, 23 According to a meta-analysis, subjective sleep disturbance was related to higher levels of CRP and IL-6.24 One study was conducted in late pregnancy and found that subjective sleep disturbance was related to increased circulating IL-6.12 Collectively, there is a need to investigate the relationship between sleep architecture and inflammation in pregnant women.
The relationship between sleep and inflammation seems to be reciprocal.25–27 On the one hand, changes in immune function could impact sleep depth, with both somnogenic and sleep-inhibitory effects.28, 29 On the other hand, sleep, particularly deep sleep, may restore and promote immune functions.5 Empirical evidence from humans revealed that IL-6 could enhance stage N3 sleep30 and enhanced stage N3 sleep may decrease IL-6 concentrations.25 Based on the above evidence, we hypothesized that proinflammatory cytokines before sleep were associated with sleep stages (N1, N2, and N3) differentially; and sleep stages, in turn, were associated with proinflammatory cytokines the next morning. To test these hypotheses, we obtained objective sleep parameters using PSG. We measured the plasma cytokines twice (CRP, IL-6, and soluble IL-6 receptor (sIL-6R)), once before sleep and then once in the morning upon awakening. We tested the associations in a group of pregnant women while controlling for potential confounders.
Materials and methods
Design and sample
Data were collected in a case-control study of sleep-related determinants of GDM. Women between 24 and 36 weeks of pregnancy were recruited from the obstetric clinic at the Medical Center of the University of Illinois at Chicago (UIC). The exclusion criteria were: (1) unable to read and write in English; (2) had been diagnosed with either a sleep disorder (e.g., insomnia or OSA), diabetes, or GDM (all based on their medical charts); (3) had a diagnosis of neurological or psychiatric disorder; (4) excessive alcohol intake (≥30 g/day) or illegal drug use (self-report and medical charts); (5) had a previous history of preterm delivery or other pregnancy health issues (e.g., small-for-gestational-age infant, preeclampsia, placental abruption, and neonatal death). These adverse outcomes may increase the risk of subsequent preterm birth;31 and (6) refusal to have an overnight sleep study. A total of 87 women were included in this study and 13 women withdrew or were lost to follow-up. Thus, the final sample size was 74 and included 38 women with GDM based on an oral glucose tolerance test. In the cases of GDM, eligible women with newly diagnosed GDM were recruited to the study. GDM diagnosis was based on the following criteria32: (1) Glucose challenge test ≥140 mg/dL with two or more abnormal values on 3-h oral glucose tolerance testing (OGTT) (OGTT: ≥95 mg/dL at baseline, ≥180 mg/dL at 1 h, ≥155 mg/dL at 2 h, or ≥140 mg/dL at 3 h) or (2) nonfasting 50-g OGTT≥200 mg/dL if no fasting 3-h OGTT was performed.
Procedures and measurements
The study was approved by the Institutional Review Board of UIC (protocol number 2014–0485). Women who agreed to participate provided informed consent. All participants underwent an overnight sleep assessment by PSG at the UIC Sleep Science Center. During the overnight visit, the participants completed a battery of self-report questionnaires about their demographics, sleep, and depression. Depression was measured using Patient Health Questionnaire-9 (PHQ-9). Scores of 5, 10, 15, and 20 indicate mild, moderate, moderately severe, and severe depressive symptoms, respectively.33 We also obtained self-reported information about the use of prescribed medications, such as blood pressure medication, antihistamine or decongestant, thyroid medication, or tranquilizer.
Both IL-6 and CRP demonstrate circadian rhythm. IL-6 peaked at awakening, gradually declined during the daytime, and peaked again before sleep.34 Plasma CRP also exhibited a biphasic pattern.35 In this study, all participants provided blood samples for the evaluation of inflammatory biomarkers, including CRP, IL-6, and sIL-6R, before and after the overnight PSG assessment. The evening blood sample was collected 1 h before the participants’ usual bedtime. The morning blood sample was collected within 1 h upon awakening at a fasting state. This sampling technique was consistent with previous studies.25, 36
Demographic and clinical measures
We obtained demographic and anthropometric measures during the baseline evaluation using self-report questionnaires (e.g., maternal age, race/ethnicity, employment status, smoking status, work schedule, and prepregnancy weight). Neck circumference was measured twice at the level of the superior border of the cricothyroid cartilage while participants were in the seated position. The average of the two assessments was used. The pregnancy body mass index (BMI) at the 3rd trimester was calculated from weight and height measured during the visit to Sleep Science Center using an upright scale and wall-mounted stadiometer, respectively.
Objective sleep parameters
Objective sleep parameters were measured by overnight PSG recording during the woman’s self-reported normal sleep period. PSG (Alice5, Respironics) included electroencephalogram (EEG: central, occipital leads), electrooculogram, submental and anterior tibialis electromyograms, single bipolar electrocardiogram, finger pulse oximetry, oronasal thermistor, and nasal-cannula pressure transducer, thoracoabdominal belts (piezo crystals), and snoring and body position sensors37. Two trained sleep technologists scored sleep recordings using standard criteria38 for REM sleep and NREM sleep (stage N1, N2, and N3 sleep). Sleep continuity variables included sleep duration, sleep efficiency (used as a proxy for sleep quality), wake after sleep onset (WASO), and arousal index (number of arousals per hour of sleep). Respiratory events39 were scored with apnea defined as a >90% decrease in airflow for ≥10 s and hypopnea defined as a ≥30% but ≤90% drop in respiratory flow for ≥10 s with ≥3% oxygen desaturation or an arousal. Average and nadir oxygen saturation (SaO2) were obtained. The apnea-hypopnea index (AHI) was defined as the number of apneas and hypopneas per hour of sleep. The diagnosis of OSA was based on an AHI > 5 events/hour. Based on AHI, the severity of OSA was classified into mild (5 ≤ AH < 15), moderate (15 ≤ AHI < 30), and severe OSA (AHI ≥ 30).39
Inflammatory biomarkers
The blood samples were collected through routine venipuncture. The samples were centrifuged, and 0.5-mL aliquots were frozen at −80° C until analysis. Serum levels of IL-6 and sIL-6R were determined using commercially available ultrasensitive ELISA kits (Biosource, Europe). Sensitivity for IL-6 was <0.104 pg/mL and coefficients of variation (CV) was ≤7.8%; sensitivity for sIL-6R was <0.09 pg/mL and CV was ≤9.7%. Samples were assayed in duplicate according to the manufacturer’s instructions. CRP was detected using an ultrasensitive ELISA (DSL, Webster, TX) with an enzymatically amplified “two-step” sandwich-type immunoassay. Sensitivity for CRP was <1.6 ng/mL and CV was <4.2%. All samples had detectable levels of CRP. The CRP samples were diluted by 1:100 and assayed in duplicate according to the manufacturer’s instructions.
Statistical analysis
Statistical analyses were performed using Stata 13.0 (StataCorp LP, College Station, Texas). Prior to data analyses, the normal distribution of the variables was checked. The natural log of IL-6, sIL-6R, and arousal index and the square root of CRP were sufficient to normalize the data. Data are presented as the mean and the standard deviation (SD) or the median and the interquartile range (IQR) for continuous variables, and frequency (n) and percent (%) for categorical variables. Group difference was compared using chi-square or a one-way ANOVA. Pearson correlation analysis was performed to determine the associations between sleep parameters and inflammatory biomarkers. Any variable related to the dependent variables at P < 0.2 in the bivariate analysis was considered for inclusion in the initial multivariable analysis. Age, pregnancy BMI, and race/ethnicity were included in the regression models as a priori covariates. Once the final regression model was obtained, all relevant model assumptions were checked (e.g., normality, model specification, multicollinearity, and homoscedasticity). A P < 0.05 (two-tailed) was considered statistically significant.
Results
Demographic and clinical characteristics
The participants’ demographic and clinical characteristics are presented in Table 1. The participants had an average age of 32.2 (SD = 4.1) years and the average gestational age was 32.8 (SD = 3.4) weeks. Thirty women (40.5%) were African American. Two were current smokers (2.7%). Their mean prepregnancy BMI was 30.6 (SD = 7.9) kg/m2 and BMI measured at the PSG night was 34.7 (SD = 7.5) kg/m2. The average neck circumference was 36.6 (SD = 3.1) centimeters. The mean PHQ-9 score was 4.6 (SD = 4.1) and three had moderately severe depression.
Table 1.
Demographic and clinical characteristics (n = 74)
| Variables | mean (SD)/n (%) |
|---|---|
| Age, years | 32.2 (4.1) |
| Gestational age, weeks | 32.8 (3.5) |
| Race/ethnicity, n (%) | |
| White/Others | 16.0 (21.6) |
| African American | 30.0 (40.5) |
| Hispanic | 28.0 (37.9) |
| Smoking status, n (%) | |
| Current smoker | 2.0 (2.7) |
| Ex-smoker | 10.0 (13.5) |
| No smoking | 62.0 (83.8) |
| Multiparous, n (%) | 57.0 (77.0) |
| Single/divorced, n (%) | 39.0 (52.7) |
| Higher education, n (%) | 39.0 (52.7) |
| Nightshift, n (%) | 14.0 (18.9) |
| Neck circumference, cm | 36.6 (3.1) |
| Prepregnancy BMI, kg/m2 | 30.6 (7.9) |
| Pregnancy BMI, kg/m2 | 34.7 (7.5) |
| PHQ-9 | 4.6 (4.1) |
| No, n (%) | 45.0 (61.6) |
| Mild, n (%) | 19.0 (26.0) |
| Moderate, n (%) | 6.0 (8.2) |
| Moderately severe, n (%) | 3.0 (4.2) |
| GDM, n (%) | 38.0 (51.4) |
| Use of prescription, n (%)† | |
| Blood pressure medication | 2.0 (2.8) |
| Antihistamine/decongestant | 16.0 (22.5) |
| Thyroid medication | 5.0 (5.6) |
Notes:
n = 71;
BMI, body mass index; GDM, gestational diabetes mellitus; PHQ-9, Patient Health Questionnaire-9; SD, standard deviation.
Sleep parameters and inflammatory biomarkers
Sleep- and inflammation-related characteristics are shown in Table 2. The mean sleep duration was 5.4 (SD = 0.9) h and mean sleep efficiency was 73.8% (SD = 14.0). The median arousal index was 6.0 events/hour. Stage N2 sleep accounted for 53.9% of the total sleep duration. Using AHI > 5 events/h as the cutoff point for the diagnosis of OSA, 11 (14.9%) women had OSA (mild OSA, n = 8; moderate, n = 3). Overall, evening cytokine levels were slightly higher than fasting levels the next morning.
Table 2.
Description of sleep parameters and inflammatory biomarkers (n = 74)
| Variables | mean (SD)/n (%) |
|---|---|
| Time in bed, h | 7.3 (0.4) |
| Sleep duration, h | 5.4 (0.9) |
| Sleep efficiency, % | 73.8 (14.0) |
| WASO, min | 84.1 (52.0) |
| Arousal index, events/h† | 6.0 (5.5) |
| Stage N1 sleep, % | 9.2 (4.2) |
| Stage N2 sleep, % | 53.9 (10.0) |
| Stage N3 sleep, % | 17.9 (6.0) |
| REM sleep, % | 19.0 (6.0) |
| Non-REM sleep, % | 81.0 (6.0) |
| OSA, n (%) | 11.o (14.9) |
| AHI, events/h† | 0.7 (1.9) |
| Average SaO2, % | 96.4 (1.1) |
| Nadir SaO2, %† | 90.8 (4.2) |
| Evening CRP, ng/mL† | 17.1 (15.0) |
| Evening IL-6, pg/mL† | 0.44 (0.74) |
| Evening sIL-6R, pg/mL† | 28.8 (13.4) |
| Morning CRP, ng/mL#,† | 16.4 (16.0) |
| Morning IL-6, pg/mL#,† | 0.34 (0.59) |
| Morning sIL-6R, pg/mL#,† | 27.7 (14.2) |
Notes.
n = 70;
data were presented as median and interquartile range; sleep stages were presented as percent of total sleep duration; AHI, apnea-hypopnea index; NREM, nonrapid eye movement sleep; OSA, obstructive sleep apnea; REM sleep, rapid eye movement sleep; SaO2, oxygen saturation; SD, standard deviation; WASO, wake after sleep onset.
Associations of evening inflammatory biomarkers with nocturnal sleep parameters
The bivariate associations between evening levels of CRP, IL-6, and sIL-6R with nocturnal sleep parameters are shown in Table 3. Evening IL-6 and sIL-6R were not associated with any sleep architecture parameters. Evening IL-6 was associated with arousal index at P < 0.20 (r = 0.16). Evening CRP was associated with stage N3 sleep (r = −0.24, P < 0.05) and stage N2 sleep at P < 0.20 (r = 0.2) (Fig. 1A and 1B). Table 3 also shows the relationships between covariates with nocturnal sleep parameters. Arousal index was associated with age (r = 0.36, P < 0.01), prepregnancy BMI (r = 0.24, P < 0.05) and pregnancy BMI (r = 0.30, P < 0.01). Neck circumference was significantly related to sleep continuity variables, including sleep duration, sleep efficiency, WASO, and arousal index. Table S1 (online only) shows the group difference in nocturnal sleep parameters. Overall, race/ethnicity, parity, GDM, night shift, and use of antihistamine or decongestant were not related to nocturnal sleep parameters. OSA diagnosis was significantly associated with arousal index and different stages of sleep (P < 0.05).
Table 3.
Bivariate associations (r) for nocturnal sleep parameters (n = 74)
| Variables | Sleep duration | Sleep efficiency | WASO | Arousal index | Stage N1 sleep | Stage N2 sleep | Stage N3 sleep | REM sleep | NREM sleep |
|---|---|---|---|---|---|---|---|---|---|
| Evening CRP, ng/mL | 0.02 | 0.03 | −0.02 | 0.12 | −0.06 | 0.20§ | −0.24* | 0.05 | −0.07 |
| Evening IL-6, pg/mL | 0.09 | 0.14 | −0.08 | 0.16§ | 0.10 | 0.01 | −0.06 | −0.09 | 0.09 |
| Evening sIL-6R, pg/mL | 0.05 | 0.06 | −0.05 | 0.06 | −0.06 | 0.14 | −0.07 | −0.09 | 0.06 |
| Age, years | −0.07 | −0.07 | 0.19§ | 0.36** | 0.10 | −0.06 | 0.11 | −0.13 | 0.11 |
| Gestational age, weeks | −0.16§ | −0.17§ | 0.17§ | 0.14 | 0.01 | 0.10 | −0.11 | −0.03 | 0.08 |
| Prepregnancy BMI, kg/m2 | −0.12 | −0.13 | 0.10 | 0.24* | 0.03 | 0.07 | −0.14 | −0.02 | 0.03 |
| Pregnancy BMI, kg/m2 | −0.11 | −0.12 | 0.10 | 0.30** | 0.04 | 0.11 | −0.16§ | −0.08 | 0.09 |
| Neck circumference, cm | −0.27* | −0.29* | 0.23* | 0.28* | 0.19 | 0.07 | −0.23§ | −0.14 | 0.12 |
| PHQ-9 | 0.19§ | 0.21§ | −0.14 | 0.02 | −0.05 | 0.09 | −0.19§ | 0.10 | −0.09 |
Note:
P < 0.2;
P < 0.05;
P < 0.01;
BMI, body mass index; NREM, nonrapid eye movement sleep; PHQ-9, Patient Health Questionnaire-9; REM sleep, rapid eye movement sleep; SD, standard deviation; WASO, wake after sleep onset; natural log of IL-6, sIL-6R, and arousal index and square root of CRP were used.
Figure 1.
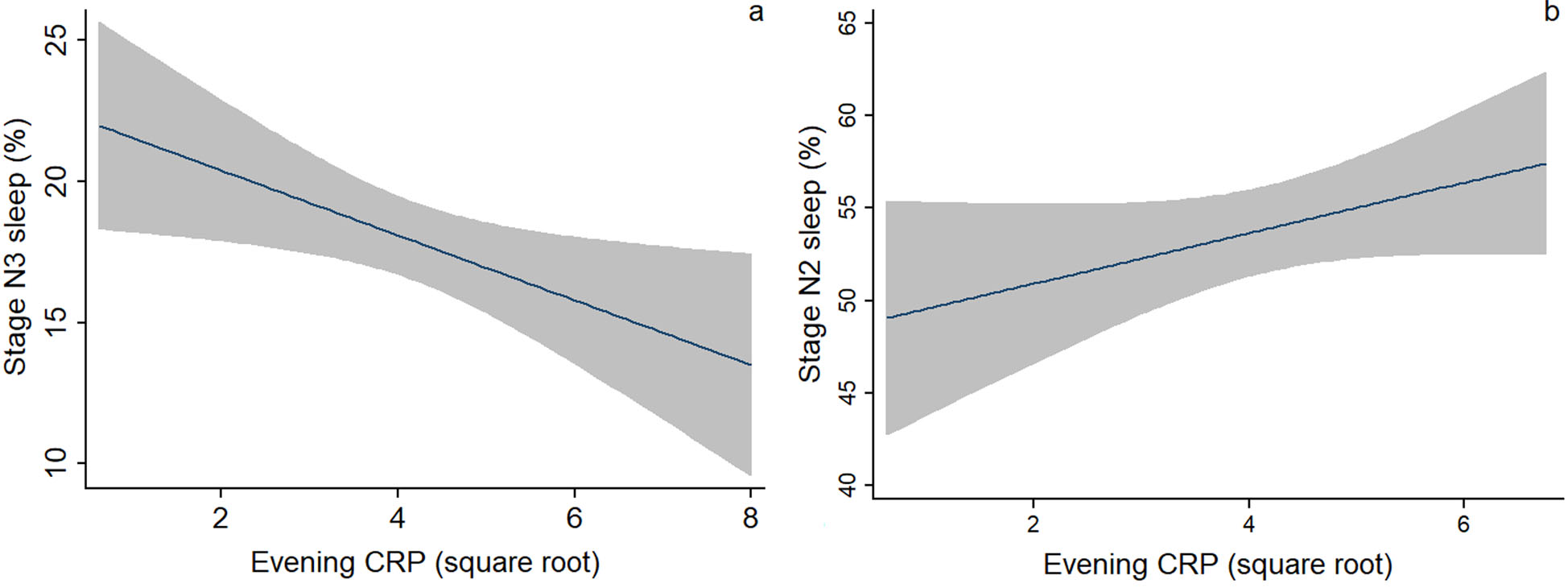
Bivariate associations between evening levels of CRP and (A) stage N3 sleep and (B) stage N2 sleep. Fitted line with a 95% confidence interval (shaded area) was presented.
We created multivariable models while controlling for a priori covariates, including age, pregnancy BMI, race/ethnicity, as well as the presence of OSA and PHQ-9 score. Table 4 shows the final regression models for stage N3 sleep and arousal index. In the model predicting stage N3 sleep, controlling for the covariates, evening CRP was negatively associated with the percentage of N3 sleep (β = −0.30, P = 0.01). In the model predicting arousal index, evening IL-6 was not associated with arousal index (β = 0.15, P = 0.12), after controlling for the covariates, such as age and the presence of OSA.
Table 4.
Multiple linear regression models predicting nocturnal sleep from inflammatory biomarkers
| Predictors | Stage N3 sleep (Model 1) | Arousal index (Model 2) | ||||
|---|---|---|---|---|---|---|
| Coefficient (95% CI) | β | P | Coefficient (95% CI) | β | P | |
| Age, years | 0.39 (0.08 to 0.70) | 0.27 | 0.02 | 0.05 (0.02 to 0.08) | 0.27 | 0.005 |
| Pregnancy BMI, kg/m2 | 0.13 (−0.08 to 0.34) | 0.17 | 0.22 | −0.003 (−0.03 to 0.02) | −0.03 | 0.77 |
| Race/ethnicity# | ||||||
| African American | −1.71 (−5.38 to 1.97) | −0.15 | 0.36 | 0.18 (−0.23 to 0.58) | 0.12 | 0.38 |
| Hispanic | −1.03 (−4.38 to 2.32) | −0.09 | 0.54 | −0.09 (−0.45 to 0.27) | −0.07 | 0.64 |
| PHQ-9 | −0.18 (−0.50 to 0.13) | −0.13 | 0.26 | −0.02 (−0.05 to 0.02) | −0.09 | 0.34 |
| OSA | −6.36 (−10.10 to −2.62) | −0.40 | 0.001 | 1.21 (0.77 to 1.65) | 0.57 | <0.001 |
| CRP, ng/ml or IL-;6, pg/mL† | −1.17 (−2.06 to −0.28) | −0.30 | 0.01 | 0.09 (−0.02 to 0.21) | 0.15 | 0.12 |
Notes: Model 1 stats: F(7,65)=3.65, P = 0.002, R2 = 0.28, adjusted R2 = 0.21; Model 2 stats: F(7,63)=8.43, P < 0.001, R2 = 0.48, adjusted R2 = 0.42;
CRP was used in Model 1 and IL-6 was used in Model 2
using Asian/Others as comparison; 95% CI, 95% confidence interval;
BMI, body mass index; OSA, obstructive sleep apnea; PHQ-9, Patient Health Questionnaire-9.
Associations of nocturnal sleep parameters with morning inflammatory biomarkers
The bivariate associations between nocturnal sleep parameters and morning levels of CRP, IL-6, and sIL-6R are shown in Table 5. Sleep stages were significantly associated with inflammatory biomarkers. Stage N1 sleep was associated with morning IL-6 (r = 0.25, P < 0.05). Stage N3 sleep (r = −0.31, P < 0.05) and N2 sleep (r = 0.25, P < 0.05) were associated with morning CRP (Fig. 2A and 2B). However, there were no significant associations between morning inflammatory biomarkers and sleep continuity parameters, including sleep duration, sleep efficiency, WASO, or arousal index. None of the sleep parameters were also associated with morning sIL-6R. In terms of the covariates, PHQ-9 was related to morning CRP (r = 0.24, P < 0.05); prepregnancy (r = −0.28, P < 0.05) and pregnancy BMI (r = −0.24, P < 0.05) were significantly related to morning sIL-6R. Parity, GDM, OSA, night shift, and use of antihistamine or decongestant were not related to morning inflammatory biomarkers (Table S2, online only). Race/ethnicity was related to morning sIL-6R (P < 0.05), with Hispanic women having higher levels of sIL-6R than African American women (P < 0.05).
Table 5.
Bivariate associations (r) for morning inflammatory biomarkers (n = 70)
| Variables | Morning CRP | Morning IL-6 | Morning sIL-6R |
|---|---|---|---|
| Age, years | −0.06 | −0.003 | −0.06 |
| Gestational age, weeks | −0.06 | 0.16 | 0.18 |
| Prepregnancy BMI, kg/m2 | 0.18§ | 0.15 | −0.28* |
| Pregnancy BMI, kg/m2 | 0.18§ | 0.17§ | −0.24* |
| Neck circumference, cm | 0.23§ | 0.09 | 0.05 |
| PHQ-9 | 0.24* | 0.23§ | 0.06 |
| Sleep duration, h | 0.02 | 0.11 | −0.01 |
| Sleep efficiency, % | 0.06 | 0.12 | −0.02 |
| WASO, min | −0.03 | −0.02 | −0.02 |
| Arousal index, events/h | 0.08 | 0.08 | −0.14 |
| Stage N1 sleep, % | −0.06 | 0.25* | 0.09 |
| Stage N2 sleep, % | 0.25* | −0.01 | 0.02 |
| Stage N3 sleep, % | −0.31* | −0.11 | −0.10 |
| REM sleep, % | −0.009 | −0.15 | 0.005 |
| NREM sleep, % | −0.007 | 0.17§ | −0.04 |
| Evening CRP | 0.88** | 0.09 | −0.05 |
| Evening IL-6 | 0.09 | 0.51** | −0.03 |
| Evening sIL-6R | 0.13 | −0.14 | 0.71** |
Note: Morning inflammatory biomarkers were not obtained from 4 of 74 participants;
P < 0.2;
P < 0.05;
P < 0.01;
BMI, body mass index; NREM, nonrapid eye movement sleep; PHQ-9, Patient Health Questionnaire-9; REM sleep, rapid eye movement sleep; SD, standard deviation; WASO, wake after sleep onset; natural log of IL-6, sIL-6R and arousal index, and square root of CRP were used.
Figure 2.
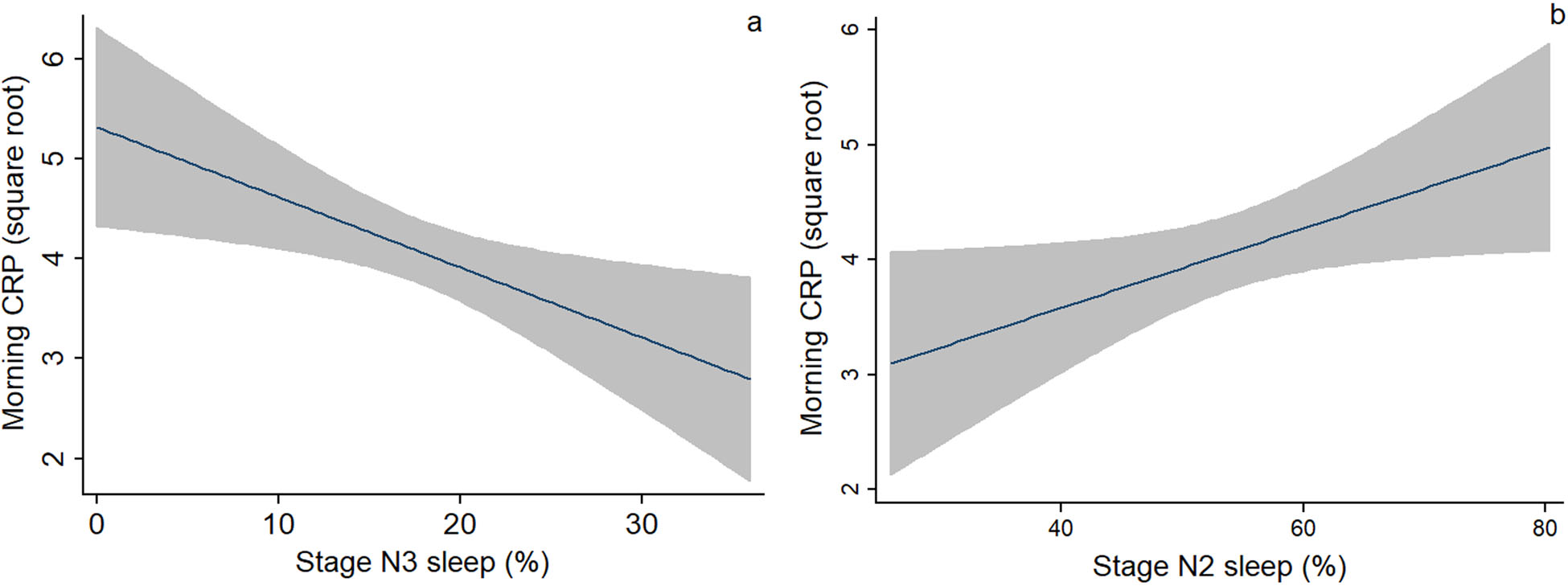
Bivariate associations between (A) stage N3 sleep and (B) stage N2 sleep and morning levels of CRP. Fitted line with a 95% confidence interval (shaded area) was presented.
We further examined the relationship between sleep parameters and morning inflammatory biomarkers using multivariable regression analyses (Table 6). In Model 1, there was a negative association between stage N3 sleep and morning CRP (β = −0.26, P = 0.036), with a higher percentage of N3 sleep associated with a lower level of morning CRP. Contrarily, in Model 2, there was a tendency for a positive association between stage N2 sleep and morning CRP (β = 0.23, P = 0.065), with a higher percentage of N2 sleep associated with a higher level of morning CRP. Stage N1 sleep was associated with morning IL-6 (β = 0.28, P = 0.021) after controlling for the covariates (Model 3).
Table 6.
Multiple linear regression models predicting morning inflammatory biomarkers from nocturnal sleep
| Predictors | CRP (Model 1) | CRP (Model 2) | IL-6 (Model 3) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Coefficient (95% CI) | β | P | Coefficient (95% CI) | β | P | Coefficient (95% CI) | β | P | |
| Age, years | −0.02 (−0.11 to 0.06) | −0.07 | 0.56 | −0.03 (−0.11 to 0.06) | −0.08 | 0.52 | −0.04 (−0.11 to 0.03) | −0.13 | 0.30 |
| Pregnancy BMI to kg/m2 | 0.01 (−0.04 to 0.06) | 0.04 | 0.76 | 0.01 (−0.04 to 0.06) | 0.06 | 0.70 | 0.04 (−0.01 to 0.07) | 0.21 | 0.15 |
| Race/ethnicity# | |||||||||
| African American | 0.22 (−0.79 to 1.24) | 0.08 | 0.66 | 0.49 (−0.54 to 1.53) | 0.17 | 0.35 | −0.50(−1.37 to 0.37) | −0.21 | 0.26 |
| Hispanic | −0.03 (−0.95 to 0.89 | −0.01 | 0.94 | 0.14 (−0.78 to 1.06) | 0.05 | 0.76 | −0.28 (−1.05 to 0.50) | −0.12 | 0.48 |
| PHQ-9 | 0.06 (−0.02 to 0.14) | 0.18 | 0.14 | 0.07 (−0.01 to 0.15) | 0.20 | 0.11 | 0.07 (−0.001 to 0.14) | 0.24 | 0.052 |
| Stage N3, N2, or N1 sleep, %† | −0.06 (−0.12 to −0.01) | −0.26 | 0.036 | 0.03 (−0.002 to 0.06) | 0.23 | 0.065 | 0.08 (0.01 to 0.14) | 0.28 | 0.021 |
Note: Model 1 stats: F(6,63) = 1.93, P = 0.090, R2 = 0.16, adjusted R2 = 0.08; Model 2 stats: F(6,63) = 1.86, P = 0.10, R2 = 0.15, adjusted R2 = 0.07; Model 3 stats: F(6,63) = 1.89, P = 0.096, R2 = 0.15, adjusted R2 = 0.07;
Stage N3 sleep was used in Model 1, stage N2 sleep was used Model 2, and stage N1 sleep was used in Model 3;
using Asian/Others as comparison; 95% CI, 95% confidence interval; BMI, Body mass index; PHQ-9, Patient Health Questionnaire-9.
Discussion
The aim of this study was to investigate the relationships between PSG-measured sleep parameters and inflammatory biomarkers in pregnant women. We did not find significant relationships between sleep continuity variables (e.g., sleep efficiency and arousal index) and inflammatory biomarkers. In the bivariate analysis, stage N2 sleep was positively related to CPR. Controlling for the confounders (e.g., age, pregnancy BMI, and OSA), stage N3 sleep had a bidirectional, negative association with CPR. However, no significant association between the sleep stage and IL-6 was observed. These findings partially supported our hypotheses and added to current literature, suggesting a close interaction between sleep and the immune system.
Stage N3 sleep is a reliable biomarker of sleep homeostasis,40 which could promote the immune function.5, 41 Compared with healthy adults, patients with OSA had a lower amount of stage N3 sleep.42 In this study, we controlled for the presence of OSA and observed a reciprocal relationship between stage N3 sleep and CRP. Specifically, a lower level of CRP before bedtime was related to a higher percentage of stage N3 sleep during the night and a higher percentage of stage N3 sleep during the night was related to a lower level of morning CRP. These findings suggest that a night of deeper sleep may decrease inflammation upon awakening, which, in turn, would facilitate deeper sleep. Similarly, a previous review reported that subjective sleep disturbance was associated with higher CRP.24 Sleep plays an important role in the formation of immunological memory. Particularly, this role is related to stage N3 sleep and the accompanying proinflammatory environment characterized by increased growth hormone and decreased cortisol.43, 44 Stage N3 sleep thus can exert a restorative effect to reduce sympathetic nervous activity. Previous evidence has demonstrated that proinflammatory cytokines may act on the brain to cause behavior changes, such as abnormal sleep.45 Recent evidence has shown communication between the nervous system and the immune system by using common molecular signals.46 Sympathetic activation could upregulate the inflammatory signaling pathway and increase cellular inflammation. Thus, the sympathetic activity might be a mechanism through which stage N3 sleep is related to inflammation. CRP is a biomarker of acute-phase response that has been used widely as a measure of low-grade inflammation.47 In women with normal pregnancy, serum CRP demonstrated a gestational age-dependent increase, suggesting a shift towards an inflammatory state.17, 48 In this study, we included women at their late pregnancy. These women were likely in a higher inflammatory state. Thus, obtaining an adequate amount of deep sleep may have a protective effect on inflammation during late pregnancy.
We did not find a significant association between stage N3 sleep and IL-6. This finding is in contrast to current evidence found in other populations. Stage N3 sleep was negatively related to morning levels of IL-6 in patients with rheumatoid arthritis.25 In adults with insomnia, nocturnal circulating IL-6 was negatively related to stage N3 sleep.49 Likewise, in healthy adults, evening stimulated monocyte production of IL-6 was negatively associated with stage N3 sleep.36 More relevantly, in pregnant women, poor subjective sleep quality was related to higher IL-6 levels during mid and late pregnancy.12 The relationship between sleep and IL-6 production has been suggested bidirectional: disturbed sleep may increase IL-6 production, which, in turn, might further have a negative impact on sleep quality.49, 50 In an animal study, blockage of peripheral and central IL-6 trans-signaling differentially affected sleep stages, mainly by enhancing stage N3 sleep.51 Collectively, IL-6 is likely involved in the modulation of sleep. Several reasons may account for the null finding related to IL-6 in this study. IL-6 is secreted in a biphasic pattern, with two peaks around 5 AM and 7 PM and two nadirs at 8 AM and 9 PM.52 In this study, the two blood samples were collected 1 h before the usual bedtime and within 1 h upon awakening, respectively. Depending on the individual’s habitual sleep/wake schedule, there were variations in sample collection time. Overall, the morning sample was collected between the peak and nadir; the evening sample was collected after the nadir of 9 PM. This sampling technique may have influenced our findings. In healthy adults, the negative association between stage N3 sleep and morning IL-6 became nonsignificant after adjusting for covariates, such as sex, age, and race.53 This finding suggests that demographics could modify the relationship between sleep and cytokines. However, in this study, none of the demographics, except age, were related to sleep stages or cytokines. These nonsignificant relationships could be due to the homogeneous sample of females recruited. Similarly, depression might modify the association between sleep and inflammation. An earlier study reported that among depressed pregnant women, poor sleep efficiency was associated with higher IL-6.54 In the current study, most of the women did not have depressive symptoms as measured by the PHQ-9. The relationship may not be strong enough for the relatively small sample to capture. In this study, sleep was assessed using PSG instead of subjective instruments used in previous studies conducted in pregnant women.12, 54 Discrepancies between PSG- and instrument-measured sleep (e.g., sleep duration, sleep efficiency, and onset latency) have been reported.55, 56 PSG-measured sleep is well conserved in pregnancy. It is possible that sleep stages do not provide a useful biomarker with which to identify risk.
In the unadjusted models, stage N2 sleep was positively related to morning CRP; stage N1 sleep was positively related to morning IL-6. Compared with healthy adults (5.3%),25 women in this study had a higher percentage of stage N1 sleep (8.6%), suggesting poorer sleep quality. This finding confirms the notion that poor sleep quality may induce increased inflammation. However, when the covariates were controlled (e.g., age, race, and BMI), the association become nonsignificant, consistent with a previous study conducted in healthy adults.53 This null finding might be explained by the small sample size. Similarly, we did not find significant associations between sleep continuity variables (e.g., sleep duration and arousal index) and inflammatory biomarkers. The relationship between sleep duration and IL-6 has been mixed. In a systematic review, extreme long sleep duration was associated with higher levels of CRP and IL-6,24 while objective short sleep duration was associated with a higher level of IL-6.24 Patel et al. controlled for covariates (obesity and apnea severity) and found that for every 1-h increase in habitual sleep duration there was a 7% increase in IL-6 (P = 0.0003).57 In a study of postpartum women, IL-6 was associated with longer sleep duration.58 These findings are in contrast with our findings in a pregnancy sample. The existence of potential confounding conditions predisposing to both increased sleep duration and elevated IL-6 may explain this finding. Although we controlled for known covariates (e.g., BMI, race/ethnicity, OSA, and GDM), important covariates may have been missed.
In this study, we collected the inflammatory biomarkers twice (e.g., before sleep and upon awakening the next morning). This sampling technique created a time-lag that enabled us to examine the temporal association between sleep and the immune system. Despite this strength, we still cannot determine their causal relationship due to the correlational design. In order to examine the causality, future interventional studies using the administration of cytokines (or their antagonists) or improving sleep are needed. Our findings need to be interpreted with caution as we did not capture the onset of systemic inflammation. Inflammatory processes could result in and from overt diseases and may affect and be affected by sleep. Relatedly, women in this study were in their third trimester, where inflammation tends to increase as the body prepares for parturition. Thus, findings cannot be extrapolated to women during early- or mid-trimester. Longitudinal studies, including assessment of sleep and inflammation at different stages of pregnancy or after gestation, are needed to shed more light on the change of inflammation over time. It is worth mentioning that around 19% of the women reported a night shift. Although no significant difference in sleep and inflammatory biomarkers was found between the night shift and no night shift, shift work can influence the circadian rhythm of sleep. Futures research should take into account this factor. Another limitation is related to the PSG assessment. In this study, the PSG monitor was performed in the laboratory that is different from the participants’ usual sleep environment. Thus, worse sleep quality may be observed (first night effect). There were women who declined to participate because of the laboratory setting. Findings may be biased toward poor sleepers looking for answers or great sleepers who are not affected by the sleep environment.
Sleep disturbance in pregnancy could skew the proinflammatory cytokine profile leading to an inflammatory state instead of the desired anti-inflammatory state, resulting in poor maternal health and negative pregnancy outcomes.13 Thus, the immune system may be an underlying pathway linking sleep disturbance and pregnancy-related outcomes. Building upon previous studies, we examined the associations between PSG-measured sleep and inflammatory biomarkers in women at late pregnancy. We found that a higher percentage of sleep spent in light sleep in pregnancy is likely associated with higher levels of CRP and IL-6. In comparison, more deep sleep (stage N3 sleep) is bidirectionally associated with lower inflammation. Our findings add to current literature about the reciprocal interaction between sleep and the immune system. They also have important implications. Elevated CRP has been associated with maternal-fetal outcomes (e.g., preeclampsia, GDM, and lower birthweight).17, 18, 59 Thus, increasing the amount of deep sleep might have a protective effect on both the mother and the baby by preventing an increase in inflammatory biomarkers such as CRP. Future studies with longitudinal design may help to reveal the detrimental effects of inflammation by collecting pregnancy-related outcomes (e.g., preeclampsia and preterm birth). Sleep disturbance could also directly increase the risk for adverse maternal and fetal outcomes (e.g., preterm birth gestational length, and death).9, 60, 61 Therefore, continuing efforts are needed to examine the effect of improving sleep quality using sleep-related intervention, on inflammation and associated outcomes in pregnant women.
Supplementary Material
Table S1. Group differences in nocturnal sleep parameters (n = 74).
Table S2. Group differences in morning inflammatory biomarkers (n = 70).
Acknowledgments
This research was supported by a Grant from the National Institutes of Health (R00-NR013187). We would like to thank Jon Balserak for his careful reading of this article.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Izci-Balserak B, Keenan BT, C. C, et al. 2018. Changes in Sleep characteristics and breathing parameters during sleep in early and late pregnancy. J Clin Sleep Med. 14: 1161–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sedov ID, Cameron EE, Madigan S, et al. 2018. Sleep quality during pregnancy: A meta-analysis. Sleep Med Rev. 38: 168–176. [DOI] [PubMed] [Google Scholar]
- 3.Reid KJ, Facco FL, Grobman WA, et al. 2017. Sleep during pregnancy: The nuMoM2b pregnancy and sleep duration and continuity study. Sleep. 40: zsx045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilson DL, Barnes M, Ellett L, et al. 2011. Decreased sleep efficiency, increased wake after sleep onset and increased cortical arousals in late pregnancy. The Australian & New Zealand Journal of Obstetrics & Gynaecology. 51: 38–46. [DOI] [PubMed] [Google Scholar]
- 5.Bryant PA, Trinder J & Curtis N. 2004. Sick and tired: Does sleep have a vital role in the immune system? Nat Rev Immunol. 4: 457–467. [DOI] [PubMed] [Google Scholar]
- 6.Berry RB, Brooks R, Gamaldo CE, et al. 2012. The AASM manual for the scoring of sleep and associated events. Rules, Terminology and Technical Specifications, Darien, Illinois, American Academy of Sleep Medicine. 176. [Google Scholar]
- 7.Lee KA, Zaffke ME & McEnany G. 2000. Parity and sleep patterns during and after pregnancy. Obstetrics and Gynecology. 95: 14–18. [DOI] [PubMed] [Google Scholar]
- 8.Garbazza C, Hackethal S, Riccardi S, et al. 2020. Polysomnographic features of pregnancy: A systematic review. Sleep Med Rev. 50: 101249. [DOI] [PubMed] [Google Scholar]
- 9.Warland J, Dorrian J, Morrison JL, et al. 2018. Maternal sleep during pregnancy and poor fetal outcomes: A scoping review of the literature with meta-analysis. Sleep Med Rev. 41: 197–219. [DOI] [PubMed] [Google Scholar]
- 10.Facco FL, Parker CB, Hunter S, et al. 2018. Association of adverse pregnancy outcomes with self-reported measures of sleep duration and timing in women who are nulliparous. J Clin Sleep Med. 14: 2047–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bublitz MH, Carpenter M, Amin S, et al. 2018. The role of inflammation in the association between gestational diabetes and obstructive sleep apnea: A pilot study. Obstetric Medicine (1753–495X). 11: 186–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okun ML, Hall M & Coussons-Read ME. 2007. Sleep disturbances increase interleukin-6 production during pregnancy: Implications for pregnancy complications. Reproductive Sciences. 14: 560–567. [DOI] [PubMed] [Google Scholar]
- 13.Okun ML 2019. Sleep disturbances and modulations in inflammation: Implications for pregnancy health. Social and Personality Psychology Compass. 13: e12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graham C, Chooniedass R, Stefura WP, et al. 2017. In vivo immune signatures of healthy human pregnancy: Inherently inflammatory or anti-inflammatory? PloS One. 12: e0177813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abell SK, De Courten B, Boyle JA, et al. 2015. Inflammatory and other biomarkers: Role in pathophysiology and prediction of gestational diabetes mellitus. International Journal of Molecular Sciences. 16: 13442–13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holingue C, Owusu JT, Feder KA, et al. 2018. Sleep duration and C-reactive protein: Associations among pregnant and non-pregnant women. Journal of Reproductive Immunology. 128: 9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raio L, Bersinger NA, Malek A, et al. 2019. Ultra-high sensitive C-reactive protein during normal pregnancy and in preeclampsia: A pilot study. Journal of Hypertension. 37: 1012–1017. [DOI] [PubMed] [Google Scholar]
- 18.Yeates AJ, McSorley EM, Mulhern MS, et al. 2020. Associations between maternal inflammation during pregnancy and infant birth outcomes in the Seychelles Child Development Study. Journal of Reproductive Immunology. 137: 102623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alamolhoda SH, Yazdkhasti M, Namdari M, et al. 2020. Association between C-reactive protein and gestational diabetes: A prospective study. Journal of Obstetrics and Gynaecology. 40: 349–353. [DOI] [PubMed] [Google Scholar]
- 20.Menon R, Torloni MR, Voltolini C, et al. 2011. Biomarkers of spontaneous preterm birth: An overview of the literature in the last four decades. Reproductive Sciences. 18: 1046–1070. [DOI] [PubMed] [Google Scholar]
- 21.Sorokin Y, Romero R, Mele L, et al. 2010. Maternal serum interleukin-6, C-reactive protein, and matrix metalloproteinase-9 concentrations as risk factors for preterm birth <32 weeks and adverse neonatal outcomes. American Journal of Perinatology. 27: 631–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nowakowski S, Matthews KA, von Känel R, et al. 2018. Sleep characteristics and inflammatory biomarkers among midlife women. Sleep. 41: zsy049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller MA, Kandala NB, Kivimaki M, et al. 2009. Gender differences in the cross-sectional relationships between sleep duration and markers of inflammation: Whitehall II study. Sleep. 32: 857–864. [PMC free article] [PubMed] [Google Scholar]
- 24.Irwin MR, Olmstead R & Carroll JE. 2016. Sleep disturbance, sleep duration, and inflammation: A systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol Psychiatry. 80: 40–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bjurstrom MF, Olmstead R & Irwin MR. 2017. Reciprocal relationship between sleep macrostructure and evening and morning cellular inflammation in rheumatoid arthritis. Psychosomatic Medicine. 79: 24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Besedovsky L, Lange T & Haack M. 2019. The sleep-immune crosstalk in health and disease. Physiological Reviews. 99: 1325–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Irwin MR & Opp MR. 2017. Sleep health: Reciprocal regulation of sleep and innate immunity. Neuropsychopharmacology. 42: 129–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krueger JM, Takahashi S, Kapas L, et al. 1995. Cytokines in sleep regulation. Adv Neuroimmunol. 5: 171–188. [DOI] [PubMed] [Google Scholar]
- 29.Gómez-González B, Domínguez-Salazar E, Hurtado-Alvarado G, et al. 2012. Role of sleep in the regulation of the immune system and the pituitary hormones. Annals of the New York Academy of Sciences. 1261: 97–106. [DOI] [PubMed] [Google Scholar]
- 30.Benedict C, Scheller J, Rose-John S, et al. 2009. Enhancing influence of intranasal interleukin-6 on slow-wave activity and memory consolidation during sleep. FASEB J. 23: 3629–3636. [DOI] [PubMed] [Google Scholar]
- 31.Baer RJ, Berghella V, Muglia LJ, et al. 2019. Previous adverse outcome of term pregnancy and risk of preterm birth in subsequent pregnancy. Maternal and Child Health Journal. 23: 443–450. [DOI] [PubMed] [Google Scholar]
- 32.Vandorsten JP, Dodson WC, Espeland MA, et al. 2013. NIH consensus development conference: Diagnosing gestational diabetes mellitus. NIH Consens State Sci Statements. 29: 1–31. [PubMed] [Google Scholar]
- 33.Kroenke K, Spitzer RL & Williams JB. 2001. The PHQ-9: Validity of a brief depression severity measure. Journal of General Internal Medicine. 16: 606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Izawa S, Miki K, Liu X, et al. 2013. The diurnal patterns of salivary interleukin-6 and C-reactive protein in healthy young adults. Brain, Behavior, and Immunity. 27: 38–41. [DOI] [PubMed] [Google Scholar]
- 35.Agorastos A, Hauger RL, Barkauskas DA, et al. 2014. Circadian rhythmicity, variability and correlation of interleukin-6 levels in plasma and cerebrospinal fluid of healthy men. Psychoneuroendocrinology. 44: 71–82. [DOI] [PubMed] [Google Scholar]
- 36.Thomas KS, Motivala S, Olmstead R, et al. 2011. Sleep depth and fatigue: Role of cellular inflammatory activation. Brain Behav Immun. 25: 53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pien GW, Pack AI, Jackson N, et al. 2014. Risk factors for sleep-disordered breathing in pregnancy. Thorax. 69: 371–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rechtschaffen A & Kales A. 1973. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Brain Information Service/Brain Research Institute, University of California. National Institute of Health (U.S.). Publication; no. 204 Los Angeles, Calif. [Google Scholar]
- 39.Iber CA-I S, Chesson AL & Jr QSF. 2007. The AASM manual for the scoring of sleep and associated events: Rules, terminology and technical specifications. American Academy of Sleep Medicine; Westchester, IL. [Google Scholar]
- 40.Borbely AA 2001. From slow waves to sleep homeostasis: New perspectives. Archives Italiennes De Diologie. 139: 53–61. [PubMed] [Google Scholar]
- 41.Léger D, Debellemaniere E, Rabat A, et al. 2018. Slow-wave sleep: From the cell to the clinic. Sleep Med Rev. 41: 113–132. [DOI] [PubMed] [Google Scholar]
- 42.Shahveisi K, Jalali A, Moloudi MR, et al. 2018. Sleep architecture in patients with primary snoring and obstructive sleep apnea. Basic and Clinical Neuroscience. 9: 147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Besedovsky L, Lange T & Born J. 2012. Sleep and immune function. Pflugers Archiv : European Journal of Physiology. 463: 121–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Cauter E, Spiegel K, Tasali E, et al. 2008. Metabolic consequences of sleep and sleep loss. Sleep Med. 9 Suppl 1 : S23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dantzer R, O’Connor JC, Freund GG, et al. 2008. From inflammation to sickness and depression: When the immune system subjugates the brain. Nature Reviews. Neuroscience 9: 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Veiga-Fernandes H & Pachnis V. 2017. Neuroimmune regulation during intestinal development and homeostasis. Nature Immunology. 18: 116–122. [DOI] [PubMed] [Google Scholar]
- 47.Sproston NR & Ashworth JJ. 2018. Role of C-reactive protein at sites of inflammation and infection. Frontiers in Immunology. 9: 754–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Oliveira LC, Franco-Sena AB, Rebelo F, et al. 2015. Factors associated with maternal serum C-reactive protein throughout pregnancy: A longitudinal study in women of Rio de Janeiro, Brazil. Nutrition. 31: 1103–1108. [DOI] [PubMed] [Google Scholar]
- 49.Burgos I, Richter L, Klein T, et al. 2006. Increased nocturnal interleukin-6 excretion in patients with primary insomnia: A pilot study. Brain Behav Immun. 20: 246–253. [DOI] [PubMed] [Google Scholar]
- 50.Rohleder N, Aringer M & Boentert M. 2012. Role of interleukin-6 in stress, sleep, and fatigue. Annals of the New York Academy of Sciences. 1261: 88–96. [DOI] [PubMed] [Google Scholar]
- 51.Oyanedel CN, Kelemen E, Scheller J, et al. 2015. Peripheral and central blockade of interleukin-6 trans-signaling differentially affects sleep architecture. Brain Behav Immun. 50: 178–185. [DOI] [PubMed] [Google Scholar]
- 52.Mills PJ, von Känel R, Norman D, et al. 2007. Inflammation and sleep in healthy individuals. Sleep. 30: 729–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hong S, Mills PJ, Loredo JS, et al. 2005. The association between interleukin-6, sleep, and demographic characteristics. Brain Behav Immun. 19: 165–172. [DOI] [PubMed] [Google Scholar]
- 54.Okun ML, Luther JF, Wisniewski SR, et al. 2013. Disturbed sleep and inflammatory cytokines in depressed and nondepressed pregnant women: An exploratory analysis of pregnancy outcomes. Psychosomatic Medicine. 75: 670–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McIntyre JP, Ingham CM, Hutchinson BL, et al. 2016. A description of sleep behaviour in healthy late pregnancy, and the accuracy of self-reports. BMC Pregnancy and Childbirth. 16: 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wilson DL, Fung A, Walker SP, et al. 2013. Subjective reports versus objective measurement of sleep latency and sleep duration in pregnancy. Behavioral Sleep Medicine. 11: 207–221. [DOI] [PubMed] [Google Scholar]
- 57.Patel SR, Zhu X, Storfer-Isser A, et al. 2009. Sleep duration and biomarkers of inflammation. Sleep. 32: 200–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Paul S & Corwin EJ. 2019. Identifying clusters from multidimensional symptom trajectories in postpartum women. Research in Nursing & Health. 42: 119–127. [DOI] [PubMed] [Google Scholar]
- 59.Qiu C, Sorensen TK, Luthy DA, et al. 2004. A prospective study of maternal serum C-reactive protein (CRP) concentrations and risk of gestational diabetes mellitus. Paediatric and Perinatal Epidemiology. 18: 377–384. [DOI] [PubMed] [Google Scholar]
- 60.Carroll JE, Teti DM, Hall MH, et al. 2019. Maternal sleep in pregnancy and postpartum Part II: Biomechanisms and intervention strategies. Current Psychiatry Reports. 21: 19. [DOI] [PubMed] [Google Scholar]
- 61.Okun ML, Schetter CD & Glynn LM. 2011. Poor sleep quality is associated with preterm birth. Sleep. 34: 1493–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Group differences in nocturnal sleep parameters (n = 74).
Table S2. Group differences in morning inflammatory biomarkers (n = 70).


