Abstract
The near universal presence of chronic low-grade systemic inflammation among patients with severe obesity disrupts iron homeostasis and underlies the association between obesity and iron deficiency. Immune activation and inflammation results in a reduction in circulating iron and diminished iron bioavailability for erythropoiesis. Inflammation also alters blood levels of commonly measured markers of iron nutrition status, which makes the diagnosis of iron deficiency difficult and has led to new recommendations regarding laboratory markers for the diagnosis. Recent evidence utilizing these newly recommended laboratory markers which include levels of ferritin, C Reactive Protein, and Transferrin Saturation suggests that the actual prevalence of iron deficiency among candidates for metabolic surgery may be double or triple the prevalence identified by low levels of ferritin alone. Thus, large numbers of surgical candidates have iron deficiency which has been heretofore largely unrecognized and inadequately treated. The assessment of iron status utilizing the currently recommended markers in the presence of chronic inflammatory diseases, and repletion of depleted stores for surgical candidates with deficiency during the pre-operative period presents an important opportunity for mitigating this condition in post-operative patients.
Keywords: Severe Obesity, Iron Deficiency, Nutrition and Metabolic Surgery, Anemia and Metabolic Surgery
INTRODUCTION
Accumulating clinical evidence substantiating disordered iron nutrition in most patients with severe obesity has raised concern about validity of the traditional approach to the diagnosis and management of iron deficiency and its associated anemia in candidates for metabolic surgery. The near universal prevalence of chronic low-grade systemic inflammation in patients with severe obesity,1–3 which results in reduced iron absorption from the intestine, enhanced iron sequestration in macrophages, and reduced iron bioavailability accounts for the common occurrence of iron deficiency (ID) in patients with severe obesity.4 The presence of inflammation also alters the direct relationship between the blood level of ferritin and available iron stores, because ferritin is an acute phase reactant which is elevated by inflammation whatever the iron status.5 Furthermore, inflammation may influence the optimal strategies for iron repletion among patients with ID.
The purpose of this narrative review is (1) to describe the prevalence of inflammation and its consequences in candidates for metabolic surgery (2) to review the effects of this chronic low-grade systemic inflammation on traditional diagnostic thresholds for the usual markers of ID and (3) to propose a new diagnostic algorithm for the diagnosis of ID that will more accurately identify the high prevalence of ID among candidates for metabolic surgery. We propose that these new concepts in iron nutrition provide an opportunity to improve overall outcome as well as to reduce the risk of ID and anemia which is known to complicate metabolic surgery procedures.
THE ASSOCIATION OF SYSTEMIC INFLAMMATION AND OBESITY
Obesity is one of the many chronic diseases characterized by a low-level activation and/or insufficient resolution of an inflammatory response producing a state of chronic, mild systemic inflammation.1–3 Inflammation in severe obesity is thought to be activated by excessive adipocyte enlargement (primarily in visceral adipose tissue1,7,8) in response to caloric excess leading to immune activation, macrophage infiltration into predominantly visceral adipocytes, enhanced release of pro-inflammatory cytokines, a reduction in anti-inflammatory adipokine levels,9 and dysregulated release of free fatty acids.6,7
Activation of the inflammatory response stimulates the synthesis and release of acute phase reactants which are markers of inflammation. C-Reactive Protein (CRP) is the inflammatory biomarker which has been most extensively studied.10 It is produced in the liver in response to an increase in IL-6. The plasma half-life of CRP is 19 hours, which does not vary with differing disease conditions, thus rendering the blood level an accurate measure of the intensity of the inflammatory response.10 In healthy individuals from the general population, the median CRP level is 0.8 mg/L. Levels up to 3mg/L are considered normal, and levels of 3–8mg/L represent subclinical inflammation. Clinically significant inflammation is identified by levels above 8 mg/L.11
Several studies have documented a direct correlation between BMI and CRP levels.12–15 In addition, a number of studies have reported CRP levels in patients with severe obesity undergoing metabolic surgery showing a high prevalence of clinically significant inflammation. The findings in 4 studies totaling 2246 patients are shown in Table 1. Note that the mean levels and range of CRP reflect a mild to modest level of inflammation.
TABLE 1.
CRP levels in studies of patients prior to Metabolic Surgery
| AUTHOR | NUMBER OF PATIENTS | BMI (KG/M2) | MEAN CRP LEVEL (MG/L) | PER CENT WITH ELEVATED CRP |
|---|---|---|---|---|
| CHEN16 | 640 | 41.5 ± 6.7 | 8.4 ± 1.1 (mean ± SD) | 74% |
| CAREAGA17 | 803 | 47.4 ± .2 | 12.4 ± .4 (mean ±. SD) | 88% |
| ARISMENDI2 | 129 | 46 ± .6 | 7.8 (4.3–14.5) (mean, range) | NOT REPORTED |
| PAEPEGAEY15 | 674 | 46 | 6.9 (0.2–55.9) (mean, range | NOT REPORTED |
IRON HOMEOSTASIS AND INFLAMMATION
Iron homeostasis is tightly regulated in the absence of an excretory pathway. In times of need when stores are depleted, absorption is increased, and when stores are replete, absorption from enterocytes and release of iron from stores is reduced. The primary regulator of circulating iron availability is hepcidin, a peptide hormone produced in the liver.18 Hepcidin acts to limit circulating iron by inhibiting iron exporters that transport ingested iron absorbed from the diet by enterocytes into the circulation and export from stored iron in hepatocyte ferritin and in macrophages of the reticuloendothelial system.18 When iron stores are replete, hepcidin levels are increased as production is upregulated and circulating iron is reduced. With increased hepcidin levels, transferrin-bound iron in the circulation is also reduced resulting in decreased iron availability for hematopoiesis and other functions.19 Iron depletion or a demand for erythropoiesis down regulates hepcidin gene expression, and levels fall resulting in enhanced iron absorption from enterocytes and increased release from storage sites.20 More detailed information about iron homeostasis and its regulation can be obtained from several excellent reviews.21,22
Immune activation and the resultant inflammatory response leads to disruption of the normal regulation of iron homeostasis. Hepcidin is also an acute phase reactant and its production is upregulated in response to the release of interleukin 6 (IL6) which occurs with immune activation and inflammation.19 In obesity accompanied by systemic inflammation, hepcidin levels are increased,19 which can lead to reduced levels of circulating transferrin-bound iron and the anemia of inflammation (also called the anemia of chronic disease) where iron availability for hematopoiesis is limited despite the presence of what would normally be adequate total body iron stores.23 In diseases like obesity, chronic inflammation may remain for an extended period of time. The associated sustained elevations in levels of hepcidin will reduce intestinal iron absorption increasing the risk of absolute ID and potentially worsening the anemia of inflammation.23
INFLAMMATION AND THE DIAGNOSIS OF IRON DEFICIENCY
Inflammation, when present, also complicates the diagnosis of ID because many of the laboratory markers of iron status such as serum iron, ferritin, transferrin (iron binding capacity) and transferrin saturation (T Sat) are also affected by the presence of inflammation. Levels of iron, transferrin and T Sat in blood fall and levels of ferritin increase with increasing degrees of inflammation.24 In the presence of inflammation, the ferritin level thus loses its specificity as a predictor of iron stores,25 and levels extending into the normal range can be consistent with iron deficiency.26,27 The high prevalence of systemic inflammation and dysregulated iron homeostasis among patients with severe obesity argues for the assessment of the degree of inflammation when measuring iron status in patients.
Although the blood hepcidin level is a potential marker of iron homeostasis, the hepcidin level is also a marker of inflammation, and each stimulus influences the level in opposite directions. Thus, an elevated hepcidin level can be consistent with normal iron nutrition with abundant stores or with inflammation, and a normal hepcidin level can indicate iron deficiency complicated by inflammation. Furthermore, this test is not generally clinically available with the standard reference range relating to age and gender currently under investigation.28 At the present time, the most widely tested marker of the presence of inflammation is the CRP level.
IRON DEFICIENCY DIAGNOSIS AND PREVALENCE AMONG CANDIDATES FOR SURGERY
Recognition of the high prevalence of inflammation in patients with severe obesity, the competing effects of inflammation and iron status on the availability of iron, and the altered relationship between levels of ferritin and iron stores in the presence of inflammation has led to investigative efforts to redefine more accurate laboratory thresholds for the diagnosis of ID in obesity and other chronic inflammatory diseases.29 For patients with heart failure, chronic kidney disease and inflammatory bowel disease with associated inflammation, the following laboratory cut-off values for ID have been proposed: Serum ferritin < 100 ng/dL or T Sat < 20%.29 Additional recommendations suggest that when clinical inflammation is present in patients with severe obesity as determined by a CRP level > 8 mg/L (the traditional level defining clinical inflammation), or if T Sat < 20%, serum ferritin levels between 30-<100 ng/ml suggest absolute ID. CRP levels between 3 and 8 mg/L using the highly sensitive CRP determination reflect lower degrees of inflammation. These levels do have clinical relevance, as they are known to increase cardiovascular risk.30 Levels of CRP >5mg/L have been proposed as a level of inflammation above which measures of ID are affected,29 but additional study of CRP levels between 3–8mg/L and their relation to iron homeostasis are needed.
When ferritin levels equal or exceed 100 ng/mL in association with T Sat < 20%,these findings suggest iron sequestration as seen in the anemia of inflammation,31,32. However this remains controversial because in heart failure, chronic renal disease, and inflammatory bowel disease, the combination of a ferritin level of 100–300ng/dL with T Sat < 20% is also considered consistent with ID.29,33, However the level of inflammation in severe obesity is generally milder than in the above conditions when they are severe.
The prevalence of ID among candidates for metabolic surgery has been extensively studied in the past with reported findings of 5.7–19% (Table 2).34–40 These studies are likely to reflect underestimates, because the diagnostic thresholds for ID are based solely on low levels of ferritin or iron without consideration of the effects of systemic inflammation on these parameters. Several more recent studies have incorporated an assessment of inflammation by either measuring CRP levels, or by adjustment of current laboratory thresholds and have proposed that the prevalence may be much higher, approximating 20–50%.17,31,41 Thus, it appears that when the high prevalence of inflammation in patients with severe obesity is assessed, the actual prevalence of ID may actually be double or triple the prevalence shown in Table 2 determined using only low levels of ferritin.
Table 2.
Reported rates of anemia and iron deficiency in pre-bariatric surgery patients (Benotti et al)40 (ID: iron deficiency; Hgb: hemoglobin; gm: grams; ♀: female; ♂ male; mmole: millimoles; l,L: liter; dl: deciliter; pmol: picomole; ng: nanogram; μmol; micromole)
| AUTHOR | YEAR | NUMBER | % ANEMIA | % ID | ANEMIA diagnosis | IRON DEFICIENCY diagnosis |
|---|---|---|---|---|---|---|
| SKROUBIS34 | 2002 | 79 | 18% | 16% | Hgb<13.4gm/dl ♂; <12.5 gm/dl ♀ | Ferritin ≲ 9 ng/ml |
| FLANCBAUM35 | 2006 | 379 | 22% | 8.4% | Hgb below normal; (not provided) | Ferritin below normal |
| TOH36 | 2009 | 232 | 6.4% | 15.7% | Hgb<13.4gm/dl ♂; <12.5 gm/dl ♀ | Serum Iron ≲ 9 μmol/L |
| ERNST37 | 2009 | 232 | 6.9% | 6.9% | Hgb < 8.7mmol/l ♂; < 7.5 mmol/l ♀ | Serum Ferritin < 18 pmol/l |
| SCHWEIGER38 | 2010 | 114 | 18.4 | 24% | Hgb < 12gm/dl ♂; < 14 gm/dl ♂ | Serum ferritin <20ng/ml. |
| LEFEBVRE39 | 2014 | 267 | 6.1% | 5.7% | Hgb < 13gm/dl ♂; < 12ng/dl ♀ | Serum Ferritin < 24 ng/ml ♂; <11 ng/ml ♀ |
A new diagnostic algorithm for the diagnosis of ID in patients with inflammation utilizing levels of ferritin and T Sat, recently proposed by a consensus panel of experts is shown in Figure 1.29,42 In order to show the potential impact of this new diagnostic algorithm, we have utilized our established clinical registry of research-consented patients undergoing metabolic surgery43 with iron status data from n= 3723 of such patients from our previous studies of perioperative iron nutrition.44. When these proposed diagnostic thresholds for ID are tested in this registry, and the prevalence of clinically significant inflammation is recognized at 76–88% as shown in Figure 1,16,17 the actual prevalence of ID in our cohort of pre-operative patients with severe obesity is consistent with recent studies.17,31,41 Thus, the use of a low level of ferritin alone as an indicator of ID vastly underestimates the actual prevalence.
Fig. 1.
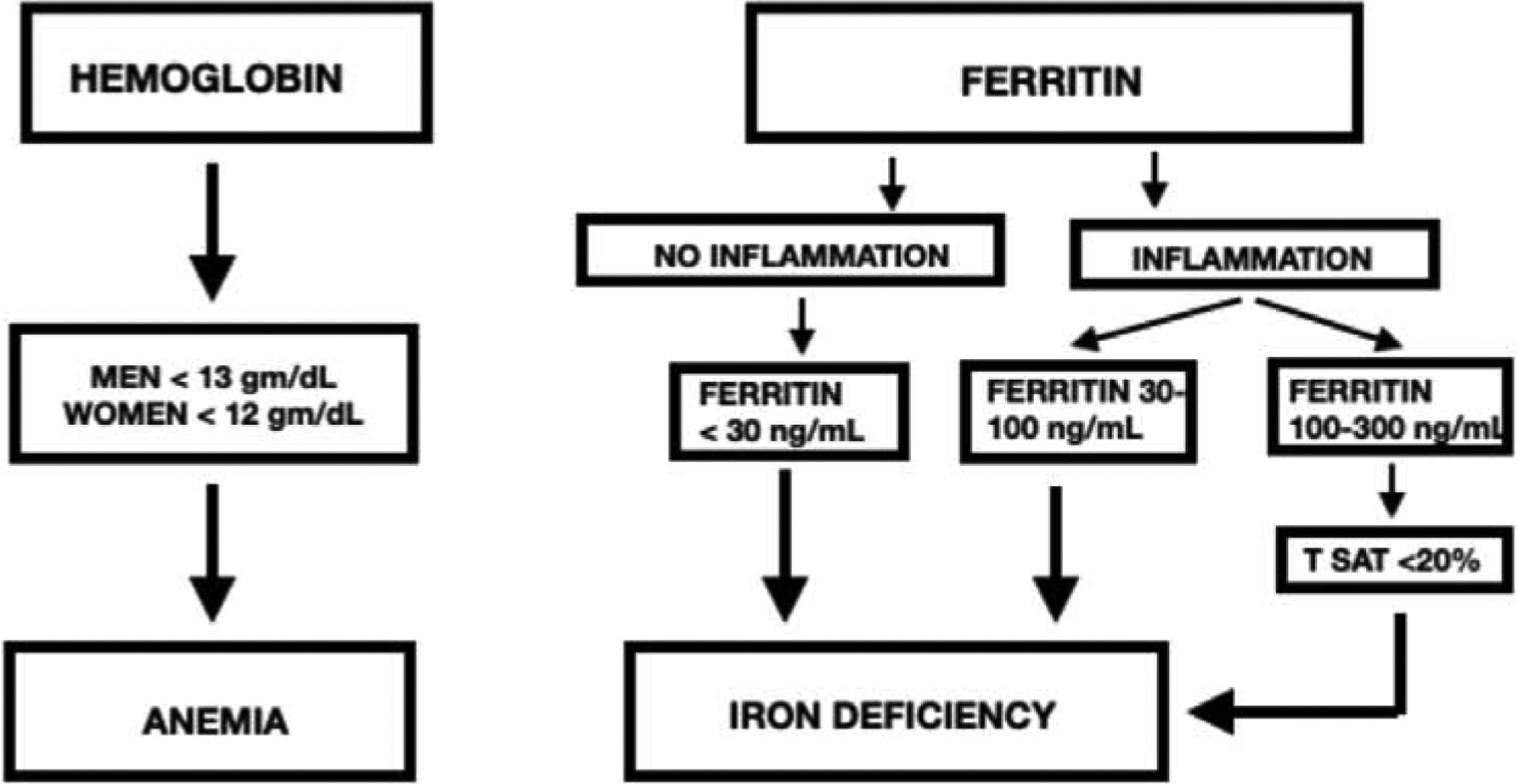
Laboratory thresholds for the diagnosis of anemia and iron deficiency proposed by an expert panel. Modified from Cappellini et al.25,27 T SAT = transferrin saturation.
These findings suggest that, if the level of ferritin is used as the only indicator of iron nutrition status in patients with severe obesity, the majority of patients with ID would be missed and untreated. This also suggests that there are large numbers of patients with unrecognized depletion of iron stores which will likely be worsened significantly when they subsequently undergo either a Roux-en-Y gastric bypass or sleeve gastrectomy, both of which sharply reduce gastrointestinal iron absorption. This impaired iron status would be further compromised postoperatively by dramatic reduction of dietary iron intake and absorption in the first postoperative year when dietary intake is severely restricted and accelerated weight loss occurs. It is likely that the lack of provider recognition of disordered iron status among preoperative patients may be a major contributor to the high prevalence of ID (13–53%) and anemia (16–54%), both of which increase with time after surgery.40,44 It is evident from this preliminary work that additional investigation is needed to assess the impact of systemic inflammation on iron nutrition status in severe obesity, and to confirm that obesity warrants consideration as a chronic inflammatory disease requiring new diagnostic thresholds for the definition of ID.
MANAGEMENT OF IRON DEFICIENCY IN CANDIDATES FOR METABOLIC SURGERY
All candidates for surgery should undergo laboratory assessment of iron nutrition at least 30 days before surgery in order to allow a time period for treatment.31,45 Laboratory studies should include serum ferritin, iron and iron binding capacity (for calculation of T Sat) as well as a standard CRP level to assess the presence and extent of inflammation. Although the introduction of multidisciplinary care has improved the preoperative nutritional screening, a recent large study reviewing claims data from 21,345 surgery eligible patients suggested that preoperative nutritional assessment occurred in < 25% of patients.46 In the presence of inflammation, the diagnosis of ID is challenging. If inflammation is present and there are findings suggestive of ID, further clarification can be obtained by measuring levels of soluble transferrin receptor where levels increase in ID, but are not affected by inflammation.47,48 A reasonable option if there is strong suspicion of ID is to provide supplementation and check for a response. When anemia is present, the amount of iron to be provided can be estimated based on the hemoglobin deficit and body weight.23 However in most circumstances use of the diagnostic algorithm in Figure 1 is a reasonable guide to define the presence of likely ID for patients with severe obesity.
ID is now increasingly recognized as a disorder needing treatment in the absence of anemia because of increased awareness of its involvement in mitochondrial electron transport, energy production, the immune response, muscle oxygen utilization, and other vital functions.21,49,50 ID with symptoms can occur in the absence of anemia, because iron stores must be significantly depleted before erythropoiesis is substantially reduced.31 A cardinal clinical symptom of ID in this situation is chronic fatigue.31,49 Non-anemic candidates for surgery who have laboratory evidence of ID with decreased stores should receive treatment for deficiency preoperatively and possibly receive a higher dose of supplementation with the recognition that tolerance to oral iron is often limited to moderate doses particularly in the postoperative period. A good example of such a patient is a non-anemic premenopausal woman with a ferritin level <30 ng/mL. Iron replacement, both oral and parenteral has been demonstrated as effective in the treatment of non-anemic ID fatigue in menstruating women and in other populations.49,51,52 Aggressive attention to the restoration and maintenance of iron stores in this situation may attenuate the major risk of ID and severe ID anemia which commonly occur after surgery, and patients with preoperative ID are at the highest risk.44 This is an important area in need of further study, as treatment needs for iron deficient metabolic surgery candidates with a relatively brief interval before surgery may be different from those of the general population.
The highest risk patients for postoperative iron deficiency and anemia after surgery are women with preoperative evidence of low iron stores or anemia.40,44 Obesity is a major risk factor for abnormal menstrual bleeding,53 a condition with a prevalence of 5–52% in the general population.54 Body iron in males can be up to 6 grams and 2 grams in females. The majority of iron (1–2 grams) is in red blood cells as hemoglobin, with 0.5–1 gram as storage iron in bone marrow, spleen and liver. Before Hemoglobin begins to fall, a large portion of body iron stores must be lost.49 A non-anemic female candidate for surgery with a ferritin level < 30 ng/L is likely to have a body iron deficit of 300–500 grams. The presence of anemia will add to this deficit. Normal iron absorption is about 1–2 mg per day55 which suggests that replacement of such a large iron deficit will be slow and poses challenges related to the time needed for repletion, patient compliance issues, and decisions regarding the optimal mode of repletion.
Current guidelines suggest routine screening of iron nutrition prior to metabolic surgery.47 Oral iron supplementation is currently considered as the first line treatment for ID for the general population.23 Specific recommendations for the treatment of ID in candidates for metabolic surgery are lacking. Adults should ideally receive 100–200 mg of elemental iron ideally in the ferrous state in divided doses daily.26 The most commonly available oral iron preparations include Ferrous Sulfate (65 mg iron per tablet), ferrous gluconate (35 mg iron per tablet), and ferrous fumarate (100 mg iron per tablet). Ferrous sulfate is the least expensive and most commonly used. Since the recommended dietary allowance (RDA) in normal healthy individuals is 8 mg/day orally, these amounts if taken should easily meet daily plus replacement needs in metabolic surgical patients with impaired iron uptake by surgery and inflammation. Interested providers should refer to information from the Society for the Advancement of Blood Management, A Physicians Guide to Oral iron Supplements for additional information and recommendations regarding oral iron supplementation.56
Patients receiving oral supplementation must be followed closely because compliance with treatment is a major problem primarily because up to 70% of patients will experience significant gastrointestinal side effects including dyspepsia, constipation, abdominal pain, and nausea.57 Alternate dosing strategies involving lower doses initially with graded increases can be used with fewer side effects.56, 58 In addition, recent evidence from randomized controlled trials suggest that newer iron preparations like Sucrasomial iron with an altered chemical structure and a different pathway for absorption may offer another option for patients who do not tolerate ferrous sulfate and other salt-based oral iron preparation.59 Preliminary studies primarily in Europe suggest that this preparation may be associated with improved gastrointestinal tolerance and bioavailability.59
Oral supplementation should be closely monitored and supervised by a dietitian, as iron is best administered between meals and in the absence of certain foods and medications.31,32,60 Patients with higher levels of inflammation, as has been recently shown in patients with central obesity,61 may not respond to oral iron due to the inhibitory effects of hepcidin on iron export from enterocytes. Patients with ID or ID anemia who cannot take or do not respond to oral supplementation should be considered for parenteral iron where the response is prompt, effective in the presence of inflammation, and, with newer formulations of parenteral iron, much better tolerated.62 Parenteral iron is now the treatment of choice for ID in patients with chronic renal disease requiring dialysis and in patients with heart failure, conditions associated with levels of inflammation comparable to patients with severe obesity.33, 63 As intravenous iron has demonstrated its efficacy and safety in these diseases complicated by inflammation, it should be considered and studied for iron repletion in preoperative patients with depleted iron stores who cannot tolerate or do not respond to oral iron supplementation, and when depleted patients have limited time prior to surgery associated with the risk of major blood loss. Comparisons between oral vs parenteral iron replacement are summarized in Table 3.26,29
Table 3.
Comparisons between oral iron replacement vs. parenteral replacement regarding indications, advantages and disadvantages. (adapted from Cappellini et al29 and Camaschella et al.26)
| IRON REPLACEMENT MODALITY | ||
|---|---|---|
| ORAL | PARENTERAL | |
| CURRENT INDICATIONS | Preferred replacement mode Current standard of care |
Failure of oral replacement Intolerance to oral replacement |
| EMERGING INDICATIONS | Presence of inflammation (reduced absorption). functional iron deficiency Need for quick response (pre-operative patients) |
|
| ADVANTAGES | Ease of use Low cost |
Rapid response to treatment Effective when inflammation present Eliminates need for patient compliance |
| DISADVANTAGES | Limited absorption Slow efficacy Unpredictable in inflammatory conditions Gastrointestinal side effects common Poor patient adherence to treatment |
Requires specialty care Must be administered in a medical facility Hypersensitivity reactions Cost |
| NEWER TREATMENT ALTERNATIVES | Sucrasomial Iron (different absorption mechanism, possibly reduced side effects) | Improved safety with the newer formulations |
Iron repletion in preoperative patients with ID is associated with improved surgical outcomes,45 and current perioperative guidelines recommend the use of parenteral iron as front line treatment when surgery with the potential for major blood loss is planned for less than 6 weeks.64 A parenteral dose of 800–1000 mg or iron would provide sufficient iron to meet the daily needs of iron (1 mg/day) for about 3 years, and has the potential to avoid to a great extent the development of iron deficiency during the phase of rapid weight loss characterized by decline in inflammation, inadequate dietary intake, and poor tolerance to oral iron.
For most patients undergoing metabolic surgery for severe obesity, the procedure is permanent with its potential impact on dietary iron intake and absorption rates long lasting, and with residual obesity present in many having potential effects on iron metabolism through continued inflammation, although presumably of a much lower degree. Whether full iron repletion in the initial preoperative and/or early postoperative period could improve subsequent outcomes including greater net weight loss with better iron status and improved physical stamina as well as less likelihood for the development of ID anemia over the long-term will require clinical study. In this regard, there is some evidence that preoperative ID has a negative association with weight loss and that preoperative anemia is associated with reduced short-term weight loss.65,66
An important limitation of this review is the fact that the concept of systemic inflammation as it relates to the pathophysiology of severe obesity, its effect on iron homeostasis, and its clinical implications has not been well studied to date. A growing number of studies do acknowledge that obesity has joined the list of chronic diseases characterized by altered iron homeostasis related to the presence of systemic inflammation,23–27,29,32,42,49,67 and that updated diagnostic markers of iron nutrition are indicated for identification of the true prevalence of ID27,29,32,42,67–70 which is underdiagnosed in the previously reported prevalences (5.7–19%, Table 2) based solely on low blood levels of ferritin or iron.34–39 These updated algorithms for the diagnostic approach for ID are based on expert opinion and not on systematic review of published material. In view of the high prevalence of ID among surgical candidates and current guidelines regarding the best practices for pre-operative management of these conditions, this is important information for providers in metabolic surgery programs.
Areas where gaps in knowledge exist include defining the role of inflammatory markers like CRP in the diagnostic algorithm for ID. An algorithm including the presence or absence of inflammation has recently been proposed (Figure 1), and a suggested cutoff for the CRP level of >5mg/L has been proposed, but this has not been confirmed.27,32,68 In addition, the role of hepcidin levels in the evaluation of iron status beyond predicting the optimal route of repletion in patients with inflammation needs clarification.
The concept of improved early recognition and management of ID and ID anemia in candidates for metabolic surgery provides an opportunity for improvement in surgical safety and to possibly mitigate the alarming prevalence of ID and ID anemia occurring longer-term after surgery. A recent retrospective study of 80 patients undergoing sleeve gastrectomy found that preoperative correction of micronutrient deficiencies was protective in the short-term (one year) after surgery.71 The known challenges with oral iron replacement and the emerging evidence for the safety and efficacy of parenteral iron argue for additional study of this modality in surgical candidates. Preoperative restoration of iron stores for metabolic surgery candidates at high risk for postoperative ID and severe anemia after surgery, and validation of alternate diagnostic criteria for ID are important areas in need of further study to establish evidence-based guidelines for the perioperative management of ID.
Obesity is associated with an increased risk of iron deficiency
Chronic low-grade systemic inflammation is present in the vast majority of patients with severe obesity and leads to reduced iron availability despite adequate stores.
inflammation affects the blood levels of markers of iron nutrition status which complicates the assessment of iron nutrition and has led to new recommendations for laboratory markers for the assessment of iron nutrition status.
Current estimates for the prevalence of iron deficiency among candidates for metabolic surgery utilizing the newer diagnostic criteria which include an assessment of inflammation suggest that the prevalence may be as high as 60%.
many candidates for metabolic surgery have undiagnosed and untreated iron deficiency which contributes to the alarming prevalence after surgery
Funding information
National Institute of Health, Grant/Award
Number: DK072488
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
None of the authors has any conflicts of interest to disclose.
Contributor Information
Jila Kaberi-Oterod, Geisinger Medical Center Wilkes-Barre PA..
Christopher D Still, Geisinger Obesity Institute, Danville PA..
Glenn S Gerhard, Department of Medical Genetics and Molecular Biology Temple University School of Medicine, Philadelphia PA..
Bruce R Bistrian, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston MA.
REFERENCES
- 1.de Heredia F, Gomez-Martinez S, Ascension M. Chronic and degenerative diseases obesity, inflammation and the immune system. Proceedings Nut Soc. 2012; 71:332–338 [DOI] [PubMed] [Google Scholar]
- 2.Arismendi E, Rivas E, Agusti A et al. The systemic inflammome of severe obesity before and after bariatric surgery. Plos One. 2014; 9(9): e107859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cox A, West N, Cripps A. Obesity, inflammation, and the gut microbiota. Lancet Diabetes Indocrinol. 2015; 3:207–215. [DOI] [PubMed] [Google Scholar]
- 4.Zhao L, Zhang X, Shen X, et al. Obesity and iron Deficiency: a quantitative meta-analysis. Obesity Reviews 2015; 16:1081–1093. [DOI] [PubMed] [Google Scholar]
- 5.Daru J, Colman K, Stanworth S et al. Serum ferritin as an indicator of iron status: What do we need to Know? Am J. Clin Nutr 2017; 106(suppl):1634S–1639S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wellen K, Hotamisligil G. Obesity-induced inflammatory changes in adipose tissue. J Clin Invest. 2003;112:1785–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greenberg A, Obin M. Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr. 2006;83(suppl):461W–465S. [DOI] [PubMed] [Google Scholar]
- 8.Harman-Boehm I, Bluher M, Redel H, et al. Macrophage infiltration into omental versus subcutaneous fat across different populations: effect of regional adiposity and the comorbidities of obesity. J Clin Endocrinol Metabol. 2007; 92(6):2240–2247. [DOI] [PubMed] [Google Scholar]
- 9.Wang Z, Scherer P. Adiponectin, the past two decades. J Mol Cell Biol. 2016; 8:93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pepys M, Hirschfield G. C-reactive protein: a critical update. J Clin Invest. 2003;111:1805–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bassuk S, Rifai N, Ridker P. High sensitivity C-Reactive Protein: clinical Importance. Curr Probl Cardiol. 2004;29:439–493. [PubMed] [Google Scholar]
- 12.Khaodhiar L, Ling PR, Blackburn GL, Bistrian BR. Serum levels of interleukin-6 and C-reactive protein correlate with body mass index across the broad range of obesity. J Parenter Enteral Nutr. 2004; 28:410–415. [DOI] [PubMed] [Google Scholar]
- 13.Visser M, Bouter L, McQuillan G, Wener M, Harris T. Elevated Creactive protein levels in overweight and obese adults. JAMA. 1999; 282:2131–2135. [DOI] [PubMed] [Google Scholar]
- 14.Khan A, Khan W, Ayub M, Humayun M, Haroon M. Ferritin is a marker of inflammation rather than Iron deficiency in overweight and obese people. J Obese. 2016; Article ID 1937320, 7 pages 10.1155/2016/1937320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paepegaey A, Genser L, Bouillot J et al. High Levels of CRP in morbid obesity: the central Rrole of adipose tissue and Lessons for clinical practice before and after bariatric surgery. Surg Obes Rel Dis. 2015; 11:148–154. [DOI] [PubMed] [Google Scholar]
- 16.Chen S, Lee Y, Ser K et al. Serum C-reactive protein and white blood cell count in morbidly obese surgical patients. Obes Surg. 2009; 19:461–466. [DOI] [PubMed] [Google Scholar]
- 17.Careaga M, Moize V, Flores L, et al. Inflammation and iron status in bariatric surgery candidates. Surg Obes Relat Dis. 2015;11:906–911. [DOI] [PubMed] [Google Scholar]
- 18.Collins J,Wessling-Resnick M, Knutson M. Hepcidin regulation of iron transport. J Nutr. 2008; 138:2284–2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pagani A, Nai A, Silvestri L et al. Hepcidin and anemia: A tight relationship. Front. Physiol 2019; 10:1294. doi: 10.3389/fphys.2019.01294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ganz T, Nemeth E. Hepcidin and iron homeostasis. Biochem Biophys Acta. 2012;1823:1434–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson G, Frazer D. Current understanding of iron homeostasis. Am J Clin Nutr. 2017;106(suppl):1559S–1566S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Waldvogel-Abramowski S, Waeber G, Gassner G, et al. Physiology of iron metabolism. Transfus Med Hemoth. 2014;41:213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ganz T Anemia of inflammation. N Engl J Med. 2019;381:1148–57. [DOI] [PubMed] [Google Scholar]
- 24.McSorley S, Jones I, McMillan D et al. Quantitative data on the magnitude of the systemic inflammatory response and its relationship with serum measures of iron status. Translational Research 2016; 176:119–126. [DOI] [PubMed] [Google Scholar]
- 25.Peng Y, Uprichard J. Ferritin and iron studies in anemia and chronic disease. Ann Clin Biochem. 2017; 54:43–48. [DOI] [PubMed] [Google Scholar]
- 26.Camaschella C Iron deficiency: new insights into diagnosis and treatment. Hematology Am Soc Hematol Educ Program 2015;2015:8–143. doi: 10.1182/asheducation-2015.1.8. [DOI] [PubMed] [Google Scholar]
- 27.Cappellini M, Musallam K, Taher A. Iron deficiency anemia revisited. J Intern Med. 2020; 287:153–170. [DOI] [PubMed] [Google Scholar]
- 28.Girelli D, Nemetjh E, Swinkels D. Hepcidin in the diagnosis of iron disorders. Blood 2016; 127:2809–2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cappellini M, Comin Colet J, de Francisco A et al. Iron deficiency across chronic inflammatory conditions: international expert opinion on definition, diagnosis and management. Am J Hematol. 2017; 92:1068–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bassuk S, Rifai N, Ridker P. High sensitivity C-reactive protein: clinical importance. Curr Probl Cardiol. 2004;29:439–493. [PubMed] [Google Scholar]
- 31.Munoz m, Botella-Romero F, Gomez-Ramirez S et al. Iron deficiency and anemia in bariatric surgical patients: causes, diagnosis and proper management. Nutr Hosp. 2009; 24:640–654. [PubMed] [Google Scholar]
- 32.Lopez A, Cacoub P, Macdougall I, et al. Iron Deficiency anaemia. Lancet 2016; 387:907–916. [DOI] [PubMed] [Google Scholar]
- 33.von Haehling S, Ebner N, Evertz R et al. Iron deficiency in heart failure. J Am Coll Cardiol Heart Failure. 2019; 7:36–46. [DOI] [PubMed] [Google Scholar]
- 34.Skroubis G, Sakellaropoulos G, Pouggouras K, Mead N, Nikifordis G, Kalfarentzos F. Comparison of nutritional deficiencies after Roux-en-Y gastric bypass and after biliopancreatic diversion with Roux-en-Y gastric bypass. Obes Surg. 2002;12:551–558. [DOI] [PubMed] [Google Scholar]
- 35.Flancbaum L, Belsley S, Drake V, Colarusso T, Tayler E. Preoperative nutritional status of patients undergoing Roux-en-Y gastric bypass for morbid obesity. J Gastrointest Surg. 2006;10(7):1033–1037. [DOI] [PubMed] [Google Scholar]
- 36.Toh S, Zarshenas N, Jorgensen J. Prevalence of nutrient deficiencies in bariatric patients. Nutrition. 2009;25:1150–11516. [DOI] [PubMed] [Google Scholar]
- 37.Ernst B, Thurnheer M, Schmid SM, Schultes B. Evidence for the necessity to systematically assess micronutrient status prior to bariatric Surgery. Obes Surg. 2009;19(1):66–73. [DOI] [PubMed] [Google Scholar]
- 38.Schweiger C, Weiss R, Berry E, Keidar A. Nutritional deficiencies in bariatric surgery candidates. Obes Surg. 2010;20(2):193–197. [DOI] [PubMed] [Google Scholar]
- 39.Lefebvre P, Letois F, Sultan A, Nocca D, Mura T, Galtier F. Nutrient Deficiencies in patients with obesity considering bariatric surgery: a Cross-sectional study. Surg Obes Relat Dis. 2014;10(3):540–546. [DOI] [PubMed] [Google Scholar]
- 40.Benotti PN, Wood GC, Still CD,et al. Metabolic Surgery and iron homeostasis. Obesity Reviews. 2018;1–9. 10.1111/obr.12811 [DOI] [PubMed] [Google Scholar]
- 41.Salgado W, Modotti C, Nonino C et al. Anemia and iron deficiency before and after bariatric surgery. Surg Obes Rel Dis. 2014; 10:49–54 [DOI] [PubMed] [Google Scholar]
- 42.Cappellini M, Musallam K, Taher A. Iron deficiency anaemia revisited. J Intern Med. 2019. October 30. doi: 10.1111/joim.13004. [DOI] [PubMed] [Google Scholar]
- 43.Wood GC, Chu X, Manney C, et al. An electronic health record- enabled obesity database. BMC Med Informatics Dec Mak. 2012;12:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McCracken E, Wood GC, Prichard W, et al. Severe anemia after Roux-en-Y gastric bypass: a cause for concern. Surg Obes Relat Dis. 2018;14:902–909. [DOI] [PubMed] [Google Scholar]
- 45.Cleland S, Thomas W. Iron homeostasis and perioperative managememt of Iron Deficiency. Brit J Anesth. 2019; 19:390–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gudzune K, Huizinga M, Chang H, Asamoah V, Gadgil M, Clark J. Screening and diagnosis of micronutrient deficiencies before and after bariatric surgery. Obes Surg. 2013;23:1581–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parrott J, Frank L, Rabena R, Craggs-Dino L, Isom K, Greiman L. American Society for Metabolic and Bariatric Surgery integrated health guidelines for the surgical weight loss patient 2016 update: micronutrients. Surg Obes Relat Dis. 2017;13:727–741. [DOI] [PubMed] [Google Scholar]
- 48.Skikne B Serum transferrin receptor. Am J Hematol. 2008;83:872–875. [DOI] [PubMed] [Google Scholar]
- 49.Munoz M, Gopmez-Ramirez S, Besser M et al. Current misconceptions in Ddagnosis and management of iron deficiency. Blood Transfus. 2017; 15:422–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Musallam K, Taher A. Iron deficiency beyond erythropoiesis: should we be concerned? Curr Med Res Opin. 2018; 34:81–93 [DOI] [PubMed] [Google Scholar]
- 51.Vaucher P, Druais P, Waldvogel S et al. Effects of iron supplementation on fatigue in non-anemic menstruating women with low ferritin: a randomized controlled trial. CMAJ 2012; 184(11):1247–1254. doi 10.1503/cmaj.110950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arcani R, Suchon P, Venton G et al. Efficacy of intravenous iron therapy in non-anemic patients with fatigue. Netherlands J Med. 2020; 78:34–36. [PubMed] [Google Scholar]
- 53.Hapangama D, Bulmer J. Pathophysiology of heavy menstrual bleeding. Women’s Health 2016; 12(1):3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fraser I, Langham S, Uhi-Hochgraeeber K. Health-related quality of life and economic burden of abnormal uterine bleeding. Expert Review of Obstetrics & Gynecology, vol. 4, no. 2, 2009. [Google Scholar]
- 55.Harrison’s Principles of Internal Medicine, 18e. New York, NY: McGraw-Hill; 2012. April 25, 2017 [Google Scholar]
- 56.https://www.sabm.org/wp-content/uploads/2019/01/2A2-PhysiciansGuideOralIron.pdf. (accessed. March 21, 2020)
- 57.Tolkien Z, Stecher L, Mander A et al. Ferrous sulfate supplementation causes significant gastrointestinal side effects in adults: a systemic review and metra-analysis. Plos One 2015; 10:e0117383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stoffel N, Cercamondi C, Brittenham G et al. Iron absorption from oral Supplements given on Consecutive vs Alternate days and as Single Morning doses vs. twice daily split dosing in oron-depleted women: two open-label randomized controlled trials. Lancet Hematol. 2017; 4:e524–e533. [DOI] [PubMed] [Google Scholar]
- 59.Gomez-Ramirez S, Brilli E, Tarantino G et al. Sucrasomial iron: a new generation iron for improving oral supplementation. Pharmaceuticals 2018; 11, 97; doi 10.3390/ph11040097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Camaschella C Iron-deficiency anemia. New Engl J Med. 2015; 372:1832–1843. [DOI] [PubMed] [Google Scholar]
- 61.Stoffel N, El-Mallah C, Herter-Aeberli I et al. The effect of central obesity on inflammation, hepcidin, and iron metabolism in young women. Int J Obes. 10.1038/s41366-020-0522-x [DOI] [PubMed] [Google Scholar]
- 62.Auerbach M, Deloughery T. Single-dose intravenous Iron for iron deficiency: a new paradigm. Hematology Am Soc Hematol Educ Program 2016. December 2;2916(1):57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mikhail A, Brown C,Williams J. et al. Clinical practice guideline anemia of chronic kidney disease (Renal Association). Renal.org (accessed March 20, 2020).
- 64.Munoz M, Acheson G, Auerbach M et al. International consensus statement on the peri-operative management of anemia and Iron deficiency. Anaesthesia 2017; 72:233–247. [DOI] [PubMed] [Google Scholar]
- 65.Still C, Wood G, Chu X et al. Clinical factors associated with weight loss outcomes after Roux-en-Y gastric bypass Surgery. Obesity 2014; 22:888–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hung K, Chun-Ning H, Chen J et al. Association of preoperative hemoglobin with weight loss after bariatric surgery: a retrospective study. Surg Obese Rel Dis. (2019), doi: 10.1016/j.soard.2019.06.016. [DOI] [PubMed] [Google Scholar]
- 67._.Weiss G, Ganz T, Goodnough L. Anemia of inflammation. Blood 2019; 133:40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Peyrin-Biroulet L, Williet N, Cacoub P. Guidelines on the diagnosis and treatment of iron deficiency across indications: a systematic review. Am J Clin Nut. 2015; 102:1585–1594. [DOI] [PubMed] [Google Scholar]
- 69.Cacoub P, Vanderwalle C, Peoc’h K et al. Using transferrin saturation as a diagnostic criterion for iron deficiency: A systematic review Critical Reviews in Clinical Laboratory Sciences. Taylor & Francis; 2019, pp 526–532. 10.1080/10408363.2019.16538s0. hal-02303354. [DOI] [PubMed] [Google Scholar]
- 70.Dignass A, Farrag K, Stein J. Limitations of serum ferritin in diagnosing iron deficiency in inflammatory conditions. Int J Chr Dis. volume 2018, Article ID 9394060. 10.1155/2018/9394060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schiavo L, Pilone V, Rosetti G et al. Correcting micronutrient deficiencies before sleeve gastrectomy may be useful in preventing early postoperative micronutrient deficiencies. Int J. Vitam Res 2019; 89(1–2):22–28. [DOI] [PubMed] [Google Scholar]


