Abstract
Background
The PROspective Cutaneous Lymphoma International Prognostic Index (PROCLIPI) Study is a prospective analysis of an international database and here we examine front-line treatments and quality-of-life in patients with newly diagnosed Mycosis Fungoides (MF).
Objectives
a) differences in first-line approach according to the TNMB staging; b) parameters related to a first-line systemic approach; c) response rates and quality of life (QoL) measures.
Patients and Methods
395 newly diagnosed patients with early-stage MF (IA-IIA) were recruited from 41 centers in 17 countries between 1/1/2015–31/12/2018 following central clinicopathological review.
Results
First-line therapy was skin directed therapy (SDT) (81.6%) whilst a smaller percentage (44 cases;11.1%) received systemic therapy. Expectant observation was 7.3%. In univariate analysis, the use of systemic therapy was significantly associated with higher clinical stage (IA: 6%; IB: 14%; IIA:20%; IA-IB vs IIA: p<0.0001), presence of plaques (T1a+T2a: 5%; T1b+T2b: 17%; p<0.001), higher mSWAT (>10: 15%; <=10: 7%; p=0.01) and folliculotropic MF (FMF) (24% vs 12%; p=0.001). Multivariate analysis demonstrated significant associations with the presence of plaques (T1b/T2b vs T1a/T2a: OR: 3.07) and FMF (OR: 2.82). The overall response rate (ORR) to first-line SDT was 73% whilst the ORR to first-line systemic treatments was lower (57%) (p=0.027). Health related QoL improved significantly in both patients with responsive and stable disease.
Conclusions
Disease characteristics such as presence of plaques and FMF influence physician treatment choices and that SDT was superior to systemic therapy even in patients with such disease characteristics. Consequently, future treatment guidelines for early-stage MF need to address these issues.
INTRODUCTION
Mycosis fungoides (MF) is characterized by long-standing, scaly, patch lesions preferentially involving the buttocks and body areas infrequently exposed to sunlight (“bathing trunk”) and slow evolution over years from patches to plaques (early-stage) and in some patient to tumors or erythroderma (advanced-stage).1,2
Early-stage MF has a good prognosis (median survival >15 years, 5-year survival >80%)3–5 compared to advanced-stage disease which has a median survival of 4–5 years and a predicted 5-year survival of approximately 50%3–7. A recent meta-analysis reported a 5-year survival of 85.8% for stage IB, 62.2% for IIB, 59.7% for IIA, 54% for IIB, 52.5% for IVA1, 34% for IVA2 and 23.3% for stage IVB8. Moreover, even in early-stage disease, morbidity can be considerable with pain, pruritus, disfigurement and poor quality of life (QoL)9–12. Progression to advanced stages (IIB-IVB) occurs in 20–25% of patients with early-stage disease and is associated with increased mortality3–5, 13.
International treatment guidelines do not recommend any particular order of treatment and there is a lack of specific data to confirm the appropriateness of current guidelines14–19. Furthermore, cross study comparisons have been difficult because of the lack of well-established response criteria which have only been developed relatively recently20.
The PROspective Cutaneous Lymphoma International Prognostic Index (PROCLIPI) database opened in January 2015 to prospectively collect data on international patients with MF and to investigate the disease course and its prognostic factors. The current analysis focuses on the treatments used for early-stage MF. The objectives are to analyze the differences in first-line treatment approach and in particular to compare the patient characteristics according to first-line therapy choice - systemic versus observation versus SDT; the overall response rate (ORR) according to different treatments and stages; the health-related quality of life (HRQoL).
MATERIALS AND METHODS
Study Design & Patients
The PROCLIPI study database has been previously described21. The study was reviewed and approved by local ethics committees/institutional review boards prior to recruitment. Written consent for participation, analysis of data and use of tissue or blood samples for translational research was obtained at study entry. Data cut-off point for this interim analysis was December 2018.
All patients included in the PROCLIPI database that had a diagnosis of “early-stage MF” (stage IA, IB, IIA) based on a central clinicopathological review process to confirm diagnosis and stage were included in the present study21. For each patient clinical, hematological, pathological and treatment data were collected at the time of diagnosis and updated annually or earlier in the event of stage progression or death. HRQoL was captured using the Skindex-29 test as already reported9. Response to treatment was evaluated according to standard consensus guidelines20 The ORR was defined as the proportion of patients with a Complete Response (CR)(100% clearance of skin lesions) and Partial Response (PR) defined as 50% - 99% clearance of skin disease based on the modified Severity Weighted Assessment Tool (mSWAT) score without new tumors in patients with T1,T2, T4 only skin disease, lasting for at least four weeks.
Treatment approaches
Treatment approaches were grouped into two categories after consensus across the participating centers as previously reported6: (1) Skin-directed therapies (SDT): topical corticosteroids, phototherapy (UVA, broad-band UVB, narrow-band-UVB, NB-UVB), local radiotherapy, total-skin electron beam therapy (TSEBT), topical nitrogen mustard, topical carmustine;
(2) Biological response modifiers: interferon (IFN), retinoids, bexarotene, extracorporeal photochemotherapy (ECP), low-dose methotrexate.
Topical corticosteroids were considered as a treatment only if performed as single therapy, whilst not recorded when in association with other treatments since they were prescribed in the majority of patients.
Statistical Analysis
The chi-square test was used to assess the associations categorical variables. Non-parametric continuous variables are presented with their medians and ranges. The Wilcoxon matched pairs signed rank test and the Kruskal-Wallis tests were used to analyse differences in the distributions of continuous variables.
Logistic regression analysis was carried out to investigate predictors of first-line systemic approach. The end-point was first-line systemic approach with respect to SDT and expectant policy. Multivariate analysis included as variables: geographical site (Europe vs outside Europe), gender, age at diagnosis, TNMB stage (stratified as IA-IB vs IIA), T-class (only patches versus plaques: T1a/T2a vs T1b/T2b), FMF, mSWAT. Age and mSWAT were included as continuous variables.
Analyses were performed using STATA SEv15 (StataCorp LP, College Station, Texas, USA).
RESULTS
Patient characteristics
A total of 395 patients were included, recruited from 41 centers in 17 countries (UK, Germany, France, Netherland, Belgium, Spain, Italy, Greece, Finland, Hungary, Switzerland, Austria, Israel, Argentina, Brazil, USA and Australia). European centers accounted for 88% of the patients. The median age at first diagnosis was 57 years (range: 5–97). (Supplementary Table 2).
Stage distribution showed 50% IA and 42% IB whilst stage IIA was represented in 8% of patients. At diagnosis, 49% of patients had only patches (29% T1a and 20% T2a) whilst 51% showed also plaques (24% T1b and 27% T2b). Folliculotropic MF (FMF) was diagnosed in 18% of cases. The majority (79%) had plaque disease (T1b=24, T2b=32), whilst a minority only patches (T1a=7; T2a=8). B1 as B-class22 was found in 30 patients (7.6%): 14 had stage IA, 14 IB and 2 IIA.
The median mSWAT was 10 (range:0.3–120). The mSWAT increased paralleling the T-classification: median values were 4 (range: 0.3–9) for T1a, 6.5 (0.5–24) for T1b, 18 (10–71.5) for T2a up to 34 (12.4–120) for T2b (Kruskal-Wallis test p<0.001). mSWAT values were lower for stage IA (median: 4, range: 0.3–24) whilst similar for stage IB (26; 10–112) and IIA (30; 1.8–120) (Kruskal-Wallis test: IA vs IB-IIA p<0.0001)
The median follow-up is 1.3 years (range: 1 month – 4.7 years).
First-line and subsequent treatment lines
The first-line therapy was SDT in the large majority of patients (n=322; 81.5%), whilst 11.1% (n=44) received a systemic treatment (Table1 and Supplementary Figure1). An expectant policy was initially adopted for 7.3% (n=29); the majority of these patients had stage IA (n=16) or IB (n=10); only 3 stage IIA patients received expectant policy respectively for 3, 4 and 5.5 months after completing diagnostic and staging procedures. 13/29 patients (45%) who initially had expectant policy received a subsequent treatment after a median of 7.5 months (range: 3– 34).
Table 1.
Summary of first treatment approaches in the patient cohort.
| Drug / treatment | No. patients | % | |
|---|---|---|---|
| EXPECTANT POLICY | "wait and see" | 29 | 7.3% |
| SDT | Topical steroids | 155 | 39.2% |
| UVB | 73 | 18.4% | |
| PUVA | 75 | 18.5% | |
| Topical nitrogen mustard | 5 | 1.3% | |
| Topical BiCNU | 2 | 0.5% | |
| Local RT | 12 | 3% | |
| Total SDT | 322 | 81.5% | |
| SYSTEMIC | Phototherapy + IFN and/or retinoids | 16 | 4% |
| ECP | 1 | 0.3% | |
| Oral retinoids | 15 | 3.8% | |
| Oral bexarotene | 4 | 1% | |
| MTX | 4 | 1% | |
| IFN | 4 | 1% | |
| Total systemic | 44 | 11.1% |
SDT= Skin Directed Therapies
UVB= Phototherapy with Ultraviolet B rays
PUVA= Phototherapy with Psoralens plus Ultraviolet A rays
BiCNU= bis-chloroethylnitrosourea, carmustine
RT= Radiotherapy
ECP= Extracorporeal Photochemotherapy
MTX= Methotrexate
IFN= Interferon
The most frequently used SDTs were topical steroids (39.2%) and phototherapy (36.9%; 18.5%=PUVA and 18.4%= UVB,). Topical steroids were more frequently used in stage IA (48%vs32% in IB;chi-square:9.643, p=0.002), whilst phototherapy in IB (47%vs29%;chi-square:12.693,p<0.0001). Steroids were more frequently used than phototherapy in T1a (55%) compared with other T-scores (T1b:39%; T2a:34%; T2b:37%) (chi-square:11.061,p<0.0001) (Supplementary Figure2). Patients with patches only (T1a/T2a) were more likely to receive UVB (22%) than PUVA (13%) whilst patients with plaques were statistically more frequently treated with PUVA (25%vs15% UVB;chi-square:5.098,p=0.024). No patients received TSEBT as first-line therapy.
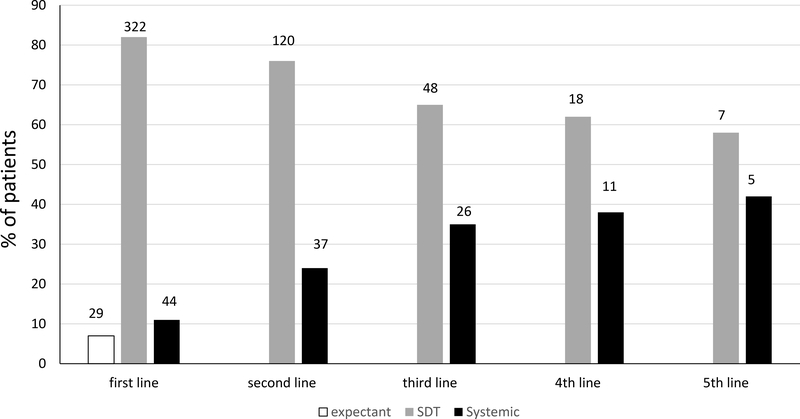
Forty-four patients (11.1%) received systemic therapy as first-line treatment: retinoids (19 patients), IFN-2alpha (n=4), methotrexate (n=4), ECP (n=1); the remaining 16 patients received a combination of phototherapy with oral retinoids and/or interferon. The utilization of systemic treatment increased with the number of treatment lines (Figure1). A systemic treatment was adopted as second-line treatment in 24% of patients, as 3rd line in 35% and as 4th line in 38% of patients (1stvs2nd line; chi-square: 11.188; p<0.001).
Figure 1.
Percentages of patients treated according to a different approach (expectant policy, SDT, systemic) across the therapy lines. Numbers at the top of each bar represent absolute number of patients treated by the respective therapeutical approach.
Parameters associated with a first systemic approach
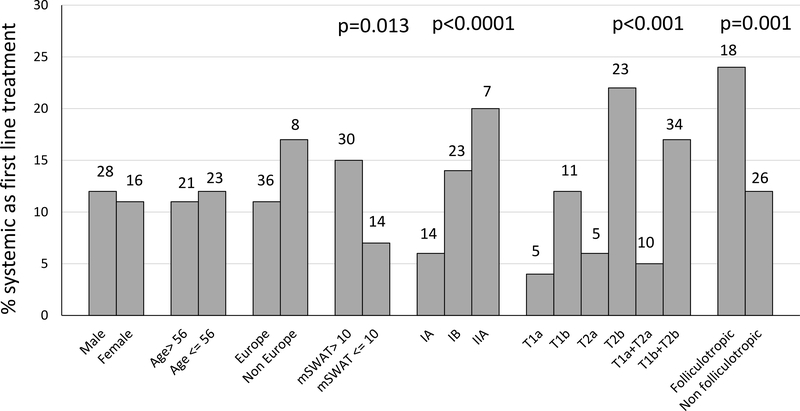
The factors significantly associated with a first-line systemic therapy in univariate analysis were clinical stage (IA: 6%; IB: 14%; IIA: 20%; IA vs IB: chi- square: 4.465;p=0.035; IA-IB vs IIA: chi-square:15.398;p<0.0001); T-classification (T1a+T2a:5%;T1b+T2b: 17%; chi-square:13.159;p<0.001); FMF (24%vs12% in classic MF; chi-square=10.779;p=0.001); higher mSWAT (7% when mSWAT<=10 and 15% with higher values) (chi-square:6.222;p=0.013) (Figure2).
Figure 2.
Clinico-pathologic characteristics associated with first systemic approach. Bars represents percentage values of patients treated with a first systemic approach according to the different clinic-pathologic characteristics. Numbers at the top of each bar represent absolute numbers of patients. P values of parameters with a statistically significant difference are reported at the top of the graph.
No differences were found according to age, gender, duration of MF lesions before diagnosis, B-class, geographical site (17% outside Europevs10% Europe) and low versus high volume centers (less or more than 10 patients; 12.5%vs11.1%).
Parameters associated with a statistically significant increased use of systemic therapy as first-line in multivariate analysis were: presence of plaques (OR:3.07, 95%CI=1.35–6.98) and FMF (OR:2.82, 95% CI=1–5.77) (Table 2). Overall stage (IA–IB–IIA) was not an independent predictor of systemic therapy as first-line therapy.
Table 2.
Multivariate analysis of parameters associated with first systemic approach.
| Variable | Coefficient | Standard error | p | O.R | 95% CI low | 95% CI high |
|---|---|---|---|---|---|---|
| Geographical | 0.7711 | 0.4636 | 0.0962 | 2.1622 | 0.8715 | 5.3643 |
| Age | −0.0011 | 0.0103 | 0.9146 | 0.9989 | 0.9790 | 1.0192 |
| Gender | −0.0219 | 0.3543 | 0.9508 | 0.9784 | 0.4886 | 1.9593 |
| mSWAT | 0.1683 | 0.4283 | 0.6943 | 1.1833 | 0.5111 | 2.7395 |
| TNM stage | 0.4363 | 0.3003 | 0.1463 | 1.5470 | 0.8587 | 2.7871 |
| Plaques | 1.1221 | 0.4186 | 0.0074 | 3.0712 | 1.3521 | 6.9761 |
| FMF | 1.0391 | 0.3641 | 0.0043 | 2.8268 | 1.3846 | 5.7709 |
OR odds ratio
CI Confidence Interval
FMF: Folliculotropic mycosis fungoides
Response rate
CR was achieved in 26% and PR in 41% of patients, accounting for a 67% ORR. Moreover, 31% (n=123) of patients achieved stable disease and only 6 had disease progression during their first-line treatment (Table 3). The ORR decreased with increasing T-class, from 74% for T1a to 61% for T2b (T1avsT2b: chi-square:4.260,p=0.039). Higher mSWAT values and FMF were associated with a trend towards lower ORR without statistical significance. Patients with Stage IIA disease had a significantly lower ORR (39%) compared to IA (73%) and IB (66%) (chi-square:12.788,p<0.001); the Stage IIA patients (n=33) did have a high tumour burden with 19 having stage T2b disease and 22 having an mSWAT greater than 10.
Table 3.
Response to selected SDTs according to the main clinico-pathologic predictors.
| FIRST LINE | ORR | |||||
|---|---|---|---|---|---|---|
| SDT+expectant+ systemic | SDT | Systemic | Topical corticosteroids | UVB | PUVA | |
| Total | 266/ 395 (67%) | 235/322 (73%) | 25/44 (57%) | 106/155 (68%) | 54/73 (74%) | 62/75 (83%) |
| IA | 145/198 (73%) | 131/168 (78%) | 11/14 (79%) | 71/95 (75%) | 26/34 (76%) | 21/23 (91%) |
| IB | 108/164 (66%) | 94/131 (72%) | 11/23 (48%) | 33/53 (62%) | 25/34 (74%) | 36/43 (84%) |
| IIA | 13/33 (39%) | 10/23 (43%) | 3/7 (43%) | 2/7 (29%) | 3/5 (60%) | 5/9 (56%) |
| T1a | 84/113 (74%) | 78/100 (78%) | 4/5 (80%) | 47/62 (76%) | 16/21 (76%) | 7/7 (100%) |
| T2a | 53/80 (66%) | 51/67 (76%) | 1/5 (20%) | 18/27 (67%) | 17/22 (78%) | 16/18 (89%) |
| T1b | 64/96 (66%) | 55/76 (72%) | 8/11 (73%) | 26/37 (70%) | 11/15 (73%) | 13/16 (81%) |
| T2b | 65/106 (61%) | 51/79 (65%) | 12/23 (52%) | 15/29 (52%) | 10/15 (67%) | 26/34 (76%) |
| T1a+T2a | 137/193 (71%) | 129/167 (77%) | 5/10 (50%) | 66/89 (74%) | 33/43 (77%) | 23/25 (92%) |
| T1b+T2b | 129/202 (64%) | 106/155 (68%) | 20/34 (59%) | 41/66 (62%) | 21/30 (70%) | 39/50 (78%) |
| mSWAT>10 | 125/197 (63%) | 107/155 (69%) | 15/30 (50%) | 31/54 (57%) | 29/41 (71%) | 46/57 (81%) |
| mSWAT <=10 | 141/198 (71%) | 128/167 (77%) | 10/14 (71%) | 75/101 (74%) | 25/32 (78%) | 16/18 (89%) |
| FMF | 43/71 (60%) | 32/49 (65%) | 9/18 (50%) | 16/21 (76%) | 2/5 (40) | 11/17 (65%) |
| Not FMF | 223/324 (69%) | 203/273 (74%) | 16/26 (62%) | 90/134 (69%) | 52/68 (76%) | 51/58 (88%) |
ORR: overall response rate
SDT: Skin-directed therapies
FMF: Folliculotropic Mycosis Fungoides
The ORR to first-line therapy was 73% for SDT and 57% for systemic treatments (chi-square:4.915,p=0.027) (Table 3). Indeed, the ORR of systemic treatments was similar or even lower than SDT in patients even with those with adverse prognostic factors such as higher stage, presence of plaques, FMF and higher mSWAT.
Among SDTs, phototherapy was associated with slightly higher ORR (UVB 77%, PUVA 83%) compared to topical steroids (70%). Lower ORR for topical steroids were particularly relevant for stage IIA (ORR: 29%) (chi-square:5.375,p=0.020vsIA-IB) and T2b patients (ORR: 52%) (chi-square:4.581,p=0.032 with vs other T-classes).
First-line treatment is ongoing in 39% of patients. In the remaining, reasons for stopping were complete remission (21%), completion of the treatment schedule (17%), inadequate or no response (11%), worsening disease and/or stage progression (2%), toxicity (3%) or other reason (7%).
Stage progression and treatment
Stage progression occurred in 39 patients (18-stage IA, 14=IB and 7=IIA), 22 of whom progressed to an advanced-stage (13 stage IIB, 5 stage III, 4 stage IVA1). Thirty-one progressed patients had plaques (T1b/T2b) (chi-square:13.881;p<0.001) (OR 4.19; 95% CI=1.8–9.3), 9/39 FMF (23%) and 24/39 >10 mSWAT (61%). B1 at initial diagnosis was found in 3 progressed patients, 2 of whom progressed to stage IIIB and one to stage IVA1. The median time to stage progression from diagnosis was 13 months (1–41 months). (Supplementary Figure 3).
First-line treatment was SDT in 29 and systemic therapies in 7 patients (3 had a wait and see policy): 22/39 (56%) progressed did not respond to first-line treatment (chi-square:11.072;p=0.001)(OR 3.012; 95%CI= 1.5–5.9).
HRQoL
Skindex-29 data were available at diagnosis in 121 patients. A second evaluation was retrieved in 56 of them after a median of 13 months (range: 1–43). The selection of patients for HRQoL tended to be on the basis of the participating centre rather than the particular patient characteristics.
The first-line treatment in these latter patients was expectant policy (n=10), SDT (n=43) and systemic therapy (n=3) achieving 9 CR, 20 PR, 25 SD and 2 PD.
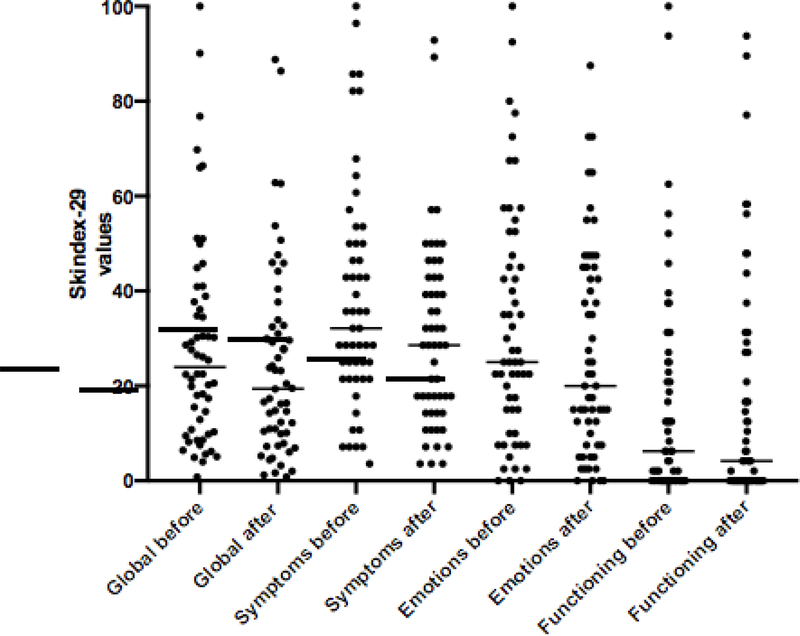
At baseline the median global Skindex-29 score was 23.95 (95%CI=18.3– 30.2); 18 patients (32%) had values exceeding 32 (moderate impairment)23 and 11 (20%) exceeding 44 (severe).
A statistically significant reduction in the median global Skindex-29 score was found between the first and the second evaluation (19.41, 95% CI: 14.29–27.62) (p=0.006). The reduction was confirmed for the subscales symptoms (p=0.003) and emotions (p=0.008) but not for functioning (p=0.926) (Figure 3). The reduction in the global Skindex-29 occurred not only in responding (Wilcoxon paired signed ranked test p=0.05) but also in SD patients (p= 0.024).
Figure 3.
HRQoL Global Skindex before and after treatment
DISCUSSION
This study reports treatment data on 395 patients with confirmed early-stage MF prospectively enrolled into the PROCLIPI database. This is the largest prospective series of patients with early-stage MF reported in terms of treatment data and outcomes.
The first major conclusion is that the first therapy was SDT in most patients (81.5%), although a minority received systemic therapy as their first therapy (usually retinoids or IFN +/−phototherapy). Although we recognize that a physician’s decision to choose a therapy may be influenced by external factors other than direct disease-related factors (i.e.regulatory status and health insurance reimbursement), we have focused our analyses on the clinical parameters related to ‘real-life’ decision-making. (data collection didn’t include a ‘reason’ to choose one therapy over another). Features associated with selecting systemic treatment first-line were clinical stage (20% of stage IIA patients), presence of plaques (17% of patients with plaques T1b/T2b), FMF (24%), and higher mSWAT (15% in patients with values>10). By multivariate analysis, T-classification and FMF remained independent factors. Among SDTs, stage and T-score both modifiedthe treatment decision. Topical steroids were more frequently used for patch-stage disease and limited cutaneous involvement (stage IA and T1a), whilst phototherapy was selected for limited plaque-disease (T1b) or extended skin involvement (T2). PUVA was preferred in plaque disease (22%vs15% UVB) whilst UVB was used mainly for patch MF (22%vs13% PUVA). This real-life scenario reflects European and US guidelines14,15, 17,19,24 which recommend NB-UVB for patch MF and PUVA for plaque disease, given the UVA potential to penetrate deeper into the dermis than UVB. Moreover, NCCN 19, ESMO15 and USCLC 24 guidelines consider NB-UVB indicated for patients with patch/flat plaque while PUVA for thick plaques or FMF. Plaque stage patients treated by UVB may have had a preponderance of thin/flat plaques. Moreover, the use of UVB could also be due to the lack of availability of PUVA in some centers.
The second main observation was that the ORR to first-line therapy was relatively high (67%) but the CR rate was low (26%). However, maximum responses may not have been achieved given that a substantial proportion (39%) of patients are still receiving therapy.Moreover, patients with stage IIA, T2b score and, to a lesser extent, FMF and high mSWAT (which are also the patient group more commonly receiving front-line systemic treatment), showed lower ORR, similar to responses in advanced-stage MF6,27. Notably, these specific features which have the potential to result in a different clinical course are not captured by the classic TNMB staging system (in which the presence of patches vs. plaques does not modify the overall stage). Of interest, skin plaques (T1b/T2b) also appear to predict a high risk to progression to advanced-stage disease.
Another important observation is that the ORR for systemic therapies (57%) was significantly lower than SDTs (73%). Moreover, a lower ORR to SDTwas also observed in patients with adverse prognostic factors such as higher stage, FMF and higher T scores which was the subgroup most likely to receive a first line systemic; for example, the ORR in T2b was 65% with SDTs and 52% with systemic therapies. It is important to recognize that some of these patients may have received SDT prior to their diagnosis of MF as early-stage MF is often misdiagnosed as eczema or psoriasis and there can be a substantial delay in confirming a diagnosis of MF. This has been demonstrated in previous PROCLIPI reports21 and also confirmed in the present analysis at a median of 32months. Nonetheless, given that the ORR of systemic treatments was similar or even lower than SDT (even in those with adverse prognostic factors), our data suggests that it is generally preferable to initiate therapy with SDT in most cases. We acknowledge that the inferior ORR with systemic therapy is likely to be due to the pre-selection of early-stage patients with more aggressive disease characteristics not captured by TNMB and this emphasizes the need for more effective treatments and better clinical markers beyond TNMB to predict the variation in clinical outcomes. For example, the treatment strategy for MF patients with high-risk features could be improved through the development of combination strategies or new drugs such as brentuximab vedotin and mogamulizumab earlier in the treatment of MF28,29.
FMF is generally poorly responsive to first-line SDTs and may run a more aggressive course30–32. Recent studies from Hodak et al.33 and the Dutch group34 showed that FMF present with 2 distinct patterns, the early (follicle-based patch/flat plaques) and the advanced (follicle-based infiltrated plaques and/or tumors). The good prognosis of early-stage FMF implies that these patients should benefit from SDT 32–35. In our study, 18% of early stage MF had FMF and these patients were more likely to receive systemic first-line therapies. It is conceivable that some of these FMF cases had infiltrated rather than thin plaques, thus representing advanced stage FMF33–35.
We have shown that the majority of early-stage MF patients have persistent skin lesions after their first-line treatment (CR 26%) which could potentially impact on their QoL. Our results indicate that half of the patients with early-stage disease (52%) suffer from a moderate to severe QoL reduction, in agreement with our recent report from the PROCLIPI database9. The reduction of Skindex-29 and thus the improvement in HRQoL, demonstrate the positive impact of treatment even if a minority of patients had only 2 time-points available for analysis. Finally, the finding of improved Skindex-29 scores in SD patients is in concordance with previous data showing that improved HRQoL scores were observed in patients despite the lack of an objective response36. This supports the need to incorporate HRQoL as part of standard patient evaluation and response criteria becoming a 5th compartment (TNMBQ). Consequently, we may find that patients with SD patients who have an improved HRQoL could be objectively identified as obtaining a clinical benefit despite failing to achieve a formal response.
In conclusion, this PROCLIPI study reports that real-life treatment decisions by clinicians for early-stage MF are not only based on stage, but also take into account presence of plaques, FMF disease and mSWAT; treatment outcomes such as ORR and progression to higher stages are adversely affected by these factors. Our study also highlights that the early use of systemic therapy does not achieve better outcomes than SDT and the importance of incorporating QoL into assessments of treatment activity. Potential limitations are short follow-up time (median: 1.3 years), the low number of patients with HRQoL data available and the relatively lower number of patients included in centers outside Europe thus limiting the capacity to extend the conclusions to geographical areas. The ongoing enrollment in PROCLIPI will allow subsequent analyses to involve a larger patient cohort with longer follow-up. Overall, this study strongly supports that the current “early-stage” grouping is too simplistic and next-generation management guidelines need to be developed incorporating predictive high-risk features to drive treatment decisions.
Supplementary Material
Supplementary figure 1: Summary of first-line therapies. Numbers represent absolute number of patients treated by each therapy
Supplementary figure 2. Topical steroids and phototherapy according to stage and T score
Supplementary figure 3. Disease-stage progression curve (included only patients who developed a disease progression)
What’s already known about this topic?
Early-stage Mycosis Fungoides is characterised by a good prognosis. The first-line treatment approach is typically stage-based and usually skin-directed therapy
What does this study add?
This multi-center prospective international study reports that real life treatment decisions are not limited to a stage-based approach but also influenced by the presence of plaques and folliculotropic MF disease. Approximately half the patients with early-stage disease experienced a moderate or severe impact on their quality of life at diagnosis. This study suggests that treatment guidelines in patients with early stage disease should incorporate high-risk features and quality of life evaluation.
Acknowledgement
We would like to acknowledge all the Centers who included early stage patients who passed Central Pathology Review (listed as authors) and all the Centers who participate in PROCLIPI (listed in Supplementary Table 1).
Funding: This work was supported by
Cancer Research UK (50763/A18021; J.J.J.)
European Academy Dermatology Venerology (2014-23; J.J.J.)
Spatz Foundation; Sundown Endowment Legacy (Y.K.)
Krebsliga Schweiz (KFS-4243-08-2017; E.G.); Promedica Stiftung 1406/M and 1412/M (E. G.)
FIS PI17/00957 (P.O.R.)
NIH/NCI Cancer Center Support Grant (P30CA033572) to the City of Hope, NIH/NCI grant (R01 CA229510-01) and Leukemia Lymphoma Society Clinical Scholar Award (CDP-14110-18) (C.Q.)
Footnotes
Conflict of Interest Disclosures
SW: consultant/advisory/honoraria: Galderma
JS: honoraria: Therakos, 4SC, Millennium/Takeda, Kiowa-Kirin, Innate Pharma, Actelion, Helsinn-Recordati
RK: consultant/advisory/honoraria: Therakos, 4SC, Millennium/Takeda
PQ: consultant/advisory/honoraria: Therakos, 4SC, Millennium/Takeda, Actelion, Kiowa-Kirin, Innate Pharma, Helsinn-Recordati
HMP: consultant/advisory/honoraria: Millennium/Takeda, Celgene, Eisai
RW: consultant/advisory: Millennium/Takeda; honoraria: Millennium/Takeda, Actelion.
RS: consultant/advisory/honoraria: Millennium/Takeda, 4SC
MV: consultant/advisory: Kiowa, Innate
POR: consultant: Recordati
EG: consultant: Helsinn-Recordati
REFERENCES
- 1.Willemze R, Cerroni L, Kempf W et al. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood 2019; 133:1703–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pimpinelli N, Olsen EA, Santucci M et al. Defining early mycosis fungoides. J Am Acad Dermatol 2005; 53:1053–63. [DOI] [PubMed] [Google Scholar]
- 3.Agar NS, Wedgeworth E, Crichton S et al. Survival outcomes and prognostic factors in mycosis fungoides/Sézary syndrome: validation of the revised International Society for Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer staging proposal. J Clin Oncol 2010;28:4730–9. [DOI] [PubMed] [Google Scholar]
- 4.Quaglino P, Pimpinelli N, Berti E et al. Time course, clinical pathways, and long-term hazards risk trends of disease progression in patients with classic mycosis fungoides: a multicenter, retrospective follow-up study from the Italian Group of Cutaneous Lymphomas. Cancer 2012; 118:5830–9. [DOI] [PubMed] [Google Scholar]
- 5.Talpur R, Singh L, Daulat S et al. Long-term outcomes of 1,263 patients with mycosis fungoides and Sézary syndrome from 1982 to 2009. Clin Cancer Res 2012;18:5051–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quaglino P, Maule M, Prince HM et al. Global patterns of care in advanced stage mycosis fungoides/Sezary syndrome: a multicenter retrospective follow-up study from the Cutaneous Lymphoma International Consortium. Ann Oncol 2017; 28:2517–2525. [DOI] [PubMed] [Google Scholar]
- 7.Scarisbrick JJ, Prince HM, Vermeer MH, et al. Cutaneous Lymphoma International Consortium Study of Outcome in Advanced Stages of Mycosis Fungoides and Sézary Syndrome: Effect of Specific Prognostic Markers on Survival and Development of a Prognostic Model. J Clin Oncol 2015; 33:3766–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mourad A, Gniadecki R. Overall Survival in Mycosis Fungoides: A Systematic Review and Meta-Analysis. J Invest Dermatol 2019; 19: 33145–8. [DOI] [PubMed] [Google Scholar]
- 9.Molloy K, Jonak C, Woei-A-Jin FJSH et al. Characteristics associated with significantly worse quality of life in mycosis fungoides/Sézary syndrome from the Prospective Cutaneous Lymphoma International Prognostic Index (PROCLIPI) study. Br J Dermatol 2019. May 2 doi: 10.1111/bjd.18089. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 10.Demierre MF, Gan S, Jones J et al. Significant impact of cutaneous T-cell lymphoma on patients’ quality of life: results of a 2005 National Cutaneous Lymphoma Foundation Survey. Cancer 2006; 107:2504–11. [DOI] [PubMed] [Google Scholar]
- 11.Wright A, Wijeratne A, Hung T et al. Prevalence and Severity of Pruritus and Quality of Life in Patients With Cutaneous T-Cell Lymphoma. J Pain Symptom Manage 2013; 45:114–9. [DOI] [PubMed] [Google Scholar]
- 12.Porkert S, Lehner-Baumgartner E, Valencak J et al. Patients’ Illness Perception as a Tool to Improve Individual Disease Management in Primary Cutaneous Lymphomas. Acta Derm Venereol 2018; 98:240–245. [DOI] [PubMed] [Google Scholar]
- 13.Wernham AG, Shah F, Amel-Kashipaz R et al. Stage I mycosis fungoides: frequent association with a favourable prognosis but disease progression and disease specific mortality may occur. Br J Dermatol 2015; 173:1295–7, 2015. [DOI] [PubMed] [Google Scholar]
- 14.Trautinger F, Eder J, Assaf C et al. European Organisation for Research and Treatment of Cancer consensus recommendations for the treatment of mycosis fungoides/Sézary syndrome - Update 2017. Eur J Cancer 2017; 77:57–74. [DOI] [PubMed] [Google Scholar]
- 15.Willemze R, Hodak E, Zinzani PL et al. ESMO Guidelines Committee. Primary cutaneous lymphomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018; 29: iv30–iv40. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Bagot M. Updates in cutaneous lymphoma: evidence-based guidelines for the management of cutaneous lymphoma 2018. Br J Dermatol 2019; 180: 443–444. [DOI] [PubMed] [Google Scholar]
- 17.Gilson D, Whittaker SJ, Child FJ et al. British Association of Dermatologists and U.K. Cutaneous Lymphoma Group guidelines for the management of primary cutaneous lymphomas 2018. Br J Dermatol 2019; 180:496–526. [DOI] [PubMed] [Google Scholar]
- 18.Horwitz SM, Olsen EA, Duvic M et al. Review of the treatment of mycosis fungoides and Sézary syndrome: a stage-based approach. J Natl Compr Canc Netw 2008; 6:436–42. [DOI] [PubMed] [Google Scholar]
- 19.National Comprehensive Cancer Network: https://www.nccn.org/store/login/login.aspx?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/primary_cutaneous.pdf. Version 2.2019, December 2018. [DOI] [PubMed]
- 20.Olsen EA, Whittaker S, Kim YH et al. Clinical end points and response criteria in mycosis fungoides and sezary syndrome: a consensus statement of the international society for cutaneous lymphomas, the United States cutaneous lymphoma consortium, and the cutaneous lymphoma task force of the European organisation for research and treatment of cancer. J Clin Oncol 2011; 29:2598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scarisbrick JJ, Quaglino P, Prince HM et al. The PROCLIPI international registry of early-stage mycosis fungoides identifies substantial diagnostic delay in most patients. Br J Dermatol 2019; 181:350–357. [DOI] [PubMed] [Google Scholar]
- 22.Scarisbrick JJ, Hodak E, Bagot M et al. Developments in the understanding of blood involvement and stage in mycosis fungoides/Sezary syndrome. Eur J Cancer 2018;101:278–280. [DOI] [PubMed] [Google Scholar]
- 23.Prinsen CA, Lindeboom R, de Korte J. Interpretation of Skindex-29 scores: cutoffs for mild, moderate, and severe impairment of health-related quality of life. J Invest Dermatol 2011;131:1945–7. [DOI] [PubMed] [Google Scholar]
- 24.Olsen EA, Hodak E, Anderson T et al. Guidelines for phototherapy of mycosis fungoides and Sézary syndrome: A consensus statement of the United States Cutaneous Lymphoma Consortium. J Am Acad Dermatol 2016;74:27–58. [DOI] [PubMed] [Google Scholar]
- 25.Nikolaou V, Sachlas A, Papadavid E et al. Phototherapy as a first-line treatment for early-stage mycosis fungoides: The results of a large retrospective analysis. Photodermatol Photoimmunol Photomed 2018;34:307–313. [DOI] [PubMed] [Google Scholar]
- 26.Phan K, Ramachandran V, Fassihi H et al. Comparison of Narrowband UV-B With Psoralen-UV-A Phototherapy for Patients With Early-Stage Mycosis Fungoides: A Systematic Review and Meta-analysis. JAMA Dermatol 2019; 155:335–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hughes CF, Khot A, McCormack C et al. Lack of durable disease control with chemotherapy for mycosis fungoides and Sézary syndrome: a comparative study of systemic therapy. Blood 2015; 125:71–81. [DOI] [PubMed] [Google Scholar]
- 28.Stadler R, Otte HG, Luger T et al. Prospective randomized multicenter clinical trial on the use of interferon −2a plus acitretin versus interferon −2a plus PUVA in patients with cutaneous T-cell lymphoma stages I and II. Blood 1998; 92:3578–81. [PubMed] [Google Scholar]
- 29.Prince HM, Querfeld C. Integrating novel systemic therapies for the treatment of mycosis fungoides and Sézary syndrome. Best Pract Res Clin Haematol 2018; 31:322–335. [DOI] [PubMed] [Google Scholar]
- 30.van Doorn R, Scheffer E, Willemze R. Follicular mycosis fungoides, a distinct disease entity with or without associated follicular mucinosis: a clinicopathologic and follow-up study of 51 patients. Arch Dermatol 2002; 138:191–8. [DOI] [PubMed] [Google Scholar]
- 31.Gerami P, Rosen S, Kuzel T et al. Folliculotropic mycosis fungoides: an aggressive variant of cutaneous T-cell lymphoma. Arch Dermatol 2008; 144:738–46. [DOI] [PubMed] [Google Scholar]
- 32.Wieser I, Wang C, Alberti-Violetti S et al. Clinical characteristics, risk factors and long-term outcome of 114 patients with folliculotropic mycosis fungoides. Arch Dermatol Res 2017; 309:453–459. [DOI] [PubMed] [Google Scholar]
- 33.Hodak E, Amitay-Laish I, Atzmony L et al. New insights into folliculotropic mycosis fungoides (FMF): A single-center experience. J Am Acad Dermatol 2016; 75:347–55. [DOI] [PubMed] [Google Scholar]
- 34.van Santen S, van Doorn R, Neelis KJ et al. Recommendations for treatment in folliculotropic mycosis fungoides: report of the Dutch Cutaneous Lymphoma Group. Br J Dermatol 2017; 177: 223–228. [DOI] [PubMed] [Google Scholar]
- 35.Amitay-Laish I, Prag-Naveh H, Dalal A et al. Treatment of early folliculotropic Mycosis Fungoides with special focus on psoralen plus Ultraviolet-A. Acta Derm Venereol 2018; 98: 951–955. [DOI] [PubMed] [Google Scholar]
- 36.Jonak C, Porkert S, Oerlemans S et al. Health-related Quality of Life in Cutaneous Lymphomas: Past, Present and Future. Acta Derm Venereol 2019; 99: 640–646. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figure 1: Summary of first-line therapies. Numbers represent absolute number of patients treated by each therapy
Supplementary figure 2. Topical steroids and phototherapy according to stage and T score
Supplementary figure 3. Disease-stage progression curve (included only patients who developed a disease progression)