Abstract
Background and Objective
Type 1 diabetes is a disease characterized by lifelong insulin administration to compensate for the autoimmune destruction of insulin-producing pancreatic beta-cells. Optimal insulin dosing presents a challenge for individuals with type 1 diabetes, as the amount of insulin needed for optimal blood glucose control depends on each subject’s varying needs. In this context, physical activity represents one of the main factors altering insulin requirements and complicating treatment decisions. This work aims to develop and test in simulation a datadriven method to automatically incorporate physical activity into daily treatment decisions to optimize mealtime glycemic control in individuals with type 1 diabetes.
Methods
We leveraged glucose, insulin, meal and physical activity data collected from twenty-three individuals to develop a method that (i) tracks and quantifies the accumulated glycemic impact from daily physical activity in real-time,(ii) extracts an individualized routine physical activity profile, and (iii) adjusts insulin doses according to the prolonged changes in insulin needs due to deviations in daily physical activity in a personalized manner. We used the data replay simulation framework developed at the University of Virginia to “re-simulate” the clinical data and estimate the performances of the new decision support system for physical activity informed insulin dosing against standard insulin dosing. The paired t-test is used to compare the performances of dosing methods with p <0.05 as the significance threshold.
Results
Simulation results show that, compared with standard dosing, the proposed physical-activity informed insulin dosing could result in significantly less time spent in hypoglycemia (15.3±8% vs. 11.1±4%, p=0.007) and higher time spent in the target glycemic range (66.1±11.7% vs. 69.6±12.2%, p < 0.01) and no significant difference in the time spent above the target range(26.6±1.4 vs. 27.4±0.1, p=0.5).
Conclusions
Integrating daily physical activity, as measured by the step count, into insulin dose calculations has the potential to improve blood glucose control in daily life with type 1 diabetes.
Keywords: activity on board, diabetes, physical activity, insulin dosing
1. Introduction
In health, the human body’s blood glucose (BG) regulation is accomplished via various feedback mechanisms that govern the secretion and action of insulin – the main BG lowering hormone. The destruction of insulin-secreting beta cells in type 1 diabetes (T1D) results in the break-down of the endogenous BG regulation1. Consequently, exogenous insulin injections and careful BG monitoring are required to maintain glycemic levels within a target range (generally 70-180 mg/dL) and avoid potentially severe complications2. Carbohydrate intake increases glucose levels and is required to be matched by insulin injection for proper BG control. In standard therapy, the amount of insulin required at mealtime is broken down into two components: the amount required to compensate for the carbohydrates ingested during the meal, and the amount required to correct for any current elevated BG level. Additionally, people with T1D also need to consider the previously injected insulin still in circulation when calculating the total dose to be administered. The prevalent method used to calculate the required dosage of insulin can be explicitly formalized as follows3:
| (1) |
where CHO is the amount of meal carbohydrates (g), CR is a person’s carbohydrate-to-insulin ratio (g/U) used to determine the appropriate dose of insulin that compensates for the estimated increase in BG from the ingested CHO, Gtarget is the target BG value (mg/dL), CF is the BG correction factor (mg/dL/U) to account for BG excursions away from this target, and G is the BG value at the time of the meal bolus (mg/dL). Since insulin affects BG concentrations for several hours following its injection4, the active insulin in circulation from the previous insulin injections is tracked by a concept called insulin on board (IOB). IOB is computed as the convolution of insulin injections within the past four-hours and insulin action curve obtained from a previous study by Swan et al.4.
Insulin needs vary among people with T1D, and hence the therapy is tailored to the individual through patient-specific treatment parameters (e.g., CR and CF). The treatment parameters can also be adjusted to account for systematic diurnal variations in BG dynamics. Deviation from these patterns, however, require additional care. Existing literature on physical activity (PA) related BG control in T1D can be classified into two categories based on their methodologies: (a) studies based on dose-response experiments, that explore the BG responses to various insulin and carbohydrate doses surrounding an exercise bout5-7, and (b) studies based on algorithms that use biosensors to take/suggest actions for an ongoing exercise bout8-10. Conversely, non-exercise PA has seen little attention, and its effects on BG metabolism have been presumed to be minor. However, recent studies show that even short bouts of walking in the course of otherwise sedentary days significantly affect glucose metabolism11-13, and thus require treatment adjustments to improve overall BG control14-16. In the present work, we propose a method that extends the mealtime insulin bolus calculation to account for the accumulated prolonged glycemic impact of the daily PA
2. Materials and Methods
In the development of the PA informed insulin dosing method, we utilize retrospective data collected from individuals with T1D under their free-living conditions. We quantify PA through “step count” recorded via an off-the-shelf PA tracker and inform the insulin dosing by (i) a quantified accumulated glycemic impact of prior PA in real-time (ii) a PA profile extracted from retrospective data, that is representative of systematic glycemic disturbances resulting from the individual’s routine PA, and (iii) a PA correction parameter that accounts for the altered insulin needs due to deviations from the routine PA profile.
2.1. Tracking and quantifying the prolonged glycemic impact of prior PA in real-time
The quantification of the glycemic impact of PA performed prior to the point of assessmentis inspired by the “insulin on board” concept17 and is conceived analogously as “activity on board” (AOB). The AOB is designed as a quantitative representation of the previously performed PA that is still affecting the BG levels and is calculated as follows:
| (2) |
where the “s” components maintain a historical record of step-count assessed as sums over non-overlapping 5-minute time-intervals (aligning with common CGM sampling intervals), and the AOBcurve is a one-dimensional vector of weights corresponding to the theorized time-decay dynamics of the previously performed PA’s glycemic impact, assuming that the effect of PA over time is additive. The values and length of the AOBcurve are chosen based on the findings from a previous study that investigated changes in the glucose uptake following a structured bout of PA18. These changes were shown to persist for up to 13.5 hours, and the AOB encompasses all the PA within this time frame via the s vector. The weighting components of the AOBcurve are obtained by:
| (3) |
where kPA is the time index of the performed PA, ΔkPA is the discrete-time difference passed after the PA is performed, and ΔGU(η) is the PA-induced increase in glucose uptake at stage η (adapted from 18), demonstrated in Figure 1A.
Figure 1—
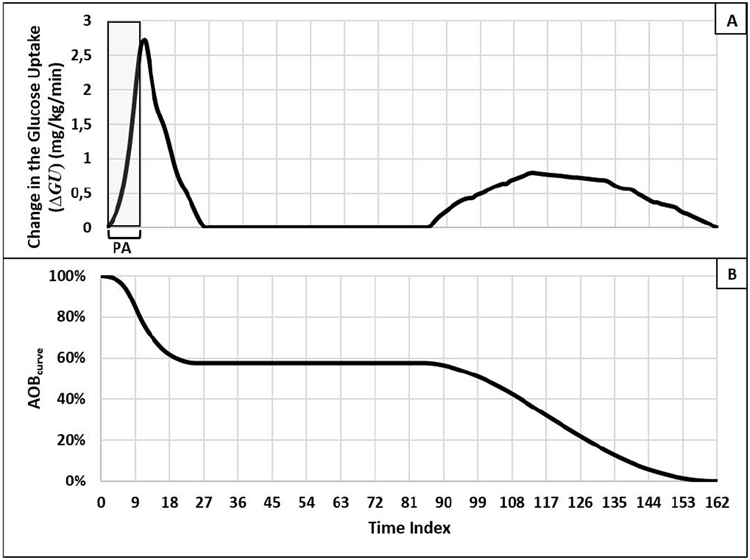
(A) Observed average change in glucose uptake, as a result of a 45-minutes (nine five-minute time intervals) bout of moderate-intensity PA extracted from18 and (B) AOBcurve representing the evolution of the percent of glycemic impact left from the performed PA -obtained from (A) using Equation (4). The x-axis is time index in both panels (A) and (B), depicting 13.5 hours in 162 5-minute intervals.
2.2. Extracting a patient-specific mealtime AOB profile
We define a patient-specific mealtime AOB profile that captures the systematic glycemic impact from routinely performed PA. The AOB profile is obtained for meals that are often consumed approximately around the same time every day (e.g., breakfast, lunch, dinner). The fundamental insight behind the formation of the “AOB profile” is that the systematic glycemic impact from routine daily PA is already accounted for in the standard daily treatment design in T1D. Because an individual’s routine PA is likely to be within an interval rather than a set value, the AOB profile consists of an upper and a lower bound. These bounds form an interval of systematically accumulated glycemic impact from the daily PA at the time of the selected meal. We compute these bounds using meal and PA data collected for three to four weeks from individuals with T1D under free-living conditions as follows:
| (4) |
| (5) |
where τhigh,m and τlow,m are the upper and lower bounds of the AOB profile for the chosen meal m with km,d being the time index that the meal m is eaten on day d, nd is the total number of days in the dataset. As a result, AOB(km,d) is the AOB observed at the time of meal m on day d. Finally, MAD is the median absolute deviation.
2.3. PA informed mealtime insulin dose calculations
Significant deviations from the routine PA likely alter the insulin needs of an individual with T1D due to PA’s effect on BG dynamics. To compensate for the altered insulin needs, we augment the standard bolus formula with a PA related correction as follows:
| (6) |
where the standard bolus B is corrected based on the deviation between AOB at the time of the meal bolus, AOB(km,d), and the corresponding bound of the AOB profile. When the AOB (km,d) exceeds the upper bound, τhigh,m , we decrease the insulin dose to compensate for the glucose-lowering effects of the excess PA relative to the routine amount. Likewise, in the case that the AOB(km,d) is below the lower bound, τlow,m, we increase insulin dose to compensate for the reduced glucose uptake due to the subject’s lower than usual accumulated PA. Any AOB(km,d) within the bounds of the AOB profile is considered within the routine daily PA range and no PA related correction is applied. The magnitude of any PA-related correction depends on the magnitude of the deviation from routine PA and the patient-specific parameters AF1 and AF2 are called activity factors. These activity factors translate the anticipated glycemic impact of deviations from the routine PA into insulin unit equivalents in terms of the expected impact on the BG. Due to the lack of concise biological information on the relationship between the rates of change in the insulin needs in response to either an increase or a decrease in the performed PA, we search for an optimal pair of activity factors without any assumed connection between these parameters.
2.3. Optimization of Activity Factors
We determine the values of AF1 and AF2 for each individual by assessing the BG excursions following dinners across days in their dataset in order to capture the impact from a wide range of PA accumulated throughout the day. To accomplish this, we label dinners, denoted by ρ, in our dataset through a time and meal-size based dinner detection algorithm. Then, the glycemic risk associated with the post-dinner BG behavior is assessed according to the well-recognized glucose control metrics19, known as low blood glucose index (LBGI) and high blood glucose index (HBGI) and described below:
| (8) |
| (9) |
where d represents the day, G(k) is the BG reading at time k, kρ,d is the time of the dinner that is also the start time of the analysis, klast,d is the time of the last BG reading in the postmeal analysis window, both on day d, and rl and rh are the mapping functions that translate the G(k) into the risk space with the details provided in a previous work by Kovatchev et al.20.
The AF1 and AF2 are obtained as a pair that minimizes the total glycemic risk as evaluated on the BG traces simulated with “replayed” insulin boluses with PA correction, using the following cost function:
| (10) |
2.4. In silico testing
We test, in silico, PA informed insulin dosing method’s ability to improve BG control on postmeal BG excursions on a retrospective dataset. To capture the prolonged glycemic impact from all the PA performed throughout the day, dinner meals are selected for the testing.
2.4.1. Data:
We use retrospective data collected from 29 individuals with T1D in two clinical studies conducted at the University of Virginia (clinicaltrials.gov: NCT02558491, NCT03394352). Participants with T1D on insulin pump therapy with the age range 15-65 years, HbA1c range 6-10% were enrolled. Both studies had the same free-living data collection period lasting approximately one month during which the participants followed their regular therapy for a month while wearing a CGM G4 Platinum (Dexcom Inc., San Diego, CA) and a wristband PA tracker (Fitbit Charge HR and HR2, Fitbit Inc., San Francisco CA). Participants recorded consumed carbohydrates grams (meals, snacks, and hypoglycemia treatment) via the pump bolus wizard. Data from all devices were downloaded at regular intervals during the study period. The free-living data from these trials were employed in the analyses of this manuscript after the following pre-processing procedure:
Data relevant to a meal was rejected from analysis if more than 24 CGM values were missing between 4:30 pm and 10 pm or if no insulinized meal was present in this interval. When there was a CGM gap that did not violate this criterion, we replaced the missing values by linear interpolation of the closest available CGM data.
PA data (i.e., heart rate and step count) had to be available the morning (6 am to noon), afternoon (noon to 5 pm), and evening (5 pm to 10 pm), as defined by no more than 2-hour missing data in any of these three intervals.
Participants with less than 15 days of valid data were rejected from the analysis.
2.4.2. Simulations:
The treatment parameters used in daily life are often sub-optimal due to the absence of formal optimization tools in current clinical practices. Therefore, as a simulation setup, we first rebalance the main meal bolus parameter (i.e., CR) to study the effect of PA isolated from carbohydrates. In principle, the CR in the standard therapy is designed to provide a CHO /insulin ratio that compensates for the expected BG increase from the carbohydrates in a meal. By using a PA informed cost function in the optimization of CR, we separate the compensation for the PA from the one for the carbohydrates. For this purpose, we use the extracted AOB profile for each individual such that:
When the AOB(kρ,d) is higher than the τhigh,ρ, we expect lower than usual BG excursion due to the increased glucose uptake associated with elevated PA. Thus, we down-weight the LBGI proportional to the deviation from routine PA.
When the AOB(kρ,d) is lower than the τlow,ρ, we expect higher than usual BG excursion due to the decreased glucose uptake associated with lower than usual PA. Thus, we down-weight the HBGI proportional to the deviation from routine PA.
When AOB (kρ,d is within [τlow,ρ, τhigh,ρ], the performed PA is within the limits of the individual’s routine PA and the CR is expected to provide optimum BG control without needing a PA correction. Thus, we assign the same weights to both LBGI and HBGI in the cost function.
These insights yield the following cost function:
| (11) |
| (12) |
where α ∈ [0,1] is an empirically chosen regularization term, RiskCR* is calculated as a weighted sum of the glycemic risk indices associated with meal m, LBGI and HBGI, across available days in the dataset obtained from an individual. The weights are calculated relative to the corresponding bound of the individual’s AOB profile and the AOB value at the time of the meal for each day.
We utilize the net effect simulation procedure to “replay” the post-dinner BG traces yielded by different CR* values, hence, mealtime insulin doses21. Through numerical optimization, CR* is obtained as the value that minimizes the RiskCR*.
Using the extracted AOB profiles, we obtained CR*, AF1 and AF2 for each patient by analyzing the BG excursions associated with the dinner meals. Using these parameters, we re-simulated the post-dinner BG traces with insulin doses calculated according to PA informed bolus at dinnertimes (and for any subsequent meal until 1 am). For comparison, we also re-simulated these BG traces with insulin doses calculated according to the standard dosing. Optimum parameters were obtained using Isqnonlin in Matlab with the search range of [1000, 25000] for activity factors and [70%*CR,130%*CR] for CR*. As a safety saturation, we limit the PA related dose adjustment to the ±50% of the meal component of the bolus (i.e., CHO/CR*). The target BG was set to 110 mg/dl in all simulations. We compared the BG control performances of the PA informed vs. standard dosing methods based on the percentage time spent in hypoglycemia (< 70 mg/dl), target glycemic range (70-180 mg/dl), time above 180 mg/dL, time above 250 mg/dL, and the mean BG in the analysis window. The paired t-test was used for comparison with the p-value threshold of p<0.05.
3. Results
Representative case
To provide a comparable example while evaluating the performances of standard vs. PA-informed boluses, we select two days that belong to the same participant and satisfy the following criteria:
similar BG traces that do not exceed the target BG at the dinnertime
same amounts of carbohydrate intake at the dinnertime,
no residual insulin from previous boluses at the dinner time (i.e., at least four hours have passed since the last bolus insulin),
same amounts of insulin injection at the dinnertime,
different PA behavior prior to the dinnertime.
Figure 2 presents the selected days for comparison. The AOB profile bounds at the dinnertime for this subject were calculated as τlow,ρ = 1922 and τhigh,ρ = 3078. Following the standard therapy boluses on these days, the subject experienced hypoglycemia (i.e., BG<70 mg/dL) on day #13 (AOB(kρ,13 =7734) while being exposed to hyperglycemia (i.e., BG>180 mg/dL) in response to the same treatment under similar conditions on day #15 (AOB(kρ,15)=3743).
Figure 2—
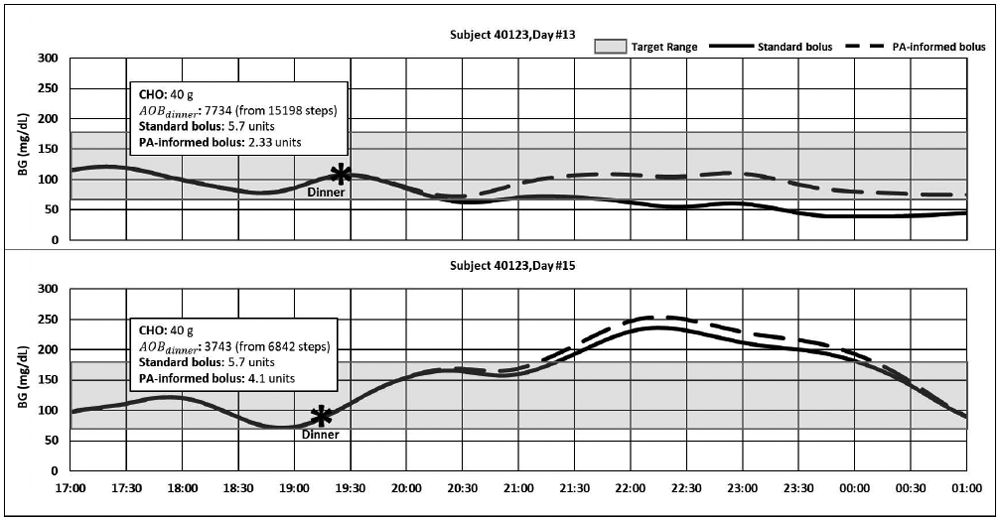
Comparison of BG traces resulting from the standard vs. PA informed dinner boluses (standard bolus parameters: CR = 7g/unit; PA informed bolus parameters: CR* = 8.6 g/unit AF1 = 1171 and AF2 = 2657).
On day #13, the PA informed insulin dosing method adjusted the dinner bolus for the impact of the excess AOB. The time spent in hypoglycemia decreased from 77.9 % to 0%, eliminating 4.3 hours of hypoglycemia exposure, and increasing the total time spent in the target range from 22.1% to 100%.
On day #15, the AOB (kρ,15) ρ was slightly higher than the τhigh,ρ. Time spent in hypoglycemia remained unchanged as 0% for both methods. A decrease of 1.4% was observed in the time spent in the target range (from 48.5% to 47.1%) due to a 25-minute increase in exposure to hyperglycemia resulting from the PA correction in the bolus.
Neither of the presented days had insulin on board at the time of dinner, indicating that the hypoglycemia on day #13 was not due to higher insulin in the circulation compared to day #15. While the AOB(kρ,d) was higher than τhigh,ρ on both days, it was more than twice of τhigh,ρ on day #13.
Population results
Out of 29 subjects, six subjects were excluded from the analysis for having less than 15 valid days in their dataset. Each participant had a unique PA behavior, and personalized PA informed treatment parameters were obtained for each of them. There was no statistically significant difference between CR and CR* (CR=10.6±3.3 and CR* =11.3±4.5, p=0.1). In the remaining dataset of 23 subjects, no optimum AF pair was found within the search range for four subjects. Other two subjects did not have an optimum AF1 while another subject did not have an optimum AF2 value. These subjects were still included in the analyses using their CR* for PA informed boluses, but no additional PA correction was performed when AOBρ was outside of their corresponding AOB profiles.
Overall, dinner boluses on 651 days from 23 subjects were replayed with standard vs. PA informed boluses. Resulting post-dinner BG traces until 1am were compared and reported as mean ± std hereafter. As Table 1 presents, the observed average time spent in hypoglycemia reduced significantly with PA informed boluses (p=0.007) with no significant increase in time above the target range (p=0.5 for >180 mg/dL, p=0.87 for >250 mg/dL) and mean BG (p=0.1). No significant increase was observed in the mean BG (140.8±20.3 vs. 145.4±13.3 mg/dL, p=0.1). Finally, there was no significant difference between the average insulin bolus administered per subject (p=0.45).
Table 1.
Paired t-test results for average CGM and bolus insulin outcomes by bolusing method
| Standard Bolus |
PA-informed Bolus |
p-value | |
|---|---|---|---|
| % time of CGM < 70 mg/dL | 15.3±8 | 11.1±4 | p=0.007 |
| % time of CGM 70-180 mg/dL | 66.1±11.7 | 69.6±12.2 | p<0.01 |
| % time of CGM > 180 mg/dL | 26.6±1.4 | 27.4±0.1 | p=0.5 |
| % time of CGM > 250 mg/dL | 7.2±0.3 | 7.3±0.3 | p=0.87 |
| Mean CGM (mg/dL) | 140.8±20.3 | 145.4±13.3 | p=0.1 |
| Total bolus insulin administered | 10.1±4.2 | 9.9±4.4 | p=0.45 |
4. Discussion
Our results indicate a significantly improved glycemic control using the PA-informed bolus when compared to the standard bolus method. This improvement was achieved by reducing exposure to hypoglycemia and increasing the time spent in the target BG range. Note that in our simulations, no additional carbohydrate intake or basal insulin dose reduction was applied to treat hypoglycemia events as opposed to the real-life practice. This potentially resulted in higher than clinically expected hypoglycemia outcomes in the results for both bolusing methods. The PA-related increases (informed by low PA) and decreases (informed by high PA) in insulin boluses approximately cancel each other when evaluated across simulation days per each subject. Hence, the average insulin use per subject in our method does not significantly differ from the standard method, while the glycemic control performance is substantially improved.Using representative case day #15 (figure 2 lower panel) we can observe a key challenge of PA-related insulin bolus adjustments: potential for increased postprandial hyperglycemia. We hypothesize that this increase could be partly due the different pharmacodynamics of insulin and PA. More specifically, in this study, PA informed boluses were adjusted for the entire glycemic impact of PA (~13.5 hours), while the influence of insulin on BG levels lasts four to six hours4. Such temporal difference could result in overcompensation of physical activity.. Similarly, previously reported potential changes in the circulating insulin levels during and after PA in individuals with T1D may affect the outcomes in real-life applications of the developed method 22,23. Additionally, PA adjustments for the meals earlier in the day as well as for prospective PA were not assessed in this work. Furthermore, profile and optimal treatment parameters were estimated from a rich but rather short (approximately 3 −4 weeks per participants) database, which could lead to sub-optimal estimates considering the innate variability in T1D glycemic control. On a similar note, due to the limited amount of data per participant, we used all available data for both optimization and performance evaluation.
Another limitation of the presented method is the lack of specificity to the type and intensity of PA. Unfortunately currently available information is limited about the prolonged glycemic impact of PA performed at different times, intensities, durations, and metabolic states. Therefore, we assumed a similar time-decay in the glycemic impact of daily PA, ranging from slow walks to running and for all durations. As different intensity of PA may lead to different glucose uptake profiles (e.g., immediate increase in the BG followed by a precipitous decrease for anaerobic PA) which may differ both in timing and amplitude of the effect, adding PA intensity as a part of the method can widen its domain of use. Future work may explore the use of different AOB curves, nonlinear forms of convolution, and other PA indicators (e.g., heart rate), including their combinations that can also take the intensity of the PA into account.
Nonetheless, PA-related treatment adjustments in T1D have long been proven necessary, and there exist algorithms for optimum dose corrections surrounding an exercise bout8-10. In a previous proof-of-concept in silico study, we have shown that our method can be used to correct the insulin dose following an exercise bout24. In this work, we offer a more holistic perspective on the topic of PA-related adjustments in T1D treatment by automatically adjusting mealtime insulin doses for the overall daily PA, instead of practical guidelines focusing on a structured exercise bout only25. While the range of PA that affects the insulin requirements is broader11-13,16, to our knowledge, no other formal methods exist to adapt insulin doses to the daily PA-induced changes in the insulin needs of individuals with T1D. By leveraging readily available step count information from a wearable PA tracker, our method bridges this gap with the potential to improve daily BG control. For exercise-specific insulin adjustment version of our method that also integrates prospective exerciseestimations in a closed-loop control framework is presented in our previous work10. The novelties of our personalized PA informed insulin bolusing method lie in (1) the introduction of a technique that quantifies PA in a way that factors in its glycemic impact, (2) the inclusion of all PA (exercise and non-exercise) in insulin dose adjustments, and (3) the consideration of the prolonged impact of PA in these adjustments. Our results indicate that accounting for the glycemic impact of prior PA in insulin treatment can improve BG control in individuals with T1D. Although the presented in silico experiment was conducted in an open-loop BG control setting, the proposed method can also be implemented in a closed-loop BG control system.
Highlights.
Step-count data from wearable physical activity trackers is leveraged to track and quantify daily physical activity.
An approach to calculate the accumulated glycemic impact from prior physical activity is presented.
A physical activity informed mealtime insulin bolus calculator is developed.
Simulation results suggest that the proposed physical activity informed insulin dosing could result in significantly improved postprandial glucose control in individuals with type 1 diabetes, compared with the standard dosing.
Acknowledgements including declarations:
B.O has no conflict of interest to disclose. S.D.P is employed by Dexcom, Inc. whose sensors were used in NCT02558491, NCT03394352; SDP reports royalties from IP licenses in this field, managed by the University of Virginia. C.F reports consulting fees from Epsilon (Abbott). MDB reports research support from Dexcom, Sanofi, and Tandem Diabetes Care; MDB reports consulting fees and honoraria from Air Liquide, Dexcom, and Tandem Diabetes Care; MDB reports royalties from IP licenses in this field, managed by the University of Virginia. Funding: NIH NIDDK DP3DK106826.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Registry numbers of clinical trials of the data used in the analyses of this manuscript: clinicaltrials.gov: NCT02558491, NCT03394352
REFERENCES
- 1.Aronoff SL, Berkowitz K, Shreiner B & Want L Glucose Metabolism and Regulation: Beyond Insulin and Glucagon. Diabetes Spectr. 17, 183–190 (2004). [Google Scholar]
- 2.Nathan DM Long-Term Complications of Diabetes Mellitus. N. Engl. J. Med 328, 1676–1685 (1993). [DOI] [PubMed] [Google Scholar]
- 3.Walsh J, Roberts R & Bailey T Guidelines for Optimal Bolus Calculator Settings in Adults. J. Diabetes Sci. Technol 5, 129–135 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swan KL et al. Effect of Age of Infusion Site and Type of Rapid-Acting Analog on Pharmacodynamic Parameters of Insulin Boluses in Youth With Type 1 Diabetes Receiving Insulin Pump Therapy. Diabetes Care 32, 240–244 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reducing Basal Insulin 90 Minutes before Exercise Protects Against Hypoglycemia Better than Insulin Suspension at Exercise Onset in T1D—The OmniTIME Results ∣ Diabetes. http://diabetes.diabetesjournals.org/content/67/Supplement_1/65-OR.abstract. [Google Scholar]
- 6.Grimm J, Ybarra J, Berne C, Muchnick S & Golay A A new table for prevention of hypoglycaemia during physical activity in type 1 diabetic patients. Diabetes Metab. 30, 465–470 (2004). [DOI] [PubMed] [Google Scholar]
- 7.McCarthy O, Bain SC & Deere R Basal insulin reductions in anticipation of multiple exercise sessions in people with type 1 diabetes—a clinical perspective. Ann. Transl. Med 6, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adding Heart Rate Signal to a Control-to-Range Artificial Pancreas System Improves the Protection Against Hypoglycemia During Exercise in Type 1 Diabetes ∣ Diabetes Technology & Therapeutics. https://www.liebertpub.com/doi/full/10.1089/dia.2013.0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turksoy K et al. Classification of Physical Activity: Information to Artificial Pancreas Control Systems in Real Time. J. Diabetes Sci. Technol 9, 1200–1207 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia-Tirado J, Colmegna P, Corbett J, Ozaslan B & Breton MD Ensemble Model Predictive Control Strategies Can Reduce Exercise Hypoglycemia in Type 1 Diabetes: In Silico Studies. in 2019 American Control Conference (ACC) 4752–4758 (2019). doi: 10.23919/ACC.2019.8814728. [DOI] [Google Scholar]
- 11.Peddie MC et al. Breaking prolonged sitting reduces postprandial glycemia in healthy, normal-weight adults: a randomized crossover trial. Am. J. Clin. Nutr 98, 358–366 (2013). [DOI] [PubMed] [Google Scholar]
- 12.Bailey DP & Locke CD Breaking up prolonged sitting with light-intensity walking improves postprandial glycemia, but breaking up sitting with standing does not. J. Sci. Med. Sport 18, 294–298 (2015). [DOI] [PubMed] [Google Scholar]
- 13.Manohar C et al. The Effect of Walking on Postprandial Glycemic Excursion in Patients With Type 1 Diabetes and Healthy People. Diabetes Care 35, 2493–2499 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robertson K, Adolfsson P, Riddell MC, Scheiner G & Hanas R Exercise in children and adolescents with diabetes. Pediatr. Diabetes 9, 65–77 (2008). [DOI] [PubMed] [Google Scholar]
- 15.Colberg SR et al. Physical Activity/Exercise and Diabetes: A Position Statement of the American Diabetes Association. Diabetes Care 39, 2065–2079 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ozaslan B, Patek SD & Breton MD Impact of Daily Physical Activity as Measured by Commonly Available Wearables on Meal Time Glucose Control in Type 1 Diabetes. Diabetes Technol. Ther (2020) doi: 10.1089/dia.2019.0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campbell R & Abramovich A Calculating insulin on board for extended bolus being delivered by an insulin delivery device. (2012). [Google Scholar]
- 18.McMahon SK et al. Glucose Requirements to Maintain Euglycemia after Moderate-Intensity Afternoon Exercise in Adolescents with Type 1 Diabetes Are Increased in a Biphasic Manner. J. Clin. Endocrinol. Metab 92, 963–968 (2007). [DOI] [PubMed] [Google Scholar]
- 19.Metrics for glycaemic control — from HbA 1c to continuous glucose monitoring ∣ Nature Reviews Endocrinology. https://www.nature.com/articles/nrendo.20173. [DOI] [PubMed] [Google Scholar]
- 20.Kovatchev BP, Straume M, Cox DJ & Farhy LS Risk Analysis of Blood Glucose Data: A Quantitative Approach to Optimizing the Control of Insulin Dependent Diabetes. Computational and Mathematical Methods in Medicine https://www.hindawi.com/journals/cmmm/2000/208936/abs/ (2000) doi: 10.1080/10273660008833060. [DOI] [Google Scholar]
- 21.Patek SD et al. Empirical Representation of Blood Glucose Variability in a Compartmental Model in Prediction Methods for Blood Glucose Concentration: Design, Use and Evaluation (eds. Kirchsteiger H, Jørgensen JB, Renard E & del Re L) 133–157 (Springer International Publishing, 2016). doi: 10.1007/978-3-319-25913-0_8. [DOI] [Google Scholar]
- 22.Mallad A et al. Exercise effects on postprandial glucose metabolism in type 1 diabetes: a triple-tracer approach. Am. J. Physiol.-Endocrinol. Metab 308, E1106–E1115 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McAuley SA et al. Insulin pump basal adjustment for exercise in type 1 diabetes: a randomised crossover study. Diabetologia 59, 1636–1644 (2016). [DOI] [PubMed] [Google Scholar]
- 24.Fabris C, Ozaslan B & Breton MD Continuous Glucose Monitors and Activity Trackers to Inform Insulin Dosing in Type 1 Diabetes: The University of Virginia Contribution. Sensors 19, 5386 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riddell MC et al. Exercise management in type 1 diabetes: a consensus statement. Lancet Diabetes Endocrinol. 5, 377–390 (2017). [DOI] [PubMed] [Google Scholar]


