Abstract
Background:
Few studies investigated whether late preterm infants might have developmental delays in several domains in early life and how stable the lag in developmental status might be.
Aim:
We aimed to examine the stability of potential delays across developmental domains at 24 and 36 months of age in late preterm (340-366 weeks) and term (≥37 weeks) children and whether the risk of delays remained high at 36 months.
Study Design, Subjects, and Outcome Measure:
We conducted a prospective cohort analysis of the children of pregnant women participating in the Vitamin Antenatal Asthma Reduction Trial (VDAART). 652 children who were prospectively followed up and had parent-completed Ages Stages Questionnaires (ASQ-3) questionnaires at both 24 and 36 months were analyzed to assess their domain-specific developmental status.
Results:
6.61% (42/635) of children had a late preterm birth. Developmental delays were stable between 24 and 36 months on all 5 domains for the children born preterm and on 4/5 domains for those born at term. The developmental domains with the status stability at 24 and 36 months in both late preterm and term children were the gross motor, communication, personal-social skills, and problem-solving. Late preterm children compared with term children remained at higher risk of delays at 36 months for gross motor, communication, and problem-solving skills (aOR=4.54, 95%CI: 1.81–10.79; aOR=8.60, 95%CI: 3.10–23.28 and aOR=3.80, 95%CI: 1.58–8.73, respectively).
Conclusion:
Late preterm birth is associated with suboptimal development and stability in several domains at both 24 and 36 months and compared with term birth, requiring early monitoring and assessment of the developmental lag to avoid potential long-term implications.
Keywords: late preterm birth, neurodevelopment delay, early life, Ages Stages Questionnaires
1. Introduction
Preterm birth (gestational age at delivery <37 weeks) occurs in approximately 12% of all live births in the US and prematurity is the leading cause of neonatal morbidity and mortality in children younger than 5 years worldwide (American College of, Gynecologists, & Committee on Practice, 2012; Blencowe, et al., 2012). The incidence and survival rates of preterm births have increased, due to the advancement of scientific knowledge along with technology in obstetrics and neonatology, which allow preterm infants to survive at earlier gestational ages (Shapiro-Mendoza & Lackritz, 2012). Preterm neonates who survive are at greater risk of several short-term and long-term morbidities. 25–50% of children born preterm are predisposed to developmental delays (de Kieviet, Piek, Aarnoudse-Moens, & Oosterlaan, 2009; Shah, Kaciroti, Richards, Oh, & Lumeng, 2016) such that developmental impairment rather than survival is now recognized as the main problem in preterm infants (Institute of Medicine (US) Committee on Understanding Premature Birth and Assuring Healthy Outcomes; Behrman RE; Pierrat, et al., 2017; Quigley, et al., 2012). Late preterm infants (340/7-366/7 weeks of gestation) account for about 75% of all preterm births (Arpino, et al., 2010). The studies of preterm birth and developmental impairments mostly investigated very early and moderate preterm infants (<34 weeks) (Allotey, et al., 2018; Halbwachs, et al., 2014; Hornman, de Winter, Kerstjens, Bos, & Reijneveld, 2017). Similar to early preterm infants, the late-preterm infants might be at higher risk of morbidity and developmental disabilities than term infants (gestational age ≥37 weeks) due to their relative physiologic and metabolic immaturity (“ACOG Committee Opinion No 579: Definition of term pregnancy,” 2013; Barfield WD). The rates of short and long-term neurodevelopmental problems and their domain specificity in late preterm infants an in comparison to term infants are less certain. In a Norwegian prospective study, the proportion of children with communication impairments at age 18 months with persistent problems at age 36 months, assessed by the Ages and Stages Questionnaire (ASQ), was reported 21% and 32% in term-born and late-preterm children, respectively (Stene-Larsen, et al., 2014).
Few studies have investigated the population of late preterm infants to address two important questions (i) whether they have a common specific developmental delay in early life or the risk for developmental delays might be global and (ii) how stable the lag in developmental status is in early life? Furthermore, several factors related to developmental delays may moderate the risk associated with preterm infants’ developmental delays. Are there modifiable risk factors that could reduce the risk of potential delays independently of late preterm birth? Improvement of our knowledge regarding these questions could support early screening and timely professional assessment, monitoring, and targeted interventions, if needed, to prevent later disabilities (Agarwal, et al., 2017; Schonhaut, Armijo, Schonstedt, Alvarez, & Cordero, 2013; Simard, Luu, & Gosselin, 2012).
Parental questionnaires have shown feasibility and efficacy in screening and screen and longitudinally assessing a child’s development. (Blaggan, et al., 2014; Squires & Bricker, 2009) The Ages and Stages Questionnaire (ASQ-3rd edition) is the most commonly used parent-completed developmental screening tool worldwide, and it is accepted by the American Academy of Pediatrics as a valid tool in both term and preterm infants as an effective early life developmental screening tool for children at risk of long-term developmental impairments (Agarwal, et al., 2017; Council on Children With, Section on Developmental Behavioral, Bright Futures Steering, & Medical Home Initiatives for Children With Special Needs Project Advisory, 2006; Kerstjens, et al., 2015; Schonhaut, et al., 2013; Simard, et al., 2012; Steenis, Verhoeven, Hessen, & van Baar, 2015). It has been suggested that a developmental co-occurrence pattern might begin at a very early age (Roberts, et al., 2018). Using data from the Vitamin Antenatal Asthma Reduction Trial (VDAART) and parental-reported ASQ-3 score, we first examined the frequency and stability of developmental delay status across five developmental domains at 24 and 36 months among children born late preterm (340/7 to 366/7weeks of gestation) and term (≥37 weeks of gestation). Thereafter, we investigated whether the risk of developmental delays at 24 months across developmental domains in late preterm children remained high at 36 months relative to those born at term and whether a priori risk factors for developmental impairments may differ across the domains and the study time points.
2. Methods
2.1. Participants
This study was an ancillary study of VDAART, a randomized, double-blind, placebo-controlled clinical trial of daily vitamin D supplementation (4,400 International Units) versus placebo (400 IU) initiated at 10–18 weeks of gestation in pregnant women to prevent the development of pregnancy complications and asthma or atopy in their children by age of 3 years. Details of the trial design and the protocol are published (Litonjua, et al., 2014). Overall, 806 mother-offspring pairs were included in the VDAART intent-to-treat analysis for the primary outcome of the trial (Litonjua, et al., 2016). For this secondary analysis, we included those offspring who had completed ASQ-3 questionnaires during their follow-ups and had ASQ assessment at both 24 and 36 months. Among these, offspring with preterm birth before 34 weeks of gestation (early preterm birth) were excluded. All mothers who participated in the study provided written informed consent. The VDAART protocol and ancillary studies were approved by the institutional review boards at each participating institution and the Brigham and Women’s Hospital (ClinicalTrials.gov #NCT00920621)..
2.2. Ages and Stages Questionnaire and Primary Outcome
ASQ-3 was used to determine the developmental level of children through parent report. At the 24 and 36 months of age, study researchers administered the age-specific questionnaires to parents or primary caregivers during a study clinic visit or phone interview (Squires & Bricker, 2009). The ASQ-3 includes 30 questions regarding a child’s development divided into five domains; gross motor skills, fine motor skills, problem-solving ability, communication, and personal and social skills. Each developmental domain is assessed by 6 questions ascertaining the achievement of relevant skills and answered as ‘yes’ (10 points), ‘sometimes’ (5 points), or ‘not yet’ (0 points). Screening scores for individual items are summed to give a total score for each of the five domains with a range of 0–60. If all total domain scores are above a predetermined age-specific monitoring cutoff, a child’s development is considered to be on schedule (typical development). An ASQ-3 domain score below a priori recommended cut-offs (obtained from a large sample of US children) in one or more areas indicates potential atypical development or developmental delay in that domain (suboptimal): 1) child needs monitoring on developmental advancement (scores between 1 and 2 standard deviations below mean performance in each developmental area; or 2) child needs a referral for further assessment by specialist and potentially an early intervention (scores 2 standard deviations below mean performance in each developmental area) (Squires & Bricker, 2009). Accordingly, we used a dichotomized variable for each domain indicating children with typical development vs those at a risk range for developmental delay (suboptimal). i.e., with a need for monitoring or further assessment (i.e., potential atypical or delayed development) as the primary outcome for this prospective cohort analysis (Squires & Bricker, 2009). The chronological age of infants born at ≤366/7 weeks was corrected by subtracting the weeks remaining to complete 400/7 weeks.
2.3. Assessment of the Main and Additional Study Variables
The primary risk factor of interest was live birth with late preterm birth (340/7 to 366/7weeks of gestation) compared to live birth at term (≥37 weeks of gestation) among the pregnant women participating in the VDAART. A priori covariates known or reported to be risk factors for a child’s developmental impairment and potential confounders of the association between preterm delivery and child’s development status were obtained from the study questionnaires and data records. Maternal variables included the number of prior pregnancies (none or ≥1), maternal age, gestational diabetes during pregnancy, study center, marital status, and maternal educational status, preeclampsia during pregnancy, maternal asthma and maternal plasma vitamin D (25-hydroxyvitamin D [25OHD], ng/mL) level at 32–38 weeks of gestation, measured per trial protocol. (Litonjua, et al., 2014) Offspring’s variables included child’s race and child sex, child’s birth weight, and child’s breastfeeding during the first six months of life. Breastfeeding was assessed by maternal questionnaire 6 months after birth.
2.4. Statistical Analysis
Study group subjects’ characteristics (late preterm and term birth) were compared using a Student’s t-test or Chi-square test, as deemed appropriate. In each study group, the differences in paired proportions of children with an ASQ developmental score below vs above the normally recommended binary cutoff in each developmental domain were compared at 24 months and 36 months using McNemar’s test. Multivariable logistic regression models with adjustment for potential confounders were used to examine the relationship of preterm birth with suboptimal developmental status for each ASQ developmental domain at 24 and 36 months. Due to the high correlation between preterm birth and low birth weight (r=0.7, Table 1), birth weight was not included in the multivariable model to avoid collinearity and the effect on the risk estimates. Adjusted odds ratios (aOR), 95% confidence intervals (CI), and corresponding P values were calculated using a logistic regression model. R version 3.5 was used for all analyses (R Foundation for Statistical Computing). All tests were 2-sided, and the significance level was pre-specified at P<0.05. Odds ratio with a raw P-value smaller than its Holm’s-corrected critical P-value at each study time point were considered statistically significant (Holm S).
Table 1.
Characteristics of mothers and infants in the VDAART cohort with ASQ-3 assessment at both 24 and 36 months (N=635)
| Birth Status | ||
|---|---|---|
| Late Preterm (N=42) | Term (N=593) | |
| Gestational age at delivery (weeks)* | ||
| Mean (SD) | 35.72 (0.82) | 39.29 (1.11) |
| Range | 34.0–36.6 | 37.0–42.3 |
| Maternal age at 10–18 weeks of gestation | ||
| Mean (SD) | 28.37 (5.52) | 27.51 (5.52) |
| <30 years old | 25 (59.52) | 376 (63.4) |
| ≥30 years old | 17 (40.48) | 217 (36.6) |
| Clinical center | ||
| Boston | 13 (30.95) | 193 (32.55) |
| San Diego | 6 (14.29) | 152 (25.63) |
| St. Louis | 23 (54.76) | 248 (41.82) |
| Gestational diabetes | ||
| Yes | 4 (9.52) | 32 (5.40) |
| No | 38 (90.48) | 560 (94.44) |
| Child’s Race | ||
| African American | 22 (52.38) | 281 (47.40) |
| Non-African American | ||
| White | 15 (35.71) | 202 (34.06) |
| Asian | 3 (7.14) | 39 (6.58) |
| Native Hawaiian | 0 (0.00) | 5 (0.84) |
| Other | 2 (4.76) | 66 (11.12) |
| Maternal education completed | ||
| Less than college | ||
| less than high school | 5 (11.90) | 76 (12.82) |
| high school, technical school | 10 (23.81) | 171 (28.84) |
| some college | 9 (21.43) | 131 (22.10) |
| Above college | ||
| college graduate /graduate school | 18 (42.86) | 215 (36.26) |
| Income | ||
| <50,000$/y | 16 (38.10) | 245 (41.32) |
| ≥50,000$/y | 16 (38.10) | 207 (34.91) |
| Unknown or refused | 10 (23.81) | 141 (23.78) |
| Child’s Sex | ||
| Male | 21 (50.00) | 320 (53.96) |
| Female | 21 (50.00) | 273 (46.04) |
| Delivery mode | ||
| Cesarean | 14 (33.33) | 174 (29.34) |
| Vaginal | 28 (66.67) | 418 (70.49) |
| Missing | - | 1 (0.17) |
| Child’s breast feeding during first 6 month | ||
| Yes | 22 (52.38) | 325 (54.81) |
| No | 15 (35.71) | 235 (39.63) |
| Missing | 5 (11.90) | 33 (5.56) |
| Child’s birth weight (kilogram)*& | ||
| Mean (SD) | 2.62 (0.38) | 3.36 (0.46) |
| <2.5 | 15 (35.71) | 21 (3.54) |
| ≥2.5 | 27 (64.29) | 571 (96.29) |
| Missing | - | 1 (0.17) |
| Maternal marital status | ||
| Married | 19 (45.24) | 276 (46.54) |
| Not married or divorced | 23 (54.76) | 317 (53.46) |
| Maternal asthma | ||
| Yes | 14 (33.33) | 239 (40.30) |
| No | 28 (66.67) | 354 (59.70) |
| Preeclampsia* | ||
| Yes | 9 (21.42) | 42 (7.08) |
| No | 33 (78.57) | 551 (92.92) |
| Number of prior pregnancies | ||
| 0 (1st) | 15 (35.71) | 207 (34.91) |
| >1 | 27 (64.29) | 386 (65.09) |
| Maternal 25OHD at 32–38 weeks of | ||
| gestation (ng/mL) | ||
| Mean (SD)** | 28.13 (14.08) | 33.55 (14.82) |
| Sufficient (≥30 ng/mL) | 18 (42.86) | 327 (55.14) |
| Insufficient (<30 ng/mL) | 20 (47.62) | 254 (42.83) |
| Missing | 4 (9.52) | 12 (2.02) |
| Trial arm | ||
| Intervention (4400 IU vitamin D) | 25 (59.52) | 297 (50.08) |
| Placebo (400 IU vitamin D) | 17 (40.48) | 296 (49.92) |
| ASQ-3 score at child’s 2nd year, mean (SD) | ||
| Gross motor skills* | 51.55 (10.15) | 56.2 (6.60) |
| Fine motor skills | 50.0 (9.17) | 51.10 (7.37) |
| Communication | 51.31 (11.95) | 54.06 (7.80) |
| Personal and social skills | 51.43 (10.49) | 52.36 (8.96) |
| Problem solving skills | 50.95 (10.37) | 50.91 (9.80) |
| ASQ-3 score at 3rd year, mean (SD) | ||
| Gross motor skills* | 51.79(11.68) | 56.45 (6.11) |
| Fine motor skills | 40.60 (18.42) | 44.15(14.70) |
| Communication* | 50.60 (10.37) | 53.75 (7.27) |
| Personal and social skills | 52.74 (8.78) | 54.49 (7.41) |
| Problem solving skills | 50.48 (11.47) | 53.0 (9.38) |
ASQ-3: Ages and Stages Questionnaire, 3rd Edition; ASQ-3 score ranges from 0 to 60 across each domain; higher score indicates better performance.
VDAART: Vitamin Antenatal Asthma Reduction Trial
Data are given as number (percentage) of individuals, unless otherwise specified. SD: Standard Deviation P>0.05 for all between study group comparisons, unless otherwise indicated:
P<0.05
Birth weight (kilograms) correlation with gestational age (weeks) was 0.71 (95%CI: 0.67–0.74; P<0.001)
3. Results
3.1. Participants
Six hundred fifty-two offspring (81%) had completed ASQ-3 questionnaires at both 24 and 36 months. Of these children, 17 had preterm birth before 34 weeks of gestation (early preterm birth) and were excluded. Accordingly, 635 children were included in this analysis, of whom 42 (6.6%) were born late preterm. A comparison of the characteristics of children included (N=635) and excluded from this analysis (N=171) is summarized in Supplemental Table S1. The baseline characteristics of the study groups (late preterm children vs term children) are provided in Table 1. Mean gestational ages among preterm and term study groups were 36 and 39 weeks, respectively. Low birth weight (<2.5 kg) was more frequent in late preterm children compared with those born at term (late preterm group 15/42[35.71%] vs the term group 21/593[3.54%], relative risk “RR” =7.8, 95%CI: 4.3–14.1). The mean of ASQ-3 scores for the domains per study group at 24 and 36 months are provided in Table 1.
3.2. Stability (Concordance) of ASQ Developmental Status at 24 and 36 Months
We examined the differences in paired proportions of the suboptimal developmental status of each domain at 24 months versus the corresponding domain at 36 months by study groups (children with late preterm and term birth). Among children with late preterm birth, the proportions of suboptimal developmental status at 36 months were not significantly different from those at 24-months across five developmental domains (36 months vs 24 months-gross motor: 28.6% (12/42) vs. 23.8% (10/42), McNemar P=0.77; fine motor: 28.6.0% (12/42) vs. 19.0% (8/42), McNemar P=0.39; communication: 14.30% (6/42) vs. 21.43% (9/42), McNemar P=0.39; personal and social skills: 9.50% (4/42) vs 14.2% (6/42), McNemar P=0.63; problem-solving skills: 26.2 (11/42) vs. 12% (5/42) McNemar P=0.11; Supplemental Table S2A). By contrast, in term children, the proportions of suboptimal developmental status were higher for fine motor and lower for problem-solving skills at 36 months compared to the prior assessment at 24 months (36 months vs 24 months-gross motor: 9.78% (58/593) vs 8.94% (53/593), McNemar P=0.66; fine motor: 21.08% (125/593) vs. 11.30% (65/593), McNemar P<0.001; communication: 6.60% (39/593) vs. 6.90% (41/593), McNemar P=0.89; personal-social skills: 8.80% (52/593)vs. 7.80% (46/593), McNemar P=0.54; problem-solving skills: 12.70% (75/593) vs 10.12% (60/593), McNemar P=0.14; Supplemental Table S2B).
3.3. Late Preterm Birth and Risk of Developmental Delays at 24 and 36 Months
Table 2 provides the proportions of preterm and term children with optimal and suboptimal development across the ASQ-3 domains and the odds of delays for late preterm birth at 24 and 36 months. With multivariable adjustment for imbalances in baseline characteristics and other prespecified covariates, late preterm birth remained the most consistent domain predictor of the developmental status of children aged 24 and 36 months. At 24 months, preterm birth relative to term birth was associated with an increased odds of child’s atypical development or potential developmental delay in developmental domains of gross and fine motor skills (aOR=2.89, 95%CI: 1.08–6.96 and aOR=2.69, 95%CI: 0.92–6.94, respectively), communication (aOR=4.98, 95%CI: 1.44–15.49), and personal-social skills (aOR=3.10, 95%CI: 1.25–7.76) (Table 3A). Similarly, at 36 months, a child with late preterm birth had higher odds of having a potential developmental delay in areas of gross motor skills (aOR=4.54, 95%CI: 1.81–10.79), communication skills (aOR=8.60, 95%CI: 3.10–23.28), and problem-solving (aOR=3.80, 1.58–8.73) compared with a term child (Table 3B). 24-month-old boys had higher odds of atypical development than girls in the area of personal-social skills (aOR=2.33, 95%CI: 1.43–3.89) but lower odds at problem-solving skills (aOR=0.52, 95%CI: 0.29–0.92). At 36 months, the odds of less optimal development in boys remained higher in personal-social skills (aOR=1.40, 95%CI: 1.26–4.81; Table 3A). 36-month-old boys also had a trend towards higher odds of being behind typical progress in communication skills than girls (aOR=1.86, 95%CI: 0.96–3.71; Table 3B).
Table 2.
Late Preterm Birth and Odds of Less Optimal Development Across ASQ Developmental Domains at 24 and 36 Months of Age (N=635 at each time point).
| Gross Motor Skills | Fine Motor Skills | Communication | Problem Solving Skills | Personal-Social Skill | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| At 24 Months | Needs monitoring or further assessment | Typical | OR (95% Cl) P-value | Needs monitoring or further assessment | Typical | OR (95% Cl) P-value | Needs monitoring or further assessment | Typical | OR (95% Cl) P-value | Needs monitoring or further assessment | Typical | OR (95% Cl) P-value | Needs monitoring or further assessment | Typical | OR (95% Cl) P-value |
| Late preterm | 10 | 32 | 3.18 (1.48–6.83) | 8 | 34 | 1.84 (0.82–4.15) | 6 | 36 | 2.24 (0.90–5.60) | 5 | 37 | 1.20 (0.46–3.17) | 11 | 31 | 1.98 (0.96–3.30) |
| Term | 53 | 540 | 0.003 | 67 | 526 | 0.138 | 41 | 552 | 0.08 | 60 | 533 | 0.712 | 90 | 503 | 0.09 |
| At 36 months | |||||||||||||||
| Late preterm | 12 | 30 | 3.69 (1.79–7.60) | 12 | 30 | 1.50 (0.745–3.01) | 9 | 33 | 3.90 (1.73–8.66) | 11 | 31 | 2.45 (1.18–5.08) | 4 | 38 | 1.10 (0.38–3.19) |
| Term | 58 | 535 | <0.001 | 125 | 468 | 0.257 | 39 | 554 | <0.001 | 75 | 518 | 0.016 | 52 | 541 | 0.87 |
Table 3:
A and B. Multivariable Logistic Regression Models for Association of Late Preterm Birth, and Maternal and Neonatal Characteristics with ASQ-3 Developmental Domains at 24 and 36 Months.
| Characteristics (N=635) | A. Adjusted odds ratios for suboptimal development at 24 months | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gross Motor Skills | Fine Motor Skills | Communication | Problem Solving Skills | Personal and Social Skills | ||||||
| aOR (95% CI) | P-Value | aOR (95% CI) | P-Value | aOR (95% CI) | P-Value | aOR (95% CI) | P-Value | aOR (95% CI) | P-Value | |
| Gestational age at delivery | ||||||||||
| Late preterm vs Term (34–37 vs ≥37 weeks of gestation) | 2.89 (1.10–6.90) | 0.017 | 2.69 (0.92–6.94) | 0.051 | 4.98 (1.44–15.13) | 0.006 | 1.26 (0.34–3.65) | 0.69 | 3.10 (1.25–7.76) | 0.012 |
| Maternal age | ||||||||||
| <30 vs ≥30 years old | 0.76 (0.39–1.49) | 0.42 | 1.03 (0.54–2.00) | 0.93 | 1.16 (0.54–2.59) | 0.70 | 0.70 (0.35–1.39) | 0.31 | 0.49 (0.28–0.87) | 0.014 |
| Clinical center | ||||||||||
| Boston | Ref | Ref | Ref | |||||||
| San Diego | 2.07 (0.88–4.92) | 0.09 | 2.83 (1.23–6.65) | 0.02 | 10.9 (3.93–33.54) | <0.001 | 1.30 (0.52–3.20) | 0.57 | 2.87 (1.42–5.88) | 0.004 |
| St. Louis | 1.40 (0.56–3.32) | 0.49 | 0.74 (0.29–1.88) | 0.53 | 2.39 (0.72–8.28) | 0.16 | 0.68 (0.25–1.76) | 0.43 | 0.52 (0.23–1.16) | 0.12 |
| Gestational diabetes | ||||||||||
| Yes vs No | 0.68 (0.11–2.50) | 0.62 | 1.90 (0.52–5.55) | 0.28 | 0.30 (0.015–1.80) | 0.28 | 1.19 (0.26–3.94) | 0.80 | 0.42 (0.06–1.51) | 0.25 |
| Child Race | ||||||||||
| African American | Ref | Ref | Ref | Ref | ||||||
| White | 1.90 (0.87–4.14) | 0.11 | 0.73 (0.35–1.50) | 0.40 | 1.57 (0.65–3.76) | 0.31 | 0.86 (0.36–2.01) | 0.73 | 1.08 (0.56–2.06) | 0.82 |
| Other | 1.39 (0.53–3.41) | 0.48 | 0.74 (0.28–1.88) | 0.53 | 6.26 (2.11–19.00) | 0.001 | 1.02 (0.36–2.76) | 0.97 | 1.28 (0.57–2.89) | 0.55 |
| Maternal education | ||||||||||
| Less than college vs College or above | 1.39 (0.64–3.02) | 0.41 | 1.24 (0.60–2.60) | 0.56 | 2.11 (0.88–5.23) | 0.10 | 1.29 (0.59–2.87) | 0.53 | 1.57 (0.83–2.98) | 0.17 |
| Child Sex | ||||||||||
| Male vs Female | 0.75 (0.43–1.31) | 0.32 | 1.23 (0.72–2.13) | 0.46 | 1.73 (0.88–3.56) | 0.12 | 0.52 (0.29–0.92) | 0.025 | 2.33 (1.43–3.89) | <0.001 |
| Delivery mode | ||||||||||
| Cesarean vs Vaginal | 0.91 (0.47–1.66) | 0.76 | 0.97 (0.53–1.73) | 0.92 | 2.11 (1.05–4.21) | 0.034 | 0.87 (0.45–1.61) | 0.67 | 0.91 (0.53–1.53) | 0.71 |
| Child breast feeding during first 6 month | ||||||||||
| Yes vs No | 1.10 (0.56–2.18) | 0.79 | 0.74 (0.39–1.40) | 0.35 | 1.35 (0.62–3.05) | 0.45 | 0.34 (0.17–0.67) | 0.002 | 0.51 (0.28–0.89) | 0.019 |
| Maternal 25OHD at 32–38 weeks of gestation (ng/mL) | 0.98 (0.96–0.997) | 0.036 | 1.0 (0.98–1.02) | 0.82 | 0.996 (0.97–1.02) | 0.81 | 0.98 (0.959–0.997) | 0.05 | 0.99 (0.98–1.01) | 0.68 |
| Maternal marital status | ||||||||||
| Married vs not | 0.66 (0.31–1.42) | 0.30 | 0.93 (0.45–1.91) | 0.85 | 2.94 (0.84–4.45) | 0.12 | 1.14 (0.52–2.47) | 0.60 | 1.11 (0.58–2.12) | 0.74 |
| married or divorced | ||||||||||
| Preeclampsia | ||||||||||
| Yes vs No | 0.79 (0.23–2.14) | 0.68 | 0.43 (0.10–1.30) | 0.18 | 1.02 (0.23–3.27) | 0.99 | 0.15 (0.01–0.74) | 0.07 | 0.74 (0.26–1.82) | 0.54 |
| Number of prior pregnancies | ||||||||||
| 1 vs >1 | 1.47 (0.81–2.65) | 0.20 | 2.57 (1.45–4.61) | 0.001 | 1.20 (0.58–2.46) | 0.62 | 0.99 (0.51–1.85) | 0.97 | 2.23 (1.34–3.74) | 0.002 |
| Maternal asthma | ||||||||||
| Yes vs. No | 0.81 (0.44–1.44) | 0.47 | 0.99 (0.57–1.72) | 0.98 | 1.33 (0.66–2.66) | 0.41 | 0.83 (0.46–1.49) | 0.54 | 0.82 (0.49–1.33) | 0.42 |
| Characteristics (N=635) | B. Adjusted odds ratios for suboptimal development at 36 months | |||||||||
| Gross Motor Skills | Fine Motor Skills | Communication | Problem Solving Skills | Personal and Social Skills | ||||||
| aOR (95% CI) | P-Value | aOR (95% CI) | P-Value | aOR (95% CI) | P-Value | aOR (95% CI) | P-Value | aOR (95% CI) | P-Value | |
| Gestational age at delivery | ||||||||||
| Late preterm vs Term (34–37 vs ≥37 weeks of gestation) | 4.54 (1.81–10.79) | <0.001 | 1.52 (0.61–3.48) | 0.34 | 8.60 (3.10–23.28) | <0.001 | 3.80 (1.58–8.73) | 0.002 | 1.35 (0.30–4.35) | 0.64 |
| Maternal age | ||||||||||
| <30 vs ≥30 years old | 1.33 (0.68–2.68) | 0.41 | 1.43 (0.85–2.43) | 0.18 | 2.05 (0.90–5.02) | 0.10 | 1.47 (0.80–2.75) | 0.22 | 1.07 (0.52–2.25) | 0.85 |
| Clinical center | ||||||||||
| Boston | Ref | Ref | Ref | Ref | ||||||
| San Diego | 1.80 (0.76–4.30) | 0.18 | 1.61 (0.82–3.18) | 0.17 | 2.94 (1.0–9.26) | 0.055 | 1.74 (0.78–3.90) | 0.16 | 4.62 (1.87–11.8) | 0.001 |
| St. Louis | 1.16 (0.48–2.82) | 0.74 | 0.72 (0.36–1.51) | 0.41 | 2.00 (0.65–6.62) | 0.24 | 1.30 (0.52–2.81) | 0.65 | 0.60 (0.20–1.77) | 0.36 |
| Gestational diabetes | ||||||||||
| Yes vs No | 2.05 (0.56–6.06) | 0.23 | 0.64 (0.18–1.79) | 0.43 | 1.13 (0.16–4.59) | 0.88 | 0.00 (0.00–1380.0) | 0.98 | 1.22 (0.26–4.21) | 0.78 |
| Child Race | ||||||||||
| African American | Ref | Ref | Ref | Ref | ||||||
| White | 1.91 (0.88–4.16) | 0.10 | 0.69 (0.37–1.26) | 0.23 | 1.62 (0.66–3.93) | 0.29 | 1.08 (0.52–2.21) | 0.83 | 1.36 (0.59–3.12) | 0.47 |
| Other | 1.29 (0.45–3.49) | 0.63 | 0.69 (0.31–1.50) | 0.36 | 1.96 (0.55–6.47) | 0.28 | 2.00 (0.82–4.87) | 0.13 | 2.38 (0.87–6.67) | 0.09 |
| Maternal education | ||||||||||
| College or above vs less than college | 0.94 (0.44–2.01) | 0.87 | 1.18 (0.66–2.11) | 0.58 | 2.21 (0.87–6.10) | 0.11 | 1.07 (0.55–2.13) | 0.84 | 1.15 (0.52–2.59) | 0.73 |
| Child Sex | ||||||||||
| Male vs Female | 0.63 (0.36–1.07) | 0.10 | 1.50 (0.98–2.30) | 0.064 | 1.86 (0.96–3.71) | 0.07 | 1.15 (0.71–1.89) | 0.57 | 1.40 (1.26–4.81) | 0.01 |
| Delivery mode | ||||||||||
| Cesarean vs Vaginal | 0.69 (0.36–1.27) | 0.25 | 1.27 (0.81–2.00) | 0.29 | 1.66 (0.84–3.23) | 0.14 | 1.18 (0.68–1.98) | 0.55 | 2.61 (1.38–4.98) | 0.003 |
| Child breast feeding during first 6 month | ||||||||||
| Yes vs No | 0.53 (0.27–1.01) | 0.056 | 0.54 (0.33–0.90) | 0.017 | 0.83 (0.39–1.76) | 0.62 | 0.73 (0.41–1.30) | 0.29 | 0.43 (0.21–0.89) | 0.02 |
| Maternal 25OHD at 32–38 weeks of gestation (ng/mL) | 1.0 (0.99–1.03) | 0.24 | 1.0 (0.99–1.02) | 0.80 | 1.0 (0.98–1.02) | 0.89 | 0.99 (0.98–1.01) | 0.48 | 1.0 (0.99–1.04) | 0.068 |
| Maternal marital status | ||||||||||
| Married vs Not | 0.28 (0.22–1.02) | 0.061 | 1.24 (0.69–2.23) | 0.47 | 0.98 (0.40–2.28) | 0.96 | 0.92 (0.46–1.80) | 0.80 | 1.10 (0.49–2.41) | 0.82 |
| married or divorced | ||||||||||
| Preeclampsia | ||||||||||
| Yes vs No | 0.28 (0.04–0.98) | 0.091 | 1.03 (0.47–2.10) | 0.94 | 0.19 (0.01–0.93) | 0.11 | 0.83 (0.30–1.96) | 0.69 | 0.40 (0.06–1.51) | 0.27 |
| Number of prior pregnancies | ||||||||||
| 1 vs>1 | 1.28 (0.71–2.28) | 0.41 | 0.83 (0.52–1.32) | 0.44 | 0.70 (0.33–1.44) | 0.35 | 0.66 (0.37–1.13) | 0.14 | 1.90 (0.97–3.77) | 0.062 |
| Maternal asthma | ||||||||||
| Yes vs. No | 0.87 (0.49–1.52) | 0.64 | 0.67 (0.43–1.04) | 0.08 | 0.89 (0.45–1.72) | 0.73 | 0.81 (0.48–1.33) | 0.41 | 1.00 (0.51–1.90) | 0.99 |
The effect of modifiable developmental risk factors such as prenatal vitamin D and early life breastfeeding was age-dependent and developmental domain-specific, independently of late preterm birth. Breastfeeding during a child’s first six months of life was associated with on-schedule developmental advancement to meet typical milestones independently of preterm birth, maternal socioeconomic status, and education. At 24 months, children who were breastfed were at lower odds of being identified with suboptimal development in personal-social skills and problem-solving skills adjusted for preterm birth status and other potential covariates (aOR=0.51, 95%CI: 0.28–0.89 and aOR=0.34, 95%CI: 0.17–0.67, respectively, Table 3A). Furthermore, these children also were less likely to demonstrate suboptimal development in the domains of fine motor and personal and social areas at 36 months (aOR=0.54, 95%CI: 0.33–0.90 and aOR=0.43, 95%CI: 0.21–0.89, respectively; Table 3B). Higher maternal 25OHD level (10 ng/ml increase) at 32–38 of gestation was associated with an 18% reduction in odds of suboptimal development in gross motor and problem-solving skills at 24 months (aORunit_change=0.98, 95%CI: 0.96–0.997 and aORunit_change=0.98, 95%CI: 0.96–0.997, respectively) (Table 3A).
4. Discussion
In the VDAART cohort, developmental delays were stable between 24 and 36 months on all 5 domains for the children born preterm and on 4/5 domains for those born at term. The developmental domains with the status stability in both late preterm and term children at 24 and 36 months were gross motor, communication, personal-social skills and problem solving. The late-preterm children remained at a higher risk of delay in the developmental domains of communication, gross motor, and problem-solving skills compared to term children at 36 months. However, the developmental domains with a higher risk of delays at both 24 and 36 months were the communication and gross motor skills (∼30% at 36 months). This observation suggests that while majority of late preterm infants might have typical development in early life, some could remain at risk for physical and cognitive impairments. While different risk factors contributed to a potential delay in each developmental domain by the age of 3 years, preterm birth was the main risk factor for suboptimal developmental outcomes across five ASQ-3 domains at 24-and 36-month-old children. Our prospective study highlights the importance of early screening (≤ 2 years) to potentially avert the lag in development and long-term developmental impairments in children born late preterm. These children might benefit from early screening, professional assessment of the impaired developmental domains, and focused interventions according to the deficits in their early years of life.
Children with late preterm birth are at risk of short and long-term developmental delays compared with term children. Our study suggests that risk and stability could be different across developmental domains by age while several developmental domains could be affected. This observation might be of growing importance as the rate of late preterm births continues to increase (McGowan, Alderdice, Holmes, & Johnston, 2011; Woythaler, 2019).
In a French population-based study (1997–2011), Pierrat and colleagues demonstrated that children born at 32–34 weeks of gestation had 2-year ASQ scores below the threshold and at risk for developmental delay (Pierrat, et al., 2017). The domains of communication, gross motor, and personal-social skills were assessed with a potential delay at age 2 which is similar to affected domains in our study at age 2, suggesting similarity of the neurodevelopmental outcomes of moderate and late preterm birth. However, our results are in contrast with those from Shah and colleagues who found the lack of suboptimal development at 24 months using Bayley Scales of Infant Development, Second Edition (BSID-II) but the reemergence of suboptimal development at preschool (Shah, et al., 2016). Conversely, Woythaler and colleagues showed late preterm infants with a low Mental Development Index of Bayley-II at 24 months would continue to be delayed and perform less than term children at kindergarten (61–69 months) (Woythaler, McCormick, Mao, & Smith, 2015). Hornman and colleagues reported more frequent abnormal ASQ scores among late-preterm children than full-term children. The authors’ report suggested that the relation between the preterm births and the stability of impairments varies by the developmental domain (Hornman, et al., 2017). Despite a decrease in rates of developmental problems between ages 4, 8, and 18 months among late preterm born children at 340/7-366/7 weeks of gestational age, the authors observed both early and late preterm born children had greater rates of developmental problems than full term-born children before school entry (Hornman, et al., 2017). These observations along with our findings suggest that the chance of improvement in developmental impairments might reduce after 24 months before school and follow-up of developmental deficits in late preterm children is critical.
Using the Dutch version of the age 48-month form of the ASQ, Kerstjens and colleagues found an almost 2-fold greater prevalence of developmental delay at preschool age in moderately preterm‒born children (32–36 weeks of gestation) compared with term-born children. These infants were more likely to have problems with fine motor, communication, personal-social skills compared with full-term infants (Kerstjens, et al., 2011). The differences in the observed developmental domains and risk estimates in comparison to our study could be attributed to the contrasts in the study population characteristics and the fact that we included children that might require monitoring of a potential developmental delay in addition to those in need of a professional evaluation. Nevertheless, in late preterm children, when a child’s score on any given ASQ-3 domain falls in the monitoring zone or a potential developmental delay by age 2, a child could benefit from professional assessment for confirmation of a potential developmental delay and focused support as opposed to rescreening in a few months. In our study, children born preterm demonstrated less optimal development in multiple developmental domains at both 24 and 36 months. The fact that 24-month preterm children with the need for monitoring or further assessment were at higher risk of remaining in the suboptimal categories at 36 months highlights the importance of screening, Intervention in the early life of preterm children, particularly those with late preterm birth that ideally will prevent or reduce developmental impairments are desired.
Our study also suggests that the effect of other developmental risk factors could be age-dependent and domain-specific. Kerstjens and colleagues reported the association of child’s sex with abnormal ASQ scores in moderate preterm (32–36 weeks of gestation) infants (Kerstjens, et al., 2011). Using the Griffiths Mental Development Scale, Di Rosa and colleagues similarly demonstrated that sex and gestational age could influence a child’s neurodevelopment and result in different temporal profiles of neurodevelopment in early and late preterm and term infants. More specifically, they demonstrated that preterm girls (32–36 weeks) better performed on personal–social skills at 30–36 months compared with preterm boys and boys were at higher risk of suboptimal development in their personal and social skills at both 24 and 36 months (Di Rosa, Pironti, Cucinotta, Alibrandi, & Gagliano, 2019). Exclusive breastfeeding in infants for at least six months, as recommended by the AAP, has shown several short and long-term advantages including improved neurodevelopmental outcomes such as cognitive disorders and attention-deficit/hyperactivity disorder in both preterm and term children (Bar, Milanaik, & Adesman, 2016; Horta, de Sousa, & de Mola, 2018; Koo, Tank, Martin, & Shi, 2014). Our study implicates a protective effect of breastfeeding during the first six months of infants’ life on motor skills as well as personal-social and problem-solving skills at 24 and 36 months. The fact that this effect might not be similar across all developmental domains and whether early-life breastfeeding might have long term effects on only specific developmental domains such as cognitive outcomes requires further investigation.
4.1. Limitations
There were a higher number of pregnant women with asthma in the VDAART cohort as opposed to the prevalence of asthma among pregnant women in the general population. This study characteristic might limit the generalizability of the results. Nevertheless, the observed incidence of preterm children, including those born late preterm (6–7%), was consistent with the expected incidence of this condition in the general population (Arpino, et al., 2010; Davidoff, et al., 2006) and asthma was not associated with preterm birth or developmental delay in this cohort. A low number of late-preterm infants in this study is a major limitation. Parents of children with low ASQ scores in the VDAART were notified and recommended for professional assessment by their health provider per VDAART protocol but were not follow-up with their developmental status at later childhood. A study with larger sample size, professional assessment, and longer follow-ups with more frequent evaluation could shed light upon which late preterm infants are at higher risk for developmental delays. Such a study should also address whether a lag in a specific developmental domain by the age of 3 might be resolved with early identification and targeted intervention to avoid lifelong issues or decrease the risk of impairments.
4.2. Conclusion
Late preterm birth was associated with suboptimal development in several domains at 24 and 36 months compared with term birth, requiring monitoring and further assessment for the outcome and resolution of developmental lag. The late preterm children’s developmental status was more stable at 24 and 36 months than that of children born at term. The risk of suboptimal development varied according to the age and developmental domains at which children were assessed. Therefore, identification of areas of development that might be less optimal across a range of skills as early as possible is crucial and prompts appropriate monitoring, professional and targeted assessment, or early therapeutic intervention. Domain-specific risk factors other than preterm birth, particularly the ones that can be modified, should be acknowledged and further investigated. Future studies are required to investigate the effect on parent-infant relationships over time and the long-term benefits to a child’s health using the support programs (Institute of Medicine (US) Committee on Understanding Premature Birth and Assuring Healthy Outcomes; Behrman RE). These studies should also address potential differences that might be needed in supportive programs in early versus late preterm infants.
Supplementary Material
Figure 1.
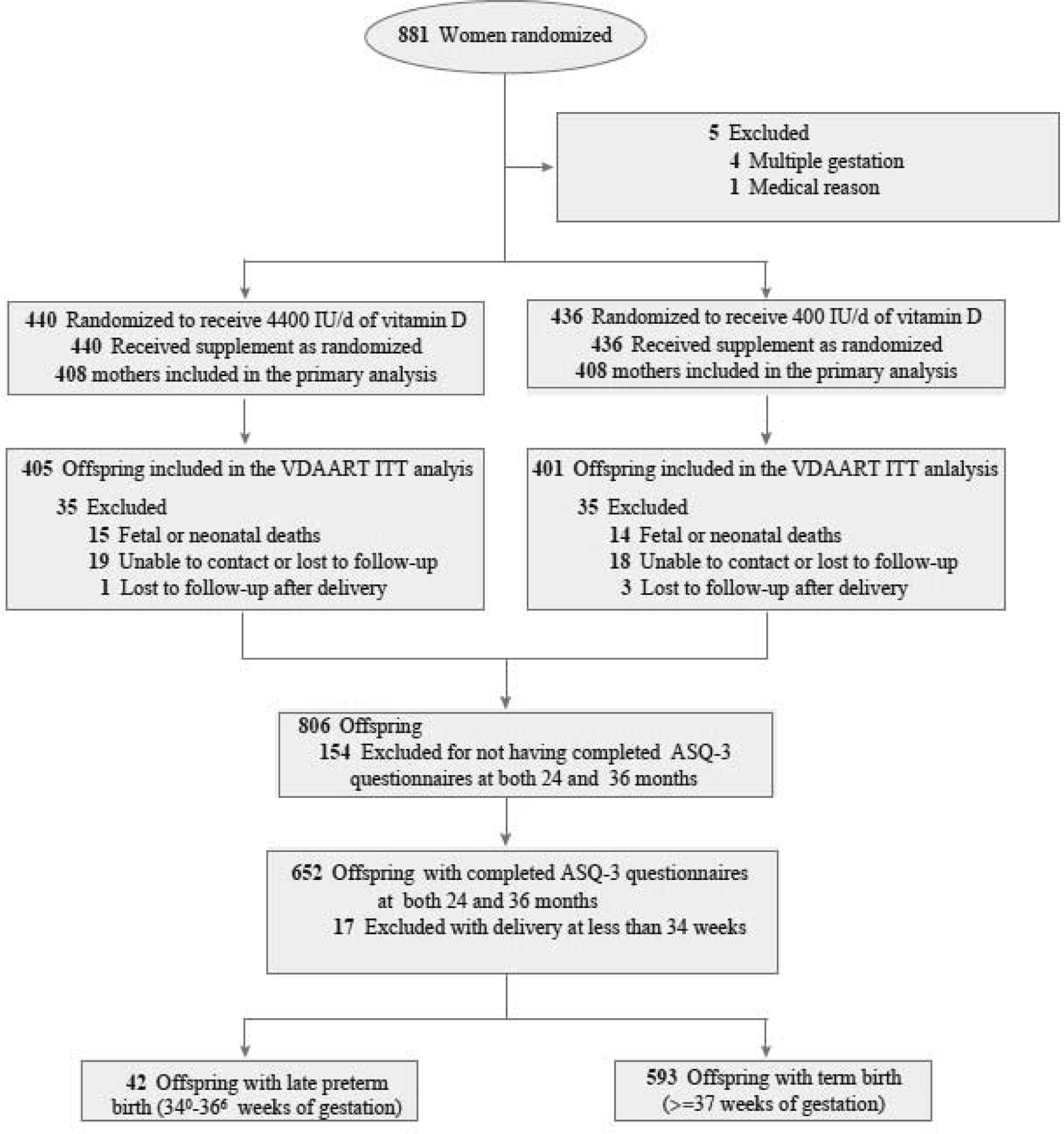
The VDAART and Ancillary Study Participant Flow
Highlights.
Late preterm infants are at higher risk of not meeting the typical developmental milestones at both 24 and 36 months compared to infants born term.
Late preterm infants could have developmental delays in several domains in early life.
The lag in developmental status are stable at 24 and 36 months in late preterm infants.
Modifiable risk factors (e.g., breastfeeding) could reduce the risk of potential delays, independent of late preterm birth.
Acknowledgment
The authors thank the women and their children who participated in the trial and all the study staff for their contributions to the trial and ancillary investigations.
Funding:
This study is ancillary in the Vitamin D Antenatal Asthma Reduction Trial (VDAART). VDAART was supported by U01HL091528 and R01HL091528 from the National Heart, Lung, and Blood Institute (NHLBI, to STW and AAG). HM was supported by 1 K01HL146977 01A1, L30-HL129467-01, 2L30 HL129467-02A1, 5 UH3OD023268 from NHLBI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of Interests
The authors have no potential conflicts of interest relevant to this article to disclose.
Reference
- ACOG Committee Opinion No 579: Definition of term pregnancy. (2013). Obstet Gynecol, 122, 1139–1140. [DOI] [PubMed] [Google Scholar]
- Agarwal PK, Shi L, Daniel LM, Yang PH, Khoo PC, Quek BH, Zheng Q, & Rajadurai VS (2017). Prospective evaluation of the Ages and Stages Questionnaire 3rd Edition in very-low-birthweight infants. Dev Med Child Neurol, 59, 484–489. [DOI] [PubMed] [Google Scholar]
- Allotey J, Zamora J, Cheong-See F, Kalidindi M, Arroyo-Manzano D, Asztalos E, van der Post J, Mol BW, Moore D, Birtles D, Khan KS, & Thangaratinam S (2018). Cognitive, motor, behavioural and academic performances of children born preterm: a meta-analysis and systematic review involving 64 061 children. BJOG, 125, 16–25. [DOI] [PubMed] [Google Scholar]
- American College of, O., Gynecologists, & Committee on Practice, B.-O. (2012). ACOG practice bulletin no. 127: Management of preterm labor. Obstet Gynecol, 119, 1308–1317. [DOI] [PubMed] [Google Scholar]
- Arpino C, Compagnone E, Montanaro ML, Cacciatore D, De Luca A, Cerulli A, Di Girolamo S, & Curatolo P (2010). Preterm birth and neurodevelopmental outcome: a review. Childs Nerv Syst, 26, 1139–1149. [DOI] [PubMed] [Google Scholar]
- Bar S, Milanaik R, & Adesman A (2016). Long-term neurodevelopmental benefits of breastfeeding. Curr Opin Pediatr, 28, 559–566. [DOI] [PubMed] [Google Scholar]
- Barfield WD, Lee KG (2019). Late preterm infants UpToDate, Weismen LE, Kim MS (Eds.) Retrieved April 28, 2019, from https://www.uptodate.com/contents/late-preterm-infants. [Google Scholar]
- Blaggan S, Guy A, Boyle EM, Spata E, Manktelow BN, Wolke D, & Johnson S (2014). A parent questionnaire for developmental screening in infants born late and moderately preterm. Pediatrics, 134, e55–62. [DOI] [PubMed] [Google Scholar]
- Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, Adler A, Vera Garcia C, Rohde S, Say L, & Lawn JE (2012). National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet, 379, 2162–2172. [DOI] [PubMed] [Google Scholar]
- Council on Children With, D., Section on Developmental Behavioral, P., Bright Futures Steering, C., & Medical Home Initiatives for Children With Special Needs Project Advisory, C. (2006). Identifying infants and young children with developmental disorders in the medical home: an algorithm for developmental surveillance and screening. Pediatrics, 118, 405–420. [DOI] [PubMed] [Google Scholar]
- Davidoff MJ, Dias T, Damus K, Russell R, Bettegowda VR, Dolan S, Schwarz RH, Green NS, & Petrini J (2006). Changes in the gestational age distribution among U.S. singleton births: impact on rates of late preterm birth, 1992 to 2002. Semin Perinatol, 30, 8–15. [DOI] [PubMed] [Google Scholar]
- de Kieviet JF, Piek JP, Aarnoudse-Moens CS, & Oosterlaan J (2009). Motor development in very preterm and very low-birth-weight children from birth to adolescence: a meta-analysis. JAMA, 302, 2235–2242. [DOI] [PubMed] [Google Scholar]
- Di Rosa G, Pironti E, Cucinotta F, Alibrandi A, & Gagliano A (2019). Gender affects early psychomotor milestones and long-term neurodevelopment of preterm infants. Infant and Child Development, 28, e2110. [Google Scholar]
- Halbwachs M, Muller JB, Nguyen The Tich S, Gascoin G, Chauty-Frondas A, Branger B, Rouger V, Roze JC, & Flamant C (2014). Predictive value of the parent-completed ASQ for school difficulties in preterm-born children <35 weeks’ GA at five years of age. Neonatology, 106, 311–316. [DOI] [PubMed] [Google Scholar]
- Holm S A simple sequentially rejective multiple test procedure. Scand J Stat 1979;6:65–70. [Google Scholar]
- Hornman J, de Winter AF, Kerstjens JM, Bos AF, & Reijneveld SA (2017). Stability of Developmental Problems after School Entry of Moderately-Late Preterm and Early Preterm-Born Children. J Pediatr, 187, 73–79. [DOI] [PubMed] [Google Scholar]
- Horta BL, de Sousa BA, & de Mola CL (2018). Breastfeeding and neurodevelopmental outcomes. Curr Opin Clin Nutr Metab Care, 21, 174–178. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine (US) Committee on Understanding Premature Birth and Assuring Healthy Outcomes; Behrman RE, B A, editors. Preterm Birth: Causes, Consequences, and Prevention Washington (DC): National Academies Press (US); 2007. 11, Neurodevelopmental, Health, and Family Outcomes for Infants Born Preterm. Available from: https://www.ncbi.nlm.nih.gov/books/NBK11356/. [PubMed] [Google Scholar]
- Kerstjens JM, de Winter AF, Bocca-Tjeertes IF, ten Vergert EM, Reijneveld SA, & Bos AF (2011). Developmental delay in moderately preterm-born children at school entry. J Pediatr, 159, 92–98. [DOI] [PubMed] [Google Scholar]
- Kerstjens JM, Nijhuis A, Hulzebos CV, van Imhoff DE, van Wassenaer-Leemhuis AG, van Haastert IC, Lopriore E, Katgert T, Swarte RM, van Lingen RA, Mulder TL, Laarman CR, Steiner K, & Dijk PH (2015). The Ages and Stages Questionnaire and Neurodevelopmental Impairment in Two-Year-Old Preterm-Born Children. PLoS One, 10, e0133087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo W, Tank S, Martin S, & Shi R (2014). Human milk and neurodevelopment in children with very low birth weight: a systematic review. Nutr J, 13, 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litonjua AA, Carey VJ, Laranjo N, Harshfield BJ, McElrath TF, O’Connor GT, Sandel M, Iverson RE Jr., Lee-Paritz A, Strunk RC, Bacharier LB, Macones GA, Zeiger RS, Schatz M, Hollis BW, Hornsby E, Hawrylowicz C, Wu AC, & Weiss ST (2016). Effect of Prenatal Supplementation With Vitamin D on Asthma or Recurrent Wheezing in Offspring by Age 3 Years: The VDAART Randomized Clinical Trial. JAMA, 315, 362–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litonjua AA, Lange NE, Carey VJ, Brown S, Laranjo N, Harshfield BJ, O’Connor GT, Sandel M, Strunk RC, Bacharier LB, Zeiger RS, Schatz M, Hollis BW, & Weiss ST (2014). The Vitamin D Antenatal Asthma Reduction Trial (VDAART): rationale, design, and methods of a randomized, controlled trial of vitamin D supplementation in pregnancy for the primary prevention of asthma and allergies in children. Contemp Clin Trials, 38, 37–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan JE, Alderdice FA, Holmes VA, & Johnston L (2011). Early childhood development of late-preterm infants: a systematic review. Pediatrics, 127, 1111–1124. [DOI] [PubMed] [Google Scholar]
- Pierrat V, Marchand-Martin L, Arnaud C, Kaminski M, Resche-Rigon M, Lebeaux C,Bodeau-Livinec F, Morgan AS, Goffinet F, Marret S, Ancel PY, & group E. w. (2017). Neurodevelopmental outcome at 2 years for preterm children born at 22 to 34 weeks’ gestation in France in 2011: EPIPAGE-2 cohort study. BMJ, 358, j3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley MA, Poulsen G, Boyle E, Wolke D, Field D, Alfirevic Z, & Kurinczuk JJ (2012). Early term and late preterm birth are associated with poorer school performance at age 5 years: a cohort study. Arch Dis Child Fetal Neonatal Ed, 97, F167–173. [DOI] [PubMed] [Google Scholar]
- Roberts MY, Curtis P, Estabrook R, Norton ES, Davis MM, Burns J, Briggs-Gowan M, Petitclerc A, & Wakschlag LS (2018). Talking Tots and the Terrible Twos: Early Language and Disruptive Behavior in Toddlers. J Dev Behav Pediatr, 39, 709–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonhaut L, Armijo I, Schonstedt M, Alvarez J, & Cordero M (2013). Validity of the ages and stages questionnaires in term and preterm infants. Pediatrics, 131, e1468–1474. [DOI] [PubMed] [Google Scholar]
- Shah P, Kaciroti N, Richards B, Oh W, & Lumeng JC (2016). Developmental Outcomes of Late Preterm Infants From Infancy to Kindergarten. Pediatrics, 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro-Mendoza CK, & Lackritz EM (2012). Epidemiology of late and moderate preterm birth. Semin Fetal Neonatal Med, 17, 120–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard MN, Luu TM, & Gosselin J (2012). Concurrent validity of ages and stages questionnaires in preterm infants. Pediatrics, 130, e108–114. [DOI] [PubMed] [Google Scholar]
- Squires J, & Bricker D (2009). Ages & Stages Questionnaires®, Third Edition (ASQ®−3): A Parent-Completed Child Monitoring System. Baltimore: Paul H. Brookes Publishing Co., Inc. [Google Scholar]
- Steenis LJ, Verhoeven M, Hessen DJ, & van Baar AL (2015). Parental and professional assessment of early child development: the ASQ-3 and the Bayley-III-NL. Early Hum Dev, 91, 217–225. [DOI] [PubMed] [Google Scholar]
- Stene-Larsen K, Brandlistuen RE, Lang AM, Landolt MA, Latal B, & Vollrath ME (2014). Communication impairments in early term and late preterm children: a prospective cohort study following children to age 36 months. J Pediatr, 165, 1123–1128. [DOI] [PubMed] [Google Scholar]
- Woythaler M (2019). Neurodevelopmental outcomes of the late preterm infant. Semin Fetal Neonatal Med, 24, 54–59. [DOI] [PubMed] [Google Scholar]
- Woythaler M, McCormick MC, Mao WY, & Smith VC (2015). Late Preterm Infants and Neurodevelopmental Outcomes at Kindergarten. Pediatrics, 136, 424–431. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


