Abstract
Neurogenic orthostatic hypotension (OH) is a disabling disorder caused by impairment of the normal autonomic compensatory mechanisms that maintain upright blood pressure. Nonpharmacologic treatment is always the first step in the management of this condition, but a considerable number of patients will require pharmacologic therapies. Denervation hypersensitivity and impairment of baroreflex buffering makes these patients sensitive to small doses of pressor agents. Understanding the underlying pathophysiology can help in selecting between treatment options. In general, patients with low “sympathetic reserve”, i.e., those with peripheral noradrenergic degeneration (pure autonomic failure, Parkinson’s disease) and low plasma norepinephrine, tend to respond better to “norepinephrine replacers” (midodrine and droxidopa). On the other hand, patients with relatively preserved “sympathetic reserve”, i.e., those with impaired central autonomic pathways but spared peripheral noradrenergic fibers (multiple system atrophy) and normal or slightly reduced plasma norepinephrine, tend to respond better to “norepinephrine enhancers” (pyridostigmine, atomoxetine, and yohimbine). There is, however, a spectrum of responses within these extremes, and treatment should be individualized. Other nonspecific treatments include fludrocortisone and octreotide. The presence of associated clinical conditions, such as supine hypertension, heart failure, and postprandial hypotension, need to be considered in the pharmacologic management of these patients.
Keywords: orthostatic hypotension, pharmacologic treatment
1. Introduction
Orthostatic hypotension (OH), defined as a sustained reduction of systolic blood pressure (BP) of at least 20 mmHg, or of diastolic BP of 10 mmHg within 3 minutes of standing or at least 60 degree head up tilt (Freeman et al., 2011), is a disorder particularly prevalent in the elderly population and a cause of significant disability for those affected. Its presence is evidence of a failure of the compensatory autonomic mechanisms that normally maintain upright BP, most often as a consequence of systemic illnesses causing autonomic neuropathies (e.g., diabetes, amyloid, autoimmune or paraneoplastic disorders) or neurodegenerative disorders (pure autonomic failure [PAF], Parkinson’s disease [PD], and multiple system atrophy [MSA]) (Arnold et al., 2013b; Robertson, 2008). In all cases, we first need to consider the possibility that OH is precipitated or worsened by reversible factors that overwhelm compensatory autonomic mechanisms already impaired by aging or disease. Hypertension, diabetes mellitus and heart failure are the most common comorbidities associated with OH. Thus, the initial treatment of OH should always be the removal of these aggravating factors. Medications are the most common culprit, in particular alpha-blockers (including tamsulosin, carvedilol), sympatholytics (including clonidine, tizanidine), vasodilators (nitrates, sildenafil citrate), and certain antidepressants (tricyclic antidepressants). This should be followed by educating the patients about conservative countermeasures, such as avoid standing motionless, wearing compression garments (Jordan et al., 2019; Okamoto et al., 2016), and increasing intake of water (1.5–2 L per day) and sodium (6–10 g per day) (Shibao et al., 2012). Water boluses can be effective also (Shannon et al., 2002). Still, these treatments may not be sufficient in a substantial proportion of patients, and additional pharmacological treatments, the focus of this review, may be needed.
2. Basic autonomic concepts that influence the selection of pharmacological treatment
There is little empirical evidence on which to base guidelines regarding the selection of the optimal pharmacologic treatment in a given patient, and this is left mostly to the clinician’s experience and preference (Biaggioni, 2017). Nonetheless, understanding the basic concepts of the underlying pathophysiology of the patient’s condition, and the clinical pharmacology of available therapies, is valuable in the management of these patients (Biaggioni, 2017). We will discuss the disease characteristics that affect the response to pharmacological treatment, followed by a discussion of the clinical pharmacology of individual medications.
2.1. Denervation hypersensitivity
Patients with autonomic impairment have an exaggerated response to both pressor and depressor stimuli because of denervation hypersensitivity. This explains the profound drop in BP in response to venous pooling induced by standing (orthostatic hypotension) or digestion (postprandial hypotension), but also the dramatic increase in BP observed after seemingly trivial stimuli (e.g., the water pressor reflex (Shannon et al., 2002)). Denervation hypersensitivity can also contribute to supine hypertension commonly seen in these patients (Arnold et al., 2012).
Two mechanisms can account for this hyperresponsiveness. First, and perhaps the most important, is the loss of baroreflex buffering that normally counteracts any change in BP. The second is upregulation of adrenergic receptors in response to the decreased exposure to norepinephrine (NE) resulting from the loss of noradrenergic nerve fibers (classical denervation hypersensitivity) (Benarroch, 2020; Jacob et al., 1999). Accordingly, intravenous administration of very low doses of alpha- or beta-adrenoreceptor agonists triggers exaggerated increases in BP and heart rate (HR), respectively (Nakamura et al., 2011; Niimi et al., 1999). This denervation hypersensitivity is easily explained in patients with peripheral neurodegeneration (i.e., PAF and PD), characterized by loss of postganglionic noradrenergic fibers and low plasma NE. However, it is also observed in patients with central neurodegeneration (i.e., MSA) and relatively preserved postganglionic fibers and plasma NE (Coon et al., 2018). The patient’s hyperresponsiveness to pressor stimuli can be used to their advantage when developing treatments.
2.2. Residual sympathetic function (“sympathetic reserve”)
Ultimately, OH is due to the inability of sympathetic nerves to release sufficient NE required to maintain upright BP. This is caused either by a loss of peripheral noradrenergic fibers (PAF and PD) or by impairment of central pathways that normally trigger sympathetic activation on standing (MSA). The former is characterized by low plasma NE (low sympathetic reserve) and the latter by normal or only slightly reduced plasma NE (residual sympathetic reserve). The functional relevance of these differences is evidenced by the observation that the ganglionic blocker trimethaphan, which interrupts the pathways that release NE in the peripheral nervous system, resulting in a profound decrease in of BP in MSA patients (Jordan et al., 2015). On the other hand, the ganglionic blockade has little if any effect in patients with severe peripheral postganglionic denervation (e.g., PAF) (Jordan et al., 2015; Shannon et al., 1997). In clinical practice, most patients with OH are in the middle of this spectrum of responses and have some degree of sympathetic reserve.
Understanding these concepts can help us in the selection of medications. The logical treatment for patients with impaired sympathetic reserve (PAF) would be to restore low plasma NE with “norepinephrine replacers” (direct adrenergic agonists such as midodrine and droxidopa). On the other hand, in patients with impaired central autonomic pathways (MSA), we can harness their preserved sympathetic reserve with “norepinephrine enhancers” (“indirect sympathomimetics”) either by facilitating cholinergic ganglionic transmission with the cholinesterase inhibitor pyridostigmine (Singer et al., 2006), or increasing synaptic NE with the NE transporter blocker atomoxetine (Shibao et al., 2007c). It is important to consider that denervation hypersensitivity will magnify the effect of even small increases in synaptic NE, resulting in significant increases in BP.
2.3. Estimation of sympathetic reserve
There are indirect and noninvasive clinical indicators that can help us estimate individual sympathetic reserve (Biaggioni, 2014; Biaggioni, 2017). The magnitude of the compensatory orthostatic tachycardia is one of them. Patients with neurogenic OH are unable to appropriately increase HR in response to the drop in BP, so that on average, for every two mmHg drop in BP, patients with autonomic failure only increase their HR by one beat per minute (Norcliffe-Kaufmann et al., 2018). A greater orthostatic tachycardia implies either intact cardiovagal function or relative preserved sympathetic reserve. Of greater practical importance, it can alert the physician of the presence of factors that trigger or worsen OH such as medications. The BP response to the Valsalva maneuver can also provide an estimate of the severity of sympathetic impairment (Low et al., 2013). Autonomic failure is characterized by an exaggerated and sustained decrease in BP during strain (phase 2) and the absence of overshoot after release (phase 4). The time it takes for BP to return to baseline during phase 4 can be an indicator of disease severity (Vogel et al., 2005). However, this method requires continuous BP monitoring which is not always available in general practice. Measurement of plasma NE is arguably a more direct method to assess sympathetic reserve of peripheral postganglionic neurons; plasma NE is normal or only slightly low in OH patients with central nervous lesions, whereas patients with diffuse peripheral nervous lesions typically show reduced or very low plasma NE levels. In a research setting, samples are drawn after patients rest supine for at least 10 minutes to avoid any unintentional stimulation of NE (Grouzmann et al., 2013), but even when measured in these carefully-controlled conditions, there is a substantial overlap between patients with central or peripheral disease. Nonetheless, in clinical practice even measuring random seated NE in a regular clinical laboratory may provide useful information.
3. Pharmacologic treatments of OH
3.1. Physiologic targets to increase blood pressure
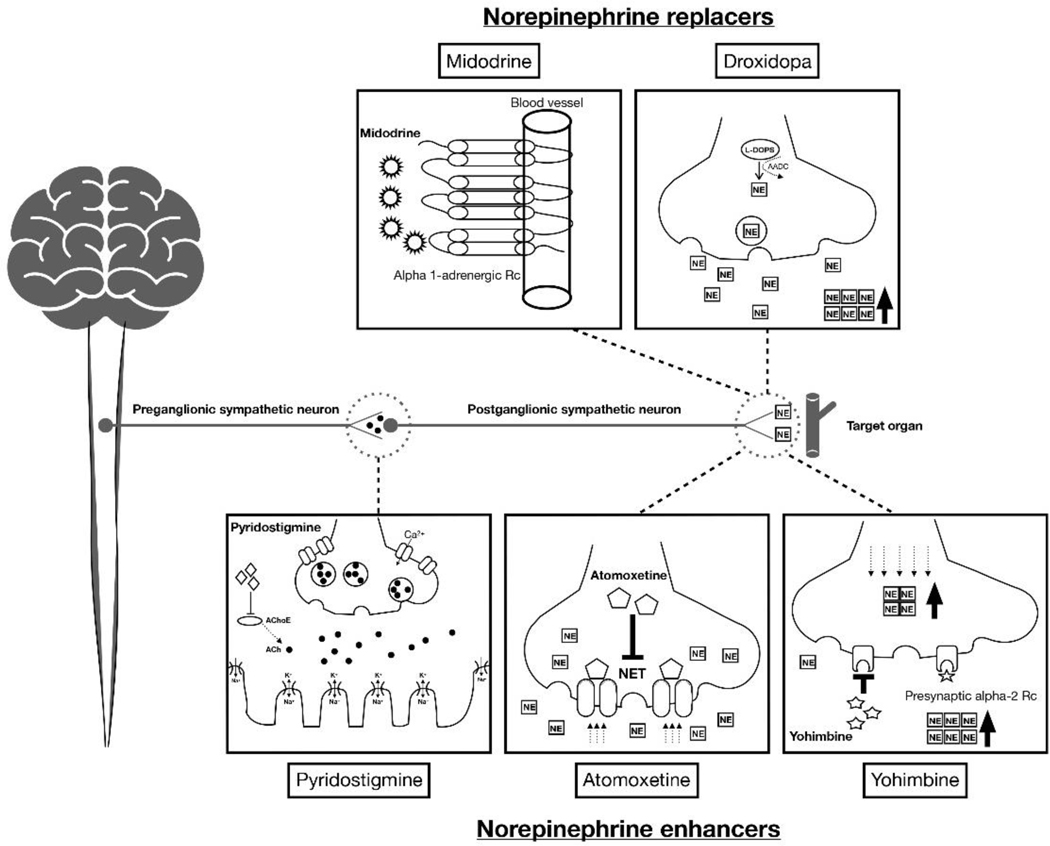
The activation of sympathetic pathways and thereby stimulating autonomic effectors (e.g., smooth and cardiac muscles) via alpha or beta receptor activation is the major mechanism to increase the blood pressure either by contraction of the blood vessel (i.e., increasing total peripheral resistance), or increasing the heart rate and contractility (i.e., increasing cardiac output). As discussed above, NE plays the most essential role in this mechanism. Therefore, the major pharmacologic treatments targets to replace or enhance NE which is mostly deficient in OH patients (Figure 1). These treatments will eventually be attributed to direct alpha-receptor activation (NE replacement) or more physiologically enhance the remaining potential NE function by stimulating the secretion, or preventing the degradation of NE (NE enhancement).
Figure 1.
Schematic mechanism of action in norepinephrine replacers and enhancers.
3.2. Norepinephrine replacers
3.2.1. Midodrine
Midodrine is an oral prodrug that is metabolized to the alpha 1-adrenoreceptor agonist desglymidodrine. Midodrine activates alpha 1A and 1B receptors to trigger peripheral arterial and venous constriction, thus increasing peripheral vascular resistance and elevating BP without increasing HR. The active metabolite of midodrine rapidly reaches peak blood concentrations (Cmax) in 1–2 hours (i.e., tmax), and has an elimination half-life of approximately 3–4 hours (Lamarre-Cliche et al., 2008). The initial dose is 2.5 mg, three times daily, and can be escalated up to 10 mg (Gibbons et al., 2017; Palma et al., 2020a). Standing systolic BP can increase by 10 to 30 mmHg after a 10 mg dose of midodrine within an hour, and the effect persists for another 2–3 hours (Palma et al., 2017; Zachariah et al., 1986). The short-term efficacy of midodrine has been proven in several clinical research studies (Byun et al., 2017; Low et al., 1997; Singer et al., 2014). In a randomized, double-blind multicenter clinical trial for neurogenic OH, midodrine was safe and effective both in improving upright BP and orthostatic symptoms during a 6-week study period (10 mg, three times per day) (Low et al., 1997). However, there is still uncertainty about its long-term efficacy. The major adverse effects using midodrine include urinary symptoms (retention, hesitation, urgency), goosebumps and piloerection (“itchy scalp”) and, importantly, worsening of supine hypertension (Wright et al., 1998).
3.2.2. Droxidopa
Droxidopa (L-threo-3,4-dihydroxyphenylserine, L-DOPS) is an oral synthetic NE prodrug approved by the FDA in 2014 for the treatment OH. It is converted to NE by L-aromatic-amino-acid decarboxylase (AAAD), the same enzyme that converts levodopa to dopamine, which is widely expressed in most organs (Berry et al., 1996). Droxidopa can penetrate the blood-brain barrier (BBB) but its pressor effect is due to its peripheral actions because it is blocked by high-dose carbidopa (an inhibitor of AAAD that does not cross the BBB) (Kaufmann et al., 2003). An integrated analysis of three randomized double-blinded clinical trials that included a total of 460 patients demonstrated an improvement in OH symptoms compared to placebo (Biaggioni et al., 2017). Open-label observations suggest durability of efficacy after 6-month use, with improvement of symptoms, functionality, and quality of life, including a reduction in falls (Francois et al., 2019). The tmax of droxidopa is 2 hours, and its elimination half-life is around 2.5 hours. The dose should be titrated from 100 to 600 mg three times during the daytime with the last dose given earlier to avoid supine hypertension (8 am, noon, and 4 pm) (Chen et al., 2018; Kaufmann et al., 2003). A potential consideration is the use of droxidopa in patients with PD taking carbidopa in combination with levodopa. Whereas high-doses of carbidopa can reduce the conversion of droxidopa to NE (Kaufmann et al., 2003), in clinical practice, dose titration of droxidopa seems to overcome this limitation, and thus droxidopa can be effective in PD patients with OH (Biaggioni et al., 2017). The main adverse effects of using droxidopa include headache, dizziness, nausea, and supine hypertension (Palma et al., 2020a), but in general, it seems to have a better side effect profile than midodrine. In particular, urinary retention has not been reported, and the incidence of supine hypertension seems to be lower. The integrated analysis of the pivotal clinical trials of this drug suggested that droxidopa is not as effective in controlling OH symptoms in MSA compared to PAF and PD patients (Biaggioni et al., 2017), in agreement with our concept that “norepinephrine replacers” will be more effective in patients with low sympathetic reserve. This phenomenon likely contributes to the interindividual variability in response to droxidopa observed in NOH.
3.3. Norepinephrine enhancers
3.3.1. Pyridostigmine
Pyridostigmine is an orally active inhibitor of cholinesterase, the enzyme that hydrolyzes acetylcholine in the synaptic cleft, thus terminating its action. The use of this medication for the treatment of OH is based on the concept that it facilitates cholinergic neurotransmission in autonomic ganglia thereby increasing sympathetic drive. It has the theoretical advantage that this effect will be greater during the standing position when neurotransmission at sympathetic autonomic ganglia is increased, thus preferentially increasing upright BP without worsening supine hypertension (Singer et al., 2003). Based on this mechanism of action, pyridostigmine may be more effective in patients with sympathetic reserve. In patients with severe OH the effect tends to be modest (Singer et al., 2003; Singer et al., 2006). The suggested dosage for pyridostigmine is 30 to 60 mg, 2 to 3 times per day (tmax: 1.5 hours, elimination half-life: 3 to 4 hours) (Agarwal et al., 2007; Palma et al., 2020a; Singer et al., 2006). Currently, this medication is used “off-label” and not specifically approved for the treatment of OH. Dose-limiting side effects are mostly gastrointestinal, including abdominal cramps, nausea, and diarrhea (Palma et al., 2020a).
3.3.2. Atomoxetine
Atomoxetine is an oral NE transporter (NET) blocker. NET is a monoamine transporter located presynaptically in postganglionic sympathetic nerves. By reuptaking NE from the synapse, it terminates its actions and restores intracellular NE (Hahn, 2004). Inhibition of NET increases NE concentrations in the synaptic cleft leading to a pressor response. Among patients with severe autonomic failure, atomoxetine has been shown to be effective in those with preserved peripheral sympathetic fibers function (i.e., MSA) but not in those with low sympathetic reserve (i.e., PAF) (Biaggioni, 2017). In patients with milder forms of peripheral autonomic impairment, however, atomoxetine can be effective likely due to denervation hypersensitivity (Shibao et al., 2007c).
Although the use of atomoxetine for OH is not yet approved by the FDA, several clinical trials suggested the efficacy and safety of this approach (Byun et al., 2020; Okamoto et al., 2019; Palma et al., 2020a; Ramirez et al., 2014). The drug is effective at pediatric doses of 10 to 18 mg twice per day, underscoring the hypersensitivity of these patients. A comparative study found atomoxetine superior to midodrine in improving orthostatic symptoms (Ramirez et al., 2014). Finally, there is a synergistic interaction between atomoxetine and pyridostigmine so that even patients with a low sympathetic reserve who do not respond to either drug alone, may respond to the combination (Okamoto et al., 2019). Atomoxetine reaches a tmax in 1 to 2 hours and has an elimination half-life of 5 hours (Simpson et al., 2004). Side effects of dry mouth, insomnia, loss of appetite, supine hypertension, suicidal ideation have been reported (Palma et al., 2020a).
3.3.3. Yohimbine
Yohimbine is an oral alpha-2 adrenergic antagonist (Tam et al., 2001). It is the pharmacological opposite to the alpha-2 agonist clonidine; in the CNS it increases central sympathetic outflow and in peripheral noradrenergic nerves it potentiates the release of NE. Given this mechanism of action, yohimbine should be more effective in the treatment of OH patients with preserved sympathetic function, but empirically it is also helpful in patients with peripheral disease. Efficacy data is limited; a clinical study showed short-term efficacy and safety with the administration of 5.4 mg oral dose, up to three times per day (Shibao et al., 2010). Another study showed a synergistic pressor effect between yohimbine and atomoxetine in patients with low sympathetic reserve who do not respond to either drug alone, suggesting that increasing residual sympathetic outflow with yohimbine can potentiate the pressor effect of atomoxetine (Okamoto et al., 2012). Both tmax and elimination half-life are less than one hour, but the duration of action is longer. Side effects of yohimbine include sweating, insomnia, palpitation, suicidal ideation, and supine hypertension (Tam et al., 2001). Yohimbine is no longer available as a commercial pharmaceutical but can be compounded in specialty pharmacies.
3.4. Nonspecific treatments
3.4.1. Fludrocortisone
Fludrocortisone acetate is an oral synthetic corticosteroid with potent mineralocorticoid effects and comparatively weak glucocorticoid activity. It has been clinically used in the treatment of OH by over 40 years, based on its role as an interstitial volume expander by promoting sodium reabsorption in the kidney. The increase in plasma volume, however, is transient and the sustained increase in BP in patients with OH is possibly due to potentiation of the pressor effects of endogenous NE and angiotensin II (Hickler et al., 1959; Ten Harkel et al., 1992). The observation that mineralocorticoid receptor blockade acutely lowers BP in OH patients, independent from volume regulation, also suggests off-target actions (Arnold et al., 2016). Its tmax is 2 hours with an elimination half-life of 4 to 6 hours (Ribot et al., 2013). Usual doses are 0.1–0.2 mg once daily (Palma et al., 2020a). Evidence of efficacy is largely based on clinical experience; there are only a series of case reports for the efficacy of fludrocortisone in the treatment of OH in the 1970s (Campbell et al., 1976; Hoehn, 1975). A recent efficacy study of fludrocortisone in 13 Parkinson’s patients with OH showed that fludrocortisone (0.2 mg per day) was beneficial in reducing the orthostatic fall in diastolic BP and improving standing mean BP (Schreglmann et al., 2017). Hypokalemia and hypomagnesemia occurs in a significant percentage of patients (Robertson, 2004). Other potential adverse effects include headache, edema, and supine hypertension (Robertson, 2004). Adrenal suppression can occur if higher doses (>0.3 mg a day) are used. Compared with midodrine, fludrocortisone has been shown to increase all-cause hospitalization in patients with OH (Grijalva et al., 2017). This medication should not be used in patients with hypertension or congestive heart failure.
3.4.2. Octreotide
Octreotide is a stable somatostatin analog that inhibits the release of a number of gastrointestinal vasodilating peptides, increasing cardiac output by acting as a splanchnic vasoconstrictor. Its effect was found to be comparable to midodrine and was particularly effective for the prevention of postprandial hypotension (Hoeldtke et al., 1998). The recommended doses are 12.5 to 25 μg subcutaneously (tmax: 30 minutes, elimination half-life: 2 hours) (Harris, 1994; Hoeldtke et al., 1998) prior to meals. Adverse effects include nausea, abdominal cramps, diarrhea, flatulence, and fat malabsorption (Lamberts et al., 1996). In our experience, octreotide is particularly useful in patients that are refractory to other treatments.
4. Pharmacologic treatment in specific clinical conditions associated with OH
4.1. Supine hypertension
Supine hypertension is present in the majority of OH patients, even in those with no previous history of essential hypertension. Thus, supine hypertension and OH may be a hemodynamically opposite manifestation of the same disease as the baroreflex is not able to counteract either one. Its presence complicates the management of OH, and the argument has been made that the treatment of OH should take precedence over that of supine hypertension (Espay et al., 2016). However, supine hypertension is associated with end-organ damage and renal impairment (Garland et al., 2009; Vagaonescu et al., 2000). Furthermore, supine hypertension induces pressure diuresis, a normal renal compensatory mechanism aimed at normalizing BP. Thus, nocturnal supine hypertension begets early morning OH by causing nighttime volume depletion.
Attempts should be made, therefore, to manage supine hypertension without worsening OH. One approach is to use short-acting antihypertensives at bedtime to reduce daytime carryover effects. OH patients are sensitive to the vasodilatory effects of nitric oxide (NO) (Gamboa et al., 2012) and several treatment options related to NO mechanisms are effective in controlling supine hypertension, including nitroglycerine patch (0.1 mg per hour) (Shibao et al., 2006), nebivolol (Okamoto et al., 2014), and sildenafil (Okamoto et al., 2014). Targeting the angiotensin-aldosterone system with losartan (Arnold et al., 2013a) and eplerenone (Arnold et al., 2016) can also be effective. The efficacy of these treatments, however, has only been assessed in acute trials under controlled conditions.
4.2. Heart failure
Heart failure is one of the most common comorbidities associated with OH (Ricci et al., 2015; Shibao et al., 2007b), and one that requires a distinct approach. In general, fludrocortisone should be avoided in this patient population; fludrocortisone increases the risk of hospitalizations not only for heart failure but for all causes (Grijalva et al., 2017). Midodrine is often used in the treatment of OH because of its short duration of action (Arnold et al., 2013b), but concerns have been raised because of supine hypertension (Olshansky et al., 2020). We prefer droxidopa for the treatment of OH in heart failure patients. In a small cohort of patients, we found droxidopa to be well-tolerated, and the persistence on medication was more than 60% at 6 months (McDonell et al., 2019). Beta-blockers can be combined with droxidopa if indicated for cardioprotection, because they do not lower BP significantly in autonomic failure patients (Okamoto et al., 2014). However, vasodilating beta-blockers with alpha-blocking actions (e.g., carvedilol) should be avoided if possible.
4.3. Postprandial hypotension
Postprandial hypotension is defined as a fall in systolic BP greater than 20 mmHg within 2 hours after meals (Jansen et al., 1995). It likely represents normal splanchnic venous pooling, and a small effect can be demonstrated in healthy individuals, but hypotension is made evident by autonomic impairment (Luciano et al., 2010). Postprandial hypotension can increase the risk of syncope and falls. Pharmacological treatments include octreotide and caffeine (Hoeldtke et al., 1998; Onrot et al., 1985) based on their possible roles in antagonizing vasodilatory peptide release, and adenosine antagonist effect, respectively. The alpha-glucosidase inhibitor acarbose has been shown the be effective in attenuating postprandial hypotension and we consider it the preferred initial therapy. Doses are 25–50 mg, given 10 minutes prior to meals. Contraindications include co-morbid conditions such as inflammatory bowel diseases. The mechanism of action is the decrease in absorption of simple carbohydrates that triggers the release of GUT peptide (Shibao et al., 2007a; Zhang et al., 2017).
4.4. Parkinson’s disease
OH is one of the premotor symptoms of the PD, which may manifest with or prior to classic PD symptoms such as tremor, bradycardia, and rigidity. The prevalence of OH in PD is very frequent. In an investigation of over 7 years from the time of diagnosis, more than 65% of patients experienced OH during this period (Hiorth et al., 2019). A substantial portion of these patients also suffered supine hypertension (41.3%). Clinicians should also aware that such medications used to treat PD can cause OH (e.g., dopamine agonists). Therefore, it is important to speculate and investigate the underlying OH symptoms in PD patients and provide adequate treatment options based on this. As discussed above, PD patients are more likely to benefit by use of NE replacers considering their loss of function in postganglionic neurons. However, there are still some portion of patients who are non-neurogenic orthostatic hypotension (Palma et al., 2020b). In these patients, the sympathetic reserve is relatively preserved with normal or enhanced NE level upon standing, and should be more benefited from non-pharmacologic treatment and volume expanders.
4.5. Multiple system atrophy
MSA is a progressive neurodegenerative disorder and central autonomic failure is one of the hallmarks of MSA in addition to motor symptoms which results in severe neurogenic OH. More than 50% of patients are known to manifest orthostatic hypotension without profound impairment of sympathetic reserve. Pathologically, peripheral sympathetic fibers are relatively spared and sympathetic reserve can be harnessed in these patients as discussed above (Jordan et al., 2015). Therefore, ideally, NE enhancers can be used as a treatment of choice to start with, if non-pharmacologic treatment are not sufficient. We have shown a greater pressor effect of atomoxetine in MSA patients compared to patients with peripheral autonomic failure (Shibao et al., 2012). However, the clinical diagnosis of MSA is challenging in the early stages of disease; therefore, estimation of sympathetic reserve can be useful to select a proper initial treatment. Denervation hypersensitivity and supine hypertension in these patients should be carefully monitored before pharmacologic treatment. It has been reported that 37% of patients with MSA possesses supine hypertension and there is a loss of nocturnal dipping in up to 75% of patients (Fanciulli et al., 2018).
4.6. Diabetes
Autonomic neuropathy is a common complication in both type 1 and type 2 diabetes mellitus (DM). In the 10 years of the follow-up study, OH was present in 31.7 of patients with type 1 DM and 32.3% with type 2 DM, and prevalence was correlated with other DM associated complications (Gaspar et al., 2016). Because cardiovascular autonomic neuropathy and the presence of OH is known to be an independent risk factor for mortality in OH, the early diagnosis of these complications is important (Soedamah-Muthu et al., 2008). The immediately optimized blood glucose control and lifestyle modification is the first step to prevent worsening of OH symptoms and disease progression (Pop-Busui et al., 2017), especially in type 1 DM. Certain medications such as tricyclic antidepressants, diuretics, and alpha-adrenoreceptor antagonists, which are commonly used in diabetic patients for various purposes should be carefully removed based on risk-benefit considerations before starting pharmacologic treatment (Fisher et al., 2017).
5. Conclusion
It is important to emphasize that nonpharmacologic treatment is always the first step in the management of OH. However, many patients will require pharmacological support. The pharmacologic treatment of OH may seem daunting, but understanding the underlying pathophysiology, and the basic clinical pharmacology of available therapies, can guide us to individualized optimal treatments.
Table 1.
Current pharmacologic treatment options for orthostatic hypotension.
| Treatment | Mechanism of action | Dosage recommendation | Administration | Adverse effects |
|---|---|---|---|---|
| Norepinephrine replacers | ||||
| Midodrine | Alpha-1 adrenoceptor agonist | Start with a 2.5 to 5 mg dose, increase up to 10 mg, up to 3 times/day | orally | Urinary retention, piloerection, supine hypertension |
| Droxidopa | Prodrug, converted to norepinephrine | 100 to 600 mg, 3 times/day | orally | Headache, nausea, supine hypertension |
| Norepinephrine enhancers | ||||
| Pyridostigmine | Cholinesterase inhibitor | 30 to 60 mg, up to 3 times/day | orally | Abdominal discomfort, diarrhea, nausea, urinary frequency |
| Atomoxetine | NE transporter (NET) inhibitor | 10 to 18 mg, 3 times/day | orally | Dry mouth, insomnia, loss of appetite, supine hypertension, suicidal ideation |
| Yohimbine | Alpha-2 adrenoreceptor antagonist | 5.4 mg, up to 3 times/day (available through compounding pharmacies only) | orally | Sweating, insomnia, palpitation, suicidal ideation, supine hypertension |
| Nonspecific treatments | ||||
| Fludrocortisone | Increasing renal sodium reabsorption and alpha-1 adrenoceptor sensitization | 0.05 to 0.2 mg/day | orally | Hypokalemia, headache, edema, adrenal suppression, supine hypertension |
| Octreotide | Somatostatin analogue | 12.5–25 μg, up to 1–3 times/day | subcutaneously | Nausea, abdominal cramps, diarrhea, flatulence, fat malabsorption |
Highlights.
-
►
Nonpharmacologic countermeasures are always the first step in the management of OH, and pharmacologic treatments are then added to the management of OH.
-
►
Treatment selection should consider the underlying pathophysiology. Patients with degeneration of peripheral noradrenergic neurons and low plasma norepinephrine (pure autonomic failure, Parkinson disease) tend to respond better to “norepinephrine replacers” (midodrine, droxidopa).
-
►
Patients with relatively spared sympathetic reserve and normal or mildly reduced plasma norepinephrine (multiple system atrophy) tend to respond better to “norepinephrine enhancers” (pyridostigmine, atomoxetine, yohimbine).
-
►
Associated clinical conditions such as supine hypertension, heart failure, and postprandial hypotension need to be considered in the pharmacological management of these patients.
Acknowledgements
Funded in part by HL122847, HL149386, HL144568 and DK11175 from the National Institutes of Health, FD0004778 from the Food and Drug Administration, and by a KNA-20-Neuro Frontier Fellowship Award from the Korean Neurological Association.
Disclosures
IB and CAS have received consultant fees and research support from Lundbeck and Theravance Biopharma, Inc. IB and LO are patent holders for the use of an automated binder in the treatment of orthostatic hypotension.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. References
- Agarwal S, Gowda KV, Mandal U, Ghosh D, Bose A, Sarkar AK, Pal TK, Chattaraj TK 2007. Analysis of pyridostigmine bromide in human plasma and its application in bioequivalence studies. Journal of liquid chromatography & related technologies 30, 2605–2615. [Google Scholar]
- Arnold AC, Biaggioni I. 2012. Management approaches to hypertension in autonomic failure. Curr Opin Nephrol Hypertens 21, 481–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AC, Okamoto LE, Gamboa A, Black BK, Raj SR, Elijovich F, Robertson D, Shibao CA, Biaggioni I. 2016. Mineralocorticoid Receptor Activation Contributes to the Supine Hypertension of Autonomic Failure. Hypertension 67, 424–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AC, Okamoto LE, Gamboa A, Shibao C, Raj SR, Robertson D, Biaggioni I. 2013a. Angiotensin II, independent of plasma renin activity, contributes to the hypertension of autonomic failure. Hypertension 61, 701–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AC, Shibao C. 2013b. Current concepts in orthostatic hypotension management. Curr Hypertens Rep 15, 304–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarroch EE 2020. Physiology and Pathophysiology of the Autonomic Nervous System. Continuum (Minneap Minn) 26, 12–24. [DOI] [PubMed] [Google Scholar]
- Berry MD, Juorio AV, Li XM, Boulton AA 1996. Aromatic L-amino acid decarboxylase: a neglected and misunderstood enzyme. Neurochem Res 21, 1075–1087. [DOI] [PubMed] [Google Scholar]
- Biaggioni I. 2014. New developments in the management of neurogenic orthostatic hypotension. Curr Cardiol Rep 16, 542. [DOI] [PubMed] [Google Scholar]
- Biaggioni I. 2017. The Pharmacology of Autonomic Failure: From Hypotension to Hypertension. Pharmacol Rev 69, 53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biaggioni I, Arthur Hewitt L, Rowse GJ, Kaufmann H. 2017. Integrated analysis of droxidopa trials for neurogenic orthostatic hypotension. BMC Neurol 17, 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byun JI, Kim DY, Moon J, Shin HR, Sunwoo JS, Lee WJ, Lee HS, Park KI, Lee ST, Jung KH, Jung KY, Kim M, Lee SK, Chu K. 2020. Efficacy of atomoxetine versus midodrine for neurogenic orthostatic hypotension. Ann Clin Transl Neurol 7, 112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byun JI, Moon J, Kim DY, Shin H, Sunwoo JS, Lim JA, Kim TJ, Lee WJ, Lee HS, Jun JS, Park KI, Lee ST, Jung KH, Jung KY, Lee SK, Chu K. 2017. Efficacy of single or combined midodrine and pyridostigmine in orthostatic hypotension. Neurology 89, 1078–1086. [DOI] [PubMed] [Google Scholar]
- Campbell IW, Ewing DJ, Clarke BF 1976. Therapeutic experience with fludrocortisone in diabetic postural hypotension. Br Med J 1, 872–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JJ, Hewitt LA 2018. Comparison of the Pharmacokinetics of Droxidopa After Dosing in the Fed Versus Fasted State and with 3-Times-Daily Dosing in Healthy Elderly Subjects. Drugs R D 18, 77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coon EA, Cutsforth-Gregory JK, Benarroch EE 2018. Neuropathology of autonomic dysfunction in synucleinopathies. Mov Disord 33, 349–358. [DOI] [PubMed] [Google Scholar]
- Espay AJ, LeWitt PA, Hauser RA, Merola A, Masellis M, Lang AE 2016. Neurogenic orthostatic hypotension and supine hypertension in Parkinson’s disease and related synucleinopathies: prioritisation of treatment targets. Lancet Neurol 15, 954–966. [DOI] [PubMed] [Google Scholar]
- Fanciulli A, Jordan J, Biaggioni I, Calandra-Buonaura G, Cheshire WP, Cortelli P, Eschlboeck S, Grassi G, Hilz MJ, Kaufmann H, Lahrmann H, Mancia G, Mayer G, Norcliffe-Kaufmann L, Pavy-Le Traon A, Raj SR, Robertson D, Rocha I, Struhal W, Thijs R, Tsioufis KP, van Dijk JG, Wenning GK 2018. Consensus statement on the definition of neurogenic supine hypertension in cardiovascular autonomic failure by the American Autonomic Society (AAS) and the European Federation of Autonomic Societies (EFAS) : Endorsed by the European Academy of Neurology (EAN) and the European Society of Hypertension (ESH). Clin Auton Res 28, 355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher VL, Tahrani AA 2017. Cardiac autonomic neuropathy in patients with diabetes mellitus: current perspectives. Diabetes Metab Syndr Obes 10, 419–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francois C, Shibao CA, Biaggioni I, Duhig AM, McLeod K, Ogbonnaya A, Quillen A, Cannon J, Padilla B, Yue B, Orloski L, Kymes SM 2019. Six-Month Use of Droxidopa for Neurogenic Orthostatic Hypotension. Mov Disord Clin Pract 6, 235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman R, Wieling W, Axelrod FB, Benditt DG, Benarroch E, Biaggioni I, Cheshire WP, Chelimsky T, Cortelli P, Gibbons CH, Goldstein DS, Hainsworth R, Hilz MJ, Jacob G, Kaufmann H, Jordan J, Lipsitz LA, Levine BD, Low PA, Mathias C, Raj SR, Robertson D, Sandroni P, Schatz IJ, Schondorf R, Stewart JM, van Dijk JG 2011. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Auton Neurosci 161, 46–48. [DOI] [PubMed] [Google Scholar]
- Gamboa A, Okamoto LE, Diedrich A, Choi L, Robertson D, Farley G, Paranjape S, Biaggioni I. 2012. Sympathetic activation and nitric oxide function in early hypertension. Am J Physiol Heart Circ Physiol 302, H1438–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EM, Gamboa A, Okamoto L, Raj SR, Black BK, Davis TL, Biaggioni I, Robertson D. 2009. Renal impairment of pure autonomic failure. Hypertension 54, 1057–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar L, Kruzliak P, Komornikova A, Celecova Z, Krahulec B, Balaz D, Sabaka P, Caprnda M, Kucera M, Rodrigo L, Uehara Y, Dukat A. 2016. Orthostatic hypotension in diabetic patients-10-year follow-up study. J Diabetes Complications 30, 67–71. [DOI] [PubMed] [Google Scholar]
- Gibbons CH, Schmidt P, Biaggioni I, Frazier-Mills C, Freeman R, Isaacson S, Karabin B, Kuritzky L, Lew M, Low P, Mehdirad A, Raj SR, Vernino S, Kaufmann H. 2017. The recommendations of a consensus panel for the screening, diagnosis, and treatment of neurogenic orthostatic hypotension and associated supine hypertension. J Neurol 264, 1567–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grijalva CG, Biaggioni I, Griffin MR, Shibao CA 2017. Fludrocortisone Is Associated With a Higher Risk of All-Cause Hospitalizations Compared With Midodrine in Patients With Orthostatic Hypotension. J Am Heart Assoc 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grouzmann E, Lamine F. 2013. Determination of catecholamines in plasma and urine. Best Pract Res Clin Endocrinol Metab 27, 713–723. [DOI] [PubMed] [Google Scholar]
- Hahn MK 2004. Norepinephrine transporter dysfunction, Primer on the Autonomic Nervous System. Elsevier; pp. 280–282. [Google Scholar]
- Harris AG 1994. Somatostatin and somatostatin analogues: pharmacokinetics and pharmacodynamic effects. Gut 35, S1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickler RB, Thompson GR, Fox LM, Hamlin JT 3rd. 1959. Successful treatment of orthostatic hypotension with 9-alpha-fluorohydrocortisone. N Engl J Med 261, 788–791. [DOI] [PubMed] [Google Scholar]
- Hiorth YH, Pedersen KF, Dalen I, Tysnes OB, Alves G. 2019. Orthostatic hypotension in Parkinson disease: A 7-year prospective population-based study. Neurology 93, e1526–e1534. [DOI] [PubMed] [Google Scholar]
- Hoehn MM 1975. Levodopa-induced postural hypotension. Treatment with fludrocortisone. Arch Neurol 32, 50–51. [DOI] [PubMed] [Google Scholar]
- Hoeldtke RD, Horvath GG, Bryner KD, Hobbs GR 1998. Treatment of orthostatic hypotension with midodrine and octreotide. J Clin Endocrinol Metab 83, 339–343. [DOI] [PubMed] [Google Scholar]
- Jacob G, Shannon JR, Costa F, Furlan R, Biaggioni I, Mosqueda-Garcia R, Robertson RM, Robertson D. 1999. Abnormal norepinephrine clearance and adrenergic receptor sensitivity in idiopathic orthostatic intolerance. Circulation 99, 1706–1712. [DOI] [PubMed] [Google Scholar]
- Jansen RW, Lipsitz LA 1995. Postprandial hypotension: epidemiology, pathophysiology, and clinical management. Ann Intern Med 122, 286–295. [DOI] [PubMed] [Google Scholar]
- Jordan J, Fanciulli A, Tank J, Calandra-Buonaura G, Cheshire WP, Cortelli P, Eschlboeck S, Grassi G, Hilz MJ, Kaufmann H, Lahrmann H, Mancia G, Mayer G, Norcliffe-Kaufmann L, Pavy-Le Traon A, Raj SR, Robertson D, Rocha I, Reuter H, Struhal W, Thijs RD, Tsioufis KP, Gert van Dijk J, Wenning GK, Biaggioni I. 2019. Management of supine hypertension in patients with neurogenic orthostatic hypotension: scientific statement of the American Autonomic Society, European Federation of Autonomic Societies, and the European Society of Hypertension. J Hypertens 37, 1541–1546. [DOI] [PubMed] [Google Scholar]
- Jordan J, Shibao C, Biaggioni I. 2015. Multiple system atrophy: using clinical pharmacology to reveal pathophysiology. Clin Auton Res 25, 53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann H, Saadia D, Voustianiouk A, Goldstein DS, Holmes C, Yahr MD, Nardin R, Freeman R. 2003. Norepinephrine precursor therapy in neurogenic orthostatic hypotension. Circulation 108, 724–728. [DOI] [PubMed] [Google Scholar]
- Lamarre-Cliche M, Souich P, Champlain J, Larochelle P. 2008. Pharmacokinetic and pharmacodynamic effects of midodrine on blood pressure, the autonomic nervous system, and plasma natriuretic peptides: a prospective, randomized, single-blind, two-period, crossover, placebo-controlled study. Clin Ther 30, 1629–1638. [DOI] [PubMed] [Google Scholar]
- Lamberts SW, van der Lely AJ, de Herder WW, Hofland LJ 1996. Octreotide. N Engl J Med 334, 246–254. [DOI] [PubMed] [Google Scholar]
- Low PA, Gilden JL, Freeman R, Sheng KN, McElligott MA 1997. Efficacy of midodrine vs placebo in neurogenic orthostatic hypotension. A randomized, double-blind multicenter study. Midodrine Study Group. Jama 277, 1046–1051. [PubMed] [Google Scholar]
- Low PA, Tomalia VA, Park KJ 2013. Autonomic function tests: some clinical applications. J Clin Neurol 9, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciano GL, Brennan MJ, Rothberg MB 2010. Postprandial hypotension. Am J Med 123, 281.e281–286. [DOI] [PubMed] [Google Scholar]
- McDonell KE, Preheim BA, Diedrich A, Muldowney JAS 3rd, Peltier AC, Robertson D, Biaggioni I, Shibao CA 2019. Initiation of droxidopa during hospital admission for management of refractory neurogenic orthostatic hypotension in severely ill patients. J Clin Hypertens (Greenwich) 21, 1308–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Hirayama M, Hara T, Hama T, Watanabe H, Sobue G. 2011. Does cardiovascular autonomic dysfunction contribute to fatigue in Parkinson’s disease? Mov Disord 26, 1869–1874. [DOI] [PubMed] [Google Scholar]
- Niimi Y, Ieda T, Hirayama M, Koike Y, Sobue G, Hasegawa Y, Takahashi A. 1999. Clinical and physiological characteristics of autonomic failure with Parkinson’s disease. Clin Auton Res 9, 139–144. [DOI] [PubMed] [Google Scholar]
- Norcliffe-Kaufmann L, Kaufmann H, Palma JA, Shibao CA, Biaggioni I, Peltier AC, Singer W, Low PA, Goldstein DS, Gibbons CH, Freeman R, Robertson D. 2018. Orthostatic heart rate changes in patients with autonomic failure caused by neurodegenerative synucleinopathies. Ann Neurol 83, 522–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto LE, Diedrich A, Baudenbacher FJ, Harder R, Whitfield JS, Iqbal F, Gamboa A, Shibao CA, Black BK, Raj SR, Robertson D, Biaggioni I. 2016. Efficacy of Servo-Controlled Splanchnic Venous Compression in the Treatment of Orthostatic Hypotension: A Randomized Comparison With Midodrine. Hypertension 68, 418–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto LE, Gamboa A, Shibao CA, Arnold AC, Choi L, Black BK, Raj SR, Robertson D, Biaggioni I. 2014. Nebivolol, but not metoprolol, lowers blood pressure in nitric oxide-sensitive human hypertension. Hypertension 64, 1241–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto LE, Shibao CA, Gamboa A, Diedrich A, Raj SR, Black BK, Robertson D, Biaggioni I. 2019. Synergistic Pressor Effect of Atomoxetine and Pyridostigmine in Patients With Neurogenic Orthostatic Hypotension. Hypertension 73, 235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olshansky B, Muldowney J. 2020. Cardiovascular Safety Considerations in the Treatment of Neurogenic Orthostatic Hypotension. The American Journal of Cardiology. [DOI] [PubMed] [Google Scholar]
- Onrot J, Goldberg MR, Biaggioni I, Hollister AS, Kingaid D, Robertson D. 1985. Hemodynamic and humoral effects of caffeine in autonomic failure. Therapeutic implications for postprandial hypotension. N Engl J Med 313, 549–554. [DOI] [PubMed] [Google Scholar]
- Palma JA, Kaufmann H. 2017. Epidemiology, Diagnosis, and Management of Neurogenic Orthostatic Hypotension. Mov Disord Clin Pract 4, 298–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma JA, Kaufmann H. 2020a. Management of Orthostatic Hypotension. Continuum (Minneap Minn) 26, 154–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma JA, Kaufmann H. 2020b. Orthostatic Hypotension in Parkinson Disease. Clin Geriatr Med 36, 53–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pop-Busui R, Boulton AJ, Feldman EL, Bril V, Freeman R, Malik RA, Sosenko JM, Ziegler D. 2017. Diabetic Neuropathy: A Position Statement by the American Diabetes Association. Diabetes Care 40, 136–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez CE, Okamoto LE, Arnold AC, Gamboa A, Diedrich A, Choi L, Raj SR, Robertson D, Biaggioni I, Shibao CA 2014. Efficacy of atomoxetine versus midodrine for the treatment of orthostatic hypotension in autonomic failure. Hypertension 64, 1235–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribot M, Polito A, Grassin-Delyle S, Annane D, Alvarez JC 2013. Human plasma quantification of fludrocortisone using liquid chromatography coupled with atmospheric pressure chemical ionization mass spectrometry after low-dosage administration. Clin Chim Acta 420, 109–113. [DOI] [PubMed] [Google Scholar]
- Ricci F, Fedorowski A, Radico F, Romanello M, Tatasciore A, Di Nicola M, Zimarino M, De Caterina R. 2015. Cardiovascular morbidity and mortality related to orthostatic hypotension: a meta-analysis of prospective observational studies. Eur Heart J 36, 1609–1617. [DOI] [PubMed] [Google Scholar]
- Robertson D. 2008. The pathophysiology and diagnosis of orthostatic hypotension. Clin Auton Res 18 Suppl 1, 2–7. [DOI] [PubMed] [Google Scholar]
- Robertson RM 2004. Fludrocortisone, Primer on the Autonomic Nervous System. Elsevier; pp. 411–412. [Google Scholar]
- Schreglmann SR, Buchele F, Sommerauer M, Epprecht L, Kagi G, Hagele-Link S, Gotze O, Zimmerli L, Waldvogel D, Baumann CR 2017. Pyridostigmine bromide versus fludrocortisone in the treatment of orthostatic hypotension in Parkinson’s disease - a randomized controlled trial. Eur J Neurol 24, 545–551. [DOI] [PubMed] [Google Scholar]
- Shannon J, Jordan J, Costa F, Robertson RM, Biaggioni I. 1997. The hypertension of autonomic failure and its treatment. Hypertension 30, 1062–1067. [DOI] [PubMed] [Google Scholar]
- Shannon JR, Diedrich A, Biaggioni I, Tank J, Robertson RM, Robertson D, Jordan J. 2002. Water drinking as a treatment for orthostatic syndromes. Am J Med 112, 355–360. [DOI] [PubMed] [Google Scholar]
- Shibao C, Gamboa A, Abraham R, Raj SR, Diedrich A, Black B, Robertson D, Biaggioni I. 2006. Clonidine for the treatment of supine hypertension and pressure natriuresis in autonomic failure. Hypertension 47, 522–526. [DOI] [PubMed] [Google Scholar]
- Shibao C, Gamboa A, Diedrich A, Dossett C, Choi L, Farley G, Biaggioni I. 2007a. Acarbose, an alpha-glucosidase inhibitor, attenuates postprandial hypotension in autonomic failure. Hypertension 50, 54–61. [DOI] [PubMed] [Google Scholar]
- Shibao C, Grijalva CG, Raj SR, Biaggioni I, Griffin MR 2007b. Orthostatic hypotension-related hospitalizations in the United States. Am J Med 120, 975–980. [DOI] [PubMed] [Google Scholar]
- Shibao C, Okamoto L, Biaggioni I. 2012. Pharmacotherapy of autonomic failure. Pharmacol Ther 134, 279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibao C, Okamoto LE, Gamboa A, Yu C, Diedrich A, Raj SR, Robertson D, Biaggioni I. 2010. Comparative efficacy of yohimbine against pyridostigmine for the treatment of orthostatic hypotension in autonomic failure. Hypertension 56, 847–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibao C, Raj SR, Gamboa A, Diedrich A, Choi L, Black BK, Robertson D, Biaggioni I. 2007c. Norepinephrine transporter blockade with atomoxetine induces hypertension in patients with impaired autonomic function. Hypertension 50, 47–53. [DOI] [PubMed] [Google Scholar]
- Simpson D, Plosker GL 2004. Atomoxetine: a review of its use in adults with attention deficit hyperactivity disorder. Drugs 64, 205–222. [DOI] [PubMed] [Google Scholar]
- Singer W, Joyner MJ, Sandroni P, Benarroch EE, Fealey RD, Mandrekar J, Low PA 2014. Midodrine efficacy in orthostatic hypotension. J Gen Intern Med 29, 1440–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer W, Opfer-Gehrking TL, McPhee BR, Hilz MJ, Bharucha AE, Low PA 2003. Acetylcholinesterase inhibition: a novel approach in the treatment of neurogenic orthostatic hypotension. J Neurol Neurosurg Psychiatry 74, 1294–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer W, Sandroni P, Opfer-Gehrking TL, Suarez GA, Klein CM, Hines S, O’Brien PC, Slezak J, Low PA 2006. Pyridostigmine treatment trial in neurogenic orthostatic hypotension. Arch Neurol 63, 513–518. [DOI] [PubMed] [Google Scholar]
- Soedamah-Muthu SS, Chaturvedi N, Witte DR, Stevens LK, Porta M, Fuller JH 2008. Relationship between risk factors and mortality in type 1 diabetic patients in Europe: the EURODIAB Prospective Complications Study (PCS). Diabetes Care 31, 1360–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam SW, Worcel M, Wyllie M. 2001. Yohimbine: a clinical review. Pharmacol Ther 91, 215–243. [DOI] [PubMed] [Google Scholar]
- Ten Harkel AD, Van Lieshout JJ, Wieling W. 1992. Treatment of orthostatic hypotension with sleeping in the head-up tilt position, alone and in combination with fludrocortisone. J Intern Med 232, 139–145. [DOI] [PubMed] [Google Scholar]
- Vagaonescu TD, Saadia D, Tuhrim S, Phillips RA, Kaufmann H. 2000. Hypertensive cardiovascular damage in patients with primary autonomic failure. Lancet 355, 725–726. [DOI] [PubMed] [Google Scholar]
- Vogel ER, Sandroni P, Low PA 2005. Blood pressure recovery from Valsalva maneuver in patients with autonomic failure. Neurology 65, 1533–1537. [DOI] [PubMed] [Google Scholar]
- Wright RA, Kaufmann HC, Perera R, Opfer-Gehrking TL, McElligott MA, Sheng KN, Low PA 1998. A double-blind, dose-response study of midodrine in neurogenic orthostatic hypotension. Neurology 51, 120–124. [DOI] [PubMed] [Google Scholar]
- Zachariah PK, Bloedow DC, Moyer TP, Sheps SG, Schirger A, Fealey RD 1986. Pharmacodynamics of midodrine, an antihypotensive agent. Clin Pharmacol Ther 39, 586–591. [DOI] [PubMed] [Google Scholar]
- Zhang J, Guo L. 2017. Effectiveness of acarbose in treating elderly patients with diabetes with postprandial hypotension. J Investig Med 65, 772–783. [DOI] [PubMed] [Google Scholar]