Abstract
Background:
Adolescent depression varies considerably in the course. However, there are no biobehavioral predictors of illness trajectories, and follow-up studies in depressed youth are sparse. Here we sought to examine whether reward function would predict future clinical outcomes in adolescents with depressive symptoms. We utilized the reward flanker fMRI task to assess brain function during distinct reward processes of anticipation, attainment and positive prediction error (PPE, i.e. receiving uncertain rewards).
Methods:
Subjects were 29 psychotropic-medication-free participants with mood and anxiety symptoms and 14 healthy controls (HC). All had psychiatric evaluations at baseline and approximately 24-month follow-up. Thirty-two adolescents (10 HC) had usable fMRI data. Correlation and hierarchical regression models examined symptom severity as predictors for follow-up clinical outcomes. Whole-brain analyses examined the relationships between neural reward processes and follow-up outcomes.
Results:
Clinically, anhedonia, but not irritability, predicted future depression and suicidal ideations. Among reward processes, only neural activation during PPE was correlated with future depression and anhedonia severity. Specifically, activation in the left angular gyrus—a component of default mode network—was associated with future depression, while activation in the dorsal anterior cingulate, operculum and left insula—key regions within the salience and pain networks—was associated with future anhedonia, even when controlling baseline anhedonia.
Limitations:
Small sample size and variability in follow-up intervals limit the generalizability of conclusions.
Conclusions:
This research suggests the anhedonia and reward dysfunction may predict a worse course in adolescent depression. The adolescents with anhedonia should be monitored more carefully for a longer period.
Keywords: adolescent depression, anhedonia, reward function, fMRI
Introduction
Adolescent depression is a devastating illness associated with significant morbidity, particularly suicide, the second leading cause of death in this age group (Heron, 2018; Whiteford et al., 2013). Importantly, adolescent depression varies considerably in course and severity, wherein some depressed youths fully recover, while others have a more severe and persistent illness or relapse in adulthood despite successful initial treatment (Andersen and Teicher, 2008; Birmaher and Axelson, 2006). For example, the multisite longitudinal NIMH “Treatment for Adolescents with Depression Study (TADS)” documented depression recurrence in 47% of remitted patients and 67% of non-responders among 196 participants (Curry et al., 2011). Other longitudinal studies have also found that only 37% of depressed adolescents will not experience a relapse in adulthood (Weissman et al., 1999). At present, there are no reliable bio-behavioral predictors of illness trajectory for adolescent depression.
One challenge is the heterogeneity of depression, which has impeded the development of reliable biomarkers. To address this obstacle, our laboratory and others have examined dimensions of behavioral symptoms instead of solely investigating outcomes based on categorical DSM diagnoses such as depression. We previously found that adolescents with moderate-to-severe depression exhibited a wide severity range of anhedonia and irritability, core symptoms of adolescent depression, emphasizing the importance of studying inter-individual differences in symptom severity (Gabbay et al., 2015). Additionally, only anhedonia, but not irritability, was associated with worse outcomes, including suicidality and chronicity (Gabbay et al., 2015). As anhedonia reflects deficits in reward function, these findings suggest that impaired neural reward activity may contribute to the maintenance of depression in youth. Indeed, a recent meta-analysis of fMRI and EEG studies in depression documented that reward system alterations preceded the onset of depression in adolescents (Keren et al., 2018b). Importantly, reward function is a complex construct involving reward anticipation, attainment and valuation phases (Lambert et al., 2018; Rømer Thomsen et al., 2015). Though longitudinal imaging studies remain sparse, a handful have suggested reduced fMRI brain activation during both reward expectation and reward attainment as predictors for future depression in non-depressed adolescents (Morgan et al., 2013; Stringaris et al., 2015). Moreover, a pair of recent EEG studies based on a large sample of 444 healthy 13–15 year old girls reported that blunted reward positivity and reduced delta band amplitude during reward attainment predicted future depressive symptoms (Nelson et al., 2018; Nelson et al., 2016). However, to date, no longitudinal studies have attempted to investigate multiple reward processes as predictors of outcomes in adolescents with depressive symptoms at baseline.
Building upon these observations, we sought to examine whether neural activation during the distinct reward processes would predict future clinical outcomes in depressed adolescents and healthy controls. To probe reward circuitry, we utilized the Reward Flanker Task (RFT; Bradley et al., 2017), which is a combination of the Monetary Incentive Delay (Knutson et al., 2000) and Flanker (Eriksen and Eriksen, 1974) tasks that allows the assessment of brain function during reward expectancy (i.e. anticipation of a reward), reward attainment (i.e. receiving a reward) and positive prediction error (i.e. receiving an unexpected/uncertain reward). The primary outcome measure was overall depression severity, while anhedonia severity was a secondary outcome that more directly reflects reward deficits. As reward function is highly relevant to many psychiatric conditions (Sharma et al., 2017), we adopted a Research Domain Criteria (RDoC) approach (Insel et al., 2010) in this pilot study and recruited a diverse sample of adolescents with mood and anxiety symptoms, as well as healthy controls. The inclusion of healthy controls was not for the purpose of group comparison, but rather allowing us to examine a full range of symptom severity and a larger variability of reward functions. As anhedonia has been associated with suicidal behavior, we further explored measures of reward function as predictors for future suicidal ideations. In line with our previous work (Gabbay et al., 2015), irritability severity was also examined as a predictor for clinical outcomes as it represents another core symptom of depression in youth. Based on prior findings, we hypothesized that anhedonia severity and all three RFT-derived measures of neural reward processes at baseline would predict anhedonia severity as well as clinical outcomes of depression, and suicidality severity at follow-up.
Methods
Participants
Participants consisted of 43 youths (age, M ± SD: 14.91 ± 2.10, range: 12–20 years; 26 females), of whom 29 participants were with diverse mood and anxiety symptoms and 14 were healthy controls (HC) with no significant presentation of psychiatric symptomatology or history of mental illness at the first visit. Participants were recruited from the New York metropolitan area through the Mount Sinai Child and Adolescent Psychiatry Outpatient Clinic, physician referrals, and advertisements in the community. Clinical follow-up averaged at approximately 2 years (M ± SD: 21.05 ± 10.91, range: 11–61 months). This follow-up study builds on our earlier cross-sectional studies examining a broad range of psychiatric conditions in youth. We invited back clinical subjects with a primary presentation of mood and/or anxiety symptoms as well as healthy controls (HC); clinical subjects with a primary presentation of externalizing disorder symptoms were not included. Approximately 67% of eligible subjects from our original cross-sectional cohort (N = 64) participated in this follow-up study. Depression severity and demographic characteristics did not differ between eligible participants and non-participants.
A subset of 32 youth (age, M ± SD: 14.88 ± 2.08, range: 12–20 years; 19 females) performed the RFT at baseline with usable MRI data collected. The remaining 11 participants were not included due to incomplete MRI acquisition (n = 5) and over 25% unusable runs (n = 6). Among the 32 included participants, 22 presented mood and anxiety symptoms and 10 were HC. Clinical data and demographics are compiled in Table 1.
Table 1.
Demographic and Clinical Characteristics
| Whole Group (N = 43) | RFT Group (N = 32) | |||
|---|---|---|---|---|
| Demographics | Baseline | Follow-up | Baseline | Follow-up |
| Age [M ± SD] (Range) | 14.91 ± 2.10 (12–20) | 16.70 ± 2.21 (13–21) | 14.88 ± 2.08 (12–20) | 16.72 ± 2.07 (13–21) |
| Gender [n Female/Male] (%) | 26/17 (60.47/39.53) | 19/13 (59.38/40.62) | ||
| Ethnicity [n Caucasian/African/Hispanic/Other] (%) | 17/17/5/4 (39.53/39.53/11.63/9.3) | 12/12/4/4 (37.5/37.5/12.5/12.5) | ||
| Months until follow-up [M ± SD] (Range) | 21.05 ± 10.91 (11–61) | 21.69 ± 11.79 (12–61) | ||
| Psychiatric Profile [n] (%) | ||||
| MDD | 16 (37.21) | 14 (32.56) | 11 (34.38) | 11 (34.38) |
| Dysthymia | 4 (9.3) | 2 (4.65) | 1 (3.13) | 1 (3.13) |
| DDNOS | 1 (2.33) | 0 (0) | 0 (0) | 0 (0) |
| Bipolar Disorder II | 1 (2.33) | 2 (4.65) | 2 (6.25) | 3 (9.38) |
| Anxiety | 19 (44.19) | 16 (37.21) | 15 (46.88) | 13 (40.62) |
| OCD | 1 (2.33) | 0 (0) | 1 (3.13) | 0 (0) |
| ODD | 5 (11.63) | 4 (9.3) | 1 (3.13) | 2 (6.25) |
| ADHD | 8 (18.6) | 10 (23.26) | 3 (9.38) | 6 (18.75) |
| Substance Use | 0 (0) | 4 (9.30) | 0 (0) | 2 (6.25) |
| Other | 0 (0) | 1 (2.33) | 0 (0) | 1 (3.13) |
| HC | 14 (32.56) | 14 (32.56) | 10 (31.25) | 10 (31.25) |
| Clinical Assessments [M ± SD] (Range) | ||||
| Med-naïve/Med-free/Medicated [n] (%) | 37/6/0 (86.05/13.96/0) | 30/9/4 (69.77/20.93/9.3) | 29/3/0 (90.62/9.38/0) | 22/6/4 (68.75/18.75/12.5) |
| CDRS-R | 32.60 ± 16.33 (17–72) | 33.88 ± 15.85 (17–71) | 31.31 ± 15.63 (17–72) | 34.91 ± 16.09 (17–71) |
| Anhedonia | 3.45 ± 2.88 (1–11)a | 3.19 ± 2.80 (1–11)a | 3.42 ± 2.87 (1–11)a | 3.47 ± 2.86 (1–11) |
| Irritability | 3.24 ± 2.44 (1–9)a | 2.69 ± 2.25 (1–8)a | 3.00 ± 2.28 (1–9)a | 2.88 ± 2.39 (1–8) |
| SHAPS | 22.98 ± 7.15 (14–43) | 21.14 ± 6.17 (14–38)a | 23.19 ± 7.60 (14–43) | 22.00 ± 6.34 (14–38) |
| Clinical Assessments in Mood and Anxiety Disorder Patients [M ± SD] (Range) | ||||
| CDRS-R | 39.55 ± 15.66 (18–72) | 40.86 ± 14.83 (19–71) | 37.32 ± 15.45 (18–72) | 41.95 ± 14.64 (19–71) |
| Anhedonia | 4.50 ± 2.89 (1–11)a | 4.18 ± 2.97 (1–11)a | 4.33 ± 2.92 (1–1)a | 4.45 ± 2.96 (1–11) |
| Irritability | 4.21 ± 2.41 (1–9)a | 3.50 ± 2.36 (1–8)a | 3.76 ± 2.36 (1–9)a | 3.68 ± 2.50 (1–8) |
| SHAPS | 23.66 ± 7.07 (15–43) | 22.32 ± 6.70 (14–38)a | 24.1o ± 7.89 (15–43) | 23.36 ± 6.80 (14–38) |
| BSSI | 3.10 ± 5.52 (0–20) | 4.21 ± 6.65 (0–23) | 3.64 ± 6.18 (0–20) | 5.09 ± 7.31 (0–23) |
Note: diagnoses and assessments were based on the DSM-IV to keep consistency across all participants over time. As participants could meet criteria for more than one disorder, totals not sum to 100%. MDD: major depressive disorder; DDNOS: depressive disorder not otherwise specified; Anxiety: includes generalized anxiety, social anxiety, phobia, post-traumatic stress disorder, panic, anxiety disorder not otherwise specified; OCD: obsessive compulsive disorder; ODD: oppositional defiant disorder; ADHD: attention-deficit hyperactivity disorder; HC: healthy controls, no history of psychiatric illness. CDRS-R: Children’s Depression Rating Scale-Revised; Anhedonia: sum of anhedonia-related items from CDRS-R (item 2) and BDI-II (item 4 and item 12); Irritability: sum of irritability-related items from CDRS-R (item 8) and BDI-II (item 17); SHAPS: Snaith-Hamilton Pleasure Scale; BSSI: Beck Scale for Suicide Ideation.
data missing from 1 participant.
The Institutional Review Board (IRB) at Icahn School of Medicine at Mount Sinai approved the study, and written informed consent was obtained from participants age 18 and older; those under the age of 18 provided signed assent, and a parent or legal guardian provided signed informed consent.
Inclusion and exclusion criteria
Inclusion criteria:
Healthy controls did not meet criteria for any lifetime psychiatric disorder at baseline and were psychotropic-medication naive. Subjects within the mood and anxiety group presented with mood and/or anxiety symptoms, either above or sub- threshold, based on clinical diagnosis.
Exclusionary criteria for all subjects included:
1) any physical or neurological conditions; 2) a low IQ (< 80) as assessed by the Kaufman Brief Intelligence Test (KBIT; Kaufman, 1990); 3) For those enrolled at the fMRI study additional exclusionary criteria included MRI contraindications, a positive drug toxicology at the day of the scan, and a positive pregnancy test in females. Mood and Anxiety group: additional exclusionary criteria included 1) current psychosis, pervasive developmental disorder, and substance abuse disorders; 2) use of any psychotropic medications at baseline visit for at least a month (3 months for medications with long half life time). However, psychotropic medication use was allowed at follow-up study.
Clinical assessments
All clinical assessments were conducted both at baseline and follow-up visits.
Clinical diagnostic procedures:
All participants were assessed using the Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version (KSADS-PL; Kaufman et al., 1997). A board-certified child/adolescent psychiatrist or a licensed clinical psychologist trained in administering the KSADS-PL carried out the diagnostic evaluation, with the final clinical report discussed between the Primary Investigator (a licensed child/adolescent psychiatrist) and the assessor.
Depression severity was measured by the clinician-rated Children’s Depression Rating Scale-Revised (CDRS-R; Poznanski et al., 1985) administered to the participant—as well as a parent when the participant was under the age of 18, with a score range of 17 to 113. The self-rated Beck Depression Inventory-Second Edition (BDI-II; Beck et al., 1996) was also collected but not directly used in analyses.
Anhedonia severity was assessed using the self-rated Snaith-Hamilton Pleasure Scale (SHAPS; Snaith et al., 1995), a 14 item scale constructed to minimize age, sex, and cultural influences, with a total score range of 14 to 56. The SHAPS is widely used and has been validated in children and adults (De Berardis et al., 2013; Farabaugh et al., 2015). In addition, a secondary measure of anhedonia severity was derived from answers to specific items on the clinician-rated CDRS-R and the self-rated BDI-II in order to have comparable measures for both anhedonia and irritability (see below). This approach has been used by our group and other laboratories (Gabbay et al., 2012a; Gabbay et al., 2013; Gabbay et al., 2015; Gabbay et al., 2012b; Henderson et al., 2013; McMakin et al., 2012). Specifically, anhedonia was measured based on the sum of one item from the CDRS-R (item 2: “Difficulty having fun,” rated 1–7) and two items from the BDI-II (item 4: “Loss of pleasure,” rated 0–3, and item 12: “Loss of interest,” rated 0–3), yielding a total score range of 1 to 13.
Suicidality severity was assessed by the 19 items self-rated Beck Scale for Suicide Ideation (BSSI; Beck et al., 1979) which evaluates suicidal thinking, with a total score range of 0 to 38.
Irritability severity was quantified by summing one item reflecting irritability from CDRS-R (item 8: “Irritability,” rated 1–7) and one from the BDI-II (item 17: “Irritability,” rated 0–3), with a total score range of 1 to 10.
MRI acquisition
Imaging data were acquired during baseline visit at Mount Sinai’s Brain Imaging Center on a 3T Skyra scanner (Siemens, Germany) with a 16+4 head-neck coil. Imaging parameters were similar to those used for the Human Connectome Project (HCP) LifeSpan protocols (Harms et al., 2018). High resolution (0.9mm isotropic) T1-weighted anatomical images were acquired using a MPRAGE sequence with the following parameters: TR = 2400 ms, TI = 1000 ms, TE = 2.06 ms, flip angle = 8°, FOV = 256 mm × 256 mm, 224 sagittal slices, 0.9 mm slice thickness (no gaps). Matched 0.9mm isotropic T2-weighted anatomical images were acquired using a SPACE sequence with the following parameters: TR = 3200 ms; TE = 566ms, flip angle = 120°, FOV = 256 mm × 256 mm, 224 sagittal slices, 0.9 mm slice thickness (no gaps). Functional T2*-weighted gradient echo multiband echo planar images (EPI) were acquired at 2.3mm isotropic resolution with alternating LR/RL phase-encoding directions and the following parameters: TR = 1000 ms, TE = 31.4 ms, flip angle = 60°, FOV = 624 mm × 720 mm, 60 transverse slices 2.3 mm slice thickness (no gaps), in-plane resolution = 2.3 mm × 2.3 mm, multiband factor = 5, 374 volumes in each of 4 runs. Additionally, a pair of spin-echo fieldmap images with LR/RL phase-encoding directions were acquired with the following parameters: TR = 6150 ms, TE = 57 ms, flip angle = 80°, FOV = 624 mm × 720 mm, 60 transverse slices, 2.3 mm slice thickness (no gaps), in-plane resolution = 2.3 mm × 2.3 mm. All participants completed a RFT training session in a mock scanner before the MRI scanning session.
Reward Flanker Task
During the RFT, participants were presented with a monetary cue, then made button presses and earned the cued reward amount if they correctly identified a target letter surrounded by four flanking letters during an allotted response interval (Bradley et al., 2017). During each trial, the monetary cue was presented for 4–6 s. Four cues were used: low reward (“10¢”), high reward (“50¢”), no reward (“0¢”), and uncertain reward (“?”). Uncertain reward cues (“?”) led to high (50¢), low (10¢) or no (0¢) reward with equal probability. After the cue, flanker stimulus was presented for 300 ms, followed by a response interval that was calibrated for each participant based on performance during the pre-scan training session (maximum 1700 ms). Participants then received feedback for 2 s informing them of the value of the obtained or unobtained reward. A total of 120 trials were presented in a pseudo-random event-related design over four runs, with 30 trials per run. After each run, participants were told how much money they had earned. Participants were informed of the performance-based bonus prior to RFT in order to increase motivation.
Statistical analysis for clinical measurements
All statistical analyses for clinical measures were performed in Matlab 2018b (The MathWorks, Inc.). To investigate the relationship between baseline anhedonia and clinical outcomes, we calculated the Pearson partial correlation coefficients between baseline SHAPS and follow-up CDRS-R, SHAPS, and BSSI while controlling for sex and baseline age. As suicidality is minimal in healthy adolescents (all BSSI = 0 in the current sample), predictors for suicidality were only examined in the clinical cohort.
Hierarchical regression was also used to study the unique contribution of anhedonia and irritability to long-term illness progression. For each regression analysis, the dependent variable was the follow-up CDRS-R score. There were three hierarchical models in each analysis: Model 1 only included the covariates of age and sex as predictors; Model 2 included one additional symptom, either irritability or anhedonia; and Model 3 included all predictors: age, sex, and both symptoms (anhedonia and irritability). Thus the improvement in R2 (i.e. the proportion of explained variance) from Model 2 to Model 3 reflected the unique contribution of the recently added anhedonia or irritability predictor, and its statistical significance was examined. Within the clinical cohort, additional hierarchical regression models were constructed to examine effect of baseline anhedonia and irritability on follow-up depression severity and suicidality ideation.
To evaluate the effect of variable follow-up interval, follow-up interval was also included as an additional covariates in both correlation analyses and hierarchical regression model with results detailed in supplementary materials.
RFT behavioral data analysis
Methods and results were detailed in supplementary materials.
MRI data analysis
MRI analyses followed the standard Human Connectome Project (HCP) minimal preprocessing pipeline (Glasser et al., 2013), including gradient non-linearity and fieldmap-based EPI distortion correction, realignment, and template normalization to standard Montreal Neurological Institute (MNI) space. Then, structured noise components were identified and removed using an automated independent components analysis classifier, ICA-FIX (Griffanti et al., 2014; Salimi-Khorshidi et al., 2014), which we verified as having high denoising sensitivity (>95%) and specificity (>99%) for our locally-acquired fMRI data. ICA-FIX was run on the concatenated RFT scans plus a 10 minute (600 volumes) resting-state fMRI scan collected immediately prior to RFT. All components identified as “unknown” were reviewed and, where appropriate, reclassified as “noise”; all final “noise” components were then regressed out of the concatenated timeseries, with all final “signal” and “unknown” components were retained. Runs with excessive motion (both RFT and resting-state), defined as more than 3% of frames with relative motion greater than 1 mm, were excluded from ICA-FIX and further analyses. Runs with empty response-dependent regressors (see below) were also eliminated from further analyses.
Spatial smoothing (4mm FWHM) and first-level (subject-level) analysis were performed in Statistical Parametric Mapping (SPM) version 12 (Wellcome Trust Centre for Neuroimaging, London, UK) running on Matlab 2015a. Eleven task-based regressors were specified: four for cues (high, low, no reward, and uncertain reward cues), six for feedback (high, low, and no reward feedback on correct trials, separately for certain and uncertain cues), and one for error feedback (incorrect trials, if applicable). Each regressor was convolved with the canonical hemodynamic response function using the general linear model (GLM). First-level contrasts examined: a) Reward Expectancy, defined as differential neural activation during reward cues (10¢ + 50¢) versus no reward cues (0¢); b) Reward Attainment, defined as differential neural activation while receiving reward feedback (10¢ + 50¢) versus no reward feedback (0¢) regardless of cue, and; c) positive prediction error (PPE), defined as neural activation receiving reward feedback (10¢ + 50¢) after uncertain cues (?) versus reward feedback (10¢ + 50¢) after certain cue.
For the group-level analyses, relationship between whole-brain activation during reward expectancy, reward attainment, and PPE at baseline and follow-up illness severity (CDRS-R) and anhedonia severity (SHAPS) were examined, including sex and baseline age as covariates of no interest. The associations between baseline neural reward activity and follow-up suicidality severity (BSSI) in the clinical cohort were also examined. Finding were repeated controlling baseline CDRS-R and SHAPS and are presented in supplementary materials. To evaluate the effect of variable follow-up interval, it was also included as an additional covariate, with results detailed in the supplementary materials. All group-level analyses were performed in FSL PALM (Winkler et al., 2014) using Threshold-Free Cluster Enhancement (TFCE) and permutation-based non-parametric statistics to controlled the family-wise error (FWE) rate. Results for main analyses were considered significant at the two-tailed pTFCE-FWE < 0.05 level; the exploratory suicidality analysis used a less stringent single-tailed pTFCE-FWE < 0.05 threshold. Significant clusters over 10 voxels and local peaks with minimum distance 15 mm were identified with the FSL cluster command. The brain regions were reported according to Harvard-Oxford atlases.
Results
Demographic and clinical features
Demographic and clinical characteristics of the whole group (N = 43) and fMRI RFT subgroup (n = 32) during both baseline and follow-up evaluations are presented in Table 1. As noted in the Methods, all participants were psychotropic-medication-free at baseline; 9 subjects started psychotropic medications after the baseline visit, of whom 4 subjects remained on medications while 5 subjects discontinued. Of the 4 patients who were taking medications, 3 had significant depressive symptoms while the fourth subject had CDRS-R score under 28 at both baseline and follow-up. See Supplementary Table 1 for medication details at follow-up. We also detail the breakdown of the clinical group into mood and anxiety subgroups in Supplementary Table 2, including baseline and follow-up assessment scores.
Clinical follow-up predictors
In the entire group of youths, depression severity was relatively stable across time. Baseline CDRS-R scores were highly correlated with follow-up CDRS-R scores (rho = 0.813, p = 1.096×10−10). In addition, baseline SHAPS scores were significantly correlated with follow-up CDRS-R scores (Figure 1 left, rho = 0.410, p = 0.008), as well as with follow-up SHAPS scores (Figure 1 center, rho = 0.662, p = 3.332×10−6) and, in the clinical cohort, BSSI scores (Figure 1 right, rho = 0.748, p = 4.656 ×10−6). Baseline SHAPS and follow-up CDRS-R scores remained correlated significantly after excluding the anhedonia-related question in the CDRS-R (item 2; see Methods) from the CDRS-R (rho = 0.417, p = 0.007).
Figure 1.
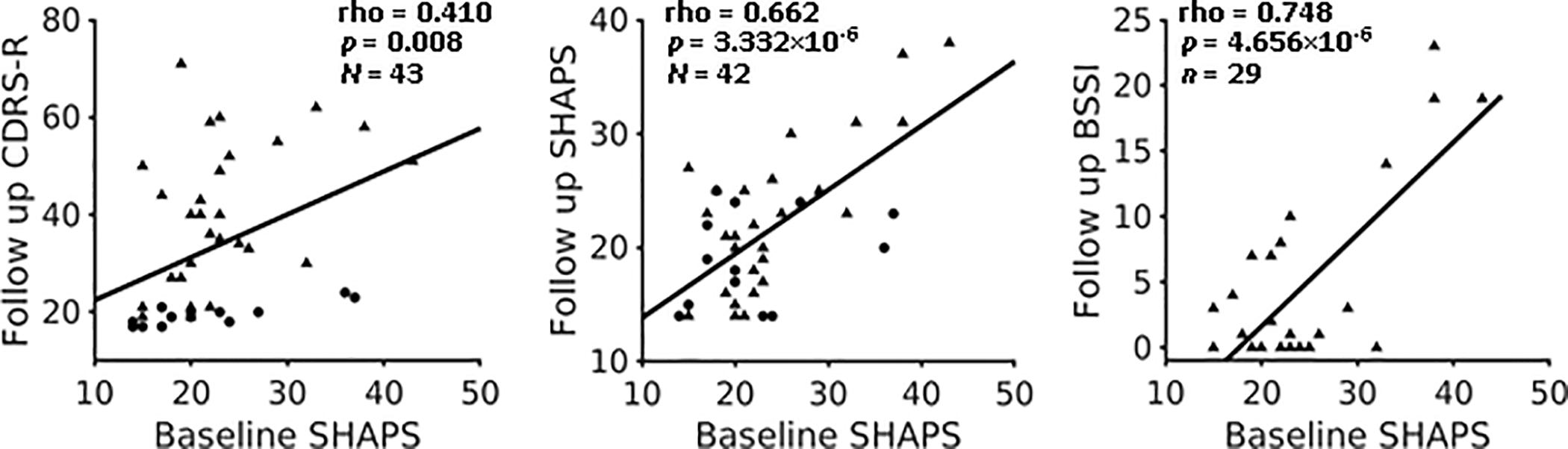
Anhedonia severity at baseline correlated with follow-up depression, anhedonia and suicidality severity. Dots represent healthy subject, and triangles represent subject with mood and anxiety symptoms.
Hierarchical regression analysis of baseline anhedonia and irritability for follow-up illness and suicidality severity
We used hierarchical regression to study the unique contribution of each baseline symptom to depression progression in youth (findings are presented in Table 2). In the whole sample, irritability scores alone could predict follow-up CDRS-R (compared to demographic variables sex and baseline age, ΔR2 = 43.77%, p = 4.747×10−7), and the model was significantly improved after adding anhedonia scores (ΔR2 = 16.46%, p = 7.432×10−5). When examining anhedonia alone as a predictor, anhedonia scores were more predictive of follow-up CDRS-R compared to demographic variables (ΔR2 = 59.02%, p = 2.985×10−10). Importantly, when irritability was added as an additional variable, the model was not significantly better (ΔR2 = 0.12%, p = 0.241). These results indicate that, although baseline anhedonia and irritability levels both significantly predicted follow-up depression severity in youth, anhedonia provided unique predictive power beyond what was captured by irritability, whereas nearly all the predictive power of irritability was encompassed by anhedonia.
Table 2.
Predictive power of baseline anhedonia and irritability on follow-up outcome
| Irritability first | Anhedonia first | |||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2i | Model 3 | Model 1 | Model 2a | Model 3 | |
| Prediction for FU CDRS-R in whole group (N = 42) | ||||||
| Sex (β) | −4.101 | −1.729 | −1.084 | −4.101 | −1.201 | −1.084 |
| Age (β) | 0.940 | 0.150 | −0.376 | 0.940 | −0.402 | −0.376 |
| Irritability (β) | 4.385*** | 1.142 | 1.142 | |||
| Anhedonia (β) | 3.618*** | 4.373*** | 3.618*** | |||
| R2 (%) | 3.45 | 47.22 | 63.68 | 3.45 | 62.47 | 6’.68 |
| ΔR2 (%) | 43.77*** | 16.46*** | 59.02*** | 1.21 | ||
| Prediction for FU CDRS-R in clinical cohort (n = 28) | ||||||
| Sex (β) | −6.405 | −3.967 | −1.033 | −6.405 | −1.029 | −1.033 |
| Age (β) | 0.327 | −0.059 | −0.882 | 0.327 | −0.890 | −0.882 |
| Irritability (β) | 3.065** | 0.137 | 0.137 | |||
| Anhedonia (β) | 3.745*** | 3.825*** | 3.745*** | |||
| R2 (%) | 4.74 | 28.05 | 52.94 | 4. 74 | 52.91 | 52.94 |
| ΔR2 (%) | 23.31** | 24.89** | 48.17*** | 0.03 | ||
| Prediction for FU BSSI in clinical cohort (n = 28) | ||||||
| Sex (β) | −2.391 | 0.019 | 3.270 | −2.391 | 3.263 | 3.270 |
| Age (β) | 0.992 | 0.611 | −0.301 | 0.992 | −0.288 | −0.301 |
| Irritability (β) | 3.029** | −0.217 | −0.217 | |||
| Anhedonia (β) | 4.151*** | 4.023*** | 4.151*** | |||
| R2 (%) | 3.12 | 31.36 | 69.29 | 3.12 | 69.21 | 69.29 |
| ΔR2 (%) | 28.24** | 37.93*** | 66.09*** | 0.08 | ||
p < 0.05;
p < 0.01;
p < 0.001.
One subject was not included for irritability and anhedonia score missing.
In light of the significantly different distribution ranges of follow-up CDRS-R in clinical subjects (n = 28, Median = 40) vs. HC (n = 14, Median = 19, Wilcoxon Rank-Sum Test Z = 4.625, p = 3.754×10−6), hierarchical regression analyses were repeated within the psychiatric group only (Table 2 middle). The results remained the same, such that the addition of baseline anhedonia scores significantly improved prediction of follow-up CDRS-R compared to irritability scores and demographics (ΔR2 = 24.89%, p = 5.657×10−4), whereas the addition of irritability scores did not appreciably change CDRS-R prediction relative to anhedonia scores and demographics (ΔR2 = 0.03%, p = 0.903).
Findings were similar for follow-up BSSI in the clinical cohort (Table 2 lower, N = 28) as baseline anhedonia significantly improved prediction of follow-up BSSI compared to irritability and demographic variables (ΔR2 = 37.93%, p = 2.083×10−6), while baseline irritability did not affect significance of BSSI prediction compared to anhedonia and demographic variables (ΔR2 = 0.08%, p = 0.790).
Neural reward processes as predictors for follow-up clinical outcomes
Whole group (combined clinical and HC subjects):
Whole-brain analyses identified that several activation clusters at baseline during PPE (i.e. contrast of uncertain > certain reward feedback) were significantly (two-tailed pTFCE-FWE < 0.05) correlated with both depression and anhedonia severity at follow-up. Specifically, CDRS- R scores were positively correlated with activation in the left angular gyrus (AG, Figure 2, Table 3), and SHAPS scores positively correlated with activation in the bilateral dorsal anterior cingulate cortex (dACC), supplementary motor area, operculum; left anterior insula and frontal pole; and right planum temporale (Figure 2, Table 3). No significant correlation was observed between brain activation during reward expectancy (i.e. contrast of reward cues > no reward cues) or reward attainment (i.e. contrast of reward > no reward feedback) at baseline and follow-up clinical outcomes.
Figure 2.
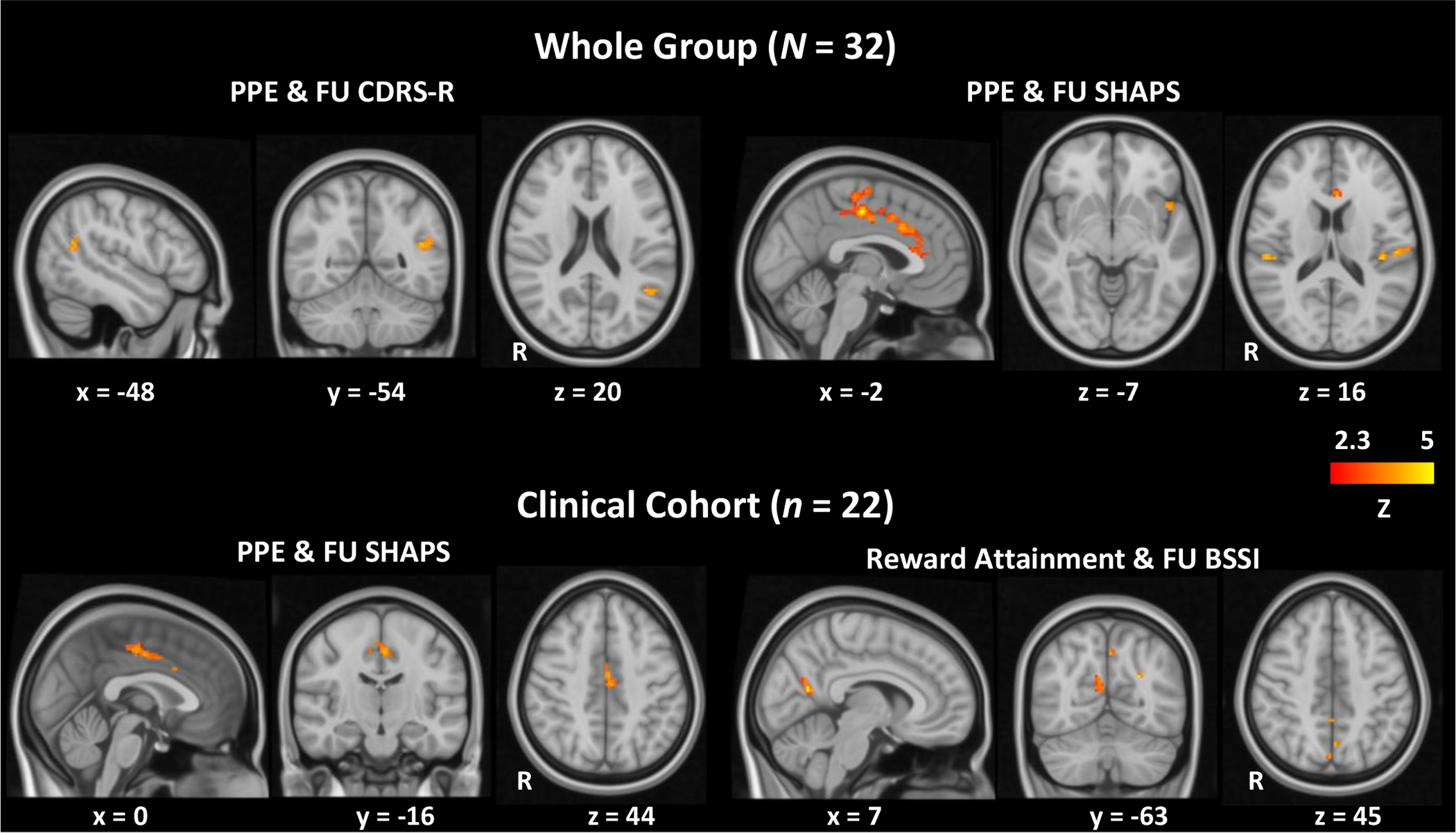
Baseline brain activation during reward processes correlated with follow-up clinical outcomes.
Table 3.
neural reward activity correlated with follow-up clinical outcomes
| cluster size (voxels) | Z | MNI coordinates (peak X, Y, Z) | |
|---|---|---|---|
| Baseline PPE & Follow-up CDRS-R (N = 32) | |||
| Left angular gyrus | 41 | 4.84 | (−38.8, −59.3, 15.4) |
| Baseline PPE & Follow-up SHAPS (N = 32) | |||
| Bilateral Anterior Cingulate; | 605 | 5.13 | (−2, −17.9, 47.6) |
| Bilateral Precentral Gyrus; | 4.31 | (−4.3, 23.5, 15.4) | |
| Bilateral Supplementary Motor Cortex | 4.03 | (−2, 16.6, 33.8) | |
| 3.87 | (0.3, 39.6, 3.9) | ||
| 3.63 | (−2, −13.3, 63.7) | ||
| 3.51 | (9.5, −40.9, 56.8) | ||
| Left Central and Parietal Opercular Cortex | 108 | 5.64 | (−59.5, −6.4, 8.5) |
| Left anterior Insular Cortex | 33 | 4.24 | (−45.7, 7.4, −5.3) |
| Left Parietal Opercular Cortex | 21 | 4.69 | (−41.1, −27.1, 15.4) |
| Right Planum Temporale | 20 | 4.44 | (53.2, −27.1, 15.4) |
| Left frontal pole | 10 | 5.21 | (-25, 39.6, 45.3) |
| Baseline PPE & Follow-up SHAPS in Clinical Cohort (n = 22) | |||
| Bilateral Anterior Cingulate | 78 | 4.48 | (−2, −15.6, 45.3) |
| Baseline Reward Attainment & Follow-up BSSI in Clinical Cohort (n = 22)a | |||
| Right Precuneous | 90 | 4.43 | (7.2, −61.6, 15.4) |
| 3.3 | (18.7, −68.5, 24.6) | ||
| 3.18 | (14.1, −47.8, 15.4) | ||
| Left Precuneous | 14 | 4.04 | (0.3, −68.5, 49.9) |
single-tailed pTFCE-FWE < 0.05.
Clinical cohort:
The same whole-brain analyses were repeated in the psychiatric group (n = 22). Results were similar to those obtained in the whole cohort but less significant (Supplementary Figure 1), with only the positive correlation between dACC activation during PPE and follow-up SHAPS scores surviving FWE correction (Figure 2, Table 3). Clinical outcome correlations with neural activation during reward expectancy and reward attainment were again non-significant.
As suicidality is not present in healthy controls and to avoid a floor effect, the BSSI analyses were limited to the clinical cohort. At the slightly relaxed threshold (single-tailed pTFCE-FWE < 0.05) used for our exploratory suicidality analysis, bilateral precuneus activation during reward attainment was found to correlate positively with follow-up BSSI scores (Figure 2, Table 3).
Discussion
The current pilot study investigated reward functions as neural and behavioral predictors of adolescent depression outcome. As hypothesized, we demonstrated that decreased hedonic capacity at baseline predicted future depression, anhedonia and suicidality severity in youth. When we examined both baseline anhedonia and irritability in the same hierarchical regression model, only anhedonia predicted future depression and suicidality severity outcomes. When we probed reward circuitry using the RFT fMRI task, we found that neural activation to uncertain rewards (PPE) was also predictive of future depression and anhedonia severity. Specifically, activation in the left angular gyrus (AG) predicted depression severity, while activation in dACC, bilateral operculum and left insula predicted anhedonia severity. Importantly, activation in the dorsal ACC remained a significant predictor for anhedonia severity even when HC subjects were excluded from the analysis. Exploratory analysis within the clinical sample further suggested that precuneus activation during reward attainment might be related to the development of suicidality ideation.
Anhedonia, but not irritability, predicted depression persistence in youth
In an effort to overcome the inherent heterogeneity of psychiatric disorders like adolescent depression, which are typically defined based on clusters of potentially disparate symptoms, researchers have increasingly turned to study more specific clinical features such as anhedonia and irritability rather than limiting the research to categorical DSM disorders (Gabbay et al., 2012a; Gabbay et al., 2013; Gabbay et al., 2012b; Henderson et al., 2013; Sanislow et al., 2019). Our current longitudinal finding that anhedonia, compared to irritability, uniquely predicted subsequent depression and suicidality severity fits with our prior cross-sectional work showing that anhedonia, but not irritability, was associated with chronicity, illness severity, and suicidality in depressed youths (Gabbay et al., 2015). These results are also consistent with independent research documenting that depressed youths with or without significant irritability did not significantly differ in illness severity, number of depressive episodes, anhedonia severity, or suicidality (Stringaris et al., 2013). Anhedonia was also suggested as a predictor for worse outcome by the multisite Treatment of Resistant Depression in Adolescents (TORDIA) study, which examined longitudinal treatment outcomes for 334 adolescents with selective serotonin reuptake inhibitor (SSRI) treatment-resistant depression for 24 weeks. They found that, of the five CDRS-R symptom dimensions (i.e. depressed mood, anhedonia, somatic symptoms, morbid thoughts, and observed depression), only anhedonia predicted longer time to remission and fewer depression-free days (McMakin et al., 2012). Thus, our findings support the overall hypothesis that reward dysfunction, reflected by anhedonia, plays a particularly important role the development and persistence of depression in adolescents. However, despite our findings, interpretation of anhedonia as a predictor needs to be cautious, as anhedonia is not a pre-morbid trait.
Brain activation during uncertain reward predicted follow-up depression and anhedonia severity
While anhedonia predict future clinical depression and suicidality, it is also clear that not all anhedonic patients will experience the same course of illness or become suicidal. In this study, we adopted fMRI RFT task to identify neuroimaging signatures that differentiate adolescents who will go on to become depressive and suicidal from those who will not. Our fMRI analysis provided complimentary evidence that localized neural activation during the receipt of uncertain vs. certain rewards (i.e. PPE) predicted future depression and anhedonia severity. Neural activation during PPE that significantly predicted future depression severity was highly specific to the left AG, a cross-modal hub within the default mode network (DMN) which is usually deactivated by external, goal-oriented tasks (Fox et al., 2005). The AG is also involved in a wide variety of cognitive functions including language, memory retrieval, attention, spatial cognition, and social cognition (Seghier, 2012). In adolescents, lack of deactivation in the temporoparietal junction (comprising the AG and Wernicke’s area) is associated with impaired fear extinction (Ganella et al., 2018). The association between failure to deactivate the AG during RFT performance and future depression severity in the current study suggests possible impairment in fear extinction in future depressive youth.
Our findings differ somewhat from prior longitudinal studies of reward processing in non-depressed youths, which have reported that brain activity during reward anticipation and reward attainment predicted future depression symptomatology (Morgan et al., 2013; Nelson et al., 2018; Nelson et al., 2016; Stringaris et al., 2015). By contrast, in our study neural responses to reward expectancy and reward attainment were not found to significantly predict future depression outcomes. A key methodological difference is that these studies were carried out in healthy cohorts, whereas our sample primarily consisted of clinical subjects with modest depressive symptoms at baseline (CDRS-R: M ± SD = 37.32 ± 15.45). Our task design also differed from these prior studies in several regards: although it is similar to the monetary incentive delay task (Stringaris et al., 2015), the RFT also includes additional, explicitly uncertain cues (i.e. ? as well as 0¢, 10¢, and 50¢), and trial success was based on flanker task performance rather than entirely probabilistic card guessing (Morgan et al., 2013) or door guessing (Nelson et al., 2018; Nelson et al., 2016). In the context of prior literature, our results therefore suggest that the neural mechanisms contributing to depression persistence are likely distinct from those underlying initial disease onset in youth.
Notably, PPE was also the only reward process that predicted future anhedonia severity. Predictive neural activation during PPE was more widespread for anhedonia than depression severity, including the dACC within the medial prefrontal cortex (mPFC), the posterior operculum, and the anterior insula. The dACC plays a key role in reward-based decision-making and learning (Bush et al., 2002; Taylor et al., 2006), error detection and conflict monitoring (Botvinick et al., 1999), as well as pain processing (Jahn et al., 2016; Lieberman and Eisenberger, 2015). Increased dACC and mPFC activity is also among the most consistently reported findings in fMRI studies of depression (Nejad et al., 2013; Rive et al., 2013), including a recent report of enhanced connectivity between the mPFC and nucleus accumbens, a core subcortical reward structure within the ventral striatum, during reward attainment in adolescent boys with a history of depression (Morgan et al., 2016). Moreover, we also found that anterior insula activation during PPE predicted downstream anhedonia levels, consistent with the close functional relationship between the dACC and anterior insula as the primary structures of the salience network responsible for evaluating and directing attention toward important stimuli (Medford and Critchley, 2010). The anterior insula supports subjective emotional awareness (Craig, 2009), reliably represents negative reward prediction error signals in youth (Keren et al., 2018a), and is involved in the memory consolidation phase of inhibitory avoidance (Fornari et al., 2012). Finally, we observed that the anhedonia-predictive brain regions activated during PPE are also closely associated with pain processing. In particular, the posterior operculum has been identified as critical for the perception of painful stimuli (Garcia-Larrea, 2012), which also elicit strong activation throughout the salience network (Borsook et al., 2013; Legrain et al., 2011). Importanly, this area remained significant when controlling baseline anhedonia severity (Supplementary Figure 2; Supplementary Table 3), emphasizing its possible importance in anhedonia maintenance. Clinical studies consistently report comorbid depression and pain symptoms that respond similarly to treatment (Bair et al., 2003; Goesling et al., 2013), while the presence of pain predicted worse treatment outcomes and slower remission in depressed adults (Karp et al., 2005). Our study similarly found that hyper-activation in pain processing regions predicted more severe anhedonia in medication-free youth. Although uncertain RFT rewards elicit widespread neural activation in healthy and depressed adolescents (Bradley et al., 2017), only activation in areas that are also involved in aversion processing predicted future anhedonia severity. These findings suggest a potential link between early negative valence system (NVS, particularly pain) activity and later positive valence system (PVS) deficits such as anhedonia.
Importantly, dACC activity remained predictive for anhedonia even when HC were excluded from the analysis, the only predictive result that still met significance. As anhedonia predicted depression severity, we expected that the neural predictors of anhedonia would also predict depression severity, which were not supported by our data. One explanation may be that depression is a heterogeneous disorder comprised of multiple discrete behavioral constructs, which may have limited our ability to identify the same neural predictors for anhedonia and depression severity particularly in a small sample. Another possible explanation for not predicting depression severity when HC were excluded was the reduced statistical power in the smaller cohort, as the unthresholded Z-map pattern was quite similar to the full sample (Supplementary Figure 1). An additional reason might be that AG activity was more related to baseline diagnosis, as most of our clinical subjects persisted their symptoms at follow-up. Additionally, depression encompasses a constellation of various PVS and NVS abnormalities, while anhedonia more specifically reflects PVS deficiencies. This might also be the reason distinct brain regions were predictive of depression and anhedonia: the dACC, which is heavily involved in reward processing, predicted anhedonia; while the AG, a cross-modal hub, predicted general depression severity. A previous study looking into depression symptoms in a large-scale community sample of healthy adolescents found that disruptions in neural reward system were associated with anhedonia but not low mood (Stringaris et al., 2015). The current work extends this conclusion to psychiatric youths and suggests that the relationship of reward system disturbances with future anhedonia is stronger and more stable than with future depression as a whole.
Taken together, the whole brain analysis results suggested a possible role of the AG, dACC, anterior insula and operculum, brain areas involved in reward learning and NVS, as predictors for worse clinical outcome. In particular, stronger neural activation differences between reward receipt after uncertain vs. certain cues were related to more severe future depression and anhedonia. This contrast of uncertain vs. certain rewards is not a classic “prediction error” which is calculated using a computational approach and to our knowledge has not been investigated previously in longitudinal studies on depression. Our task adopted fixed reward valences and excludes possible contamination of outcome unpredictability and other cognitive processes such as decision-making and learning, which may induce anxiety. By avoiding these stresses, our design may provide a cleaner estimate of PPE, especially in the trans-diagnostic psychiatric sample. Clinically, this contrast falls under evaluation of reward possibility, which is characterized by the over-/under-estimation of positive outcome probabilities under uncertain conditions. We found that youths who responded to uncertain rewards with neural activation similar to those for a certain rewards had better outcomes while those who activated the negative valence network during the uncertain conditions had worse outcome. While no studies have examined this construct in fMRI studies, depression is characterized in negative future view, as well as hypersensitivity to punishment and pain (Eshel and Roiser, 2010; Martin-Soelch et al., 2007; Moore and Fresco, 2012; Strunk et al., 2006). Similarly, a recent computational study found that maladaptive features of depression could emerge from negatively biased expectation at evaluation in goal-directed decision making (Huys et al., 2015). While more is needed to generate adequate fMRI data to investigate this construct, our findings suggest that reward processes during uncertainty may play a role in illness progression.
Neural responses to reward attainment predicted follow-up suicidality in youths with psychiatric symptoms
Finally, we found evidence that future suicidality severity in clinical subjects was associated with stronger activation during reward attainment in the precuneus, a region involves in a range of functions related to episodic memory retrieval and self-referential processing (Kim, 2012; Leech and Sharp, 2014; Maddock et al., 2003). The precuneus is also well-studied as a core component of the DMN (Fox et al., 2005). Although our exploratory suicidality analysis was conducted under a relatively liberal threshold (single-tailed pTFCE-FWE < 0.05, equivalent to two-tailed pTFCE-FWE < 0.1), accumulating evidence implicates precuneus and DMN dysfunction in a variety of mental disorders that emerge during adolescence, including depression (Leech and Sharp, 2014). Crucially, two recent cross-sectional studies have specifically linked stronger resting-state functional connectivity within the DMN to suicidality in depressed youths (Schreiner et al., 2019; Zhang et al., 2016). Though tentative, our results are highly consistent with these findings, suggesting that alterations in precuneus function during reward processing may also be associated with the development of suicidality symptoms.
Limitations
Several limitations should be noted in this study. Foremost is the relatively small sample size, which limits the generalizability of our conclusions, particularly for the RFT fMRI analysis. Although we had a high participation rate of approximately 67% among subjects eligible for follow-up, our sample size was nevertheless reduced through attrition, and only a subset of participants had useable RFT data at baseline. Another limitation was the variability in interval time from baseline to follow-up visit. Importantly, our clinical predictors remain the same when we controlled for the interval but not all neuroimaging findings remain significant (details in supplementary materials). Another potential limitation is that as the cohort was small, the broad spectrum of mood and anxiety symptom severity might have contributed to Type II error particularly in regard to the fMRI findings not detecting relationship for reward expectancy and attainment. Similarly, a follow-up scan which was not done in this study should also have improved our study. Additionally, although all participants were medication-free at baseline, subsequent use of medication was permitted and varied between subjects which was likely to affect findings. However, such approach allows a better generalization of our findings as depression exacerbation occurs despite successful medication treatment.
Conclusion
In this study, we combined behavioral and neuroimaging techniques to study reward processes as predictors of illness outcomes in adolescent depression. Our results revealed that only anhedonia severity, the most direct clinical measure of reward dysfunction, uniquely predicted future depression and suicidality in youth. Furthermore, we found that neural responses to uncertain reward attainment in brain regions within salience network and NVS predicted anhedonia later on, while the key region of default mode network, angular gyrus, was associated with more severe depression at follow-up. Clinically, the study suggests that youth who present with anhedonia are at higher risk for illness progression and should be monitored carefully for suicide risk and chronicity. Future studies should expand this focus to examine the closely related construct of reward learning, as well as the interface between NVS and PVS in a larger sample, with the ultimate goal of identifying early targets for therapeutic intervention.
Supplementary Material
Highlights.
Anhedonia but not irritability predicted follow-up depression and suicidality
Activation in left angular gyrus during PPE predicted future depression severity
dACC, right insula & bilateral operculum activation during PPE predicted anhedonia
Reward dysfunction is a potential biobehavioral predictor for adolescent depression
Acknowledgments
We would like to thank Lushna Mehra for research coordination and assistance in preparing database for the manuscript.
Role of the funding source
This study was supported by the National Institute of Mental Health (NIMH) grants R01MH101479 and R01MH095807 to VG (PI). This work was also supported in part through the computational resources and staff expertise provided by ISMMS Scientific Computing, with additional resource support provided by the ISMMS Brain Imaging Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing Interest
The authors declare no conflict of interests.
Reference
- Andersen SL, Teicher MH, 2008. Stress, sensitive periods and maturational events in adolescent depression. Trends in Neurosciences 31, 183–191. [DOI] [PubMed] [Google Scholar]
- Bair MJ, Robinson RL, Katon W, Kroenke K, 2003. Depression and Pain Comorbidity: A Literature Review. JAMA Internal Medicine 163, 2433–2445. [DOI] [PubMed] [Google Scholar]
- Beck AT, Kovacs M, Weissman A, 1979. Assessment of suicidal intention: the Scale for Suicide Ideation. Journal of consulting and clinical psychology 47, 343. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer R, Brown G, 1996. Beck depression inventory–second edition Manual San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Birmaher B, Axelson D, 2006. Course and outcome of bipolar spectrum disorder in children and adolescents: A review of the existing literature. Development and Psychopathology 18, 1023–1035. [DOI] [PubMed] [Google Scholar]
- Borsook D, Edwards R, Elman I, Becerra L, Levine J, 2013. Pain and analgesia: The value of salience circuits. Progress in Neurobiology 104, 93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD, 1999. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature 402, 179–181. [DOI] [PubMed] [Google Scholar]
- Bradley KAL, Case JAC, Freed RD, Stern ER, Gabbay V, 2017. Neural correlates of RDoC reward constructs in adolescents with diverse psychiatric symptoms: A Reward Flanker Task pilot study. Journal of Affective Disorders 216, 36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Vogt BA, Holmes J, Dale AM, Greve D, Jenike MA, Rosen BR, 2002. Dorsal anterior cingulate cortex: A role in reward-based decision making. Proceedings of the National Academy of Sciences 99, 523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD, 2009. How do you feel — now? The anterior insula and human awareness. Nature Reviews Neuroscience 10, 59–70. [DOI] [PubMed] [Google Scholar]
- Curry J, Silva S, Rohde P, Ginsburg G, Kratochvil C, Simons A, Kirchner J, May D, Kennard B, Mayes T, Feeny N, Albano AM, Lavanier S, Reinecke M, Jacobs R, Becker-Weidman E, Weller E, Emslie G, Walkup J, Kastelic E, Burns B, Wells K, March J, 2011. Recovery and recurrence following treatment for adolescent major depression. Archives of general psychiatry 68, 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Berardis D, Marini S, Fornaro M, Srinivasan V, Iasevoli F, Tomasetti C, Valchera A, Perna G, Quera-Salva M-A, Martinotti G, di Giannantonio M, 2013. The melatonergic system in mood and anxiety disorders and the role of agomelatine: implications for clinical practice. International journal of molecular sciences 14, 12458–12483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen BA, Eriksen CW, 1974. Effects of noise letters upon the identification of a target letter in a nonsearch task. Perception & psychophysics 16, 143–149. [Google Scholar]
- Eshel N, Roiser JP, 2010. Reward and Punishment Processing in Depression. Biological Psychiatry 68, 118–124. [DOI] [PubMed] [Google Scholar]
- Farabaugh A, Fisher L, Nyer M, Holt D, Cohen M, Baer L, Shapero BG, Huz I, Cardoos A, Fava M, Alpert JE, 2015. Similar changes in cognitions following cognitive-behavioral therapy or escitalopram for major depressive disorder: Implications for mechanisms of change. Annals of Clinical Psychiatry 27, 118–126. [PubMed] [Google Scholar]
- Fornari RV, Wichmann R, Atucha E, Desprez T, Eggens-Meijer E, Roozendaal B, 2012. Involvement of the insular cortex in regulating glucocorticoid effects on memory consolidation of inhibitory avoidance training. Frontiers in behavioral neuroscience 6, 10–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME, 2005. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A 102, 9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbay V, Ely BA, Babb J, Liebes L, 2012a. The possible role of the kynurenine pathway in anhedonia in adolescents. Journal of neural transmission (Vienna, Austria : 1996) 119, 253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbay V, Ely BA, Li Q, Bangaru SD, Panzer AM, Alonso CM, Castellanos FX, Milham MP, 2013. Striatum-Based Circuitry of Adolescent Depression and Anhedonia. Journal of the American Academy of Child and Adolescent Psychiatry 52, 628–641. e613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbay V, Johnson AR, Alonso CM, Evans LK, Babb JS, Klein RG, 2015. Anhedonia, but not Irritability, Is Associated with Illness Severity Outcomes in Adolescent Major Depression. Journal of Child and Adolescent Psychopharmacology 25, 194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbay V, Mao X, Klein RG, Ely BA, Babb JS, Panzer AM, Alonso CM, Shungu DC, 2012b. Anterior Cingulate Cortex γ-Aminobutyric Acid in Depressed Adolescents: Relationship to Anhedonia. Archives of general psychiatry 69, 139–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganella DE, Drummond KD, Ganella EP, Whittle S, Kim JH, 2018. Extinction of Conditioned Fear in Adolescents and Adults: A Human fMRI Study. Frontiers in human neuroscience 11, 647–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Larrea L, 2012. The posterior insular-opercular region and the search of a primary cortex for pain. Neurophysiologie Clinique/Clinical Neurophysiology 42, 299–313. [DOI] [PubMed] [Google Scholar]
- Glasser MF, Sotiropoulos SN, Wilson JA, Coalson TS, Fischl B, Andersson JL, Xu J, Jbabdi S, Webster M, Polimeni JR, Van Essen DC, Jenkinson M, 2013. The minimal preprocessing pipelines for the Human Connectome Project. NeuroImage 80, 105–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goesling J, Clauw DJ, Hassett AL, 2013. Pain and Depression: An Integrative Review of Neurobiological and Psychological Factors. Current Psychiatry Reports 15, 421. [DOI] [PubMed] [Google Scholar]
- Griffanti L, Salimi-Khorshidi G, Beckmann CF, Auerbach EJ, Douaud G, Sexton CE, Zsoldos E, Ebmeier KP, Filippini N, Mackay CE, Moeller S, Xu J, Yacoub E, Baselli G, Ugurbil K, Miller KL, Smith SM, 2014. ICA-based artefact removal and accelerated fMRI acquisition for improved resting state network imaging. NeuroImage 95, 232–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms MP, Somerville LH, Ances BM, Andersson J, Barch DM, Bastiani M, Bookheimer SY, Brown TB, Buckner RL, Burgess GC, Coalson TS, Chappell MA, Dapretto M, Douaud G, Fischl B, Glasser MF, Greve DN, Hodge C, Jamison KW, Jbabdi S, Kandala S, Li X, Mair RW, Mangia S, Marcus D, Mascali D, Moeller S, Nichols TE, Robinson EC, Salat DH, Smith SM, Sotiropoulos SN, Terpstra M, Thomas KM, Tisdall MD, Ugurbil K, van der Kouwe A, Woods RP, Zöllei L, Van Essen DC, Yacoub E, 2018. Extending the Human Connectome Project across ages: Imaging protocols for the Lifespan Development and Aging projects. NeuroImage 183, 972–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson SE, Johnson AR, Vallejo AI, Katz L, Wong E, Gabbay V, 2013. A Preliminary Study of White Matter in Adolescent Depression: Relationships with Illness Severity, Anhedonia, and Irritability. Frontiers in Psychiatry 4, 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heron M, 2018. Deaths: Leading Causes for 2016. National Vital Statistics Reports 67, 1–77. [PubMed] [Google Scholar]
- Huys QJ, Daw ND, Dayan P, 2015. Depression: a decision-theoretic analysis. Annual review of neuroscience 38, 1–23. [DOI] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Sanislow C, Wang P, 2010. Research Domain Criteria (RDoC): Toward a New Classification Framework for Research on Mental Disorders. American Journal of Psychiatry 167, 748–751. [DOI] [PubMed] [Google Scholar]
- Jahn A, Nee DE, Alexander WH, Brown JW, 2016. Distinct Regions within Medial Prefrontal Cortex Process Pain and Cognition. The Journal of Neuroscience 36, 12385–12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner T, 2007. Why people die by suicide. Harvard University Press. [Google Scholar]
- Karp JF, Scott J, Houck P, Reynolds CF III, Kupfer DJ, Frank E, 2005. Pain predicts longer time to remission during treatment of recurrent depression. The Journal of clinical psychiatry 66, 591–597. [DOI] [PubMed] [Google Scholar]
- Kaufman AS, 1990. Kaufman brief intelligence test: KBIT. AGS, American Guidance Service Circle Pines, MN. [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao UMA, Flynn C, Moreci P, Williamson D, Ryan N, 1997. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial Reliability and Validity Data. Journal of the American Academy of Child & Adolescent Psychiatry 36, 980–988. [DOI] [PubMed] [Google Scholar]
- Keren H, Chen G, Benson B, Ernst M, Leibenluft E, Fox NA, Pine DS, Stringaris A, 2018a. Is the encoding of Reward Prediction Error reliable during development? NeuroImage 178, 266–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren H, O’Callaghan G, Vidal-Ribas P, Buzzell GA, Brotman MA, Leibenluft E, Pan PM, Meffert L, Kaiser A, Wolke S, Pine DS, Stringaris A, 2018b. Reward Processing in Depression: A Conceptual and Meta-Analytic Review Across fMRI and EEG Studies. American Journal of Psychiatry 175, 1111–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, 2012. A dual-subsystem model of the brain’s default network: Self-referential processing, memory retrieval processes, and autobiographical memory retrieval. NeuroImage 61, 966–977. [DOI] [PubMed] [Google Scholar]
- Knutson B, Westdorp A, Kaiser E, Hommer D, 2000. FMRI Visualization of Brain Activity during a Monetary Incentive Delay Task. NeuroImage 12, 20–27. [DOI] [PubMed] [Google Scholar]
- Lambert C, Da Silva S, Ceniti AK, Rizvi SJ, Foussias G, Kennedy SH, 2018. Anhedonia in depression and schizophrenia: A transdiagnostic challenge. CNS Neuroscience & Therapeutics 24, 615–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R, Sharp DJ, 2014. The role of the posterior cingulate cortex in cognition and disease. Brain : a journal of neurology 137, 12–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrain V, Iannetti GD, Plaghki L, Mouraux A, 2011. The pain matrix reloaded: A salience detection system for the body. Progress in Neurobiology 93, 111–124. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Eisenberger NI, 2015. The dorsal anterior cingulate cortex is selective for pain: Results from large-scale reverse inference. Proc Natl Acad Sci U S A 112, 15250–15255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddock RJ, Garrett AS, Buonocore MH, 2003. Posterior cingulate cortex activation by emotional words: fMRI evidence from a valence decision task. Human brain mapping 18, 30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Soelch C, Linthicum J, Ernst M, 2007. Appetitive conditioning: neural bases and implications for psychopathology. Neuroscience and biobehavioral reviews 31, 426–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMakin DL, Olino TM, Porta G, Dietz LJ, Emslie G, Clarke G, Wagner KD, Asarnow JR, Ryan ND, Birmaher B, Shamseddeen W, Mayes T, Kennard B, Spirito A, Keller M, Lynch FL, Dickerson JF, Brent DA, 2012. Anhedonia predicts poorer recovery among youth with selective serotonin reuptake inhibitor treatment-resistant depression. Journal of the American Academy of Child and Adolescent Psychiatry 51, 404–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medford N, Critchley HD, 2010. Conjoint activity of anterior insular and anterior cingulate cortex: awareness and response. Brain structure & function 214, 535–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore MT, Fresco DM, 2012. Depressive realism: A meta-analytic review. Clinical Psychology Review 32, 496–509. [DOI] [PubMed] [Google Scholar]
- Morgan JK, Olino TM, McMakin DL, Ryan ND, Forbes EE, 2013. Neural response to reward as a predictor of increases in depressive symptoms in adolescence. Neurobiol Dis 52, 66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JK, Shaw DS, Olino TM, Musselman SC, Kurapati NT, Forbes EE, 2016. History of Depression and Frontostriatal Connectivity During Reward Processing in Late Adolescent Boys. Journal of Clinical Child & Adolescent Psychology 45, 59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nejad AB, Fossati P, Lemogne C, 2013. Self-referential processing, rumination, and cortical midline structures in major depression. Frontiers in human neuroscience 7, 666–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson BD, Infantolino ZP, Klein DN, Perlman G, Kotov R, Hajcak G, 2018. Time-Frequency Reward-Related Delta Prospectively Predicts the Development of Adolescent-Onset Depression. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging 3, 41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson BD, Perlman G, Klein DN, Kotov R, Hajcak G, 2016. Blunted Neural Response to Rewards as a Prospective Predictor of the Development of Depression in Adolescent Girls. American Journal of Psychiatry 173, 1223–1230. [DOI] [PubMed] [Google Scholar]
- Poznanski EO, Freeman LN, Mokros HB, 1985. Children’s depression rating scale-revised Psychopharmacology Bulletin 21, 979–989. [Google Scholar]
- Rive MM, van Rooijen G, Veltman DJ, Phillips ML, Schene AH, Ruhé HG, 2013. Neural correlates of dysfunctional emotion regulation in major depressive disorder. A systematic review of neuroimaging studies. Neuroscience & Biobehavioral Reviews 37, 2529–2553. [DOI] [PubMed] [Google Scholar]
- Rømer Thomsen K, Whybrow PC, Kringelbach ML, 2015. Reconceptualizing anhedonia: novel perspectives on balancing the pleasure networks in the human brain. Frontiers in behavioral neuroscience 9, 49–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salimi-Khorshidi G, Douaud G, Beckmann CF, Glasser MF, Griffanti L, Smith SM, 2014. Automatic denoising of functional MRI data: Combining independent component analysis and hierarchical fusion of classifiers. NeuroImage 90, 449–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanislow CA, Ferrante M, Pacheco J, Rudorfer MV, Morris SE, 2019. Advancing Translational Research Using NIMH Research Domain Criteria and Computational Methods. Neuron 101, 779–782. [DOI] [PubMed] [Google Scholar]
- Schreiner MW, Klimes-Dougan B, Cullen KR, 2019. Neural Correlates of Suicidality in Adolescents with Major Depression: Resting-State Functional Connectivity of the Precuneus and Posterior Cingulate Cortex. Suicide and Life-Threatening Behavior 49, 899–913. [DOI] [PubMed] [Google Scholar]
- Seghier ML, 2012. The Angular Gyrus: Multiple Functions and Multiple Subdivisions. The Neuroscientist 19, 43–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Wolf DH, Ciric R, Kable JW, Moore TM, Vandekar SN, Katchmar N, Daldal A, Ruparel K, Davatzikos C, Elliott MA, Calkins ME, Shinohara RT, Bassett DS, Satterthwaite TD, 2017. Common Dimensional Reward Deficits Across Mood and Psychotic Disorders: A Connectome-Wide Association Study. American Journal of Psychiatry 174, 657–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snaith R, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P, 1995. A scale for the assessment of hedonic tone the Snaith–Hamilton Pleasure Scale. The British Journal of Psychiatry 167, 99–103. [DOI] [PubMed] [Google Scholar]
- Stringaris A, Maughan B, Copeland WS, Costello EJ, Angold A, 2013. Irritable Mood as a Symptom of Depression in Youth: Prevalence, Developmental, and Clinical Correlates in the Great Smoky Mountains Study. Journal of the American Academy of Child & Adolescent Psychiatry 52, 831–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringaris A, Vidal-Ribas Belil P, Artiges E, Lemaitre H, Gollier-Briant F, Wolke S, Vulser H, Miranda R, Penttila J, Struve M, Fadai T, Kappel V, Grimmer Y, Goodman R, Poustka L, Conrod P, Cattrell A, Banaschewski T, Bokde AL, Bromberg U, Buchel C, Flor H, Frouin V, Gallinat J, Garavan H, Gowland P, Heinz A, Ittermann B, Nees F, Papadopoulos D, Paus T, Smolka MN, Walter H, Whelan R, Martinot JL, Schumann G, Paillere-Martinot ML, 2015. The Brain’s Response to Reward Anticipation and Depression in Adolescence: Dimensionality, Specificity, and Longitudinal Predictions in a Community-Based Sample. The American journal of psychiatry 172, 1215–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strunk DR, Lopez H, DeRubeis RJ, 2006. Depressive symptoms are associated with unrealistic negative predictions of future life events. Behaviour Research and Therapy 44, 861–882. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Martis B, Fitzgerald KD, Welsh RC, Abelson JL, Liberzon I, Himle JA, Gehring WJ, 2006. Medial Frontal Cortex Activity and Loss-Related Responses to Errors. The Journal of Neuroscience 26, 4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman MM, Wolk S, Goldstein RB, Moreau D, Adams P, Greenwald S, Klier CM, Ryan ND, Dahl RE, Wickramaratne P, 1999. Depressed adolescents grown up. Jama 281, 1707–1713. [DOI] [PubMed] [Google Scholar]
- Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, Charlson FJ, Norman RE, Flaxman AD, Johns N, Burstein R, Murray CJ, Vos T, 2013. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet (London, England) 382, 1575–1586. [DOI] [PubMed] [Google Scholar]
- Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE, 2014. Permutation inference for the general linear model. NeuroImage 92, 381–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Chen J-M, Kuang L, Cao J, Zhang H, Ai M, Wang W, Zhang S-D, Wang S-Y, Liu S-J, Fang W-D, 2016. Association between abnormal default mode network activity and suicidality in depressed adolescents. BMC Psychiatry 16, 337–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


