Abstract
Age-related hearing loss (AHL) is the most common form of hearing impairment. AHL is thought to be a multifactorial condition resulting from the interaction of numerous causes including aging, genetics, exposure to noise, and exposure to endogenous and exogenous toxins. Cells possess many detoxification enzymes capable of removing thousands of cytotoxic xenobiotics and endogenous toxins such as 4-hydroxynonenal (4-HNE), one of the most abundant cytotoxic end products of lipid peroxidation. The cellular detoxification system involves three phases of enzymatic detoxification. Of these, the glutathione transferase (GST) detoxification system converts a toxic compound into a less toxic form by conjugating the toxic compound to reduced glutathione by GST enzymes. In this review, we describe the current understanding of the cochlear detoxification system and examine the growing link between GST detoxification, oxidative lipid damage, ototoxicity, and cochlear aging with a particular focus on the alpha-class GSTs (GSTAs). We also describe how exposure to ototoxic drugs, exposure to noise, or aging results in increased 4-HNE levels, how 4-HNE damages various cell components under stress conditions, and how GSTAs detoxify 4-HNE in the auditory system.
Keywords: detoxification, 4-HNE, GST, NRF2, estrogen, cochlea, aging
1. Introduction
Hearing loss is the third most prevalent chronic health condition affecting older adults and age-related hearing loss (AHL) is the most common form of hearing impairment (Gates & Mills, 2005; Ozmeral, Eddins, Frisina, & Eddins, 2016; Yamasoba et al., 2013). AHL is thought to be a multifactorial condition resulting from the interaction of numerous causes including aging, exposure to noise and ototoxic chemicals, genetics, epigenetic variables, comorbidities, and lifestyle (Gates & Mills, 2005; Yamasoba et al., 2013). The inner ear contains two sensory systems: the auditory system (cochlea) which detects sound waves (Hudspeth, 1997) and the vestibular system (three semicircular canals, utricle, and saccule) which detects head movements and linear motion or gravity (Angelaki & Cullen, 2008). The major sites of age-related cochlear pathology include inner hair cells (IHC), outer hair cells (OHC), spiral ganglion neurons (SGN), synaptic loss, and stria vascularis (SV) cells (Liberman & Kujawa, 2017; Liu & Yan, 2007; Someya & Prolla, 2010; Yamasoba et al., 2013). The IHCs are the true sound receptors that relay sound wave information to the central auditory system through the SGNs (Hudspeth, 1997). Post-mitotic hair cells and SGNs are particularly susceptible to injury from a combination of noise exposure, ototoxic chemicals, and oxidative damage (Someya & Prolla, 2010; Yamasoba et al., 2013). The blood vessels coursing through the cochlea are also essential for transporting oxygen and nutrients such as calcium, potassium, amino acids, cholesterol, and glucose into the cochlea. Therefore, age-related changes in the structures and functions of those cochlear cells typically disrupt auditory function and result in irreversible and permanent hearing impairment.
Cells possess many detoxification enzymes capable of removing thousands of cytotoxic xenobiotics and endogenous toxins such as 4-hydroxynonenal (4-HNE) (Henderson & Wolf, 2011; Nebert & Dalton, 2006; Simic, Savic-Radojevic, Pljesa-Ercegovac, Matic, & Mimic-Oka, 2009). The cellular detoxification system involves three phases of enzymatic detoxification. Of these, the glutathione transferase (GST) detoxification system converts a toxic compound into a less toxic form by conjugating the toxic compound to reduced glutathione (GSH) by GST enzymes. In this review, we describe the current understanding of the cochlear detoxification system and examine the growing link between GST detoxification, oxidative lipid damage, ototoxicity, and aging with a particular focus on the alpha-class GSTs (GSTAs). We also describe how exposure to ototoxic drugs, exposure to noise, or aging results in increased levels of 4-HNE, one of the most abundant cytotoxic end products of lipid peroxidation (Awasthi et al., 2004; Dalleau, Baradat, Gueraud, & Huc, 2013; Di Domenico, Tramutola, & Butterfield, 2017; Jaganjac et al., 2019; Singhal et al., 2015), how 4-HNE damages various cell components under stress conditions, and how GSTAs detoxify 4-HNE in the auditory system.
2. Role of GST detoxification in protection against aging and hearing loss
2.1. Cellular detoxification system
Cells are continuously exposed to endogenous toxins such as superoxide, hydrogen peroxide (H2O2), and 4-HNE and thousands of xenobiotic cytotoxic chemicals such as formaldehyde and pesticide chemicals throughout the course of life. Cells possess many detoxification enzymes capable of removing those toxins. The cellular detoxification system is highly complex, and involves three phases of enzymatic detoxification (Figure 1) (Henderson & Wolf, 2011; Nebert & Dalton, 2006; Simic, Savic-Radojevic, Pljesa-Ercegovac, Matic, & Mimic-Oka, 2009): In phase I (functionalization reactions), the initial bioactivation or the formation of reactive intermediates of thousands of endogenous and exogenous toxins primarily occurs in the liver, although it can also take place in kidneys and other tissues. The phase I detoxification enzymes include cytochrome P450 (CYP), hydroxylases, reductases, aldoketoreductases, flavin-containing monooxygenases, lipooxygenases, cyclooxygenases, peroxidases, epoxygenases, and oxidases (Nebert & Dalton, 2006). Of these, CYPs comprise 70–80% of all phase I detoxification enzymes. Humans possess 57 CYP genes in 18 families. The members of the CYP1 to CYP4 families oxygenate thousands of xenobiotics, whereas all members of the CYP5 family primarily metabolize endogenous toxins in a highly substrate-specific manner. Most phase I enzymes are capable of both detoxification and metabolic activation. Phase II detoxification (conjugation reactions) involves conjugation of toxic compounds, potential carcinogens, and pharmacologically active compounds, to the -SH groups of reduced glutathione (GSH) by GST enzymes (Figure 2) (Henderson & Wolf, 2011; McLaren & Moroi, 2003; Simic et al., 2009). Glutathione conjugates are metabolized further by cleavage of the glutamate and glycine residues, followed by acetylation of the resultant free amino group of the cysteinyl residue to produce a very hydrophilic product, mercapturic acid.
Fig 1. Cellular detoxification system.
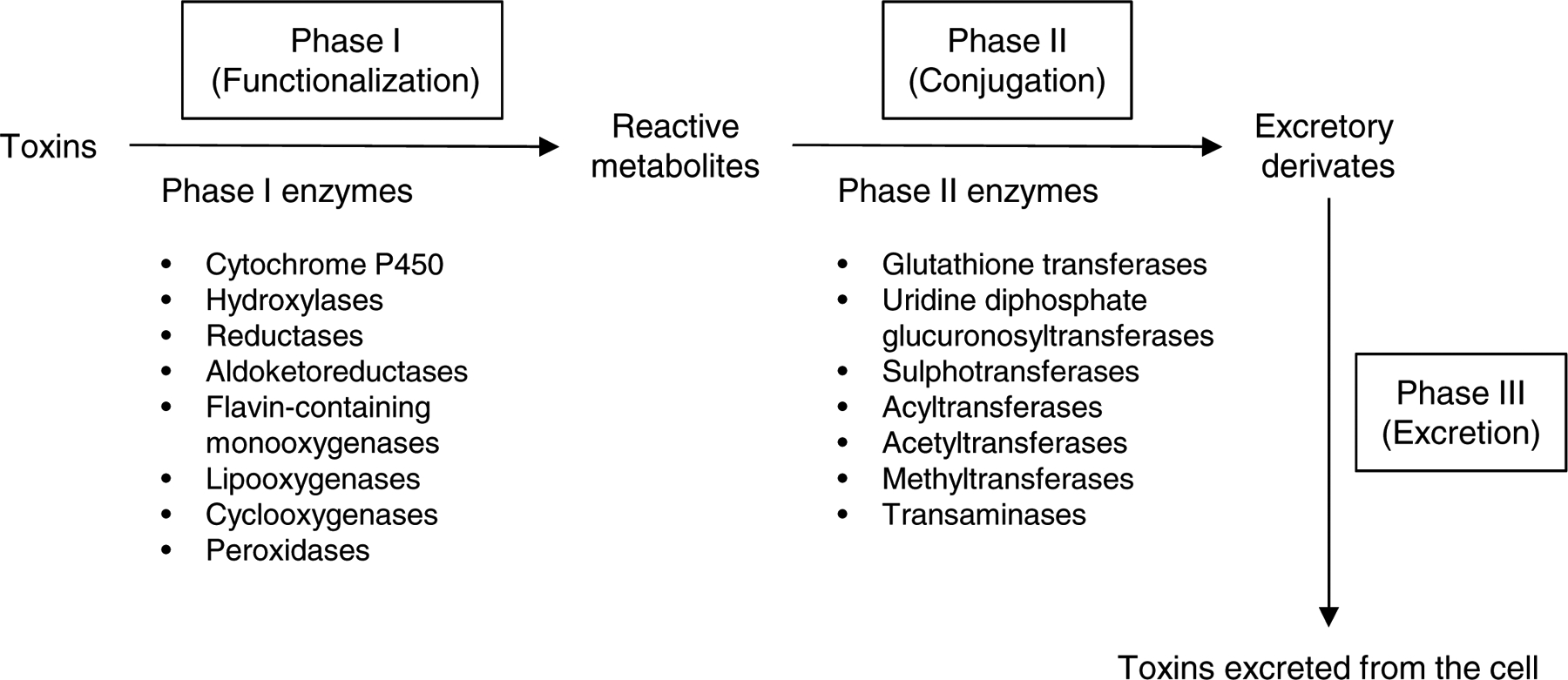
The cellular detoxification system involves three phases of detoxification. Phase I is the initial bioactivation or the formation of reactive intermediates of thousands of endogenous and exogenous toxins, and primarily occurs in the liver. The phase I detoxification enzymes include cytochrome P450, hydroxylases, reductases, aldoketoreductases, flavin-containing monooxygenases, lipooxygenases, cyclooxygenases, and peroxidases. Phase II detoxification involves conjugation of toxic compounds to the -SH groups of reduced glutathione (GSH) by GST enzymes. The phase II detoxification enzymes include glutathione transferases, uridine diphosphate glucuronosyltransferases, sulphotransferases, acyltransferases, acetyltransferases, methyltransferases, and transaminases. Phase III detoxification involves the elimination of mercapturic acid from the cell through the transmembrane transporters, completing the detoxification cycle.
Fig 2. GST detoxification.
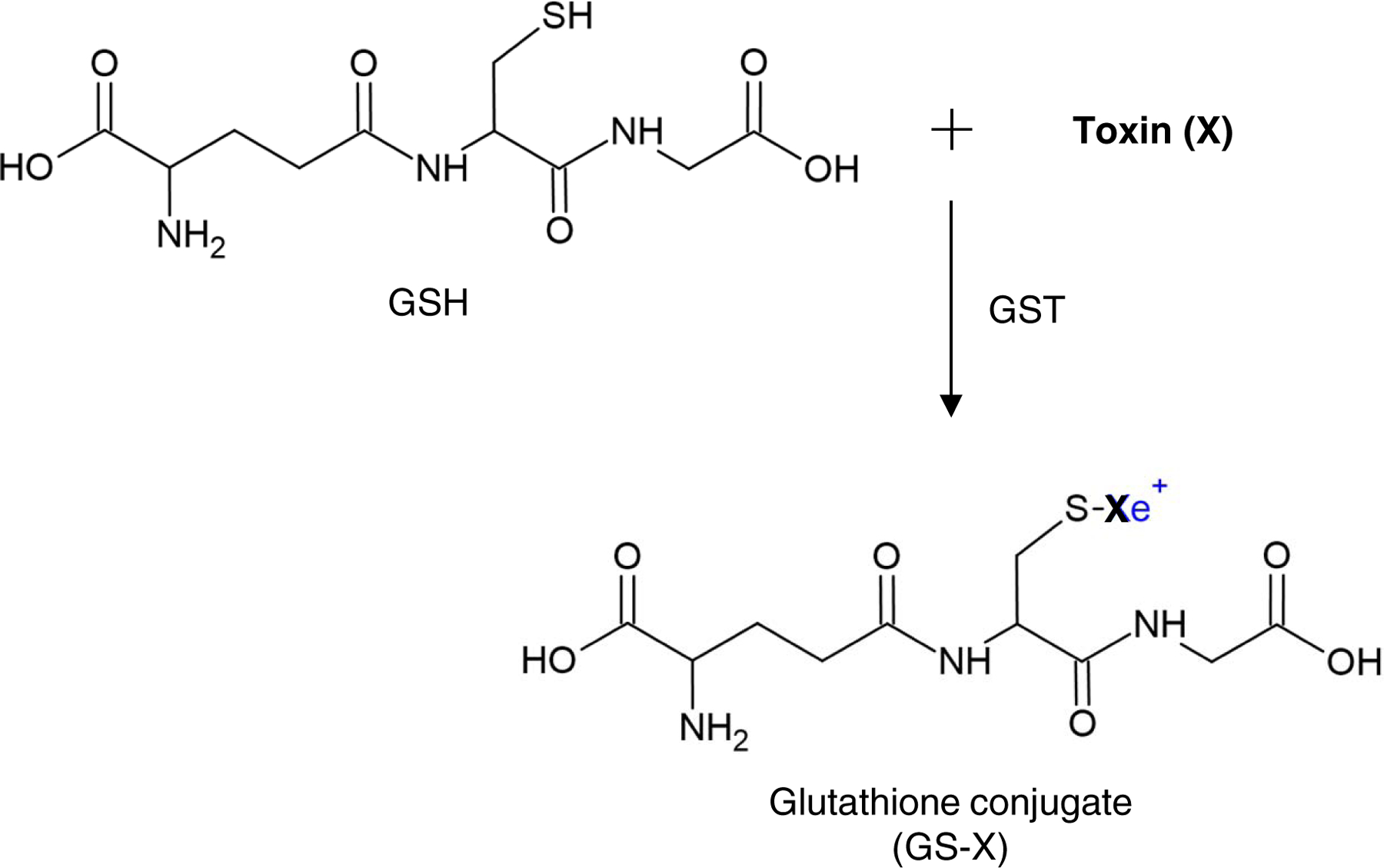
GSTs are a family of phase II detoxification enzymes that catalyze the conjugation of a wide variety of endogenous and xenobiotic toxins (X), to the -SH group of reduced glutathione (GSH), resulting in the formation of glutathione conjugate (GS-X). Glutathione conjugates are metabolized further by cleavage of the glutamate and glycine residues, followed by acetylation of the resultant free amino group of the cysteinyl residue to produce a very hydrophilic product, mercapturic acid, which is then eliminated from the cell through the transmembrane transporters.
The phase II detoxification enzymes include GSTs, uridine diphosphate glucuronosyltransferases, sulphotransferases, acyltransferases, acetyltransferases, methyltransferases, and transaminases. Phase III detoxification involves the elimination of mercapturic acid from the cell through the transmembrane transporters, completing the detoxification cycle (Nebert & Dalton, 2006; Simic et al., 2009). The combined effects of phase I and phase II detoxification enzymes usually result in the detoxification of xenobiotic chemicals. However, CYP-mediated formation of reactive oxygenated intermediates produce some highly electrophilic compounds which can readily bind covalently to DNA or proteins, causing genotoxicity and mutation (Nebert & Dalton, 2006). Hydrophobic xenobiotics can enter the cell passively, while others enter the cell through receptors, membrane-bound transporters or ion channels. Most detoxification enzymes, including GSTs have both cytosolic and membrane-bound forms, while some phase I and phase II detoxification enzymes such as CYPs are membrane-bound in the endoplasmic reticulum, mitochondria, or plasma membrane (Nebert & Dalton, 2006; Simic et al., 2009). Moreover, many phase I and II enzymes, including CYPs, do not show substrate-specific binding or have greater flexibility in substrate binding. Detoxification metabolism of endogenous and xenobiotic chemicals is thought to occur in all eukaryotic cells. Importantly, each detoxification gene shows a high level of time-specific, organ-specific, tissue-specific, cell type-specific, and/or sex-specific expression (Nebert & Dalton, 2006; Simic et al., 2009).
2.2. GST detoxification system
GSTs are a family of phase II detoxification enzymes that catalyze the conjugation of a wide variety of endogenous and xenobiotic toxins, including potential carcinogens, to the -SH groups of GSH, resulting in the formation of a glutathione conjugate, a more water-soluble form compared with the original compound (Figure 2) (Henderson & Wolf, 2011; McLaren & Moroi, 2003; Simic et al., 2009). GSTs are divided into three main families: cytosolic, mitochondrial, and microsomal (membrane-associated eicosanoid/glutathione metabolism (MAPEG) protein superfamily). Of these families, the cytosolic GSTs constitute the largest family. Cytosolic GSTs are divided into several classes on the basis of their primary structure (McLaren & Moroi, 2003; Simic et al., 2009).
Over 20 mammalian cytosolic GSTs have been identified to date. The most well characterized cytosolic GST classes have been named alpha (GSTA), mu (GSTM), pi (GSTP), and theta (GSTT) (Table 1). Cytosolic GSTs are dimeric, with subunit molecular weights of approximately 25 kDa. Each subunit contains a catalytically independent active site that consists of a GSH-binding site in the amino-terminal domain, and a site that binds the xenobiotic substrate in the carboxyl-terminal domain (Laborde, 2010). Most cytosolic GST classes show a high degree of polymorphism (Simic et al., 2009). Because of the diversity of potential xenobiotics and stressors, GSTs display extensive functional diversification in gene expression, enzyme activities, and substrate specificities. Most GSTs can bind structurally diverse non-substrate ligands such as steroids, heme, and bilirubin.
Table 1.
Classes of the most well characterized cytosolic GSTs.
| Class | Symbol | Subunit |
|---|---|---|
| Alpha | GSTA | 1, 2, 3, 4, 5 |
| Mu | GSTM | 1, 2, 3, 4, 5 |
| Pi | GSTP | 1 |
| Theta | GSTT | 1,2 |
2.3. Effects of GSTs on cancer drugs
The action of GSTs can lead to resistance to a range of anticancer drugs in tumor cells. For example, GSTP1 has a role in the regulation of mitogen-activated protein kinases (MAPK), which are involved in the induction of apoptosis (Laborde, 2010; Simic et al., 2009). A variety of stresses can activate c-Jun N-terminal kinase (JNK), a member of the MAPK family, which in turn, phosphorylates c-Jun, a component of the activator protein-1 (AP-1) transcription factor, leading to induction of AP-1-mediated target genes involved in apoptosis (Adler et al., 1999). An earlier study has shown that GSTP1 inhibits JNK through direct protein–protein interaction, forming a c-Jun–JNK complex (Adler et al., 1999). In this non-enzymatic role, GSTP1 sequesters JNK in the complex, inhibiting the phosphorylation of c-Jun by JNK, thereby blocking JNK-mediated apoptosis and leading to resistance to a range of anticancer drugs.
2.4. Roles of GSTs in GH/IGF-1 signaling, aging, and age- and noise-related hearing loss
A growing body of evidence indicates a role for GST detoxification in aging and longevity. Adequate amounts of growth hormone (GH) and insulin-like growth factor-1 (IGF-1), the main mediator of GH actions, are essential for normal growth and development for children (Rogol, 2010; Rogol, Roemmich, & Clark, 2002). Accordingly, the levels of GH and IGF-1 in circulating blood are higher early in life and begin to decline soon after physical and reproductive maturation (Bartke, 2008). In line with these observations, suppression of GH/IGF-1 signaling leads to lifespan extension in worms, fruit flies, and mice (Bartke et al., 1998; Berryman, Christiansen, Johannsson, Thorner, & Kopchick, 2008). Thus, GH/IGF-1 signaling is thought to be an evolutionarily conserved regulator for aging in a variety of species.
Ames dwarf mice lack GH, prolactin, and thyroid-stimulating hormone, exhibit a 50% increase in lifespan compared to their normal littermates and many symptoms of delayed aging (Brown-Borg & Rakoczy, 2005). Interestingly, both young and old Ames dwarf mice (Prop1df/df) display increased levels of liver glutamate-cysteine ligase (GCL), the rate-limiting enzyme in the glutathione biosynthesis pathway, and increased total GST activity in the kidney. McElwee and co-workers (McElwee et al., 2007) conducted a multi-level cross-species comparative analysis to compare gene expression changes accompanying increased longevity in mutant C. elegans (daf-2), D. melanogaster (chico1/+), Ames dwarf mice (Prop1df/df), and Little dwarf mice (Ghrhrlit/lit), well-established models of longevity associated with reduced GH/IGF-1 signaling. Interestingly, a number of gene categories were significantly enriched for genes whose expression changes in long-lived animals of all three species: up-regulated categories include GSTs and several other categories linked to CYP metabolism of endogenic and xenobiotic toxins, suggesting that enhanced GST detoxification is associated with reduced GH/IGF-1 signalling and longevity.
In humans, earlier studies suggested a role for GST detoxification in protection against age-related and noise-induced hearing loss: A significant association between AHL and GSTT1 and GSTM1 null polymorphisms was found in a Finnish population (Van Eyken et al., 2007) and a Hispanic population (Bared et al., 2010). A significant association between noise-induced hearing loss and GSTM1, GSTP1, and GSTT1 null polymorphisms was also detected in a population of noise-exposed workers in Taiwan (C. Y. Lin et al., 2009). Moreover, a GSTM1 null polymorphism was associated with poorer high-frequency otoacoustic emissions in individuals who have been working in noise exposure jobs, suggesting the protective role of GSTM1 against noise-induced hearing loss (Rabinowitz et al., 2002). Collectively, a decline in GST detoxification function may increase susceptibility to age- and noise-related hearing loss.
3. Roles of GSTAs in reducing oxidative lipid damage and ototoxicity and slowing cochlear aging
3.1. Role of GSTAs in protection against oxidative lipid damage
Up to today, ~20 mammalian GSTs have been identified (Laborde, 2010; McLaren & Moroi, 2003; Simic et al., 2009). The alpha-class GSTs consist of 5 distinct members, GSTA1, GSTA2, GSTA3, GSTA4, and GSTA5 (Awasthi et al., 2004; Balogh & Atkins, 2011; Henderson & Wolf, 2011; Simic et al., 2009; Singh, Zimniak, & Zimniak, 2010). Of these, GSTA1, GSTA2, GSTA4, and GSTA5 are thought to be the major determinants of the intracellular concentration of 4-HNE, one of the most abundant cytotoxic end products of lipid peroxidation and which contributes to neurodegenerative diseases and age-related diseases (Awasthi et al., 2004; Dalleau, Baradat, Gueraud, & Huc, 2013; Di Domenico, Tramutola, & Butterfield, 2017; Jaganjac et al., 2019; Singhal et al., 2015). GSTA1 and GSTA2 have selenium-independent glutathione peroxidase (GPX) activity and can catalyze GSH-dependent reduction of fatty acid hydroperoxides (FA-OOH) and phospholipid hydroperoxides (PL-OOH) (Figure 3) (Awasthi et al., 2004; Balogh & Atkins, 2011; Singh et al., 2010; Singhal et al., 2015). FA-OOH and or PL-OOH are then reduced to the corresponding alcohols with oxidized glutathione (GSSG) and water as by- products, thereby blocking the formation of 4-HNE. GSTA4 and GSTA5 conjugate 4-HNE to GSH, forming a GSH-4-HNE conjugate (GS-HNE), which is then eliminated through the transmembrane transporters (Figure 3). Unlike the other GSTA isoforms, GSTA3 catalyzes the double bond isomerization of precursors for progesterone and testosterone during the biosynthesis of steroid hormones (Dourado, Fernandes, Mannervik, & Ramos, 2014). Of these GSTA isoforms, conjugation to GSH by GSTA4 is thought to be a major route of 4-HNE elimination. Therefore, GSTAs play important roles in reducing oxidative lipid damage.
Fig 3. Detoxification of 4-HNE by GSTA isoforms.
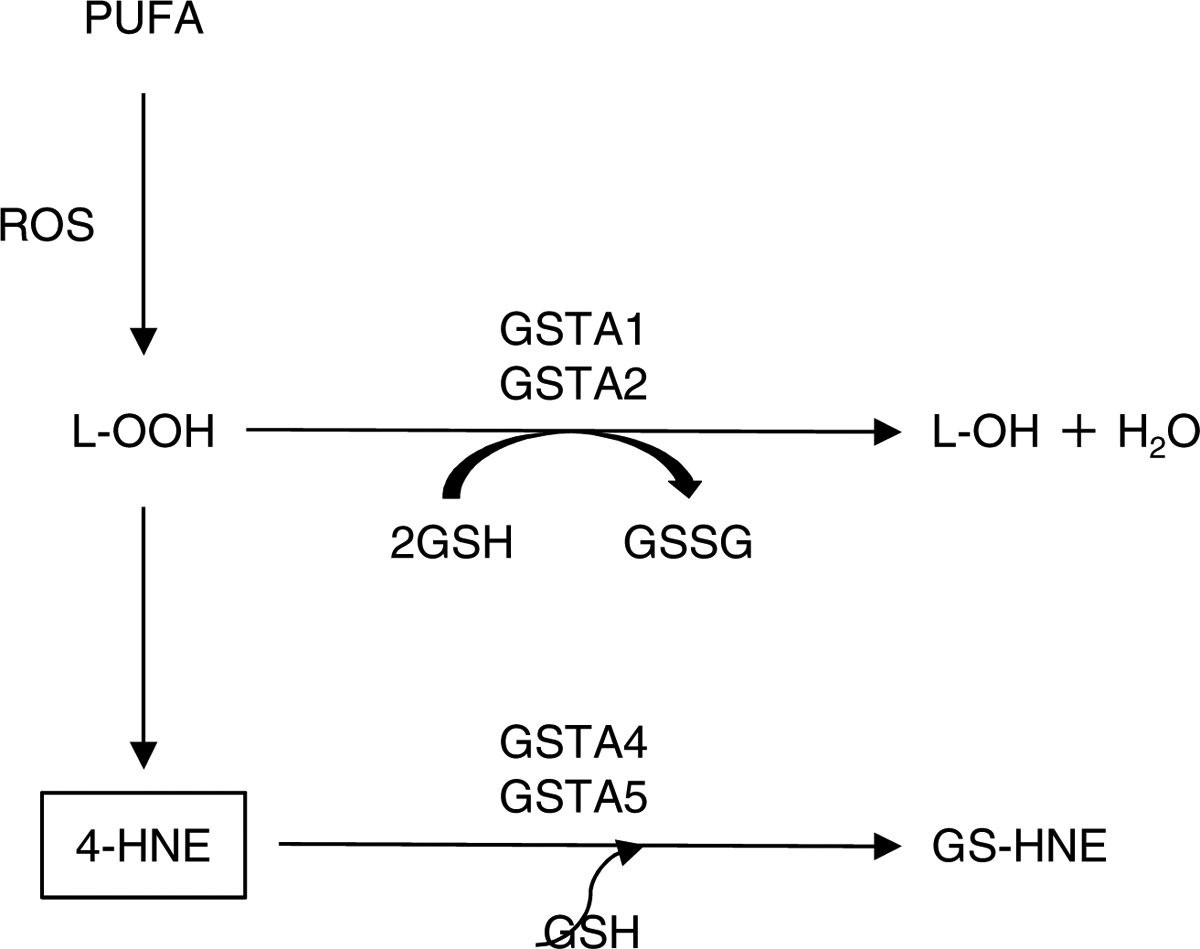
GSTA1, GSTA2, GSTA4, and GSTA5 are thought to be the major determinants of the intracellular concentration of 4-HNE. GSTA1 and GSTA2 can catalyze GSH-dependent reduction of lipid hydroperoxide (L-OOH). L-OOH is then reduced to the corresponding alcohol (L-OH) with oxidized glutathione (GSSG) and water as by-products, thereby blocking the formation of 4-HNE. GSTA4 and GSTA5 conjugate 4-HNE to GSH, forming a GSH-4-HNE conjugate (GS-HNE), which is then eliminated from the cell through the transmembrane transporters. PUFA: polyunsaturated fatty acid (i.e., linoleic acid, linolenic acid, and arachidonic acid).
3.2. The cytotoxic lipid peroxidation product 4-HNE
4-HNE is an α,β- unsaturated hydroxyalkenal mostly formed by the peroxidation of linoleic acid, linolenic acid, and arachidonic acid (Figure 4) (Dalleau et al., 2013; Di Domenico et al., 2017; Jaganjac et al., 2019). 4-HNE readily reacts with various cellular components, such as DNA, proteins and lipids, containing nucleophilic thiol (−SH) or amino (−NH2) groups. The chemical structure of 4-HNE possesses three reactive functions: a C2=C3 double bond, a C1=O carbonyl group and a hydroxyl group on C4. These reactive functions make this electrophilic molecule highly reactive toward nucleophilic thiol and amino groups. 4-HNE can react with proteins, particularly those containing histidine, cysteine and lysine residues (Di Domenico et al., 2017; Jaganjac et al., 2019). This includes plasma membrane transporters, growth factor receptors, neurotransmitters, mitochondrial electron transport chain proteins, chaperones, proteasomal proteins and cytoskeletal proteins. It is estimated that 1–8% of the 4-HNE formed in cells target proteins. 4-HNE can also react with lipids containing amino groups and nucleic acids, mostly with the guanosine moiety of DNA.
Fig 4. Role of NRF2 in GSTA detoxification of 4-HNE in cochlea.
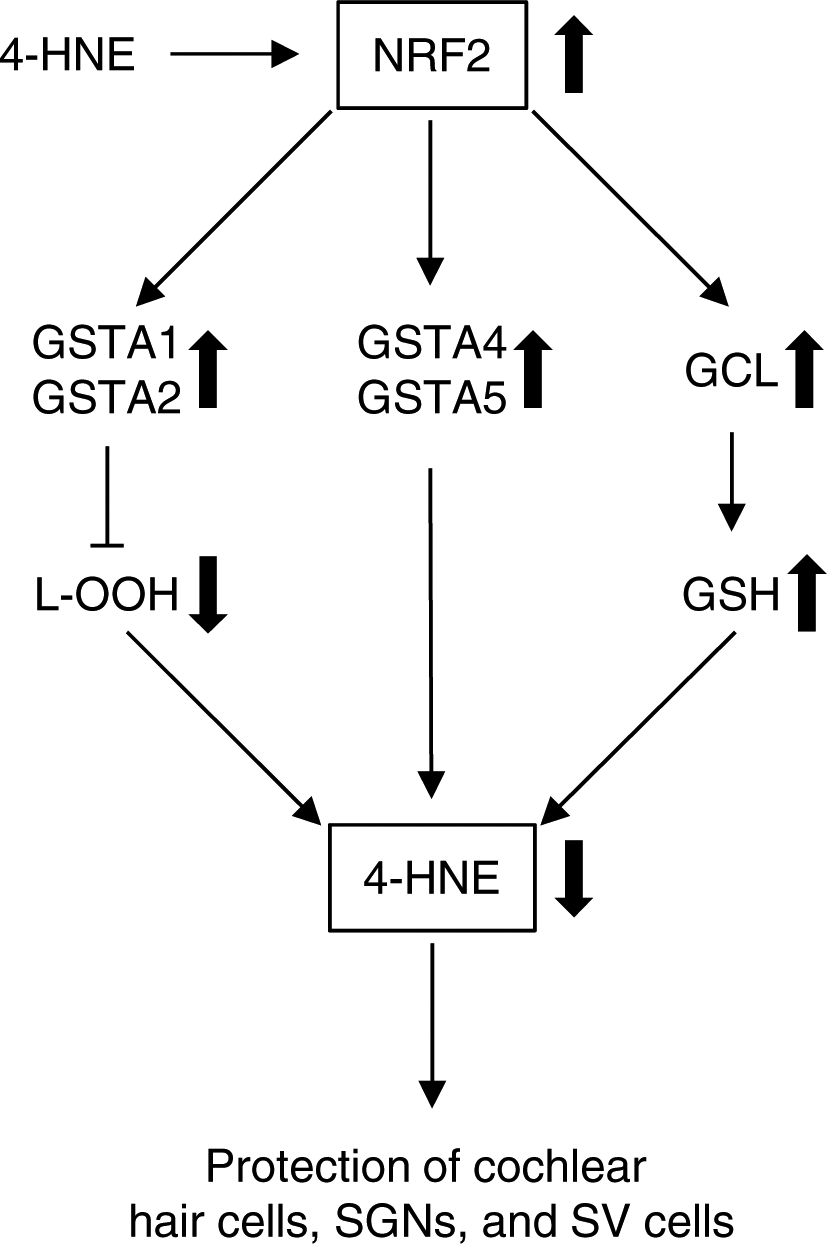
NRF2 promotes the transcriptional induction of antioxidant genes such as the subunits of glutamate-cysteine ligase (GCL), the rate-limiting enzyme in glutathione biosynthesis, and phase II detoxification genes, including GSTA genes, including GSTA1, GSTA2, GSTA4, and GSTA5. 4-HNE can also act as a direct activator of NRF2. When exposed to noise or ototoxic drug or in aged cochlear tissues, higher oxidative stress likely leads to accumulation of 4-HNE. This can trigger the activation of NRF2, which in turn activates GSTA1 and GSTA2, blocking the formation of 4-HNE, and or activates GSTA4 and GSTA5, conjugating 4-HNE to GSH. This results in elimination of 4-HNE and protecting cochlear hair cells, SGNs, and SV cells. In addition, GSH can directly sequester 4-HNE through its cysteine residues by the formation of GS-HNE adducts.
The 4-HNE concentration in human blood is estimated to be about 0.05–0.15 mM, but under pathological conditions, it can increase to over 100 mM (Dalleau et al., 2013). Thus, higher concentrations of 4-HNE cause the alteration of a wide range of biological activities, including disruption of glutamate transport, impairment of Na+/K+ ATPase activity, activation of caspase pathways, disruption of Ca2+ homeostasis, the suppression of nuclear factor-kappa B (NF-kB) activity or altered protein homeostasis.
3.3. Role of 4-HNE in age-related neurodegenerative diseases
The primary cause of biological aging is thought to be accumulation of oxidative damage at the molecular level (Balaban, Nemoto, & Finkel, 2005; Beckman & Ames, 1998; Finkel & Holbrook, 2000). In support of this view, reactive oxygen species (ROS) cause the oxidation of polyunsaturated fatty acids, leading to the formation of 4-HNE (Dalleau et al., 2013; Di Domenico et al., 2017; Jaganjac et al., 2019). The central nervous system and the peripheral nervous system are particularly sensitive to ROS damage because of the high levels of polyunsaturated fatty acids (PUFA) in neuronal cell membranes. Elevated levels of 4-HNE have been observed in brain tissues of Alzheimer disease, Parkinson disease, and Huntington disease patients and animal models of these neurodegenerative diseases (Di Domenico et al., 2017). Accumulation of 4-HNE is also linked to age-related diseases associated with increased levels of oxidative stress or redox imbalance, including cancer, atherosclerosis, and liver diseases. In addition, 4-HNE is considered as an apoptosis inducer in vitro and in vivo (Dalleau et al., 2013). These reports suggest that 4-HNE plays a role in the development of age-related neurodegenerative diseases.
3.4. Effects of Gsta4 deficiency and the resultant increase in 4-HNE levels on cell death and age- and noise-related hearing loss
GSTA4 possesses high catalytic efficiency toward 4-HNE (Balogh & Atkins, 2011) and is thought to be the major determinant of the intracellular concentration of 4-HNE. In support of this this view, knockdown of either gst-5 or gst-10, which have high catalytic activity toward 4-HNE, shortens lifespan, while overexpression of gst-10 or murine Gsta4 extends lifespan in C. elegans (Ayyadevara et al., 2007; Ayyadevara et al., 2005). In mice, short-term CR up-regulated mRNA expression of phase 2 detoxification genes, including Gsta4, in the liver (Fu & Klaassen, 2014), while treatment with paraquat significantly shortened the survival of Gsta4 KO mice compared to wild-type mice (Zimniak et al., 1994). In human erythroleukemia cell lines, overexpression of GSTA4 decreased the levels of 4-HNE (Cheng et al., 1999) and protected cells against apoptosis (Yang, Sharma, Sharma, Awasthi, & Awasthi, 2003). 4-HNE can also be detoxified by aldose reductase (AR) and aldehyde dehydrogenase (ALDH) (Black et al., 2012; Singhal et al., 2015; Srivastava, Chandra, Bhatnagar, Srivastava, & Ansari, 1995): elderly patents show lower activities of aldehyde- and lipid hydroperoxide-detoxifying enzymes, including ALDH1, ALDH2, ALDH3, GSTA4, and AR, in the thyroid arterial compared to young adult controls (Lapenna et al., 2019). Moreover, our lab has recently shown that elevated levels of 4-HNE were observed in the cochlea of aged mice compared to young controls (Park et al., 2020). This was associated with decreased SGN density, and reduced hair cells and SV thickness in the cochlea of aged mice. Interestingly, prolonged noise exposure also resulted in hair cell loss and increased levels of 4-HNE in the cochlea of rodents (Du et al., 2013; Fetoni et al., 2009; Fetoni et al., 2015; Xiong, He, Lai, & Wang, 2011; Yamashita, Jiang, Schacht, & Miller, 2004). Together, we speculate that GSTA4-mediated detoxification of 4-HNE likely plays a role in protection against cell death, noise exposure, and age-related hearing loss.
3.5. Effects of Gsta4 deficiency and the resultant increase in 4-HNE levels on cisplatin ototoxicity
Cisplatin is one of the most widely used chemotherapeutic agents for the treatment of a broad spectrum of cancers (Ding, Allman, & Salvi, 2012; Roy, Ryals, Van den Bruele, Fitzgerald, & Cunningham, 2013; Rybak & Ramkumar, 2007). However, cisplatin chemotherapy commonly causes permanent hearing loss in 40–80% of patients of all ages. Cisplatin-induced hearing loss is generally dose-dependent, irreversible, and associated with loss of sensory hair cells, SGNs and/or SV cells. Cisplatin is thought to exert its cytotoxic effects through DNA crosslinking and generation of ROS following binding to cytoplasmic proteins, leading to increased oxidative damage and cell death (Ding et al., 2012; Ober & Lippard, 2008; Rybak & Ramkumar, 2007). Previous studies have shown that cisplatin cytotoxicity is associated with increased levels of 4-HNE and malondialdehyde (MDA), major end products of lipid peroxidation, and decreased levels of GSH in the kidney of ICB mice (W. Li et al., 2016) and Wistar albino rats (Noori & Mahboob, 2010). Cisplatin administration also resulted in increased levels of 4-HNE in the cochlea of Wistar rats (Fetoni et al., 2014).
An earlier study has shown that cisplatin administration resulted in degeneration of SV associated with a decline in the endocochlear potential and degeneration of marginal cells from guinea pigs (Kohn et al., 1988). A subsequent study (Breglio et al., 2017) has shown that while cisplatin was eliminated in most organs over days to weeks, cisplatin remained in the cochlea for months to years in both mice and humans. Importantly, cisplatin levels were higher in the SV region compared to the organ of Corti or SGN region. Our lab has recently shown that GSTA4 immunostaining was prominent in the stria vascularis of Gsta4+/+ mice, suggesting that SV is likely a major site for GSTA4-mediated detoxification of 4-HNE in the cochlea (Park et al., 2019). Cisplatin treatment also stimulated GST activity toward 4-HNE in the inner ear of female Gsta4+/+ mice. In contrast, Gsta4 deficiency resulted in increased levels of 4-HNE and more profound loss of SGNs and SV atrophy in the cochlea of cisplatin-treated female mice. Together, these reports suggest that GSTA4 plays an essential role in protection against cisplatin ototoxicity.
4. Potential molecular mechanisms underlying GSTA detoxification of 4-HNE in cochlea
4.1. Role of NRF2 in GSTA detoxification of 4-HNE in cochlea
A growing body of evidence suggests that under physiological conditions, during normal aerobic metabolism, or low levels of ROS, 4-HNE is involved in the intracellular signaling mechanisms for determining whether cells undergo apoptosis, differentiation, or proliferation (Jaganjac et al., 2019; Luczaj, Gegotek, & Skrzydlewska, 2017; Yang et al., 2003). In support of this view, lower intracellular concentrations of 4-HNE (i.e., < 2 μM) appear to be beneficial to cells. The nuclear transcription factor E2-related factor 2 (NRF2) promotes the transcriptional induction of antioxidant genes such as the subunits of GCL, the rate-limiting enzyme in glutathione biosynthesis, and phase II detoxification genes, including GSTA and GSTP genes (Blackwell, Steinbaugh, Hourihan, Ewald, & Isik, 2015; Itoh et al., 1997; Shen & Kong, 2009). 4-HNE can also act as a direct activator of NRF2 (Boerma et al., 2015). Thus, under physiological conditions, 4-HNE may be involved in modulating NRF2-mediated glutathione synthesis and GSTA detoxification in cochlea. Under pathological conditions or in aged tissues, 4-HNE concentration can increase to over 100 mM, which in turn causes the alteration of a wide range of biological activities (Dalleau et al., 2013). Accordingly, when exposed to noise or ototoxic drug, or under pathological conditions, higher oxidative stress likely leads to accumulation of 4-HNE. This can also trigger the activation of NRF2, which in turn activates GSTA1 and GSTA2, blocking the formation of 4-HNE, and or activates GSTA4 and GSTA5, conjugating 4-HNE to GSH. This results in elimination of 4-HNE and protection of cochlear hair cells, SGNs, and SV cells (Figure 4). In addition, GSH can directly sequester 4-HNE through its cysteine residues by the formation of GS-HNE adducts (Figure 4).
4.2. Effects of estrogen on GSTA detoxification of 4-HNE in cochlea
Evidence indicates that constitutive gene expression of GST isoforms is tissue- and sex-specific (Benbrahim-Tallaa, Tabone, Tosser-Klopp, Hatey, & Benahmed, 2002; Faustino et al., 2012; Gupta, Medh, Leal, & Awasthi, 1990; D. J. Harrison, Kharbanda, Cunningham, McLellan, & Hayes, 1989; Knight, Choudhuri, & Klaassen, 2007; Park et al., 2019). For example, mRNA expression of Gsta4 was observed to be the highest in the stomach, while lower levels of Gsta4 mRNA were observed in the liver and kidney of mice (Knight et al., 2007). Female mice also showed higher mRNA expression levels of Gsta1, Gsta2, Gsta3, and Gsta4 in the kidneys, higher mRNA expression levels of Gsta4 in the heart (Knight et al., 2007), and higher mRNA expression of Gsta4 in the inner ears compared to males (Park et al., 2019). In contrast, ovariectomy downregulated 25 genes involved in Phase II detoxification, including Gsta4 in the inner ears. Ovariectomized mice also had significantly lower GSTA4 protein levels compared to ovary-intact female mice, suggesting that ovarian estrogen may modulate gene expression of GST genes.
It is well-documented that women live longer than men in every country in the world (Austad, 2006). A similar pattern of sex differences in longevity is found in many other animals. Numerous studies have also reported gender differences in human auditory perception (Caras, 2013; Chung, Mason, Gannon, & Willson, 1983; Dehan & Jerger, 1990; Jerger & Johnson, 1988; Jonsson, Rosenhall, Gause-Nilsson, & Steen, 1998; F. R. Lin, Niparko, et al., 2011; McFadden & Champlin, 2000). In general, the results of these studies show that the prevalence of hearing loss is lower in women than in men in virtually all age groups. Considerable evidence also suggests that auditory function is diminished following menopause (Hederstierna, Hultcrantz, Collins, & Rosenhall, 2007; Khaliq, Tandon, & Goel, 2003, 2005), whereas estrogen replacement therapy lowers hearing thresholds, shortens auditory brainstem response (ABR) latencies, and increases ABR amplitudes in postmenopausal women (Caruso et al., 2003; Hederstierna et al., 2007; Kilicdag et al., 2004). Numerous studies indicate that estrogen has neuroprotective actions (Bean, Ianov, & Foster, 2014; Bean et al., 2015; Borras et al., 2003; Sherwin, 2009; Torres et al., 2018). For example, liver mitochondria from female rodents produce fewer ROS, had higher activity of GPX, and increased glutathione redox state compared to males, suggesting that female tissues have higher activities of glutathione antioxidant defense enzymes and increased glutathione redox state compared to males (Borras et al., 2003; Torres et al., 2018).
There are three major forms of estrogen: estrone (E1), estradiol (E2), and estriol (E3) (Cui, Shen, & Li, 2013): E2 is the most potent and predominant form of estrogen during the premenopausal or reproductive period, while E1 is the dominant form of estrogen after reproductive cessation or menopause. E3 plays larger roles during pregnancy. E1, E2, and E3 are monophenolic compounds, similar to α-tocopherol (vitamin E), which can act as free-radical scavengers (Behl, 2002; Cui et al., 2013; Moosmann & Behl, 1999). The hydroxyl group on ring A of E1, E2, or E3 could donate a hydrogen, thereby detoxifying free radicals such as hydroxyl radical. This direct free-radical scavenging activity of E1, E2, or E3 is independent of the activation of estrogen receptors or of any other estrogen-mediated signaling pathways (Behl, 2002). Estrogen also modulates cell survival by inducing the transcription of neurotrophic factors and their receptors, including nerve growth factor, brain-derived neurotrophic factor (BDNF), neurotrophin 3, and IGF1 (Behl, 2002; Sohrabji, Miranda, & Toran-Allerand, 1995), and BCL2 and BCLX, inhibitors of apoptosis (Pike, 1999; Singer, Rogers, & Dorsa, 1998). Furthermore, estrogen can directly affect neurotransmission by binding to transmembrane ion channels such as GABAA (γ-aminobutyric acid type A) receptor and or NMDA (N-methyl-D-aspartate) receptor, a glutamate and ion channel protein receptor (Behl, 2002; Woolley, 1999). Glutamate is the major excitatory neurotransmitter, whereas GABA is the major inhibitory neurotransmitter in the auditory system (Puel, 1995). Given that females have higher levels of estrogen particularly during the reproduction period, we speculate that estrogen-mediated GSTA detoxification of 4-HNE may play a role in neuroprotection against oxidative lipid damage in cochlea.
5. Concluding remarks
There is a growing consensus that the primary cause of biological aging is accumulation of oxidative damage at the molecular level (Balaban et al., 2005; Beckman & Ames, 1998; Finkel & Holbrook, 2000; McElwee et al., 2007). We speculate that accumulation of oxidative lipid damage and the resultant increase in 4-HNE levels may play a causal role in age-related diseases associated with increased levels of oxidative damage, including AHL (Balogh & Atkins, 2011; Dalleau et al., 2013; Di Domenico et al., 2017; Jaganjac et al., 2019; Park et al., 2020; Singhal et al., 2015). In addition, the fact that cells possess many detoxification enzymes and antioxidants capable of removing 4-HNE or blocking its formation (i.e., GSTA1, GSTA2, GSTA4, GSTA5, AR, ALDH, glutathione, and carnosine) (Awasthi et al., 2004; Black et al., 2012; Di Domenico et al., 2017; Singh et al., 2010; Singhal et al., 2015; Srivastava et al., 1995) broadly supports the view that elevated levels of 4-HNE play a major role in the pathogenesis of a variety of age-related diseases, including AHL. Of these detoxification enzymes and antioxidants, the GSH/GSSG redox couple is thought to be an intracellular determinant of the antioxidant capacity because the abundance of GSH (10–15 mM) is three to four orders of magnitude higher than the other reductants, including NADPH and NADH (Mari, Morales, Colell, Garcia-Ruiz, & Fernandez-Checa, 2009; Rebrin & Sohal, 2008). Importantly, GSH has the ability to rapidly bind 4-HNE through its cysteine residues and detoxify 4-HNE by the formation of GS-HNE adducts (Figure 4) (Di Domenico et al., 2017). GSH can also detoxify 4-HNE through serving as a co-factor for GSTA1, GSTA2, GSTA4, and GSTA5 (Figure 4) (Awasthi et al., 2004; Balogh & Atkins, 2011; Di Domenico et al., 2017; Singh et al., 2010; Singhal et al., 2015; Yang et al., 2003). Therefore, we speculate that a combination of GSTA-mediated detoxification and glutathione antioxidant defense against 4-HNE plays a major role in protecting cochlear structure and function against oxidative lipid damage, ototoxic drug, noise, or aging.
Highlights.
A growing body of evidence indicates a role for GST detoxification in aging and longevity.
GSTA1, GSTA2, GSTA4, and GSTA5 are thought to be the major determinants of the intracellular concentration of 4-HNE.
Elevated levels of 4-HNE may play a role in the pathogenesis of noise-, drug-, and or age-related hearing loss.
GSTA-mediated detoxification may play a major role in protecting cochlear structure and function against oxidative lipid damage, ototoxic drug, noise, or aging.
Acknowledgements
This research was supported by R03 DC011840 (SS), R01 DC012552 (SS) and R01 DC014437 (SS) from the National Institute of Health and National Institute on Deafness and Communication Disorders, and the Claude D. Pepper Older Americans Independence Centers at the University of Florida (P30 AG028740) from the National Institute of Health and National Institute on Aging.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler V, Yin Z, Fuchs SY, Benezra M, Rosario L, Tew KD, … Ronai Z (1999). Regulation of JNK signaling by GSTp. EMBO J, 18(5), 1321–1334. doi: 10.1093/emboj/18.5.1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelaki DE, & Cullen KE (2008). Vestibular system: the many facets of a multimodal sense. Annu Rev Neurosci, 31, 125–150. doi: 10.1146/annurev.neuro.31.060407.125555 [DOI] [PubMed] [Google Scholar]
- Austad SN (2006). Why women live longer than men: sex differences in longevity. Gend Med, 3(2), 79–92. doi: 10.1016/s1550-8579(06)80198-1 [DOI] [PubMed] [Google Scholar]
- Awasthi YC, Yang Y, Tiwari NK, Patrick B, Sharma A, Li J, & Awasthi S (2004). Regulation of 4-hydroxynonenal-mediated signaling by glutathione S-transferases. Free Radic Biol Med, 37(5), 607–619. doi: 10.1016/j.freeradbiomed.2004.05.033 [DOI] [PubMed] [Google Scholar]
- Ayyadevara S, Dandapat A, Singh SP, Siegel ER, Shmookler Reis RJ, Zimniak L, & Zimniak P (2007). Life span and stress resistance of Caenorhabditis elegans are differentially affected by glutathione transferases metabolizing 4-hydroxynon-2-enal. Mech Ageing Dev, 128(2), 196–205. doi: 10.1016/j.mad.2006.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayyadevara S, Engle MR, Singh SP, Dandapat A, Lichti CF, Benes H, … Zimniak P (2005). Lifespan and stress resistance of Caenorhabditis elegans are increased by expression of glutathione transferases capable of metabolizing the lipid peroxidation product 4-hydroxynonenal. Aging Cell, 4(5), 257–271. doi: 10.1111/j.1474-9726.2005.00168.x [DOI] [PubMed] [Google Scholar]
- Balaban RS, Nemoto S, & Finkel T (2005). Mitochondria, oxidants, and aging. Cell, 120(4), 483–495. doi: 10.1016/j.cell.2005.02.001 [DOI] [PubMed] [Google Scholar]
- Balogh LM, & Atkins WM (2011). Interactions of glutathione transferases with 4-hydroxynonenal. Drug Metab Rev, 43(2), 165–178. doi: 10.3109/03602532.2011.558092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bared A, Ouyang X, Angeli S, Du LL, Hoang K, Yan D, & Liu XZ (2010). Antioxidant enzymes, presbycusis, and ethnic variability. Otolaryngol Head Neck Surg, 143(2), 263–268. doi: 10.1016/j.otohns.2010.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartke A (2008). Growth hormone and aging: a challenging controversy. Clin Interv Aging, 3(4), 659–665. doi: 10.2147/cia.s3697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartke A, Brown-Borg HM, Bode AM, Carlson J, Hunter WS, & Bronson RT (1998). Does growth hormone prevent or accelerate aging? Exp Gerontol, 33(7–8), 675–687. doi: 10.1016/s0531-5565(98)00032-1 [DOI] [PubMed] [Google Scholar]
- Bean LA, Ianov L, & Foster TC (2014). Estrogen receptors, the hippocampus, and memory. Neuroscientist, 20(5), 534–545. doi: 10.1177/1073858413519865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean LA, Kumar A, Rani A, Guidi M, Rosario AM, Cruz PE, … Foster TC (2015). Re-Opening the Critical Window for Estrogen Therapy. J Neurosci, 35(49), 16077–16093. doi: 10.1523/JNEUROSCI.1890-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman KB, & Ames BN (1998). The free radical theory of aging matures. Physiol Rev, 78(2), 547–581. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9562038 [DOI] [PubMed] [Google Scholar]
- Behl C (2002). Oestrogen as a neuroprotective hormone. Nat Rev Neurosci, 3(6), 433–442. doi: 10.1038/nrn846 [DOI] [PubMed] [Google Scholar]
- Benbrahim-Tallaa L, Tabone E, Tosser-Klopp G, Hatey F, & Benahmed M (2002). Glutathione S-transferase alpha expressed in porcine Sertoli cells is under the control of follicle-stimulating hormone and testosterone. Biol Reprod, 66(6), 1734–1742. doi: 10.1095/biolreprod66.6.1734 [DOI] [PubMed] [Google Scholar]
- Berryman DE, Christiansen JS, Johannsson G, Thorner MO, & Kopchick JJ (2008). Role of the GH/IGF-1 axis in lifespan and healthspan: lessons from animal models. Growth Horm IGF Res, 18(6), 455–471. doi: 10.1016/j.ghir.2008.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black W, Chen Y, Matsumoto A, Thompson DC, Lassen N, Pappa A, & Vasiliou V (2012). Molecular mechanisms of ALDH3A1-mediated cellular protection against 4-hydroxy-2-nonenal. Free Radic Biol Med, 52(9), 1937–1944. doi: 10.1016/j.freeradbiomed.2012.02.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell TK, Steinbaugh MJ, Hourihan JM, Ewald CY, & Isik M (2015). SKN-1/Nrf, stress responses, and aging in Caenorhabditis elegans. Free Radic Biol Med, 88(Pt B), 290–301. doi: 10.1016/j.freeradbiomed.2015.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerma M, Singh P, Sridharan V, Tripathi P, Sharma S, & Singh SP (2015). Effects of Local Heart Irradiation in a Glutathione S-Transferase Alpha 4- Null Mouse Model. Radiat Res, 183(6), 610–619. doi: 10.1667/RR13979.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borras C, Sastre J, Garcia-Sala D, Lloret A, Pallardo FV, & Vina J (2003). Mitochondria from females exhibit higher antioxidant gene expression and lower oxidative damage than males. Free Radic Biol Med, 34(5), 546–552. doi: 10.1016/s0891-5849(02)01356-4 [DOI] [PubMed] [Google Scholar]
- Breglio AM, Rusheen AE, Shide ED, Fernandez KA, Spielbauer KK, McLachlin KM, … Cunningham LL (2017). Cisplatin is retained in the cochlea indefinitely following chemotherapy. Nat Commun, 8(1), 1654. doi: 10.1038/s41467-017-01837-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown-Borg HM, & Rakoczy SG (2005). Glutathione metabolism in long-living Ames dwarf mice. Exp Gerontol, 40(1–2), 115–120. doi: 10.1016/j.exger.2004.11.004 [DOI] [PubMed] [Google Scholar]
- Caras ML (2013). Estrogenic modulation of auditory processing: a vertebrate comparison. Front Neuroendocrinol, 34(4), 285–299. doi: 10.1016/j.yfrne.2013.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso S, Maiolino L, Agnello C, Garozzo A, Di Mari L, & Serra A (2003). Effects of patch or gel estrogen therapies on auditory brainstem response in surgically postmenopausal women: a prospective, randomized study. Fertil Steril, 79(3), 556–561. doi: 10.1016/s0015-0282(02)04763-5 [DOI] [PubMed] [Google Scholar]
- Cheng JZ, Singhal SS, Saini M, Singhal J, Piper JT, Van Kuijk FJ, … Awasthi S (1999). Effects of mGST A4 transfection on 4-hydroxynonenal mediated apoptosis and differentiation of K562 human erythroleukemia cells. Arch Biochem Biophys, 372(1), 29–36. doi: 10.1006/abbi.1999.1479 [DOI] [PubMed] [Google Scholar]
- Chung DY, Mason K, Gannon RP, & Willson GN (1983). The ear effect as a function of age and hearing loss. J Acoust Soc Am, 73(4), 1277–1282. doi: 10.1121/1.389276 [DOI] [PubMed] [Google Scholar]
- Cui J, Shen Y, & Li R (2013). Estrogen synthesis and signaling pathways during aging: from periphery to brain. Trends Mol Med, 19(3), 197–209. doi: 10.1016/j.molmed.2012.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalleau S, Baradat M, Gueraud F, & Huc L (2013). Cell death and diseases related to oxidative stress: 4-hydroxynonenal (HNE) in the balance. Cell Death Differ, 20(12), 1615–1630. doi: 10.1038/cdd.2013.138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehan CP, & Jerger J (1990). Analysis of gender differences in the auditory brainstem response. Laryngoscope, 100(1), 18–24. doi: 10.1288/00005537-199001000-00005 [DOI] [PubMed] [Google Scholar]
- Di Domenico F, Tramutola A, & Butterfield DA (2017). Role of 4-hydroxy-2-nonenal (HNE) in the pathogenesis of alzheimer disease and other selected age-related neurodegenerative disorders. Free Radic Biol Med, 111, 253–261. doi: 10.1016/j.freeradbiomed.2016.10.490 [DOI] [PubMed] [Google Scholar]
- Ding D, Allman BL, & Salvi R (2012). Review: ototoxic characteristics of platinum antitumor drugs. Anat Rec (Hoboken), 295(11), 1851–1867. doi: 10.1002/ar.22577 [DOI] [PubMed] [Google Scholar]
- Dourado DF, Fernandes PA, Mannervik B, & Ramos MJ (2014). Isomerization of Delta5-androstene-3,17-dione into Delta4-androstene-3,17-dione catalyzed by human glutathione transferase A3–3: a computational study identifies a dual role for glutathione. J Phys Chem A, 118(31), 5790–5800. doi: 10.1021/jp410810q [DOI] [PubMed] [Google Scholar]
- Du X, Ewert DL, Cheng W, West MB, Lu J, Li W, … Kopke RD (2013). Effects of antioxidant treatment on blast-induced brain injury. PLoS One, 8(11), e80138. doi: 10.1371/journal.pone.0080138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faustino LC, Almeida NA, Pereira GF, Ramos RG, Soares RM, Morales MM, … Ortiga-Carvalho TM (2012). Thyroid hormone and estradiol have overlapping effects on kidney glutathione S-transferase-alpha gene expression. Am J Physiol Endocrinol Metab, 303(6), E787–797. doi: 10.1152/ajpendo.00223.2012 [DOI] [PubMed] [Google Scholar]
- Fetoni AR, Eramo SL, Paciello F, Rolesi R, Podda MV, Troiani D, & Paludetti G (2014). Curcuma longa (curcumin) decreases in vivo cisplatin induced ototoxicity through heme oxygenase-1 induction. Otol Neurotol, 35(5), e169–177. doi: 10.1097/MAO.0000000000000302 [DOI] [PubMed] [Google Scholar]
- Fetoni AR, Ferraresi A, Picciotti P, Gaetani E, Paludetti G, & Troiani D (2009). Noise induced hearing loss and vestibular dysfunction in the guinea pig. Int J Audiol, 48(11), 804–810. doi: 10.3109/14992020903023140 [DOI] [PubMed] [Google Scholar]
- Fetoni AR, Paciello F, Rolesi R, Eramo SL, Mancuso C, Troiani D, & Paludetti G (2015). Rosmarinic acid up-regulates the noise-activated Nrf2/HO-1 pathway and protects against noise-induced injury in rat cochlea. Free Radic Biol Med, 85, 269–281. doi: 10.1016/j.freeradbiomed.2015.04.021 [DOI] [PubMed] [Google Scholar]
- Finkel T, & Holbrook NJ (2000). Oxidants, oxidative stress and the biology of ageing. Nature, 408(6809), 239–247. doi: 10.1038/3504168739 [DOI] [PubMed] [Google Scholar]
- Fu ZD, & Klaassen CD (2014). Short-term calorie restriction feminizes the mRNA profiles of drug metabolizing enzymes and transporters in livers of mice. Toxicol Appl Pharmacol, 274(1), 137–146. doi: 10.1016/j.taap.2013.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates GA, & Mills JH (2005). Presbycusis. Lancet, 366(9491), 1111–1120. doi: 10.1016/S0140-6736(05)67423-5 [DOI] [PubMed] [Google Scholar]
- Gupta S, Medh RD, Leal T, & Awasthi YC (1990). Selective expression of the three classes of glutathione S-transferase isoenzymes in mouse tissues. Toxicol Appl Pharmacol, 104(3), 533–542. doi: 10.1016/0041-008x(90)90175-t [DOI] [PubMed] [Google Scholar]
- Harrison DJ, Kharbanda R, Cunningham DS, McLellan LI, & Hayes JD (1989). Distribution of glutathione S-transferase isoenzymes in human kidney: basis for possible markers of renal injury. J Clin Pathol, 42(6), 624–628. doi: 10.1136/jcp.42.6.624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hederstierna C, Hultcrantz M, Collins A, & Rosenhall U (2007). Hearing in women at menopause. Prevalence of hearing loss, audiometric configuration and relation to hormone replacement therapy. Acta Otolaryngol, 127(2), 149–155. doi: 10.1080/00016480600794446 [DOI] [PubMed] [Google Scholar]
- Henderson CJ, & Wolf CR (2011). Knockout and transgenic mice in glutathione transferase research. Drug Metab Rev, 43(2), 152–164. doi: 10.3109/03602532.2011.562900 [DOI] [PubMed] [Google Scholar]
- Hudspeth AJ (1997). How hearing happens. Neuron, 19(5), 947–950. doi: 10.1016/s0896-6273(00)80385-2 [DOI] [PubMed] [Google Scholar]
- Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, … Nabeshima Y (1997). An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun, 236(2), 313–322. doi: 10.1006/bbrc.1997.6943 [DOI] [PubMed] [Google Scholar]
- Jaganjac M, Milkovic L, Gegotek A, Cindric M, Zarkovic K, Skrzydlewska E, & Zarkovic N (2019). The relevance of pathophysiological alterations in redox signaling of 4-hydroxynonenal for pharmacological therapies of major stress-associated diseases. Free Radic Biol Med. doi: 10.1016/j.freeradbiomed.2019.11.023 [DOI] [PubMed] [Google Scholar]
- Jerger J, & Johnson K (1988). Interactions of age, gender, and sensorineural hearing loss on ABR latency. Ear Hear, 9(4), 168–176. doi: 10.1097/00003446-198808000-00002 [DOI] [PubMed] [Google Scholar]
- Jonsson R, Rosenhall U, Gause-Nilsson I, & Steen B (1998). Auditory function in 70- and 75-year-olds of four age cohorts. A cross-sectional and time-lag study of presbyacusis. Scand Audiol, 27(2), 81–93. doi: 10.1080/010503998420324 [DOI] [PubMed] [Google Scholar]
- Khaliq F, Tandon OP, & Goel N (2003). Auditory evoked responses in postmenopausal women on hormone replacement therapy. Indian J Physiol Pharmacol, 47(4), 393–399. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/15266950 [PubMed] [Google Scholar]
- Khaliq F, Tandon OP, & Goel N (2005). Differential effects of exogenous estrogen versus a estrogen-progesterone combination on auditory evoked potentials in menopausal women. Indian J Physiol Pharmacol, 49(3), 345–352. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/16440855 [PubMed] [Google Scholar]
- Kilicdag EB, Yavuz H, Bagis T, Tarim E, Erkan AN, & Kazanci F (2004). Effects of estrogen therapy on hearing in postmenopausal women. Am J Obstet Gynecol, 190(1), 77–82. doi: 10.1016/j.ajog.2003.06.001 [DOI] [PubMed] [Google Scholar]
- Knight TR, Choudhuri S, & Klaassen CD (2007). Constitutive mRNA expression of various glutathione S-transferase isoforms in different tissues of mice. Toxicol Sci, 100(2), 513–524. doi: 10.1093/toxsci/kfm233 [DOI] [PubMed] [Google Scholar]
- Kohn S, Fradis M, Pratt H, Zidan J, Podoshin L, Robinson E, & Nir I (1988). Cisplatin ototoxicity in guinea pigs with special reference to toxic effects in the stria vascularis. Laryngoscope, 98(8 Pt 1), 865–871. doi: 10.1288/00005537-198808000-00015 [DOI] [PubMed] [Google Scholar]
- Laborde E (2010). Glutathione transferases as mediators of signaling pathways involved in cell proliferation and cell death. Cell Death Differ, 17(9), 1373–1380. doi: 10.1038/cdd.2010.80 [DOI] [PubMed] [Google Scholar]
- Lapenna D, Ciofani G, Obletter G, Pierdomenico SD, Cipollone F, Cotellese R, … Porreca E (2019). Impaired enzymatic reactive aldehyde-detoxifying capacity and glutathione peroxidase activity in the aged human arterial tissue. Exp Gerontol, 116, 7–13. doi: 10.1016/j.exger.2018.11.013 [DOI] [PubMed] [Google Scholar]
- Liberman MC, & Kujawa SG (2017). Cochlear synaptopathy in acquired sensorineural hearing loss: Manifestations and mechanisms. Hear Res, 349, 138–147. doi: 10.1016/j.heares.2017.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Yan MH, Liu Y, Liu Z, Wang Z, Chen C, … Sun YS (2016). Ginsenoside Rg5 Ameliorates Cisplatin-Induced Nephrotoxicity in Mice through Inhibition of Inflammation, Oxidative Stress, and Apoptosis. Nutrients, 8(9). doi: 10.3390/nu8090566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CY, Wu JL, Shih TS, Tsai PJ, Sun YM, & Guo YL (2009). Glutathione S-transferase M1, T1, and P1 polymorphisms as susceptibility factors for noise-induced temporary threshold shift. Hear Res, 257(1–2), 8–15. doi: 10.1016/j.heares.2009.07.008 [DOI] [PubMed] [Google Scholar]
- Liu XZ, & Yan D (2007). Ageing and hearing loss. J Pathol, 211(2), 188–197. doi: 10.1002/path.2102 [DOI] [PubMed] [Google Scholar]
- Luczaj W, Gegotek A, & Skrzydlewska E (2017). Antioxidants and HNE in redox homeostasis. Free Radic Biol Med, 111, 87–101. doi: 10.1016/j.freeradbiomed.2016.11.033 [DOI] [PubMed] [Google Scholar]
- Mari M, Morales A, Colell A, Garcia-Ruiz C, & Fernandez-Checa JC (2009). Mitochondrial glutathione, a key survival antioxidant. Antioxid Redox Signal, 11(11), 2685–2700. doi: 10.1089/ARS.2009.2695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElwee JJ, Schuster E, Blanc E, Piper MD, Thomas JH, Patel DS, … Gems D (2007). Evolutionary conservation of regulated longevity assurance mechanisms. Genome Biol, 8(7), R132. doi: 10.1186/gb-2007-8-7-r132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden D, & Champlin CA (2000). Comparison of auditory evoked potentials in heterosexual, homosexual, and bisexual males and females. J Assoc Res Otolaryngol, 1(1), 89–99. doi: 10.1007/s101620010008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren NC, & Moroi SE (2003). Clinical implications of pharmacogenetics for glaucoma therapeutics. Pharmacogenomics J, 3(4), 197–201. doi: 10.1038/sj.tpj.6500181 [DOI] [PubMed] [Google Scholar]
- Moosmann B, & Behl C (1999). The antioxidant neuroprotective effects of estrogens and phenolic compounds are independent from their estrogenic properties. Proc Natl Acad Sci U S A, 96(16), 8867–8872. doi: 10.1073/pnas.96.16.8867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebert DW, & Dalton TP (2006). The role of cytochrome P450 enzymes in endogenous signalling pathways and environmental carcinogenesis. Nat Rev Cancer, 6(12), 947–960. doi: 10.1038/nrc2015 [DOI] [PubMed] [Google Scholar]
- Noori S, & Mahboob T (2010). Antioxidant effect of carnosine pretreatment on cisplatin-induced renal oxidative stress in rats. Indian J Clin Biochem, 25(1), 86–91. doi: 10.1007/s12291-010-0018-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ober M, & Lippard SJ (2008). A 1,2-d(GpG) cisplatin intrastrand cross-link influences the rotational and translational setting of DNA in nucleosomes. J Am Chem Soc, 130(9), 2851–2861. doi: 10.1021/ja710220x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HJ, Kim MJ, Han C, White K, Ding D, Boyd K, … Someya S (2020). Effects of Gsta4 deficiency on age-related cochlear pathology and hearing loss in mice. Exp Gerontol, 133, 110872. doi: 10.1016/j.exger.2020.110872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HJ, Kim MJ, Rothenberger C, Kumar A, Sampson EM, Ding D, … Someya S (2019). GSTA4 mediates reduction of cisplatin ototoxicity in female mice. Nat Commun, 10(1), 4150. doi: 10.1038/s41467-019-12073-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike CJ (1999). Estrogen modulates neuronal Bcl-xL expression and beta-amyloid-induced apoptosis: relevance to Alzheimer’s disease. J Neurochem, 72(4), 1552–1563. doi: 10.1046/j.1471-4159.1999.721552.x [DOI] [PubMed] [Google Scholar]
- Puel JL (1995). Chemical synaptic transmission in the cochlea. Prog Neurobiol, 47(6), 449–476. doi: 10.1016/0301-0082(95)00028-3 [DOI] [PubMed] [Google Scholar]
- Rabinowitz PM, Pierce Wise J Sr., Hur Mobo B, Antonucci PG, Powell C, Slade M (2002). Antioxidant status and hearing function in noise-exposed workers. Hear Res, 173, 164–71. doi: 10.1016/s0378-5955(02)00350-7 [DOI] [PubMed] [Google Scholar]
- Rebrin I, & Sohal RS (2008). Pro-oxidant shift in glutathione redox state during aging. Adv Drug Deliv Rev, 60(13–14), 1545–1552. doi: 10.1016/j.addr.2008.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogol AD (2010). Sex steroids, growth hormone, leptin and the pubertal growth spurt. Endocr Dev, 17, 77–85. doi: 10.1159/000262530 [DOI] [PubMed] [Google Scholar]
- Rogol AD, Roemmich JN, & Clark PA (2002).Growth at puberty. J Adolesc Health, 31(6 Suppl), 192–200. doi: 10.1016/s1054-139x(02)00485-8 [DOI] [PubMed] [Google Scholar]
- Roy S, Ryals MM, Van den Bruele AB, Fitzgerald TS, & Cunningham LL (2013). Sound preconditioning therapy inhibits ototoxic hearing loss in mice. J Clin Invest, 123(11), 4945–4949. doi: 10.1172/JCI71353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybak LP, & Ramkumar V (2007). Ototoxicity. Kidney Int, 72(8), 931–935. doi: 10.1038/sj.ki.5002434 [DOI] [PubMed] [Google Scholar]
- Shen G, & Kong AN (2009). Nrf2 plays an important role in coordinated regulation of Phase II drug metabolism enzymes and Phase III drug transporters. Biopharm Drug Dispos, 30(7), 345–355. doi: 10.1002/bdd.680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin BB (2009). Estrogen therapy: is time of initiation critical for neuroprotection? Nat Rev Endocrinol, 5(11), 620–627. doi: 10.1038/nrendo.2009.193 [DOI] [PubMed] [Google Scholar]
- Simic T, Savic-Radojevic A, Pljesa-Ercegovac M, Matic M, & Mimic-Oka J (2009). Glutathione S-transferases in kidney and urinary bladder tumors. Nat Rev Urol, 6(5), 281–289. doi: 10.1038/nrurol.2009.49 [DOI] [PubMed] [Google Scholar]
- Singer CA, Rogers KL, & Dorsa DM (1998). Modulation of Bcl-2 expression: a potential component of estrogen protection in NT2 neurons. Neuroreport, 9(11), 2565–2568. doi: 10.1097/00001756-199808030-00025 [DOI] [PubMed] [Google Scholar]
- Singh SP, Zimniak L, & Zimniak P (2010). The human hGSTA5 gene encodes an enzymatically active protein. Biochim Biophys Acta, 1800(1), 16–22. doi: 10.1016/j.bbagen.2009.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal SS, Singh SP, Singhal P, Horne D, Singhal J, & Awasthi S (2015). Antioxidant role of glutathione S-transferases: 4-Hydroxynonenal, a key molecule in stress-mediated signaling. Toxicol Appl Pharmacol, 289(3), 361–370. doi: 10.1016/j.taap.2015.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrabji F, Miranda RC, & Toran-Allerand CD (1995). Identification of a putative estrogen response element in the gene encoding brain-derived neurotrophic factor. Proc Natl Acad Sci U S A, 92(24), 11110–11114. doi: 10.1073/pnas.92.24.11110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Someya S, & Prolla TA (2010). Mitochondrial oxidative damage and apoptosis in age-related hearing loss. Mech Ageing Dev, 131(7–8), 480–486. doi: 10.1016/j.mad.2010.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S, Chandra A, Bhatnagar A, Srivastava SK, & Ansari NH (1995). Lipid peroxidation product, 4-hydroxynonenal and its conjugate with GSH are excellent substrates of bovine lens aldose reductase. Biochem Biophys Res Commun, 217(3), 741–746. doi: 10.1006/bbrc.1995.2835 [DOI] [PubMed] [Google Scholar]
- Torres MJ, Kew KA, Ryan TE, Pennington ER, Lin CT, Buddo KA, … Neufer PD (2018). 17beta-Estradiol Directly Lowers Mitochondrial Membrane Microviscosity and Improves Bioenergetic Function in Skeletal Muscle. Cell Metab, 27(1), 167–179 e167. doi: 10.1016/j.cmet.2017.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eyken E, Van Camp G, Fransen E, Topsakal V, Hendrickx JJ, Demeester K, … Van Laer L (2007). Contribution of the N-acetyltransferase 2 polymorphism NAT2*6A to age-related hearing impairment. J Med Genet, 44(9), 570–578. doi: 10.1136/jmg.2007.049205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS (1999). Electrophysiological and cellular effects of estrogen on neuronal function. Crit Rev Neurobiol, 13(1), 1–20. doi: 10.1615/critrevneurobiol.v13.i1.10 [DOI] [PubMed] [Google Scholar]
- Xiong M, He Q, Lai H, & Wang J (2011). Oxidative stress in spiral ganglion cells of pigmented and albino guinea pigs exposed to impulse noise. Acta Otolaryngol, 131(9), 914–920. doi: 10.3109/00016489.2011.577448 [DOI] [PubMed] [Google Scholar]
- Yamashita D, Jiang HY, Schacht J, & Miller JM (2004). Delayed production of free radicals following noise exposure. Brain Res, 1019(1–2), 201–209. doi: 10.1016/j.brainres.2004.05.104 [DOI] [PubMed] [Google Scholar]
- Yamasoba T, Lin FR, Someya S, Kashio A, Sakamoto T, & Kondo K (2013). Current concepts in age-related hearing loss: epidemiology and mechanistic pathways. Hear Res, 303, 30–38. doi: 10.1016/j.heares.2013.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Sharma R, Sharma A, Awasthi S, & Awasthi YC (2003). Lipid peroxidation and cell cycle signaling: 4-hydroxynonenal, a key molecule in stress mediated signaling. Acta Biochim Pol, 50(2), 319–336. doi:035002319 [PubMed] [Google Scholar]
- Zimniak P, Singhal SS, Srivastava SK, Awasthi S, Sharma R, Hayden JB, & Awasthi YC (1994). Estimation of genomic complexity, heterologous expression, and enzymatic characterization of mouse glutathione S-transferase mGSTA4–4 (GST 5.7). J Biol Chem, 269(2), 992–1000. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/7904605 [PubMed] [Google Scholar]


