Abstract
Background:
Sleeve gastrectomy (SG), the most commonly performed metabolic and bariatric surgery (MBS), is associated with reductions in areal bone mineral density (aBMD) at multiple sites, and changes in bone structure at the distal radius and tibia without reductions in strength estimates at these peripheral sites. Data are lacking regarding effects on hip strength estimates.
Objective:
To evaluate effects of SG on measures of hip structural analysis (HSA) in adolescents and young adults over 12 months using dual energy x-ray absorptiometry (DXA).
Settings:
Translational and Clinical Research Center
Methods:
We enrolled 48 youth 14–22 years old with moderate-to-severe obesity; 24 underwent SG and 24 controls were followed without surgery (18 females, 6 males in each group). Hip structure was assessed using DXA at baseline and 12 months. Analyses are adjusted for age, sex, race and the baseline bone measure.
Results:
The SG group lost 25.9% body weight versus 0.3% in controls. Compared to controls, SG had reductions in narrow neck, intertrochanteric and femoral shaft BMD Z-scores (p≤ 0.012). Further, SG had greater reductions in narrow neck and intertrochanteric region (but not femoral shaft) cross-sectional area, cortical thickness, cross-sectional moment of inertia and section modulus, and increases in buckling ratio (p≤0.039). Differences were attenuated after adjusting for 12-month body mass index (BMI) change. At 12 months, differences were minimal after adjusting for age, sex, race and weight.
Conclusions:
Over 12 months, SG had negative effects at the narrow neck and intertrochanteric regions of the hip, but not the femoral shaft. Reduced BMI may compensate for these deleterious effects on bone.
Keywords: Hip structural analysis, adolescents, metabolic and bariatric surgery, sleeve gastrectomy, bone strength, bone structure
1. Introduction
In adolescents and young adults with moderate to severe obesity in whom lifestyle and pharmacological interventions are not effective in achieving weight loss, metabolic and bariatric surgery (MBS) is a very effective strategy to achieve sustained weight loss (1–4), with improvements reported in most metabolic parameters. As a consequence, a marked increase in MBS in adolescents has been reported in recent times in the United States (5, 6), with almost a doubling in its reported utilization between 2012–2016. However, a known deleterious effect of MBS is its impact on bone health, as reported in studies of adults (7–12) and adolescents (13, 14) undergoing gastric bypass, and adults undergoing sleeve gastrectomy (8, 15–17).
Some (8, 10, 18), but not all (16, 17, 19), studies in adults suggest that sleeve gastrectomy may have a lesser impact on bone than gastric bypass, attributed to less marked hormonal changes and less malabsorption following sleeve gastrectomy vs. gastric bypass procedures. This is particularly important given the increasing use of sleeve gastrectomy in individuals with moderate to severe obesity, especially in younger populations, in whom body mass index (BMI) reductions appear to be similar following sleeve gastrectomy as with gastric bypass (20), with a similar extent of skeletal unloading following surgery. We have recently reported significant reductions in areal bone mineral density (aBMD) measures at the hip (but not the spine) using dual energy x-ray absorptiometry (DXA) in adolescents and young adults following sleeve gastrectomy, mostly related to skeletal unloading from weight loss (21). However, using high resolution peripheral quantitative computed tomography (HRpQCT) and microfinite element analysis, we found no change in total volumetric BMD (vBMD) or strength estimates at the distal radius and tibia over 12 months (21), suggesting that at least in the short-term, sleeve gastrectomy may not increase fracture risk at these sites.
Importantly, our previous study did not examine proximal femur geometry or strength estimates, and this is relevant because studies in adults following MBS have reported reductions in vBMD of the hip assessed by quantitative computed tomography (QCT) (8), and bone geometry is an additional important contributor to fracture risk (22). Given the significant morbidity associated with hip fractures, if this site is impacted deleteriously in adolescents and young adults undergoing sleeve gastrectomy, this will need to be monitored and preventive strategies identified. QCT of the hip involves significant radiation exposure, limiting its use in youth. Hip structural analysis (HSA) is a validated DXA-based technique to assess proximal femoral geometry and could be employed for a preliminary understanding of the impact of skeletal unloading on the proximal femur following sleeve gastrectomy. Strong correlations have been reported between HSA and QCT of the hip for cross-sectional area, cross-sectional moment of inertia (CSMI) and section modulus (23), and studies have demonstrated that HSA measures predict hip fractures (24–26).
In order to address these knowledge gaps, we examined HSA measures in youth aged 14–22 years old (a critical period of bone acquisition (27)) with moderate-to-severe obesity undergoing sleeve gastrectomy, as well as non-surgical controls of comparable body size matched for age and sex. Based on DXA data indicating a reduction in hip BMD following such surgery, we hypothesized that participants undergoing sleeve gastrectomy would demonstrate alterations in proximal femur geometry and strength estimates commensurate with the degree of skeletal unloading following weight loss.
2. Participants and Methods
2.1. Participant Selection:
We enrolled 75 adolescents and young adults aged 14–22 years old with moderate to severe obesity in an ongoing longitudinal study examining bone outcomes following sleeve gastrectomy versus routine care. Data at baseline and 12-months were available for 48 matched participants, 24 of whom underwent sleeve gastrectomy (18 female and 6 male) and 24 non-surgical controls (18 female and 6 male). Data for DXA measures of aBMD have been previously reported in a subset of this sample (21). Participants met BMI criteria for MBS, namely BMI of ≥35 kg/m2 (Class II or moderate obesity) with one or more obesity related complications, or a BMI of ≥40 kg/m2 (Class III or severe obesity). Exclusion criteria included conditions or medications that may affect bone metabolism (other than use of calcium and/or vitamin D supplementation or hormonal contraception) within eight weeks of the baseline visit. Use of estrogen-progesterone combination preparations was not an exclusion criterion for concern that the sample would not be representative of the general population of females with obesity in this age range, as many are on such medications for management of polycystic ovarian disease and/or for contraception. We did exclude participants on depot medroxyprogesterone injections, given their marked deleterious effects on bone heath, but not those on the progestin releasing intrauterine device given limited systemic effects, or those with progestin implants given limited bone effects. We recruited participants from area hospitals and specialized weight regulation programs.
The Partners Institutional Review Board approved this Health Insurance Portability and Accountability Act compliant study. We obtained informed consent from participants 18 years and older, or parents of participants < 18 years. Informed assent was obtained from participants younger than 18 years.
2.2. Experimental Protocol:
Eligible participants were assessed at baseline and 12-month follow-up visits. The baseline visit occurred shortly before surgery in the sleeve gastrectomy (surgical) group. We measured height on a wall mounted stadiometer as the average of three measurements, and weight to the nearest 0.1 kg using an electronic scale. Body mass index (BMI) was calculated as weight in kg/(height in meters)2. Standard deviation scores (SDS) or z-scores for anthropometric measurements were determined using CDC 2000 databases (28). Fasting blood samples were obtained for calcium, phosphate, and 25(OH) vitamin D (25OHD) levels and measured by a reference laboratory (LabCorp, Burlington, NC, USA). Hemoglobin A1C (HbA1C) was assayed in the Diabetes Core Laboratory. The Paffenbarger questionnaire was used to determine hours per week of moderate to vigorous physical activity (29, 30). Diet and exercise counseling for controls was provided throughout the study by Translational and Clinical Research Center (TCRC) dieticians, specialized weight regulation programs, or their physicians. Calcium and vitamin D intake was assessed by TCRC dieticians using a calcium and vitamin D food frequency questionnaire (31). Participants were offered at least 1200 mg elemental calcium and 800 IUs vitamin D daily to optimize calcium intake and absorption. Additional recommendations for vitamin D supplementation (based on 25OHD levels) were based on standard guidelines (32, 33).
2.3. Dual Energy X-Ray Absorptiometry:
DXA (Hologic 4500 A, Waltham, MA) was used for hip structural analysis, as well as measures of fat and lean mass at the baseline visit and one-year post gastrectomy in the surgical group, and one year following the baseline visit in the non-surgical group by an International Society of Clinical Densitometry (ISCD) certified DXA technologist on a single instrument (coefficients of variation for areal BMD, fat and lean mass 0.8%, 2.1% and 1.0%, respectively; least significant change 0.024 g/cm2 and 0.048 g/cm2 for total hip and femoral neck respectively). All scans were analyzed using the same software.
HSA provides data for three proximal femoral sites (Figure 1) using averages from five parallel lines 1 pixel apart across the cross-section of these sites: (i) narrow neck (NN), the narrowest point of the femoral neck, (ii) the trochanteric region, along the bisector of the angle of the axes of the neck and femoral shaft, and (iii) the femoral shaft (FS), a site across the shaft at a distance of 1.5 cm minimum neck width distal to the intersection of the neck and shaft axes (34). The specific measures obtained using HSA include (i) subperiosteal (or outer) diameter, (ii) estimated endosteal (or inner) diameter, (iii) cross-sectional area, an index of resistance to axial forces (excludes soft spaces in marrow and pores), (iv) estimated cortical thickness, (v) cross-sectional moment of inertia, an estimate of resistance to bending forces in a cross-section, (vi) section modulus, an index of strength of bending (vii) buckling ratio, an index of susceptibility to local cortical buckling under compressive loads, (viii) neck shaft angle, and (ix) hip axis length, the distance in mm from the inferolateral aspect of the greater trochanter to the pelvic inner rim, measured along the long axis of the femoral neck (34–36).
Figure 1:
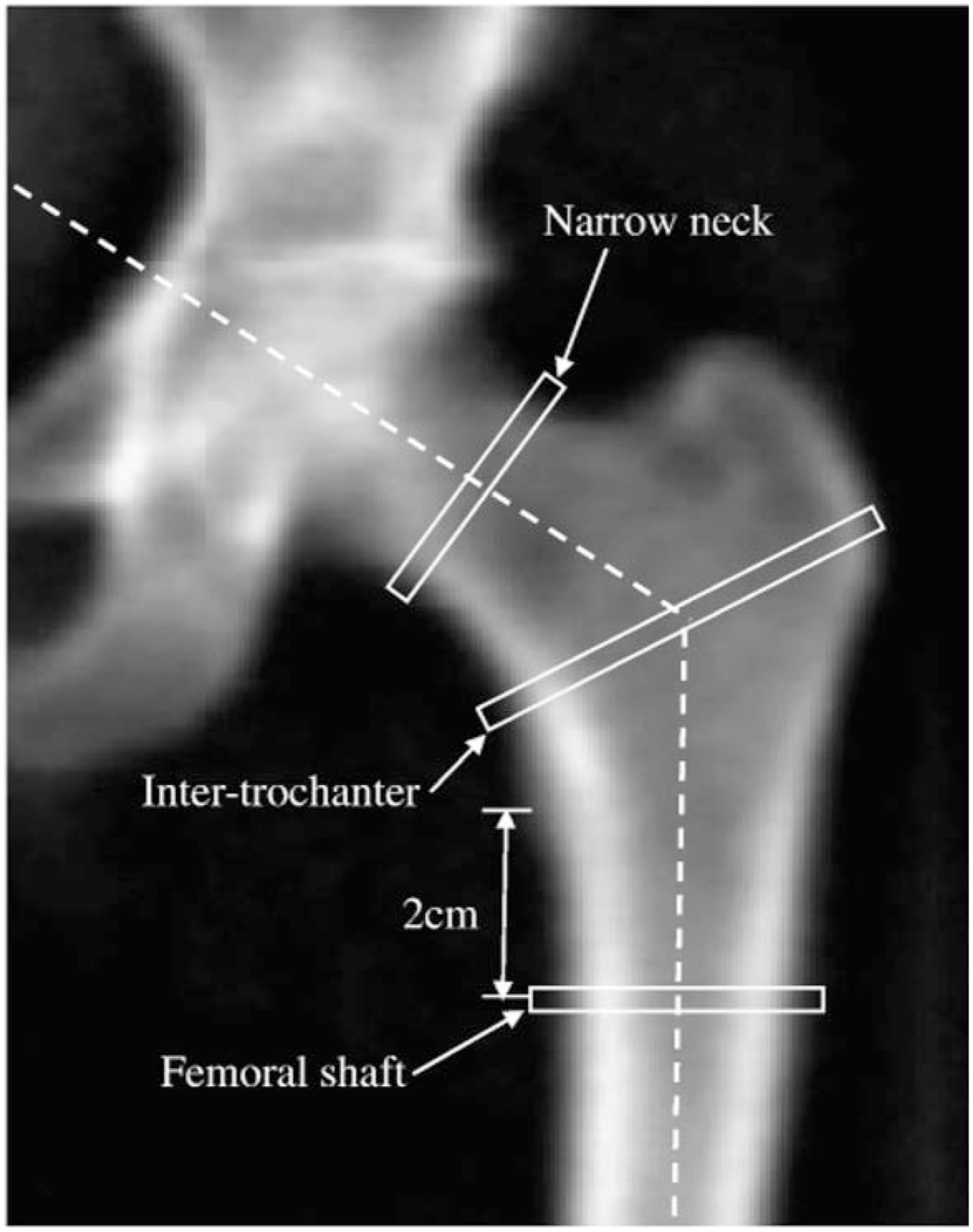
Hip image from Hologic DXA scanner showing locations of HSA narrow neck, intertrochanteric and femoral shaft regions of interest (ROI). From Laskey MA, Price RI, Khoo BCC, Prentice A. Proximal femur structural geometry changes during and following lactation. Bone. 2011 Apr 1; 48(4): 755–759. Copyright Elsevier 2011.
2.4. Statistical Analysis:
We present data as mean+/−SE or median (interquartile range) unless otherwise indicated. JMP Statistical Discovery Software (Version 14, SAS Institute, Carey, NC) was used for statistical analysis. The study was powered (based on preliminary data) at 97% and 90%, respectively, to detect a 3.5% difference between groups for changes in specific bone parameters over time at an alpha level of .05, based on an estimated standard deviation of change of 2.85%. We used the Student t-test or the Wilcoxon Rank Sum test to compare differences between surgical and non-surgical groups depending on the data distribution. Within group comparisons were performed using the paired t-test. We used multivariable analysis to determine differences between groups after controlling for possible covariates (age, sex, race and baseline bone measure +/−12-month change in BMI). Pearson correlations were used to determine associations of covariates known to impact bone (i.e. change in BMI, lean mass, fat mass, 25OHD levels, HbA1C, and physical activity) (37–39) with HSA parameters. A p value of <0.05 was considered to be statistically significant.
3. Results
3.1. Baseline Characteristics:
The sleeve gastrectomy (surgical) and non-surgical groups did not differ for baseline characteristics except for weight, BMI and fat mass, which were higher in the surgical group. However, BMI standard deviation score (SDS) and percent fat mass did not differ across groups (Table 1). Also, the proportion of study participants with HbA1C levels in the normal (<5.7%), prediabetes (5.7–6.4%) and diabetes ranges (≥ 6.5%) did not differ across groups (75.0, 20.8 and 4.2% respectively in the surgical group, and 58.3, 33.3 and 8.3% respectively in the non-surgical group, p=0.492). Race was self-reported by study participants and did not differ across groups (19 non-Black and 5 Black participants in each group).
TABLE 1:
Clinical Characteristics and Body Composition Measures at Baseline and Changes over 12 Months in the Surgical and Non- Surgical Groups
| Clinical Characteristics | Baseline Measure (Mean ± standard error of mean) | Within group change over 12-months [Mean (95% confidence interval)] | P-value comparing changes over 12 months in surgical vs. non-surgical groups | ||
|---|---|---|---|---|---|
| Non-Surgical (n=24) | Surgical (n=24) | Non-Surgical (n=24) | Surgical (n=24) | ||
| Age (years) | 17.1 ± 0.5 | 17.8 ± 0.4 | - | - | |
| Height (cm) | 167.1 ± 1.6 | 168.6 ±1.8 | - | - | - |
| Weight (kg) | 120.7 ± 4.8 | 134.4 ± 3.7* | −1.0 (−5.0, 3.1) | −39.0 (−46.1, −32.0) | <0.0001 |
| Body mass index (kg/m2) | 43.0 ± 1.2 | 47.4 ± 1.3* | −0.8 (−2.3, 0.8) | −13.7 (−16.1, −11.2) | <0.0001 |
| Body mass index z-score | 2.5 ± 0.1 | 2.6 ± 0.1 | −0.1 (−0.2, −0.0) | −0.8 (−1.0, −0.5) | <0.0001 |
| 25-hydroxy vitamin D (ng/ml) | 23.4 ± 1.5 | 23.9 ± 2.0 | −1.5 (−4.8, 1.8) | 5.5 (−1.0, 12.0) | 0.054 |
| Calcium (mg/dl) | 9.3 ± 0.1 | 9.3± 0.1 | −0.1 (−0.2, 0.1) | 0.0 (−0.1, 0.2) | 0.963 |
| Phosphorus (mg/dl) | 3.5 ± 0.1 | 3.5 ± 0.2 | 0.1 (−0.3, 0.4) | 0.2 (−0.1, 0.5) | 0.329 |
| Hemoglobin A1c** (%) | 5.5 (5.2, 5.7) | 5.6 (5.4, 5.8) | −0.7 (−1.8, 0.3) | −0.7 (−1.2, −0.2) | 0.003 |
| Moderate to vigorous physical activity (hours/week) | 27.5 ± 4.9 | 29.6 ± 4.3 | 4.0 (−10.5, 18.4) | 3.0 (−10.0, 16.1) | 0.868 |
| Dual Energy X-Ray Absorptiometry (DXA) Measures of Body Composition | |||||
| Lean Mass (kg) | 64.5 ± 2.3 | 68.8 ± 1.9 | 1.5 (0.5, 2.5) | −9.2 (−12.3, −6.1) | <0.0001 |
| Lean Mass (%) | 53.3 ± 0.9 | 51.5 ± 1.0 | 1.4 (−0.3, 3.1) | 7.6 (3.9, 11.3) | 0.0002 |
| Fat Mass (kg) | 57.1 ± 2.8 | 65.5 ± 2.6* | −1.5 (−5.1, 2.2) | −23.1 (−31.4, −14.7) | <0.0001 |
| Fat Mass (%) | 46.7 ± 0.9 | 48.5 ± 1.0 | −1.4 (−3.1, 0.3) | −7.6 (−11.3, −3.9) | 0.0002 |
p<0.05 for baseline differences between groups; significant changes from baseline, and significant differences between groups are bolded
Baseline measure indicated as median (interquartile range)
3.2. Changes in Anthropometric Measures and Body Composition over 12 Months:
The surgical group had greater reductions in weight, BMI, BMI z-scores, fat and lean mass, and HbA1C over 12 months compared to the non-surgical group (Table 1). Total body weight loss was greater in the surgical [−25.9% (−29.2%, −22.6%)] vs. non-surgical group [−0.30% (−3.58%, 2.99%)] (p<0.0001). Percent excess BMI loss was also greater in the surgical (−55.8±4.4%) vs. non-surgical group (−2.6±4.5%) (p<0.0001). The surgical group had greater reductions in percent fat mass and greater increases in percent lean mass than the non-surgical group (Table 1).
3.3. Changes in Hip Structural Analysis Measures over 12 Months:
Table 2 shows baseline measures for study participants, the within group change over 12 months, and significances for differences between groups after adjusting for age, sex, race, baseline bone measure +/−12-month BMI change. The surgical and non-surgical groups did not differ for baseline measures of HSA at any site. Similar to reductions in total hip aBMD following surgery, the surgical group had significant within group reductions from baseline in narrow neck and intertrochanteric region BMD and BMD Z-scores, cross-sectional area, CSMI, average cortical thickness and section modulus, and differed significantly from the non-surgical group for these changes over 12 months after adjusting for baseline covariates (age, sex, race and baseline bone measure) (Table 2). The surgical group also had significant within group increases in buckling ratio at the intertrochanteric region, and differed significantly from non-surgical controls for changes in this measure over 12-months at both the narrow neck and inter-trochanteric region. Groups did not differ for changes over 12 months in any HSA measure at the femoral shaft except BMD Z-scores. Of note, there was a within group increase in hip axis length in the surgical group, with a between group difference when compared with non-surgical controls. Results did not change after also controlling for height.
TABLE 2:
Hip Structural Analysis Parameters: Baseline Values and Changes over 12 Months in the Surgical and Non-Surgical Groups
| Baseline Measures (Mean ± standard error of mean) | Change over 12-months [Mean (95% confidence interval)] | P-value comparing changes over 12 months in surgical vs. non- surgical groups | ||||
|---|---|---|---|---|---|---|
| Non-Surgical (n=24) | Surgical (n=24) | Non-Surgical (n=24) | Surgical (n=24) | Adjusted for age, sex, race and baseline | Adjusted for age, sex, race, baseline and change in BMI | |
| Total hip BMD (g/cm2) | 1.17 ±0.02 | 1.18 ± 0.03 | 0.00 (−0.01, 0.02) | −0.07 (−0.10, −0.04) | 0.849 | 0.0003 |
| Total hip BMD Z-score | 1.78 ± 0.21 | 1.81 ± 0.27 | −0.15 (−0.28, −0.03) | −0.88 (−1.21, −0.55) | 0.931 | <0.0001 |
| Femoral neck BMD (g/cm2) | 1.07 ± 0.03 | 1.09 ± 0.02 | 0.00 (−0.02, 0.03) | −0.08 (−0.13, −0.04) | 0.685 | <0.0001 |
| Femoral neck BMD Z-score | 1.70 ± 0.23 | 1.88 ± 0.26 | −0.104(−0.32, 0.11) | −1.00 (−1.36, −0.63) | 0.605 | <0.0001 |
| NARROW NECK | ||||||
| BMD (g/cm2) | 1.23± 0.04 | 1.22 ± 0.03 | 0.01 (−0.03, 0.06) | −0.10 (−0.16, −0.04) | 0.003 | 0.031 |
| BMD Z-score | 0.06 ± 0.04 | −0.16 ± 0.07 | 0.06 (−0.02, 0.14) | −0.16 (−0.32, −0.01) | 0.012 | 0.174 |
| Cross sectional area (cm2) | 3.98 ± 0.11 | 4.15 ± 0.10 | 0.03 (−0.07, 0.14) | −0.38 (−0.52, −0.24) | <0.0001 | 0.051 |
| CSMI (cm4) | 3.17 ± 0.16 | 3.67 ± 0.22 | 0.11 (−0.01, 0.23) | −0.36 (−0.57, −0.15) | 0.0005 | 0.304 |
| Subperiosteal width (cm) | 3.43 ± 0.08 | 3.60 ± 0.08 | −0.02 (−0.09, 0.06) | −0.03 (−0.16, 0.10) | 0.650 | 0.090 |
| Endocortical diameter (cm) | 2.95 ± 0.09 | 3.12 ± 0.08 | −0.02 (−0.11, 0.07) | 0.01 (−0.14, 0.16) | 0.346 | 0.067 |
| Average cortical thickness (cm) | 0.24 ± 0.01 | 0.24 ± 0.01 | 0.00 (−0.01, 0.01) | −0.02 (−0.03, −0.01) | 0.005 | 0.035 |
| Section modulus (cm3) | 1.76 ± 0.09 | 1.92 ± 0.11 | 0.06 (−0.01, 0.14) | −0.16 (−0.32, −0.01) | 0.011 | 0.176 |
| Buckling ratio | 7.99 ± 0.53 | 8.47 ± 0.47 | −0.25 (−0.88, 0.38) | 0.91 (−0.17, 1.98) | 0.029 | 0.022 |
| INTERTROCHANTERIC REGION | ||||||
| BMD (g/cm2) | 1.22 ± 0.03 | 1.21 ± 0.04 | −0.02 (−0.05, 0.02) | −0.09 (−0.16, −0.03) | 0.026 | 0.481 |
| BMD Z-score | −0.02 ± 0.07 | −0.55 ± 0.16 | −0.02 (−0.18, 0.13) | −0.55 (−0.88, −0.22) | 0.011 | 0.375 |
| Cross sectional area (cm2) | 6.44 ± 0.17 | 6.54 ± 0.24 | −0.06 (−0.22, 0.09) | −0.60 (−0.94, −0.26) | 0.003 | 0.313 |
| CSMI (cm4) | 16.55 ± 0.75 | 17.83 ± 1.08 | 0.07 (−0.42, 0.56) | −1.60 (−2.78, −0.41) | 0.039 | 0.445 |
| Subperiosteal width (cm) | 5.55 ± 0.09 | 5.68 ± 0.10 | 0.02 (−0.02, 0.07) | −0.08 (−0.18, 0.01) | 0.063 | 0.385 |
| Endocortical diameter (cm) | 4.56 ± 0.10 | 4.69 ± 0.11 | 0.04 (−0.02, 0.10) | 0.02 (−0.09, 0.13) | 0.820 | 0.718 |
| Average cortical thickness (cm) | 0.50 ± 0.01 | 0.50 ± 0.02 | −0.01 (−0.02, 0.01) | −0.05 (−0.08, −0.03) | 0.004 | 0.295 |
| Section modulus (cm3) | 5.48 ± 0.18 | 5.71 ± 0.27 | −0.02 (−0.17, 0.14) | −0.55 (−0.88, −0.22) | 0.011 | 0.366 |
| Buckling ratio | 6.17 ± 0.20 | 6.37 ± 0.25 | 0.15 (−0.05, 0.34) | 0.80 (0.44, 1.16) | 0.004 | 0.250 |
| FEMORAL SHAFT | ||||||
| BMD (g/cm2) | 1.70 ± 0.03 | 1.73 ± 0.04 | 0.01 (−0.03, 0.05) | −0.02 (−0.06, 0.02) | 0.409 | 0.999 |
| BMD Z-score | 0.07 ± 0.04 | −0.02 ± 0.06 | 0.07 (−0.02, 0.16) | −0.02 (−0.31, 0.09) | 0.012 | 0.891 |
| Cross sectional area (cm2) | 5.18 ± 0.13 | 5.31 ± 0.17 | 0.06 (−0.06, 0.18) | −0.05 (−0.20, 0.11) | 0.625 | 0.603 |
| CSMI (cm4) | 4.60 ± 0.27 | 4.83 ± 0.33 | 0.13 (−0.03, 0.28) | −0.02 (−0.26, 0.22) | 0.602 | 0.733 |
| Subperiosteal width (cm) | 3.20 ± 0.06 | 3.23 ± 0.06 | 0.02 (−0.03, 0.07) | 0.02 (−0.02, 0.06) | 0.470 | 0.408 |
| Endocortical diameter (cm) | 1.89 ± 0.08 | 1.90 ± 0.07 | 0.02 (−0.07, 0.10) | 0.05 (−0.00, 0.01) | 0.353 | 0.676 |
| Average Ct. Thickness (cm) | 0.66 ± 0.02 | 0.67 ± 0.02 | 0.00 (−0.02, 0.02) | −0.02 (−0.03, 0.00) | 0.375 | 0.970 |
| Section modulus (cm3) | 2.77 ± 0.12 | 2.78 ± 0.18 | 0.07 (−0.02, 0.16) | 0.08 (−0.16, 0.33) | 0.693 | 0.123 |
| Buckling ratio | 2.58 ± 0.11 | 2.60 ± 0.13 | −0.03 (−0.19, 0.14) | 0.04 (−0.07, 0.15) | 0.418 | 0.744 |
| Shaft neck angle (degrees) | 131.5 ± 0.8 | 130.5 ± 1.2 | −1.0 (−3.1, 1.0) | 0.1 (−2.2, 2.3) | 0.668 | 0.610 |
| Hip axis length (mm) | 104.1 ± 1.4 | 104.9 ± 2.4 | 0.6 (−0.8, 2.0) | 3.2 (1.4, 4.9) | 0.006 | 0.543 |
BMD: bone mineral density; CSMI: cross-sectional moment of inertia; Ct.: cortical
Significant changes from baseline, and significant differences between groups are bolded.
Percent changes for HSA measures at the narrow neck and intertrochanteric region are shown in Figure 2 for the surgical and non-surgical groups. No differences were noted between groups at the femoral shaft (not shown).
Figure 2:
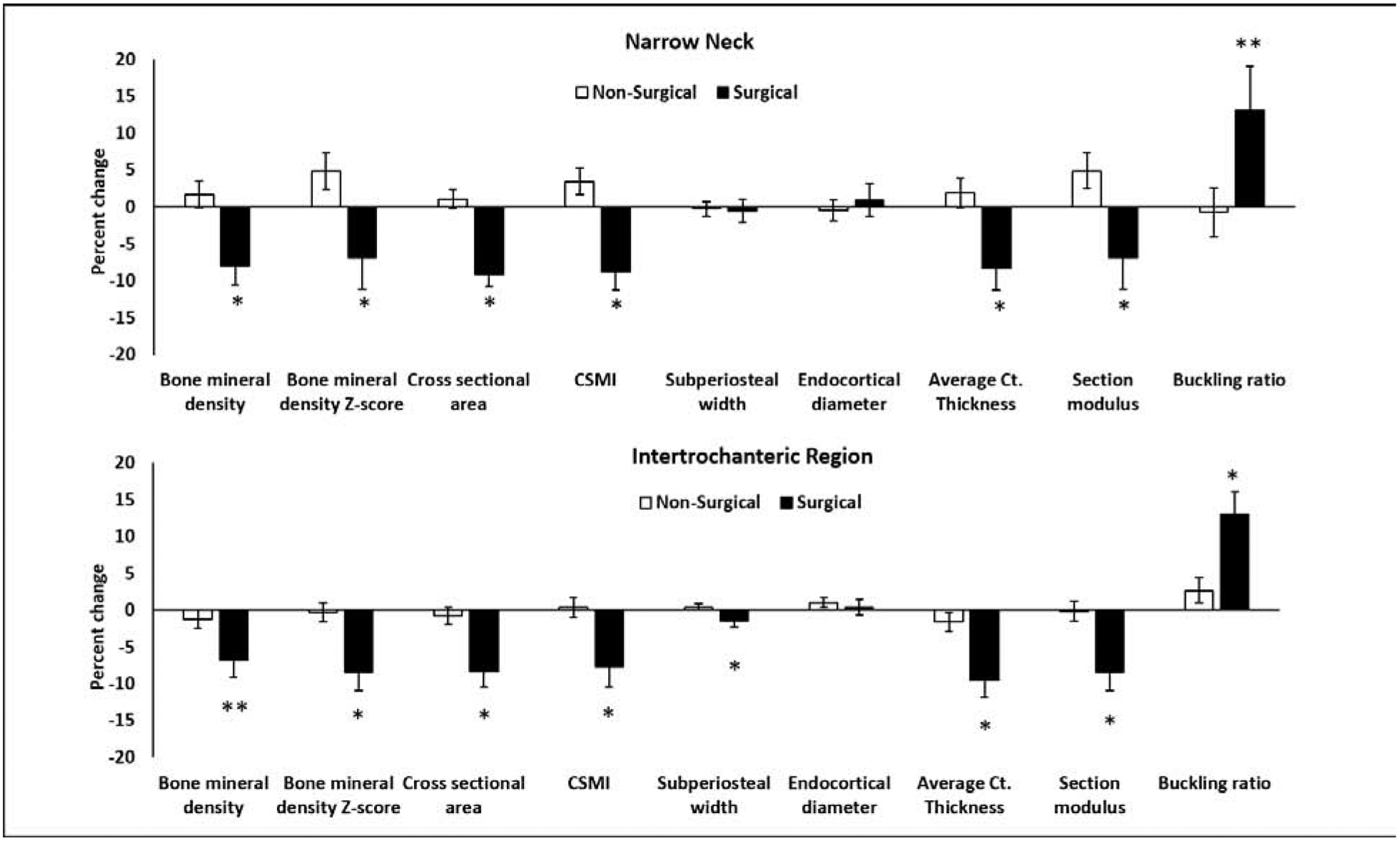
Percent change in hip structural analysis (HSA) measures at the narrow neck and intertrochanteric region in the surgical vs. non-surgical groups over 12 months. The groups differed for percent change in several measures at the narrow neck and intertrochanteric region. No differences were noted in percent changes in HSA measures at the femoral shaft (not shown). CSMI: cross sectional moment of inertia; Ct.: cortical; *P<0.05; **P<0.10 (both after controlling for age, sex and race).
Differences between groups for 12-month change and percent change in narrow neck BMD, average cortical thickness and buckling ratio remained significant even after controlling for 12-month change in BMI. However, all significant differences for 12-month change (and percent change) in HSA measures at the intertrochanteric region and femoral shaft, and for changes in hip axis length were lost after controlling for 12-month change in BMI. Study participants did not report any new fractures during the study duration.
3.4. Hip Structural Analysis Measures at 12 Months after Controlling for Body Weight:
We also examined differences in HSA measures between groups at 12 months after controlling for age, sex, race and weight at 12 months, and found no differences between the groups except for narrow neck subperiosteal width (p=0.009), endocortical diameter (p=0.014) and buckling ratio (p=0.024), all of which were higher in the surgical group.
3.5. Determinants of Changes in Hip Structural Analysis Parameters:
For all participants taken together, Table 3 shows associations of changes in BMI z-scores, lean mass, fat mass, 25OHD and physical activity levels with changes in bone parameters between surgical and non-surgical groups over time.
Table 3:
Associations of Changes in Anthropometric Measures and Body Composition with Changes in Bone Parameters Over 12 Months
| Δ BMI z-score | Δ Lean Mass | Δ Fat Mass | Δ 25OHD | Δ Physical activity | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | r | p | |
| HSA measures | ||||||||||
| NARROW NECK | ||||||||||
| Δ Bone mineral density (g/cm2) | 0.298 | 0.059 | 0.309 | 0.032 | 0.246 | 0.092 | −0.342 | 0.020 | −0.214 | 0.157 |
| Δ Bone mineral density Z-score | 0.384 | 0.013 | 0.335 | 0.020 | 0.294 | 0.043 | −0.377 | 0.010 | −0.132 | 0.389 |
| Δ Cross sectional area (cm2) | 0.491 | 0.001 | 0.600 | <.0001 | 0.468 | 0.0008 | −0.290 | 0.051 | 0.006 | 0.970 |
| Δ CSMI (cm4) | 0.540 | 0.0003 | 0.574 | <.0001 | 0.460 | 0.001 | −0.322 | 0.029 | 0.076 | 0.621 |
| Δ Subperiosteal width (cm) | 0.114 | 0.480 | 0.236 | 0.106 | 0.179 | 0.224 | 0.221 | 0.140 | 0.348 | 0.019 |
| Δ Endocortical diameter (cm) | 0.038 | 0.815 | 0.140 | 0.342 | 0.104 | 0.484 | 0.258 | 0.084 | 0.338 | 0.023 |
| Δ Average cortical thickness (cm) | 0.285 | 0.071 | 0.292 | 0.044 | 0.234 | 0.110 | −0.337 | 0.022 | −0.221 | 0.146 |
| Δ Section modulus (cm3) | 0.388 | 0.012 | 0.336 | 0.020 | 0.294 | 0.042 | −0.379 | 0.009 | −0.131 | 0.393 |
| Δ Buckling ratio | −0.155 | 0.334 | −0.121 | 0.412 | −0.083 | 0.575 | 0.345 | 0.019 | 0.292 | 0.052 |
| INTERTROCHANTERIC REGION | ||||||||||
| Δ Bone mineral density (g/cm2) | 0.228 | 0.152 | 0.524 | 0.0001 | 0.286 | 0.049 | −0.050 | 0.741 | 0.096 | 0.532 |
| Δ Bone mineral density Z-score | 0.214 | 0.179 | 0.630 | <.0001 | 0.364 | 0.011 | −0.102 | 0.500 | 0.046 | 0.762 |
| Δ Cross sectional area (cm2) | 0.244 | 0.125 | 0.639 | <.0001 | 0.371 | 0.010 | −0.105 | 0.490 | 0.082 | 0.594 |
| Δ CSMI (cm4) | 0.136 | 0.397 | 0.596 | <.0001 | 0.321 | 0.026 | −0.116 | 0.444 | −0.022 | 0.884 |
| Δ Subperiosteal width (cm) | 0.004 | 0.983 | 0.354 | 0.014 | 0.223 | 0.128 | −0.182 | 0.227 | −0.124 | 0.419 |
| Δ Endocortical diameter (cm) | −0.151 | 0.347 | −0.003 | 0.987 | 0.008 | 0.960 | −0.145 | 0.336 | −0.155 | 0.308 |
| Δ Average cortical thickness (cm) | 0.278 | 0.078 | 0.606 | <.0001 | 0.365 | 0.011 | −0.040 | 0.793 | 0.102 | 0.507 |
| Δ Section modulus (cm3) | 0.214 | 0.178 | 0.632 | <.0001 | 0.367 | 0.010 | −0.103 | 0.496 | 0.038 | 0.802 |
| Δ Buckling ratio | −0.366 | 0.019 | −0.552 | <.0001 | −0.362 | 0.011 | 0.071 | 0.639 | −0.109 | 0.477 |
| FEMORAL SHAFT | ||||||||||
| Δ Bone mineral density (g/cm2) | 0.182 | 0.254 | 0.243 | 0.096 | 0.119 | 0.419 | 0.006 | 0.967 | 0.285 | 0.058 |
| Δ Bone mineral density Z-score | 0.111 | 0.491 | 0.336 | 0.020 | 0.168 | 0.253 | −0.056 | 0.714 | 0.144 | 0.345 |
| Δ Cross sectional area (cm2) | 0.133 | 0.408 | 0.293 | 0.043 | 0.119 | 0.423 | −0.075 | 0.621 | 0.215 | 0.156 |
| Δ CSMI (cm4) | 0.053 | 0.744 | 0.302 | 0.037 | 0.121 | 0.415 | −0.127 | 0.400 | 0.074 | 0.628 |
| Δ Subperiosteal width (cm) | −0.077 | 0.632 | 0.097 | 0.510 | −0.007 | 0.962 | −0.140 | 0.354 | −0.127 | 0.405 |
| Δ Endocortical diameter (cm) | −0.171 | 0.285 | −0.079 | 0.596 | −0.087 | 0.557 | −0.106 | 0.482 | −0.255 | 0.091 |
| Δ Average cortical thickness (cm) | 0.195 | 0.221 | 0.228 | 0.119 | 0.133 | 0.369 | 0.021 | 0.889 | 0.283 | 0.059 |
| Δ Section modulus (cm3) | 0.107 | 0.506 | 0.097 | 0.511 | 0.080 | 0.588 | −0.089 | 0.558 | 0.163 | 0.284 |
| Δ Buckling ratio | −0.162 | 0.312 | −0.134 | 0.362 | −0.058 | 0.695 | −0.103 | 0.498 | −0.255 | 0.091 |
| Δ Shaft neck angle (degrees) | −0.185 | 0.247 | −0.130 | 0.380 | −0.036 | 0.809 | 0.337 | 0.022 | −0.092 | 0.546 |
| Δ Hip axis length (mm) | −0.431 | 0.005 | −0.319 | 0.027 | −0.414 | 0.004 | 0.206 | 0.169 | −0.195 | 0.199 |
Δ: Change; CSMI: cross sectional moment of inertia; BMD: bone mineral density; 25OHD: 25-hydroxy vitamin D. Significant p values are bolded
Narrow Neck:
Changes in BMI z-scores, lean mass and fat mass were associated positively with changes in narrow neck BMD Z-scores, cross-sectional area, CSMI and section modulus. Further, a change in 25OHD levels correlated inversely with changes in narrow neck BMD, BMD Z-scores, CSMI, average cortical thickness and section modulus, and positively with changes in buckling ratio. Changes in physical activity were associated positively with increases in subperiosteal width and endocortical diameter.
Intertrochanteric Region:
Changes in lean mass and fat mass correlated positively with changes in BMD, BMD Z-score, cross-sectional area, CSMI, average cortical thickness and section modulus at the intertrochanteric region. Changes in BMI, lean mass and fat mass were associated inversely with changes in the buckling ratio. No associations were noted for changes in 25OHD levels of in physical activity levels with any measure at the intertrochanteric region.
Femoral Shaft:
Changes in lean mass were positively associated with changes in femoral shaft BMD Z-scores, cross sectional area and CSMI; no other significant associations were noted.
Shaft Neck Angle and Hip Axis Length:
Changes in BMI, lean mass and fat mass were associated inversely with changes in hip axis length, and changes in 25OHD correlated positively with changes in shaft neck angle.
4. Discussion
This is the first report of hip structural analysis following sleeve gastrectomy in adolescents and young adults, demonstrating changes at the narrow neck and intertrochanteric region, such as reductions in BMD Z-scores, cross-sectional area, CSMI and section modulus, and an increase in the buckling ratio following surgery. Most of these changes were attributable to 12-month reductions in BMI following surgery, as differences between groups were no longer significant after controlling for 12-month change in BMI. The femoral shaft, however, was spared for the most part.
HSA uses properties of DXA to derive geometrical parameters for the hip associated with bone strength (40). In a study of 7474 women with 635 incident hip fractures over 13 years, women with fracture had greater neck-shaft angles, subperiosteal and endosteal diameters, and buckling ratios, and lower areal hip BMD, cross-sectional area, cortical thickness, CSMI, and section modulus than those without fracture (26). Another large study of postmenopausal women also reported that buckling ratio was an independent predictor of fracture risk even after controlling for age, body size, clinical risk factors and areal BMD (34). Further, hip axis length has been implicated in fracture risk, particularly in younger women without osteoporosis, even after controlling for aBMD and Fracture Risk Assessment Tool (FRAX) measures (41). HSA has been used in studies assessing the impact of exercise and mechanical loading on bone (40, 42), and in large and longitudinal studies in children (43, 44).
We found alterations in several HSA parameters at the narrow neck and intertrochanteric region in the surgical group over 12 months vs. the non-surgical group, including in specific parameters implicated in fracture risk in the studies described above in other populations. These include reductions in aBMD, cortical thickness, cross-sectional area, CSMI and section modulus, all of which have been implicated in fracture risk in studies of postmenopausal women (26), and increases in the buckling ratio (implicated in fracture risk in postmenopausal women (34)) and hip axis length (implicated in fracture risk in younger women without osteoporosis (41)). However, periosteal width and endocortical diameter, associated with fracture risk in postmenopausal women (26), were spared at these sites in our participants, and the femoral shaft was mostly spared, with the only significant reduction being in aBMD. Similar to our data, a study examining hip structural analysis endpoints in patients enrolled in a multidisciplinary weight loss program involving nutritional and exercise intervention reported a sparing of the femoral shaft, although in that study, the intertrochanteric region was also spared (45). The extent of weight loss was much less in this study, which did not involve MBS.
Overall, these data are concerning for a decrease in bone strength at the hip following sleeve gastrectomy, attributable to the reduction in BMI (and skeletal unloading) following surgery, or metabolic and hormonal changes (12, 46) associated with weight reduction (9), given that many differences in HSA parameters were no longer significant after adjusting for 12-month change in BMI. This is of potential concern because hip geometry parameters in childhood correlate with adult hip geometry (47); thus deficits incurred during adolescence and young adulthood may persist over the lifespan. However, we found that at 12-months, most HSA measures (other than narrow neck width and buckling ratio) did not differ between groups after adjusting for body weight at 12-months, suggesting that despite reductions in these HSA measures over time following surgery, fracture risk may not differ significantly from controls because of the concurrent reduction in body weight and therefore the force of a fall. Thus, changes in HSA measures may merely reflect an appropriate adaptation to skeletal unloading from reduced body weight.
Changes in BMI, lean mass and fat mass were positively associated with changes in HSA measures at the narrow neck and intertrochanteric region, consistent with the known impact of these measures on bone outcomes. Clinical protocols for vitamin D supplementation are now in place at centers performing MBS to maintain 25OHD levels in the normative range and reduce the impact on bone (9, 46). Inverse associations of changes in 25OHD levels with changes in HSA measures in this study may reflect the impact of changes in fat mass following MBS on 25OHD levels. Specifically, reductions in fat mass following surgery (which reflect reductions in body weight), would be associated with increases in systemic 25OHD levels (as 25OHD is sequestered in adipose tissue). Thus, inverse associations of changes in 25OHD levels with HSA measures may merely reflect positive associations of changes in fat mass with changes in HSA measures. Regardless, we found no difference between surgical and non-surgical groups for changes in 25OHD levels over time. Changes in physical activity correlated only with changes in subperiosteal width and endocortical diameter at the narrow neck, and suggest that changes in weight and body composition may be greater drivers of change in bone outcomes following surgery than physical activity.
Study limitations include a relatively small number of participants and lack of data for fractures. Studies are necessary in a larger group of adolescents over a longer duration following sleeve gastrectomy as well as gastric bypass to assess the impact of these two surgical techniques on bone outcomes, particularly fracture risk. Of note, although HSA does not directly assess bone geometry but converts two dimensional data to make inferences about geometry using engineering models, HSA measures correlate strongly with volumetric measures obtained by quantitative computed tomography (2323). A study limitation is the use of self-report data for assessment of exercise activity, calcium and vitamin D intake in study participants. It is reassuring though that these data did not differ across study groups; thus, errors from self-report are less likely to contribute to observed differences across groups. Finally, it will be important to determine how and whether changes in gut hormones, gonadal steroids, 1,25(OH)2D or PTH impact bone outcomes. An important strength is that this is the first detailed analysis of HSA endpoints in adolescents and young adults following sleeve gastrectomy.
Overall, our study indicates a deterioration in hip structural measures at the narrow neck and intertrochanteric region following sleeve gastrectomy in adolescents and young adults. However, reduced body mass may compensate for the observed deleterious effects on bone following metabolic and bariatric surgery.
Highlights:
Sleeve gastrectomy (SG) in youth results in reduced hip bone density over 12 months
Narrow neck and intertrochanteric region geometry and strength are impacted negatively
These effects are attenuated after controlling for changes in body mass index
After adjusting for weight, groups did not differ for bone measures at 12 months
Acknowledgements:
This work was supported by the NIH NIDDK R01 DK103946–01A1 (MM, MAB), NIH K23DK110419–01(VS), P30-DK040561 (VS, FCS), K24DK109940 (MAB), L30 DK118710 (FCS), NIH P30-DK057521 (VS), UL1T R002541 (MM)
Abbreviations:
- 25OHD
- 1,25(OH)2D
1,25 dihydroxy vitamin D
- BMD
Bone mineral density
- aBMD
Areal bone mineral density
- BMI
Body mass index
- CSMI
Cross-sectional moment of inertia
- DXA
Dual energy x-ray absorptiometry
- FRAX
Fracture Risk Assessment Tool
- HbA1C
Hemoglobin A1C
- HRpQCT
High resolution peripheral quantitative computed tomography
- HSA
Hip structural analysis
- MBS
Metabolic and bariatric surgery
- PTH
Parathyroid hormone
- QCT
Quantitative computed tomography
- SDS
Standard deviation score
- vBMD
Volumetric bone mineral density
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Tsai WS, Inge TH, Burd RS, Bariatric surgery in adolescents: recent national trends in use and in-hospital outcome, Arch Pediatr Adolesc Med 161(3) (2007) 217–21. [DOI] [PubMed] [Google Scholar]
- [2].Pratt JSA, Browne A, Browne NT, et al. , ASMBS pediatric metabolic and bariatric surgery guidelines, 2018, Surg Obes Relat Dis 14(7) (2018) 882–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Griggs CL, Perez NP Jr., Goldstone RN, et al. , National Trends in the Use of Metabolic and Bariatric Surgery Among Pediatric Patients With Severe Obesity, JAMA Pediatr 172(12) (2018) 1191–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Armstrong SC, Bolling CF, Michalsky MP, Reichard KW, Pediatric Metabolic and Bariatric Surgery: Evidence, Barriers, and Best Practices, Pediatrics 144(6) (2019). [DOI] [PubMed] [Google Scholar]
- [5].Humayon S, Altieri MS, Yang J, Nie L, Spaniolas K, Pryor AD, Recent trends of bariatric surgery in adolescent population in the state of New York, Surg Obes Relat Dis 15(8) (2019) 1388–1393. [DOI] [PubMed] [Google Scholar]
- [6].Kyler KE, Bettenhausen JL, Hall M, Fraser JD, Sweeney B, Trends in Volume and Utilization Outcomes in Adolescent Metabolic and Bariatric Surgery at Children’s Hospitals, J Adolesc Health 65(3) (2019) 331–336. [DOI] [PubMed] [Google Scholar]
- [7].Lindeman KG, Greenblatt LB, Rourke C, Bouxsein ML, Finkelstein JS, Yu EW, Longitudinal 5-Year Evaluation of Bone Density and Microarchitecture After Roux-en-Y Gastric Bypass Surgery, J Clin Endocrinol Metab 103(11) (2018) 4104–4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bredella MA, Greenblatt LB, Eajazi A, Torriani M, Yu EW, Effects of Roux-en-Y gastric bypass and sleeve gastrectomy on bone mineral density and marrow adipose tissue, Bone 95 (2017) 85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Stein EM, Silverberg SJ, Bone loss after bariatric surgery: causes, consequences, and management, Lancet Diabetes Endocrinol 2(2) (2014) 165–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Stein EM, Carrelli A, Young P, et al. , Bariatric surgery results in cortical bone loss, J Clin Endocrinol Metab 98(2) (2013) 541–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Frederiksen KD, Hanson S, Hansen S, et al. , Bone Structural Changes and Estimated Strength After Gastric Bypass Surgery Evaluated by HR-pQCT, Calcif Tissue Int 98(3) (2016) 253–62. [DOI] [PubMed] [Google Scholar]
- [12].Shanbhogue VV, Stoving RK, Frederiksen KH, et al. , Bone structural changes after gastric bypass surgery evaluated by HR-pQCT: a two-year longitudinal study, Eur J Endocrinol 176(6) (2017) 685–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kaulfers AM, Bean JA, Inge TH, Dolan LM, Kalkwarf HJ, Bone loss in adolescents after bariatric surgery, Pediatrics 127(4) (2011) e956–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Beamish AJ, Gronowitz E, Olbers T, Flodmark CE, Marcus C, Dahlgren J, Body composition and bone health in adolescents after Roux-en-Y gastric bypass for severe obesity, Pediatr Obes 12(3) (2017) 239–246. [DOI] [PubMed] [Google Scholar]
- [15].Jaruvongvanich V, Vantanasiri K, Upala S, Ungprasert P, Changes in bone mineral density and bone metabolism after sleeve gastrectomy: a systematic review and meta-analysis, Surg Obes Relat Dis 15(8) (2019) 1252–1260. [DOI] [PubMed] [Google Scholar]
- [16].Vilarrasa N, de Gordejuela AG, Gomez-Vaquero C, et al. , Effect of bariatric surgery on bone mineral density: comparison of gastric bypass and sleeve gastrectomy, Obes Surg 23(12) (2013) 2086–91. [DOI] [PubMed] [Google Scholar]
- [17].Tian Z, Fan XT, Li SZ, Zhai T, Dong J, Changes in Bone Metabolism After Sleeve Gastrectomy Versus Gastric Bypass: a Meta-Analysis, Obes Surg 30(1) (2020) 77–86. [DOI] [PubMed] [Google Scholar]
- [18].Fashandi AZ, Mehaffey JH, Hawkins RB, Schirmer B, Hallowell PT, Bariatric surgery increases risk of bone fracture, Surg Endosc 32(6) (2018) 2650–2655. [DOI] [PubMed] [Google Scholar]
- [19].Javanainen M, Pekkarinen T, Mustonen H, Scheinin T, Leivonen M, Two-Year Nutrition Data in Terms of Vitamin D, Vitamin B12, and Albumin After Bariatric Surgery and Long-term Fracture Data Compared with Conservatively Treated Obese Patients: a Retrospective Cohort Study, Obes Surg 28(9) (2018) 2968–2975. [DOI] [PubMed] [Google Scholar]
- [20].Inge TH, Courcoulas AP, Jenkins TM, et al. , Weight Loss and Health Status 3 Years after Bariatric Surgery in Adolescents, N Engl J Med 374(2) (2016) 113–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Misra M, Singhal V, Carmine B, et al. , Bone outcomes following sleeve gastrectomy in adolescents and young adults with obesity versus non-surgical controls, Bone 134 (2020) 115290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bouxsein ML, Technology insight: noninvasive assessment of bone strength in osteoporosis, Nat Clin Pract Rheumatol 4(6) (2008) 310–8. [DOI] [PubMed] [Google Scholar]
- [23].Ramamurthi K, Ahmad O, Engelke K, et al. , An in vivo comparison of hip structure analysis (HSA) with measurements obtained by QCT, Osteoporos Int 23(2) (2012) 543–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Faulkner KG, Wacker WK, Barden HS, et al. , Femur strength index predicts hip fracture independent of bone density and hip axis length, Osteoporos Int 17(4) (2006) 593–9. [DOI] [PubMed] [Google Scholar]
- [25].Leslie WD, Pahlavan PS, Tsang JF, Lix LM, Prediction of hip and other osteoporotic fractures from hip geometry in a large clinical cohort, Osteoporos Int 20(10) (2009) 1767–74. [DOI] [PubMed] [Google Scholar]
- [26].Kaptoge S, Beck TJ, Reeve J, et al. , Prediction of incident hip fracture risk by femur geometry variables measured by hip structural analysis in the study of osteoporotic fractures, J Bone Miner Res 23(12) (2008) 1892–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gordon CM, Zemel BS, Wren TA, et al. , The Determinants of Peak Bone Mass, J Pediatr 180 (2017) 261–269. [DOI] [PubMed] [Google Scholar]
- [28].Ogden CL, Kuczmarski RJ, Flegal KM, et al. , Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version, Pediatrics 109(1) (2002) 45–60. [DOI] [PubMed] [Google Scholar]
- [29].Paffenbarger RS Jr., Blair SN, Lee IM, Hyde RT, Measurement of physical activity to assess health effects in free-living populations, Med Sci Sports Exerc 25(1) (1993) 60–70. [DOI] [PubMed] [Google Scholar]
- [30].Simpson K, Parker B, Capizzi J, et al. , Validity and reliability question 8 of the Paffenbarger Physical Activity Questionnaire among healthy adults, J Phys Act Health 12(1) (2015) 116–23. [DOI] [PubMed] [Google Scholar]
- [31].Taylor C, Lamparello B, Kruczek K, Anderson EJ, Hubbard J, Misra M, Validation of a food frequency questionnaire for determining calcium and vitamin D intake by adolescent girls with anorexia nervosa, J Am Diet Assoc 109(3) (2009) 479–85, 485 e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Parrott J, Frank L, Rabena R, Craggs-Dino L, Isom KA, Greiman L, American Society for Metabolic and Bariatric Surgery Integrated Health Nutritional Guidelines for the Surgical Weight Loss Patient 2016 Update: Micronutrients, Surg Obes Relat Dis 13(5) (2017) 727–741. [DOI] [PubMed] [Google Scholar]
- [33].Kearns MD, Alvarez JA, Tangpricha V, Large, single-dose, oral vitamin D supplementation in adult populations: a systematic review, Endocr Pract 20(4) (2014) 341–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].LaCroix AZ, Beck TJ, Cauley JA, et al. , Hip structural geometry and incidence of hip fracture in postmenopausal women: what does it add to conventional bone mineral density?, Osteoporos Int 21(6) 919–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Beck TJ, Extending DXA beyond bone mineral density: understanding hip structure analysis, Curr Osteoporos Rep 5(2) (2007) 49–55. [DOI] [PubMed] [Google Scholar]
- [36].Ackerman KE, Pierce L, Guereca G, et al. , Hip structural analysis in adolescent and young adult oligoamenorrheic and eumenorrheic athletes and nonathletes, J Clin Endocrinol Metab 98(4) (2013) 1742–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Muschitz C, Kocijan R, Haschka J, et al. , The Impact of Vitamin D, Calcium, Protein Supplementation, and Physical Exercise on Bone Metabolism After Bariatric Surgery: The BABS Study, J Bone Miner Res 31(3) (2016) 672–82. [DOI] [PubMed] [Google Scholar]
- [38].Singhal V, Sanchita S, Malhotra S, et al. , Suboptimal bone microarchitecure in adolescent girls with obesity compared to normal-weight controls and girls with anorexia nervosa, Bone 122 (2019) 246–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Mitchell DM, Caksa S, Joseph T, Bouxsein ML, Misra M, Elevated HbA1c is associated with altered cortical and trabecular microarchitecture in girls with type 1 diabetes, J Clin Endocrinol Metab (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Hind K, Gannon L, Whatley E, Cooke C, Sexual dimorphism of femoral neck cross-sectional bone geometry in athletes and non-athletes: a hip structural analysis study, J Bone Miner Metab 30(4) (2012) 454–60. [DOI] [PubMed] [Google Scholar]
- [41].Leslie WD, Lix LM, Morin SN, et al. , Hip axis length is a FRAX-and bone density-independent risk factor for hip fracture in women, J Clin Endocrinol Metab 100(5) (2015) 2063–70. [DOI] [PubMed] [Google Scholar]
- [42].Petit MA, Beck TJ, Lin HM, Bentley C, Legro RS, Lloyd T, Femoral bone structural geometry adapts to mechanical loading and is influenced by sex steroids: the Penn State Young Women’s Health Study, Bone 35(3) (2004) 750–9. [DOI] [PubMed] [Google Scholar]
- [43].Alwis G, Karlsson C, Stenevi-Lundgren S, Rosengren BE, Karlsson MK, Femoral neck bone strength estimated by hip structural analysis (HSA) in Swedish Caucasians aged 6–90 years, Calcif Tissue Int 90(3) (2012) 174–85. [DOI] [PubMed] [Google Scholar]
- [44].Jackowski SA, Kontulainen SA, Cooper DM, Lanovaz JL, Baxter-Jones AD, The timing of BMD and geometric adaptation at the proximal femur from childhood to early adulthood in males and females: a longitudinal study, J Bone Miner Res 26(11) (2011) 2753–61. [DOI] [PubMed] [Google Scholar]
- [45].Chaplais E, Naughton G, Dutheil F, et al. , Geometric and Mechanical Bone Response to a Multidisciplinary Weight Loss Intervention in Adolescents With Obesity: The ADIBOX Study, J Clin Densitom 23(2) (2020) 254–263. [DOI] [PubMed] [Google Scholar]
- [46].Canales BK, Schafer AL, Shoback DM, Carpenter TO, Gastric bypass in obese rats causes bone loss, vitamin D deficiency, metabolic acidosis, and elevated peptide YY, Surg Obes Relat Dis 10(5) (2014) 878–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Erlandson MC, Runalls SB, Jackowski SA, Faulkner RA, Baxter-Jones ADG, Structural Strength Benefits Observed at the Hip of Premenarcheal Gymnasts Are Maintained Into Young Adulthood 10 Years After Retirement From the Sport, Pediatr Exerc Sci 29(4) (2017) 476–485. [DOI] [PubMed] [Google Scholar]


