Abstract
The American Heart Association’s Life’s Simple 7 (LS7) metric consists of 7 modifiable risk factors. Although a more favorable LS7 risk factor profile is associated with lower AF incidence, this relationship is unknown in regard to AF burden. We assessed the prospective association of overall LS7 score and individual LS7 risk factors in midlife with AF burden in late-life in the Atherosclerosis Risk in Communities Study. LS7 components were assessed at Visit 3 (1993-95) and a composite score ranging from 0 to 14 was calculated. A higher score indicates better cardiovascular health. AF burden was measured at Visit 6 (2016-17) with a 2-week Zio® XT Patch. AF burden, defined as the percent of time a participant was in AF, was categorized as none, intermittent (>0 to <100%), or continuous (100%). Weighted multinomial logistic regression was used. Of the 2,363 participants, 58% were female and 24% were black. Participants were aged 57±5 years at Visit 3 and 79±5 years at Visit 6. From the Zio® XT Patch, 5% had continuous AF, 4% had intermittent AF, and 91% had none. After multivariable adjustment, each 1-point increase in LS7 score had 0.87 (95% CI: 0.79-0.95) higher odds of continuous AF than no AF. Individually, poor levels of physical activity, BMI, and fasting blood glucose were associated with greater AF burden. In conclusion, this population-based prospective cohort study reports that unfavorable cardiovascular health profile in midlife is associated with higher AF burden in late-life and future research to evaluate the effectiveness of optimizing physical activity, BMI, and fasting blood glucose in lowering AF burden is warranted.
Keywords: atrial fibrillation, Life’s Simple 7, cardiovascular health
INTRODUCTION
Atrial fibrillation (AF) is a significant issue as its prevalence is expected to increase 3-fold in the next 3 decades.1 A recent American Heart Association (AHA) Scientific Statement highlights the importance of considering the quantity of AF (i.e., AF burden) rather than just AF presence or absence.2 Higher AF burden is associated with adverse outcomes, but determinants of AF burden are not clearly defined.2 The AHA’s Life’s Simple 7 (LS7) promotes ideal cardiovascular health and includes 7 modifiable health behaviors and factors (physical activity, total cholesterol, blood pressure, body mass index (BMI), fasting blood glucose, smoking, and diet).3 A more favorable LS7 risk factor profile is associated with lower AF incidence,4 but this has not yet been reported with respect to AF burden. The recent availability of an ECG recording device that records heart rhythm continuously for 2 weeks—Zio® XT Patch (iRhythm Technologies, Inc, San Francisco, CA)—facilitates AF burden measurement. We leveraged this technology to test the hypothesis that better LS7 score and individual LS7 risk factors in midlife are associated with lower AF burden in late life among participants in the Atherosclerosis Risk in Communities (ARIC) study.
METHODS
The ARIC Study5 is a prospective, community-based cohort, which began in 1987-89. At baseline, the 15,792 participants were aged 45-64 years and recruited from 4 US communities (Forsyth County, NC; Jackson, MS; Washington County, MD; selected suburbs of Minneapolis, MN). Several additional clinical exams have since taken place. Visit 3 (1993-95) served as the baseline for this analysis. At Visit 6 (2016-17), 4,003 participants aged 75-94 years attended and were invited to wear a Zio® XT Patch. The prescribed wear time was 14 days. Exclusion criteria included history of cardiac electronic device implantation or skin allergic reaction to adhesive tape. After the recording period, participants mailed the device to iRhythm Technologies Inc., where recorded ECG data were processed using a proprietary algorithm and a report was generated. Of the 2,650 participants who wore the device, 34 (1.3%) were lost or returned without data resulting in 2,616 devices with analyzable data. Exclusion criteria for this analysis included races other than black or white and non-whites in the MN and MD centers (due to small numbers), wearing the Zio® XT Patch <48 hours or missing LS7 covariates, leaving an analytic sample of 2,363 (Figure 1). Institutional review boards at each center approved the study.
Figure 1.
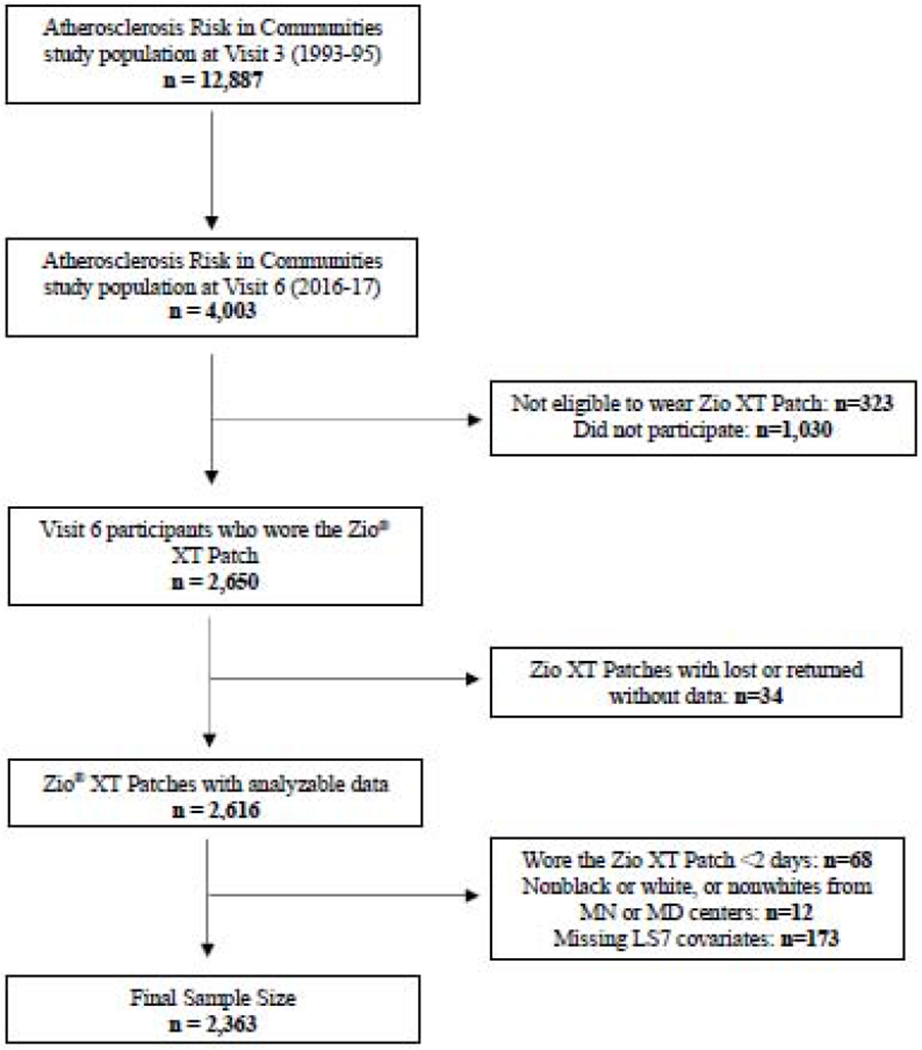
Study Sample Inclusion Flowchart
LS7 components were assessed at midlife at Visit 3. Physical activity was measured using the modified Baecke questionnaire6 and responses were converted to minutes per week of moderate or vigorous exercise.7 Sitting blood pressure were measured 3 times and the final 2 measures were averaged. BMI was calculated from weight and height. Enzymatic procedures were used to assess total blood cholesterol concentration.8 Fasting blood glucose was measured by the modified hexokinase/glucose-6-phosphate dehydrogenase method. Participants self-reported cigarette smoking status as current, former, or never. Former smokers were categorized based on smoking quit date (>12 vs. ≤12 months). Diet was measured using a 66-item food frequency questionnaire.9 The healthy diet score was calculated by meeting nutritional guidelines in 5 areas: fruits and vegetables, fish, sodium, sugar-sweetened beverages, and whole grains.
Each LS7 component was categorized as poor (0 points), intermediate (1 point), or ideal (2 points) based on the AHA’s LS7 criteria,3 as has been done previously in ARIC.10 Briefly, ideal levels were: ≥150 min/week of physical activity, total cholesterol <200 mg/dL, blood pressure <120/80 mmHg, BMI <25 kg/m2, fasting blood glucose <100 mg/dL, nonsmokers or quit >12 months ago, and a healthy diet score (≥4 components). Supplemental Table 1 shows the intermediate and poor levels for each component, respectively. Participants taking medications to achieve target levels for cholesterol, blood pressure, or blood glucose were classified as intermediate for each respective health factor. An overall LS7 composite score ranging from 0 to 14 was calculated. A higher score indicates better health. The composite score was categorized as inadequate (0-4), average (5-9), or optimum (10-14) cardiovascular health.10
AF burden was defined as the percent of time a participant was in AF, as computed from the Zio® XT Patch data using iRhythm’s proprietary algorithm. AF burden was categorized into no AF (0%), intermittent AF (>0 to <100%), or continuous AF (100%).
Other self-reported covariates measured at Visit 3 included age, sex, race (black, white), ARIC field center, and drinking status (current, never). Educational attainment was obtained from Visit 1. Coronary heart disease (CHD), stroke, and heart failure (HF) events were identified through Visit 6 and used as mediators in this analysis. Beta-blocker and calcium-channel blocker medication use was collected at Visit 6. Technicians recorded medication use via review of medication bottles. HF was identified by the Gothenburg criteria (Visit 1 only), self-report of HF medication use within the past 2 weeks, or presence of ICD codes for HF in any hospitalization or during follow-up prior to Visit 6.11 CHD was defined by self-reported physician diagnosis at visit 1, history of myocardial infarction on ECG, or adjudicated cases following visit 1.11,12 Stroke was defined as the self-reported history of a physician diagnosis of a stroke prior to visit 1; following visit 1, stroke was adjudicated from diagnosis codes indicative of cerebrovascular disease using criteria adapted from the National Survey of Stroke.13
Baseline characteristics of participants who wore the Zio® XT Patch were described as mean (standard deviation [SD]) for continuous variables and percentages for categorical variables. Multinomial logistic regression was used to compute the odds ratios (OR) and 95% confidence intervals (CI) for the association of LS7 composite score with AF burden. ORs for AF burden were also calculated for each LS7 risk factor individually and per 1-unit higher LS7 score. Multivariable models were used: Model 1 adjusted for age, sex, race/center (5 levels); Model 2 additionally adjusted for education and drinking status; Model 3 further adjusted for CHD, stroke, HF, beta-blockers, and calcium-channel blockers (all collected through Visit 6). To account for potential survival bias, we used inverse probability weighting (IPW) to account for attrition due to death, failure to attend Visit 6, or not wearing the Zio® XT Patch.14 The ideal overall health category or optimum risk factor level was used as the reference group. Analyses were conducted using SAS software (version 9.4; SAS Institute Inc., Cary, NC).
RESULTS
Visit 3 participant characteristics, stratified by Visit 6 AF burden categories, are presented in Table 1. The 2,363 participants included in this analysis were on average (SD), 56.7 (4.6) years at Visit 3 (baseline) and 79.2 (4.7) year at Visit 6; 58% were female, while 24% were black. Mean LS7 score at Visit 3 was 8.6 (2.1) and mean Zio® XT Patch wear time at Visit 6 was 12.6 (2.6) days. Visit 6 took place an average (SD) of 22.5 (0.9) years after Visit 3. Among those who wore the Zio® XT Patch, 117 (5%) had continuous, 83 (4%) had intermittent, and 2,163 (91%) had no AF. Those with continuous AF at Visit 6 were at Visit 3 older, more likely to be male, and had more prevalent CHD than those with intermittent or no AF at Visit 6.
Table 1.
Midlife (1993-1995) Characteristics of Study Participants by Late Life (2016-2017) Atrial Fibrillation Burden Categories: The Atherosclerosis Risk in Communities Study*
| Atrial Fibrillation Burden in 2016-17 | |||
|---|---|---|---|
| None | Intermittent | Continuous | |
| (n=2,163) | (n=83) | (n=117) | |
| Variable | |||
| Age (years) | 56.6 (5) | 57.5 (4) | 59.3 (5) |
| Men | 41% | 41% | 60% |
| Black | 24% | 13% | 14% |
| Education, < high school degree | 12% | 6% | 13% |
| Current alcohol drinkers | 57% | 65% | 58% |
| Body mass index (kg/m2) | 27.8 (5) | 28.5 (6) | 29.7 (6) |
| Systolic blood pressure (mmHg) | 118.8 (16) | 119.0 (18) | 122.3 (16) |
| Diastolic blood pressure (mmHg) | 71.8 (10) | 70.7 (10) | 71.8 (9) |
| Total cholesterol (mg/dL) | 204.3 (36) | 206.6 (41) | 199.7 (36) |
| Fasting blood glucose (mg/dL) | 102.3 (26) | 99.8 (20) | 108.6 (34) |
| Coronary heart disease | 3% | 4% | 6% |
| Heart failure | 1% | 0% | 1% |
| Stroke | 1% | 0% | 0% |
| Beta-blockers | 11% | 18% | 12% |
| Calcium-channel blockers | 10% | 18% | 16% |
Data are expressed as mean (SD) or %.
Overall, 36% of participants were in the ideal LS7 composite category, 3% in the intermediate category, and 61% in the poor category. The most common ideal LS7 factors were fasting blood glucose (58%) and smoking status (87%), while ideal diet was the least common ideal LS7 factor (2%). The distribution of the LS7 composite and individual factors, stratified by the AF burden categories, is shown in Table 2.
Table 2.
Life’s Simple 7 (LS7) Distribution by Atrial Fibrillation Burden Categories: The Atherosclerosis Risk in Communities Study, 1993–1995
| Atrial Fibrillation Burden in 2016-2017 | |||
|---|---|---|---|
| Variable | None (n=2,163) | Intermittent (n=83) | Continuous (n=117) |
| LS7 Health Categories | |||
| Inadequate | 58 (3%) | 4 (5%) | 4 (3%) |
| Average | 1,323 (61%) | 44 (53%) | 82 (70%) |
| Optimum | 782 (36%) | 35 (42%) | 31 (27%) |
| Individual LS7 Components | |||
| Physical activity | |||
| Poor | 718 (33%) | 21 (25%) | 46 (39%) |
| Intermediate | 524 (24%) | 28 (34%) | 26 (22%) |
| Ideal | 921 (43%) | 34 (41%) | 45 (39%) |
| Total cholesterol (mg/dL) | |||
| Poor (≥ 240) | 320 (15%) | 18 (22%) | 13 (11%) |
| Intermediate (200-239 or treated to <200) | 861 (40%) | 27 (32%) | 46 (39%) |
| Ideal (<200, without medication) | 982 (45%) | 38 (46%) | 58 (50%) |
| Blood pressure (mmHg) | |||
| Poor (SBP ≥140 or DBP ≥90) | 248 (12%) | 11 (13%) | 15 (13%) |
| Intermediate (SBP 120-139 or DBP 80-89 or treated to <120/<80) | 871 (40%) | 33 (40%) | 57 (49%) |
| Ideal (<120/<80, without medication) | 1,044 (48%) | 39 (47%) | 45 (38%) |
| Body mass index (kg/m2) | |||
| Poor (≥30) | 589 (27%) | 33 (40%) | 43 (37%) |
| Intermediate (25 - <30) | 935 (43%) | 24 (29%) | 47 (40%) |
| Ideal (<25) | 639 (30%) | 26 (31%) | 27 (23%) |
| Fasting blood glucose (mg/dL) | |||
| Poor (≥126) | 131 (6%) | 6 (7%) | 10 (9%) |
| Intermediate (100-125 or treated to <100) | 763 (35%) | 19 (23%) | 52 (44%) |
| Ideal (<100, without medication) | 1,269 (59%) | 58 (70%) | 55 (47%) |
| Smoking status | |||
| Poor | 262 (12%) | 11 (13%) | 7 (6%) |
| Intermediate | 26 (1%) | 2 (3%) | 5 (4%) |
| Ideal | 1,875 (87%) | 70 (84%) | 105 (90%) |
| Diet | |||
| Poor | 903 (42%) | 35 (42%) | 51 (43%) |
| Intermediate | 1,211 (56%) | 43 (52%) | 65 (56%) |
| Ideal | 49 (2%) | 5 (6%) | 1 (1%) |
The ORs (95% CIs) of Visit 3 LS7 and Visit 6 AF burden are shown in Table 3. We did not observe any statistically significant associations between LS7 categories and intermittent AF vs. no AF. However, compared to optimal LS7 category, those with inadequate and average LS7 had 3.95 and 1.82 times the odds of continuous AF than no AF. Similarly, for each 1-point higher LS7 score, the odds of continuous AF in Model 2 was 0.87 relative to no AF, indicating that those with more favorable LS7 score in mid-life had lower odds of continuous AF than no AF in late-life. These associations were attenuated with further adjustment for potential mediators (cardiovascular events and medications). There was no association between any of the LS7 score categories when comparing the odds of continuous versus intermittent AF.
Table 3.
Weighted* Odds Ratios (95% Confidence Intervals) of Atrial Fibrillation Burden with Overall Life’s Simple 7 (LS7) Score: the Atherosclerosis Risk in Communities Study, 1993–2017
| Atrial Fibrillation Burden | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Intermittent vs. none | Continuous vs. none | Continuous vs. intermittent | |||||||
| Model 1 | Model 2 | Model 3 | Model 1 | Model 2 | Model 3 | Model 1 | Model 2 | Model 3 | |
| LS7 health categories | |||||||||
| Inadequate | 2.42 (0.92-6.37) | 2.38 (0.90-6.33) | 2.18 (0.81-5.85) | 3.41 (1.38-8.41) | 3.95 (1.58-9.88) | 2.07 (0.79-5.39) | 1.41 (0.40-5.01) | 1.66 (0.46-6.01) | 0.95 (0.25-3.59) |
| Average | 0.92 (0.56-1.50) | 0.89 (0.54-1.46) | 0.83 (0.50-1.37) | 1.72 (1.08-2.75) | 1.82 (1.14-2.92) | 1.32 (0.81-2.16) | 1.87 (0.97-3.62) | 2.04 (1.05-3.98) | 1.60 (0.80-3.18) |
| Optimum | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| Per 1-point increase | 0.92 (0.83-1.02) | 0.92 (0.83-1.02) | 0.96 (0.84-1.10) | 0.89 (0.81-0.97) | 0.87 (0.79-0.95) | 0.94 (0.82-1.08) | 0.96 (0.84-1.09) | 0.94 (0.82-1.07) | 1.02 (0.88-1.17) |
Inverse-probability weighting was used.
Model 1: adjusted for age, sex, race/center.
Model 2: adjusted for model 1 plus education and drinking status.
Model 3: adjusted for model 2 plus coronary heart disease, stroke, heart failure, beta-blockers, and calcium-channel blockers (all measured through Visit 6).
To determine the association between individual LS7 components and AF burden, separate models were analyzed. Table 4 shows the odds of AF burden for each LS7 factor. No associations were noted when assessing the relationship between any of the individual LS7 components and intermittent AF compared to no AF. Poor BMI was associated with 2.36-fold increased odds of continuous AF as compared to ideal BMI in Model 2. Those with poor levels of physical activity had 1.64 times higher odds of continuous AF than no AF. Both poor and intermediate levels of fasting blood glucose had 2.28 and 1.71 times the odds of continuous AF than no AF, respectively. Further adjustment for potential mediators (cardiovascular events and medications) attenuated these associations. There were no consistent significant associations between individual LS7 components and continuous AF vs. intermittent AF noted.
Table 4.
Weighted* Odds Ratios (95% Confidence Intervals) of Atrial Fibrillation Burden with Individual Life’s Simple 7 Components: The Atherosclerosis Risk in Communities Study, 1993–2017
| Atrial Fibrillation Burden | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Intermittent vs. none | Continuous vs. none | Continuous vs. intermittent | |||||||
| Model 1 | Model 2 | Model 3 | Model 1 | Model 2 | Model 3 | Model 1 | Model 2 | Model 3 | |
| Physical activity | |||||||||
| Poor | 1.10 (0.62-1.94) | 1.09 (0.61-1.95) | 1.08 (0.60-1.92) | 1.51 (0.99-2.32) | 1.64 (1.06-2.56) | 1.56 (0.98-2.47) | 1.38 (0.69-2.77) | 1.51 (0.74-3.07) | 1.45 (0.70-2.99) |
| Intermediate | 1.85 (1.09-3.15) | 1.83 (1.08-3.12) | 1.84 (1.08-3.13) | 1.09 (0.66-1.82) | 1.12 (0.67-1.86) | 1.08 (0.63-1.82) | 0.59 (0.29-1.21) | 0.61 (0.30-1.25) | 0.59 (0.28-1.22) |
| Ideal | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| Total cholesterol | |||||||||
| Poor | 1.59 (0.89-2.86) | 1.58 (0.88-2.85) | 1.53 (0.85-2.76) | 0.85 (0.47-1.53) | 0.88 (0.49-1.57) | 0.71 (0.39-1.30) | 0.54 (0.24-1.20) | 0.55 (0.25-1.24) | 0.46 (0.20-1.06) |
| Intermediate | 0.93 (0.56-1.55) | 0.92 (0.55-1.54) | 0.89 (0.53-1.49) | 0.99 (0.67-1.49) | 1.01 (0.67-1.51) | 0.89 (0.59-1.36) | 1.07 (0.57-2.02) | 1.09 (0.58-2.06) | 1.00 (0.52-1.92) |
| Ideal | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| Blood pressure | |||||||||
| Poor | 1.22 (0.60-2.47) | 1.20 (0.59-2.44) | 0.98 (0.46-2.07) | 1.22 (0.67-2.23) | 1.29 (0.70-2.36) | 0.56 (0.29-1.09) | 1.00 (0.41-2.48) | 1.08 (0.43-2.67) | 0.57 (0.21-1.52) |
| Intermediate | 1.18 (0.72-1.92) | 1.15 (0.70-1.89) | 1.00 (0.60-1.68) | 1.61 (1.05-2.45) | 1.65 (1.08-2.52) | 1.14 (0.72-1.80) | 1.37 (0.72-2.57) | 1.43 (0.76-2.70) | 1.13 (0.58-2.22) |
| Ideal | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| Body mass index | |||||||||
| Poor | 1.67 (0.96-2.90) | 1.64 (0.94-2.86) | 1.61 (0.92-2.81) | 2.30 (1.37-3.84) | 2.36 (1.41-3.97) | 1.83 (1.08-3.13) | 1.38 (0.66-2.87) | 1.44 (0.69-3.01) | 1.14 (0.54-2.42) |
| Intermediate | 0.60 (0.33-1.10) | 0.60 (0.33-1.10) | 0.59 (0.32-1.07) | 1.03 (0.62-1.73) | 1.04 (0.62-1.73) | 0.93 (0.55-1.57) | 1.71 (0.79-3.72) | 1.74 (0.80-3.78) | 1.59 (0.72-3.49) |
| Ideal | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| Fasting blood glucose | |||||||||
| Poor | 1.12 (0.52-2.39) | 1.19 (0.56-2.56) | 1.13 (0.53-2.44) | 2.26 (1.25-4.11) | 2.28 (1.26-4.16) | 1.70 (0.91-3.19) | 2.02 (0.80-5.15) | 1.91 (0.75-4.90) | 1.50 (0.58-3.93) |
| Intermediate | 0.55 (0.32-0.95) | 0.54 (0.31-0.92) | 0.53 (0.31-0.91) | 1.64 (1.09-2.47) | 1.71 (1.13-2.58) | 1.48 (0.97-2.27) | 2.97 (1.54-5.76) | 3.20 (1.65-6.22) | 2.80 (1.43-5.49) |
| Ideal | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| Smoking status | |||||||||
| Poor | 1.27 (0.73-2.23) | 1.28 (0.73-2.26) | 1.28 (0.73-2.26) | 0.63 (0.35-1.15) | 0.65 (0.36-1.18) | 0.57 (0.30-1.06) | 0.50 (0.22-1.10) | 0.51 (0.23-1.13) | 0.44 (0.19-1.02) |
| Intermediate | 2.19 (0.49-9.77) | 2.23 (0.50-9.99) | 2.27 (0.50-10.3) | 4.55 (1.75-11.8) | 4.60 (1.76-12.1) | 3.86 (1.39-10.7) | 2.09 (0.39-10.9) | 2.07 (0.39-11.0) | 1.70 (0.31-9.45) |
| Ideal | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| Diet | |||||||||
| Poor | 0.42 (0.15-1.20) | 0.46 (0.16-1.33) | 0.49 (0.17-1.43) | 1.80 (0.26-12.4) | 1.74 (0.25-12.0) | 2.21 (0.30-16.0) | 4.31 (0.50-37.2) | 3.76 (0.43-32.7) | 4.47 (0.50-40.4) |
| Intermediate | 0.36 (0.13-1.01) | 0.40 (0.14-1.13) | 0.41 (0.14-1.17) | 1.94 (0.28-13.3) | 1.85 (0.27-12.7) | 2.18 (0.30-15.6) | 5.43 (0.64-46.2) | 4.62 (0.54-39.6) | 5.31 (0.60-47.2) |
| Ideal | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
Inverse probability weighting was used.
Model 1: adjusted for age, sex, race/center.
Model 2: adjusted for model 1 plus education and drinking status.
Model 3: adjusted for model 2 plus coronary heart disease, stroke, heart failure, beta-blockers, and calcium-channel blockers (measured at Visit 6).
DISCUSSION
In this population-based prospective cohort study, we investigated the relationship between modifiable lifestyle behaviors and factors in midlife and AF burden in late life. Our study, which followed a large cohort of individuals from midlife for over 20 years, advances the field on several fronts. Our principal findings are: (1) a more favorable LS7 score was associated with lower odds of continuous vs. no AF; and (2) individually, poor physical activity, BMI, and fasting blood glucose were associated with higher odds of continuous vs. no AF. Collectively, our findings suggest that having an optimal lifestyle in middle age may result in lower AF burden in older age. This expands upon prior work which has shown a healthful lifestyle to be associated with lower AF incidence.4
Increased cardiorespiratory fitness is associated with a reduced risk of incident AF.15 Patients with permanent AF who participated in an exercise program reported an improvement in quality of life and reduction in AF-related symptoms.16 However, the relationship between physical activity and AF burden have produced mixed results. A Norwegian clinical trial showed that 12 weeks of aerobic interval training reduces AF burden in AF patients, indicating an increase in physical activity reduces AF burden,17 similar to our results. On the other hand, a clinical trial in Copenhagen found that high intensity exercise was not superior to low intensity exercise in decreasing AF burden among AF patients.18 Though our study only showed a significant increase in odds of intermittent AF, the measures of association comparing the odds of continuous AF and no AF were in the hypothesized direction for physical activity.
Obesity has been found to be the most prominent modifiable risk factor that influences lifetime AF risk.19 The LEGACY study reported that among patients referred for symptomatic AF treatment, weight loss results in a reduction of AF burden.20 Similarly, a clinical trial found that overweight and obese patients with symptomatic AF who participated in a structured weight management program had fewer AF episodes seen on 7-day Holter monitors and reduced AF symptom burden.21 An increase in cardiorespiratory fitness is associated with a decreased risk of incident AF, with the association strongest in obese patients,15 while a dose-dependent reduction in AF burden was noted among obese patients.22 These prior findings, in addition to our results, suggest a combination of exercise training and weight loss may further reduce AF burden.
Diabetes is an established risk factor for AF.23 A prior ARIC study found that individuals with diabetes have a 30% greater risk of incident AF than those without diabetes,24 similar to results from a meta-analysis.23 AF risk is further increased in those with longer diabetes duration and worse glycemic control,25 with fasting blood glucose independently associated with a higher risk of AF among those with diabetes.24 This association was not seen in those without diabetes, suggesting that AF may be related to cumulative exposure to hyperglycemia.24 Better glycemic control prior to ablation in patients with diabetes is associated with a reduction in AF recurrence after AF ablation,26 highlighting the importance of screening for diabetes early. To our knowledge, our study is the first to show that compared with intermediate and poor levels, ideal fasting blood glucose in midlife is associated with lower AF burden in older age. Achieving good glycemic control may be an effective strategy to lower AF burden in late life.
Studies assessing the effect of a combination of risk factors on AF burden are scarce. The ARREST-AF study found that patients who participated in a risk factor management program had a decrease in AF burden after AF ablation.27 Many risk factors in this program are included in LS7, suggesting that overall healthy cardiovascular behaviors may lower AF burden. This was supported by our study, which showed that a more favorable LS7 score was associated with lower odds of continuous AF vs. no AF. In addition, evidence is accumulating to indicate that structured risk factor management is not only clinically effective, but also more cost-effective than usual care.28 Our findings that physical activity, BMI, and fasting blood glucose are the LS7 factors most consistently associated with lower AF burden form the basis to test the efficacy and cost-effectiveness of a structured program encompassing these 3 factors in lowering AF burden. Interestingly, no consistent association between elevated blood pressure (an established AF risk factor)29 and AF burden was seen. As few studies have assessed the relationship between blood pressure and AF burden,30 more research to confirm this finding is needed.
Strengths of this study include the prospective design, long follow-up, representation of black and white men and women, and high percent analyzable time achieved with the Zio® XT Patch. However, limitations exist. First, some LS7 and AF burden categories were small (e.g., inadequate LS7, intermittent or continuous AF), resulting in imprecise effect estimates. Second, although we utilized IPW, selection bias in regard to visit non-attendance or not agreeing to wear the Zio® XT Patch may have occurred. Third, only 83 participants had intermittent AF (AF burden <100%), which did not provide the spread of data and power to adequately evaluate percent of time spent in AF. Fourth, although it would be of interest to evaluate the trajectories of LS7 and its components over time, it is not possible to calculate a complete LS7 score at each visit given that some variables (e.g., diet, physical activity) were not assessed at all visits. Finally, similar to other observational studies, residual confounding cannot be excluded.
In conclusion, this population-based prospective cohort study indicates that lower levels of overall cardiovascular health in midlife (as defined by LS7) is associated with higher AF burden in older age. Individually, poor levels of physical activity, BMI, and fasting blood glucose were each associated with higher AF burden. Selective targeting of these lifestyle factors in midlife may help to lower AF burden in older age. Future research to evaluate the effectiveness of optimizing these 3 factors in lowering AF burden is warranted.
Supplementary Material
ACKNOWLEDGEMENTS
The Atherosclerosis Risk in Communities study has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract nos. (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, HHSN268201700004I). The authors thank the staff and participants of the ARIC study for their important contributions. This work was also supported by grants from the National Heart Lung and Blood Institute [R01HL126637-01A1 (LYC), R01HL141288 (LYC), K24HL14852 (AA)] and the American Heart Association [16EIA26410001 (AA)].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper
References
- 1.Morin DP, Bernard ML, Madias C, Rogers PA, Thihalolipavan S, Estes NA. The State of the Art: Atrial Fibrillation Epidemiology, Prevention, and Treatment. Mayo Clin Proc 2016;91:1778–1810. [DOI] [PubMed] [Google Scholar]
- 2.Chen LY, Chung MK, Allen LA, Ezekowitz M, Furie KL, McCabe P, Noseworthy PA, Perez MV, Turakhia MP, Cardiology AHACoC, Nursing CoCaS, Research CoQoCaO, Council aS. Atrial Fibrillation Burden: Moving Beyond Atrial Fibrillation as a Binary Entity: A Scientific Statement From the American Heart Association. Circulation 2018;137:e623–e644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD, Committee AHASPTFaS. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation 2010;121:586–613. [DOI] [PubMed] [Google Scholar]
- 4.Garg PK, O’Neal WT, Chen LY, Loehr LR, Sotoodehnia N, Soliman EZ, Alonso A. American Heart Association’s Life Simple 7 and Risk of Atrial Fibrillation in a Population Without Known Cardiovascular Disease: The ARIC (Atherosclerosis Risk in Communities) Study. J Am Heart Assoc 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol 1989;129:687–702. [PubMed] [Google Scholar]
- 6.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr 1982;36:936–942. [DOI] [PubMed] [Google Scholar]
- 7.Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, O’Brien WL, Bassett DR, Schmitz KH, Emplaincourt PO, Jacobs DR, Leon AS. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc 2000;32:S498–504. [DOI] [PubMed] [Google Scholar]
- 8.Siedel J, Hägele EO, Ziegenhorn J, Wahlefeld AW. Reagent for the enzymatic determination of serum total cholesterol with improved lipolytic efficiency. Clin Chem 1983;29:1075–1080. [PubMed] [Google Scholar]
- 9.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol 1985;122:51–65. [DOI] [PubMed] [Google Scholar]
- 10.Folsom AR, Shah AM, Lutsey PL, Roetker NS, Alonso A, Avery CL, Miedema MD, Konety S, Chang PP, Solomon SD. American Heart Association’s Life’s Simple 7: Avoiding Heart Failure and Preserving Cardiac Structure and Function. Am J Med 2015;128:970–976.e972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study). Am J Cardiol 2008;101:1016–1022. [DOI] [PubMed] [Google Scholar]
- 12.Rosamond WD, Chambless LE, Heiss G, Mosley TH, Coresh J, Whitsel E, Wagenknecht L, Ni H, Folsom AR. Twenty-two-year trends in incidence of myocardial infarction, coronary heart disease mortality, and case fatality in 4 US communities, 1987–2008. Circulation 2012;125:1848–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosamond WD, Folsom AR, Chambless LE, Wang CH, McGovern PG, Howard G, Copper LS, Shahar E. Stroke incidence and survival among middle-aged adults: 9-year follow-up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke; a journal of cerebral circulation 1999;30:736–743. [DOI] [PubMed] [Google Scholar]
- 14.Weuve J, Tchetgen Tchetgen EJ, Glymour MM, Beck TL, Aggarwal NT, Wilson RS, Evans DA, Mendes de Leon CF. Accounting for bias due to selective attrition: the example of smoking and cognitive decline. Epidemiology 2012;23:119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qureshi WT, Alirhayim Z, Blaha MJ, Juraschek SP, Keteyian SJ, Brawner CA, Al-Mallah MH. Cardiorespiratory Fitness and Risk of Incident Atrial Fibrillation: Results From the Henry Ford Exercise Testing (FIT) Project. Circulation 2015;131:1827–1834. [DOI] [PubMed] [Google Scholar]
- 16.Osbak PS, Mourier M, Kjaer A, Henriksen JH, Kofoed KF, Jensen GB. A randomized study of the effects of exercise training on patients with atrial fibrillation. Am Heart J 2011;162:1080–1087. [DOI] [PubMed] [Google Scholar]
- 17.Malmo V, Nes BM, Amundsen BH, Tjonna AE, Stoylen A, Rossvoll O, Wisloff U, Loennechen JP. Aerobic Interval Training Reduces the Burden of Atrial Fibrillation in the Short Term: A Randomized Trial. Circulation 2016;133:466–473. [DOI] [PubMed] [Google Scholar]
- 18.Skielboe AK, Bandholm TQ, Hakmann S, Mourier M, Kallemose T, Dixen U. Cardiovascular exercise and burden of arrhythmia in patients with atrial fibrillation - A randomized controlled trial. PLoS One 2017;12:e0170060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Staerk L, Wang B, Preis SR, Larson MG, Lubitz SA, Ellinor PT, McManus DD, Ko D, Weng LC, Lunetta KL, Frost L, Benjamin EJ, Trinquart L. Lifetime risk of atrial fibrillation according to optimal, borderline, or elevated levels of risk factors: cohort study based on longitudinal data from the Framingham Heart Study. BMJ 2018;361:k1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pathak RK, Middeldorp ME, Meredith M, Mehta AB, Mahajan R, Wong CX, Twomey D, Elliott AD, Kalman JM, Abhayaratna WP, Lau DH, Sanders P. Long-Term Effect of Goal-Directed Weight Management in an Atrial Fibrillation Cohort: A Long-Term Follow-Up Study (LEGACY). J Am Coll Cardiol 2015;65:2159–2169. [DOI] [PubMed] [Google Scholar]
- 21.Abed HS, Wittert GA, Leong DP, Shirazi MG, Bahrami B, Middeldorp ME, Lorimer MF, Lau DH, Antic NA, Brooks AG, Abhayaratna WP, Kalman JM, Sanders P. Effect of weight reduction and cardiometabolic risk factor management on symptom burden and severity in patients with atrial fibrillation: a randomized clinical trial. JAMA 2013;310:2050–2060. [DOI] [PubMed] [Google Scholar]
- 22.Pathak RK, Elliott A, Middeldorp ME, Meredith M, Mehta AB, Mahajan R, Hendriks JM, Twomey D, Kalman JM, Abhayaratna WP, Lau DH, Sanders P. Impact of CARDIOrespiratory FITness on Arrhythmia Recurrence in Obese Individuals With Atrial Fibrillation: The CARDIOFIT Study. J Am Coll Cardiol 2015;66:985–996. [DOI] [PubMed] [Google Scholar]
- 23.Huxley RR, Filion KB, Konety S, Alonso A. Meta-analysis of cohort and case-control studies of type 2 diabetes mellitus and risk of atrial fibrillation. Am J Cardiol 2011;108:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huxley RR, Alonso A, Lopez FL, Filion KB, Agarwal SK, Loehr LR, Soliman EZ, Pankow JS, Selvin E. Type 2 diabetes, glucose homeostasis and incident atrial fibrillation: the Atherosclerosis Risk in Communities study. Heart 2012;98:133–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dublin S, Glazer NL, Smith NL, Psaty BM, Lumley T, Wiggins KL, Page RL, Heckbert SR. Diabetes mellitus, glycemic control, and risk of atrial fibrillation. J Gen Intern Med 2010;25:853–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Donnellan E, Aagaard P, Kanj M, Jaber W, Elshazly M, Hoosien M, Baranowski B, Hussein A, Saliba W, Wazni O. Association Between Pre-Ablation Glycemic Control and Outcomes Among Patients With Diabetes Undergoing Atrial Fibrillation Ablation. JACC Clin Electrophysiol 2019;5:897–903. [DOI] [PubMed] [Google Scholar]
- 27.Pathak RK, Middeldorp ME, Lau DH, Mehta AB, Mahajan R, Twomey D, Alasady M, Hanley L, Antic NA, McEvoy RD, Kalman JM, Abhayaratna WP, Sanders P. Aggressive risk factor reduction study for atrial fibrillation and implications for the outcome of ablation: the ARREST-AF cohort study. J Am Coll Cardiol 2014;64:2222–2231. [DOI] [PubMed] [Google Scholar]
- 28.Pathak RK, Evans M, Middeldorp ME, Mahajan R, Mehta AB, Meredith M, Twomey D, Wong CX, Hendriks JML, Abhayaratna WP, Kalman JM, Lau DH, Sanders P. Cost-Effectiveness and Clinical Effectiveness of the Risk Factor Management Clinic in Atrial Fibrillation: The CENT Study. JACC Clin Electrophysiol 2017;3:436–447. [DOI] [PubMed] [Google Scholar]
- 29.Huxley RR, Lopez FL, Folsom AR, Agarwal SK, Loehr LR, Soliman EZ, Maclehose R, Konety S, Alonso A. Absolute and attributable risks of atrial fibrillation in relation to optimal and borderline risk factors: the Atherosclerosis Risk in Communities (ARIC) study. Circulation 2011;123:1501–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deftereos S, Giannopoulos G, Kossyvakis C, Efremidis M, Panagopoulou V, Raisakis K, Kaoukis A, Karageorgiou S, Bouras G, Katsivas A, Pyrgakis V, Stefanadis C. Effectiveness of moxonidine to reduce atrial fibrillation burden in hypertensive patients. Am J Cardiol 2013;112:684–687. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


