Abstract
Aging-associated microglial priming results in the potential for an exaggerated neuroinflammatory response to a subsequent inflammatory challenge in regions of the brain known to support learning and memory. This excessive neuroinflammation in the aging brain is known to occur following a variety of peripheral insults, including infection and surgery, where it has been associated with precipitous declines in cognition and memory. As the average lifespan increases worldwide, identifying interventions to prevent and treat aging-associated excessive neuroinflammation and ensuing cognitive impairments is of critical importance. Lifestyle has emerged as a potential non-pharmacological target in this endeavor. Here, we review important and recent preclinical and clinical literature demonstrating the anti-inflammatory effects of lifestyle modifications such as exercise, diet, and environmental enrichment in the context of aging and memory. Importantly, we focus on research indicating that these lifestyle modifications do not need to be lifelong, suggesting that such interventions may be efficacious in the prevention and treatment of aging- and neuroinflammation-associated cognitive impairment, even when initiated in older age.
Keywords: aging, neuroinflammation, post-operative cognitive dysfunction, lifestyle, diet, exercise, environmental enrichment
1. Introduction
Older individuals are particularly susceptible to precipitous memory impairments following a variety of insults, including peripheral surgeries and infections, unhealthy diets, and injury, and deleterious neuroinflammation has emerged as a critical component underlying these memory deficits. During normal aging, the brain’s resident immune cells – microglia – become sensitized (Rozovsky et al., 1998), priming them to mount prolonged and exaggerated inflammatory responses (Barrientos et al., 2015). It is hypothesized that when these already sensitized microglia are activated in response to peripheral insults the significant neuroinflammatory reaction that results leads to downstream changes that are damaging to the brain’s synaptic plasticity functions, ensuing in memory impairment. It should be noted that other cell types, namely astrocytes, play a profound role in neuroinflammation and brain aging as well (Salminen et al., 2011; Colombo and Farina, 2016; Boisvert et al., 2018). However, a preponderance of research indicates that in aging, microglial activation promotes subsequent astrocytic activation. That is, the aging-induced increase of reactive astrocyte genes is reduced in mice lacking microglial-secreted cytokines known to induce reactive astrocyte formation (namely interleukin 1α [IL-1α] and tumor necrosis factor [TNF]), implicating microglia as the primary driver of exaggerated neuroinflammation in aging (Clarke et al., 2018). Indeed, extensive research has implicated this exaggerated neuroinflammation resulting from aging-associated microglial priming in a number of memory impairments.
In the aging population, the peripheral insults of surgery and infection are known to result in detrimental cognitive deficits. We and others have demonstrated that a variety of surgeries, including various abdominal, orthopedic, and cardiac surgeries, result in exaggerated neuroinflammation and memory impairments (Moller et al., 1998; Monk et al., 2008; Barrientos et al., 2012; Alam et al., 2018; Safavynia and Goldstein, 2018; Lu et al., 2019). Similarly, peripheral infections, including E. coli, influenza A, and human immunodeficiency virus (HIV) have been shown to disproportionately cause memory impairments in aged systems as compared to young adult ones; again, excessive neuroinflammation resulting from normal aging-associated microglial priming is implicated in the underlying processes (Barrientos et al., 2006; Barrientos et al., 2009; Jurgens et al., 2012; Tanaka et al., 2018; Sparkman et al., 2019). Likewise, diets high in fat and sugar are now understood to impact cognition, including learning and memory. In fact, a high fat diet (HFD) alone is sufficient to cause cognitive deficits in aged rodents, but not young adults, in as a little as 3 days (Spencer et al., 2017). One mechanism of diet’s effects on cognition is its ability to induce a robust inflammatory response in the aged brain (Spencer et al., 2017; Cavaliere et al., 2019; Noronha et al., 2019; Butler et al., 2020), and recent work by our group implicates marked increases in pro-inflammatory saturated fatty acids and decreases in anti-inflammatory polyunsaturated fatty acids following short-term HFD in this phenomenon (Butler et al., 2020). Indeed, elevated expression of pro-inflammatory cytokines and reduced phagocytic activity by microglia are seen to parallel HFD-induced memory deficits in aged rodents (Spencer et al., 2019; Xu et al., 2019). Furthermore, neuroinflammation has been implicated in a variety of aging-associated cognitive diseases, most notably Alzheimer’s disease and other dementias, as well as in the pathology of traumatic brain injury (Cai et al., 2014; Calsolaro and Edison, 2016; Chiu et al., 2016; Loane and Kumar, 2016; Bright et al., 2019).
Given the prevalence of neuroinflammation in memory impairments which disproportionately inflict aged individuals, interventions which facilitate anti-neuroinflammatory responses are of critical importance. In recent years, lifestyle has emerged as a potential non-pharmacological target for achieving such results. In particular, lifestyle modifications such as diet, exercise, and environmental enrichment have been found to elicit anti-inflammatory effects in aging. Thus, lifestyle modification presents a promising potential method for preventing and treating aging- and neuroinflammation-associated memory deficits, including those induced by peripheral insults (Fig. 1). Here we review recent and important preclinical and clinical findings on the impact and efficacy of lifestyle modifications initiated specifically in middle to older age in the prevention and treatment of aging-associated and neuroinflammation-mediated memory impairments.
Figure 1.
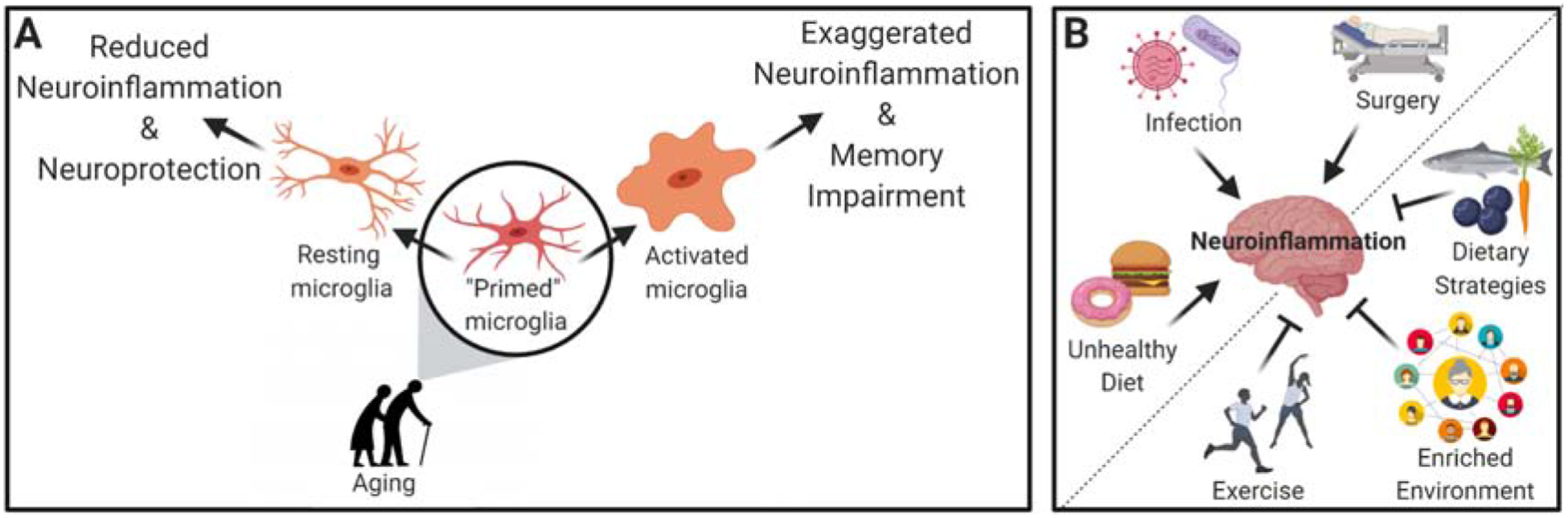
Schematic depiction of the current literature. (A) Normal aging causes microglial priming in key memory-mediating regions of the brain such that, upon proinflammatory challenge, exaggerated activation occurs, leading to deleterious neuroinflammation and memory impairment. In contrast, anti-inflammatory modifications reduce microglial activation, effectively reducing levels of neuroinflammation and leading to neuroprotection. (B) Examples of proinflammatory challenges (infection, surgery, or unhealthy diet) that compound the aging-associated microglial priming to exacerbate neuroinflammation; and examples of lifestyle modifications (exercise, dietary strategies, and environmental enrichment) demonstrated to have anti-neuroinflammatory benefits.
2. Dietary Strategies
Diet elicits profound effects on inflammation, including in the brain. For example, overfeeding or consuming diets high in saturated fats and refined sugars are known to robustly increase saturated fatty acids in the brain and neuroinflammatory responses (Milanski et al., 2009; Clarke et al., 2012; Thaler et al., 2012; Miller and Spencer, 2014; Sobesky et al., 2014; Beilharz et al., 2016; Cai et al., 2016; Sobesky et al., 2016; Spencer et al., 2017; Cavaliere et al., 2019; Noronha et al., 2019; Butler et al., 2020), while other dietary strategies have been shown to reduce levels of neuroinflammation (Fig. 1). Dietary restriction encompasses any reduction in the intake of certain nutrients through diet and can be further classified as caloric restriction – a reduction in total food intake without inducing malnutrition; and intermittent fasting – a dietary strategy that cycles between fasting and non-fasting physiological states. Other dietary strategies include specific nutritional consumption patterns (e.g. protein restriction, Mediterranean diet, or veganism). Since the pivotal 1935 study by McCay and colleagues, in which it was first demonstrated that caloric restriction extended lifespan (McCay et al., 1935), extensive research has been done to elucidate the mechanisms by which dietary restriction induces beneficial and protective effects, including on the brain. Interestingly, anti-inflammatory mechanisms have emerged as a critical benefit of dietary restriction.
2.1. Preclinical Evidence: Dietary Strategies Reduce Glial Activation & Neuroinflammation
Recently, a detailed multi-tissue, transcriptomic study identified systemic effects of both aging and caloric restriction on cell type composition, molecular programs, regulatory transcription factors, and cellular communication networks (Ma et al., 2020). In this study, 30% caloric restriction was initiated in middle aged mice (18 months) and maintained for 9 months. The results strongly corroborate previous work showing that aging is associated with a chronic inflammatory status in multiple tissues, including heart, liver, serum, and brain, and, most interestingly, revealed that these pro-inflammatory changes can be repressed or even reversed by 30% caloric restriction, even when initiated in middle age. Similarly, another study demonstrated that in various brain regions, including the hippocampus and corpus collosum, aging-associated astrocyte and microglial activation was attenuated by 30–40% caloric restriction when initiated in young adulthood (3 months) and continued into late middle-age (24 months) (Morgan et al., 1999). The authors note that these changes were paralleled by mitigation of aging related changes to both learning and long-term potentiation (LTP) (a critical mechanism underlying learning and memory) in the hippocampus following caloric restriction.
Other work indicates that more short-term dietary restriction is sufficient to reduce levels of neuroinflammation, suggesting that diet modification implemented later in life can still be an effective non-pharmacological intervention. One study (Vasconcelos et al., 2014) examined the effects of intermittent fasting on an adult rat model of sepsis, particularly on neuroinflammation and cognitive deficits associated with the condition. In this study, intermittent fasting consisted of 24 hours of food deprivation every other day for 30 days prior to infection induction. This regimen ameliorated spatial memory and inhibitory avoidance impairments. The authors demonstrated that intermittent fasting modulated the NFκB transcription factor and reduced mRNA expression of toll-like receptor 4 (TLR4) within the hippocampus, likely explaining the ameliorated LPS-induced levels of hippocampal pro-inflammatory cytokines interleukin 1-alpha (IL-1α), interleukin 1-beta (IL-1β), and tumor necrosis factor-alpha (TNFα). Finally, the authors reported that intermittent fasting prevented LPS-induced reduction of brain derived neurotrophic factor (BDNF), a protein critical to long-term memory consolidation (Barco et al., 2005) with a known vulnerability to neuroinflammation (Barrientos et al., 2003; Barrientos et al., 2004). Similarly, another study examined the effects of intermittent fasting pretreatment (for 12 weeks) on cognitive function (object and spatial memory) in an adult rodent model of vascular dementia (Hu et al., 2017). Intermittent fasting robustly reduced oxidative stress and neuroinflammation, prevented BNDF levels from plummeting, and prevented cognitive deficits, effects all normally associated with the disease.
This dietary strategy initiated in middle to old age has also been shown to be neuroprotective. A series of studies by Singh and colleagues has demonstrated that intermittent fasting begun either in middle or old age can prevent behavioral impairment in male rats (Singh et al., 2012; Singh et al., 2015). In their 2012 study of late-onset intermittent fasting, 21 month-old rats were subjected to periodic fasting, in which they were without access to food for 24 hours every other day, for 3 months. Following the intervention, motor coordination and spatial memory function were assessed via rotarod and Morris water maze, respectively. The authors found that the fasting regimen resulted in improved age-related motor and memory function compared to ad libitum-fed aged controls. These findings were paralleled by an attenuation of age-related increases in protein carbonylation and partial recovery of mitochondrial electron chain complexes (ETC) activity in the fasted rats, both of which are indicative of reduced oxidative damage. Furthermore, in the hippocampus, intermittent fasting mitigated the aging-associated decrease of key synaptic proteins synaptophysin and CaM kinase II, which are known to be negatively affected by oxidative damage. The 2015 study extended these findings with the observation that NFκB was significantly reduced in the fasted rats, suggestive of a role for intermittent fasting’s anti-inflammatory effects in the observed benefits.
Likewise, in a study of senescence-accelerated prone 8 (SAMP8) mice, which have a reduced lifespan and neurological changes characteristic of advanced aging, intermittent fasting for 8 weeks was found to normalize expression of BNDF and Sirtuin 1 (Sirt1) (Tajes et al., 2010). Sirt1 is a nicotinamide adenine dinucleotide (NAD+)-dependent protein deacetylase with a myriad of functions, including in memory formation and synaptic plasticity; additionally, elevated Sirt1 has been associated with reduced microglial expression of NFκB, thus promoting an anti-inflammatory role for the molecule (Chen et al., 2005). Indeed, Tajes et al demonstrated that increased Sirt1 following fasting was paralleled by reduced NFκB expression.
Taken together, these studies suggest that caloric restriction and intermittent fasting confer neuroprotection against a variety of aging-related insults via anti-inflammatory and pro-BDNF mechanisms, thus preserving memory functions. Intermittent fasting may be particularly relevant to post-operative cognitive dysfunction, a disorder disproportionately afflicting older adults, as it could potentially be included in pre-operative patient regimens in an effort to ameliorate surgery-induced exaggerated neuroinflammation and memory impairments.
2.2. Clinical Evidence: Dietary Strategies Mitigate Aging-Associated Cognitive Impairments
A number of clinical studies have ascertained a positive correlation between more prudent or restrictive dietary strategies, initiated in late-middle- to old-age, and better cognitive outcomes in older age. In one study (Shakersain et al., 2016), dementia-free individuals over the age of 65 were followed for up to 6 years and their diet and cognitive abilities were assessed. Using a food frequency questionnaire to determine diet intake and mini-mental state examination (MMSE) to assess global cognition, including attention, calculation, and memory, the authors found that high adherence to a more prudent, Mediterranean-like diet (i.e. high in fruits, vegetables, fish, and low-fat dairy) was significantly correlated to less cognitive decline, as determined by the MMSE. The group reported that those who maintained a Western diet (generally considered a diet high in fats and sugars) were more highly associated with cognitive decline. Interestingly, the Western diet-associated cognitive decline became less pronounced and non-significant for individuals who maintained a mix of prudent and Western diets, suggesting that a more restrictive dietary pattern may attenuate the adverse effects of an unhealthy one. Similarly, another study (Sindi et al., 2018) found that implementing a healthier diet in midlife reduced one’s risk of dementia in later-life. Following an initial self-report of diet at midlife (mean age=56), study participants returned for two late life reexaminations (mean age=70 and 78). The group found that greater midlife healthy dietary changes were associated with reduced risk of dementia later in life.
Likewise, more specific nutritional patterns have also been shown to elicit beneficial cognitive effects in older humans. For example, emerging evidence suggests that plant-based diets, such as vegetarianism and veganism, elicit anti-inflammatory effects in part due to the high amounts of antioxidant-rich foods in plant-based diets, such as nuts and berries. Additionally, multiple lines of research suggest that plant-based diets or the addition of more plant-based foods can improve cognitive function, such as learning memory, visuospatial orientation, executive function, and logical memory, and reduce risk of cognitive decline in old age, even when initiated in late middle- to old-age (see (Jamshed et al., 2019) for a detailed review). Similarly, the Mediterranean diet – a diet pattern typically involving high intake of plant-based foods (e.g. nuts, fruit, and vegetables) and olive oil, moderate intake of dairy, eggs, fish and poultry, and low intake of red meats – has been associated with healthy cognitive aging, including improved working and short term memory, retrieval fluency, inhibition, visuospatial memory, attention and executive function and reduced risk of cognitive decline (Petersson and Philippou, 2016; Masana et al., 2017). These human studies suggest that dietary modifications beyond simple calorie restriction or intermittent fasting may also be neuroprotective in older age, indicating a complex role for diet – and specifically diet composition – in cognitive function and aging. It should be noted that these studies are limited to assessing estimates of particular dietary components, i.e. fats, sugars, meats, and vegetables, and did not measure caloric intake. Thus, it cannot be ascertained if the observed effects are due to changes in nutrient intake or the reduction in caloric intake often associated with a more restrictive diet. Future studies should aim to determine the specific contributions of these factors on cognition in older age.
Clinical studies aimed at understanding the mechanisms underlying some of these neuroprotective dietary strategies have revealed several anti-inflammatory effects. For example, many studies have shown that supplementation with poly-unsaturated fatty acids (PUFA) significantly reduce circulating C-reactive protein, TNFα and IL-6 concentrations (Ferrucci et al., 2006; Browning et al., 2007; Kelley et al., 2009; Satoh et al., 2009; Ramel et al., 2010; Lopez-Alarcon et al., 2011; Nobili et al., 2011; Skulas-Ray et al., 2011). Additionally, intermittent fasting may be neuroprotective by influencing circadian rhythm. A growing body of literature indicates that the circadian clock regulates activity and function of neuroimmune cells (Fonken et al., 2015; Fonken et al., 2016; Segal et al., 2018). Indeed, in a clinical study on the effects of early time restricted feeding – a form of intermittent fasting in which participants ate only between 8am and 2pm everyday for 4 days – fasting was found to have widespread effects on circadian clock gene expression in whole blood cells and these were associated with increases in expression of Sirt1, BDNF, and mTOR (Jamshed et al., 2019), supporting a critical role for circadian rhythm in the neuroprotective effects of intermittent fasting.
Taken together, these clinical studies demonstrate the powerful impact of diet on cognitive aging (Fig. 1), even when implemented at an older age. It is likely that these neuroprotective effects are mediated at least in part by reductions in neuroinflammation, strongly suggesting that diet modification may be an efficacious non-pharmacological intervention to reduce aging-associated exaggerated neuroinflammation and ensuing memory impairments.
3. Exercise
Exercise can be broadly defined as any physical activity, and is further classified as either aerobic, anaerobic, or flexibility exercise. Aerobic exercise consists of physical activities which involve large muscle groups and that require the body to use more oxygen than it does at rest; walking, running, swimming, biking, and rowing are classic examples of aerobic exercise. Anaerobic exercise involves strength and resistance training (e.g. weightlifting and interval training) and is known for improving muscle mass, strength, and overall function. Lastly, flexibility exercise increases muscle length and thus joint range of motion, typically via stretching. In the present review, we will focus on aerobic exercise and its effect on neuroinflammation and cognition in aging, primarily due to a lack of current research on anaerobic and flexibility exercise’s effects on these processes. Significant work has been done to evaluate the anti-inflammatory effects of voluntary aerobic exercise in rodent models, especially in aging, and to delineate the underlying molecular mechanisms, as well as to understand the impact of regular exercise on cognition in older humans.
3.1. Preclinical Evidence: Exercise is Neuroprotective & Anti-Neuroinflammatory in Aging
Our group has shown that a modest amount of voluntary wheel running over a 6 week period was robustly effective at preventing peripheral infection-induced exaggerated neuroinflammation in the hippocampus of aged rats (Barrientos et al., 2011). Blunted BDNF mRNA induction and hippocampal-dependent long-term memory deficits, which were also observed in the infection model, were reversed with exercise. Furthermore, exercise abrogated age-related microglial priming, suggesting a mechanism underlying exercise-induced neuroprotection in aging. Importantly, rats did not begin voluntary exercise until 22 months of age, representing late middle-age. Similarly, others have shown that, in aged mice, 8 weeks of voluntary wheel running decreased the number of new microglia while increasing the number of microglia expressing insulin-like growth factor 1 (IGF-1), a protein produced in the brain which is critical to brain development, synapse formation, adult neurogenesis, and cognition (Kohman et al., 2012; Labandeira-Garcia et al., 2017); importantly, IGF-1 is thought to exert neuroprotective effects via inhibition of neuroinflammation (Sukhanov et al., 2007; Park et al., 2011). Interestingly, IGF-1 expression normally decreases with age. Thus, these data suggest that exercise shifts microglia towards a less inflammatory phenotype, which may underlie, at least in part, its neuroprotective effects in cognitive aging.
Another study found that six weeks of voluntary wheel running among aged mice led to improved spatial learning and memory performance, accelerated glymphatic clearance, and a reduced number of microglia and astrocytes (He et al., 2017). The authors also assessed synaptic structures (dendrites, dendritic spines, and post-synaptic density 95 [PSD95]), and found that running significantly increased expression of all three compared to that of sedentary mice. Based on these results, the authors concluded that exercise attenuated neuroinflammation and that this was associated with protection from synaptic dysfunction and cognitive decline. Taken together, these studies suggest that exercise exerts neuroprotective effects via anti-inflammatory mechanisms and can protect against neuroinflammation-associated cognitive deficits, even when that exercise is initiated later in life, indicating that these mechanisms remain plastic into older age.
3.2. Clinical Evidence: Exercise Protects Cognitive Function in Older Humans
Clinical studies examining the effects of exercise on cognitive aging are also promising. Lin and colleagues recently carried out a longitudinal study on the effects of self-selected exercise on cognition in a non-dementia aging Chinese population (Lin et al., 2019). The study reported a significant improvement from baseline in cognitive function, particularly visuospatial function, in the self-selected exercise group compared to the non-exercise group at a one-year cognitive evaluation follow-up. Similarly, other clinical studies found that, in cognitively intact older adults, higher levels of aerobic fitness were associated with larger hippocampi and better spatial memory performance (Erickson et al., 2009; Erickson et al., 2011). In an interesting long-term exercise study examining the combination of physical activity and cognitive enrichment, researchers assessed various measures of neuroplasticity in older adults assigned to either a dance exercise group (in which participants had to constantly learn new movement patterns) or a conventional strength and endurance exercise group (Muller et al., 2017). Both groups exhibited significant improvements from baseline in attention after six months, and attention and verbal memory after eighteen months. However, the dancers exhibited increased plasma BDNF concentrations and greater gray matter volume in the precentral gyrus and parahippocampal regions compared to baseline values, effects not observed in the conventional exercise group. These findings suggest that combining physical activity with new learning may be even more neuroprotective than conventional exercise alone.
In a study of the acute effects of aerobic exercise, it was demonstrated that just 30 minutes of moderate-intensity cycling (65% of their maximum heart rate) promoted a change in functional connectivity of networks (as assessed via fMRI analysis) in the brains of older individuals, particularly in brain regions associated with learning and memory (hippocampus), affect and reward (nucleus accumbens and amygdala), and executive control (frontal gyrus, dorsolateral and ventrolateral prefrontal cortex) (Weng et al., 2017). Additionally, while both older participants and the younger controls experienced this increased functional connectivity, older adults underwent a greater change in aging-sensitive regions such as the hippocampus and executive control networks. In a follow-up study, these results were extended to show that, in older adults, the changes to functional connectivity which occur following a single session of aerobic exercise reflect initial adaptations experienced with prolonged exercise training (12 weeks) (Voss et al., 2020). Importantly, the group also found that these functional changes, specifically those within the right lateralized prefrontal cortex, were associated with improved working memory. The results of these studies strongly suggest that even acute aerobic exercise in older age elicits neuroprotective effects, and that more prolonged exercise regimes can positively influence memory function.
4. Environmental Enrichment
Environmental enrichment (EE) is classically defined as a combination of social and inanimate stimulation, and generally refers to conditions that promote increased social, cognitive, and physical engagement (Rosenzweig and Bennett, 1996; Jurgens and Johnson, 2012). It is well-established that EE positively impacts cognition, including learning and memory (Nilsson et al., 1999; Bruel-Jungerman et al., 2005; Leggio et al., 2005), and research has implicated reduced microglia-mediated neuroinflammation as a crucial mechanism underlying EE’s neuroprotective effects.
4.1. Preclinical Evidence: Environmental Enrichment Is Anti-Neuroinflammatory
In a study aimed at evaluating EE’s effects on the innate immune system of the brain, particularly in the context of Alzheimer’s disease (AD), researchers subjected wild-type young adult mice to 7–8 weeks of standard or EE housing (consisting of large cages with 8 mice, a running wheel, tunnels, and objects of various sizes and colors; mice were rotated through 4 different cages daily) (Xu et al., 2016). This was followed by exposure to human amyloid beta (Aβ) oligomers, the primary component of amyloid plaques associated with AD, via intracerebroventricular microinjection. Mice that received Aβ injections and were housed in standard conditions exhibited greater immunological and morphological markers of microglial activation and increased cytokine expression, including TNFα, IL-6, and IL-1β. Importantly, EE-housed counterparts demonstrated a robust suppression of this activated microglial and inflammatory phenotype. In another model of AD utilizing 12-month old APP/PS1 mice, 6 months of EE (3–4 mice housed in a large cage with blocks of various shapes and material, a ball, a running wheel, platforms, and a hut) was shown to improve spatial short-term, but not long-term memory (Stuart et al., 2019). Additionally, an increased number of phagocytic microglia following EE were observed compared to APP/PS1 mice in standard housing; a higher percentage of phagocytic microglia has also been observed in other AD mouse models (5xFAD) following EE (Ziegler-Waldkirch et al., 2018). Importantly, aging-related microglial activation is associated with reduced phagocytosis, thus observations of increased phagocytic microglia following EE are suggestive of a potential mechanism underlying the intervention’s beneficial effects on cognition (Koellhoffer et al., 2017).
In an elegant series of studies by Cao and colleagues, it has been revealed that EE, when implemented in middle-age, can promote healthy aging in mice (McMurphy et al., 2018), and that this is mediated in part by reduced neuroinflammation and effects on microglial morphology (Ali et al., 2019). The group found that long-term EE (7.5–12 months), consisting of 5 mice group-housed in large cages with running wheels, tunnels, igloos, huts and other retreats, wooden toys, and a maze, resulted in decreased expression of inflammatory cytokines and MHC-II in hippocampus, amygdala, and hypothalamus. These findings were accompanied by ramification of microglia in those same brain regions. Typically, microglia from aged rodents present a more ameboid-like morphology compared to microglia in young rodents (Rawji et al., 2016), and thus these EE-induced changes represent a shift in microglia towards a more youthful and less inflammatory phenotype.
The functional implications of EE’s anti-inflammatory effects have also begun to be examined. A recent study has revealed that in aged mice, non-social EE (i.e. housed alone but with a running wheel and novel toys) mitigates the detrimental effects of social isolation in aging on spatial and social memory, and that this attenuation is associated with partial amelioration of glial cell and NOD-like receptor protein-3 (NLRP3) inflammasome activation in the hippocampus (Wang et al., 2018). Together, these preclinical studies suggest that enriching one’s environment may be beneficial in the prevention and even treatment of aging- and neuroinflammation-associated cognitive impairment.
4.2. Clinical Evidence: Environmental Enrichment, Aging, and Cognition
To date, little clinical work has been done on the topic of EE and aging-related cognition. Instead, much of the available research has focused on the benefits of social networks and healing spaces. For instance, living in close proximity to green spaces and other amenities such as parks, libraries, and cafes has been associated with reduced risk of dementia, certain mental health disorders, and slowed cognitive decline in old age (Wu et al., 2015; Cherrie et al., 2018; Wu et al., 2020).
Similarly, several longitudinal clinical studies have identified social network size and composition as correlates to cognitive function (Kim and Lee, 2019; Sharifian et al., 2019). In one study of cognitively intact older adults (>65 years old) it was found that cognitive decline 2 years after initial assessment was least pronounced in married individuals living only with their spouse, but who frequently interacted with their children and friends and partook in social groups compared to individuals with a more restricted social network (Kim and Lee, 2019). Another study found similar results when looking at global cognition, as opposed to cognitive decline overtime (Sharifian et al., 2019). It was observed that social networks which included a high number of friends, as opposed to a greater proportion of family, were associated with greater global cognition, regardless of network size.
Despite limited clinical studies relating EE and aging-associated cognitive declines, the current body of both preclinical and clinical research suggests that EE elicits anti-neuroinflammatory effects which are protective against cognitive impairment in rodent models, and which might underlie the observed benefits of EE/EE-like activities on cognition in aging. Future studies should aim to assess the effects of EE beyond just social networks; for instance, reading, game play, and puzzles are all well-regarded for the cognitive stimulation they provide. However, despite a lack of clinical studies on true EE, the present data is suggestive of its efficacy in reducing basal neuroinflammation and protecting against aging- and neuroinflammation-associated cognitive impairments.
5. Conclusions and Future Directions
Precipitous cognitive declines during normal aging have often been observed to occur following peripheral inflammatory challenges such as infection or surgery, and are more devastating than gradual declines over time. Given that aging is normally associated with microglial priming, it is likely that the brain’s immune cells mount a heightened response following such peripheral immune challenges, resulting in exaggerated and deleterious neuroinflammation leading to downstream effects on cognition and memory (Fig. 1). With the average human life-expectancy increasing worldwide (WHO, 2020), it is imperative to identify preventative and therapeutic interventions aimed at ameliorating this excessive neuroinflammation and preventing such inflammation-associated cognitive deficits.
Lifestyle has become a popular research topic in recent years, and a variety of studies, reviewed here, suggest that dietary strategies, voluntary physical exercise, and environmental enrichment are among the most effective lifestyle modifications to elicit various neuroprotective effects in the aged brain. Reductions in neuroinflammatory markers and increases in BDNF and synaptic markers critical for memory function have emerged as the most robust mechanisms underlying these effects. Importantly, these findings strongly suggest that these lifestyle modifications need not be life-long, but instead could be initiated in middle to old age and still elicit beneficial effects. Despite the abundance of evidence supporting these effects, the available clinical studies are still limited in their assessment of the inflammatory response to such lifestyle modifications. Future studies should aim to further characterize how such interventions impact basal inflammation levels, especially in the nervous system, in humans. Additionally, it should be noted that the preponderance of preclinical studies have used only male subjects. Given that the aging female brain undergoes tremendous change during menopause, older women are particularly vulnerable to cognitive impairments following inflammatory insults (Phillips Bute et al., 2003). Thus, future preclinical studies examining the effects of lifestyle modifications on neuroinflammation and neuroplasticity in older subjects should systematically examine sex differences. Fortunately, from the clinical studies that examined both sexes, no pronounced sex differences were noted, however, whether the underlying mechanisms of neuroprotection are the same or different in males and females is important to understand. Taken together, the available data favors lifestyle modification as a promising non-pharmacological intervention for preventing or treating neuroinflammation-associated cognitive deficits which disproportionately affect older individuals.
Highlights.
Aging individuals are vulnerable to cognitive deficits following a variety of peripheral insults
Exaggerated neuroinflammation mediates such impairments
Exercise, diet, and environmental enrichment elicit anti-neuroinflammatory effects
These non-pharmacological interventions protect against aging- and neuroinflammation-associated cognitive impairments
Acknowledgements:
This work is supported in part by grants from the National Institute on Aging AG028271 and AG067061 to R.M.B.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Alam A, Hana Z, Jin Z, Suen KC, Ma D (2018) Surgery, neuroinflammation and cognitive impairment. EBioMedicine 37:547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S, Liu X, Queen NJ, Patel RS, Wilkins RK, Mo X, Cao L (2019) Long-term environmental enrichment affects microglial morphology in middle age mice. Aging (Albany NY) 11:2388–2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barco A, Patterson SL, Alarcon JM, Gromova P, Mata-Roig M, Morozov A, Kandel ER (2005) Gene expression profiling of facilitated L-LTP in VP16-CREB mice reveals that BDNF is critical for the maintenance of LTP and its synaptic capture. Neuron 48:123–137. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, Kitt MM, Watkins LR, Maier SF (2015) Neuroinflammation in the normal aging hippocampus. Neuroscience 309:84–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Hein AM, Frank MG, Watkins LR, Maier SF (2012) Intracisternal interleukin-1 receptor antagonist prevents postoperative cognitive decline and neuroinflammatory response in aged rats. J Neurosci 32:14641–14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Sprunger DB, Campeau S, Watkins LR, Rudy JW, Maier SF (2004) BDNF mRNA expression in rat hippocampus following contextual learning is blocked by intrahippocampal IL-1beta administration. J Neuroimmunol 155:119–126. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, Sprunger DB, Campeau S, Higgins EA, Watkins LR, Rudy JW, Maier SF (2003) Brain-derived neurotrophic factor mRNA downregulation produced by social isolation is blocked by intrahippocampal interleukin-1 receptor antagonist. Neuroscience 121:847–853. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, Frank MG, Hein AM, Higgins EA, Watkins LR, Rudy JW, Maier SF (2009) Time course of hippocampal IL-1 beta and memory consolidation impairments in aging rats following peripheral infection. Brain Behav Immun 23:46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Higgins EA, Biedenkapp JC, Sprunger DB, Wright-Hardesty KJ, Watkins LR, Rudy JW, Maier SF (2006) Peripheral infection and aging interact to impair hippocampal memory consolidation. Neurobiol Aging 27:723–732. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, Frank MG, Crysdale NY, Chapman TR, Ahrendsen JT, Day HE, Campeau S, Watkins LR, Patterson SL, Maier SF (2011) Little exercise, big effects: reversing aging and infection-induced memory deficits, and underlying processes. J Neurosci 31:11578–11586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beilharz JE, Maniam J, Morris MJ (2016) Short-term exposure to a diet high in fat and sugar, or liquid sugar, selectively impairs hippocampal-dependent memory, with differential impacts on inflammation. Behav Brain Res 306:1–7. [DOI] [PubMed] [Google Scholar]
- Boisvert MM, Erikson GA, Shokhirev MN, Allen NJ (2018) The Aging Astrocyte Transcriptome from Multiple Regions of the Mouse Brain. Cell Rep 22:269–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright F, Werry EL, Dobson-Stone C, Piguet O, Ittner LM, Halliday GM, Hodges JR, Kiernan MC, Loy CT, Kassiou M, Kril JJ (2019) Neuroinflammation in frontotemporal dementia. Nat Rev Neurol 15:540–555. [DOI] [PubMed] [Google Scholar]
- Browning LM, Krebs JD, Moore CS, Mishra GD, O’Connell MA, Jebb SA (2007) The impact of long chain n-3 polyunsaturated fatty acid supplementation on inflammation, insulin sensitivity and CVD risk in a group of overweight women with an inflammatory phenotype. Diabetes Obes Metab 9:70–80. [DOI] [PubMed] [Google Scholar]
- Bruel-Jungerman E, Laroche S, Rampon C (2005) New neurons in the dentate gyrus are involved in the expression of enhanced long-term memory following environmental enrichment. Eur J Neurosci 21:513–521. [DOI] [PubMed] [Google Scholar]
- Butler MJ, Cole RM, Deems NP, Belury MA, Barrientos RM (2020) Fatty food, fatty acids, and microglial priming in the adult and aged hippocampus and amygdala. Brain Behav Immun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai G, Ziko I, Barwood J, Soch A, Sominsky L, Molero JC, Spencer SJ (2016) Overfeeding during a critical postnatal period exacerbates hypothalamic-pituitary-adrenal axis responses to immune challenge: a role for adrenal melanocortin 2 receptors. Sci Rep 6:21097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai G, Dinan T, Barwood JM, De Luca SN, Soch A, Ziko I, Chan SM, Zeng XY, Li S, Molero J, Spencer SJ (2014) Neonatal overfeeding attenuates acute central pro-inflammatory effects of short-term high fat diet. Frontiers in neuroscience 8:446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calsolaro V, Edison P (2016) Neuroinflammation in Alzheimer’s disease: Current evidence and future directions. Alzheimers Dement 12:719–732. [DOI] [PubMed] [Google Scholar]
- Cavaliere G, Trinchese G, Penna E, Cimmino F, Pirozzi C, Lama A, Annunziata C, Catapano A, Mattace Raso G, Meli R, Monda M, Messina G, Zammit C, Crispino M, Mollica MP (2019) High-Fat Diet Induces Neuroinflammation and Mitochondrial Impairment in Mice Cerebral Cortex and Synaptic Fraction. Front Cell Neurosci 13:509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Zhou Y, Mueller-Steiner S, Chen LF, Kwon H, Yi S, Mucke L, Gan L (2005) SIRT1 protects against microglia-dependent amyloid-beta toxicity through inhibiting NF-kappaB signaling. J Biol Chem 280:40364–40374. [DOI] [PubMed] [Google Scholar]
- Cherrie MPC, Shortt NK, Mitchell RJ, Taylor AM, Redmond P, Thompson CW, Starr JM, Deary IJ, Pearce JR (2018) Green space and cognitive ageing: A retrospective life course analysis in the Lothian Birth Cohort 1936. Soc Sci Med 196:56–65. [DOI] [PubMed] [Google Scholar]
- Chiu CC, Liao YE, Yang LY, Wang JY, Tweedie D, Karnati HK, Greig NH, Wang JY (2016) Neuroinflammation in animal models of traumatic brain injury. J Neurosci Methods 272:38–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke LE, Liddelow SA, Chakraborty C, Munch AE, Heiman M, Barres BA (2018) Normal aging induces A1-like astrocyte reactivity. Proc Natl Acad Sci U S A 115:E1896–E1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke MA, Stefanidis A, Spencer SJ (2012) Postnatal overfeeding leads to obesity and exacerbated febrile responses to lipopolysaccharide throughout life. J Neuroendocrinol 24:511–524. [DOI] [PubMed] [Google Scholar]
- Colombo E, Farina C (2016) Astrocytes: Key Regulators of Neuroinflammation. Trends Immunol 37:608–620. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Prakash RS, Voss MW, Chaddock L, Hu L, Morris KS, White SM, Wojcicki TR, McAuley E, Kramer AF (2009) Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus 19:1030–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF (2011) Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrucci L, Cherubini A, Bandinelli S, Bartali B, Corsi A, Lauretani F, Martin A, Andres-Lacueva C, Senin U, Guralnik JM (2006) Relationship of plasma polyunsaturated fatty acids to circulating inflammatory markers. J Clin Endocrinol Metab 91:439–446. [DOI] [PubMed] [Google Scholar]
- Fonken LK, Frank MG, Kitt MM, Barrientos RM, Watkins LR, Maier SF (2015) Microglia inflammatory responses are controlled by an intrinsic circadian clock. Brain Behav Immun 45:171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonken LK, Kitt MM, Gaudet AD, Barrientos RM, Watkins LR, Maier SF (2016) Diminished circadian rhythms in hippocampal microglia may contribute to age-related neuroinflammatory sensitization. Neurobiol Aging 47:102–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He XF, Liu DX, Zhang Q, Liang FY, Dai GY, Zeng JS, Pei Z, Xu GQ, Lan Y (2017) Voluntary Exercise Promotes Glymphatic Clearance of Amyloid Beta and Reduces the Activation of Astrocytes and Microglia in Aged Mice. Front Mol Neurosci 10:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Yang Y, Zhang M, Deng M, Zhang JJ (2017) Intermittent Fasting Pretreatment Prevents Cognitive Impairment in a Rat Model of Chronic Cerebral Hypoperfusion. J Nutr 147:1437–1445. [DOI] [PubMed] [Google Scholar]
- Jamshed H, Beyl RA, Della Manna DL, Yang ES, Ravussin E, Peterson CM (2019) Early Time-Restricted Feeding Improves 24-Hour Glucose Levels and Affects Markers of the Circadian Clock, Aging, and Autophagy in Humans. Nutrients 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurgens HA, Johnson RW (2012) Environmental enrichment attenuates hippocampal neuroinflammation and improves cognitive function during influenza infection. Brain Behav Immun 26:1006–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurgens HA, Amancherla K, Johnson RW (2012) Influenza infection induces neuroinflammation, alters hippocampal neuron morphology, and impairs cognition in adult mice. J Neurosci 32:3958–3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley DS, Siegel D, Fedor DM, Adkins Y, Mackey BE (2009) DHA supplementation decreases serum C-reactive protein and other markers of inflammation in hypertriglyceridemic men. J Nutr 139:495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YB, Lee SH (2019) Social network types and cognitive decline among older Korean adults: A longitudinal population-based study. Int J Geriatr Psychiatry 34:1845–1854. [DOI] [PubMed] [Google Scholar]
- Koellhoffer EC, McCullough LD, Ritzel RM (2017) Old Maids: Aging and Its Impact on Microglia Function. Int J Mol Sci 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohman RA, DeYoung EK, Bhattacharya TK, Peterson LN, Rhodes JS (2012) Wheel running attenuates microglia proliferation and increases expression of a proneurogenic phenotype in the hippocampus of aged mice. Brain Behav Immun 26:803–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labandeira-Garcia JL, Costa-Besada MA, Labandeira CM, Villar-Cheda B, Rodriguez-Perez AI (2017) Insulin-Like Growth Factor-1 and Neuroinflammation. Front Aging Neurosci 9:365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggio MG, Mandolesi L, Federico F, Spirito F, Ricci B, Gelfo F, Petrosini L (2005) Environmental enrichment promotes improved spatial abilities and enhanced dendritic growth in the rat. Behav Brain Res 163:78–90. [DOI] [PubMed] [Google Scholar]
- Lin S, Yang Y, Qi Q, Wei L, Jing N, Jie Z, Xia L, Shifu X (2019) The Beneficial Effect of Physical Exercise on Cognitive Function in a Non-dementia Aging Chinese Population. Front Aging Neurosci 11:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loane DJ, Kumar A (2016) Microglia in the TBI brain: The good, the bad, and the dysregulated. Exp Neurol 275 Pt 3:316–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Alarcon M, Martinez-Coronado A, Velarde-Castro O, Rendon-Macias E, Fernandez J (2011) Supplementation of n3 long-chain polyunsaturated fatty acid synergistically decreases insulin resistance with weight loss of obese prepubertal and pubertal children. Arch Med Res 42:502–508. [DOI] [PubMed] [Google Scholar]
- Lu Y, Xu X, Dong R, Sun L, Chen L, Zhang Z, Peng M (2019) MicroRNA-181b-5p attenuates early postoperative cognitive dysfunction by suppressing hippocampal neuroinflammation in mice. Cytokine 120:41–53. [DOI] [PubMed] [Google Scholar]
- Ma S, Sun S, Geng L, Song M, Wang W, Ye Y, Ji Q, Zou Z, Wang S, He X, Li W, Esteban CR, Long X, Guo G, Chan P, Zhou Q, Belmonte JCI, Zhang W, Qu J, Liu GH (2020) Caloric Restriction Reprograms the Single-Cell Transcriptional Landscape of Rattus Norvegicus Aging. Cell 180:984–1001 e1022. [DOI] [PubMed] [Google Scholar]
- Masana MF, Koyanagi A, Haro JM, Tyrovolas S (2017) n-3 Fatty acids, Mediterranean diet and cognitive function in normal aging: A systematic review. Exp Gerontol 91:39–50. [DOI] [PubMed] [Google Scholar]
- McCay CM, Crowell MF, Maynard LA (1935) The effect of retarded growth upon the length of life span and upon the ultimate body size. Journal of Nutrition 10:63–79. [PubMed] [Google Scholar]
- McMurphy T, Huang W, Queen NJ, Ali S, Widstrom KJ, Liu X, Xiao R, Siu JJ, Cao L (2018) Implementation of environmental enrichment after middle age promotes healthy aging. Aging (Albany NY) 10:1698–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milanski M, Degasperi G, Coope A, Morari J, Denis R, Cintra DE, Tsukumo DM, Anhe G, Amaral ME, Takahashi HK, Curi R, Oliveira HC, Carvalheira JB, Bordin S, Saad MJ, Velloso LA (2009) Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: implications for the pathogenesis of obesity. J Neurosci 29:359–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AA, Spencer SJ (2014) Obesity and neuroinflammation: a pathway to cognitive impairment. Brain Behav Immun 42:10–21. [DOI] [PubMed] [Google Scholar]
- Moller JT, Cluitmans P, Rasmussen LS, Houx P, Rasmussen H, Canet J, Rabbitt P, Jolles J, Larsen K, Hanning CD, Langeron O, Johnson T, Lauven PM, Kristensen PA, Biedler A, van Beem H, Fraidakis O, Silverstein JH, Beneken JE, Gravenstein JS (1998) Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International Study of Post-Operative Cognitive Dysfunction. Lancet 351:857–861. [DOI] [PubMed] [Google Scholar]
- Monk TG, Weldon BC, Garvan CW, Dede DE, van der Aa MT, Heilman KM, Gravenstein JS (2008) Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology 108:18–30. [DOI] [PubMed] [Google Scholar]
- Morgan TE, Xie Z, Goldsmith S, Yoshida T, Lanzrein AS, Stone D, Rozovsky I, Perry G, Smith MA, Finch CE (1999) The mosaic of brain glial hyperactivity during normal ageing and its attenuation by food restriction. Neuroscience 89:687–699. [DOI] [PubMed] [Google Scholar]
- Muller P, Rehfeld K, Schmicker M, Hokelmann A, Dordevic M, Lessmann V, Brigadski T, Kaufmann J, Muller NG (2017) Evolution of Neuroplasticity in Response to Physical Activity in Old Age: The Case for Dancing. Front Aging Neurosci 9:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson M, Perfilieva E, Johansson U, Orwar O, Eriksson PS (1999) Enriched environment increases neurogenesis in the adult rat dentate gyrus and improves spatial memory. J Neurobiol 39:569–578. [DOI] [PubMed] [Google Scholar]
- Nobili V, Bedogni G, Alisi A, Pietrobattista A, Rise P, Galli C, Agostoni C (2011) Docosahexaenoic acid supplementation decreases liver fat content in children with non-alcoholic fatty liver disease: double-blind randomised controlled clinical trial. Arch Dis Child 96:350–353. [DOI] [PubMed] [Google Scholar]
- Noronha SSR, Lima PM, Campos GSV, Chirico MTT, Abreu AR, Figueiredo AB, Silva FCS, Chianca DA Jr., Lowry CA, De Menezes RCA (2019) Association of high-fat diet with neuroinflammation, anxiety-like defensive behavioral responses, and altered thermoregulatory responses in male rats. Brain Behav Immun 80:500–511. [DOI] [PubMed] [Google Scholar]
- Park SE, Dantzer R, Kelley KW, McCusker RH (2011) Central administration of insulin-like growth factor-I decreases depressive-like behavior and brain cytokine expression in mice. J Neuroinflammation 8:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersson SD, Philippou E (2016) Mediterranean Diet, Cognitive Function, and Dementia: A Systematic Review of the Evidence. Adv Nutr 7:889–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips Bute B, Mathew J, Blumenthal JA, Welsh-Bohmer K, White WD, Mark D, Landolfo K, Newman MF (2003) Female gender is associated with impaired quality of life 1 year after coronary artery bypass surgery. Psychosom Med 65:944–951. [DOI] [PubMed] [Google Scholar]
- Ramel A, Martinez JA, Kiely M, Bandarra NM, Thorsdottir I (2010) Effects of weight loss and seafood consumption on inflammation parameters in young, overweight and obese European men and women during 8 weeks of energy restriction. Eur J Clin Nutr 64:987–993. [DOI] [PubMed] [Google Scholar]
- Rawji KS, Mishra MK, Michaels NJ, Rivest S, Stys PK, Yong VW (2016) Immunosenescence of microglia and macrophages: impact on the ageing central nervous system. Brain 139:653–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig MR, Bennett EL (1996) Psychobiology of plasticity: effects of training and experience on brain and behavior. Behav Brain Res 78:57–65. [DOI] [PubMed] [Google Scholar]
- Rozovsky I, Finch CE, Morgan TE (1998) Age-related activation of microglia and astrocytes: in vitro studies show persistent phenotypes of aging, increased proliferation, and resistance to down-regulation. Neurobiol Aging 19:97–103. [DOI] [PubMed] [Google Scholar]
- Safavynia SA, Goldstein PA (2018) The Role of Neuroinflammation in Postoperative Cognitive Dysfunction: Moving From Hypothesis to Treatment. Front Psychiatry 9:752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen A, Ojala J, Kaarniranta K, Haapasalo A, Hiltunen M, Soininen H (2011) Astrocytes in the aging brain express characteristics of senescence-associated secretory phenotype. Eur J Neurosci 34:3–11. [DOI] [PubMed] [Google Scholar]
- Satoh N, Shimatsu A, Kotani K, Himeno A, Majima T, Yamada K, Suganami T, Ogawa Y (2009) Highly purified eicosapentaenoic acid reduces cardio-ankle vascular index in association with decreased serum amyloid A-LDL in metabolic syndrome. Hypertens Res 32:1004–1008. [DOI] [PubMed] [Google Scholar]
- Segal JP, Tresidder KA, Bhatt C, Gilron I, Ghasemlou N (2018) Circadian control of pain and neuroinflammation. J Neurosci Res 96:1002–1020. [DOI] [PubMed] [Google Scholar]
- Shakersain B, Santoni G, Larsson SC, Faxen-Irving G, Fastbom J, Fratiglioni L, Xu W (2016) Prudent diet may attenuate the adverse effects of Western diet on cognitive decline. Alzheimers Dement 12:100–109. [DOI] [PubMed] [Google Scholar]
- Sharifian N, Manly JJ, Brickman AM, Zahodne LB (2019) Social network characteristics and cognitive functioning in ethnically diverse older adults: The role of network size and composition. Neuropsychology 33:956–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindi S, Kareholt I, Eskelinen M, Hooshmand B, Lehtisalo J, Soininen H, Ngandu T, Kivipelto M (2018) Healthy Dietary Changes in Midlife Are Associated with Reduced Dementia Risk Later in Life. Nutrients 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Lakhanpal D, Kumar S, Sharma S, Kataria H, Kaur M, Kaur G (2012) Late-onset intermittent fasting dietary restriction as a potential intervention to retard age-associated brain function impairments in male rats. Age (Dordr) 34:917–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Manchanda S, Kaur T, Kumar S, Lakhanpal D, Lakhman SS, Kaur G (2015) Middle age onset short-term intermittent fasting dietary restriction prevents brain function impairments in male Wistar rats. Biogerontology 16:775–788. [DOI] [PubMed] [Google Scholar]
- Skulas-Ray AC, Kris-Etherton PM, Harris WS, Vanden Heuvel JP, Wagner PR, West SG (2011) Dose-response effects of omega-3 fatty acids on triglycerides, inflammation, and endothelial function in healthy persons with moderate hypertriglyceridemia. Am J Clin Nutr 93:243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobesky JL, Barrientos RM, De May HS, Thompson BM, Weber MD, Watkins LR, Maier SF (2014) High-fat diet consumption disrupts memory and primes elevations in hippocampal IL-1beta, an effect that can be prevented with dietary reversal or IL-1 receptor antagonism. Brain Behav Immun 42:22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobesky JL, D’Angelo HM, Weber MD, Anderson ND, Frank MG, Watkins LR, Maier SF, Barrientos RM (2016) Glucocorticoids Mediate Short-Term High-Fat Diet Induction of Neuroinflammatory Priming, the NLRP3 Inflammasome, and the Danger Signal HMGB1. eNeuro 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparkman NL, Buchanan JB, Dos Santos NL, Johnson RW, Burton MD (2019) Aging sensitizes male mice to cognitive dysfunction induced by central HIV-1 gp120. Exp Gerontol 126:110694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer SJ, D’Angelo H, Soch A, Watkins LR, Maier SF, Barrientos RM (2017) High-fat diet and aging interact to produce neuroinflammation and impair hippocampal- and amygdalar-dependent memory. Neurobiol Aging 58:88–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer SJ, Basri B, Sominsky L, Soch A, Ayala MT, Reineck P, Gibson BC, Barrientos RM (2019) High-fat diet worsens the impact of aging on microglial function and morphology in a region-specific manner. Neurobiol Aging 74:121–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart KE, King AE, King NE, Collins JM, Vickers JC, Ziebell JM (2019) Late-life environmental enrichment preserves short-term memory and may attenuate microglia in male APP/PS1 mice. Neuroscience 408:282–292. [DOI] [PubMed] [Google Scholar]
- Sukhanov S, Higashi Y, Shai SY, Vaughn C, Mohler J, Li Y, Song YH, Titterington J, Delafontaine P (2007) IGF-1 reduces inflammatory responses, suppresses oxidative stress, and decreases atherosclerosis progression in ApoE-deficient mice. Arterioscler Thromb Vasc Biol 27:2684–2690. [DOI] [PubMed] [Google Scholar]
- Tajes M, Gutierrez-Cuesta J, Folch J, Ortuno-Sahagun D, Verdaguer E, Jimenez A, Junyent F, Lau A, Camins A, Pallas M (2010) Neuroprotective role of intermittent fasting in senescence-accelerated mice P8 (SAMP8). Exp Gerontol 45:702–710. [DOI] [PubMed] [Google Scholar]
- Tanaka N, Cortese GP, Barrientos RM, Maier SF, Patterson SL (2018) Aging and an Immune Challenge Interact to Produce Prolonged, but Not Permanent, Reductions in Hippocampal L-LTP and mBDNF in a Rodent Model with Features of Delirium. eNeuro 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, Zhao X, Sarruf DA, Izgur V, Maravilla KR, Nguyen HT, Fischer JD, Matsen ME, Wisse BE, Morton GJ, Horvath TL, Baskin DG, Tschop MH, Schwartz MW (2012) Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest 122:153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasconcelos AR, Yshii LM, Viel TA, Buck HS, Mattson MP, Scavone C, Kawamoto EM (2014) Intermittent fasting attenuates lipopolysaccharide-induced neuroinflammation and memory impairment. J Neuroinflammation 11:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss MW, Weng TB, Narayana-Kumanan K, Cole RC, Wharff C, Reist L, Dubose L, Sigurdsson G, Mills JA, Long JD, Magnotta VA, Pierce GL (2020) Acute Exercise Effects Predict Training Change in Cognition and Connectivity. Med Sci Sports Exerc 52:131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Cao M, Pu T, Huang H, Marshall C, Xiao M (2018) Enriched Physical Environment Attenuates Spatial and Social Memory Impairments of Aged Socially Isolated Mice. Int J Neuropsychopharmacol 21:1114–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng TB, Pierce GL, Darling WG, Falk D, Magnotta VA, Voss MW (2017) The Acute Effects of Aerobic Exercise on the Functional Connectivity of Human Brain Networks. Brain Plast 2:171–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2020) World health statistics 2020: monitoring health for the SDGs, sustainable development goals. In: (Organization WH, ed). [Google Scholar]
- Wu YT, Prina AM, Jones A, Matthews FE, Brayne C, Mrc C (2015) Older people, the natural environment and common mental disorders: cross-sectional results from the Cognitive Function and Ageing Study. BMJ Open 5:e007936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YT, Brayne C, Liu Z, Huang Y, Sosa AL, Acosta D, Prina M (2020) Neighbourhood environment and dementia in older people from high-, middle- and low-income countries: results from two population-based cohort studies. BMC Public Health 20:1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Gelyana E, Rajsombath M, Yang T, Li S, Selkoe D (2016) Environmental Enrichment Potently Prevents Microglia-Mediated Neuroinflammation by Human Amyloid beta-Protein Oligomers. J Neurosci 36:9041–9056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Gao H, Zhang L, Rong S, Yang W, Ma C, Chen M, Huang Q, Deng Q, Huang F (2019) Melatonin alleviates cognition impairment by antagonizing brain insulin resistance in aged rats fed a high-fat diet. J Pineal Res 67:e12584. [DOI] [PubMed] [Google Scholar]
- Ziegler-Waldkirch S, d’Errico P, Sauer JF, Erny D, Savanthrapadian S, Loreth D, Katzmarski N, Blank T, Bartos M, Prinz M, Meyer-Luehmann M (2018) Seed-induced Abeta deposition is modulated by microglia under environmental enrichment in a mouse model of Alzheimer’s disease. EMBO J 37:167–182. [DOI] [PMC free article] [PubMed] [Google Scholar]


