Abstract
Neuroimaging studies of childhood onset schizophrenia (COS), a rare yet severe form of schizophrenia with an onset before the age of 13 years, have shown continuity with adult onset schizophrenia. Previous research in adult patients has shown reduced sleep spindle activity, transient oscillations in the sleep electroencephalogram (EEG) generated through thalamocortical loops. The current study examines sleep spindle activity in patients with COS. Seventeen children and adolescents with COS (16 years ± 6.6) underwent overnight sleep EEG recordings. Sleep spindle activity was compared between patients with COS and age and gender matched controls and correlated with clinical symptom severity. We found pronounced deficits in sleep spindle amplitude, duration, density and frequency in patients with COS (effect size = 0.61 to 1.96; dependent on metric and EEG derivation). Non-rapid eye movement (NREM) sleep EEG power and coherence in the sigma band (11–16 Hz) corresponding to spindle activity were also markedly diminished in patients with COS as compared to controls. Furthermore, the degree of deficit in power and coherence of spindles was strongly associated with clinician rated hallucinations and positive symptoms over widespread cortical regions. Our finding of diminished spindle activity and its association with hallucinations likely reflect dysfunction of the thalamocortical circuits in children and adolescents with COS. Given the relative ease of sleep EEG recordings in vulnerable populations, this study highlights the potential of such recordings to characterize brain function in schizophrenia.
Keywords: schizophrenia, sleep, spindle, adolescent, biomarker
1. Introduction
An association between disrupted sleep and schizophrenia has been described since the early days of modern psychiatry (Bleuler, 1911; Kraepelin, 1919). Recently, the potential of sleep oscillations to gain insight into not only the sleep process, but also into the pathophysiology of schizophrenia has been recognized (Manoach et al., 2016; Ferrarelli and Tononi, 2017). In contrast to waking measures of neural activity, sleep oscillations are not influenced by fluctuations in attention, motivation, or the active presence of positive or negative symptoms and thus provide an “unbiased” measure of brain activity in psychiatric populations.
Sleep spindles are cardinal sleep oscillations that have been the focus of scientific inquiry in recent years and have been strongly implicated in the pathophysiology of schizophrenia. Sleep spindles are burst-like oscillations between 11 and 16 Hz generated through the interaction between inhibitory thalamic reticular nucleus (TRN) neurons and excitatory thalamocortical neurons (Krosigk et al., 1993), and synchronized by both thalamocortical and corticothalamic neurons (Fuentealba and Steriade, 2005). Therefore, sleep spindles are a unique measure of the integrity of the thalamocortical system (Steriade et al., 1993; Contreras et al., 1996; Timofeev and Steriade, 1996; Sanchez-Vives and McCormick, 2000; Steriade, 2006). Deficits in sleep spindles have been consistently reported in adult patients with schizophrenia. These deficits include diminished spindle amplitude (Ferrarelli et al., 2010, 2007; Manoach et al., 2014), spindle duration (Ferrarelli et al., 2010, 2007) and spindle density (Ferrarelli et al., 2007, 2010; Manoach et al., 2010; Wamsley et al., 2012; Manoach et al., 2014; Göder et al., 2015). Therefore, several features of sleep spindles are compromised in adult onset schizophrenia, and are often collectively referred to as sleep spindle activity. In addition to reduced sleep spindle activity, Wamsley et al. (2012) found sleep spindles across EEG derivations to be less coherent (i.e., less synchronized across regions) in adult patients with schizophrenia as compared to healthy controls, further indication of an anomaly in the thalamocortical system.
The degree to which spindle activity is diminished in adults with schizophrenia may be of functional significance as an association between spindle deficits and positive symptoms in patients has been demonstrated (Ferrarelli et al., 2010; Wamsley et al., 2012; Manoach et al., 2014). In particular, diminished spindle amplitude and spindle density have been associated with more severe hallucinations (Ferrarelli et al., 2010).
Importantly, current evidence suggests that the spindle deficits in schizophrenia are independent of medication status. For example, in the study by Ferrarelli et al. (2010), patients receiving antipsychotic medication not diagnosed with schizophrenia did not demonstrate a change in spindle activity suggesting that the observed effects in schizophrenia are not related to antipsychotic medication. In addition, a trend towards reduced sleep spindle activity in unaffected and unmedicated first-degree relatives of patients with schizophrenia has also been reported implying that reduced spindles in schizophrenia might be an endophenotype, independent of medication status, and confers susceptibility to developing the disorder (Manoach et al., 2014; D’Agostino et al., 2018).
In line with findings in adult schizophrenia, Tesler et al. (2015) reported reduced spindle density in a sample of nine 14- to 18-year-old adolescents with early onset schizophrenia (EOS), a form of the disorder with an onset before the age of 18 years. Like EOS, childhood onset schizophrenia (COS), defined as an onset before 13 years of age, usually presents with a more severe course and greater progressive brain abnormalities as compared to adult onset schizophrenia (Childs and Scriver, 1986; Sporn et al., 2003; Greenstein et al., 2006; Gogtay, 2008). Though rare, with a prevalence of 1 in 40’000 individuals suffering from this disorder, neuroimaging (reviewed in Gogtay, 2008; Ordóñez et al., 2016) and genetic (reviewed in Addington and Rapoport, 2009; Asarnow and Forsyth, 2013) studies of COS have furthered our understanding of the pathophysiology of schizophrenia.
Thus, the aim of the current study is to examine sleep spindle activity and its relation to clinical symptoms in children and adolescents diagnosed with COS as part of the National Institute of Mental Health (NIMH) COS study (Gordon et al., 1994; McKenna et al., 1994; Kumra et al., 1996; Nicolson and Rapoport, 1999; Driver et al., 2013). As in previous studies on the neurobiology of COS, which show that COS is continuous with adult onset schizophrenia, we hypothesize that sleep spindle activity will be diminished in those with COS. Based on previous findings in adult patients with schizophrenia (Ferrarelli et al., 2010; Wamsley et al., 2012; Manoach et al., 2014), we hypothesize that lower sleep spindle activity will be associated with greater severity of positive symptoms and hallucinations in particular. Furthermore, we expect that exploratory correlations of sleep spindle activity with general functioning as measured via Children’s Global Assessment Scale (CGAS) will result in positive correlation coefficients suggesting that greater reductions in the spindle range are associated with greater impairment in overall functioning.
2. Materials and Methods
2.1. Patients with COS
The current study included 17 children and adolescents aged 9 to 21 years (mean age = 16; SD = 3.6; 12 females; for age and gender distribution see Table 1) who were recruited as part of the longitudinal study of COS at the NIMH (Gordon et al., 1994; McKenna et al., 1994; Kumra et al., 1996; Nicolson and Rapoport, 1999; Driver et al., 2013). Potential participants who met DSM-III-R or DSM-IV (American Psychiatric Association, 2013) criteria for schizophrenia with the onset of psychotic symptoms before the age of 13 years were nationally recruited. At the screening interview, patients who were likely to meet criteria for COS were offered an in-patient stay to confirm diagnosis. Two child psychiatrists confirmed diagnosis after extensive in-patient observation, which included a one- to three-week medication washout period. During the stay, structured psychiatric interviews were conducted using Children’s Global Assessment Scale (CGAS) and Scale for the Assessment of Positive Symptoms (SAPS). Individual values for these scales along with demographic information (e.g., age, gender, duration of illness and age at onset) are available in Table 1. Individuals with a pre-psychotic IQ of less than 70 or major neurological or medical conditions or other psychiatric diagnoses as the main focus of treatment were excluded. Whole-night polysomnography (PSG) recordings were performed at the National Institute of Mental Health in Bethesda. Eleven patients were assessed with the TWin system (Astromed, Grass, West Warwick, RI) and six with the Nihon Kohden system (Nihon Kohden, Tokyo, Japan), both with a sampling rate of 200 Hz. At the time of PSG assessments, all but one patient were visiting the NIMH for a follow-up visit, and therefore receiving antipsychotic medication, typically clozapine, with a chlorpromazine equivalent ranging between 150 and 1300 mg daily (mean = 677; SD = 325; for individual values see Table 1). The institutional review board of the NIMH approved the study. Written assent was obtained from all patients and informed consent was obtained from their parents or legal guardians.
Table 1:
Age, gender, chlorpromazine equivalents of medication (mg), age at onset of psychosis, duration of illness (years) and clinical ratings of individual subjects. The order of the patients is as shown in Figure 2 (i.e., the first bar in these plots corresponds to a 13-year-old female). Mean and standard deviation are depicted in the last row. PSG: polysomnography; CGAS: Children’s Global Assessment Scale (higher score means better overall functioning, i.e. 100 = Superior functioning, 50 = Moderate degree of interference in functioning, 10 = Needs constant supervision); SAPS: Scale for the Assessment of Positive Symptoms (0 = None/Not at all, 1 = Questionable, 2 = Mild, 3 = Moderate, 4 = Marked, 5 = Severe; 34 items).
| Patient | Gender | Age at PSG | Medication (mg) | Age at Onset | Duration of Illness (y) | CGAS | SAPS Sum | SAPS Global Rating of Hallucinations |
|---|---|---|---|---|---|---|---|---|
| 1 | Female | 13 | 900 | 9 | 4 | 8 | 28 | 3 |
| 2 | Male | 17 | 1200 | 5 | 12.9 | 33 | 41 | 5 |
| 3 | Female | 15 | 600 | 11 | 4.6 | 35 | 23 | 1 |
| 4 | Female | 13 | 550 | 11 | 2.6 | 36 | 23 | 4 |
| 5 | Female | 18 | 700 | 9 | 9.5 | 35 | 32 | 3 |
| 6 | Female | 9 | 900 | 8 | 1.7 | 22 | 22 | 5 |
| 7 | Male | 21 | 250 | 11 | 10.1 | 55 | 1 | 0 |
| 8 | Male | 12 | None | 11 | 1.7 | 10 | 40 | 5 |
| 9 | Female | 17 | 400 | 10 | 7.6 | 35 | 5 | 0 |
| 10 | Female | 19 | 750 | 12 | 7.2 | 45 | 10 | 2 |
| 11 | Female | 12 | 150 | 9 | 3.8 | 40 | 17 | 3 |
| 12 | Female | 20 | 1000 | 10 | 10.7 | 55 | 20 | 4 |
| 13 | Female | 15 | 300 | 10 | 5.3 | 45 | 0 | 0 |
| 14 | Male | 21 | 1300 | 11 | 11 | 50 | 9 | 0 |
| 15 | Female | 15 | 650 | 11 | 4.1 | 45 | 10 | 2 |
| 16 | Female | 21 | 1100 | 10 | 11.3 | 60 | 54 | 4 |
| 17 | Male | 14 | 600 | 8 | 6.3 | 35 | 36 | 3 |
| Mean (±SD) | 16.00 (±3.64) | 709.38 (±340.69) | 9.76 (±1.68) | 6.73 (±3.63) | 37.88 (±14.50) | 21.82 (±15.29) | 2.59 (±1.84) |
2.2. Healthy Control
Seventeen age and gender matched healthy controls were selected from two studies. Nine controls’ PSGs were recorded via a Geodesics EEG system (GSN300; Electrical Geodesic Inc., Eugene, OR, USA) with a sampling rate of 1000 Hz (down-sampled to 250 Hz for analysis). The remaining 8 controls’ PSGs were recorded on the Synamps EEG system (Neuroscan Inc., El Paso, TX, USA) with a sampling rate of 500 Hz. All data were processed and analyzed at the University Hospital of Child and Adolescent Psychiatry and Psychotherapy in Bern using the same procedures. To ensure compatibility across systems, all EEG data were re-referenced to linked mastoids (i.e., the average of the signals at the left and the right mastoid). This adjustment allowed for the comparison of sleep spindle characteristics obtained from PSG across recording sites, as has been previously demonstrated (Purcell et al., 2017). All study procedures were approved by the responsible ethics committee and performed according to the Declaration of Helsinki.
2.3. PSG Data Analysis
PSG data were visually scored in 30-s epochs applying standard criteria (Rechtschaffen and Kales, 1968) with consensus agreement between two scorers. Ten derivations (F3, FZ, F4, C3, C4, P3, PZ, P4, O1, O2) were included in the analyses. Epochs with artifacts were detected and excluded using a semi-automated procedure based on power in the low (0.8–4.6 Hz) and high (20–40 Hz) frequencies (Buckelmüller et al., 2006). All EEG signal processing was performed in MATLAB (Mathworks, Natick MA, USA).
2.4. Quantification of Sleep Spindles
We used several methods to quantify the strength of sleep spindle activity. First, power density spectra were calculated for each 30-s epoch (average of six 5-s windows; Hanning window; no overlap; frequency resolution 0.2 Hz) and averaged across NREM sleep. NREM sleep epochs were defined as all epochs scored as either stage 2 or SWS, as the differences between these stages can be minimal and highly subjective. Furthermore, sleep spindles may also occur during SWS. Because the duration of sleep will impact the quantification of sleep oscillations, the maximal common number of NREM sleep epochs for each patient-control pair was used for analysis. Power in the sigma frequency band (11 to 16 Hz), which has been shown to correspond to sleep spindle activity (Warby et al., 2014), was quantified. In addition to this measure, we analyzed sigma power at each derivation normalized by the total power across derivations to examine topographic characteristics of sigma power that may have been masked by absolute power.
We used a similar procedure to calculate coherence, a measure of connectivity, in the sigma frequency band for all possible channel pairs (i.e., 30-s epochs; 5-s Hanning windows with no overlap). Coherence of two signals (i.e., EEG derivations), and , is defined as where is the cross-spectral density and and are the auto-spectral density functions of the two signals (Bendat and Piersol, 2010).
In addition to power and coherence in the sigma band during NREM sleep, we used a measure of sigma peak power/coherence which quantifies the sigma peak relative to the background spectrum (modified from Gottselig et al. (2002)). An algorithm was used to detect the peak and the local minima of the NREM power/coherence spectra in the sigma band. A line was fit between the minimum before the peak and the last point in the frequency range of interest (i.e., 16.2 Hz; Suppl. Figure S1). The distance between peak power/coherence and this line was used as an index of relative sigma peak power/coherence and will be referred to as NREM sigma peak power/coherence throughout this manuscript.
In a next step, we used an automatic spindle detection algorithm as described by Ferrareli et al. (2007) to detect and characterize individual spindles. The frequency, amplitude and duration of each sleep spindle were computed for each EEG derivation and a mean value in addition to density (spindles/min) was calculated for each subject. This analysis was performed for slow (10–12 Hz) and fast (12–16 Hz) spindles as well as the whole range (10–16 Hz) separately, because functional and topographic differences have previously been observed with regards to these spindle classes (Ferrarelli et al., 2010). Since children demonstrate lower spindle frequencies as compared to adults, we set the lower limit at 10 Hz based on these observations (Shinomiya et al., 1999; Chatburn et al., 2013; Sheldon, 2014; Rusterholz et al., 2018).
2.5. Statistical Analysis
A one-way analysis of variance (ANOVA) was performed on the following sleep stage variables to assess differences between patients and controls: total sleep time (time in minutes scored as stage 2, slow wave or REM sleep), wake after sleep onset (wake time in minutes between the first and the last sleep epoch), sleep latency (time in minutes until the first occurrence of stage 2 sleep), sleep efficiency (total sleep time divided by total time in bed), REM sleep latency (time in minutes from sleep onset until the first occurrence of REM sleep), and percent of stage 2, slow wave and REM sleep.
A bootstrap analysis was used to compare patients with COS to controls for the sleep EEG measures described in 2.4 (NREM sigma power, NREM sigma peak power, NREM sigma coherence, NREM sigma peak coherence, as well as spindle amplitude, frequency, duration and density) at each derivation. The bootstrap statistic is based on random sampling from the original data pool, which included both patients and controls. In an iterative procedure two subsets of data with the same size as our original groups (i.e., n = 17) were randomly resampled from this data pool and the difference between them was calculated to create a distribution of differences. The actual difference was then compared to the corresponding bootstrap distribution obtained from random sampling. The difference between patients and controls was considered statistically significant if the actual difference was larger than 95% of the distribution. This procedure was used for all EEG comparisons. The bootstrap statistic is often used in EEG analysis and controls for multiple comparisons (Maris and Oostenveld, 2007). Effect sizes were calculated by means of Cohen’s d (Cohen, 1992).
We also examined the association between clinical symptom severity and sleep spindle activity in the patient group only. We correlated clinical ratings obtained closest to the time point of the PSG assessment by means of the partial r while controlling for age, gender and IQ. In order to control for multiple comparisons, a bootstrap statistic was used to compare the obtained r-value to a randomly generated distribution of r-values obtained through random sampling. For the majority of patients with COS (n = 12) the time between the clinical assessment and the PSG recording was between 0 and 3 days (mean = 1; SD = 1.28), however for 5 patients we only had older clinical ratings 647 to 1661 days before the PSG assessment (mean = 1012; SD = 411). To limit the number of statistical tests, we focused on symptoms that have previously been shown to be associated with sleep spindles. These include positive symptom severity (Ferrarelli et al., 2010; Wamsley et al., 2012; Manoach et al., 2014; Tesler et al., 2015) as assessed by the sum score on the SAPS, and global ratings of hallucinations (Ferrarelli et al., 2010) from the SAPS. In addition, we also examined the total score for general functioning reported by the CGAS in order to examine the degree to which sleep spindles were associated with specific symptoms versus overall functioning.
In order to test the impact of medication, duration of illness and age of onset of psychosis on sleep spindle activity, we performed correlations between these demographic variables and sleep EEG measures. A bootstrap statistic was used to control for multiple comparisons as described above with a correlation instead of a subtraction as the core operation. The results were considered significant if the magnitude of the observed r-value was greater than 95% of the bootstrap distribution of r-values. We made use of either Pearson’s or Spearman’s correlation coefficient, dependent on the distribution of data. We interpret findings occurring at more than a single derivation as biologically meaningful and only those are reported here.
3. Results
3.1. Sleep Stage Variables
ANOVA analysis of sleep stage variables revealed no significant differences between patients and controls (Suppl. Table S1).
3.2. Sleep Spindle Activity
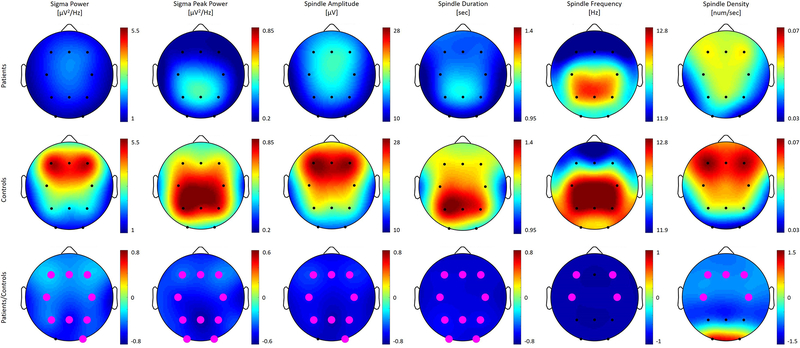
Controls had significantly more NREM sigma power at all derivations (effect size = 0.66 to 1.39) except for O1 (p = 0.07), which showed a trend towards significance (first column of Figure 1). The analysis of normalized NREM sigma power revealed a topographic shift towards central regions in patients with significant differences at all derivations except for O1 (p = 0.15) and O2 (p = 0.06) (Suppl. Figure S2; effect size = 0.04 to 1.24).
Figure 1:
Topographic distribution across derivations for all metrics of spindle activity, i.e. non-rapid eye movement (NREM) sigma power, NREM sigma peak power, spindle amplitude, duration, frequency and density. The first and the second row depict power values averaged for patients and controls separately and plotted on the same scale. The third row shows the ratio of average power for patients divided by control with statistically significant electrodes in magenta. Note that these plots are on a different scale with negative values (in cool colors) representing decreased power in patients and positive values (in warm colors) indicating enhanced power in patients as compared to controls. We observed significant decreases in patients across the brain for all metrics shown.
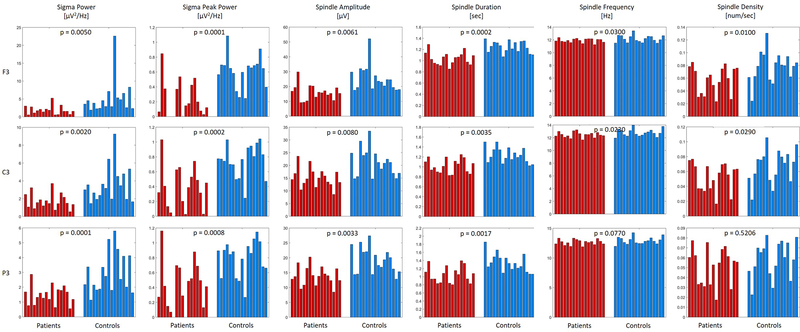
We found no significant differences between the two spindle classes – slow and the fast spindles – with regards to our group comparisons, and, therefore, focus on combined data from both classes (i.e., 10–16 Hz). NREM sigma peak power, spindle amplitude, and spindle duration were significantly diminished in patients as compared to controls across nearly all scalp derivations (Figure 1; second to fourth column; effect size = 0.63 to 1.96). In contrast to the topographically widespread differences with regards to the aforementioned spindle parameters, spindle density (number of spindles/sec) and spindle frequency significantly differed between patients and controls only at frontal and central derivations (F3, FZ, F4, C3 and C4; Figure 1; fifth and sixth column; effect size = 0.62 to 0.92). In order to illustrate these differences on a subject level, we plot individual values for both groups in Figure 2.
Figure 2:
Individual values for spindle metrics in patients (shown in red) and controls (shown in blue) for three derivations over the left hemisphere (Frontal = F3, Central = C3, and Parietal = P3). The p-values of the bootstrap statistic between patients and controls are shown at the top of every chart. The columns are ordered such that the patients correspond to the age and gender matched control (e.g., the first column in the patient and control group are age and gender matched).
3.3. Sleep Spindle Coherence
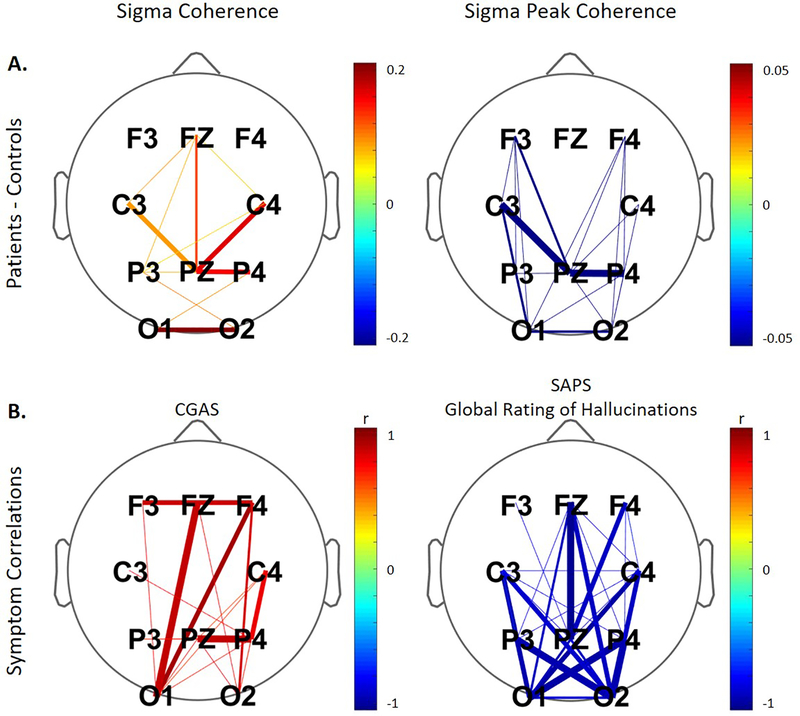
NREM sigma coherence was significantly increased in patients as compared to controls across regions reflecting a pattern of hyper-connectivity in patients (Figure 3; panel A left; 11 significant connections; 0.001 ≤ p ≤ 0.045; effect size = 0.66 to 1.31). In contrast, NREM sigma peak coherence, which only takes into account the sigma peak in the coherence spectrum, was significantly diminished in patients across derivations reflecting hypo-connectivity in patients (Figure 3; panel A right; 20 significant connections; 0.0001 ≤ p ≤ 0.044; effect size = 0.61 to 1.34). Thus, although sigma coherence was increased in patients, the sigma peak typically seen in the coherence spectrum of healthy populations is reduced in patients with COS. Therefore, when background activity is subtracted and only the sigma peak is taken into account, patients show diminished coherence.
Figure 3:
A. Connections significantly different between patients and controls for non-rapid eye movement (NREM) sigma coherence and NREM sigma peak coherence. Negative values (in cool colors) represent decreased coherence in patients and positive values (in warm colors) indicating enhanced coherence in patients as compared to controls. B. Significant correlations between coherence and clinical ratings for the patient group. The color corresponds to the direction of correlation with warm tones for positive and cool tones for negative correlations. The line thickness reflects the four levels of significance (p < 0.05; p < 0.01; p < 0.005; p < 0.001) with increasing thickness for higher significance in all plots. Non-significant connections are not depicted. We note that for global ratings of hallucinations from the Assessment of Positive Symptoms (SAPS) larger values indicate more impairment, while on the Children’s Global Assessment Scale (CGAS), smaller numbers correspond to greater impairment.
3.4. Association between Sleep Spindles and Clinical Ratings
We examined the association between the following symptoms and sleep EEG measures in the patient sample: global rating of hallucinations from the SAPS, total score from the SAPS and the CGAS. The individual values for these measures are shown in Table 1, while the results from the correlation analysis are summarized in Tables 2 and 3. Because in five of our subjects clinical ratings took place more than three days before the PSG, we additionally conducted all analyses excluding these subjects, which yielded similar results.
Table 2:
Significant correlations of sleep EEG power and coherence with clinical measures including all patients. For every correlation, the range of r- and p-values with the number of significant electrodes in brackets are reported. Ranges for positive and negative r-values and the corresponding p-values are depicted separately (only applicable for NREM Sigma Peak Coherence and SAPS Sum). NREM: non-rapid eye movement sleep; CGAS: Children’s Global Assessment Scale (higher score means better overall functioning, i.e. 100 = Superior functioning, 50 = Moderate degree of interference in functioning, 10 = Needs constant supervision); SAPS: Scale for the Assessment of Positive Symptoms (0 = None/Not at all, 1 = Questionable, 2 = Mild, 3 = Moderate, 4 = Marked, 5 = Severe; 34 items).
| NREM Sigma Power | NREM Sigma Peak Power | NREM Sigma Coherence | NREM Sigma Peak Coherence | |||||
|---|---|---|---|---|---|---|---|---|
| r-value | p-value | r-value | p-value | r-value | p-value | r-value | p-value | |
| Age of Onset | / | / | / | / | 0.52 | 0.03 (1) | / | / |
| Duration of Illness | −0.57 ≤ r ≤ −0.55 | 0.02 ≤ p ≤ 0.03 (2) | / | / | −0.67 ≤ r ≤ −0.49 | 0.005 ≤ p ≤ 0.04 (11) | / | / |
| Medication Dosage | / | / | / | / | / | / | / | / |
| CGAS | 0.71 | 0.03 (1) | / | / | 0.64 ≤ r ≤ 0.94 | 0.0001 ≤ p ≤ 0.049 (18) | 0.71 ≤ r ≤ 0.83 | 0.01 ≤ p ≤ 0.04 (3) |
| SAPS Sum | −0.71 | 0.03 (1) | −0.85 ≤ r ≤ −0.69 | 0.001 ≤ p ≤ 0.04 (10) | −0.83 ≤ r ≤ −0.73 | 0.01 ≤ p ≤ 0.04 (8) | −0.94 ≤ r ≤ −0.72; r = 0.76 | 0.001 ≤ p ≤ 0.04; p = 0.046 (20) |
| SAPS Global Rating of Hallucinations | / | / | −0.89 ≤ r ≤ −0.73 | 0.001 ≤ p ≤ 0.04 (9) | −0.74 ≤ r ≤ −0.70 | 0.02 ≤ p ≤ 0.03 (2) | −0.98 ≤ r ≤ −0.67 | 0.0001 ≤ p ≤ 0.0496 (27) |
Table 3:
Significant correlations between spindle features and clinical measures including all patients. For every correlation, the range of r- and p-values with the number of significant electrodes in brackets are reported. NREM: non-rapid eye movement sleep; CGAS: Children’s Global Assessment Scale (higher score means better overall functioning, i.e. 100 = Superior functioning, 50 = Moderate degree of interference in functioning, 10 = Needs constant supervision); SAPS: Scale for the Assessment of Positive Symptoms (0 = None/Not at all, 1 = Questionable, 2 = Mild, 3 = Moderate, 4 = Marked, 5 = Severe; 34 items).
| Spindle Amplitude | Spindle Duration | Spindle Frequency | Spindle Density | |||||
|---|---|---|---|---|---|---|---|---|
| r-value | p-value | r-value | p-value | r-value | p-value | r-value | p-value | |
| Age of Onset | / | / | / | / | / | / | / | / |
| Duration of Illness | −0.65 ≤ r ≤ −0.59 | 0.006 ≤ p ≤ 0.01 (2) | / | / | / | / | / | / |
| Medication Dosage | / | / | / | / | −0.49 | 0.04 (1) | / | / |
| CGAS | 0.74 | 0.02 (1) | 0.678 ≤ r ≤ 0.683 | 0.038 ≤ p ≤ 0.041 (2) | / | / | / | / |
| SAPS Sum | −0.68 | 0.04 (1) | −0.67 | 0.04 (1) | −0.83 ≤ r ≤ −0.76 | 0.002 ≤ p ≤ 0.02 (2) | −0.67 | 0.047 (1) |
| SAPS Global Rating of Hallucinations | / | / | −0.76 ≤ r ≤ −0.68 | 0.02 ≤ p ≤ 0.03 (4) | −0.71 ≤ r ≤ −0.66 | 0.041 ≤ p ≤ 0.047 (2) | −0.73 | 0.02 (1) |
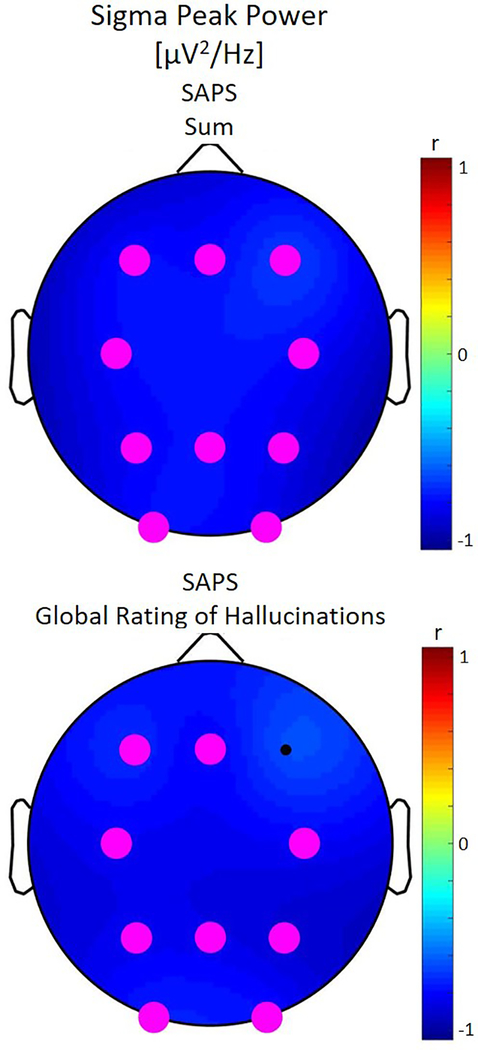
Sigma power was not correlated with any clinical symptoms, however, lower sigma peak power was associated with higher global ratings of hallucinations and higher scores of the SAPS total score across derivations (Figure 4). At a few derivations, patients with shorter duration (derivations F3, FZ, P3 and P4) and slower frequency (derivations P4 and O2) of spindles had more hallucinations.
Figure 4:
Correlations between non-rapid eye movement (NREM) sigma peak power and clinical ratings for the patient group. The color corresponds to the direction of correlation with cool tones for negative correlations. Statistically significant electrodes are depicted in magenta. We note that for global ratings of hallucinations and the sum from the Assessment of Positive Symptoms (SAPS) larger values indicate more impairment. Lower sigma peak power was significantly associated with more severe hallucinations and positive symptoms across the brain.
Panel B in Figure 3 shows findings with regards to coherence and those connections that manifested significant correlations with clinical ratings. Higher NREM sigma coherence was associated with better global functioning as assessed by the CGAS, but not correlated with hallucinations or positive symptoms. On the other hand, less sigma peak coherence was associated with more severe hallucinations and more positive symptoms (sum of SAPS; data not shown; 20 significant connections) across regions. We observed no further significant correlations of sleep EEG measures with clinical ratings.
We found no significant correlations between sleep EEG measures and age of onset, duration of illness or medication dosage. The only exception was the negative correlation of spindle amplitude, as well as NREM sigma power and coherence with duration of illness. Lower amplitude, power and coherence were associated with longer duration of illness.
4. Discussion
In this study we examine sleep spindle activity in a sample of children and adolescents with childhood onset schizophrenia and find marked reduction in spindle activity, the magnitude of which is associated with clinical rating of positive symptoms. Our study adds to and extends the existing literature on sleep in early onset and adult onset schizophrenia by showing that sleep deficits in COS are more pronounced, thus, supporting the notion that COS is a continuous but more severe form of the adolescent and adult manifestation of the disorder.
Although disrupted sleep is commonly reported by patients with schizophrenia, when examining PSG derived sleep architecture, we found no significant differences between the two groups. We note that a previous study (Mattai et al., 2006) of sleep in COS based on nursing staff observations found evidence of disrupted and short sleep during a medication free period and therefore it is likely that the medications taken in the current sample increase sleep duration and diminish sleep disruption.
With regards to sleep neurophysiology, deficits in sleep spindles have previously been reported in adults with schizophrenia. Similar to studies in adults, we found diminished sigma power (Ferrarelli et al., 2007, 2010; Manoach et al., 2010; Wamsley et al., 2012; Manoach et al., 2014; Göder et al., 2015), spindle duration (Ferrarelli et al., 2010, 2007), spindle amplitude (Ferrarelli et al., 2010, 2007; Manoach et al., 2014), and spindle density (Ferrarelli et al., 2007, 2010; Manoach et al., 2010; Wamsley et al., 2012; Manoach et al., 2014; Göder et al., 2015) in patients with COS as compared to controls. However, in contrast to studies in adults with schizophrenia (Ferrarelli et al., 2007, 2010; Manoach et al., 2010; Wamsley et al., 2012; Manoach et al., 2014) and patients with EOS (Tesler et al., 2015), which find diminished spindle activity over prescribed regions, the deficits observed in patients with COS were topographically widespread. This finding is in line with the current understanding of COS as a more severe form of schizophrenia that is nonetheless continuous with adult onset schizophrenia (e.g., Driver et al., 2013). Moreover, we observed a topographic shift of normalized sigma power towards central regions in patients as compared to controls. As the topography observed in patients can be described as more developmentally advanced (Kurth et al., 2010), the observed shift may further index the accelerated development in schizophrenia as has previously been posited (Feinberg, 1982; Gogtay, 2008; Driver et al., 2013; Rapoport and Gogtay, 2011).
The spindle deficits in schizophrenia suggest dysfunction in the thalamocortical pathways responsible for the generation of spindles (Steriade et al., 1993). Specifically, the thalamic reticular nucleus (TRN) serves as a pacemaker for spindles (Fuentealba and Steriade, 2005), but both thalamocortical and corticothalamic neurons are involved in the initiation and synchronization of spindles. Therefore, as hypothesized by Ferrarelli et al. (2017), we conclude that the deficits in spindles indicate dysfunction of the TRN in patients with COS.
Despite this, a small subset of patients showed spindle integrity on a level similar to their age and gender matched peers (Figure 2). The variability in spindle activity across patients is mirrored in the clinical ratings and may reflect heterogeneous presentation of the disorder, which are rooted in distinct neuronal dysfunctions. For example, we found lower spindle activity (peak power and duration) was associated with higher hallucination scores. This finding is in line with previous work in adults reporting a negative correlation between measures of spindle activity and hallucinations (Ferrarelli et al., 2010). In addition to playing a pivotal role in the generation of spindles, the TRN is involved in sensory gating of auditory and visual inputs (Krause et al., 2003; McAlonan et al., 2008). Therefore, the association between spindle activity and hallucinations may also reflect TRN function (Ferrarelli and Tononi, 2011).
We found increased levels of absolute NREM sigma coherence concurrent with reduced relative NREM sigma coherence in patients with COS as compared to controls. We note that absolute and relative NREM sigma coherence measure two distinct phenomena. The reduction in sleep spindle activity in patients with COS, reflected in decreased NREM sleep sigma power, sigma peak power, and spindle amplitude, duration, frequency and density, is most likely mirrored in decreased NREM sigma peak coherence. On the other hand, absolute NREM sleep EEG coherence in all bands, including the sigma band, is increased in patients with COS as compared to controls (Markovic et al., 2020) and, therefore, likely reflects increased background coherence. This greater absolute coherence in patients may signal a pattern of hyper-connectivity and is in agreement with wake EEG coherence studies in adult patients with schizophrenia (Ford et al., 1986; Merrin et al., 1989; Nagase et al., 1992).
Greater sigma peak coherence, in addition to greater NREM sigma peak power, was associated with fewer hallucinations and positive symptoms, adding further support for aberrant TRN functioning in schizophrenia (reviewed in Ferrarelli and Tononi, 2011). In contrast, we observed a positive correlation between absolute NREM sigma coherence and better global functioning (CGAS) scores suggesting that background levels of coherence may play a role in overall wellbeing and serve as a compensatory mechanism as previously suggested in MRI connectivity studies in adult patients with schizophrenia (Bracht et al., 2014; Cavelti et al., 2018).
In an exploratory report (Markovic et al., 2020), we present data for other frequency bands (i.e., delta (1.6 – 4.8 Hz), theta (5 – 8.4 Hz), alpha (8.6 – 11 Hz), beta 1 (16.4 – 20.2 Hz) and beta 2 (20.4 – 24.2 Hz)) and sleep states (i.e., NREM and REM sleep) in the same sample of participants. We found decreased sleep EEG power in patients compared to controls in both states (i.e., NREM and REM sleep) for beta 1 and beta 2 bands. The state non-specificity of this finding suggests that the decline in beta power may be due to anatomical changes in COS patients compared to controls. On the other hand, sleep EEG coherence was increased in patients across frequencies and in both states; thus, the increase in absolute coherence observed in the NREM sigma band in the current study is likely a more global phenomenon, reflecting overall hyper-connectivity in patients with COS compared to controls.
Our findings may also be of clinical relevance. COS is difficult to diagnose with approximately 30 to 50% of patients with affective disorders misdiagnosed as COS (Driver et al., 2013). At the NIMH more than 90% of patients referred to the study of COS receive alternate diagnoses, some only after rigorous testing and interviews following a medication washout period (Driver et al., 2013). Given that sleep EEG recordings are generally well tolerated in vulnerable populations and non-invasive, they may represent a powerful adjunct diagnostic tool readily available to most clinicians. One caveat is that although deficits in spindles are absent in affective disorders (Ferrarelli et al., 2010), preliminary evidence suggests that disorders, such as autism, which share genetic (e.g., Gandal et al., 2018) and neurobiological (e.g., de Lacy and King, 2013) bases with schizophrenia, also manifest aberrant spindles (Godbout et al., 2000; Limoges et al., 2013; Tessier et al., 2015). Future studies directly comparing spindles in COS and other disorders are needed to determine whether there are markers unique to COS (e.g., coherence).
Several limitations of the study are important to note. For one, our sample size and the broad age range may have masked weaker effects. The fact that we find large effect sizes across a broad age range is noteworthy given the marked developmental decline in power across adolescence (Feinberg et al., 1990; Campbell and Feinberg, 2009; Tarokh and Carskadon, 2010; Carskadon and Tarokh, 2014). Medication effects pose another limitation to this study given that all except one patient were taking medication at the time of the sleep study. This confound was unavoidable given that a diagnosis of COS is accompanied by significant impairment and the questionable ethics of washing out subjects once they have such a diagnosis. However, we find no association with medication dosage for any spindle metric, which is in agreement with previous research showing that diminished sleep spindle activity and coherence are highly unlikely to be due to medication (Ferrarelli et al., 2007, 2010; Wamsley et al., 2012; Merrin et al., 1989) (for a summary, see Castelnovo et al. (2017)). The few studies demonstrating an effect of such medication on sleep spindles have found increases in spindle activity (Tsekou et al., 2015) associated with the medication, which, thus, cannot account for the dramatic reduction of sleep spindle activity we observed in patients with COS. Furthermore, correlating sleep EEG measures with clinical ratings more than a few days prior to the sleep recording in a subset of our subjects might be problematic due to possible symptom fluctuations over the time period between clinical assessments and the PSG. However, after excluding these subjects we observed similar but less significant correlations implying that the correlations we report including all subjects are rather reliable and we only lack statistical power to detect them in the subset of patients with clinical ratings closely matching the timing of the PSG assessments. Finally, we acknowledge that data was acquired on four different EEG systems, however, since we only examine the sigma band and not low or high frequencies (these frequencies may be affected by differential filters), the data across all recording systems are directly comparable. Previous studies have shown the success of combining data across multiple sites to examine sleep spindle activity (Purcell et al., 2017). Despite these factors we believe that this study makes a significant scientific contribution to the literature on schizophrenia due to the rarity of COS and the large effect sizes observed.
To conclude, we describe marked deficits in the amplitude, duration, density and frequency of sleep spindles in patients with COS, similar to but more pronounced than those observed in adult onset schizophrenia. This finding adds to the mounting literature characterizing COS as a continuous but more severe form of adult onset schizophrenia. Furthermore, the strong association of sleep spindles with hallucinatory symptoms highlights the potential role of the thalamocortical system in general, and TRN neurons in particular, in hallucinatory phenomena. Given the relative ease of sleep EEG recordings in vulnerable populations, this study highlights the potential of such recordings to characterize brain function and potentially aid in diagnosis of schizophrenia.
Supplementary Material
Supplementary Figure S1: The non-rapid eye movement (NREM) log-transformed power spectrum (left panel) and the NREM coherence spectrum (right panel) for one of the control subjects demonstrating the procedure to calculate sigma peak power/coherence. The function findpeaks in MATLAB was used to find the peak in the sigma range (11.2 to 16.2 Hz). The local minimum before the peak was then detected and served as the starting point of the fitting line (shown in red) while the endpoint of the line was the last point in the frequency range of interest (i.e., 16.2 Hz). The distance between this line and the peak point was calculated (shown in black) and this served as a measure of relative sigma peak power/coherence.
Supplementary Figure S2: Topographic distribution of sigma power normalized by the total power across derivations during non-rapid eye movement (NREM) sleep. The first and the second row depict power values averaged for patients and controls separately and plotted on the same scale. The third row shows the ratio of average power for patients divided by control with statistically significant electrodes in magenta. Note that this plot is on a different scale with negative values (in cool colors) representing decreased power in patients and positive values (in warm colors) indicating enhanced power in patients as compared to controls. We observed a topographic shift towards central regions in patients as compared to controls.
References
- Addington AM, Rapoport JL, 2009. The genetics of childhood-onset schizophrenia: When madness strikes the prepubescent. Curr. Psychiatry Rep. 11, 156–161. 10.1007/s11920-009-0024-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association, 2013. Diagnostic and Statistical Manual of Mental Disorder. American Psychiatric Association, Washington, DC. [Google Scholar]
- Asarnow RF, Forsyth JK, 2013. Genetics of Childhood-onset Schizophrenia. Child Adolesc. Psychiatr. Clin 22, 675–687. 10.1016/j.chc.2013.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendat JS, Piersol AG, 2010. Random Data: Analysis and Measurement Procedures, Wiley Series in Probability and Statistics. John Wiley & Sons, Inc, Hoboken, NJ, USA: 10.1002/9781118032428 [DOI] [Google Scholar]
- Bleuler E, 1911. Dementia Praecox or the Group of Schizophrenias. International Universities Press, New York. [Google Scholar]
- Bracht T, Horn H, Strik W, Federspiel A, Razavi N, Stegmayer K, Wiest R, Dierks T, Müller TJ, Walther S, 2014. White matter pathway organization of the reward system is related to positive and negative symptoms in schizophrenia. Schizophr. Res 153, 136–142. 10.1016/j.schres.2014.01.015 [DOI] [PubMed] [Google Scholar]
- Buckelmüller J, Landolt H-P, Stassen HH, Achermann P, 2006. Trait-like individual differences in the human sleep electroencephalogram. Neuroscience 138, 351–356. 10.1016/j.neuroscience.2005.11.005 [DOI] [PubMed] [Google Scholar]
- Campbell IG, Feinberg I, 2009. Longitudinal trajectories of non-rapid eye movement delta and theta EEG as indicators of adolescent brain maturation. Proc. Natl. Acad. Sci. U. S. A. 106, 5177–5180. 10.1073/pnas.0812947106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carskadon MA, Tarokh L, 2014. Developmental changes in sleep biology and potential effects on adolescent behavior and caffeine use. Nutr. Rev 72 Suppl 1, 60–64. 10.1111/nure.12147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelnovo A, Graziano B, Ferrarelli F, D’Agostino A, 2017. Sleep spindles and slow waves in Schizophrenia and related disorders: main findings, challenges, and future perspectives. Eur. J. Neurosci 48, 2738–2758. 10.1111/ejn.13815 [DOI] [PubMed] [Google Scholar]
- Cavelti M, Winkelbeiner S, Federspiel A, Walther S, Stegmayer K, Giezendanner S, Laimböck K, Dierks T, Strik W, Horn H, Homan P, 2018. Formal thought disorder is related to aberrations in language-related white matter tracts in patients with schizophrenia. Psychiatry Res. Neuroimaging 279, 40–50. 10.1016/j.pscychresns.2018.05.011 [DOI] [PubMed] [Google Scholar]
- Chatburn A, Coussens S, Lushington K, Kennedy D, Baumert M, Kohler M, 2013. Sleep Spindle Activity and Cognitive Performance in Healthy Children. Sleep 36, 237–243. 10.5665/sleep.2380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs B, Scriver CR, 1986. Age at onset and causes of disease. Perspect. Biol. Med 29, 437–460. [DOI] [PubMed] [Google Scholar]
- Cohen J, 1992. A power primer. Psychol. Bull 112, 155–159. [DOI] [PubMed] [Google Scholar]
- Contreras D, Destexhe A, Sejnowski TJ, Steriade M, 1996. Control of spatiotemporal coherence of a thalamic oscillation by corticothalamic feedback. Science 274, 771–774. [DOI] [PubMed] [Google Scholar]
- D’Agostino A, Castelnovo A, Cavallotti S, Casetta C, Marcatili M, Gambini O, Canevini M, Tononi G, Riedner B, Ferrarelli F, Sarasso S, 2018. Sleep endophenotypes of schizophrenia: slow waves and sleep spindles in unaffected first-degree relatives. NPJ Schizophr. 4, 2 10.1038/s41537-018-0045-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lacy N, King BH, 2013. Revisiting the relationship between autism and schizophrenia: toward an integrated neurobiology. Annu. Rev. Clin. Psychol. 9, 555–587. 10.1146/annurev-clinpsy-050212-185627 [DOI] [PubMed] [Google Scholar]
- Driver DI, Gogtay N, Rapoport JL, 2013. Childhood Onset Schizophrenia and Early Onset Schizophrenia spectrum disorders. Child Adolesc. Psychiatr. Clin. N. Am 22, 539–555. 10.1016/j.chc.2013.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg I, 1982. Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence? J. Psychiatr. Res 17, 319–334. [DOI] [PubMed] [Google Scholar]
- Feinberg I, March JD, Flach K, Maloney T, Chern J-W, Travis F, 1990. Maturational changes in amplitude, incidence and cyclic pattern of the 0 to 3 Hz (Delta) electroencephalogram of human sleep. Brain Dysfunct. 3, 183–192. [Google Scholar]
- Ferrarelli F, Huber R, Peterson MJ, Massimini M, Murphy M, Riedner BA, Watson A, Bria P, Tononi G, 2007. Reduced Sleep Spindle Activity in Schizophrenia Patients. Am. J. Psychiatry 164, 483–492. 10.1176/ajp.2007.164.3.483 [DOI] [PubMed] [Google Scholar]
- Ferrarelli F, Peterson MJ, Sarasso S, Riedner BA, Murphy MJ, Benca RM, Bria P, Kalin NH, Tononi G, 2010. Thalamic dysfunction in schizophrenia suggested by whole-night deficits in slow and fast spindles. Am. J. Psychiatry 167, 1339–1348. 10.1176/appi.ajp.2010.09121731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrarelli F, Tononi G, 2017. Reduced sleep spindle activity point to a TRN-MD thalamus-PFC circuit dysfunction in schizophrenia. Schizophr. Res 180, 36–43. 10.1016/j.schres.2016.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrarelli F, Tononi G, 2011. The Thalamic Reticular Nucleus and Schizophrenia. Schizophr. Bull 37, 306–315. 10.1093/schbul/sbq142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MR, Goethe JW, Dekker DK, 1986. EEG coherence and power in the discrimination of psychiatric disorders and medication effects. Biol. Psychiatry 21, 1175–1188. 10.1016/0006-3223(86)90224-6 [DOI] [PubMed] [Google Scholar]
- Fuentealba P, Steriade M, 2005. The reticular nucleus revisited: intrinsic and network properties of a thalamic pacemaker. Prog. Neurobiol 75, 125–141. 10.1016/j.pneurobio.2005.01.002 [DOI] [PubMed] [Google Scholar]
- Gandal MJ, Haney JR, Parikshak NN, Leppa V, Ramaswami G, Hartl C, Schork AJ, Appadurai V, Buil A, Werge TM, Liu C, White KP, CommonMind Consortium, PsychENCODE Consortium, iPSYCH-BROAD Working Group, Horvath S, Geschwind DH, 2018. Shared molecular neuropathology across major psychiatric disorders parallels polygenic overlap. Science 359, 693–697. 10.1126/science.aad6469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godbout R, Bergeron C, Limoges E, Stip E, Mottron L, 2000. A laboratory study of sleep in Asperger’s syndrome. Neuroreport 11, 127–130. [DOI] [PubMed] [Google Scholar]
- Göder R, Graf A, Ballhausen F, Weinhold S, Baier PC, Junghanns K, Prehn-Kristensen A, 2015. Impairment of sleep-related memory consolidation in schizophrenia: relevance of sleep spindles? Sleep Med. 16, 564–569. 10.1016/j.sleep.2014.12.022 [DOI] [PubMed] [Google Scholar]
- Gogtay N, 2008. Cortical brain development in schizophrenia: insights from neuroimaging studies in childhood-onset schizophrenia. Schizophr. Bull 34, 30–36. 10.1093/schbul/sbm103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon CT, Frazier JA, McKenna K, Giedd J, Zametkin A, Zahn T, Hommer D, Hong W, Kaysen D, Albus KE, 1994. Childhood-onset schizophrenia: an NIMH study in progress. Schizophr. Bull 20, 697–712. [DOI] [PubMed] [Google Scholar]
- Gottselig JM, Bassetti CL, Achermann P, 2002. Power and coherence of sleep spindle frequency activity following hemispheric stroke. Brain 125, 373–383. 10.1093/brain/awf021 [DOI] [PubMed] [Google Scholar]
- Greenstein D, Lerch J, Shaw P, Clasen L, Giedd J, Gochman P, Rapoport J, Gogtay N, 2006. Childhood onset schizophrenia: cortical brain abnormalities as young adults. J. Child Psychol. Psychiatry 47, 1003–1012. 10.1111/j.1469-7610.2006.01658.x [DOI] [PubMed] [Google Scholar]
- Kraepelin E, 1919. Dementia praecox and paraphrenia. E. S. Livingston, Edinburgh, Scotland. [Google Scholar]
- Krause M, E Hoffmann W, Hajós M, 2003. Auditory sensory gating in hippocampus and reticular thalamic neurons in anesthetized rats. Biol. Psychiatry 53, 244–53. 10.1016/S0006-3223(02)01463-4 [DOI] [PubMed] [Google Scholar]
- Krosigk M, Bal T, McCormick DA, 1993. Cellular mechanisms of a synchronized oscillation in the thalamus. Science 261, 361–364. [DOI] [PubMed] [Google Scholar]
- Kumra S, Frazier JA, Jacobsen LK, McKenna K, Gordon CT, Lenane MC, Hamburger SD, Smith AK, Albus KE, Alaghband-Rad J, Rapoport JL, 1996. Childhood-onset schizophrenia. A double-blind clozapine-haloperidol comparison. Arch. Gen. Psychiatry 53, 1090–1097. [DOI] [PubMed] [Google Scholar]
- Kurth S, Ringli M, Geiger A, LeBourgeois M, Jenni OG, Huber R, 2010. Mapping of Cortical Activity in the First Two Decades of Life: A High-Density Sleep Electroencephalogram Study. J. Neurosci 30, 13211–13219. 10.1523/JNEUROSCI.2532-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limoges É, Bolduc C, Berthiaume C, Mottron L, Godbout R, 2013. Relationship between poor sleep and daytime cognitive performance in young adults with autism. Res. Dev. Disabil 34, 1322–1335. 10.1016/j.ridd.2013.01.013 [DOI] [PubMed] [Google Scholar]
- Manoach DS, Demanuele C, Wamsley EJ, Vangel M, Montrose DM, Miewald J, Kupfer D, Buysse D, Stickgold R, Keshavan MS, 2014. Sleep spindle deficits in antipsychotic-naïve early course schizophrenia and in non-psychotic first-degree relatives. Front. Hum. Neurosci 8, 762 10.3389/fnhum.2014.00762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoach DS, Pan JQ, Purcell SM, Stickgold R, 2016. Reduced Sleep Spindles in Schizophrenia: A Treatable Endophenotype That Links Risk Genes to Impaired Cognition? Biol. Psychiatry 80, 599–608. 10.1016/j.biopsych.2015.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoach DS, Thakkar KN, Stroynowski E, Ely A, McKinley SK, Wamsley E, Djonlagic I, Vangel MG, Goff DC, Stickgold R, 2010. Reduced overnight consolidation of procedural learning in chronic medicated schizophrenia is related to specific sleep stages. J. Psychiatr. Res 44, 112 10.1016/j.jpsychires.2009.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maris E, Oostenveld R, 2007. Nonparametric statistical testing of EEG- and MEG-data. J. Neurosci. Methods 164, 177–190. 10.1016/j.jneumeth.2007.03.024 [DOI] [PubMed] [Google Scholar]
- Markovic A, Buckley A, Driver DI, Dillard‐Broadnax D, Gochman PA, Hoedlmoser K, Rapoport JL, Tarokh L, 2020. Sleep neurophysiology in childhood onset schizophrenia. J. Sleep Res e13039 10.1111/jsr.13039 [DOI] [PubMed] [Google Scholar]
- Mattai AA, Tossell J, Greenstein DK, Addington A, Clasen LS, Gornick MC, Seal J, Inoff-Germain G, Gochman PA, Lenane M, Rapoport JL, Gogtay N, 2006. Sleep disturbances in childhood-onset schizophrenia. Schizophr. Res 86, 123–129. 10.1016/j.schres.2006.04.020 [DOI] [PubMed] [Google Scholar]
- McAlonan K, Cavanaugh J, Wurtz RH, 2008. Guarding the gateway to cortex with attention in visual thalamus. Nature 456, 391–394. 10.1038/nature07382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna K, Gordon CT, Lenane M, Kaysen D, Fahey K, Rapoport JL, 1994. Looking for childhood-onset schizophrenia: the first 71 cases screened. J. Am. Acad. Child Adolesc. Psychiatry 33, 636–644. 10.1097/00004583-199406000-00003 [DOI] [PubMed] [Google Scholar]
- Merrin EL, Floyd TC, Fein G, 1989. EEG coherence in unmedicated schizophrenic patients. Biol. Psychiatry 25, 60–66. [DOI] [PubMed] [Google Scholar]
- Nagase Y, Okubo Y, Matsuura M, Kojima T, Toru M, 1992. EEG coherence in unmedicated schizophrenic patients: Topographical study of predominantly never medicated cases. Biol. Psychiatry 32, 1028–1034. 10.1016/0006-3223(92)90064-7 [DOI] [PubMed] [Google Scholar]
- Nicolson R, Rapoport JL, 1999. Childhood-onset schizophrenia: rare but worth studying. Biol. Psychiatry 46, 1418–1428. [DOI] [PubMed] [Google Scholar]
- Ordóñez AE, Luscher ZI, Gogtay N, 2016. Neuroimaging findings from childhood onset schizophrenia patients and their non-psychotic siblings. Schizophr. Res 173, 124–131. 10.1016/j.schres.2015.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell SM, Manoach DS, Demanuele C, Cade BE, Mariani S, Cox R, Panagiotaropoulou G, Saxena R, Pan JQ, Smoller JW, Redline S, Stickgold R, 2017. Characterizing sleep spindles in 11,630 individuals from the National Sleep Research Resource. Nat. Commun 8, 15930 10.1038/ncomms15930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport JL, Gogtay N, 2011. Childhood onset schizophrenia: support for a progressive neurodevelopmental disorder. Int. J. Dev. Neurosci. Off. J. Int. Soc. Dev. Neurosci 29, 251–258. 10.1016/j.ijdevneu.2010.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechtschaffen A, Kales A, 1968. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. UCLA Brain Information Service/Brain Research Institute, Los Angeles. [Google Scholar]
- Rusterholz T, Hamann C, Markovic A, Schmidt SJ, Achermann P, Tarokh L, 2018. Nature and Nurture: Brain region specific inheritance of sleep neurophysiology in adolescence. J. Neurosci. Off. J. Soc. Neurosci 38, 9275–9285. 10.1523/JNEUROSCI.0945-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Vives MV, McCormick DA, 2000. Cellular and network mechanisms of rhythmic recurrent activity in neocortex. Nat. Neurosci 3, 1027–1034. 10.1038/79848 [DOI] [PubMed] [Google Scholar]
- Sheldon SH, 2014. Development of Sleep in Infants and Children, in: Sheldon SH, Ferber R, Kryger MH, Gozal D (Eds.), Principles and Practice of Pediatric Sleep Medicine (Second Edition). Saunders, Philadelphia, pp. 17–23. 10.1016/B978-1-4557-0318-0.00003-6 [DOI] [Google Scholar]
- Shinomiya S, Nagata K, Takahashi K, Masumura T, 1999. Development of sleep spindles in young children and adolescents. Clin. EEG Electroencephalogr. 30, 39–43. [DOI] [PubMed] [Google Scholar]
- Sporn AL, Greenstein DK, Gogtay N, Jeffries NO, Lenane M, Gochman P, Clasen LS, Blumenthal J, Giedd JN, Rapoport JL, 2003. Progressive brain volume loss during adolescence in childhood-onset schizophrenia. Am. J. Psychiatry 160, 2181–2189. 10.1176/appi.ajp.160.12.2181 [DOI] [PubMed] [Google Scholar]
- Steriade M, 2006. Grouping of brain rhythms in corticothalamic systems. Neuroscience 137, 1087–1106. 10.1016/j.neuroscience.2005.10.029 [DOI] [PubMed] [Google Scholar]
- Steriade M, Nuñez A, Amzica F, 1993. Intracellular analysis of relations between the slow (< 1 Hz) neocortical oscillation and other sleep rhythms of the electroencephalogram. J. Neurosci. Off. J. Soc. Neurosci 13, 3266–3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarokh L, Carskadon MA, 2010. Developmental changes in the human sleep EEG during early adolescence. Sleep 33, 801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesler N, Gerstenberg M, Franscini M, Jenni OG, Walitza S, Huber R, 2015. Reduced sleep spindle density in early onset schizophrenia: A preliminary finding. Schizophr. Res 166, 355–357. 10.1016/j.schres.2015.04.042 [DOI] [PubMed] [Google Scholar]
- Tessier S, Lambert A, Chicoine M, Scherzer P, Soulières I, Godbout R, 2015. Intelligence measures and stage 2 sleep in typically-developing and autistic children. Int. J. Psychophysiol. Off. J. Int. Organ. Psychophysiol 97, 58–65. 10.1016/j.ijpsycho.2015.05.003 [DOI] [PubMed] [Google Scholar]
- Timofeev I, Steriade M, 1996. Low-frequency rhythms in the thalamus of intact-cortex and decorticated cats. J. Neurophysiol 76, 4152–4168. [DOI] [PubMed] [Google Scholar]
- Tsekou H, Angelopoulos E, Paparrigopoulos T, Golemati S, Soldatos CR, Papadimitriou GN, Ktonas PY, 2015. Sleep EEG and spindle characteristics after combination treatment with clozapine in drug-resistant schizophrenia: a pilot study. J. Clin. Neurophysiol. Off. Publ. Am. Electroencephalogr. Soc 32, 159–163. 10.1097/WNP.0000000000000145 [DOI] [PubMed] [Google Scholar]
- Wamsley EJ, Tucker MA, Shinn AK, Ono KE, McKinley SK, Ely AV, Goff DC, Stickgold R, Manoach DS, 2012. Reduced Sleep Spindles and Spindle Coherence in Schizophrenia: Mechanisms of Impaired Memory Consolidation? Biol. Psychiatry 71, 154–161. 10.1016/j.biopsych.2011.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warby SC, Wendt SL, Welinder P, Munk EGS, Carrillo O, Sorensen HBD, Jennum P, Peppard PE, Perona P, Mignot E, 2014. Sleep-spindle detection: crowdsourcing and evaluating performance of experts, non-experts and automated methods. Nat. Methods 11, 385–392. 10.1038/nmeth.2855 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1: The non-rapid eye movement (NREM) log-transformed power spectrum (left panel) and the NREM coherence spectrum (right panel) for one of the control subjects demonstrating the procedure to calculate sigma peak power/coherence. The function findpeaks in MATLAB was used to find the peak in the sigma range (11.2 to 16.2 Hz). The local minimum before the peak was then detected and served as the starting point of the fitting line (shown in red) while the endpoint of the line was the last point in the frequency range of interest (i.e., 16.2 Hz). The distance between this line and the peak point was calculated (shown in black) and this served as a measure of relative sigma peak power/coherence.
Supplementary Figure S2: Topographic distribution of sigma power normalized by the total power across derivations during non-rapid eye movement (NREM) sleep. The first and the second row depict power values averaged for patients and controls separately and plotted on the same scale. The third row shows the ratio of average power for patients divided by control with statistically significant electrodes in magenta. Note that this plot is on a different scale with negative values (in cool colors) representing decreased power in patients and positive values (in warm colors) indicating enhanced power in patients as compared to controls. We observed a topographic shift towards central regions in patients as compared to controls.