Abstract
Various bioactive ingredients have been extracted from Chinese herbal medicines (CHMs) that affect tumor progression and metastasis. To further understand the mechanisms of CHMs in cancer therapy, this article summarizes the effects of five categories of CHMs and their active ingredients on tumor cells and the tumor microenvironment. Despite their treatment potential, the undesirable physicochemical properties (poor permeability, instability, high hydrophilicity or hydrophobicity, toxicity) and unwanted pharmacokinetic profiles (short half-life in blood and low bioavailability) restrict clinical studies of CHMs. Therefore, development of liposomes through relevant surface modifying techniques to achieve targeted CHM delivery for cancer cells, i.e., extracellular and intracellular targets and targets in tumor microenvironment or vasculature, have been reviewed. Current challenges of liposomal targeting of these phytoconstituents and future perspective of CHM applications are discussed to provide an informative reference for interested readers.
Keywords: Chinese herbal medicines, Liposomes, Anti-tumor effect, Targeting strategies, Tumor microenvironment
1. Introduction
Cancer is a leading cause of death worldwide. It is an uncontrolled and excessive growth of cells that can metastasize to a number of organs [1]. Cancer therapy has remained challenging for centuries, although therapeutic strategies such as surgery, chemotherapy and radiotherapy, have been developed. Until now, the main clinical treatment has been chemotherapy in addition to surgery. However, most chemotherapy drugs have high toxicity, low specificity, and are accompanied by painful side effects [2]. In addition, multidrug resistance could be induced by chemotherapeutics, causing treatment failure upon disease recurrence [3].
Phytoconstituents have emerged as a prospective approach for cancer treatment and are also widely used, especially in Asia [4,5]. These bioactive molecules are of great interest due to their high range of biological activity, minimal side effects, and low cost [6].
The current reviews on the developments of phytochemical delivery systems for cancer treatment mainly focus on specific types of delivery systems or compounds, e.g. polyphenols [5,7,8]. Bahrami’s group have provided a summary of the anticancer effect of natural agents, either alone or in combination with natural drugs, by modulation of regulatory T cells (Tregs) [9]. However, to the best of our knowledge, there is no comprehensive review using the syndrome differentiation, and treatment of traditional Chinese medicine (TCM) as the guiding principle which classifies plants according their functions and indications in Pharmacopoeia of the People’s Republic of China (ChP) 2015 edition, and covers preclinical stage liposomal targeting compound delivery strategies. Moreover, this review is both limited to the anti-tumoral efficacy of plant-derived medicines, and also includes their effects on the tumor microenvironment (TME), which is subdivided into tumor immune and non-immune microenvironments [10].
The aims of this review were to summarize the history of treating cancer in Chinese medicine and to critically analyze the potential anti-tumoral effects of Chinese medical plants recorded in ChP 2015 edition and their active components, which function in counteracting toxin with toxin, heat-clearing and detoxifying, promoting blood circulation and removing blood stasis, resolving phlegm and eliminating dampness, strengthening healthy energy and consolidating body resistance, and suppressing tumor aerobic glycolysis, by comparing the treatment theory of TCM with modern medicine. Because modulating the stromal TME (tumor associated macrophages (TAMs), TAFs, and endothelial cells) or immune microenvironment by either small molecules or nanodrugs can facilitate the remodeling of blood vessels or extracellular matrix (ECM) at tumor sites [11], we also summarize recent developments and strategies for liposomal delivery systems of promising anticancer phytoconstituents and discuss the opportunities of further improvement.
It is worth mentioning that our review summarizes the anti-tumor effects of natural products and their impact on the TME, together with the targeted liposomal drug delivery system. In fact, the specific pharmacological effects of each monomer compound on tumor cells and TME, and liposomal targeting formulations in this review are all based on the Western medicine theory and summarized by searching for literatures with systematic data sets. The research on the action mechanism of monoconstituents and their delivery system is the same as that of Western medicine, except that we use the traditional Chinese medicine classification standard for the classification of Chinese medicines. This classification is secondary, not the focus of our review. In fact, traditional Chinese medicine and Western medicine have their own characteristics in diagnosis and treatment. Not every concept of traditional Chinese medicine treatment can be explained in terms of Western medicine theory. In this review, the classification we use is an attempt to combine the research of traditional Chinese medicine monomers with the theory of traditional Chinese medicine.
2. The connotation of TCM in tumor treatment
“Tumor” was recorded in the oracle bone inscriptions of the Yin and Zhou era more than 3,500 years ago. Miraculous Pivot in the Han Dynasty mentioned the pathogenesis and symptoms of “carbuncle”. Until the Ming Dynasty, the occurrence and development of breast tumors were discussed in detail [12]. Physician Zhang Xichun explained the treatment and prescription of phrenic disease syndromes (mainly including esophageal cancer or gastric cardia cancer) in detail in his book “Records of Tradition Chinese and Western Medicine in Combination” at the end of the Qing Dynasty. As it was stated in Yellow Emperor’s Inner Cannon (written from the Warring States period to the Qin and Han Dynasties), deficient vital qi and excess pathogenic qi is the most important basic pathogenesis of cancer. Qi refers to the nutritive and refined substances circulating in the body and the functional state of organs and tissues [13]. Qi correlates with longevity and qi depletion is linked to death [14]. Qi is divided into vital qi and pathogenic qi. The so-called “vital qi” is the body’s resistance to pathogenic microorganisms and its ability to adjust and adapt. Deficiency of vital energy is deficiency of vital qi [15]. Pathogenic qi refers to various pathogenic factors, including wind, cold, summer-heat, dampness, dryness, and heat. Disease results from the struggle between the vital qi and the pathogenic qi in the body [16]. Therefore, treatment is divided into two aspects: removing pathogenic factors and strengthening the vital qi. Using the syndrome differentiation and treatment of TCM as the guiding principle, treatment to eliminate pathogens refers to counteracting toxin with toxin, heat-clearing and detoxifying, promoting blood circulation and removing blood stasis, resolving phlegm and eliminating dampness; treatment to reinforce healthy qi refers to strengthening healthy energy and consolidating body resistance [17–20]. Here, the TCM classification is not independent or irrelevant. On the contrary, the use and function of TCM are often intersected and need to be used as a whole.
Among the natural products eliminating pathogenic factors, counteracting toxin with toxin, and heat-clearing and detoxifying herbal medicines are most extensively used. According to the pathogenesis theory of cancerous toxins [21], it is suitable to use heat-clearing and detoxification therapy to eliminate cancer toxins at the early stages of cancer. If the cancer toxin has the potential to spread, then the method of attacking poison with poison should be used to attack and stop the development of cancer toxins; in the middle stages of cancer, it is better to use the synergistic therapy of heat-clearing and detoxification, and attacking toxin with toxin; for patients with advanced cancer, heat-clearing and detoxification is the only appropriate method to remove cancer toxins continuously.
3. Anti-tumor mechanism of different classification of Chinese herbal medicines (CHMs) and their active components
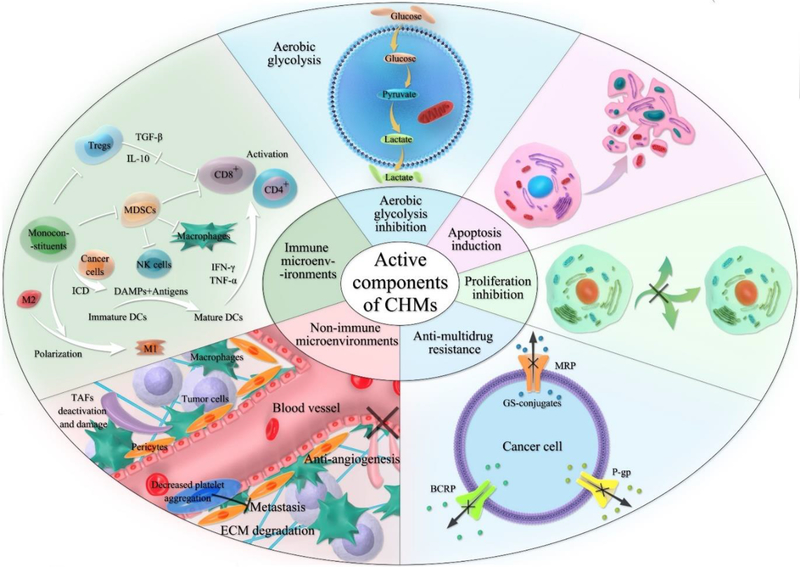
In the treatment of tumors, CHMs can play multi-channel and multi-target roles, and have the advantages of less adverse reactions and good tolerance. We summarized the anti-tumor efficacies of the following five categories CHMs and their active components in the aspects of inducing apoptosis, inhibiting cell proliferation, regulating autophagy, reversing multidrug resistance, inhibiting angiogenesis, targeting cancer stem cells (CSCs), blocking tumor invasion and metastasis, and regulating immune state (Figure 1). Some natural products target glycolysis pathway in cancer cells, which could control cancer growth, multidrug resistance, and metastasis. The regulatory effect of CHMs on TME including inhibiting tumor-associated fibroblasts (TAFs) and their released cytokines, decreasing angiogenic cells and their pro-angiogenic factors, degrading ECM and regulating on immune cells and their cytokines. The involving immune cells include T lymphocytes (T cells), B lymphocytes, natural killer cells (NK cells), tumor-associated macrophages (TAMs), dendritic cells (DCs) and myeloid-derived suppressor cells (MDSCs). Some monoconstituents could induce immunogenic cell death (ICD) on cancer cells, which mediated by damage-associated molecular patterns (DAMPs) and thus increased the levels of costimulatory signals on DCs to promote the antitumor T-cell response.
Figure 1:
Overview of mechanisms for monoconstituents of CHMs on tumor cells and the TME (Abbreviation: MRP, multidrug resistance-associated protein; GS-conjugates, glutathione conjugates; P-gp, P-glycoprotein; BCRP, breast cancer resistance-relative protein; TGF-β, transforming growth factor-β; IL-10, interleukin-10; IFN-γ, interferon gamma; TNF-α, tumor necrosis factor-alpha).
3.1. CHMs that counteract toxin with toxin and their active components
There are 70 kinds of toxic herbs and decoction pieces are recorded in ChP (2015 edition, volume I). Of these, eight had great toxicity, 33 had general toxicity, and 29 had small toxicity. Among them, 52 are reported to exhibit anti-cancer effects, 30 are used as extraction, and 22 as both extraction and active components.
The “cancer toxin” is thought to be an important reason for the formation of cancer. TCM uses the theory of the cancer toxin to describe viruses, bacteria, chemical pollution, and invisible carcinogens. Cancer toxins can directly lead to dysfunction of viscera, induce phlegm, blood stasis, heat toxin and other pathological factors similar to inflammation. Heat toxin refers to external toxin characterized by redness, heat pain, hemorrhage, dizziness, abnormal movement of limbs, and sudden illness [22]. Due to the slow process of tumor formation, and the deep accumulation of toxins, conventional Chinese medicine is challenging. As such, some toxic products, by virtue of their fierce nature, are needed attack the cancer poison in the so-called “fight fire with fire” approach. TCM that uses poison to attack poison can activate blood stagnation, soften hardness, and dissolve lumps [23]. The active components of the crude drugs CHMs for counteracting toxin with toxin and their effects on tumor cells are summarized in Table 1.
Table 1.
Mechanism of the CHMs that counteract toxin with toxin and their active components on tumor cells.
| CHMs | Active components | Tumor cells | TME | References | ||||
|---|---|---|---|---|---|---|---|---|
| Apoptosis induction | Proliferation inhibition | Autophagy regulation | Anti-multidrug resistance | Migration and invasion inhibition | Anti-angiogenesis | |||
| Radix aconiti kusnezoffii (cao wu) | Aconitine | √ | √ | √ | √ | [30–33] | ||
| Hypaconitine | √ | √ | [33,34] | |||||
| Mesaconitine | √ | √ | [33,35] | |||||
| Nux vomica (ma qian zi) | Strychnine | √ | √ | [36] | ||||
| Brucine | √ | √ | √ | √ | √ | [37,38] | ||
| Stellera chamaejasme (lang du) | Chamaejasmenin B and Neochamaejasmin C | √ | √ | √ | [39,40] | |||
| Fructus gleditsiae sinensis (zao jiao) | Echinocystic acid | √ | √ | [41] | ||||
| Fructus toosendan (chuan lian zi) | Toosendanin and Other triterpenoids | √ | [42] | |||||
| Sinopodophylli fructus (xiao ye lian) | Podophyllotoxin, Deoxypodophyllotoxin, 8-Isopentenyl kaempferol, and Kaempferol | √ | √ | [43,44] | ||||
| Zanthoxylum nitidum (liang mian zhen) | Nitidine chloride and Zanthobungeanine | √ | √ | √ | [45] | |||
| Fructus evodiae (wu zhu yu) | Evodiamine and Rutaecarpine | √ | √ | √ | √ | [46] | ||
| Semen armeniacae amarae (ku xing ren) | Amygdalin | √ | √ | [47] | ||||
| Fructus cnidii (she chuang zi) | Osthole | √ | √ | √ | √ | √ | [48] | |
| Cortex pseudolaricis (tu jing pi) | Pseudolaric acid B | √ | √ | [49] | ||||
| Momordicae semen (mu bie zi) | p-Hydroxylcinnamaldehyde | √ | [50] | |||||
| Herba chelidonii (bai qu cai) | Chelidonine and Sanguinarine | √ | [51] | |||||
| Chelerythrine | √ | [52] | ||||||
| Fructus xanthii (cang er zi) | Xanthium | √ | √ | [53] | ||||
| Cortex periplocae (xiang jia pi) | Periplocin | √ | √ | √ | [54] | |||
| Baohuoside I | √ | [55] | ||||||
From Table 1, most of the CHMs that counteract toxin with toxin function by directly damaging tumor cell DNA, inhibiting tumor cell division and gene expression to induce tumor cell apoptosis. Apoptosis mainly includes the cell death receptor-mediated extrinsic and mitochondrion-mediated intrinsic pathways [24,25], which are affected by the B-cell lymphama-2 (Bcl-2) protein family.
The cell cycle can be divided into G1 (preceding initiation of DNA synthesis), S (DNA synthesis), G2 (preceding mitosis), and M (mitosis) stages. G1/S and G2 /M are the most important steps of cell cycle regulation, while cyclin dependent kinases (CDKs) are key cell cycle regulators [26]. CHM active components could decrease expression of proliferative genes, such as cyclin, protein 53 (p53), and k167, proliferating cell nuclear antigen (PCNA), telomerase, and CDKs [27].
The signaling pathways for inducing apoptosis and inhibiting proliferation include the Wnt pathway (such as resveratrol, berberine, and curcumin), transforming growth factor-β (TGF-β) pathway (such as resveratrol and baicalein), signal transducer and activator of transcription 3 (STAT3) pathway (such as nitidine chloride, scutellarin, isoliquiritigenin and cryptotanshinone), p53 pathway (such as magnolol, ligustrazine, emodin, and evodiamine), nuclear transcription factor-κB (NF-κB) pathway (such as evodiamine), p38 mitogen activated protein kinase (MAPK) pathway (such as matrine and baicalein), extra cellular signal regulated kinase (ERK) pathway (such as scutellarin, luteolin, curcumin and pistil isoflavone), c-Jun N-terminal kinase (c-Jun) pathway (such as tanshinone ⅡA and celastrol), and phosphatidylinositol-3-kinases/protein kinase B/mammalian target of rapamycin (PI3K/Akt/mTOR) pathway (such as resveratrol glycoside) [28,29].
3.2. CHMs that clear heat and detoxify and their active components
There are 115 kinds of heat-clearing and detoxifying CHMs and slices recorded in ChP (2015 edition, volume I). Of these, 88 are reported to exhibit antitumor effects in vitro or in vivo. Eight are used as crude drugs, 37 as extraction, and 43 as active components.
Zhongying Zhou, a master of Chinese medicine, reported that heat is an important pathogenic factor for tumor development, as cancer patients often have fever, pain, thirst, local burning, constipation, red tongue with yellowish fur, and so on. Clinical manifestations of heat syndrome in microenvironments include increased oxygen consumption, higher leukocyte and neutrophil count, and higher consumption of cortical alcohols and catecholamines [56,57]. As recorded in Yellow Emperor’s Inner Cannon, body’s organs, limbs, skin, muscles and bones are connected into an organic whole through meridians and keep it relatively coordinated and unified to complete various functions, where meridians refer to channels that provide energy for human metabolism, treat diseases, and transmit diseases. “Heat toxin” will block the meridians of the viscera and heat-clearing and detoxification must continue through the whole process of cancer treatment. It is clearly put forward in the Yellow Emperor’s Inner Cannon that “treating heat syndrome with drugs of cold nature and treating warm syndrome with drugs of cool nature” is the proper approach [23]. Experimental results show that the most effective way to clear away heat and detoxify is to use cold and cool natured drugs to eliminate or degrade the heat toxin in the body and control inflammation.
Chronic “non-resolving inflammation” contributes significantly to the pathogenesis of malignancies. This relationship between inflammation and cancer was first put forward by Rudolph Virchow. The process consists of two parts, initiation and promotion. First, precancerous inflammation can cause irreversible DNA alteration in proliferating cells. Second, in the persistent presence of aberrant activation of inflammatory oncogenes in chronically inflamed tissues, the abnormal cell replication and proliferation continues, and achieve the full malignant phenotype, such as ECM remodeling, angiogenesis, metastasis, and suppressed innate immune responses [58–62].
Inflammation is recognized to play a major role in the development and progression of malignancies and is closely related to the heat toxin of TCM in disease occurrence and development [63–65]. Modern studies have shown that heat-clearing and detoxifying drugs exhibit as anti-inflammatory properties to clear heat, detoxify, resist bacteria, and improve immunity. They can control and eliminate inflammation of the tumor and its surroundings, proving that heat-clearing and detoxifying drugs used in tumor treatment are key to inhibiting tumor development [66]. The active components of crude drugs of heat-clearing and detoxifying CHMs and their effects on tumor cells and the TME are summarized in Table 2.
Table 2.
Mechanism of the CHMs that clear heat and detoxify and their active components on tumor cells and the TME.
| CHMs | Active components | Tumor cells | TME | References | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Apoptosis induction | Proliferation inhibition | Autophagy regulation | Anti-multidrug resistance | Migration and invasion inhibition | TAFs | Anti-angiogenesis | TAMs | NK cells | T cells | DCs | MDSCs | |||
| Saururus chinensis (san bai cao) | Sauchinone | √ | √ | √ | √ | [67–69] | ||||||||
| Solidago decurrens (yizhi huang hua) | 6β-Angeloyloxykolavenic acid and 6β-Tigloyloxykolavenic acid | √ | [70] | |||||||||||
| Rhizoma coptidis (huang lian) | Berberine | √ | √ | √ | √ | √ | √ | √ | √ | √ | [71–74] | |||
| Sargentglory vine stem (da xue teng) | Chlorogenic acid | √ | √ | √ | √ | √ | √ | √ | √ | [75–79] | ||||
| Folium isatidis (da qing ye) | Indirubin | √ | √ | √ | √ | √ | [80–83] | |||||||
| Rheum palmatum (da huang) | Emodin | √ | √ | √ | √ | √ | √ | √ | [84–88] | |||||
| Rhein | √ | [89] | ||||||||||||
| Radix sophorae tonkinensis (shan dou gen) | Cytisine | √ | [90] | |||||||||||
| Matrine and Oxymatrine | √ | √ | √ | √ | √ | √ | [91–95] | |||||||
| Oxysophocarpine | √ | [96] | ||||||||||||
| Flos lonicerae (shan yin hua) | Macranthoside B | √ | √ | √ | [97,98] | |||||||||
| Portulace oleracea (ma chi xian) | Betacyanins | √ | √ | [99,100] | ||||||||||
| Fructus arctii (niu bang zi) | Arctiin and Arctigenin | √ | √ | √ | √ | [101–104] | ||||||||
| Belleric terminalia fruit (mao he zi) | Gallic acid | √ | √ | √ | [105–107] | |||||||||
| Rhizoma cimicifugae (sheng ma) | Actein and 23-epi-26-Deoxyactein | √ | √ | √ | √ | [108,109] | ||||||||
| Cimigenol | √ | [110] | ||||||||||||
| Ferulic acid and Isoferulic acid | √ | √ | √ | √ | √ | [111–114] | ||||||||
| Silybum marianum (shui fei ji) | Taxifolin | √ | √ | √ | √ | [115–118] | ||||||||
| Isosilybin A/B | √ | √ | [119,120] | |||||||||||
| Silibinin | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | [121–130] | ||
| Caulis mahoniae (gong lao mu) | Jatrorrhizin | √ | √ | √ | [131–133] | |||||||||
| Radix liquiritiae (gan cao) | Liquiritigenin and Liquirtin | √ | √ | √ | [134] | |||||||||
| Isoliquiritigenin, Soliquiritin | √ | √ | √ | √ | √ | [135,136] | ||||||||
| Licochalcone A/E | √ | √ | [137] | |||||||||||
| Glabridin | √ | √ | [138] | |||||||||||
| Glycyrrhetinic acid | √ | √ | √ | √ | √ | √ | √ | [139–141] | ||||||
| Glycyrrhizic acid | √ | √ | [142] | |||||||||||
| Pulsatilla chinensis (bai tou weng) | Isorhamnetin | √ | √ | √ | [143,144] | |||||||||
| Quercetin and Isoquercitin | √ | √ | √ | √ | √ | √ | √ | √ | √ | [145–150] | ||||
| Oleanolic acid | √ | √ | √ | √ | [151–153] | |||||||||
| Hederagenin | √ | √ | √ | [154,155] | ||||||||||
| Anemoside B4 | √ | √ | [156] | |||||||||||
| Pulsatilla saponin A | √ | [157] | ||||||||||||
| Pulsatilla saponin D | √ | √ | [158] | |||||||||||
| 23-Hydroxyl betulinic acid | √ | [159] | ||||||||||||
| Rhizoma imperatae (bai mao gen) | Caffeic acid | √ | √ | √ | [160] | |||||||||
| Arundoin | √ | [161] | ||||||||||||
| Radix ampelopsis (bai lian) | Catechin | √ | √ | √ | √ | √ | [162–164] | |||||||
| Resveratrol | √ | √ | √ | √ | √ | √ | √ | √ | √ | [165–168] | ||||
| Rabdosia rubescens (dong ling cao) | Oridonin | √ | √ | √ | √ | [169] | ||||||||
| Radix scrophulariae (xuan shen) | Cinnamic acid | √ | [170] | |||||||||||
| Radix sanguisorbae (diyu) | Ellagic acid | √ | √ | √ | [171] | |||||||||
| Ziyuglycoside I and II | √ | √ | √ | [172] | ||||||||||
| Polygonum cuspidatum (hu zhang) | Polydatin | √ | √ | [173] | ||||||||||
| Fagopyrum dibotrys (jin qiao mai) | Rutin | √ | [174] | |||||||||||
| Luteolin | √ | √ | √ | √ | √ | √ | [175,176] | |||||||
| Procyanidin B1 and B2 | √ | √ | √ | √ | [177] | |||||||||
| Radix scutellariae (huang qin) | Baicalein | √ | √ | √ | √ | √ | √ | [178,179] | ||||||
| Baicalin | √ | √ | √ | √ | [178] | |||||||||
| Wogonin | √ | √ | √ | √ | √ | √ | √ | [180] | ||||||
| Wogonoside | √ | √ | √ | √ | [181,182] | |||||||||
| Calyx seu fructus physalis (jin deng long) | Physalins A and B | √ | √ | √ | [183–185] | |||||||||
| Radix arnebiae (zi cao) | Shikonin | √ | √ | √ | √ | √ | [186–188] | |||||||
| Radix bupleuri (chai hu) | Saikosaponins A and D | √ | √ | √ | √ | √ | √ | √ | [189] | |||||
| Centella asiatica (ji xue cao) | Asiaticoside | √ | √ | √ | √ | [190] | ||||||||
| Asiatic acid | √ | √ | √ | √ | [191] | |||||||||
| Herba leonuri (yi mu cao) | Leonurine hydrochloride | √ | [192] | |||||||||||
| Andrographis paniculata (chuan xin lian) | Andrographolide | √ | √ | √ | √ | √ | [193,194] | |||||||
| Neoandrographolide | √ | √ | [195] | |||||||||||
| Cortex fraxini (qin pi) | Esculetin | √ | √ | √ | √ | √ | [196] | |||||||
| Aesculin | √ | √ | [197] | |||||||||||
| Fructus brucea (ya dan zi) | Brusatol, Bruceantinol, Bruceine A/D, Bruceantin | √ | √ | √ | √ | √ | [198–201] | |||||||
| Fructus gardeniae (zhi zi) | Genipin | √ | √ | [202] | ||||||||||
| Ursolic Acid | √ | √ | √ | √ | √ | [203] | ||||||||
| Apigenin | √ | √ | √ | √ | [204] | |||||||||
| β-Sitosterol | √ | √ | √ | [205] | ||||||||||
| Scutellaria barbata (ban zhi lian) | Aloe emodin | √ | √ | √ | [206] | |||||||||
| Rhein | √ | √ | √ | √ | √ | [207] | ||||||||
Heat-clearing and detoxifying plant-derived drugs can directly inhibit tumor cell proliferation, induce cell apoptosis, regulate and enhance immune levels, induce differentiation and reversion, regulate cell signaling pathways and transduction, resist mutation, inhibit angiogenesis, and reverse multidrug resistance. The possible mechanisms for their antitumor effects mainly derived from their anti-inflammatory properties. Anti-inflammatory drugs might suppress the release of inflammatory cytokines, inhibit protein kinases and growth factors, then retard tumor cell proliferation and transformation. Flavonoids have the abilities to inhibit proinflammatory enzymes, including inducible nitric oxide synthase and cyclooxygenase-2 (COX-2), and suppress COX-2-induced prostaglandin E2 (PGE2) expression. The combination of baicalin and baicalein can promote apoptosis of human breast cancer cells through the ERK/p38 MAPK pathway. Most phenylpropanoids are reported to suppress proinflammatory cytokines (tumor necrosis factor-alpha (TNF-α), interleukin-1 (IL-1), IL-6, IL-12 and IL-2). Saponins might induce cleavage of poly-ADP ribose polymerase and activate of caspases by downregulating inflammatory factors. They would also inhibit tumor growth, invasion and metastasis through matrix metalloproteinases (MMP-2, −9 and −14), STAT3, and NF-κB. Quinones suppress cell proliferation by inhibiting of protein kinase C and epidermal growth factor-receptor tyrosine kinase, associating cyclin D1 with cyclin-dependent kinases, downregulating protein activated kinase 1, and phosphorylating CDK-mediated retinoblastoma protein. Alkaloids inhibit angiogenesis by modulating the expression of vascular endothelial growth factor. The broad range of molecular targets provides a molecular basis for the therapeutic action of these anti-inflammatory plant medicines.
3.3. CHMs that promote blood circulation and remove blood stasis and their active components
There are 54 blood circulation and removing blood stasis herbs and decoction pieces recorded in ChP (2015 edition, volume I). Of these, 42 are reported to exhibit anticancer effects, 16 are used as extraction, and 26 as both extraction and active components.
According to the theory of TCM, tumor occurrence caused by the deficiency of vital energy, phlegm coagulation, blood stasis, toxins, etc. Among them, blood stasis is one of the main pathological mechanisms of tumor formation and development, and can be seen in all stages of tumor course. Modern research confirmed that the hemorheology of tumor patients shows high coagulation, high viscosity, and peripheral microcirculation disorders [208,209]. Most TCM for promoting blood circulation and removing stasis function by regulating vascular growth factor, improving microcirculation, and improving hemorheology and coagulation [210]. The active components of the crude drugs promoting blood circulation and removing blood stasis and their effects on tumors are summarized in Table 3.
Table 3.
Mechanism of the CHMs that promote blood circulation and remove blood stasis and their active components on tumor cells and the TME.
| CHMs | Active components | Tumor cells | TME | Blood circulation | References | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Apoptosis induction | Proliferation inhibition | Autophagy regulation | Anti-multidrug resistance | Migration and invasion inhibition | Anti-angiogenesis | Tregs | Platelet aggregation | |||
| Panax notoginseng (san qi) | Notoginsenoside R1, R7, and Ft1 | √ | √ | √ | √ | [218–221] | ||||
| Rhizome chuanxiong (chuan xiong) | Ligustrazine | √ | √ | √ | √ | √ | √ | √ | [222,223] | |
| Salvia miltiorrhiza (dan shen) | Tanshinone I and IIA | √ | √ | √ | √ | √ | √ | [224–226] | ||
| Cryptotanshinone | √ | √ | √ | √ | √ | [226,227] | ||||
| Rosmarinic acid | √ | √ | √ | √ | √ | √ | [228–230] | |||
| Danshensu | √ | √ | √ | [231] | ||||||
| Salvianolic acid | √ | √ | √ | √ | [232,233] | |||||
| Dihydrotanshinone | √ | √ | √ | √ | √ | √ | [234–236] | |||
| Crocus sativus (xi hong hua) | Crocin and Crocetin | √ | √ | √ | [237–239] | |||||
| Erigeron breviscapus (deng zhan xi xin) | Scutellarin | √ | √ | √ | √ | √ | [240,241] | |||
| Flos carthami (hong hua) | Hydroxysafflor yellow A | √ | √ | √ | √ | [242,243] | ||||
| Rhodiola rosea (hong jing tian) | Salidroside | √ | √ | √ | √ | √ | [244,245] | |||
| Cortex moutan radicis (mu dan pi) | Paeonol | √ | √ | √ | [246,247] | |||||
| Ardisia japonica (ai di cha) | Bergenin | √ | √ | [248] | ||||||
| Peach kernel (tao ren) | Amygdalin | √ | √ | √ | √ | [249,250] | ||||
| Naringenin | √ | √ | √ | √ | √ | [9,251,252] | ||||
| Rhizoma curcumae (e zhu) | Curcumin | √ | √ | √ | √ | √ | √ | √ | √ | [9,253–256] |
| Curcumol | √ | √ | √ | √ | √ | [257,258] | ||||
The possible mechanisms of their antitumor effects are mainly through the following aspects. First is the direct cytotoxic and antimutagenic effects, which can inhibit tumor cell proliferation and induce differentiation. Second is the down regulation of vascular endothelial growth factor and its receptor, leading to inhibition, promotion, and normalization of angiogenesis. Angiogenesis is a tightly regulated process integral to tumor growth, involving endothelial cell growth, differentiation, and migration [211]. The mechanisms of CHMs exhibiting anti-angiogenesis effects include the following models: (1) direct inhibition of vascular endothelial cell proliferation and migration; (2) inhibiting MMP activity; (3) inhibiting signal transduction of tumor angiogenic factors; (4) promoting tumor angiogenesis inhibitor expression [212–214]. Third is to weaken platelet aggregation; decreased platelet activation and platelet aggregation inhibits tumor progression [215]. Attenuated platelet aggregation and blood stasis prevent cancer cells from staying, adhering, aggregating, and planting in the blood. By avoiding of the shear forces or attack of the immune system in the blood stream, less than 0.01% of tumor cells are needed to cause successful hematogenous metastasis by increasing tumor cell emboli arrest in microcirculation. Platelet aggregation is also proposed to protect tumor cells from immunological assault in circulation [216]. Therefore, phytochemicals that promote blood circulation and remove blood stasis were used to reduce metastasis and affect microcirculation.
However, there are several shortcomings in tumor treatment by targeting the blood microenvironment alone: (1) if only a single target is inhibited, the rest of the signaling pathway will compensate and reduce the curative effect; (2) tumor cells may be dormant, preventing eradication [217]. Therefore, the promoting blood circulation and removing blood stasis treatment needs to be combined with other drugs to cure cancer.
3.4. CHMs that resolve phlegm and eliminate dampness and their active components
There are 21 kinds of resolving phlegm and eliminating dampness herbs and decoction pieces recorded in ChP (2015 edition, volume I). Of these, 15 are reported to exhibit anti-cancer effect, 6 are used as extraction, and 9 as both extraction and active components.
Phlegm coagulation is a main pathological factor of tumors. While dampness evil invades the body, phlegm and dampness gather together, leading to stagnation of qi and blood stasis, followed by accumulation in tumors which improves their invasive and metastasis abilities [259]. The relative lack of oxygen, high glucose absorption rate, glycolysis rate, and accumulation of acid metabolites or other abnormal intercellular components in tumors are manifestations of phlegm caused by the abnormal metabolism of body fluids and accumulation of dampness. The active components of the crude drugs resolving phlegm and eliminating dampness and their effects on tumors are summarized in Table 4.
Table 4.
Mechanism of the CHMs that resolve phlegm and eliminate dampness and their active components on tumor cells and the TME.
| CHMs | Active components | Tumor cells | TME | References | ||||
|---|---|---|---|---|---|---|---|---|
| Apoptosis induction | Proliferation inhibition | Autophagy regulation | Anti-multidrug resistance | Migration and invasion inhibition | Anti-angiogenesis | |||
| Acorus tatarinowii schott (shi chang pu) | Alpha-asarone | √ | √ | √ | √ | √ | [263,264] | |
| Rhizoma alismatis (ze xie) | Alisol B and Alisol B23-acetate | √ | √ | √ | √ | √ | [265,266] | |
| Angelica dahurica (bai zhi) | Imperatorin and Isoimperatorin | √ | √ | √ | √ | [267–270] | ||
| Fructus citri sarcodactylis (fo shou) | Bergapten | √ | √ | √ | [271,272] | |||
| Diosmetin | √ | √ | √ | √ | [273,274] | |||
| Diosmin | √ | √ | [275] | |||||
| Hesperidin and Hesperetin | √ | √ | √ | √ | √ | [276–278] | ||
| Dried tangerine peel (chen pi) | Nobiletin | √ | √ | √ | √ | √ | [279–281] | |
| Tangeretin | √ | √ | √ | √ | [282–284] | |||
| Magnolia bark (hou pu) | Magnolol and Honokiol | √ | √ | √ | √ | √ | √ | [285,286] |
| Pipernigrum (hu jiao) | Piperine, Citronellal, and Piperlongumine | √ | √ | √ | √ | √ | √ | [287–290] |
The underlying mechanism includes regulating immunity, inhibiting tumor growth and angiogenesis, and inducing tumor cell apoptosis. Furthermore, resolving phlegm and eliminating dampness CHMs can effectively inhibit tumor metastasis by: (1) regulating expression of cell adhesion molecules (such as E-cadherin and vimentin) [260]; (2) inhibiting expression of MMP-2 and −9 proteases to delay extracellular matrix degradation [261]; (3) regulating abnormal neovascularization; (4) reversing the epithelial mesenchymal transition of tumor cells through the Wnt/β-catenin signaling pathway [262], ERK pathway, and TGF-β pathway to inhibit tumor metastasis.
3.5. CHMs that strengthen vital energy and consolidate body resistance and their active components
There are 53 kinds of strengthening vital energy and consolidating body resistance herbs and decoction pieces recorded in ChP (2015 edition, volume I). Of these, 47 are reported to exhibit anti-cancer effect, 2 are used as crude materials, 20 as extraction, and 25 as both extraction and active components.
According to TCM, after radiotherapy, chemotherapy or surgery, the human body is in a state of qi and blood deficiency. If we continue to use the “attack” method, the burden on the immune system will increase. Therefore, the treatment should change to maximize the recovery of bone marrow and immune function, that is, reconstruction of positive qi [61]. Chinese medical plants can regulate the immune response of hosts by elevating the proportion of effector T cells (T lymphocytes) to Tregs, inducing interferon gamma (IFN-γ) production in effector T cell, the phagocytic function of macrophages, the activity of natural killer cells (NK cells), promoting spleen dendritic cell (DC) differentiation, reducing myeloid-derived suppressor cells (MDSCs), decreasing TGF-β and Interleukin (IL)-10 levels, prevention of both IL-2 consumption and IL-2 expansion, regulating the balance of immune cell differentiation, and enhancing immune ability [291,292]. The active components of the crude drugs strengthening vital energy and consolidating body resistance and their effects on tumors are summarized in Table 5.
Table 5.
Mechanism of the CHMs that strengthen vital energy and consolidate body resistance and their active components on tumor cells and the TME.
| CHMs | Active components | Tumor cells | TME | References | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Apoptosis induction | Proliferation inhibition | Autophagy regulation | Anti-multidrug resistance | Migration and invasion inhibition | CSCs population reduction | Anti-angiogenesis | TAFs | DCs | TAMs | NK cells | T cells | MDSCs | |||
| Panax ginseng (ren shen) | Ginsenoside Rg3 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | [218,293–297] | ||
| Ginsenoside Rg1, Rh2, Rg5, Rh4, Rk3, Rb2, Rb1, Rd, F2, and CK | √ | √ | √ | √ | √ | √ | √ | √ | [218,293–302] | ||||||
| Radix polygalae (Yuan zhi) | Onjisaponin B | √ | [303] | ||||||||||||
| Ganoderma lucidum (lingzhi) | Ganoderic acid | √ | √ | √ | √ | √ | √ | [304,305] | |||||||
| Semen ziziphi spinosae (suan zao ren) | Jujuboside A/B | √ | [306] | ||||||||||||
| Dioscorea opposite (shan yao) | Diosgenin | √ | √ | √ | √ | √ | [307] | ||||||||
| Fructus ligustri lucidi (nv zhen zi) | Oleuropein | √ | √ | √ | √ | [308] | |||||||||
| Verbascoside | √ | √ | √ | [309,310] | |||||||||||
| Fructus schisandrae chinensis (wu wei zi) | Kadsuphilactone B | √ | [311] | ||||||||||||
| Deoxyschizandrin | √ | √ | [312] | ||||||||||||
| Schisandrin B | √ | √ | √ | √ | √ | [313] | |||||||||
| Atractylodes macrocephalae (bai zhu) | Atractylone, Atractylenolides I and III | √ | √ | √ | √ | √ | √ | [314] | |||||||
| β-Eudesmol | √ | √ | √ | [315] | |||||||||||
| β-Elemene | √ | √ | √ | √ | √ | √ | √ | √ | √ | [316] | |||||
| Ophiopogon japonicus (mai dong) | Ophiopogonin D | √ | √ | √ | √ | √ | [317] | ||||||||
| Ophiopogonin B | √ | √ | √ | √ | [318] | ||||||||||
| Herba epimedium (yin yang huo) | Icariin | √ | √ | √ | √ | √ | √ | √ | √ | [319,320] | |||||
| Baohuoside I | √ | √ | √ | [321,322] | |||||||||||
| Fructus psoraleae (bu gu zhi) | Psoralen and Isopsoralen | √ | √ | √ | √ | √ | √ | √ | [323,324] | ||||||
| Bakuchiol | √ | √ | √ | [325] | |||||||||||
| Isobavachalcone | √ | √ | √ | [326] | |||||||||||
| Acanthopanax senticosus (ci wu jia) | Sesamin | √ | √ | √ | √ | √ | [327,328] | ||||||||
| Puerarin | √ | √ | √ | √ | √ | [329–331] | |||||||||
| Radix astragali (huang qi) | Astragaloside I/II/III | √ | √ | √ | √ | [332] | |||||||||
| Astragaloside IV | √ | √ | √ | √ | √ | √ | √ | √ | [333,334] | ||||||
| Dendrobii caulis (shi hu) | Gigantol | √ | √ | √ | [335,336] | ||||||||||
| Dendrofalconerol A | √ | √ | [337] | ||||||||||||
| Erianin | √ | √ | √ | √ | √ | [338–341] | |||||||||
| Radix angelicae sinensis (dang gui) | Imperatorin, Decursin, and Imperatorol | √ | √ | √ | √ | √ | [342–344] | ||||||||
| Ferulic acid | √ | √ | √ | √ | √ | √[ | √[ | [345–347] | |||||||
3.6. Active components that suppress aerobic glycolysis in tumor cells
Metabolic reprogramming, especially switching to aerobic glycolysis, is a hallmark property of the cancer cells [348,349]. Aerobic glycolysis, also refers to the Warburg effect, could (1) provide tumor cells with the energy they need for survival. Although less adenosine triphosphate (ATP) is produced for per mole glucose through aerobic glycolysis (18~19-fold less than that of glucose metabolism under sufficient oxygen in normal cells), the speed of ATP generation is faster for per unit time, compared to oxidative metabolism of glucose [350]; (2) convert glucose in tumor cells to pyruvate and lactate even in the presence of oxygen. The lactate accumulation in the extracellular space results in an acidic TME (pH<6.8). The acidification of TME enhances chemotherapy resistance, induces epithelial-to-mesenchymal transition and promotes tumor migration and metastasis [351]; and (3) provides a large amount of glycolytic intermediates for the synthesis and metabolism of the tumor cells, including nucleotides, lipids and nonessential amino acids [352].
Acceleration of the glycolysis is triggered by transcription factor hypoxia-inducible factor-1α (HIF-1α) through PI3K/Akt/mTOR pathway, which associates with increased glucose transport through glucose transporters (GLUT-1 and GLUT-3) and upregulated glycolytic enzymes, such as hexokinase (HK), pyruvate dehydrogenase kinase 1 (PDK-1), lactate dehydrogenase A (LDH-A) and phosphofructokinase (PFK). Although the internal mechanism of aerobic glycolysis is still elusive, glycolysis suppression has been considered as a potential strategy for metastasis inhibition and anti-tumor treatment. The clinical application of current glycolysis inhibitors (such as 2-deoxyglucose and 3-bromopyruvic acid) are limited due to the serious systemic adverse effects [353]. Therefore, discovering natural products with high safety targeting glycolysis pathway is highly appreciated. The active components from CHMs for cancer cells aerobic glycolysis inhibition and their mechanism are summarized in Table 6.
Table 6.
The active components from CHMs for cancer aerobic glycolysis inhibition and their mechanism.
| CHMs | Active components | Mechanism | Targets | Cancer model | Routes | References |
|---|---|---|---|---|---|---|
| Glycyrrhiza glabra (guang guo gan cao) | Glabridin | Inhibition of glucose transport and lactate formation | Downregulation of the GLUT-1 and LDH-A |
In vitro MDA-MB-231 cells |
\ | [354] |
| Pulsatilla chinensis (bai tou weng) | Quercetin | Inhibition of glucose transport and lactate formation | Downregulation of the GLUT-1, LDH-A and pyruvate kinase M2 (PKM2) |
In vivo Balb/c mice xenografted breast cancer (MCF-7 cells) In vitro MCF-7 and MDA-MB-231 cells |
Intraperitoneal injection | [355] |
| Oleanolic acid | Blockade of HIF-1α-mediated aerobic glycolysis | Reduction of the nuclear abundance of yes-associated protein |
In vitro MKN-45 and SGC-7901 cells |
\ | [356] | |
| Salvia miltiorrhiza (dan shen) | Rosmarinic acid | Suppression of glucose consumption and lactate generation | Inhibition of the expression of HIF-1α |
In vitro HCT-8, HCT-116, Ls174-T, and Lovo cells |
\ | [357] |
| Birch bark (hua shu pi) | Betulinic acid | Inhibition of lactate production, glucose uptake and extracellular acidification rate | Suppression cancer glycolysis via caveolin-1/NF-κB/c-Myc pathway and upregulation of glucose-regulated protein 78 |
In vivo Mammary-tumor-prone transgenic mice/ Balb/c mice lung colonization breast cancer (MDA-MB-231 cells) In vitro MDA-MB-231, BT-549 and MCF-7 cells |
Intraperitoneal injection | [349,358] |
| Cortex pseudolaricis (tu jing pi) | Pseudolaric acid B | Inhibition of glucose uptake, and ATP generation | p53 mutation or inactivation |
In vitro H1299 cells |
\ | [359] |
| Rabdosia rubescens (dong ling cao) | Oridonin | Inhibiting glucose uptake and reducing lactate export | Downregulation of the protein levels of GLUT1 and monocarboxylate transporter 1 (MCT1) |
In vivo Balb/c mice xenografted colorectal cancer (SW480 cells) In vitro p53-mutated colorectal cancer cells |
Intraperitoneal injection | [360] |
| Radix Dichroae (chang shan) | Halofuginone | Inhibition of glycolysis and lipid biosynthesis | Inhibition of protein kinase B/mammalian target of rapamycin complex 1 (Akt/mTORC1) pathway |
In vivo Balb/c nude mice xenografted colorectal cancer (HCT116 cells) In vitro SW480, HCT116, SW620, HT29 and DLD-1 cells |
Intraperitoneal injection | [361] |
| Radix liquiritiae (gan cao) | Licochalcone A | Inhibition of the glucose consumption and lactate production | Inhibition of HK2 expression and blockade of the Akt signaling pathway |
In vivo Balb/c nude mice xenografted gastric cancer (MKN45 cells) In vitro MKN45 and SGC7901 cells |
Intraperitoneal injection | [362] |
| Salvia miltiorrhiza (dan shen) | Tanshinone IIA | Dysregulation of gluconeogenesis | Suppression of the expression of lactate dehydrogenase B chains and malate dehydrogenase 1 |
In vitro CRL-1739 cells |
\ | [363] |
| Inhibition of glycolysis | Downregulation of glucose-6-phosphate isomerase (GPI) | |||||
| Cortex fraxini (qin pi) | Esculetin | Inhibition of the rates of glucose consumption and lactate production | Alteration of the conformation of glycolysis-related enzymes, phosphoglycerate kinase 2 (PGK2), glycerol-3-phosphate dehydrogenase (GPD2), and GPI |
In vivo Balb/c mice xenografted liver cancer (H22 cells) In vitro HepG2 cells |
Intraperitoneal injection | [364] |
4. Surface functionalization strategies of liposomes for monoconstituents of CHMs to treat cancer
A wide spectrum of bioactivities has been found in CHM-derived monoconstituents, and most have also shown anti-tumor efficacy through multiple mechanisms, including inhibition of angiogenesis, tumor progression, invasion and metastasis. Although they have important roles in different anti-tumor processes as previously discussed, there have been limited anticancer applications of these plant-derived medicines (alkaloids, saponins, flavonoids, organic acids, and so on) [4,365]. This is primarily due to the following. First, their low hydrophilicity (terpenoids such as oridonin, oleanolic acid and andrographolide; polyphenols such as curcumin, resveratrol, quercetin, silymarin, and puerarin; alkaloids such as tetrahydropalmatine and tetrandrine) results in low cellular uptake and low chemical stability. Second, some active components (saponins, such as saikosaponin, ginsenoside Rg1) undergo a series of reactions in animals to be transformed into water-soluble metabolites, which are then excreted in urine and bile, and some drugs are also excreted in their original form. They generally show low protein binding in blood and clearance by the reticuloendothelial system (RES) due to their hydrophilicity, related to the number and location of glycosides [366,367]. Therefore, they are rapidly eliminated, and these poor pharmacokinetic behaviors limit their effectiveness. In addition to the negative properties, side effects also play a vital role in impeding their clinical use. For example, ophiopogon, saponin, and ginsenosides cause hemolysis phenomena [368,369]. Some alkaloids produce neurotoxicity and hepatorenal toxicity [365]. Furthermore, high-frequency administration of some bioactive drugs is necessary to maintain their efficacies (such as triptolide and podophyllotoxin), which leads to low compliance and even cumulative toxicity in patients [370]. To overcome these limitations, the use of drug delivery systems has been used. In TCM, these approaches are used to guide the clinical application to expand from supporting the healthy energy and combating poison with poison to “supporting the vital qi in the disease area, and targeted combating poison with poison” during treatment development [371,372]. Nowadays, various types of drug carriers have been developed for TCM which target delivery to cancer cells or animal xenografts, including polymeric nanoparticles, iron oxide nanoparticles, liposomes, micelles, and dendrimers [373].
Liposomes have a vesicular structure composed of lipid bilayers and were discovered by Bangham et al. in 1965 [374]. Liposomal delivery is an ideal dosage form for formulations available in the market for clinical use of antitumor therapeutic compounds, such as Doxil® (PEGylated liposomal doxorubicin; Centocor Ortho Biotech Inc., USA), DaunoXome® (non-PEGylated liposomal daunorubicin; Diatos, France), and Myocet® (non-PEGylated liposomal doxorubicin; Sopherion Therapeutics, USA) [375]. Liposomes can encapsulate both hydrophobic and hydrophilic agents within the lipid bilayer and inner water phase, respectively. They are characterized by high biocompatibility, elevated encapsulation efficiency, and easy modified for optimization of their properties, such as increased blood circulation time and receptor-mediated site-specific distribution [4,376,377]. Furthermore, liposomes can reduce the incidence of adverse reactions, by reducing local drug amount distributed in normal tissues [378]. Although growing numbers of investigations of TCM-loaded liposomes have proven to produce a strong anticancer response, there is still much room for improvement of anti-tumor liposomal TCM treatment via injection, and significant advances are continuously achieved.
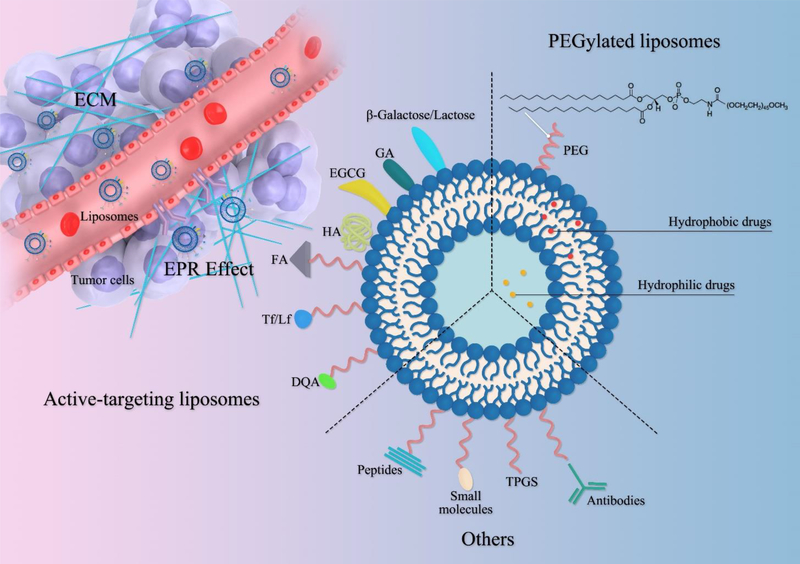
Here we focus on targeting delivery of monoconstituents of CHMs to cancer cells and overcoming the barriers of the TME, including current strategies for achieving these goals by functionalized liposomal surfaces, taking advantages of physiological and biochemical differences between cancerous tissue and normal tissue (Figure 2). External stimuli-driven tumor targeting and drug release, such as magnetism, heating, and laser, fall outside the scope of this review.
Figure 2.
Overview of tumor-specific targeting strategies of liposomes for delivery of monoconstituents of CHMs (Abbreviation: EPR effect, enhanced permeability and retention effect; PEG, polyethylene glycol; TPGS, D-alpha tocopheryl polyethylene glycol 1000 succinate; DQA, dequalinium; Tf/Lf, transferrin/lactoferrin; FA, folic acid; HA, hyaluronic acid; EGCG, epigallocatechin 3-gallate; GA, glycyrrhetinic acid).
4.1. Long-circulating liposomes
Conventional liposomes are often composed of cholesterol and soy phosphatidylcholine in specific proportions and are not modified with other moieties [365]. These vesicles always show as significant interaction with the RES, mainly residing in the liver and the spleen, due to the opsonization as foreign particles, and are rapidly cleared from the blood circulation before accumulating in tumors [379]. A widely used strategy to achieve RES-avoidance is to graft the vesicle surface with polyethylene glycol (PEG), taking advantage of steric repulsion to create a long circulating “stealth” effect. PEG creates a hydrated outer shell, which also shields the nanoparticles from recognition and clearance by the RES. PEG grafting results in an extended drug half-life and improved tissue distribution [148,380,381]. The increased blood circulation time of small-sized liposomes (40–200 nm) achieved by PEG-lipid incorporation in bilayers enhances drug accumulation in tumors, where leaky vascular structure is found. This strategy maximizes the enhanced permeability and retention (EPR) effect. This strategy is called passive targeting, which improves the therapeutic effect of entrapped agents. Various other polymers have been reported to improve blood circulation time of liposome, but PEG remains the primary choice thus far [382–385].
PEG2000 (molecular weight of 2000) is the most frequently used PEG polymer to avoid RES uptake [386,387]. PEG is usually covalently attached to a lipid (PEG-lipid), such as distearoylphosphatidylethanolamine (DSPE), which can be incorporated into the lipid bilayers through hydrophobic interaction. Due to the amphiphilicity of the PEG-lipid, the surface area available for PEGylation is quite limited, with a maximum of 7±2 mol% to maintain membrane integrity [388]. Huang’s group formulated a membrane-core structured Lipid-Calcium-Phosphate (LCP) nanoparticle with a surface that can be modified with various amounts of PEGylation, up to 20 %. It was found that a high density of PEG is required to achieve the steric stabilization and thus create the “stealth” property for particles, according to the concentration and type of PEG-lipids [386]. The long-circulating liposomes with active components of CHMs overcome their disadvantages in physicochemical properties and their applications are shown in Table 7.
Table 7.
Long-circulating liposomes with active components of CHMs overcome their disadvantages in physicochemical properties and their application
| Entrapped components | Disadvantages | Drug loading | Cancer model | Main results | Reference |
|---|---|---|---|---|---|
| β-Elemene | Phlebitis, fever, pain and induced bleeding are caused by solubilizing agents after intravenous injection | Ethanol injection method |
In vivo Kunming mice |
In vivo The size of PEGylated liposomes was 221.4 nm and the EE% was 92.7%. The AUC and half-life of PEGylated liposomes were both enhanced, being 1.5- and 6.0-fold larger than those of conventional β-elemene liposomes and free β-elemene, respectively. |
[389] |
| Brucine | The therapeutic amount of brucine is close to the toxic amount | Ethanol injection and ammonium sulphate gradient methods | \ | Different molecular weights of PEG were screened for their modification on the liposomes surface. The size of PEGylated liposomes was 120 nm with the EE% at 93.7%. The liposomes had a better sustained-released effect than free brucine and good stability in 4 °C. | [390] |
| Berberine | Hypotension and vasodilation, and a quick and wide distribution to major organs after solution injection | pH gradient-film dispersion method |
In vivo Balb/c nude mice xenografted gastric tumor (SGC-7901 cells) |
In vivo PEG-modified berberine liposomes significantly increased circulation retention compared to berberine solution and proved effective and safe in suppressing the 45.8 % tumor growth in nude mice. |
[377,391,392] |
| Oxymatrine | Short half-life (133 min) in human body after intramuscular injection | Thin-film dispersion, reversed-phase evaporation, pH gradient and ammonium-sulfate gradient methods | \ | Oxymatrine liposomes could be produced with EE% at 57.2% by ammonium-sulfate gradient method. The ammonium sulfate concentration, incubation time, and drug-to-lipid ratio greatly influenced the EE%, while the incubation temperature had almost no effect on EE%. | [393] |
| Oridonin | Low water solubility | Ethanol injection method followed by freeze-drying |
In vivo SD rats |
In vivo The levels of stealth liposomal oridonin in the blood were increased, with MRT about 2- and 6-fold longer than that of conventional liposomes and free oridonin solution, respectively. |
[394] |
| Artemisinin | Low water solubility | Ethanol injection method |
In vitro MCF-7 cells |
In vitro The cytotoxicity effect of pegylated liposomal artemisinin was greater (IC50=1.58 μg/mL) than that of liposomal artemisinin. |
[395] |
| Glycyrrhetinic acid (GA) | Low water solubility | Film-dispersion method |
In vivo SD rats |
GA-loaded PEGylated liposomes had a 2.75-fold larger AUC, a 1.7-fold longer MRT, and a 0.4-fold lower CL, compared to GA solution. | [396] |
| Oleanolic acid (OA) and Doxorubicin (DOX) | Low aqueous solubility and low permeability across the intestinal mucosa | Ethanol injection method |
In vivo Balb/c nude mice xenografted liver tumor (HepG2 cells) In vitro) HepG2 cells |
In vivo OA-loaded liposomes formulation inhibited tumor growth and OA had a protective effect to attenuate DOX toxicity.) In vivo) The IC50 value for codelivery of OA and DOX in liposomes is significantly lower than OA-loaded liposomes alone. |
[397] |
| Honokiol | Poor stability and water solubility | Ethanol injection method followed by freeze-drying |
In vivo BALB/c nude mice xenografted NSCLC tumor (HCC827 and H1975 cells) |
In vivo Liposomal honokiol showed antitumor activities in four xenografted models, including inoculating HCC827 (gefitinib-sensitive) and H1975 (gefitinib-resistant) cells. |
[398] |
| Plumbagin (PLB) | Severe side effects caused by solubilizing agents in injection | Film hydration method |
In vivo C57BL/6J mice bearing melanoma (B16F1) |
In vivo The long circulating liposomes exhibited a 36.4-fold increased plasma and significantly less CL in comparison to free PLB. The PEGylated liposomes significantly delayed tumor growth with median survival value at 27 days. |
[399] |
| Quercetin (Que) and Adriamycin (ADM) | Low aqueous solubility, low bioavailability, and instability during processing and storage | Film-ultrasound technique with ammonium sulfate gradient method |
In vivo BALB/c nude mice xenografted breast cancer (MCF-7/ADR) In vitro ADM-resistant cell strains (HL-6/ADR and MCF-7/ADR) |
In vivo Liposomes enveloping QUE and ADM extended the half-life and increased the blood concentration of ADM, with the highest inhibition rate of tumor growth. In vitro The combination of ADM and QUE can enhance cell toxicity to ADM-resistant cells, achieving 4.81- and 3.21-fold higher in HL-6/ADR and MCF-7/ADR, respectively. |
[400] |
| Crocin | Water solubility, and instability during processing and storage | Film-ultrasound technique with extrusion |
In vivo BALB/c mice xenografted colorectal cancer (CT26 cells) In vitro CT26 cells |
In vivo Liposomal crocin (50 and 100 mg/kg) significantly prolonged survival time compared with crocin and free doxorubicin, in a dose-dependent manner. In vitro PEGylated liposomes with higher cholesterol retained crocin more effectively and thus showed higher IC50 values in C26 cells compared to crocin alone. |
[401] |
| Silibinin and Glycyrrhizic acid | Poor solubility in water and oil and low bioavailability | Film hydration method with HEPES buffer and sonication |
In vitro HepG2 and fibroblast cell lines |
In vitro The IC50 for pegylated liposomal silibinin and glycyrrhizic acid was 10-times and 2.3-times lower than that of free silibinin and glycyrrhizic acid, in the HepG2 and fibroblast cells, respectively. |
[402] |
| Resveratrol (Res) and DOX | Low water solubility | Film hydration method |
In vitro NT8e cells |
In vitro The formulation exhibited a superior effect on the apoptosis proteins and cell cycle but had a higher IC50 value than that of free DOX. |
[403] |
| Celastrol | Highly hydrophobic nature | Film hydration method and extrusion |
In vitro Vertebral-cancer of the prostate (VCaP) cells |
In vitro The lipid composition influenced the EE%, serum stability and drug release of celastrol from PEGylated liposomes. |
[404] |
| Ursolic acid | Low water solubility | Film hydration method and homogenization |
In vivo Nude mice xenografted breast tumor (MCF-7 cells) |
In vivo Magnetic resonance parameters of tumor-bearing animals showed a possible antiangiogenic effect induced by ursolic acid-loaded liposomes |
[405] |
| Caffeic acid, Carvacrol, pterostilbene, Caffeic acid phenethyl ester (CAPE), Carvacrol, N-(3-oxo-dodecanoyl)-l-homoserine lactone (3-oxo-C12-HSL), Thymol, and Resveratrol | Poor water solubility or instability | Film hydration method |
In vivo Balb/c nude mice xenografted head and neck squamous-cell carcinoma (14C-tumor cells) |
In vivo These compounds were entrapped in liposomes with better stability. Liposomal 3-oxo-C12-HSL and resveratrol inhibited tumor growth significantly as compared to control group. |
[406] |
| Betulinic acid (BA) | Poor water solubility | Ethanol injection technique |
In vivo Kunming mice xenografted cervical cancer (U14 cells) In vitro HepG2 cells and HeLa cells |
In vivo The PEGylated BA-loaded liposomes with sustained release profile can effectively accumulate in tumor tissues. The tumor inhibitory rate of PEGylated BA was 1.2-fold stronger than that of BA liposomes. |
[407] |
Abbreviations: AUC, the area under the plasma concentration-time curve; MRT, mean residence time; NSCLC, non-small cell lung cancer; CL, clearance; IC50, half inhibit concentration
4.2. Active-targeting liposomes
Active-targeting liposomes exert their targeting capabilities through a series of affinity-based interactions (such as that between ligands and receptors) on the diseased cells and release drugs specifically into the target sites, with minimum deposition at healthy tissues or with less off-target effects [365,408,409]. Active- and passive-targeting liposomes are transported to tumor sites in the same manner. Only after arriving in the tumor tissue are ligands able to work, and it was found that some PEGylated liposomes were unable to release drugs at the tumor site. Moreover, with an increasing number of injections administered, PEGylated materials are prone to be cleared from the blood, which is called the accelerated blood clearance (ABC) phenomenon [410–412]. This means that long blood circulation times and efficient tumor target binding are both required for appreciable tumor accumulation of ligand-targeted liposomes [385]. Active targeting ligands could be attached directly to the liposomal surface, together with PEG-lipids, or conjugated to the distal end of PEG chains and incorporated into liposome membrane as ligand-PEG-lipids [413–416].
There are two issue to consider when taking advantage of a specific ligand to functionalize liposomes. First, it is important to optimize the ligand density on the liposome surface by relevant surface engineering techniques to properly form a targeted liposome system. High ligand density in a feasible range could promote target binding; however, issues such as aggregation may be caused by increasing ligand density over the optimal density [417]. Second, as the binding affinity for a tumor-related ligand rises, liposomes would bind tightly to the cancer cells they first encounter near blood vessels. This might block the tumor penetration of vesicles into cancer regions far away. Then subsequent vesicles are unable to reach free receptors and are thus forced back out of the tumor. It thus diminishes effective targeting [418,419]. In other words, exceedingly strong affinity ligands may impair tumor penetration and fail to increase tumor drug accumulation. Even if with increased tumor accumulation, anti-tumor efficacy is not necessarily improved if drug accumulation only takes places near blood vessels [385]. Various liposomal active-targeting ligands for active components of CHMs for solid tumor therapy are shown in Table 8.
Table 8.
Various liposomal active targeting ligands for active components of CHMs targeted to cancer cells and the TME
| Targeting ligands | Targeting site | Entrapped components | Drug loading | Cancer model | Main results | Reference |
|---|---|---|---|---|---|---|
| Over-expressed receptors on the surface of cancer cells | ||||||
| β-Galactose | Asialoglycoprotein receptors | Glycyrrhetinic acid (GA) | Film dispersion method |
In vivo Kunming mice In vitro HepG2 cells |
In vivo Galactosylated GA-liposomes (Gal-GA-LP) showed long-circulating profile in Kunming mice with the MRT of Gal-GA-LP being 1.48- and 1.3-fold increasement when compared with GA solution and nontargeting GA-loaded liposomes, respectively. In vitro Gal-GA-LP showed 1.4-fold higher drug concentration in cells than that of GA-liposomes. |
[420] |
| Oridonin (ORI) | Ethanol injection method |
In vivo Kunming mice |
In vivo The MRT of ORI-loaded liposomes modified with galactose (NOH-ORI-LP) was 5.56-fold longer than that of ORI solution in mice, with lower clearance from liver. |
[421] | ||
| Lactose | Matrine (MA) | Reversed-phase evaporation method |
In vivo Kunming mice In vitro HepG2 cells |
In vivo The accumulation of MA in targeting liposomes in the mice liver was 2.7 times higher than in the spleen, 3 times higher than in the lung, 6.6 times higher than in the kidney, and 8.5 times higher than in the heart. In vitro Inhibitory rate of targeting liposomes of MA was 1.6- and 1.83-fold higher than those of conventional MA liposomes and MA solution at 0.5 mg/mL concentration, respectively. |
[422] | |
| Transferrin (Tf) | Transferrin receptors | Tetrandrine and Vincristine | Film dispersion method |
In vivo Glioma-bearing ICR mice model In vitro C6 cells, C6/ADR cells and murine brain microvascular endothelial cells (BMVECs) |
In vivo Tf targeting liposomes improved accumulation in brain tumor tissue with fluorescent signal maintained up to 48 h and animals exhibited prolonged survival time (42.67 ± 3.56 days) compared with the saline group. In vitro The targeting liposomes showed significantly prolonged circulation time and increased accumulation in tumors. |
[423] |
| Lactoferrin (Lf) | Honokiol and Daunorubicin | Film dispersion plus ammonium sulfate gradient methods |
In vivo Glioma-bearing ICR mice model In vitro C6 cells and BMVECs |
In vivo The targeting liposomes improved accumulation in brain tumor tissue indicated by fluorescence probe and conferred prolonged survival time. In vitro Lf modified daunorubicin plus honokiol liposomes enhanced drug transportation across the blood-brain barrier, inhibited C6 cells invasion, and destroyed vasculogenic mimicry channels. |
[424] | |
| Folic acid | Folate receptors (FR) | Curcumin (Cur) | Film dispersion method |
In vitro KB, Hela, and A549 cells |
In vitro FR-positive cells endocytosed more FR-targeting liposomes than nontargeted liposomal Cur. Targeted liposomes more effectively inhibited cellular proliferation and caused higher dose- and time-dependent apoptosis. KB cells were more sensitive to FR-targeting liposomal Cur than Hela cells. |
[425] |
| Baicalin (Bai) | Film hydration and homogenization |
In vitro HeLa cells |
In vitro Cytotoxicity and cellular uptake of Bai-loaded FR-targeting liposomes were higher than that of non-targeted liposomes. |
[426] | ||
| Ursolic acid (UA) | Film hydration and extrusion |
In vivo Balb/c nude mice xenografted oral cancer (KB cells) In vitro KB cells |
In vivo Compared with passive targeting liposomes, FA-PEG modified UA liposomes significantly inhibit oral tumor growth. In vitro The EE% of UA was more than 85%. FR targeting UA PEGylated liposomes showed improved tumor cell uptake, proliferation inhibition, and apoptotic effects as compared with nontargeting PEGylated liposomes. |
[427] | ||
| Glycyrrhetinic acid (GA) | GA receptors | Ginsenoside Rh2 | Film hydration method |
In vitro SMMC-7721 cells |
In vitro IC50 of GA-modified liposomes in SMMC-7721 cells increased by 0.5- and 0.6-fold compared with Rh2 solution and Rh2-loaded liposomes, respectively. |
[428] |
| Baicalin | Film hydration and ethanol injection methods | \ | The size of GA modified baicalin liposomes was 150 nm with EE% at 41.8%. | [429] | ||
| Brucine | Ethanol injection method | \ | The formulation of GA modified brucine liposomes was optimized with a lipid to cholesterol (w/w) ratio of 6, lipid to drug (w/w) ratio of 15, and lipid to GA (w/w) ratio of 35 (g/g). The size of targeted liposomes was 147.2 nm and the EE% of brucine was 82.8%. | [430] | ||
| Wogonin | Reversed-phase evaporation method |
In vivo ICR mice xenografted liver cancer (HepG2 cells) In vitro HepG2, L-02 and LX-2 cells |
In vivo GA modified liposomes rapidly and highly accumulated in the tumor and liver shortly after administration. The tumor inhibitory ratio of targeted liposomes was 1.7-fold higher than that of nontargeted liposomes. In vitro GA-modified liposomes showed 1.46-fold higher growth inhibition than that of non-targeted liposomes in HepG2 cells. |
[428] | ||
| Cur | Ethanol injection method |
In vitro HepG-2 and H22 cells |
In vivo GA modified liposomes loaded with Cur inhibited tumor growth, reduced tumor microvascular density and regulated the expression of Caspases3 and VEGF proteins in H22 tumor tissues. In vitro GA modified liposomes induced more HepG2 cellular apoptosis and tumor growth inhibition. |
[431] | ||
| Epigallocatechin 3-gallate (EGCG) | 67LR | Doxarubicin | Film hydration method |
In vivo C57BL/6 mice melanoma (B16F10 cells) |
In vivo Targeting liposomes showed superior anti-tumor effect without observed side effects. |
[432] |
| Targeting cytoplasmic organelles | ||||||
| Mitochondrion | Dequalinium (DQA) | Resveratrol | Film dispersion and extrusion |
In vivo Balb/c nude mice xenografted resistant lung cancer (A549/cDDP cells) In vitro A549 cells and A549/cDDP cells |
In vivo The combination administration of targeting resveratrol liposomes with vinorelbine liposomes exhibited superior prohibition of tumor growth. In vitro The increased mitochondrial uptake and solubility of resveratrol in targeting liposomes promoted inhibitory cell activity by triggering cytochrome C release. |
[433] |
| Berberine | Film dispersion and extrusion |
In vivo Balb/c nude mice xenografted breast cancer (MCF-7 dissociated cancer stem cells) In vitro MCF-7 cells |
In vivo The synergistic effect of targeting berberine liposomes and paclitaxel liposomes exhibited the strongest anti-tumor effect. In vitro Targeting berberine liposomes (1, 5, and 10 μM) could synergistically enhance the toxicity of paclitaxel liposomes in a dose-dependent manner, while achieving selective mitochondrial accumulation. |
[434] | ||
| 4-Carboxybutyl triphenylphosphonium bromide (TPP) or DQA | Resveratrol | Film dispersion and extrusion |
In vitro B16F10 cells |
In vitro TPP and DQA conjugated with DSPE-PEG liposomes carrying resveratrol caused better cellular uptake and selective accumulation in mitochondria. |
[435] | |
| Targeting the TME | ||||||
| Hyaluronic acid (HA) | CD44 and the receptor for hyaluronan-mediated motility | Curcumin (Cur) | Reversed-phase evaporation method |
In vitro HepG2 and A549 cells |
In vitro The binding rate of HA-lipid to Cur-liposomes was 72%. The IC50 values were found to be 0.054, 0.032 and 0.021 mol/ml for free Cur, nontargeted Cur liposomes and modified Cur liposomes, respectively, after incubation with A549 cells overexpressing the CD44 receptor. |
[436] |
| Matrine | Film dispersion, reversed-phase evaporation and ethanol injection methods |
In vivo Balb/c mice xenografted liver cancer (H22 cells) |
In vivo The inhibition rate of HA-modified MA liposomes on H22 hepatocarcinoma was similar to that of cyclophosphamide, and 2.0- and 1.4- fold higher than that of MA solution and MA conventional liposomes, respectively. |
[437] | ||
| Surface modification with antibodies | ||||||
| Anti-CD44 antibody | CD44 receptors | Timosaponin AIII (TAIII) | Film evaporation and ultrasonic methods |
In vivo Balb/c nude mice xenografted liver cancer (HepG2 cells) In vitro HepG2 cells |
In vivo CD44 targeting liposomes showed significantly inhibition on tumor growth, which was 7.2- and 1.3- fold greater than that of free drug and non-targeted liposomes, respectively. In vitro Targeting liposomes were more toxic to tumor cells with lower IC50 than non-targeting liposomes. |
[438] |
| Trastuzumab | Human epidermal growth factor receptor-2 (HER2) | Curcumin and Resveratrol | Film evaporation and extrusion method |
In vitro JIMT1 an MCF-7 cells |
In vitro The immunoliposomes of curcumin and resveratrol showed notably higher cytotoxicity, compared to free drugs. The finding was more prominent for curcumin than resveratrol. |
[439] |
| Surface modification with peptides | ||||||
| T7 (HAIYPRH) peptide | Transferrin receptors | Quercetin | Film hydration method |
In vivo Balb/c nude mice with orthotopic lung cancer (A549-Luc cells) In vitro A549-Luc cells |
In vivo T7 modified liposomes significantly delayed tumor growth and enhanced the lifespan of mice following pulmonary administration. In vitro T7 modified liposomes showed higher cellular uptake and significantly higher tumor-spheroid growth inhibition. |
[440] |
| NGR (Asn-Gly-Arg) | CD13 | Triptolide | Film hydration method |
In vitro Human umbilical vein endothelial cells (HUVEC) |
In vitro Triptolide-loaded liposomes modified with NGR showed stronger cellular toxicity with lowest IC50 on HUVECs at 11 μM. |
[441] |
| Brucine | Ammonium sulfate gradient method |
In vivo Balb/c nude mice xenografted fibrosarcoma (HT1080 cells) In vitro HT1080 cells and HUVEC |
In vivo The highest fluorescence signals in tumors were found in animals treated with NGR-modified liposomes with HSPC and SPC together than that with HSPC or SPC used alone. In vitro The composition of lipids did not exhibit significant influence on EE% of brucine. The cellular uptake of NGR-modified liposomes was about 1.5-fold higher than that of non-targeted liposomes. |
[442] | ||
| RGD (Arg-Gly-Asp) | Integrins | Tetrandrine and Vinorelbine | Film dispersion followed by ammonium sulfate gradient method |
In vivo Glioma-bearing ICR mice model (Doxorubicin-resistant C6 cells) In vitro C6 cells and BMVECs |
In vivo RGD modified vinorelbine and tetrandrine liposomes prolonged elimination half-life compared with free drug, and extended survival times. Strong fluorescent signals of targeting liposomes were observed in tumors within 24 h after administration, while the signals of free drugs was in the liver. In vitro Targeted liposomes significantly enhanced transportation across the BBB and uptake in tumor cells. |
[443] |
| Matrine | Film dispersion followed by pH-gradient method |
In vitro Bcap-37, HT-29 and A375 cells |
In vitro RGD-PEG-DSPE was employed to obtain matrine liposomes with an EE% of 83.13%. Compared with free matrine, targeting liposomes induced stronger inhibition of proliferation and induced more apoptosis in tumor cells. |
[444] | ||
| Shikonin (SHK) | Film hydration method |
In vitro MDA-MB-231 cells |
In vitro RGD modified SHK-loaded liposomes with EE% of 94.9% greatly induced cellular apoptosis by increasing the ratio of Bcl-2-associated X protein (Bax)/Bcl-2, and inhibited cellular proliferation, migration and invasion, as compared with nontargeted SHK-loaded liposomes. |
[445] | ||
| Octaarginine (R8) | Syndecan 4 | Dihydroartemisinin and Epirubicin | Film dispersion method |
In vitro A549 cells |
In vivo R8 modified liposomes increased the selective drug accumulation at tumor sites and showed a targeting capable of reducing tumor volume. In vitro R8 modified liposomes exhibited powerful cytotoxicity on A549 cells, effectively suppressed vasculogenic mimicry channels, and regulated metastasis related proteins. |
[446] |
| R8GD (RGD and R8) | Integrins | Emodin | Film dispersion method |
In vivo Balb/c nude mice xenografted breast cancer (MDA-MB-435S cells) In vitro MDA-MB-435S cells |
In vivo Strong fluorescence signals of R8GD modified liposomes were found in tumors within 24 h after administration. R8GD modified daunorubicin liposomes significantly inhibited tumor growth with the combination of R8GD modified emodin liposomes. Their efficacy was 3-fold stronger than that of nontargeting liposomes combination. In vitro The cellular uptake was increased for targeting liposomes. |
[447] |
| HIV protein transactivator of transcription (TAT) together with HA | Syndecan 4 | Curcumin and Celecoxib | Film hydration method |
In vivo Balb/c mice with orthotopic breast cancer (4T1 cells) In vitro RAW264.7, HUVEC and 4T1 cells |
In vivo NF-κB essential modulator-binding domain (NBD) fused with TAT peptide modified liposomes co-delivering curcumin and celecoxib were coated with HA (HA/TN-CCLP). HA/TN-CCLP could prolong circulation time, increase tumor accumulation, and block lung metastasis. In vitro HA/TN-CCLP improved cytotoxicity, inhibited migration, and enhanced anti-inflammation effect, when compared to nontargeted liposomes. |
[448] |
| tLyp-1 (sequence CGNKRTR) | Neuropilin-1 | Ginsenoside CK and Parthenolide | Film hydration method |
In vivo Balb/c nude mice xenografted lung cancer (A549 cells) In vitro A549 cells |
In vivo Targeting liposomes with hydrophilic PEG shell demonstrated stronger antitumor effect than free drugs combination without observed toxicity. In vitro The cellular uptake of targeted liposomes was through micropinocytosis and the liposomes were escaped from lysosome in a time-dependent manner. |
[449] |
| Surface modification with polymers | ||||||
| D-alpha tocopheryl polyethylene glycol 1000 succinate (TPGS) | \ | Luteolin | Film-dispersion method |
In vivo Balb/c nude mice xenografted lung cancer (A549 cells) In vitro A549 cells |
In vivo TPGS coated liposomes loaded with luteolin were significantly accumulated in tumor sites. The modified liposomes exhibited higher tumor inhibition rate to 51.7% than that of free luteolin. In vitro TPGS coated liposomes improved cytotoxicity in A549 cells by increasing the Bax/Bcl-2 ratio. |
[450] |
| \ | Ginsenoside compound K | Film hydration method |
In vivo Balb/c nude mice xenografted lung cancer (A549 cells) In vitro A549 cells |
In vivo Targeted liposomes delivered ginsenoside compound K into tumor sites, enhanced its permeability and retained it in tumor cells. In vitro The EE% of targeted liposomes was 98.4%, and the size was (119.3±1.4) nm at the ratio of 7:3 (phospholipid: TPGS). The IC50 of targeted liposomes was significantly reduced to 25% of that of free drugs. |
[451] | |
4.2.1. Targeting specific receptors over-expressed on the surface of cancer cells
4.2.1.1. Glycolipids
Liposomes with different glycosyl combining on their surfaces can be differentially distributed in vivo. Liposomes carrying mannose residues are enriched in sinusoids, involving Kupffer cells, endothelial cells, and stellate cells in liver. Galactosylated liposome-entrapped materials were largely distributed to hepatocytes [376,452]. To exhibit significant therapeutic efficacies, various glycolipids with low molecular weight were used to modify the liposomal surface by conjugating the sugar moieties to lipids. Galactosylated liposomes are bound specifically to asialoglycoprotein receptors (ASGPRs) to achieve hepatocyte-specific drug delivery after entering systemic circulation because (1) ASGPRs are specifically over-expressed in hepatocytes; (2) because of the rich blood flow and discontinuous endothelium of the liver, particles can easily access hepatocytes [452,453]. However, interactions between glycolipids and plasma lipoproteins or tissue lipids after intravenous injection may interfere with the integrity of liposomes by removing glycolipids from bilayers, and thus lead to reduced tumor cell selectivity [454]. Thus, cholesterol was used to introduce the galactosyl moiety to liposome surface as the hydrophobic anchor. Cholesten-5-yloxy-N-(4-((1-imino-2-L-D-thiogalactosylethyl)amino)butyl)formamide (Gal-C4-Chol), cholesten-5-yloxy-N-(4-((1-imino-2-L-D-thiomannosylethyl) amino)butyl) formamide (Man-C4-Chol), and cholesten-5-yloxy-N-(4-((1-imino-2-L-L-thiofucosylethyl)amino)-butyl)formamide (Fuc-C4-Chol) are synthesized as different efficient glycosylating agents with cell-specific hepatic targeting potential [455]. Furthermore, when coating the galactosylated liposomes with PEG to obtain the long-circulating effect, the molecular weight of PEG is important because longer PEG chains might retard asialoglycoprotein receptor-mediated uptake of galactosylated liposomes by steric hindrance [456].
4.2.1.2. Transferrin family
The blood-brain barrier (BBB) protects the brain as a highly selective barrier. Nearly all large-molecules and 98% of small-molecule drugs are unable to be transported across the barrier in the active transport manner [423,457]. Transferrin (Tf), an iron transporter, regulates free iron levels in biological fluids. Transferrin receptors (TfR1 and TfR2 or CD77) are lowly expressed in most normal cells, and overexpressed in tumor cells (~100 fold) due to increased iron demand of cancer cells, such as liver cancer cells, brain glioma cells, or endothelial cells of the BBB, thus Tf could target the transferrin receptor and exhibit tumor-specific cell binding [458]. In addition to Tf, lactoferrin (Lf) also belongs to the transferrin family. Drug carriers with Lf modifications cross the BBB via receptor-mediated endocytosis [424]. In fact, Lf is also reported to inhibit the cell cycle in G0/G1 and G2 phases of U87MG cells through downregulation of cyclin D1 and D4 [459].
4.2.1.3. Folic acid
Folic acid (FA, folate or vitamin B9) is a crucial vitamin for nucleotide synthesis in all living cells [385]. Living cells take in FA through folate receptors (FRs)-mediated endocytosis by stimulating the clathrin-independent pathway, which is a characteristic used for macromolecular drug delivery. FRs are highly expressed on lung, ovarian, kidney, breast, brain, endometrial, and colon cancer cells. Limited expression of FRs on most normal tissues, but overexpression on cancer cells are observed. Further, FR density increases with the deterioration of tumor grade or stage [460]. As such, it is suggested that FA can be used as a potential targeting molecule for active drug delivery to tumor cells [425,461]. FA was also reported to be conjugated with chitosan by an acylation reaction to obtain folic acid-chitosan, which could form a coating liposomal system by electrostatic interactions on the surface of the negatively charged nano-liposomes with better stability and sustained release of curcumin [462].
4.2.1.4. Glycyrrhetinic acid (GA)
GA, a pentacyclic triterpeneglycoside, is the hydrolysis product of glycyrrhizin. In addition to its effective anticancer effects against hepatocellular carcinoma, GA was also reported to specifically target hepatocytes where the GA receptors (GA-R) are abundantly expressed on hepatocyte membranes [463,464]. Both GA-R and glycyrrhizin receptor (GL-R) could achieve active hepatic targeting of drug delivery. However, GA-R is more effective because GA binding sites outnumber GL binding sites [465]. Recently, growing interest has developed on carriers modified with GA as hepatocyte-targeted delivery systems [428–431].
4.2.1.5. Epigallocatechin 3-gallate (EGCG)
EGCG, a polyphenol from green tea, has been reported to reduce the expression of MMP-2 and MMP-9, involving in tumor growth, angiogenesis, and metastasis. Moreover, it selectively binds the 67-kDa laminin receptor (67LR) [466], which is more highly expressed on tumor cells, such as breast, bile duct, colorectal cancers, and melanoma cells, than on normal cells [467]. Therefore, EGCG-PEG-modified liposomes loaded with doxorubicin took advantage of not only the apoptosis inducing effect of EGCG, but also the tumor-targeting of EGCG together with the PEG shielding to synergistically combine with the topisomerase II inhibition induced by doxorubicin. The dual-effect liposomes showed outstanding antitumor efficacy on melanoma [432].
4.2.1.6. Anisamide derivatives
Anisamide and its derivatives modified liposomes were proven to be highly selectively targeting to sigma receptors, leading to stronger tumor growth retardation, compared to non-targeted liposomes [468]. Sigma receptors, subdivided into sigma-1 and sigma-2 receptors, are over-expressed membrane proteins on TAFs and tumor cells, such as melanoma, breast tumors, non-small cell lung carcinoma, hepatocellular carcinoma and prostate cancer, and their expression is much higher compared to normal cells [469,470]. Aminoethyl anisamide (AEAA) is a targeting ligand for sigma receptor with high affinity (Kd=9 nM) employed for phytochemicals delivery in other lipidic formulations (emulsions and micelles). Puerarin in DSPE-PEG-AEAA modified nanoemulsions were shown to significantly facilitate chemotherapeutic effect of paclitaxel and activate immune microenvironment, compared to the control group [330]. AEAA targeting nanocarriers loaded with celastrol and mitoxantrone were also demonstrated the increased drug accumulation in tumors, compared to free drugs [471]. Icaritin was co-delivered with doxorubicin in micelles with the composition of poly lactic-co-glycolic acid (PLGA)-PEG-AEAA (PLGA-PEG-AEAA). The AEAA modified fluorescence micelles accumulated 4-fold higher than non-targeted micelles in tumor sites, with much less amount in normal livers [472].
4.2.2. Targeting specific receptors on desired organelles
Mitochondria are membrane-enclosed organelles described as “cellular power plants”. They are an ideal antitumor target due to their involvement in at least six cancer hallmarks, including deregulated cellular energetics, cell apoptosis resistance, expanded invasion and metastasis, immortal replication, genomic instability, and tumor-promoting inflammation [473]. Moreover, tumor cell mitochondria are distinct from their normal counterparts in structures and functions, showing increased reactive oxygen species production, higher mitochondrial membrane potential, and decreased oxidative phosphorylation [474–476]. Given these features, tumor cells require high energy levels for proliferation and are more susceptible to mitochondrial perturbation than healthy cells [433,477]. Consequently, mitochondria-targeting therapies emerge as an attractive means to selectively eliminate cancers [478]. TPP, rhodamine B, DQA, and mitochondrial targeting signal peptides (MTSs) were used to pack or modify onto lipid carriers (mitochondriotropic molecules) to target mitochondria [477,479,480]. Among these molecules, delocalized cationic 4-carboxybutyl triphenylphosphonium bromide facilitates mitochondrial uptake by interacting with the highly negatively charged mitochondrial membrane in cancer cells, leading to its improved accumulation or access to the mitochondria [481]. DQA, a cationic amphiphile, promotes specific mitochondrial accumulation driven by the transmembrane electrical potential [434,482].
4.2.3. Targeting specific receptors on cells in the TME
Tumors consist not only of neoplastic cells, but also of stromal cells (such as tumor-associated fibroblasts, mesenchymal cells, pericytes, occasionally adipocytes, blood and lymphatic vasculature) and immune cells that maintain the TME. The ECM and secreted extracellular molecules also bear tumor development [483–485].
Among ECM components, hyaluronic acid (HA) is the main one characterizing fibrotic process. HA is a natural acidic polysaccharide synthesized by three transmembrane HA synthases (HAS1, HAS2, and HAS3) that is non-toxic, biodegradable, and possesses a strong affinity for CD44 and the receptor for hyaluronan-mediated motility (RHAMM) [486,487]. CD44 is a non-kinase transmembrane cell-surface glycoprotein with endogenous limited expression in healthy cells and highly expressed as one of the most common cancer stem cell surface markers in various cancer types, including breast, prostate, pancreas, colon, and hepatocellular cancer. Therefore, CD44 is a potential receptor for targeting drug delivery systems, which is involved in tumor invasion and metastasis [488–491]. HA can be covalently linked to the polar headgroup of the lipid, or coats cationic liposomes via electrostatic interaction, in order to achieve CD44-receptor targeting drug delivery. A negatively charged HA-based prodrug can also be formed to insert into the lipid bilayers [492].
4.3. Others
4.3.1. Liposomal surface modifications with antibodies
Monoclonal or polyclonal antibodies conjugated on the surface of liposomes that recognize and bind to specific antigens on the surface of targeted tumor cells are called immunoliposomes. They are able to distinguish the target cell and enhance the selective targeting effects of liposomes [376].
Various tumor cell surface markers were reported as potential targets for selective targeting of therapeutic agents, such as CD147, CD133 and CD44 [438]. Antibody targeted therapies are effective for the following main reasons: (1) The over-expressed epitope is found on the cancer cell; (2) The antibody could get better access to the tumor sites than to healthy sites, due to the longer circulation time and EPR effect. Anti-CD44 antibodies modified liposomes were characterized with enhanced cellular uptake through CD44 receptor-mediated endocytosis, and increased selective targeting effect [493,494].
Human epidermal growth factor receptor-2 (HER2) is a member of epidermal growth factor receptor family. It is a transmembrane receptor tyrosine kinase, generally over-expressed in 25–30% of ovarian and breast cancer patients and normally expressed at low levels in adult cells. It is commonly targeted for breast cancer therapy. Trastuzumab (Herceptin®, Genentech, Inc., USA) was the first humanized monoclonal antibody that binds the extracellular domain of the HER2 receptor for clinical breast cancer therapy [495,496]. Trastuzumab coupled HER2-targeted immunoliposomes loaded with curcumin and resveratrol showed notably higher cytotoxicity in HER2 positive human breast cancer cells than that of non-targeting liposomes or free drugs [439].
4.3.2. Liposomal surface modifications with peptides
Peptides (a short amino acid chain) for surface functionalization are non-covalently or covalently bonded to liposome surface through the MAL linkage bond, sulfanyl bond, peptide bond, or disulfide bond [382]. They are divided into cell-targeting peptides and cell-penetrating peptides, having receptor-specific and non-specific binding and internalization, respectively.
4.3.2.1. Cell-targeting peptides (CTPs)
T7 (HAIYPRH) peptide specifically targets a small cavity on the transferrin receptor surface without interfering transferrin binding to transferrin receptor and is then transported inside the cell through endocytosis [440,497].
RGD (Arg-Gly-Asp) or NGR (Asn-Gly-Arg) are ligands for targeting angiogenic blood vessels, which have several proteins that are absent or expressed at much lower levels in established blood vessels. Targeting the tumor vasculature has some advantages, as compared to targets on the cancer cell surface. First, liposomes do not have to penetrate the tumor tissue to find and reach antigens on tumor cell surface, while they have better access to antigens on the tumor vasculature. Second, abnormal rapid proliferation of cancer cells required sufficient nutrients and oxygen for further growth. Therefore, killing of a relatively small number of vascular endothelial cells can destroy a large number of cancer cells supported by these blood vessels. Third, as compared to cancer cells, vascular endothelial cells are more genetically stable and less susceptible to develop drug resistance [385].
RGD (Arg-Gly-Asp) selectively binds to cell surface integrin αvβ3/αvβ5. Integrins are transmembrane glycoprotein receptors that play an essential role in the attachment between a cell and its extracellular matrix [498]. They are overexpressed in cancers (glioma cells, melanoma cells, lung cancer cells, breast cancer cells, and so on) and can enhance disease progression partially through angiogenesis and metastasis, making them worthy molecules for targeted drug delivery into tumor tissues [499,500]. Liposomes modified with RGD have clear benefits as anticancer drug delivery systems over non-targeted liposomes [501]. The ability of NGR peptides to target the tumor vasculature and improve therapeutic outcomes has also been demonstrated. NGR can specifically bind to CD13, which is a transmembrane metalloproteinase, involving in cell migration and angiogenesis. CD13 is not activated in normal vascular endothelial cells but is highly expressed in tumor vasculature and some tumor cells [441,502]. Therefore, the NGR peptide is a potential ligand to target the tumor vascular antigen CD13 with good selectivity and low immunogenicity [503]. The effect of phosphatidylcholine (PC) composition with different Tm used to prepare targeted liposomes has significant effects on the desirable properties of resulting liposomes. PCs with different Tms, 1,2-dipalmitoyl-sn-glycero-3-phosphatidylcholine (DPPC, Tm=41 °C), hydrogenated soy phosphatidylcholine (HSPC, Tm=53 °C), and soy phosphatidylcholine (SPC, Tm blow 0 °C) were investigated and found that the decrease of Tm of lipids facilitated drug release, and more fluidic liposomes exhibited improved cellular uptake [442].
4.3.2.2. Cell-penetrating peptides (CPPs)
CPPs are positively charged short peptides (5–30 amino acids) capable of penetrating cells across the cell membrane through endocytosis [373]. They allow a wide array of biomolecules, from peptides and proteins to nucleic acids, to rapidly and efficiently traverse cytoplasmic membranes.
For polyarginines, a popular cell penetration tool, the optimum chain length is eight arginine units (R8) [446]. The positively charged R8 peptide makes the coated particles positively charged for internalization and mediates efficient cellular uptake micropinocytosis, improving intracellular gene transportation and expression [504]. R8 with positive charges was also combined with RGD in modified emodin as a tandem peptide (R8GD) to treat breast cancer. The targeting mechanism of R8GD includes receptor-mediated drug targeting and electrostatic incorporation [505]. The R8GD-modified emodin liposomes with small particle size and high EE% exhibited a distinct antitumor effect together with R8GD modified daunorubicin-loaded liposomes [447].
HIV protein transactivator of transcription (TAT, YGRKKRRQRRR, residues 47–57 of HIV-1 Tat protein) readily passes through the plasma membrane of uninfected mammalian cells [448]. Full-length TAT could bind the extensively expressed cellular heparan sulfate proteoglycans (HSPGs), integrins and chemokine (C-X-C motif) receptor 4 (CXCR4). HSPG syndecan 4, a potential receptor, enhances the uptake of TAT through energy-dependent micropinocytosis. Recently, TAT was reported to form transient pores and translocate across the membrane by diffusing on these pores, without observed cell death due to leakage [506,507]. After delivery to the cells, endosomal entrapment leads to most of CPP cargos eventually undergo degradation in lysosome or distribute back to cell membrane for recycling and then ejection from the cell, which is called “endosomal escape problem”. It could be solved to some extent by using endosomolytic agents, reversible covalent binding and high-affinity non-covalent binding [508].
The tumor-homing peptide tLyp-1 contains the sequence motif (R/K)XX(R/K). It mediates tissue penetration and tumor targeting through neuropilin-1 dependent C-end rule internalization and overcome the barriers caused by dysfunctional tumor vasculature and high interstitial pressure. The liposomes coated with tLyp-1 initially target the receptor specifically highly expressed on the tumor cell surface, internalize through micropinocytosis and then escape from lysosome in a time-dependent manner [449,509,510].
4.3.3. Surface modifications with polymers
D-alpha tocopheryl polyethylene glycol 1000 succinate (TPGS) is an amphiphilic PEG derivative of vitamin E succinate, with hydrophilic-liphophilic balance value between 15 and 19. Therefore, it is an ideal emulsifier and solubilizer for hydrophobic drugs (67 times higher than polyvinyl alcohol) and greatly enhanced cellular uptake, in vitro cancer cell cytotoxicity [450,511], and prolonged long-circulation pharmacokinetic behaviors [148]. Moreover, TPGS also inhibited multidrug resistance by inhibiting the P-glycoprotein pump [451].
5. Conclusions
This article studied the antitumor effects of active ingredients of CHMs, counteracting toxin with toxin, heat-clearing and detoxifying, promoting blood circulation and removing blood stasis, resolving phlegm and eliminating dampness, and strengthening healthy energy and consolidating body resistance. We find that the active components function to inhibit proliferation, inhibit invasion and migration, induce apoptosis, reverse multidrug resistance, block angiogenesis, suppress aerobic glycolysis and improve immunity. Despite treatment potential, the undesirable physicochemical properties (poor permeability, instability, highly hydrophilicity or hydrophobicity, and toxicity) and unwanted pharmacokinetic profiles (short half-life in blood and low bioavailability) limit the clinical studies of CHMs. Various targeting liposomes have shown immense advantages in the delivery of active components of CHMs, involving improved physicochemical characteristics and pharmacokinetic profiles, enhanced therapeutic efficacies and reduced side effects. This review could be regarded as a useful and informative reference for these CHM components.
6. Limitations and future perspectives
TCMs are the oldest alternative and complementary medicines and there are rising interests on the investigation of CHMs. Using modern technology, the mechanism of some active components of CHMs were determined to some degree at the cellular, molecular, and pharmacological level. However, there are still some issues to consider.
First, there are only a few studies on the effect of single or combined use of monoconstituents on immunology regulation. The clinical application of CHMs are very complex as the act not only through a single factor, but also through multiple targets and based on a holistic approach, pointing to the entire human body. Current research is primarily focused on individual monoconstituent or the combination of active ingredients from CHMs with chemotherapeutic drugs and their bioactions on tumor cells and non-immune tumor microenvironment. The anti-tumor characteristics of plant-derived medicines, including inducing apoptosis, reversing multidrug resistance, regulating angiogenesis, killing TAFs, and inhibiting cell metastasis and invasion, make the combination with chemotherapeutic drugs reasonable and effective. In fact, the improved tumor immune microenvironment caused by phytochemicals is attracting and proved to enhance therapeutically effect with combination of chemo drugs or checkpoint inhibitors. Huang’s group reported that celastrol, a pentacyclic triterpene from Tripterygium wilfordii functioning in counteracting toxin with toxin, induced ICD with mitoxantrone synergistically to remodel immune-suppressive TME with a robust immune memory response in melanoma [471]. In order to avoid using the highly toxicity of CHMs, icaritin from herbs warming kidney-yang, were studied and the combination of icaritin with doxorubicin caused synergistic ICD induction through mitophagy and apoptosis, leading to improved hepatocellular carcinoma treatment [472]. Puerarin could not only downregulate intra-tumoral reactive oxygen species and deactivate TAFs, but also serves as an adjuvant therapy in nanoparticles for programmed cell death-ligand 1 (PD-L1) monoclonal antibody in triple-negative breast cancer model [330]. In fact, the combination of phytochemicals themselves (quercetin and alantolactone) was proved to induce synergistic ICD at low doses in CT26 and 4T1 cells. The micelles loaded with optimal ratio of quercetin and alantolactone stimulated the host immune response and inhibited murine orthotopic colorectal and breast tumor growth [148]. Besides using monoconstituents of CHMs, the components of TCMs could be studied at a relatively complex level, in order to make good use of the resources of TCMs, such as the classical CHM pairs (Pinellia-Aconitum, Scutellaria barbata-Oldenlandia diffusa, Rhizoma coptidis-Fructus evodiae). The effective extractions of Chinese medicine (such as essential oil) could also be potential agents for tumor treatment because their efficacy has already been clinically proven, and the herbs have relatively simple compositions.
Second, as for the targeting preparation of TCMs, the stability, metabolism, synthesis and quality evaluation need to be improved, especially for more complex combinations of ingredients of CHMs, to exhibit synergistic effects for tumor cells and for specific targeting to the TME. Liposomes can realize the co-delivery of medicinal ingredients with different pharmacological effects and physicochemical properties. In fact, by encapsulating all drug combinations in liposomes, the pharmacokinetic behavior of multiple drugs in vivo can be controlled while prolonging their circulation half-life. Multi drugs encapsulation in liposomes modified with targeting ligands makes liposomes versatile carriers, which is suitable for the application of herbal compound recipes in TCM [512,513]. There are several routes of administration for liposomes, and this review selects the route of systematic administration. Compared with the oral administration route, especially for lipophilic compounds from CHMs, the transportation of drugs from liposomal to biological membranes via intravenous injection are different from oral drug delivery [514]. The characteristics of intravenous injection of liposomes with functionalized surfaces different from other routes of administration are maintaining drugs combination at certain ratios, controlling drugs release, permitting selective delivery of drugs to targeting tissue [515–517]. Although the review demonstrated that targeting liposomal nanocarriers are effective and favorable delivery systems for CHMs to treat cancers, it is still at the exploratory stage.
Further investigations are necessary for anti-tumor mechanisms of some potential monoconstituents, especially the identification of subcellular targets and regulation of immunity through modulating immune-suppressive tumor microenvironment and priming immune responses effects. Site-specific and selective liposomal delivery enhances the accumulated drug concentrations at the site of action and comes with superior efficacy and safety in cancer treatments by reducing the cost and duration of therapy.
Acknowledgements
The work was supported by NIH grant CA198999 (L.H.), the National Natural Science Foundation of China (Nos. 81760639, 81603054), the Fund of Health and Family Planning Commission of Jiangxi Province (2016A008), the Natural Science Fund of Jiangxi Province (20171BAB215066), the Foundation of Jiangxi Provincial Education Department (GJJ190666, GJJ190663), Young Jinggang Scholar of Jiangxi Province and “1050” Talents Program (Jing Zhang and Xiang Li), Jiangxi BaiQianWan Talents Program (2017082, Xiang Li), and the Fund for First-Rate Discipline of Chinese Materia Medica (JXSYLXKZHYAO055, JXSYLXKZHYAO056, JXSYLXK-ZHYAO019).
Footnotes
Conflict of interest statement
L.H. is a consultant for Samyang Biopharmaceuticals, PDS Biotechnology, Stemirna and Beijing Inno Medicine. The rest of the authors report no conflicts of interest in this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- [1].Nosrati H, Salehiabar M, Manjili HK, Danafar H, Davaran S, Preparation of magnetic albumin nanoparticles via a simple and one-pot desolvation and co-precipitation method for medical and pharmaceutical applications, Int J Biol Macromol, 108 (2018) 909–915. [DOI] [PubMed] [Google Scholar]
- [2].Zhu DW, Tao W, Zhang HL, Liu G, Wang T, Zhang LH, Zeng XW, Mei L, Docetaxel (DTX)-loaded polydopamine-modified TPGS-PLA nanoparticles as a targeted drug delivery system for the treatment of liver cancer, Acta Biomater, 30 (2016) 144–154. [DOI] [PubMed] [Google Scholar]
- [3].Lu CS, Shieh GS, Wang CT, Su BH, Su YC, Chen YC, Su WC, Wu P, Yang WH, Shiau AL, Wu CL, Chemotherapeutics-induced Oct4 expression contributes to drug resistance and tumor recurrence in bladder cancer, Oncotarget, 8 (2017) 30844–30858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Feng T, Wei YM, Lee RJ, Zhao L, Liposomal curcumin and its application in cancer, Int J Nanomedicine, 12 (2017) 6027–6044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lagoa R, Silva J, Rodrigues JR, Bishayee A, Advances in phytochemical delivery systems for improved anticancer activity, Biotechnol Adv,38 (2020) 107382. [DOI] [PubMed] [Google Scholar]
- [6].Giri TK, Alexander A, Ajazuddin TK Barman S Maity, Infringement of the barriers of cancer via dietary phytoconstituents capsaicin through novel drug delivery system, Curr Drug Deliv, 13 (2016) 27–39. [DOI] [PubMed] [Google Scholar]
- [7].Giri TK, Breaking the barrier of cancer through liposome loaded with phytochemicals, Curr Drug Deliv, 16 (2019) 3–17. [DOI] [PubMed] [Google Scholar]
- [8].Davatgaran-Taghipour Y, Masoomzadeh S, Farzaei MH, Bahramsoltani R, Karimi-Soureh Z, Rahimi R, Abdollahi M, Polyphenol nanoformulations for cancer therapy: Experimental evidence and clinical perspective, Int J Nanomedicine, 12 (2017) 2689–2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bahrami A, Fereidouni M, Pirro M, Biancion V, Sahebkar A, Modulation of regulatory T cells by natural products in cancer, Cancer Lett, 459 (2019) 72–85. [DOI] [PubMed] [Google Scholar]
- [10].Newton JM, Hanoteau A, Sikora AG, Enrichment and characterization of the tumor immune and non-immune microenvironments in established subcutaneous murine tumors, J Vis Exp, 136 (2018) e57685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Miao L, Huang L Exploring the tumor microenvironment with nanoparticles, Cancer Treat Res, 166 (2015) 193–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zeng XJ, Progress in the treatment of cancer with traditional Chinese medicine, Nei Mongol J Tradit Chi Med, 4 (2011) 138–139. [Google Scholar]
- [13].Leong PK, Wong HS, Chen JH, Ko KM, Yang/Qi invigoration: An herbal therapy for chronic fatigue syndrome with yang deficiency? Evid Based Complement Alternat Med, 2015 (2015), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wang XM, Li XB, Peng Y, Impact of qi-invigorating traditional Chinese medicines on intestinal flora: A basis for rational choice of prebiotics, Chin J Nat Med, 15 (2015), 241–254. [DOI] [PubMed] [Google Scholar]
- [15].Lin H, Pi ZF, Men LH, Chen WJ, Liu ZQ, Liu ZY, Uinary metabonomic study of Panax ginseng in deficiency of vital energy rat using ultra performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry, J Ethnopharmacol, 184 (2016), 10–17. [DOI] [PubMed] [Google Scholar]
- [16].Wang JJ, Qi FH, Wang ZX, Zhang ZK, Pan N, Huai L, Qu SY, Zhao L, A review of traditional Chinese medicine for treatment of glioblastoma, Biosci Trends, 13 (2020), 476–487. [DOI] [PubMed] [Google Scholar]
- [17].Yang Q, Tumor microenvironment and its epigenetic mechanism and the essence of TCM syndrome, China Continuing Med Edu, 10 (2018) 151–153. [Google Scholar]
- [18].Li N, Yun KL, Self-made heat-clearing and detoxicating, blood-activating and stasis-resolving formula combined with cinobufotalin in damp heat and toxin type of cervical carcinoma, World Chin Med, 11 (2016) 2324–2326. [Google Scholar]
- [19].Zhang YH, Zhang X, Ye LH, Peng HY, Discussion on the therapeutic method of counteracting toxin with toxin for cancers, J Nanjing Univ Tradit Chin Med, 28 (2012) 105–108. [Google Scholar]
- [20].Wang H, Hou W, Sun GZ, Hua BJ, Review of reinforcing healthy qi and root method in treatment of tumor, World Chin Med, 11 (2016) 2500–2504. [Google Scholar]
- [21].Wang JY, Cheng HB, Application of clearing heat and removing toxicity therapy and counteracting toxin with toxin therapy in tumor treatment, China J Tradit Chin Med Pharm, 33 (2018) 3417–3419. [Google Scholar]
- [22].Wang ZX, Theoretical interpretation on “Toxic” of Chinese medicine and clinical efficacy observation on treatment of common cold with tonifying qi by detoxification (syndrome of qi deficiency and evil invasion) and its influence on cytokine HβD-2, Chengdu University of TCM, 2016, 27. [Google Scholar]
- [23].Shi WJ, Tan JN, Shen WX, Xu CL, Cheng HB, Comparative study of heat-clearing and detoxifying and treating the toxifying disease with poisonous agents therapy in cancer treatment, Lishizhen Med Mater Med Res, 28 (2017) 2184–2186. [Google Scholar]
- [24].Okada H, Mark TW, Pathways of apoptotic and non-apoptotic death in tumour cells, Nat Rev Cancer, 4 (2004) 592–603. [DOI] [PubMed] [Google Scholar]
- [25].Zhu YZ, Zhang GB, Zhu GF, Research progress in mechanism of anti-tumor effects of Chinese medicinal formula, Chin J Exp Tradit Med Form, 23 (2017) 227–234. [Google Scholar]
- [26].Xu W, McArthur G, Cell cycle regulation and melanoma, Curr Oncol Rep, 18 (2016) 34. [DOI] [PubMed] [Google Scholar]
- [27].Lin NM, Zhang RS, Kong WY, Research progress in the mechanism of anti-lung cancer effect of traditional Chinese medicine and its compound preparation, Her Med, 27 (2008) 767–771. [Google Scholar]
- [28].Hong ZD, Mo ZX, Eleven kinds of signal pathways in anti-tumor mechanism of traditional Chinese medicine, Chin J Exp Tradit Med Form, 24 (2018) 205–218. [Google Scholar]
- [29].Kannaiyan R, Manu KA, Chen LX, Li F, Rajendran P, Subramaniam A, Lam P, Kumar AP, Sethi G, Celastrol inhibits tumor cell proliferation and promotes apoptosis through the activation of c-Jun N-terminal kinase and suppression of PI3K/Akt signaling pathways, Apoptosis, 16 (2011) 1028–1041. [DOI] [PubMed] [Google Scholar]
- [30].Li XM, Liu J, Pan FF, Shi DD, Wen ZG, Yang PL, Quercetin and aconitine synergistically induces the human cervical carcinoma HeLa cell apoptosis via endoplasmic reticulum (ER) stress pathway, PLoS One, 13 (2018) e0191062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Wu YH, Research progress in antitumor effect of warming yang preparation of traditional Chinese medicine containing aconitine, Hebei J Tradit Chin Med, 29 (2007) 468–470. [Google Scholar]
- [32].Zhou XJ, Li YH, Tang X, Lv YB, Zhou JB, Aconitine inhibits the proliferation and invasion while induces the apoptosis of esophageal cancer EC-1 cells, Chin Med Biotechnol, 14 (2019) 335–340. [Google Scholar]
- [33].Ye L, Yang XS, Yang Z, Gao S, Yin TJ, Liu W, Wang F, Hu M, Liu ZQ, The role of efflux transporters on the transport of highly toxic aconitine, mesaconitine, hypaconitine, and their hydrolysates, as determined in cultured Caco-2 and transfected MDCKII cells, Toxicol Lett, 216 (2013) 86–99. [DOI] [PubMed] [Google Scholar]
- [34].Feng HT, Zhao WW, Lu JJ, Wang YT, Chen XP, Hypaconitine inhibits TGF-β1-induced epithelial-mesenchymal transition and suppresses adhesion, migration, and invasion of lung cancer A549 cells, Chin J Nat Med, 15 (2017) 427–435. [DOI] [PubMed] [Google Scholar]
- [35].Guan X, Yang TH, Zhang CR, Induction effect of mesaconitine on apoptosis of K562 and K562/DNR cells and related mechanism, Acta Med Univ Sci Technol Huazhong, 46 (2017) 281–284. [Google Scholar]
- [36].Saraswati S, Mathur R, Agrawal SS, Evaluation of strychnine, a plant alkaloid for in vitro antiangiogenesis, apoptosis and antioxidant potential in MCF-7 cancer cells, Eur J Cancer Supplements, 8 (2010) 204. [Google Scholar]
- [37].Zhao LM, Liu YG, Niu ZX, Anti-tumor effect of brucine, Chin J Cancer Prev Treat, 20 (2013) 877–880. [Google Scholar]
- [38].Li M, Li P, Zhang M, Ma F, Brucine suppresses breast cancer metastasis via inhibiting epithelial mesenchymal transition and matrix metalloproteinases expressions, Chin J Integr Med, 24 (2018) 40–46. [DOI] [PubMed] [Google Scholar]
- [39].Zhang C, Zhou SS, Feng LY, Zhang DY, Lin NM, Zhang LH, Pan JP, Wang JB, Li J, In vitro anti-cancer activity of chamaejasmenin B and neochamaejasmin C isolated from the root of Stellera chamaejasme L, Acta Pharmacol Sin, 34 (2013) 262–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Li Q, Wang YJ, Xiao HB, Li YJ, Kan XX, Wang XM, Zhang GL, Wang ZX, Yang Q, Chen X, Weng XG, Chen Y, Zhou BB, Gao Y, Liu XC, Zhu XX, Chamaejasmenin B, a novel candidate, inhibits breast tumor metastasis by rebalancing TGF-beta paradox, Oncotarget, 7 (2016) 48180–48192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Ma DL, Chen C, Wu J, Wang HL, Wu DM, Effect of echinocystic acid on proliferation and apoptosis of breast cancer cell line MCF-7, Chin J Gerontol, 38 (2018) 4041–4043. [Google Scholar]
- [42].Dai Y, Ai TB, Advances on the antitumor active compositions of fructus toosendan and rhizoma corydalis, J Shantou Univ (Nat Sci), 33 (2018) 57–62. [Google Scholar]
- [43].Gamage CDB, Park SY, Yang Y, Zhou R, Tas I, Bae WK, Kim KK, Shim JH, Kim E, Yoon G, Kim H, Deoxypodophyllotoxin exerts anti-cancer effects on colorectal cancer cells through induction of apoptosis and suppression of tumorigenesis, Int J Mol Sci, 20 (2019) 2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kong Y, Wang QH, Shang MY, Xiao JJ, Meng SC, Cai SQ, Effects of active constituents of sinopodophylli fructus on cell proliferation, cell cycle and mitochondrial membrane potential of human breast cancer cell, China Pharm, 28 (2017) 1368–1371. [Google Scholar]
- [45].Liu HG, Li DN, Liu LM, Research progress on the antitumour mechanism of nitidine chloride, J Guangxi Academy Sci, 25 (2009) 187–191. [Google Scholar]
- [46].Xu JJ, Yang R, Yang FJ, Jiao X, Liu YB, Recent advances in the antitumor effects of evodiamine, J Shanghai Jiaotong Univ (Med Sci), 38 (2018) 578–583. [Google Scholar]
- [47].Shi JM, Fan LH, Advances in research on anti-tumor mechanism of amygdalin, Chem Life, 39 (2019) 147–152. [Google Scholar]
- [48].Hu XJ, Zhang XN, Research advances on anti-tumor activity of osthole, Anti-Infect Pharm, 9 (2012) 248–251. [Google Scholar]
- [49].Xu JW, Hu K, Effects of pseudolaric acid B on cell proliferation and apoptosis in lung cancer A549 cells and its mechanism, China Med Herald, 12 (2015) 18–22. [Google Scholar]
- [50].Zhang LM, Geng YM, Sun SP, Zhi FZ, Han LN, Shan BN, Effect and mechanisms of p-hydroxylcinnamaldehyde from Cochinchina momordica seed on differentiation of mouse melanoma B16 cells in vitro, Chin J Cancer Biother, 21 (2014) 282–287. [Google Scholar]
- [51].Yang MH, Li JT, Zhang DD, Liu YX, Xu Y, Wang ZW, Huang XW, Recent advances in anticancer composition and effect of celandine, Ginseng Res, 29 (2017) 55–57. [Google Scholar]
- [52].Panzer A, Joubert AM, Bianchi PC, Haamel E, Seegers JC, The effects of chelidonine on tubulin polymerisation, cell cycle progression and selected signal transmission pathways, Eur J Cell Biol, 80 (2001) 111–118. [DOI] [PubMed] [Google Scholar]
- [53].Cai YY, Wu Y, Yang SY, Miao J, Cao G, Chen G, Anticancer effect of xanthium in vitro and in vivo in HepG2 of hepatocellular carcinoma, World J Integr Tradit West Med, 14 (2019) 946–949. [Google Scholar]
- [54].Lu ZJ, Zhou Y, Song Q, Qin Z, Zhang H, Zhou YJ, Gou LT, Yang JL, Luo F, Periplocin inhibits growth of lung cancer in vitro and in vivo by blocking AKT/ERK signaling pathways, Cell Physiol Biochem, 26 (2010) 609–618. [DOI] [PubMed] [Google Scholar]
- [55].Song J, Shu L, Zhang ZH, Tan XB, Sun E, Jin X, Chen Y, Jia XB, Reactive oxygen species-mediated mitochondrial pathway is involved in Baohuoside I-induced apoptosis in human non-small cell lung cancer, Chem Biol Interact, 199 (2012) 9–17. [DOI] [PubMed] [Google Scholar]
- [56].Cheng HB, Wu MH, Zhou HG, Zhou ZY, Experience of differentiation and treatment of malignant tumor from cancer toxin, BeiJing J Tradit Chin Med, 28 (2009) 844–846. [Google Scholar]
- [57].Li Y, Qian WH, Lu Y, Zheng SZ, Wang AY, Wang YY, Treatment principles and methods of traditional Chinese medicine and tumor microenvironment, J Tradit Chin Med, 52 (2011) 1801–1804. [Google Scholar]
- [58].Liao CP, Booker RC, Brosseau JP, Chen ZG, Mo J, Tchegnon E, Wang Y, Clapp DW, Le LQ, Contributions of inflammation and tumor microenvironment to neurofibroma tumorigenesis, J Clin Invest, 128 (2018) 2848–2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Lu HT, Ouyang WM, Huang CS, Inflammation, a key event in cancer development, Mol Cancer Res, 4 (2006) 221–233. [DOI] [PubMed] [Google Scholar]
- [60].Demaria S, Pikarsky E, Karin M, Coussens LM, Chen YC, El-Omar EM, Trinchieri G, Dubinett SM, Mao JT, Szabo E, Krieg A, Weiner GJ, Fox BA, Coukos G, Wang E, Abraham RT, Carbone M, Lotze MT, Cancer and inflammation, promise for biologic therapy, J Immunother, 33 (2010) 335–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Coussens LM, Zitvogel L, Palucka AK, Neutralizing tumor-promoting chronic inflammation: A magic bullet? Science, 339 (2013) 286–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Wong AST, Che CM, Leung KW, Recent advances in ginseng as cancer therapeutics: A functional and mechanistic overview, Nat Prod Rep, 32 (2015) 256–272. [DOI] [PubMed] [Google Scholar]
- [63].Balkwill F, Mantovani A, Inflammation and cancer: Back to virchow? Lancet, 2001, 357, 539–545. [DOI] [PubMed] [Google Scholar]
- [64].Diakos CI, Charles KA, McMillan DC, Clarke SJ, Cancer-related inflammation and treatment effectiveness, Lancet Oncol, 15 (2014) 493–503. [DOI] [PubMed] [Google Scholar]
- [65].Qiao LM, Zhang PT, The discrimination of clearing heat and detoxicating on lung cancer treatment, China cancer, 23 (2014) 316–321. [Google Scholar]
- [66].Ju DW, Wei PK, Mechanism and application of Chinese materia medica with heat-clearing and toxin-resolving on prevention and treatment in malignant cancer, Jilin J Tradit Chin Med, 27 (2007) 60–62. [Google Scholar]
- [67].Kim YW, Jang EJ, Kim CH, Lee JH, Sauchinone exerts anticancer effects by targeting AMPK signaling in hepatocellular carcinoma cells, Chem Biol Interact, 261 (2017) 108–117. [DOI] [PubMed] [Google Scholar]
- [68].Gong QZ, Xiao D, Feng F, Wen XD, Qu W, ent-Sauchinone as potential anticancer agent inhibiting migration and invasion of human liver cancer cells via suppressing the STAT3 signaling pathway, Chem Biodivers, 15 (2018) e1800024. [DOI] [PubMed] [Google Scholar]
- [69].He ZK, Dong WX, Li QY, Qin CJ, Li YJ, Sauchinone prevents TGF-β-induced EMT and metastasis in gastric cancer cells, Biomed Pharmacother, 101 (2018) 355–361. [DOI] [PubMed] [Google Scholar]
- [70].Liu XY, Zhu HK, Wu SH, Chen YL, Liu FY, Wu P, Antitumor activity of diterpenoids from Solidago canadensis L, J Zhejiang Univ (Sci Ed), 34 (2007) 661–664. [Google Scholar]
- [71].Casey SC, Amedei A, Aquilano K, Azmi AS, Benencia F, Bhakta D, Bilsland AE, Boosani CS, Chen S, Ciriolo MR, Crawford S, Fujii H, Georgakilas AG, Guha GJ, Halicka D, Helferich WG, Heneberg P, Honoki K, Keith WN, Kerkar SP, Mohammed SI, Niccolai E, Nowsheen S, Rupasinghe HPV, Samadi A, Singh N, Talib WH, Venkateswaran V, Whelan RL, Yang XJ, Felsher DW, Cancer prevention and therapy through the modulation of the tumor microenvironment, Semin Cancer Biol, 35 (2015) S199–S223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Eom KS, Hong JM, Youn MJ, So HS, Park R, Kim JM, Kim TY, Berberine induces G1 arrest and apoptosis in human glioblastoma T98G cells through mitochondrial/caspases pathway, Biol Pharm Bull, 31 (2008) 558–562. [DOI] [PubMed] [Google Scholar]
- [73].Li HL, Wu H, Zhang BB, Shi HL, Wu XJ, MAPK pathways are involved in the inhibitory effect of berberine hydrochloride on gastric cancer MGC 803 cell proliferation and IL-8 secretion in vitro and in vivo, Mol Med Rep, 14 (2016) 1430–1438. [DOI] [PubMed] [Google Scholar]
- [74].Tan W, Li YB, Chen MW, Wang YT, Berberine hydrochloride: Anticancer activity and nanoparticulate delivery system, Int J Nanomedicine, 6 (2011) 1773–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Wang XK, Liu JH, Xie ZX, Rao JY, Xu GR, Huang KY, Li WY, Yin ZJ, Chlorogenic acid inhibits proliferation and induces apoptosis in A498 human kidney cancer cells via inactivating PI3K/Akt/mTOR signalling pathway, J Pharm Pharmacol, 71 (2019) 1100–1109. [DOI] [PubMed] [Google Scholar]
- [76].Tajik N, Tajik M, Mack I, Enck P, The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: A comprehensive review of the literature, Eur J Nutr, 56 (2017) 2215–2244. [DOI] [PubMed] [Google Scholar]
- [77].Kang TY, Yang HR, Zhang J, Li D, Lin J, Wang L, Xu XP, The studies of chlorogenic acid antitumor mechanism by gene chip detection: The immune pathway gene expression, J Anal Methods Chem, 2013 (2013) 617243. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [78].Ahmad B, Rizwan M, Rauf A, Raza M, Bashir S, Molnar J, Csonka A, Szabo D, Mubarak MS, Noor M, Siddiqui BS, Isolation of chlorogenic acid from soil borne fungi Screlotium rolfsii, their reversal of multidrug resistance and antiproliferative in mouse lymphoma cells, Med Chem, 13 (2017) 721–726. [DOI] [PubMed] [Google Scholar]
- [79].Feng Q, Liu WS, Baker SS, Li HS, Chen C, Liu Q, Tang SJ, Guan LY, Tsompana M, Kozielski R, Baker RD, Peng JH, Liu P, Zhu RX, Hu YY, Zhu LX, Multi-targeting therapeutic mechanisms of the Chinese herbal medicine QHD in the treatment of non-alcoholic fatty liver disease, Oncotarget, 8 (2017) 27820–27838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Gao X, Zhou Y, Wu KX, Ding YH, Fan DM, Yang M, Zhang YZ, Zhang YJ, Xiong DS, Inhibitory effects of indirubin derivative PHII-7 on invasion and migration in metastatic cancer, Neoplasma, 62 (2015) 209–229. [DOI] [PubMed] [Google Scholar]
- [81].Wang J, Long L, Chen YZ, Xu YS, Zhang L, Design, synthesis and antineoplastic activity of novel hybrids of podophyllotoxin and indirubin against human leukaemia cancer cells as multifunctional anti-MDR agents, Bioorg Med Chem Lett, 28 (2018) 1817–1824. [DOI] [PubMed] [Google Scholar]
- [82].Chen LH, Wang JH, Wu JB, Zheng QM, Hu JF, Indirubin suppresses ovarian cancer cell viabilities through the STAT3 signaling pathway, Drug Des Devel Ther, 12 (2018) 3335–3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Li ZH, Zhu CF, An BP, Chen Y, He XY, Qian L, Lan L, Li SJ, Indirubin inhibits cell proliferation, migration, invasion and angiogenesis in tumor-derived endothelial cells, Onco Targets Ther, 11 (2018) 2937–2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Pooja T, Karunagaran D, Emodin suppresses Wnt signaling in human colorectal cancer cells SW480 and SW620, Eur J Pharmacol, 742 (2014) 55–64. [DOI] [PubMed] [Google Scholar]
- [85].Li WY, Ng YF, Zhang H, Guo ZD, Guo DJ, Kwan YW, Leung GPH, Lee SMY, Yu PHF, Chan SW, Emodin elicits cytotoxicity in human lung adenocarcinoma A549 cells through inducing apoptosis, Inflammopharmacol, 22 (2014) 127–134. [DOI] [PubMed] [Google Scholar]
- [86].Manu KA, Shanmugam MK, Ong TH, Subramaniam A, Siveen KS, Perumal E, Samy RP, Bist P, Lim LHK, Kumar AP, Hui KM, Sethi G, Emodin suppresses migration and invasion through the modulation of CXCR4 expression in an orthotopic model of human hepatocellular carcinoma, PLoS One, 8 (2013) e57015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Jia X, Yu F, Wang JF, Iwanowycz S, Saaoud F, Wang YZ, Hu J, Wang Q, Fan DP, Emodin suppresses pulmonary metastasis of breast cancer accompanied with decreased macrophage recruitment and M2 polarization in the lungs, Breast Cancer Res Treat, 148 (2014) 291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Ding Y, Huang ZH, A review on anti-tumor actions of emodin, Lishizhen Med Mater Med Res, 19 (2008) 1272–1274. [Google Scholar]
- [89].Wang A, Jiang HH, Liu YY, Chen J, Zhou X, Zhao CX, Chen X, Lin MB, Rhein induces liver cancer cells apoptosis via activating ROS-dependent JNK/Jun/caspase-3 signaling pathway, J Cancer, 11 (2020) 500–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Xu WT, Li TZ, Li SM, Wang C, Wang H, Luo YH, Piao XJ, Wang JR, Zhang Y, Zhang T, Xue H, Cao LK, Jin CH, Cytisine exerts anti-tumour effects on lung cancer cells by modulating reactive oxygen species-mediated signalling pathways, Artif Cells Nanomed Biotechnol, 48 (2020) 84–95. [DOI] [PubMed] [Google Scholar]
- [91].Yan PH, Huang ZQ, Zhu JB, Matrine inhibits cell proliferation and induces apoptosis of human rhabdomyosarcoma cells via downregulation of the extracellular signal regulated kinase pathway, Oncol Lett, 14 (2017) 3148–3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Li HJ, Li XJ, Bai ML, Suo Y, Zhang GH, Cao XY, Matrine inhibited proliferation and increased apoptosis in human breast cancer MCF-7 cells via upregulation of Bax and downregulation of Bcl-2, Int J Clin Exp Pathol, 8 (2015) 14793–14799. [PMC free article] [PubMed] [Google Scholar]
- [93].Cai YS, Xu P, Yang L, Xu K, Zhu JL, Wu XQ, Jiang CS, Yuan QL, Wang B, Li YB, Qiu YS, HMGB1-mediated autophagy decreases sensitivity to oxymatrine in SW982 human synovial sarcoma cells, Sci Rep, 6 (2016) 37845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Zhou BG, Wei CS, Zhang S, Zhang Z, Gao HM, Matrine reversed multidrug resistance of breast cancer MCF-7/ADR cells through PI3K/Akt signaling pathway, J Cell Biochem, 119 (2018) 3885–3891. [DOI] [PubMed] [Google Scholar]
- [95].Liu Y, Xu Y, Ji WD, Li XY, Sun B, Gao QG, Su CQ, Anti-tumor activities of matrine and oxymatrine: Literature review, Tumour Biol, 35 (2014) 5111–5119. [DOI] [PubMed] [Google Scholar]
- [96].Liu R, Peng J, Wang HL, Li L, Wen XJ, Tan Y, Zhang L, Wan HY, Chen FM, Nie X, Oxysophocarpine retards the growth and metastasis of oral squamous cell carcinoma by targeting the Nrf2/HO-1 axis, Cell Physiol Biochem, 49 (2018) 1717–1733. [DOI] [PubMed] [Google Scholar]
- [97].Wang J, Zhao XZ, Qi Q, Tao L, Zhao Q, Mu R, Gu HY, Wang M, Feng X, Guo QL, Macranthoside B, a hederagenin saponin extracted from Lonicera macranthoides and its anti-tumor activities in vitro and in vivo, Food Chem Toxicol, 47 (2009) 1716–1721. [DOI] [PubMed] [Google Scholar]
- [98].Shan Y, Guan FQ, Zhao XZ, Wang M, Chen Y, Wang QZ, Feng X, Macranthoside B induces apoptosis and autophagy via reactive oxygen species accumulation in human ovarian cancer A2780 cells, Nutr Cancer, 68 (2016) 280–289. [DOI] [PubMed] [Google Scholar]
- [99].Yang GQ, Wang CQ, Study on the anti-tumor effects of betacyanins extracted from Portulaca oleracea L, Lishizhen Med Mater Med Res, 21 (2010) 388–390. [Google Scholar]
- [100].Scarpa ES, Emanuelli M, Frati A, Pozzi V, Antonini E, Diamantini G, Di Ruscio G, Sartini D, Armeni T, Palma F, Ninfali P, Betacyanins enhance vitexin-2-O-xyloside mediated inhibition of proliferation of T24 bladder cancer cells, Food Funct, 7 (2016) 4772–4780. [DOI] [PubMed] [Google Scholar]
- [101].Wang PW, Solorzano W, Diaz T, Magyar CE, Henning SM, Vadgama JV, Arctigenin inhibits prostate tumor cell growth in vitro and in vivo, Clin Nutr Exp, 13 (2017) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Maxwell T, Lee KS, Kim S, Nam KS, Arctigenin inhibits the activation of the mTOR pathway, resulting in autophagic cell death and decreased ER expression in ER-positive human breast cancer cells, Int J Oncol, 52 (2018) 1339–1349. [DOI] [PubMed] [Google Scholar]
- [103].He YH, Fan QM, Cai TT, Huang W, Xie XZ, Wen YY, Shi Z, Molecular mechanisms of the action of arctigenin in cancer, Biomed Pharmacother, 108 (2018) 403–407. [DOI] [PubMed] [Google Scholar]
- [104].Xu YR, Lou ZY, Lee SH, Arctigenin represses TGF-β-induced epithelial mesenchymal transition in human lung cancer cells, Biochem Biophys Res Commun, 493 (2017) 934–939. [DOI] [PubMed] [Google Scholar]
- [105].Tsai CL, Chiu YM, Ho TY, Hsieh CT, Shieh DC, Lee YJ, Tsay GJ, Wu YY, Gallic acid induces apoptosis in human gastric adenocarcinoma cells, Anticancer Res, 38 (2018) 2057–2067. [DOI] [PubMed] [Google Scholar]
- [106].Liao CC, Chen SC, Huang HP, Wang CJ, Gallic acid inhibits bladder cancer cell proliferation and migration via regulating fatty acid synthase (FAS), J Food Drug Anal, 26 (2018) 620–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Lo C, Lai TY, Yang JS, Yang JH, Ma YS, Weng SW, Lin HY, Chen HY, Lin JG, Chung JG, Gallic acid inhibits the migration and invasion of A375.S2 human melanoma cells through the inhibition of matrix metalloproteinase-2 and Ras, Melanoma Res, 21 (2011) 267–273. [DOI] [PubMed] [Google Scholar]
- [108].Ji L, Zhong B, Jiang X, Mao F, Liu G, Song B, Wang CY, Jiao Y, Wang JP, Xu ZB, Li X, Zhan B, Actein induces autophagy and apoptosis in human bladder cancer by potentiating ROS/JNK and inhibiting AKT pathways, Oncotarget, 8 (2017) 112498–112515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Wu XX, Yue GGL, Dong JR, Lam CWK, Wong CK, Qiu MH, Lau CBS, Actein inhibits the proliferation and adhesion of human breast cancer cells and suppresses migration in vivo, Front Pharmacol, 9 (2018) 1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Sakurai N, Kozuka M, Tokuda H, Mukainaka T, Enjo F, Nishino H, Nagai M, Sakurai Y, Lee KH, Cancer preventive agents. Part 1: Chemopreventive potential of cimigenol, cimigenol-3,15-dione, and related compounds, Bioorg Med Chem,13 (2005) 1403–1408. [DOI] [PubMed] [Google Scholar]
- [111].Long ZG, Feng GJ, Zhao N, Wu L, Zhu HB, Isoferulic acid inhibits human leukemia cell growth through induction of G2/M phase arrest and inhibition of Akt/mTOR signaling, Mol Med Rep, 21 (2020) 1035–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Gao JH, Yu H, Guo WK, Kong Y, Gu LN, Li Q, Yang SS, Zhang YY, Wang YX, The anticancer effects of ferulic acid is associated with induction of cell cycle arrest and autophagy in cervical cancer cells, Cancer Cell Int, 18 (2018) 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Zhang X, Lin D, Jiang R, Li HZ, Wan JY, Li HY, Ferulic acid exerts antitumor activity and inhibits metastasis in breast cancer cells by regulating epithelial to mesenchymal transition, Oncol Rep, 36 (2016) 271–278. [DOI] [PubMed] [Google Scholar]
- [114].Muthusamy G, Gunaseelan S, Prasad NR, Ferulic acid reverses Pglycoprotein-mediated multidrug resistance via inhibition of PI3K/Akt/NF-κB signaling pathway, J Nutr Biochem, 63 (2019) 62–71. [DOI] [PubMed] [Google Scholar]
- [115].Fu XL, Zhao XD, Zhang Q, Weng AH, Zhou H, Effects of taxifolin on human non-small cell lung cancer A549 cells and its mechanism, Anti-Tumor Pharm, 8 (2018) 519–523. [Google Scholar]
- [116].Chen HJ, Chung YL, Li CY, Chang YT, Wang CCN, Lee HY, Lin HY, Hung CC, Taxifolin resensitizes multidrug resistance cancer cells via uncompetitive inhibition of P-glycoprotein function, Molecules, 23 (2018) 3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Kim YJ, Choi SE, Lee MW, Lee CS, Taxifolin glycoside inhibits dendritic cell responses stimulated by lipopolysaccharide and lipoteichoic acid, J Pharm Pharmacol, 60 (2008) 1465–72. [DOI] [PubMed] [Google Scholar]
- [118].Li W, Kim TI, Kim JH, Chung HS, Immune checkpoint PD-1/PD-L1 CTLA-4/CD80 are blocked by Rhus Verniciflua stokes and its active compounds, Molecules, 24 (2019) 4062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Deep G, Oberlies NH, Kroll DJ, Agarwal R, Identifying the differential effects of silymarin constituents on cell growth and cell cycle regulatory molecules in human prostate cancer cells, Int J Cancer, 123 (2008) 41–50. [DOI] [PubMed] [Google Scholar]
- [120].Deep G, Gangar SC, Oberlies NH, Kroll DJ, Agarwal R, Isosilybin A induces apoptosis in human prostate cancer cells via targeting Akt, NF-κB, and androgen receptor signaling, Mol Carcinog, 49 (2010) 902–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Deep G, Singh RP, Agarwal C, Kroll DJ, Agarwal R, Silymarin and silibinin cause G1 and G2-M cell cycle arrest via distinct circuitries in human prostate cancer PC3 cells: A comparison of flavanone silibinin with flavanolignan mixture silymarin, Oncogene, 25 (2006) 1053–1069. [DOI] [PubMed] [Google Scholar]
- [122].Jahannfrooz Z, Motamed N, Rinner B, Mokhtarzadeh A, Baradaran B, Silibinin to improve cancer therapeutic, as an apoptotic inducer, autophagy modulator, cell cycle inhibitor, and microRNAs regulator, Life Sci, 213 (2018) 236–247. [DOI] [PubMed] [Google Scholar]
- [123].Noori-Daloii MR, Saffari M, Raoofian R, Yekaninejad M, Dinehkabodi OS, Noori-Daloii AR, The multidrug resistance pumps are inhibited by silibinin and apoptosis induced in K562 and KCL22 leukemia cell lines, Leuk Res, 38 (2014) 575–580. [DOI] [PubMed] [Google Scholar]
- [124].Singh RP, Deep G, Chittezhath M, Kaur M, Dwyer-Nield LD, Malkinson AM, Agarwal R, Effect of silibinin on the growth and progression of primary lung tumors in mice, J Natl Cancer Inst, 98 (2006) 846–855. [DOI] [PubMed] [Google Scholar]
- [125].Mokhtari MJ, Motamed N, Shokrgozar MA, Evaluation of silibinin on the viability, migration and adhesion of the human prostate adenocarcinoma (PC-3) cell line, Cell Biol Int, 32 (2008) 888–892. [DOI] [PubMed] [Google Scholar]
- [126].Ting H, Deep G, Kumar S, Jain AK, Agarwal C, Agarwal R, Beneficial effects of the naturally occurring flavonoid silibinin on the prostate cancer microenvironment: Role of monocyte chemotactic protein-1 and immune cell recruitment, Carcinogenesis, 37 (2016) 589–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Forghani P, Khorramizadeh MR, Waller EK, Silibinin inhibits accumulation of myeloid-derived suppressor cells and tumor growth of murine breast cancer, Cancer Med, 3 (2014) 215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Lee JS, Kim SG, Kim HK, Lee TH, Jeong YI, Lee CM, Yoon MS, Na YJ, Soh DS, Park NC, Choi IH, Kim GY, Choi YH, Chung HY, Park YM, Silibinin polarizes Th1/Th2 immune responses through the inhibition of immunostimulatory function of dendritic cells, J Cell Physiol, 210 (2007) 385–397. [DOI] [PubMed] [Google Scholar]
- [129].Schmann J, Prockl J, Kiemer AK, Vollmar AM, Bang R, Tiegs G, Silibinin protects mice from T cell-dependent liver injury, J Hepatol, 39 (2003) 333–340. [DOI] [PubMed] [Google Scholar]
- [130].Tyagi A, Singh RP, Ramasamy K, Raina K, Redente EF, Dwyer-Nield LD, Radcliffe RA, Malkinson AM, Agarwal R, Growth inhibition and regression of lung tumors by silibinin: Modulation of angiogenesis by macrophage-associated cytokines and nuclear factor-kappaB and signal transducers and activators of transcription 3, Cancer Prev Res (Phila), 2 (2009) 74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Li K, Wang TX, Advances in mahoniae anti-tumor mechanisms, J Henan Univ (Nat Sci), 40 (2010) 399–401. [Google Scholar]
- [132].Sun YF, Gao XY, Wu PP, Wink M, Li JH, Dian LL, Liang ZS, Jatrorrhizine inhibits mammary carcinoma cells by targeting TNIK mediated Wnt/β-catenin signalling and epithelial-mesenchymal transition (EMT), Phytomedicine, 63 (2019) 153015. [DOI] [PubMed] [Google Scholar]
- [133].Liu RF, Cao ZF, Pan YY, Zhang GC, Yang P, Guo PD, Zhou QS, Jatrorrhizine hydrochloride inhibits the proliferation and neovascularization of C8161 metastatic melanoma cells, Anticancer Drugs, 24 (2013) 667–676. [DOI] [PubMed] [Google Scholar]
- [134].Huang YT, Chi ZL, Wang SM, Wu HQ, Qin KM, Li WD, Research progress on licorice flavonoids and their antitumor activities, Chin J New Drugs, 26 (2017) 1532–1537. [Google Scholar]
- [135].Zhang BY, Lai Y, Li YF, Shu N, Wang Z, Wang YP, Li YS, Chen ZJ, Antineoplastic activity of isoliquiritigenin, a chalcone compound, in androgen-independent human prostate cancer cells linked to G2/M cell cycle arrest and cell apoptosis, Eur J Pharmacol, 821 (2018) 57–67. [DOI] [PubMed] [Google Scholar]
- [136].Zhao HX, Zhang XH, Chen XW, Li Y, Ke ZQ, Tang T, Chai HY, Guo AM, Chen HL, Yang J, Isoliquiritigenin, a flavonoid from licorice, blocks M2 macrophage polarization in colitis-associated tumorigenesis through downregulating PGE2 and IL-6, Toxicol Appl Pharmacol, 279 (2014) 311–321. [DOI] [PubMed] [Google Scholar]
- [137].Yoon G, Kang BY, Cheon SH, Topoisomerase I inhibition and cytotoxicity of licochalcones A and E from glycyrrhiza inflata, Arch Pharm Res, 30 (2007) 313–316. [DOI] [PubMed] [Google Scholar]
- [138].Hsieh MJ, Lin CW, Yang SF, Chen MK, Chiou HL, Glabridin inhibits migration and invasion by transcriptional inhibition of matrix metalloproteinase 9 through modulation of NF-κB and AP-1 activity in human liver cancer cells, Br J Pharmacol, 171 (2014) 3037–3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Chen F, Xu HQ, Duan ZQ, Zhao JF, Wu P, Chen YL, Regulation effect of glycyrrhetinic acid on the expression of BLM and proliferation apoptosis and invasion migration of PC3 cells, Chin Pharm J, 53 (2018) 346–352. [Google Scholar]
- [140].Nabekura T, Yamaki T, Ueno K, Kitagawa S, Inhibition of P-glycoprotein and multidrug resistance protein 1 by dietary phytochemicals, Cancer Chemother Pharmacol, 62 (2008) 867–873. [DOI] [PubMed] [Google Scholar]
- [141].Gao ZB, kang X, Xu CL, Research progress of anticancer mechanism of glycyrrhetinic acid, China J Chin Meter Med, 36 (2011) 3213–3216. [PubMed] [Google Scholar]
- [142].Li S, The Effect and mechanism of glycyrrhizic acid on human glioma U251 cells, Southern Med Univ, 2014. [Google Scholar]
- [143].Hu S, Huang LM, Meng LW, Sun H, Zhang W, Xu YC, Isorhamnetin inhibits cell proliferation and induces apoptosis in breast cancer via Akt and mitogen activated protein kinase kinase signaling pathways, Mol Med Rep, 12 (2015) 6745–6751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Liu J, Guo WJ, Gen J, Tan DF, Tang XH, Gao J, Isorhamnetin induces autophagy in HCT116 cells, Chin Tradit Patent Med, 37 (2015) 2596–2599. [Google Scholar]
- [145].Chen C, Zhou J, Ji CY, Quercetin: A potential drug to reverse multidrug resistance, Life Sci, 87 (2010) 333–338. [DOI] [PubMed] [Google Scholar]
- [146].Niu G, Yin S, Xie S, Li Y, Nie D, Ma L, Wang X, Wu Y, Quercetin induces apoptosis by activating caspase-3 and regulating Bcl-2 and cyclooxygenase-2 pathways in human HL-60 cells, Acta Biochim Biophys Sin (Shanghai), 43 (2011) 30–37. [DOI] [PubMed] [Google Scholar]
- [147].Hu KL, Miao L, Goodwin TJ, Li J, Liu Q, Huang L, Quercetin remodels the tumor microenvironment to improve the permeation, retention, and antitumor effects of nanoparticles, ACS Nano, 11 (2017) 4916–4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Zhang J, Shen LM, Li X, Song WT, Liu Y, Huang L, Nanoformulated codelivery of quercetin and alantolactone promotes an antitumor response through synergistic immunogenic cell death for microsatellite-stable colorectal cancer, ACS Nano, 13 (2019) 12511–12524. [DOI] [PubMed] [Google Scholar]
- [149].Liu Y, Tang ZG, Yang JQ, Zhou Y, Meng LH, Wang H, Li CL, Low concentration of quercetin antagonizes the invasion and angiogenesis of human glioblastoma U251 cells, Onco Targets Ther, 10 (2017) 4023–4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Orfali GDC, Duarte AC, Bonadio V, Martinez NP, de Araújo MEMB, Priviero FBM, Carvalho PO, Priolli DG, Review of anticancer mechanisms of isoquercitin, World J Clin Oncol, 7 (2016) 189–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Zhou RP, Zhang ZM, Zhao L, Jia CH, Xu S, Mai QG, Lu M, Huang MJ, Wang L, Wang XK, Jin DD, Bai XC, Inhibition of mTOR signaling by oleanolic acid contributes to its anti-tumor activity in osteosarcoma cells, J Orthop Res, 29 (2011) 846–852. [DOI] [PubMed] [Google Scholar]
- [152].Yan YQ, Yang Y, Research progress in the anticancer mechanism of oleanolic acid, Med Recapitulate, 22 (2016) 914–917. [Google Scholar]
- [153].Lin CC, Huang CY, Mong MC, Chan CY, Yin MC, Antiangiogenic potential of three triterpenic acids in human liver cancer cells, J Agric Food Chem, 59 (2011) 755–62. [DOI] [PubMed] [Google Scholar]
- [154].Liubao XZ, Wang RP, Xi Z, Zhou JY, Effect of hederagenin on biological behaviors including proliferation, adhesion, invasion and migration capabilities of human gastric cancer cell line, Chin J Exp Tradit Med Form, 19 (2013) 212–215. [Google Scholar]
- [155].Fang LW, Liu MM, Cai LL, Hederagenin inhibits proliferation and promotes apoptosis of cervical cancer CaSki cells by blocking STAT3 pathway, Chin J Cell Mol Immunol, 35 (2019) 140–145. [PubMed] [Google Scholar]
- [156].Xue SY, Li MC, Miao Q, Zhou Y, Inhibitory effects and mechanism of anemoside B4 on hepatocellular carcinoma Huh-7 cells and tumor bearing nude mice, China Pharm, 30 (2019) 601–607. [Google Scholar]
- [157].Liu Q, Chen WC, Jiao Y, Hou JQ, Wu QY, Liu YL, Qi XF, Pulsatilla saponin A, an active molecule from pulsatilla chinensis, induces cancer cell death and inhibits tumor growth in mouse xenograft models, J Surg Res, 188 (2014) 387–395. [DOI] [PubMed] [Google Scholar]
- [158].Son MK, Jung KH, Hong SW, Lee HS, Zheng HM, Choi MJ, Seo JH, Suh JK, Hong SS, SB365, Pulsatilla saponin D suppresses the proliferation of human colon cancer cells and induces apoptosis by modulating the AKT/mTOR signalling pathway, Food Chem, 136 (2013) 26–33. [DOI] [PubMed] [Google Scholar]
- [159].Ye YY, He DW, Ye WC, Zhang QW, Zhao SX, The study of 23-hydroxyl betulinic acid against melanoma in vivo and in vitro, Chin J Clin Oncol Rehabil, 7 (2000) 7–9. [Google Scholar]
- [160].Yang Y, Li L, Research progress in molecular mechanisms of caffeic acid and its derivatives on anti-tumor function, Food Sci, 34 (2013) 341–345. [Google Scholar]
- [161].Chen DK, Apoptotic effect of arundoin on human prostatic cancer cells by activating poly ADP ribose polymeras, Chin Tradit Herbal Drugs, 48 (2017) 1183–1187. [Google Scholar]
- [162].Du PP, Liu ZW, Zhang Y, Zhang YH, Shang SF, Jia DH, Effects of catechin on invasion and metastasis in gastric carcinoma cells SGC-7901 and MGC-803, J Zhengzhou Univ (Med Sci), 51 (2016) 51–55. [Google Scholar]
- [163].Liang G, Zhang S, Huang ZM, Tang AZ, Reversal of drug resistance of human hepatoma cells by two catechins in vitro, Chin J Cancer, 23 (2004) 401–405. [Google Scholar]
- [164].Gong ZL, Zhang K, Ding YP, Advance in anticancer studies on catechins and their derivatives, China J Chin Mater Med, 37 (2012) 2510–2518. [PubMed] [Google Scholar]
- [165].Liu Y, Tong L, Luo Y, Li X, Chen GW, Wang YF, Resveratrol inhibits the proliferation and induces the apoptosis in ovarian cancer cells via Inhibiting glycolysis and targeting AMPK/mTOR signaling pathway, J Cell Biochem, 119 (2018) 6162–6172. [DOI] [PubMed] [Google Scholar]
- [166].Ko JH, Sethi G, Um JY, Shanmugam MK, Arfuso F, Kumar AP, Bishayee A, Ahn KS, The role of resveratrol in cancer therapy, Int J Mol Sci, 18 (2017) e2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Gu J, Fan YQ, Zhang HL, Pan JA, Yu JY, Zhang JF, Wang CQ, Resveratrol suppresses doxorubicin-induced cardiotoxicity by disrupting E2F1 mediated autophagy inhibition and apoptosis promotion, Biochem Pharmacol, 150 (2018) 202–213. [DOI] [PubMed] [Google Scholar]
- [168].Han YJ, Jo H, Cho JH, Dhanasekaran DN, Song YS, Resveratrol as a tumor-suppressive nutraceutical modulating tumor microenvironment and malignant behaviors of cancer, Int Mol Sci, 20 (2019) 925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Fen YR, Chen HS, Research progress in antitumor activity of oridonin, Chin J Tradit Med Sci Technol, 23 (2016) 125–127. [Google Scholar]
- [170].Guo ZY, Shi SL, Li QF, Effect of cinnamic acid on proliferation and differentiation of human osteosarcoma MG-63 cells, Chin Pharmacol Bull, 28 (2012) 1262–1266. [Google Scholar]
- [171].Ceci C, Tentori L, Atzori MG, Lacal PM, Bonanno E, Scimeca M, Cicconi R, Mattei M, Martino MGD, Vespasiani G, Miano R, Graziani G, Ellagic acid inhibits bladder cancer invasiveness and in vivo tumor growth, Nutrients, 8 (2016) 744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Jang E, Inn KS, Jang YP, Lee KT, Lee JH, Phytotherapeutic activities of sanguisorba officinalis and its chemical constituents: A review, Am J Chin Med, 46 (2018) 299–318. [DOI] [PubMed] [Google Scholar]
- [173].Li HX, Shi BY, Li YY, Yin FF, Polydatin inhibits cell proliferation and induces apoptosis in laryngeal cancer and HeLa cells via suppression of the PDGF/AKT signaling pathway, J Biochem Mol Toxicol, 31 (2017) e21900. [DOI] [PubMed] [Google Scholar]
- [174].Kong XH, Li ZX, Fang LJ, Jian JF , Reversal effect of rutin on cisplatin-resistance in human lung cancer A549/DDP cells and its mechanisms, Chin J Immunol, 35 (2019) 2332–2336. [Google Scholar]
- [175].Imran M, Rauf A, Abu-Izneid T, Nadeem M, Shariati MA, Khan IA, Imran A, Orhan IE, Rizwan M, Atif M, Gondal TA, Mubarak MS, Luteolin, a flavonoid, as an anticancer agent: A review, Biomed Pharmacother, 112 (2019) 108612. [DOI] [PubMed] [Google Scholar]
- [176].Lin Y, Shi R, Wang X, Shen HM, Luteolin, a flavonoid with potential for cancer prevention and therapy, Curr Cancer Drug Targets, 8 (2008) 634–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Lee Y, Cancer chemopreventive potential of procyanidin, Toxicol Res, 33 (2017) 273–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Dou J, Wang Z, Ma L, Peng B, Mao K, Li CQ, Su MQ, Zhou CL, Peng GY, Baicalein and baicalin inhibit colon cancer using two distinct fashions of apoptosis and senescence, Oncotarget, 9 (2018) 20089–20102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Bie BB, Sun J, Guo Y, Li J, Jiang W, Yang J, Huang C, Li ZF, Baicalein: A review of its anti-cancer effects and mechanisms in hepatocellular carcinoma, Biomed Pharmacother, 93 (2017) 1285–1291. [DOI] [PubMed] [Google Scholar]
- [180].Huynh DL, Sharma N, Singh AK, Sodhi SS, Zhang JJ, Mongre RK, Ghosh M, Kim N, Park YH, Jeong DK, Anti-tumor activity of wogonin, an extract from scutellaria baicalensis, through regulating different signaling pathways, Chin J Nat Med, 15 (2017) 15–40. [DOI] [PubMed] [Google Scholar]
- [181].Yao YY, Zhao K, Yu Z, Ren HC, Zhao L, Li ZY, Guo QL, Lu N, Wogonoside inhibits invasion and migration through suppressing TRAF2/4 expression in breast cancer, J Exp Clin Cancer Res, 36 (2017) 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Huang YJ, Zhao K, Hu Y, Zhou YX, Lou XW, Li XR, Wei LB, Li ZY, You QD, Guo QL, Lu N, Wogonoside inhibits angiogenesis in breast cancer via suppressing Wnt/β-catenin pathway, Mol Carcinog, 55 (2016) 1598–1612. [DOI] [PubMed] [Google Scholar]
- [183].Wang AQ, Wang SP, Zhou FY, Li P, Wang YT, Gan LS, Lin LG, Physalin B induces cell cycle arrest and triggers apoptosis in breast cancer cells through modulating p53-dependent apoptotic pathway, Biomed Pharmacother, 101 (2018) 334–341. [DOI] [PubMed] [Google Scholar]
- [184].Ma YM, Han W, Li J, Hu LH, Zhou YB, Physalin B not only inhibits the ubiquitin-proteasome pathway but also induces incomplete autophagic response in human colon cancer cells in vitro, Acta Pharmacol Sin, 36 (2015) 517–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Zhu FF, Dai CY, Fu YF, Loo JFC, Xia DJ, Gao SZP, Ma ZJ, Chen Z, Physalin A exerts anti-tumor activity in non-small cell lung cancer cell lines by suppressing JAK/STAT3 signaling, Oncotarget, 7 (2016) 9462–9476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Liu YK, Kang X, Niu G, He SL, Zhang TT, Bai YW, Li Y, Hao HY, Chen C, Shou ZX, Li B, Shikonin induces apoptosis and prosurvival autophagy in human melanoma A375 cells via ROS-mediated ER stress and p38 pathways, Artif Cells Nanomed Biotechnol, 47 (2019) 626–635. [DOI] [PubMed] [Google Scholar]
- [187].Zhu MY, Wang RB, Zhou W, Li SS, Antitumor effect research progress of shikonin and its derivatives, Acta Pharma Sin, 47 (2012) 588–593. [PubMed] [Google Scholar]
- [188].Yang WB, Luo XL, Hu J, Mi BZ, Qiao XX, Research status of antitumor active components and mechanism of lithospermum, Pharm J Chin People’s Liberation Army, 33 (2017) 359–362. [Google Scholar]
- [189].Liu D, Wang JH, Research progress on antitumor mechanisms of saikosaponin, Drugs Clin, 33 (2018) 203–208. [Google Scholar]
- [190].Li YC, Wang HH, Zhang R, Zhang GJ, Yang Y, Liu ZG, Antitumor activity of asiaticoside against multiple myeloma drug-resistant cancer cells is mediated by autophagy induction, activation of effector caspases, and inhibition of cell migration, invasion, and STAT-3 signaling pathway, Med Sci Monit, 25 (2019) 1355–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Guo BJ, Ling CQ, Anti-tumor effect of asiatic acid and its mechanism, Chin J Cancer Biother, 26 (2019) 597–601. [Google Scholar]
- [192].Mao F, Zhang L, Cai MH, Guo H, Yuan HH, Leonurine hydrochloride induces apoptosis of H292 lung cancer cell by a mitochondria-dependent pathway, Pharm Biol, 53 (2015) 1684–90. [DOI] [PubMed] [Google Scholar]
- [193].Islam MT, Ali ES, Uddin SJ, Islam MA, Shaw S, Khan IN, Saravi SSS, Ahmad S, Rehman S, Gupta VK, Găman MA, Găman AM, Yele S, Das AK, de Castro E Sousa JM, de Moura Dantas SMM, Rolim HML, de Carvalho Melo-Cavalcante AA, Mubarak MS, Yarla NS, Shilpi JA, Mishra SK, Atanasov AG, Kamal MA, Andrographolide, a diterpene lactone from andrographis paniculata and its therapeutic promises in cancer, 420 (2018) 129–145. [DOI] [PubMed] [Google Scholar]
- [194].Kang XJ, Zheng ZN, Liu ZH, Wang HY, Zhao YG, Zhang WY, Shi MJ, He Y, Cao Y, Xu Q, Peng CY, Huang YZ, Liposomal codelivery of doxorubicin and andrographolide inhibits breast cancer growth and metastasis, Mol Pharm, 15 (2018) 1618–1626. [DOI] [PubMed] [Google Scholar]
- [195].Lim JCW, Chan TK, Ng DSW, Sagineedu SR, Stanslas J, Wong WSF, Andrographolide and its analogues: Versatile bioactive molecules for combating inflammation and cancer, Clin Exp Pharmacol Physiol, 39 (2012) 300–310. [DOI] [PubMed] [Google Scholar]
- [196].Li ZY, Ding YJ, Li J, Zhao AG, Anti-cancer effects and mechanism of esculetin, Chin Arch Tradit Chin Med, 37 (2019) 1620–1623. [Google Scholar]
- [197].Wang J, Inhibitory effect of aesculin on mice with Lewis lung cancer, Chin Tradit Patent Med, 36 (2014) 249–252. [Google Scholar]
- [198].Ye QM, Bai LL, Hu SZ, Tian HY, Ruan LJ, Tan YF, Hu LP, Ye WC, Zhang DM, Jiang RW, Isolation, Chemotaxonomic significance and cytotoxic effects of quassinoids from brucea javanica, Fitoterapia, 105 (2015) 66–72. [DOI] [PubMed] [Google Scholar]
- [199].Lu Z, Lai ZQ, Leung AWN, Leung PS, Li ZS, Lin ZX, Exploring brusatol as a new anti-pancreatic cancer adjuvant: Biological evaluation and mechanistic studies, Oncotarget, 8 (2017) 84974–84985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Harder B, Tian W, Clair JJL, Tan AC, Ooi A, Chapma E, Zhang DD, Brusatol overcomes chemoresistance through inhibition of protein translation, Mol Carcinog, 56 (2017) 1493–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Pan L, Chin YW, Chai HB, Ninh TN, D Soejarto D, Kinghorn AD, Bioactivity-guided isolation of cytotoxic constituents of brucea javanica collected in vietnam, Bioorg Med Chem, 17 (2009) 2219–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Shanmugam MK, Shen HY, Tang FR, Arfuso F, Rajesh M, Wang LZ, Kumar AP, Bian JS, Goh BC, Bishayee A, Sethi G, Potential role of genipin in cancer therapy, Pharmacol Res, 133 (2018) 195–200. [DOI] [PubMed] [Google Scholar]
- [203].Sun CY, Qi RX, Zhang Q, Yu MH, Jin WS, Advancement for the study of ursolic acid on anti-tumor, Progr Mod Biomed, 16 (2016) 4393–4397. [Google Scholar]
- [204].Xin LL, Zhang X, Gong JN, Progress in research on anticancer mechanism of apigenin, Chin J Exp Tradit Med Form, 19 (2013) 335–341. [Google Scholar]
- [205].Sun YC, Liu XW, Pian GZ, Effect and mechanism study on the autophagy and apoptosis induced by β-sitosterol in human gastic cancer cells, J Chin Physician, 21 (2019) 866–871. [Google Scholar]
- [206].Ming Y, Liang L, Seok-Mo H, Yunjo S, Aloe-emodin induces chondrogenic differentiation of ATDC5 cells via MAP kinases and BMP-2 signaling pathways, Biomol Ther, 24 (2016) 395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Yu DQ, Liu XH, Research progress in antitumor effect of rhein, J Mod Med Health, 36 (2020) 390–392, [Google Scholar]
- [208].Letai A, Kuter DJ, Cancer, coagulation, and anticoagulation, Oncologist, 4 (1999) 443–449. [PubMed] [Google Scholar]
- [209].Zhang GD Research progress of anti-tumor effect by promoting blood circulation and removing blood stasis, Zhejiang J Tradit Chin Med, 4 (2002) 179–180. [Google Scholar]
- [210].Han SB, Lee CW, Kang MR, Yoon YD, Kang JS, Lee KH, Yoon WK, Lee K, Park SK, Kim HM, Pectic polysaccharide isolated from Angelica gigas Nakai inhibits melanoma cell metastasis and growth by directly preventing cell adhesion and activating host immune functions, Cancer Lett, 243 (2006) 264–273. [DOI] [PubMed] [Google Scholar]
- [211].Ferrara N, Vascular endothelial growth factor: Basic science and clinical progress, Endocr Rev, 25 (2004) 581–611. [DOI] [PubMed] [Google Scholar]
- [212].Zhou L, Liu X, Li J, Li W, Zhang L, Li J, Zhang J, Tetramethylpyrazine enhances the antitumor effect of paclitaxel by inhibiting angiogenesis and inducing apoptosis, Front Pharmacol, 10 (2019) 707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Li CY, Wang Q, Wang X, Li G, Shen S, Wei X, Scutellarin inhibits the invasive potential of malignant melanoma cells through the suppression epithelial-mesenchymal transition and angiogenesis via the PI3K/Akt/mTOR signaling pathway, Eur J Pharmacol, 858 (2019) 172463. [DOI] [PubMed] [Google Scholar]
- [214].Li XC, Research progress in anti -tumor angiogenesis of Chinese medicine, Mod Oncol, 22 (2014) 1993–1996. [Google Scholar]
- [215].Boone BA, Murthy P, Miller-Ocuin JL, Liang XY, Russell KL, Loughran P, Gawaz M, Lotze MT, H.J. 3rd Zeh, S. Vogel, The platelet NLRP3 inflammasome is upregulated in a murine model of pancreatic cancer and promotes platelet aggregation and tumor growth, Ann Hematol, 98 (2019) 1603–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Tsuruo T, Fujita N, Platelet aggregation in the formation of tumor metastasis, Proc Jpn Acad Ser B Biol Sci, 84 (2008) 189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Verheul HM, Voest EE, Schlingemann RO, Are tumours angiogenesis-dependent? J Pathol, 202 (2004) 5–13. [DOI] [PubMed] [Google Scholar]
- [218].Zhang JY, Zhang JB, Progress on experimental study of Panax notoginseng for anti-tumor, Global Tradit Chin Med, 8 (2015) 1008–1012. [Google Scholar]
- [219].Li L, Sun JX, Wang XQ, Liu XK, Chen XX, Zhang B, He ZD, Liu DZ, Chen LX, Wang LW, Huang Z, Notoginsenoside R7 suppresses cervical cancer via PI3K/PTEN/Akt/mTOR signaling, Oncotarget, 8 (2017) 109487–109496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Lee CY, Hsieh SL, Hsieh SC, Tsai CC, Hsieh LC, Kuo YH, Wu CC, Inhibition of human colorectal cancer metastasis by notoginsenoside R1, an important compound from Panax notoginseng, Oncol Rep, 37 (2017) 399–407. [DOI] [PubMed] [Google Scholar]
- [221].Yao Y, Wu WY, Liu AH, Deng SS, Bi KS, Liu X, Guo DA, Interaction of salvianolic acids and notoginsengnosides in inhibition of ADP-induced platelet aggregation, Am J Chin Med, 36 (2008) 313–328. [DOI] [PubMed] [Google Scholar]
- [222].Chi XY, Zhou T, Hu KW, Advances in research on the effects of chuanxiong on invasion and metastasis of malignant tumors, Acta Chin Med, 34 (2019) 495–500. [Google Scholar]
- [223].Law BYK, Mok SWF, Wu AG, Lam CWK, Yu MXY, Wong VKW, New potential pharmacological functions of Chinese herbal medicines via regulation of autophagy, Molecules, 21 (2016) 359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Jiang X, Shi L, Research progress on active constituents and pharmacological effects of Salvia miltiorrhiza, J Pharm Res, 36 (2017) 166–169. [Google Scholar]
- [225].Zhao PW, Lee DYW, Tao SY, Chen M, Niu JZ, Effect of G protein-coupled estrogen receptor on the occurrence and development of estrogen related cancer, Chin Pharmacol Bull, 30 (2014) 1037–1041. [Google Scholar]
- [226].Maione F, Cantone V, Chini MG, Feo VD, Mascolo N, Bifulco G, Molecular mechanism of tanshinone ⅡA and cryptotanshinone in platelet anti-aggregating effects: An integrated study of pharmacology and computational analysis, Fitoterapia, 100 (2015) 174–178. [DOI] [PubMed] [Google Scholar]
- [227].Ye H, Ruan JS, Wang SM, Research progress on anti-tumor metastasis of cryptotanshinone, Chin Pharmacol Bull, 30 (2014) 893–896. [Google Scholar]
- [228].Mou YS, Deng WL, Zhou LM, Research progress in antitumor effect of rosmarinic acid, Pharmacol Clin Chin Mater Med, 31 (2015) 266–269. [Google Scholar]
- [229].Feng CP, Ding HX, Liang J, Liu YX, Yao SQ, Effect of rosmarinic acid on HepG2 cell apoptosis and autophagy inhibition, Pharmacol Clin Chin Mater Med, 35 (2019) 57–63. [Google Scholar]
- [230].Zou ZW, Xu LN, Tian JY, Antithrombotic and antiplatelet effects of rosmarinic acid, a water-soluble component isolated from Radix Salviae miltiorrhizae (Danshen), Acta Pharm Sin, 28 (1993) 241–245. [PubMed] [Google Scholar]
- [231].Xu LJ, Wang Y, Progress in the study of antitumor mechanism of Danshensu, Zhejiang J Tradit Chin Med, 51 (2016) 700–701. [Google Scholar]
- [232].Jiang X, Shi L, Research progress on active constituents and pharmacological effects of Salvia miltiorrhiza, J Pharm Res, 36 (2017) 166–169. [Google Scholar]
- [233].Yao Y, Wu WY, Liu AH, Deng SS, Bi KS, Liu X, Guo DA, Interaction of salvianolic acids and notoginsengnosides in inhibition of ADP-induced platelet aggregation, Am J Chin Med, 36 (2008) 313–328. [DOI] [PubMed] [Google Scholar]
- [234].Tan T, Chen J, Hu YX, Wang N, Chen YM, Yu TT, Lin DY, Yang SD, Luo JY, Luo XJ, Dihydrotanshinone I inhibits the growth of osteosarcoma through the Wnt/β-catenin signaling pathway, Onco Targets Ther, 12 (2019) 5111–5122. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [235].Bian WP, Chen F, Bai L, Zhang P, Qin WX, Dihydrotanshinone Ⅰ inhibits angiogenesis both in vitro and in vivo, Acta Biochim Biophys Sin (Shanghai), 40 (2008) 1–6. [DOI] [PubMed] [Google Scholar]
- [236].Park JW, Lee SH, Yang MK, Lee JJ, Song MJ, Ryu SY, Chung HJ, Won HS, Lee CS, Kwon SH, Yun YP, Choi WS, Shin HS, 15,16-Dihydrotanshinone I, a major component from Salvia miltiorrhiza Bunge (Dansham), inhibits rabbit platelet aggregation by suppressing intracellular calcium mobilization, Arch Pharm Res, 31 (2008) 47–53. [DOI] [PubMed] [Google Scholar]
- [237].Hoshyar R, Bathaie SZ, Sadeghizadeh M, Crocin triggers the apoptosis through increasing the Bax/Bcl-2 ratio and caspase activation in human gastric adenocarcinoma, AGS, cells, DNA Cell Biol, 32 (2013) 50–57. [DOI] [PubMed] [Google Scholar]
- [238].Zhou Y, Luo T, Li S, Shen XY, Zhou LQ, Wang HQ, Effects of crocetin on proliferation, apoptosis and migration of human MG63 myeloma cells, Anat Res, 37 (2015) 54–58. [Google Scholar]
- [239].Thushara RM, Hemshekhar M, Sebastin Santhosh M, Jnaneshwari S, Nayaka SC, Naveen S, Kemparaju K, Girish KS, Crocin, a dietary additive protects platelets from oxidative stress-induced apoptosis and inhibits platelet aggregation, Mol Cell Biochem, 373 (2013) 73–83. [DOI] [PubMed] [Google Scholar]
- [240].Yu LY, Bao Y, Research progress of anticancer mechanisms of scutellarin, Chin J Clin Pharm, 27 (2018) 440–443. [Google Scholar]
- [241].Ding D, Cai XJ, Zheng HX, Guo SW, Liu XS, Scutellarin suppresses platelet aggregation and stalls lesional progression in mouse with induced endometriosis, Reprod Sci, 26 (2019) 1417–1428. [DOI] [PubMed] [Google Scholar]
- [242].Yuan YM, Wang RH, Yang SX, Research progress on anti-tumor effects of safflor yellow pigment, Chin J Clin Pharmacol, 33 (2017) 478–480. [Google Scholar]
- [243].Liang MM, Li SF, Wang KH, Zhang LW, Biological activity and hydroxy safflor yellow a content of intermediates in preparation of safflower injection, China J Chin Mater Med, 43 (2018) 4850–4854. [DOI] [PubMed] [Google Scholar]
- [244].Peng XL, Sun JN, Yu G, Li N, Chen L, Progress study on anti-tumor mechanism in salidroside, J Liaoning Univ Tradit Chin Med, 19 (2017) 5–8. [Google Scholar]
- [245].Zhang YJ, Zhao GA, Lin F, Yan ZG, Advances in research on antiatherosclerosis mechanism of salidroside, Chin J Arterioscler, 27 (2019) 547–552. [Google Scholar]
- [246].Zhang L, Li DC, Liu LF, Paeonol: Pharmacological effects and mechanisms of action, Int Immunopharmacol, 72 (2019) 413–421. [DOI] [PubMed] [Google Scholar]
- [247].Koo YK, Kim JM, Koo JY, Kang SS, Bae KH, Kim YS, Chung JH, Yun-Choi HS, Platelet anti-aggregatory and blood anti-coagulant effects of compounds isolated from Paeonia lactiflora and Paeonia suffruticosa, Pharmazie, 65 (2010) 624–628. [PubMed] [Google Scholar]
- [248].Chen YW, Zhang H, Gan YP, Zhang W, Wu J, Shen QM, Inhibitory role of bergenin on hepatic cancer, Chongqing Med, 47 (2018) 3365–3367. [Google Scholar]
- [249].Shi JM, Fan LH, Advances in research on anti-tumor mechanism of amygdalin, Chem Life, 39 (2019) 147–152. [Google Scholar]
- [250].Liu L, Duan JA, Tang YP, Guo JM, Yang NY, Ma HY, Shi XQ, Taoren-Honghua herb pair and its main components promoting blood circulation through influencing on hemorheology, plasma coagulation and platelet aggregation, J Ethnopharmacol, 139 (2012) 381–387. [DOI] [PubMed] [Google Scholar]
- [251].Li YD, Zhang YQ, Li JW, Research progress in the mechanism of naringenin against breast cancer, Med Innov China, 15 (2018) 145–148. [Google Scholar]
- [252].Wright B, Spencer JPE, Lovegrove JA, Gibbins JM, Flavonoid inhibitory pharmacodynamics on platelet function in physiological environments, Food Funct, 4 (2013) 1803–1810. [DOI] [PubMed] [Google Scholar]
- [253].Tomeh MA, Hadianamrei R, Zhao X, A review of curcumin and its derivatives as anticancer agents, Int J Mol Sci, 20 (2019) 1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [254].Fu HB, Wang CM, Yang DJ, Wei ZR, Xu JP, Hu ZQ, Zhang Y, Wang WM, Yan RL, Cai QP, Curcumin regulates proliferation, autophagy, and apoptosis in gastric cancer cells by affecting PI3K and P53 signaling, J Cell Physiol, 233 (2018) 4634–4642. [DOI] [PubMed] [Google Scholar]
- [255].Lopes-Rodrigues V, Oliveira A, Correia-da-Silva M, Pinto M, Lima RT, Sousa E, Vasconcelos MH, A novel curcumin derivative which inhibits P-glycoprotein, arrests cell cycle and induces apoptosis in multidrug resistance cells, Bioorg Med Chem, 25 (2017) 581–596. [DOI] [PubMed] [Google Scholar]
- [256].Li YP, Sun WX, Han N, Zou Y, Yin DX, Curcumin inhibits proliferation, migration, invasion and promotes apoptosis of retinoblastoma cell lines through modulation of miR-99a and JAK/STAT pathway, BMC Cancer, 18 (2018) 1230–1238. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [257].Zhao ZM, Zhang LJ, Xia T, Xiao Z, Advances in anti-inflammatory and anti-tumor effects of the main components of Curcumae Rhizoma, Drug Eva Res, 40 (2017) 119–124. [Google Scholar]
- [258].Zhang C, Wang LM, Inhibition of autophagy attenuated curcumol-induced apoptosis in MG-63 human osteosarcoma cells via Janus kinase signaling pathway, Oncol Lett, 14 (2017) 6387–6394. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [259].Gu KB, Zhang LN, Wu J, Zhang C, Research progress on resolving phlegm method on the treatment of malignant tumors, China J Tradit Chin Med Pharm, 32 (2017) 5468–5471. [Google Scholar]
- [260].Fang HY, Liu L, Shi H, Huang JL, Hong XH, Effect of extract of Huatan-Tongyu-Jiedu decoction on invasion and migration of human gastric carcinoma cell line SGC-7901, J Anhui Univ Chin Med, 35 (2016) 67–70. [Google Scholar]
- [261].Lou CH, Xu XT, Chen Y, Zhao HJ, Alisol a suppresses proliferation, migration, and invasion in human breast cancer MDA-MB-231 cells, Molecules, 24 (2019) 3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [262].Fan XY, Peng J, Huang JL, Wang L, Hong XH, Wang EY, Shi H, Fang HY, Extract from Huatan-Tongyu-Jiedu decoction inhibits epithelial-mesenchymal transformation and invasion/migration in human gastric cancer cell lines MKN-45 via Wnt/β-catenin signal pathway, Pharmacol Clin Chin Mater Med, 34 (2018) 163–168. [Google Scholar]
- [263].Wang BY, Han QQ, Zhu YQ, Study on the apoptosis of human lung cancer A549 cells induced by α-asarone through the Bcl-2 way, Pharmacol Clin Chin Mater Med, 32 (2016) 20–23. [Google Scholar]
- [264].Wang NB, Zhang QX, Luo LY, Ning BL, Fang YQ, β-asarone inhibited cell growth and promoted autophagy via p53/Bcl-2/Bclin-1 and p53/AMPK/mTOR pathways in human glioma U251 cells, J Cell Physiol, 233 (2017) 2434–2443. [DOI] [PubMed] [Google Scholar]
- [265].Lou CH, Xu XT, Chen Y, Zhao HJ, Alisol a suppresses proliferation, migration, and Invasion in human breast cancer MDA-MB-231 Cells, Molecules, 24 (2019) 3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [266].Wang C, Zhang JX, Shen XL, Wan CK, Tse AK, Fong WF, Reversal of P-glycoprotein-mediated multidrug resistance by Alisol B 23-acetate, Biochem Pharmacol, 68 (2004) 843–855. [DOI] [PubMed] [Google Scholar]
- [267].Kim YK, Kim YS, Ryu SY, Antiproliferative effect of furanocoumarins from the root of Angelica dahurica on cultured human tumor cell lines, Phytother Res, 21 (2007) 288–290. [DOI] [PubMed] [Google Scholar]
- [268].Mi CL, Ma J, Wang KS, Zuo HX, Wang Z, Li MY, Piao LX, Xu GH, Li XZ, Quan ZS, Jin XJ, Imperatorin suppresses proliferation and angiogenesis of human colon cancer cell by targeting HIF-1α via the mTOR/p70S6K/4E-BP1 and MAPK pathways, J Ethnopharmacol, 2013 (2017) 27–38.. [DOI] [PubMed] [Google Scholar]
- [269].Jiang TF, Lv ZH, Wang YH, Yue ME, On-line concentration by field-enhanced sample injection with reverse migrating micelles in micellar electrokinetic capillary chromatography for the analysis of coumarins from traditional Chinese medicine and human serum, Biomed Chromatogr, 24 (2010) 581–587. [DOI] [PubMed] [Google Scholar]
- [270].Liao ZG, Tang T, Guan XJ, Dong W, Zhang J, Zhao GW, Yang M, Liang XL, Improvement of transmembrane transport mechanism study of imperatorin on P-glycoprotein-mediated drug transport, Molecules, 21(2016) 1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [271].Lin BH, Wan SW, Liu FM, Cui ZY, Zhang X, Li XY, Effect of bergapten on cells cycle in nasopharyngeal carcinoma, Chin Pharm J, 49 (2014) 837–842. [Google Scholar]
- [272].Zhang Y, Wan YZ, Huo B, Li BY, Jin Y, Hu X, Extracts and components of Ficus carica leaves suppress survival, cell cycle, and migration of triple-negative breast cancer MDA-MB-231 cells, Onco Targets Ther, 11 (2018) 4377–4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [273].M N, Li YJ, Fan JP, Research progress on pharmacological action of diosmetin, J Liaoning Univ Tradi Chin Med, 20 (2018) 214–217. [Google Scholar]
- [274].Choi J, Lee DH, Park SY, Seol JW, Diosmetin inhibits tumor development and block tumor angiogenesis in skin cancer, Biomed Pharmacother, 117 (2019) 109091. [DOI] [PubMed] [Google Scholar]
- [275].Zhang H, Zhou YY, Lu WH, Chen CL, Gao X, Shan S, Overview on anti-tumor mechanism and effect of diosmin, Sci Technol Food Industry, 37 (2016) 376–379. [Google Scholar]
- [276].Li L, Ren ZX, Zhao P, Liu XF, Research progress in antitumor pharmacological activities of hesperidin and hesperetin, Acta Chin Med, 33 (2018) 2304–2308. [Google Scholar]
- [277].Roohbakhsh A, Parhiz H, Soltani F, Rezaee R, Iranshahi M, Molecular mechanisms behind the biological effects of hesperidin and hesperetin for the prevention of cancer and cardiovascular diseases, Life Sci, 124 (2015) 64–74. [DOI] [PubMed] [Google Scholar]
- [278].Wolfram J, Scott B, Boom K, Shen J, Borsoi C, Suri K, Grande R, Fresta M, Celia C, Zhao Y, Shen H, Ferrari M, Hesperetin liposomes for cancer therapy, Curr Drug Deliv, 13 (2016) 711–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [279].Wei JH, Wei JS, Wu WB, Huang SY, Research progress in the antitumor mechanism effects of nobiletin, Chin J Hosp Pharm, 39 (2019) 1211–1216. [Google Scholar]
- [280].Sp N, Kang DY, Kim DH, Park JH, Lee HG, Kim HJ, Darvin P, Park YM, Yang YM, Nobiletin inhibits CD36-dependent tumor angiogenesis, migration, invasion, and sphere formation through the CD36/STAT3/NF-κB signaling axis, Nutrients, 10 (2018) 772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [281].Feng SL, Zhou HF, Wu DY, Zheng DC, Qu B, Liu RM, Zhang C, Li Z, Xie Y, Luo HB, Nobiletin and its derivatives overcome multidrug resistance (MDR) in cancer: Total synthesis and discovery of potent MDR reversal agents, Acta Pharm Sin B, 10 (2019) 327–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [282].Dong Y, Cao AL, Shi JR, Yin PH, Wang L, Ji G, Xie JQ, Wu DZ, Tangeretin, a citrus polymethoxyflavonoid, induces apoptosis of human gastric cancer AGS cells through extrinsic and intrinsic signaling pathways, Oncol Rep, 31 (2014) 1788–1794. [DOI] [PubMed] [Google Scholar]
- [283].Zheng J, Shao YQ, Jiang Y, Chen F, Liu SL, Yu NH, Zhang DD, Liu XH, Zou LH, Tangeretin inhibits hepatocellular carcinoma proliferation and migration by promoting autophagy-related Beclin1, Cancer Manag Res, 11 (2019) 5231–5242. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [284].Feng SL, Yuan ZW, Yao XJ, Ma WZ, Liu L, Liu ZQ, Xie Y, Tangeretin, a citrus pentamethoxyflavone, antagonizes ABCB1-mediated multidrug resistance by inhibiting its transport function, Pharmacol Res, 110 (2016) 193–204. [DOI] [PubMed] [Google Scholar]
- [285].Qin J, Li XQ, Chun CJ, Qi ZY, Chen J, Yu ZD, Nie GH, Mechanism of anti-nasopharyngeal carcinoma effect of magnolol and honokiol, Chin Tradit Herbal Drugs, 46 (2015) 226–230. [Google Scholar]
- [286].Han HK, Anh LTV, Modulation of P-glycoprotein expression by honokiol, magnolol and 4-O-methylhonokiol, the bioactive components of Magnolia officinalis, Anticancer Res, 32 (2012) 4445–4452. [PubMed] [Google Scholar]
- [287].Zhou Y, Wang T, Zhao JJ, Wang L, Jiang XH, The reversal effects of piperine and (R) -(+)-citronellal on multidrug resistant breast cancer cells, J Chin Pharm Sci, 25 (2016) 351–356. [Google Scholar]
- [288].Liu P, Suo JX, Yu TF, Progress in the pharmacological action of piperine, Chin J Drug Appl Monit, 3 (2007) 7–9. [Google Scholar]
- [289].Wang F, Mao Y, You QJ, Hua D, Cai DY, Piperlongumine induces apoptosis and autophagy in human lung cancer cells through inhibition of PI3K/Akt/mTOR pathway, Int J Immunopathol Pharmacol, 28 (2015) 362–373. [DOI] [PubMed] [Google Scholar]
- [290].Xu SQ, Xiao YJ, Zeng S, Zou YY, Qiu Q, Huang MC, Zhan ZP, Liang LQ, Yang XY, Xu HS, Piperlongumine inhibits the proliferation, migration and invasion of fibroblast-like synoviocytes from patients with rheumatoid arthritis, Inflamm Res, 67 (2018) 233–243. [DOI] [PubMed] [Google Scholar]
- [291].Lin J, Li P, Chen KS, Advance in studies on anti-tumor activity of polysaccharides in latest five years, China J Chin Mater Med, 38 (2013) 1116–1125. [PubMed] [Google Scholar]
- [292].Liu QY, Yao YM, Zhang SW, Sheng ZY, Astragalus polysaccharides regulate T cell-mediated immunity via CD11c (high) CD45RB(low) DCs in vitro, J Ethnopharmacol, 136 (2011) 457–464. [DOI] [PubMed] [Google Scholar]
- [293].Zheng X, Chen W, Hou HL, Li J, Li HJ, Sun XM, Zhao L, Li X, Ginsenoside 20(S)-Rg3 induced autophagy to inhibit migration and invasion of ovarian cancer, Biomed Pharmacother, 85 (2017) 620–626. [DOI] [PubMed] [Google Scholar]
- [294].Tang YC, Zhang Y, Zhou J, Zhi QM, Wu MY, Gong FR, Shen M, Liu L, Tao M, Shen BR, Gu DM, Yu J, Xu MD, Gao Y, Li W, Ginsenoside Rg3 targets cancer stem cells and tumor angiogenesis to inhibit colorectal cancer progression in vivo, Int J Oncol, 52 (2017) 127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [295].Sun MY, Ye Y, Xiao L, Duan XY, Zhang YM, Zhang H, Anticancer effects of ginsenoside Rg3 (Review), Int J Mol Med, 39 (2017) 507–518. [DOI] [PubMed] [Google Scholar]
- [296].Zhang YH, Li HD, Li B, Jiang SD, Jiang LS, Ginsenoside Rg3 induces DNA damage in human osteosarcoma cells and reduces MNNG-induced DNA damage and apoptosis in normal human cells, Oncol Rep, 31 (2014) 919–925. [DOI] [PubMed] [Google Scholar]
- [297].Zhang YF, Qiu ZD, Qiu Y, Su T, Qu P, Jia AL, Functional regulation of ginsenosides on myeloid immunosuppressive cells in the tumor microenvironment, Integr Cancer Ther, 18 (2019) 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [298].Li HL, Huang N, Zhu WK, Wu JC, Yang XH, Teng WJ, Tian JH, Fang ZH, Luo YB, Chen M, Li Y, Modulation the crosstalk between tumor-associated macrophages and non-small cell lung cancer to inhibit tumor migration and invasion by ginsenoside Rh2, BMC Cancer, 18 (2018) 579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [299].Liu YN, Fan DD, Ginsenoside Rg5 induces apoptosis and autophagy via inhibition of PI3K/Akt pathway against breast cancer in mouse model, Food Funct, 9 (2018) 5513–5527. [DOI] [PubMed] [Google Scholar]
- [300].Liu HH, Zhao JQ, Fu RZ, Zhu CH, Fan DD, The ginsenoside Rk3 exerts anti-esophageal cancer activity in vitro and in vivo by mediating apoptosis and autophagy through regulation of the PI3K/Akt/mTOR pathway, PLoS One, 14 (2019) e0216759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [301].Dai GL, Sun BT, Gong T, Pan ZH, Meng QH, Ju WZ, Ginsenoside Rb2 inhibits epithelial-mesenchymal transition of colorectal cancer cells by suppressing TGF-β/SMAD signaling, Phytomed, 56 (2018) 126–135. [DOI] [PubMed] [Google Scholar]
- [302].Luo LM, Shi YN, Jiang YN, Zhan JH, Qin L, Chen NH, Advance in components with antitumor effect of Panax ginseng and their mechanisms, Chin Tradit Herbal Drugs, 48 (2017) 582–596. [Google Scholar]
- [303].Wu AG, Wong VKW, Xu SW, Chan WK, Ng CI, Liu L, Law BYK, Onjisaponin B derived from Radix Polygalae enhances autophagy and accelerates the degradation of mutant α-synuclein and huntingtin in PC-12 cells, Int J Mol Sci, 14 (2013) 22618–22641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [304].Law BYK, Mok SWF, Wu AG, Lam CWK, Yu MXY, Wong VKW, New potential pharmacological functions of Chinese herbal medicines via regulation of autophagy, Molecules, 21 (2016) 359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [305].Bryant JM, Bouchard M, Haque A, Anticancer activity of ganoderic acid DM: Current status and future perspective, J Clin Cell Immunol, 8 (2017) 535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [306].Madeo F, Tavernarakis N, Kroemer G, Can autophagy promote longevity? Nat Cell Biol, 12 (2010) 842–846. [DOI] [PubMed] [Google Scholar]
- [307].Li YJ, Wang XR, Cheng SL, Du J, Deng ZT, Zhang YN, Liu Q, Gao JD, Cheng BB, Ling CQ, Diosgenin induces G2/M cell cycle arrest and apoptosis in human hepatocellular carcinoma cells, Oncol Rep, 33 (2015) 693–698. [DOI] [PubMed] [Google Scholar]
- [308].Hamdi HK, Castellon R, Oleuropein, a non-toxic olive iridoid, is an anti-tumor agent and cytoskeleton disruptor, Biochem Biophys Res Commun, 334 (2005) 769–778. [DOI] [PubMed] [Google Scholar]
- [309].Chen RC, Su JH, Yang SM, Li J, Wang TJ, Zhou H, Effect of isoverbascoside, a phenylpropanoid glycoside antioxidant, on proliferation and differentiation of human gastric cancer cell, Acta Pharmacol Sin, 23 (2002) 997–1001. [PubMed] [Google Scholar]
- [310].Yuan Y, Han X, Xue MF, Ji MY, Shu W, Verbascoside inhibits migration and invasion of oral squamous cell carcinoma cells by upregulating Max protein, J Nanjing Med Univ (Nat Sci), 39 (2019) 32–36. [Google Scholar]
- [311].Jeong M, Kim HM, Kim HJ, Choi JH, Jang DS, Kudsuphilactone B, a nortriterpenoid isolated from Schisandra chinensis fruit, induces caspase-dependent apoptosis in human ovarian cancer A2780 cells, Arch Pharm Res, 40 (2017) 500–508. [DOI] [PubMed] [Google Scholar]
- [312].Lee K, Ahn JH, Lee KT, Jang DS, Choi JH, Deoxyschizandrin, isolated from Schisandra berries, induces cell cycle arrest in ovarian cancer cells and inhibits the protumoural activation of tumour-associated macrophages, Nutrients, 10 (2018) 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [313].Wu HX, Hou XJ, Xu XN, Liu YQ, Tao YL, Qi L, The antitumor multi-mechanism of Schisandrin, J Jilin Med Univ, 2 (2012) 107–110. [Google Scholar]
- [314].Zhu B, Zhang QL, Hua JW, Cheng WL, Qin LP, The traditional uses, phytochemistry, and pharmacology of Atractylodes macrocephala Koidz.: A review. J Ethnopharmacol, 226 (2018) 143–167. [DOI] [PubMed] [Google Scholar]
- [315].Kanawut K, Wanna C, Phunuch M, Kesara NB, Cytotoxic activities and effects of atractylodin and β-eudesmol on the cell cycle arrest and apoptosis on cholangiocarcinoma cell line, J Pharmacol Sci, 36 (2018) 51–56. [DOI] [PubMed] [Google Scholar]
- [316].Ma J, Chen J, Zhao BJ, Jiang ZY, Feng L, Jia XB, Advance in research on anticancer drug β-elemene and its derivatives, Chin Tradit Herbal Drugs, 49 (2018) 1184–1191. [Google Scholar]
- [317].Wang YH, Miao J, Tang RR, Ophiopogonin D inhibited the proliferation, migration and invasion of ovarian carcinoma 8910 cells by targeting PI3K/Akt pathway, Anti-Tumor Pharm, 9 (2019) 865–869. [Google Scholar]
- [318].He BQ, Zhou XJ, Shi DL, Chen GL, Progress on anti-tumor effects and mechanisms of Ophiopogonin B, Chin Arch Tradit Chin Med, 36 (2018) 2911–2914. [Google Scholar]
- [319].Lu TT, Ke LL, Tang LL, Wang LY, Study on the icariin targeting tumor immunomodulation and its anti-tumor mechanism, J Gansu Univ Chin Med, 35 (2018) 82–85. [Google Scholar]
- [320].He W, Sun H, Yang B, Zhang D, Kabelitz D, Immunoregulatory effects of the Herba Epimediia glycoside icariin, Arzneimittelforschung, 45 (1995) 910–913. [PubMed] [Google Scholar]
- [321].Wei YJ, Lou B, Wang XD, Preparation of Baohuoside I-loaded mixed micelles and its anti-tumor activity, J Nanjing Univ Tradit Chin Med, 32 (2016) 62–66. [Google Scholar]
- [322].Ma AL, Qi SJ, Xu DS, Daloze P, Chen HF, Baohuoside-1 inhibits activated T cell proliferation at G1-S phase transition, Transpl Immunol, 15 (2005) 55–62. [DOI] [PubMed] [Google Scholar]
- [323].Wang XH, Cheng K, Han Y, Zhang GQ, Dong JL, Cui YZ, Yang ZL, Effects of psoralen as an anti-tumor agent in human breast cancer MCF-7/ADR cells, Biol Pharm Bull, 39 (2016) 815–822. [DOI] [PubMed] [Google Scholar]
- [324].Yuan XF, Sun YM, Zhang ZY, Li CL, Guo Q, Song GH, Shi MY, Yu LC, Ren X, Jiang GS, Molecular mechanism of apoptosis of HL-60 cells induced by angelicin, Chin J Cancer Prev Treat, 22 (2015) 1675–1679. [Google Scholar]
- [325].Lv L, Liu B Anti-tumor effects of bakuchiol on human gastric carcinoma cell lines are mediated through PI3K/Akt and MAPK signaling pathways, Mol Med Rep, 16 (2017) 8977–8982. [DOI] [PubMed] [Google Scholar]
- [326].Zhang C, Zhang XD, Zhao J, Research status of semen psoraleae anti-tumor effect, China Foreign Med Treat, 36 (2017) 193–195. [Google Scholar]
- [327].Bian HL, Effect of sesamin on immune function and proliferating cell nuclear antigen in S180 sarcoma-bearing mice, China J Modern Med, 18 (2008) 3411–3413. [Google Scholar]
- [328].Kim JH, K Lee J, Naringenin enhances NK cell lysis activity by increasing the expression of NKG2D ligands on Burkitt’s lymphoma cells, Arch Pharm Res, 38 (2015) 2042–2048. [DOI] [PubMed] [Google Scholar]
- [329].Hu YL, Lin ZX, Liu J, Shi ZQ, Tang J, Li GL, Research progress of puerarin in tumor mechanism, Syst Med, 3 (2018) 184–186. [Google Scholar]
- [330].Xu H, Hu MY, Liu MR, An S, Guan KY, Wang ML, Li L, Zhang J, Li J, Huang L, Nano-puerarin regulates tumor microenvironment and facilitates chemo-and immunotherapy in murine triple negative breast cancer model, Biomaterials, 235 (2020) 119769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [331].Kang HG, Zhang J, Wang BZ, Liu MR, Zhao J, Yang MX, Li YX, Puerarin inhibits M2 polarization and metastasis of tumor-associated macrophages from NSCLC xenograft model via inactivating MEK/ERK ½ pathway, Int J Oncol, 50 (2017) 545–554. [DOI] [PubMed] [Google Scholar]
- [332].Tang M, Xu XF, Advances in studies on chemical constituents and pharmacological effects of medicinal astragalus, Guid J Tradit Chin Med Pharm, 24 (2018) 117–122. [Google Scholar]
- [333].Zou H, L Ding, Li JJ, Li YL, Progress in AS-IV anti-cancer research, Pharm Biotechnol, 26 (2019) 547–551. [Google Scholar]
- [334].Zhang Q, Zhang Q, Li J, Anti-tumor effects of Astragaloside Ⅳ: Research progress study, Inf Tradit Chin Med, 36 (2019) 129–132. [Google Scholar]
- [335].Unahabhokha T, Chanvorachote P, Pongrakhananon V, The attenuation of epithelial to mesenchymal transition and induction of anoikis by gigantol in human lung cancer H460 cells, Tumour Biol, 37 (2016) 8633–8641. [DOI] [PubMed] [Google Scholar]
- [336].Bhummaphan N, Chanvorachote P, Gigantol suppresses cancer stem cell-like phenotypes in lung cancer cells, Evid Based Complement Alternat Med, 2015 (2015) 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [337].Pengpaeng P, Sritularak B, Chanvorachote P, Dendrofalconerol A sensitizes anoikis and inhibits migration in lung cancer cells, J Nat Med, 69 (2015) 178–190. [DOI] [PubMed] [Google Scholar]
- [338].Wang H, Zhang T, Sun W, Wang Z, Zuo D, Zhou Z, Li S, Xu J, Yin F, Hua Y, Cai Z, Erianin induces G2/M-phase arrest, apoptosis and autophagy via the ROS/JNK signaling pathway in human osteosarcoma cells in vitro and in vivo, Cell Death Dis, 7 (2016) e2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [339].Gong YQ, Fan Y, Wu DZ, Yang H, Hu ZB, Wang ZT, In vivo and in vitro evaluation of erianin, a novel anti-angiogenic agent, Eur J Cancer (Oxford, England: 1990), 40 (2004) 1554–1565. [DOI] [PubMed] [Google Scholar]
- [340].Sun J, Fu X, Wang Y, Liu Y, Zhang Y, Hao T, Hu X, Erianin inhibits the proliferation of T47D cells by inhibiting cell cycles, inducing apoptosis and suppressing migration, Am J Transl Res, 8 (2016) 3077–3086. [PMC free article] [PubMed] [Google Scholar]
- [341].Wang XY, Meng CW, Zhou QM, Research progress of sesquiterpenoids from Dendrobium nobile, Nat Prod Res Dev, 31 (2019) 1837–1845. [Google Scholar]
- [342].Jung SY, Choi JH, Kwon SM, Masuda H, Asahara T, Lee YM, Decursin inhibits vasculogenesis in early tumor progression by suppression of endothelial progenitor cell differentiation and function, J Cell Biochem, 113 (2012) 1478–1487. [DOI] [PubMed] [Google Scholar]
- [343].Chen H, Imperatorin induction of apoptosis in human breast cancer cell line MCF-7 in vitro, Zhejiang Practical Med, 20 (2015) 177–179. [Google Scholar]
- [344].Kim BS, Seo H, Kim HJ, Bae SM, Son HN, Lee YJ, Ryu S, Park RW, Nam JO, Decursin from Angelica gigas Nakai inhibits B16F10 melanoma growth through induction of apoptosis, J Med Food, 18 (2015) 1121–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [345].Lee CC, Wang CC, Huang HM, Lin CL, Leu SJ, Lee YL, Ferulic acid induces Th1 responses by modulating the function of dendritic cells and ameliorates Th2-mediated allergic airway inflammation in mice, Evid Based Complement Alternat Med, 2015 (2015) 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [346].Wang T, Gong X, Jiang R, Li H, Du W, Kuang G, Ferulic acid inhibits proliferation and promotes apoptosis via blockage of PI3K/Akt pathway in osteosarcoma cell, Am J Transl Res, 8 (2016) 968–980. [PMC free article] [PubMed] [Google Scholar]
- [347].Du KW, Li ZQ, Fang XC, Cao TW, Xu YZ, Ferulic acid promotes osteogenesis of bone marrow-derived mesenchymal stem cells by inhibiting microRNA-340 to induce β-catenin expression through hypoxia, Eur J Cell Biol, 96 (2017) 496–503. [DOI] [PubMed] [Google Scholar]
- [348].Hanahan D, Weinberg RA, Hallmarks of cancer: The next generation, Cell, 144 (2011) 646–674. [DOI] [PubMed] [Google Scholar]
- [349].Zheng YF, Liu PX, Wang N, Wang SQ, Yang BW, Li M, Chen JP, Situ HL, Xie MQ, Liu Y, Wang ZY, Betulinic acid suppresses breast cancer metastasis by targeting GRP78-mediated glycolysis and ER stress apoptotic pathway, Oxid Med Cell Longev, 2019 (2019) 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [350].Shestov AA, Liu XJ, Ser Z, Cluntun AA, Hung YP, Huang L, Kim D, Le A, Yellen G, Albeck JG, Locasale JW, Quantitative determinants of aerobic glycolysis identify flux through the enzyme GADH as a limiting step, Elife, 3 (2014) 3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [351].Vaupel P, Schmidberger H, Mayer A, The Warburg effect: Essential part of metabolic reprogramming and central contributor to cancer progression, Int J Radiat Biol, 95 (2019) 912–919. [DOI] [PubMed] [Google Scholar]
- [352].Wu J, Zhang XX, Wang YH, Sun QM, Chen M, Liu SL, Zou X, Licochalcone A suppresses hexokinase 2-mediated tumor glycolysis in gastric cancer via downregulation of the Akt signaling pathway, Oncol Rep, 39 (2018) 1181–1190. [DOI] [PubMed] [Google Scholar]
- [353].Hamanaka RB, Chandel NS, Targeting glucose metabolism for cancer therapy, J Exp Med, 209 (2012) 211–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [354].Li LJ, Li GW, Xie Y, Regulatory effects of glabridin and quercetin on energy metabolism of breast cancer cells, China J Chin Meter Med, 44 (2019) 3786–3791. [DOI] [PubMed] [Google Scholar]
- [355].Jia LJ, Huang S, Yin XR, Zan Y, Guo Y, Han LL, Quercetin suppresses the mobility of breast cancer by suppressing glycolysis through Akt-mTOR pathway mediated autophagy induction, Life Sci, 208 (2018) 123–130. [DOI] [PubMed] [Google Scholar]
- [356].Li YY, Xu QF, Yang W, Wu TL, Lu XR, Oleanolic acid reduces aerobic glycolysis-associated proliferation by inhibiting yes-associated protein in gastric cancer cells, Gene, 712 (2019) 143956. [DOI] [PubMed] [Google Scholar]
- [357].Xu YC, Han S, Lei KS, Chang XN, Wang K, Li Z, Liu JW, Anti-Warburg effect of rosmarinic acid via miR-155 in colorectal carcinoma cells, Eur J Cancer Prev, 25 (2016) 481–489. [DOI] [PubMed] [Google Scholar]
- [358].Jiao L, Wang SQ, Zheng YF, Wang N, Yang BW, Wang DM, Yang DP, Mei WJ, Zhao ZM, Wang ZY, Betulinic acid suppresses breast cancer aerobic glycolysis via caveolin-1/NF-κB/c-Myc pathway, Biochem Pharmacol, 161 (2019) 149–162. [DOI] [PubMed] [Google Scholar]
- [359].Yao GD, Yang J, Li XX, Song XY, Hayashi T, Tashiro SI, Onodera S, Song SJ, Ikejima T, Blocking the utilization of glucose induces the switch from senescence to apoptosis in pseudolaric acid B-treated human lung cancer cells in vitro, Acta Pharmacol Sin, 38 (2017) 1401–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [360].Yao Z, Xie FH, Li M, Liang ZR, Xu WL, Yang JH, Liu C, Li HWW, Zhou H, Qu LH, Oridonin induces autophagy via inhibition of glucose metabolism in p53-mutated colorectal cancer cells, Cell Death Dis, 8 (2017) 2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [361].Chen GQ, Tang CF, Shi XK, Lin CY, Fatima S, Pan XH, Yang DJ, Zhang G, Lu AP, Lin SH, Bian ZX, Halofuginone inhibits colorectal cancer growth through suppression of Akt/mTORC1 signaling and glucose metabolism, Oncotarget, 6 (2015) 24148–24162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [362].Wu J, Zhang XX, Wang YH, Sun QM, Chen M, Liu SL, Zou X, Licochalcone A suppresses hexokinase 2-mediated tumor glycolysis in gastric cancer via downregulation of the Akt signaling pathway, Oncol Rep, 39 (2018) 1181–1190. [DOI] [PubMed] [Google Scholar]
- [363].Lin LL, Hsia CR, Hsu CL, Huang HC, Juan HF, Integrating transcriptomics and proteomics to show that tanshinone IIA suppresses cell growth by blocking glucose metabolism in gastric cancer cells, BMC Genomics, 16 (2015) 41–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [364].Wu ST, Liu B, Ai ZZ, Hong ZC, You PT, Wu HZ, Yang YF, Esculetin inhibits cancer cell glycolysis by binding tumor PGK2, GPD2, and GPI, Front Pharmacol, 11 (2020) 379–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [365].Zheng X, Wu F, Lin X, Shen L, Feng Y, Developments in drug delivery of bioactive alkaloids derived from traditional Chinese medicine, Drug Deliv, 25 (2018) 398–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [366].Zhan SY, Shao Q, Li Z, Wang Y, Fan XH, Study on PK-PD characteristics of ginsenoside Rg1 and Rb1 in rats with myocardial ischemia following intravenous administration of Shengmai injection, China J Chin Mater Med, 39 (2014) 1300–1305. [PubMed] [Google Scholar]
- [367].Liu ZY, Jiang WX, Wu B, Advance in studies on ADME of saponins, Chin J Mod Drug Appl, 6 (2012) 121–124. [Google Scholar]
- [368].Han SX, You Y, Balance between cardiovascular pharmacological and hemolytic effects osaponins of Panax notogenseng, China J Chin Mater Med, 41 (2016) 818–822. [DOI] [PubMed] [Google Scholar]
- [369].Xu YL, Study on hemolytic effect of ophiopogon saponin in vitro, J Chengdu Univ (Nat Sci), 37 (2018) 125–128. [Google Scholar]
- [370].Wei L, Marasini N, Li G, Yong CS, Kim JO, Quan Q, Development of ligustrazine-loaded lipid emulsion: Formulation optimization, characterization and biodistribution, Int J Pharm, 437 (2012) 203–212. [DOI] [PubMed] [Google Scholar]
- [371].Zhang J, Zhang Y, Wang YL, Gao WY, Liu Z, Study on anticancer activity and correlation with meridian tropism of toxic Chinese materia medica. Chin Tradit Herbal Drugs, 44 (2013) 1852–1858. [Google Scholar]
- [372].Tang ZH, Sanmamed MF, Chen LP, A paradigm shift in cancer immunotherapy: From enhancement to normalization, Cell, 175 (2018) 313–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [373].Bahrami B, Hojjat-Farsangi M, Mohammadi H, Anvari E, Ghalamfarsa G, Yousefi M, Jadidi-Niaragh F, Nanoparticles and targeted drug delivery in cancer therapy, Immunol Lett, 190 (2017) 64–83. [DOI] [PubMed] [Google Scholar]
- [374].Bangham AD, Standish MM, Watkins JC, Diffusion of univalent ions across lamellae of swollen phospholipids, J Mol Biol, 13 (1965) 238–258. [DOI] [PubMed] [Google Scholar]
- [375].Misra R, Acharya S, Sahoo SK, Cancer nanotechnology: Application of nanotechnology in cancer therapy, Drug Discov Today, 15 (2010) 842–850. [DOI] [PubMed] [Google Scholar]
- [376].Xu W, Xing FJ, Dong K, You C, Yan Y, Zhang L, Zhao G, Chen Y, Wang K, Application of traditional Chinese medicine preparation in targeting drug delivery system, Drug Deliv, 22 (2015) 258–265. [DOI] [PubMed] [Google Scholar]
- [377].Wang X, Wang Q, Liu Z, Zheng X, Preparation, pharmacokinetics and tumour-suppressive activity of berberine liposomes, J Pharm Pharmacol, 69 (2017) 625–632. [DOI] [PubMed] [Google Scholar]
- [378].Nekkanti V, Kalepu S, Recent advances in liposomal drug delivery: A review, Pharm Nanotechnol, 3 (2015) 35–55. [Google Scholar]
- [379].Yan XD, Scherphof GL, Kamps J, Liposome opsonization, J Liposome Res, 15 (2005) 109–139. [DOI] [PubMed] [Google Scholar]
- [380].Otsuka H, Nagasaki Y, Kataoka K, PEGylated nanoparticles for biological and pharmaceutical applications, Adv Drug Deliv Rev, 55 (2003) 403–419. [DOI] [PubMed] [Google Scholar]
- [381].Blume G, Cevc G, Molecular mechanism of the lipid vesicle longevity in vivo, Biochim Biophys Acta, 1146 (1993) 157–168. [DOI] [PubMed] [Google Scholar]
- [382].Riaz MK, Riaz MA, Zhang X, Lin CC, Wong KH, Chen XY, Zhang G, Lu AP, Yang ZJ, Surface functionalization and targeting strategies of liposomes in solid tumor therapy: a review, Int J Mol Sci, 19 (2018) 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [383].Whiteman KR, Subr V, Ulbrich K, Torchilin VP, Poly(HPMA)-coated liposomes demonstrate prolonged circulation in mice, J Liposome Res, 11 (2001) 153–164. [DOI] [PubMed] [Google Scholar]
- [384].Zhang Jing J, Chen YC, Li X, Liang XL, Luo XJ, The influence of different long-circulating materials on the pharmacokinetics of liposomal vincristine sulfate, Int J Nanomedicine, 11 (2016) 4187–4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [385].Kaasgaard T, Andresen TL, Liposomal cancer therapy: Exploiting tumor characteristics, Expert Opin Drug Deliv, 7 (2010), 225–243. [DOI] [PubMed] [Google Scholar]
- [386].Liu Y, Hu Y, Huang L, Influence of polyethylene glycol density and surface lipid on pharmacokinetics and biodistribution of lipid-calcium-phosphate nanoparticles, Biomaterials, 35 (2014) 3027–3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [387].Moghimi SM, Szebeni J, Stealth liposomes and long circulating nanoparticles: Critical issues in pharmacokinetics, opsonization and protein-binding properties, Prog Lipid Res, 42 (2003) 463–478. [DOI] [PubMed] [Google Scholar]
- [388].Garbuzenko O, Barenholz Y, Priev A, Effect of grafted PEG on liposome size and on compressibility and packing of lipid bilayer, Chem Phys Lipids, 135 (2005) 117–129. [DOI] [PubMed] [Google Scholar]
- [389].Zhang BJ, Li WC, Liu XP, The pharmacokinetics of long-circulating β-elemene liposomes, Journal of Chinese Medicinal Materials, 35 (2012) 1464–1468. [PubMed] [Google Scholar]
- [390].Wang L, Cai BC, Li WD, Deng XK, Preparation and quality evaluation of brucine long-circulating liposomes, China Pharm J, 41 (2006) 1397–1400. [Google Scholar]
- [391].Kheir MM, Wang YG, Hua L, Hu J, Li LL, Lei F, Du LJ, Acute toxicity of berberine and its correlation with the blood concentration in mice, Food Chem Toxicol, 48 (2010) 1105–1110. [DOI] [PubMed] [Google Scholar]
- [392].Zhang JW, Zhou F, Lu M, Ji W, Niu F, Zha WB, Wu XL, Hao HP, Wang GJ, Pharmacokinetics-pharmacology disconnection of herbal medicines and its potential solutions with cellular pharmacokinetic-pharmacodynamic strategy. Curr Drug Metab, 13 (2012) 558–576. [DOI] [PubMed] [Google Scholar]
- [393].Li SM, Li JM, Yin ZQ, Study on preparation of oxymatrine PEGs liposomes, Heilongjiang Med J, 23 (2010) 871–874. [Google Scholar]
- [394].Lin H, Qu CX, Yu YJ, Tang YN, Sun XY, Preparation of freeze-dried long-circulation oridonin liposomes and their pharmacokinetics in rats, J Zhejiang Univ (Med Sci), 42 (2013) 638–643. [DOI] [PubMed] [Google Scholar]
- [395].Dadgar N, Esfahani MKM, Torabi S, Alavi SE, Akbarzadeh A, Effects of nanoliposomal and pegylated nanoliposomal artemisinin in treatment of breast cancer, Indian J Clin Biochem, 29 (2014) 501–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [396].Lu Y, Li J, Wang G, In vitro and in vivo evaluation of mPEG-PLA modified liposomes loaded glycyrrhetinic acid, Int J Pharm, 356 (2008) 274–281. [DOI] [PubMed] [Google Scholar]
- [397].Sarfraz M, Afzal A, Raza SM, Bashir S, Madni A, Khan MW, Ma X, Xiang GY, Liposomal co-delivered oleanolic acid attenuates doxorubicin induced multi-organ toxicity in hepatocellular carcinoma, Oncotarget, 8 (2017) 47136–47153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [398].Yang JH, Wu WS, Wen JL, Ye HY, Luo H, Bai P, Tang MH, Wang F, Zheng L, Yang SY, Li WM, Peng AH, Yang L, Wan L, Chen LJ, Liposomal honokiol induced lysosomal degradation of Hsp90 client proteins and protective autophagy in both gefitinib-sensitive and gefitinib-resistant NSCLC cells, Biomaterials, 141 (2017) 188–198. [DOI] [PubMed] [Google Scholar]
- [399].Kumar MR, Aithal BK, Udupa N, Reddy MS, Raakesh V, Murthy RS, Raju DP, Rao BS, Formulation of plumbagin loaded long circulating pegylated liposomes: In vivo evaluation in C57BL/6J mice bearing B16F1 melanoma, Drug Deliv, 18 (2011) 511–522. [DOI] [PubMed] [Google Scholar]
- [400].Yu JQ, Chen H, Jiang LX, Wang JH, Dai JD, Wang J, Codelivery of adriamycin and P-gp inhibitor quercetin using PEGylated liposomes to overcome cancer drug resistance, J Pharm Sci, 108 (2019) 1788–1799. [DOI] [PubMed] [Google Scholar]
- [401].Rastgoo M, Hosseinzadeh H, Alavizadeh H, Abbasi A, Ayati Z, Jaafari MR, Antitumor activity of PEGylated nanoliposomes containing crocin in mice bearing C26 colon carcinoma, Planta Med, 79 (2013) 447–451. [DOI] [PubMed] [Google Scholar]
- [402].Ochi MM, Amoabediny G, Rezayat SM, Akbarzadeh A, Ebrahimi B, In vitro co-delivery evaluation of novel pegylated nano-liposomal herbal drugs of silibinin and glycyrrhizic acid (nano-phytosome) to hepatocellular carcinoma cells. Cell J, 18 (2016) 135–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [403].Mohan A, Narayanan S, Balasubramanian G, Sethuraman S, Krishnan UM, Dual drug loaded nanoliposomal chemotherapy: A promising strategy for treatment of head and neck squamous cell carcinoma, Eur J Pharm Biopharm, 99 (2016) 73–83. [DOI] [PubMed] [Google Scholar]
- [404].Wolfram J, Suri K, Huang Y, Molinaro R, Borsoi C, Scott B, Boom K, Paolino D, Fresta M, Wang J, Ferrari M, Celia C, Shen H, Evaluation of anticancer activity of celastrol liposomes in prostate cancer cells, J Microencapsul, 31 (2014) 501–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [405].Rocha TGR, de Araújo Lopes SC, Cassali GD, Ferreira Ê, Veloso ES, Leite EA, Braga FC, Ferreira LAM, Balvay D, Garofalakis A, Oliveira MC, Tavitian B, Evaluation of antitumor activity of long-circulating and pH-sensitive liposomes containing ursolic acid in animal models of breast tumor and gliosarcoma, Integr Cancer Ther, 15 (2016) 512–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [406].Coimbra M, Isacchi B, van Bloois L, Torano JS, Ket A, Wu X, Broere F, Metselaar JM, Rijcken CJ, Storm G, Bilia R, Schiffelers RM, Improving solubility and chemical stability of natural compounds for medicinal use by incorporation into liposomes, Int J Pharm, 416 (2011) 433–442. [DOI] [PubMed] [Google Scholar]
- [407].Liu Y, Gao D, Zhang X, Liu Z, Dai K, Ji B, Wang Q, Luo L, Antitumor drug effect of betulinic acid mediated by polyethylene glycol modified liposomes, Mater Sci Eng C Mater Biol Appl, 64 (2016) 124–132. [DOI] [PubMed] [Google Scholar]
- [408].Gómez-Hens A, Fernández-Romero JM, Analytical methods for the control of liposomal delivery systems, Trends Anal Chem, 25 (2006) 167–178. [Google Scholar]
- [409].Lim SB, Banerjee A, Önyüksel H, Improvement of drug safety by the use of lipid- based nanocarriers, J Control Release, 163 (2012) 34–45. [DOI] [PubMed] [Google Scholar]
- [410].Zhai B, Zeng Y, Zeng Z, Zhang N, Li C, Zeng Y, You Y, Wang S, Chen X, Sui X, Xie T, Drug delivery systems for elemene, its main active ingredient beta-elemene, and its derivatives in cancer therapy, Int J Nanomedicine, 13 (2018) 6279–6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [411].Blenke EO, Mastrobattista E, Schiffelers RM, Strategies for triggered drug release from tumor targeted liposomes, Expert Opin Drug Deliv, 10 (2013) 1399–1410. [DOI] [PubMed] [Google Scholar]
- [412].Belfiore L, Saunders DN, Ranson M, Thurecht KJ, Storm G, Vine KL, Towards clinical translation of ligand-functionalized liposomes in targeted cancer therapy: Challenges and opportunities. J Control Release, 277 (2018) 1–13. [DOI] [PubMed] [Google Scholar]
- [413].Patel JD, O’Carra R, Jones J, Woodward JG, Mumper RJ, Preparation and characterization of nickel nanoparticles for binding to HIS-TAG proteins and antigens, Pharm Res, 24 (2007) 343–352. [DOI] [PubMed] [Google Scholar]
- [414].Torchilin VP, Klibanov AL, Huang L, O’Donnell S, Nossiff ND, Khaw BA, Targeted accumulation of polyethylene glycol-coated immunoliposomes in infarcted rabbit myocardium, FASEB J, 6 (1992) 2716–2719. [DOI] [PubMed] [Google Scholar]
- [415].Zhang J, Zhang P, Zou Q, Li X, Fu JJ, Luo Y, Liang XL, Jin Y, Co-delivery of gemcitabine and paclitaxel in cRGD-modified long circulating nanoparticles with asymmetric lipid layers for breast cancer treatment, Molecules, 23 (2018) 2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [416].Lee RJ, Low PS,, Delivery of liposomes into cultured KB cells via folate receptor-mediated endocytosis, J Biol Chem, 269 (1994) 3198–3204. [PubMed] [Google Scholar]
- [417].Feng L, Mumper RJ, A critical review of lipid-based nanoparticles for taxane delivery, Cancer Lett, 334 (2013) 157–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [418].Adams GP, Schier R, McCall AM, Simmons HH, Horak EM, Alpaugh RK, Marks JD, Weiner LM, High affinity restricts the localization and tumor penetration of single-chain Fv antibody molecules, Cancer Res, 61 (2001) 4750–4755. [PubMed] [Google Scholar]
- [419].Emanuel N, Kedar E, Bolotin EM, Smorodinsky NI, Barenholz Y, Targeted delivery of doxorubicin via sterically stabilized immunoliposomes: pharmacokinetics and biodistribution in tumor-bearing mice, Pharm Res, 13 (1996) 861–868. [DOI] [PubMed] [Google Scholar]
- [420].Chen J, Chen YC, Cheng Y, Gao YH, Zheng PJ, Li CN, Tong YD, Li Z, Luo WH, Chen Z, Modifying glycyrrhetinic acid liposomes with liver-targeting ligand of galactosylated derivative: Preparation and evaluations, Oncotarget, 8 (2017) 102046–102066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [421].Guo B, Cheng Y, Li N, Li X, Jin M, Li T, Li J, In vitro and in vivo studies of galactose-modified liver-targeting liposomes, J Drug Target, 21 (2012) 257–264. [DOI] [PubMed] [Google Scholar]
- [422].Zhang FY, He S, Study on targetability of lactosaminated matrine liposomes to liver in vivo and inhibition to HepG2 cells in vitro, J Chongqing Med Univ, 34 (2009) 747–751. [Google Scholar]
- [423].Song XL, Liu S, Jiang Y, Gu LY, Xiao Y, Wang X, Cheng L, Li XT, Targeting vincristine plus tetrandrine liposomes modified with DSPE-PEG2000-transferrin in treatment of brain glioma, Eur J Pharm Sci, 96 (2017) 129–140. [DOI] [PubMed] [Google Scholar]
- [424].Liu S, Zhang SM, Ju RJ, Xiao Y, Wang X, Song XL, Gu LY, Cheng L, Li XT, Chen GR, Antitumor efficacy of Lf modified daunorubicin plus honokiol liposomes in treatment of brain glioma, Eur J Pharm Sci, 106 (2017) 185–197. [DOI] [PubMed] [Google Scholar]
- [425].Lu Y, Ding N, Yang C, Huang L, Liu J, Xiang G, Preparation and in vitro evaluation of a folate-linked liposomal curcumin formulation, J Liposome Res, 22 (2012) 110–119. [DOI] [PubMed] [Google Scholar]
- [426].Chen Y, Minh LV, Liu J, Angelov B, Drechsler M, Garamus VM, Willumeit-Romer R, Zou A, Baicalin loaded in folate-PEG modified liposomes for enhanced stability and tumor targeting, Colloids Surf B Biointerfaces, 140 (2016) 74–82. [DOI] [PubMed] [Google Scholar]
- [427].Yang G, Zhang T, Chen XQ, Preparation of targeted ursolic acid liposomes modified with folate derivative and their inhibitory effect on human epidermoid carcinoma, Chin J New Drugs, 24 (2015) 2708–2714. [Google Scholar]
- [428].Tian J, Wang L, Wang L, Ke X, A wogonin-loaded glycyrrhetinic acid-modified liposome for hepatic targeting with anti-tumor effects, Drug Deliv, 21 (2014) 553–559. [DOI] [PubMed] [Google Scholar]
- [429].Zhou R, Zhou LL, Zhong SY, Yuan L, Zhou W, Li C, Xia XH, The study on preparation of baicalin liposomes modified by glycyrrhetinic acid, J Hunan Univ Chin Med, 37 (2017) 373–377. [Google Scholar]
- [430].Xiao L, Chen ZP, Xiao YY, Cai BC, Li WD, Optimization of brucine liposomes modified with glycyrrhetinic acid by central composite design, J Nanjing TCM Univ, 27 (2011) 451–454. [Google Scholar]
- [431].Chang M, Wu M, Li H, Antitumor activities of novel glycyrrhetinic acid-modified curcumin-loaded cationic liposomes in vitro and in H22 tumor-bearing mice, Drug Deliv, 25 (2018) 1984–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [432].Sugiyama I, Kaihatsu K, Soma Y, Kato N, Sadzuka Y, Dual-effect liposomes with increased antitumor effects against 67-kDa laminin receptor-overexpressing tumor cells, Int J Pharm, 541 (2018) 206–213. [DOI] [PubMed] [Google Scholar]
- [433].Wang XX, Li YB, Yao HJ, Ju RJ, Zhang Y, Li RJ, Yu Y, Zhang L, Lu WL, The use of mitochondrial targeting resveratrol liposomes modified with a dequalinium polyethylene glycol-distearoylphosphatidyl ethanolamine conjugate to induce apoptosis in resistant lung cancer cells, Biomaterials, 32 (2011) 5673–5687. [DOI] [PubMed] [Google Scholar]
- [434].Ma X, Zhou J, Zhang CX, Li XY, Li N, Ju RJ, Shi JF, Sun MG, Zhao WY, Mu LM, Yan Y, Lu WL, Modulation of drug-resistant membrane and apoptosis proteins of breast cancer stem cells by targeting berberine liposomes, Biomaterials, 34 (2013) 4452–4465. [DOI] [PubMed] [Google Scholar]
- [435].Kang JH, Ko YT, Enhanced subcellular trafficking of resveratrol using mitochondriotropic liposomes in cancer cells, Pharmaceutics, 11 (2019) 423–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [436].Li JL, Meng LH, Zhao YX, Pan LJ, Wang C, Preparation and cytotoxicity of hyaluronic acid phosphatidyl derivative modified curcumin liposomes, Chin J Pharm, 44 (2013) 471–475. [Google Scholar]
- [437].Zhao YX, Zhang SP, Zhang F, Liu N, Liu F, Inhibition effect of hyaluronic acid targeting modified matrine liposome on murine H22 hepatocarcinoma, China Pharm, 27 (2018) 5–7. [Google Scholar]
- [438].Lu L, Ding Y, Zhang Y, Ho RJ, Zhao Y, Zhang T, Guo C, Antibody-modified liposomes for tumor-targeting delivery of timosaponin AIII, Int J Nanomedicine, 13 (2018) 1927–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [439].Catania A, Barrajón-Catalán E, Nicolosi S, Cicirata F, Micol V, Immunoliposome encapsulation increases cytotoxic activity and selectivity of curcumin and resveratrol against HER2 overexpressing human breast cancer cells, Breast Cancer Res Treat, 141 (2013) 55–65. [DOI] [PubMed] [Google Scholar]
- [440].Riaz MK, Zhang X, Wong KH, Chen H, Liu Q, Chen X, Zhang G, Lu A, Yang Z, Pulmonary delivery of transferrin receptors targeting peptide surface-functionalized liposomes augments the chemotherapeutic effect of quercetin in lung cancer therapy, Int J Nanomedicine, 14 (2019) 2879–2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [441].Wang J, Cai XJ, Ni JJ, Preparation of triptolide liposomes modified by NGR polypeptide and its inhibitory effect on human umbilical vein endothelial cells, China Pharmacist, 22 (2019) 1141–1143. [Google Scholar]
- [442].Chen J, Lin A, Peng P, Wang Y, Gu W, Liu Y, Lipid composition has significant effect on targeted drug delivery properties of NGR-modified liposomes, Drug Deliv, 23 (2016) 1426–1433. [DOI] [PubMed] [Google Scholar]
- [443].Li XT, Tang W, Xie HJ, Liu S, Song XL, Xiao Y, Wang X, Cheng L, Chen GR, The efficacy of RGD modified liposomes loaded with vinorelbine plus tetrandrine in treating resistant brain glioma, J Liposome Res, 29 (2019) 21–34. [DOI] [PubMed] [Google Scholar]
- [444].Liu XY, Ruan LM, Mao WW, Wang JQ, Shen YQ, Sui MH, Preparation of RGD-modified long circulating liposome loading matrine, and its in vitro anti-cancer effects, Int J Med Sci, 7 (2010) 197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [445].Wen X, Li J, Cai D, Yue L, Wang Q, Zhou L, Fan L, Sun J, Wu Y, Anticancer efficacy of targeted shikonin liposomes modified with RGD in breast cancer cells, Molecules, 23 (2018) 268–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [446].Liu JJ, Tang W, Fu M, Gong XQ, Kong L, Yao XM, Jing M, Cai FY, Li XT, Ju RJ, Development of R8 modified epirubicin-dihydroartemisinin liposomes for treatment of non-small-cell lung cancer, Artif Cells Nanomed Biotechnol, 47 (2019) 1947–1960. [DOI] [PubMed] [Google Scholar]
- [447].Fu M, Tang W, Liu JJ, Gong XQ, Kong L, Yao XM, Jing M, Cai FY, Li XT, Ju RJ, Combination of targeted daunorubicin liposomes and targeted emodin liposomes for treatment of invasive breast cancer, J Drug Target, 28 (2019) 245–258. [DOI] [PubMed] [Google Scholar]
- [448].Sun Y, Li X, Zhang L, Liu X, Jiang B, Long Z, Jiang Y, Cell permeable NBD peptide-modified liposomes by hyaluronic acid coating for the synergistic targeted therapy of metastatic inflammatory breast cancer, Mol Pharm, 16 (2019) 1140–1155. [DOI] [PubMed] [Google Scholar]
- [449].Jin X, Zhou J, Zhang Z, Lv H, The combined administration of parthenolide and ginsenoside CK in long circulation liposomes with targeted tLyp-1 ligand induce mitochondria-mediated lung cancer apoptosis, Artif Cells Nanomed Biotechnol, 46 (2018) S931–S942. [DOI] [PubMed] [Google Scholar]
- [450].Li J, Cheng X, Chen Y, He W, Ni L, Xiong P, Wei M, Vitamin E TPGS modified liposomes enhance cellular uptake and targeted delivery of luteolin: An in vivo/in vitro evaluation, Int J Pharm, 512 (2016) 262–272. [DOI] [PubMed] [Google Scholar]
- [451].Yang L, Xin J, Zhang Z, Yan H, Wang J, Sun E, Hou J, Jia X, Lv H, TPGS-modified liposomes for the delivery of ginsenoside compound K against non-small cell lung cancer: Formulation design and its evaluation in vitro and in vivo, J Pharm Pharmacol, 68 (2016) 1109–1118. [DOI] [PubMed] [Google Scholar]
- [452].Dube D, Khatri K, Goyal AK, Mishra N, Vyas SP, Preparation and evaluation of galactosylated vesicular carrier for hepatic targeting of silibinin, Drug Dev Ind Pharm, 36 (2010) 547–555. [DOI] [PubMed] [Google Scholar]
- [453].Murao A, Nishikawa M, Managit C, Wong J, Kawakami S, Yamashita F, Hashida M, Targeting efficiency of galactosylated liposomes to hepatocytes in vivo: Effect of lipid composition, Pharm Res, 19 (2002) 1808–1814. [DOI] [PubMed] [Google Scholar]
- [454].Sliedregt LAJM, Rensen PCN, Rump ET, van Santbrink PJ, Bijsterbosch MK, Valentijn ARPM, van der Marel GA, van Boom JH, van Berkel TJC, Biessen EAL, Design and synthesis of novel amphiphilic dendritic galactosides for selective targeting of liposomes to the hepatic asialoglycoprotein receptor, J Med Chem, 42 (1999) 609–618. [DOI] [PubMed] [Google Scholar]
- [455].Kawakami S, Wong J, Sato A, Hattori Y, Yamashita F, Hashida M, Biodistribution characteristics of mannosylated, fucosylated, and galactosylated liposomes in mice, Biochim Biophys Acta, 1524 (2000) 258–265. [DOI] [PubMed] [Google Scholar]
- [456].Managit C, Kawakami S, Nishikawa M, Yamashita F, Hashida M, Targeted and sustained drug delivery using PEGylated galactosylated liposomes, Int J Pharm, 266 (2003) 77–84. [DOI] [PubMed] [Google Scholar]
- [457].Gao H, Jiang X, Progress on the diagnosis and evaluation of brain tumors, Cancer Imaging, 13 (2013) 466–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [458].Jones AR, Shusta EV, Blood-brain barrier transport of therapeutics via receptor-mediation, Pharma Res, 24 (2007) 1759–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [459].Arcella A, Oliva MA, Staffieri S, Aalberti S, Grillea G, Madonna M, Bartolo M, Pavone L, Giangaspero F, Cantore G, Frati A, In vitro and in vivo effect of human lactoferrin on glioblastoma growth, J Neurosurg, 123 (2015) 1026–1035. [DOI] [PubMed] [Google Scholar]
- [460].Sega EI, Low PS, Tumor detection using folate receptor targeted imaging agents, Cancer Metastasis Rev, 27 (2008) 655–664. [DOI] [PubMed] [Google Scholar]
- [461].Gupta A, Kaur CD, Saraf S, Saraf S, Targeting of herbal bioactives through folate receptors: A novel concept to enhance intracellular drug delivery in cancer therapy, J Recept Signal Transduct Res, 37 (2017) 314–323. [DOI] [PubMed] [Google Scholar]
- [462].Zhu YQ, Liu W, Chen X, Cheng C, Zou LQ, Preparation and properties of curcumin nanoliposomes modified by folic acid-chitosan complex, Food Sci, 39 (2018) 7–13. [Google Scholar]
- [463].Cai Y, Xu Y, Chan HF, Fang X, He C, Chen M, Glycyrrhetinic acid mediated drug delivery carriers for hepatocellular carcinoma therapy, Mol Pharm, 13 (2016) 699–709. [DOI] [PubMed] [Google Scholar]
- [464].Negishi M, Irie A, Nagata N, Ichikawa A, Specific binding of glycyrrhetinic acid to the rat liver membrane, Biochim Biophys Acta, 1066 (1991) 77–82. [DOI] [PubMed] [Google Scholar]
- [465].Negishi M, Irie A, Nagata N, Ichikawa A, Specific binding of glycyrrhetinic acid to the rat liver membrane, Biochim Biophys Acta Biomembr, 1066 (1991) 77–82. [DOI] [PubMed] [Google Scholar]
- [466].Umeda D, Yano S, Yamada K, Tachibana H, Green tea polyphenol epigallocatechin-3-gallate signaling pathway through 67-kDa laminin receptor, J Biol Chem, 283 (2008) 3050–3058. [DOI] [PubMed] [Google Scholar]
- [467].Menaed S, Castronovo V, Tagliabue E, Sobel ME, New insights into the metastasis-associated 67 kD laminin receptor, J Cell Biochem, 67 (1997) 155–165. [PubMed] [Google Scholar]
- [468].Banerjee R, Tyagi P, Li S, Huang L, Anisamide-targeted stealth liposomes: A potent carrier for targeting doxorubicin to human prostate cancer cells, Int J Cancer, 112 (2004) 693–700. [DOI] [PubMed] [Google Scholar]
- [469].Luan X, Rahme K, Cong ZC, Wang LM, Zou YF, He Y, Yang H, Holmes JD, O’Driscoll CM, Guo JF, Anisamide-targeted PEGylated gold nanoparticles designed to target prostate cancer mediate: Enhanced systemic exposure of siRNA, tumour growth suppression and a synergistic therapeutic response in combination with paclitaxel in mice, Eur J Pharm Biopharm, 137 (2019) 56–67. [DOI] [PubMed] [Google Scholar]
- [470].Chen YC, Bathula SR, Yang Q, Huang L, Targeted nanoparticles deliver siRNA to melanoma, J Invest Dermatol, 130 (2010) 2790–2798. [DOI] [PubMed] [Google Scholar]
- [471].Liu Q, Chen FQ, Hou L, Shen LM, Zhang XQ, Wang DG, Huang L, Nanocarrier-mediated chemo-immunotherapy arrested cancer progression and induced tumor dormancy in desmoplastic melanoma, ACS Nano,12 (2018) 7812–7825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [472].Yu Z, Guo JF, Hu MY, Gao YQ, Huang L, Icaritin exacerbates mitophagy and synergizes with doxorubicin to induce immunogenic cell death in hepatocellular carcinoma, ACS Nano, 2019, DOI: 10.1021/acsnano.0c00708. [DOI] [PubMed] [Google Scholar]
- [473].Milane L, Trivedi M, Singh A, Talekar M, Amiji M, Mitochondrial biology, targets, and drug delivery, J Control Release 207 (2015) 40–58. [DOI] [PubMed] [Google Scholar]
- [474].Song J, Lin C, Yang X, Xie Y, Hu P, Li H, Zhu W, Hu H, Mitochondrial targeting nanodrugs self-assembled from 9-O-octadecyl substituted berberine derivative for cancer treatment by inducing mitochondrial apoptosis pathways, J Control Release, 294 (2019) 27–42. [DOI] [PubMed] [Google Scholar]
- [475].Hanahan D, Weinberg RA, Hallmarks of cancer: The next generation, Cell, 144 (2011) 646–674. [DOI] [PubMed] [Google Scholar]
- [476].Gogvadze V, Orrenius S, Zhivotovsky B, Mitochondria in cancer cells: What is so special about them? Trends Cell Biol, 18 (2008) 165–173. [DOI] [PubMed] [Google Scholar]
- [477].Dutta S, Moses JA, Anandharamakrishnan C, Encapsulation of nutraceutical ingredients in liposomes and their potential for cancer treatment, Nutr Cancer, 70 (2019) 1184–1198. [DOI] [PubMed] [Google Scholar]
- [478].Gogvadze V, Orrenius S, Zhivotovsky B, Mitochondria as the targets for chemotherapy, Apoptosis, 14 (2009) 624–640. [DOI] [PubMed] [Google Scholar]
- [479].Yamada Y, Akita H, Kogure K, Kamiya H, Harashima H, Mitochondrial drug delivery and mitochondrial disease therapy--an approach to liposome-based delivery targeted to mitochondria, Mitochondrion, 7 (2007) 63–71. [DOI] [PubMed] [Google Scholar]
- [480].Wang ZJ, Guo WL, Kuang X, Hou SS, Liu HZ, Nanopreparations for mitochondria targeting drug delivery system: Current strategies and future prospective, Asian J Pharm Sci, 12 (2017) 498–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [481].Battogtokh G, Gotov O, Kang JH, Cho J, Jeong TH, Chimed G, Ko YT, Triphenylphosphine-docetaxel conjugate-incorporated albumin nanoparticles for cancer treatment, Nanomedicine, 13 (2018) 325–338. [DOI] [PubMed] [Google Scholar]
- [482].Pathak RK, Kolishetti N, Dhar S, Targeted nanoparticles in mitochondrial medicine, Wiley Interdiscip Rev Nanomed Nanobiotechnol, 7 (2015) 315–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [483].Chen F, Zhuang X, Lin L, Yu P, Wang Y, Shi Y, Hu G, Sun Y, New horizons in tumor microenvironment biology: Challenges and opportunities, BMC Med, 13 (2015) 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [484].Roma-Rodrigues C, Mendes R, Baptista PV, Fernandes AR, Targeting tumor microenvironment for cancer therapy, Int J Mol Sci, 20 (2019) 840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [485].Wu T, Dai Y, Tumor microenvironment and therapeutic response, Cancer Lett, 387 (2017) 61–68. [DOI] [PubMed] [Google Scholar]
- [486].Li Y, Jiang D, Liang J, Meltzer EB, Gray A, Miura R, Wogensen L, Yamaguchi Y, Noble PW, Severe lung fibrosis requires an invasive fibroblast phenotype regulated by hyaluronan and CD44, J Exp Med, 208 (2011) 1459–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [487].Pandolfi L, Frangipane V, Bocca C, Marengo A, Tarro Genta E, Bozzini S, Morosini M, D’Amato M, Vitulo S, Monti M, Comolli G, Scupoli MT, Fattal E, Arpicco S, Meloni F, Hyaluronic acid-decorated liposomes as innovative targeted delivery system for lung fibrotic cells, Molecules, 24 (2019) 3291–3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [488].Cichy J, Pure E, The liberation of CD44, J Cell Biol, 161 (2003) 839–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [489].Mackay CR, Terpe HJ, Stauder R, Marston WL, Stark H, Günthert U, Expression and modulation of CD44 variant isoforms in humans, J Cell Biol, 124 (1994) 71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [490].Paulis YW, Huijbers EJM, van der Schaft DWJ, Soetekouw PMMB, Pauwels P, Tjan-Heijnen VCG, Griffioen AW, CD44 enhances tumor aggressiveness by promoting tumor cell plasticity, Oncotarget, 6 (2015) 19634–19646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [491].Naor D, Wallach-Dayan SB, M.A, Zahalka, R.V. Sionov, Involvement of CD44, a molecule with a thousand faces, in cancer dissemination, Semin Cancer Biol, 18 (2008) 260–267. [DOI] [PubMed] [Google Scholar]
- [492].Lv Y, Xu C, Zhao X, Lin C, Yang X, Xin X, Zhang L, Qin C, Han X, Yang L, He W, Yin L, Nanoplatform assembled from a CD44-targeted prodrug and smart liposomes for dual targeting of tumor microenvironment and cancer cells, ACS Nano, 12 (2018) 1519–1536. [DOI] [PubMed] [Google Scholar]
- [493].Sneath RJS, Mangham DC, The normal structure and function of CD44 and its role in neoplasia, Mol Pathol, 51 (1998) 191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [494].Platt VM, Szoka FC Jr, Anticancer therapeutics: Targeting macromolecules and nanocarriers to hyaluronan or CD44, a hyaluronan receptor, Mol Pharm, 5 (2008) 474–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [495].Press MF, Cordon-Cardo C, Slamon DJ, Expression of the HER-2/neu proto-oncogene in normal human adult and fetal tissues, Oncogene, 5 (1990) 953–962. [PubMed] [Google Scholar]
- [496].Pegram MD, Konecny G, Slamon DJ, The molecular and cellular biology of HER2/neu gene amplification/overexpression and the clinical development of herceptin (trastuzumab) therapy for breast cancer, Cancer Treat Res, 103 (2000) 57–75. [DOI] [PubMed] [Google Scholar]
- [497].Lee JH, Engler JA, Collawn JF, Moore BA, Receptor mediated uptake of peptides that bind the human transferrin receptor, Eur J Biochem, 268 (2001) 2004–2012. [DOI] [PubMed] [Google Scholar]
- [498].Deshpande PP, Biswas S, Torchilin VP, Current trends in the use of liposomes for tumor targeting, Nanomedicine, 8 (2013) 1509–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [499].Schwartz MA, Integrins and extracellular matrix in mechanotransduction, Cold Spring Harb Perspect Biol, 2 (2010) 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [500].Brooks PC, Clark RA, Cheresh DA, Requirement of vascular integrin alphav-beta3 for angiogenesis, Science, 264 (1994) 569–571. [DOI] [PubMed] [Google Scholar]
- [501].Zhu R, Tian Y, Preparation and evaluation of RGD and TAT co-modified docetaxel-loaded liposome, Drug Des Devel Ther, 11 (2017) 3481–3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [502].Wang Y, Chen J, Lin AH, Fang Y, Advance in studies on NGR peptide modified liposome and its anti-tumor performance, China J Chin Mater Med, 38 (2013) 2041–2045. [PubMed] [Google Scholar]
- [503].Di Matteo P, Curnis F, Longhi R, Colombo G, Sacchi A, Crippa L, Protti MP, Ponzoni M, Toma S, Corti A, Immunogenic and structural properties of the Asn-Gly-Arg (NGR) tumor neovasculature-homing motif, Mol Immunol, 43 (2006) 1509–1518. [DOI] [PubMed] [Google Scholar]
- [504].Deshpande P, Jhaveri A, Pattni B, Biswas S, Torchilin V, Transferrin and octaarginine modified dual-functional liposomes with improved cancer cell targeting and enhanced intracellular delivery for the treatment of ovarian cancer, Drug Deliv, 25 (2018) 517–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [505].Gao H, Zhang Q Yu Z, He Q, Cell-penetrating peptide-based intelligent liposomal systems for enhanced drug delivery. Curr Pharm Biotechnol, 15 (2014) 210–219. [DOI] [PubMed] [Google Scholar]
- [506].Letoha T, Keller-Pintér A, Kusz E, Kolozsi C, Bozsó Z, Tóth G, Vizler C, Oláh Z, Szilák L, Cell-penetrating peptide exploited syndecans, Biochim Biophys Acta, 1798 (2010) 2258–2265. [DOI] [PubMed] [Google Scholar]
- [507].Herce HD, Garcia AE, Molecular dynamics simulations suggest a mechanism for translocation of the HIV-1 TAT peptide across lipid membranes, Proc NatlAcad Sci USA, 104 (2007) 20805–20810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [508].LeCher JC, Nowak SJ, McMurry JL, Breaking in and busting out: Cell-penetrating peptides and the endosomal escape problem, Biomol Concepts, 8 (2017) 13–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [509].Wang W, Li M, Zhang Z, Cui C, Zhou J, Yin L, Lv H, Design, synthesis and evaluation of multi-functional tLyP-1-hyaluronic acid-paclitaxel conjugate endowed with broad anticancer scope, Carbohydr Polym, 156 (2017) 97–107. [DOI] [PubMed] [Google Scholar]
- [510].Teesalu T, Sugahara KN, Kotamraju VR, Ruoslahti E, C-end rule peptides mediate neuropilin-1-dependent cell, vascular, and tissue penetration, Proc Natl Acad Sci USA, 106 (2009) 16157–16162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [511].Mu L, Feng SS, Vitamin E TPGS used as emulsifier in the solvent evaporation/extraction technique for fabrication of polymeric nanospheres for control release of paclitaxel (Taxol?), J Control Release, 80 (2002) 129–144. [DOI] [PubMed] [Google Scholar]
- [512].Wu D, Pusuluri A, Vogus D, Krishnan V, Shields IV CW, Kim J, Razmi A, Mitragotri S, Design principles of drug combinations for chemotherapy, J Control Release, 323 (2020) 36–46. [DOI] [PubMed] [Google Scholar]
- [513].Guo JF, Yu Z, Das M, Huang L, Nano codelivery of oxaliplatin and folinic acid achieves synergistic chemo-immunotherapy with 5-fluorouracil for colorectal cancer and liver metastasis, ACS Nano, 14 (2020) 5075–5089. [DOI] [PubMed] [Google Scholar]
- [514].Fahr A, van Hoogevest P, Kuntsche J, Leigh MLS, Lipophilic drug transfer between liposomal and biological membranes: What does it mean for parenteral and oral drug delivery? J Liposome Res, 16 (2006) 281–301. [DOI] [PubMed] [Google Scholar]
- [515].Barui S, Saha S, Mondal G, Haseena S, Chaudhuri A, Simultaneous delivery of doxorubicin and curcumin encapsulated in liposomes of pegylated RGDK-lipopeptide to tumor vasculature, Biomaterials, 35 (2014) 1643–1656. [DOI] [PubMed] [Google Scholar]
- [516].O Harasym T, Tardi PG, Harasym NL, Harvie P, Johnstone SA, Mayer LD, Increased preclinical efficacy of irinotecan and floxuridine coencapsulated inside liposomes is associated with tumor delivery of synergistic drug ratios, Oncol Res, 16 (2007) 361–374. [DOI] [PubMed] [Google Scholar]
- [517].Abu Lila AS, Ishida T, Liposomal delivery systems: Design optimization and current applications, Biol Pharm Bull, 40 (2017) 1–10. [DOI] [PubMed] [Google Scholar]