Abstract
This comparative effectiveness trial compared the longer-term effectiveness (12 and 18 months) of the standard Fit & Strong! physical activity program to Fit & Strong! Plus, which combined physical activity and dietary weight loss. Outcomes were weight, diet quality, physical activity, osteoarthritis symptoms, performance measures, and anxiety/depression. In this study, 413 overweight/obese participants with OA, ≥60 years old and primarily African American, were randomly assigned to Fit & Strong! (F&S!) or Fit & Strong! Plus (F&S! Plus), with outcomes assessed at 2, 6, 12, and 18 months. 356 (86%) participants completed the 18-month visit. Compared with participants randomized to standard F&S!, F&S! Plus participants maintained longer-term benefits at 12 months in weight (mean change ± SE: −1.7 ± 0.3 kg for F&S! Plus vs −0.9 ± 0.3 kg for F&S!, p = 0.049), BMI (−0.6 ± 0.1 vs −0.3 ± 0.1 kg/m2, p = 0.04), waist circumference (−2.7 ± 0.6 vs −0.4 ± 0.6 cm, p = 0.004), and lower extremity strength (1.6 ± 0.2 vs 1.0 ± 0.2 chair stands, p = 0.046). At 18 months, F&S! Plus participants showed improved lower extremity strength (1.4 ± 0.2 vs. 0.7 ± 0.2 chair stands, p = 0.045. African American older adults in the F&S! Plus arm showed sustained modest improvements in weight, waist circumference, and lower extremity strength at 12 months and in lower extremity strength at 18 months compared to F&S!. Implications for the translation of evidence-based programs into community settings to support healthy behaviors in older adults are discussed.
Keywords: Osteoarthritis, Weight management, Diet, Physical activity, Comparative effectiveness trial
1. Introduction
The prevalence of obesity has more than doubled over the past several decades. (Hales et al., 2018; Fryar et al., 2018) This has led to significant growth in obesity-related chronic diseases, including osteoarthritis (OA) (Safiri et al., 2020). OA affects approximately 32.5 million individuals in the US (Vaughn et al., 2019). Lower extremity (LE) OA, in particular, is predicted to grow due to an aging population, but also a population that is increasingly overweight /obese (Cross et al., 2014). The prevalence of OA varies by racial/ethnic groups, with African American women having a slightly higher prevalence (23%) than non-Hispanic white women (22%) (Jordan et al., 2009) African American women, however, do report higher rates of functional limitations (Jordan et al., 2009; Walker et al., 2016; Jordan, 2015), as well as twice the rate of disability compared to non-Hispanic white women (Dunlop et al., 2002). This could be related to the fact that older African American women also have the highest rate of obesity compared to non-Hispanic white women (57.5% vs. 38.2%) (Deshpande et al., 2016).
Obese individuals with OA are usually advised to lose weight. (Deshpande et al., 2016; Zhang et al., 2008; March et al., 2010) The benefits of weight loss among individuals with OA have been examined, including a systematic review concluding that a > 5% reduction in body weight has a clinically meaningful effect on OA symptoms, including pain and disability (Christensen et al., 2007). Several randomized controlled trials (RCTs) have tested the combined effect of modest weight loss with physical activity (PA). However, these interventions have been conducted primarily under highly controlled conditions, have not included a high percentage of African American participants, and have not yet shown long-term improvements in community settings or shown the potential for greater public health impact (Messier et al., 2004; Messier et al., 2013a). These efficacy trials highlight the need to assess the long-term outcomes of evidence-based programs that combine PA and weight management for older adults with OA. To our knowledge, no studies have tested the combined effect of weight loss with PA, compared to PA alone, among a high-risk group of overweight/obese African Americans who are disproportionately affected by OA compared to other racial/ethnic groups (Bolen et al., 2010).
Therefore, we conducted a comparative effectiveness trial by adding a dietary weight loss component to standard Fit & Strong! (F&S!). Standard F&S! focused on OA symptom management through physical activity but does not incorporate any dietary weight-loss strategies. This study was meant to test whether the addition of a dietary weight loss component was superior or caused any adverse effects (Lie et al., 2017).
We previously reported that F&S! Plus at both post-intervention (2 months) and follow-up (6 months) was more effective than standard F&S! in producing modest weight loss and body composition changes, as well as clinically meaningful improvements in LE pain, physical function, and mobility (Fitzgibbon et al., 2018; Hughes et al., 2018; Smith-Ray et al., 2014). However, the challenge of weight loss maintenance and the question as to whether sustained weight loss can influence OA pain, function, and mobility is critical in understanding the two linked conditions of obesity and OA. This paper reports overall results from the completed study, focusing on the final 12 and 18-month follow-up visits.
The primary hypothesis was that Fit &Strong! Plus (F&S! Plus) would produce statistically significant positive dietary changes compared to F&S! at 2 months, accompanied by a 5% weight loss at 6 months that would be maintained at 12 and 18 months. The secondary hypotheses were that physical activity and OA symptoms would be significantly improved compared to F&S! at 2 months and maintained at 12 and 18 months. Given that obesity is a known risk factor for both the incidence and progression of OA (19,20), this comparative effectiveness trial, if successful, could point to strategies for improved management of OA (Roos and Arden, 2016).
1.1. Design and methods
The trial design is described in detail elsewhere (Fitzgibbon et al., 2018; Hughes et al., 2018; Smith-Ray et al., 2014). Briefly, we employed a randomized comparative effectiveness design to test whether F &S! Plus produces significantly greater improvements in weight loss, waist circumference, diet quality, PA, OA pain, physical function, mobility, LE strength, and anxiety/depression. The study was approved by the Institutional Review Board at the University of Illinois at Chicago (#2012–0277), and all participants provided written informed consent.
1.1.1. Setting
Both F&S! and F&S! Plus were conducted at 10 unique local community settings such as churches and community park districts that regularly deliver programming to older and primarily African American adults.
1.1.2. Recruitment
Study staff made initial contacts and presentations at local community churches and community park districts that served African American communities in an effort to advertise the program. Participants were also recruited through advertisements at the intervention sites and in the surrounding neighborhood, recruitment presentations to local groups that offer programming in primarily minority neighborhoods, and email messages sent by the Arthritis Foundation. Potential participants were screened over the phone, and if they were eligible, they were scheduled for an in-person assessment.
1.1.3. Participants
Study participants were randomly assigned to either F&S! (n = 210) or F&S! Plus (n = 203) using the Research Electronic Data Capture (REDCap) randomization module (Harris et al., 2009). Randomization was stratified by iteration, WOMAC physical function score (0–19 vs. 20–68) and BMI (< 35 vs. ≥35 kg/m2). Data were collected at baseline, post-intervention (2 months), and at 6, 12, and 18 months. Participants who missed a visit were encouraged to complete later visits, as long as they had not formally withdrawn from the study.
1.1.4. Inclusion criteria
The study definition of LE OA was based on self-reported pain or stiffness: pain in or around one or both knees or hips on most days in the past month and/or pain or stiffness in or around hips, knees ankles, feet or lower back on most days of at least 1 month during the last 6 months. Eligible participants were 60 or older and had a BMI of 25–50 kg/m2.
1.1.5. Exclusion criteria
Participants were excluded if they were unable to attend class sessions at the scheduled time and location, engaged in ≥150 min/week of aerobic activity, had three or more errors on the Mental Status Questionnaire (MSQ), had uncomplicated hip or knee surgery within the previous six months, surgery with complications within the past year, or plans for hip or knee surgery within the next year, steroid injections in either knee or hip within the previous three months, rheumatoid arthritis, uncontrolled diabetes, or health conditions that might interfere with exercise. Participants who identified one or more potential contraindications to exercise on the Exercise and Screening for You (EASY) screener were required to obtain a physician’s approval before participating (Smith-Ray et al., 2014).
1.1.6. Interventions
Both F&S! and F&S! Plus were grounded in the Social Cognitive Theory (SCT), which aids in the development of self-regulatory skills (Bandura, 1989). The interventions were both 90 min in length and were both conducted three times per week for eight weeks, for a total of 24 sessions. The initial 60 min of both interventions consist of stretching, low-impact aerobics, and strength training, with a primary focus on the lower extremities. Following each 60-min PA session, the F &S! group participated in a 30-min health education session that included topics such as using PA to manage OA symptoms and exercising safely with OA.
The curriculum for F&S! Plus retained the core physical activity and OA material included in standard F&S!. Sixteen weight and diet-related topics were added to F&S! Plus, with weight loss and diet quality concepts included in 22 of the 24 sessions. The diet quality information followed the Group Lifestyle Balance curriculum, adapted from the Diabetes Prevention Program and the 2010–2015 Dietary Guidelines for Americans (Dietary Guidelines for Americans, 2010) and USDA My-Plate eating plan (Choose My Plate, n.d.). Content was designed to produce 5% weight loss at 6 months and improve diet quality. Together, the curriculum, homework, and weekly weight checks were designed to enhance social support (e.g., group problem solving) and self-regulation (e.g., goal setting, planning, self-monitoring) to build self-efficacy for weight loss and improve overall diet quality. The boosters sought to help participants continue to adhere to their dietary and physical activity goals.
1.1.7. Maintenance boosters
Maintenance of behavior was reinforced in months 3–18 in both groups through telephone reinforcement sessions in months 4, 8, and 15. The health educators who conducted these sessions were trained in motivational interviewing techniques, and separate educators were assigned to F&S! and F&S! Plus.
1.2. Measures
1.2.1. Primary outcomes
1.2.1.1. Anthropometrics.
Height was measured using a portable stadiometer (Seca, United Kingdom), and weight was measured using a calibrated digital scale (Tanita Worldwide). Body mass index (BMI) was calculated as weight (kg) divided by height (m) squared. To assess waist circumference, a Gulick 150-cm anthropometric tape (Country Technology, Inc.; Gays Mills, WI, USA) was used.
1.2.1.2. Dietary intake.
Dietary intake was measured using the 110-item Block Food Frequency Questionnaire (FFQ) created by NutritionQuest (Berkeley, CA). Completed FFQs were sent to NutritionQuest for scoring. The scoring algorithm included calculation of the Healthy Eating Index (HEI) 2010, a measure of overall diet quality based on adherence to the 2010 Dietary Guidelines for Americans (Guenther et al., 2013; HEI-2010 Total and Component Scores for Children, Adults, and Older Adults during 2011–2012. [Internet], 2012). A higher HEI score indicates higher diet quality.
1.2.2. Secondary outcomes
1.2.2.1. Physical activity.
PA was measured using the Physical Activity Scale for the Elderly (Washburn et al., 1999). The PASE has been used in other studies of older adults with OA, including the Osteoarthritis Initiative (Dunlop et al., 2011) and the Multicenter Osteoarthritis Study (Segal et al., 2013). A higher score indicates more PA.
1.2.2.2. OA symptomatology.
OA symptoms were measured using the Western Ontario and McMaster Universities Arthritis Index (WOMAC). The WOMAC is a self-report instrument that has been well-validated and used widely with individuals with OA (Bellamy et al., 1988). Participants are asked to rate their pain (5 items), physical function (17 items) and stiffness (2 items) on a Likert scale ranging from 0 (none) to four (extreme) (Bellamy et al., 1988). Higher scores indicate more severe OA symptoms. For both pain and function, a 20% decrease is considered clinically important (Dougados et al., 2000).
1.2.2.3. Performance measures.
Functional LE strength was measured using the 30-s Chair Stand (Jones et al., 1999). This test measures the number of full stands from a seated position a participant can complete in 30 s with arms folded across the chest (Jones et al., 1999). Mobility was assessed using the 6-Minute Walk Test (Guyatt et al., 1985; Naylor et al., 2014), which is a self-paced timed test of the total distance a person can walk in six minutes. For both measures, a higher score reflects better performance.
1.2.2.4. Depression and anxiety.
These outcomes were measured with the GERI-AIMS, a version of the Arthritis Impact Measurement Scale, which was adapted for an older adult population (Hughes et al., 1991). Three items measure anxiety and three measure depression. The items are summed to create a 0–10 scale, with higher scores reflecting increased anxiety/depression.
1.3. Statistical analysis
We used chi-square tests for group differences in retention and in the percentage of participants who lost ≥5% of baseline weight or whose OA pain or function improved by ≥20%. To test for differences between participants with and without 18-month data, we used t-tests and chi-square tests, plus a logistic regression model including demographics, intervention group, and baseline BMI, HEI-2010, and WOMAC function as covariates. To test for group differences in completion of telephone reinforcement, we used chi-square tests for the individual sessions and a Wilcoxon rank sum test for a difference in number of sessions completed.
To test the effects of the interventions on primary and secondary outcome measures, we used repeated-measures linear models in SAS PROC MIXED with a fully specified (unstructured) covariance matrix and the baseline value included in the outcome vector (Mallinckrod et al., 2008). All models included intervention group, visit as a set of indicator variables with baseline as the reference group, and a group*visit interaction term. Stratification variables were included as covariates. If the baseline or a closely related value was included in the outcome vector (the weight, BMI, and WOMAC physical function models), that covariate was omitted from the model. For HEI-2010 outcomes, records with implausible values for energy (< 500 or > 5000 kcal) were excluded. The models for weight and BMI were also run for African American women separately for comparison with other trials (N = 328).
Within the repeated-measures models, we estimated adjusted means for each group at each visit and tested for within-group and between-group change at each visit using SAS LSMESTIMATE statements (see Table 2). To estimate the adjusted percentage change at each visit, we divided the estimated change by the adjusted baseline mean and multiplied by 100.
Table 2.
Adjusteda mean outcome measures at each visit and mean change from baseline to 2, 6, 12, and 18 months.
| F&S! Plus | F&S! | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcomes over time | Outcome measure | Change from baseline | Outcome measure | Change from baseline | Significance of difference in mean change between groups | Significance of overall group*visit interaction | ||||||
| Mean ± SE | Meanb ± SE | %c | pd | (N) | Mean ± SE | Meanb ± SE | %c | pd | (N) | pe | p | |
| Weight, kg | < .0001 | |||||||||||
| Baseline | 93.1 ± 1.1 | (N = 203) | 93.7 ± 1.1 | (N = 210) | – | |||||||
| 2 Mon | 91.2 ± 1.1 | −2.0 ± 0.2 | −2.1% | *** | (N = 191) | 93.2 ± 1.1 | −0.5 ± 0.2 | −0.6% | ** | (N = 191) | < 0.0001 | |
| 6 Mon | 91.1 ± 1.1 | −2.0 ± 0.3 | −2.2% | *** | (N = 175) | 92.9 ± 1.1 | −0.8 ± 0.3 | −0.9% | ** | (N = 171) | 0.0009 | |
| 12 Mon | 91.4 ± 1.1 | −1.7 ± 0.3 | −1.8% | *** | (N = 154) | 92.8 ± 1.1 | −0.9 ± 0.3 | −1.0% | ** | (N = 168) | 0.049 | |
| 18 Mon | 91.8 ± 1.2 | −1.3 ± 0.3 | −1.4% | *** | (N = 174) | 92.8 ± 1.2 | −0.9 ± 0.3 | −1.0% | ** | (N = 181) | 0.40 | |
| BMI, kg/m2 | < 0.0001 | |||||||||||
| Baseline | 34.7 ± 0.4 | (N = 203) | 35.0 ± 0.4 | (N = 210) | ||||||||
| 2 Mon | 34.0 ± 0.4 | −0.7 ± 0.1 | −2.1% | *** | (N = 191) | 34.8 ± 0.4 | −0.2 ± 0.1 | −0.5% | ** | (N = 191) | < 0.0001 | |
| 6 Mon | 34.0 ± 0.4 | −0.8 ± 0.1 | −2.2% | *** | (N = 175) | 34.7 ± 0.4 | −0.3 ± 0.1 | −0.9% | ** | (N = 171) | 0.0009 | |
| 12 Mon | 34.1 ± 0.4 | −0.6 ± 0.1 | −1.9% | *** | (N = 154) | 34.7 ± 0.4 | −0.3 ± 0.1 | −0.9% | ** | (N = 168) | 0.04 | |
| 18 Mon | 34.2 ± 0.4 | −0.5 ± 0.1 | −1.4% | *** | (N = 174) | 34.7 ± 0.4 | −0.3 ± 0.1 | −0.9% | ** | (N = 181) | 0.39 | |
| Waist circumference, cm | 0.0003 | |||||||||||
| Baseline | 113.9 ± 0.5 | (N = 201) | 111.8 ± 0.5 | (N = 210) | ||||||||
| 2 Mon | 111.1 ± 0.5 | −2.9 ± 0.4 | −2.5% | *** | (N = 191) | 111.3 ± 0.5 | −0.5 ± 0.4 | −0.4% | (N = 191) | < 0.0001 | ||
| 6 Mon | 110.5 ± 0.6 | −3.4 ± 0.5 | −3.0% | *** | (N = 175) | 110.8 ± 0.6 | −1.0 ± 0.5 | −0.9% | (N = 171) | 0.0006 | ||
| 12 Mon | 111.2 ± 0.7 | −2.7 ± 0.6 | −2.4% | *** | (N = 155) | 111.4 ± 0.6 | −0.4 ± 0.6 | −0.3% | (N = 168) | 0.004 | ||
| 18 Mon | 111.9 ± 0.7 | −2.0 ± 0.5 | −1.8% | *** | (N = 174) | 110.4 ± 0.6 | −1.4 ± 0.5 | −1.2% | * | (N = 181) | 0.39 | |
| Healthy Eating Index 2010f | 0.004 | |||||||||||
| Baseline | 65.9 ± 0.7 | (N = 197) | 66.7 ± 0.7 | (N = 203) | ||||||||
| 2 Mon | 70.6 ± 0.7 | 4.7 ± 0.7 | 7.1% | *** | (N = 183) | 68.8 ± 0.7 | 2.1 ± 0.7 | 3.1% | ** | (N = 185) | 0.005 | |
| 6 Mon | 67.9 ± 0.8 | 2.0 ± 0.7 | 3.1% | ** | (N = 166) | 67.5 ± 0.8 | 0.8 ± 0.7 | 1.2% | (N = 161) | 0.24 | ||
| 12 Mon | 66.4 ± 0.7 | 0.6 ± 0.7 | 0.9% | (N = 150) | 68.0 ± 0.7 | 1.3 ± 0.7 | 2.0% | (N = 158) | 0.47 | |||
| 18 Mon | 66.9 ± 0.8 | 1.0 ± 0.7 | 1.5% | (N = 164) | 66.7 ± 0.8 | 0.0 ± 0.7 | 0.0% | (N = 169) | 0.33 | |||
| PASE physical activity scoreg | 0.23 | |||||||||||
| Baseline | 99.6 ± 4.2 | (N = 199) | 99.2 ± 4.2 | (N = 210) | ||||||||
| 2 Mon | 128.7 ± 4.9 | 29.1 ± 4.8 | 29.2% | *** | (N = 184) | 113.3 ± 5.0 | 14.1 ± 4.7 | 14.2% | ** | (N = 184) | 0.03 | |
| 6 Mon | 117.1 ± 5.2 | 17.5 ± 5.0 | 17.6% | *** | (N = 172) | 114.6 ± 5.2 | 15.4 ± 5.0 | 15.5% | ** | (N = 169) | 0.77 | |
| 12 Mon | 111.2 ± 5.0 | 11.6 ± 4.9 | 11.7% | * | (N = 153) | 106.2 ± 4.9 | 7.0 ± 4.8 | 7.0% | (N = 163) | 0.50 | ||
| 18 Mon | 111.7 ± 4.8 | 12.2 ± 4.9 | 12.2% | * | (N = 171) | 104.4 ± 4.8 | 5.2 ± 4.8 | 5.3% | (N = 178) | 0.32 | ||
| WOMAC pain (0−20)h | 0.004 | |||||||||||
| Baseline | 5.4 ± 0.2 | (N = 203) | 5.7 ± 0.2 | (N = 210) | ||||||||
| 2 Mon | 4.1 ± 0.2 | −1.4 ± 0.2 | −25.1% | *** | (N = 191) | 5.2 ± 0.2 | −0.5 ± 0.2 | −8.8% | * | (N = 192) | 0.01 | |
| 6 Mon | 3.9 ± 0.3 | −1.5 ± 0.3 | −27.2% | *** | (N = 174) | 5.1 ± 0.3 | −0.6 ± 0.3 | −11.1% | * | (N = 173) | 0.03 | |
| 12 Mon | 4.5 ± 0.3 | −0.9 ± 0.3 | −16.7% | ** | (N = 156) | 5.0 ± 0.3 | −0.7 ± 0.3 | −12.0% | * | (N = 169) | 0.57 | |
| 18 Mon | 4.5 ± 0.3 | −0.9 ± 0.3 | −16.4% | ** | (N = 174) | 4.8 ± 0.3 | −1.0 ± 0.3 | −16.7% | ** | (N = 182) | 0.88 | |
| WOMAC physical function (0–68)h | 0.004 | |||||||||||
| Baseline | 17.7 ± 0.9 | (N = 203) | 17.8 ± 0.9 | (N = 210) | ||||||||
| 2 Mon | 12.2 ± 0.9 | −5.5 ± 0.8 | −31.0% | *** | (N = 191) | 15.8 ± 0.9 | −1.9 ± 0.7 | −10.8% | * | (N = 191) | 0.0009 | |
| 6 Mon | 12.8 ± 1.0 | −4.9 ± 0.9 | −27.5% | *** | (N = 174) | 16.1 ± 1.0 | −1.7 ± 0.9 | −9.4% | * | (N = 173) | 0.01 | |
| 12 Mon | 13.6 ± 0.9 | −4.1 ± 0.9 | −23.4% | *** | (N = 156) | 15.7 ± 0.9 | −2.0 ± 0.9 | −11.5% | (N = 169) | 0.09 | ||
| 18 Mon | 14.2 ± 1.0 | −3.5 ± 0.9 | −19.5% | *** | (N = 174) | 15.2 ± 1.0 | −2.6 ± 0.9 | −14.7% | ** | (N = 182) | 0.48 | |
| WOMAC stiffness (0–8)h | 0.049 | |||||||||||
| Baseline | 3.1 ± 0.1 | (N = 203) | 3.2 ± 0.1 | (N = 210) | ||||||||
| 2 Mon | 2.4 ± 0.1 | −0.6 ± 0.1 | −21.2% | *** | (N = 191) | 2.9 ± 0.1 | −0.3 ± 0.1 | −10.2% | ** | (N = 192) | 0.06 | |
| 6 Mon | 2.6 ± 0.1 | −0.5 ± 0.1 | −15.5% | *** | (N = 174) | 2.7 ± 0.1 | −0.5 ± 0.1 | −15.9% | *** | (N = 173) | 0.81 | |
| 12 Mon | 2.6 ± 0.1 | −0.5 ± 0.1 | −15.2% | *** | (N = 156) | 2.7 ± 0.1 | −0.6 ± 0.1 | −17.6% | *** | (N = 169) | 0.57 | |
| 18 Mon | 2.6 ± 0.1 | −0.4 ± 0.1 | −13.4% | ** | (N = 174) | 2.6 ± 0.1 | −0.6 ± 0.1 | −18.3% | *** | (N = 182) | 0.32 | |
| Six-minute walk, m | 0.0005 | |||||||||||
| Baseline | 360.8 ± 6.2 | (N = 201) | 357.7 ± 6.2 | (N = 208) | ||||||||
| 2 Mon | 411.2 ± 6.8 | 50.5 ± 4.4 | 14.0% | *** | (N = 190) | 380.6 ± 6.8 | 22.9 ± 4.4 | 6.4% | *** | (N = 189) | < 0.0001 | |
| 6 Mon | 390.1 ± 7.3 | 29.3 ± 5.1 | 8.1% | *** | (N = 173) | 371.0 ± 7.3 | 13.3 ± 5.2 | 3.7% | * | (N = 165) | 0.03 | |
| 12 Mon | 384.0 ± 7.5 | 23.2 ± 5.3 | 6.4% | *** | (N = 154) | 367.7 ± 7.4 | 10.0 ± 5.3 | 2.8% | (N = 164) | 0.08 | ||
| 18 Mon | 373.5 ± 7.7 | 12.8 ± 5.8 | 3.5% | * | (N = 168) | 357.2 ± 7.7 | −0.6 ± 5.8 | −0.2% | (N = 173) | 0.10 | ||
| Chair stands in 30 s | 0.26 | |||||||||||
| Baseline | 8.8 ± 0.2 | (N = 203) | 8.8 ± 0.2 | (N = 209) | ||||||||
| 2 Mon | 10.6 ± 0.2 | 1.8 ± 0.2 | 20.4% | *** | (N = 187) | 10.2 ± 0.2 | 1.3 ± 0.2 | 15.0% | *** | (N = 188) | 0.08 | |
| 6 Mon | 10.4 ± 0.3 | 1.7 ± 0.2 | 18.8% | *** | (N = 172) | 10.0 ± 0.3 | 1.1 ± 0.2 | 13.0% | *** | (N = 165) | 0.07 | |
| 12 Mon | 10.4 ± 0.3 | 1.6 ± 0.2 | 18.3% | *** | (N = 152) | 9.8 ± 0.3 | 1.0 ± 0.2 | 11.4% | *** | (N = 162) | 0.046 | |
| 18 Mon | 10.2 ± 0.3 | 1.4 ± 0.2 | 15.5% | *** | (N = 170) | 9.6 ± 0.3 | 0.7 ± 0.2 | 8.2% | ** | (N = 176) | 0.045 | |
| Anxiety and depression (0−10)i | 0.26 | |||||||||||
| Baseline | 2.5 ± 0.1 | (N = 203) | 2.6 ± 0.1 | (N = 210) | ||||||||
| 2 Mon | 2.0 ± 0.1 | −0.5 ± 0.1 | −19.9% | *** | (N = 191) | 2.2 ± 0.1 | −0.4 ± 0.1 | −13.8% | *** | (N = 190) | 0.26 | |
| 6 Mon | 2.1 ± 0.1 | −0.4 ± 0.1 | −15.9% | *** | (N = 174) | 2.4 ± 0.1 | −0.1 ± 0.1 | −5.8% | (N = 173) | 0.07 | ||
| 12 Mon | 2.1 ± 0.1 | −0.4 ± 0.1 | −15.1% | *** | (N = 156) | 2.4 ± 0.1 | −0.1 ± 0.1 | −5.0% | (N = 169) | 0.08 | ||
| 18 Mon | 2.2 ± 0.1 | −0.3 ± 0.1 | −12.2% | ** | (N = 174) | 2.3 ± 0.1 | −0.2 ± 0.1 | −9.3% | * | (N = 182) | 0.66 | |
From repeated-measures linear models with a fully specified (unstructured) covariance matrix and the baseline value included in the outcome vector. Covariates included in models: Iteration, baseline WOMAC physical function (except for the WOMAC physical function model), and baseline BMI (except for the weight and BMI models).
Estimated mean change is the difference between the follow-up and baseline adjusted means.
Percent change is mean change divided by the adjusted baseline mean.
Test for within-group change different from 0
p < 0.05
p < 0.01
p < 0.001.
Test for difference in change between groups.
A higher score indicates higher diet quality. Records with energy values < 500 or > 5000 kcal were excluded from the analysis: 13 at baseline and 2 months, 20 at 6 months, 16 at 12 months, 22 at 18 months.
A higher score indicates greater physical activity.
A higher score indicates greater difficulties due to OA.
A higher score indicates greater anxiety/depression.
Our planned sample size of 400 was selected to allow us to detect an effect size of 0.2 (based on a 5% endpoint difference in mean weight between groups) with power > 0.9, assuming 5% attrition at each visit and a cross-time correlation for weight of 0.9 (Smith-Ray et al., 2014).
A repeated-measures analysis using restricted maximum likelihood and unstructured covariance has been found to give unbiased estimates in cases with missing data, as long as the data are missing at random (Mallinckrod et al., 2008). Therefore, the analysis described above is an intention-to-treat analysis.
All statistical analyses were performed using SAS v9.4 (SAS Institute, Cary, NC).
2. Results
As shown in Fig. 1, 1379 potential participants were screened in order to randomize 413 participants, 210 to standard F&S! and 203 to F &S! Plus. Baseline demographics are shown in Table 1. The mean age for the entire sample was 67.9 years (SD = 5.9 years). Most of the participants were female (86%) and African American (92%).
Fig. 1.
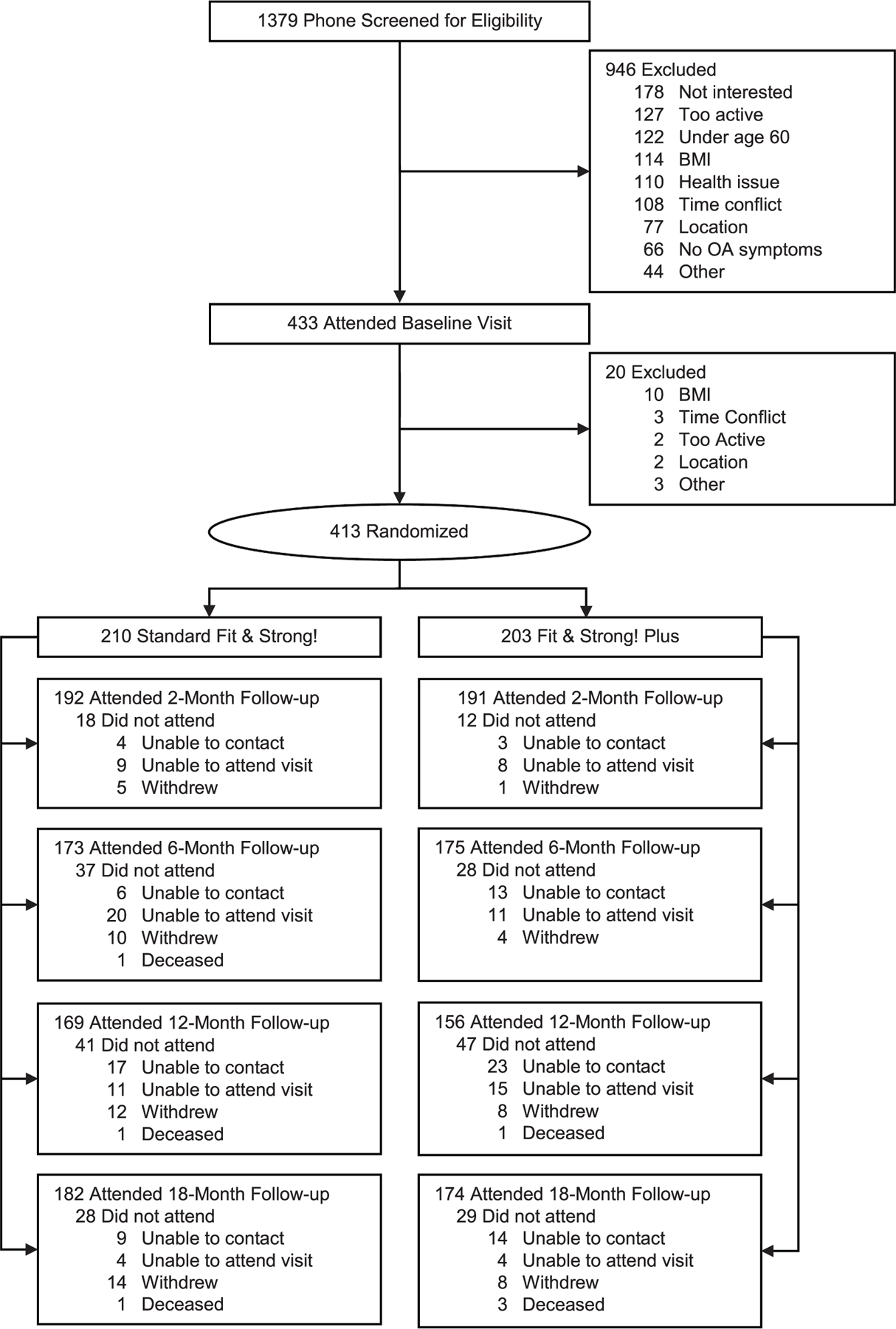
Participant flow.
Table 1.
Participant characteristics at baseline.
| F&S! Plus N = 203 |
F&S! N = 210 |
All N = 413b |
||||
|---|---|---|---|---|---|---|
| Mean or % | SD or N | Mean or % | SD or N | Mean or % | SD or N | |
| Age, y | 67.7 | 6.0 | 68.1 | 5.8 | 67.9 | 5.9 |
| Sex | ||||||
| Female | 87% | 177 | 85% | 178 | 86% | 355 |
| Male | 13% | 26 | 15% | 32 | 14% | 58 |
| Race | ||||||
| Black or African-American, not Hispanic | 92% | 187 | 92% | 193 | 92% | 380 |
| Other | 8% | 16 | 8% | 17 | 8% | 33 |
| Education, y | 14.3 | 1.9 | 14.1 | 2.0 | 14.2 | 1.9 |
| Not HS graduate, n (%) | 4% | 9 | 7% | 15 | 6% | 24 |
| HS graduate/GED | 13% | 27 | 16% | 34 | 15% | 61 |
| Some college | 45% | 91 | 40% | 84 | 42% | 175 |
| College graduate | 37% | 76 | 37% | 77 | 37% | 153 |
| Employed full or part-time | 15% | 30 | 11% | 23 | 13% | 53 |
| Married or member of unmarried couple | 26% | 52 | 26% | 54 | 26% | 106 |
| Income, mediana | 25,000 | 25,000 | 25,000 | |||
| < $20,000 | 37% | 64 | 34% | 58 | 35% | 122 |
| $20,000 – < $40,000 | 26% | 45 | 28% | 48 | 27% | 93 |
| ≥$40,000 | 37% | 64 | 38% | 66 | 38% | 130 |
| BMI category, kg/m2 | ||||||
| Overweight (25− < 30) | 21% | 43 | 19% | 39 | 20% | 82 |
| Obese (≥ 30) | 79% | 160 | 81% | 171 | 80% | 331 |
N = 345 for income
Of the 413 randomized participants, 383 (93%) completed the 2-month visit; 348 (84%) completed the 6-month visit; 325 (79%) completed the 12-month visit, and 356 (86%) completed the 18-month visit. There were no significant differences in retention between groups. The 57 participants who did not have 18-month data were older than those who did (mean = 69.6 vs. 67.7 yr, p = 0.02), but there were no other significant differences in sex, race, education, employment, marital status, income, or baseline BMI, HEI-2010, or WOMAC function. Similarly, age was the only significant predictor in a multiple logistic regression model of missing 18-month data (b = 0.07, p = 0.005).
F&S! Plus participants completed slightly fewer of the three maintenance booster sessions than F&S! participants: mean (SD) = 1.9 (1.0) vs. 2.0 (1.0), p = 0.04. This was largely due to a difference in completion of the 4-month booster session: 63.5% for F&S! Plus vs. 76.7% for F&S!, p = 0.004. Both groups were equally likely to complete the other maintenance sessions: 64.0% vs. 65.2%, p = 0.80 at 8 months and 58.6% vs. 62.9%, p = 0.38 at 15 months. Most participants completed at least one booster session: 89.7% for F&S! Plus and 91.0% for F &S!.
Table 2 shows adjusted mean change from baseline to 2, 6, 12, and 18 months for all outcome variables.
2.1. Primary outcomes
2.1.1. Body weight
The group*visit interaction term was significant for weight (p < 0.0001), indicating an overall difference in weight between groups across all visits. Results through 6 months have been described elsewhere (Hughes et al., 2018). Briefly, F&S! Plus participants lost 2.0 kg (SE = 0.2), or 2.1% of body weight on average during the intervention, compared to 0.5 ± 0.2 kg in F&S!, p < 0.0001. Weight loss was maintained at 6 months: 2.0 ± 0.3 vs 0.8 ± 0.3 kg, p = 0.0009. Results were similar when the analysis was restricted to African-American women; at 6 months, weight loss was 2.1 ± 0.3 (2.2%) vs. 1.0 ± 0.3 kg (1.1%), p = 0.009. At 12 months, there was still a significant difference in weight loss between groups, but the difference had narrowed: 1.7 ± 0.3 kg vs. 0.9 ± 0.3 kg, p = 0.049. By 18 months, we no longer observed a significant difference between groups, either in the sample as a whole or among African American women.
Among participants with weight measured at 2 months, 14% (27/191) of F&S! Plus participants had lost ≥5% of baseline weight, compared to 4% (7/191) in F&S!, p = 0.0003. At 6 months, the percentage of F&S! Plus participants with ≥5% weight loss had increased to 25% (43/175), compared to 9% (15/171) in F&S!, p < 0.0001. There was still a significant difference between groups at 12 months: 21% (33/154) vs 12% (21/168), p = 0.03, but not at 18 months: 23% (40/174) vs 17% (30/181), p = 0.13.
Results for BMI and waist circumference were similar, with a significant group*visit interaction and significant differences in change between groups at 2, 6, and 12 months, but not at 18 months.
2.1.2. Diet quality
The overall group*visit interaction was significant (p = 0.004). From baseline to 2 months, diet quality improved significantly more in the F&S! Plus group: 4.7 ± 0.7 for F&S! Plus vs. 2.1 ± 0.7 for F&S!, p = 0.005. However, we saw no significant differences between groups at 6, 12, or 18 months.
2.2. Secondary outcomes
2.2.1. Physical activity (PA)
PASE scores showed greater improvement in the F&S! Plus group from baseline to post-intervention: 29.1 ± 4.8 vs. 14.1 ± 4.7, p = 0.03. However, there were no significant differences between groups at 6, 12, or 18 months, and the overall group*visit interaction was not significant (p = 0.23).
2.2.2. Osteoarthritis symptomatology
Participants’ unadjusted mean baseline score was 5.6 (SE = 0.2) on the pain subscale, 18.0 ± 0.6 for physical function, and 3.2 ± 0.1 for stiffness, indicating a moderate degree of OA-related symptoms. The group*visit interaction was significant for pain (p = 0.004), function (p = 0.004), and stiffness (p = 0.049). Self-reported pain showed significantly greater improvement at 2 and 6 months in the F&S! Plus group: −1.4 ± 0.2 vs −0.5 ± 0.2, p = 0.01 at 2 months and −1.5 ± 0.3 vs −0.6 ± 0.3, p = 0.03 at 6 months. There was no longer a significant difference between groups at 12 or 18 months. Physical function scores showed a similar pattern, with significant differences between groups at 2- and 6-months favoring F&S! Plus. There was no longer a significant difference between groups at 12 or 18 months. For stiffness, there was not a significant difference between groups at any individual visit.
Post-intervention, 55% (106/191) of F&S! Plus participants and 43% (83/192) of F&S! reported a clinically important improvement in pain of ≥20% (p = 0.02 for difference between groups), and results were similar at 6 months. There was no significant difference between groups at 12 or 18 months; at 18 months, clinically important improvement was 47% (82/174) for F&S! Plus and 46% (83/182) for F& S!, p = 0.77. For physical function, clinically important improvement was similar in both groups at 2 months: 53% (101/191) for F&S! Plus participants and 48% (91/191) for F&S!, p = 0.31. At 6 months, there was a significant difference between groups: 57% (99/174) for F&S! Plus and 40% (70/173) for F&S!, p = 0.002. As with pain, there was no longer a significant difference between groups at 12 or 18 months: 49% (86/174) for F&S! Plus and 46% (84/182) for F&S!, p = 0.54 at 18 months.
2.2.3. Performance measures
The overall group*visit interaction was significant for mobility (p = 0.0005). At both 2 and 6 months, there was significantly greater improvement in the F&S! Plus group than in F&S!: mean change in six-minute walk distance was 50.5 ± 4.4 m vs. 22.9 ± 4.4 m, p < 0.0001 at 2 months and 29.3 ± 5.1 m vs. 13.3 ± 5.2 m at 6 months, p = 0.03. There was no longer a significant difference between groups at 12 and 18 months.
The pattern was different for LE strength. The overall interaction term was not statistically significant (p = 0.26), and there was no significant difference between groups at 2 or 6 months. However, at 12 and 18 months, the F&S! Plus group showed a somewhat higher change from baseline than F&S: 1.6 ± 0.2 vs 1.0 ± 0.2, p = 0.046 at 12 months and 1.4 ± 0.2 vs 0.7 ± 0.2, p = 0.045 at 18 months.
2.2.4. Anxiety/depression
There was not a significant difference between groups overall (p = 0.26) or at any visit.
3. Discussion
African-American women experience higher rates of functional limitations and disabilities from OA compared to other subgroups, but to our knowledge, no other comparative effectiveness trials have addressed the longer-term impact of a combined theory-driven behavioral PA/weight loss intervention among older overweight/obese African American women with OA in community settings (Fitzgibbon et al., 2018; Hughes et al., 2018; Smith-Ray et al., 2014).
3.1. Primary outcomes
We hypothesized that weight loss in the F&S! Plus group would reach 5% at 6 months, but the actual mean weight loss was 2.2% for both the sample as a whole and for the African American women, with similar modest effects on waist circumference. However, 25% of F&S! Plus participants did lose ≥5% of their baseline weight at 6 months, compared to 9% of F&S! participants (p < 0.0001). At 18 months, there was no longer a significant difference between groups, but 23% of F&S! Plus participants continued to maintain weight loss ≥5%, compared to 17% of F&S! participants (p = 0.13).
Although the weight loss was less than initially hypothesized, weight change between F&S! Plus and F&S! at 12 months remained significant, with losses of 1.8% and 1.0%, respectively. Our weight loss results are smaller than those reported by other trials such as the IDEA trial (Messier et al., 2013b) or Lifestyle Intervention Trial in Obese Elderly (LITOE) (Villareal et al., 2017). However, these trials were not only longer, but far more intensive, and delivered by trained dietitians, using meal replacements to enhance weight loss. Also, neither trial focused on African American women, who traditionally lose less weight in weight loss trials than non-Hispanic whites (West et al., 2008). Several possibilities have been put forth for why African American women lose less weight in traditional weight loss programs. They include: socioeconomic status (Harvey and Hill, 2004; Constantine et al., 2002), less availability of and access to high-quality food (Zenk et al., 2005; Powell et al., 2007), reduced access to PA resources (Gordon-Larsen et al., 2006; Ainsworth et al., 2003), neighborhood safety (Wilcox et al., 2003), and stress and discrimination (Wilcox et al., 2003).
We did not see longer-term differences between groups on our measure of diet quality, HEI 2010 (HEI-2010 Total and Component Scores for Children, Adults, and Older Adults during 2011–2012. [Internet], 2012) after our 2-month assessment (Fitzgibbon et al., 2018; Hughes et al., 2018; Smith-Ray et al., 2014). We speculate that the short duration of the intervention (8 weeks) did not allow participants to thoroughly build the skills necessary to maintain dietary changes. Additionally, our health educators were trained to deliver the nutrition curriculum but were not trained diet professionals. Their use in this effort increases the ability to translate this type of intervention more broadly to other community settings but may reduce the likelihood of longer-term behavior change.
3.2. Secondary outcomes
Self-reported physical activity on the PASE showed significant differences favoring F&S! Plus only at 2 months (post-intervention. Even at post-intervention, when the self-reported PA was highest (128.7 ± 4.9), it was somewhat lower than the score reported in another study of healthy community-dwelling older adults (M = 131.4) (Washburn et al., 1999). This consistent with other data showing disparities in ability to meet PA guidelines, with African-Americans being the least likely (43.4%) to meet PA guidelines compared to non-Hispanic whites who were most likely (51.2%) (Mama et al., 2015; Matthews et al., 2008). Women are also less likely to meet guidelines than men (CDC, 2014).
Among overweight/obese African-American older adults following an 8-week (2 month) intervention and at 6, 12, and 18-month follow-up visits, participants in F&S! Plus showed modest but positive changes in weight (12 months) BMI (12 months), waist circumference (12 months), and LE strength (12 and 18 months), compared to F&S!, which addressed PA alone. The findings of this randomized comparative effectiveness trial provide support for the addition of a weight management component to an evidence-based PA intervention to reduce OA symptomatology. Given that African Americans report higher average levels of pain and disability related to OA (Allen et al., 2009) and that obesity has been linked to worse self-reported WOMAC scores, providers should be cognizant of non-pharmacologic strategies that can be delivered in “real world” settings in order to provide sustained benefits to high-risk populations with OA.
3.3. Limitations and strengths
The results of the current study are limited to overweight/obese older adults and to a primarily female and African American population. We also used self-reported LE pain and stiffness to define the presence of OA and self-reported PA and dietary intake. We did not measure pro-inflammatory cytokines, including IL-6 and C-reactive protein, that have been shown to influence both the incidence (Livshits et al., 2009) and progression of OA (Sharif et al., 2000; Spector et al., 1997). We did not assess whether OA was primary or secondary.
However, to our knowledge, this is one of the first trials of a PA/weight management intervention to have successfully recruited and retained a relatively large sample of community-dwelling overweight/obese older African American adults, delivered a highly scalable intervention, and objectively measured height, body weight, waist circumference, mobility, and LE strength.
Funding
This study was supported by the National Institute on Aging of the National Institutes of Health (R01AG039374), the American Cancer Society of Illinois (261775), and the American Cancer Society Mentored Research Scholar (MRSG014–025-01-CNE). The trial is registered at clinicaltrials.gov (NCT03180008).
References
- Ainsworth BE, Wilcox S, Thompson WW, Richter DL, Henderson KA, 2003. Personal, social, and physical environmental correlates of physical activity in African-American women in South Carolina. Am. J. Prev. Med 25 (3), 23–29. [DOI] [PubMed] [Google Scholar]
- Allen KD, Helmick CG, Schwartz TA, DeVellis RF, Renner JB, Jordan JM, 2009. Racial differences in self-reported pain and function among individuals with radiographic hip and knee osteoarthritis: the Johnston County osteoarthritis project. Osteoarthr. Cartil 17 (9), 1132–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandura A, 1989. Human agency in social cognitive theory. Am. Psychol. 44 (9), 1175–1184. [DOI] [PubMed] [Google Scholar]
- Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW, 1988. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J. Rheumatol 15 (12), 1833–1840. [PubMed] [Google Scholar]
- Bolen SD, Clark JM, Richards TM, Shore AD, Goodwin SM, Weiner JP, 2010. Trends in and patterns of obesity reduction medication use in an insured cohort. Obesity 18 (1), 206–209. [DOI] [PubMed] [Google Scholar]
- CDC, 2014. National Health Interview Survey 2014: National Center for Health Statistics. Available from: https://www.cdc.gov/nchs/nhis/nhis_2014_data_release.htm.
- Choose My Plate US Department of Agriculture. Available from: https://www.choosemyplate.gov/resources/MyPlatePlan.
- Christensen R, Bartels EM, Astrup A, Bliddal H, 2007. Effect of weight reduction in obese patients diagnosed with knee osteoarthritis: a systematic review and meta-analysis. Ann. Rheum. Dis 66 (4), 433–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantine MG, Donnelly PC, Myers LJ, 2002. Collective self-esteem and Africultural coping styles in African American adolescents. J. Black Stud. 32 (6), 698–710. [Google Scholar]
- Cross M, Smith E, Hoy D, Nolte S, Ackerman I, Fransen M, et al. , 2014. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann. Rheum. Dis 73 (7), 1323–1330. [DOI] [PubMed] [Google Scholar]
- Deshpande BR, Katz JN, Solomon DH, Yelin EH, Hunter DJ, Messier SP, et al. , 2016. Number of persons with symptomatic knee osteoarthritis in the US: impact of race and ethnicity, age, sex, and obesity. Arthritis Care Res 68 (12), 1743–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietary Guidelines for Americans, 2010. US Department of Agriculture US Department of Health and Human Services. Available from: https://health.gov/dietaryguidelines/dga2010/DietaryGuidelines2010.pdf.
- Dougados M, Leclaire P, Bloch D, Bellamy N, Altman R, 2000. Response criteria for clinical trials on osteoarthritis of the knee and hip: a report of the osteoarthritis research society international standing Committee for Clinical Trials response criteria initiative. Osteoarthr. Cartil 8 (6), 395–403. [DOI] [PubMed] [Google Scholar]
- Dunlop DD, Manheim LM, Sohn M-W, Liu X, Chang RW, 2002. Incidence of functional limitation in older adults: the impact of gender, race, and chronic conditions. Arch. Phys. Med. Rehabil 83 (7), 964–971. [DOI] [PubMed] [Google Scholar]
- Dunlop DD, Song J, Semanik PA, Sharma L, Chang RW, 2011. Physical activity levels and functional performance in the osteoarthritis initiative: a graded relationship. Arthritis Rheum 63 (1), 127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgibbon M, Tussing-Humphreys L, Schiffer L, Smith-Ray R, Demott A, Martinez M, et al. , 2018. Fit & strong! Plus: descriptive demographic and risk characteristics in a comparative effectiveness trial for older African-American adults with osteoarthritis. J. Aging Res. Clin. Pract 7 (1), 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryar CD, Carroll MD, Ogden CL, 2018. Prevalence of Overweight, Obesity, and Severe Obesity among Adults Aged 20 and over: United States, 1960–1962 through 2015–2016.
- Gordon-Larsen P, Nelson MC, Page P, Popkin BM, 2006. Inequality in the built environment underlies key health disparities in physical activity and obesity. Pediatrics 117 (2), 417–424. [DOI] [PubMed] [Google Scholar]
- Guenther PM, Casavale KO, Reedy J, Kirkpatrick SI, Hiza HA, Kuczynski KJ, et al. , 2013. Update of the healthy eating index: HEI-2010. J. Acad. Nutr. Diet. 113 (4), 569–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyatt GH, Sullivan MJ, Thompson PJ, Fallen EL, Pugsley SO, Taylor DW, et al. , 1985. The 6-minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can. Med. Assoc. J 132 (8), 919. [PMC free article] [PubMed] [Google Scholar]
- Hales CM, Fryar CD, Carroll MD, Freedman DS, Ogden CL, 2018. Trends in obesity and severe obesity prevalence in US youth and adults by sex and age, 2007–2008 to 2015–2016. JAMA 319 (16), 1723–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG, 2009. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform 42 (2), 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey AR, Hill RB, 2004. Africentric youth and family rites of passage program: promoting resilience among at-risk African American youths. Soc. Work 49 (1), 65–74. [DOI] [PubMed] [Google Scholar]
- HEI-2010 Total and Component Scores for Children, Adults, and Older Adults during. 2011–2012 [Internet]. Available from: https://www.cnpp.usda.gov/sites/default/files/healthy_eating_index/HEI-2010-During-2011-.
- Hughes SL, Edelman P, Chang RW, Singer RH, Schuette P, 1991. The geri-aims. Reliability and validity of the arthritis impact measurement scales adapted for elderly respondents. Arthritis Rheum 34 (7), 856–865. [DOI] [PubMed] [Google Scholar]
- Hughes SL, Tussing-Humphreys L, Schiffer L, Smith-Ray R, Marquez DX, DeMott AD, et al. , 2018. Fit & Strong! Plus Trial Outcomes for Obese Older Adults with Osteoarthritis. The Gerontologist. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CJ, Rikli RE, Beam WC, 1999. A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Res. Q. Exerc. Sport 70 (2), 113–119. [DOI] [PubMed] [Google Scholar]
- Jordan JM, 2015. An ongoing assessment of osteoarthritis in African Americans and Caucasians in North Carolina: the Johnston County osteoarthritis project. Trans. Am. Climatol. Assoc 126, 77. [PMC free article] [PubMed] [Google Scholar]
- Jordan JM, Helmick CG, Renner JB, Luta G, Dragomir AD, Woodard J, et al. , 2009. Prevalence of hip symptoms and radiographic and symptomatic hip osteoarthritis in African Americans and Caucasians: the Johnston County osteoarthritis project. J. Rheumatol 36 (4), 809–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie RK, Chan FKL, Grady C, Ng VH, Wendler D, 2017. Comparative effectiveness research: what to do when experts disagree about risks. BMC Med. Ethics 18 (1), 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livshits G, Zhai G, Hart DJ, Kato BS, Wang H, Williams FM, et al. , 2009. Interleukin-6 is a significant predictor of radiographic knee osteoarthritis: the Chingford study. Arthritis Rheum 60 (7), 2037–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallinckrod CH, Lane PW, Schnell D, Peng Y, Mancuso JP, 2008. Recommendations for the primary analysis of continuous endpoints in longitudinal clinical trials. Drug Inform. J 42 (4), 303–319. [Google Scholar]
- Mama SK, McCurdy SA, Evans AE, Thompson DI, Diamond PM, Lee RE, 2015. Using community insight to understand physical activity adoption in overweight and obese African American and Hispanic women: a qualitative study. Health Educ. Behav 42 (3), 321–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March L, Amatya B, Osborne RH, Brand C, 2010. Developing a minimum standard of care for treating people with osteoarthritis of the hip and knee. Best Pract. Res. Clin. Rheumatol 24 (1), 121–145. [DOI] [PubMed] [Google Scholar]
- Matthews CE, Chen KY, Freedson PS, Buchowski MS, Beech BM, Pate RR, et al. , 2008. Amount of time spent in sedentary behaviors in the United States, 2003–2004. Am. J. Epidemiol 167 (7), 875–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messier SP, Loeser RF, Miller GD, Morgan TM, Rejeski WJ, Sevick MA, et al. , 2004. Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis: the arthritis, diet, and activity promotion trial. Arthritis Rheum 50 (5), 1501–1510. [DOI] [PubMed] [Google Scholar]
- Messier SP, Mihalko SL, Legault C, Miller GD, Nicklas BJ, DeVita P, et al. , 2013a. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the IDEA randomized clinical trial. JAMA 310 (12), 1263–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messier SP, Mihalko SL, Legault C, Miller GD, Nicklas BJ, DeVita P, et al. , 2013b. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the IDEA randomized clinical trial. JAMA 310 (12), 1263–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor JM, Hayen A, Davidson E, Hackett D, Harris IA, Kamalasena G, et al. , 2014. Minimal detectable change for mobility and patient-reported tools in people with osteoarthritis awaiting arthroplasty. BMC Musculoskelet. Disord 15 (1), 235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell LM, Slater S, Mirtcheva D, Bao Y, Chaloupka FJ, 2007. Food store availability and neighborhood characteristics in the United States. Prev. Med 44 (3), 189–195. [DOI] [PubMed] [Google Scholar]
- Roos EM, Arden NK, 2016. Strategies for the prevention of knee osteoarthritis. Nat. Rev. Rheumatol 12 (2), 92. [DOI] [PubMed] [Google Scholar]
- Safiri S, Kolahi A-A, Smith E, Hill C, Bettampadi D, Mansournia MA, et al. , 2020. Global, regional and national burden of osteoarthritis 1990–2017: a systematic analysis of the global burden of disease study 2017. Ann. Rheum. Dis 79 (6), 819–828. [DOI] [PubMed] [Google Scholar]
- Segal NA, Nevitt MC, Gross KD, Hietpas J, Glass NA, Lewis CE, et al. , 2013. The multicenter osteoarthritis study (MOST): opportunities for rehabilitation research. PM&R 5 (8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharif M, Shepstone L, Elson C, Dieppe P, Kirwan J, 2000. Increased serum C reactive protein may reflect events that precede radiographic progression in osteoarthritis of the knee. Ann. Rheum. Dis 59 (1), 71–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Ray RL, Fitzgibbon ML, Tussing-Humphreys L, Schiffer L, Shah A, Huber GM, et al. , 2014. Fit and strong! Plus: design of a comparative effectiveness evaluation of a weight management program for older adults with osteoarthritis. Contemp. Clin. Trials 37 (2), 178–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector TD, Hart DJ, Nandra D, Doyle DV, Mackillop N, Gallimore JR, et al. , 1997. Low-level increases in serum C-reactive protein are present in early osteoarthritis of the knee and predict progressive disease. Arthritis Rheum 40 (4), 723–727. [DOI] [PubMed] [Google Scholar]
- Vaughn IA, Terry EL, Bartley EJ, Schaefer N, Fillingim RB, 2019. Racial-ethnic differences in osteoarthritis pain and disability: a meta-analysis. J. Pain 20 (6), 629–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villareal DT, Aguirre L, Gurney AB, Waters DL, Sinacore DR, Colombo E, et al. , 2017. Aerobic or resistance exercise, or both, in dieting obese older adults. N. Engl. J. Med 376 (20), 1943–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JL, Harrison TC, Brown A, Thorpe RJ Jr., Szanton SL, 2016. Factors associated with disability among middle-aged and older African American women with osteoarthritis. Dis. Health J 9 (3), 510–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn RA, McAuley E, Katula J, Mihalko SL, Boileau RA, 1999. The physical activity scale for the elderly (PASE): evidence for validity. J. Clin. Epidemiol 52 (7), 643–651. [DOI] [PubMed] [Google Scholar]
- West DS, Elaine Prewitt T, Bursac Z, Felix HC, 2008. Weight loss of black, white, and Hispanic men and women in the diabetes prevention program. Obesity (Silver Spring) 16 (6), 1413–1420. [DOI] [PubMed] [Google Scholar]
- Wilcox S, Bopp M, Oberrecht L, Kammermann SK, McElmurray CT, 2003. Psychosocial and perceived environmental correlates of physical activity in rural and older African American and white women. J. Gerontol. B Psychol 58 (6), P329–P337. [DOI] [PubMed] [Google Scholar]
- Zenk SN, Schulz AJ, Hollis-Neely T, Campbell RT, Holmes N, Watkins G, et al. , 2005. Fruit and vegetable intake in African Americans: income and store characteristics. Am. J. Prev. Med 29 (1), 1–9. [DOI] [PubMed] [Google Scholar]
- Zhang W, Moskowitz R, Nuki G, Abramson S, Altman RD, Arden N, et al. , 2008. OARSI recommendations for the management of hip and knee osteoarthritis, part II: OARSI evidence-based, expert consensus guidelines. Osteoarthr. Cartil 16 (2), 137–162. [DOI] [PubMed] [Google Scholar]


