Abstract
Isocitrate dehydrogenase 1 (IDH1) mutational status is an important prognostic biomarker in gliomas. γ-aminobutyric acid (GABA) and reduced glutathione (GSH) play an important role in energy production, which is related to tumor progression. Hadamard Encoding and Reconstruction of Mega-Edited Spectroscopy (HERMES) is able to detect GABA and GSH in healthy controls. This study aims to examine GABA and GSH alterations in IDH1-mutated low-grade gliomas using HERMES. We prospectively enrolled 14 suspected low-grade gliomas and 6 healthy control patients in this study, all cases underwent a 3T MRI scan, including T1-weighted imaging and HERMES acquisition with a volume of interest 3×3×3 cm3. HERMES detects a “GABA+” signal that includes contributions from macromolecules and homocarnosine. GABA+ and GSH in tumor foci (group 1), contralateral cerebral regions (group 2) and healthy controls (group 3) were quantified using Gannet. The fitting errors and SNR of HERMES for GABA+ and GSH were analyzed; FWHM of the unsuppressed water signal was also recorded. The Wilcoxon signed-rank test was performed to test for differences between contralateral GABA+ and GSH levels, and differences in GABA+, GSH and fitting errors/SNR between the three groups were analyzed using analysis of variance (ANOVA). Eleven IDH1-mutant low-grade gliomas (5 Female and 6 Male, age 33–69) and 6 healthy subjects (2 Female and 4 Male, age 35–60) were finally enrolled this study. The mean water linewidth across all subjects was 9.67 ± 2.28 Hz. The Wilcoxon signed-rank test revealed that GABA+ and GSH were decreased significantly in glioma foci compared with contralateral regions, whereas no differences were seen between the left and right regions in healthy controls. ANOVA showed that GABA+ and GSH levels in tumor were lower than contralaterally and in healthy controls, while no differences were observed between the contralateral healthy tissue and healthy controls. No differences of fitting errors or SNR were found between tumors, contralateral regions or healthy controls. Our results suggest that HERMES is a reliable tool to simultaneously measure GABA and GSH alterations in low-grade gliomas with IDH1 mutations.
Keywords: glioma, isocitrate dehydrogenase, HERMES, GABA, glutathione
Introduction
Glioma is the most common type of primary brain tumor, classified as either low-grade (WHO grade I and II) or high-grade (WHO grade III and IV) based on the histopathological and clinical criteria, and the prognosis remains poor (Louis et al., 2007; Wen and Kesari, 2008). The incidence of isocitrate dehydrogenase 1 (IDH1) mutations is very high in low-grade gliomas (Cancer Genome Atlas Research et al., 2015), and IDH1-mutant gliomas are associated with much better prognosis than IDH1 wild-type (Sanson et al., 2009). IDH1 mutational status was integrated into the 2016 WHO classification as a vital biomarker of gliomas (Louis et al., 2016).
The metabolic alterations seen in gliomas with IDH1 mutation are the subject of much recent interest, including gamma-aminobutyric acid (GABA) (Jalbert et al., 2017) and GSH (Gu et al., 2015). GABA is extensively metabolized in astrocytes, playing an important role in providing carbon backbone for glutamine, supporting and regulating neuronal functions of glioma-related astrocytes, and it can be also used as an alternative fuel to support astrocyte oxidative metabolism(Andersen et al., 2020). GABA is derived from the tricarboxylic acid (TCA) cycle intermediate alpha-ketoglutarate, which is itself formed by IDH1catalyzed oxidative decarboxylation of isocitrate (Turkalp et al., 2014). Cells with mutant IDH1 produce 2-HG rather than alpha-ketoglutarate (Gelman et al., 2018), and we hypothesis that the lack of availability of this important GABA precursor in IDH1 glioma would affect the GABA concentration.
GSH is the most abundant intracellular antioxidant (Gu et al., 2015), functioning in the protection of cells against oxidative damage caused by reactive oxygen species (ROS) (Juan et al., 2008), and low GSH was reported in gliomas before (Kudo et al., 1990). NADPH is a required cofactor to reduce oxidized glutathione (GSSG) back to GSH (Ren et al., 2017). Mutant IDH1 is a cytosolic enzyme that draws upon the cellular pool of NADPH, oxidizing it to NADP+ while converting alpha-ketoglutarate to the metabolite 2-hydroxyglutarate (2-HG) (Dang et al., 2009). Altered NADPH might change the GSH/GSSG redox balance but may not necessarily lead to less actual glutathione, and we hypothesize the decrease of NADPH in mutant IDH1 glioma may result in the imbalance of GSH/GSSG, exhausting the levels of GSH.
The concentration of GABA and GSH in the brain is too low to be detected using conventional single-voxel magnetic resonance spectroscopy (MRS). However, edited MRS, e.g. Mescher-Garwood Point-resolved Spectroscopy (MEGA-PRESS), is able to detect GABA+(Gong et al., 2018b; Mescher et al., 1998) (an edited signal combining GABA and co-edited macromolecular and homocarnosine signals) and GSH (Dhamala et al., 2019; Sanaei Nezhad et al., 2017). Typically only one metabolite can be edited at a time with relatively long acquisition times. Hadamard Encoding and Reconstruction of Mega-Edited Spectroscopy (HERMES) applies orthogonal editing encoding, allowing both GSH and GABA+ spectra to be reconstructed from a single sequence (Chan et al., 2019; Saleh et al., 2016).
Although previous studies have indicated that GABA and GSH levels are altered in low-grade gliomas, the relatively small number of publications is sparse offer conflicting information(Hujber et al., 2018; Hulsey et al., 2015; Jalbert et al., 2017; Lai et al., 2018; Moren et al., 2015; Shi et al., 2015) and no studies have sought to investigate both metabolites using HERMES. Thus this study set out to examine GABA+ and GSH alterations in IDH1-mutated low-grade gliomas using HERMES at 3T magnetic field in vivo.
Methods and materials
Human subjects
This study was performed under the approval of the local ethical committee; all subjects provided written informed consent. Glioma subjects, enrolled at Shandong Provincial Hospital, met these criteria: suspected low-grade glioma; age over 18 years old; tumor volume >30 cm3; candidate for resection or biopsy. IDH mutational status for glioma was determined by Sanger sequencing combined with automated immune histochemical analysis. Additionally, 6 age-matched healthy subjects were enrolled in this study as controls.
Magnetic Resonance Imaging and Spectroscopy
All MR data were acquired using a 32-channel phased-array head coil on a 3T MR scanner (Siemens, Erlangen, Germany), including three-dimensional anatomical images: T1-weighted magnetization-prepared rapid gradient-echo; repetition time/echo time (TR/TE) 2300/2.29 msec; slice thickness 1 mm; field of view 24×24 cm2; matrix size 256×256; flip angle 8°, acquisition time was ~5 min
Edited MRS was acquired using the HERMES experiment, which allows simultaneous editing of multiple metabolite targets (Saleh et al., 2016); in this study, HERMES was acquired with the ‘universal’ sequence timings(Saleh et al., 2019), with parameters: TR/TE 2000/80 ms; 320 averages; voxel size 3×3×3 cm3; ~10 min acquisition time (per region). HERMES ROIs were set in the glioma foci and contralateral control regions (Figure 1a and b) in patients; for healthy controls, six pairs of ROIs were set in the region of most common glioma onset: white and gray matter junctions of frontal, temporal or parietal lobes, as shown in Figure 1d and e.
Figure 1.
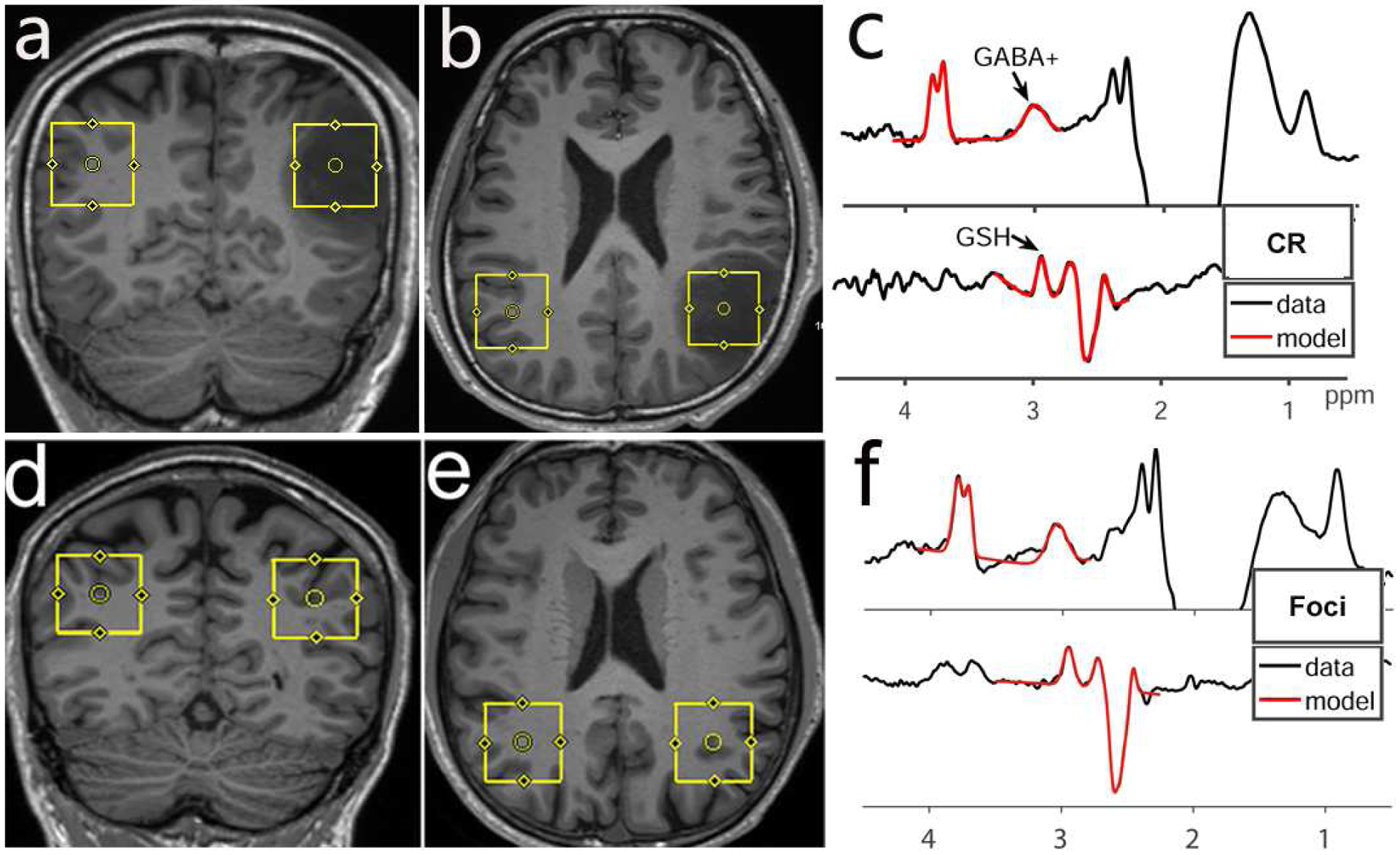
Voxel position and HERMES fitting results in a patient with diffuse astrocytoma (WHO II, IDH1-mutant) and in a control subject. The location and size of the VOIs were shown on coronal (a/d) and axial (b/e) T1 weighted images; MR spectra data (black lines) of the glioma foci and healthy control (left region) are shown with Gannet model (red lines). Fitting errors for contralateral region (c) and foci spectra (f) were 5.4% (GABA+) / 4.2% (GSH) and 9.6% (GABA+) / 5.9% (GSH), respectively. CR = contralateral region.
MRS data were analyzed using the Gannet 3.0 analysis toolkit (Edden et al., 2014). The edited 3-ppm signal also contains contributions from macromolecules (MM) and homocarnosine in addition to GABA (Oeltzschner et al., 2018), so we refer to it as GABA+. Gannet-standard preprocessing was performed, followed by nonlinear least-squares modeling to quantify the GABA+ and GSH peaks (as shown in Figure 1 c and f) (Gong et al., 2018b; Mikkelsen et al., 2018; Saleh et al., 2016). GABA+ and GSH were quantified relative to the unsuppressed water signal, as a concentration in institutional units (i.u.), assuming a bulk water concentration of 55.5 mM, and a water transverse relaxation time constant of 150 ms. The relative fitting error (FitError) and signal-to-noise ratio (SNR) of GABA and GSH in Hermes determination by GannetFit, together with the full width at half maximum (FWHM) of the water signal were also recorded.
Statistical Analysis
Quantitative results are presented as mean ± standard deviation (SD). Wilcoxon signed-rank test was used to test for differences in GABA+ and GSH levels between the tumor foci (group 1) and contralateral regions (group 2), and between left and right regions in healthy controls (group 3). The GABA+, GSH, fitting errors and SNR generated by GannetFit of GABA + and GSH in three groups were also analyzed using ANOVA and least significant difference (LSD) tests. Statistical analyses were carried out by GraphPad Prism 7.00, the threshold of significance was set at P < 0.05.
Results
Fourteen patients (5 Female and 9 Male, aged 33–69 years), with a suspected diagnosis of low-grade glioma and who were scheduled to receive surgery, were prospectively enrolled in this study. All subjects were at first diagnosis without prior treatment. Histopathologic findings indicated that one case was inflammatory pseudotumor (IPT), not a tumor. The genotyping assay for IDH1 indicated 13 IDH1-mutant and 1 IDH1 wild-type cases; one IDH1-mutant tumor was classified as high-grade (WHO III) according to the histopathological result. Thus, 11 IDH1-mutant low-grade gliomas were finally enrolled this study, and the subject information was listed in Table 1. Another 6 healthy subjects (2 Female and 4 Male, age 35–60) were enrolled as controls.
Table 1.
Demographic, histomolecular features and HERMES data of the cohort
| Subject # | Histological Diagnosis | IDH1 status | GABA+(i.u.) Foci/CR | GSH(i.u.) Foci/CR | Age | Sex |
|---|---|---|---|---|---|---|
| Subject 1 | DA grade II | Mutation | 0.802/1.162 | 0.220/0.522 | 46 | F |
| Subject 2 | DA grade II | Mutation | 1.205/1.623 | 0.603/0.886 | 33 | F |
| Subject 3 | OD grade II | Mutation | 0.510/2.006 | 1.089/1.343 | 50 | M |
| Subject 4 | DA grade II | Mutation | 0.565/0.803 | 0.726/1.269 | 35 | M |
| Subject 5 | DA grade II | Mutation | 1.309/1.218 | 0.379/0.895 | 49 | F |
| Subject 6 | DA grade II | Mutation | 1.256/1.807 | 0.324/0.540 | 35 | M |
| Subject 7 | OD grade II | Mutation | 1.324/1.623 | 0.573/0.896 | 69 | M |
| Subject 8 | DA grade II | Mutation | 1.567/2.072 | 0.876/1.026 | 40 | M |
| Subject 9 | DA grade II | Mutation | 1.807/2.308 | 1.076/1.560 | 38 | F |
| Subject 10 | OD grade II | Mutation | 0.934/1.537 | 0.638/1.153 | 46 | M |
| Subject 11 | DA grade II | Mutation | 1.143/1.736 | 0.576/1.254 | 39 | F |
| Subject 12 | AA grade III | Mutation | — | — | 40 | M |
| Subject 13 | OD grade II | Wild-type | — | — | 50 | M |
| Subject 14 | IPT | — | — | — | 53 | M |
Abbreviations: CR = contralateral region; DA = diffuse astrocytoma; OD = oligodendroglioma; AA = anaplastic astrocytoma; IPT = inflammatory pseudotumor.
HERMES could perform simultaneous measurement of GABA+ and GSH levels in glioma patients, with edited spectra shown in Figure 1. Water linewidths were 9.67 ± 2.28 Hz, all smaller than 15 Hz. HERMES data of the tumor foci, contralateral regions, and right/left regions in healthy controls was plotted using PaperPlot in Gannet, as illustrated in Figure 2. Wilcoxon signed-rank test revealed that GABA+ and GSH were significantly lower in glioma foci compared with contralateral regions (p=0.011, t=2.781; p=0.007, t=2.977 respectively), while no differences were seen between left and right regions in healthy controls, as shown in Figure 3. The ANOVA showed no differences of fitting errors and SNR between three groups, as listed in Table 2. ANOVA did indicate that both GABA+ and GSH levels in tumor were lower than contralaterally or in controls (p=0.023/0.005; p=0.001/0.047, respectively), while no differences between CR and control subjects was observed (p=0.065/0.619), as shown in Figure 3.
Figure 2.
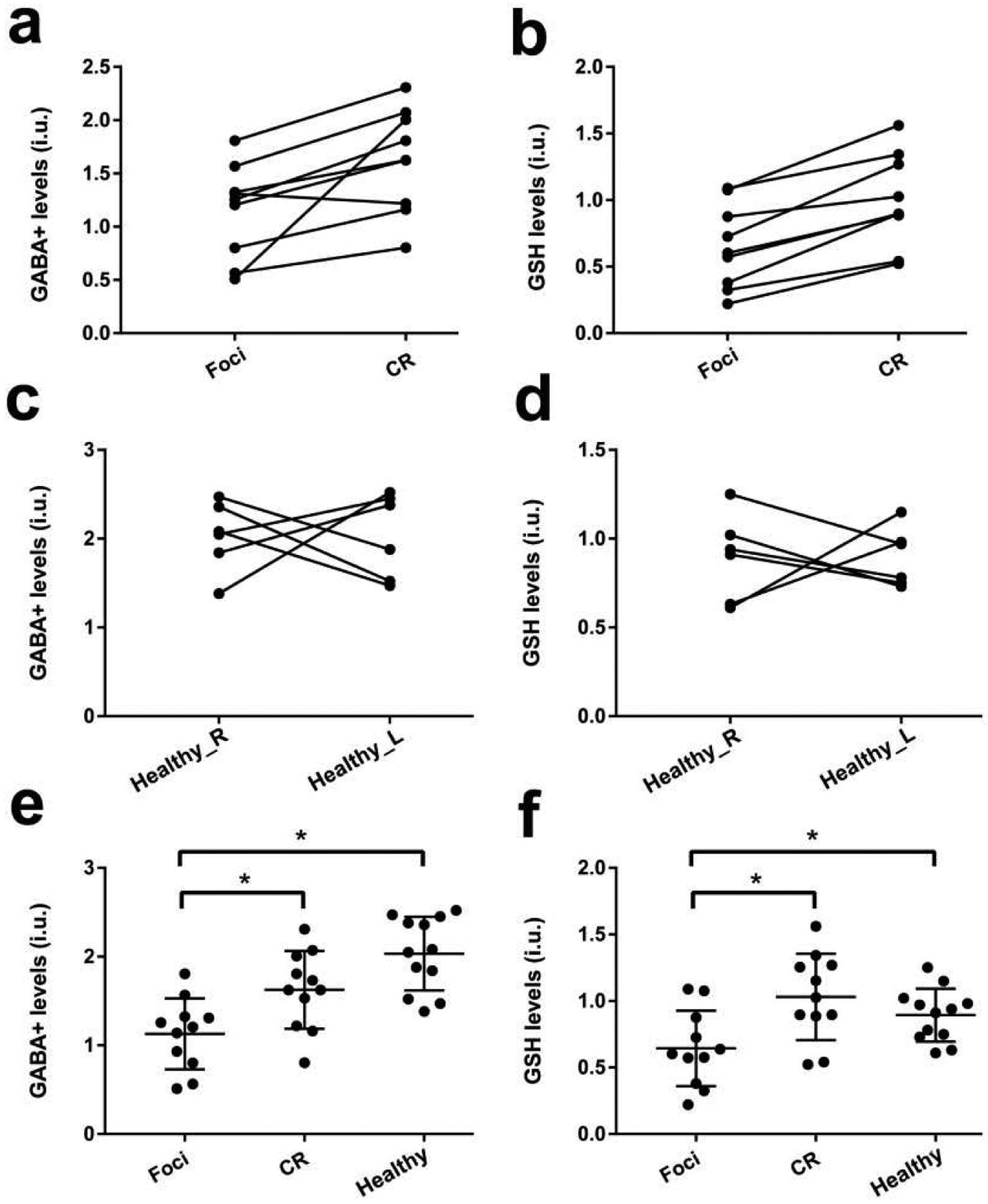
The mean (± standard deviation) GABA-edited spectra (a) and GSH-edited spectra (b) from HERMES in tumor foci, contralateral regions, right and left regions in healthy controls. CR = contralateral region; Healthy_L = left regions in healthy controls, Healthy_R = right regions in healthy controls.
Figure 3.
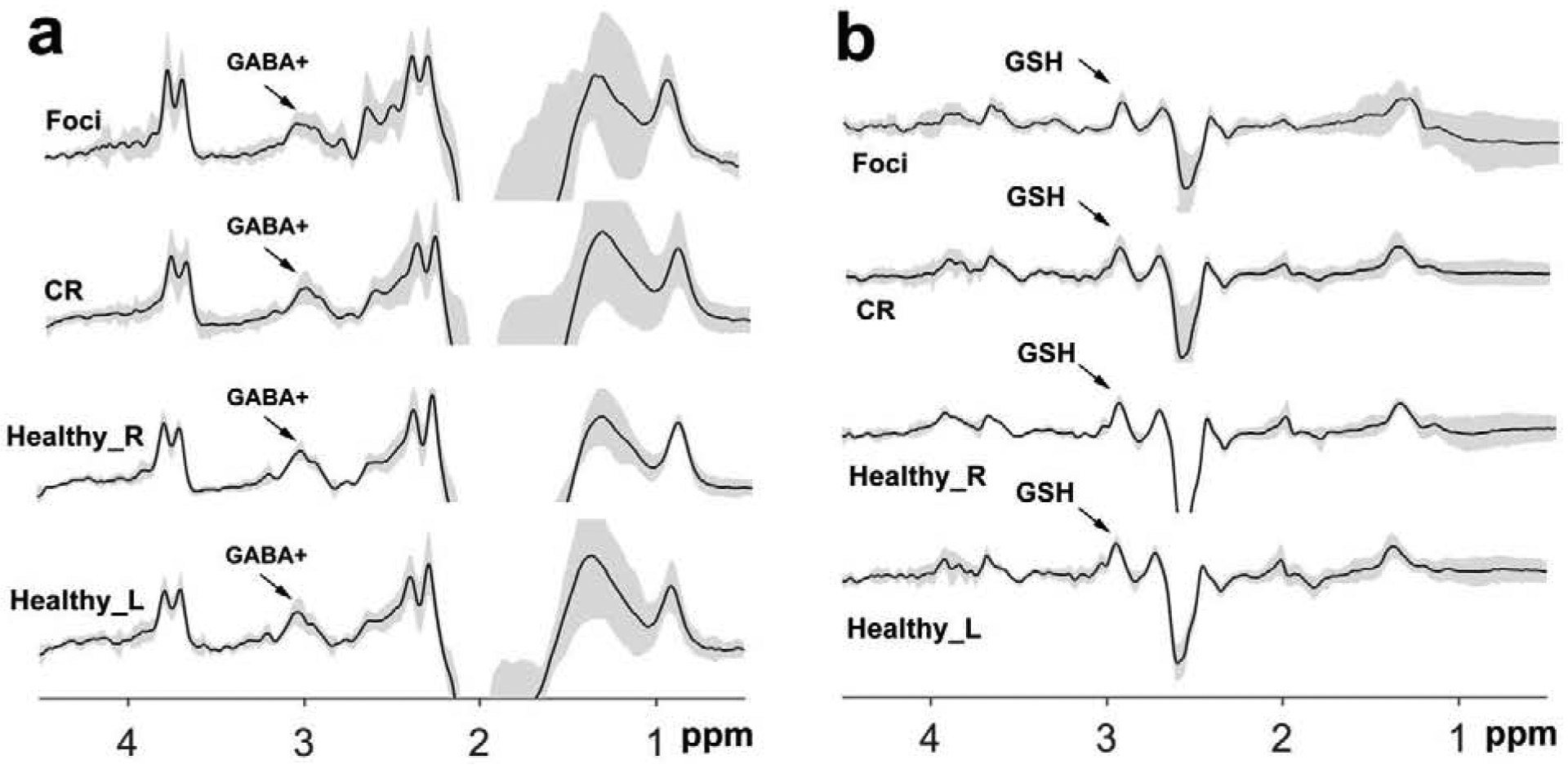
GABA+ and GSH levels in gliomas (foci and contralateral region) and healthy controls (left and right region). Wilcoxon signed-rank test in gliomas (a and b) and healthy controls (c and d), results showed GABA+ and GSH decreased significantly in glioma foci compared with contralateral regions (p=0.004, Z=−2.845; p=0.003, Z=−2.934 respectively), while no differences was found between left and right regions in controls (p=0.843; p=0.844). ANOVA revealed GABA+ and GSH levels in foci region were lower than CR and Healthy groups (p=0.023/0.005; p=0.001/0.047, respectively), while no differences between CR and Healthy was observed (p=0.065/0.619). Healthy_L = left region in healthy controls, Healthy_R = right region in healthy controls; Healthy = Left + right regions in healthy controls; CR = tumor-contralateral regions.
Table 2.
The GABA+ and GSH levels, Fitting Errors, and SNR of HERMES in IDH1-mutant low-grade gliomas
| GABA+(i.u.) | GSH(i.u.) | GABA+FitError (%) | GSH FitError (%) | GABA+SNR | GSH SNR | |
|---|---|---|---|---|---|---|
| Glioma foci | 1.15 ± 0.44 | 0.65 ± 0.32 | 9.83 ± 4.66 | 7.27 ± 3.23 | 11.58 ± 2.78 | 12.47 ± 4.27 |
| Contralateral region | 1.62 ± 0.47 | 0.99 ± 0.35 | 9.08 ± 2.31 | 6.45 ± 1.86 | 10.69 ± 2.68 | 13.76 ± 5.16 |
| Healthy right and left | 2.03 ± 0.41 | 0.89 ± 0.19 | 8.87 ± 2.36 | 6.78 ± 1.75 | 9.49 ± 2.87 | 12.31 ± 4.56 |
| P values | 0.001 | 0.006 | 0.43 | 0.96 | 0.32 | 0.73 |
ANOVA, p < 0.05 was considered significant. Abbreviations: i.u.= institutional units; FitError = fitting error; SNR = signal-to-noise ratio.
Discussion
This study has shown that HERMES can be applied for the simultaneous detection of GABA and GSH signals in low-grade gliomas with IDH1 mutations; we further found that GABA and GSH levels were both significantly lower in the region of tumor foci compared with contralateral cerebral regions and healthy controls, while no differences were found contralaterally in controls or between controls and tumor-contralateral tissue.
HERMES of GABA and GSH (Saleh et al., 2016) uses Hadamard encoding to perform two MEGA-PRESS experiments simultaneously. Most studies (Berrington et al., 2018; Gong et al., 2018a; Saleh and Mikkelsen, 2018; Saleh et al., 2019) applying HERMES have involved further methodological development, and the method has not yet been widely applied for clinical studies. So, this is the first time to prove the capability of HERMES in detecting the GABA and GSH alterations in gliomas, and to illustrate that it is sufficiently robust for clinical research studies in general.
GABA is the main inhibitory neurotransmitter and extensively metabolized in astrocytes, serving a key role in shaping and regulating patterns of neuronal activity, providing carbon backbone for the synthesis of glutamine in Glutamine-Glutamate/GABA Cycle(Walls et al., 2015), and can also be as an alternative fuel to support astrocyte oxidative metabolism (Andersen et al., 2020). There is growing evidence (El-Habr et al., 2017; Hujber et al., 2018; Smits et al., 2012) indicating that GABA could drive the proliferation of tumor by regulation of neuronal stem cells and tumor stem cells. As in astrocytes, GABA is assumed to enter the mitochondria in exchange with glutamate, and both glutamate and GABA are derived from the TCA cycle intermediate alpha-ketoglutarate. IDH1 catalyzes the oxidative decarboxylation of isocitrate to alpha-ketoglutarate (Turkalp et al., 2014), while mutant IDH1 consumes alpha-ketoglutarate in production of the metabolite 2-HG while oxidizing NADPH to NADP+ (Gelman et al., 2018). The alteration of alpha-ketoglutarate level in IDH1 glioma would affect the GABA concentration. Records on GABA in glioma in previous research are sparse and inconsistent. Lina et al. (Moren et al., 2015) found that GABA concentration in foci were lower in GBM than that in oligodendroglioma, while another study (Faria et al., 2011) failed to detect GABA in high-grade tumors. In low-grade gliomas, Faria et al. (Faria et al., 2011) showed GABA levels decreased in tumors compared to healthy brain, in line with the results of this study. Similarly, lower GABA levels in tumors than healthy brain have been reported in animal studies (Hulsey et al., 2015; Lai et al., 2018). One hypothesis (Faria et al., 2011) is that the GABA decrease is driven by the absence of mature and/or well-differentiated neurons and glial cells in these tumors; and the IDH-mutated status would further decrease the GABA levels (Jalbert et al., 2017).
GSH is the primary redox compound in the central nervous system, functioning in reducing the damage caused by ROS. In the production of GSH, NADPH is a required cofactor for the reduction of glutathione disulfide (GSSG) back to GSH (Ren et al., 2017); while mutant IDH1 results in the loss of the enzyme’s ability to produce NADPH and confers a gain of enzyme function that oxidizes NADPH to NADP+ (Dang et al., 2009). Lower NADPH slows the production of GSH in IDH1-mutated gliomas, lowering the GSH level accordingly. The results of this study suggests the decrease of GSH in IDH1-mutated gliomas consistent with several pioneering human MRS studies (Andronesi et al., 2018; Bisdas et al., 2016; Pope et al., 2012) and animal or cell studies (Shi et al., 2015; Shi et al., 2014a; Shi et al., 2014b; Tang et al., 2020; Yu et al., 2020). To our knowledge, this is the first study conducted to determine GSH variations in low-grade gliomas using edited MRS at 3T MRI.
Previous studies (Dubbink et al., 2009; Sanson et al., 2009) have confirmed that IDH1 mutation in low-grade gliomas could result in better prognosis compared with wild-type, but the mechanisms remain largely unknown. GABA and GSH play a role in energy production through reducing oxidative stress, which is related with the neoplastic behavior of a tumor. One limitation of this study is that it only enrolled IDH1-mutant gliomas, the incidence of IDH1 wild-type gliomas is very low, only 1 case in the prospectively enrolled 12 patients. More data is needed to further evaluate the differences of GABA and GSH between IDH1-mutant and wild-type gliomas.
In conclusion, HERMES can noninvasively detect GABA and GSH alterations in low-grade gliomas with IDH1 mutation; it may be a valuable tool in the search for latent biomarkers characterizing of low-grade glioma. Noninvasive detection of GSH and GABA may prove to be a valuable diagnostic and prognostic biomarker of IDH1-mutant low-grade glioma.
Highlights.
HERMES is a reliable tool to simultaneously measure GABA and GSH levels in IDH1-mutant low-grade gliomas;
GABA and GSH both decreased in IDH1-mutant low-grade glioma foci compared with contralateral regions
Acknowledgments
This project was funded by National Natural Science Foundation of China (81671668; 81371534), Major research project of Shandong province (2016ZDJS07A16). This study applies tools developed under NIH grants R01 EB016089, P41 EB015909 and R01 EB023693; RAEE also receives salary support from these grants. We would like to thank all volunteers and patients for their participation.
Abbreviations
- GABA
γ-aminobutyric acid
- GSH
reduced glutathione
- IDH1
in isocitrate dehydrogenase 1
- HERMES
Hadamard Encoding and Reconstruction of Mega-Edited Spectroscopy
- ROS
reactive oxygen species
- MEGA-PRESS
Mescher-Garwood Point-resolved Spectroscopy
- 3D
three-dimensional
- ROI
region of interest
- CR
contralateral region
- DA
diffuse astrocytoma
- OD
oligodendroglioma
- AA
anaplastic astrocytoma
- IPT
inflammatory pseudotumor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersen JV, Jakobsen E, Westi EW, Lie MEK, Voss CM, Aldana BI, Schousboe A, Wellendorph P, Bak LK, Pinborg LH, Waagepetersen HS, 2020. Extensive astrocyte metabolism of gamma-aminobutyric acid (GABA) sustains glutamine synthesis in the mammalian cerebral cortex. Glia. [DOI] [PubMed] [Google Scholar]
- Andronesi OC, Arrillaga-Romany IC, Ly KI, Bogner W, Ratai EM, Reitz K, Iafrate AJ, Dietrich J, Gerstner ER, Chi AS, Rosen BR, Wen PY, Cahill DP, Batchelor TT, 2018. Pharmacodynamics of mutant-IDH1 inhibitors in glioma patients probed by in vivo 3D MRS imaging of 2-hydroxyglutarate. Nat Commun 9, 1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrington A, Barker PB, Edden RAE, Mikkelsen M, 2018. Frequency and phase correction for multiplexed edited MRS of GABA and glutathione. Magn Reson Med 80, 21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisdas S, Chadzynski GL, Braun C, Schittenhelm J, Skardelly M, Hagberg GE, Ethofer T, Pohmann R, Shajan G, Engelmann J, Tabatabai G, Ziemann U, Ernemann U, Scheffler K, 2016. MR spectroscopy for in vivo assessment of the oncometabolite 2-hydroxyglutarate and its effects on cellular metabolism in human brain gliomas at 9.4T. J Magn Reson Imaging 44, 823–833. [DOI] [PubMed] [Google Scholar]
- Cancer Genome Atlas Research, N., Brat DJ, Verhaak RG, Aldape KD, Yung WK, Salama SR, Cooper LA, Rheinbay E, Miller CR, Vitucci M, Morozova O, Robertson AG, Noushmehr H, Laird PW, Cherniack AD, Akbani R, Huse JT, Ciriello G, Poisson LM, Barnholtz-Sloan JS, Berger MS, Brennan C, Colen RR, Colman H, Flanders AE, Giannini C, Grifford M, Iavarone A, Jain R, Joseph I, Kim J, Kasaian K, Mikkelsen T, Murray BA, O’Neill BP, Pachter L, Parsons DW, Sougnez C, Sulman EP, Vandenberg SR, Van Meir EG, von Deimling A, Zhang H, Crain D, Lau K, Mallery D, Morris S, Paulauskis J, Penny R, Shelton T, Sherman M, Yena P, Black A, Bowen J, Dicostanzo K, Gastier-Foster J, Leraas KM, Lichtenberg TM, Pierson CR, Ramirez NC, Taylor C, Weaver S, Wise L, Zmuda E, Davidsen T, Demchok JA, Eley G, Ferguson ML, Hutter CM, Mills Shaw KR, Ozenberger BA, Sheth M, Sofia HJ, Tarnuzzer R, Wang Z, Yang L, Zenklusen JC, Ayala B, Baboud J, Chudamani S, Jensen MA, Liu J, Pihl T, Raman R, Wan Y, Wu Y, Ally A, Auman JT, Balasundaram M, Balu S, Baylin SB, Beroukhim R, Bootwalla MS, Bowlby R, Bristow CA, Brooks D, Butterfield Y, Carlsen R, Carter S, Chin L, Chu A, Chuah E, Cibulskis K, Clarke A, Coetzee SG, Dhalla N, Fennell T, Fisher S, Gabriel S, Getz G, Gibbs R, Guin R, Hadjipanayis A, Hayes DN, Hinoue T, Hoadley K, Holt RA, Hoyle AP, Jefferys SR, Jones S, Jones CD, Kucherlapati R, Lai PH, Lander E, Lee S, Lichtenstein L, Ma Y, Maglinte DT, Mahadeshwar HS, Marra MA, Mayo M, Meng S, Meyerson ML, Mieczkowski PA, Moore RA, Mose LE, Mungall AJ, Pantazi A, Parfenov M, Park PJ, Parker JS, Perou CM, Protopopov A, Ren X, Roach J, Sabedot TS, Schein J, Schumacher SE, Seidman JG, Seth S, Shen H, Simons JV, Sipahimalani P, Soloway MG, Song X, Sun H, Tabak B, Tam A, Tan D, Tang J, Thiessen N, Triche T Jr., Van Den Berg DJ, Veluvolu U, Waring S, Weisenberger DJ, Wilkerson MD, Wong T, Wu J, Xi L, Xu AW, Yang L, Zack TI, Zhang J, Aksoy BA, Arachchi H, Benz C, Bernard B, Carlin D, Cho J, DiCara D, Frazer S, Fuller GN, Gao J, Gehlenborg N, Haussler D, Heiman DI, Iype L, Jacobsen A, Ju Z, Katzman S, Kim H, Knijnenburg T, Kreisberg RB, Lawrence MS, Lee W, Leinonen K, Lin P, Ling S, Liu W, Liu Y, Liu Y, Lu Y, Mills G, Ng S, Noble MS, Paull E, Rao A, Reynolds S, Saksena G, Sanborn Z, Sander C, Schultz N, Senbabaoglu Y, Shen R, Shmulevich I, Sinha R, Stuart J, Sumer SO, Sun Y, Tasman N, Taylor BS, Voet D, Weinhold N, Weinstein JN, Yang D, Yoshihara K, Zheng S, Zhang W, Zou L, Abel T, Sadeghi S, Cohen ML, Eschbacher J, Hattab EM, Raghunathan A, Schniederjan MJ, Aziz D, Barnett G, Barrett W, Bigner DD, Boice L, Brewer C, Calatozzolo C, Campos B, Carlotti CG Jr., Chan TA, Cuppini L, Curley E, Cuzzubbo S, Devine K, DiMeco F, Duell R, Elder JB, Fehrenbach A, Finocchiaro G, Friedman W, Fulop J, Gardner J, Hermes B, Herold-Mende C, Jungk C, Kendler A, Lehman NL, Lipp E, Liu O, Mandt R, McGraw M, McLendon R, McPherson C, Neder L, Nguyen P, Noss A, Nunziata R, Ostrom QT, Palmer C, Perin A, Pollo B, Potapov A, Potapova O, Rathmell WK, Rotin D, Scarpace L, Schilero C, Senecal K, Shimmel K, Shurkhay V, Sifri S, Singh R, Sloan AE, Smolenski K, Staugaitis SM, Steele R, Thorne L, Tirapelli DP, Unterberg A, Vallurupalli M, Wang Y, Warnick R, Williams F, Wolinsky Y, Bell S, Rosenberg M, Stewart C, Huang F, Grimsby JL, Radenbaugh AJ, Zhang J, 2015. Comprehensive, Integrative Genomic Analysis of Diffuse Lower-Grade Gliomas. N Engl J Med 372, 2481–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan KL, Oeltzschner G, Saleh MG, Edden RAE, Barker PB, 2019. Simultaneous editing of GABA and GSH with Hadamard-encoded MR spectroscopic imaging. Magn Reson Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang L, White DW, Gross S, Bennett BD, Bittinger MA, Driggers EM, Fantin VR, Jang HG, Jin S, Keenan MC, Marks KM, Prins RM, Ward PS, Yen KE, Liau LM, Rabinowitz JD, Cantley LC, Thompson CB, Vander Heiden MG, Su SM, 2009. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 462, 739–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhamala E, Abdelkefi I, Nguyen M, Hennessy TJ, Nadeau H, Near J, 2019. Validation of in vivo MRS measures of metabolite concentrations in the human brain. NMR Biomed 32, e4058. [DOI] [PubMed] [Google Scholar]
- Dubbink HJ, Taal W, van Marion R, Kros JM, van Heuvel I, Bromberg JE, Zonnenberg BA, Zonnenberg CB, Postma TJ, Gijtenbeek JM, Boogerd W, Groenendijk FH, Smitt PA, Dinjens WN, van den Bent MJ, 2009. IDH1 mutations in low-grade astrocytomas predict survival but not response to temozolomide. Neurology 73, 1792–1795. [DOI] [PubMed] [Google Scholar]
- Edden RA, Puts NA, Harris AD, Barker PB, Evans CJ, 2014. Gannet: A batch-processing tool for the quantitative analysis of gamma-aminobutyric acid-edited MR spectroscopy spectra. J Magn Reson Imaging 40, 1445–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Habr EA, Dubois LG, Burel-Vandenbos F, Bogeas A, Lipecka J, Turchi L, Lejeune FX, Coehlo PL, Yamaki T, Wittmann BM, Fareh M, Mahfoudhi E, Janin M, Narayanan A, Morvan-Dubois G, Schmitt C, Verreault M, Oliver L, Sharif A, Pallud J, Devaux B, Puget S, Korkolopoulou P, Varlet P, Ottolenghi C, Plo I, Moura-Neto V, Virolle T, Chneiweiss H, Junier MP, 2017. A driver role for GABA metabolism in controlling stem and proliferative cell state through GHB production in glioma. Acta Neuropathol 133, 645–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria AV, Macedo FC Jr., Marsaioli AJ, Ferreira MM, Cendes F, 2011. Classification of brain tumor extracts by high resolution (1)H MRS using partial least squares discriminant analysis. Braz J Med Biol Res 44, 149–164. [DOI] [PubMed] [Google Scholar]
- Gelman SJ, Naser F, Mahieu NG, McKenzie LD, Dunn GP, Chheda MG, Patti GJ, 2018. Consumption of NADPH for 2-HG Synthesis Increases Pentose Phosphate Pathway Flux and Sensitizes Cells to Oxidative Stress. Cell Rep 22, 512–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong T, Harris AD, Oeltzschner G, Puts NAJ, Cecil KM, Wilkinson ID, Edden RAE, Oeltzschner G, 2018a. Hadamard editing of glutathione and macromolecule-suppressed GABA. Magn Reson Med 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong T, Xiang Y, Saleh MG, Gao F, Chen W, Edden RAE, Wang G, 2018b. Inhibitory motor dysfunction in parkinson’s disease subtypes. J Magn Reson Imaging 47, 1610–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu F, Chauhan V, Chauhan A, 2015. Glutathione redox imbalance in brain disorders. Curr Opin Clin Nutr Metab Care 18, 89–95. [DOI] [PubMed] [Google Scholar]
- Hujber Z, Horvath G, Petovari G, Krencz I, Danko T, Meszaros K, Rajnai H, Szoboszlai N, Leenders WPJ, Jeney A, Tretter L, Sebestyen A, 2018. GABA, glutamine, glutamate oxidation and succinic semialdehyde dehydrogenase expression in human gliomas. J Exp Clin Cancer Res 37, 271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulsey KM, Mashimo T, Banerjee A, Soesbe TC, Spence JS, Vemireddy V, Maher EA, Bachoo RM, Choi C, 2015. (1)H MRS characterization of neurochemical profiles in orthotopic mouse models of human brain tumors. NMR Biomed 28, 108–115. [DOI] [PubMed] [Google Scholar]
- Jalbert LE, Elkhaled A, Phillips JJ, Neill E, Williams A, Crane JC, Olson MP, Molinaro AM, Berger MS, Kurhanewicz J, Ronen SM, Chang SM, Nelson SJ, 2017. Metabolic Profiling of IDH Mutation and Malignant Progression in Infiltrating Glioma. Sci Rep 7, 44792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juan ME, Wenzel U, Daniel H, Planas JM, 2008. Resveratrol induces apoptosis through ROS-dependent mitochondria pathway in HT-29 human colorectal carcinoma cells. J Agric Food Chem 56, 4813–4818. [DOI] [PubMed] [Google Scholar]
- Kudo H, Mio T, Kokunai T, Tamaki N, Sumino K, Matsumoto S, 1990. Quantitative analysis of glutathione in human brain tumors. J Neurosurg 72, 610–615. [DOI] [PubMed] [Google Scholar]
- Lai M, Vassallo I, Lanz B, Poitry-Yamate C, Hamou MF, Cudalbu C, Gruetter R, Hegi ME, 2018. In vivo characterization of brain metabolism by (1) H MRS, (13) C MRS and (18) FDG PET reveals significant glucose oxidation of invasively growing glioma cells. Int J Cancer 143, 127–138. [DOI] [PubMed] [Google Scholar]
- Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P, 2007. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114, 97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW, 2016. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 131, 803–820. [DOI] [PubMed] [Google Scholar]
- Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R, 1998. Simultaneous in vivo spectral editing and water suppression. NMR Biomed 11, 266–272. [DOI] [PubMed] [Google Scholar]
- Mikkelsen M, Saleh MG, Near J, Chan KL, Gong T, Harris AD, Oeltzschner G, Puts NAJ, Cecil KM, Wilkinson ID, Edden RAE, 2018. Frequency and phase correction for multiplexed edited MRS of GABA and glutathione. Magn Reson Med 80, 21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moren L, Bergenheim AT, Ghasimi S, Brannstrom T, Johansson M, Antti H, 2015. Metabolomic Screening of Tumor Tissue and Serum in Glioma Patients Reveals Diagnostic and Prognostic Information. Metabolites 5, 502–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeltzschner G, Chan KL, Saleh MG, Mikkelsen M, Puts NA, Edden RAE, 2018. Hadamard editing of glutathione and macromolecule-suppressed GABA. NMR Biomed 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope WB, Prins RM, Albert Thomas M, Nagarajan R, Yen KE, Bittinger MA, Salamon N, Chou AP, Yong WH, Soto H, Wilson N, Driggers E, Jang HG, Su SM, Schenkein DP, Lai A, Cloughesy TF, Kornblum HI, Wu H, Fantin VR, Liau LM, 2012. Non-invasive detection of 2-hydroxyglutarate and other metabolites in IDH1 mutant glioma patients using magnetic resonance spectroscopy. Journal of Neuro-Oncology 107, 197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X, Zou L, Zhang X, Branco V, Wang J, Carvalho C, Holmgren A, Lu J, 2017. Redox Signaling Mediated by Thioredoxin and Glutathione Systems in the Central Nervous System. Antioxid Redox Signal 27, 989–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh MG, Mikkelsen M, 2018. Simultaneous editing of GABA and glutathione at 7T using semi-LASER localization. 80, 474–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh MG, Oeltzschner G, Chan KL, Puts NAJ, Mikkelsen M, Schar M, Harris AD, Edden RAE, 2016. Simultaneous edited MRS of GABA and glutathione. Neuroimage 142, 576–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh MG, Rimbault D, Mikkelsen M, Oeltzschner G, Wang AM, Jiang D, Alhamud A, Near J, Schar M, Noeske R, Murdoch JB, Ersland L, Craven AR, Dwyer GE, Gruner ER, Pan L, Ahn S, Edden RAE, 2019. Multi-vendor standardized sequence for edited magnetic resonance spectroscopy. Neuroimage 189, 425–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanaei Nezhad F, Anton A, Parkes LM, Deakin B, Williams SR, 2017. Quantification of glutathione in the human brain by MR spectroscopy at 3 Tesla: Comparison of PRESS and MEGA-PRESS. Magn Reson Med 78, 1257–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanson M, Marie Y, Paris S, Idbaih A, Laffaire J, Ducray F, El Hallani S, Boisselier B, Mokhtari K, Hoang-Xuan K, Delattre JY, 2009. Isocitrate dehydrogenase 1 codon 132 mutation is an important prognostic biomarker in gliomas. J Clin Oncol 27, 4150–4154. [DOI] [PubMed] [Google Scholar]
- Shi J, Sun B, Shi W, Zuo H, Cui D, Ni L, Chen J, 2015. Decreasing GSH and increasing ROS in chemosensitivity gliomas with IDH1 mutation. Tumour Biol 36, 655–662. [DOI] [PubMed] [Google Scholar]
- Shi J, Zuo H, Ni L, Xia L, Zhao L, Gong M, Nie D, Gong P, Cui D, Shi W, Chen J, 2014a. An IDH1 mutation inhibits growth of glioma cells via GSH depletion and ROS generation. Neurol Sci 35, 839–845. [DOI] [PubMed] [Google Scholar]
- Shi J, Zuo H, Ni L, Xia L, Zhao L, Gong M, Nie D, Gong P, Cui D, Shi W, Chen J, 2014b. An IDH1 mutation inhibits growth of glioma cells via GSH depletion and ROS generation. Neurological Sciences 35, 839–845. [DOI] [PubMed] [Google Scholar]
- Smits A, Jin Z, Elsir T, Pedder H, Nister M, Alafuzoff I, Dimberg A, Edqvist PH, Ponten F, Aronica E, Birnir B, 2012. GABA-A channel subunit expression in human glioma correlates with tumor histology and clinical outcome. PLoS One 7, e37041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Fu X, Liu Y, Yu D, Cai SJ, Yang C, 2020. Blockade of Glutathione Metabolism in IDH1-Mutated Glioma. Mol Cancer Ther 19, 221–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkalp Z, Karamchandani J, Das S, 2014. IDH mutation in glioma: new insights and promises for the future. JAMA Neurol 71, 1319–1325. [DOI] [PubMed] [Google Scholar]
- Walls AB, Waagepetersen HS, Bak LK, Schousboe A, Sonnewald U, 2015. The glutamineglutamate/GABA cycle: function, regional differences in glutamate and GABA production and effects of interference with GABA metabolism. Neurochem Res 40, 402–409. [DOI] [PubMed] [Google Scholar]
- Wen PY, Kesari S, 2008. Malignant gliomas in adults. N Engl J Med 359, 492–507. [DOI] [PubMed] [Google Scholar]
- Yu D, Liu Y, Zhou Y, Ruiz-Rodado V, Larion M, Xu G, Yang C, 2020. Triptolide suppresses IDH1-mutated malignancy via Nrf2-driven glutathione metabolism. Proc Natl Acad Sci U S A 117, 9964–9972. [DOI] [PMC free article] [PubMed] [Google Scholar]


