Abstract
Background:
Cognitive deficits, particularly in processing speed, are widely recognized as a critical feature of schizophrenia, and are also present across schizophrenia spectrum disorders. A number of important confounders, however, such as hospitalization effects and antipsychotic medication, have been shown to affect processing speed, causing debate as to the core cognitive deficits of schizophrenia. The study of individuals who are not clinically psychotic but have schizotypal traits allows investigation of cognitive deficits associated with both positive and negative schizotypy dimensions while excluding potential confounds.
Methods:
A population-based community sample of 242 healthy adult volunteers assessed using the Structured Interview of Schizotypy – Revised (SIS-R) scale, and a neuropsychological testing battery that included measures of verbal ability, visual and verbal memory, verbal fluency, working memory, executive functions and processing speed. Participants were classified in High or Low Positive Schizotypy (H-PST or L-PST), High or Low Paranoia-like traits (H-PAR or L-PAR) and High or Low Negative Schizotypy (H-NST or L-NST) groups, respectively.
Results:
Individuals with H-PST performed significantly (p<0.05) worse than L-PST on measures of processing speed and executive functions. Processing speed deficits were also observed in individuals with H-PAR compared to L-PAR (p<0.05). There were no statistically significant differences in neuropsychological performance between H-NST and L-NST on any measure.
Conclusions:
In a population-based community sample, individuals with high positive schizotypal traits or paranoia-like traits show impairments in processing speed. Consistent with a dimensional view of psychosis, this supports the hypothesis that processing speed represents a core deficit of schizophrenia-like mental states.
Keywords: schizotypy, schizophrenia, neurocognition, processing speed, executive functions
1. Introduction
Impaired neuropsychological function is frequently observed in schizophrenia patients and is characterized by severe deficits in memory, learning, executive functions, attention, and processing speed, which are evident against a background of a generalized cognitive deficit (Dickenson et al., 2007; Zanelli et ah, 2010). Processing speed, as measured by the digit-symbol task in particular, has been consistently shown to be the most impaired cognitive ability in schizophrenia (Dickenson et al., 2007). Schizotypal Personality Disorder (SPD) shares common etiological and clinical features with schizophrenia (Nelson et al., 2013), and similarly, studies examining the neuropsychological characteristics of SPD have reported impairments in executive functions (Voglmaier et al., 2005), working memory (McClure et al., 2007), language and verbal learning, attention, and processing speed (Matsui et al., 2007).
Subtle neuropsychological impairments have also been reported in non-clinical samples of individuals with schizotypal traits (Matheson & Langdon, 2008). Schizotypy is a multidimensional construct, initially defined by Bleuler (1911) who observed the schizophrenia-like sub-clinical symptoms observed in relatives of people with schizophrenia. As such, schizotypal traits are believed to represent a fundamental liability to schizophrenia that underlie a range of clinical manifestations ranging between healthy variation and severe mental illness (Rado, 1960; Lenzenweger et al., 2010). The evidence for neuropsychological impairments in schizotypy is mixed. A mild generalized impairment has been described by some (Kim et al., 2011), and specific neuropsychological deficits have been reported by others e.g. in executive functions (e.g. Chang et al., 2011), working memory processing speed (Hori et al., 2014) and sustained attention (Gooding et al., 1999). A recent systematic review (Siddi et al., 2017) reports that several neurocognitive impairments were related to schizotypy, in particular cognitive flexibility and verbal and visual working memory. Alternatively, different dimensions of schizotypy may be associated with differential profiles of cognitive impairment (Dinn et al., 2002). Executive function and verbal IQ impairments have been shown to be associated with positive schizotypy (Chang et al., 2011; Noguchi et al., 2008), while attention (Sarkin et al., 1998), working memory (Park & McTigue, 1997), and executive function deficits (Daly et al., 2012) have, for example, been linked to negative schizotypy. However, others report that similar cognitive impairments relate to both dimensions suggesting common cognitive factors may be involved in both the positive and negative dimensions of schizotypy (Gooding et al., 1999; Matheson & Langdon, 2008).
While findings which speak to the relationship between specific schizotypy dimensions and cognition are inconsistent, several methodological limitations might contribute to some degree account for this lack of consistency. Studies have predominantly relied on self-report measures of schizotypy (Nelson et al., 2013) such as questionnaires, which are prone to bias such as social desirability bias or subjective interpretation of the questions (Cohen et al., 2014). Studies often used small samples consisting of university students, and a limited neuropsychological assessment battery (Dinn et al., 2002; Daly et al., 2012; Cohen et al., 2014).
Investigating schizotypal traits, or schizotypy, in non-clinical and community samples is valuable because it allows the study of neuropsychological impairment without the presence of potentially confounding factors such as medication, hospitalization and developmental course. In addition, the value of non-clinical, community samples is that they can be informative as to the range of symptoms and associated neuropsychological impairments in the population, including their presence in individuals who do not seek psychiatric care (Gur, 2016).
In the present study we systematically investigated the neuropsychological impairment associated with schizotypy, and tested (a) the presence of a generalized neuropsychological impairment, and (b) if processing-speed represents a core dysfunction - consistent with a robust meta-analytic evidence found in schizophrenia (Dickinson et al., 2007). Three features of the present study are especially important. First, we studied a large community sample. Second, participants were administered a broad neuropsychological assessment enabling detailed comparison of multiple cognitive domains. Finally, schizotypal traits were assessed using a validated structured interview, not self-report or questionnaire measure. Taken together these study characteristics enable more reliable and detailed comparisons and greater generalizability than in previous research.
2. Methods and Materials
The study took place at the Sheba Medical Centre (Tel Aviv, Israel). Ethics committees in Israel and the US approved the study, and each participant gave written informed consent. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
2.1. Participants
Participants were ascertained from a well-defined catchment area in the central coastal area of Israel using methods aimed at limiting sampling bias. Briefly, recruitment procedures adhered to a scientific survey sampling method. Residential telephone numbers in the catchment area were randomly sampled. Research staff members called these numbers, conducted preliminary screening for demographic characteristics, and using a prepared script explaining the purpose of the study, asked whether respondents would consider participating. Persons who passed the initial screening and who were interested in participating were invited for a clinical and neuropsychological assessment. 272 volunteer controls were recruited and consented. Due to incomplete information, 242 participants were included in the final analysis.
2.2. Assessment of Schizotypy
The modified Structured Interview for Schizotypy (SIS-R; Vollema & Ormel, 2000) which is part of the Diagnostic Interview for Genetic Studies was administered to assess schizotypy. Interviewers were psychology graduates trained in SIS-R administration. The modified SIS-R is a reliable measure of 11 schizotypy traits: Ideas of Reference, Suspiciousness, Magical Thinking, Illusions, Psychotic-like Symptoms, Social Isolation, Introversion, Sensitivity, Anger in Response to Perceived Offense, Social Anxiety and Narrow Affect. The scores range from zero (Virtually no evidence of symptoms’) to six (‘symptoms present and quite severe’), where a score of three represents clearly present symptoms with some clinical significance. Studies report that the SIS-R is a valid and reliable measure (Vollema & Ormel, 2000; Tsuang et al., 2002;).
2.3. Neuropsychological assessment
A comprehensive neuropsychological battery was administered. Verbal fluency was assessed using the Delis-Kaplan Executive Function System (D-KEFS; Delis et al., 2001) Verbal Fluency (letter fluency) and Semantic Fluency (category fluency) subtests. Verbal ability was evaluated using the Word-reading and Spelling subtests of the Wide Range Achievement Test (WRAT-IV; Wilkinson & Robertson, 2006) and the Information subtests of the Wechsler Adult Intelligence Scale (WAIS-III; Wechsler, 1997a). Visuospatial problem solving was assessed using the Block Design subtest of the WAIS-III. Working memory was measured using the Wechsler Memory Scale (WMS-III; Wechsler, 1997b) Spatial Span Forward and Backward subtests, WAIS-III Digit Span Forward and Letter-Number Sequencing subtests, Visual Patterns Test (VPT; Della Sala et al., 1997) and Listening Memory Span (LMS; Daneman & Carpenter, 1980) tests. Visual memory was evaluated using WMS-III Visual Reproduction immediate and delayed subtest. Verbal memory was assessed using WMS-IV Logical Memory subtest (immediate, delayed, and recognition scores; Wechsler, 2009) and California Verbal Learning Test (CVLT; Delis et al., 2000) immediate, delayed and recognition conditions. Processing speed was assessed using the Number Sequencing, Letters Sequencing and Motor Speed subcomponents of the D-KEFS Trail Making Test (TMT-2; TMT-3; TMT-5; Delis et al., 2001) and the WAIS-IV Digit Symbol Coding subtest. Executive functioning was measured using category Switching fluency test (total correct response and accuracy), Card Sorting Test (free sorting description score and sort recognition score), Tower test and D-KEFS Trail Making Test Number-Letter shifting test subcomponent (TMT-4; Delis et al., 2001).
2.4. Data Analysis
A principal axis factor analysis with promax rotation was performed with 3 factors (as suggested by eigenvalues>1 and parallel analysis). The three factors were labelled as Negative schizotypy (Narrow Affect, Social Isolation, Introversion), Paranoia-like traits (Sensitivity, Social Anxiety, Anger in Response to Perceived Offense, Ideas of Reference, Suspiciousness) and Positive schizotypy (Magical Thinking, Illusions, Psychotic-Like Symptoms). Participants were classified as having High or Low scores within each of these three groups (based on a sum of scores of all traits within each of the factors). These were split by approximately top third of the scorers versus the others, consistent with quasi-dimensional conceptualisation of schizotypy. The following subgroups were formed: High Positive Schizotypal Traits (H-PST) vs. Low Positive Schizotypal Traits (L-PST), High Paranoia-like Traits (H-PAR) vs. Low Paranoia-like Traits (L-PAR) and High Negative Schizotypal Traits (H-NST) vs. Low Negative Schizotypal Traits (L-NST).
Demographic and other background characteristics of the High and Low scoring individuals within each of the three groups were compared using univariate ANOVA and Chi-square tests. The distributions of raw scores were assessed for normality and outliers. To allow for comparisons between different cognitive measures, neuropsychological test raw scores were converted to standardized Z-scores so that comparisons between different cognitive measures could be made and represents a standard way of reporting across a large cognitive battery.
A repeated-measures analysis of covariance (ANCOVA) was used to compare neuropsychological performance between H-PST and L-PST, H-PAR and L-PAR and between H-NST and L-NST. Neuropsychological tests were the within-subject factor, and schizotypy subgroup was the between-subject factor. Covariates were sex and age. For descriptive purposes, post-hoc univariate ANCOVA was applied to each individual test. Cohen’s d effect sizes were computed based on differences in standardized scores. Effect sizes of 0.2, 0.5 and 0.8 reflect ‘small’, ‘medium’ and ‘large’ effects respectively (Cohen, 1988). Due to the exploratory nature of our analysis and the non-independence of the neurocognitive domains, we also used the False Discovery Rate (FDR) method to control for multiple comparisons. The FDR procedure was carried out separately for each of the three schizotypy factors Following McDonald (2014) results with p-values < 0.05 and q-values < 0.25 were retained as significant.
In addition, in order to attempt to ‘isolate’ the neurocognitive factors associated with scoring highly on each of the positive, negative, and paranoid dimensions separately, a sensitivity analysis was carried out (using ANCOVA). Firstly, we compared neurocognitive performance of individuals who scored low across all three schizotypy domains (Negative, Positive, Paranoia-like traits) with those who scored high on Negative Schizotypy but low on Positive Schizotypy and Paranoia. Secondly, we compared neurocognitive performance of individuals who scored low across all three factors with those who scored high on either Positive Schizotypy or Paranoia (but low on Negative Schizotypy). Both analyses were adjusted for sex and age. Due to limited data, the Positive Schizotypy and Paranoia domain were combined for the purpose of this analysis - consistent with the SIS-R literature (Vollema & Ormel, 2000). Analyses were carried using IBM Statistical Package v22.
3. Results
3.1. Demographic characteristics
The average age of the sample (N=242) was 40.7 (SD=13.5, range 17-65) years of age (35.2% male and 64.8% female) and had on average 14.3 years of education. 13% of sample had at least one schizotypal symptom with clinical significance present (a score of 3 or more). Overall, the most frequently reported schizotypal trait was ‘sensitivity’ (reported by 88% of the sample, 22% above clinical significant level), the least frequently reported trait was ‘ideas of reference’ (reported by 18% of the total sample, 3% above clinical significance).
87 participants (36%) were classified as H-PST, and 155 as L-PST. 81 participants (34%) were classified as H-PAR, 154 as L-PAR. 102 (42%) participants were classified as H-NST, and 139 as L-NST. Demographic characteristics are shown in Table 1.
Table 1.
Demographic characteristics of high vs low schizotypal groups.
| Positive schizotypy (N=242) | Paranoia (N=235) | Negative schizotypy (N=241) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Low (N=155) | High (N=87) | p-value | Low (N=154) | High (N=81) | p-value | Low (N=139) | High (N=102) | p-value | |
| Gender (%Male) | 42.9 | 21.8 | P<0.01 | 37.7 | 25.9 | 0.07 | 34.5 | 35.3 | 0.90 |
| Age (Mean, (SD)) | 40.1(13.3) | 40.4(13.9) | 0.43 | 41.8(14.0) | 38.9(12.4) | 0.06 | 38.3(12.9) | 43.7(14.1) | 0.99 |
| Years of Education (Mean, SD) | 14.3(3.1) | 14.4(13.1) | 0.63 | 14.4 (3.4) | 14. (2.4) | 0.30 | 14.5 (3.2) | 14.2(2.8) | 0.25 |
3.2. Positive Schizotypy and neurocognitive performance
The repeated-measures ANCOVA analysis did not show a statistically significant difference in neuropsychological performance between H-PST and L-PST groups (unadjusted F(30,162)=1.27, p=.311, adjusted F(30,155)=1.23, p=.205). For the group x neuropsychological test interaction term a trend for statistical significance was observed (adj. F(27,158)=1.42, p=.070). Inspection of Figure 1 revealed that effect sizes varied between individual neuropsychological measures. To explore this further, individual ANCOVA comparing high and low positive schizotypy were conducted for each cognitive test. Differences between high and low positive schizotypy were evident for the processing speed domain (Digit symbol: F(1,229)=8.55, p=.004, Cohen’s d=0.27, D-KEFS TMT3: F(1,216)=5.55, p=.019, Cohen’s d=.33) and executive function when measured by D-KEFS TMT4 (F(1,215)=6.41, p=.012, Cohen’s d=.31). These measures also reached the FDR level of significance. There were no statistically significant differences between high and low positive schizotypy groups in any of the measures of working memory, verbal or visual memory, verbal knowledge or verbal fluency.
Figure 1.

Neuropsychological functioning (in standardized scores) of low and high positive schizotypy groups. (L-PST denotes Low Positive Schizotypy group; H-PST denotes High Positive Schizotypy group; * p <0.05)
D-KEFS: Delis-Kaplan Executive Function System; WRAT: Wide Range Achievement Test; WAIS: Wechsler Adult Intelligence Scale; WMS: Wechsler Memory Scale; CVLT: California Verbal Learning Test; TMT: Trails Making Test.
3.3. Paranoia-like traits and neurocognitive performance
Individuals scoring high on paranoia-like traits differed in overall neuropsychological performance compared to those with low paranoia-like traits (unadjusted F(30,154)=1.64, p=0.049, adjusted F(30,148)=1.96, p=.010). A group x neuropsychological test interaction term was also statistically significant (adj. F(27,151)=1.82, p=.013). Individual ANCOVAs showed that high paranoia-like traits were associated with impairments in the processing speed domain (Digit symbol: F(l,223)=6.94, p=.009; Cohen’s d=.27; TMT2: (F(1,216)=4.31, p=.039, Cohen’s d=.24) (Figure 2). However, these measures did not reach the FDR level of significance.
Figure 2.

Neuropsychological functioning (in standardized scores) of low and high negative paranoia groups. (L-PAR denotes Low Paranoia group; H-PAR denotes High Paranoia group; * p <0.05)
D-KEFS: Delis-Kaplan Executive Function System; WRAT: Wide Range Achievement Test; WAIS: Wechsler Adult Intelligence Scale; WMS: Wechsler Memory Scale; CVLT: California Verbal Learning Test; TMT: Trails Making Test.
3.4. Negative Schizotypy and neurocognitive performance
There were no significant differences between the high and low negative schizotypy groups in overall neuropsychological performance (unadjusted F(30,162)=1.11, p=.33), adjusted F(30,156)=1.02, p=.417) (Figure 3). The group x neuropsychological test interaction term was not statistically significant (adj. F(27,159)=1.08, p=.368), and there was no statistically significant group difference observed for any individual test (Figure 3).
Figure 3.
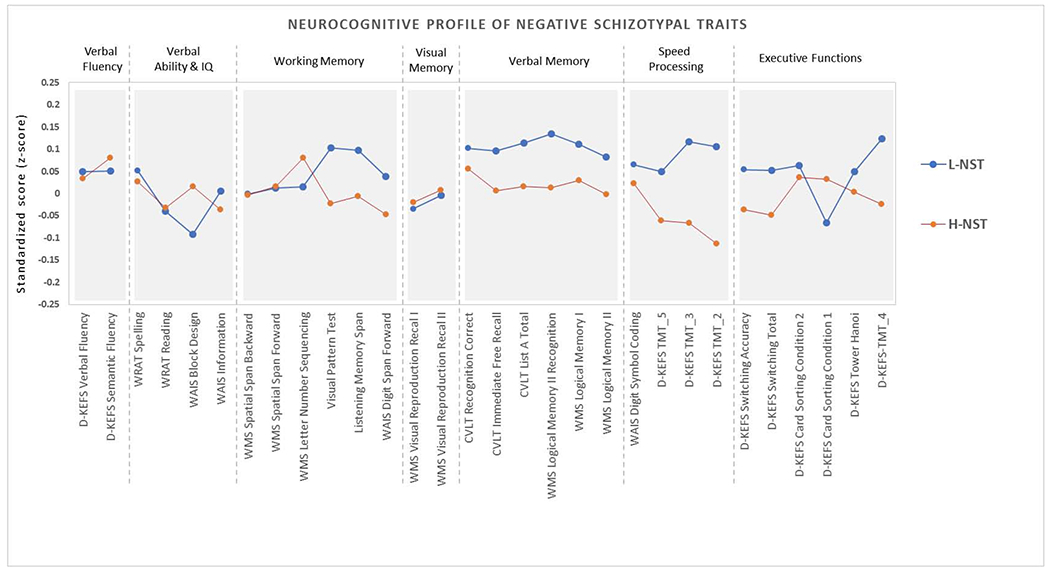
Neuropsychological functioning (in standardized scores) of low and high negative schizotypy groups. (L-NST denotes Low Negative Schizotypy group; H-NST denotes High Negative Schizotypy group; * p <0.05)
D-KEFS: Delis-Kaplan Executive Function System; WRAT: Wide Range Achievement Test; WAIS: Wechsler Adult Intelligence Scale; WMS: Wechsler Memory Scale; CVLT: California Verbal Learning Test; TMT: Trails Making Test.
3.5. Sensitivity analysis
The difference between neurocognitive performance of individuals scoring high on Negative Schizotypy (and low on Positive Schizotypy and Paranoia) compared to group of individuals scoring low across all three domains was not statistically significant (adjusted F(1,78)=1.097, p=.298, Figure 4). The inspection of Figure 4 reveals similar patterns of neurocognitive performance of individuals scoring high on either Positive Schizotypy or Paranoia (and low on Negative Schizotypy) when compared to performance of individuals observed in Figures 1 and 2. However, the difference between high Positive Schizotypy/Paranoia and those individuals scoring low across schizotypy domains was not statistically significant (adj. F(1,81)=0.149, p=.700).
Figure 4.


Neurocognitive functioning (in standardized scores) of individuals who scored low on all three schizotypy domains vs those who a) scored high on Negative Schizotypy and low on Positive Schizotypy and Paranoia, and b) scored high on either Positive Schizotypy or Paranoia and low on Negative Schizotypy. (H-NST denotes High Negative Schizotypy group; H-PPT denotes High Positive Schizotypy or High Paranoia; All Low denotes individuals scoring low across schizotypy domains)
D-KEFS: Delis-Kaplan Executive Function System; WRAT: Wide Range Achievement Test; WAIS: Wechsler Adult Intelligence Scale; WMS: Wechsler Memory Scale; CVLT: California Verbal Learning Test; TMT: Trails Making Test.
Finally, for descriptive purpose we examined whether there was an interaction of gender and schizotypy dimensions on cognitive scores. Results were not statistically significant but for completeness are reported in supplementary materials.
4. Discussion
In this community-based sample, high positive schizotypal traits and paranoia-like traits were associated with neuropsychological impairment, particularly in processing speed, while high negative schizotypal traits were not associated with any cognitive deficits. A generalized deficit was not detected. The current findings are consistent with a recent study (Karagiannopoulou et al., 2016) that reported processing speed deficits in high schizotypy, in particular among individuals with high paranoid schizotypy. In contrast, some previous studies suggested processing speed to be unimpaired in individuals with high schizotypy (Badcock et al., 2105). Discrepancies between studies could be due to methodological differences, including sample size and methods for assessment of schizotypy.
The results highlight similarities, as well as differences, between neuropsychological dysfunction associated with schizotypy and schizophrenia. We found a negative association between positive schizotypy / paranoia-like traits and digit symbol coding test performance. Such an association between dimensions of schizotypy and neuropsychological functioning was not observed for any other domain, suggesting a potential selective impairment in processing speed. Impairments in processing speed have been consistently reported in schizophrenia, for example, two meta-analyses (Dickenson et al., 2007; Henry & Crawford, 2005) indicated that processing speed (as measured by the same (Digit Symbol coding test) is the most severe cognitive impairment in schizophrenia. Processing speed deficits are also a marker of risk as processing speed dysfunction has been found in ultra-high risk groups (Kelleher et al., 2013). Relatives of schizophrenia patients show deficits of similar magnitude in processing speed to the ones observed in this study (Dickenson et al., 2007; Snitz et al., 2006), thus taken together, these findings support the importance of processing speed deficits in schizophrenia and spectrum disorders, and suggests there may be a trait-like quality of digit symbol coding impairment within patients and at-risk individuals (Dickenson et al., 2007). The discrepancy between a substantial coding impairment in schizophrenia and small impairment in positive schizotypy and paranoia, similar to the deficits in processing speed in relatives of schizophrenia patients suggests that schizophrenia may be associated with increasing abnormality in processing speed (Mollon et al., 2016).
In schizophrenia, studies report small to moderate correlations between severity of cognitive impairment and severity of negative symptoms (Rabinowitz et al., 2002). In addition, some studies observed (Dinn et al., 2002; Delawalla et al., 2006) that only negative and disorganized schizotypy, but not positive schizotypy, were related to cognitive impairments. This however was not supported in this study, as we did not find a relationship between negative schizotypy and cognitive impairment. It is also worth noting that these studies had much smaller sample sizes, and one (Dinn et al., 2002) utilised a short self-rating questionnaire to measure schizotypy. Consistent with our present results, Noguchi et al. (2008) did not find an association between cognitive functioning and negative schizotypy.
Whilst this study has a number of strengths, including the use of a structured interview to measure schizotypy and a community sample, which allows for greater generalizability on the relation between schizotypy and associated neuropsychological impairments in the population, it also has some potential limitations. One potential limitation of the present study is the differences in gender distribution in high and low schizotypy groups. However, there is some evidence that individuals with high schizotypy load performed remarkably similar across genders (Dinn et al., 2002). Gender analyses are included in supplementary materials however with low sample sizes these analyses are tentative though may inform future research. Also, the current results were similar with or without adjustment for age and sex.
Another potential limitation is that many of the included measures are from the D-KEFS (Delis et al., 2001). However, there was considerable variability in the effect sizes observed for different measures from the D-KEFS, including for multiple measurements from the same test, which provides evidence that the observed results are not due to measurement selection only. It is also worth noting that the primary analyses examined specific trait factors/dimensions in isolation. However, in the sensitivity analysis, which compared participants who scored low on all dimensions to those who scored high specifically on positive/paranoid schizotypy, whilst group differences were non-significant, they showed a very similar pattern and magnitude of impairment. These deficits are though significantly different and demonstrated more clearly in the more statistically-powered primary analysis.
Lastly, drug use (Thames et al., 2014) and affective states (e.g. depression, anxiety; Carrigan & Barkus, 2017) have been found to impact neurocognitive functioning, but such data was not available for our analysis. Finally, it is possible that in a different measure of schizotypy the neuropsychological impairment may be characterized differently. The version of the SIS-R instrument used here has also replaced a 7-point scale with a revised 4-point scale for increased reliability of ratings.
In summary, in a population based community sample we found that positive schizotypy and paranoia-like schizotypal traits are associated with a cognitive impairment in processing speed, supporting the hypothesis that processing speed impairment may be a core deficit associated with schizophrenia-like mental states.
Supplementary Material
Acknowledgements
We thank all our participants for taking time to participate in this research.
Funding body agreements and policies
This research was supported by The National Institute of Mental Health (NIMH) grant MH066105 and by the UK Department of Health via the National Institute for Health Research (NIHR) Specialist Biomedical Research Centre for Mental Health award to South London and Maudsley NHS Foundation Trust (SLaM) and the Institute of Psychiatry, Psychology and Neuroscience at King’s College, London. The work was also supported, in part, by the Beatrice and Samuel L Seaver Foundation. Dr. Velikonja is a Seaver Post Doctoral Fellow.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
All authors report no conflicts of interest.
References
- Badcock JC, Clark ML, Pedruzzi RA, Morgan VA, Jablensky A, 2015. Intact speed of processing in a community-based sample of adults with high schizotypy: a marker of reduced psychosis risk? Psychiatry Res. 228(3) 531–7. [DOI] [PubMed] [Google Scholar]
- Bleuler E, 1911. Dementia Praecox oder Gruppe der Schizophrenien. Deuticke, Leipzig, Germany. [Google Scholar]
- Carrigan N, Barkus E, 2017. Schizotypy and cognitive failures: a mediating role for affect. Psychopathology 50(3) 195–202. [DOI] [PubMed] [Google Scholar]
- Chang TG, Lee IH, Chang CC, Yang YK, Huang SS, Chen KC, Wang CH, Chang YH, 2011. Poorer Wisconsin card-sorting test performance in healthy adults with higher positive and negative schizotypal traits. Psy. Clin. Neurosci 65(6) 596–9. [DOI] [PubMed] [Google Scholar]
- Cohen J, 1988. Statistical Power Analysis For The Behavioral Sciences, third ed. New York: Academic Press. [Google Scholar]
- Cohen AS, Auster TL, MacAulay RK, McGovern JE, 2014. Illusory superiority and schizotypal personality: Explaining the discrepancy between subjective/objective psychopathology. Person. Disord. 5(4) 413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly MP, Afroz S, Walder DJ, 2012. Schizotypal traits and neurocognitive functioning among nonclinical young adults. Psychiatry Res. 200(2-3) 635–40. [DOI] [PubMed] [Google Scholar]
- Daneman M, Carpenter PA, 1980. Individual differences in working memory and reading. J. Verb. Learn. Verb Behav. 19(4) 450–66. [Google Scholar]
- Delawalla Z, Barch DM, Fisher Eastep JL, Thomason ES, Hanewinkel MJ, Thompson PA, 2006. Factors mediating cognitive deficits and psychopathology among siblings of individuals with schizophrenia. Schiz. Bull. 32(3) 525–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA, 2000. Manual for the California Verbal Learning Test, (CVLT-II). San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Delis DC, Kaplan E, Kramer J, 2001. Delis-Kaplan Executive Function Scale. San Antonio: The Psychological Corporation. [Google Scholar]
- Della Sala S, Gray C, Baddeley A, and Wilson JTL (1997). Visual patterns test: a test of short-term visual recall. Thames Valley Test Company. [Google Scholar]
- Dickinson D, Ramsey ME, Gold JM, 2007. Overlooking the obvious: a meta-analytic comparison of digit symbol coding tasks and other cognitive measures in schizophrenia. Arch. Gen. Psy. 64(5) 532–42. [DOI] [PubMed] [Google Scholar]
- Dinn WM, Harris CL, Aycicegi A, Greene P, Andover MS, 2002. Positive and negative schizotypy in a student sample: neurocognitive and clinical correlates. Schiz. Res 56(1-2) 171–85. [DOI] [PubMed] [Google Scholar]
- Gooding DC, Kwapil TR, Tallent KA, 1999. Wisconsin Card Sorting Test deficits in schizotypic individuals. Schiz. Res 40(3) 201–9. [DOI] [PubMed] [Google Scholar]
- Gur RC, 2016. Prospective Community Studies Linking Cognitive Deficits to Subclinical Symptoms and a Step Toward Precision Medicine. JAMA Psych. 73(2) 109–10. [DOI] [PubMed] [Google Scholar]
- Henry J Crawford J, 2005. A meta-analytic review of verbal fluency deficits in schizophrenia relative to other neurocognitive deficits. Cogn. Neuropsychiatry 10(1) 1–33. [DOI] [PubMed] [Google Scholar]
- Hori H, Teraishi T, Sasayama D, Matsuo J, Kinoshita Y, Ota M, Hattori K, Kunugi H, 2014. A latent profile analysis of schizotypy, temperament and character in a nonclinical population: association with neurocognition. J. Psychiatry Res. 48(1) 56–64. [DOI] [PubMed] [Google Scholar]
- Karagiannopoulou L, Karamaouna P, Zouraraki C, Roussos P, Bitsios P, Giakoumaki SG, 2016). Cognitive profiles of schizotypal dimensions in a community cohort: Common properties of differential manifestations. J. Clin Exp. Neuropsy. 38(9) 1050–63. [DOI] [PubMed] [Google Scholar]
- Kelleher I, Murtagh A, Clarke MC, Murphy J, Rawdon C, Cannon M, 2013. Neurocognitive performance of a community-based sample of young people at putative ultra high risk for psychosis: support for the processing speed hypothesis. Cogn. Neuropsychiatry 18(1–2) 9–25. [DOI] [PubMed] [Google Scholar]
- Kim M-S., Oh SH, Hong M-H, Choi DB, 2011. Neuropsychologic profile of college students with schizotypal traits. Compreh. Psych. 52(5) 511–6. [DOI] [PubMed] [Google Scholar]
- Lenzenweger MF, 2010. Schizotypy and Schizophrenia. Guilford Press, New York. [Google Scholar]
- Matheson S, Langdon R, 2008. Schizotypal traits impact upon executive working memory and aspects of IQ. Psychiatry Res. 159(1–2) 207–14. [DOI] [PubMed] [Google Scholar]
- Matsui M, Yuuki H, Kato K, Takeuchi AI, Nishiyama S, Bilker WB, Kurachi M, 2007. Schizotypal disorder and schizophrenia: a profile analysis of neuropsychological functioning in Japanese patients. J. Int. Neuropsy. Soc. 13(4) 672–82. [DOI] [PubMed] [Google Scholar]
- McClure MM, Romero MJ, Bowie CR, Reichenberg A, Harvey PD, Siever LJ, 2007. Visual-spatial learning and memory in schizotypal personality disorder: continued evidence for the importance of working memory in the schizophrenia spectrum. Arch. Clin. Neuropsy 22(1) 109–16. [DOI] [PubMed] [Google Scholar]
- McDonald JH, 2014. Handbook of Biological Statistics, 3rd Edn Sparky House Publishing. Baltimore, MD: sparky house publishing. [Google Scholar]
- Mollon J, David AS, Morgan C, Frissa S, Glahn D, Pilecka I, 2016. Psychotic experiences and neuropsychological functioning in a population-based sample. JAMA Psych. 73(2) 129–38. [DOI] [PubMed] [Google Scholar]
- Nelson MT, Seal ML, Pantelis C, Phillips LJ, 2013. Evidence of a dimensional relationship between schizotypy and schizophrenia: a systematic review. Neurosci. Biobehav. Rev 37(3) 317–27. [DOI] [PubMed] [Google Scholar]
- Noguchi H, Hori H, Kunugi H, 2008. Schizotypal traits and cognitive function in healthy adults. Psychiatry. Res. 161(2) 162–9. [DOI] [PubMed] [Google Scholar]
- Park S, McTigue K, 1997. Working memory and the syndromes of schizotypal personality. Schiz. Res. 26(2-3) 213–20. [DOI] [PubMed] [Google Scholar]
- Rabinowitz J, De Smedt G, Harvey PD, Davidson M, 2002. Relationship between premorbid functioning and symptom severity as assessed at first episode of psychosis. Am. J. Psy. 159(12) 2021–6. [DOI] [PubMed] [Google Scholar]
- Rado S, 1960. Theory and therapy: The theory of schizotypal organization and its application to the treatment of decompensated schizotypal behaviour In The outpatient treatment of schizophrenia, Scher SC, & Davis HR,( eds.). Grune and Stratton, New York, pp. 87–101. [Google Scholar]
- Sarkin AJ, Dionisio DP, Hillix WA, Granholm E, 1998. Positive and negative schizotypal symptoms relate to different aspects of crossover reaction time task performance. Psychiatry. Res 81(2) 241–9. [DOI] [PubMed] [Google Scholar]
- Siddi S, Petretto DR, Preti A, 2017. Neuropsychological correlates of schizotypy: a systematic review and meta-analysis of cross-sectional studies. Cogn Neuropsychiatry 22(3) 186–212. [DOI] [PubMed] [Google Scholar]
- Snitz BE, MacDonald AW, Carter CS, 2006. Cognitive deficits in unaffected first-degree relatives of schizophrenia patients: a meta-analytic review of putative endophenotypes. Schiz. Bull. 32(1) 179–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thames AD, Arbid N, Sayegh P, 2014. Cannabis use and neurocognitive functioning in a non-clinical sample of users. Addict. Behav 39(5) 994–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuang MT, Stone WS, Tarbox SI, & Faraone SV, 2002. An integration of schizophrenia with schizotypy: identification of schizotaxia and implications for research on treatment and prevention. Schiz Res. 54(1-2) 169–175. [DOI] [PubMed] [Google Scholar]
- Voglmaier MM, Seidman LJ, Niznikiewicz MA, Dickey CC, Shenton ME, McCarley RW, 2005. A comparative profile analysis of neuropsychological function in men and women with schizotypal personality disorder. Schiz. Res. 74(1) 43–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollema MG, Ormel J, 2000. The reliability of the structured interview for schizotypy-revised. Schiz. Bull 26(3) 619–629. [DOI] [PubMed] [Google Scholar]
- Wechsler D, 1997. WAIS-III administration and scoring manual. The Psychological Corporation, San Antonio, TX. [Google Scholar]
- Wechsler D, 1997. Wechsler Memory Scale, third Edition The Psychological Corporation, San Antonio, TX. [Google Scholar]
- Wechsler D, 2009. Wechler Memory Scale, fourth edition (WMS-IV UK) Administration and Scoring Manual. The Psychological Corporation, Pearson, Inc; San Antonio, TX. [Google Scholar]
- Wilkinson GS, Robertson GJ, 2006. Wide Range Achievement Test 4 (WRAT4). Psychological Assessment Resources. [Google Scholar]
- Zanelli J, Reichenberg A, Morgan K, Fearon P, Kravariti E, Dazzan P, Morgan C, Zanelli C, Demjaha A, Jones PB, Doody GA, 2010. Specific and generalized neuropsychological deficits: a comparison of patients with various first-episode psychosis presentations. Am. J. Psy. 167(1) 78–85. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


