Abstract
Functional neuroimaging research has consistently associated brain structures within the default mode network (DMN) and frontoparietal network (FPN) with mind-wandering. Targeted lesion research has documented impairments in mind-wandering after damage to the medial prefrontal cortex (mPFC) and hippocampal regions associated with the DMN. However, no lesion studies to date have applied lesion network mapping to identify common networks associated with deficits in mind-wandering. In lesion network mapping, resting-state functional connectivity data from healthy participants are used to infer which brain regions are functionally connected to each lesion location from a sample with brain injury. In the current study, we conducted a lesion network mapping analysis to test the hypothesis that lesions affecting the DMN and FPN would be associated with diminished mind-wandering. We assessed mind-wandering frequency on the Imaginal Processes Inventory (IPI) in participants with brain injury (n = 29) and healthy comparison participants without brain injury (n = 19). Lesion network mapping analyses showed the strongest association of reduced mind-wandering with the left inferior parietal lobule within the DMN. In addition, traditional lesion symptom mapping results revealed that reduced mind-wandering was associated with lesions of the dorsal, ventral, and anterior sectors of mPFC, parietal lobule, and inferior frontal gyrus in the DMN (p < 0.05 uncorrected). These findings provide novel lesion support for the role of the DMN in mind-wandering and contribute to a burgeoning literature on the neural correlates of spontaneous cognition.
Keywords: default mode network, frontoparietal network, lesion network mapping, mind-wandering, self-generated cognition, spontaneous thought
1 |. INTRODUCTION
In our daily lives–whether we are driving to work, riding on a bus, or reading a book–our minds are often occupied by a continuous flow of thoughts. Researchers estimate that adults spend as much as 25%-50% of their waking day engaged in this experience of mind-wandering (Kane et al., 2007; Killingsworth & Gilbert, 2010). The term mind-wandering has been used by various authors to describe several partially distinct but overlapping processes, including task-unrelated, stimulus independent, and intentional or unintentional thoughts (Sell, Kane, Smallwood, et al., 2018; Smallwood & Schooler, 2015). Although debate remains regarding the precise definition of mind-wandering (Christoff et al., 2018; Seli, Kane, Metzinger, et al., 2018), in the current study we concentrate on task-unrelated thought, or the process by which one’s attention turns inward to focus on self-generated thoughts or feelings instead of stimuli in the external world (Killingsworth & Gilbert, 2010; Smallwood & Schooler, 2015). Empirical research on mind-wandering has concentrated on the negative consequences of mind-wandering related to cognition and mood, such as the association between frequent mind-wandering and impaired driving ability (He, Becic, Lee, & McCarley, 2011; Yanko & Spalek, 2013), diminished reading comprehension (Franklin, Broadway, Mrazek, Smallwood, & Schooler, 2013), decreased academic performance (Foulsham, Farley, & Kingstone, 2013), and negative mood (Killingsworth & Gilbert, 2010; Smallwood, O’Connor, Sudbery, & Obonsawin, 2007). Other studies suggest that mind-wandering may also have benefits, such as enhancing creative problem-solving (Baird et al., 2012; Leszczynski et al., 2017; Mooneyham & Schooler, 2013; Smallwood & Schooler, 2015). Given the high frequency of mind-wandering and its relationship with negative and positive consequences, it may be important to understand the neural systems necessary for mind-wandering.
Functional neuroimaging research has consistently associated mind-wandering with the default mode network (DMN), comprised of a set of brain regions including the medial prefrontal cortex (mPFC), posterior cingulate cortex, posterior inferior parietal lobule (pIPL/angular gyrus), lateral temporal cortex, hippocampus, and parahippocampal gyrus (Andrews-Hanna, Reidler, Huang, Reidler, Huang, & Buckner, 2010; Christoff, Gordon, Smallwood, Smith, & Schooler, 2009; Fox, Spreng, Ellamil, Andrews-Hanna, & Christoff, 2015; Mason et al., 2007; McKiernan, D’Angelo, Kaufman, & Binder, 2006; Stawarczyk, Majerus, Maquet, & D’Argembeau, 2011; Stawarczyk & D’Argembeau, 2015). For example, a functional magnetic resonance imaging (fMRI) study found a correlation between greater self-reported mind-wandering frequency and elevated activity in DMN regions, including the mPFC and posterior cingulate cortex, during practiced relative to novel working memory tasks (Mason et al., 2007). Another fMRI study reported increased activity in DMN regions during the periods of mind-wandering using an experience sampling mind-wandering task in the scanner (Christoff et al., 2009). More recent research indicates that resting-state functional connectivity (rsFC) between the hippocampus and other regions of the DMN may facilitate mind-wandering (Karapanagiotidis, Bernhardt, Jefferies, & Smallwood, 2017; Kucyi & Davis, 2014) and determine the content and form of mind-wandering episodes (Smallwood et al., 2016). Beyond the DMN, functional neuroimaging studies have also implicated the frontoparietal network (FPN), including the dorsolateral prefrontal cortex and anterior inferior parietal lobule (supramarginal gyrus), in mind-wandering (Christoff et al., 2009; Fox et al., 2015 for review). Brain regions within the FPN contribute to goal-directed cognition and through interactions with the DMN may direct attention to select and monitor internal thoughts (Fox et al., 2015; Spreng, Sepulcre, Turner, Stevens, & Schacter, 2013; Spreng, Stevens, Chamberlain, Gilmore, & Schacter, 2010).
While existing functional neuroimaging research has identified brain regions within the DMN and FPN that are active or coactive during mind-wandering, complementary human lesion work is also essential to help determine whether those brain regions are necessary for mind-wandering. Several lesion studies have implicated the mPFC and other regions within the DMN in self-related memory processes such as autobiographical memory, future thinking, and the self-reference effect (Bertossi, Aleo, Braghittoni, & Ciaramelli, 2016; Bertossi, Candela, Candela, De Luca, & Ciaramelli, 2017; Philippi, Duff, Denburg, Tranel, & Rudrauf, 2011; Philippi, Tranel, Duff, & Rudrauf, 2015). However, to our knowledge, only two focal lesion studies have been conducted that explicitly investigate mind-wandering after brain injury in humans (Bertossi & Ciaramelli, 2016; McCormick, Rosenthal, Miller, & Maguire, 2018; but see also Robertson, Manly, Andrade, Baddeley, & Yiend, 1997). Bertossi and Ciaramelli (2016) found significantly diminished mind-wandering frequency in individuals with ventral mPFC damage during three behavioral tasks and a self-report measure of mind-wandering as compared with other brain-injured and healthy control participants. In a separate study, McCormick and colleagues (2018) reported alterations in the content of mind-wandering after bilateral hippocampal damage, with episodes characterized by reduced episodic detail and visual imagery and a greater focus on the present than control participants (McCormick et al., 2018). These studies were important in establishing the critical role of the ventral mPFC and hippocampus in the frequency and content of mind-wandering. However, it is not known whether mind-wandering would be impaired by lesions to other key nodes of the DMN, such as the pIPL/angular gyrus. Another unanswered question is whether disrupted network connectivity within the DMN or FPN following focal brain lesions may contribute to mind-wandering deficits. A promising new method called lesion network mapping can be used to identify common brain networks affected by focal lesions in different locations that may result in the same neurological, psychiatric, or cognitive symptoms (Boes et al., 2015; Fox, 2018; Sutterer et al., 2016). This method makes use of rsFC data from healthy participants to infer which brain regions are functionally connected to each lesion location from a sample of individuals with brain injury. As compared with traditional lesion symptom mapping, one advantage of lesion network mapping is that it can be used to identify an association between a functional brain network and a lesion syndrome, such as impaired mind-wandering, even if the lesions themselves occur at different nodes within the network. However, no lesion network mapping studies have yet investigated the common lesion-derived networks related to impaired mind-wandering.
To address this knowledge gap, we performed a lesion network mapping analysis to identify brain regions and common networks associated with deficits in mind-wandering frequency. A traditional lesion-symptom mapping approach was also utilized. We predicted that lesions affecting the DMN and FPN would be related to reduced mind-wandering.
2 |. METHODS AND MATERIALS
2.1 |. Participants
Participants with brain injury (n = 29) and healthy comparison participants without brain injury (n = 19) took part in the present study. See Table 1 for participant characteristics. All brain-injured patients had stable (nonprogressive) and circumscribed brain lesions and were from the Iowa Patient Registry (n = 23) or from an Italian patient sample (n = 6; see Bertossi & Ciaramelli, 2016 for a detailed description of these mPFC lesion patients). The Iowa lesion participants were characterized neuropsychologically and neuroanatomically in the chronic epoch (≥3 months post lesion onset) according to the standard protocols of the Benton Neuropsychology Laboratory (Tranel, 2009) and the Laboratory of Human Neuroanatomy and Neuroimaging (Damasio & Damasio, 1989; Frank, Damasio, & Grabowski, 1997). Participants with any psychiatric disorders or other neurological illnesses were excluded. For the entire sample of 29 lesion participants, etiology included the following: stroke (n = 12 hemorrhagic or ischemic; n = 9 anterior communicating artery rupture or subarachnoid hemorrhage), surgical resection (n = 4 benign tumor; n = 2 arteriovenous malformation; n = 1 epilepsy), and focal contusion from head trauma (n = 1). Healthy comparison participants were recruited from the local community in Iowa to be matched in average age and education to the lesion patients (Table 1). All participants gave informed consent according to a protocol approved by the institutional review board at the University of Iowa or the Ethical Committee of the Department of Psychology at the University of Bologna.
TABLE 1.
Participant group characteristics
| Group | n | Age (Years) | Educ. (Years) | Sex | Chron. (Months) | Lat. | Lesion Volume (mm3) | TMT-A | MI | Rey AVLT: T5/Recall | Token test | BDI | IPI (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BDa | 29 | 60.0 (11.9) | 13.4 (2.2) | 10F/19M | 37.0 (43.3) | 7L/9R/13B | 56,507 (54,627) | 34.6 (13.2) | 100.1 (14.7) | 12.2 (2.4)/9.5 (3.2) | 41.6 (4.3) | 5.8 (5.3) | 52 (19) |
| NC | 19 | 63.6 (7.1) | 14.6 (1.9) | 13F/6M | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 65 (18) |
Notes: Means and standard deviations (in parentheses) are reported for demographic and neuropsychological variables. Note, neuropsychological variables and lesion-specific measures (laterality and chronicity) were only available for the lesion patient groups. There were no significant differences between NC and BD groups in age (t(46) = 1.31, p = 0.20) or education (t(46) = 1.83, p = 0.07), but there was a significant difference in sex (p = 0.02).
Abbreviations/variable descriptions: B, bilateral; BD, participants with brain injury; BDI, Beck Depression Inventory-II; Chron., chronicity or time between lesion onset and experimental testing in months; F, female; IPI, Imaginal Processes Inventory–daydreaming subscale percentage score; L, left; Lat., laterality of lesion; M, male; N/A, not applicable; NC, healthy comparison; R, right.
Neuropsychological data were not available for all mPFC participants from Bertossi and Ciaramelli (2016). Specifically, these participants were missing data for: TMT-A (n = 2); WMI (n = 6); Rey AVLT T5/Recall (n = 6); Token test (n = 3); BDI (n = 1).
2.2 |. Lesion mapping procedures
All participants with brain damage underwent structural scanning procedures. For Iowa participants, magnetic resonance imaging (MRI) scans were acquired in a 1.5-T General Electric Signa scanner with a 3-D Spoiled Gradient Echo sequence (1.5 mm contiguous T1-weighted coronal cuts). Computerized axial tomography (CT) data were collected for those subjects who were unable to undergo MRI scanning. Lesion maps were generated using the MAP-3 method (Fiez, Damasio, & Grabowski, 2000; Frank et al., 1997), in which boundaries of the lesions of a given subject are visually identified on MRI or CT scans and manually transferred onto a normal reference brain (PC local standard space, resolution, 0.94 × 0.94 × 1.6 mm) based on the delineation of homologous landmarks. This mapping procedure requires anatomical expertise but circumvents the problems of interindividual registration encountered with lesion data and the problems of combining subjects scanned with different imaging modalities. Lesion delineation and transfer were done using Brainvox (Frank et al., 1997).
All Iowa participant lesion masks were warped and resampled from PC local standard space to Montreal Neurological Institute (MNI-152) template space (resolution, 1 mm3) using a Symmetric Normalization algorithm in ANTS (Avants & Gee, 2004; as in Sutterer et al., 2016). For participants from Italy, MRI or CT scanning was performed and lesions were mapped using MRICron (Bertossi & Ciaramelli, 2016). All lesions were also aligned to same MNI-152 template space (as described above).
2.3 |. Mind-wandering frequency
Given that previous neuroimaging research has reported robust associations between self-reported mind-wandering frequency and activity in DMN regions (Mason et al., 2007; Wang et al., 2009) and lesion studies have documented reduced mind-wandering after mPFC damage (Bertossi & Ciaramelli, 2016), we chose to administer the same daydreaming scale from the IPI (Singer & Antrobus, 1972) that was used in those studies. The daydreaming scale from the IPI is a 12-item questionnaire. All questions from this scale require multiple choice answers, with five possible answers for each question. For example, one question states, “Recalling things from my past, thinking of the future, or imagining unusual kinds of events occupies […]”. The total possible score on the daydreaming scale is 60; higher scores indicate greater daydreaming frequency. A daydreaming percentage score was used for each subject (e.g., 50% for an IPI score = 30/60).
2.4 |. Neuropsychological variables
All Iowa patients with brain injury were tested on various neuropsychological measures including processing speed (Trail Making Test Part A, TMT-A), working memory (Wechsler Memory Scale-Third Edition: working memory index reported), verbal memory (Rey Auditory Verbal Learning Test, AVLT: scores for trial 5 and 30-min delayed recall reported), language (Token Test score from the Multilingual Aphasia examination reported), and general mood (Beck Depression Inventory-ll, total raw score reported). Some neuropsychological data were missing for mPFC participants from Bertossi and Ciaramelli (2016). Specifically, these participants were missing data for: TMT-A (n = 2); WMI (n = 6); Rey AVLT T5/Recall (n = 6); Token test (n = 3); BDI (n = 1). These neuropsychological variables were selected to examine potential relationships between processing speed, working memory, verbal memory, language or mood and mind-wandering performance in the present study. Since five participants were missing WAIS-III working memory index scores, WAIS-IV working memory index scores were used instead. In addition, the same five participants were missing language scores for the Token test. Notably, all other evaluations indicated that these participants had normal language comprehension (Token test scores = 44, 42, 40, 44, 44 as estimated by a board-certified neuropsychologist).
2.5 |. Lesion overlap map
To visualize the distribution of lesion locations across our sample, we generated a lesion overlap map by summing the binarized lesion masks for all brain-injured participants (Lesion overlap map; Figure 1).
FIGURE 1.

Lesion overlap map for brain-injured participants. Lesion overlap map displays lesions for all brain-injured participants. Color bars show the number of overlapping lesions from 0 to 10. The map is displayed on the MNI-152 template
2.6 |. Lesion network mapping analysis
We first evaluated the functional connectivity network derived from the lesion location, which was represented as a three-dimensional lesion volume, or mask. This analysis relied on a recently developed method that infers the lesion-associated networks using normative resting-state functional MRI (rs-fMRI) data (Lesion network mapping analysis; Boes et al., 2015; Fischer et al., 2016). A publicly available rs-fMRI data set was used that included 98 healthy right-handed subjects (age 22 ± 3.2 years, 50 females) (Buckner, Roffman, & Smoller, 2014), as used previously for similar analyses (Boes et al., 2015; Fischer et al., 2016). Rs-fMRI data were processed in accordance with the strategy of Fox et al., (2005) as implemented in Van Dijk et al., (2009) and described in detail elsewhere (Fox, Buckner, White, Greicius, & Pascual-Leone, 2012). Briefly, subjects completed two 6.2-min rs-fMRI scans during which they were asked to rest in the scanner (3T, Siemens) with their eyes open (TR = 3,000 ms,TE = 30 ms, FA = 85°, 3 × 3 × 3 mm voxels, FOV = 216,47 axial slices with interleaved acquisition and no gap). Functional data were acquired at 3 × 3 × 3 mm voxel size and spatially smoothed using a Gaussian kernel of 4 mm full-width at half-maximum. The data were spectrally filtered (0.009 Hz < f < 0.08 Hz) and several nuisance variables were removed by regression, including: (a) six movement parameters computed by rigid body translation and rotation during preprocessing, (b) mean whole brain signal, (c) mean brain signal within the lateral ventricles, and (d) the mean signal within a deep white matter ROI. Inclusion of the first temporal derivatives of these regressors within the linear model accounted for the time-shifted versions of spurious variance.
After preprocessing, we performed lesion network mapping to identify the lesion-associated networks for all participants with brain injury (n = 29). For each participant, the lesion mask was used as a seed region to calculate rsFC in the healthy participant sample with rs-fMRI data (n = 98). To compute rsFC, the mean BOLD time series for each lesion mask seed region was correlated with all other voxels in the brain for all healthy participants, yielding 98 rsFC maps of voxelwise correlation values. The 98 rsFC maps (transformed to Z-scores) were then thresholded to examine only positive correlations (Z-scores) and entered into a voxelwise one-sample t test (FLAME in FSL). Similar to a previous study (Albazron et al., 2019), the Z-score maps associated with each lesion were thresholded at a value of 8 and binarized. This lesion network mapping analysis resulted in binarized lesion-associated network maps for all brain-injured participants (n = 29).
In order to identify networks associated with reduced mind-wandering in our brain-injured participants, we performed a voxel-based lesion network mapping analysis using npm (Rorden, Karnath, & Bonilha, 2007; https://people.cas.sc.edu/rorden/mricron/stats.html). We used the voxelwise Brunner and Munzel test with binary lesion-associated network masks and continuous self-reported trait daydreaming scores from each of our brain-injured participants. In contrast to the lesion symptom mapping analysis, this test shows the lesion-associated networks that are related to reduced trait daydreaming. We also performed a power analysis to evaluate our ability to detect significant associations based on the lesion-derived network distribution of the sample. Power maps were family-wise error (FWE) corrected at a threshold of pFWE < 0.05 (Figure S1).
2.7 |. Lesion symptom mapping analysis
In order to identify brain regions associated with reduced mind-wandering in our brain-injured participants, we then performed a voxel-based lesion symptom mapping analysis with the nonparametric mapping program (npm) available within MRIcron (Rorden et al., 2007; https://people.cas.sc.edu/rorden/mricron/stats.html). Specifically, we used the voxelwise Brunner and Munzel test with binary lesion masks and continuous self-reported trait daydreaming scores from each of our brain-injured participants. The results of this test show the lesioned brain regions that are related to reduced trait daydreaming. We also performed a power analysis to evaluate our ability to detect significant lesion-deficit associations based on the lesion distribution of the sample. Power maps were FWE corrected at a threshold of pFWE < 0.05 (Figure S2).
3 |. RESULTS
3.1 |. Demographic and neuropsychological variables
Within the brain-injured group, there were no significant correlations between self-reported trait daydreaming and demographic or neuropsychological variables (ps = 0.06–0.52), except for working memory index scores (r = 0.46, p = 0.03; see below for a followup analysis of working memory). There were no significant differences between male and female participants in trait daydreaming (t(27) = 0.11, p = 0.91). As noted earlier, neuropsychological data were not available for all of the mPFC lesion participants (n = 6) from the study by Bertossi and Ciaramelli (2016; Table 1). Lastly, there was no significant correlation between total lesion volume and daydreaming across participants (r = −0.27, p = 0.15).
3.2 |. Lesion overlap map
The lesion overlap map shows the distribution of brain regions damaged across all brain-injured participants (Figure 1). Lesion coverage across the sample overlapped with several brain structures within the DMN and FPN, including dorsal and ventral mPFC, lateral prefrontal cortex, and IPL (both anterior and posterior regions). There were also participants with lesions outside of these networks, such as the visual cortex.
3.3 |. Lesion network mapping analysis
We performed a voxel-based lesion network mapping analysis to investigate the lesion-associated networks related to diminished trait daydreaming. Consistent with our prediction, reduced self-reported trait daydreaming was associated with connectivity with brain regions located primarily within the DMN, with the strongest effects in the IPL (angular gyrus), middle frontal gyrus, inferior frontal gyrus, middle temporal gyrus, ventral and dorsal mPFC, precuneus, and parahippocampal gyrus (p < 0.05, uncorrected; Figure 2, Table 2).There were also smaller clusters of lesion-associated network effects that occur within the FPN and in regions outside of these networks including thalamus and dorsal striatum (Figure 2, Table 2). However, only the findings in the left IPL within the DMN survived multiple comparisons correction (pFWE < 0.05).
FIGURE 2.

Voxel-based lesion network mapping results. Voxel-based lesion network mapping results from the Brunner and Munzel statistical test are displayed for self-reported trait daydreaming and are thresholded at p < 0.05 uncorrected. Results indicate that those lesion-associated networks that are associated with reduced trait daydreaming scores. Findings are displayed on the MNI-152 template
TABLE 2.
Voxel-based lesion-associated network results
| Cluster location | MNI coordinates (x, y, z) | Cluster size (voxels) |
|---|---|---|
| Left angular gyrus | −46, −65, 34 | 11,888 |
| Left inferior frontal gyrus | −26, 25, −21 | 6,112 |
| Left middle temporal gyrus | −54, −9, −19 | 2,408 |
| Left dorsal mPFC | −8, 45, 27 | 1,704 |
| Right ventral mPFC | 2, 29, −16 | 1,432 |
| Right anterior mPFC extending to superior frontal gyrus | 14, 65, −3 | 936 |
| Left middle temporal gyrus | −40, −61, 7 | 896 |
| Left ventral precuneus | −14, −55, 35 | 544 |
| Left middle temporal gyrus | 50, −23, −11 | 488 |
| Left thalamus | −1, −10, 3 | 488 |
| Left middle frontal gyrus | −40, 11, 43 | 480 |
| Left middle frontal gyrus | −34, −1, 49 | 328 |
| Left middle temporal gyrus | −58, −65, −7 | 312 |
| Right putamen | 20, 14, −8 | 192 |
| Right pallidum extending to parahippocampal gyrus | 13, −4, −8 | 168 |
| Right inferior frontal gyrus | 46, 25, −9 | 136 |
| Right superior temporal gyrus | 58, −53, 15 | 120 |
| Left superior parietal lobule | −34, −59, 59 | 120 |
| Left fusiform gyrus | −44, −55, −15 | 112 |
Notes: These voxel-based lesion-associated network mapping results results were thresholded at p < 0.05 uncorrected. Only the angular gyrus results (in bold) survived FWE corrected pFWE < 0.05. Clusters less than 100 voxels and/or only in white matter are not listed.
To further illustrate that our results were located within the DMN and FPN, we overlaid our thresholded lesion-derived network findings onto the DMN and FPN masks from a rsfMRI study of 1,000 participants (Yeo et al., 2011; Figure 3). Our results in the thalamus also intersected with thalamic nuclei shown to be functionally connected to the DMN and FPN (Hwang, Bertolero, Liu, & D’Esposito, 2017; Figure 3b).
FIGURE 3.
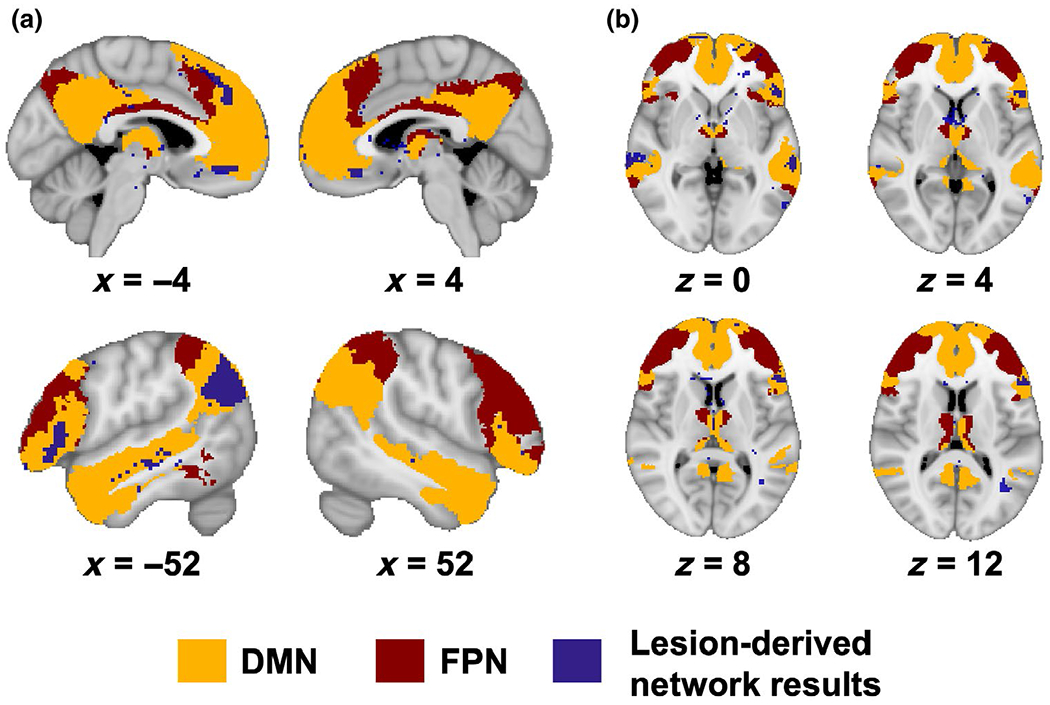
Lesion network mapping results overlap with default mode and frontoparietal network masks. Thresholded voxel-based lesion network mapping results (p < 0.05, uncorrected; purple) are overlaid on masks of the default mode network (DMN) (orange) and frontoparietal network (FPN) (maroon) from a resting-state fMRI study of 1,000 participants (Yeo et al., 2011) and a study examining resting-state functional connectivity of thalamic nuclei major cortical networks (Hwang et al., 2017). (a) There is considerable overlap between the network results and regions in the DMN, and to a lesser extent with FPN regions as well as areas outside the DMN. (b) The network results intersect with thalamic nuclei functionally connected with the DMN and FPN. Results are displayed on the MNI-152 template
3.4 |. Lesion symptom mapping analysis
Using voxel-based lesion symptom mapping, we found associations between reduced self-reported trait daydreaming and brain injury to right dorsal and anterior mPFC, right ventral mPFC, left IPL, and right inferior frontal gyrus (p < 0.05, uncorrected; Figure 4, Table 3). However, these lesion symptom mapping results did not survive multiple comparisons correction (pFWE < 0.05).
FIGURE 4.

Voxel-based lesion symptom mapping results. Voxel-based lesion symptom mapping results from the Brunner and Munzel statistical test are displayed for self-reported trait daydreaming and are thresholded at p < 0.05 uncorrected. Results indicate that those lesion locations are associated with reduced trait daydreaming scores. Findings are displayed on the MNI-152 template
TABLE 3.
Voxel-based lesion symptom mapping results
| Cluster location | MNI coordinates (x, y, z) | Cluster size (voxels) |
|---|---|---|
| Right mPFC extending to inferior frontal gyrus | 8, 50, −4 | 8,834 |
| Left IPL | −36, −41, 48 | 2,915 |
| Right ventral mPFC | 14, 34, −24 | 250 |
| Left precuneus | −40, −78, 36 | 231 |
| Right dorsal mPFC | 15, 50, 22 | 173 |
| Right middle frontal gyrus | 36, 42, 19 | 107 |
Notes: These voxel-based lesion symptom mapping results results were thresholded at p < 0.05 uncorrected. These results did not survive multiple comparisons correction pFWE < 0.05. Clusters less than 100 voxels and/or only in white matter are not listed.
3.5 |. Follow-up analysis of working memory and daydreaming
Given that working memory index scores were correlated with daydreaming, we also conducted the same voxel-based lesion symptom mapping and lesion network mapping analyses with working memory index scores to determine whether the findings overlapped with our daydreaming results. Importantly, we did not find evidence for substantial overlap between the brain regions associated with both working memory and daydreaming (Figure 5).
FIGURE 5.
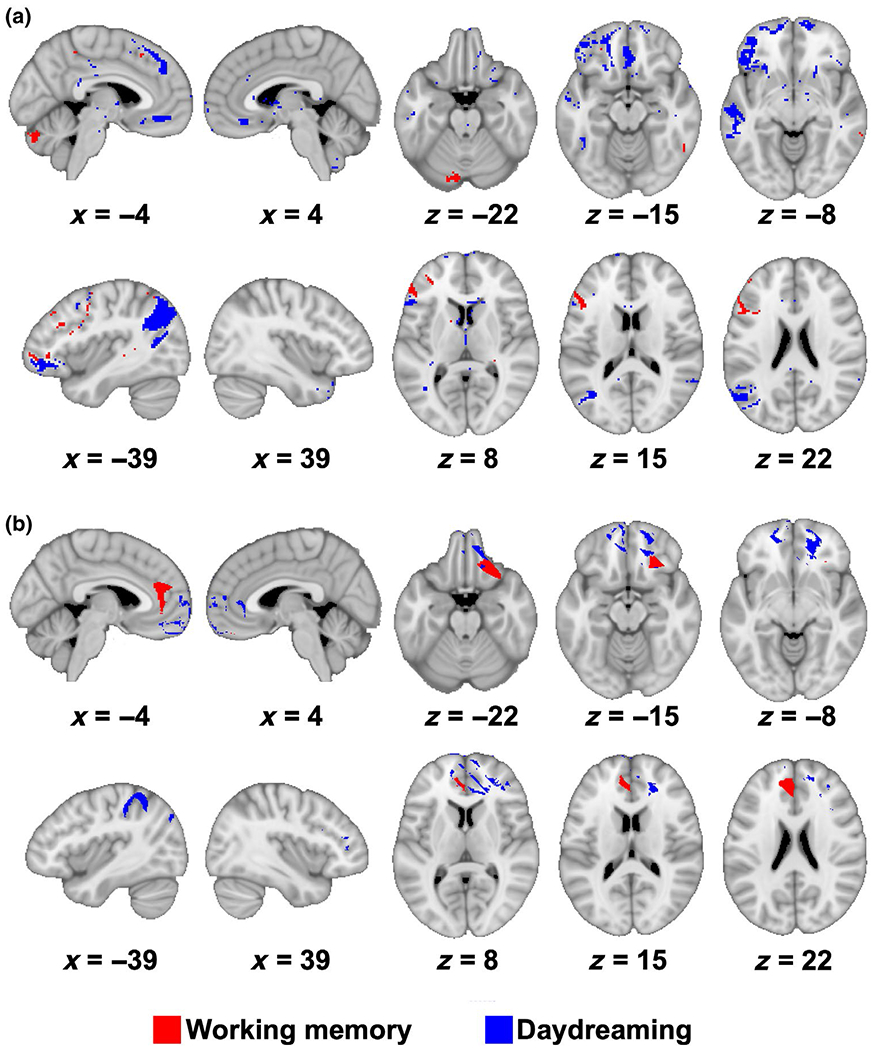
Results show minimal overlap between working memory and daydreaming. (a) Overlap of voxel-based lesion network mapping results are displayed for working memory (red) and self-reported trait daydreaming (blue). (b) Overlap of voxel-based lesion symptom mapping results are displayed for working memory (red) and self-reported trait daydreaming (blue). These figures show minimal overlap between these results. Results are thresholded at p < 0.05 uncorrected. Maps are displayed on the MNI-152 template
4 |. DISCUSSION
We applied a lesion network mapping approach to investigate brain regions and networks associated with deficits in mind-wandering. Our findings revealed that reduced mind-wandering, as assessed with a self-reported trait daydreaming measure, was related to brain lesions that either directly affect the DMN or are functionally connected with the DMN. The results also indicated that brain regions outside of the DMN, including the FPN, may contribute to diminished mind-wandering.
Our findings from the lesion network mapping analysis are convergent with existing functional neuroimaging (Andrews-Hanna, Reidler, Huang, et al., 2010; Christoff et al., 2009; Fox et al., 2015; Mason et al., 2007; McKiernan et al., 2006; Stawarczyk et al., 2011; Stawarczyk & DArgembeau, 2015) and lesion work (Bertossi & Ciaramelli, 2016; McCormick et al., 2018) implicating brain regions primarily within the DMN in mind-wandering. Specifically, we found the strongest association between reduced daydreaming and lesion sites that affect or are functionally connected with the left IPL of the DMN. Although to a lesser extent than DMN, lesions also impacted connectivity with the main components of the FPN including the middle frontal gyrus, dorsal mPFC, and supramarginal gyrus. These findings provide support for neuroimaging research suggesting that connectivity within and between these networks may contribute to various aspects of mind-wandering from the initiation of spontaneous cognition to the content and form of thoughts (Dixon et al., 2018; Karapanagiotidis et al., 2017; Smallwood et al., 2013, 2016). For example, Dixon and colleagues (2018) identified an anterior FPN subnetwork that was functionally connected with the DMN and may be involved in the modulation of introspective cognition. However, the findings within the FPN in the present study did not survive correction for multiple comparisons, even though our power maps suggested that we had statistical power to detect findings in FPN regions in our lesion network mapping analysis (Figure S1). Given that the present study only examined the frequency of self-reported trait daydreaming, future lesion network mapping studies should also assess the content of mind-wandering using self-report and experience sampling measures. For example, it is possible that experience sampling measures of mind-wandering (as compared to daydreaming measures) may be more likely to engage the FPN (Christoff et al., 2009).
Findings from invasive and noninvasive brain stimulation research also align with our lesion network mapping results implicating the DMN in spontaneous thought. Direct electrical stimulation of the medial temporal lobe, including the hippocampus associated with the DMN, tends to produce more instances of spontaneous cognition and dream-like experiences than any other brain region evaluated (Selimbeyoglu & Parvizi, 2010). Other regions of the DMN, the mPFC and IPL, when undergoing transcranial direct current stimulation are associated with decreases in mind-wandering (Bertossi, Peccenini, Peccenini, Solmi, Avenanti, & Ciaramelli, 2017; Kajimura, Kochiyama, Nakai, Abe, & Nomura, 2016; Kajimura & Nomura, 2015). Overall, these studies further highlight the potential influence of connectivity between DMN in impeding internal thought.
We also found reduced mind-wandering linked to lesions affecting brain structures outside the major nodes of the DMN and FPN such as the dorsal striatum and thalamus. While not contained within these networks, it is possible that the dorsal striatum was identified in our study due to its connectivity with other FPN and DMN regions lesioned in the brain-injured participants. For example, there is some evidence that regions within the dorsal striatum are functionally connected to DMN and FPN regions (Choi, Yeo, & Buckner, 2012).
Recent rsFC research suggests that the thalamus may serve as a critical hub that integrates multiple cognitive functions through connectivity with several cortical networks (Hwang et al., 2017). In line with findings from Hwang and colleagues (2017), our results intersected with thalamic nuclei shown to be functionally connected to the DMN and FPN. In addition, both functional neuroimaging and lesion studies indicate that the thalamus is involved in various aspects of attention, including spatial and selective attention (Rafal & Posner, 1987; Rees, 2009; Shipp, 2004; Wright, Vann, Aggleton, & Nelson, 2015). In terms of mind-wandering, the thalamus may provide a spotlight to increase attention to specific thoughts, visual images, or memories. However, further research is needed to investigate the role of the thalamus and striatum in mind-wandering, as these findings were not as robust as those in the DMN.
Based on the lesion symptom mapping analysis, reduced trait daydreaming was associated with lesions of the dorsal, anterior, and ventral sectors of mPFC, angular gyrus, and inferior frontal gyrus of the DMN. Recent neuroimaging research indicates that the DMN is not a unitary network, but instead is composed of multiple subnetworks, including a core DMN subsystem (anterior mPFC and posterior cingulate) that is active during self-relevant cognition (Andrews-Hanna, Reidler, Sepulcre, Reidler, Sepulcre, Poulin, & Buckner, 2010; Buckner & DiNicola, 2019). In the present study, diminished daydreaming was associated with lesions overlapping with the anterior mPFC of the core DMN but not the posterior ventral mPFC of the medial temporal lobe subsystem. However, these lesion symptom mapping results did not survive multiple comparisons correction, so conclusions based on these analyses are preliminary at this point.
There are several limitations to the current study that should be addressed. First, we only assessed brain regions and networks related to reduced mind-wandering frequency using self-report measures. A previous lesion study also used behavioral tasks, which may provide a broader measurement of mind-wandering (Bertossi & Ciaramelli, 2016). This may have emphasized the contribution of those regions that are more crucially connected with the awareness of mind-wandering and of internal/memory contents, such as the left IPL and anterior mPFC (Cabeza, Ciaramelli, & Moscovitch, 2012; Smallwood & Schooler, 2015). Subsequent research could further investigate the dynamics of mind-wandering and determine whether damage to DMN structures similarly disrupts the moment-to-moment triggering and detection of mental states, or their prominent contents. Future studies could also examine whether abnormally increased mind-wandering or specific deficits in mind-wandering content relate to distinct lesion-associated networks. For instance, reduced episodic detail and vividness may be associated with lesions affecting hippocampal networks or the medial temporal lobe subsystem of the DMN. Second, our sample was relatively small for a lesion network mapping study and thus we did not have complete lesion coverage of the brain, although we can infer which, among the covered regions, is more strongly tied to mind-wandering measures. Additional research in a larger sample is warranted. Third, lesion network mapping relies on rsFC from healthy participants to generate inferences about how lesions affect functional connectivity of particular networks. It is possible that lesions could change connectivity in ways that are different from network connectivity in healthy participants (Rudrauf, 2014). Additional research will be required to determine how lesions directly impact functional and structural connectivity.
5 |. CONCLUSION
In summary, traditional lesion symptom mapping results confirmed an association between mPFC and reduced mind-wandering, and extended the association to other nodes of the DMN, including the IPL and inferior frontal gyrus. Moreover, we reported novel lesion network mapping findings associating reduced mind-wandering with lesions that affect the DMN, in particular the left IPL. Our results provide new neuropsychological evidence for the role of the DMN in spontaneous cognition.
Supplementary Material
FIGURE S1 Power maps for lesion network mapping analysis of daydreaming. The results of the power analysis for self-reported trait daydreaming for the lesion network mapping analysis, where at least 10% of participants had lesion-associated network connectivity (FWE corrected p < 0.05). Maps are displayed on the MNI-152 template
FIGURE S2 Power maps for lesion symptom mapping analysis of daydreaming. The results of the power analysis for self-reported trait daydreaming for the lesion symptom mapping analysis, where at least 10% of participants had a lesion (FWE corrected p < 0.05). Maps are displayed on the MNI-152 template
Significance.
Adults spend up to 50% of their waking day mind-wandering, which is the process of turning one’s attention inward to focus on self-generated thoughts or feelings. Mind-wandering can have both costs and benefits, such as increased negative mood or enhanced creative problem-solving. In this study, we report novel findings linking reduced mind-wandering with brain injury located within the default mode network. This work is important because it can help us to determine which brain networks are necessary for self-generated cognition, which may improve our understanding of neuropsychiatric conditions associated with altered self-focused thought.
ACKNOWLEDGMENTS
This work was supported by the National Institute of Health (P50 MH0942581, U01 NS103780, K12NS098482) and the Kiwanis International Neuroscience Research Foundation. We thank the participants for their time and effort in making this research possible.
Funding information
Kiwanis International Neuroscience Research Foundation; National Institutes of Health, Grant/Award Number: K12NS098482, P50 MH0942581 and U01 NS103780
Footnotes
The peer review history for this article is available at https://publons.com/publon/10.1002/jnr.24648.
DECLARATION OF TRANSPARENCY
The authors, reviewers, and editors affirm that in accordance with the policies set by the Journal of Neuroscience Research this manuscript presents an accurate and transparent account of the study being reported and that all critical details describing the methods and results are present.
CONFLICT OF INTEREST
The authors have no conflict of interest to disclose.
DATA AVAILABILITY STATEMENT
Data are available to other researchers upon request from the authors.
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the Supporting Information section.
Transparent Science Questionnaire for Authors
Transparent Peer Review Report
REFERENCES
- Albazron FM, Bruss J, Jones RM, Yock TI, Pulsifer MB, Cohen AL, … Boes AD (2019). Pediatric postoperative cerebellar cognitive affective syndrome follows outflow pathway lesions. Neurology, 93(16), e1561–e1571. 10.1212/WNL.0000000000008326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Huang C, & Buckner RL (2010). Evidence for the default network’s role in spontaneous cognition. Journal of Neurophysiology, 104(1), 322–335. 10.1152/jn.00830.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, & Buckner RL (2010). Functional-anatomic fractionation of the brain’s default network. Neuron, 65(4), 550–562. 10.1016/j.neuron.2010.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants B, & Gee JC (2004). Geodesic estimation for large deformation anatomical shape averaging and interpolation. Mathematics in Brain Imaging, 23, S139–S150. 10.1016/j.neuroimage.2004.07.010 [DOI] [PubMed] [Google Scholar]
- Baird B, Smallwood J, Mrazek MD, Kam JWY, Franklin MS, & Schooler JW (2012). Inspired by distraction: Mind wandering facilitates creative incubation. Psychological Science, 23(10), 1117–1122. 10.1177/0956797612446024 [DOI] [PubMed] [Google Scholar]
- Bertossi E, Aleo F, Braghittoni D, & Ciaramelli E (2016). Stuck in the here and now: Construction of fictitious and future experiences following ventromedial prefrontal damage. Neuropsychologia, 81, 107–116. 10.1016/j.neuropsychologia.2015.12.015 [DOI] [PubMed] [Google Scholar]
- Bertossi E, Candela V, De Luca F, & Ciaramelli E (2017). Episodic future thinking following vmPFC damage: Impaired event construction, maintenance, or narration? Neuropsychology, 31(3), 337–348. 10.1037/neu0000345 [DOI] [PubMed] [Google Scholar]
- Bertossi E, & Ciaramelli E (2016). Ventromedial prefrontal damage reduces mind-wandering and biases its temporal focus. Social Cognitive and Affective Neuroscience, 11(11), 1783–1791. 10.1093/scan/nsw099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertossi E, Peccenini L, Solmi A, Avenanti A, & Ciaramelli E (2017). Transcranial direct current stimulation of the medial prefrontal cortex dampens mind-wandering in men. Scientific Reports, 7(1), 16962 10.1038/s41598-017-17267-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boes AD, Prasad S, Liu H, Liu Q, Pascual-Leone A, Caviness VS Jr., & Fox MD (2015). Network localization of neurological symptoms from focal brain lesions. Brain, 138(10), 3061–3075. 10.1093/brain/awv228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, & DiNicola LM (2019). The brain’s default network: Updated anatomy, physiology and evolving insights. Nature Reviews Neuroscience, 20(10), 593–608. 10.1038/s41583-019-0212-7 [DOI] [PubMed] [Google Scholar]
- Buckner RL, Roffman JL, & Smoller JW (2014). Brain genomics superstruct project (GSP). Cambridge, MA: Harvard Dataverse; 10.7910/DVN/25833 [DOI] [Google Scholar]
- Cabeza R, Ciaramelli E, & Moscovitch M (2012). Cognitive contributions of the ventral parietal cortex: An integrative theoretical account. Trends in Cognitive Sciences, 16(6), 338–352. 10.1016/j.tics.2012.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi EY, Yeo BTT, & Buckner RL (2012). The organization of the human striatum estimated by intrinsic functional connectiveity. Journal of Neurophysiology, 108(8), 2242–2263. 10.1152/jn.00270.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoff K, Gordon AM, Smallwood J, Smith R, & Schooler JW (2009). Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proceedings of the National Academy of Sciences of the United States of America, 106(21), 8719–8724. 10.1073/pnas.0900234106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoff K, Mills C, Andrews-Hanna JR, Irving ZC, Thompson E, Fox KCR, & Kam JWY (2018). Mind-wandering as a scientific concept: Cutting through the definitional haze. Trends in Cognitive Sciences, 22(11), 957–959. 10.1016/j.tics.2018.07.004 [DOI] [PubMed] [Google Scholar]
- Damasio H, & Damasio A (1989). Lesion analysis in neuropsychology. Cambridge, UK: Oxford University Press. [Google Scholar]
- Dixon ML, De La Vega A, Mills C, Andrews-Hanna J, Spreng RN, Cole MW, & Christoff K (2018). Heterogeneity within the frontoparietal control network and its relationship to the default and dorsal attention networks. Proceedings of the National Academy of Sciences of the United States of America, 115(7), E1598–E1607. 10.1073/pnas.1715766115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiez JA, Damasio H, & Grabowski TJ (2000). Lesion segmentation and manual warping to a reference brain: Intra- and interobserver reliability. Human Brain Mapping, 9(4), 192–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer DB, Boes AD, Demertzi A, Evrard HC, Laureys S, Edlow BL, … Geerling JC (2016). A human brain network derived from coma-causing brainstem lesions. Neurology, 87(23), 2427–2434. 10.1212/WNL.0000000000003404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulsham T, Farley J, & Kingstone A (2013). Mind wandering in sentence reading: Decoupling the link between mind and eye. Canadian Journal of Experimental Psychology/Revue Canadienne de Psychologie Experimentale, 67(1), 51–59. 10.1037/a0030217 [DOI] [PubMed] [Google Scholar]
- Fox KCR, Spreng RN, Ellamil M, Andrews-Hanna JR, & Christoff K (2015). The wandering brain: Meta-analysis of functional neuroimaging studies of mind-wandering and related spontaneous thought processes. Neuroimage, 111, 611–621. 10.1016/j.neuroimage.2015.02.039 [DOI] [PubMed] [Google Scholar]
- Fox MD (2018). Mapping symptoms to brain networks with the human connectome. New England Journal of Medicine, 379(23), 2237–2245. 10.1056/NEJMra1706158 [DOI] [PubMed] [Google Scholar]
- Fox MD, Buckner RL, White MP, Greicius MD, & Pascual-Leone A (2012). Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biological Psychiatry, 72(7), 595–603. 10.1016/j.biopsych.2012.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, & Raichle ME (2005). The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America, 102(27), 9673–9678. 10.1073/pnas.0504136102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank RJ, Damasio H, & Grabowski TJ (1997). Brainvox: An interactive, multimodal visualization and analysis system for neuroanatomical imaging. Neuroimage, 5(1), 13–30. 10.1006/nimg.1996.0250 [DOI] [PubMed] [Google Scholar]
- Franklin MS, Broadway JM, Mrazek MD, Smallwood J, & Schooler JW (2013). Window to the wandering mind: Pupillometry of spontaneous thought while reading. Quarterly Journal of Experimental Psychology, 66(12), 2289–2294. 10.1080/17470218.2013.858170 [DOI] [PubMed] [Google Scholar]
- He J, Becic E, Lee Y-C, & McCarley JS (2011). Mind wandering behind the wheel: Performance and oculomotor correlates. Human Factors, 53(1), 13–21. 10.1177/0018720810391530 [DOI] [PubMed] [Google Scholar]
- Hwang K, Bertolero MA, Liu WB, & D’Esposito M (2017). The human thalamus is an integrative hub for functional brain networks. Journal of Neuroscience, 37(23), 5594–5607. 10.1523/JNEUROSCI.0067-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajimura S, Kochiyama T, Nakai R, Abe N, & Nomura M (2016). Causal relationship between effective connectivity within the default mode network and mind-wandering regulation and facilitation. Neuroimage, 133, 21–30. 10.1016/j.neuroimage.2016.03.009 [DOI] [PubMed] [Google Scholar]
- Kajimura S, & Nomura M (2015). Decreasing propensity to mind-wander with transcranial direct current stimulation. Neuropsychologia, 75, 533–537. 10.1016/j.neuropsychologia.2015.07.013 [DOI] [PubMed] [Google Scholar]
- Kane MJ, Brown LH, McVay JC, Silvia PJ, Myin-Germeys I, & Kwapil TR (2007). For whom the mind wanders, and when: An experience-sampling study of working memory and executive control in daily life. Psychological Science, 18(7), 614–621. 10.1111/j.1467-9280.2007.01948.x [DOI] [PubMed] [Google Scholar]
- Karapanagiotidis T, Bernhardt BC, Jefferies E, & Smallwood J (2017). Tracking thoughts: Exploring the neural architecture of mental time travel during mind-wandering. Neuroimage, 147, 272–281. 10.1016/j.neuroimage.2016.12.031 [DOI] [PubMed] [Google Scholar]
- Killingsworth MA, & Gilbert DT (2010). A wandering mind is an unhappy mind. Science, 330(6006), 932 10.1126/science.1192439 [DOI] [PubMed] [Google Scholar]
- Kucyi A, & Davis KD (2014). Dynamic functional connectivity of the default mode network tracks daydreaming. Neuroimage, 100, 471–480. 10.1016/j.neuroimage.2014.06.044 [DOI] [PubMed] [Google Scholar]
- Leszczynski M, Chaieb L, Reber TP, Derner M, Axmacher N, & Fell J (2017). Mind wandering simultaneously prolongs reactions and promotes creative incubation. Scientific Reports, 7(1), 10197 10.1038/s41598-017-10616-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason ME, Norton MI, Van Horn JD, Wegner DM, Grafton ST, & Macrae CN (2007). Wandering minds: The default network and stimulus-independent thought. Science, 315(5810), 393–395. 10.1126/science.1131295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick C, Rosenthal CR, Miller TD, & Maguire EA (2018). Mind-wandering in people with hippocampal damage. Journal of Neuroscience, 38(11), 2745–2754. 10.1523/JNEUROSCI.1812-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKiernan KA, D’Angelo BR, Kaufman JN, & Binder JR (2006). Interrupting the “stream of consciousness”: An fMRI investigation. Neuroimage, 29(4), 1185–1191. 10.1016/j.neuroimage.2005.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooneyham BW, & Schooler JW (2013). The costs and benefits of mind-wandering: A review. Canadian Journal of Experimental Psychology, 67(1), 11–18. 10.1037/a0031569 [DOI] [PubMed] [Google Scholar]
- Philippi CL, Duff MC, Denburg NL, Tranel D, & Rudrauf D (2011). Medial PFC damage abolishes the self-reference effect. Journal of Cognitive Neuroscience, 24(2), 475–481. 10.1162/jocn_a_00138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippi CL, Tranel D, Duff M, & Rudrauf D (2015). Damage to the default mode network disrupts autobiographical memory retrieval. Social Cognitive and Affective Neuroscience, 10(3), 318–326. 10.1093/scan/nsu070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafal RD, & Posner MI (1987). Deficits in human visual spatial attention following thalamic lesions. Proceedings of the National Academy of Sciences of the United States of America, 84(20), 7349–7353. 10.1073/pnas.84.20.7349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees G (2009). Visual attention: The thalamus at the centre? Current Biology, 19(5), R213–R214. 10.1016/j.cub.2009.01.011 [DOI] [PubMed] [Google Scholar]
- Robertson IH, Manly T, Andrade J, Baddeley BT, & Yiend J (1997). Oops!’: Performance correlates of everyday attentional failures in traumatic brain injured and normal subjects. Neuropsychologia, 35(6), 747–758. 10.1016/S0028-3932(97)00015-8 [DOI] [PubMed] [Google Scholar]
- Rorden C, Karnath H-O, & Bonilha L (2007). Improving lesion-symptom mapping. Journal of Cognitive Neuroscience, 19(7), 1081–1088. 10.1162/jocn.2007.19.7.1081 [DOI] [PubMed] [Google Scholar]
- Rudrauf D (2014). Structure-function relationships behind the phenomenon of cognitive resilience in neurology: Insights for neuroscience and medicine. Advances in Neuroscience, 2014, 1–28. 10.1155/2014/462765 [DOI] [Google Scholar]
- Seli P, Kane MJ, Metzinger T, Smallwood J, Schacter DL, Maillet D, … Smilek D (2018). The family-resemblances framework for mind-wandering remains well clad. Trends in Cognitive Sciences, 22(11), 959–961. 10.1016/j.tics.2018.07.007 [DOI] [PubMed] [Google Scholar]
- Sell P, Kane MJ, Smallwood J, Schacter DL, Maillet D, Schooler JW, & Smilek D (2018). Mind-wandering as a natural kind: A family-resemblances view. Trends in Cognitive Sciences, 22(6), 479–490. 10.1016/j.tics.2018.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selimbeyoglu A, & Parvizi J (2010). Electrical stimulation of the human brain: Perceptual and behavioral phenomena reported in the old and new literature. Frontiers in Human Neuroscience, 4, 46 10.3389/fnhum.2010.00046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipp S (2004). The brain circuitry of attention. Trends in Cognitive Sciences, 8(5), 223–230. 10.1016/j.tics.2004.03.004 [DOI] [PubMed] [Google Scholar]
- Singer JL, & Antrobus JS (1972). Daydreaming, imaginal processes, and personality: A normative study In Sheehan P (Ed.), The function and nature of imagery (pp. 175–202). New York, NY: Academic Press. [Google Scholar]
- Smallwood J, Gorgolewski K, Golchert J, Ruby F, Engen H, Baird B, … Margulies D (2013). The default modes of reading: Modulation of posterior cingulate and medial prefrontal cortex connectiveity associated with comprehension and task focus while reading. Frontiers in Human Neuroscience, 7, 734 10.3389/fnhum.2013.00734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallwood J, Karapanagiotidis T, Ruby F, Medea B, de Caso I, Konishi M, … Jefferies E (2016). Representing representation: Integration between the temporal lobe and the posterior cingulate influences the content and form of spontaneous thought. PLoS One, 11(4), e0152272 10.1371/journal.pone.0152272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallwood J, O’Connor RC, Sudbery MV, & Obonsawin M (2007). Mind-wandering and dysphoria. Cognition and Emotion, 21(4), 816–842. 10.1080/02699930600911531 [DOI] [Google Scholar]
- Smallwood J, & Schooler JW (2015). The science of mind wandering: Empirically navigating the stream of consciousness. Annual Review of Psychology, 66(1), 487–518. 10.1146/annurev-psych-010814-015331 [DOI] [PubMed] [Google Scholar]
- Spreng RN, Sepulcre J, Turner GR, Stevens WD, & Schacter DL (2013). Intrinsic architecture underlying the relations among the default, dorsal attention, and frontoparietal control networks of the human brain. Journal of Cognitive Neuroscience, 25(1), 74–86. 10.1162/jocn_a_00281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Stevens WD, Chamberlain JP, Gilmore AW, & Schacter DL (2010). Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. Neuroimage, 53(1), 303–317. 10.1016/j.neuroimage.2010.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stawarczyk D, & D’Argembeau A (2015). Neural correlates of personal goal processing during episodic future thinking and mind-wandering: An ALE meta-analysis. Human Brain Mapping, 36(8), 2928–2947. 10.1002/hbm.22818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stawarczyk D, Majerus S, Maquet P, & D’Argembeau A (2011). Neural correlates of ongoing conscious experience: Both task-unrelatedness and stimulus-independence are related to default network activity. PLoS One, 6(2), el6997 10.1371/journal.pone.0016997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutterer MJ, Bruss J, Boes AD, Voss MW, Bechara A, & Tranel D (2016). Canceled connections: Lesion-derived network mapping helps explain differences in performance on a complex decision-making task. Cortex, 78, 31–43. 10.1016/j.cortex.2016.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranel D (2009). The lowa-Benton school of neuropsychological assessment In Grant T & Adams KM (Eds.), Neuropsychological assessment of neuropsychiatric disorders (3rd ed., pp. 66–83). New York, NY: Oxford University Press. [Google Scholar]
- Van Dijk KRA, Hedden T, Venkataraman A, Evans KC, Lazar SW, & Buckner RL (2009). Intrinsic functional connectivity as a tool for human connectomics: Theory, properties, and optimization. Journal of Neurophysiology, 103(1), 297–321. 10.1152/jn.00783.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Yu C, Xu L, Qin W, Li K, Xu L, & Jiang T (2009). Offline memory reprocessing: Involvement of the brain’s default network in spontaneous thought processes. PLoS One, 4(3), e4867 10.1371/journal.pone.0004867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright NF, Vann SD, Aggleton JP, & Nelson AJD (2015). A critical role for the anterior thalamus in directing attention to task-relevant stimuli. Journal of Neuroscience, 35(14), 5480–5488. 10.1523/JNEUROSCI.4945-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanko MR, & Spalek TM (2013). Driving with the wandering mind: The effect that mind-wandering has on driving performance. Human Factors, 56(2), 260–269. 10.1177/0018720813495280 [DOI] [PubMed] [Google Scholar]
- Yeo BTT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, … Buckner RL (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology, 106(3), 1125–1165. 10.1152/jn.00338.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1 Power maps for lesion network mapping analysis of daydreaming. The results of the power analysis for self-reported trait daydreaming for the lesion network mapping analysis, where at least 10% of participants had lesion-associated network connectivity (FWE corrected p < 0.05). Maps are displayed on the MNI-152 template
FIGURE S2 Power maps for lesion symptom mapping analysis of daydreaming. The results of the power analysis for self-reported trait daydreaming for the lesion symptom mapping analysis, where at least 10% of participants had a lesion (FWE corrected p < 0.05). Maps are displayed on the MNI-152 template


