Abstract
A longitudinal study of a sample of women and their offspring from two urban areas (N=233) was conducted to test whether maternal prenatal anxiety trajectories from early to late pregnancy are associated with 12-month infant developmental outcomes, independent of maternal postpartum anxiety symptoms, prenatal and postpartum depressive symptoms, parity, birth outcomes and maternal education. Three types of maternal anxiety trajectories over the course of pregnancy were identified and labeled increasing, decreasing, and stable-low. Only increasing maternal prenatal anxiety was associated with 12-month infant outcomes, specifically lower Bayley-III scores on receptive language and gross motor skills. Maternal anxiety measured at each individual timepoint in pregnancy was not associated with infant Bayley-III outcomes, highlighting the importance of examining trajectories of maternal affect.
Keywords: Pregnancy, Anxiety, Trajectories, Infancy, Development
1. Introduction
Research with human and animal models has identified several critical periods of fetal growth and development in which the fetus is particularly sensitive to teratogens and other harmful exposures, with negative associations extending well into childhood through adulthood (Davis, Head, Buss, & Sandman, 2017; Davis, Sandman, Buss, Wing, & Head, 2013; Davis & Sandman, 2010; Doyle & Cicchetti, 2018; Sandman, Davis, Buss, & Glynn, 2012). Pregnancy is also a time of drastic changes in maternal physiology, psychosocial stress, and associated risk of depression and anxiety (O’Hara & Wisner, 2014; Woods, Melville, Guo, Fan, & Gavin, 2010), which according to the fetal programming hypothesis could each affect the developmental course beginning in utero (Van den Bergh et al., 2017). Clinical and subclinical anxiety symptoms are common during pregnancy with prevalence ranging from 18% in the first trimester to 25% in the third trimester (Dennis, Falah-Hassani, & Shiri, 2017). Despite this, much of the research has focused on associations between maternal depression during pregnancy and pre-term birth and low birthweight (Accortt, Cheadle, & Dunkel Schetter, 2015; Jarde et al., 2016), and infant and child development across domains (Kingston, Tough, & Whitfield, 2012).
Researchers have, however, grown increasingly interested in different types of maternal psychological distress, such as maternal anxiety in general or anxiety specifically about pregnancy, the timing of effects during pregnancy, and developmental endpoints from birth through adulthood (Field, 2017; Kingston et al., 2012; Mughal et al., 2018; Pearson et al., 2016; Van den Bergh et al., 2005). For example, one study found that elevated pregnancy-specific anxiety in mid-gestation was associated with lower mental and motor development scores in 8-month-old infants (Huizink, Robles De Medina, Mulder, Visser, & Buitelaar, 2003), and in another study, higher pregnancy-specific anxiety, but not general anxiety, early in gestation was associated with lower mental development scores in 12-month-old infants (Davis & Sandman, 2010). In a Dutch sample, 3-week-old infants of prenatally anxious mothers scored significantly lower on the Orientation cluster of the Neonatal Behavioral Assessment Scale, and had significantly lower motor development scores at 12 mos and mental development scores at 24 mos compared to infants of mothers without anxiety during pregnancy (Brouwers, Van Baar, & Pop, 2001). Looking further into childhood, Buss and colleagues (2011) found that pregnancy-specific anxiety was associated with impaired executive function among 6- to 9-year-old offspring.
In addition to the associations with cognitive and motor development, the literature also indicates that there are significant associations between prenatal anxiety and child anxiety, temperament and internalizing behaviors. Elevated pregnancy-specific anxiety early in gestation was associated with increased negative affectivity in 2-year-old children (Blair, Glynn, Sandman, & Davis, 2011). Also, Davis and colleagues (2007) assessed anxiety at 19, 25, and 31 wks gestation and found that prenatal anxiety across gestation predicted child temperament at 2 mos child age, and in a similar study (Davis & Sandman, 2012), pregnancy-specific anxiety predicted anxiety symptoms in children aged 6 to 9 years.
Despite the attention prenatal anxiety has received in the literature, not all studies in this area have identified significant effects of maternal prenatal anxiety on developmental outcomes (Brouwers et al., 2001; DiPietro, Novak, Costigan, Atella, & Reusing, 2006; Martini, Knappe, Beesdo-Baum, Lieb, & Wittchen, 2010; Van den Bergh, 1990), and most have focused on its association with child anxiety, temperament and internalizing behaviors, and not on child cognitive, language, and motor development. In addition, the ways in which anxiety and development were measured varied, limiting the ability to make comparisons across studies. Moreover, many of the studies on the effects of prenatal anxiety do not analyze data longitudinally, with some notable exceptions (Betts, Williams, Najman, & Alati, 2014; Blair et al., 2011; Davis et al., 2007; Davis, Glynn, Waffarn, & Sandman, 2011; Davis & Sandman, 2010; Glynn, Dunkel Schetter, Hobel, & Sandman, 2008; Glynn et al., 2018; Mughal et al., 2018).
Thus, there is a need for more research on the chronicity and severity of prenatal and postnatal maternal distress, which can be examined in longitudinal studies with repeated measures and analysis of trajectories (Kingston et al., 2012). One of the studies mentioned above, from Mughal and colleagues (2018), investigated types of prenatal/postnatal trajectories of maternal anxiety (measured at <25 wks and 34–36 wks gestation, and 4 mos, 1, 2, and 3 yrs after birth) and effects on child outcomes, using the State scale of the State-Trait Anxiety Inventory (STAI-S). Mughal and colleagues (2018) found three distinct anxiety trajectories they labeled persistently high, persistently moderate, and persistently low. When examining whether these trajectories predicted child outcomes, they found only high persistent anxiety to be predictive of developmental delay measured with the Ages and Stages maternal self-report at 3 years (Mughal et al., 2018).
The current study tested two aims that contribute to gaps in the literature. First, we aimed to determine whether there are distinct types of anxiety trajectories in women across pregnancy, as measured by the Overall Anxiety Severity and Impairment Scale (OASIS). The second aim was to determine whether there was an association between type of maternal anxiety trajectory and 12-month infant developmental outcomes, independent of maternal postpartum anxiety symptoms, and maternal prenatal and postpartum depressive symptoms, multiparity, preterm or low birthweight status, and maternal education. This study adds to the work by Mughal and colleagues (2018) in three ways. First, it examines anxiety trajectories from early to late gestation, as opposed to mid-to late gestation. Second, it includes a dimensional measure of infant developmental outcomes (Bayley-III) that was administered in person with the child by trained examiners, as opposed to a caregiver reported composite representing general developmental delay across domains. Third, it makes use of a well-validated screener for anxiety (OASIS) that evaluates frequency, intensity, and functional impairment associated anxiety symptoms (Norman et al., 2011), compared to the STAI, which measures symptoms of distress associated with anxiety and not functional or behavioral difficulties (Spielberger, Gorsuch, & Lushene, 1970).
2. Materials and Methods
2.1. Participants
Data for the current study were collected as part of a longitudinal study designed to test the impact of antenatal maternal mood on birth outcomes and early infant development. This longitudinal study included a total of 233 pregnant women enrolled from 2014 to 2018 in two urban areas. Participants were 18 years of age or older with singleton intrauterine pregnancies, and gave birth to liveborn infants. All were receiving prenatal care in Denver, Colorado, or Los Angeles, California, primarily at prenatal clinics, in medical centers, and private practices. Denver participants were included if they spoke English or Spanish as their primary language, while only English-speaking participants were included in Los Angeles. Women were recruited into the study at less than 16 weeks of gestation and were excluded if they were currently engaging in substance use (including nicotine or cannabis) or had a current substance abuse diagnosis, were HIV-positive, and/or pregnant with multiple gestation. Informed consent was obtained from all participants. This research was conducted within prevailing ethical principles and was reviewed and approved by all relevant Institutional Review Boards at the respective institutions.
After the recruitment visit (T0) which occurred prior to the completion of their 16th week of gestation, subsequent study prenatal visits took place between 8 to 16 wks gestation (T1), 20 to 26 wks gestation (T2), and 30 to 36 wks gestation (T3). Postpartum visits took place between 4 to 8 weeks after birth (P1), at 5 to 7 mos (P2), and 11 to 13 mos (P3). Each visit included interviews and collection of biological samples. Medical records were abstracted for relevant variables from prenatal, labor and delivery, and neonatal charts. The one-year post-birth visit included a direct assessment of infant development via the Bayley Scales of Infant and Toddler Development – Third Edition (Bayley-III; Bayley, 2006).
2.2. Measures
2.2.1. Maternal prenatal anxiety.
The Overall Anxiety Severity and Impairment Scale (OASIS; Norman, Cissell, Means-Christensen, & Stein, 2006) is a five-item, self-report measure that assesses frequency and severity of anxiety, whether anxiety interferes with their daily functioning or social relationships, and whether they avoid anxiety-provoking situations, places, objects, or activities. It was designed to measure anxiety symptoms of all kinds, which makes it useful for capturing anxiety severity in those who experience multiple types of anxiety, clinical or subclinical. Responses are coded on a 5-point Likert-type scale and are summed to obtain a total score with a possible range of 0 to 20. The OASIS has demonstrated strong internal consistency, test-retest reliability, and convergent and discriminant validity in prior studies of its psychometric properties for clinical (Campbell-Sills et al., 2009) and nonclinical samples (Norman et al., 2006).
For the current study, the OASIS scores were obtained at three time points during pregnancy (T0: < 16 wks gestation; T2: 20 – 26 wks gestation; and T3: 30 – 36 wks gestation), and one time point postpartum (P2: 5 – 7 months postpartum; used as a covariate). Cronbach’s alpha coefficients for the OASIS at T0, T2, and T3 were .86, .82, and .85, for each time point respectively. Correlations among the OASIS total scores across timepoints in pregnancy ranged from .40 to .54.
2.2.2. Developmental assessment.
The Bayley Scales of Infant and Toddler Development –Third Edition (Bayley-III; Bayley, 2006) is a widely used, comprehensive assessment of development for infants and toddlers aged 1 to 42 months, and is directly administered by trained examiners. The domains of functioning assessed by the Bayley-III include cognitive, language, and motor skills, as well as social-emotional and adaptive behavior skills as assessed via parent questionnaires. Assessed at 12 months (P3), Bayley-III cognitive, language, and motor composite scores (age-referenced and scaled to a mean of 100 and standard deviation of 15; possible range of 40 – 160), as well as scaled scores for receptive and expressive communication, and fine and gross motor skills (age-referenced and scaled to a mean of 10 and standard deviation of 3; possible range of 1 – 19), were used in the current study.
2.2.3. Covariates.
Tests of the second aim adjusted for covariates based on their known or suspected associations with child developmental outcomes. Multiparity (parity dichotomized into nulliparous = 0, multiparous = 1) was covaried because of possible effects of birth order and sibling status (Sutton-Smith, 2014). Birth outcome was covaried given the possible effects of preterm birth or low birthweight on Bayley-III at one year, and was scored by assigning a 1 for each participant that delivered preterm and/or whose infant was born low birthweight according to medical records obtained at childbirth; all others received a 0. Maternal prenatal depressive symptoms (measured with the 9-item Patient Health Questionnaire [PHQ-9] at time of study screening, which was prior to 16 weeks gestation) was included as a covariate because of the known comorbidity of depression and anxiety. Maternal postpartum depressive symptoms (Edinburgh Postnatal Depression Scale; EPDS) and anxiety symptoms (OASIS) at 5 to 7 months after childbirth (P2) were included as covariates because of the potential of maternal affect at time of testing to affect Bayley-III scores (Kingston et al., 2012). Maternal education in years and annual household income as proxies for socioeconomic status (SES) were obtained by interview and were examined as possible covariates in preliminary analyses, but annual household income was not retained as a covariate because it was not significantly associated with any other variable to be used in analyses. Maternal education was not included as a covariate in models with a motor variable as the outcome, as there was no theoretical justification to do so and it was not significantly associated with the motor variables.
2.3. Statistical Analysis Plan
Prior to conducting the main analyses, bivariate correlations and measures of central tendency were calculated on OASIS scores across pregnancy, Bayley-III scores at 12 months child age, and covariates. In addition, analyses of missing values were undertaken, including a series of independent sample t-tests and chi-square tests of independence. These analyses were conducted using SPSS, Version 25.
2.3.1. Aim 1.
Latent class growth analysis with latent trajectory classes was conducted to determine whether there were distinct groups of homogeneous trajectories of maternal anxiety across pregnancy on the OASIS total scores at T0, T2, and T3. Full-information maximum likelihood (FIML) with robust standard errors was used as the estimator to address missing data (which amounted to 0.9%, 12.4%, and 16.7% missing at T0, T2, and T3, respectively). This is considered the ideal approach when conducting analyses with incomplete and non-normal data (Newman, 2014; Yuan, 2009).
Based on prior research (Mughal et al., 2018), it was hypothesized that three distinct trajectory classes would be identified: stable-high, stable-moderate, and stable-low (Mughal et al., 2018). As such, models were tested fitting one to four classes successively, comparing fit indices for each model to identify the model that is the best fit to the data. These indices included the Bayesian Information Criterion (BIC; model with the lowest BIC is preferred) and the bootstrapped parametric likelihood ratio test, which statistically compares the model with K classes to a model with K-1 classes and provides a p value representing whether there is a statistically significant improvement in fit for the model with one more class (Nylund, Asparouhov, & Muthén, 2007). Other measures of model fit included high entropy and high diagonal posterior probabilities (values above .80 indicate a clear delineation of classes), and no less than 1% of participants in a single class (Jung & Wickrama, 2008). Predicted trajectory class membership for each participant from the model judged to be the best fit to the data was saved and used for the second aim of the current study. These analyses were conducted using Mplus, version 8 (Muthén & Muthén, 1998-2017).
2.3.2. Aim 2.
To examine the associations between membership in each prenatal anxiety trajectory class and Bayley-III scores at 12 months, linear regression models were conducted with dummy-coded latent trajectory class groups as the predictors and each continuous Bayley-III score as the outcome, in separate analyses per Bayley-III outcome, adjusting for maternal prenatal depressive symptoms, maternal postpartum depressive and anxiety symptoms, multiparity, low birthweight (< 2500 grams) or preterm birth (≤ 37 wks gestation) status, and maternal education (in years, but not included in models with motor outcomes). FIML with robust standard errors was used as the estimator to address missing data on the predictors, only for those with data for the Bayley-III (N = 124). These analyses were conducted using Mplus, version 8 (Muthén & Muthén, 1998-2017).
3. Results
Descriptive statistics for the entire sample revealed that participants were on average 30 years old (SD = 5.96), slightly more than half were first time mothers (54.5%), and more than two-thirds married or cohabitating (68.2%). Median per capita household income adjusted for cost of living in Los Angeles or Denver was $16,916 (Interquartile range = $7,133 – $36,105). Mean years of maternal education was 15.5 years (Range = 5 – 26 yrs). Thus, this sample is low to middle SES in general. The largest proportion (45.1%) of the women identified as non-Hispanic White and a majority spoke English (94.4%) as their primary language. Fourteen out of 209 infants in the sample with available birth outcome data were born preterm and 8 were low birthweight. A total of 19 out of 209 (9.1%) had an adverse outcome on one or both of these variables. Overall, Bayley-III cognitive (M (SD) = 105.24 (10.95)), language (M (SD) = 96.72 (9.92)), and motor scores (M (SD) = 97.69 (12.56)) were comparable to norms for average infants, although there was considerable variability. Maternal prenatal anxiety on the OASIS was low on average during pregnancy (OASIS T0 M (SD) = 3.41 (3.19); T2 M (SD) = 3.56 (2.68); T3 M (SD) = 3.74 (3.34)), although scores spanned the full possible range at each time point.
G*Power 3 (Faul, Erdfelder, Buchner, & Lang, 2009) was used to estimate what sample size would be necessary to detect a significant effect, if one exists. With power set at 80% and a two-tailed significance level (α) of 0.05, a sample size of 103 would be needed to detect a significant effect. An effect size (f2) of 0.15 was used in the calculations, which Cohen (1992) defined as a medium effect size. This indicates that the current study has sufficient power to evaluate the proposed hypotheses with a sample of 124 (corresponding to the number of participants with Bayley-III data).
A missing values analysis, including a series of independent sample t tests and chi-square tests of independence, indicated that there was a statistically significant difference in years of maternal education (p < .001) and multiparity (p = .006) between those who did and did not have Bayley-III data. The mothers whose children were missing Bayley-III data had 14.24 mean years of education as compared to 16.60 years of education for those with Bayley-III data, and were more likely to have given birth previously with 55% of those missing Bayley-III data having prior births vs 37.1% of those with Bayley-III data. There were no statistically significant differences in OASIS or EPDS scores, nor preterm or low birthweight status, between those who were included in the final regression analyses and those who were excluded due to incomplete Bayley-III data.
3.1. Aim 1
Model fit information for each latent class growth analysis is presented in Table 1. The model with three classes was determined to be the best fit to the data based on its lower Bayesian Information Criterion (BIC) value, bootstrapped parametric likelihood ratio test p value of < .001 (indicating a statistically significant improvement over the model with one fewer class), and entropy and diagonal posterior probabilities above .80 (indicating a clear delineation of classes). The BIC value for the 4-class model was slightly lower than the BIC value for the 3-class model, its bootstrapped parametric likelihood ratio test p value was also < .001, and it also had entropy and diagonal posterior probabilities above .80, but one of its classes was comprised of less than 1% of participants and thus was determined to be inferior to the 3-class model.
Table 1.
Model fit information for latent class growth analyses specifying 1–4 classes.
| # Classes | BIC | BPLRT p value | Entropy | Proportions within each class, n (%) | |||
|---|---|---|---|---|---|---|---|
| Class 1 | Class 2 | Class 3 | Class 4 | ||||
| 1 | 3229.44 | - | - | 233 (100%) | |||
| 2 | 3179.86 | < .001 | .90 | 195 (83.69%) | 38 (16.31%) | ||
| 3 | 3144.24 | < .001 | .89 | 182 (78.11%) | 36 (15.45%) | 15 (6.44%) | |
| 4 | 3139.91 | < .001 | .90 | 182 (78.11%) | 34 (14.59%) | 15 (6.44%) | 2 (0.86%) |
Note. BIC: Bayesian Information Criterion. BPLRT: Bootstrapped Parametric Likelihood Ratio Test.
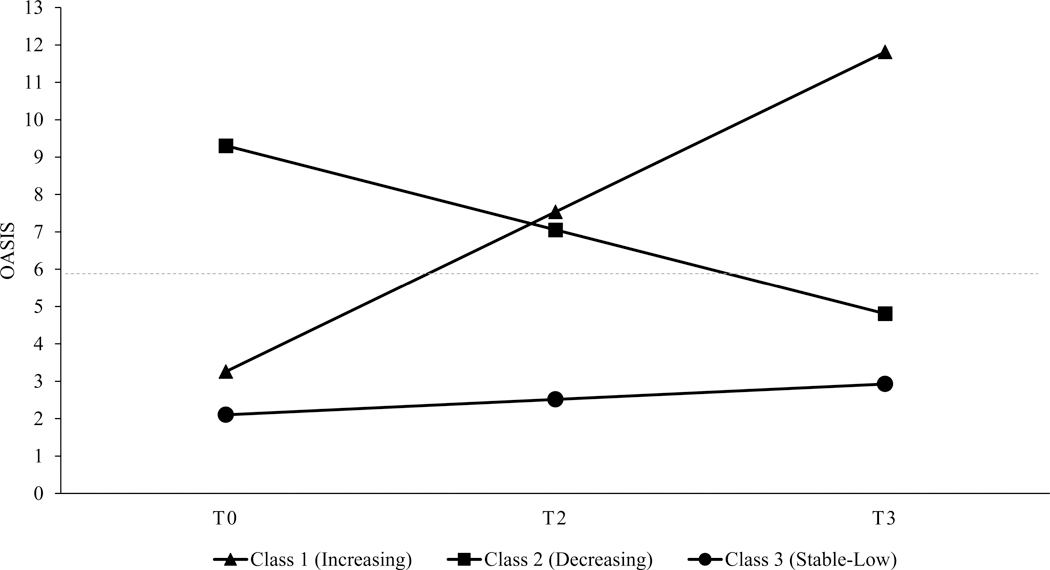
Visual inspection of the mean trajectory for each class (see Figure 1) indicated that the largest class (n = 182, 78.11%) was comprised of women who had stable-low anxiety across pregnancy, the second largest class (n = 36, 15.45%) had women who had high anxiety early in pregnancy that decreased to a low level by the end of pregnancy, and the smallest class (n = 15, 6.44%) of women had low anxiety early in pregnancy that increased to a high level by the end of pregnancy.
Figure 1.
Estimated mean latent class trajectories of OASIS scores across pregnancy (N = 233)
Note. The dotted line represents the OASIS clinical cut-off (≥ 7) to differentiate those with clinically significant anxiety from those without clinically significant anxiety in a non-clinical research sample.
3.2. Aim 2
The prenatal anxiety class membership variable was dummy coded into variables representing the stable-low anxiety class, increasing anxiety class, and decreasing anxiety class, which were used in regression models predicting Bayley-III scores at 12 months with the stable-low anxiety class as the reference group. Covariates included maternal postpartum anxiety symptoms, prenatal and postpartum depressive symptoms, multiparity, and preterm or low birthweight status, and maternal education (in years). FIML with robust standard errors was used as the estimator to address missing data on the predictors, only for those with data for the Bayley-III (N = 124).
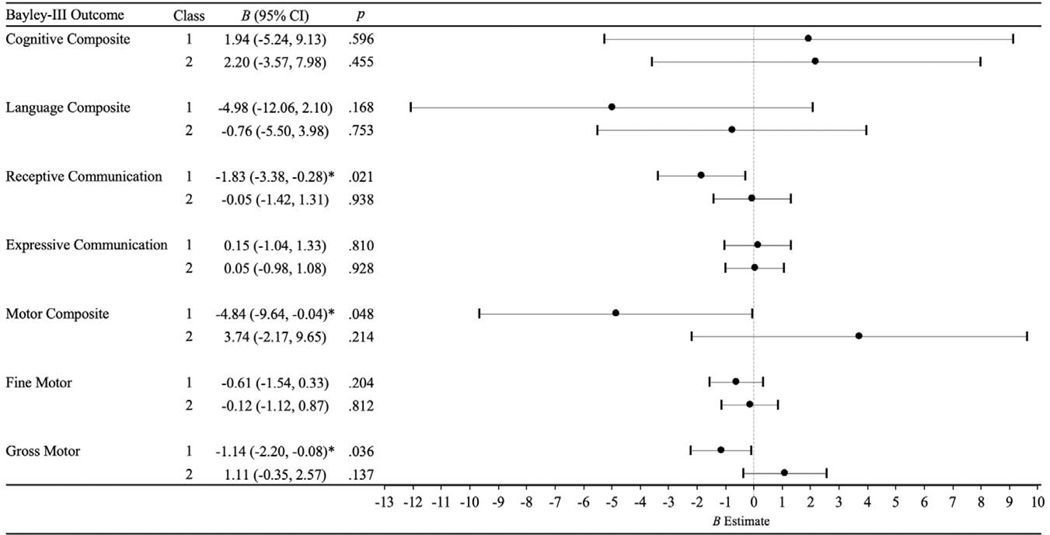
Results of these regression models are presented in Figure 2. Increasing maternal anxiety across pregnancy was associated with lower receptive communication skills (B [95% CI] = −1.83 [−3.38, −0.28], p = .021) and gross motor skills (B [95% CI] = −1.14 [−2.20, −0.08], p = .036) scores at 12 months. Stable-low and decreasing maternal anxiety during pregnancy were not significantly associated with any Bayley-III developmental outcomes at 12 months. The negative effects of increasing maternal prenatal anxiety on receptive communication and gross motor skills at 12 months held after adjustment for the aforementioned factors with known or suspected associations with child development.
Figure 2.
Regression models for OASIS trajectory classes predicting Bayley-III scores at 12 months (N = 124)
Note. Unstandardized regression coefficients and corresponding 95% confidence intervals are shown, after adjustment for the following covariates: maternal depressive symptoms at 12 weeks’ gestation and 6 months postpartum, maternal anxiety symptoms at 6 months postpartum, parity, preterm or low birthweight status, and maternal education (except for motor outcomes). Class 1: increasing anxiety across pregnancy. Class 2: decreasing anxiety across pregnancy. Class 3 (stable-low anxiety across pregnancy) is the reference group. * p < .05; ** p < .01
To determine whether it was the increase in anxiety across pregnancy that was associated with lower receptive communication and gross motor skills, and not due to the fact that anxiety was high at the end of pregnancy for those in the increasing anxiety group, regression analyses were run with the continuous OASIS scores at each time point (T0, T2, and T3) predicting receptive communication and gross motor skills (in three separate models per outcome) after adjusting for the same covariates as in the previous models. Results indicated that, although the pattern of findings were in the same direction, mean levels of anxiety symptoms at T0, T2, and T3 were not significantly associated with receptive communication (p values were .84, .69, and .13) nor gross motor skills (p values were .93, .52, and .18) at 12 months, after adjusting for maternal postpartum anxiety symptoms, prenatal and postpartum depressive symptoms, multiparity, preterm or low birthweight status, and maternal education in years (only for the model with receptive communication).
4. Discussion
The current study examined whether there are distinct types of anxiety trajectories experienced by women over the course of pregnancy, and tested whether these trajectories are associated with 12-month infant developmental outcomes, independent of many relevant covariates in a racially diverse and predominantly low-to-moderate-income sample of women and their children from two urban areas. Based on prior research, it was hypothesized that three distinct trajectory classes would be identified (Mughal et al., 2018). As such, models were tested fitting one to four classes successively, and the results supported a 3-class model. However, in contrast to the three patterns found previously that were all stable over pregnancy (Mughal et al., (2018), the three anxiety trajectory classes identified in the current study were stable-low, decreasing (high anxiety early in pregnancy that decreased to a low level by the end of pregnancy, and increasing referring to low anxiety early in pregnancy rising to a high level by the end of pregnancy. Our study design differed in that we examined trajectories from first to third trimester, whereas the prior study started in the second trimester and continued through postpartum (with measurements at roughly 25 wks and 34–36 wks gestation, and 4 mos, 1, 2, and 3 yrs after birth, thus more closely representing trajectories in infancy and early childhood, not pregnancy).
In addition, the earlier studies measured anxiety with the STAI whereas this study used the OASIS, which captures the frequency, severity, and functional impairment associated with anxiety symptoms in the previous week. It is possible that someone could have a stable number of anxiety symptoms over time on general distress measures such as the STAI-State version, while also experiencing an increase in the severity of these symptoms or the degree to which the symptoms impact their daily lives over time. Here, we find that for some women, anxiety and functional impairment associated with these symptoms increases over time. This may contribute to the differences between our study and those studies using the STAI.
The prior study found that pregnant women with persistently high anxious mood (using the STAI) across six time points from mid-pregnancy to three years post-birth had children with delayed child developmental outcomes at three years of age, measured by maternal report on the Ages and Stages Questionnaire-Third Edition (Mughal et al., 2018). Results of the current study were similar in that increasing maternal prenatal anxiety over three trimesters was negatively associated with infant developmental outcomes but, in this case, at an earlier age (12 months) using the Bayley-III and, specifically, in the domains of receptive communication and gross motor skills. In addition, the outcomes were evaluated objectively by trained examiners, not by maternal self-report. Furthermore, the adverse effects of increasing maternal prenatal anxiety on receptive communication and gross motor skills at 12 months held after adjustment for several factors associated with infant development, namely low birthweight or preterm birth, multiparity, maternal postpartum depressive symptoms, and maternal education. In addition, there were no statistically significant associations with infant outcomes at one year of age between anxiety scores on the OASIS at any one time point in pregnancy. These results strongly suggest that it is indeed the increase in anxiety during pregnancy quantified in analyses of trajectories that is associated with lower receptive communication and gross motor skills, and not anxiety at the end of pregnancy for those in the increasing anxiety group, or high anxiety at any other time point. Anxiety, or its physiological correlates, may have a cumulative teratogenic effect, meaning that increasing exposure to anxiety across gestation, rather than level of symptoms at any one time point, may be more strongly associated with adverse receptive language and gross motor outcomes. Thus, risk may be compounded by the trajectory of anxiety symptoms during pregnancy. These results are consistent with the work of others such as Glynn and colleagues (2008, 2018) who find patterns of anxiety (i.e., increase in anxiety across gestation versus a decrease) in pregnancy are more strongly associated with risk for adverse outcomes (i.e., preterm birth) than levels of anxiety. This suggests that mothers who are not receiving support or treatment for anxiety, or whose life circumstances lead to increasingly anxiety provoking experiences, are most at risk and warrant clinical attention.
Additional research is needed to fully understand the precise mechanisms by which prenatal maternal anxiety influences infant receptive language and gross motor development. One possibility is that increasing anxiety across gestation has an effect on the development of brain regions responsible for processing of auditory information, as receptive language is the one developmental domain in this study dependent on accurate and efficient processing of auditory cues. Research has found that chronic or extreme maternal anxiety may restrict blood flow to the fetus, impairing delivery of oxygen and other important nutrients to the developing fetal organs (Hobel & Culhane, 2003). In rat models, deprivation of oxygen during prenatal development is associated with lasting deficits in auditory processing (Holly Fitch, Alexander, & Threlkeld, 2013). In addition, maternal anxiety during human pregnancy is related to alterations in brain morphology, and among the regions most affected by high levels of anxiety are the middle temporal gyrus, the superior temporal gyrus, and the angular gyrus (Claudia Buss, Davis, Muftuler, Head, & Sandman, 2010), which have been shown to be important for auditory language processing in children (Ahmad, Balsamo, Sachs, Xu, & Gaillard, 2003). Buss and colleagues (2010) also found that development of the premotor cortex, medial temporal lobe, and cerebellum, areas involved in motor planning, execution, and control (Tankus & Fried, 2012), are affected by prenatal maternal anxiety.
This study has several distinct strengths, including that mothers in the study were followed from early pregnancy through birth, and the mothers and infants were followed for one year after birth in two urban sites. Also its use of an established assessment of infant development (Bayley-III) administered by trained examiners, and of a well-validated measure of anxiety (OASIS) that evaluates the frequency of anxiety, intensity of anxiety symptoms, and functional impairment associated with the full range of anxiety disorders, even for those with subsyndromal anxiety (Norman et al., 2011). To our knowledge, this is also the first study to measure the chronicity and severity of anxiety and functional impairment associated with anxiety from early to late pregnancy, and then test prediction of infant development at 12 months. In addition, models were run with a number of key confounding or indicated covariates including maternal depressive symptoms at six months. This is important because prior research examining the effects of prenatal maternal anxiety has often ignored the potential impact of maternal postpartum mental health, and is therefore unable to tease apart their respective effects on infant developmental outcomes (Kingston et al., 2012).
Despite these strengths, the current study also has limitations. The OASIS was only given at three time points during pregnancy, which necessitates linear as opposed to nonlinear modeling. Future research aimed at identifying the effects of anxiety during pregnancy on child developmental outcomes may select to have several different measures of anxiety at multiple time points (preferably four or more) in order to examine linear and curvilinear trajectories of anxiety across pregnancy. In addition, it would be useful to identify early predictors of an increasing anxiety trajectory to identify those at greatest risk as in prior work (Dunkel Schetter, Niles, Guardino, Khaled, & Kramer, 2016), and intervene accordingly. Furthermore, it would be helpful to identify the characteristics of the children whose development is most affected versus seemingly unaffected by increasing maternal prenatal anxiety.
Finally, in one of the two sites (Denver) in this study participants had to return to a different university-based location for the Bayley-III rather than the hospital where they received prenatal care, which contributed to attrition at the last visit when the Bayley was administered. The missing values analysis indicated that the mothers whose children were missing Bayley-III data were more likely to be from Denver than LA and also had fewer years of education (14.2 vs 16.6 years) and were more likely to have given birth previously (55% vs 37.1%), indicating that our results may underestimate the effects for mothers with fewer years of education and more children in the home.
In sum, the findings of the current study demonstrate the importance of not only depression screening, which is now common in postnatal clinics, but also anxiety screening in prenatal clinics and throughout gestation, not on a single occasion. Furthermore, these results suggest that mental health services are indicated for women who experience increasing anxiety during pregnancy in order to lessen its potential effects on child development.
Highlights.
Pregnant women had increasing, decreasing, or stable-low anxiety across pregnancy
Only increasing prenatal anxiety was associated with 12-month infant outcomes
It was specifically associated with worse receptive language and gross motor skills
Anxiety at each individual point in pregnancy was not associated with any outcomes
This highlights the importance of examining changes in anxiety across pregnancy
Acknowledgements
This work was supported by the National Institutes of Health R01 HD073491 to Mary Coussons-Read and Christine Dunkel Schetter (Joint PIs). The study sponsors had no role in the design, collection, analysis, or interpretation of the data; in the writing of the report; nor in the decision to submit the article for publication.
Footnotes
We confirm that this work is original and has not been published elsewhere, nor is it currently under consideration for publication elsewhere. All authors have reviewed and approved the manuscript and have no conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Accortt EE, Cheadle AC, & Dunkel Schetter C. (2015). Prenatal Depression and Adverse Birth Outcomes: An Updated Systematic Review. Maternal and Child Health Journal, 19(6), 1306–1337. 10.1007/s10995-014-1637-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad Z, Balsamo LM, Sachs BC, Xu B, & Gaillard WD (2003). Auditory comprehension of language in young children: Neural networks identified with fMRI. Neurology, 60(10), 1598–1605. 10.1212/01.WNL.0000059865.32155.86 [DOI] [PubMed] [Google Scholar]
- Bayley N. (2006). Bayley scales of infant and toddler development–Third edition. San Antonio, TX: Pearson Education, Inc. [Google Scholar]
- Betts KS, Williams GM, Najman JM, & Alati R. (2014). Maternal depressive, anxious, and stress symptoms during pregnancy predict internalizing problems in adolescence. Depression and Anxiety, 31, 9–18. 10.1002/da.22210 [DOI] [PubMed] [Google Scholar]
- Blair MM, Glynn LM, Sandman CA, & Davis EP (2011). Prenatal maternal anxiety and early childhood temperament. Stress, 14(6), 644–651. 10.3109/10253890.2011.594121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwers EPM, Van Baar AL, & Pop VJM (2001). Maternal anxiety during pregnancy and subsequent infant development. Infant Behavior and Development, 24(1), 95–106. 10.1016/S0163-6383(01)00062-5 [DOI] [Google Scholar]
- Buss C, Davis EP, Hobel CJ, & Sandman CA (2011). Maternal pregnancy-specific anxiety is associated with child executive function at 69 years age. Stress, 14(6), 665–676. 10.3109/10253890.2011.623250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss Claudia, Davis EP, Muftuler LT, Head K, & Sandman CA (2010). High pregnancy anxiety during mid-gestation is associated with decreased gray matter density in 6–9-year-old children. Psychoneuroendocrinology. 10.1016/j.psyneuen.2009.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell-Sills L, Norman SB, Craske MG, Sullivan G, Lang AJ, Chavira DA, … Stein MB (2009). Validation of a brief measure of anxiety-related severity and impairment: The Overall Anxiety Severity and Impairment Scale (OASIS). Journal of Affective Disorders, 112, 92–101. 10.1016/j.jad.2008.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. (1992). Quantitative methods in psychology: A power primer. Psychological Bulletin, 112(1), 155–159. 10.1038/141613a0 [DOI] [PubMed] [Google Scholar]
- Davis EP, Glynn LM, Schetter CD, Hobel C, Chicz-Demet A, & Sandman CA (2007). Prenatal exposure to maternal depression and cortisol influences infant temperament. Journal of the American Academy of Child and Adolescent Psychiatry, 46(6), 737–746. 10.1097/chi.0b013e318047b775 [DOI] [PubMed] [Google Scholar]
- Davis EP, Glynn LM, Waffarn F, & Sandman CA (2011). Prenatal maternal stress programs infant stress regulation. Journal of Child Psychology and Psychiatry and Allied Disciplines, 52(2), 119–129. 10.1111/j.1469-7610.2010.02314.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EP, Head K, Buss C, & Sandman CA (2017). Prenatal maternal cortisol concentrations predict neurodevelopment in middle childhood. Psychoneuroendocrinology, 75, 56–63. 10.1016/j.psyneuen.2016.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EP, & Sandman CA (2010). The timing of prenatal exposure to maternal cortisol and psychosocial stress is associated with human infant cognitive development. Child Development, 81(1), 131–148. 10.1111/j.1467-8624.2009.01385.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EP, & Sandman CA (2012). Prenatal psychobiological predictors of anxiety risk in preadolescent children. Psychoneuroendocrinology, 37(8), 1224–1233. 10.1016/j.psyneuen.2011.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EP, Sandman CA, Buss C, Wing DA, & Head K. (2013). Fetal glucocorticoid exposure is associated with preadolescent brain development. Biological Psychiatry, 74(9), 647–655. 10.1016/j.biopsych.2013.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis CL, Falah-Hassani K, & Shiri R. (2017). Prevalence of antenatal and postnatal anxiety: Systematic review and meta-analysis. British Journal of Psychiatry, 210(5), 315–323. 10.1192/bjp.bp.116.187179 [DOI] [PubMed] [Google Scholar]
- DiPietro JA, Novak MFSX, Costigan KA, Atella LD, & Reusing SP (2006). Maternal psychological distress during pregnancy in relation to child development at age two. Child Development, 77(3), 573–587. 10.1111/j.1467-8624.2006.00891.x [DOI] [PubMed] [Google Scholar]
- Doyle C, & Cicchetti D. (2018). Future directions in prenatal stress research: Challenges and opportunities related to advancing our understanding of prenatal developmental origins of risk for psychopathology. Development and Psychopathology, 30, 721–724. 10.1017/S095457941800069X [DOI] [PubMed] [Google Scholar]
- Dunkel Schetter C, Niles AN, Guardino CM, Khaled M, & Kramer MS (2016). Demographic, Medical, and Psychosocial Predictors of Pregnancy Anxiety. Paediatric and Perinatal Epidemiology. 10.1111/ppe.12300 [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Buchner A, & Lang A-G (2009). Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behavior Research Methods, 41(4), 1149–1160. 10.3758/BRM.41.4.1149 [DOI] [PubMed] [Google Scholar]
- Field T. (2017). Prenatal anxiety effects: A review. Infant Behavior and Development, 49, 120–128. 10.1016/j.infbeh.2017.08.008 [DOI] [PubMed] [Google Scholar]
- Glasheen C, Richardson GA, & Fabio A. (2010). A systematic review of the effects of postnatal maternal anxiety on children. Archives of Women’s Mental Health, 13, 61–74. 10.1007/s00737-009-0109-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn LM, Dunkel Schetter C, Hobel CJ, & Sandman CA (2008). Pattern of Perceived Stress and Anxiety in Pregnancy Predicts Preterm Birth. Health Psychology. 10.1037/0278-6133.27.1.43 [DOI] [PubMed] [Google Scholar]
- Glynn LM, Howland MA, Sandman CA, Davis EP, Phelan M, Baram TZ, & Stern HS (2018). Prenatal maternal mood patterns predict child temperament and adolescent mental health. Journal of Affective Disorders, 228, 83–90. 10.1016/j.jad.2017.11.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobel C, & Culhane J. (2003). Role of Psychosocial and Nutritional Stress on Poor Pregnancy Outcome. The Journal of Nutrition, 133(5), 1709S–1717S. 10.1093/jn/133.5.1709s [DOI] [PubMed] [Google Scholar]
- Holly Fitch R, Alexander ML, & Threlkeld SW (2013). Early neural disruption and auditory processing outcomes in rodent models: Implications for developmental language disability. Frontiers in Systems Neuroscience, 7(58), 1–16. 10.3389/fnsys.2013.00058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizink AC, Robles De Medina PG, Mulder EJH, Visser GHA, & Buitelaar JK (2003). Stress during pregnancy is associated with developmental outcome in infancy. Journal of Child Psychology and Psychiatry and Allied Disciplines, 44(1025–1036). 10.1111/1469-7610.00166 [DOI] [PubMed] [Google Scholar]
- Jarde A, Morais M, Kingston D, Giallo R, MacQueen GM, Giglia L, … McDonald SD (2016). Neonatal outcomes in women with untreated antenatal depression compared with women without depression: A systematic review and meta-analysis. JAMA Psychiatry, 73(8), 826–837. 10.1001/jamapsychiatry.2016.0934 [DOI] [PubMed] [Google Scholar]
- Jung T, & Wickrama KAS (2008). An Introduction to Latent Class Growth Analysis and Growth Mixture Modeling. Social and Personality Psychology Compass, 302–317. 10.1111/j.1751-9004.2007.00054.x [DOI] [Google Scholar]
- Kingston D, Tough S, & Whitfield H. (2012). Prenatal and postpartum maternal psychological distress and infant development: A systematic review. Child Psychiatry and Human Development, 43, 683–714. 10.1007/s10578-012-0291-4 [DOI] [PubMed] [Google Scholar]
- Martini J, Knappe S, Beesdo-Baum K, Lieb R, & Wittchen HU (2010). Anxiety disorders before birth and self-perceived distress during pregnancy: Associations with maternal depression and obstetric, neonatal and early childhood outcomes. Early Human Development, 86, 305–310. 10.1016/j.earlhumdev.2010.04.004 [DOI] [PubMed] [Google Scholar]
- Mughal MK, Giallo R, Arnold P, Benzies K, Kehler H, Bright K, & Kingston D. (2018). Trajectories of maternal stress and anxiety from pregnancy to three years and child development at 3 years of age: Findings from the All Our Families (AOF) pregnancy cohort. Journal of Affective Disorders. 10.1016/j.jad.2018.02.095 [DOI] [PubMed] [Google Scholar]
- Muthén LK, & Muthén BO (1998). Mplus User’s Guide. Eighth Edition. Los Angeles, CA: Muthén & Muthén; 10.1111/j.1600-0447.2011.01711.x [DOI] [Google Scholar]
- Newman DA (2014). Missing data: Five practical guidelines. Organizational Research Methods, 17(4), 372–411. 10.1177/1094428114548590 [DOI] [Google Scholar]
- Norman SB, Campbell-Sills L, Hitchcock CA, Sullivan S, Rochlin A, Wilkins KC, & Stein MB (2011). Psychometrics of a brief measure of anxiety to detect severity and impairment: the Overall Anxiety Severity and Impairment Scale (OASIS). Journal of Psychiatric Research, 45(2), 262–268. 10.1016/j.jpsychires.2010.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman SB, Cissell SH, Means-Christensen AJ, & Stein MB (2006). Development and validation of an Overall Anxiety Severity and Impairment Scale (OASIS). Depression and Anxiety, 23(4), 245–249. 10.1002/da.20182 [DOI] [PubMed] [Google Scholar]
- Nylund KL, Asparouhov T, & Muthén BO (2007). Deciding on the number of classes in latent class analysis and growth mixture modeling: A Monte Carlo simulation study. Structural Equation Modeling, 14(4), 535–569. 10.1080/10705510701575396 [DOI] [Google Scholar]
- O’Hara MW, & Wisner KL (2014). Perinatal mental illness: Definition, description and aetiology. Best Practice and Research: Clinical Obstetrics and Gynaecology, 28, 3–12. 10.1016/j.bpobgyn.2013.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson RM, Bornstein MH, Cordero M, Scerif G, Mahedy L, Evans J, … Stein A. (2016). Maternal perinatal mental health and offspring academic achievement at age 16: The mediating role of childhood executive function. Journal of Child Psychology and Psychiatry and Allied Disciplines, 57, 491–501. 10.1111/jcpp.12483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandman CA, Davis EP, Buss C, & Glynn LM (2012). Exposure to prenatal psychobiological stress exerts programming influences on the mother and her fetus. Neuroendocrinology, 95(1), 8–21. 10.1159/000327017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, & Lushene RE (1970). The State-Trait Anxiety Inventory Manual. Palo Alto, Cal.: Consulting Psychologists. 10.1037/t06496-000 [DOI] [Google Scholar]
- Sutton-Smith B. (2014). Birth Order and Sibling Status Effects In Lamb ME & Sutton-Smith B. (Eds.), Sibling Relationships: Their Nature and Significance Across the Lifespan (1st ed.). New York, NY: Taylor & Francis Group; [Google Scholar]
- Tankus A, & Fried I. (2012). Visuomotor coordination and motor representation by human temporal lobe neurons. Journal of Cognitive Neuroscience, 24(3), 600–610. 10.1162/jocn_a_00160 [DOI] [PubMed] [Google Scholar]
- Tarabulsy GM, Pearson J, Vaillancourt-Morel MP, Bussières EL, Madigan S, Lemelin JP, … Royer F. (2014). Meta-analytic findings of the relation between maternal prenatal stress and anxiety and child cognitive outcome. Journal of Developmental and Behavioral Pediatrics, 35, 38–43. 10.1097/DBP.0000000000000003 [DOI] [PubMed] [Google Scholar]
- Van den Bergh BRH (1990). The influence of maternal emotions during pregnancy on fetal and neonatal behavior. Journal of Prenatal & Perinatal Psychology & Health, 5(2), 119–130. [Google Scholar]
- Van den Bergh BRH, Mennes M, Oosterlaan J, Stevens V, Stiers P, Marcoen A, & Lagae L. (2005). High antenatal maternal anxiety is related to impulsivity during performance on cognitive tasks in 14- and 15-year-olds. Neuroscience and Biobehavioral Reviews, 29(2), 259–269. 10.1016/j.neubiorev.2004.10.010 [DOI] [PubMed] [Google Scholar]
- Van den Bergh BRH, van den Heuvel MI, Lahti M, Braeken M, de Rooij SR, Entringer S, … Schwab M. (2017). Prenatal developmental origins of behavior and mental health: The influence of maternal stress in pregnancy. Neuroscience and Biobehavioral Reviews. 10.1016/j.neubiorev.2017.07.003 [DOI] [PubMed] [Google Scholar]
- Woods SM, Melville JL, Guo Y, Fan MY, & Gavin A. (2010). Psychosocial stress during pregnancy. American Journal of Obstetrics and Gynecology, 202(1), 61.e1–61.e7. 10.1016/j.ajog.2009.07.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan K-H (2009). Normal distribution based pseudo ML for missing data: With applications to mean and covariance structure analysis. Journal of Multivariate Analysis, 100, 1900–1918. 10.1016/j.jmva.2009.05.001 [DOI] [Google Scholar]