Abstract
To evaluate the temporal relationships of cardiovascular disease in oncology patients referred to cardio-oncology and describe the impact of cardiovascular disease and cardiovascular risk factors on outcomes. All adult oncology patients referred to the cardio-oncology service at the Cleveland Clinic from January 2011 up to June 2018 were included in the study. Comprehensive clinical information were collected. The impact on survival of temporal trends of cardiovascular disease in oncology patients were assessed with a Cox proportional hazards model and time-varying covariate adjustment for confounders. In total, 6,754 patients were included in the study (median age, 57 years; [interquartile range, 47–65 years]; 3,898 women [58%]; oncology history [60% - breast cancer, lymphoma and leukemia]). Mortality and diagnosis of clinical cardiac disease peaked around the time of chemotherapy. 2,293 patients (34%) were diagnosed with a new cardiovascular risk factor after chemotherapy, over half of which were identified in the first year after cancer diagnosis. Patients with pre-existing and post-chemotherapy cardiovascular disease had significantly worse outcomes than patients that did not develop any cardiovascular disease (p<0.0001). The highest one-year hazard ratios [HR] of post-chemotherapy cardiovascular disease were significantly associated with male [HR 1.81; 95% CI 1.55–2.11; p<0.001] and diabetes [HR 1.51; 95% CI 1.26–1.81; p<0.001]. In conclusion, patients referred to cardio-oncology, first diagnosis of cardiac events peaked around the time of chemotherapy. Those with pre-existing or post-chemotherapy cardiovascular disease had worse survival. In addition to a high rate of cardiovascular risk factors at baseline, risk factor profile worsened over course of follow-up.
Keywords: Cardio-Oncology, Chemotherapy, Clinical Oncology, Cardiovascular Diseases, Survival Analysis, Risk Factors
INTRODUCTION
The association between cardiovascular disease (CVD) and cancer is increasingly recognized1–8. Cardiovascular (CV) risk factors are more common in cancer survivors as compared to the general population9. In general, co-morbidity has been associated with worse survival in oncology patients10,11. Similarly, the nature and number of CV risk factors is adversely related with survival12,13. To date, there is limited published data regarding the frequency and temporal relationships of clinical cardiovascular risk factors and cardiac outcomes to chemotherapy start date. A previous study that examined the impact on survival of new-onset CVD in oncology patients reported significantly worse longer-term (at 8yrs) mortality, but excluded those with pre-existing CVD and those that died within two years of their cancer diagnosis14. This study aims to define the frequency and temporal relationships of CVD with regard to chemotherapy start date in a large contemporaneous cohort of oncology patients referred to cardio-oncology and to describe the impact of pre-existing CVD and CV risk factors on such outcomes.
METHODS
All adult patients with cancer referred to the cardio-oncology service at the Cleveland Clinic from January 2011 up to June 2018 were included. The study protocol was reviewed and approved by the Cleveland Clinic Institutional Review Board with waiver of individual informed consent. Patients or public were not directly involved in the design, conduct, reporting or dissemination plans of our research.
The patient pool in this study represents oncology patients seen by oncology specialists at our institution undergoing cancer therapeutics and referred for cardiology evaluation/testing based upon cardiac risk factor profile or cardiac comorbidity. Patients at any point on the cancer continuum were indeed considered. Cancer types are detailed in Table 1. Patients with recurrent cancers or second cancers were not excluded but likely represent a small minority of patients. We are not aware of any patients lost to follow up due to insurance reasons. Our institution accepts Medicare and Medicaid patients in addition to those with private insurance and financial counsellors are available to all patients to assist with coverage requirements. Comprehensive clinical information was collected using Cleveland Clinic electronic medical health record database (EPIC) by use of International Classification of Diseases (ICD 9/10) codes. Data was also cross-checked with the prospective Cleveland Clinic Tumor Registry which includes chemotherapy treatment details and mortality information. This registry has coordinators following up patients via phone call as per Ohio regulation and is updated annually15. Presence of traditional risk factors such as diabetes, hypertension, history of smoking, elevated body mass index (BMI), family history of heart disease and hyperlipidemia were also collected also by use of International Classification of Diseases (ICD 9/10) codes. All ICD 9/10 codes were manually checked by looking at patient charts on EPIC for accuracy.
Table 1:
Baseline Patient Characteristics
| Characteristic | Total Cohort N = 6,754 |
|---|---|
| Age of cancer diagnosis (years) | |
| Median (IQR*) | 57 (47–65) |
| Female | 3,898 (58%) |
| Male | 2,856 (42%) |
| White | 5,762 (85%) |
| Black | 703 (10%) |
| Unknown | 109 (2%) |
| Multiracial/Multicultural | 93 (1%) |
| Asian | 75 (1%) |
| American Indian/Alaska Native | 8 (<1%) |
| Native Hawaiian/Pacific Islander | 4 (<1%) |
| Median body mass index (kg/m2) | 27 |
| ≥30 | 2,314 (34%) |
| 25–29.9 | 2,091 (31%) |
| <25 | 2,349 (35%) |
| Hypertension | 1,898 (28%) |
| Current/ previous smoker | 3,380 (50%) |
| Hyperlipidemia | 1,555 (23%) |
| Diabetes mellitus | 698 (10%) |
| Cancer type | |
| Breast | 1,999 (30%) |
| Lymphoma | 1,246 (18%) |
| Leukemia | 842 (12%) |
| Gastro-Intestinal | 614 (9%) |
| Multiple myeloma | 606 (9%) |
| Genito-Urinary | 541 (8%) |
| Lung | 280 (4%) |
| Myelodysplastic syndrome | 190 (3%) |
| Sarcoma | 168 (2%) |
| Other | 144 (2%) |
| Head and Neck | 124 (2%) |
| Chemotherapy type | |
| Anthracycline | 2,768 (41%) |
| HER2 Neu inhibitor | 914 (14%) |
| Kinase inhibitor | 794 (12%) |
| Other | 2,278 (34%) |
| Radiation | 2,908 (43%) |
| Medications | |
| Aspirin | 1,650 (24%) |
| †ACE inhibitor/ ‡ ARB | 1,729 (26%) |
| Beta blocker | 3,272 (48%) |
| Statin | 1,480 (22%) |
Abbreviations:
IQR, inter-quartile range
ACE, angiotensin converting enzyme
ARB, angiotensin receptor blocker.
CVD endpoints (including diagnosis of heart failure (HF), HF admissions, myocardial infarction (MI), stroke, atrial fibrillation, coronary artery disease (CAD), all-cause mortality and in-patient cardiac death) were extracted based upon ICD 9/10 coding and verified manually in the clinical notes. HF with preserved ejection fraction was defined clinically as symptoms and signs of HF, a left ventricular ejection fraction (LVEF) ≥50 percent. Mortality information was also cross-checked with online obituary records where available. In-hospital deaths were adjudicated into cardiac versus non-cardiac death. “All CVD” includes first diagnosis of CAD, atrial fibrillation, HF, stroke and MI. Survival outcomes were analyzed at 1-year, 5-year and long term follow up after starting chemotherapy.
Descriptive statistics were computed to summarize the data. Continuous non-normal variables were presented by medians with interquartile range [IQR], and categorical or ordinal variables were presented as number (percentage). Pearson χ2 tests were used for categorical variable comparisons and the Wilcoxon rank sum test were used for continuous and ordinal variable comparisons.
Unadjusted Kaplan-Meier survival curves were built to describe all-cause mortality after chemotherapy initiation 1-year, 5-year and long-term separately in three different CVD associated subgroups, no CVD, pre-CVD and post-CVD. The date of the last known follow-up was identified as the last date of observation for censored patients. The final date to adjudicate overall mortality follow-up was February 1st, 2019. The log-rank test with the Benjamini & Hochberg adjustment was used for survival comparisons among three CVD associated subgroups16. Comparator age- and sex-matched survival curves for the general population were added to the survival graphs17.
Cox proportional hazards models were used to test associations between with multiple covariates and all-cause mortality after chemotherapy initiation 1-year, 5-year and long-term respectively. Fixed covariates included age, gender, BMI, smoking, and family history. Time varying covariates included hypertension, hyperlipidemia and diabetes. The adjusted effects of covariates with all-cause mortality were estimated with hazard ratios (and 95% confidence interval) and the Wald χ2 test was used to evaluate the covariates with statistically significant coefficients. Cox models were used to determine covariate-adjusted survival probabilities. After this adjusted analysis, patients were stratified into three subgroups no CVD, pre-CVD and post-CVD to compare each as a predictor of survival. The covariates dataset creation for time-varying regression were generated by ‘lifelines’ packages on python 3.7 platform. All survival analyses were performed using the survival (v2.44–1.1) and survminer (v0.4.6) packages on R version 3.5.2 (R Foundation for Statistical Computing). The code written for and used in this study is available from the corresponding author upon reasonable request.
RESULTS
6,754 oncology patients referred to the cardio-oncology service at the Cleveland Clinic from January 2011 up to June 2018 were analyzed. Table 1 details baseline patient characteristics for the total cohort relative to start of chemotherapy (time zero). Despite a relatively young cohort (median age was 57 (SD, 47–65 years), 5,144 patients (76%) had one or more of these risk factors at baseline; 2,767 patients (41%) had two or more, while 908 patients (13%) had pre-existing CVD. Breast cancer, lymphoma and leukemia consisted of 60% of cancers in the total cohort. Total cohort follow-up after chemotherapy start date was a median of 40 months (interquartile range, 19–83 months).
Temporal trends of CVD relative to start of chemotherapy are illustrated in Figure 1a and detailed in Table 2a. Temporal trends of all CVD within 1 year of chemotherapy is shown in Supplemental Figure 1. 1,929 (29%) patients from the total cohort (n = 6,754) were diagnosed with CVD. 1,021 patients (15%) had first CVD diagnosis made post-chemotherapy, of which 425 patients (6%) within the first year. 678 patients (10%) had a diagnosis of HF at any time-point, mainly associated with HF with reduced ejection fraction, occurring post-chemotherapy and treated in the outpatient setting. Of these 678 patients, only 87 (1%) had a hospital admission due to HF. 2,293 patients (34%) were diagnosed with a new CV risk factor after chemotherapy, over half of which were identified in the first year (see Table 2a). CVD across cancer subtypes is included in Supplemental Table 1.
Figure 1. (a). Temporal trends of clinical cardiovascular disease diagnoses from time of starting chemotherapy.
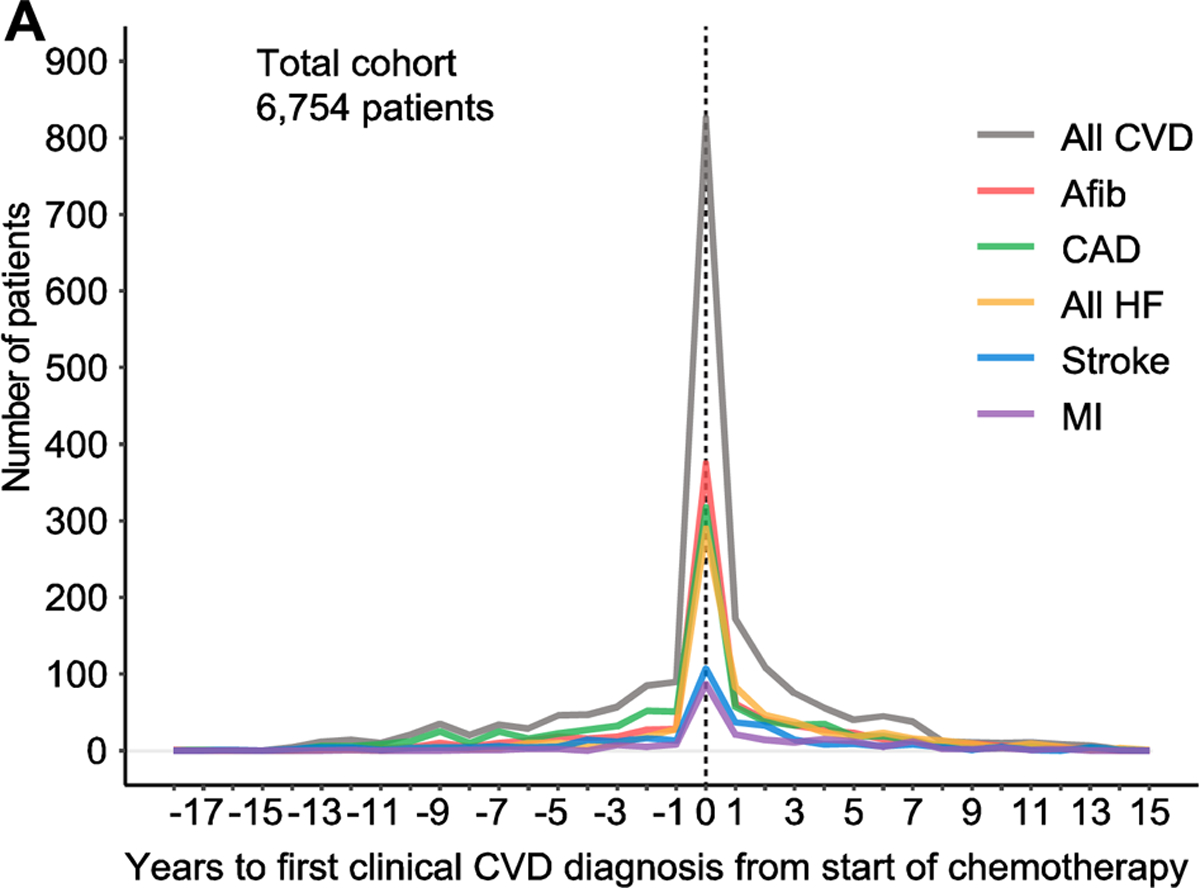
Graph illustrates time to first diagnosed clinical cardiovascular disease (CVD) relative to time of starting chemotherapy. Clinical CVD includes atrial fibrillation (Afib), coronary artery disease (CAD), all heart failure (HF), stroke and myocardial infarction (MI). All CVD includes Afib, CAD, all HF, stroke and MI.
Table 2 (a): Rates of clinical cardiovascular disease (CVD) diagnoses and risk factors pre-existing chemotherapy, post-chemotherapy and in the first year before and after chemotherapy.
All CVD includes coronary artery disease (CAD), atrial fibrillation, all heart failure (HF), HF with reduced ejection fraction (HFrEF), HF admission, stroke and myocardial infarction (MI). Cardiovascular risk factors includes hypertension, hyperlipidemia and diabetes mellitus. Abbreviations: Chemotx, chemotherapy.
| Total Cohort (N = 6,754 (%)) | Total number of patients | Pre-existing Comorbidity (Any) | Pre-existing Comorbidity (in 1 year prior to chemotx) | Post chemotx Comorbidity (in 1 year post chemotx) | Post chemotx Comorbidity (Any) |
|---|---|---|---|---|---|
| All CVD | 1,929 (29%) | 908 (13%) | 401 (6%) | 425 (6%) | 1,021 (15%) |
| CAD | 848 (13%) | 461 (7%) | 168 (2%) | 157 (2%) | 387 (6%) |
| Atrial fibrillation | 783 (12%) | 318 (5%) | 156 (2%) | 221 (3%) | 465 (7%) |
| A11HF | 678 (10%) | 189 (3%) | 94 (1%) | 204 (3%) | 489 (7%) |
| HFrEF | 462 (7%) | 130 (2%) | 65 (1%) | 140 (2%) | 332 (5%) |
| HF Admission | 87 (1%) | 16 (0.2%) | 7 (0.1%) | 27 (0.4%) | 71 (1%) |
| Stroke | 331 (5%) | 134 (2%) | 41 (1%) | 67 (1%) | 197 (3%) |
| MI | 214 (3%) | 62 (1%) | 35 (1%) | 54 (1%) | 152 (2%) |
| Hypertension | 3,403 (50%) | 1,898 (28%) | 805 (12%) | 821 (12%) | 1,505 (22%) |
| Hyperlipidemia | 2,570 (38%) | 1,555 (23%) | 429 (6%) | 370 (5%) | 1,015 (15%) |
| Diabetes Mellitus | 1,333 (20%) | 698 (10%) | 341 (5%) | 308 (5%) | 635 (9%) |
Post-chemotherapy mortality rates in the total cohort (all-cause death, in-hospital death and in-hospital cardiac death respectively) are illustrated in Figure 1b and detailed in Table 2b. All mortality rates peaked around the time of starting chemotherapy. 2,391 patients (35%) died during the follow-up period, of which 731 patients (11%) died within the first year of starting chemotherapy.
Figure 1. (b). Temporal trends of all-cause mortality from time of starting chemotherapy.
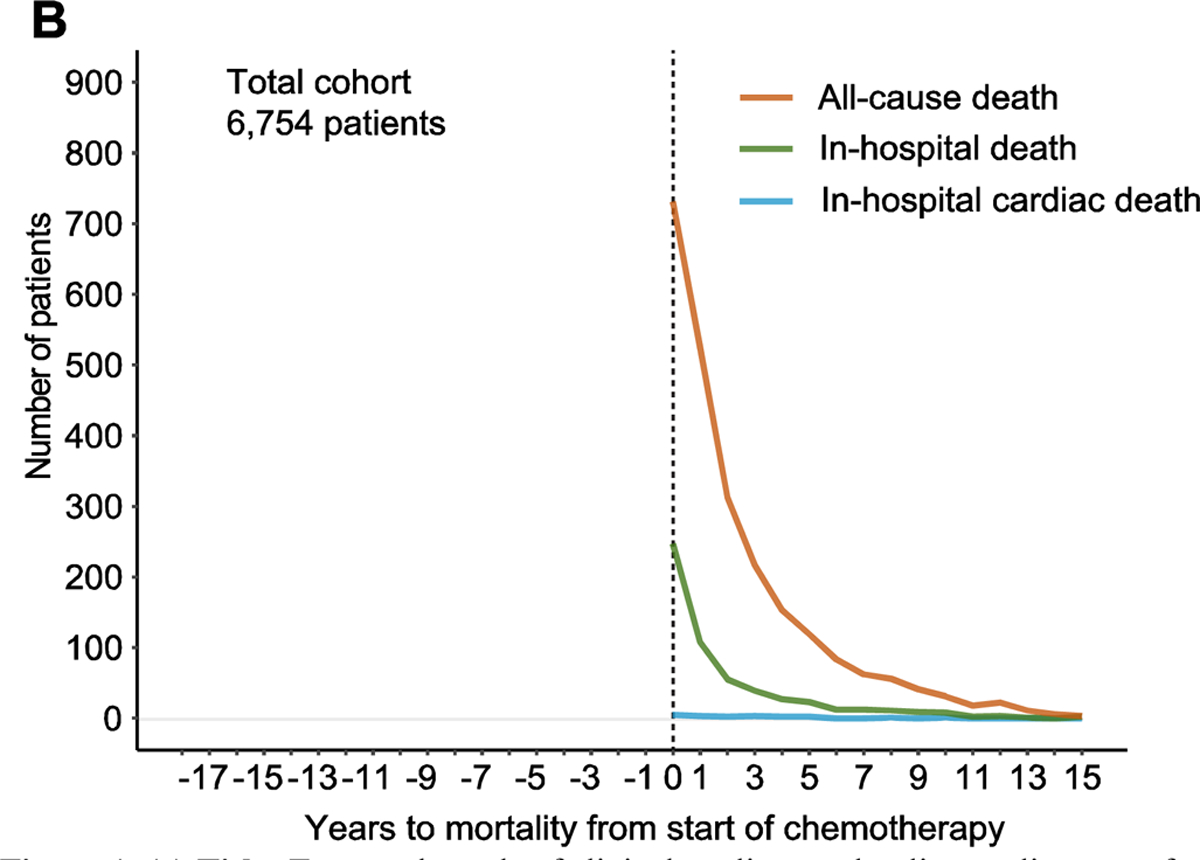
Mortality is sub-classified as all-cause death, in-hospital death and in-hospital cardiac death.
Table 2 (b): Rates of mortality in the total cohort.
Mortality is sub-classified as all-cause death, in-hospital death and in-hospital death.
| Variable | Total number of patients (N = 6,754) | Within first year Post Chemotx* |
|---|---|---|
| All-cause mortality | 2,391 (35%) | 731 (11%) |
| In-hospital mortality | 559 (8%) | 247 (4%) |
| In-hospital cardiac mortality | 19 (0.3%) | 5 (0.07%) |
Abbreviations:
Chemotx, chemotherapy.
Survival curves are illustrated in Figure 2 at 1-year, 5-years and long-term. Supplemental Figure 2 details breakdown of CVD groupings. The presence of CVD (either pre- or post-chemotherapy) was associated with significantly worse mortality at 1-year and 5-years after commencing chemotherapy compared to patients that never developed CVD, independent of CV risk factor adjustment (p < 0.0001). In general, post-chemotherapy CVD was associated with the worst adjusted survival at 1-year even when compared to those with pre-existing CVD; this difference was not evident beyond 5-years.
Figure 2. 1-year, 5-years and long-term survival in patients without cardiovascular disease (CVD) “no-CVD”; patients with pre-existing “CVD pre-chemo”; and those that develop CVD post-chemotherapy “CVD post-chemo”.
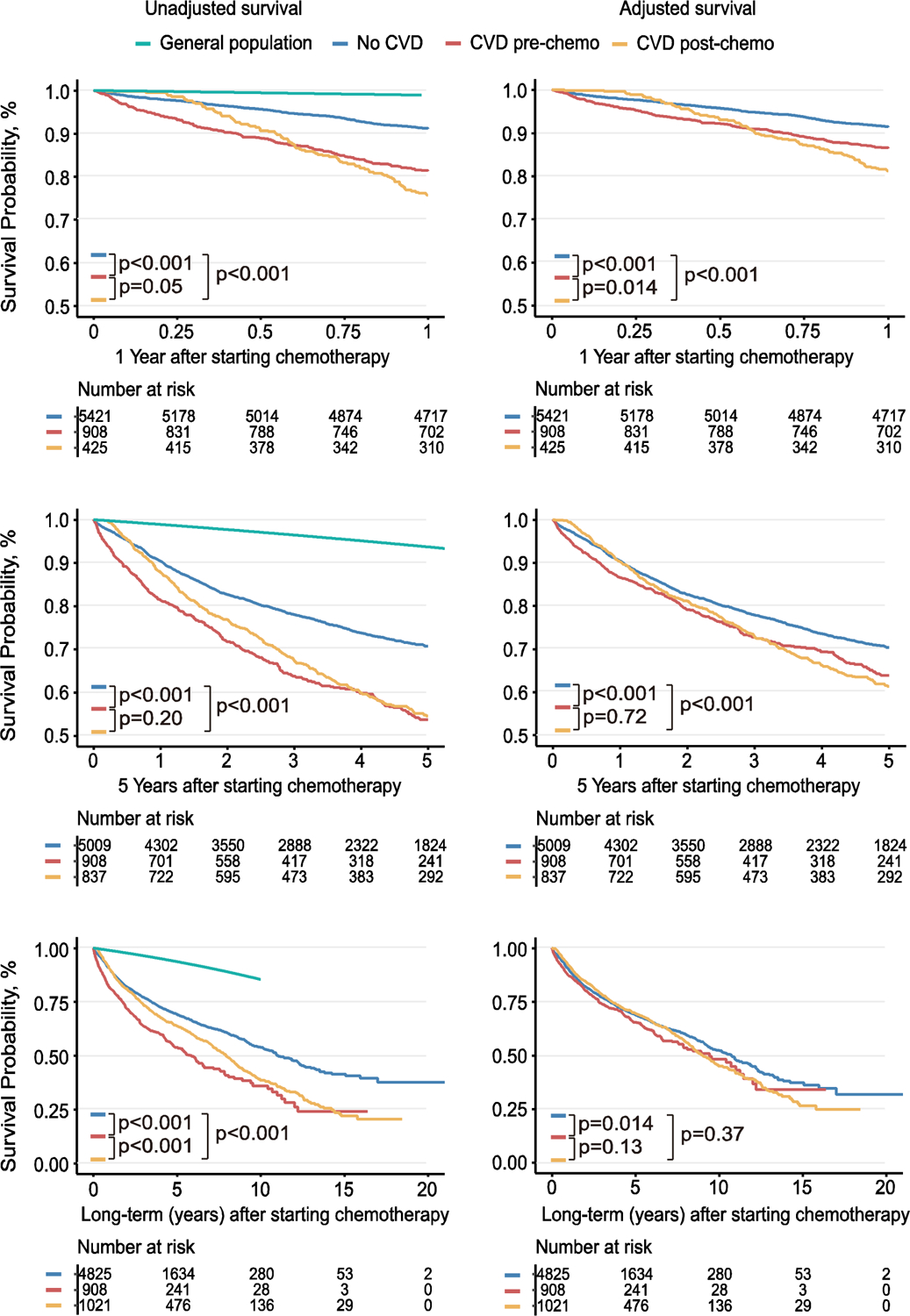
Time zero is chemotherapy start date. Unadjusted and adjusted survival graphs shown at 1-year, 5-year and long-term mortality. Adjustment made for CVD confounders include age, gender, hypertension, hyperlipidemia, diabetes mellitus, smoking status, body mass index and family history of heart disease. Age-matched and sex-matched survival of the general population is also shown.
Hazard ratios (HR) of traditional CV risk factors all-cause mortality at 1-year, 5-years and long-term post-chemotherapy are demonstrated in Figure 3. Amongst standard cardiovascular risk factors, the highest 1-year risk-adjusted hazard ratios [HR] of post-chemotherapy CVD were associated with male sex [HR 1.81; 95% CI 1.55–2.11; p<0.001], diabetes [HR 1.51; 95% CI 1.26–1.81; p<0.001] and hypertension [HR 1.43; 95% CI 1.21–1.69; p<0.001]. The hazard ratio for post-chemotherapy CVD compared to those with pre-existing CVD was significant at 1 year [HR 1.37; 95% CI 1.07–1.77; p=0.014]; this difference was not evident beyond 5-years. Hazard ratio for post-chemotherapy CVD risk is illustrated in Supplemental Figure 3.
Figure 3. 1-year, 5-years and long-term hazard ratios of all-cause mortality.
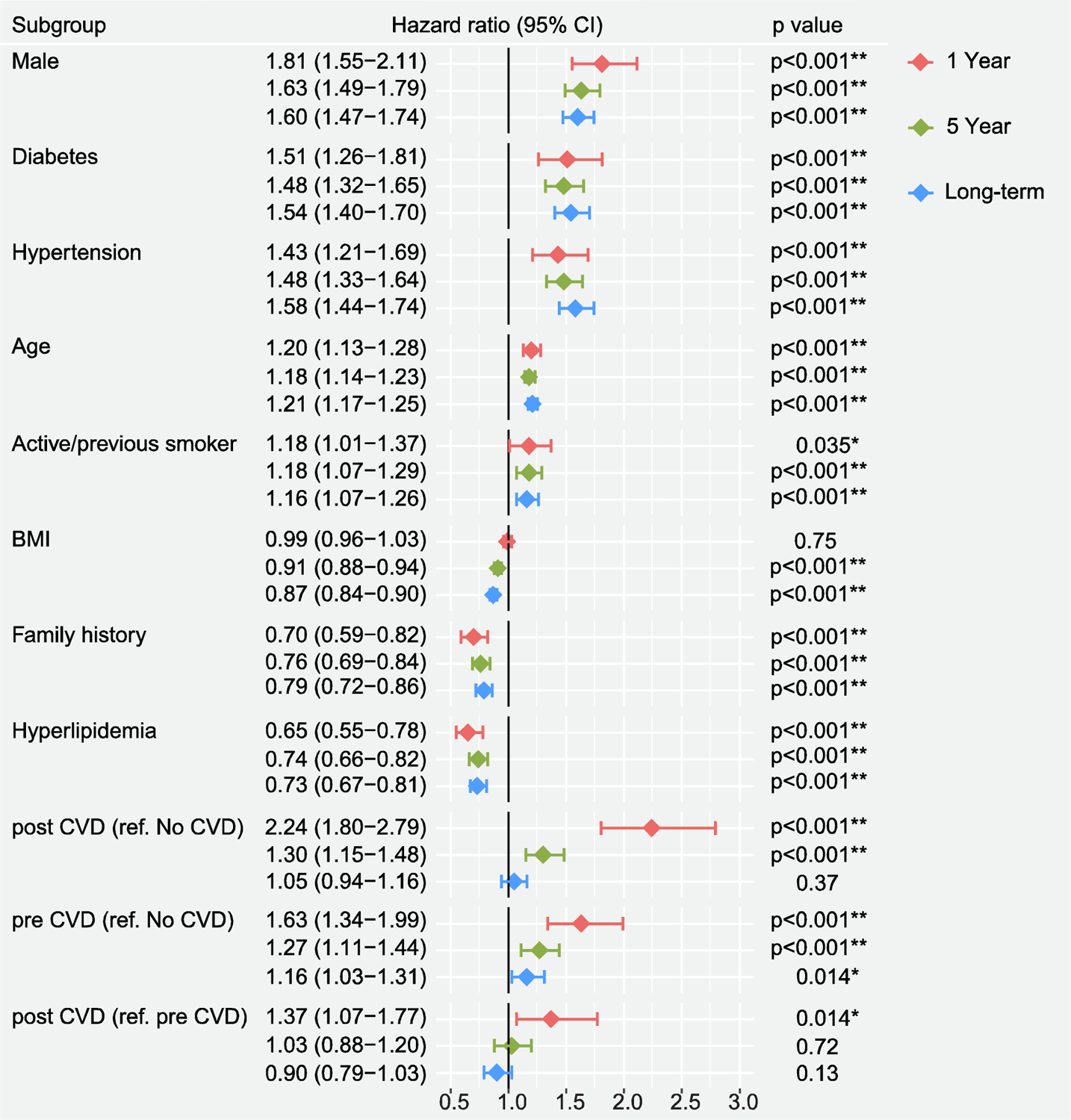
Time zero is chemotherapy start date. Forest plot of hazard ratios for all-cause mortality at 1-year, 5-years and long-term post-chemotherapy. CI - Confidence interval, BMI - body mass index, CVD - cardiovascular disease *Denotes significant association (p<0.05).
Around 1 in 8 patients had pre-existing CVD (prior to chemotherapy) and these patients had a higher rate of another subsequent post-chemotherapy CVD and higher all-cause mortality (p < 0.001) compared to those without pre-existing CVD as shown in Supplemental Table 2. Amongst post-chemotherapy CVD, atrial fibrillation and MI carry the worst outcomes in terms of both all-cause mortality as shown in Supplemental Figure 4.
DISCUSSION:
First diagnosis of CVD events in patients referred to cardio-oncology peaked around the time of chemotherapy. In Figure 1A, it appears that vast majority of events occurred at time zero - i.e. at start of chemotherapy, but when time on the x-axis is spread out over months (Supplemental Figure 1), it can be seen that this increased event rate is normally distributed. The number of echocardiograms performed also dramatically peaked around this time which would account for some new diagnoses (Supplemental Figure 5). How much of this peak in CVD diagnosis reflected pre-existing disease / latent disease discovered at the time of increased physician visits, investigations and hospitalizations versus de novo disease in a susceptible, high risk population with a high burden of pre-existing CV risk factors in the face of extensive testing and therapeutics (not limited to biopsy, staging, chemotherapy, radiotherapy, surgery, subsequent re-staging etc.) is unclear. Regardless, it does not take away from the central finding of this paper that there was high burden of manifest concomitant CVD peaking around the time of cancer treatment. We believe these data offer further justification for the burgeoning subspecialist discipline of cardio-oncology to support multi-disciplinary management of oncology patients. The rapid rise in cardio-oncology clinics across the world may reflect a practical recognition of these trends18–21. Our data would suggest that the highest risk period was the first year post-chemotherapy where arguably focused optimal CV preventative and treatment strategies may provide the highest yield. Survival analyses demonstrated the precise extent to which concomitant CVD (either pre-existing or post-chemotherapy) compounded the already reduced survival of oncology patients compared to age, sex-matched population. Similar prior work to our study also reported reduced overall survival in cancer survivors who develop CVD but included only those that had survived cancer by 1–2 years)14,22. Our data supports and expands on the findings of that data especially given the early peak in CV event rate as seen in our study. How did type of post-chemotherapy CVD diagnosis affect survival? MI and AF carried the worst outcomes in terms of all-cause mortality, even worse than (any stage) HF. There is limited data regarding survival post MI in cancer patients, although it is recognized oncology patients that experience acute coronary syndrome were less likely to undergo percutaneous coronary intervention than those without23. AF in most cohorts is strongly prognostic echoing what we saw in our data24. How did timing of CVD diagnosis relative to chemotherapy start time affect survival? Those with pre-existing CVD clearly had the worst initial outcomes but shortly after 6 months, the survival of those who suffered a post-chemotherapy CVD diagnosis (without pre-existing CVD) became relatively worse. There may have been many underlying reasons as to why this happened. We hypothesize that the ability to cope with a new CVD diagnosis may be lower in patients with established cancer due to lack of pre-conditioning effect, increased frailty and a deficiency of “clinical reserve”25–28. Male sex, diabetes and hypertension were the standard cardiovascular risk factors associated with the highest 1-year risk-adjusted HR of post-chemotherapy CVD. In contrast, as adjusted risk factors, family history and hyperlipidemia were more favorable which we speculate may relate to better primary prevention and an apparent high rate of statin use in these sub-groups. Risk factor profile worsened over time. Over a third of the total cohort were subsequently diagnosed with a new CV risk factor. Rates of diabetes and hypertension nearly doubled. One might expect a population with cancer to experience a drop in average BMI during follow-up; however, over the course of this study, the BMI was relatively unchanged and actually rose slightly. These findings expose an unmet need for better prevention in this population. It is likely that some of this exacerbation of risk factor profile was related to cancer treatment itself as many cancer chemotherapeutics have been shown to have adverse effects on both lipid metabolism and rates of hypertension29–31.
This is an observational study involving oncology patients referred to cardiology. While this methodology introduces referral bias, the study population is reflective of practical real-world patients with a wide variety of cancers and treatment types seen by cardio-oncology. Data collection involved using electronic health records and ICD 9/10 coding; each event was verified by a physician via retrospective chart review. In order to try and minimize screening bias, definition of CVD was limited to clinically overt disease and the dates of all events were reviewed for accuracy. Change in CV risk factor profile during follow-up was mitigated for using time-varying covariate adjustment (smoking status change was not available). To minimize reporting bias, outcome data was manually crosschecked with clinical events. Mortality data was cross-referenced with an Intuitional Tumor Registry and obituary data. Socio-economic status, diet, exercise, impact of either chemotherapy or medical therapy were not examined in this study. All-cause mortality was examined in this study which we felt was the least biased metric given the universal difficulty in acquiring accurate cause of death32. Despite competing risk of cancer-related death as a cause for death, there was no difference in rates of advanced staged disease (stage 4) between those with pre-existing CVD vs those without (staging data available in 52% of the cohort).
Amongst patients referred to cardio-oncology, first diagnosis of cardiac events peaked around the time of chemotherapy. Those with pre-existing or post-chemotherapy cardiovascular events had worse survival. In addition to a high rate of cardiovascular risk factors at baseline, this risk factor profile worsened over the course of follow-up. Focused preventative cardiology input is likely warranted for patients with cancer particularly around the time of and in the first year after chemotherapy.
Supplementary Material
Funding:
This work was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health (NIH) under Award Number K99 HL138272 and R00 HL138272 to F.C.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Ethics approval: Reviewed and approved by the Institutional Review Board with waiver of individual informed consent
Consent to participate: Yes
Consent for publication: Yes
Availability of data and material: Upon request
Code availability: Upon request
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Koene RJ, Prizment AE, Blaes A, Konety SH. Shared Risk Factors in Cardiovascular Disease and Cancer. Circulation 2016;133(11):1104–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hancock SL, Tucker MA, Hoppe RT. Factors affecting late mortality from heart disease after treatment of Hodgkin’s disease. JAMA 1993;270(16):1949–1955. [PubMed] [Google Scholar]
- 3.Steinherz LJ, Steinherz PG, Tan CT, Heller G, Murphy ML. Cardiac toxicity 4 to 20 years after completing anthracycline therapy. JAMA 1991;266(12):1672–1677. [PubMed] [Google Scholar]
- 4.Fedele P, Orlando L, Schiavone P. Clinical outcomes and cardiac safety of continuous antiHer2 therapy in c-erbB2-positive metastatic breast cancer patients. J Chemother 2013;25(6):369–375. [DOI] [PubMed] [Google Scholar]
- 5.Lee J, Hur H, Lee JW. Long-term risk of congestive heart failure in younger breast cancer survivors: A nationwide study by the SMARTSHIP group. Cancer 2019. [DOI] [PubMed] [Google Scholar]
- 6.Pituskin E, Mackey JR, Koshman S. Multidisciplinary Approach to Novel Therapies in Cardio-Oncology Research (MANTICORE 101-Breast): A Randomized Trial for the Prevention of Trastuzumab-Associated Cardiotoxicity. J Clin Oncol 2017;35(8):870–877. [DOI] [PubMed] [Google Scholar]
- 7.Cardinale D, Colombo A, Bacchiani G. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation 2015;131(22):1981–1988. [DOI] [PubMed] [Google Scholar]
- 8.Hussain M, Collier P. Chemotherapy-Related Cardiovascular Complications 2019:1–23.31234166 [Google Scholar]
- 9.Weaver KE, Foraker RE, Alfano CM. Cardiovascular risk factors among long-term survivors of breast, prostate, colorectal, and gynecologic cancers: a gap in survivorship care? J Cancer Surviv 2013;7(2):253–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Land LH, Dalton SO, Jensen MB, Ewertz M. Impact of comorbidity on mortality: a cohort study of 62,591 Danish women diagnosed with early breast cancer, 1990–2008. Breast Cancer Res Treat 2012;131(3):1013–1020. [DOI] [PubMed] [Google Scholar]
- 11.Piccirillo JF, Tierney RM, Costas I, Grove L, Spitznagel EL Jr., Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA 2004;291(20):2441–2447. [DOI] [PubMed] [Google Scholar]
- 12.Hershman DL, Till C, Shen S. Association of Cardiovascular Risk Factors With Cardiac Events and Survival Outcomes Among Patients With Breast Cancer Enrolled in SWOG Clinical Trials. J Clin Oncol 2018;36(26):2710–2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barone BB, Yeh HC, Snyder CF. Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: a systematic review and meta-analysis. JAMA 2008;300(23):2754–2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Armenian SH, Xu L, Ky B. Cardiovascular Disease Among Survivors of Adult-Onset Cancer: A Community-Based Retrospective Cohort Study. J Clin Oncol 2016;34(10):1122–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sidhu RS, Sharma A, Paterson ID, Bainey KR. Influenza H1N1 Infection Leading To Cardiac Tamponade in a Previously Healthy Patient: A Case Report. Res Cardiovasc Med 2016;5(3):e31546–e31546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the royal statistical society. Series B (Methodological) 1995:289–300. [Google Scholar]
- 17.Arias E, Heron M, Xu J. United States Life Tables, 2014. Natl Vital Stat Rep 2017;66(4):1–64. [PubMed] [Google Scholar]
- 18.Hampton T Cardio-Oncology Programs Strive to Balance Cancer Care With Heart Health. Circulation 2016;134(4):353–354. [DOI] [PubMed] [Google Scholar]
- 19.Gujral DM, Manisty C, Lloyd G, Bhattacharyya S. Organisation & models of cardio-oncology clinics. Int J Cardiol 2016;214:381–382. [DOI] [PubMed] [Google Scholar]
- 20.Lancellotti P, Suter TM, Lopez-Fernandez T. Cardio-Oncology Services: rationale, organization, and implementation. Eur Heart J 2019;40(22):1756–1763. [DOI] [PubMed] [Google Scholar]
- 21.Yeh ET, Chang HM. Oncocardiology-Past, Present, and Future: A Review. JAMA Cardiol 2016;1(9):1066–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strongman H, Gadd S, Matthews A. Medium and long-term risks of specific cardiovascular diseases in survivors of 20 adult cancers: a population-based cohort study using multiple linked UK electronic health records databases. Lancet 2019;394(10203):1041–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rohrmann S, Witassek F, Erne P, Rickli H, Radovanovic D. Treatment of patients with myocardial infarction depends on history of cancer. Eur Heart J Acute Cardiovasc Care 2018;7(7):639–645. [DOI] [PubMed] [Google Scholar]
- 24.Odutayo A, Wong CX, Hsiao AJ, Hopewell S, Altman DG, Emdin CA. Atrial fibrillation and risks of cardiovascular disease, renal disease, and death: systematic review and meta-analysis. BMJ 2016;354:i4482. [DOI] [PubMed] [Google Scholar]
- 25.Fidler MM, Reulen RC, Henson K. Population-Based Long-Term Cardiac-Specific Mortality Among 34 489 Five-Year Survivors of Childhood Cancer in Great Britain. Circulation 2017;135(10):951–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Honda T, He Q, Wang F, Redington AN. Acute and chronic remote ischemic conditioning attenuate septic cardiomyopathy, improve cardiac output, protect systemic organs, and improve mortality in a lipopolysaccharide-induced sepsis model. Basic Res Cardiol 2019;114(3):15. [DOI] [PubMed] [Google Scholar]
- 27.Maulik A, Davidson SM, Piotrowska I, Walker M, Yellon DM. Ischaemic Preconditioning Protects Cardiomyocytes from Anthracycline-Induced Toxicity via the PI3K Pathway. Cardiovasc Drugs Ther 2018;32(3):245–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mauguen A, Collette S, Pignon JP, Rondeau V. Concordance measures in shared frailty models: application to clustered data in cancer prognosis. Stat Med 2013;32(27):4803–4820. [DOI] [PubMed] [Google Scholar]
- 29.Huang C, Freter C. Lipid metabolism, apoptosis and cancer therapy. Int J Mol Sci 2015;16(1):924–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sagstuen H, Aass N, Fossa SD. Blood pressure and body mass index in long-term survivors of testicular cancer. J Clin Oncol 2005;23(22):4980–4990. [DOI] [PubMed] [Google Scholar]
- 31.Herrmann J, Yang EH, Iliescu CA. Vascular Toxicities of Cancer Therapies: The Old and the New--An Evolving Avenue. Circulation 2016;133(13):1272–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lund JL, Harlan LC, Yabroff KR, Warren JL. Should cause of death from the death certificate be used to examine cancer-specific survival? A study of patients with distant stage disease. Cancer Invest 2010;28(7):758–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


