Abstract
Left bundle branch block (LBBB) increases the likelihood of developing reduced left ventricular (LV) ejection fraction (EF) but predicting which patients with LBBB and normal LVEF will develop decreased LVEF remains challenging. Fifty patients with LBBB and normal LVEF were retrospectively identified. Clinical, electrocardiographic (ECG), and echocardiographic variables were compared between patients who developed a decreased LVEF and those who did not. A total of 16/50 patients developed reduced LVEF after 4.3 (SD=2.8) years of follow-up. Baseline patient and ECG variables were similar between patients who did and did not develop decreased LVEF. Baseline LVEF was lower in patients who developed decreased LVEF than in those who did not [51.9% (SD=2.2%) vs. 54.9% (SD=4.4%), P<0.01.] Diastolic filling time (DFT) accounted for a significantly smaller percentage of the cardiac cycle in patients who developed decreased LVEF than in those who did not [35.9%, (SD=6.9%) vs. 44.4% (SD=4.5%) P<0.01]. In univariable logistic regression, DFT had a C-statistic of 0.86 (P<0.0001) for prediction of development of decreased LVEF. In conclusion, patients in whom DFT accounted for <38% of the cardiac cycle had a relative risk of developing decreased LVEF of 7.0 (95% CI 3.0–16.0) compared to patients with DFT accounting for ≥38% of the cardiac cycle.
Keywords: Left bundle branch block, left ventricular ejection fraction, echocardiography
Up to one-third of otherwise healthy patients with left bundle branch block (LBBB) go on to develop decreased left ventricular (LV) ejection fraction (EF) a unique clinical entity defined by LV dilation occurring in the setting of a LBBB with evident dyssynchrony1. Three retrospective studies show that decreased LVEF is less responsive to ACC/AHA HF guideline directed medical therapies compared to decreased LVEF occurring in the setting of normal conduction2–4. Cardiac resynchronization therapy (CRT) improves heart failure (HF) symptoms and survival among patients with LBBB, symptomatic HF, and LVEF ≤ 35%5,6. Early reports suggest that both systolic and diastolic function are impaired in patients with LBBB and symptomatic HF with preserved LVEF and both systolic and diastolic function can be improved with CRT7. CRT implantation ≤ 9 months after the diagnosis of decreased LVEF in patients with LBBB is associated with a higher likelihood of LV reverse remodeling compared to implantation >9 months after diagnosis4 but it is unclear if prophylactic CRT implantation could prevent decreased LVEF in patients with LBBB. The purpose of this study is to determine if electrocardiographic and echocardiographic assessments of systolic and diastolic LV performance in patients with LBBB can predict the likelihood of developing decreased LVEF.
Methods
This study complied with the Declaration of Helsinki and was approved by the Duke Institutional Review Board. The study cohort was identified in the Duke Echocardiographic Laboratory Database8,9 after linking to the electrocardiogram database. The study population consisted of patients aged ≥ 18 years with a standard 12 lead electrocardiogram obtained within 30 days of a clinically obtained echocardiogram and a follow-up echocardiogram performed > 6 months later. Baseline echocardiograms were obtained between January 1, 1998 and December 31, 2013. The baseline echocardiogram was the earliest qualifying echocardiogram after the first electrocardiogram diagnosis of LBBB which was defined using ACC/AHA criteria10.
To reduce the frequency of competing sources of decreased LVEF, patients were excluded if they had a history of previous valve intervention, end-stage renal disease, prior left ventricular assist device, heart transplantation, metastatic cancer, moderate or severe valve disease, atrial fibrillation, previous myocardial infarction, previous implantable cardiac device, or conduction abnormality other than LBBB. Patients were also excluded if echocardiogram images were inadequate to measure diastolic function using tissue Doppler imaging (due to sinus tachycardia, a rhythm other than sinus or inadequate spectral tissue Doppler images with sampling at the septal mitral annulus), or if they had an LVEF < 50% at the time of the baseline echocardiogram. In cases where multiple echocardiograms were obtained after baseline, endpoint ascertainment was performed using the study with the lowest LVEF measurement. If the LVEF remained unchanged, the latest echocardiogram was used.
Decreased LVEF was defined as a new reduction in LVEF to ≤ 45% at least 6 months after the baseline echocardiogram without having had an intercurrent myocardial infarction. All echocardiogram data were abstracted from the Duke Echocardiogram Laboratory Database which contains clinical interpretations by cardiologists with level III training in echocardiography. All baseline echocardiograms were re-analyzed by a single investigator (KE) for this study and verified by other investigators (JK, LL).
Echocardiographic Analysis:
All measurements were performed in Xcelera R3.2LA SP2 (Phillips Medical Systems Nederland B.V., Best, The Netherlands). The presence and severity of valvular disease were evaluated as described in the echocardiographic guidelines.11–13 Diastolic dysfunction grading was based on the latest recommendations from the American Society of Echocardiography.14 Transmitral flow was recorded from the apical 4-chamber view at end expiration at the mitral leaflet tips. Pulse wave (PW) Doppler recordings were made at a sweep speed of 100mm/s with an electrocardiogram superimposed. The E wave amplitude and duration, E wave deceleration time (DT) and A wave amplitude and duration were measured manually and the E/A ratio was calculated. Isovolumic contraction time (ICT), ejection time (ET), isovolumic relaxation time (IRT) and diastolic filling time (DFT) were measured on spectral tissue Doppler images with sampling at the septal mitral annulus in the apical 4-chamber view.15 The ICT was defined as the interval from the end of the a’-wave to the beginning of the s’-wave, ET was defined as the interval from the onset to end of the s’-wave, and IRT was defined as the interval from the end of the s’-wave to the beginning of the e’-wave. The DFT was defined as the interval from the beginning of the e’-wave to the end of the a’-wave. The duration of each phase was then expressed as a percentage of the RR interval measured from the peak of the QRS complex on the measured electrocardiogram recording. All measurements were repeated on up to 3 consecutive cardiac cycles and averaged.
Continuous variables with a normal distribution were presented as mean with standard deviation (SD) while variables with a non-Gaussian distribution were presented as median with interquartile range. Categorical variables were presented as n (%). Groups were compared using Student’s t-test for continuous variables and Fisher’s exact test for categorical variables. Logistic regression modeling was performed to identify variables that predicted the development of decreased LVEF and receiver operator characteristic curve analysis was performed to identify the variable threshold value that optimized prediction of decreased LVEF. Variables were included in the multivariable model if they differed between groups. The number of variables entered into the model was limited based on the number of observed endpoints (in a 1:10 ratio) to avoid overfitting. The outcome of interest was a reduction in LVEF to ≤45% on follow up echocardiography at least 6 months after the baseline echocardiogram. A two-sided p-value of <0.05 was considered statistically significant.
Results
A total of 50 patients fulfilled eligibility criteria and were included in the analysis (Figure 1), 16 patients developed decreased LVEF and 34 patients did not. The mean time to follow-up echocardiogram was 4.3 years (SD=2.8 years) among both patients with decreased LVEF and those without unchanged follow-up LVEF, P=0.95 for difference between groups. Patients who developed decreased LVEF had a mean follow-up LVEF of 37.0% (SD=8.1%) with a mean LVEF change of −14.9% (SD=8.3%) while patients who had normal follow-up LVEF had a mean follow-up LVEF of 54.5% (SD=2.5%) with a mean change in LVEF of −0.4% (SD=4.5%).
Figure 1:

Cohort derivation. DELD= Duke Echocardiographic Laboratory Database, Echo=echocardiogram, LVAD= left ventricular assist device, ICD=implantable cardioverterdefibrillator, ECG= electrocardiogram, LVEF= left ventricular ejection fraction, MI= myocardial infarction, AF= atrial fibrillation, ESRD= end stage renal disease, CRT= cardiac resynchronization therapy, LBBB= left bundle branch block.
Baseline patient characteristics stratified by outcome are summarized in Table 1. Patients who developed decreased LVEF were similar to those who did not in all respects. Nearly half the population had a diagnosis of HF with preserved EF at the time of the baseline echocardiogram. Baseline echocardiogram findings stratified by outcome are summarized in Table 2. Patients who developed decreased LVEF had a lower baseline LVEF than patients who did not (51.9%, SD=2.2% vs. 54.9%, SD=4.4%, P<0.01 for difference). Isovolumic contraction made up a larger percentage of the cardiac cycle in patients who later developed decreased LVEF (13.8%, SD=3.9%) than in those who did not (11.5%, SD=2.5%, P=0.04 for difference). Isovolumic relaxation also made up a larger percentage of the cardiac cycle in patients who later developed decreased LVEF than in those who did not (16.7% SD=3.6% vs. 13.2% SD=2.2%, P<0.01 for difference). As a consequence, DFT accounted for a smaller percentage of the cardiac cycle in patients who developed decreased LVEF (35.9%, SD=6.9%) compared to those who did not (44.4%, SD=4.5%) P<0.01 for difference, (Figure 2). ET was prolonged in patients who later developed decreased LVEF compared to those who did not, but this difference was not significant (P=0.07). The distributions of DFT among patients with and without development of decreased LVEF are shown in Figure 3.
Table 1:
Baseline Characteristics
| Variable | Decreased f/u Left Ventricular Ejection Fraction (N=16) | Normal f/u Left Ventricular Ejection Fraction (N=34) | P-Value |
|---|---|---|---|
| Age (years) mean | 65 ± 12 | 68 ± 15 | 0.43 |
| Men | 7 (44%) | 13 (38%) | 0.76 |
| Height (inches) mean | 66 ± 4 | 66 ± 5 | 0.89 |
| Weight (pounds) mean | 178 ± 46 | 198 ± 47 | 0.17 |
| Hypertension | 13 (81%) | 25 (74%) | 0.73 |
| Diabetes mellitus | 5 (31%) | 9 (26%) | 0.75 |
| Coronary artery disease | 3 (19%) | 13 (38%) | 0.21 |
| Heart failure | 7 (44%) | 15 (44%) | 1.0 |
| Medications | |||
| Beta-Blockers | 6 (38%) | 11 (32%) | 0.76 |
| Angiotensin converting enzyme | 8 (50%) | 11 (32%) | 0.35 |
| inhibitor/Angiotensin receptor blocker | |||
| Aldosterone Antagonist | 2 (13%) | 3 (9%) | 0.65 |
| ECG Findings | |||
| Heart Rate (beats per minute) mean | 70 ± 16 | 76 ± 15 | 0.28 |
| PR | 193 ± 22 | 179 ± 34 | 0.08 |
| QRS | 146 ± 14 | 141 ± 14 | 0.19 |
| QT | 448 ± 39 | 438 ± 39 | 0.47 |
Table 2:
Baseline Echocardiogram Characteristics
| Variable | Decreased f/u Left Ventricular Ejection Fraction (N=16) | Normal f/u Left Ventricular Ejection Fraction (N=34) (N=34) | P-Value |
|---|---|---|---|
| Left ventricular ejection fraction (%) mean | 51.9 ± 2.2 | 54.9 ± 4.4 | 0.003 |
| Global longitudinal strain (%) mean | −16.7 ± 3.0 | −16.7 ± 3.1 | 0.99 |
| Dyssynchrony pattern | 0.19 | ||
| Classical | 8 (50%) | 14 (41%) | |
| Transitional | 2 (13%) | 12 (35%) | |
| Other | 6 (37%) | 8 (24%) | |
| Septal thickness (mm) mean | 11.9 ± 3.0 | 12.5 ± 3.4 | 0.57 |
| Lateral thickness (mm) mean | 11.4 ± 1.6 | 10.9 ± 3.0 | 0.41 |
| Left ventricular hypertrophy | 6 (32%) | 10 (32%) | 1.0 |
| E wave amplitude (m/s) mean | 78.3 ± 27 | 80.9 ± 30 | 0.76 |
| A wave amplitude (m/s) mean | 94.3 ± 30 | 93.8 ± 31 | 0.96 |
| E/A ratio mean | 0.88 ± 0.4 | 0.90 ± 0.3 | 0.85 |
| E/e’ ratio mean | 13.’ ± 4.6 | 15.4 ± 6.9 | 0.28 |
| Diastolic dysfunction grade | 0.25 | ||
| 0 | 1 | 7 | |
| I | 10 | 18 | |
| II | 4 | 9 | |
| III | 1 | 0 | |
| Heart rate (beats per minute) mean | 72 ± 11 | 67 ± 8 | 0.34 |
| Isovolumic contraction time (% PR) mean | 13.8 ± 3.9 | 11.5 ± 2.5 | 0.04 |
| Ejection time (% RR) mean | 33.4 ± 3.4 | 31.3 ± 4.2 | 0.07 |
| Isovolumic relaxation the (% RR) mean | 16.7 ± 3.6 | 13.2 ± 2.3 | 0.002 |
| Diastolic filling time (% RR) mean | 35.9 ± 6.8 | 44.4 ± 4.5 | 0.0002 |
| 0.92 ± 0.23 | 0.80 ± 0.2 | 0.05 |
Figure 2:

Box plots comparing the mean percentage of the cardiac cycle occupied by each of the 4 cardiac phases by study group. Patients who did not develop left bundle branch block associated decreased left ventricular ejection fraction (top) are compared to patients who did develop left bundle branch block associated decreased ejection fraction (bottom). Differences in the mean isovolumic contraction time, isovolumic relaxation time, and diastolic filling time were statistically significant (P<0.05 for all). ECG lead I demonstrates left bundle branch block. LVEF= left ventricular ejection fraction, f/u= follow-up ICT= isovolumic contraction time, ET= ejection time, IRT= isovolumic relaxation time, DFT= diastolic filling time, ECG= electrocardiogram
Figure 3:
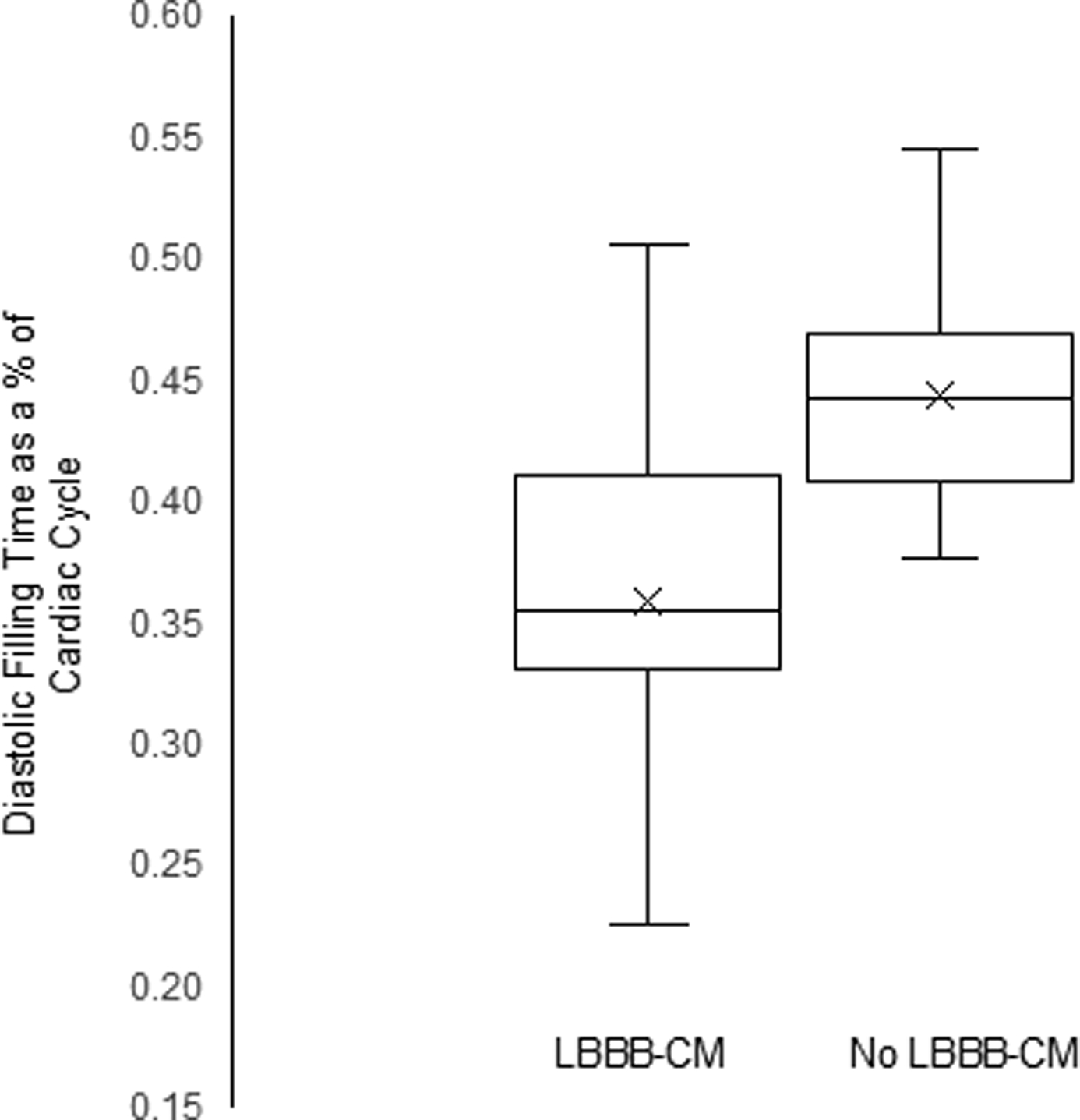
Box and whisker plot of diastolic filling time as a % of the cardiac cycle among patients who developed left bundle branch block-associated decreased left ventricular ejection fraction and those who did not. Median is solid line, box demarcates the 25th and 75th percentiles, and whiskers mark the minimum and maximum values, the X marks the mean. LVEF= left ventricular ejection fraction.
Logistic regression modeling including DFT as a percentage of the cardiac cycle as the sole variable had a Wald Chi-square of 22.7 and a C-statistic of 0.86 (P<0.0001) for prediction of decreased LVEF. A DFT interval <38% of the cardiac cycle had a sensitivity of 68.8%, a specificity of 97.3%, a positive predictive value of 91.7% and a negative predictive value of 86.8% for the development of decreased LVEF. Patients with a DFT interval accounting for <38% of the cardiac cycle had a relative risk of developing decreased LVEF of 6.97 (95% CI 3.0–16.0) compared to patients with a DFT interval accounting for ≥38% of the cardiac cycle.
Logistic regression modeling including DFT as a % of the cardiac cycle and baseline LVEF as the only included variables had a Wald Chi-square of 27.0 and a C-statistic of 0.90, (P<0.0001) for prediction of decreased LVEF. The multivariable model had a sensitivity of 93.8%, specificity of 82.3%, positive predictive value of 71.4% and negative predictive value of 96.6% for the development of decreased LVEF.
Discussion
In our cohort of 50 patients with LBBB and normal LVEF, 16 (32%) developed decreased LVEF. Baseline patient characteristics were not associated with the risk of developing decreased LVEF, however baseline echocardiographic variables including lower LVEF, longer ICT and IRT, and shorter DFT were associated with the risk of developing decreased LVEF.
The current ACCF/HRS/AHA/ASE/HFSA/SCAI/SCCT/SCMR appropriate use criteria statement supports the use of CRT as a class I indication for treatment of HF in patients with LBBB, QRS duration ≥150ms, NYHA class II-IV HF, and LVEF ≤35%16 Few data exist supporting the use of CRT in patients with an LVEF >35%17, despite this, CRT implantation receives a class IIb indication among patients with LBBB, LVEF 36–50%, and QRSd >150ms18. LBBB associated decreased LVEF rarely improves with the use of guideline directed medical therapies but has a high likelihood of improving with CRT, particularly if it is implemented early after diagnosis2,4,19. Our data suggest that echocardiographic assessment of DFT using tissue Doppler imaging may facilitate the identification of patients with LBBB at high risk for developing decreased LVEF. Based on these findings, patients with LBBB and LVEF ≥50% who have a DFT that accounts for <38% of the cardiac cycle may benefit from close clinical and echocardiographic follow-up to facilitate early CRT implantation. Additionally, future studies evaluating whether prophylactic CRT implantation may prevent decreased LVEF in this high-risk group may be warranted.
Recently a variety of clinical20 and echocardiographic21 characteristics have been evaluated to determine if it is possible to predict the development of LBBB associated reduced LVEF. LV hypertrophy, defined as LV mass >300g predicted a higher likelihood of developing decreased LVEF in one series21. Dyssynchronous LV contraction results in early septal contraction against a passive lateral wall resulting in stretching of the contractile apparatus of the lateral wall. This additional stretch leads to abnormally vigorous late contraction of the lateral wall by the Starling mechanism22. Over time this vigorous contraction can lead to lateral wall hypertrophy23. We did not find any association of LV hypertrophy or HF with preserved EF with the likelihood of development of decreased LVEF. Specifically, we found no association between the lateral wall thickness and risk of development of decreased LVEF. This may be due to the strict entry criteria employed in our study, designed to reduce the likelihood of including patients at high risk for developing decreased LVEF from causes other than LBBB.
Isovolumic contraction, ejection, isovolumic relaxation and diastolic filling must all occur within the fixed period of the cardiac cycle as determined by the heart rate. Assuming the heart rate remains fixed, prolongation of one cardiac phase necessitates shortening of others (Figure 2). When LBBB initially develops, the newly dyssynchronous LV contraction results in immediate attenuation of dP/dTmaximum24 and prolongation of isovolumic contraction. Similarly, dyssynchronous LV relaxation results in immediate attenuation of dP/dTminimum23 and prolongation of isovolumic relaxation. Prolongation of the isovolumic phases with an unchanged heart rate may then result in compression of the DFT as observed in our patients. It is conceivable that chronically compressed DFT may cause chronic LV underfilling and symptomatic HF with preserved EF in patients with LBBB, particularly at faster heart rates. Chronic LV underfilling due to compressed DFT may also contribute to the protein dysregulation and Ca++ handling abnormalities observed among patients who go on to develop LV dilation and reduced LVEF25. Conversely, compressed DFT may simply be an effective marker of the severity of LV dysfunction occurring as a result of LBBB induced dyssynchrony. Further experimental work evaluating the effect of DFT compression on the fundamental mechanisms of LV remodeling are warranted.
This is a single center retrospective series designed to explore whether baseline echocardiographic data could predict subsequent development of decreased LVEF. As such, it is limited by the referral and ascertainment biases of this study design. The patients were carefully selected and had few cardiovascular comorbidities to try to ensure we captured LBBB associated decreased LVEF and minimized the frequency of other causes of decreased LVEF. The findings should be verified in larger datasets from other locations. The small sample size is a result of the strict entry criteria applied to increase the specificity of the findings for prediction of LBBB associated decreased LVEF over other causes of decreased LVEF. This small sample size limited our ability to perform extensive multivariable analyses and may have prevented us from identifying other weaker associations between echocardiographic, electrocardiographic, and clinical variables and the development of LBBB associated decreased LVEF. The derived predictive model should be validated in an independent validation cohort.
In conclusion, echocardiographic tissue Doppler imaging assessment of DFT predicted the risk of developing decreased LVEF among patients with LBBB and normal baseline LVEF.
Disclosures:
Dr. Atwater receives research support from Boston Scientific, Abbott Medical and Honoraria from Abbott Medical, Medtronic, and Biotronik. Dr. Sze has received research funding from Medtronic. Dr. Black-Maier receives research support from Boston Scientific. Dr. Loring is supported in part by an NIH T32 training grant (#5T32HL069749) and receives research support from Boston Scientific, and serves as a consultant to Huxley Medical. All other authors have reported no relevant disclosures to this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Sze E, Dunning A, Loring Z, Atwater BD, Chiswell K, Daubert JP, Kisslo JA, Mark DB, Velazquez EJ, Samad Z. Comparison of Incidence of Left Ventricular Systolic Dysfunction Among Patients With Left Bundle Branch Block Versus Those With Normal QRS Duration. Am J Cardiol 2017;120:1990–1997. [DOI] [PubMed] [Google Scholar]
- 2.Sze E, Samad Z, Dunning A, Campbell KB, Loring Z, Atwater BD, Chiswell K, Kisslo JA, Velazquez EJ, Daubert JP. Impaired Recovery of Left Ventricular Function in Patients With Cardiomyopathy and Left Bundle Branch Block. J Am Coll Cardiol 2018;71:306–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang NC, Singh M, Adelstein EC, Jain SK, Mendenhall GS, Shalaby AA, Voigt AH, Saba S. New-onset left bundle branch block-associated idiopathic nonischemic cardiomyopathy and left ventricular ejection fraction response to guideline-directed therapies: The NEOLITH study. Heart Rhythm 2016;13:933–942. [DOI] [PubMed] [Google Scholar]
- 4.Wang NC, Li JZ, Adelstein EC, Althouse AD, Sharbaugh MS, Jain SK, Mendenhall GS, Shalaby AA, Voigt AH, Saba S. New-onset left bundle branch block-associated idiopathic nonischemic cardiomyopathy and time from diagnosis to cardiac resynchronization therapy: The NEOLITH II study. Pacing Clin Electrophysiol 2018;41:143–154. [DOI] [PubMed] [Google Scholar]
- 5.Moss AJ, Hall WJ, Cannom DS, Klein H, Brown MW, Daubert JP, Estes NA 3rd, Foster E, Greenberg H, Higgins SL, Pfeffer MA, Solomon SD, Wilber D, Zareba W, Investigators M-CT. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med 2009;361:1329–1338. [DOI] [PubMed] [Google Scholar]
- 6.Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L, Cardiac Resynchronization-Heart Failure Study I. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 2005;352:1539–1549. [DOI] [PubMed] [Google Scholar]
- 7.Friedman DJ, Emerek K, Sogaard P, Vejdani-Jahromi M, Kisslo J, Atwater BD. The mechanical and hemodynamic effects of left ventricular pacing in heart failure with preserved ejection fraction and left bundle branch block. J Electrocardiol 2018;51:859–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ersboll M, Schulte PJ, Al Enezi F, Shaw L, Kober L, Kisslo J, Siddiqui I, Piccini J, Glower D, Harrison JK, Bashore T, Risum N, Jollis JG, Velazquez EJ, Samad Z. Predictors and progression of aortic stenosis in patients with preserved left ventricular ejection fraction. Am J Cardiol 2015;115:86–92. [DOI] [PubMed] [Google Scholar]
- 9.Horvath MM, Winfield S, Evans S, Slopek S, Shang H, Ferranti J. The DEDUCE Guided Query tool: providing simplified access to clinical data for research and quality improvement. J Biomed Inform 2011;44:266–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Surawicz B, Childers R, Deal BJ, Gettes LS, Bailey JJ, Gorgels A, Hancock EW, Josephson M, Kligfield P, Kors JA, Macfarlane P, Mason JW, Mirvis DM, Okin P, Pahlm O, Rautaharju PM, van Herpen G, Wagner GS, Wellens H, American Heart Association E, Arrhythmias Committee CoCC, American College of Cardiology F, Heart Rhythm S. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part III: intraventricular conduction disturbances: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol 2009;53:976–981. [DOI] [PubMed] [Google Scholar]
- 11.Lancellotti P, Moura L, Pierard LA, Agricola E, Popescu BA, Tribouilloy C, Hagendorff A, Monin JL, Badano L, Zamorano JL, European Association of E. European Association of Echocardiography recommendations for the assessment of valvular regurgitation. Part 2: mitral and tricuspid regurgitation (native valve disease). Eur J Echocardiogr 2010;11:307–332. [DOI] [PubMed] [Google Scholar]
- 12.Lancellotti P, Tribouilloy C, Hagendorff A, Moura L, Popescu BA, Agricola E, Monin JL, Pierard LA, Badano L, Zamorano JL, European Association of E. European Association of Echocardiography recommendations for the assessment of valvular regurgitation. Part 1: aortic and pulmonary regurgitation (native valve disease). Eur J Echocardiogr 2010;11:223–244. [DOI] [PubMed] [Google Scholar]
- 13.Baumgartner H, Hung J, Bermejo J, Chambers JB, Evangelista A, Griffin BP, Iung B, Otto CM, Pellikka PA, Quinones M, American Society of E, European Association of E. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr 2009;22:1–23; quiz 101–102. [DOI] [PubMed] [Google Scholar]
- 14.Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Alexandru Popescu B, Waggoner AD, Houston T, Oslo N, Phoenix A, Nashville T, Hamilton OC, Uppsala S, Ghent Liege B, Cleveland O, Novara I, Rochester M, Bucharest R, St. Louis M. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2016;17:1321–1360. [DOI] [PubMed] [Google Scholar]
- 15.Kim H, Yoon HJ, Park HS, Cho YK, Nam CW, Hur SH, Kim YN, Kim KB. Usefulness of tissue Doppler imaging-myocardial performance index in the evaluation of diastolic dysfunction and heart failure with preserved ejection fraction. Clin Cardiol 2011;34:494–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Russo AM, Stainback RF, Bailey SR, Epstein AE, Heidenreich PA, Jessup M, Kapa S, Kremers MS, Lindsay BD, Stevenson LW. ACCF/HRS/AHA/ASE/HFSA/SCAI/SCCT/SCMR 2013 appropriate use criteria for implantable cardioverter-defibrillators and cardiac resynchronization therapy: a report of the American College of Cardiology Foundation appropriate use criteria task force, Heart Rhythm Society, American Heart Association, American Society of Echocardiography, Heart Failure Society of America, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance. Heart rhythm : the official journal of the Heart Rhythm Society 2013;10:e11–58. [DOI] [PubMed] [Google Scholar]
- 17.Witt CM, Wu G, Yang D, Hodge DO, Roger VL, Cha YM. Outcomes With Left Bundle Branch Block and Mildly to Moderately Reduced Left Ventricular Function. JACC Heart Fail 2016;4:897–903. [DOI] [PubMed] [Google Scholar]
- 18.Kusumoto FM, Schoenfeld MH, Barrett C, Edgerton JR, Ellenbogen KA, Gold MR, Goldschlager NF, Hamilton RM, Joglar JA, Kim RJ, Lee R, Marine JE, McLeod CJ, Oken KR, Patton KK, Pellegrini CN, Selzman KA, Thompson A, Varosy PD. 2018 ACC/AHA/HRS Guideline on the Evaluation and Management of Patients With Bradycardia and Cardiac Conduction Delay: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2019;74:e51–e156. [DOI] [PubMed] [Google Scholar]
- 19.Wang NC, Adelstein EC, Singh M, Voigt AH, Saba S. Left Bundle Branch Block-Associated Cardiomyopathies and Early Cardiac Resynchronization Therapy: Conceptualizing a Tailored Approach. J Am Coll Cardiol 2018;71:1943–1944. [DOI] [PubMed] [Google Scholar]
- 20.Hashemi Jazi M, Nilforoush P, Gharipour M, Batvandi A, Mohammadi R, Najafi R. Clinical determinants of left ventricular ejection fraction deterioration in patients suffered from complete left bundle branch block. Iran Red Crescent Med J 2015;17:e16570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Angheloiu GO, Saul M, Edelman K, Shah H, Mezu UL, Saba S. Predictors of left ventricular function deterioration in patients with left bundle branch block and ejection fraction >50%. Congest Heart Fail 2013;19:E1–4. [DOI] [PubMed] [Google Scholar]
- 22.Sarnoff SJ, Berglund E. Ventricular function. I. Starling’s law of the heart studied by means of simultaneous right and left ventricular function curves in the dog. Circulation 1954;9:706–718. [DOI] [PubMed] [Google Scholar]
- 23.Vernooy K, Verbeek XA, Peschar M, Crijns HJ, Arts T, Cornelussen RN, Prinzen FW. Left bundle branch block induces ventricular remodelling and functional septal hypoperfusion. Eur Heart J 2005;26:91–98. [DOI] [PubMed] [Google Scholar]
- 24.Byrne MJ, Helm RH, Daya S, Osman NF, Halperin HR, Berger RD, Kass DA, Lardo AC. Diminished left ventricular dyssynchrony and impact of resynchronization in failing hearts with right versus left bundle branch block. Journal of the American College of Cardiology 2007;50:1484–1490. [DOI] [PubMed] [Google Scholar]
- 25.Spragg DD, Leclercq C, Loghmani M, Faris OP, Tunin RS, DiSilvestre D, McVeigh ER, Tomaselli GF, Kass DA. Regional alterations in protein expression in the dyssynchronous failing heart. Circulation 2003;108:929–932. [DOI] [PubMed] [Google Scholar]


