Abstract
The oncometabolite L-2-hydroxyglutarate (L-2HG) is considered an abnormal product of central carbon metabolism that is capable of disrupting chromatin architecture, mitochondrial metabolism, and cellular differentiation. Under most circumstances, mammalian tissues readily dispose of this compound, as aberrant L-2HG accumulation induces neurometabolic disorders and promotes renal cell carcinomas. Intriguingly, Drosophila melanogaster larvae were recently found to accumulate high L-2HG levels under normal growth conditions, raising the possibility that L-2HG plays a unique role in insect metabolism. Here we explore this hypothesis by analyzing L-2HG levels in 18 insect species. While L-2HG was present at low-to-moderate levels in most of these species (<100 pmol/mg; comparable to mouse liver), dipteran larvae exhibited a tendency to accumulate high L-2HG concentrations (>100 pmol/mg), with the mosquito Aedes aegypti, the blow fly Phormia regina, and three representative Drosophila species harboring concentrations that exceed 1 nmol/mg - levels comparable to those measured in mutant mice that are unable to degrade L-2HG. Overall, our findings suggest that one of the largest groups of animals on earth commonly generate high concentrations of an oncometabolite during juvenile growth, hint at a role for L-2HG in the evolution of dipteran development, and raise the possibility that L-2HG metabolism could be targeted to restrict the growth of key disease vectors and agricultural pests.
Keywords: L-2-hydroxyglutarate, oncometabolite, hypoxia, Drosophila
Graphical Abstract

INTRODUCTION
The field of cancer metabolism has become increasingly focused on how small molecule metabolites regulate cell proliferation and promote cancer progression (Martinez-Reyes and Chandel, 2020). In this regard, a number of compounds have emerged as oncometabolites - pro-growth molecules that enhance tumor growth by interfering with gene expression, epigenetic modifications, mitochondrial physiology, and signal transduction cascades (Mullen and DeBerardinis, 2012; Yang et al., 2013; Ye et al., 2018). These compounds, however, are not simply cancer-causing molecules, but also serve essential roles in normal metabolism and physiology. For example, the first molecules implicated as oncometabolites were the tricarboxylic acid intermediates fumarate and succinate (Raimundo et al., 2011), both of which play ancient and essential roles in energy production. Similarly, the oncometabolite D-2-hydroxyglutarate (D-2HG), which is perhaps best known for promoting glioblastoma (Ye et al., 2018), also serves normal metabolic roles in bacteria, yeast, and even humans (Becker-Kettern et al., 2016; Struys et al., 2005; Zhang et al., 2017). Such examples illustrate how oncometabolites act in diverse and important metabolic mechanism across all kingdoms of life and suggest that studying normal oncometabolite function can advance our understanding of how these molecules induce human disease.
Among known oncometabolites, the compound L-2HG stands out as unusual because eukaryotes lack enzymes dedicated to L-2HG production. Mammalian cells synthesize L-2HG as a result of promiscuous Lactate Dehydrogenase (Ldh) and Malate Dehydrogenase (Mdh) activity and degrade this compound via the enzyme L-2HG dehydrogenase (L2HGDH) (Ye et al., 2018). Most mammalian tissues, with the exception of the mouse testis, maintain low L-2HG concentrations and inappropriate L-2HG accumulation can induce dramatic changes in epigenetic modifications, central carbon metabolism, and growth factor signaling (Ma et al., 2017; Teng et al., 2016; Ye et al., 2018). As a result, nearly all in vivo studies of L-2HG focus on the detrimental effects of this compound in neurological disorders and renal cell carcinoma, thus the question remains as to whether L-2HG, like the other oncometabolites, serves a normal physiological role (Ma et al., 2017; Shim et al., 2014; Ye et al., 2018). In this regard, several studies have observed that cultured mammalian cells accumulate excess L-2HG in response to oxidative stress, with hypoxia, acidic pH, and elevated NADH levels enhancing L-2HG synthesis and accumulation (Intlekofer et al., 2015; Intlekofer et al., 2017; Mullen et al., 2014; Nadtochiy et al., 2016; Oldham et al., 2015; Reinecke et al., 2012; Teng et al., 2016). These cell culture studies suggest that L-2HG metabolism could act as a metabolic signaling molecule that helps cellular physiology adapt to redox stress; however, little is known about L-2HG function in vivo.
One of the only examples of a healthy animal accumulating high L-2HG levels in a regulated and predictable manner is during larval development of the fruit fly Drosophila melanogaster (Li et al., 2017). While the exact reason for why Drosophila melanogaster larvae accumulate L-2HG remains to be elucidated, this observation raises the possibility that L-2HG serves a unique role in insects and suggests that comparative studies of insect metabolism could illuminate the endogenous function of this oncometabolite. Towards this goal, we used gas chromatography-mass spectrometry to quantify L- and D-2HG levels in 18 species of insects. Our analysis revealed that representative species from order Diptera seem particularly adept at accumulating very high L-2HG concentrations during larval development. Moreover, we demonstrate that while dipteran larvae can generate excess L-2HG in response to hypoxia, larvae generate high concentrations of this compound regardless of oxygen concentration. This finding indicates that one of the largest and most diverse animal orders on the planet commonly produces high concentrations of an oncometabolite in a developmentally-regulated manner and suggests that further studies of dipteran L-2HG metabolism could help elucidate the endogenous functions of this compound. In addition, our study suggests the L-2HG metabolism serves a unique role in the dipteran development, thus raising the possibility that production of this compounds could be used to control populations of common disease vectors and agricultural pests.
METHODS
Insect Husbandry
Aedes aegypti:
RexD (Puerto Rico) derived Higgs White Eye (HWE) strain were maintained in an insect incubator (Percival Model I-36VL, Perry, IA, USA) at 28°C and 75% relative humidity with a 12h:12h light:dark cycle. Larvae were reared in freshwater (dH2O) at a density of 200 larvae/L of water. Water was changed every other day. Each larval cup was fed a 4:1 mixture of finely ground fish pellets to baker’s yeast a day. Adult mosquitos were fed 10mL of 10% sucrose daily via cotton balls. Samples contained of 10–20 mg of third instar larvae.
Apis mellifera:
Bee samples were collected from a colony established and maintained by Dr. Irene Newton’s lab at Indiana University-Bloomington. Samples were collected in 2018 during the months of June, July, and August. For both larvae and adults, each sample contained an individual animal. Adult samples consisted of workers.
Drosophila species:
All Drosophila species were maintained Drosophila Stock Center (BDSC) media at 25°C. The Drosophila melanogaster strain w1118 was used for all experiments. Drosophila hydei and Drosophila busckii cultures, which were kindly provided by Dr. Irene Newton’s lab (Indiana University-Bloomington, USA), are derived from wild isolates collected in Brown County, Indiana.
For all species, virgin males and females were collected immediately following eclosion and aged for 3 days on BDSC media prior to collection or treatment. For larval analyses, embryos were collected on molasses agar with covered with yeast paste as previously described (Li and Tennessen, 2018). Larvae were allowed to develop for 60 hrs (D. melanogaster and D. busckii) or 84 hrs (D. hydei) prior to collection. For hypoxic and hyperoxic treatments, larvae were placed in 35mm plates with Whatman filter paper at the bottom that was wetted with 2 ml of phosphate buffer saline (PBS; pH 7.4) and contained approximately 500 mg of yeast paste in the center. Regardless of treatment or age, larval samples were collected by placing ~20 mg of larvae in a 1.5 ml microfuge tube on ice. Larvae were washed at least three times using ice-cold PBS to remove all yeast and debris from the sample. Following the final wash, all PBS was removed from the sample and the tube was flash frozen in liquid nitrogen.
Galleria mellonella:
Larvae were purchased from Carolina Biological Supply (Item # 143928) and raised on the provided media according to the distributors care sheet. All samples contained a single individual.
Libellulida species and Enallagma species.:
Nymphs were purchased from Carolina Biological Supply (Libellulida species, 143526; Enallagma species, 143520) and maintained following the supplier’s recommendation. For collections, individual nymphs were removed from the culture, briefly patted dry, placed in a pre-tared 2 mL tube containing 1.4 mm ceramic beads, massed, and flash frozen in liquid nitrogen.
Phormia regina:
All flies were collected from a laboratory colony (> 5 generations) that was generated from wild-caught P. regina (collected from Military Park, Indianapolis, IN, USA) and maintained in a 30 × 30 × 30cm cage (Bioquip, Rancho Dominguez, CA) within the IUPUI “fly room.” The colony was reared at ~25°C ambient temperature, 60% ambient humidity and 24 hr light and were provided sugar and water ad libitum. Chicken liver was provided to the colony ~ 1 week post-eclosion for ovary maturation. 2–4 days following ovary maturation, chicken liver (25g) was provided as the egg oviposition substrate for a period of 4–6 hrs. Following oviposition, the cup containing the chicken liver and eggs were placed in a one-quart glass jar half-filled with fine pine shavings (Lanjay Inc., Montreal, QC). Larvae were allowed to develop under ambient conditions. For sample collection, an individual third instar larva was placed in a 1.5 ml microfuge tube on ice. Samples were washed as described for the Drosophila species (see above). For adult analysis, adult flies were randomly collected 3 – 5 days post-emergence from the lab colony.
Oncopeltus fasciatus:
Milkweed bugs were obtained from Carolina Biological Supply (Item # 143800) and maintained on organic sunflower seeds.
Hermetia illucens:
Larval cultures were shipped from Dr. Jeffery Tomberlin’s lab (Texas A&M University; College Station, Texas, United States) and collected upon arrival. Each sample contained an individual larva. Cultures were maintained at ambient temperature and adults were collected upon emergence.
Musca domestica:
Larvae were purchased from Carolina Biological Supply (Item #144410) and raised on Instant House Fly Medium (Item # 144424) at 25°C. Individual larva were collected in a 1.5 ml microfuge tube and immediately placed on ice. Samples were washed as described for the Drosophila species (see above). All samples contained a single individual.
Manduca sexta:
Larvae were obtained from Carolina Biological Supply (#143886) and maintained at room temperature on hornworm diet (Carolina Biological Supply; #143910). Animals were collected throughout the L3 stage. Each sample consisted of an individual animal. For larvae with a mass greater than 50 mg, individual animals were homogenized in 800 μl of methanol extraction buffer as described below (see Sample collection and L-2HG Quantification) and the homogenate was immediately diluted 1:2 or 1:4 with additional extraction buffer, depending on the mass of the larvae.
Onthophagus taurus:
Adults were collected from cow dung pads at Busselton, Western Australia (−33° 39’ 8” S, 115° 20’ 43” E) in January 2016 and shipped to Bloomington, IN, for rearing. All beetles were maintained as a single colony in the laboratory at 24°C on a 16 L : 8 D cycle, and fed homogenized cow dung ad libitum following an established protocol (Moczek et al., 2002). In order to obtain offspring, beetles were allowed to breed in plastic containers (25 cm tall × 20 cm in diameter) and filled ~75% with a moist sand:soil mixture. For each round of breeding, six female and three male beetles were added to one breeding container and provisioned with ~0.5 L of homogenized cow dung. Beetles were allowed to breed for one week, at which point they were recaptured and brood balls containing offspring were collected and placed in plastic containers. All samples contained a single individual.
Tribolium castaneum:
Cultures were maintained on King Arthur whole wheat flour supplemented with active dry yeast at 28°C and 55–65% humidity. For larval analysis, 3rd and 4th instar larvae were collected. For adult hypoxia experiment, recently emerged adults were collected and adults were kept on new flour for the duration of the treatment. The strain Vermillion white was used for all the studies in this report.
Gryllodes sigillatus:
Nymphs were obtained from Carolina Biology (item #143558). Upon arrival, cultures were maintained at ambient temperature and fed a diet of dried dog food, lettuce, and apple slices. All samples contained a single individual.
Tenebrio molitor:
Larvae (~100–200 mg; Item #144274 and ~20 mg; Item #144287) and adults (Item #144270) were purchased from Carolina Biological Supply and fed whole wheat flour and apple slices, as per the distributors care sheet. All samples contained an individual animal. For larvae of a mass >100 mg, individual animals were homogenized in 800 ml of methanol extraction buffer as described below (see Sample collection and L-2HG Quantification) and the homogenate was immediately diluted 1:2 or 1:4 with additional extraction buffer, depending on the mass of the larvae. All samples contained a single individual.
Vanessa cardui:
Larvae were obtained from Carolina Biology (Item # 144076) and maintained on the culture media provided by the distributor at ambient temperature. All samples contained a single individual.
Method for Mouse Liver Harvest:
Liver tissue was harvested from male and female C57BL/6J control mice and a previously described L2hgdh knockout (KO) mutant (Brinkley et al., 2020). All mice were aged 2–4 months were given normal chow (Labdiet) ad libitum. For mouse starvation, chow was removed in the evening, 14 hrs prior to tissue harvest. At time of harvest, mice were anesthetized using isoflourane (Vetone) and blood was collected. Mice were then euthanized. Tissues were briefly washed in chilled DPBS (Corning), dried using kimwipes (Kimberly-Clark) and snap frozen using liquid nitrogen. All animal studies were approved by institutional animal care and use committee (IACUC) as previously described (Brinkley et al., 2020).
Sample collection and L-2HG Quantification
For pooled samples that contained multiple individuals, samples were collected in 1.5 ml microfuge tubes and flash frozen in liquid nitrogen. Prior to homogenization, tubes were removed from liquid nitrogen, the cap end swiftly pounded against the desktop to dislodge the sample, and the pellet was transferred into a pre-tared 2 ml screwcap tube containing 1.4 mm ceramic beads. The sample mass was immediately measured with an analytical balance, the tube was flash frozen in liquid nitrogen, and samples were stored at −80°C. For samples that contained an individual insect, the animal was collected and immediately transferred into a pre-tared 2 ml screwcap tube containing 1.4 mm ceramic beads. The sample mass was measured using an analytical balance, the tube was flash frozen in liquid nitrogen, and samples were stored at −80 °C.
Homogenization and metabolite extractions were conducted as previously described (Li and Tennessen, 2019). Briefly, samples were removed from the −80°C freezer and placed into a benchtop enzyme cooler pre-chilled to −20°C. 800 μl of pre-chilled methanol extraction buffer (−20°C; 90% methanol diluted with HPLC-grade water + 8 μg (RS)-2-Hydroxy-1,5-pentanedioate-2,3,3-d3) was added to individual sample tubes. Sample were homogenized in an Omni Beadruptor 24. Homogenized samples were returned to a −20°C benchtop enzyme cooler and incubated in a −20°C freezer for one hour. Samples were then centrifuged at ~20,000 × g in a refrigerated centrifuge for 5 minutes to pellet insoluble debris. 600 μl of the supernatant was removed and transferred to a 1.5 ml microfuge tube and dried overnight in a vacuum centrifuge. Samples were then derivatized using a two-step method involving R-2-butanol and acetic anhydride.
Derivatized samples were injected (1.5 μL, 1:5 split ratio) into an Agilent GC6890–5973i instrument using a Gerstel MPS autosampler. Separation of compounds was achieved by gas chromatography (GC) with a Phenomex ZB5–5 MSi column. The GC was programmed to increase temperature as follows: (1) Inlet temperature was set to 250 °C and initial temperature was set to 95 °C with a one-minute hold. (2) Temperature was increased at a rate of 40 °C per minute until it reached 110 °C with a two-minute hold. (3) Temperature was increased a rate of 5 °C per minute to 200 °C. (4) Temperature was increased at a rate of 5 °C per minute to 330 °C followed by a three-minute hold. Selected ion monitoring (SIM mode) was programmed to record m/z ion values 173 for endogenous D-/L-2HG, and 176 for the deuterated D-/L-2HG internal heavy standard. Concentration in each sample was calculated by comparison to the internal standard and normalized to the sample mass.
Hypoxia and Hyperoxia Treatments
For all manipulations of atmospheric oxygen concentration, samples were placed in an air sealed plexiglass chamber equipped with a pressure release valve that was located within a 25°C temperature-controlled room. A Sable systems ROXY-4 gas regulator was used to control oxygen concentration within the chamber. Desired oxygen concentrations were maintained by the ROXY-4 system by injecting either N2 or O2 gas into the chamber.
Statistical Analysis
All data analyses were conducted using JMP v. 14. Prior to analysis, variables were evaluated for normality and homoscedasticity using Shapiro-Wilk and Levene’s tests, respectively. Where these assumptions were met, we used two-tailed pooled t-tests or ANOVA followed by Tukey-Kramer HSD test to determine differences among treatment groups. Where these assumptions were not met, we conducted non-parametric Wilcoxon rank sum or Kruskal-Wallis tests. To compare L-2HG titers across larval insect species with those of mouse samples (see Fig. 1 and 2), we conducted Dunnett’s post hoc test with a Bonferroni adjustment for multiple comparisons.
Figure 1. Adult insects have low levels of L- and D-2HG.
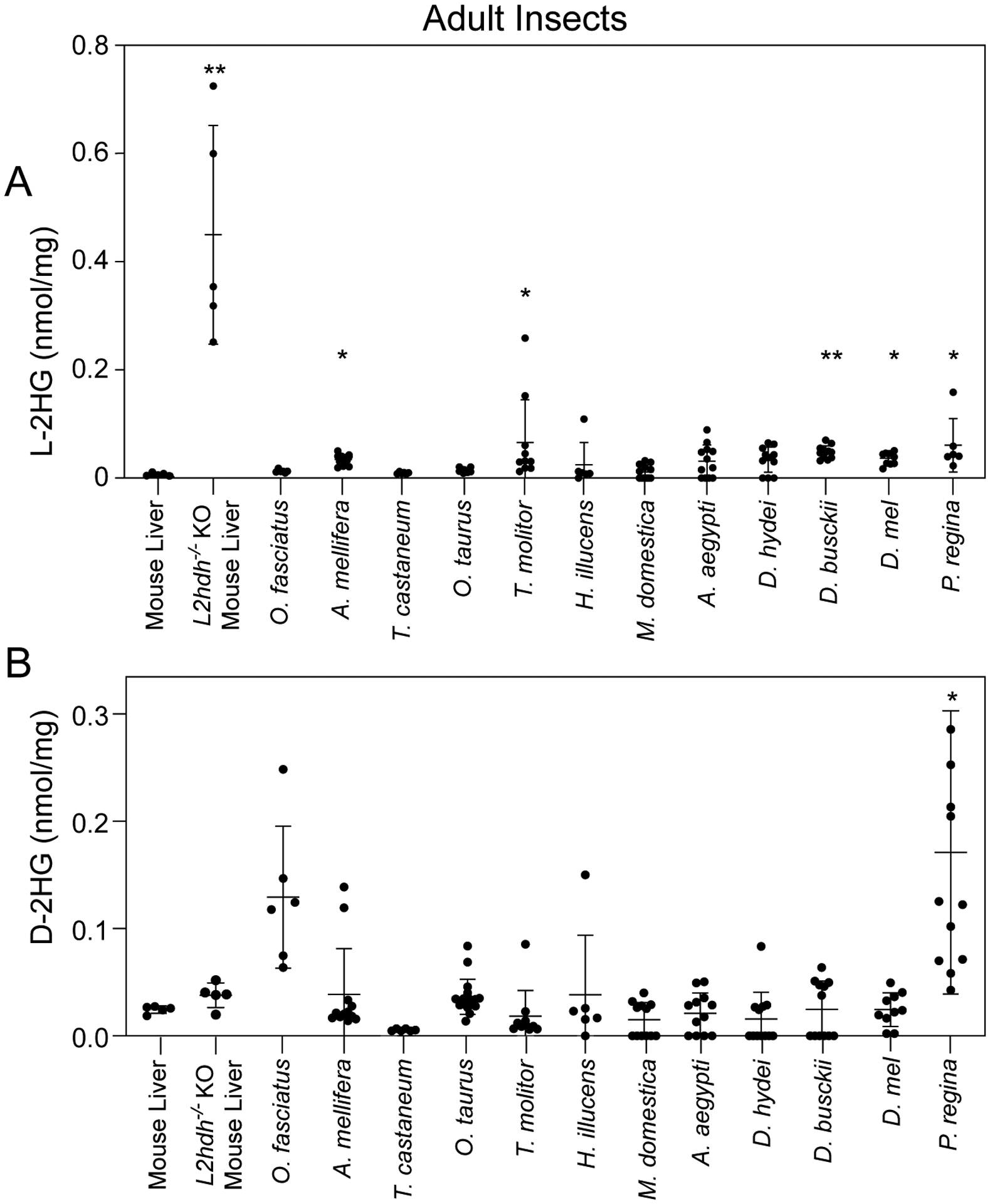
L-2HG (A) and D-2HG (B) levels were measured in adult insects as well as mouse liver samples harvested from wild-type controls and L2ghdh KO mutants. Asterisks indicate that L- or D-2HG levels are significantly higher than those measured in mouse liver. Data are presented in scatter plots with mean ± SD and P-values represent significance relative to wild-type mouse liver samples. *P<0.05. **P<0.01. ***P<0.001. Statistical analysis was conducted using a Kruskal-Wallis test followed by a Dunnett’s post hoc test with a Bonferroni adjustment for multiple comparisons.
Figure 2. Dipteran larvae accumulate high L-2HG levels.
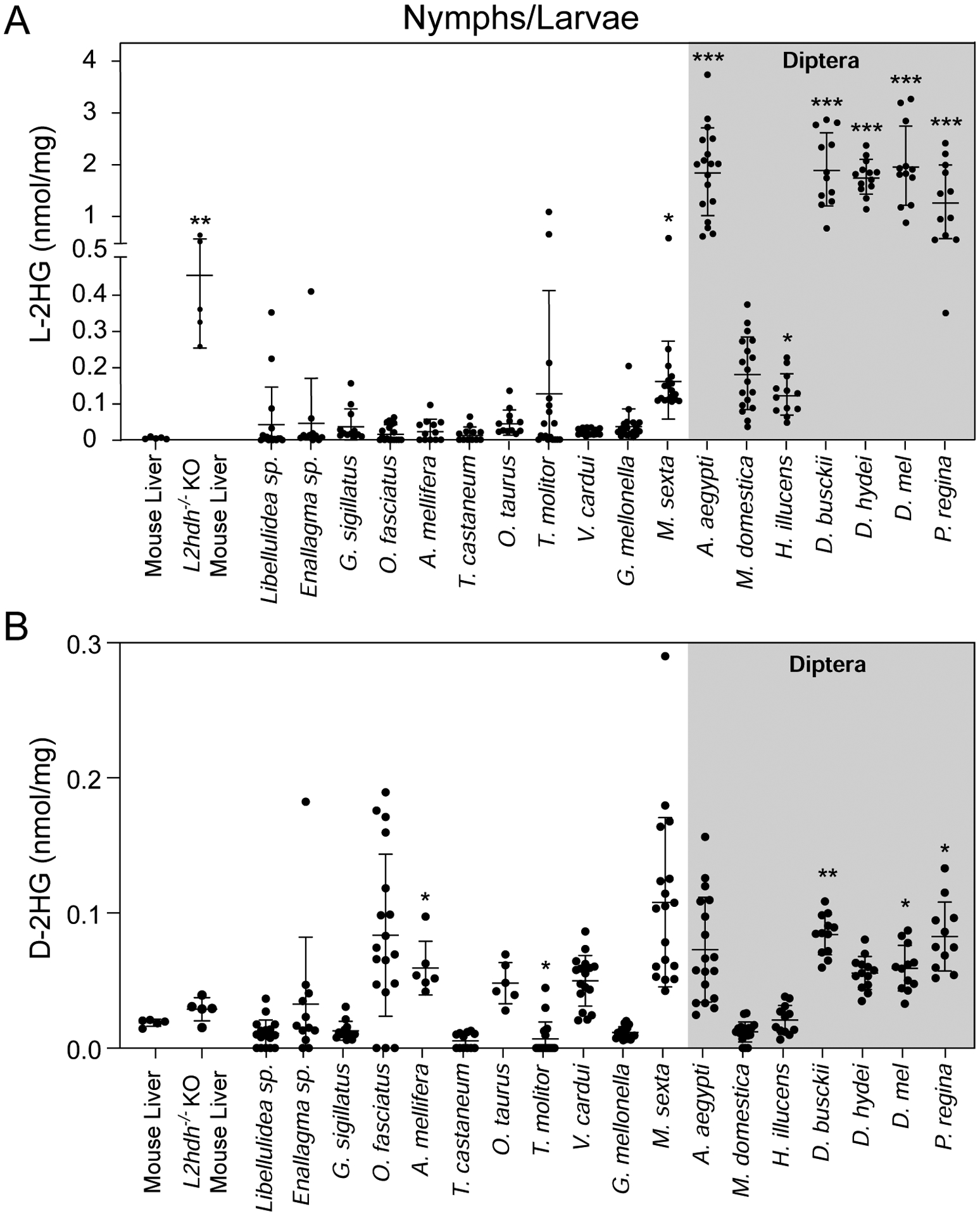
L-2HG (A) and D-2HG (B) levels were measured in juvenile insects as well as mouse liver samples harvested from wild-type controls and L2ghdh KO mutants. Asterisks indicate that L- or D-2HG levels are significantly higher than those measured in mouse liver. Data are presented in scatter plots with mean ± SD. *P<0.05. ***P<0.001. Statistical analysis was conducted using a Kruskal-Wallis test followed by a Dunnett’s post hoc test with a Bonferroni adjustment for multiple comparisons.
RESULTS
Several dipteran species accumulate high L-2HG levels during larval development
Recent findings that D. melanogaster larvae accumulate high L-2HG levels motivated us to determine if this molecule is abundant in other insects. Towards this goal, we used a chiral derivatization method coupled with gas chromatography-mass spectrometry (GC-MS) to quantify L- and D-2HG levels in a diversity of insect species, C57BL/6J mouse liver, which possesses L-2HG levels that are comparable to other mammalian tissues and served as a baseline control in our study, as well as liver tissue from L2hgdh−/− mutant mice that accumulates abnormally high L-2HG levels (Brinkley et al., 2020; Ma et al., 2017; Rzem et al., 2015). We first set out to determine if adult insects of selected species harbored high concentrations of either 2HG enantiomer. Although several species accumulated L- or D-2HG levels that were significantly elevated compared with wild-type mouse liver (Figure 1A and B), the concentration in these animals remained an order of magnitude less than the L-2HG concentration found in D. melanogaster larvae (~ 2 nmol/mg).
Considering that D. melanogaster only generates high L-2HG levels during larval development, we examined the possibility that insect metabolism is predisposed to generating these molecules during juvenile stages (Figure 2A and B). Similar to adult insects, both L-2HG and D-2HG were present at low levels in nymphal stages of four hemimetabolous species (i.e., insects that do not undergo metamorphosis; Figure 2A,B) and larval stages of holometabolous species including the European honey bee (Apis mellifera), representative species from the insect order Coleoptera (beetles), and two lepidopteran (moths and butterflies) species (Vanessa cardui and Galleria mellonella). Moreover, timecourse analyses of L-2HG concentrations within Tenebrio molitor, Vanessa cardui and Galleria mellonella suggest that L-2HG levels remain relatively low throughout the larval growth phase of these insects (Supplemental Figure 1A–C). The only exception among members of these insect orders was the moth Manduca sexta, which accumulated L-2HG levels that were slightly, but significantly, higher than mouse liver (Figure 2A).
In contrast to the insects described above, a majority of the dipteran species examined harbored notably elevated larval L-2HG levels, with the mosquito Aedes aegypti, the blow fly Phormia regina, and multiple members of genus Drosophila (D. melanogaster, D. busckii, and D. hydei) accumulating L-2HG levels that exceeded 1 nmol/mg. These L-2HG concentrations are comparable to those measured in both humans and mice lacking the enzyme L-2-hydroxyglutarate dehydrogenase, which is responsible for degrading L-2HG (Figure 2A,Brinkley et al., 2020; Ma et al., 2017; Rzem et al., 2015; Rzem et al., 2004). In fact, of the seven dipteran species examined in this study, only the house fly, Musca domestica, maintained larval L-2HG levels that were not significantly higher than those observed in normal mouse liver tissue (Figure 2A); however, we note that even these samples contained an average of >100 pmol/mg.
Our findings suggest that dipteran larval metabolism is predisposed towards generating L-2HG. To expand upon these observations, we measured L-2HG concentrations at multiple timepoints during Aedes aegypti and Phormia regina larval development. Consistent with previously studies of Drosophila melanogaster (Li et al., 2017), L-2HG levels in Aedes aegypti larvae remained at concentrations >0.5 nmol/mg at all stages surveyed (Figure 3A). Similarly, L-2HG levels in three of four timepoints sampled during Phormia regina development exceeded 1 nmol/mg (Figure 3B). However, Phormia regina samples from a single timepoint, 96 hr after egg-laying, contained L-2HG concentrations ranging from ~0.2–0.8 nmol/mg – a level significantly below those concentrations measured in other larval timepoints (Figure 3B). Such results raise the possibility that Phormia regina L-2HG levels fluctuate in a developmentally-regulated manner and should be the subject of future investigations. Regardless, even the lowest L-2HG level measured in 96 hr Phormia regina larval samples (0.22 nmol/mg) exceeds the maximum L-2HG concentration measured in nearly all non-dipteran juvenile samples assayed in this study as well as all previously analyzed mammalian tissue samples (Figure 2A, Brinkley et al., 2020; Ma et al., 2017).
Figure 3. L-2HG are elevated at multiple timepoints during Aedes aegypti and Phormia regina larval development.
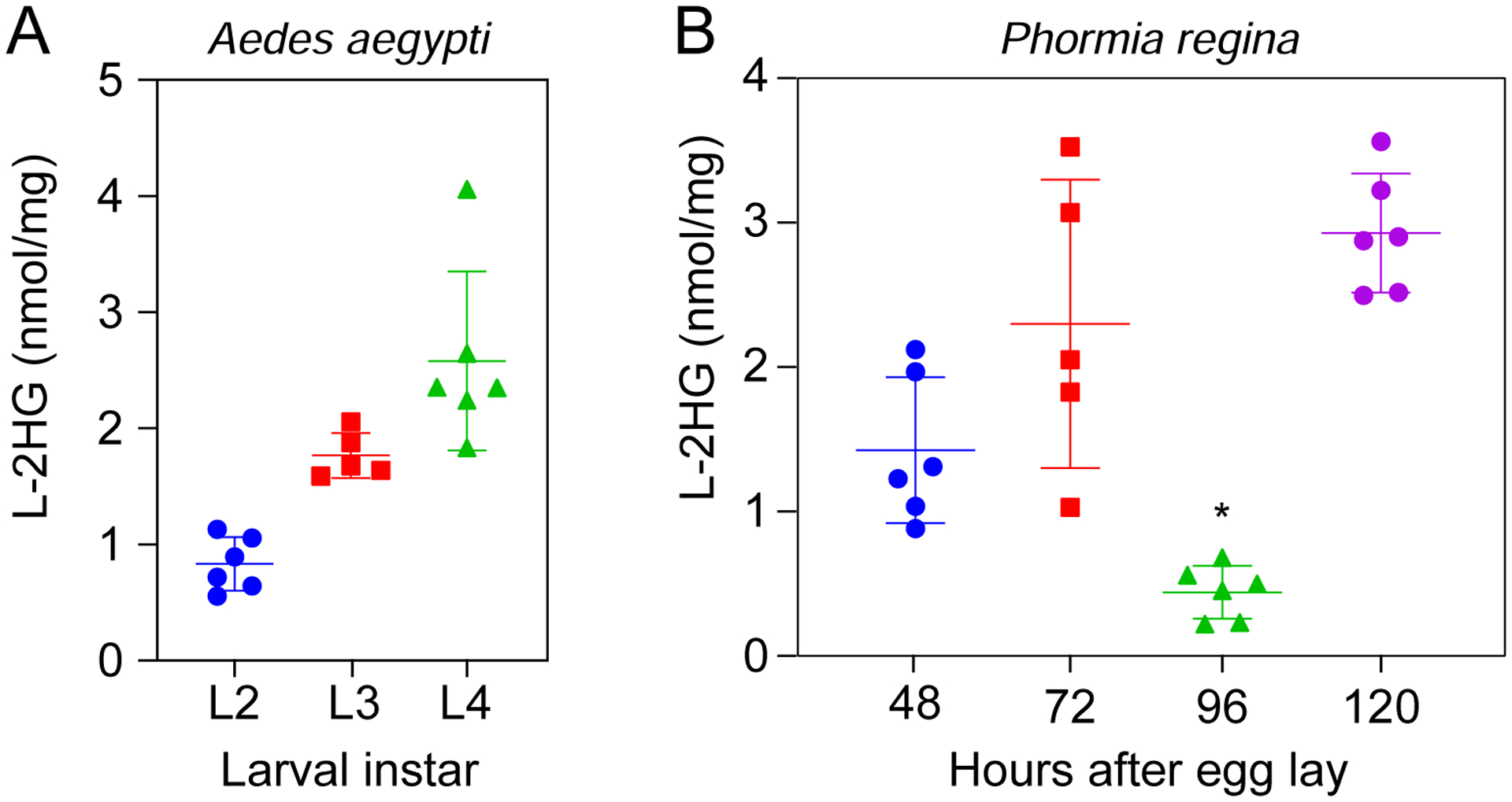
L-2HG levels were measured in (A) Aedes aegypti and (B) Phormia regina at the indicated timepoints during larval development. For Phormia regina (B), samples collected at the 48 hr timepoint contained L2 larvae while those collected at 72 hr, 96 hr, and 120 hr timepoints contained L3 larvae. *P<0.05 for comparisons with all other individual timepoints. Statistical analysis was conducted using a Kruskal-Wallis test followed by a Dunnett’s post hoc test with a Bonferroni adjustment for multiple comparisons.
When compared with L-2HG, D-2HG was present at relatively low levels in all dipteran samples (Figure 2B and Supplemental Figure 2C,D). However, it is noteworthy that three out of five species that have significantly higher D-2HG levels were members of the order Diptera (Figure 2B). Overall, our observations suggest that dipterans exhibit a unique tendency to accumulate L-2HG during larval development.
Dipterans generate excess L-2HG in response to hypoxia
Dipteran larvae develop in moist environments. Since human cells accumulate L-2HG in response to hypoxia and decreased electron transport chain activity (Intlekofer et al., 2015; Mullen et al., 2014; Oldham et al., 2015), we examined the possibility that the dipteran species analyzed herein accumulate high L-2HG levels as the result of growing within a potentially hypoxic environment (e.g., yeast paste, water, and rotting chicken liver). To test this hypothesis, we first determined if hypoxia is capable of inducing L-2HG accumulation in adult males of these species, which normally harbor low L-2HG levels (Figure 1A). Consistent with studies of mammalian cells, adult male Aedes aegypti, D. melanogaster, D. hydeii, and Phormia regina accumulated excess L-2HG when exposed to 1% O2 (~1 kPa O2) for 6 hrs (Figure 4A). Intriguingly, we also observed a slight but significant increase in D-2HG production (Figure 4B), suggesting that hypoxia-dependent production of these two molecules is linked by a yet to be determined mechanism.
Figure 4. Dipteran insects accumulate L-2HG in response to hypoxia.
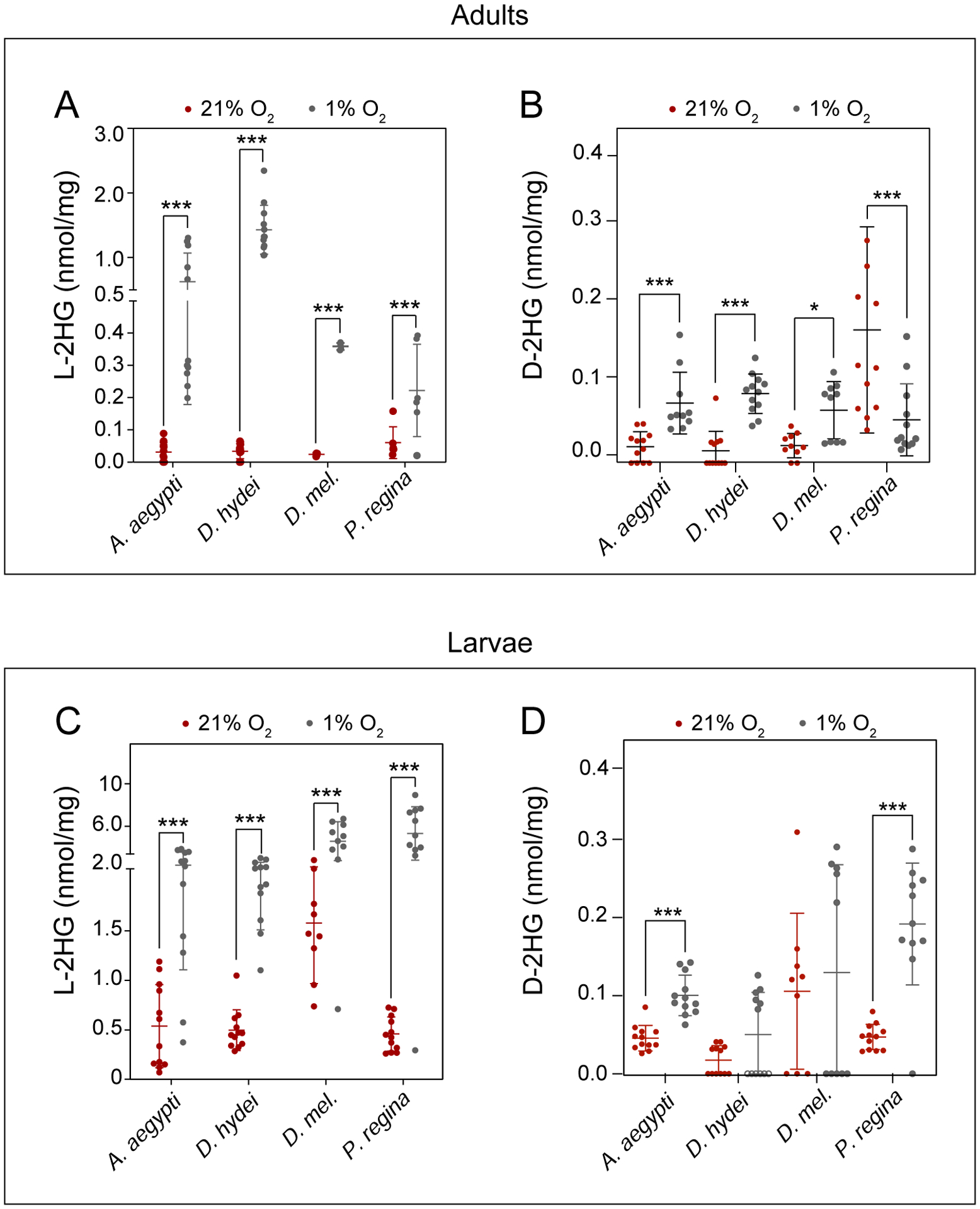
L-2HG and D-2HG levels were measured in select dipteran adults (A, B) or larvae (C, D) following a 6 hr incubation in the presence of 1% O2. Data are presented in scatter plots with mean ± SD. *P<0.05. ***P<0.001. Statistical analysis was conducted using a Kruskal-Wallis test followed by a Dunnett’s post hoc test with a Bonferroni adjustment for multiple comparisons, except for D. mel in (C) and A. aegypti in (B) and (D), which were calculated using a pooled t-test.
We next examined if dipteran larvae, similar to adults, produce L-2HG when exposed to hypoxia. Indeed, when compared with normoxic controls (~20 kPa O2), third instar larvae of Aedes aegypti, D. melanogaster, D. hydeii, and Phormia regina generate significantly higher L-2HG levels than normoxic controls when exposed to 1% O2 for 6 hrs (Figure 4C). In fact, several individual blowfly larvae harbored L-2HG concentrations that exceeded 6 nmol/mg, which are among the highest levels ever recorded in animal tissues, even exceeding the L-2HG levels observed in L2hgdh mutant mouse brains and testis (Figure 4C,Brinkley et al., 2020; Ma et al., 2017; Rzem et al., 2015). Moreover, we also observed a slight but significant increase in D-2HG in hypoxia treated Aedes aegypti and Phormia regina (Figure 4D), reinforcing the possibility that D-2HG levels are sensitive to oxygen concentration. Overall, our observations demonstrate that, similar to mammalian cells, dipterans seem capable of accumulating relatively large quantities of L-2HG in response to hypoxia.
Influence of mild hypoxia and hyperoxia on larval L-2HG accumulation
Our findings that a low oxygen environment results in elevated L-2HG levels raises the question as to whether the high L-2HG concentrations observed in dipteran larvae are simply the result of transient exposure to hypoxia. We tested this possibility by measuring L-2HG levels in larvae following 6 hr exposures to mild hypoxia (5%, an O2 level that delays, but does not arrest larval development, Zhou et al., 2008), normoxia, or hyperoxia (30% O2, 50% O2). If the high L-2HG levels observed in these larvae simply result from hypoxic stress, we predict that L-2HG levels would inversely correlate with O2 concentration. Instead, we observed that larval L-2HG concentrations in Drosophila hydeii, Drosophila melanogaster, and Phormia regina remained unchanged following exposure to mild hypoxia or hyperoxia (Figure 5A). Moreover, a longer 24 hr exposure to the same oxygen concentrations failed to induce changes in L-2HG concentration relative to normoxic controls (Supplemental Figure 3A), suggesting that oxygen availability is not the primary driving force behind L-2HG accumulation in these species. Moreover, our finding that 1 % O2, but not 5 % O2, induces excess L-2HG accumulation supports previous observations in Drosophila melanogaster that larvae mount different physiological responses depending on the severity of hypoxic conditions (Lavista-Llanos et al., 2002).
Figure 5. The effects of mild hypoxia and hyperoxia on larval L-2HG accumulation.
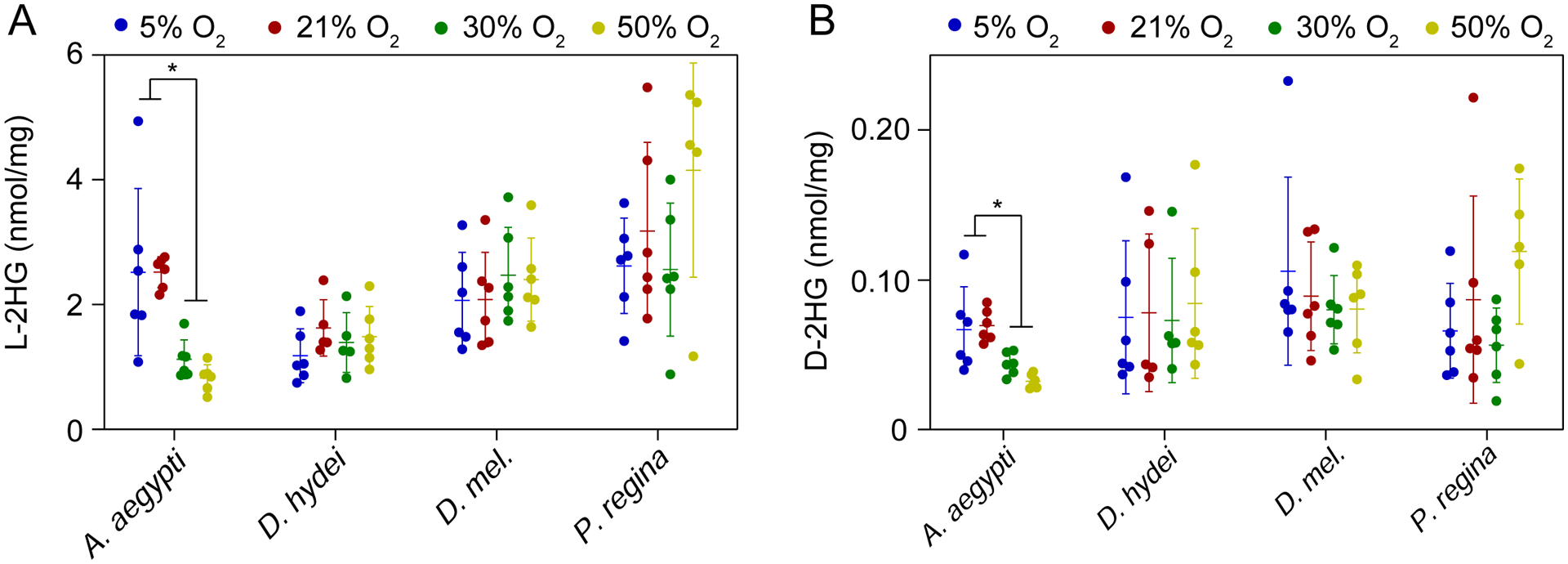
L-2HG (A) and D-2HG (B) levels were measured in select dipteran larvae following a 6 hr incubation in the presence of 5%, 21%, 30% or 50% O2. Data are presented in scatter plots with mean ± SD. *P<0.05. **P<0.01. Statistical analysis was conducted using a Kruskal-Wallis test followed by a Dunnett’s post hoc test with a Bonferroni adjustment for multiple comparisons.
In contrast to Drosophila hydeii, Drosophila melanogaster, and Phormia regina, L-2HG concentrations in Aedes aegypti were significantly lower in the 6 hr hyperoxia treated samples as compared with controls (Figure 5A), suggesting that L-2HG accumulation in these animals are due, in part, to limited oxygen availability within larval tissues. However, despite this difference, the amount present within hyperoxic larvae remain at relatively high levels relative to non-dipteran species, indicating that L-2HG accumulation in Aedes aegypti is likely complex and involves both developmental and physiological causes. This hypothesis would be consistent with recent observations that the Aedes aegypti microbiome generates a hypoxic state within the larval intestine that influences growth and molting (Coon et al., 2017). Intriguingly, we observed no difference in L-2HG concentration among samples collected following 24 hr exposure to either hypoxia or hyperoxia (Supplemental Figure 3A), suggesting that mosquito larval metabolism adapts to the hyperoxic state over extended periods of time. Future studies should examine the significance of this difference as well as the potential role of the gut microbiome in regulating redox metabolism during larval development.
During the course of these experiments, we also observed that concentrations of D-2HG remained relatively unchanged at every O2 condition tested (Figure 5B and Supplemental Figure 3B). Of all the oxygen concentrations measured, the only Aedes aegypti (hypoxia/normoxia vs hyperoxia) and Phormia regina (21% O2 vs 50% O2) displayed any O2 dependent changes in D-2HG levels, and these changes were of modest amounts.
Manduca sexta L-2HG accumulation is sensitive to mild hypoxia
Our survey of 2HG metabolism reveals that Manduca sexta larvae also accumulate significant amounts of L-2HG when compared to mouse liver (Figure 2A). Considering that Manduca sexta larvae become hypoxic during the instar, when growth outpace the tracheal systems ability to exchange oxygen (Callier and Nijhout 2012), L-2HG production in these animals could also be related to hypoxic stress. To determine if Manduca sexta larvae accumulate L-2HG due to growth-induced hypoxia, we first determined if these animals are capable of generating either 2HG enantiomer in response to low O2 levels. Indeed, L3 larvae exposed to 5% O2 for 24 hrs exhibited a dramatic 7-fold increase in L-2HG (Figure 6A), as well as a modest, but statistically significant increase in D-2HG (Figure 6B), thus demonstrating that Manduca sexta larvae generate these molecules in response to hypoxia. Based on this finding, we examined the possibility that Manduca sexta larvae accumulate L-2HG due to hypoxic stress resulting from increased body mass. Our analysis, however, revealed no correlation between L3 body mass and the concentrations of either 2HG enantiomer (Figure 7A,B), suggesting that the L-2HG produced in these animals is likely not the result of changes in body size.
Figure 6. Manduca sexta larvae accumulate L-2HG in response to mild hypoxia.
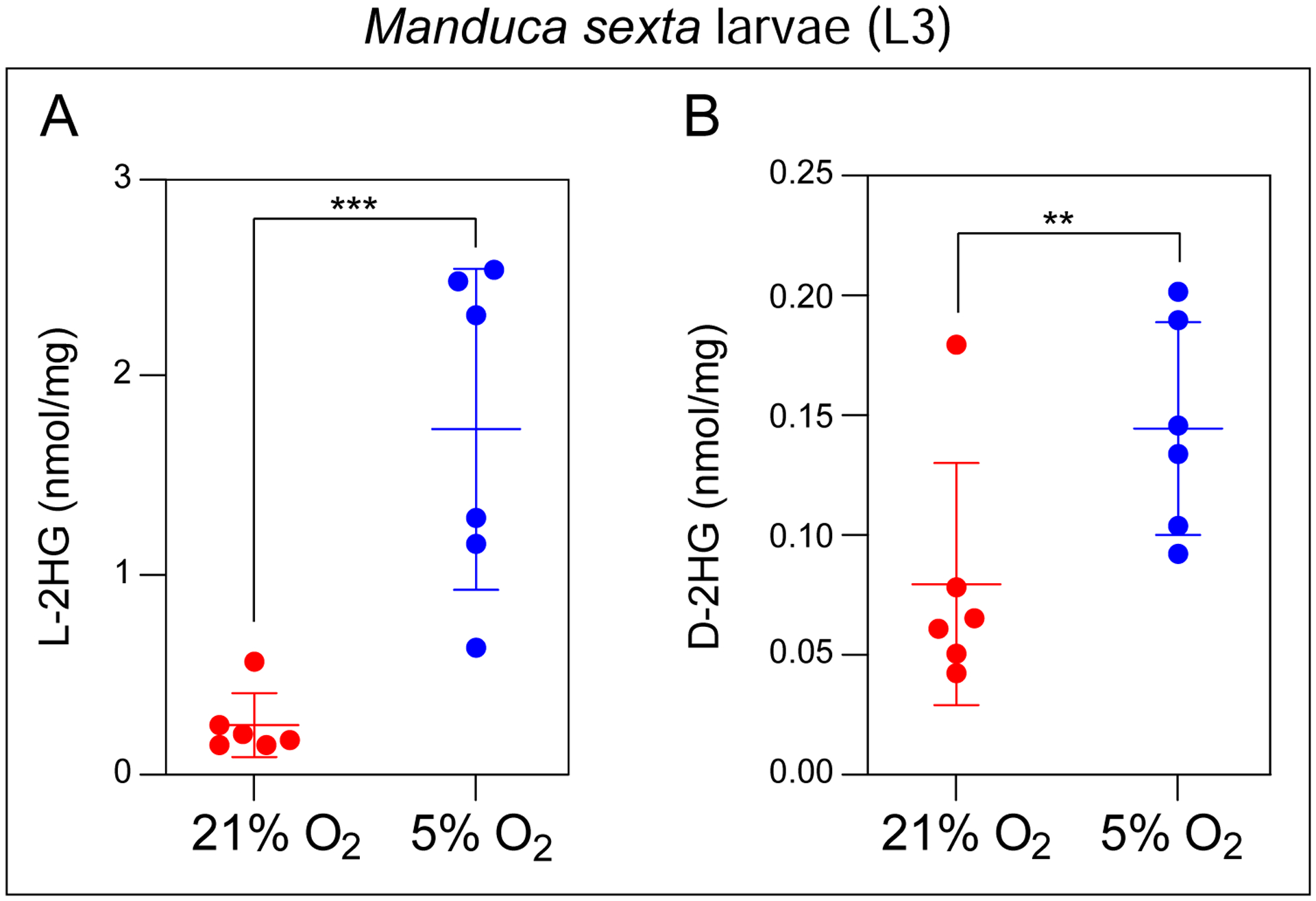
Concentrations of L-2HG (A) and D-2HG (B) were measured in M. sexta larvae following a 24 hr incubation in the presence of 5%, 21% O2. Data are presented in scatter plots with mean ± SD. **P<0.01. ***P<0.001. Statistical analysis was conducted using a Mann-Whitney test.
Figure 7. 2HG concentrations remain constant during Manduca sexta L3 development.
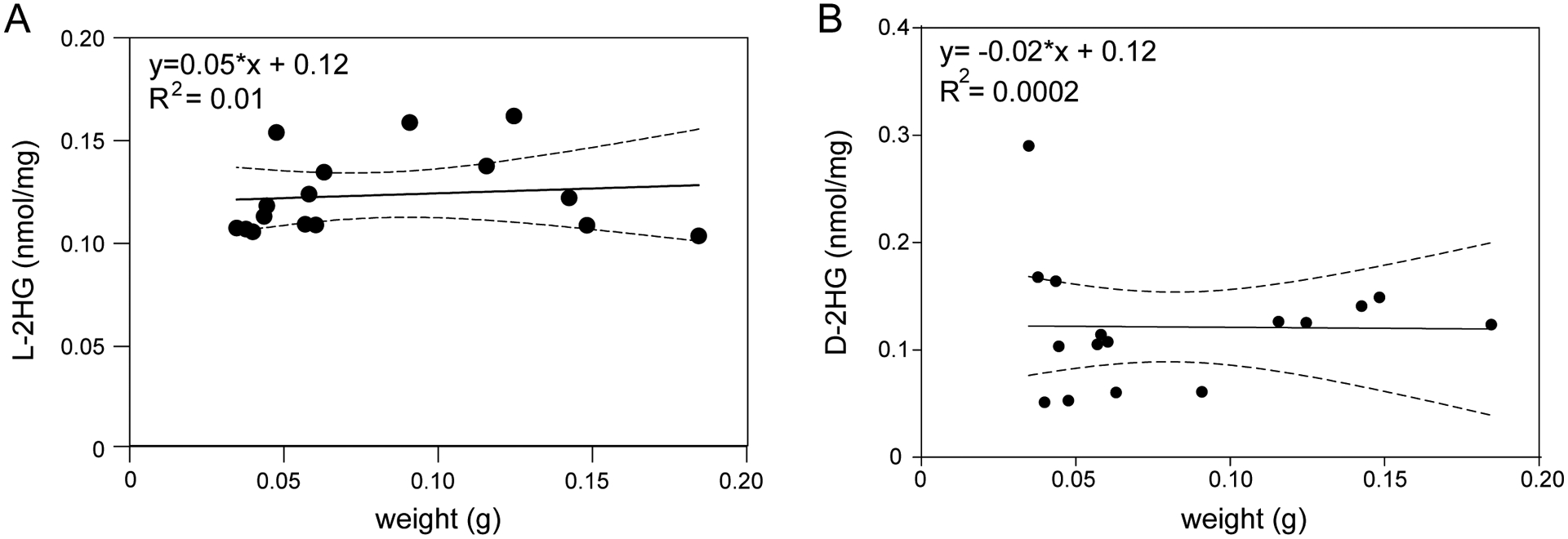
Concentrations of L-2HG (A) and D-2HG (B) plotted relative to body mass in M. sexta third instar larvae. Dashed lines represent the 95% confidence interval.
DISCUSSION
Our results demonstrate that several dipteran species accumulate high levels of the oncometabolite L-2HG during normal larval development. While the endogenous functions of L-2HG within these insects remains to be elucidated, our observations raise important considerations. Insects are among the most diverse groups of animals on the planet, display complex life histories, and are adaptable to a wide range of environmental conditions. When considered in this context, our survey of L-2HG metabolism is small in both the number of species and life-stages surveyed. Despite this limitation, we uncovered several instances where larvae generated relatively high L-2HG concentrations - a result which implies that this compound is not simply an oncometabolite or a waste product of the TCA cycle, but rather accumulates during normal development of a potentially large number of animal species.
The amount of L-2HG found within dipteran larvae is striking, as similar L-2HG concentrations in mammals are associated with severe neurometabolic defects and renal tumors (Shim et al., 2014; Ye et al., 2018), suggesting that this compound serves a unique role in dipteran physiology when compared to other animals. One explanation for our observations is that developmentally regulated L-2HG accumulation acts as part of a metabolic program that protects dipteran larvae from unpredictable fluctuations in oxygen availability. L-2HG has been repeatedly observed to be produced in animal cells exposed to hypoxic conditions and the production of this molecule is thought to play a role in the cellular hypoxia response (Intlekofer et al., 2015; Intlekofer et al., 2017; Nadtochiy et al., 2016; Oldham et al., 2015). Moreover, dipteran larvae have evolved to be exceptionally tolerant of hypoxia and anoxia, as evident by the ability of D. melanogaster larvae to remain motile for over 30 minutes under anoxic conditions (Callier et al., 2015). Based on these observations, we propose that dipteran larvae accumulate L-2HG in a developmentally-regulated oxygen-independent manner that preadapts larval development to survive transient exposure to mild hypoxic conditions that are common in the larval environment. This hypothesis is also supported by our finding that only other insect to accumulate significant L-2HG levels is Manduca sexta (Figure 2A). During the larval stage of this insect, dramatic increases in body mass induces growth-induced bouts of hypoxia (Callier and Nijhout, 2012). Our studies, however, suggest that L-2HG production within this insect is not simply the result of changes in oxygen availability and raise the possibility that Manduca sexta, similar to dipteran larvae, generate L-2HG in preparation for inevitable bouts of hypoxic stress.
The outlier during our studies of O2-dependent L-2HG accumulation was Aedes aegypti, wherein larval L-2HG levels significantly decreased following a 6 hr bout of hyperoxia. Moreover, while this hyperoxia-induced decrease in L-2HG levels was significant, a very large L-2HG pool remained, suggesting Aedes aegypti L-2HG production results from both O2-dependent and independent mechanisms within larval tissues. These finding are notable because bacteria within the Aedes aegypti larval gut induces a hypoxic microenvironment that regulates growth and molting (Coon et al., 2017). Future studies should explore this potential link between microbiome metabolism and L-2HG accumulation and signaling.
Our findings also raise the question as to how the dipteran species analyzed in this study have evolved to tolerate such high L-2HG levels. L-2HG is a potent inhibitor of enzymes that use α-ketoglutarate (α-KG) as a substrate and high concentrations of this molecule interfere with a diversity of α-KG-dependent processes (Chowdhury et al., 2011; Ye et al., 2018), which include mitochondrial metabolism, the removal of methyl groups for DNA and histones, and stabilization of the transcription factor HIF1α. Considering that the L-2HG concentration observed in Aedes aegypti, Phormia regina, and the three Drosophila species used in this study exceeds all previous reported Ki values for α-KG-dependent enzymes (Chowdhury et al., 2011), the cellular physiology of these systems must be uniquely adapted to the presence of this oncometabolite - a hypothesis that is partially supported by a recent study of DNA methylation in insects (Provataris et al., 2018). While the gene encoding the DNA methyltransferase enzyme DMNT1 was found to be present within representative species of nearly all insect orders and is thus assumed to have been present in the ancestor of all insects, Diptera was one of only three orders found to have lost this enzyme secondarily. This finding is consistent with the low levels of methylated DNA present within dipteran genomes and raises questions as to why flies largely abandoned this ancient mechanism of regulating gene expression (Bewick et al., 2016; Provataris et al., 2018; Zemach et al., 2010).
Considering that L-2HG interferes with the ability of cells to modify methylated nucleotides in DNA (Li et al., 2017; Shim et al., 2014; Ye et al., 2018), we speculate that the loss of Dmnt1 during dipteran evolution and the resulting reduction in DNA methylation may have allowed larvae to accumulate excess L-2HG with minimal disruption of gene expression programs. One intriguing possibility is that this tradeoff would have provided early dipterans with the metabolic capacity to colonize hypoxic environments that were inaccessible to other insects that rely on DNA methylation as a means of regulating gene expression. Consistent with this model, the only other holometabolous order that seem to lack Dmnt1 homologs are the Strepsiptera (Provataris et al., 2018), whose larvae develop, and female adults live, as endoparasites in diverse Hymenoptera (McMahon et al., 2011), and thus also have to contend with potentially low oxygen environments.
Our findings also raise questions as to how dipterans control L-2HG production in a developmentally-regulated manner and why is accumulation of this compound restricted to the larval stage? In regard to both mechanisms, work in Drosophila melanogaster indicates that the larval L-2HG pool is largely the result of two metabolic mechanisms: (1) LDH converts α-ketoglutarate to L-2HG in a NADH-dependent manner (Li et al., 2017). (2) Lactate inhibits the ability of L2HGDH to degrade L-2HG (Li et al., 2017; Li et al., 2018). Since LDH is expressed at high levels during the larval stages and is down-regulated at the onset of metamorphosis, larval L-2HG accumulation within Drosophila melanogaster is likely due to developmental expression pattern of this enzyme. Future studies should determine if the elevated L-2HG levels observed in other dipterans is also results from larval-specific increases in LDH activity and lactate-induced inhibition of L2HGDH.
Regardless of the dipteran larvae accumulate L-2HG, we observed that all adult insects maintain low L-2HG levels under normoxic conditions, raising the possibility that this molecule can be detrimental to adult physiology. This hypothesis is supported by a recent study which suggests that L-2HG induces oxidative stress within the brain of adult Drosophila melanogaster (Hunt et al., 2019). Since L-2HG is known to inhibit activity of both ATP synthase activity and α-ketoglutarate dehydrogenase (Brinkley et al., 2020; Fu et al., 2015), inappropriate L-2HG production in adults might interfere with oxidative phosphorylation and ATP production. We hypothesize that larvae might generate L-2HG for exactly the same reasons - if L-2HG dampens larval mitochondrial function in a predictable and consistent manner, perhaps unexpected bouts of mild hypoxia will induce less oxidative stress in growing tissues. Moreover, such a larval function for L-2HG could also have a secondary effect of rewiring intermediary metabolism in a way that might promote biosynthetic reactions and support the rapid growth rate associated with dipteran larval development. Our model would also explain why L-2HG levels are reduced in adult flies, where the energetic demands of flight require high levels of oxidative phosphorylation. These possibilities should be tested by examining metabolic flux in both larvae lacking L-2HG and adult flies harboring excess L-2HG.
Finally, our limited survey of insect 2HG metabolism reveals that while D-2HG levels remain at relatively low levels at all life stages and environmental conditions surveyed, a clear correlation emerged between decreased oxygen availability and elevated D-2HG accumulation. While the significance of this change remains to be determined, future studies should investigate the potential relationship between the two 2HG enantiomers. We would note that unlike L-2HG, D-2HG is normally produced by the enzyme hydroxyacid-oxoacid-transhydrogenase (HOT) and as the result of noncanonical phosphoglycerate dehydrogenase (PHGDH) activity (Fan et al., 2015; Struys et al., 2005). Yet, despite the presence of both enzymes within dipteran genomes (Garapati et al., 2019), the function of D-2HG in flies or any other insect order remains largely unknown. There are indications, however, that unlike L-2HG, high levels of D-2HG are detrimental to Drosophila melanogaster larvae, as ectopic D-2HG production induces the formation of melanotic masses (Reitman et al., 2015). Why excess D-2HG is detrimental to larval development while L-2HG appears beneficial remains unknown, however, we note a controversial study in human cells suggests that D-2HG induces degradation of the transcription factor HIF1a while L-2HG stabilizes this protein (Koivunen et al., 2012). We are uncertain as to whether D-2HG and L-2HG regulate Drosophila melanogaster HIF1α stability in opposing manners, but this possibility leads to an interesting hypothesis where D-2HG acts in a negative feedback loop that dampens the hypoxia response by inhibiting HIF1α stability. Therefore, despite the relatively low levels of D-2HG found in our survey, future studies should examine the role of this molecule in the dipteran hypoxia response.
But regardless of the endogenous L-2HG function and the underappreciated role of D-2HG in the hypoxia response, our study highlights how the natural diversity of insects presents an underappreciated resource for discovering and exploring the metabolic mechanisms that support animal growth. And beyond the exploring the metabolic parallels between insect development and cancer, our findings demonstrate how studying dipteran development can identify unique metabolic features that could be targeted for controlling both agricultural pests and human disease vectors.
Supplementary Material
Supplemental Figure 1. L-2HG levels during larval development of select insects. Concentrations of L-2HG plotted relative to body mass in T. molitor, V. cardui, and G. mellonella larvae. Dashed lines represent the 95% confidence interval.
Supplemental Figure 2. D-2HG levels during Aedes aegypti and Phormia regina larval development. D-2HG levels were measured in (A) Aedes aegypti and (B) Phormia regina at the indicated timepoints during larval development. For Phormia regina (B), samples collected at the 48 hr timepoint contained L2 larvae while those collected at 72 hr, 96 hr, and 120 hr timepoints contained L3 larvae.
Supplemental Figure 3. Larval L-2HG levels do not change in response to mild hypoxia or hyperoxia. L-2HG (A) and D-2HG (B) levels were measured in select dipteran larvae following a 24 hr incubation in the presence of 5%, 21%, 30% or 50% O2. Data are presented in scatter plots with mean ± SD. *P<0.05. **P<0.01. P-values calculated using a Wilcoxon/Kruskal-Wallis tests followed by Tukey-Kramer HSD test in (A) and Dunn’s test in (B).
Research Highlights.
A survey of 18 insect species reveals that dipteran larvae accumulate high concentrations of the oncometabolite L-2-hydroxyglutarate (L-2HG).
Dipteran larvae accumulate high concentrations of L-2HG in a developmentally regulated manner.
Adult dipterans maintain low levels of L-2HG but increase L-2HG production in response to hypoxia.
Our findings indicate that L-2HG is more than an oncometabolite and serves a normal purpose in animal development.
ACKNOWLEDGEMENTS
We thank the Newton, Zelhof, and Tomberlin labs for providing for samples and advice. S.S. is supported by NCI R01CA200653 and NCI F30CA232397. J.A.K. and JMT are supported by NSF 1726633. J.M.T. is supported by NIH 1R35GM119557.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LITERATURE CITED
- Becker-Kettern J, Paczia N, Conrotte JF, Kay DP, Guignard C, Jung PP and Linster CL (2016). Saccharomyces cerevisiae Forms D-2-Hydroxyglutarate and Couples Its Degradation to D-Lactate Formation via a Cytosolic Transhydrogenase. J Biol Chem 291, 6036–6058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewick AJ, Vogel KJ, Moore AJ and Schmitz RJ (2016). Evolution of DNA Methylation across Insects. Molecular Biology and Evolution 34, 654–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkley G, Nam H, Shim E, Kirkman R, Kundu A, Karki S, Heidarian Y, Tennessen JM, Liu J, Locasale JW, et al. (2020). Teleological Role of L-2-Hydroxyglutarate Dehydrogenase in the Kidney. Dis Model Mech, dmm.045898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callier V, Hand SC, Campbell JB, Biddulph T and Harrison JF (2015). Developmental changes in hypoxic exposure and responses to anoxia in Drosophila melanogaster. J Exp Biol 218, 2927–2934. [DOI] [PubMed] [Google Scholar]
- Callier V and Nijhout HF (2012). Supply-side constraints are insufficient to explain the ontogenetic scaling of metabolic rate in the tobacco Hornworm, Manduca sexta. PLoS One 7, e45455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury R, Yeoh KK, Tian Y-M, Hillringhaus L, Bagg EA, Rose NR, Leung IKH, Li XS, Woon ECY, Yang M, et al. (2011). The oncometabolite 2-hydroxyglutarate inhibits histone lysine demethylases. EMBO reports 12, 463–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coon KL, Valzania L, McKinney DA, Vogel KJ, Brown MR and Strand MR (2017). Bacteria-mediated hypoxia functions as a signal for mosquito development. Proc Natl Acad Sci U S A 114, E5362–E5369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Teng X, Liu L, Mattaini KR, Looper RE, Vander Heiden MG and Rabinowitz JD (2015). Human phosphoglycerate dehydrogenase produces the oncometabolite D-2-hydroxyglutarate. ACS Chem Biol 10, 510–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X, Chin RM, Vergnes L, Hwang H, Deng G, Xing Y, Pai MY, Li S, Ta L, Fazlollahi F, et al. (2015). 2-Hydroxyglutarate Inhibits ATP Synthase and mTOR Signaling. Cell Metab 22, 508–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garapati PV, Zhang J, Rey AJ and Marygold SJ (2019). Towards comprehensive annotation of Drosophila melanogaster enzymes in FlyBase. Database 2019, bay144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt RJ, Granat L, McElroy GS, Ranganathan R, Chandel NS and Bateman JM (2019). Mitochondrial stress causes neuronal dysfunction via an ATF4-dependent increase in L-2-hydroxyglutarate. J Cell Biol 218, 4007–4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intlekofer AM, Dematteo RG, Venneti S, Finley LW, Lu C, Judkins AR, Rustenburg AS, Grinaway PB, Chodera JD, Cross JR, et al. (2015). Hypoxia Induces Production of L-2-Hydroxyglutarate. Cell Metabolism 22, 304–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intlekofer AM, Wang B, Liu H, Shah H, Carmona-Fontaine C, Rustenburg AS, Salah S, Gunner MR, Chodera JD, Cross JR, et al. (2017). L-2-Hydroxyglutarate production arises from noncanonical enzyme function at acidic pH. Nature chemical biology 13, 494–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koivunen P, Lee S, Duncan CG, Lopez G, Lu G, Ramkissoon S, Losman JA, Joensuu P, Bergmann U, Gross S, et al. (2012). Transformation by the (R)-enantiomer of 2-hydroxyglutarate linked to EGLN activation. Nature 483, 484–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavista-Llanos S, Centanin L, Irisarri M, Russo DM, Gleadle JM, Bocca SN, Muzzopappa M, Ratcliffe PJ and Wappner P (2002). Control of the hypoxic response in Drosophila melanogaster by the basic helix-loop-helix PAS protein similar. Mol Cell Biol 22, 6842–6853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Chawla G, Hurlburt AJ, Sterrett MC, Zaslaver O, Cox J, Karty JA, Rosebrock AP, Caudy AA and Tennessen JM (2017). Drosophila larvae synthesize the putative oncometabolite L-2-hydroxyglutarate during normal developmental growth. Proc Natl Acad Sci U S A 114, 1353–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Hurlburt AJ and Tennessen JM (2018). A Drosophila model of combined D-2- and L-2-hydroxyglutaric aciduria reveals a mechanism linking mitochondrial citrate export with oncometabolite accumulation. Dis Model & Mech 11, dmm035337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H and Tennessen JM (2018). Preparation of Drosophila Larval Samples for Gas Chromatography-Mass Spectrometry (GC-MS)-based Metabolomics. J Vis Exp, 57847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H and Tennessen JM (2019). Quantification of D- and L-2-Hydroxyglutarate in Drosophila melanogaster Tissue Samples Using Gas Chromatography-Mass Spectrometry. Methods Mol Biol 1978, 155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, Sun R, Jiang B, Gao J, Deng W, Liu P, He R, Cui J, Ji M, Yi W, et al. (2017). L2hgdh Deficiency Accumulates l-2-Hydroxyglutarate with Progressive Leukoencephalopathy and Neurodegeneration. Mol Cell Biol 37, e00492–00416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Reyes I and Chandel NS (2020). Mitochondrial TCA cycle metabolites control physiology and disease. Nat Commun 11, 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon DP, Hayward A and Kathirithamby J (2011). Strepsiptera. Curr Biol 21, R271–272. [DOI] [PubMed] [Google Scholar]
- Moczek AP, Hunt J, Emlen DJ and Simmons LW (2002). Threshold evolution in exotic populations of a polyphenic beetle. Evol Ecol Res 4, 587–601. [Google Scholar]
- Mullen AR and DeBerardinis RJ (2012). Genetically-defined metabolic reprogramming in cancer. Trends Endocrinol Metab 23, 552–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen AR, Hu Z, Shi X, Jiang L, Boroughs LK, Kovacs Z, Boriack R, Rakheja D, Sullivan LB, Linehan WM, et al. (2014). Oxidation of alpha-ketoglutarate is required for reductive carboxylation in cancer cells with mitochondrial defects. Cell Rep 7, 1679–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadtochiy SM, Schafer X, Fu D, Nehrke K, Munger J and Brookes PS (2016). Acidic pH Is a Metabolic Switch for 2-Hydroxyglutarate Generation and Signaling. J Biol Chem 291, 20188–20197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldham WM, Clish CB, Yang Y and Loscalzo J (2015). Hypoxia-Mediated Increases in L-2-hydroxyglutarate Coordinate the Metabolic Response to Reductive Stress. Cell Metab 22, 291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provataris P, Meusemann K, Niehuis O, Grath S and Misof B (2018). Signatures of DNA Methylation across Insects Suggest Reduced DNA Methylation Levels in Holometabola. Genome Biology and Evolution 10, 1185–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raimundo N, Baysal BE and Shadel GS (2011). Revisiting the TCA cycle: signaling to tumor formation. Trends Mol Med 17, 641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinecke CJ, Koekemoer G, van der Westhuizen FH, Louw R, Lindequie JZ, Mienie LJ and Smuts I (2012). Metabolomics of urinary organic acids in respiratory chain deficiencies in children. Metabolomics 8, 264–283. [Google Scholar]
- Reitman ZJ, Sinenko SA, Spana EP and Yan H (2015). Genetic dissection of leukemia-associated IDH1 and IDH2 mutants and D-2-hydroxyglutarate in Drosophila. Blood 125, 336–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rzem R, Achouri Y, Marbaix E, Schakman O, Wiame E, Marie S, Gailly P, Vincent MF, Veiga-da-Cunha M and Van Schaftingen E (2015). A mouse model of L-2-hydroxyglutaric aciduria, a disorder of metabolite repair. PLoS One 10, e0119540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rzem R, Veiga-da-Cunha M, Noel G, Goffette S, Nassogne MC, Tabarki B, Scholler C, Marquardt T, Vikkula M and Van Schaftingen E (2004). A gene encoding a putative FAD-dependent L-2-hydroxyglutarate dehydrogenase is mutated in L-2-hydroxyglutaric aciduria. Proc Natl Acad Sci U S A 101, 16849–16854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim EH, Livi CB, Rakheja D, Tan J, Benson D, Parekh V, Kho EY, Ghosh AP, Kirkman R, Velu S, et al. (2014). L-2-Hydroxyglutarate: an epigenetic modifier and putative oncometabolite in renal cancer. Cancer Discov 4, 1290–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struys EA, Verhoeven NM, Ten Brink HJ, Wickenhagen WV, Gibson KM and Jakobs C (2005). Kinetic characterization of human hydroxyacid-oxoacid transhydrogenase: relevance to D-2-hydroxyglutaric and gamma-hydroxybutyric acidurias. J Inherit Metab Dis 28, 921–930. [DOI] [PubMed] [Google Scholar]
- Teng X, Emmett MJ, Lazar MA, Goldberg E and Rabinowitz JD (2016). Lactate Dehydrogenase C Produces S-2-Hydroxyglutarate in Mouse Testis. ACS Chem Biol 11, 2420–2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Soga T and Pollard PJ (2013). Oncometabolites: linking altered metabolism with cancer. J Clin Invest 123, 3652–3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye D, Guan KL and Xiong Y (2018). Metabolism, Activity, and Targeting of D- and L-2-Hydroxyglutarates. Trends Cancer 4, 151–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemach A, McDaniel IE, Silva P and Zilberman D (2010). Genome-Wide Evolutionary Analysis of Eukaryotic DNA Methylation. Science 328, 916–919. [DOI] [PubMed] [Google Scholar]
- Zhang W, Zhang M, Gao C, Zhang Y, Ge Y, Guo S, Guo X, Zhou Z, Liu Q, Zhang Y, et al. (2017). Coupling between d-3-phosphoglycerate dehydrogenase and d-2-hydroxyglutarate dehydrogenase drives bacterial l-serine synthesis. Proc Natl Acad Sci U S A 114, E7574–E7582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Xue J, Lai JC, Schork NJ, White KP and Haddad GG (2008). Mechanisms underlying hypoxia tolerance in Drosophila melanogaster: hairy as a metabolic switch. PLoS Genet 4, e1000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. L-2HG levels during larval development of select insects. Concentrations of L-2HG plotted relative to body mass in T. molitor, V. cardui, and G. mellonella larvae. Dashed lines represent the 95% confidence interval.
Supplemental Figure 2. D-2HG levels during Aedes aegypti and Phormia regina larval development. D-2HG levels were measured in (A) Aedes aegypti and (B) Phormia regina at the indicated timepoints during larval development. For Phormia regina (B), samples collected at the 48 hr timepoint contained L2 larvae while those collected at 72 hr, 96 hr, and 120 hr timepoints contained L3 larvae.
Supplemental Figure 3. Larval L-2HG levels do not change in response to mild hypoxia or hyperoxia. L-2HG (A) and D-2HG (B) levels were measured in select dipteran larvae following a 24 hr incubation in the presence of 5%, 21%, 30% or 50% O2. Data are presented in scatter plots with mean ± SD. *P<0.05. **P<0.01. P-values calculated using a Wilcoxon/Kruskal-Wallis tests followed by Tukey-Kramer HSD test in (A) and Dunn’s test in (B).


