Abstract
Feather pecking and cannibalism are 2 major problems in laying hens' husbandry. Although additional environmental enrichment material (EM) supply is thought to lessen these problems, consistent evidences are lacking. This study examined the effects of EM supply (pecking stones and alfalfa bales) on biological performance, carcass composition, and animal losses in a littered housing system. 2,000 brown-egg and 2,000 white-egg layer hen pullets of the genetic strains Lohmann Brown classic and Lohmann Selected Leghorn classic, respectively, were reared separately in a 16-compartment aviary system until week 18. 1,320 remaining laying hens were then transferred to a 44-compartment laying stable (weeks 19–48). Both strains were tested under 4 treatment variants (n = 150–180 per strain and per variant): V1—no EM over the entire study period; V2—the rearing period with and the laying period without EM; V3—the rearing period without and the laying period with EM; V4—EM over the entire study period. Data on development, performance, egg quality, feed intake, EM consumption, animal losses, carcass composition, and economic traits were collected. Enrichment material supply during rearing (V2 and V4, both strains) was found to globally increase not only hens' relative gizzard mass (P = 0.036) but also the cracked eggs' percentage (compared with V3; P = 0.008) and to decrease the body mass in weeks 6 (P = 0.023) and 8 (P = 0.023) and the uniformity in week 16 (P = 0.011). Enrichment material provision during the laying period (V3 and V4, both strains) increased egg weights (P = 0.028) and the mean body mass (P = 0.036); however, continuous provision of EM (V4, both strains) increased the floor eggs' percentage (P = 0.019). The EM supply did not affect mortality, loss of production days, losses due to skin and toe cannibalism, or the income over feed costs. However, the income over feed and enrichment costs of V1 hens was higher by 0.55 €/hen than that of V4 hens (P = 0.022). Therefore, EM supply cannot be recommended as a measure to increase laying performance and reduce animal losses, but its positive effects on animal welfare should be considered.
Key words: enrichment, laying hen, performance, mortality, economics
Introduction
Feather pecking and cannibalism are 2 major problems in laying hens' husbandry that have considerable impact on animal welfare and performance. Their prevalence increases significantly when beak trimming is abandoned (Niebuhr et al., 2006; Sepeur et al., 2015). With the loss of feathers caused by feather pecking, the plumage loses its insulation function because heat loss increases via the body surface, which results in an increased feed intake to maintain energy balance (Damme and Pirchner, 1984). Cannibalistic pecking can cause severe bloody injuries to the skin, which may lead to the victims' death (Spindler et al., 2016). Pecking injuries to the skin and toes are a major cause of laying hen losses (Cronin et al., 2018; Damme et al., 2018) and lead to an increased mortality on the herd level (Johnsen et al., 1998; Hartini et al., 2002; Cronin et al., 2018). It is beyond dispute that animals identified as injured, ill, dying, or otherwise adversely affected likely experience compromised welfare (Brambell 1965; Farm Animal Welfare Council 1979). Moreover, cannibalism may result in reduced laying performance (El-Lethey et al., 2000; Niebuhr et al., 2006). Correlations between floor and feather pecking and characteristics of egg quality have been demonstrated (Buitenhuis et al., 2004), whereby selection for feather pecking leads to changes in the egg quality in these lines (Su et al., 2006).
The supply of additional environmental enrichment materials (EM) is an approach thought to reduce feather pecking and cannibalism (reviewed by Schreiter et al., 2019). However, consistent evidence of the effect of additional EM on animal losses and biological performance in littered housing systems is lacking. To date, as regard to mortality, Steenfeldt et al. (2007) found that the use of carrots and silage as EM reduced animal losses during the laying period, while Cronin et al. (2018), that offered straw as EM, obtained an increased mortality. Freytag et al. (2016) did not find unidirectional effects of EM on animal losses. In a few studies only, positive effects of EM were observed on body mass (Steenfeldt et al., 2007) and weight gain (Cronin et al., 2018). Effects of EMs on laying performance, egg weights, egg mass production, and feed consumption could not be identified in Hartcher et al. (2015) and Cronin et al. (2018). Steenfeldt et al. (2007) found differences in laying performance when providing different EM but not in comparison with control (CON) groups (without enrichment). Compound feed consumption was reduced with the supply of carrots and pea-barley silage compared with CON groups, and silage EM were found to increase gizzard mass. Recently, Bari et al. (2020) investigated the effects of an EM offered during the rearing period on the egg quality in the subsequent laying period. Littered CON groups were compared with structural environmental enrichment groups (perches) and groups supplied with peckable but not edible EM (e.g., balls, brooms, buckets, ropes, dog toys, pipes). Between the rearing treatments, no differences in egg weight, albumen consistency, egg shell thickness, and egg anomalies were observed during the laying period. In contrast, the CON groups showed a faster reduction of the egg shell reflectivity and a paler yolk color than the groups with environmental enrichment. In addition, the EM offer led to a reduction of floor eggs.
The aim of the present study was to examine the effects of edible EM supplied during the rearing and/or laying period on the biological performance, carcass composition, and animal losses. The following hypotheses were formulated: (1) The provision of EM affects laying performance, egg weight, and/or animal losses, and (2) there are differences between partial and permanent EM supply as regard to production performance and animal losses.
Material and methods
This comprehensive study was carried out at the Bavarian State Research Center for Agriculture, Department of Applied Research and Education in Poultry in Kitzingen/Germany on non–beak-trimmed pullets and laying hens from October 2017 to September 2018. In this study, we present the effect of additional EM on biological performance and animal development in white-egg and brown-egg layers. The effect of additional EM on the integument condition was also investigated, and results of this first part of the study are presented in the study by Schreiter et al. (2020).
Animal Husbandry and Management
Husbandry and management during the rearing and laying period are described in detail in the study by Schreiter et al. (2020). Briefly, chicks were housed from their first day of life in the lower aviary level of a rearing stable divided into 16 identical compartments (250 chicks per compartment), each one equipped with a double-decked aviary system (i.e., 12.9 m2 of floor area, thereof 6.6-m2 grids and 6.3-m2 litter). On day 35, aviary segments were opened so that chicks could access the littered floor. In week 19, hens were moved to the laying stable consisting of 44 identical compartments with a floor area of 4.07 m2 each (30 hens housed per compartment). Each compartment was equipped with a storage feeding trough, nipple drinkers, perches, and a family nest. One-third of the floor was littered with soft wood shavings, and the other two-thirds consisted of perforated flooring (i.e., metal grids). For demand-oriented feeding, a four-phase feeding program with chick starter feed, complete chick feed, complete young hen feed, and a prelaying feed was used. From week 21 onward, a complete phase-I feed for laying hens was given until the end of the study on week 48. To control the animal development and laying maturity, a regulated step-down step-up light program using a high frequency was applied (Lohmann Tierzucht, 2017).
Animals, Study Design, and Data Collection
Details on the animals and study design are available in the study by Schreiter et al. (2020). In brief, 2,000 chicks of the white-egg–laying hybrid strain Lohmann Selected Leghorn classic (LSL, Lohmann Tierzucht, Cuxhaven/DE) and 2,000 chicks of the brown-egg laying hybrid strain Lohmann Brown classic (LB, Lohmann Tierzucht, Cuxhaven/DE) were housed in the rearing stable in alternating compartments (of 250 chicks each, 516 cm2 per animal, i.e., 19.4 animals/m2) and study blocks. Animals of both strains were divided into 2 different study groups. In the CON group, no EM was supplied apart from the chick paper and litter. In the experimental (EXP) group, additional edible EMs (i.e., 2 pecking stones (VILOlith medium, Deutsche Vilomix Tierernährung GmbH, Neuenkirchen-Vörden/DE) and 4 hard-pressed alfalfa bales (Einstreuprofis, Seelingstädt/DE) per compartment) were available to the animals from the first day of life throughout the entire rearing period. Sample sizes per study group were calculated using a web-based tool (http://imsieweb.uni-koeln.de/beratung/rechner/t2.html). During rearing, the body mass was defined as the main outcome and the individual compartment as the observation unit. Based on preliminary investigations (data not shown), we chose a sample size of 4 compartments per study group to be able to detect differences in the body mass of 30 g at an SD from 15 g with a statistical power of 0.80 and a type-I error of α ≤ 0.05. During the rearing period, 4 replicates for each of the study groups and for each strain were available.
In week 19, the animals were transferred to the laying house, and the CON and EXP groups from the rearing period were further split into 2 groups, with or without access to EM during the subsequent laying period, resulting in 4 different groups: variant 1 (V1)—no EM over the entire study period; variant 2 (V2)—the rearing period with and the laying period without EM; variant 3 (V3)—the rearing period without and the laying period with EM; and variant 4 (V4)—EM over the entire study period. In V3 and V4, EM (i.e., one pecking stone and ¼ of a hard-pressed alfalfa bale) were permanently available in the scratching area, from the first day of housing throughout the entire laying period. The same web-based tool as for the rearing period was used to calculate sample sizes for each variant of the laying period. In that period, the laying performance per average hen was chosen as the main outcome, and the compartment was defined as the observation unit. Based on preliminary investigations (data not shown), we defined a sample size of at least 5 compartments per study group (i.e., 5 or 6 repetitions per variant and per strain was used) to be able to detect differences in laying performance per average hen of 5% (88% vs. 93%) at an SD of 2.8% with a statistical power of 0.80 and a type-1 error of α ≤ 0.05. A total of 660 LB and 660 LSL hens were equally distributed into the 44 compartments (1,356 cm2 per animal, i.e., 7.4 animals/m2) of the laying stable (Schreiter et al., 2020).
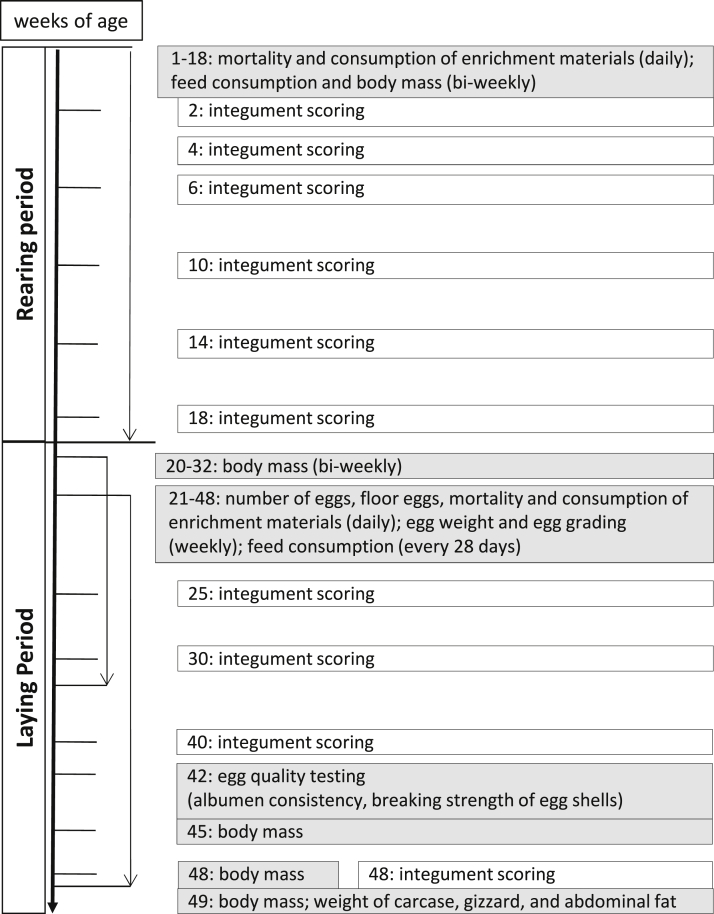
For this part of the study, we collected information on pullets' biological performance (body mass, body mass gain, uniformity), laying performance, egg quality, body mass, feed intake, consumption of EM, animal losses, carcass composition, and economic traits (for details on data collection, see Figure 1).
Figure 1.
Study design including the scoring schedule and data collection (for integument scoring information, see the study by Schreiter et al. (2020)).
During the laying period, the number of total eggs and floor eggs (i.e., found in the litter) was recorded daily. The laying performance per average hen and per hen housed was calculated according to Damme et al. (2018). Eggs were sorted (sorting machine, Staalkat International, Aalten, the Netherlands) according to their weight class, dirtiness, and whether they were cracked, conforming to the Commission Regulation (EC, No. 589/2008). The average egg weight was determined weekly by weighing with a digital scale (Kern DE6 K2N, Kern, Balingen/DE) all individual eggs of one daily clutch of each compartment. Egg quality traits were assessed in week 42 in 15 randomly selected eggs per compartment, in the same way as reported by Damme et al. (2018) for the egg quality assessments in laying hen performance tests. The egg weight (Navigator NV1101 digital scale, Ohaus, Parsippany, NJ), breaking strength of the egg shells (FEST V2.0 egg shell tester, Futura, Lohne/DE), and the albumen height (1/A 2001 albumin altimeter, Futura, Lohne, DE) were measured. Based on the egg weight and albumen height, and according to Eisen et al. (1962), the Haugh unit (Haugh unit = 100log [albumen height − 1.7 × egg weight0.37 + 7.69]) for characterizing albumen consistency were calculated.
The body mass of the pullets was determined during rearing in weeks 2, 6, 10, 14, and 18 (scale: Defender 3000, Ohaus, Parsippany, NJ) by weighing groups of 40 animals per compartment (10 hens each from the lower and upper aviary levels, from the middle and outer scratching areas; 4 compartments per strain and variant, n = 160 animals). In weeks 4, 8, 12, and 16, the individual animal masses were determined using the FlexScale (Big Dutchman AG, Vechta-Calveslage, DE) by weighing 50 animals per compartment (4 compartments per strain and variant, n = 200 animals). At the same time, the uniformity for each compartment was calculated on the basis of these individual animal masses. The uniformity indicates the proportion of animals weighed in a sample with respect to the body mass that lies within ±10% of the arithmetic mean of the sample (Jeroch and Müller, 2018). During the laying period, the body mass of all hens of 2 compartments per strain and variant (16 compartments) was measured in groups in weeks 20, 22, 24, 26, 28, and 32 using a Defender 3000 table scale. Individual animal masses were recorded in week 48 for all hens in the study (44 compartments) and in weeks 30 and 45 for all hens from 4 compartments per strain and variant (32 compartments) using a FlexScale (Big Dutchman AG). In addition, the metabolic body mass (body mass0.75) was calculated according to Jeroch et al. (2013).
During the rearing period (weeks 1–18), the feed consumption was determined in each compartment at the end of the 14-d period by recording the quantity consumed using a feeding computer (Fancom 743, Fancom, Panningen, the Netherlands) and a manual back weighing (Defender 3000 scale). From week 21 onward (i.e., the laying period), the feed consumption was determined by continuous feed and back weighing (Defender 3000 scale) in 4 wk periods. Each pecking stone and alfalfa bale of each compartment were weighed (DE6 K2N scale, Kern, Balingen, DE).
Animal losses were recorded daily. Dead hens with bloody skin injuries, or skin injuries and bloody surrounding plumage, were classified as mortality because of skin cannibalism, and those with toe injuries as mortality due to toe cannibalism. According to Damme et al. (2018), and based on the realized hen days, the loss of production days was calculated. Three hens per compartment (n = 132) were slaughtered at the end of week 48 to measure their live mass after 8 h of sobriety (FlexScale) and carcass mass 12 h after slaughter (Navigator NV1101 scale, Ohaus, Parsippany/NJ) and thus calculate their individual carcass yield (i.e., the percentage of carcass mass to live mass; Damme et al., 2015). Furthermore, the gizzard and abdominal fat masses of each of these hens were determined according to Halle et al. (2012), to calculate their relation to the carcass mass.
Income over feed costs (IOFCs, i.e., egg revenue minus feed costs) were calculated for each compartment according to Damme et al. (2018), with assumed compound feed costs of 26.00 €/dt and the following revenues per egg: S–5.20 ct, M–7.05 ct, L–7.63 ct, and XL–12.50 ct (Market Info Eggs and Poultry, 2017). Furthermore, income over feed and enrichment costs (IOFECs, i.e., egg revenue minus feed costs and costs for additional EMs) were calculated as an economic parameter, which takes into account not only feed costs but also costs for EMs (IOFEC = IOFC − costs for EMs during rearing − costs for EMs during the laying period). Costs for EMs resulted from the consumption of pecking stones and alfalfa bales in the compartment, and from the additional costs of these materials during rearing for V2 and V4 only (LB: 17.70 ct/laying hen, LSL: 15.70 ct/laying hen). Costs of 0.94 €/kg VILOlith medium, 0.78 €/kg VILOlith hard, and 1.14 €/kg alfalfa bale were assumed.
Statistical Analyses
Microsoft Excel (version 2013, Microsoft Corporation, Redmond) was used for the data collection, processing, and creation of selected diagrams. For further descriptive and inferential statistical analyses, the Standard SAS program package (version 9.4., SAS Institute Inc., Cary, NC) and the IBM SPSS Statistics program (version 23, SPSS Inc., Chicago, IL) were used. The normal distribution of the residuals was tested using the Kolmogorov-Smirnov test (Weiß, 1999). The gizzard mass and its relative proportion to the carcass mass, body mass, and metabolic body mass, as well as abdominal fat proportion to the carcass mass were log10-transformed (Rasch et al., 2010).
For normally distributed data (i.e., egg number per hen housed, egg number per average hen, egg mass per hen housed, egg mass per average hen, egg weight, body mass, body mass gain, uniformity, feed and EM consumption, feed conversion, albumen consistency, breaking strength, IOFC, IOEFC, gizzard mass, gizzard percentage of carcass/body/metabolic body mass, abdominal fat percentage of carcass mass), a two-factorial ANOVA linear model was calculated (du Prel et al., 2010) with the fixed-effect strain, group, and the interaction strain∗group. For post hoc pairwise comparisons the Generalized Tukey 2 test according to Hochberg was used (Weiß, 1999; Rasch et al., 2010). The same ANOVA linear model was used for the rearing and laying periods, but the number of study groups differed:
yij: observed trait (dependent variable); μ: model constant; Hi: effect of strain (i = 1–2); Vj: effect of the study group (j = 1–2 for the rearing period and j = 1–4 for the laying period); (H∗V)ij: interaction strain∗study group; eij: residual error.
For individual animal body mass analysis during the laying period, an ANOVA linear model with the group and strain as between-subject effects and age as a within-subject effect was used because of repeated measurements (Rasch et al., 2010). Metrically scaled traits without normal distribution of residuals (i.e., cumulative mortality, loss of production days, mortality due to skin cannibalism, mortality due to toe cannibalism, percentage of cracked/dirty and floor eggs, and carcass yield) were tested using a Mann-Whitney U-test (for the rearing period) or Kruskal-Wallis test (for the laying period) (du Prel et al., 2010). For the characteristics of the laying period, a post hoc pairwise comparison was performed when necessary using a Mann-Whitney U-test (du Prel et al., 2010). Differences were considered statistically significant for P ≤ 0.05. To control for the false discovery rate due to multiple testing, the Benjamini-Hochberg procedure was used (Victor et al., 2010).
Results
Rearing Period
The LB pullets' mean body mass was higher than that of LSL pullets (P < 0.01; Table 1). However, in both strains, the body mass was lower in weeks 6 (P = 0.023) and 8 (P = 0.028; data not shown) when EM were provided (EXP). A group effect was found on the weekly weight gain of weeks 1 to 6 only (P = 0.031, Table 1), with EXP weight gain being lower. Compared with LB pullets, LSL pullets were less uniform in week 4 (P = 0.012) but more uniform in weeks 8 (P = 0.035), 12 (P < 0.001), and 16 (P < 0.001; data not shown). An effect of the strain∗group interaction was observed in week 12 (P = 0.008; Table 1), with CON pullets from the LSL strain being the most uniform (P = 0.014), and in week 16 (P = 0.032; Table 1), with CON pullets being more uniform than EXP pullets (P = 0.011) and EXP pullets from the LB strain the least uniform (P = 0.005). Feed consumption and feed conversion were not influenced by EM supply (P = 0.582 and P = 0.571, respectively; Table 1). During rearing, an average consumption of 43.5 (4.2) g (mean (SD) in g/hen) of pecking stones and 110.9 (14.1) g of alfalfa bales per hen was recorded. The consumption of alfalfa bales was lower in LSL (100.6 (4.1) g/hen) than in LB pullets (121.1 (12.9) g/hen; P = 0.023; data not shown), while the consumption of pecking stones was not influenced by strains (P = 0.330; data not shown).
Table 1.
Effect of edible enrichment materials on performance and feed consumption during the rearing period (weeks 1–18).
| Trait (unit) | Strain | Study group (mean ± SD) |
P-value |
||
|---|---|---|---|---|---|
| CON | EXP | Group | Strain∗group | ||
| Weight gain (weeks 1–6) (g BM/WA) | LB + LSL | 61.3 ± 2.4 | 59.2 ± 2.0 | 0.031 | 0.472 |
| LB | 63.0 ± 1.3 | 60.3 ± 1.3 | 0.027 | ||
| LSL | 59.6 ± 1.9 | 58.2 ± 2.1 | 0.346 | ||
| Weight gain (weeks 7–12) (g BM/WA) | LB + LSL | 110.9 ± 10.6 | 111.1 ± 9.5 | 0.856 | 0.684 |
| LB | 120.3 ± 4.8 | 119.9 ± 1.5 | 0.899 | ||
| LSL | 101.5 ± 2.2 | 102.3 ± 1.6 | 0.550 | ||
| Weight gain (weeks 13–18) (g BM/WA) | LB + LSL | 67.1 ± 14.2 | 68.4 ± 12.5 | 0.542 | 0.517 |
| LB | 79.7 ± 4.6 | 79.7 ± 2.9 | 0.977 | ||
| LSL | 54.4 ± 4.8 | 57.1 ± 4.0 | 0.424 | ||
| Uniformity (week 4) (%) | LB + LSL | 68.8 ± 6.5 | 70.0 ± 9.9 | 0.723 | 0.831 |
| LB | 73.5 ± 3.0 | 75.5 ± 8.5 | 0.674 | ||
| LSL | 64.0 ± 5.4 | 64.5 ± 8.9 | 0.926 | ||
| Uniformity (week 8) (%) | LB + LSL | 73.3 ± 10.3 | 71.0 ± 8.4 | 0.592 | 0.377 |
| LB | 66.5 ± 7.7 | 68.0 ± 6.3 | 0.774 | ||
| LSL | 80.0 ± 8.2 | 74.0 ± 10.1 | 0.390 | ||
| Uniformity (week 12) (%) | LB + LSL | 81.0 ± 10.8 | 78.8 ± 5.0 | 0.274 | 0.008 |
| LB | 71.5 ± 5.0 | 75.5 ± 3.4 | 0.235 | ||
| LSL | 90.5 ± 2.5 | 82.0 ± 4.3 | 0.014 | ||
| Uniformity (week 16) (%) | LB + LSL | 92.0 ± 4.0 | 84.8 ± 7.9 | 0.011 | 0.032 |
| LB | 89.0 ± 2.6 | 78.0 ± 4.3 | 0.005 | ||
| LSL | 95.0 ± 2.6 | 91.5 ± 2.5 | 0.100 | ||
| Cumulative feed consumption (kg/pullet) | LB + LSL | 6,838 ± 197 | 6,787 ± 159 | 0.582 | 0.818 |
| LB | 6,910 ± 236 | 6,838 ± 205 | 0.663 | ||
| LSL | 6765.6 ± 143.8 | 6736.1 ± 98.2 | 0.746 | ||
| Feed conversion (kg feed/kg weight gain) | LB + LSL | 4,690 ± 0.448 | 4,659 ± 0.401 | 0.571 | 0.479 |
| LB | 4,285 ± 0.127 | 4,292 ± 0.108 | 0.929 | ||
| LSL | 5,096 ± 0.111 | 5,027 ± 0.066 | 0.327 | ||
Abbreviations: BM, body mass; CON, control group (without additional enrichment materials); EXP, enrichment group (supply of additional enrichment materials); LB, Lohmann Brown classic; LSL, Lohmann Selected Leghorn classic; WA, week of age.
The cumulative mortality during the rearing period (weeks 1–18) was 1.2 [0.6; 1.9] % (median [lower; upper quartile]) in the LB pullets, which was lower than in LSL pullets with 2.1 [1.7; 2.1] % (P = 0.028). Remarkably, the main difference was the mortality rate in the first 2 wk, which was 0.1% in LB and 0.8% in LSL (P = 0.002). There was no group effect on mortality rates (with 1.2 [1.2; 2.0] % in CON and 2.0 [1.6; 2.3] % in EXP; P = 0.130).
Laying Period
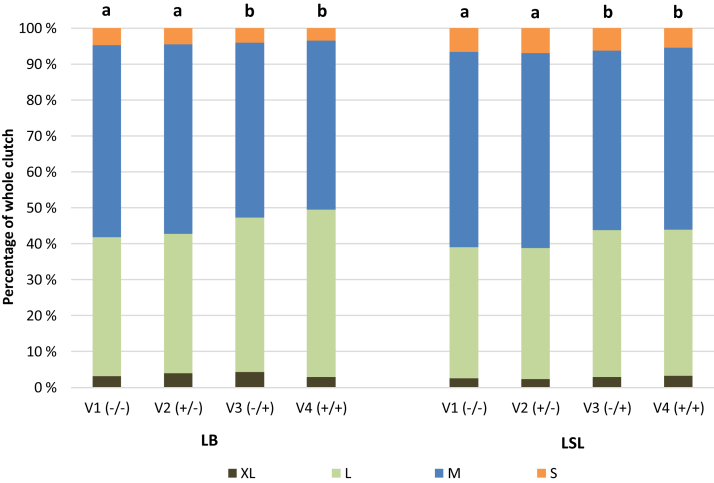
During the laying period (weeks 21–48), an average hen laid 170.2 eggs, equating to a laying performance of 86.8%. No variant effect was observed on the laying performance and egg mass production (Table 2). However, the egg mass production of LSL hens tended to be lower in V2 than in the other variants. The average egg weight was influenced by the variant (P = 0.028; Table 2), with the provision of EM during the laying period (V3 and V4), increasing egg weights. This was accompanied by differences in the egg weight classes' distribution (Figure 2), that is, a higher proportion of L-eggs (P = 0.021) and a lower proportion of M-eggs (P = 0.048) in V3 and V4. Differences between strains were observed in egg weights (P = 0.027; Table 2) and thus in egg weight class distribution, but not in laying performance per average hen (P = 0.105) or per hen housed (P = 0.487; data not shown). No effect of EM supply was found in the albumen consistency (P = 0.214) and egg shell stability (P = 0.162; Table 2). Enrichment material provision throughout the study (V4) resulted in a higher percentage of floor eggs than the other 3 variants over both strains confounded (P = 0.019), and in LSL hens (P = 0.021; Table 3). 1.4 [1.1; 1.9] % (median [lower; upper quartile]) of dirty eggs from the total clutch was recorded, with no variant effect (Table 3). The percentage of cracked eggs was higher in the variants providing EM during rearing (V2 and V4) than in V3 (P = 0.008; Table 3). Across all variants, 0.3 [0.1; 0.6] % (median [lower; upper quartile]) of the laid eggs classified as cracked eggs were not due to a strain effect (P = 0.778; data not shown).
Table 2.
Effect of edible enrichment materials on biological performance (weeks 21–48) and egg characteristics (at week 42) during the laying period.
| Trait (unit) | Strain | Study group (mean ± SD) |
P-value |
||||
|---|---|---|---|---|---|---|---|
| V1 (−/−) | V2 (+/−) | V3 (−/+) | V4 (+/+) | Group | Strain∗Group | ||
| Number of eggs per hen housed | LB + LSL | 168.1 ± 5.0 | 164.7 ± 9.1 | 167.1 ± 5.9 | 167.5 ± 7.7 | 0.641 | 0.098 |
| LB | 165.3 ± 4.5 | 168.2 ± 6.0 | 165.9 ± 7.0 | 164.4 ± 9.0 | |||
| LSL | 170.4 ± 4.4 | 160.6 ± 11.3 | 168.5 ± 4.5 | 170.2 ± 6.1 | |||
| Number of eggs per average hen | LB + LSL | 171.4 ± 5.9 | 169.1 ± 6.1 | 170.8 ± 4.8 | 169.2 ± 8.5 | 0.714 | 0.208 |
| LB | 169.1 ± 6.2 | 170.8 ± 4.8 | 168.8 ± 5.0 | 165.1 ± 9.3 | |||
| LSL | 173.2 ± 5.3 | 167.2 ± 7.6 | 173.3 ± 3.3 | 172.6 ± 6.6 | |||
| Individual egg mass (g) | LB + LSL | 61.5 ± 0.7a | 61.5 ± 1.0a | 62.2 ± 0.8b | 62.2 ± 0.5b | 0.028 | 0.952 |
| LB | 61.6 ± 0.4 | 61.8 ± 1.1 | 62.5 ± 0.8 | 62.5 ± 0.3 | |||
| LSL | 61.3 ± 0.8 | 61.1 ± 1.0 | 61.9 ± 0.8 | 62.0 ± 0.2 | |||
| Egg mass production per hen housed (kg) | LB + LSL | 10.3 ± 0.3 | 10.1 ± 0.6 | 10.4 ± 0.4 | 10.4 ± 0.5 | 0.375 | 0.111 |
| LB | 10.2 ± 0.2 | 10.4 ± 0.3 | 10.4 ± 0.5 | 10.3 ± 0.5 | |||
| LSL | 10.4 ± 0.3 | 9.8 ± 0.8 | 10.4 ± 0.4 | 10.6 ± 0.4 | |||
| Egg mass per average hen (kg) | LB + LSL | 10.5 ± 0.3 | 10.4 ± 0.4 | 10.6 ± 0.4 | 10.5 ± 0.5 | 0.586 | 0.230 |
| LB | 10.4 ± 0.3 | 10.6 ± 0.2 | 10.5 ± 0.3 | 10.3 ± 0.5 | |||
| LSL | 10.6 ± 0.3 | 10.2 ± 0.5 | 10.7 ± 0.3 | 10.7 ± 0.4 | |||
| Albumen consistency (week 42) (HU) | LB + LSL | 88.9 ± 5.8 | 87.7 ± 7.0 | 89.0 ± 6.4 | 89.2 ± 5.9 | 0.214 | 0.504 |
| LB | 86.5 ± 6.1 | 85.8 ± 6.5 | 86.5 ± 6.6 | 86.0 ± 6.4 | |||
| LSL | 90.9 ± 4.8 | 90.0 ± 7.0 | 91.9 ± 4.6 | 91.8 ± 3.7 | |||
| Breaking strength of egg shells (week 42) (N) | LB + LSL | 42.3 ± 9.4 | 41.6 ± 9.9 | 43.9 ± 9.8 | 42.2 ± 9.9 | 0.162 | 0.194 |
| LB | 43.5 ± 11.1 | 44.6 ± 11.0 | 46.1 ± 10.6 | 44.5 ± 11.6 | |||
| LSL | 41.3 ± 7.5 | 40.8 ± 6.8 | 41.1 ± 7.8 | 40.2 ± 7.9 | |||
| Daily feed intake (g/hen day) | LB + LSL | 119.5 ± 3.1 | 119.6 ± 2.8 | 120.3 ± 2.7 | 119.3 ± 2.6 | 0.835 | 0.881 |
| LB | 119.1 ± 4.8 | 119.6 ± 1.7 | 119.4 ± 3.0 | 119.0 ± 1.2 | |||
| LSL | 119.8 ± 0.5 | 119.6 ± 4.0 | 121.3 ± 2.2 | 119.5 ± 3.5 | |||
| Feed conversion (kg feed/kg egg mass) | LB + LSL | 2,225 ± 0.064 | 2,256 ± 0.090 | 2,220 ± 0.085 | 2,224 ± 0.096 | 0.629 | 0.179 |
| LB | 2,239 ± 0.075 | 2,223 ± 0.047 | 2,221 ± 0.062 | 2,264 ± 0.110 | |||
| LSL | 2,213 ± 0.073 | 2,297 ± 0.117 | 2,219 ± 0.060 | 2,190 ± 0.076 | |||
| Consumption of pecking stones (g/hen day) | LB + LSL | n.a. | n.a. | 190.2 ± 55.6 | 183.7 ± 65.8 | 0.863 | 0.353 |
| LB | n.a. | n.a. | 211.0 ± 61.8 | 181.2 ± 100.0 | |||
| LSL | n.a. | n.a. | 165.4 ± 39.1 | 186.0 ± 25.6 | |||
| Consumption of alfalfa bales (g/hen day) | LB + LSL | n.a. | n.a. | 172.4 ± 21.9a | 264.6 ± 85.9b | <0.001 | <0.001 |
| LB | n.a. | n.a. | 183.7 ± 23.7a | 349.5 ± 31.9b | |||
| LSL | n.a. | n.a. | 158.9 ± 8.8a | 193.8 ± 26.9b | |||
Abbreviations: HU, Haugh unit; LB, Lohmann Brown classic; LSL, Lohmann Selected Leghorn classic; variants: V1, no additional enrichment materials supplied over the whole study period; V2, the rearing period with and the laying period without supply of additional enrichment materials; V3, the rearing period without and the laying period with supply of additional enrichment materials; V4, supply of additional enrichment materials over the whole study period (as defined in the study by Eisen et al., 1962).
Figure 2.
Distribution of the egg weight classes (weeks 21–48) in the whole clutch of brown-egg and white-egg layer hens depending on the supply of edible enrichment materials. Egg classification was according to the Commission Regulation (EG, No. 589/2008; XL—individual egg mass ≥73 g, L—63 g ≤ individual egg mass <73 g, M—53 g ≤ individual egg mass <63 g, S—individual egg mass <53 g). Abbreviations: LB, Lohmann Brown classic; LSL, Lohmann Selected Leghorn classic; variants: V1, no additional enrichment materials supplied over the whole study period; V2, the rearing period with and the laying period without supply of additional enrichment materials; V3, the rearing period without and the laying period with supply of additional enrichment materials; V4, supply of additional enrichment materials over the whole study period. Different indices indicate differences concerning the proportion of eggs classified as M or L between variants within a genetic strain (P < 0.05).
Table 3.
Effect of edible enrichment materials on laid eggs and animal losses during the laying period (weeks 21–48).1
| Trait (unit) | Strain | Study group (median [lower; upper quartile]) |
P-value | |||
|---|---|---|---|---|---|---|
| V1 (−/−) | V2 (+/−) | V3 (−/+) | V4 (+/+) | |||
| Cracked eggs (%) | LB + LSL | 0.3 [0.0; 0.4]a,b | 0.4 [0.3; 0.7]b,c | 0.2 [0.1; 0.3]a | 0.5 [0.4; 0.8]c | 0.008 |
| LB | 0.3 [0.1; 0.9] | 0.4 [0.2; 0.7] | 0.3 [0.2; 0.4] | 0.4 [0.1; 0.6] | 0.757 | |
| LSL | 0.2 [0.0; 0.4]a | 0.5 [0.3; 1.0]a,b | 0.1 [0.1; 0.3]a | 0.8 [0.5; 1.7]b | 0.008 | |
| Dirty eggs (%) | LB + LSL | 1.3 [0.9; 1.7] | 1.3 [0.7; 2.0] | 1.3 [1.1; 1.8] | 1.7 [1.2; 2.1] | 0.606 |
| LB | 1.7 [1.4; 2.0] | 1.5 [0.6; 2.4] | 1.8 [1.2; 2.7] | 2.1 [1.5; 2.6] | 0.652 | |
| LSL | 1.1 [0.7; 1.3] | 1.2 [0.9; 1.6] | 1.2 [0.9; 1.3] | 1.4 [1.1; 1.7] | 0.364 | |
| Floor eggs (%) | LB + LSL | 0.8 [0.6; 1.4]a | 0.9 [0.7; 1.5]a | 0.9 [0.8; 2.1]a | 2.6 [1.3; 5.0]b | 0.019 |
| LB | 1.1 [0.8; 2.6] | 1.0 [0.7; 1.5] | 1.9 [1.0; 3.0] | 1.3 [1.2; 4.0] | 0.230 | |
| LSL | 0.7 [0.6; 1.2]a | 0.9 [0.7; 2.1]a | 0.8 [0.7; 1.0]a | 3.3 [2.2; 5.2]b | 0.021 | |
| Mortality (total) (%) | LB + LSL | 4.1 [3.3; 6.7] | 3.3 [0.0; 10.0] | 4.4 [0.0; 6.7] | 1.9 [0.0; 3.3] | 0.429 |
| LB | 4.2 [1.7; 6.7] | 3.3 [0.0; 7.5] | 2.5 [0.0; 8.3] | 0.7 [0.0; 1.7] | 0.294 | |
| LSL | 4.0 [2.5; 6.7] | 3.3 [0.0; 9.0] | 5.8 [3.3; 8.3] | 3.3 [0.0; 7.5] | 0.728 | |
| Lost hen days (%) | LB + LSL | 1.6 [0.6; 2.7] | 1.0 [0.0; 4.0] | 2.5 [0.0; 3.3] | 0.1 [0.0; 1.8] | 0.360 |
| LB | 1.4 [0.3; 4.5] | 0.7 [0.0; 3.7] | 1.1 [0.0; 3.8] | 0.4 [0.0; 1.1] | 0.360 | |
| LSL | 1.9 [0.7; 2.5] | 2.7 [0.0; 8.5] | 2.6 [1.9; 3.7] | 0.9 [0.0; 2.5] | 0.454 | |
| Mortality (skin cannibalism) (%) | LB + LSL | 0.9 [0.0; 3.3] | 0.7 [0.0; 1.2] | 1.7 [0.0; 3.3] | 0.3 [0.0; 0.4] | 0.258 |
| LB | 2.0 [0.0; 3.3] | 0.6 [0.0; 0.8] | 2.0 [0.0; 4.2] | 0.0 [0.0; 0.0] | 0.144 | |
| LSL | 0.0 [0.0; 0.0] | 2.7 [0.0; 6.7] | 1.3 [0.0; 3.3] | 0.6 [0.0; 0.8] | 0.454 | |
| Mortality (toe cannibalism) (%) | LB + LSL | 0.0 [0.0; 0.9] | 0.0 [0.0; 0.4] | 0.0 [0.0; 0.5] | 0.0 [0.0; 0.4] | 1.000 |
| LB | 0.0 [0.0; 0.0] | 0.0 [0.0; 0.0] | 0.0 [0.0; 0.0] | 0.0 [0.0; 0.0] | 1.000 | |
| LSL | 1.1 [0.0; 1.7] | 0.7 [0.0; 1.7] | 0.7 [0.0; 1.7] | 0.6 [0.0; 0.8] | 0.990 | |
Abbreviations: LB, Lohmann Brown classic; LSL, Lohmann Selected Leghorn classic; variants: V1, no additional enrichment materials supplied over the whole study period; V2, the rearing period with and the laying period without supply of additional enrichment materials; V3, the rearing period without and the laying period with supply of additional enrichment materials; and V4, supply of additional enrichment materials over the whole study period.
Different indices indicate differences between study groups (P < 0.05).
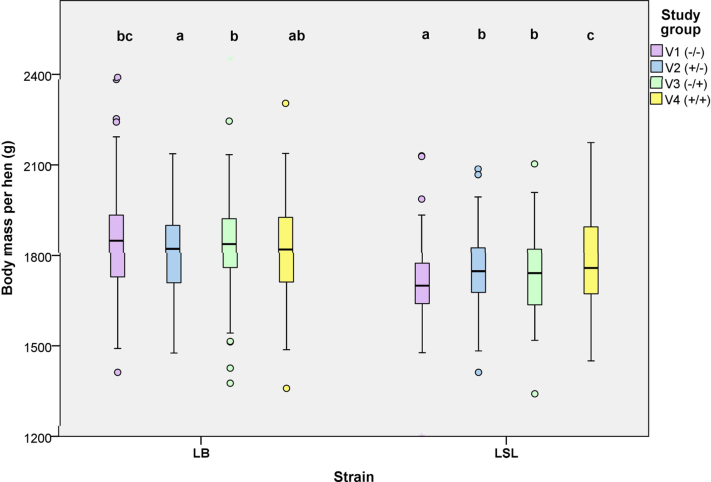
When analyzing the course of body mass during the laying period, the variant had as expected a significant effect over both strains (P < 0.001) as well as within LB (P = 0.001) and LSL (P < 0.001), with LB hens being heavier than LSL hens (P < 0.001; Figure 3). Overall, EM supply during the laying period (V3 and V4) increased the mean body mass, but an effect of the strain∗group interaction (P < 0.001; Figure 3) led to different variants' ranking between the 2 strains. The LB hens in V2 and V4 (i.e., hens reared with EM) had the lowest mean body mass and those in V1 the highest, while on the contrary in LSL hens, V4 hens had the highest mean body mass, followed by V2 and V3 hens, and then V1 hens (Figure 3). A strain effect was observed on the hens' body weight gain in weeks 21 to 26 and weeks 27 to 32 (P < 0.001; data not shown). A variant effect could additionally be observed in weeks 21 to 26, where the weekly weight gain in V4 (34.6 (9.9) g; mean (SD)) was higher than in V2 (27.7 (9.5) g; P = 0.015; data not shown). Feed consumption and feed conversion were unaffected by the strains and variants (Table 2). The pecking stone consumption during the laying period was unaffected by the EM supply during rearing (P = 0.863; Table 3), or by the strain (P = 0.450; data not shown). Conversely, the consumption of alfalfa was found to be higher in V4 (+92.2 g/hen in laying hens that had already access to EM during rearing) than in V3 (group and strain∗group interaction: P < 0.001; Table 2), and more pronounced in LB hens (+165.8 g/hen) than in LSL (+34.9 g/hen).
Figure 3.
Effect of edible enrichment materials on the laying hens' body mass in week 48. Boxplots indicate the data range as well as median, and lower and upper quartiles. Different indices indicate differences between variants within a genetic strain. Abbreviations: LB, Lohmann Brown classic; LSL, Lohmann Selected Leghorn classic; variants: V1, no additional enrichment materials supplied over the whole study period; V2, the rearing period with and the laying period without supply of additional enrichment materials; V3, the rearing period without and the laying period with supply of additional enrichment materials; V4, supply of additional enrichment materials over the whole study period.
Differences in animal losses due to toe cannibalism were found between strains (Table 3). Toe cannibalism was not the cause of any animal loss in LB hens, whereas 0.6 [0.0; 0.8] % (median [lower; upper quartile]) of the LSL hens died because of toe injuries (P = 0.038; Table 3). The total mortality (P = 0.132), loss of production days (P = 0.170), and losses due to skin cannibalism (P = 0.335; data not shown) did not differ between strains. During the laying period, 3.3 [0.0; 6.7] % (median [lower; upper quartile]) of the laying hens of all groups died, which resulted in a loss of production days of 1.4 [0.0; 3.0] %. As a result of pecking injuries to the skin, 0.7 [0.0: 2.5] % of the hens over all groups died (i.e., cannibalism loss). Enrichment material supply did not affect the total mortality (P = 0.429; Table 3), loss of production days (P = 0.360), and losses due to skin (P = 0.258) and toe (P = 1.000) cannibalism.
The strain had an effect on the percentage of carcass yield, gizzard mass (in g, in the percentage of the carcass mass and in the percentage of the body mass), and percentage of abdominal fat mass of the carcass mass (P < 0.001; data not shown), all higher in LB than LSL strain except for the abdominal fat mass. The variant had an effect on the carcass yield (P = 0.004; Table 4), which was the lowest in V4, and within the LB strain, also lower in V2 than V1 (P = 0.005). The absolute gizzard mass was 28.0 [24.1; 30.2] g (median [lower; upper quartile]), and represented 2.56 [2.29; 2.91] % of the carcass mass, 1.54 [1.35-; 1.75] % of the body mass, and 1.78 [1.57; 2.01] % of the metabolic body mass. After logarithmic transformation, a variant effect was observed on the relative gizzard mass to the carcass mass (P = 0.013; Table 4), body mass (P = 0.036), and metabolic body mass (P = 0.048), being higher in V2 and V4 (i.e., in hens supplied with EM during rearing), but not on the absolute gizzard mass (P = 0.412) and relative abdominal fat mass to the carcass mass (P = 0.144).
Table 4.
Effect of enrichment materials (alfalfa bales and pecking stones) on carcass yield, gizzard mass, and abdominal fat in laying hens at the time of slaughter (day of life 336).1
| Trait (unit) | Strain | Study group (median [lower; upper quartile]) |
P-value | |||
|---|---|---|---|---|---|---|
| V1 (−/−) | V2 (+/−) | V3 (−/+) | V4 (+/+) | |||
| Carcass yield (%) | LB + LSL | 60.3 (58.8; 61.3)b | 59.4 (58.0; 60.9)b | 60.5 (58.8; 62.2)b | 58.5 (57.6; 59.8)a | 0.004 |
| LB | 61.3 (59.4; 62.1)c | 60.7 (59.3; 61.2)b | 61.9 (60.2; 63.6)b,c | 59.4 (58.3; 60.0)a | 0.005 | |
| LSL | 59.5 (58.1; 60.5) | 58.0 (56.6; 58.9) | 59.2 (57.1; 59.9) | 57.9 (57.3; 59.2) | 0.060 | |
| Study group (mean ± SD) |
P-value |
||||||
|---|---|---|---|---|---|---|---|
| V1 (−/−) | V2 (+/-) | V3 (−/+) | V4 (+/+) | Group | Strain∗group | ||
| Log gizzard mass (g) | LB + LSL | 1.42 ± 0.07 | 1.45 ± 0.09 | 1.44 ± 0.06 | 1.44 ± 0.08 | 0.412 | 0.680 |
| LB | 1.46 ± 0.05 | 1.49 ± 0.07 | 1.47 ± 0.05 | 1.50 ± 0.07 | |||
| LSL | 1.38 ± 0.07 | 1.39 ± 0.08 | 1.40 ± 0.06 | 1.39 ± 0.05 | |||
| Log gizzard mass/carcass mass (%) | LB + LSL | 0.38 ± 0.07a | 0.43 ± 0.08b | 0.41 ± 0.07a | 0.43 ± 0.08b | 0.013 | 0.107 |
| LB | 0.40 ± 0.05a | 0.46 ± 0.07a,b | 0.42 ± 0.07a | 0.49 ± 0.07b | |||
| LSL | 0.37 ± 0.07 | 0.39 ± 0.08 | 0.39 ± 0.07 | 0.39 ± 0.06 | |||
| Log gizzard mass/body mass (%) | LB + LSL | 0.16 ± 0.07a | 0.20 ± 0.08b | 0.19 ± 0.07a,b | 0.20 ± 0.08b | 0.036 | 0.135 |
| LB | 0.18 ± 0.07a | 0.24 ± 0.07b | 0.21 ± 0.06a | 0.26 ± 0.07b | |||
| LSL | 0.14 ± 0.07 | 0.16 ± 0.07 | 0.16 ± 0.07 | 0.15 ± 0.05 | |||
| Log gizzard mass/metabolic body mass (%) | LB + LSL | 0.22 ± 0.07a | 0.26 ± 0.08b | 0.25 ± 0.07a,b | 0.26 ± 0.08b | 0.048 | 0.220 |
| LB | 0.25 ± 0.06a | 0.30 ± 0.07b | 0.28 ± 0.06a | 0.32 ± 0.07b | |||
| LSL | 0.20 ± 0.07 | 0.21 ± 0.07 | 0.22 ± 0.06 | 0.21 ± 0.05 | |||
| Log abdominal fat mass/carcass mass (%) | LB + LSL | 0.67 ± 0.16 | 0.59 ± 0.21 | 0.59 ± 0.17 | 0.59 ± 0.18 | 0.144 | 0.144 |
| LB | 0.63 ± 0.18 | 0.50 ± 0.21 | 0.51 ± 0.14 | 0.46 ± 0.18 | |||
| LSL | 0.70 ± 0.13 | 0.70 ± 0.17 | 0.68 ± 0.16 | 0.71 ± 0.08 | |||
Abbreviations: LB, Lohmann Brown classic; LSL, Lohmann Selected Leghorn classic; variants: V1, no additional enrichment materials supplied over the whole study period; V2, the rearing period with and the laying period without supply of additional enrichment materials; V3, the rearing period without and the laying period with supply of additional enrichment materials; V4, supply of additional enrichment materials over the whole study period.
Different indices indicate differences between study groups (P < 0.05).
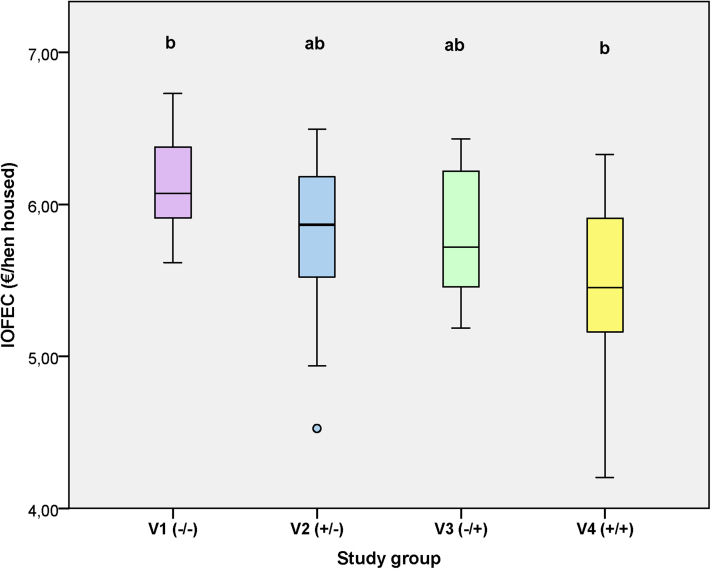
No differences in the economic parameters IOFC (P = 0.872) and IOFEC (P = 0.624) were found between strains. Although the IOFC was not influenced by the variant, the IOFEC of V1 hens was higher by 0.55 €/hen housed compared with the one of V4 hens (P = 0.022; Figure 4).
Figure 4.
Effect of edible enrichment materials on the income over feed and enrichment costs in brown-egg and white-egg layer hens during the laying period (weeks of age 21–48). Boxplots indicate the data range as well as median, and lower and upper quartiles. Different indices indicate differences between variants (P < 0.05). Abbreviations: IOFEC, income over feed and enrichment costs; variants: V1, no additional enrichment materials supplied over the whole study period; V2, the rearing period with and the laying period without supply of additional enrichment materials; V3, the rearing period without and the laying period with supply of additional enrichment materials; V4, supply of additional enrichment materials over the whole study period.
Discussion
In this study, we assessed the effect of edible EM during the rearing and/or laying periods on pullets' development and laying hens' performance, egg quality, carcass composition, and mortality.
Although the overall provision of EM did not influence the laying performance and mortality, it did increase the egg weight and relative gizzard mass and decreased the carcass yield with differences found between the partial and permanent supply of EMs.
In agreement with previous studies (Steenfeldt et al., 2007; Hartcher et al., 2015; Cronin et al., 2018), the laying performance was found not to be influenced by the supply of EM. The targets of the breeding company (Lohmann Tierzucht, 2017) regarding the laying performance of the hybrids used were not achieved. Conversely, the eggs' weight was above the target value and was increased by the supply of EM during the laying period (V3 and V4), regardless of the EM supply during rearing. Thus, the egg weight class distribution changed with fewer M- and more L-eggs in V3 and V4. Although a higher nutrient intake from the complete feed might have been an explanation, no differences in feed consumption between the variants were noted. Indeed, the intake of methionine, lysine, energy, and linoleic acid as nutritive factors is known to influence the eggs' weight (Thiele, 2012; Schreiter and Damme, 2017). However, the consumption of pecking stones solely cannot explain to such an extent the observed effects on eggs' weight. Because the exact amount of alfalfa consumed by hens has not been quantified, the additional substrates' intake is thus a possible cause for the differences in eggs' weight (and egg weight classes) measured. In the study by Laudadio et al. (2014), partial replacement of soy meal by alfalfa in the laying hens' diet resulted in a shift in the egg weight classes, with a higher proportion of heavy and a lower proportion of medium-weighted eggs.
On the other hand, the supply of EM increased the prevalence of eggs laid on the floor, especially in white-egg–laying hens provided continuously with EM during the rearing and laying periods. Explanatory approaches include the prolonged use of the scratching area because of the EM supply (Freytag et al., 2016; Cronin et al., 2018), a preferred oviposition in the corners, and a preference for the additional objects in the litter (Martin et al., 2005). Consequently, the proportion of cracked eggs increased simultaneously with the proportion of floor eggs (in V4), which can be explained by the fact that eggs placed outside the nests are usually dirty and have a defective shell (Appleby, 1984). However, an effect on the proportion of dirty eggs could not be identified in this study.
The weekly body mass gain during rearing was partially below the reference values (Lohmann Tierzucht, 2017) until week 9 but exceeded the specifications after that age until the end of the rearing. An effect of EM supply during rearing resulting in a lower body mass in weeks 6 and 8 and a reduced uniformity in week 16 was observed. It can be speculated that EM-supplemented pullets consumed excessive amounts of the EM, resulting in a lower body mass and uniformity. Moreover, a strong deviation from the reference body mass (Lohmann Tierzucht, 2017) was found in the LB hens during the laying period, with a loss of body mass between weeks 24 and 32, before it slightly reincreased. A possible cause of body mass loss may be the insufficient increase in food intakes at the laying maturity (Schreiter and Damme, 2017). When testing the effect of the variant in the laying period over both strains, the body mass was higher if EMs were provided in this period. Body mass gain was also higher in the period after transfer to the laying stable (weeks 21–26) in the groups with EMs in the laying period. In the further course of the study, no additional differences due to EM supply were observed. Cronin et al. (2018) also found higher body mass gains when straw was provided as EM. They attributed it to the possible improved nutrient availability when feeding fiber-containing diet components.
Throughout the study, the feed consumption was not influenced by the strain or variant. This is in agreement with the study by Cronin et al. (2018) that found no compound feed intake suppression when offering straw as EM, but in contradiction with the study by Steenfeldt et al. (2007) that offered silage and carrots as EM and observed a reduction of the compound feed consumption (EM accounting for up to 49% of the total feed intake). In our study, alfalfa (as EM) consumption was higher during the laying period when hens already had access to it during rearing, confirming the practical recommendation assumptions (Keppler et al., 2017).
When EM were available, both during the rearing and laying periods (V4), the mass of the gastrointestinal tract and other organs removed at slaughter was higher than in the other variants. This is consistent with the higher proportion of relative gizzard mass to the body mass or metabolic body mass measured in the LB hens reared with EM (V2 and V4) than in hens reared without EMs (V1 and V3), regardless of the EM supply during the laying period. Therefore, the gizzard development seems to be promoted by the EM supply during rearing, possibly due to the intake of fiber-containing alfalfa material. Indeed, several studies found an increase of the relative gizzard mass when providing fiber-containing EM, for example, wood shavings (Hetland et al., 2005; Amerah et al., 2009), straw and sugar beet pulp (Guzman et al., 2015), or silage (Steenfeldt et al., 2007). The higher consumption of alfalfa during rearing recorded in LB than in LSL pullets may explain why a greater gizzard mass was only observed in the LB hens. The EM effects on the egg weight and body mass, as well as on the integument condition (Schreiter et al., 2020) observed in this study may also be attributed to the better availability of nutrients because of the intake of coarsely structured fiber (Amerah et al., 2009; Svihus, 2011).
Animal losses were not reduced by the provision of EM during either rearing or the laying period. This is in line with the study by Freytag et al. (2016), although previous studies could not provide consistent results on the question. Steenfeldt et al. (2007) reduced the mortality by offering silage and carrots, whereas Cronin et al. (2018) observed a higher mortality when offering straw.
With reference to working hypothesis 1 of this study, an increase in egg weight by the provision of EM could be detected, but no effects on laying performance and animal losses. Working hypothesis 2, according to differences in laying performance and animal losses between the groups with permanent and temporary EM access, could not be confirmed.
Income over feed costs did not differ between the tested variants. However, IOFECs, which also takes into account the additional EM costs into the egg selling price, were considerably lower when EMs were permanently provided (during both the rearing and laying periods, V4; i.e., −0.55 €/hen housed). The latter economically greatly impacts the egg producing farms. Such high additional costs for EM supply can thus not be compensated by improved performance.
In regard to the generalizability of our results to more practical laying hen farm conditions, the group sizes in particular must be considered as a deviating factor. In contrast to our study, the group size in farms is ten to hundred times higher, that is, from 250 pullets or 30 hens per compartment in our study compared with groups of several thousands of hens per compartment in a typical floor housing system (Pottgüter et al., 2018). A higher group size results in a reduced availability of the EM per individual hen. According to a survey by Spindler (2019), only one enrichment element for 500 up to 1,500 hens is a common practice. In this view, a validation under practical conditions of the effects of the 4 tested EM variants on pullets' development and laying hens' performance and mortality is recommended. Further studies should enclose the period from the first day of life to the end of a complete laying period (>70 wk of life). In addition, it should be explored whether similar effects on the gizzard development can be achieved with enrichment substrates other than the pecking stones and alfalfa bales we used and whether the observed effects of EM on the performance, egg quality, and carcass composition are applicable to other hybrid strains.
Conclusions
Pecking stones and alfalfa bales as EMs did not affect animal losses or the laying rate. However, the eggs' weight was increased when EMs were provided during the laying period but not the cumulative egg mass production. A reduced compound feed intake was not observed, although the consumption of alfalfa during the laying period was higher when the hens had access to EM during rearing. The provision of EM during rearing was found to reduce the body mass gain in the first weeks of life and the 16th wk uniformity. During the laying period, on the other hand, positive effects of the EM supply on the body mass development can be expected. With the additional intake of EM, particularly during the rearing period, the development of the gizzard was promoted. However, a permanent supply of EM greatly decreases the economic outcome of laying hen farming. Therefore, the supply of EM in pullets and laying hens cannot be recommended as a measure to increase the laying performance and reduce animal losses. Nonetheless, not only these requirements should be taken into account in the decision-making process of farmers but also animal welfare aspects with reduced occurrence of feather pecking and cannibalism when EMs are supplied. Thus, further research is needed to achieve a synopsis between economic requirements and animal welfare.
Acknowledgments
The authors thank the animal caretakers for their expert technical support.
The work described in this article with research on live animals was conducted in accordance with the principles and specific guidelines presented in the Guide for the Care and Use of Agricultural Animals in Research and Teaching, third edition, 2010.
Conflict of Interest Statement: The authors did not provide a conflict of interest statement.
References
- Amerah A.M., Ravindran V., Lentle R.G. Influence of insoluble fiber and whole wheat inclusion on the performance, digestive tract development and ileal microbiota profile of broiler chickens. Br. Poult. Sci. 2009;50:366–375. doi: 10.1080/00071660902865901. [DOI] [PubMed] [Google Scholar]
- Appleby M. Factors affecting floor laying by domestic hens: a review. Worlds Poult. Sci. J. 1984;40:241–249. [Google Scholar]
- Bari M.S., Cohen-Barnhouse A.M., Campbell D.L.M. Early rearing enrichments influenced nest use and egg quality in free-range laying hens. Animal. 2020;14:1249–1257. doi: 10.1017/S1751731119003094. [DOI] [PubMed] [Google Scholar]
- Brambell R. H.M. Stationery Office; London, UK: 1965. Report of the Technical Committee to Enquire into the Welfare of Animals Kept under Intensive Livestock Husbandry Systems, Cmd. (Great Britain. Parliament) pp. 1–84. [Google Scholar]
- Buitenhuis A.J., Rodenburg T.B., Rodenburg B., Wissink P.H., Visscher J., Koene P., Bovenhuis H., Ducro B.J., van der Poel J.J. Genetic and phenotypic correlations between feather pecking behavior, stress response, immune response, and egg quality traits in laying hens. Poult. Sci. 2004;83:1077–1082. doi: 10.1093/ps/83.7.1077. [DOI] [PubMed] [Google Scholar]
- Cronin G.M., Hopcroft R.L., Groves P.J., Hall E.J.S., Phalen D.N., Hemsworth P.H. Why did severe feather pecking and cannibalism outbreaks occur? An unintended case study while investigating the effects of forage and stress on pullets during rearing. Poult. Sci. 2018;97:1484–1502. doi: 10.3382/ps/pey022. [DOI] [PubMed] [Google Scholar]
- Damme K., Keppler C., Haustleitner M., Bachmeier J., Hartmann J., Louton H., Rauch E. Test of different premium broiler genotypes under Animal Welfare Label conditions. Part I: fattening and slaughter yield. Eur. Poult. Sci. 2015;79 [Google Scholar]
- Damme K., Pirchner F. Genetic differences of feather-loss in layers and effects on production traits. Arch. Geflügelk. 1984;48:215–222. [Google Scholar]
- Damme K., Schreiter R., Schneider M., Hildebrand R.A. 13. Bavarian benchmarking of laying hybrids in barn housing systems. 2018. https://www.lfl.bayern.de/mam/cms07/lvfz/kitzingen/dateien/13._bayerischer_herkunftsvergleich.pdf
- du Prel J.-B., Röhrig B., Hommel G., Blettner M. Choosing statistical tests. Dtsch. Aerztebl. Int. 2010;107:343–348. doi: 10.3238/arztebl.2010.0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen E.J., Bohren B.B., Mckean H.E. Haugh unit as a measure of egg albumen quality. Poult. Sci. 1962;41:1361–1368. [Google Scholar]
- El-Lethey H., Aerni V., Jungi T.W., Wechsler B. Stress and feather pecking in laying hens in relation to housing conditions. Br. Poult. Sci. 2000;41:22–28. doi: 10.1080/00071660086358. [DOI] [PubMed] [Google Scholar]
- Farm Animal Welfare Council Farm Animal Welfare Council Press statement. 1979. https://webarchive.nationalarchives.gov.uk/20121010012428/http://www.fawc.org.uk/pdf/fivefreedoms1979.pdf
- Freytag S., Kemper N., Spindler B. Effects of access to enrichment materials on behaviour and flock health of pullets and laying hens on farms - final project report. Institut für Tierhygiene, Tierschutz und Nutztierethologie, Stiftung Tierärztliche Hochschule Hannover. 2016. https://www.google.de/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&ved=2ahUKEwi0sJ_XnfXhAhWKUBUIHSsNAvIQFjAAegQIAhAC&url=https%3A%2F%2Fwww.ml.niedersachsen.de%2Fdownload%2F107762%2FAbschlussbericht_TiHo_-_Ausstieg_Schnabelkuerzung_Einfluss_des_Zugangs_zu_Beschaeftigungsmaterial.pdf&usg=AOvVaw08urgjDm7zz5ID6F87tKel
- Guzman P., Saldana B., Kimiaeitalab M.V., Garcia J., Mateos G.G. Inclusion of fiber in diets for brown-egg laying pullets: effects on growth performance and digestive tract traits from hatching to 17 weeks of age. Poult. Sci. 2015;94:2722–2733. doi: 10.3382/ps/pev288. [DOI] [PubMed] [Google Scholar]
- Halle I., Kluth H., Dänicke S. Effect of a graded dietary protein-energy-concentration on the growth performance of laying-type cockerels of different strains. Arch. Geflügelk. 2012;76:223–229. [Google Scholar]
- Hartcher K.M., Tran K.T.N., Wilkinson S.J., Hemsworth P.H., Thomson P.C., Cronin G.M. The effects of environmental enrichment and beak-trimming during the rearing period on subsequent feather damage due to feather-pecking in laying hens. Poult. Sci. 2015;94:852–859. doi: 10.3382/ps/pev061. [DOI] [PubMed] [Google Scholar]
- Hartini S., Choct M., Hinch G., Kocher A., Nolan J.W. Effects of light intensity during rearing and beak trimming and dietary fiber sources on mortality, egg production, and performance of ISA Brown laying hens. J. Appl. Poult. Res. 2002;11:104–110. [Google Scholar]
- Hetland H., Choct M., Svihus B. Role of insoluble fiber on gizzard activity in layers. J. Appl. Poult. Res. 2005;14:38–46. [Google Scholar]
- Jeroch H., Müller R. Nutritional Recommendations for Laying Hens Including the Rearing Period. In: Damme K., Mayer A., editors. Geflügeljahrbuch 2019. Ulmer; Stuttgart, Germany: 2018. pp. 201–227. [Google Scholar]
- Jeroch H., Simon A., Zentek J. Ulmer; Stuttgart, Germany: 2013. Poultry Nutrition. [Google Scholar]
- Johnsen P.F., Vestergaard K.S., Norgaard-Nielsen G. Influence of early rearing conditions on the development of feather pecking and cannibalism in domestic fowl. Appl. Anim. Behav. Sci. 1998;60:25–41. [Google Scholar]
- Keppler C., Fetscher S., Hilmes N., Knierim U. Management aid for rearing and keeping laying hens. 2017. https://www.mud-tierschutz.de/fileadmin/SITE_MASTER/content/Dokumente/Downloads/MTool/2018-09-25_Basiswissen_MTool_web.pdf
- Laudadio V., Ceci E., Lastella N.M.B., Introna M., Tufarelli V. Low-fiber alfalfa (Medicago sativa L.) meal in the laying hen diet: effects on productive traits and egg quality. Poult. Sci. 2014;93:1868–1874. doi: 10.3382/ps.2013-03831. [DOI] [PubMed] [Google Scholar]
- Lohmann Tierzucht . Lohmann Tierzucht GmbH; Cuxhaven: 2017. Management Guide Alternative Systems – Management Recommendations for Barn, Aviary & Free-Range Systems.www.ltz.de/de-wAssets/docs/management-guides/de/Legehennen/Alternativ/LTZ_MG-AlternHaltung_DE.pdf [Google Scholar]
- Market Info Eggs and Poultry Listing of the egg packing stations association Weser-Ems of 27.07.2017 – Brown eggs from husbandry type 2. DGS-Magazin. 2017;31:31. [Google Scholar]
- Martin G., Sambraus H.H., Steiger A. Verlag Uni Kassel; Germany: 2005. Welfare of Laying Hens in Europe: Reports, Analyses and Conclusions. [Google Scholar]
- Niebuhr K., Zaludik K., Gruber B., Thenmaier I., Lugmair A., Troxler J. University Vienna; Vienna, Austria: 2006. Epidemiological Studies on the Occurrence of Cannibalism and Feather Pecking in Alternative Laying Hen Farms in Austria. Final Project Report Nr. 1313 ITT 2006. [Google Scholar]
- Pottgüter R., Schreiter R., van der Linde J. Management recommendations for rearing and husbandry of laying hens in floor, aviary and free range systems. In: Damme K., Mayer A., editors. Poultry Annual 2019. Ulmer; Stuttgart, Germany: 2018. pp. 104–155. [Google Scholar]
- Rasch B., Friese M., Hofmann W.J., Naumann E. 3th rev. ed. volume 1. Springer; Heidelberg, Germany: 2010. (Quantitative Methods). [Google Scholar]
- Schreiter R., Damme K. LfL-Information; Freising, Germany: 2017. Nutrition of Laying Hens - Use of Domestic Feed and Feeding of Laying Hens with Untrimmed Beaks. [Google Scholar]
- Schreiter R., Damme K., Klunker M., Raoult C., von Borell E., Freick M. Effects of edible environmental enrichments during the rearing and laying periods in a littered aviary—Part 1: integument condition in pullets and laying hens. Poult. Sci. 2020;99:5184–5196. doi: 10.1016/j.psj.2020.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiter R., Damme K., von Borell E., Vogt I., Klunker M., Freick M. Effects of litter and additional enrichment elements on the occurrence of feather pecking in pullets and laying hens – a focused review. Vet. Med. Sci. 2019;5:500–507. doi: 10.1002/vms3.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepeur S., Spindler B., Schulze-Bisping M., Habig C., Andersson R., Beyerbach M., Kemper N. Comparison of plumage condition of laying hens with intact and trimmed beaks kept on commercial farms. Eur. Poult. Sci. 2015;79 [Google Scholar]
- Spindler B. Beschäftigung von Jung- und Legehennen. DGS-Magazin. 2019;5:16–19. [Google Scholar]
- Spindler B., Giersberg M.F., Andersson R., Kemper N. Keeping laying hens with untrimmed beaks – a review of the status quo in practice and science. Züchtungskunde. 2016;88:475–493. [Google Scholar]
- Steenfeldt S., Kjaer J.B., Engberg R.M. Effect of feeding silages or carrots as supplements to laying hens on production performance, nutrient digestibility, gut structure, gut microflora and feather pecking behaviour. Br. Poult. Sci. 2007;48:454–468. doi: 10.1080/00071660701473857. [DOI] [PubMed] [Google Scholar]
- Su G., Kjaer J.B., Sorensen P. Divergent selection on feather pecking behavior in laying hens has caused differences between lines in egg production, egg quality, and feed efficiency. Poult. Sci. 2006;85:191–197. doi: 10.1093/ps/85.2.191. [DOI] [PubMed] [Google Scholar]
- Svihus B. The gizzard: function, influence of diet structure and effects on nutrient availability. Worlds Poult. Sci. J. 2011;67:207–223. [Google Scholar]
- Thiele H.H. Management tools to influence egg weight in commercial layers. Lohmann Inf. 2012;47:21–31. [Google Scholar]
- Victor A., Elsäßer A., Hommel G., Blettner M. Judging a plethora of p-values: how to contend with the problem of multiple testing? Dtsch. Aerztebl. Int. 2010;107:50–56. doi: 10.3238/arztebl.2009.0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiß C. Springer; Heidelberg, Germany: 1999. Basic Knowledge of Medical Statistics. [Google Scholar]