Abstract
Background:
Bronchiolitis is the leading cause of infant hospitalizations in the U.S. Growing evidence supports the heterogeneity of bronchiolitis. However, little is known about the interrelationships between major respiratory viruses (and their species), host systemic metabolism, and disease pathobiology.
Methods:
In an ongoing multicenter prospective cohort study, we profiled the serum metabolome in 113 infants (63 RSV-only, 21 RV-A, and 29 RV-C) hospitalized with bronchiolitis. We identified serum metabolites that are most discriminatory in the RSV—RV-A and RSV—RV-C comparisons using sparse partial least squares discriminant analysis. We then investigated the association between discriminatory metabolites with acute and chronic outcomes.
Results:
In 113 infants with bronchiolitis, we measured 639 metabolites. Serum metabolome profiles differed in both comparisons (Ppermutation<0.05). In the RSV—RV-A comparison, we identified 30 discriminatory metabolites, predominantly in lipid metabolism pathways (e.g., sphingolipids and carnitines). In multivariable models, these metabolites were significantly associated with the risk of clinical outcomes (e.g., tricosanoyl sphingomyelin, OR for recurrent wheezing at age 3 years = 1.50; 95%CI 1.05–2.15). In the RSV—RV-C comparison, the discriminatory metabolites were also primarily involved in lipid metabolism (e.g., glycerophosphocholines [GPCs], 12,13-DiHome). These metabolites were also significantly associated with the risk of outcomes (e.g., 1-stearoyl-2-linoleoyl-GPC, OR for positive pressure ventilation use during hospitalization=0.47; 95%CI 0.28–0.78).
Conclusion:
Respiratory viruses and their species had distinct serum metabolomic signatures that are associated with differential risks of acute and chronic morbidities of bronchiolitis. Our findings advance research into the complex interrelations between viruses, host systemic response, and bronchiolitis pathobiology.
Keywords: serum metabolome, bronchiolitis, infant, rhinovirus, rhinovirus species, respiratory syncytial virus
INTRODUCTION
Bronchiolitis is the most common lower respiratory infection in infants.1,2 In addition to its acute morbidity (e.g., the leading cause of infant hospitalization in the US),3 infants with severe bronchiolitis (bronchiolitis requiring hospitalization) are at high risk for developing chronic morbidities, such as recurrent wheeze and asthma in childhood.2 Severe bronchiolitis is predominantly caused by two viruses; the most common virus is respiratory syncytial virus (RSV), followed by rhinovirus (RV).2 RVs are RNA viruses with >160 recognized genotypes that are classified into three species (A, B, and C).4
Bronchiolitis has been traditionally considered a single, relatively homogenous disease entity; its management and prognostication do not currently differ by viral etiology.1,2 However, several recent studies demonstrate the heterogeneity of bronchiolitis by infecting virus. For example, research has shown that RSV and RV infection are associated with differential acute (e.g., disease severity)5 and chronic (e.g., incident asthma)6,7 morbidity burdens, host response (through transcriptomics,8,9 cytokines,10 and metabolomics11,12), and airway microbiome profiles.9,13,14 Despite the clinical and research relevance, it remains unclear how different viruses (including RV species) systemically contribute to downstream molecules that influence clinical outcomes in infants with bronchiolitis. Metabolomics addresses this knowledge gap through comprehensively profiling functional metabolites that are a function of the child’s genetic make-up and environmental factors,2 such as respiratory viruses.
In this context, by comprehensively profiling the serum metabolome from a nested subset of multicenter bronchiolitis cohort, we examined the relationships between respiratory viruses (including RV species), metabolome, and acute and chronic morbidities of bronchiolitis.
METHODS
Study design, setting, and participants
We analyzed data from the 35th Multicenter Airway Research Collaboration (MARC-35)—a multicenter prospective cohort study of infants hospitalized with bronchiolitis.6–13,15,16 Site investigators enrolled infants (age <1 year) hospitalized with bronchiolitis at 17 sites across 14 U.S. states (Supplemental Table E1) during three consecutive bronchiolitis seasons (from November through April) in 2011–2014. Bronchiolitis was defined by the American Academy of Pediatrics guidelines1 as an acute respiratory illness (ARI) with some combination of rhinitis, cough, tachypnea, wheezing, crackles, and retractions and was diagnosed by an attending physician. We excluded infants who were transferred to a participating hospital >24 hours after the original hospitalization or those with known heart-lung disease, immunodeficiency, immunosuppression, or gestational age of <32 weeks. All patients were treated at the discretion of the treating physicians. The institutional review board at each of the participating hospitals approved the study. Written informed consent was obtained from the parent or guardian. The current analysis investigated infants with RSV or RV infection who underwent serum metabolomics testing. By design, infants with RV infection were overrepresented in this substudy (Supplemental Table E2).
Data collection, virus testing, and serum metabolomics testing
Clinical data (patients’ demographic characteristics, and family, environmental, and medical history, and details of the acute illness) were collected via structured interview and chart reviews using a standardized protocol. 6–13,15,16 In addition to the clinical data, nasopharyngeal and serum specimens were collected by trained site investigators within 24 hours of hospitalization.15 These specimens underwent the following virus and metabolomics testing.
Nasopharyngeal samples were first tested for 17 respiratory viruses (including RSV and RV) by using real-time polymerase chain reaction (PCR) assays at Baylor College of Medicine (Houston, TX, USA). For RV detection, complementary DNA was generated using gene-specific primers for RV and singleplex real-time PCR was used. The details of the RV primers and probes have been described elsewhere.17 Next, to identify the RV species (A, B, and C), RV-positive samples were genotyped by molecular typing assay using partial sequencing18 at the University of Wisconsin (Madison, WI, USA).
The details of metabolomic profiling may be found in Supplemental Methods. In brief, metabolome profiling used ultrahigh-performance liquid chromatography–tandem mass spectrometry. Instrument variability was 4%, as determined by calculating the median relative standard deviation for the internal standards that were added to each sample before the sample was injected into the mass spectrometer.
Outcomes
The clinical outcomes were acute severity of bronchiolitis (as measured by positive pressure ventilation (PPV) use), as well as chronic morbidities (as measured by recurrent wheezing by age three years and asthma by age five years). PPV use was defined as the use of continuous positive airway pressure and/or intubation—at any time during the index hospitalization.16 Recurrent wheezing was defined—according to the 2007 NIH asthma guidelines19—as having at least two corticosteroid-requiring exacerbations in six months or at least four wheezing episodes in one year that last at least one day and affect sleep. Asthma was defined using an established epidemiologic definition of asthma20—i.e., physician-diagnosis of asthma by age five years, plus either asthma medication use (e.g., albuterol inhaler, inhaled corticosteroids, montelukast) or asthma-related symptoms in the preceding year.
Statistical analyses
In the current analysis, we categorized the patients into three mutually-exclusive groups: RSV-only (reference) and two RV species (A and C) groups, excluding infants with RSV infection with non-RV pathogen(s), those with RV-B infection, and those with coinfection of multiple RV species. RV-B infections were excluded because of its clinical insignificance in ARI5 and the small number of RV-B (n=3) infection in the cohort. To investigate the association of respiratory viruses with serum metabolome, we compared metabolites between RSV and RV-A as well as between RSV and RV-C groups. RSV group was chosen as the reference given the relatively large sample size. First, to derive metabolites that best discriminate the virus groups, we performed sparse partial least squares discriminate analysis (sPLS-DA) with Lasso penalization and 5-fold cross-validation (repeated 50 times) to minimize potential overfitting, using R mixOmics package.21 We also tested for the between-virus differences by performing permutation test (2000 random permutations). For the downstream analyses, we selected the top 30 metabolites that had a high variable importance in each of the between-virus comparisons (i.e., RSV vs. RV-A and RSV vs. RV-C comparisons).
Next, we computed the Spearman`s correlation between viral genomic load (measured as the inverse cycle threshold) and the selected 30 discriminatory metabolites. We then tested for the association of respiratory viruses (RSV as the reference group) with metabolites by constructing multivariable linear regression models adjusting for five potential confounders (age, sex, prematurity, feeding status, and batch of metabolomics testing) and patient clustering within hospitals using generalized estimating equations. To identify the underlying causal relations between respiratory viruses and metabolites—e.g., infection to unique RV species perturbates the downstream metabolites (or alerted metabolomic profile changes the risk of infection to specific virus), we utilized the PC algorithm implemented in R pcalg package. This causal structure learning approach recovers the underlying causal pathways through the conditional independence relationships in the empirical data. To detect biologically meaningful pathways, we also performed metabolite set enrichment analysis using MetaboAnalyst 4.0.
Lastly, we also examined the association of discriminatory metabolites (Benjamini-Hochberg false discovery rate [FDR] of <0.05) with each of the three clinical outcomes (PPV, recurrent wheezing, asthma). We constructed multivariable logistic regression models with generalized estimating equations adjusting for age, detected virus, potential batch effect, and patient clustering within sites. In the sensitivity analysis, we repeated the analysis using sole RV-A and RV-C infection data. To examine the relations between serum and nasopharyngeal metabolites, we examined the within-individual correlations of the discriminatory metabolites. We performed the analysis using R version 3.6.1.
RESULTS
As part of a 17-center prospective cohort study, 140 infants with bronchiolitis underwent serum metabolomics testing. Of these, we excluded 23 infants with RSV infection with co-infection, 3 with RV-B infection, and 1 with coinfection of multiple RV species, leaving 113 infants (63 RSV-only, 21 RV-A, and 29 RV-C) eligible for the current analysis. The analytic (113 eligible infants) and non-analytic (903 infants who were not eligible) cohorts did not differ in patient characteristics, except for the overrepresentation of RV infection in the analytic cohort (Supplemental Table E2). Overall, the median age was 3.0 (IQR, 1.3–5.9) months and 62% were male. Infants with RSV-only infection were younger than those with RV-A or RV-C (P<0.05; Table 1). In contrast, there were no significant between-group differences in pre-hospitalization treatments (e.g., systemic corticosteroids, antibiotics). While infants with RSV-only had the highest proportion of PPV use during the bronchiolitis hospitalization, those with RV-C group had the highest risk of developing recurrent wheezing by age 3 years (both P<0.05).
Table 1.
Patient characteristics of infants hospitalized for bronchiolitis, according to respiratory virus group
| RSV (n=63; 56%) | RV-A (n=21; 19%) | RV-C (n=29; 26%) | P-value | ||||
|---|---|---|---|---|---|---|---|
| Baseline characteristics | |||||||
| Age (month), median (IQR) | 2.0 (1.0–4.5) | 3.5 (2.3–6.6) | 5.4 (3.3–8.0) | <0.001 | |||
| Male sex | 39 | (62) | 13 | (62) | 19 | (66) | 0.94 |
| Race/ethnicity | 0.89 | ||||||
| Non-Hispanic white | 27 | (43) | 9 | (43) | 9 | (31) | |
| Non-Hispanic black | 15 | (24) | 4 | (19) | 8 | (28) | |
| Hispanic | 19 | (30) | 8 | (38) | 11 | (38) | |
| Other or unknown | 2 | (3) | 0 | 0 | 1 | (3) | |
| Maternal smoking during pregnancy | 6 | (10) | 5 | (24) | 3 | (10) | 0.26 |
| C-section delivery | 24 | (38) | 9 | (43) | 9 | (31) | 0.68 |
| Prematurity (32–37 weeks) | 13 | (21) | 5 | (24) | 6 | (21) | 0.95 |
| History of eczema | 7 | (11) | 4 | (19) | 6 | (21) | 0.42 |
| Mostly breastfed for the first 3 month of age | 30 | (48) | 10 | (48) | 15 | (52) | 0.95 |
| Cigarette smoke exposure at home | 5 | (8) | 3 | (14) | 3 | (10) | 0.63 |
| Corticosteroid use before the index hospitalization | 5 | (8) | 2 | (10) | 4 | (14) | 0.69 |
| Antibiotic use before the index hospitalization | 17 | (27) | 1 | (5) | 8 | (28) | 0.09 |
| Clinical presentation | |||||||
| Weight at presentation (kg), median (IQR) | 5.2 (4.2–6.9) | 6.1 (4.9–7.2) | 7.5 (6.1–8.5) | 0.003 | |||
| Respiratory rate at presentation (per minute), median (IQR) | 48 (40–60) | 46 (42–52) | 50 (40–60) | 0.82 | |||
| Oxygen saturation at presentation | 0.58 | ||||||
| <90% | 6 | (10) | 3 | (14) | 1 | (3) | |
| 90–93% | 12 | (19) | 4 | (19) | 4 | (14) | |
| ≥94% | 43 | (68) | 13 | (62) | 24 | (83) | |
| Missing | 2 | (3) | 1 | (5) | 0 | 0 | |
| Detected pathogen | |||||||
| RSV | 63 | (100) | 6 | (29) | 8 | (28) | - |
| RV | 0 | (0) | 21 | (100) | 29 | (100) | - |
| Other pathogen* | 0 | (0) | 7 | (33) | 10 | (34) | <0.001 |
| No pathogen | 0 | (0) | 0 | (0) | 0 | (0) | - |
| Clinical outcomes | |||||||
| Positive pressure ventilation during hospitalization† | 26 | (41) | 5 | (24) | 8 | (28) | 0.004 |
| Hospital length of stay (day), median (IQR) | 4 (2–8) | 2 (1–3) | 2 (1–3) | <0.001 | |||
| Recurrent wheezing by 3-years‡ | 25 | (40) | 12 | (57) | 23 | (79) | 0.002 |
| Asthma by 5-years§ | 22 | (35) | 7 | (33) | 16 | (55) | 0.16 |
Abbreviations: RSV, respiratory syncytial virus; RV, rhinovirus; IQR, interquartile range.
Data are n (%) of infants unless otherwise indicated.
Adenovirus, bocavirus, Bordetella pertussis, enterovirus, human coronavirus NL63, OC43, 229E, or HKU1, human metapneumovirus, influenza A or B virus, Mycoplasma pneumoniae, parainfluenza virus 1–3.
Defined as use of invasive and/or non-invasive mechanical ventilation (e.g., continuous positive airway pressure ventilation).
Defined by parental report of at least two corticosteroid-requiring breathing problems in six months or at least four wheezing episodes in one year that last at least one day and affect sleep.
Defined by physician-diagnosis of asthma by age five years, plus either asthma medication use (e.g., albuterol inhaler, inhaled corticosteroids, montelukast) or asthma-related symptoms in the preceding year.
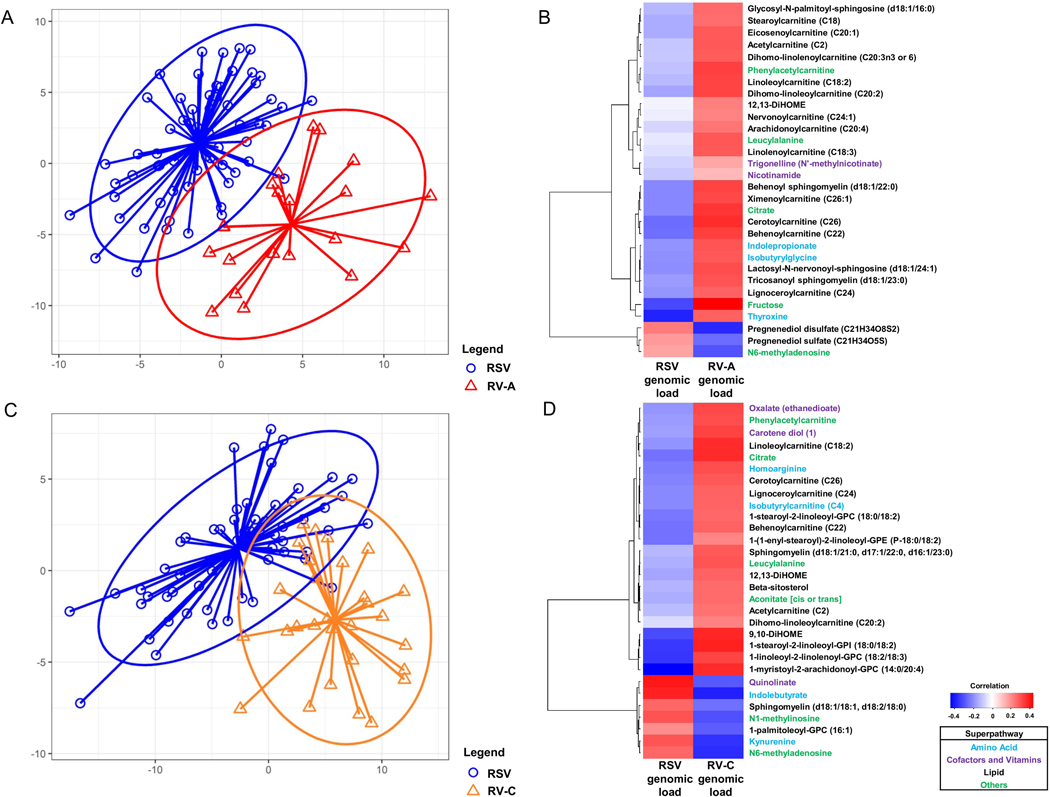
Serum metabolomic profiling identified a total of 639 metabolites from 87 sub-pathways within 7 super-pathways. Based on the sPLS-DA, the overall metabolome profiles distinctly clustered both in the RSV—RV-A (Ppermutation=0.004; Figure 1A) and RSV—RV-C comparisons (Ppermutation<0.001; Figures 1C). In the RSV—RV-A comparison, sPLS-DA selected 30 discriminatory metabolites—primarily involved in lipid metabolism—such as sphingolipids and carnitines (Table 2). In the RSV—RV-C comparison, while the most discriminatory metabolite was homo-arginine, the metabolites chiefly consisted of other families of lipids—such as glycerophospholipids (e.g., glycerophosphocholines [GPCs], glycerophosphoethanolamines [GPEs]) and 9,10/12,13-DiHOMEs; Table 3). These differences between qualitative virus status (presence vs. absence of corresponding virus) were consistent with the correlations between quantitative virus status (viral genomic load [measured as the inverse cycle threshold value]) and metabolite intensities (Figure 1B and D). In the multivariable models, the between-virus differences remained significant for 23 metabolites when comparing with RV-A (e.g., sphingolipids, carnitines) and 20 metabolites when comparing with RV-C (e.g., homo-arginine, glycerophospholipids) (FDR<0.05; Tables 2 and 3). These results were consistent in the sensitivity analysis using sole RV data (Tables E3 and E4). In contrast, there was no significant within-individual correlation between the intensity of serum discriminatory metabolites and those in nasopharyngeal metabolites in either between-virus comparison (all P>0.05; Supplemental Figure E1).
Figure 1.
Associations of respiratory syncytial virus and rhinovirus species with serum metabolites in infants with severe bronchiolitis
A. Sparse partial least squares discriminant analysis (sPLS-DA) score plot of serum metabolome according to RSV-only (blue) vs. RV-A (red) infection (Ppermutation=0.004 with 2000 random permutations). Each dot represents the serum metabolome profile of a single infant, by plotting the component scores in the smaller subspace spanned by latent variables of sPLS-DA. The eclipses are 90% confidence intervals. The arrows start from the centroid of each virus group and end for each patient belonging to each group.
B. Heatmap of Spearman’s correlations between the genomic load (measured as the inverse cycle threshold) of RSV and RV-A and the 30 most discriminatory serum metabolites in the corresponding sPLS-DA model (Table 2). Clustering is based on Euclidean distance and Ward’s minimum variance linkage algorithm. The color bar indicates the correlation coefficients—red color indicates a positive correlation while blue color indicates a negative correlation. Superpathway of each metabolite is color-coded for each function.
C. sPLS-DA score plot in the comparison between RSV (blue) and RV-C (orange) infection (Ppermutation<0.001 with 2000 random permutations).
D. Heatmap of Spearman’s correlations between the genomic load of RSV and RV-C and the 30 most discriminatory serum metabolites in the corresponding sPLS-DA model (Table 3).
Abbreviations: RSV, respiratory syncytial virus; RV, rhinovirus
Table 2.
Multivariable associations of respiratory syncytial virus and rhinovirus-A with serum metabolites
| Metabolites* | Superpathway | Subpathway | Coefficient† | Standard error† | P-value† | FDR† |
|---|---|---|---|---|---|---|
| Tricosanoyl sphingomyelin (d18:1/23:0) | Lipid | Sphingolipid metabolism | 0.34 | 0.06 | <0.001 | <0.001 |
| Fructose | Carbohydrate | Fructose, mannose and galactose metabolism | 0.58 | 0.10 | <0.001 | <0.001 |
| Behenoyl sphingomyelin (d18:1/22:0) | Lipid | Sphingolipid metabolism | 0.19 | 0.04 | <0.001 | <0.001 |
| Lignoceroylcarnitine (C24) | Lipid | Fatty acid metabolism | 0.42 | 0.08 | <0.001 | <0.001 |
| Leucylalanine | Peptide | Dipeptide | 1.34 | 0.29 | <0.001 | <0.001 |
| Cerotoylcarnitine (C26) | Lipid | Fatty acid metabolism | 0.54 | 0.12 | <0.001 | <0.001 |
| Isobutyrylglycine | Amino acid | Leucine, isoleucine and valine metabolism | 0.76 | 0.18 | <0.001 | <0.001 |
| Pregnenediol disulfate (C21H34O8S2) | Lipid | Pregnenolone steroids | −0.91 | 0.23 | <0.001 | <0.001 |
| Dihomo-linoleoylcarnitine (C20:2) | Lipid | Fatty acid metabolism | 0.44 | 0.12 | <0.001 | <0.001 |
| Nervonoylcarnitine (C24:1) | Lipid | Fatty acid metabolism | 0.33 | 0.09 | <0.001 | <0.001 |
| Behenoylcarnitine (C22) | Lipid | Fatty acid metabolism | 0.47 | 0.13 | <0.001 | <0.001 |
| Ximenoylcarnitine (C26:1) | Lipid | Fatty acid metabolism | 0.50 | 0.14 | <0.001 | 0.001 |
| Glycosyl-N-palmitoyl-sphingosine (d18:1/16:0) | Lipid | Hexosylceramides | 0.27 | 0.08 | <0.001 | 0.002 |
| Lactosyl-N-nervonoyl-sphingosine (d18:1/24:1) | Lipid | Lactosylceramides | 0.20 | 0.06 | 0.001 | 0.003 |
| Pregnenediol sulfate (C21H34O5S) | Lipid | Pregnenolone steroids | −0.74 | 0.24 | 0.002 | 0.004 |
| Arachidonoylcarnitine (C20:4) | Lipid | Fatty acid metabolism | 0.55 | 0.18 | 0.002 | 0.004 |
| Dihomo-linolenoylcarnitine (C20:3n3 or 6) | Lipid | Fatty acid metabolism | 0.50 | 0.17 | 0.003 | 0.005 |
| Citrate | Energy | TCA cycle | 0.30 | 0.10 | 0.003 | 0.005 |
| Stearoylcarnitine (C18) | Lipid | Fatty acid metabolism | 0.33 | 0.11 | 0.003 | 0.005 |
| N6-methyladenosine | Nucleotide | Purine metabolism, adenine containing | −0.38 | 0.13 | 0.004 | 0.005 |
| Thyroxine | Amino acid | Tyrosine metabolism | 0.32 | 0.11 | 0.005 | 0.005 |
| Linoleoylcarnitine (C18:2) | Lipid | Fatty acid metabolism | 0.40 | 0.17 | 0.02 | 0.03 |
| Phenylacetylcarnitine | Peptide | Acetylated peptides | 1.04 | 0.49 | 0.04 | 0.04 |
| Eicosenoylcarnitine (C20:1) | Lipid | Fatty acid metabolism | 0.31 | 0.18 | 0.07 | 0.09 |
| Indolepropionate | Amino acid | Tryptophan metabolism | 1.16 | 0.66 | 0.08 | 0.09 |
| Acetylcarnitine (C2) | Lipid | Fatty acid metabolism | 0.21 | 0.12 | 0.08 | 0.09 |
| Trigonelline (N’-methylnicotinate) | Cofactors and vitamins | Nicotinate and nicotinamide metabolism | 0.56 | 0.33 | 0.09 | 0.10 |
| Linolenoylcarnitine (C18:3) | Lipid | Fatty acid metabolism | 0.41 | 0.26 | 0.12 | 0.13 |
| 12,13-DiHOME | Lipid | Fatty acid, dihydroxy | 0.43 | 0.30 | 0.15 | 0.15 |
| Nicotinamide | Cofactors and vitamins | Nicotinate and nicotinamide metabolism | 0.31 | 0.27 | 0.25 | 0.25 |
Abbreviations: FDR, false discovery rate; TCA, tricarboxylic acid
The 30 metabolites that have a high variable importance in projection coefficients of component one in sparse partial least squares discriminate models.
The multivariable linear models adjusted for age, sex, prematurity, feeding status, and potential batch effect, as well as clustering within hospitals (with the use of generalized estimating equations). The coefficient represents the between-virus difference in the metabolite intensity (the respiratory syncytial virus group as the reference).
Table 3.
Multivariable associations of respiratory syncytial virus and rhinovirus-C with serum metabolites
| Metabolites* | Superpathway | Subpathway | Coefficient† | Standard error† | P-value† | FDR† |
|---|---|---|---|---|---|---|
| Homoarginine | Amino acid | Urea cycle; arginine and proline metabolism | 0.71 | 0.15 | <0.001 | <0.001 |
| Linoleoylcarnitine (C18:2) | Lipid | Fatty acid metabolism | 0.36 | 0.08 | <0.001 | <0.001 |
| 9,10-DiHOME | Lipid | Fatty acid, dihydroxy | 0.64 | 0.14 | <0.001 | <0.001 |
| 1-stearoyl-2-linoleoyl-GPC (18:0/18:2) | Lipid | Phosphatidylcholine | 0.24 | 0.06 | <0.001 | <0.001 |
| Oxalate (ethanedioate) | Cofactors and vitamins | Ascorbate and aldarate metabolism | 0.41 | 0.11 | <0.001 | <0.001 |
| 1-linoleoyl-2-linolenoyl-GPC (18:2/18:3) | Lipid | Phosphatidylcholine | 1.14 | 0.31 | <0.001 | 0.001 |
| 1-myristoyl-2-arachidonoyl-GPC (14:0/20:4) | Lipid | Phosphatidylcholine | 0.59 | 0.16 | <0.001 | 0.002 |
| Phenylacetylcarnitine | Peptide | Acetylated peptides | 1.02 | 0.29 | <0.001 | 0.002 |
| 1-stearoyl-2-linoleoyl-GPI (18:0/18:2) | Lipid | Phosphatidylinositol | 0.52 | 0.15 | <0.001 | 0.003 |
| 1-(1-enyl-stearoyl)-2-linoleoyl-GPE (P-18:0/18:2) | Lipid | Plasmalogen | 0.36 | 0.11 | 0.001 | 0.003 |
| Isobutyrylcarnitine (C4) | Amino acid | Leucine, isoleucine and valine metabolism | 0.57 | 0.18 | 0.002 | 0.006 |
| Sphingomyelin (d18:1/21:0, d17:1/22:0, d16:1/23:0) | Lipid | Sphingolipid metabolism | 0.40 | 0.15 | 0.007 | 0.02 |
| Beta-sitosterol | Lipid | Sterol | 0.67 | 0.26 | 0.01 | 0.02 |
| Leucylalanine | Peptide | Dipeptide | 1.09 | 0.43 | 0.01 | 0.02 |
| 12,13-DiHOME | Lipid | Fatty acid, dihydroxy | 0.47 | 0.19 | 0.01 | 0.03 |
| Aconitate | Energy | TCA cycle | 0.32 | 0.13 | 0.01 | 0.03 |
| Dihomo-linoleoylcarnitine (C20:2) | Lipid | Fatty acid metabolism | 0.29 | 0.13 | 0.02 | 0.04 |
| Indolebutyrate | Amino acid | Tryptophan metabolism | −0.63 | 0.28 | 0.03 | 0.04 |
| Quinolinate | Cofactors and vitamins | Nicotinate and nicotinamide metabolism | −0.20 | 0.09 | 0.03 | 0.04 |
| 1-palmitoleoyl-GPC (16:1) | Lipid | Lysophospholipid | −0.28 | 0.13 | 0.03 | 0.047 |
| Citrate | Energy | TCA cycle | 0.30 | 0.14 | 0.04 | 0.06 |
| Acetylcarnitine (C2) | Lipid | Fatty acid metabolism | 0.33 | 0.17 | 0.04 | 0.06 |
| N6-methyladenosine | Nucleotide | Purine metabolism, adenine containing | −0.22 | 0.11 | 0.04 | 0.06 |
| Carotene diol (1) | Cofactors and vitamins | Vitamin A metabolism | 0.81 | 0.41 | 0.05 | 0.06 |
| Behenoylcarnitine (C22) | Lipid | Fatty acid metabolism | 0.36 | 0.19 | 0.05 | 0.07 |
| Sphingomyelin (d18:1/18:1, d18:2/18:0) | Lipid | Sphingolipid metabolism | −0.21 | 0.11 | 0.06 | 0.07 |
| Kynurenine | Amino acid | Tryptophan metabolism | −0.12 | 0.07 | 0.07 | 0.07 |
| Lignoceroylcarnitine (C24) | Lipid | Fatty acid metabolism | 0.23 | 0.13 | 0.07 | 0.08 |
| Cerotoylcarnitine (C26) | Lipid | Fatty acid metabolism | 0.21 | 0.17 | 0.21 | 0.22 |
| N1-methylinosine | Nucleotide | Purine metabolism, xanthine/inosine containing | 0.01 | 0.06 | 0.89 | 0.89 |
Abbreviations: FDR, false discovery rate; GPC, glycerophosphorylcholine; GPI, glycosylphosphatidylinositol; GPE, glycerophosphorylethanolamine; TCA, tricarboxylic acid
The 30 metabolites that have a high variable importance in projection coefficients of component one in sparse partial least squares discriminate models.
The multivariable linear models adjusted for age, sex, prematurity, feeding status, and potential batch effect, as well as clustering within hospitals (with the use of generalized estimating equations). The coefficient represents the between-virus difference in the metabolite intensity (the respiratory syncytial virus group as the reference).
The metabolite-set enrichment analysis also identified differentially-enriched pathways—e.g., mitochondrial β-oxidation of long-chain saturated fatty acid and oxidation of branched-chain fatty acids pathways in the RSV—RV-A comparison, as well as arachidonic acid, arginine, and glycerophospholipid metabolism pathways in the RSV—RV-C comparison (Supplemental Figure E2). Additionally, the causal structure learning demonstrated that most metabolites were observed downstream of viruses—suggesting that viruses influenced the observed perturbations of most metabolites (Supplemental Figure E3).
Several discriminatory metabolites were significantly associated with acute and chronic clinical outcomes (Tables 4 and 5). In the RSV—RV-A comparison, for example, lignoceroyl-carnitine intensity was associated with a significantly lower risk of PPV use (OR 0.20; 95%CI 0.08–0.48; P<0.001). In contrast, tricosanoyl sphingomyelin was associated with a higher risk of developing recurrent wheeze (OR 1.50; 95%CI 1.05–2.15; P=0.03) and linoleoylcarnitine with a higher risk of asthma (OR 1.70; 95%CI 1.03–2.81; P=0.04). In the RSV—RV-C comparison, for example, 1-stearoyl-2-linoleoyl-GPC and 1-(1-enyl-stearoyl)-2- linoleoyl-GPE were associated with a lower risk of PPV use (both P<0.05). In contrast, homo-arginine was associated with a higher risk of recurrent wheezing (OR 1.94; 95%CI 1.06–3.53; P=0.03).
Table 4.
Multivariable associations of serum discriminatory metabolites (respiratory syncytial virus vs rhinovirus-A) with clinical outcomes
| Metabolites* | Superpathway | Positive pressure ventilation† | Recurrent wheezing‡ | Asthma§ | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR§ | 95% CI | P-value | OR§ | 95% CI | P-value | OR§ | 95% CI | P-value | ||
| Tricosanoyl sphingomyelin (d18:1/23:0) | Lipid | 0.99 | 0.56–1.75 | 0.97 | 1.50 | 1.05–2.15 | 0.03 | 1.15 | 0.77–1.73 | 0.50 |
| Fructose | Carbohydrate | 1.14 | 0.78–1.66 | 0.49 | 0.58 | 0.41–0.84 | 0.004 | 0.96 | 0.68–1.37 | 0.83 |
| Behenoyl sphingomyelin (d18:1/22:0) | Lipid | 0.77 | 0.46–1.29 | 0.32 | 1.54 | 1.06–2.23 | 0.02 | 1.07 | 0.70–1.64 | 0.76 |
| Lignoceroylcarnitine (C24) | Lipid | 0.20 | 0.08–0.48 | <0.001 | 0.99 | 0.68–1.44 | 0.95 | 0.73 | 0.45–1.17 | 0.19 |
| Leucylalanine | Peptide | 2.08 | 0.90–4.85 | 0.09 | 0.77 | 0.55–1.08 | 0.14 | 1.16 | 0.65–2.09 | 0.61 |
| Cerotoylcarnitine (C26) | Lipid | 0.41 | 0.19–0.93 | 0.03 | 1.01 | 0.53–1.94 | 0.97 | 1.15 | 0.55–2.40 | 0.71 |
| Isobutyrylglycine | Amino acid | 1.01 | 0.45–2.28 | 0.98 | 1.77 | 1.15–2.73 | 0.009 | 1.50 | 0.97–2.32 | 0.07 |
| Pregnenediol disulfate (C21H34O8S2) | Lipid | 0.95 | 0.56–1.59 | 0.84 | 0.67 | 0.47–0.96 | 0.03 | 1.00 | 0.65–1.54 | 0.99 |
| Dihomo-linoleoylcarnitine (C20:2) | Lipid | 0.42 | 0.15–1.20 | 0.10 | 1.11 | 0.66–1.86 | 0.69 | 1.22 | 0.63–2.37 | 0.55 |
| Nervonoylcarnitine (C24:1) | Lipid | 0.52 | 0.25–1.09 | 0.08 | 1.26 | 0.70–2.25 | 0.44 | 0.88 | 0.47–1.65 | 0.70 |
| Behenoylcarnitine (C22) | Lipid | 0.14 | 0.02–0.81 | 0.03 | 1.32 | 0.52–3.37 | 0.56 | 0.68 | 0.39–1.18 | 0.17 |
| Ximenoylcarnitine (C26:1) | Lipid | 0.51 | 0.25–1.05 | 0.07 | 1.17 | 0.73–1.89 | 0.51 | 1.04 | 0.45–2.40 | 0.93 |
| Glycosyl-N-palmitoyl-sphingosine (d18:1/16:0) | Lipid | 1.25 | 0.45–3.48 | 0.67 | 1.15 | 0.71–1.86 | 0.58 | 0.71 | 0.39–1.32 | 0.28 |
| Lactosyl-N-nervonoyl-sphingosine (d18:1/24:1) | Lipid | 1.51 | 0.84–2.72 | 0.17 | 1.41 | 0.98–2.01 | 0.06 | 1.15 | 0.74–1.77 | 0.53 |
| Pregnenediol sulfate (C21H34O5S) | Lipid | 0.72 | 0.46–1.11 | 0.14 | 0.66 | 0.50–0.88 | 0.004 | 0.88 | 0.53–1.46 | 0.62 |
| Arachidonoylcarnitine (C20:4) | Lipid | 0.42 | 0.16–1.09 | 0.08 | 1.00 | 0.64–1.54 | 0.99 | 1.09 | 0.56–2.10 | 0.81 |
| Dihomo-linolenoylcarnitine (C20:3n3 or 6) | Lipid | 0.38 | 0.20–0.70 | 0.002 | 1.11 | 0.74–1.68 | 0.61 | 1.14 | 0.61–2.15 | 0.68 |
| Citrate | Energy | 0.46 | 0.17–1.25 | 0.13 | 1.05 | 0.58–1.9 | 0.88 | 1.08 | 0.60–1.95 | 0.80 |
| Stearoylcarnitine (C18) | Lipid | 1.02 | 0.61–1.71 | 0.93 | 1.47 | 0.90–2.42 | 0.12 | 1.00 | 0.64–1.56 | 0.99 |
| N6-methyladenosine | Nucleotide | 0.85 | 0.44–1.65 | 0.64 | 0.81 | 0.54–1.21 | 0.30 | 0.75 | 0.56–1.01 | 0.06 |
| Thyroxine | Amino acid | 0.45 | 0.26–0.80 | 0.01 | 0.87 | 0.47–1.62 | 0.66 | 0.84 | 0.59–1.20 | 0.34 |
| Linoleoylcarnitine (C18:2) | Lipid | 0.39 | 0.12–1.24 | 0.11 | 1.72 | 1.28–2.32 | <0.001 | 1.70 | 1.03–2.81 | 0.04 |
| Phenylacetylcarnitine | Peptide | 0.32 | 0.07–1.55 | 0.16 | 0.97 | 0.59–1.6 | 0.91 | 1.38 | 0.89–2.13 | 0.15 |
Abbreviations: CI, confidence interval; OR, odds ratio
Bold results are statistically significant.
The discriminatory metabolites that have a false discovery rate of <0.05 in the virus-metabolite model (Table 2) are selected
Defined as use of invasive and/or non-invasive mechanical ventilation (e.g., continuous positive airway pressure ventilation).
Defined by parental report of at least two corticosteroid-requiring breathing problems in six months or at least four wheezing episodes in one year that last at least one day and affect sleep.
Defined by physician-diagnosis of asthma by age five years, plus either asthma medication use (e.g., albuterol inhaler, inhaled corticosteroids, montelukast) or asthma-related symptoms in the preceding year.
The multivariable logistic regression models adjusted for age and potential batch effect, as well as clustering within hospitals (with the use of generalized estimating equations). Odds ratio is calculated per each standard deviation of corresponding metabolite intensity.
Table 5.
Multivariable associations of serum discriminatory metabolites (respiratory syncytial virus vs rhinovirus-C) with clinical outcomes
| Metabolites* | Superpathway | Positive pressure ventilation† | Recurrent wheezing‡ | Asthma§ | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR§ | 95% CI | P-value | OR§ | 95% CI | P-value | OR§ | 95%CI | P-value | ||
| Homoarginine | Amino acid | 0.40 | 0.18–0.89 | 0.02 | 1.94 | 1.06–3.53 | 0.03 | 0.81 | 0.60–1.09 | 0.17 |
| Linoleoylcarnitine (C18:2) | Lipid | 0.62 | 0.31–1.22 | 0.16 | 1.51 | 1.12–2.03 | 0.007 | 1.64 | 1.17–2.29 | 0.004 |
| 9,10-DiHOME | Lipid | 0.91 | 0.48–1.73 | 0.77 | 1.50 | 0.94–2.38 | 0.09 | 1.03 | 0.63–1.67 | 0.91 |
| 1-stearoyl-2-linoleoyl-GPC (18:0/18:2) | Lipid | 0.47 | 0.28–0.78 | 0.004 | 1.00 | 0.67–1.48 | 0.99 | 0.85 | 0.52–1.39 | 0.52 |
| Oxalate (ethanedioate) | Cofactors and vitamins | 0.58 | 0.36–0.94 | 0.03 | 0.94 | 0.65–1.35 | 0.74 | 0.89 | 0.55–1.44 | 0.64 |
| 1-linoleoyl-2-linolenoyl-GPC (18:2/18:3) | Lipid | 0.65 | 0.37–1.14 | 0.13 | 0.89 | 0.58–1.37 | 0.59 | 1.06 | 0.66–1.70 | 0.82 |
| 1-myristoyl-2-arachidonoyl-GPC (14:0/20:4) | Lipid | 0.35 | 0.18–0.68 | 0.002 | 1.01 | 0.71–1.42 | 0.97 | 1.16 | 0.59–2.31 | 0.66 |
| Phenylacetylcarnitine | Peptide | 0.60 | 0.23–1.57 | 0.30 | 0.86 | 0.46–1.61 | 0.64 | 0.99 | 0.48–2.03 | 0.98 |
| 1-stearoyl-2-linoleoyl-GPI (18:0/18:2) | Lipid | 0.39 | 0.23–0.67 | 0.001 | 0.88 | 0.55–1.41 | 0.60 | 1.01 | 0.55–1.85 | 0.98 |
| 1-(1-enyl-stearoyl)-2-linoleoyl-GPE (P-18:0/18:2) | Lipid | 0.34 | 0.17–0.67 | 0.002 | 1.04 | 0.56–1.95 | 0.89 | 0.71 | 0.46–1.09 | 0.12 |
| Isobutyrylcarnitine (C4) | Amino acid | 0.32 | 0.09–1.16 | 0.08 | 1.04 | 0.55–1.96 | 0.90 | 0.98 | 0.58–1.64 | 0.93 |
| Sphingomyelin (d18:1/21:0, d17:1/22:0, d16:1/23:0) | Lipid | 0.71 | 0.45–1.12 | 0.14 | 1.23 | 0.87–1.72 | 0.24 | 1.09 | 0.71–1.67 | 0.70 |
| Beta-sitosterol | Lipid | 0.74 | 0.45–1.22 | 0.24 | 1.56 | 1.08–2.25 | 0.02 | 0.98 | 0.71–1.34 | 0.88 |
| Leucylalanine | Peptide | 1.50 | 0.69–3.25 | 0.31 | 1.32 | 0.86–2.04 | 0.21 | 0.48 | 0.32–0.73 | <0.001 |
| 12,13-DiHOME | Lipid | 0.47 | 0.16–1.36 | 0.16 | 1.15 | 0.67–1.96 | 0.61 | 0.94 | 0.67–1.34 | 0.75 |
| Aconitate | Energy | 0.56 | 0.24–1.29 | 0.17 | 1.28 | 0.91–1.79 | 0.16 | 0.89 | 0.49–1.64 | 0.72 |
| Dihomo-linoleoylcarnitine (C20:2) | Lipid | 0.72 | 0.44–1.20 | 0.21 | 0.98 | 0.71–1.35 | 0.90 | 1.36 | 0.96–1.94 | 0.08 |
| Indolebutyrate | Amino acid | 3.81 | 1.58–9.20 | 0.003 | 0.90 | 0.47–1.73 | 0.76 | 0.92 | 0.56–1.49 | 0.72 |
| Quinolinate | Cofactors and vitamins | 1.05 | 0.75–1.46 | 0.79 | 1.08 | 0.61–1.93 | 0.79 | 1.26 | 0.83–1.90 | 0.28 |
| 1-palmitoleoyl-GPC (16:1) | Lipid | 0.95 | 0.60–1.53 | 0.84 | 0.89 | 0.51–1.54 | 0.68 | 1.12 | 0.87–1.44 | 0.38 |
Abbreviations: CI, confidence interval; OR, odds ratio; GPC, glycerophosphorylcholine; GPI, glycosylphosphatidylinositol; GPE, glycerophosphorylethanolamine
Bold results are statistically significant.
The discriminatory metabolites that have a false discovery rate of <0.05 in the virus-metabolite model (Table 3) are selected
Defined as use of invasive and/or non-invasive mechanical ventilation (e.g., continuous positive airway pressure ventilation).
Defined by parental report of at least two corticosteroid-requiring breathing problems in six months or at least four wheezing episodes in one year that last at least one day and affect sleep.
Defined by physician-diagnosis of asthma by age five years, plus either asthma medication use (e.g., albuterol inhaler, inhaled corticosteroids, montelukast) or asthma-related symptoms in the preceding year.
The multivariable logistic regression models adjusted for age and potential batch effect, as well as clustering within hospitals (with the use of generalized estimating equations). Odds ratio is calculated per each standard deviation of corresponding metabolite intensity.
DISCUSSION
In this multicenter prospective cohort of infants with severe bronchiolitis, we tested for respiratory viruses (and their species) and comprehensively profiled the serum metabolome in a subset of 113 children. We found that overall serum metabolomic profiles differed by infecting virus. We also identified that discriminatory metabolites were primarily involved in lipid metabolism (e.g., sphingolipids and carnitines) in the RSV—RV-A comparison, and arginine and other lipid mediators (e.g., glycerophospholipids) in the RSV—RV-C comparison. The causal structure learning approach indicated that virus infections perturbated these metabolites (not vice versa). Additionally, these discriminatory metabolites were also significantly associated with the risk of acute and chronic morbidities of bronchiolitis. In contrast, there was no significant relation between the intensity of serum (systemic) discriminatory metabolites and those in nasopharyngeal airway (local) metabolites. To the best of our knowledge, this is the first investigation that has examined the interrelations between respiratory viruses, host systemic metabolism, and clinical outcomes in children with bronchiolitis.
For decades, bronchiolitis has been considered a single disease with similar mechanisms and clinical characteristics.2 However, in agreement with the current study, growing evidence in infants with bronchiolitis supports between-virus differences in clinical presentation,22 acute and chronic morbidity,5–7 upper airway microbiome,9,13,14 microRNA,8 transcriptome,9 cytokine,10 and metabolome11,12 profiles. In prior analyses without RV-species level resolution, nasopharyngeal11 and circulating12 metabolome profiles differed between RSV and RV infection. Adddtionally, our group previously reported between-virus differences in the nasopharyngeal metabolome.23 For example, in the RSV vs. RV-A comparison, the discriminatory nasopharyngeal metabolites consisted mainly of nucleic and amino acids (such as guanine and cysteine-glutathione disulfide). Interestingly, these discriminatory metabolites found in the upper airway were uniquely different from the serum discriminatory metabolites in the current study, suggesting that these infection-induced host local and systemic responses may involve different downstream molecules in infants with bronchiolitis. The present study with RV species-level characterization on the serum (circulating) metabolome corroborates these prior reports, and extends them by comprehensively examining the relationship of respiratory virus (and species) with host systemic response in infants with severe bronchiolitis and demonstrating their relationships with both acute and chronic morbidities.
The mechanisms underlying these interrelations remain to be elucidated. In the RSV—RV-A comparison, sphingolipids and carnitines were discriminatory metabolites that are associated with differential risks of acute and chronic morbidities. Sphingolipids are not only major structural component of cellular membranes but also lipid mediators that play important roles in immune response and inflammation.24 Consistent with our observations, research reported that sphingolipid metabolism is associated with bronchiolitis severity16 and childhood asthma (e.g., ORMDL3 within the 17q21 locus regulating de novo sphingolipid synthesis).25 A recent study using human epithelial cell models showed that a ORMDL3 knockdown inhibits RV-A16 replication, endoplasmic reticulum stress, and IFN-β expression.26 Furthermore, previous research using newborn screening data also reported associations of acylcarnitines—metabolites that play critical role in fatty acid metabolism—with risks of wheezing by age 3 years.27 Another experimental model also reported that RV-A infection perturbates both sphingolipid and carnitine levels, thereby leading to infection-induced metabolic repogramming.28
In the RSV—RV-C comparison, our data demonstrated that glycerophospholipids from the plasmalogen pathway metabolites (e.g., GPE) were not only identified as discriminatory metabolites but also associated with lower acute disease severity. Consistently, plasmalogens are known to function as anti-inflammatory mediators and have protective effects against oxidation of surfactant lipids.29 Additionally, studies reported decreased levels of plasmalogens in premature infants with bronchopulmonary dysplasia.30 We also found the most-discriminatory metabolite—homo-arginine—was associated with a higher risk of chronic outcome. Arginine metabolism via the arginase and nitric oxide synthase pathways is important in maintenance of airway tone and responsiveness in asthma pathobiology.31 Altered arginine metabolism leads to nitric oxide deficiency and airway dysfunction in patients with asthma.31
The current study has potential limitations. First, the study did not have “healthy controls.” However, the study objective was not to compare metabolomic profiles between healthy infants and those with bronchiolitis, but rather to examine the relations of respiratory viruses with serum metabolome within infants with bronchiolitis. Second, the metabolome was profiled at a single time point. Yet, the findings using the data in the early course of bronchiolitis are biologically and clinically significant. Third, pre-hospitalization treatments (e.g., systemic corticosteroids, antibiotics) might have altered some of the measured metabolites. However, the proportion of these treatments were not different between the virus groups. Therefore, it is unlikely that they served as confounders. Fourth, as with any observational study, causal inference may be confounded by unmeasured factors, such as nutrition and between-hospital differences in intensive care resources. However, we statistically accounted for patient clustering at the hospital level. Fifth, although we applied approaches to minimize overfitting of data (e.g., via Lasso penalization and cross-validations), an external validation is warranted. Lastly, while the cohort consisted of a racially/ethnically-diverse multicenter sample, the inferences may not be generalizable beyond infants hospitalized for bronchiolitis. Regardless, our data are directly relevant to >100,000 infants with severe bronchiolitis in the U.S. each year.3
Conclusions
Based on the multicenter prospective data of infants with severe bronchiolitis, we found that, compared to RSV-only infection, serum metabolomic signatures differed in infants with RV-A or RV-C infection. The discriminatory metabolites—primarily involved in lipid metabolism—were associated with acute and chronic morbidities of bronchiolitis. Our data facilitate further investigations into the complex interrelations between respiratory viruses (including their species) and host systemic metabolism as well as their integrated contributions to clinical outcomes. For clinicians, our inferences lend additional support to the growing evidence that bronchiolitis is not a homogeneous disease condition. Furthermore, our observations should also offer new avenues for bronchiolitis management (e.g., modification of metabolic pathways) as well as primary prevention strategies of childhood asthma (e.g., through early identification of infants at high risk for asthma) in this large population.
Supplementary Material
Impact statement:
Little had been known about the interrelationships between rhinovirus species, host systemic metabolism, and disease pathobiology in infants with bronchiolitis. The current study found that, compared to RSV-only infection, serum metabolome signatures differed in infants with RV-A or -C infection, and that discriminatory metabolites were primarily involved in lipid metabolism (e.g., sphingolipids and carnitines) in the RV-A comparison, and arginine and other lipid mediators (e.g., glycerophospholipids) in the RV-C comparison. These metabolites were also associated with clinical outcomes. Our findings advance research into the complex interrelations between viruses and host systemic response.
Acknowledgments:
We thank the MARC-35 study hospitals and research personnel for their ongoing dedication to bronchiolitis and asthma research (Supplemental Table E1 in the Online Supplement), and Janice A. Espinola, MPH and Ashley F. Sullivan, MS, MPH (Massachusetts General Hospital, Boston, MA) for their many contributions to the MARC-35 study. We also thank Alkis Togias, MD, at the National Institutes of Health (Bethesda, MD) for helpful comments about the study results.
Funding: The current study is supported by grants from the National Institutes of Health (Bethesda, MD): R01 AI-114552, R01 AI-127507, R01 AI-134940, R01 AI-137091, and UG3/UH3 OD-023253. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authorship: M.F. carried out the statistical analysis, drafted the initial manuscript, revised the manuscript, and approved the final manuscript as submitted. C.A.C. and K.H. conceptualized and designed the study, obtained the funding, reviewed and revised the manuscript, and approved the final manuscript as submitted. Y.R. carried out the statistical analysis, reviewed and revised the manuscript, and approved the final manuscript as submitted. Y.A.B., J.E.G., P.A.P. generated virus data, reviewed and revised the manuscript, and approved the final manuscript as submitted. J.M.M. collected the study data, reviewed and revised the manuscript, and approved the final manuscript as submitted.
Abbreviations:
- ARI
acute respiratory infection
- FDR
false discovery rate
- GPC
glycerophosphorylcholine
- GPE
glycerophosphorylethanolamine
- MARC
Multicenter Airway Research Collaboration
- PPV
positive pressure ventilation
- RSV
respiratory syncytial virus
- RV
rhinovirus
- sPLS-DA
sparse partial least squares discriminate analysis
Footnotes
Conflict of interests statement: Dr. Bochkov has a patent Methods of Propagating Rhinovirus C in Previously Unsusceptible Cell Lines with royalties paid, and a patent Adapted Rhinovirus C with royalties paid. Dr. Gern reports personal fees from Regeneron, personal fees and stock options from Meissa Vaccines Inc, personal fees from MedImmune/AstraZeneca and Ena Therapeutics, outside the submitted work. In addition, Dr. Gern has a patent Methods of Propagating Rhinovirus C in Previously Unsusceptible Cell Lines pending, and a patent Adapted Rhinovirus C pending. The other authors have indicated that they have no financial relationships relevant to this article to disclose.
REFERENCES
- 1.Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: The diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474-e1502. [DOI] [PubMed] [Google Scholar]
- 2.Hasegawa K, Dumas O, Hartert T V, et al. Advancing our understanding of infant bronchiolitis through phenotyping and endotyping: Clinical and molecular approaches. Expert Rev Respir Med. 2017;10(8):891–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fujiogi M, Goto T, Yasunaga H, et al. Trends in bronchiolitis hospitalizations in the United States: 2000–2016. Pediatrics. 2019;144(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basnet S, Palmenberg AC, Gern JE. Rhinoviruses and their receptors. Chest. 2019;155(5):1018–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hasegawa K, Mansbach JM, Camargo CA. Infectious pathogens and bronchiolitis outcomes. Expert Rev Anti Infect Ther. 2014;12(7):817–828. [DOI] [PubMed] [Google Scholar]
- 6.Hasegawa K, Mansbach JM, Bochkov YA, et al. Association of rhinovirus C bronchiolitis and immunoglobulin E sensitization during infancy with development of recurrent wheeze. JAMA Pediatr. 2019;173(6):544–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dumas O, Hasegawa K, Mansbach JM, Sullivan AF, Piedra PA, Camargo CA. Severe bronchiolitis profiles and risk of recurrent wheeze by age 3 years. J Allergy Clin Immunol. 2019;143(4):1371–1379.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hasegawa K, Hoptay CE, Epstein S, et al. RSV bronchiolitis versus rhinovirus: Difference in nasal airway microRNA profiles and NFκB signaling. Pediatr Res. 2018;83(3):606–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujiogi M, Camargo CA Jr, Bernot JP, et al. In infants with severe bronchiolitis: Dual-transcriptomic profiling of nasopharyngeal microbiome and host response. Pediatr Res. January 2020: 10.1038/s41390-019-0742-0748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hasegawa K, Hoptay CE, Harmon B, et al. Association of type 2 cytokines in severe rhinovirus bronchiolitis during infancy with risk of developing asthma: A multicenter prospective study. Allergy Eur J Allergy Clin Immunol. 2019;74(7):1374–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stewart CJ, Hasegawa K, Wong MC, et al. Respiratory syncytial virus and rhinovirus bronchiolitis are associated with distinct metabolic pathways. J Infect Dis. 2018;217(7):1160–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stewart CJ, Mansbach JM, Piedra PA, Toivonen L, Camargo CA, Hasegawa K. Association of respiratory viruses with serum metabolome in infants with severe bronchiolitis. Pediatr Allergy Immunol. 2019;30(8):848–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toivonen L, Camargo CA, Gern JE, et al. Association between rhinovirus species and nasopharyngeal microbiota in infants with severe bronchiolitis. J Allergy Clin Immunol. 2019;143(5):1925–1928.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosas-Salazar C, Shilts MH, Tovchigrechko A, et al. Differences in the nasopharyngeal microbiome during acute respiratory tract infection with human rhinovirus and respiratory syncytial virus in infancy. J Infect Dis. 2016;214(12):1924–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stewart CJ, Mansbach JM, Ajami NJ, et al. Serum metabolome is associated with the nasopharyngeal microbiota and disease severity among infants with bronchiolitis. J Infect Dis. 2019;219(12):2005–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stewart CJ, Mansbach JM, Wong MC, et al. Associations of nasopharyngeal metabolome and microbiome with severity among infants with bronchiolitis: A multiomic analysis. Am J Respir Crit Care Med. 2017;196(7):882–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu X, Holloway B, Dare RK, et al. Real-time reverse transcription-PCR assay for comprehensive detection of human rhinoviruses. J Clin Microbiol. 2008;46(2):533–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bochkov YA, Grindle K, Vang F, Evans MD, Gern JE. Improved molecular typing assay for rhinovirus species A, B, and C. J Clin Microbiol. 2014;52(7):2461–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.US Department of Health and Human Services. National asthma education and prevention program: Expert panel report 3 (EPR 3): Guidelines for the diagnosis and management of asthma (NIH publication 08– 4051). Bethesda, MD: Natl Institutes Heal; 2007:11. [Google Scholar]
- 20.Camargo CA, Ingham T, Wickens K, et al. Cord-blood 25-hydroxyvitamin D levels and risk of respiratory infection, wheezing, and asthma. Pediatrics. 2011;127(1). [DOI] [PubMed] [Google Scholar]
- 21.Rohart F, Gautier B, Singh A, Le Cao K-A. mixOmics: An R package for ‘omics feature selection and multiple data integration. PLoS Comput Biol. 2017;13(11):e1005752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia CG, Bhore R, Soriano-Fallas A, et al. Risk factors in children hospitalized with RSV bronchiolitis versus non-RSV bronchiolitis. Pediatrics. 2010;126(6):e1453-e1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fujiogi M, Camargo CAJ, Raita Y, et al. Association of rhinovirus species with nasopharyngeal metabolome in bronchiolitisinfants: A multicenterstudy. Allergy. April 2020: 10.1111/all.14326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sturgill JL. Sphingolipids and their enigmatic role in asthma. Adv Biol Regul. 2018;70:74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.lişkan M, Bochkov YA, Kreiner-Mløler E, et al. Rhinovirus wheezing illness and genetic risk of childhood-onset asthma. N Engl J Med. 2013;368(15):1398–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y, Bochkov YA, Eickhoff JC, et al. Orosomucoid like 3 (ORMDL3) supports rhinovirus replication in human epithelial cells. Am J Respir Cell Mol Biol. 2020; 10.1165/rcmb.2019-0237OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Donovan BM, Ryckman KK, Breheny PJ, et al. Association of newborn screening metabolites with risk of wheezing in childhood. Pediatr Res. 2018;84(5):619–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gualdoni GA, Mayer KA, Kapsch AM, et al. Rhinovirus induces an anabolic reprogramming in host cell metabolism essential for viral replication. Proc Natl Acad Sci U S A. 2018;115(30):E7158-E7165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Braverman NE, Moser AB. Functions of plasmalogen lipids in health and disease. Biochim Biophys Acta - Mol Basis Dis. 2012;1822(9):1442–1452. [DOI] [PubMed] [Google Scholar]
- 30.Rüdiger M, Von Baehr A, Haupt R, Wauer RR, Rüstow B. Preterm infants with high polyunsaturated fatty acid and plasmalogen content in tracheal aspirates develop bronchopulmonary dysplasia less often. Crit Care Med. 2000;28(5):1572–1577. [DOI] [PubMed] [Google Scholar]
- 31.Scott JA, Grasemann H. Arginine metabolism in asthma. Immunol Allergy Clin North Am. 2014;34(4):767–775. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.